Abstract
Some pathogens have evolved to produce proteins, called B-cell superantigens, that can interact with human immunoglobulin variable regions, independently of the combining site, and activate B lymphocytes that express the target immunoglobulins. However, the in vivo consequences of these interactions on human B-cell numbers and function are largely unknown. Using transgenic mice expressing fully human immunoglobulins, we studied the consequences of in vivo exposure of protein L of Peptostreptococcus magnus with human immunoglobulins. In the mature pool of B cells, protein L exposure resulted in a specific reduction of splenic marginal-zone B cells and peritoneal B-1 cells. Splenic B cells exhibited a skewed light-chain repertoire consistent with the capacity of protein L to bind specific kappa gene products. Remarkably, these two B-cell subsets are implicated in innate B-cell immunity, allowing rapid clearance of pathogens. Thus, the present study reveals a novel mechanism that may be used by some infectious agents to subvert a first line of the host's immune defense.
Throughout evolution, the continuous interactions between the mammalian host and infectious agents have produced an array of innate and adaptive immune effectors able to combat insults by pathogens (30). Reciprocally, infectious agents have developed efficient countermeasures to persist in the infected host (63). These pathogens have developed mechanisms to mutate, exchange genetic materials, multiply rapidly, vary their phenotype, and occupy diverse ecological niches (61). One intriguing feature of some infectious agents is to produce proteins able to interact specifically with the immunoglobulin (Ig) heavy (H)- or light (L)-chain variable regions, independently of the conventional binding site. They are referred to as “B-cell superantigens” (SAgs) and include protein A of Staphylococcus aureus (SpA) (22, 29, 52, 57), gp120 of human immunodeficiency virus type 1 (HIV-1) (3, 20, 32, 33, 43, 50), staphylococcal enterotoxins A and D (7, 45), and protein L of Peptostreptococcus magnus (12, 13).
Although conventional antigens stimulate a small proportion of B cells, the B lymphocytes responsive to SAgs can be orders of magnitude higher. Because the B-cell SAg interacts primarily with the VH or VL portion of the Ig molecule, it can, in principle, trigger all B cells bearing the appropriate VH or VL, regardless of the other JH, D, JL, and pairing with VH or VL segments (58, 68). Since there are a limited number of V genes, this property results in stimulation of a large proportion of the repertoire. For example, the bacterial cell wall protein SpA has sites that interact with the Fab of many IgM, IgA, IgG, and IgE, and this interaction is restricted to the VH3 gene family, leading to activation of ca. 40% of human polyclonal IgMs (58, 68).
The function of these proteins is unclear, but their ability to bind conserved portions of Igs suggests that they help the bacteria to evade the host's immune system. Through direct interaction with host Igs, they have a potential to interfere with the humoral effector arm of the immune system and to modify the antibody response of the host. Since SAg interactions with the B-cell receptor (BcR) may, in principle, lead to activation, proliferation, differentiation, anergy, or induction of programmed cell death (68), this group of microbial molecules could interfere with mechanisms that shape the B-cell repertoire and could play a role in the pathogenesis of infectious diseases in humans. In HIV infection, for example, studies revealed that subjects infected with HIV have aberrant and unstable expression of Ig genes, a finding suggestive of humoral immune disregulation and responses to HIV-associated antigens and SAgs (4, 6, 31).
Protein L is a cell wall protein produced by ca. 10% of strains of P. magnus, an anaerobic bacterial species (7). Depending on the bacterial strain from which it is isolated, protein L is a 76 to 106-kDa protein containing four or five highly homologous, consecutive extracellular Ig-binding domains (35). Protein L binds predominantly to κ-chains regardless of the H chain and consequently has affinity for all classes of Igs. Since ca. 60% of human Igs have κ-type L chains, protein L interacts with a significant proportion of Igs. Its binding does not block the antigen-binding site (45), and the affinity of the interaction ranges from 1.5 × 109 M−1 to 1 × 1010 M−1, depending on the L-chain and H-chain isotypes (1). The crystal structure of a human antibody Fab complexed through its VL region to a protein L domain revealed that P. magnus protein L interacts with the framework part of the variable regions without contacting the hypervariable loops (23). In vitro, protein L appears to act as a SAg for human B cells (2) and induces BcR downmodulation (65). It also cross-links the VL domains of IgE bound to Fcɛ receptors, resulting in the release of histamine by basophils and mast cells (49) and secretion of IL-4 and IL-13 by basophils (18).
Although the findings obtained in vitro suggest that B-cell SAgs play a pathogenic role, the impact of this group of microbial proteins on the human B-cell repertoire has been difficult to test, partly because of the lack of an experimental system. We used mice in which endogenous H- and L-chain genes were inactivated and instead were engineered to express human Igs (44). The Ig transgenes somatically recombine and mutate upon immunization to encode a functional human repertoire (44, 51). Here, we have determined the consequences of confronting B cells expressing human surface Igs with protein L in vivo. We were also able to follow the fate of various B-cell populations throughout development in the bone marrow (BM) and in secondary lymphoid organs after protein L treatment.
MATERIALS AND METHODS
Mice and immunogens.
Generation of the “five-feature” transgenic mice used in these experiments has previously been described (44). Briefly, the endogenous loci coding for the H- and L-chains have been inactivated, and human H-, κ-, and λ-chain transloci were “ knocked in.” The H-chain translocus contains the core region of the human H-chain locus with the five most 3′ VH gene segments, the complete DH and JH loci, and the Cμ and Cδ exons in correct germ line configuration. The κ-chain translocus is a construction that contains 20 repeats of five Vκ gene segments linked to the core of the germ line locus, including the first three Vκ gene segments, the complete Jκ cluster, and the Cκ coding region. It contains 82 functional Vκ gene segments. Finally, the λ-chain translocus encompasses 28 Vλ gene segments (with 16 functional) attached to the Jλ and Cλ coding regions in the correct germ line configuration. All mice used in these experiments were bred in the animal facility of Broussais Hospital and used at 7 to 9 weeks of age. Animals were housed in a specific-pathogen-free barrier facility. Groups of five mice were treated with either hen egg lysozyme (HEL; Sigma-Aldrich, St. Louis, Mo.) or the B1-B4 recombinant domains of protein L (35) that was tested to exclude lipopolysaccharide contamination. Each mouse received five intraperitoneal injections of protein (1 mg in phosphate-buffered saline [PBS]) every other day. Mice were sacrificed 21 days after the first injection, and spleens, lymph nodes, BM, and peritoneal cells were collected.
Immunofluorescence analysis.
Single-cell suspensions were isolated, and erythrocytes were lysed with Tris-buffered ammonium chloride, when necessary. Leukocytes (0.5 × 106 cells) were stained at 4°C by using predetermined optimal concentrations of different combinations of fluorochrome-labeled antibodies for 30 min. After washes, when necessary, biotin-labeled antibodies were revealed by using Streptavidin-CyChrome (BD Pharmingen, San Diego, Calif.).
Cell phenotype was determined by using the following reagents: anti-mouse CD5-phycoerythrin (PE; 53-7.3), anti-mouse CD45R/B220-fluorescein isothiocyanate (FITC), anti-mouse CD45R/B220-CyChrome (RA3-6B2), anti-mouse CD4-PE (H129.19), anti-mouse CD43-FITC (S7), anti-mouse CD21/CD35-FITC (7G6), anti-mouse CD23-PE (B3B4), anti-human IgM (HuIgM)-FITC, anti-HuIgM-PE (G20-127), anti-HuIgD-FITC (IA6-2), anti-HuIgκ-FITC (G20-193), biotinylated anti-HuIgλ (JDC-12), or Streptavidin-CyChrome. All monoclonal antibodies and their corresponding isotype controls were purchased from BD Pharmingen. Anti-mouse CD8-FITC (KT15) was obtained from Immunotech (Marseille, France).
