Abstract
The long-chain omega-3 polyunsaturated (n-3 PUFA), eicosapentaenoic (EPA) and docosahexaenoic acid (DHA), may have anti-inflammatory effects. We evaluated the dose-response effect of EPA+DHA supplementation on circulating TNF-α, IL-6, and CRP and explored associations between red blood cell (RBC) membrane PUFA content and TNF-α, IL-6, and CRP. Young adults with low fish intake (n = 116) received one of five doses (0, 300, 600, 900, or 1,800 mg/d EPA+DHA) for 5 months. There were no significant effects of supplemental EPA+DHA on IL-6 or CRP; however, there was a marginal treatment effect for TNF-α (p < 0.08). At baseline, higher quartiles of RBC DHA were associated with lower TNF-α (p = 0.001); higher quartiles of arachidonic acid were associated with higher TNF-α (p = 0.005). EPA+DHA supplementation had no dose-response effect on TNF-α, IL-6, or CRP in healthy young adults; however, associations between inflammatory markers and RBC PUFA warrant further investigation.
Keywords: eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), docosapentaenoic acid (DPA), omega-3, inflammation, C-reactive protein (CRP)
1. INTRODUCTION
Chronic inflammatory diseases are increasingly prevalent and typically managed by costly medical therapies that have significant side effects. The important role of diet in reducing low grade inflammation and thereby modulating the risk of chronic diseases, including cardiovascular disease (CVD), is supported by observational data [1]. In particular, the potential anti-inflammatory effect of increased dietary long-chain omega-3 (n-3) PUFA—specifically eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)—is demonstrated by mechanistic research [2] and population studies in which higher EPA+DHA intake is associated with lower levels of inflammation [3-6]. However, randomized controlled trials with n-3 PUFA supplementation have yielded mixed results in terms of both inflammatory markers and CVD outcomes [7-16].
Clinically evaluating the effects of n-3 PUFA interventions on inflammatory status is difficult due to considerable acute variations in inflammatory markers caused by factors unrelated to the study intervention. Blood concentrations of the pro-inflammatory cytokines tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6), as well as the acute phase reactant, C-reactive protein (CRP), are used as markers of systemic inflammatory status because they provide quantitative assessments of ongoing inflammatory processes [17, 18]. However, acute elevations in these markers can be caused by unavoidable stimuli in the daily lives of research participants, including unreported acute illness [19], changes in physical activity [20], and psychosocial stressors [21]. This poses a significant challenge for evaluating the effects of interventions by introducing extreme changes in inflammatory markers that are not due to treatment effects. Excluding these acutely elevated values according to pre-defined thresholds may enable more accurate assessment of the relationship between n-3 PUFA and chronic inflammation.
Another approach to evaluating links between PUFA and inflammation is utilizing blood biomarkers of fatty acid intake [22]. The EPA+DHA content of the RBC membrane is a useful biomarker of dietary n-3 PUFA intake as it reflects dietary intake over the course of the RBC lifespan (~120 d) and correlates with concentrations in tissues and other cells [23-25]. Inverse associations between RBC n-3 PUFA content and inflammatory marker concentrations have been reported [26-28]. However, the relationships between the content of individual n-3 and n-6 PUFA in RBC membranes and concentrations of circulating inflammatory markers is not completely understood. Additionally, whether changes in RBC PUFA relate to changes in inflammatory status has not been determined. Despite this, fish oil supplementation across a range of non-pharmacological doses (< 2 g/d) is commonly used in the U.S. for anti-inflammatory purposes [29, 30]. Understanding the links between supplemental n-3 PUFA intake, tissues levels, and markers of inflammation is important given inadequate EPA+DHA intake in the U.S. and the increasing burden of chronic inflammatory diseases.
We recently reported that EPA+DHA supplementation dose-dependently increased RBC EPA+DHA content and that additional factors, such as body weight and baseline RBC EPA+DHA, further explained variability in the RBC EPA+DHA response [31]. In the present study, we evaluated the dose-response effects of 0, 300, 600, 900, and 1800 mg/d EPA+DHA on circulating markers of inflammation (TNF-α, IL-6, and CRP) in healthy young adults. We also explored the relationships between both the baseline content and post-supplementation changes in RBC n-3 and n-6 PUFA content with circulating inflammatory marker concentrations, and assessed the potential of n-3 PUFA supplementation to alter circulating white blood cell populations as an additional immunoregulatory effect [32].
2. METHODS
2.1. Study design and intervention
Healthy young adults (n = 125) between the ages of 20-45 y and with BMI between 20-30 kg/m2 reporting no or low habitual oily fish consumption (<4 servings per month) and not taking n-3 PUFA supplements were recruited. Additional exclusion criteria included use of n-3 PUFA supplemented foods in the past 3 months, history of diabetes, serious medical conditions, smoking, chronic anti-inflammatory medications, planning to change dietary habits, and pregnant, nursing, or planning a pregnancy. Details of the study recruitment and screening have been reported previously [31]. The study was approved annually by the Pennsylvania State University Institutional Review Board.
Each participant was randomly assigned to one of five doses (0, 300, 600, 900, 1800 mg) daily of EPA+DHA as fish oil supplements, using soybean oil as the placebo (Nordic Naturals, Watsonville, CA) for approximately 5 months. The intervention provided nutritionally-achievable doses of EPA+DHA in triglyceride form equivalent to that which could be obtained by consumption of oily fish. The fatty acid profile of the supplements has been described in detail [31]. The fish oil capsules contained approximately 20% EPA, 2% docosapentaenoic acid (DPA), and 13% DHA. Participants agreed to maintain their weight, activity level, usual (limited) fish consumption, and not take any other n-3 PUFA supplements during the study. Daily log sheets and monthly check-ins were completed to verify compliance. Participants returned to the Pennsylvania State University Clinical Research Center every 8 weeks to receive new supplies, return empty containers, and provide completed log sheets.
2.2. Blood sample collection
At the beginning of the study and after the treatment period, participants reported to the Clinical Research Center after a 12-h overnight fast to provide a blood sample by venipuncture. Whole blood was centrifuged at 1500 × g for 15 min at 4°C. Serum was collected and stored at −80 °C until they were analyzed, except for the complete blood count (CBC), which was measured in EDTA anticoagulated whole blood samples using a Coulter LH 750 with VCS technology (Quest Diagnostics, Pittsburgh, PA). Red blood cells (RBC) were collected following separation from plasma by centrifugation and frozen at −80 °C until analyzed. Fatty acid analysis was performed as described previously [31].
2.3. Inflammatory marker concentrations
Serum concentrations of TNF-α and IL-6 were measured using high-sensitivity ELISA kits (R&D Systems, Minneapolis, MN) in duplicate (CV <10%). Serum high-sensitivity CRP was measured by latex-enhanced immunonephelometry (Quest Diagnostics; assay CV < 8%).
2.4. Statistical analysis
Statistical analyses were performed using Minitab (version 16.2, Minitab, State College, PA). Analytes that were assayed in duplicate (i.e., TNF-α and IL-6) were averaged before analysis. Fit statistics were assessed for each variable to identify any outliers and to test assumptions of normality. Subjects with acute inflammation (i.e., baseline or endpoint TNF-α and IL-6 values >3.0 ng/L or CRP values >3.0 mg/L), were identified as outliers and secondarily removed from the analysis to ensure that analysis was performed on samples from the target population of non-inflamed adults [33, 34]. A natural log transformation was applied to baseline and endpoint values of IL-6 and CRP because of non-normal distribution (skew > 1), and further analysis of associations and treatment effects were performed on the transformed values.
Independent two-sample t tests were used to assess sex differences in baseline inflammatory marker concentrations. Associations between baseline inflammatory marker concentrations and BMI, body weight, blood pressure, and age were assessed using Pearson correlation tests.
General linear models were used to test the effects of treatment on inflammatory marker concentrations and CBC measures. Baseline values were included as covariates. We present 4 models for the effect of supplementation on TNF-α, IL-6, and CRP concentrations: unadjusted (model 1), adjusted for baseline value (model 2), adjusted for baseline value, sex, and age (model 3), and adjusted for baseline value, sex, age, blood pressure, and body weight (model 4). Tukey-adjusted p-values were used for post-hoc comparisons between treatment groups, with adjusted p < 0.05 considered significant.
Baseline RBC membrane content of n-3 PUFA (alpha-linolenic acid [ALA], EPA, DPA, DHA) and n-6 PUFA (linoleic acid [LA], arachidonic acid [AA]) were assessed as quartiles and compared with circulating inflammatory marker concentrations using ANOVA. Tukey-adjusted p-values were used for post hoc comparisons between quartiles. Multiple regression models, with baseline inflammatory markers as the response variable and RBC PUFA as predictor variables, also were conducted to test for the combined effects of RBC PUFA on baseline inflammatory marker concentrations and to guard against spurious findings in the models that examined the relationships between baseline inflammatory marker concentrations and single RBC PUFA quartiles. Changes in RBC membrane content were compared with changes in inflammatory marker concentrations using Pearson correlation tests. Change scores were calculated as the end of supplementation value minus baseline value. Scatterplots were generated to illustrate exploratory analyses of continuous relationships between baseline RBC PUFA content and inflammatory marker concentrations with Pearson correlation coefficients and unadjusted p-values reported for each comparison.
3. RESULTS
3.1. Participant characteristics
The study design and flow of participants have been reported previously [31]. Of the 125 participants, nine withdrew from the study, leaving 116 participants who completed the study (mean compliance: 97%, range 85-100%). Two additional participants were removed from the analysis due to having either very high RBC EPA+DHA content at study entry (>8%) or an underlying health condition (leukopenia). Elevated baseline TNF-α (n=4), IL-6 (n=2), or CRP (n=12) data were excluded in order to limit our analyses to healthy non-inflamed adults and remove statistical outliers. Remaining subjects with elevated endpoint TNF-α (n=0), IL-6 (n=3), or CRP (n=4) were excluded from the treatment effect and change score analyses.
There were no significant differences between the treatment groups at baseline with respect to inflammatory marker concentrations and white blood cell populations (Table 1), RBC and platelet measures (Supplemental Table 1), or n-3 and n-6 PUFA content of RBC membranes [31]. The mean RBC EPA+DHA content at study entry (± SEM) was 4.3 ± 0.1%. Men had higher baseline TNF-α concentrations compared with women (1.5 ± 0.1 ng/L vs 1.2 ± 0.1 ng/L, p < 0.001), whereas women had higher CRP concentrations than men (1.1 ± 0.1 mg/L vs 0.6 ± 0.1 mg/L, p = 0.001). Individuals with higher baseline TNF-α also had higher body weight, diastolic blood pressure, and systolic blood pressure (p ≤ 0.001 for all), whereas CRP was associated with higher BMI (p = 0.009) (Supplemental Table 2). There were no significant associations between age and baseline inflammatory marker concentrations.
Table 1.
Baseline white blood cell count and inflammatory marker concentrations
| EPA+DHA |
|||||
|---|---|---|---|---|---|
| 0 mg/d (n=23) |
300 mg/d (n=23) |
600 mg/d (n=21) |
900 mg/d (n=24) |
1800 mg/d (n=23) |
|
| White blood cells, cells/μL a |
5400 ± 280 | 5630 ± 180 | 6000 ± 320 | 5800 ± 220 | 5800 ± 240 |
| Neutrophils | 3080 ± 230 | 3130 ± 150 | 3380 ± 270 | 3230 ± 160 | 3240 ± 170 |
| Lymphocytes | 1800 ± 90 | 1910 ± 120 | 1870 ± 80 | 1940 ± 100 | 1880 ± 100 |
| Monocytes | 395 ± 25 | 421 ± 19 | 480 ± 29 | 429 ± 23 | 454 ± 27 |
| Eosinophils | 133 ± 16 | 154 ± 26 | 168 ± 29 | 168 ± 30 | 158 ± 26 |
| Basophils | 22.2 ± 2.0 | 23.0 ± 2.7 | 28.1 ± 3.8 | 29.6 ± 3.3 | 20.4 ± 2.4 |
| Inflammatory markers | |||||
| TNF-α, ng/L b | 1.4 (1.1, 1.6) | 1.4 (1.3, 1.5) | 1.3 (1.1, 1.5) | 1.4 (1.2, 1.5) | 1.5 (1.2, 1.6) |
| IL-6, ng/L c | 0.9 (0.7, 1.1) | 0.9 (0.7, 1.1) | 1.0 (0.8, 1.2) | 0.9 (0.7, 1.1) | 1.0 (0.8, 1.2) |
| CRP, mg/L d | 0.5 (0.3, 0.8) | 0.6 (0.4, 1.1) | 0.5 (0.3, 0.6) | 0.6 (0.4, 1.0) | 0.6 (0.4, 0.9) |
Groups were not significantly different for any of these metrics at baseline (p for group effect >0.05). CRP, C-reactive protein; IL-6, interleukin-6; TNF- α, tumor necrosis factor-alpha.
Values are means ± SEM. To convert white blood cells (neutrophils, lymphocytes, monocytes, eosinophils, and basophils) from cells/μL to 109/L, multiple by 0.001.
N=110, means (95% CI).
N=112, geometric means (95% CI).
N=102, geometric means (95% CI).
3.2. Effect of supplementation on inflammatory markers
Following supplementation, there were no significant differences between treatment groups with respect to IL-6 or CRP concentrations (Table 2); however, a marginally significant treatment effect was observed for TNF-α in the adjusted models (p < 0.08). The 1,800 mg/d group experienced a 10% reduction in TNF-α concentration from baseline; however, no group was significantly different from placebo (Table 2).
Table 2.