After being washed and fixed in PBS-1% formaldehyde, the cells were analyzed by using a single laser FACScan flow cytometer (Becton Dickinson, San Jose, Calif.). A minimum of 10,000 events was collected per sample, and data were analyzed with CellQUEST (version 3.1; Becton Dickinson).
Enzyme-linked assays.
A enzyme-linked immunosorbent assay (ELISA) was used to quantify IgM(κ), IgM(λ), and IgM reactive with protein L in mouse plasma. Briefly, microtiter plates (MaxiSorp; Nunc-Immunoplate) were coated overnight with goat antibodies (1 μg/ml in borate-buffered saline [pH 8.4]) to human κ chain (K3502; Sigma-Aldrich) or to μ chain (I0759; Sigma-Aldrich). After a blocking step with 1% PBS-bovine serum albumin, plasma samples diluted in 1% PBS-BSA were incubated 2 h at 37°C with the sensitized wells. Bound antibodies were detected either by incubation with biotinylated protein L (60 ng/ml), followed by the addition of horseradish peroxidase-labeled streptavidin, or directly with horseradish peroxidase-labeled goat anti-human κ and λ antibodies (A7164 and A5175, respectively; Sigma-Aldrich). The human monoclonal antibody (MAb) M3 (IgM, VH3, and Vκ1), provided by J. P. Bouvet (60), and a human serum pool were used as calibration standards.
Single-cell PCR analysis.
B220+ fixed splenocytes from HEL- and protein L-treated mice were bulk sorted by using a Becton Dickinson FACS Vantage SE DiVa (Santa Cruz, Calif.), yielding 21,082 cells with 95% purity from the HEL-treated mice and 11,487 cells with 93% purity from the protein L-treated mice (8). B220+ cells were diluted in lysis buffer and aliquots containing 1 cell were dispensed into wells of a PCR plate (Robbins Scientific, Sunnyvale, Calif.).
Genomic DNA from cell lysates was amplified by using random 15-mers and 60 cycles of nested PCR as previously described (9, 14, 15). Sequences were analyzed on an automated capillary sequencer (ABI Prism 3100 Genetic Analyzer; Applied Biosystems, Foster City, Calif.). Germ line sequences were determined by using the JoinSolver sequence analysis program for the VH amplifications and DNAplot (http://www.mrc-cpe.cam.ac.uk/DNAPLOT) for the VL segments. A rearrangement was considered productive if the VDJ and VLJL junction maintained the reading frame into the JH or JL segment. Rearrangements that were out of frame or introduced a stop codon during the rearrangement at a junction were considered nonproductive.
Statistical analysis.
All experimental results were tested with analysis of variance (Fisher PLSD test) and Mann-Whitney nonparametric U test. The results were considered significant at a P of <0.05, to be highly significant at a P of <0.01, and to lack significance at a P of >0.05. The results of tests for significance are provided in the figure legends.
RESULTS
To investigate the in vivo consequences of confronting the human antibody repertoire with protein L, we made use of mice that were engineered to express human Igs. The transgenic mice produce B lymphocytes that secrete fully human antibodies (44). Their surface HuIgM+ (sHuIgM+) splenic B cells bind protein L in a way that is comparable to human peripheral blood B cells. Transgenic naive mice were injected intraperitoneally with 1 mg of recombinant protein L (or HEL as a control protein) on days 1, 3, 5, 7, and 9 to determine the effect on B-cell development and function and on other cells of the immune system.
Time course reduction of protein L-positive HuIgMs.
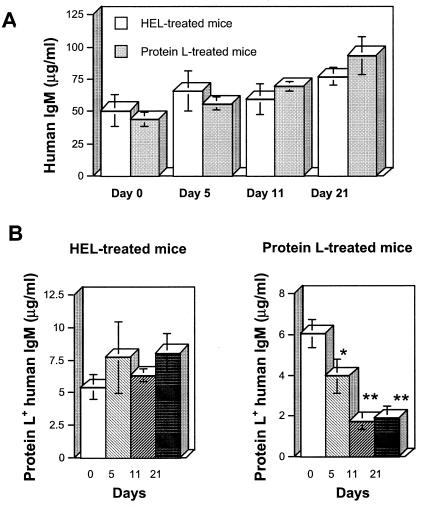
The mice were bled periodically, and the concentration of HuIgM in the plasma of each mouse was determined. Throughout the observation period, there was a progressive increase of the mean HuIgM concentrations in plasma in both the HEL- and the protein L-treated groups (Fig. 1A). Since protein L targets κ-chain-positive Igs, we determined the concentrations of HuIgM reactive with protein L in the plasma of treated mice. Beginning at day 5, protein L+ HuIgM concentrations were depressed in the protein L-treated group and, after 3 weeks, there was a 68% decline compared to the concentrations at day 0 (P < 0.005) (Fig. 1B). This dramatic decrease was specific since in the HEL-treated mice protein L+ HuIgM concentrations exhibited minimal changes. Thus, repeated injections of soluble protein L to the transgenic mice led to a decrease in protein L+ IgM levels that was not observed in mice treated with a control protein.
FIG. 1.
Kinetics of HuIgM concentrations in the plasma of the HEL- and protein L-treated transgenic mice on days 1, 3, 5, 7, and 9. HuIgM concentrations were measured in the plasma of individual mice. (A) Total human IgM concentrations show no significant variations between the two groups. (B) Protein L+ HuIgM concentrations exhibit a dramatic specific decrease in the protein L-injected mice but not in the HEL-treated mice. ✽, P < 0.05; ✽✽, P < 0.005.
Reduction of peripheral IgM(κ)+ B cells by protein L treatment.
To determine whether protein L treatment affected B-lymphocyte development, we examined the numbers of B cells (defined as B220+) in the peripheral blood of the mice over time. Cells were stained with fluorescently labeled MAbs and analyzed by immunofluorescence-activated flow cytometry (FACS). There was a progressive increase of HuIgM-expressing lymphocytes (B220+ IgM+ cells) in both HEL- and protein L-treated mice, rising from 29.8% ± 6% and 29.9% ± 8% at day 0 to 67.9% ± 5% (P < 0.0005) and 59.5 × 10% (P < 0.05) at day 21, respectively. We then quantitated protein L-specific lymphocytes (B220+ protein L+ cells) in the peripheral blood of treated mice. In mice receiving HEL, protein L+ B cells showed a slight increase, reaching 25.1% ± 2% at day 21 compared to day 0 (22.2% ± 2.3%). In contrast, protein L-treated mice exhibited a slight decrease of protein L+ B cells at day 21 (19.2% ± 2%) compared to day 0 (21.9% ± 1.5%). Thus, injection of protein L had only a modest effect on peripheral B lymphocytes that express protein L+ IgM on their cell surfaces compared to HEL-treated mice at day 21.
Protein L targets immature B cells in the BM.
To determine the developmental stage at which protein L acts in vivo, BM tissues, where early stages of B-lymphocyte development occur, were isolated, and cell populations were analyzed by FACS by triple labeling. Analysis of B220+ cells in the BM indicated no changes in the pro-B-cell (B220low CD43+ IgM−) or pre-B-cell (B220low CD43− IgM−) compartments. Thus, the treatment does not affect the generation or differentiation of BM lymphoid progenitor cells that represent the majority of the B cells in the BM and that give rise to fully committed B lymphocytes. In contrast, immature B cells (B220+ IgM+ IgD−) were increased. The average percentages of immature B cells were expanded from 5.7% ± 1% to 16.7% ± 2% in the protein L-treated group (P < 0.005) but not in HEL-treated mice. In sharp contrast to this 66% elevation of the immature population, the percentages of cells that have progressed to the recirculating B-cell stage (B220+ IgM+ IgD+) in the BM were similar in the two groups.
Protein L treatment results in a specific loss of mature B-cell populations but has little effect on other cell lineages.
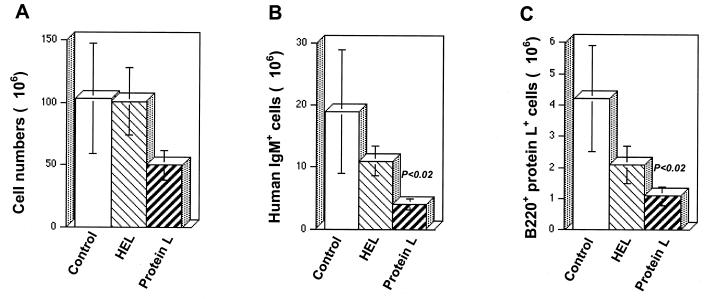
To characterize the effect of protein L on other compartments of the immune system, the spleen and lymph nodes were isolated from the mice on day 21. The numbers of cells comprising splenic peripheral lymphoid compartments were determined in each mouse. Cell populations from each tissue were then stained with fluorescently labeled MAbs and analyzed by FACS in order to identify specific cell lineages in each of these organs. In the spleen, there was a decrease in the total numbers of viable cells in protein L-treated mice compared to HEL-treated and control mice. With protein L injections, the total splenocyte numbers were 1.7-fold lower than the splenocyte numbers in control untreated mice (Fig. 2A). More importantly, protein L had a profound effect on the representation of HuIgM+ B cells in this secondary organ, decreasing them from 18.9 × 106 ± 10 × 106 (control mice) to 4.3 × 106 ± 0.9 × 106 cells (P < 0.02, Fig. 2B). This marked reduction was specific for protein L-positive B cells. In the protein L-treated group, their mean numbers dropped from 4.2 × 106 ± 1.7 × 106 cells (control mice) to 1.1 × 106 ± 0.3 × 106 cells (P < 0.02, Fig. 2C). Remarkably, despite the substantial loss of mature B cells, protein L treatment had no effect on the percentages or total numbers of mature T cells defined by CD4/CD8 surface expression in the spleen and lymph nodes (Fig. 3B). Similarly, the CD5− B220− cell population was not affected significantly by the treatment.
FIG. 2.
Specific and marked reduction in the number of protein L-reactive cells in the spleens of transgenic humanized mice. Splenic cells of control (untreated) and HEL- and protein L-treated transgenic mice were depleted of erythrocytes by using Tris-buffered ammonium chloride and stained with cell surface markers. (A) Total number of splenic cells counted by using a Malassez cell; (B) HuIgM+ B220+ cells as detected by PE-labeled anti-HuIgM and CyChrome-labeled anti-mouse B220 MAbs; (C) B220+ protein L+ cells as detected by FITC-labeled anti-mouse B220 MAb and biotinylated protein L, followed by treatment with CyChrome-labeled streptavidin.
FIG. 3.
Protein L targets B-cell subpopulations involved in innate immunity. Lymphoid cell subpopulations were identified by immunofluorescence staining in control (untreated) and in HEL- and protein L-treated transgenic mice. (A) Splenocytes were triple stained with CyChrome-labeled anti-mouse B220 MAb, FITC-labeled anti-mouse CD21 MAb, and PE-labeled anti-mouse CD23 MAb. Lymphoid cells were gated on the B220-expressing cells. MZ (B220+ CD23−/low CD21+) but not FO (B220+ CD23+ CD21+) B cells are reduced in protein L-treated mice. (B) Lymph node cells were stained with PE-labeled anti-mouse CD4 and FITC-labeled anti-mouse CD8 MAbs. The treatments do not affect T lymphocytes. (C) Peritoneal B1 cells are reduced in protein L-treated mice.
Protein L treatment results in negative selection of B cells utilizing Vκ L chains.
To determine whether protein L exposure affects the repertoire of expressed V genes, B220+ splenocytes from HEL- or protein L-treated mice were sorted and assessed for Vκ or Vλ genes from genomic DNA of individual B cells. This approach makes it possible to analyze both nonproductive and productive Ig rearrangements (8). Analysis of the nonproductive repertoire was informative to determine the frequency with which specific transgenic elements were rearranged because the nonproductive rearrangements do not encode an Ig molecule that can influence whether the B-cell is positively or negatively selected. Therefore, by comparing the nonproductive and productive repertoires, the impact of positive and negative selection of functionally rearranged B cells could be determined. A total of 82 L-chain PCR products from HEL-treated mice and 111 products from protein L-treated mice were analyzed. From either set of mice, there was a nearly equal proportion of nonproductive and productive rearrangements. From the HEL-treated mice, there were 47.6% nonproductive rearrangements and 52.4% productive rearrangements. Similarly, from the protein L-treated mice, 51.4% of the rearrangements were nonproductive and 48.7% were productive. The ratio of Vκ and Vλ gene utilization in the nonproductive repertoire was also similar in the two sets of mice. In the HEL-treated mice 48.7% of the nonproductive rearrangements utilized Vκ genes and 51.3% utilized Vλ genes, resulting in a nonproductive κ/λ ratio of nearly 1:1. In protein L-treated mice, 49.1% of the nonproductive repertoire utilized Vκ gene segments and 50.9% utilized Vλ gene segments resulting in a κ/λ ratio of nearly 1:1.
There were no significant differences between the κ/λ ratio in the nonproductive repertoires of the two sets of mice. Interestingly, the observed nonproductive κ/λ ratios were lower than expected considering the 5:1 ratio of 82 human Vκ genes and 16 human Vλ genes in the transgene loci of the mice (44).
In contrast to the nonproductive repertoire, there were marked differences in the Vκ and Vλ gene usage in the productive repertoires of the HEL- and protein L-treated mice. In the productive repertoire of HEL-treated mice, 44.2% of the L-chain sequences utilized Vκ gene rearrangements and 55.8% utilized Vλ genes, resulting in a productive κ/λ ratio of 5:6. Alternatively, in protein L-treated mice, only 33.3% of the productive rearrangements utilized Vκ gene segments, whereas 66.7% of the rearrangements utilized Vλ gene segments, resulting in a κ/λ ratio of 1:2.
Protein L binds the VL region of Igs encoded by Vκ1, Vκ3, and Vκ4 subgroups but not Vκ5 or Vλ genes. To determine whether there was Vκ family specific deletion of B cells related to protein L administration, we analyzed in detail the utilization of Vκ genes in the nonproductive and productive repertoires of HEL- and protein L-treated mice (Table 1). Among the five Vκ genes in the translocus, one gene (Vκ1D-13) was not detected in the HEL- or protein L-treated mice. Another gene, Vκ3D-11, was detected only in the productive repertoire (10.5%) of HEL-treated mice and was not detected in the protein L-treated group. Vκ4-1 was the most frequently utilized gene in both the HEL- and protein L-treated mice. In the nonproductive repertoire of HEL-treated mice, it was utilized in 73.7% Vκ rearrangements and 64.3% of the Vκ rearrangements from the nonproductive repertoire of protein L-treated mice. In the productive repertoires, Vκ4-1 was utilized in 57.9% of the Vκ rearrangements of HEL-treated mice and 50% of the Vκ rearrangements from protein L-treated mice.