Effects of treatment on inflammatory marker concentrations
| Inflammatory marker |
0 mg/d | 300 mg/d | 600 mg/d | 900 mg/d | 1800 mg/d | P value |
|---|---|---|---|---|---|---|
| TNF-α, ng/L a | ||||||
| Model 1 | 1.36 (1.20, 1.53 ) | 1.40 (1.23,1.57 ) | 1.38 (1.20, 1.56) | 1.39 (1.23, 1.55) | 1.32 (1.15, 1.49) | 0.95 |
| Model 2 | 1.38 (1.28, 1.47) | 1.38 (1.29, 1.47) | 1.43 (1.33, 1.52) | 1.41 (1.32, 1.49) | 1.26 (1.17, 1.35) | 0.07 |
| Model 3 | 1.38 (1.29, 1.47) | 1.39 (1.30, 1.48) | 1.42 (1.33, 1.51) | 1.41 (1.32, 1.49) | 1.26 (1.17, 1.35) | 0.08 |
| Model 4 | 1.39 (1.30, 1.47) | 1.39 (1.30, 1.48) | 1.42 (1.33, 1.52) | 1.41 (1.33, 1.50) | 1.24 (1.14, 1.34) | 0.04 |
| IL-6, ng/L b | ||||||
| Model 1 | 0.96 (0.80, 1.15) | 0.78 (0.65, 0.95) | 1.05 (0.86, 1.27) | 1.00 (0.82, 1.21) | 1.01 (0.84, 1.21) | 0.21 |
| Model 2 | 0.97 (0.81, 1.15) | 0.80 (0.67, 0.96) | 1.03 (0.86, 1.24) | 0.99 (0.84, 1.18) | 0.98 (0.83, 1.17) | 0.23 |
| Model 3 | 0.97 (0.81, 1.15) | 0.81 (0.67, 0.97) | 1.03 (0.86, 1.23) | 0.99 (0.84, 1.18) | 0.98 (0.83, 1.17) | 0.24 |
| Model 4 | 0.98 (0.82, 1.16) | 0.82 (0.68, 0.98) | 1.03 (0.85, 1.23) | 1.00 (0.85, 1.19) | 0.95 (0.80, 1.13) | 0.37 |
| CRP, mg/L c | ||||||
| Model 1 | 0.50 (0.34, 0.73) | 0.42 (0.29, 0.63) | 0.48 (0.33, 0.71) | 0.47 (0.31, 0.70) | 0.44 (0.30, 0.65) | 0.97 |
| Model 2 | 0.52 (0.40, 0.67) | 0.41 (0.31, 0.53) | 0.54 (0.41, 0.70) | 0.45 (0.33, 0.60) | 0.42 (0.31, 0.55) | 0.45 |
| Model 3 | 0.52 (0.40, 0.68) | 0.41 (0.31, 0.54) | 0.54 (0.41, 0.71) | 0.45 (0.33, 0.60) | 0.41 (0.31, 0.54) | 0.42 |
| Model 4 | 0.52 (0.40, 0.68) | 0.44 (0.33, 0.58) | 0.54 (0.41, 0.70) | 0.47 (0.35, 0.64) | 0.36 (0.27, 0.48) | 0.30 |
Model 1 includes unadjusted endpoint values. Model 2 includes treatment adjusted for baseline value. Model 3 includes treatment adjusted for baseline value, sex, and age; Model 4 includes treatment adjusted for baseline value, sex, age, blood pressure, and body weight. P values are for the main effect of treatment. CRP, C-reactive protein; IL-6, interleukin-6; TNF-α, tumor necrosis factor-alpha.
N=110, least squares mean (95% CI).
N=109, geometric means (95% CI).
N=98, geometric means (95% CI).
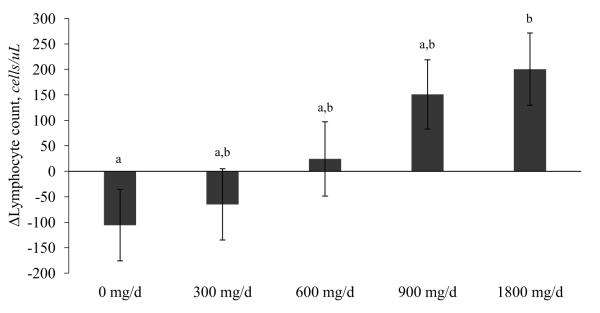
There were no significant treatment effects on CBC measures with the exception of lymphocyte concentrations (Supplemental Table 3). The 1,800 mg/d group had a 17% higher lymphocyte concentration following supplementation compared to the placebo (Tukey-adjusted p = 0.02; Figure 1).
Figure 1.
Changes in lymphocyte concentrations following 5 months of EPA+DHA supplementation. Values are means ± SEM (n=114). Values with different letters are significantly different, p < 0.05 (Tukey-adjusted values for post hoc tests).
3.3. Associations between RBC content of PUFA and circulating inflammatory markers
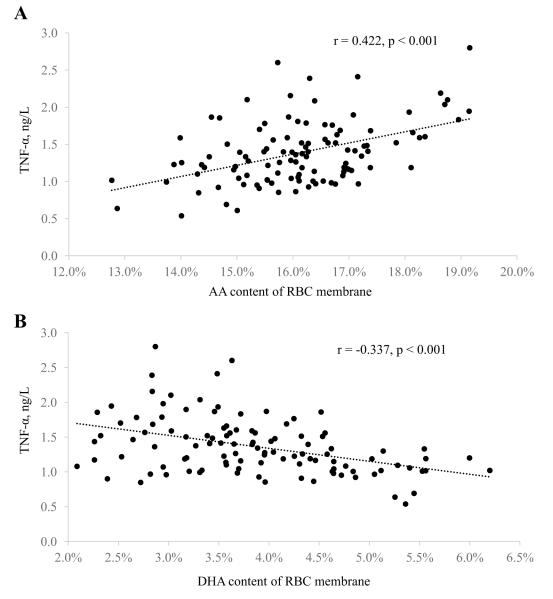
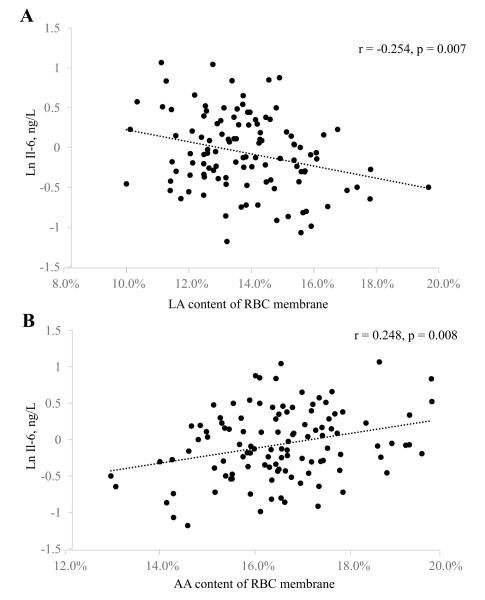
Baseline inflammatory marker concentrations were compared between quartiles of n-3 and n-6 PUFA content in RBC membranes (Table 3). There were no significant differences in any inflammatory marker concentrations across quartiles of ALA or EPA content. Higher quartiles of DHA content were associated with lower TNF-α concentrations (p = 0.001), whereas higher quartiles of AA content were associated with higher TNF-α concentrations (p = 0.005) (Table 3; Figure 2). We also found that higher (highest vs. lowest quartiles) LA levels were associated with 29% lower IL-6 concentrations (Table 3; Figure 3). Furthermore, participants in the lowest DPA quartile had approximately twice the CRP concentrations of participants in all other DPA quartiles. These findings were consistent with the multivariable regression models (Supplemental Table 4).
Table 3.