TABLE 1.
Distribution of Vκ and Vλ genes in the nonproductive and productive repertoires from B220+ splenocytes obtained from HEL- or protein L-treated mice
| Gene | % Distribution (n)a |
|||
|---|---|---|---|---|
| Nonproductive rearrangements |
Productive rearrangements |
|||
| HEL | Protein L | HEL | Protein L | |
| Vκ | ||||
| 1D-12 | 21.5 (19) | 28.6 (28) | 26.3 (19) | 16.7* (18) |
| 3D-11 | 0 (19) | 0 (28) | 10.5 (19) | 0* (18) |
| 4-1 | 73.7 (19) | 64.3 (28) | 57.9 (19) | 50* (18) |
| 5-2 | 5.3 (19) | 7.1 (28) | 5.3 (19) | 33.3* (18) |
| Vλ | ||||
| 2 | 70 (20) | 62 (29) | 41.7 (24) | 44.4 (36) |
| 3 | 10 (20) | 20.7 (29) | 54.2 (24) | 55.6 (36) |
| 4 | 20 (20) | 17.2 (29) | 4.2 (24) | 0 (36) |
*, P < 0.01. P values were determined within each type of rearrangement by the χ2 test.
Vκ1D-12 was the second most utilized gene in HEL- and protein L-treated mice. Vκ1D-12 utilization in the HEL-treated mice was similar in the nonproductive and productive repertoires; 21.5% and 26.3%, respectively. By comparison, and consistent with the ability of protein L to bind the Vκ1 subgroup, in the protein L-treated mice, 28.6% of the nonproductive Vκ rearrangements utilized Vκ1D-12, whereas only 16.7% of the productive Vκ rearrangements used Vκ1D-12, suggesting that Vκ1D-12 was deleted from the expressed repertoire.
Unlike Vκ1, Vκ3, and Vκ4, the products of Vκ5 gene do not bind protein L. Although the utilization of Vκ5-2 is infrequent in the HEL-treated mice, Vκ5-2 was utilized at the same frequency in the nonproductive and productive repertoires of HEL-treated mice (5.3%). In contrast, in protein L-treated mice 7.1% of the nonproductive and 33.3% of the productive Vκ rearrangements utilized Vκ5-2. The increased utilization of Vκ5-2 in the productive repertoire of protein L-treated mice could reflect positive selection. However, in view of its lack of reactivity with protein L, it more likely reflects a compensatory response to the deletion of Vκ1D-12 and Vκ4-1 genes, that could be exaggerated because of the limited number of human Vκ genes expressed in the transgenic mice. In marked contrast to Vκ gene expression, there was no positive or negative selection of Vλ gene rearrangements in relation to HEL or protein L treatment (Table 1). These results suggest that the cells that survive protein L treatment express a skewed L-chain repertoire consistent with the capacity of this SAg to bind specific Vκ-chain families.
Protein L specifically impairs marginal zone B-cell development in the spleen.
We examined the numbers of splenic B-cell populations (defined as B220+) to determine the populations that were affected by the treatment with protein L versus the numbers of B cells affected by HEL. We used antibodies to CD21, CD23, HuIgD, and HuIgM to identify newly formed (NF), transitional 1 (T1), transitional 2 (T2), mature (M), marginal zone (MZ), and follicular (FO) B cells. Although the number of T1 (B220+ IgM+ CD21−), T2 (B220+ IgM+ CD21+), and M (B220+ IgMlow CD21low) B cells did not vary significantly, there was a decrease in NF B-cell numbers (B220+ CD23− CD21−) in the protein L-treated group (P < 0.05). There was also a clear block in development of MZ B cells (B220+ CD23−/low CD21+) after exposure of mice to protein L, as manifested by a 70% decrease (P < 0.01). Despite these changes in MZ B cells, there was no apparent change in the mean numbers of FO B lymphocytes (B220+ CD23+ CD21+) in protein L-injected mice (Table 2 and Fig. 3A). Thus, there is a clear loss of NF B cells in the spleen of mice exposed to protein L, whereas transitional cells remain unaffected. There is also a distinct block in the development of MZ B cells.
TABLE 2.
Effect of protein L treatment on B-cell populations in the spleens of transgenic mice
| B-cell sub- population | Mean cell number (106) ± SEa |
||
|---|---|---|---|
| Control untreated mice | HEL-treated mice | Protein L-treated mice | |
| NF | 4.70 ± 2.9 | 0.98 ± 0.3 | 0.86 ± 0.3* |
| T1 | 3.29 ± 2.2 | 3.99 ± 1.3 | 2.34 ± 0.9 |
| T2 | 5.08 ± 2.2 | 3.36 ± 1 | 1.98 ± 0.4 |
| M | 1.80 ± 0.7 | 2.23 ± 0.4 | 2.12 ± 0.5 |
| MZ | 1.87 ± 0.4 | 1.17 ± 0.2 | 0.54 ± 0.1*** |
| FO | 1.74 ± 0.3 | 2.62 ± 0.4 | 1.66 ± 0.4 |
Cell numbers are shown. B-cell subpopulations were identified by the expression of cell surface markers as follows: T1, B220+ IgM+ CD21−; T2, B220+ IgM+ CD21+; M, B220+ IgMlow CD21low; MZ, B220+ CD23−/low CD21+; FO, B220+ CD23+ CD21+; and NF, B220+ CD23− CD21−. *, P < 0.05; **, P < 0.02; ***, P < 0.01. P values were determined by using the Mann-Whitney U test.
Protein L reduces the number of B-1 cells.
The recirculating B-cell population is essentially composed of CD5− B cells (called B-2 cells). In addition, a subpopulation of CD5+ B cells (termed B-1) has a unique tissue distribution and function. Most B-1 cells are located in the body cavities and make a dominant contribution to natural antibody production and T-cell-independent immune responses to exogenous antigens (27, 69). We therefore probed the B-1-cell subpopulation (B220+ CD5+) in the spleen and the peritoneal exudates. There were no apparent changes in B-1 cells in the spleen. However, there was a reduction of B-1 cells in the peritoneal cavity, dropping from 59.7% ± 5% to 35.7% ± 8% in mice that received protein L (P < 0.05). For comparison, B-1 cells averaged 56.8% ± 7% in HEL-injected mice (Fig. 3C). Given the compelling evidence that B-1 and MZ subpopulations are involved in first-line immune defense against foreign invaders (38), these findings are in line with the reduction of MZ B cells in the spleen.
DISCUSSION
Work in recent years has revealed a novel mode of binding of some microbial proteins, termed SAgs, to the variable regions of Igs, independently of the paratope. Whereas the interactions have been substantially characterized at the immunochemical and structural levels, their consequences on the immune system in vivo have remained unclear. Using transgenic mice that express human Igs, we investigated the in vivo effects of protein L, a bacterial protein with well characterized in vitro SAg properties for soluble human Igκ (23, 45) and for Igκ-positive B lymphocytes (2). The experiments demonstrate that the introduction of soluble protein L to naive mice leads to a disruption in B-cell development without substantially affecting other cell populations, such as T-lineage cells or myeloid cell populations in the spleen, lymph node, and peritoneal cavity. Given the biological properties of protein L in vitro on human B cells, we can therefore conclude that protein L acts essentially on the B-cell lineage in vivo.
Impact of protein L on immature B cells.