Inflammatory marker concentrations at baseline by quartiles of selected RBC fatty acids
| Quartiles of RBC fatty acid content |
P value | ||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||
| ALA, 18:3 n-3 | |||||
| TNF-α,ng/L 1 | 1.54 (1.37, 1.70) | 1.38 (1.22, 1.55) | 1.29 (1.12, 1.45) | 1.33 (1.16, 1.49) | 0.14 |
| IL-6, ng/L 2 | 1.01 (0.84, 1.22) | 0.99 (0.86, 1.13) | 0.83 (0.67, 1.04) | 0.94 (0.78, 1.12) | 0.74 |
| CRP, mg/L 3 | 0.43 (0.31, 0.60) | 0.52 (0.39, 0.70) | 0.60 (0.42, 0.87) | 0.73 (0.50, 1.07) | 0.15 |
|
| |||||
| EPA, 20:5 n-3 | |||||
| TNF-α, ng/L | 1.47 (1.31, 1.63) | 1.32 (1.20, 1.44) | 1.46 (1.30, 1.62) | 1.27 (1.15, 1.39) | 0.21 |
| IL-6, ng/L | 0.97 (0.79, 1.19) | 0.94 (0.79, 1.12) | 0.92 (0.77, 1.10) | 0.93 (0.77, 1.11) | 0.98 |
| CRP, mg/L | 0.66 (0.44, 0.99) | 0.46 (0.35, 0.61) | 0.67 (0.48, 0.93) | 0.49 (0.34, 0.69) | 0.26 |
|
| |||||
| DPA, 22:5 n-3 | |||||
| TNF-α, ng/L | 1.37 (1.20, 1.54) | 1.32 (1.16, 1.49) | 1.36 (1.20, 1.53) | 1.47 (1.30, 1.64) | 0.60 |
| IL-6, ng/L | 1.03 (0.83, 1.28) | 0.85 (0.71, 1.01) | 0.95 (0.80, 1.13) | 0.94 (0.79, 1.11) | 0.50 |
| CRP, mg/L | 1.05 (0.73, 1.53) a | 0.40 (0.29, 0.55) b | 0.43 (0.33, 0.57) b | 0.53 (0.41, 0.71) b | <0.001 |
|
| |||||
| DHA, 22:6 n-3 | |||||
| TNF-α, ng/L | 1.55 (1.39, 1.71) a | 1.48 (1.33, 1.63) a | 1.36 (1.21, 1.51) a,b | 1.13 (0.97, 1.29) b | 0.001 |
| IL-6, ng/L | 0.91 (0.75, 1.09) | 1.05 (0.89, 1.23) | 0.97 (0.80, 1.18) | 0.84 (0.69, 1.02) | 0.34 |
| CRP, mg/L | 0.55 (0.40, 0.75) | 0.57 (0.40, 0.81) | 0.54 (0.38, 0.75) | 0.59 (0.39, 0.89) | 0.98 |
|
| |||||
| Omega-3 index (EPA+DHA) | |||||
| TNF-α, ng/L | 1.56 (1.40, 1.72) a | 1.49 (1.34, 1.65) a | 1.33 (1.18, 1.49) a,b | 1.13 (0.98, 1.29) b | 0.001 |
| IL-6, ng/L | 0.92 (0.76, 1.10) | 0.99 (0.82, 1.19) | 1.02 (0.84, 1.22) | 0.84 (0.70, 1.01) | 0.46 |
| CRP, mg/L | 0.52 (0.37, 0.74) | 0.68 (0.48, 0.96) | 0.55 (0.39, 0.77) | 0.51 (0.36, 0.72) | 0.62 |
|
| |||||
| LA, 18:2 n-6 | |||||
| TNF-α, ng/L | 1.46 (1.29, 1.64) | 1.45 (1.32, 1.58) | 1.41 (1.27, 1.56) | 1.20 (1.09, 1.32) | 0.08 |
| IL-6, ng/L | 1.03 (0.86, 1.24) a | 1.01 (0.84, 1.22) a | 1.02 (0.85, 1.23) a | 0.73 (0.63, 0.84) b | 0.014 |
| CRP, mg/L | 0.55 (0.38, 0.80) | 0.67 (0.47, 0.94) | 0.55 (0.39, 0.78) | 0.48 (0.35, 0.67) | 0.60 |
|
| |||||
| AA, 20:4 n-6 | |||||
| TNF-α, ng/L | 1.18 (1.00, 1.33) a | 1.36 (1.20, 1.52) a,b | 1.39 (1.25, 1.53) a,b | 1.59 (1.41, 1.77) b | 0.005 |
| IL-6, ng/L | 0.79 (0.66, 0.94) | 0.97 (0.80, 1.17) | 0.91 (0.75, 1.10) | 1.11 (0.95, 1.31) | 0.06 |
| CRP, mg/L | 0.55 (0.39, 0.78) | 0.57 (0.40, 0.81) | 0.56 (0.40, 0.79) | 0.56 (0.38, 0.81) | 0.99 |
Quartiles are specific for the content of each fatty acid in RBC membranes reported as percent of total RBC fatty acids. P values are for trend across quartiles calculated using ANOVA. Values with different superscript letters are significantly different, p < 0.05 (Tukey-adjusted values for post hoc tests). AA, arachidonic acid; ALA, alpha-linolenic acid; CRP, C-reactive protein; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; DPA, docosapentaenoic acid; LA, linoleic acid; n-3, omega-3; n-6, omega-6; Q, quartile; RBC, red blood cell.
n=110, least squares means (95% CI).
n=112, geometric means (95% CI).
n=102, geometric means (95% CI).
Figure 2.
Scatterplots for baseline RBC membrane content of AA (A) and DHA (B) versus circulating TNF-α concentrations in healthy adults (n=110). Pearson correlation coefficients and unadjusted p-values are reported for each comparison. AA, arachidonic acid.
Figure 3.
Scatterplots for baseline RBC membrane content of LA (A) and AA (B) versus circulating IL-6 concentrations in healthy adults (n=112). Pearson correlation coefficients and unadjusted p-values are reported for each comparison. AA, arachidonic acid; LA, linoleic acid.
Changes in inflammatory marker concentrations were compared with changes in n-3 and n-6 PUFA content in RBC membranes following supplementation (Supplemental Table 5). No significant associations were observed between the change in inflammatory marker concentrations versus change in n-3 or n-6 PUFA content. However, we found that changes in lymphocyte concentrations were positively associated with changes in EPA and DPA content following supplementation (p = 0.001 and p = 0.008, respectively; Supplemental Figure 1).
4. DISCUSSION
The primary objective of this study was to determine the effect of supplemental EPA+DHA, across a range of non-pharmacological doses, on circulating inflammatory marker concentrations in healthy adults after 5 months of supplementation. There were no significant effects of EPA+DHA supplementation on IL-6 or CRP concentrations. However, a marginally significant treatment effect was observed for TNF-α due to reductions from baseline following 1,800 mg/d EPA+DHA. More research is needed to evaluate whether TNF-α concentrations may be modestly reduced by high doses of EPA+DHA (i.e., ≥ 1.8 g/d) administered for long periods of time. Although most clinical studies of n-3 PUFA supplementation have not demonstrated reductions in TNF-α, patients with congestive heart failure may experience a benefit [35-37]. Analytical approaches that account for acute elevations in TNF-α may improve the sensitivity of future clinical studies in both healthy individuals and those with specific inflammatory conditions, thereby helping to clarify the relationship between n-3 PUFA supplementation and TNF-α.
We also examined the relationship between RBC n-3 and n-6 PUFA content and inflammatory marker concentrations before and after supplementation to determine whether this provided insight into a more direct biological relationship between tissue PUFA and inflammation. Incorporation of dietary PUFA into membrane phospholipids can affect cell signaling and lipid mediator production [32]. However, the change in RBC n-3 and n-6 PUFA content was not related to changes in TNF-α, IL-6, or CRP concentrations following supplementation. This may be due to the absence of changes in inflammatory markers following the fish oil intervention. Our results may also suggest that RBC fatty acid content, although an important biomarker, may not play a direct role in regulating inflammatory signaling.
Of note is that there were several baseline associations between RBC n-3 PUFA content and inflammatory markers, which supports the modest anti-inflammatory effect of increasing n-3 PUFA intake in healthy adults with low habitual n-3 PUFA intake, suggesting that small differences in dietary n-3 PUFA, especially in sub-optimal ranges, may influence the development of low-grade inflammation. We found that higher RBC content of DHA was associated with lower TNF-α at baseline, while individuals with the lowest DPA content had the highest baseline CRP. Whether low RBC DHA and/or DPA content is a cause or effect of elevated inflammatory markers is unclear; however, this relationship between RBC DHA content and TNF-α lends support to the potential ability of fish oil supplementation to reduce TNF-α suggested by the trend observed in response to 1,800 mg/d supplementation. Dietary and supplemental sources of n-3 PUFA typically contain both EPA and DHA, but their accumulation and retention in tissues varies [38]. The fact that RBC membranes are more responsive to DHA may explain why only RBC DHA content was significantly related to TNF-α [31].
Clinical research on the individual roles of EPA, DPA, and DHA is relatively scarce, and the links between these individual fatty acids and inflammation remain unclear [39]. Inconsistent findings in observational studies of n-3 PUFA and inflammatory markers are likely due to differences in which fatty acid pool was assessed (e.g. plasma vs. RBC membrane), variability in subject population (e.g., age, sex, BMI, etc.) [40, 41], and genetic differences that influence PUFA metabolism and responses to supplementation [42]. Therefore, additional research is needed to prospectively assess the individual effects of EPA vs. DHA on markers of inflammation before any definitive conclusions can be made about their differential effects. Moreover, the relationship between DPA—which is typically found in animal products— and inflammation needs to be investigated further.
In the present study, the association between n-6 PUFA and inflammatory marker concentrations also varied depending upon the specific fatty acid. Individuals with the highest RBC content of AA had higher TNF-α at baseline compared to those with lower AA content. This may reflect the ability of AA to generate eicosanoids that promote pro-inflammatory cytokine production [43]. In contrast, individuals with the highest LA content had lower baseline IL-6 concentrations compared to those with the lowest LA content. Upon further investigation, we found that baseline LA and AA content were significantly inversely correlated (Supplemental Figure 2). This is particularly interesting since it has been proposed that dietary intake of LA is pro-inflammatory, and thus increases CVD risk, largely based on the fact that LA is a precursor of AA [44, 45]. However, the potential for dietary and tissue LA to influence inflammation remains unclear [46], and additional studies examining the differences between LA and AA in modulating inflammation are needed.
Exploratory analyses revealed several potential relationships between baseline inflammatory marker concentrations and participant characteristics. Being male and have higher body weight was associated with higher TNF-α concentrations, whereas being female and having higher BMI were associated with higher CRP. Gender effects remained significant after accounting for differences in bodyweight and BMI (data not shown), which has been previously reported by others [47-53]. Sex-specific differences in body fat distribution [50, 54] and hormones may account for these differences [50, 55, 56].
We also found that diastolic and systolic blood pressures were positively associated with TNF-α. Several studies have demonstrated an association between elevated TNF-α and hypertension [57-62]. Although mechanistic evidence suggests that TNF-α can directly influence hypertension [63], elevated TNF-α in observational studies could be both a cause and effect of hypertension.
Unexpectedly, we found that 1,800 mg/d EPA+DHA increased circulating lymphocyte concentrations relative to the placebo group. Greater EPA+DHA content in the RBC membrane was also associated with increasing lymphocyte concentrations following supplementation. Although few studies that we are aware of have reported the effects of EPA+DHA on circulating leukocytes, an increase in the number of circulating lymphocytes in healthy adults (n=27) has also been found after 4 weeks of supplementation with fish oil (860 mg/d EPA+DHA) and borage oil (831 mg/d gamma-LA) [64]. While this is counter to some mechanistic research on in vitro cell proliferation, we speculate that it could relate to reduced expression of the cellular adhesion molecules L-selectin and soluble intercellular adhesion molecule-1 (ICAM-1, resulting in reduced lymphocyte adhesion to the endothelium [65, 66] and thus a greater proportion freely circulating in peripheral blood. Lipid mediators formed from n-3 PUFA, which have been shown to influence hematopoietic stem cell and myeloid progenitor cell differentiation, could also potentially explain this finding [67]. However, since we did not differentiate between types of lymphocytes we are unable to determine whether the observed increase in circulating lymphocytes can be attributed to a particular cell type (i.e. T cell, B cell, NK cell). Further clinical research is needed to assess the effects of supplemental n-3 PUFA intake on sub-types of circulating lymphocytes and cellular adhesion molecules.
4.1. Strengths and limitations
The present study had a number of strengths, including the placebo-controlled, double-blind study design comparing five treatment groups, large sample size, well-characterized study cohort, relatively longer duration of supplementation (5 months), and use of a validated biomarker of cell membrane fatty acid content. The study population had a baseline RBC EPA+DHA content that is representative of the average American not taking n-3 PUFA supplements. We instructed participants to reschedule visits if they were experiencing or recovering from any acute illness or injury and secondarily excluded from analysis any participants with elevated inflammatory markers. This analytical approach eliminated highly influential outliers and increased the sensitivity of models to detect effects in our target population of healthy adults. Moreover, the study also had a dropout rate of 7%, which is relatively low considering the duration of the study intervention.
The predominantly Caucasian, young, healthy participant population as well as the absence of dietary assessment are limitations. Population subgroups may have unique responses to supplemental EPA+DHA intake. Thus, future studies should consider identifying racial differences and genetic determinants of n-3 PUFA metabolism. Participants were instructed to limit their intake of n-3 PUFA from food, specifically fatty fish, and continue their habitual diets throughout the duration of the intervention. However, we did not monitor their dietary habits and therefore their fatty acid intake from other foods, as well as non-fatty acid dietary components, could have confounded our findings. Despite this, participants were required to maintain their habitual diets and, thus, dietary changes most likely did not affect the study results.
Lastly, although TNF-α, IL-6, and CRP are well-established circulating inflammatory markers, they provide little information regarding the origin of inflammation. Consequently, we are unable to determine whether the relationships between n-3 and n-6 PUFA content of RBC membranes and these inflammatory markers reflect events originating in certain tissues (e.g., liver, adipose tissue, etc.). Omega-3 PUFA supplementation may also affect serum cytokines not measured in the present study (e.g., IL-2, IL-8, IL-10). Measuring tissue-specific inflammatory markers (e.g. resistin) as well as serum markers beyond TNF-α, IL-6, and CRP would provide greater insight.