During B-lymphocyte development, ordered rearrangements of Ig gene segments are linked with cellular expansion and differentiation events (55). Initially, pro-B cells (B220+ CD43+) undergo rearrangement of Ig H-chain D to JH gene segments, followed by VH to DJH rearrangement. The expressed μ H-chain proteins then complex with the surrogate L-chain proteins (VpreB and λ5) to form the receptor expressed on pre-B cells (B220+ CD43−). At this stage most Ig L-chain assembly occurs and the L chains produced will pair with the preexisting H chains, leading to BcR expression and differentiation of pre-B cells to immature B lymphocytes. After BcR expression, rearrangement, and negative selection of autoreactive B cells in the BM, a fraction of B cells migrates to secondary lymphoid organs. In protein L-treated mice, characterization of early B-cell development revealed a specific expansion at the immature B-cell stage in the BM. Alterations in B-cell development in this organ were restricted to the immature B-cell population and B-cell precursor populations (pro-B and pre-B cells) were unaffected by protein L administration. This stage-specific activity of protein L reflects the requirement of κ-chain expression on B cells, which marks the transition from the pre-B to the immature B-cell stage. The presence of normal B-cell precursor populations in the BM of protein L-treated mice suggests that B cells will repopulate the periphery after protein L is cleared from the animal.
NF B cells leaving the BM have a short half-life and remain largely confined to the blood circulation and defined areas of the spleen, since they lack adhesion molecules necessary for extravasation in peripheral lymphoid organs, such as lymph nodes. Only a fraction of these NF B cells eventually enter the pool of long-lived, recirculating B cells populating lymphoid organs, which are referred to as B-2 B cells. In the spleen, NF cells (B220+ IgMhigh) acquire more mature phenotypes with downregulation of IgM and upregulation of differentiation molecules, including CD21, CD23, and IgD, and transitional T1 cells (CD21low IgMhigh) give rise to transitional T2 cells (CD21high IgMhigh), which are the direct precursors of mature B cells. In marked contrast to the immature B-cell expansion in the BM, analysis of NF B cells in the spleen showed that protein L treatment resulted in a substantial loss of this immature B-cell population in the spleen. This observation has implications with regard to the mechanisms of B-cell selection. Although there is ample evidence that negative selection is an active process in the BM, it is unclear whether negative selection of immature B cells occurs in the peripheral compartment of conventional mice. The loss of NF B cells we noted supports the view that, in addition to receptor editing (42, 53), BcR-mediated affinity interactions result in apoptosis in immature peripheral B cells, probably representing a developmental checkpoint during B-cell maturation in the periphery.
The finding that protein L treatment results in a clear expansion of immature B cells in the BM and a loss of NF B cells in the periphery deserves consideration. First, it is possible that different antigen concentrations are reached in the BM and the periphery. Previous transgenic models demonstrated that deletion and anergy of autoreactive B cells are involved in B-cell tolerance, and different concentrations of antigen, or different forms, i.e., soluble versus membrane-bound, were invoked (42, 53). The concentration of protein L in our system can be estimated not to be lower than that of membrane-bound antigens, suggesting that the concentration may be high enough to cause deletion in the periphery. However, it is difficult to assess the precise, effective protein L concentrations in the BM because its tissue distribution is unknown. Second, another aspect of antigen expression that may trigger different cell fates is the timing of contact between protein L and B cells. It may be that the duration of protein-l-B-cell encounters is longer in the periphery than in the BM. Third, the role of the microenvironment is likely to play a role (55). Since B-cell commitment, proliferation, differentiation, and survival are regulated by a finely tuned balance between cell-autonomous mechanisms (pre-BcR and BcR) and signals provided by the microenvironment (IL-7, stroma cells, competitor cells, and soluble signals), the B-cell response will be determined in large part by the developmental stage of the B-cell and its location within the immune system, as illustrated here by the in vivo effects of protein L.
Impact of protein L on Vκ-expressing B cells and circulating Igs.
In addition to the expansion of the immature B-cell population in the BM, we noted that protein L induces a marked deficit in circulating protein L+ IgM. In the spleen, protein L-treatment deleted a portion of B cells utilizing Vκ1D-12 and Vκ4 genes, and the greatest impact of deletion was attributed to Vκ1D-12 rearrangements. The effect of protein L on Vκ gene expression was probably underestimated because single-cell Vκ gene analysis was performed on total splenic B-cell populations. We can postulate several mechanisms by which protein L perturbs humoral immunity. One possibility is that protein L acts on B-cell subpopulations that impart adaptive immunity. It may, for example, inhibit factors required for the maintenance of germinal centers or completely block germinal center formation. Protein L could also act directly by inhibiting the differentiation of B cells into Ig-producing plasma cells. Similarly, protein L may act by inhibiting the survival of mature B cells in the periphery, reducing the pool of cells that can develop into Ig-secreting cells. However, the characteristics of the deficit in circulating Igs suggest that protein L more likely targets B-cell subpopulations important in innate immunity. First, we observed a rapid (5 days after injection of soluble protein L) impact on Ig levels. Second, the deficit was specific for circulating κ-positive IgM, which are the targets of protein L. Third, as will be discussed below, we found no effect of protein L on FO B cells, which are involved in adaptive immunity.
Protein L affects innate B-cell immunity.
The long-lived pool of B cells is composed of mature B-2 cells that are derived from NF B cells and that recirculate among the follicules, where they are termed FO B cells. Also derived from NF B cells that mature through the transitional stage in the spleen are MZ B cells that migrate to a more static cell compartment (reviewed in references 5 and 40). MZ B cells represent a distinct subset of lymphocytes. They are located in the periphery of the splenic periarteriolar lymphoid sheath at the border of white and red pulp and can be distinguished from FO recirculating B cells by the characteristic expression of cell surface markers. They have a preactivated phenotype, express high levels of B7-1 and B7-2 and, upon stimulation in vitro, rapidly differentiate into plasma cells (48). Remarkably, the route of antigen administration and the BcR specificity determine the relative contributions of FO versus MZ B-cell subpopulations. Because of their anatomical location, MZ B cells are the first cell population to encounter blood-borne antigens and are thought to play a critical role in host defense against pathogens (41). The properties of the MZ B-cell subset are reminiscent of those of B-1 cells, which are enriched in the peritoneal cavity and derived from fetal or adult precursor populations generated early in development (28, 67). B-1 cells have the capacity of self-renewal and are responsible for secreting most of the serum preimmune IgM, with multireactive binding properties and potentially protective properties (36). This B-cell subset has been shown to be deleted by injection of the SAg S. aureus protein A that targets VH3+ Igs (59).
Whether innate B-cell immunity is also affected in humans infected with protein L-expressing strains of P. magnus remains to be demonstrated. There are, however, observations suggesting that this could be the case. P. magnus is a member of the indigenous flora of the skin, the oral cavity, and the gastrointestinal and genitourinary tracts. Only a minority (∼10%) of the P. magnus strains express protein L, and these isolates are more frequently connected with clinical infections, indicating that protein L is a virulence determinant (34). It is also noteworthy that significant amounts of protein L are found in the growth medium of protein L-expressing strains (34), suggesting that protein L also in vivo is released from the bacterial surface. Such a mechanism would allow the targeting of B-1 and MZ B cells during infection.
Analyzing various cell populations in the organs isolated from protein L-treated mice, we found no effect on mature recirculating FO B cells that have evolved to generate a huge repertoire able to mount T-cell-dependent B-cell responses with high-affinity and long-term memory (54). In contrast, we found that protein L alters the development of both MZ B cells in the spleen and B-1 B cells in the peritoneal cavity, two B-cell subsets that have evolved to provide a first line of defense against antigens acquired through the gut/peritoneum and the bloodstream. Both B-1 and MZ B cells exhibit a high antigen-presenting capacity (48, 62), preferentially secrete complement-fixing, potentially protective natural IgM (26, 46, 69). These cells proliferate rapidly and vigorously for more prolonged time periods to anti-μ and LPS stimulation than FO B cells (47). We therefore conclude that protein L acts predominantly on B-cell subpopulations with innate immune functions.
Targeting innate B cells, a novel escape mechanism used by some pathogens.
The mechanisms underlying the specific suppression of the B-cell subpopulations by protein L remain to be elucidated. A reduction in the B-1 and MZ B-cell compartments could be caused by altered development, accelerated death, or defective retention at the appropriate microenvironmental site. It is known that different cellular responses are triggered at different signaling thresholds (64) and that balanced and focused BcR signaling seems to determine the fate of both B-1- and MZ B-cell populations. Mice lacking molecules important in the BcR signalosome have altered expression of these B-cell subsets. First, CD5, expressed on B-1 cells, is physically associated with sIgM in the BcR complex and may be a negative regulator of BcR signaling by acting via a pseudoimmunoreceptor tyrosine-based activation motif (17). Second, mice lacking key signaling effectors, such as CD19 and CD45, show an absence or severe reduction of B-1 cells (5). Third, MZ B cells are markedly reduced in the absence of CD19 (37, 39), and they also fail to develop in the absence of Pyk-2 tyrosine kinase (25) and in mice lacking the transcription factors NFκBp50 (10) and Ailolos (66). Fourth, the highly motile behavior of MZ B cells is altered in Pyk-2−/−, Lsc−/−, Doc2−/−, and CD22−/− mice, leading to abnormal MZ development (16, 19, 25, 56). Fifth, enhanced BcR signaling facilitates FO B-cell activation and survival but impairs MZ B-cell generation (11). It is therefore tempting to suggest that protein L somehow delivers signals through the BcR that impact preferentially B-1 and MZ B cells. However, although a functional BcR is required for B-cell development, a variety of other receptors and signals from the microenvironment also seem essential. In addition to BcR-mediated signaling, the selecting stimuli probably depend on environmental factors, including stroma cells, competitor cells and soluble signals. For example, the TACI longevity factor influences the survival of B-1 and MZ populations (24). Therefore, we cannot formally exclude the possibility that, through indirect effects, protein L affects B-1 and MZ B-cell development.
It is notable that, compared to protein L that appears to affect innate B-cell populations specifically, another B-cell superantigen, S. aureus protein A, targets VH3+ mature B lymphocytes in all compartments, including B-1, MZ, and FO cells (21). Whether these disparities reflect differences in signals delivered by B-cell SAgs that bind VH gene products (protein A) or Vκ gene products (protein L) or differences in the capacity of human versus murine surface Ig molecules to provide signals to B cells in vivo or other features of the two B-cell SAgs remains to be determined.
Finally, as discussed above, B-cell subsets are distributed in a predetermined fashion, probably to cope selectively with different kinds of exogenous antigens. The ability to elicit adaptive and innate B-cell responses to different environmental stimuli probably requires both ligand-dependent and ligand-independent functions, providing a mechanism for enhanced flexibility in responding to exogenous insults. The ability of the microbial protein we have studied to target B cells responsible for innate immunity reveals a novel escape mechanism that may be used by some infectious agents to evade the host's immune system.
Acknowledgments
This study was supported by grants from the SIDACTION (Paris, France), the Agence Nationale de Recherches sur le Sida (ANRS; Paris, France), and the Swedish Research Council (projects 7480 and 14379). M.V. is supported by a predoctoral fellowship from ANRS. M.Z. is a senior investigator of the Institut National de la Santé et de la Recherche Médicale (INSERM, Paris, France).
We are indebted to Marianne Brüggemann for the transgenic mice used in these experiments.
Editor: F. C. Fang
REFERENCES
- 1.Akerstrom, B., and L. Bjorck. 1989. Protein L: an immunoglobulin light chain-binding bacterial protein: characterization of binding and physicochemical properties. J. Biol. Chem. 264:19740-19746. [PubMed] [Google Scholar]
- 2.Axcrona, K., L. Bjorck, and T. Leanderson. 1995. Multiple ligand interactions for bacterial immunoglobulin-binding proteins on human and murine cells of the hematopoietic lineage. Scand. J. Immunol. 42:359-367. [DOI] [PubMed] [Google Scholar]
- 3.Berberian, L., L. Goodglick, T. J. Kipps, and J. Braun. 1993. Immunoglobulin VH3 gene products: natural ligands for HIV gp120. Science 261:1588-1591. [DOI] [PubMed] [Google Scholar]
- 4.Berberian, L., J. Shukla, R. Jefferis, and J. Braun. 1994. Effects of HIV infection on VH3 (D12 idiotope) B cells in vivo. J. Acquir. Immun. Defic. Syndr. 7:641-646. [PubMed] [Google Scholar]
- 5.Berland, R., and H. H. Wortis. 2002. Origins and functions of B-1 cells with notes on the role of CD5. Annu. Rev. Immunol. 20:253-300. [DOI] [PubMed] [Google Scholar]
- 6.Bessudo, A., L. Rassenti, D. Havlir, D. Richman, E. Feigal, and T. Kipps. 1998. Aberrant and unstable expression of immunoglobulin genes in persons infected with human immunodeficiency virus. Blood 92:1317-1323. [PubMed] [Google Scholar]
- 7.Bjorck, L. 1988. Protein L: a novel bacterial cell wall protein with affinity for Ig L chains. J. Immunol. 140:1194-1197. [PubMed] [Google Scholar]
- 8.Brezinschek, H. P., R. I. Brezinschek, and P. E. Lipsky. 1995. Analysis of the heavy chain repertoire of human peripheral B cells using single-cell polymerase chain reaction. J. Immunol. 155:190-202. [PubMed] [Google Scholar]