4.2. Conclusions
Consumption of nutritionally-achievable doses of EPA+DHA for 5 months had no effect on circulating IL-6 or CRP concentrations in healthy young adults; however, 1,800 mg/d EPA+DHA may exert a modest anti-inflammatory effect as evidenced by a marginally significant reduction in TNF-α. Additional markers of inflammation, including cellular adhesion molecules, need to be examined to better understand the clinical effects of supplemental EPA+DHA intake on inflammation, as well as to clarify the observed increase in lymphocyte concentrations following 1,800 mg/d EPA+DHA. We also found that increased RBC DHA content was associated with lower TNF-α at baseline, whereas low DPA content was associated with higher CRP. In contrast, changes in RBC membrane content of n-3 or n-6 PUFA were not associated with changes in inflammatory marker concentrations following EPA+DHA supplementation, indicating a need for longer term studies and exploration of other biomarkers of fatty acid content (e.g., white blood cells) that may be more directly involved in the regulation of inflammation. Collectively, our findings indicate that EPA+DHA intake, over a range of nutritionally-achievable doses, had no effect on circulating TNF-α, IL-6, or CRP in healthy young adults after 5 months of supplementation. However, despite no relationship between changes in RBC PUFA content and inflammatory markers, the observed baseline associations warrant further investigation.
Supplementary Material
HIGHLIGHTS.
We evaluate the dose-response effect of EPA+DHA on serum inflammatory markers.
We explore associations between RBC PUFA content and inflammatory markers.
EPA+DHA supplementation had no effect on IL-6 or CRP concentrations.
High doses of EPA+DHA (≥ 1.8 g/d) may reduce TNF-α concentrations.
Higher RBC DHA was associated with lower TNF-α concentrations.
Acknowledgments
We thank our research participants for their dedication to the project. We are also grateful to the nursing and clinician staff of the Clinical Research Center, part of The Pennsylvania State University Clinical and Translational Science Institute, which was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1TR000127. This project was funded by a USDA, NIFA, Grant #2009-65200-05973 (P.M.K.E.). This research also was supported in part by an American Oil Chemists’ Society Fellowship Award (M.R.F.) and a Baxter Pharmaceuticals Postdoctoral Fellowship (A.C.S.R.). The study protocol was approved by the Institutional Review Board at the Pennsylvania State University, and registered on ClinicalTrials.gov (NCT01078909). Study capsules were donated by Nordic Naturals, Inc., Watsonville, CA.
FUNDING: This project was funded by a USDA, NIFA, Grant #2009-65200-05973 (P.M.K.E.). This research also was funded in part by an American Oil Chemists’ Society Fellowship Award (M.R.F.) and a Baxter Pharmaceuticals Postdoctoral Fellowship (A.C.S.R.). This study was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, Grant UL1TR000127. The study protocol was approved by the Institutional Review Board at the Pennsylvania State University, and registered on ClinicalTrials.gov (NCT01078909). Study capsules were donated by Nordic Naturals, Inc., Watsonville, CA.
Abbreviations
- AA
arachidonic acid
- ALA
alpha-linolenic acid
- CRP
c-reactive protein
- DHA
docosahexaenoic acid
- DPA
docosapentaenoic acid
- EPA
eicosapentaenoic acid
- IL-6
interleukin-6
- LA
linoleic acid
- n-3
omega-3
- n-6
omega-6
- PUFA
polyunsaturated fatty acid
- TNF-α
tumor necrosis factor alpha
Footnotes
PUBMED: Flock MR, Skulas-Ray AC, Harris WS, Gaugler TL, Fleming JA, Kris-Etherton PM.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Barbaresko J, Koch M, Schulze MB, Nöthlings U. Dietary pattern analysis and biomarkers of low-grade inflammation: a systematic literature review. Nutrition Reviews. 2013;71:511–527. doi: 10.1111/nure.12035. [DOI] [PubMed] [Google Scholar]
- [2].Calder PC. n-3 Fatty acids, inflammation and immunity: new mechanisms to explain old actions. Proceedings of the Nutrition Society. 2013;72:326–336. doi: 10.1017/S0029665113001031. [DOI] [PubMed] [Google Scholar]
- [3].Zampelas A, Panagiotakos DB, Pitsavos C, et al. Fish consumption among healthy adults is associated with decreased levels of inflammatory markers related to cardiovascular disease: the ATTICA study. J Am Coll Cardiol. 2005;46:120–124. doi: 10.1016/j.jacc.2005.03.048. [DOI] [PubMed] [Google Scholar]
- [4].Niu K, Hozawa A, Kuriyama S, et al. Dietary long-chain n-3 fatty acids of marine origin and serum C-reactive protein concentrations are associated in a population with a diet rich in marine products. Am J Clin Nutr. 2006;84:223–229. doi: 10.1093/ajcn/84.1.223. [DOI] [PubMed] [Google Scholar]
- [5].Pischon T, Hankinson SE, Hotamisligil GS, Rifai N, Willett WC, Rimm EB. Habitual dietary intake of n-3 and n-6 fatty acids in relation to inflammatory markers among US men and women. Circulation. 2003;108:155–160. doi: 10.1161/01.CIR.0000079224.46084.C2. [DOI] [PubMed] [Google Scholar]
- [6].Ferrucci L, Cherubini A, Bandinelli S, et al. Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. J Clin Endocrinol Metab. 2006;91:439–446. doi: 10.1210/jc.2005-1303. [DOI] [PubMed] [Google Scholar]
- [7].Yusof HM, Miles EA, Calder P. Influence of very long-chain n-3 fatty acids on plasma markers of inflammation in middle-aged men. Prostaglandins Leukot. Essent. Fatty Acids. 2008;78:219–228. doi: 10.1016/j.plefa.2008.02.002. [DOI] [PubMed] [Google Scholar]
- [8].Vedin I, Cederholm T, Freund Levi Y, et al. Effects of docosahexaenoic acid-rich n-3 fatty acid supplementation on cytokine release from blood mononuclear leukocytes: the OmegAD study. Am J Clin Nutr. 2008;87:1616–1622. doi: 10.1093/ajcn/87.6.1616. [DOI] [PubMed] [Google Scholar]
- [9].Thies F, Miles EA, Nebe-von-Caron G, et al. Influence of dietary supplementation with long-chain n-3 or n-6 polyunsaturated fatty acids on blood inflammatory cell populations and functions and on plasma soluble adhesion molecules in healthy adults. Lipids. 2001;36:1183–1193. doi: 10.1007/s11745-001-0831-4. [DOI] [PubMed] [Google Scholar]
- [10].Skulas-Ray AC, Kris-Etherton PM, Harris WS, Vanden Heuvel JP, Wagner PR, West SG. Dose-response effects of omega-3 fatty acids on triglycerides, inflammation, and endothelial function in healthy persons with moderate hypertriglyceridemia. Am J Clin Nutr. 2011;93:243–252. doi: 10.3945/ajcn.110.003871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Schubert R, Kitz R, Beermann C, et al. Influence of low-dose polyunsaturated fatty acids supplementation on the inflammatory response of healthy adults. Nutrition. 2007;23:724–730. doi: 10.1016/j.nut.2007.06.012. [DOI] [PubMed] [Google Scholar]