- 9.Brezinschek, H. P., S. J. Foster, R. I. Brezinschek, T. Dorner, R. Domiati-Saad, and P. E. Lipsky. 1997. Analysis of the human VH gene repertoire: differential effects of selection and somatic hypermutation on human peripheral CD5+/IgM+ and CD5−/IgM+ B cells. J. Clin. Investig. 99:2488-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cariappa, A., H. C. Liou, B. H. Horwitz, and S. Pillai. 2000. Nuclear factor κB is required for the development of marginal zone B lymphocytes. J. Exp. Med. 192:1175-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cariappa, A., M. Tang, C. Parng, E. Nebelitskiy, M. Carroll, K. Georgopoulos, and S. Pillai. 2001. The follicular versus marginal zone B lymphocyte cell fate decision is regulated by Aiolos, Btk, and CD21. Immunity 14:603-615. [DOI] [PubMed] [Google Scholar]
- 12.Domiati-Saad, R., J. F. Attrep, H. P. Brezinschek, A. H. Cherrie, D. R. Karp, and P. E. Lipsky. 1996. Staphylococcal enterotoxin D functions as a human B-cell superantigen by rescuing VH4-expressing B cells from apoptosis. J. Immunol. 156:3608-3620. [PubMed] [Google Scholar]
- 13.Domiati-Saad, R., and P. E. Lipsky. 1998. Staphylococcal enterotoxin A induces survival of VH3-expressing human B cells by binding to the VH region with low affinity. J. Immunol. 161:1257-1266. [PubMed] [Google Scholar]
- 14.Farner, N. L., T. Dorner, and P. E. Lipsky. 1999. Molecular mechanisms and selection influence the generation of the human V lambda J lambda repertoire. J. Immunol. 162:2137-2145. [PubMed] [Google Scholar]
- 15.Foster, S. J., H. P. Brezinschek, R. I. Brezinschek, and P. E. Lipsky. 1997. Molecular mechanisms and selective influences that shape the kappa gene repertoire of IgM+ B cells. J. Clin. Investig. 99:1614-1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukui, Y., O. Hashimoto, T. Sanui, T. Oono, H. Koga, M. Abe, A. Inayoshi, M. Noda, M. Oike, T. Shirai, and T. Sasazuki. 2001. Hematopoietic cell-specific CDM family protein DOCK2 is essential for lymphocyte migration. Nature 412:826-831. [DOI] [PubMed] [Google Scholar]
- 17.Gary-Gouy, H., P. Bruhns, C. Schmitt, A. Dalloul, M. Daeron, and G. Bismuth. 2000. The pseudo-immunoreceptor tyrosine-based activation motif of CD5 mediates its inhibitory action on B-cell receptor signaling. J. Biol. Chem. 275:548-556. [DOI] [PubMed] [Google Scholar]
- 18.Genovese, A., G. Borgia, L. Bjorck, A. Petraroli, A. de Paulis, M. Piazza, and G. Marone. 2003. Immunoglobulin superantigen protein L induces IL-4 and IL-13 secretion from human Fc epsilon RI+ cells through interaction with the kappa light chains of IgE. J. Immunol. 170:1854-1861. [DOI] [PubMed] [Google Scholar]
- 19.Girkontaite, I., K. Missy, V. Sakk, A. Harenberg, K. Tedford, T. Potzel, K. Pfeffer, and K. D. Fischer. 2001. Lsc is required for marginal zone B cells, regulation of lymphocyte motility and immune responses. Nat. Immunol. 2:855-862. [DOI] [PubMed] [Google Scholar]
- 20.Goodglick, L., N. Zevit, M. S. Neshat, and J. Braun. 1995. Mapping the Ig superantigen-binding site of HIV-1 gp120. J. Immunol. 155:5151-5159. [PubMed] [Google Scholar]
- 21.Goodyear, C. S., and G. J. Silverman. 2003. Death by a B-cell superantigen: in vivo VH-targeted apoptotic supraclonal B-cell deletion by a staphylococcal toxin. J. Exp. Med. 197:1125-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Graille, M., E. A. Stura, A. L. Corper, B. J. Sutton, M. J. Taussig, J. B. Charbonnier, and G. J. Silverman. 2000. Crystal structure of a Staphylococcus aureus protein A domain complexed with the Fab fragment of a human IgM antibody: structural basis for recognition of B-cell receptors and superantigen activity. Proc. Natl. Acad. Sci. USA 97:5399-5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graille, M., E. A. Stura, N. G. Housden, J. A. Beckingham, S. P. Bottomley, D. Beale, M. J. Taussig, B. J. Sutton, M. G. Gore, and J. B. Charbonnier. 2001. Complex between Peptostreptococcus magnus protein L and a human antibody reveals structural convergence in the interaction modes of Fab binding proteins. Structure 9:679-687. [DOI] [PubMed] [Google Scholar]
- 24.Gross, J. A., S. R. Dillon, S. Mudri, J. Johnston, A. Littau, R. Roque, M. Rixon, O. Schou, K. P. Foley, H. Haugen, S. McMillen, K. Waggie, R. W. Schreckhise, K. Shoemaker, T. Vu, M. Moore, A. Grossman, and C. H. Clegg. 2001. TACI-Ig neutralizes molecules critical for B-cell development and autoimmune disease-impaired B-cell maturation in mice lacking BLyS. Immunity 15:289-302. [DOI] [PubMed] [Google Scholar]
- 25.Guinamard, R., M. Okigaki, J. Schlessinger, and J. V. Ravetch. 2000. Absence of marginal zone B cells in Pyk-2-deficient mice defines their role in the humoral response. Nat. Immunol. 1:31-36. [DOI] [PubMed] [Google Scholar]
- 26.Hardy, R. R., and K. Hayakawa. 2001. B-cell development pathways. Annu. Rev. Immunol. 19:595-621. [DOI] [PubMed] [Google Scholar]
- 27.Hardy, R. R., R. Wasserman, Y. S. Li, S. A. Shinton, and K. Hayakawa. 2000. Response by B-cell precursors to pre-B receptor assembly: differences between fetal liver and bone marrow. Curr. Top. Microbiol. Immunol. 252:25-30. [DOI] [PubMed] [Google Scholar]
- 28.Hayakawa, K., and R. R. Hardy. 2000. Development and function of B-1 cells. Curr. Opin. Immunol. 12:346-353. [DOI] [PubMed] [Google Scholar]
- 29.Hillson, J. L., N. S. Karr, I. R. Oppliger, M. Mannik, and E. H. Sasso. 1993. The structural basis of germline-encoded VH3 immunoglobulin binding to staphylococcal protein A. J. Exp. Med. 178:331-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Janeway, C. A., Jr. 2001. How the immune system works to protect the host from infection: a personal view. Proc. Natl. Acad. Sci. USA 98:7461-7468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Juompan, L., P. Lambin, and M. Zouali. 1998. Selective deficit in antibodies specific for the superantigen binding site of gp120 in HIV infection. FASEB J. 12:1473-1480. [DOI] [PubMed] [Google Scholar]
- 32.Karray, S., L. Juompan, R. C. Maroun, D. Isenberg, G. J. Silverman, and M. Zouali. 1998. Structural basis of the gp120 superantigen-binding site on human immunoglobulins. J. Immunol. 161:6681-6688. [PubMed] [Google Scholar]
- 33.Karray, S., and M. Zouali. 1997. Identification of the B-cell superantigen-binding site of HIV-1 gp120. Proc. Natl. Acad. Sci. USA 94:1356-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kastern, W., E. Holst, E. Nielsen, U. Sjobring, and L. Bjorck. 1990. Protein L, a bacterial immunoglobulin-binding protein and possible virulence determinant. Infect. Immun. 58:1217-1222.2108927 [Google Scholar]
- 35.Kastern, W., U. Sjobring, and L. Bjorck. 1992. Structure of peptostreptococcal protein L and identification of a repeated immunoglobulin light chain-binding domain. J. Biol. Chem. 267:12820-12825. [PubMed] [Google Scholar]
- 36.Lalor, P. A., and G. Morahan. 1990. The peritoneal Ly-1 (CD5) B-cell repertoire is unique among murine B-cell repertoires. Eur. J. Immunol. 20:485-492. [DOI] [PubMed] [Google Scholar]