- [12].Rangel-Huerta OD, Aguilera CM, Mesa MD, Gil A. Omega-3 long-chain polyunsaturated fatty acids supplementation on inflammatory biomakers: a systematic review of randomised clinical trials. Br J Nutr. 2012;107(Suppl 2):S159–170. doi: 10.1017/S0007114512001559. [DOI] [PubMed] [Google Scholar]
- [13].Bouwens M, van de Rest O, Dellschaft N, et al. Fish-oil supplementation induces antiinflammatory gene expression profiles in human blood mononuclear cells. Am J Clin Nutr. 2009;90:415–424. doi: 10.3945/ajcn.2009.27680. [DOI] [PubMed] [Google Scholar]
- [14].Dewell A, Marvasti FF, Harris WS, Tsao P, Gardner CD. Low- and high-dose plant and marine (n-3) fatty acids do not affect plasma inflammatory markers in adults with metabolic syndrome. J Nutr. 2011;141:2166–2171. doi: 10.3945/jn.111.142240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Vega-Lopez S, Kaul N, Devaraj S, Cai RY, German B, Jialal I. Supplementation with omega3 polyunsaturated fatty acids and all-rac alpha-tocopherol alone and in combination failed to exert an anti-inflammatory effect in human volunteers. Metabolism. 2004;53:236–240. doi: 10.1016/j.metabol.2003.09.012. [DOI] [PubMed] [Google Scholar]
- [16].Pot GK, Brouwer IA, Enneman A, Rijkers GT, Kampman E, Geelen A. No effect of fish oil supplementation on serum inflammatory markers and their interrelationships: a randomized controlled trial in healthy, middle-aged individuals. Eur J Clin Nutr. 2009;63:1353–1359. doi: 10.1038/ejcn.2009.63. [DOI] [PubMed] [Google Scholar]
- [17].Danesh J, Kaptoge S, Mann AG, et al. Long-term interleukin-6 levels and subsequent risk of coronary heart disease: two new prospective studies and a systematic review. PLoS Med. 2008;5:e78. doi: 10.1371/journal.pmed.0050078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Clearfield MB. C-reactive protein: a new risk assessment tool for cardiovascular disease. J. Am. Osteopath. Assoc. 2005;105:409–416. [PubMed] [Google Scholar]
- [19].Falsey AR, Walsh EE, Francis CW, et al. Response of C-Reactive Protein and Serum Amyloid A to Influenza A Infection in Older Adults. J. Infect. Dis. 2001;183:995–999. doi: 10.1086/319275. [DOI] [PubMed] [Google Scholar]
- [20].Kasapis C, Thompson PD. The effects of physical activity on serum C-reactive protein and inflammatory markers: a systematic review. J Am Coll Cardiol. 2005;45:1563–1569. doi: 10.1016/j.jacc.2004.12.077. [DOI] [PubMed] [Google Scholar]
- [21].Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav Immun. 2007;21:901–912. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- [22].Baylin A, Campos H. The use of fatty acid biomarkers to reflect dietary intake. Curr Opin Lipidol. 2006;17:22–27. doi: 10.1097/01.mol.0000199814.46720.83. [DOI] [PubMed] [Google Scholar]
- [23].Pierigè F, Serafini S, Rossi L, Magnani M. Cell-based drug delivery. Adv Drug Deliv Rev. 2008;60:286–295. doi: 10.1016/j.addr.2007.08.029. [DOI] [PubMed] [Google Scholar]
- [24].Owen AJ, Peter-Przyborowska BA, Hoy AJ, McLennan PL. Dietary fish oil dose- and time-response effects on cardiac phospholipid fatty acid composition. Lipids. 2004;39:955–961. doi: 10.1007/s11745-004-1317-0. [DOI] [PubMed] [Google Scholar]
- [25].Harris WS. Omega-3 fatty acids and cardiovascular disease: a case for omega-3 index as a new risk factor. Pharmacol Res. 2007;55:217–223. doi: 10.1016/j.phrs.2007.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Makhoul Z, Kristal AR, Gulati R, et al. Associations of obesity with triglycerides and C-reactive protein are attenuated in adults with high red blood cell eicosapentaenoic and docosahexaenoic acids. Eur J Clin Nutr. 2011;65:808–817. doi: 10.1038/ejcn.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kelley DS, Siegel D, Fedor DM, Adkins Y, Mackey BE. DHA supplementation decreases serum C-reactive protein and other markers of inflammation in hypertriglyceridemic men. J Nutr. 2009;139:495–501. doi: 10.3945/jn.108.100354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Farzaneh-Far R, Harris WS, Garg S, Na B, Whooley MA. Inverse association of erythrocyte n-3 fatty acid levels with inflammatory biomarkers in patients with stable coronary artery disease: The Heart and Soul Study. Atherosclerosis. 2009;205:538–543. doi: 10.1016/j.atherosclerosis.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].The global market for EPA/DHA omega-3 products . Nutritional Supplements in the U.S. 5th edition Rockville, MD: 2012. [Google Scholar]
- [30].Health and wellness trends database (HWTD) Supplement/OTC/Rx database (SORD) National Marketing Institute. 2009.
- [31].Flock MR, Skulas-Ray AC, Harris WS, Etherton TD, Fleming JA, Kris-Etherton PM. Determinants of erythrocyte omega-3 fatty acid content in response to fish oil supplementation: a dose–response randomized controlled trial. J Am Heart Assoc. 2013;2 doi: 10.1161/JAHA.113.000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Calder PC. Immunomodulation by omega-3 fatty acids. Prostaglandins, Leukot Essent Fatty Acids. 2007;77:327–335. doi: 10.1016/j.plefa.2007.10.015. [DOI] [PubMed] [Google Scholar]
- [33].Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–1772. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- [34].Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- [35].Mehra MR, Lavie CJ, Ventura HO, Milani RV. Fish oils produce anti-inflammatory effects and improve body weight in severe heart failure. J Heart Lung Transplant. 2006;25:834–838. doi: 10.1016/j.healun.2006.03.005. [DOI] [PubMed] [Google Scholar]
- [36].Moertl D, Hammer A, Steiner S, Hutuleac R, Vonbank K, Berger R. Dose-dependent effects of omega-3-polyunsaturated fatty acids on systolic left ventricular function, endothelial function, and markers of inflammation in chronic heart failure of nonischemic origin: a double-blind, placebo-controlled, 3-arm study. Am Heart J. 2011;161:915, e911–919. doi: 10.1016/j.ahj.2011.02.011. [DOI] [PubMed] [Google Scholar]
- [37].Zhao Y, Shao L, Teng L, et al. Effects of n-3 Polyunsaturated Fatty Acid Therapy on Plasma Inflammatory Markers and N-Terminal Pro-brain Natriuretic Peptide in Elderly Patients with Chronic Heart Failure. J. Int. Med. Res. 2009;37:1831–1841. doi: 10.1177/147323000903700619. [DOI] [PubMed] [Google Scholar]
- [38].Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of n 3 fatty acids in humans. Am J Clin Nutr. 2006;83:S1467–1476S. doi: 10.1093/ajcn/83.6.1467S. [DOI] [PubMed] [Google Scholar]
- [39].Mozaffarian D, Wu JH. (n-3) fatty acids and cardiovascular health: are effects of EPA and DHA shared or complementary? J Nutr. 2012;142:614S–625S. doi: 10.3945/jn.111.149633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Sun Q, Ma J, Campos H, et al. Blood concentrations of individual long-chain n-3 fatty acids and risk of nonfatal myocardial infarction. Am J Clin Nutr. 2008;88:216–223. doi: 10.1093/ajcn/88.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Reinders I, Virtanen JK, Brouwer IA, Tuomainen TP. Association of serum n-3 polyunsaturated fatty acids with C-reactive protein in men. Eur J Clin Nutr. 2012;66:736–741. doi: 10.1038/ejcn.2011.195. [DOI] [PubMed] [Google Scholar]