- 37.Makowska, A., N. N. Faizunnessa, P. Anderson, T. Midtvedt, and S. Cardell. 1999. CD1high B cells: a population of mixed origin. Eur. J. Immunol. 29:3285-3294. [DOI] [PubMed] [Google Scholar]
- 38.Martin, F., and J. F. Kearney. 2000. B-cell subsets and the mature preimmune repertoire: marginal zone and B1 B cells as part of a “natural immune memory.” Immunol. Rev. 175:70-79. [PubMed] [Google Scholar]
- 39.Martin, F., and J. F. Kearney. 2000. Positive selection from newly formed to marginal zone B cells depends on the rate of clonal production, CD19, and btk. Immunity 12:39-49. [DOI] [PubMed] [Google Scholar]
- 40.Martin, F., and J. F. Kearney. 2000. Selection in the mature B-cell repertoire. Curr. Top. Microbiol. Immunol. 252:97-105. [DOI] [PubMed] [Google Scholar]
- 41.Martin, F., A. M. Oliver, and J. F. Kearney. 2001. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity 14:617-629. [DOI] [PubMed] [Google Scholar]
- 42.Nemazee, D., and M. Weigert. 2000. Revising B-cell receptors. J. Exp. Med. 191:1813-1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neshat, M. N., L. Goodglick, K. Lim, and J. Braun. 2000. Mapping the B-cell superantigen binding site for HIV-1 gp120 on a VH3 Ig. Int. Immunol. 12:305-312. [DOI] [PubMed] [Google Scholar]
- 44.Nicholson, I. C., X. Zou, A. V. Popov, G. P. Cook, E. M. Corps, S. Humphries, C. Ayling, B. Goyenechea, J. Xian, M. J. Taussig, M. S. Neuberger, and M. Bruggemann. 1999. Antibody repertoires of four- and five-feature translocus mice carrying human immunoglobulin heavy chain and kappa and lambda light chain yeast artificial chromosomes. J. Immunol. 163:6898-6906. [PubMed] [Google Scholar]
- 45.Nilson, B. H., A. Solomon, L. Bjorck, and B. Akerstrom. 1992. Protein L from Peptostreptococcus magnus binds to the kappa light chain variable domain. J. Biol. Chem. 267:2234-2239. [PubMed] [Google Scholar]
- 46.Ochsenbein, A. F., T. Fehr, C. Lutz, M. Suter, F. Brombacher, H. Hengartner, and R. M. Zinkernagel. 1999. Control of early viral and bacterial distribution and disease by natural antibodies. Science 286:2156-2159. [DOI] [PubMed] [Google Scholar]
- 47.Oliver, A. M., F. Martin, G. L. Gartland, R. H. Carter, and J. F. Kearney. 1997. Marginal zone B cells exhibit unique activation, proliferative and immunoglobulin secretory responses. Eur. J. Immunol. 27:2366-2374. [DOI] [PubMed] [Google Scholar]
- 48.Oliver, A. M., F. Martin, and J. F. Kearney. 1999. IgMhigh CD21high lymphocytes enriched in the splenic marginal zone generate effector cells more rapidly than the bulk of follicular B cells. J. Immunol. 162:7198-7207. [PubMed] [Google Scholar]
- 49.Patella, V., V. Casolaro, L. Bjorck, and G. Marone. 1990. Protein L: a bacterial Ig-binding protein that activates human basophils and mast cells. J. Immunol. 145:3054-3061. [PubMed] [Google Scholar]
- 50.Patella, V., G. Florio, A. Petraroli, and G. Marone. 2000. HIV-1 gp120 induces IL-4 and IL-13 release from human Fc epsilon RI+ cells through interaction with the VH3 region of IgE. J. Immunol. 164:589-595. [DOI] [PubMed] [Google Scholar]
- 51.Popov, A. V., X. Zou, J. Xian, I. C. Nicholson, and M. Bruggemann. 1999. A human immunoglobulin lambda locus is similarly well expressed in mice and humans. J. Exp. Med. 189:1611-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Potter, K. N., Y. Li, and J. D. Capra. 1996. Staphylococcal protein A simultaneously interacts with framework region 1, complementarity-determining region 2, and framework region 3 on human VH3-encoded Igs. J. Immunol. 157:2982-2988. [PubMed] [Google Scholar]
- 53.Radic, M. Z., and M. Zouali. 1996. Receptor editing, immune diversification and self-tolerance. Immunity 5:505-511. [DOI] [PubMed] [Google Scholar]
- 54.Rajewsky, K. 1996. Clonal selection and learning in the antibody system. Nature 381:751-758. [DOI] [PubMed] [Google Scholar]
- 55.Rolink, A. G., J. Andersson, and F. Melchers. 1998. Characterization of immature B cells by a novel monoclonal antibody, by turnover and by mitogen reactivity. Eur. J. Immunol. 28:3738-3748. [DOI] [PubMed] [Google Scholar]
- 56.Samardzic, T., D. Marinkovic, C. P. Danzer, J. Gerlach, L. Nitschke, and T. Wirth. 2002. Reduction of marginal zone B cells in CD22-deficient mice. Eur. J. Immunol. 32:561-567. [DOI] [PubMed] [Google Scholar]
- 57.Sasso, E. H., G. J. Silverman, and M. Mannik. 1991. Human IgA and IgG F(ab′)2 that bind to staphylococcal protein A belong to the VHIII subgroup. J. Immunol. 147:1877-1883. [PubMed] [Google Scholar]
- 58.Silverman, G. J. 1997. B-cell superantigens. Immunol. Today 18:379-386. [DOI] [PubMed] [Google Scholar]
- 59.Silverman, G. J., S. P. Cary, D. C. Dwyer, L. Luo, R. Wagenknecht, and V. E. Curtiss. 2000. A B-cell superantigen-induced persistent “hole” in the B-1 repertoire. J. Exp. Med. 192:87-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Silverman, G. J., R. Pires, and J. P. Bouvet. 1996. An endogenous sialoprotein and a bacterial B-cell superantigen compete in their VH family-specific binding interactions with human Igs. J. Immunol. 157:4496-4502. [PubMed] [Google Scholar]
- 61.Smith, J. M., N. H. Smith, M. O'Rourke, and B. G. Spratt. 1993. How clonal are bacteria? Proc. Natl. Acad. Sci. USA 90:4384-4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tanguay, D. A., T. P. Colarusso, S. Pavlovic, M. Irigoyen, R. G. Howard, J. Bartek, T. C. Chiles, and T. L. Rothstein. 1999. Early induction of cyclin D2 expression in phorbol ester-responsive B-1 lymphocytes. J. Exp. Med. 189:1685-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tortorella, D., B. E. Gewurz, M. H. Furman, D. J. Schust, and H. L. Ploegh. 2000. Viral subversion of the immune system. Annu. Rev. Immunol. 18:861-926. [DOI] [PubMed] [Google Scholar]
- 64.Tsubata, T. 1999. Co-receptors on B lymphocytes. Curr. Opin. Immunol. 11:249-255. [DOI] [PubMed] [Google Scholar]
- 65.Viau, M., B. Cholley, L. Bjorck, and M. Zouali. 2004. Down-modulation of the antigen-receptor by a superantigen for human B cells. Immunol. Lett. 92:91-96. [DOI] [PubMed]
- 66.Wang, J. H., N. Avitahl, A. Cariappa, C. Friedrich, T. Ikeda, A. Renold, K. Andrikopoulos, L. Liang, S. Pillai, B. A. Morgan, and K. Georgopoulos. 1998. Aiolos regulates B-cell activation and maturation to effector state. Immunity 9:543-553. [DOI] [PubMed] [Google Scholar]
- 67.Wortis, H. H., and R. Berland. 2001. Cutting edge commentary: origins of B-1 cells. J. Immunol. 166:2163-2166. [DOI] [PubMed] [Google Scholar]
- 68.Zouali, M. 1995. B-cell superantigens: implications for selection of the human antibody repertoire. Immunol. Today 16:399-405. [DOI] [PubMed] [Google Scholar]
- 69.Zouali, M. 2002. Natural antibodies, p. 638-643. In Encyclopedia of life sciences, vol. 12. Macmillan Reference, Ltd., London, England.