- [42].de Oliveira Otto MC, Wu JHY, Baylin A, et al. Circulating and dietary omega-3 and omega-6 polyunsaturated fatty acids and incidence of CVD in the Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2013;2 doi: 10.1161/JAHA.113.000506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Calder PC. Polyunsaturated fatty acids and inflammation. Prostaglandins Leukot Essent Fatty Acids. 2006;75:197–202. doi: 10.1016/j.plefa.2006.05.012. [DOI] [PubMed] [Google Scholar]
- [44].Hamazaki T, Okuyama H. The Japan Society for Lipid Nutrition recommends to reduce the intake of linoleic acid. A review and critique of the scientific evidence. World Rev Nutr Diet. 2003;92:109–132. doi: 10.1159/000073796. [DOI] [PubMed] [Google Scholar]
- [45].Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med. 2008;233:674–688. doi: 10.3181/0711-MR-311. [DOI] [PubMed] [Google Scholar]
- [46].Johnson GH, Fritsche K. Effect of Dietary Linoleic Acid on Markers of Inflammation in Healthy Persons: A Systematic Review of Randomized Controlled Trials. J Acad Nutr Diet. 2012;112:1029–1041. e1015. doi: 10.1016/j.jand.2012.03.029. [DOI] [PubMed] [Google Scholar]
- [47].Pieroni L, Bastard JP, Piton A, Khalil L, Hainque B, Jardel C. Interpretation of circulating C-reactive protein levels in adults: body mass index and gender are a must. Diabetes Metab. 2003;29:133–138. doi: 10.1016/s1262-3636(07)70019-8. [DOI] [PubMed] [Google Scholar]
- [48].Ford ES, Giles WH, Myers GL, Rifai N, Ridker PM, Mannino DM. C-reactive protein concentration distribution among US children and young adults: findings from the National Health and Nutrition Examination Survey, 1999-2000. Clin Chem. 2003;49:1353–1357. doi: 10.1373/49.8.1353. [DOI] [PubMed] [Google Scholar]
- [49].Khera A, McGuire DK, Murphy SA, et al. Race and Gender Differences in C-Reactive Protein Levels. J Am Coll Cardiol. 2005;46:464–469. doi: 10.1016/j.jacc.2005.04.051. [DOI] [PubMed] [Google Scholar]
- [50].Cartier A, Côté M, Lemieux I, et al. Sex differences in inflammatory markers: what is the contribution of visceral adiposity? Am J Clin Nutr. 2009;89:1307–1314. doi: 10.3945/ajcn.2008.27030. [DOI] [PubMed] [Google Scholar]
- [51].Rutter MK, Meigs JB, Sullivan LM, D’Agostino RB, Sr., Wilson PW. C-reactive protein, the metabolic syndrome, and prediction of cardiovascular events in the Framingham Offspring Study. Circulation. 2004;110:380–385. doi: 10.1161/01.CIR.0000136581.59584.0E. [DOI] [PubMed] [Google Scholar]
- [52].Imahara SD, Jelacic S, Junker CE, O’Keefe GE. The influence of gender on human innate immunity. Surgery. 2005;138:275–282. doi: 10.1016/j.surg.2005.03.020. [DOI] [PubMed] [Google Scholar]
- [53].Moxley G, Posthuma D, Carlson P, et al. Sexual dimorphism in innate immunity. Arthritis Rheum. 2002;46:250–258. doi: 10.1002/1529-0131(200201)46:1<250::AID-ART10064>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- [54].Piché M-È, Lemieux S, Weisnagel SJ, Corneau L, Nadeau A, Bergeron J. Relation of high-sensitivity C-reactive protein, interleukin-6, tumor necrosis factor-alpha, and fibrinogen to abdominal adipose tissue, blood pressure, and cholesterol and triglyceride levels in healthy postmenopausal women. Am J Cardiol. 2005;96:92–97. doi: 10.1016/j.amjcard.2005.02.051. [DOI] [PubMed] [Google Scholar]
- [55].Thorand B, Baumert J, Doring A, et al. Sex differences in the relation of body composition to markers of inflammation. Atherosclerosis. 2006;184:216–224. doi: 10.1016/j.atherosclerosis.2005.04.011. [DOI] [PubMed] [Google Scholar]
- [56].Khera A, Vega GL, Das SR, et al. Sex differences in the relationship between C-reactive protein and body fat. J Clin Endocrinol Metab. 2009;94:3251–3258. doi: 10.1210/jc.2008-2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Chrysohoou C, Pitsavos C, Panagiotakos DB, Skoumas J, Stefanadis C. Association between prehypertension status and inflammatory markers related to atherosclerotic disease: The ATTICA Study. Am J Hypertens. 2004;17:568–573. doi: 10.1016/j.amjhyper.2004.03.675. [DOI] [PubMed] [Google Scholar]
- [58].Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19:972–978. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]
- [59].Bautista LE, Vera LM, Arenas IA, Gamarra G. Independent association between inflammatory markers (C-reactive protein, interleukin-6, and TNF-alpha) and essential hypertension. J Hum Hypertens. 2005;19:149–154. doi: 10.1038/sj.jhh.1001785. [DOI] [PubMed] [Google Scholar]
- [60].Furumoto T, Saito N, Dong J, Mikami T, Fujii S, Kitabatake A. Association of cardiovascular risk factors and endothelial dysfunction in japanese hypertensive patients: implications for early atherosclerosis. Hypertens Res. 2002;25:475–480. doi: 10.1291/hypres.25.475. [DOI] [PubMed] [Google Scholar]
- [61].Ito H, Ohshima A, Tsuzuki M, et al. Association of serum tumour necrosis factor-alpha with serum low-density lipoprotein-cholesterol and blood pressure in apparently healthy Japanese women. Clin Exp Pharmacol Physiol. 2001;28:188–192. doi: 10.1046/j.1440-1681.2001.03429.x. [DOI] [PubMed] [Google Scholar]
- [62].Fernandez-Real JM, Lainez B, Vendrell J, et al. Shedding of TNF-alpha receptors, blood pressure, and insulin sensitivity in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab. 2002;282:E952–959. doi: 10.1152/ajpendo.00444.2001. [DOI] [PubMed] [Google Scholar]
- [63].Ramseyer VD, Garvin JL. Tumor necrosis factor-α: regulation of renal function and blood pressure. Am J Physiol Renal Physiol. 2013;304:F1231–F1242. doi: 10.1152/ajprenal.00557.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Weaver KL, Ivester P, Seeds M, Case LD, Arm JP, Chilton FH. Effect of dietary fatty acids on inflammatory gene expression in healthy humans. J Biol Chem. 2009;284:15400–15407. doi: 10.1074/jbc.M109.004861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Khalfoun B, Thibault G, Bardos P, Lebranchu Y. Docosahexaenoic and eicosapentaenoic acids inhibit in vitro human lymphocyte-endothelial cell adhesion. Transplantation. 1996;62:1649–1657. doi: 10.1097/00007890-199612150-00020. [DOI] [PubMed] [Google Scholar]
- [66].Yang Y, Lu N, Chen D, Meng L, Zheng Y, Hui R. Effects of n–3 PUFA supplementation on plasma soluble adhesion molecules: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2012;95:972–980. doi: 10.3945/ajcn.111.025924. [DOI] [PubMed] [Google Scholar]
- [67].Dupuis F, Desplat V, Praloran V, Denizot Y. Effects of lipidic mediators on the growth of human myeloid and erythroid marrow progenitors. J. Lipid Mediat. Cell Signal. 1997;16:117–125. doi: 10.1016/s0929-7855(97)00007-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.