Abstract
Brain networks that govern parental response to infant signals have been studied with imaging techniques over the last 15 years. The complex interaction of thoughts and behaviors required for sensitive parenting of offspring enable formation of each individual’s first social bonds and critically shape infants’ behavior. This review concentrates on magnetic resonance imaging experiments which directly examine the brain systems involved in parental responses to infant cues. First, we introduce themes in the literature on parental brain circuits studied to date. Next, we present a thorough chronological review of state-of-the-art fMRI studies that probe the parental brain with a range of baby audio and visual stimuli. We also highlight the putative role of oxytocin and effects of psychopathology, as well as the most recent work on the paternal brain. Taken together, a new model emerges in which we propose that cortico-limbic networks interact to support parental brain responses to infants for arousal/salience/motivation/reward, reflexive/instrumental caring, emotion response/regulation and integrative/complex cognitive processing. Maternal sensitivity and the quality of caregiving behavior are likely determined by the responsiveness of these circuits toward long-term influence of early-life experiences on offspring. The function of these circuits is modifiable by current and early-life experiences, hormonal and other factors. Known deviation from the range of normal function in these systems is particularly associated with (maternal) mental illnesses – commonly, depression and anxiety, but also schizophrenia and bipolar disorder. Finally, we discuss the limits and extent to which brain imaging may broaden our understanding of the parental brain, and consider a current model and future directions that may have profound implications for intervention long term outcomes in families across risk and resilience profiles.
Keywords: parent-child relationships, brain imaging, fMRI, attachment, parenting, caregiving, oxytocin, maternal, paternal
1. Introduction to the Parental Brain
Human mothers and fathers manifest a repertoire of parental behaviors, thoughts and activities that cross cultures (Hrdy, 2000) and also manifest homologues across species (Clutton-Brock, 1991). For humans, besides meeting the primal evolutionary needs for survival and continuation of our species, parenting involves interrelated biological, psychological, and behavioral caregiving mechanisms that contribute critically to the first environment the child experiences as a new family member.
The rich animal literature on parental brain (Bridges, 2008) strongly supports the notion that a coherent understanding of the physiology governing parenting in humans is also possible. Indeed, much of the research on the human parental brain involves attempts to look for human homologues of brain circuits found in animals. This approach has been initially successful: using audiovisual stimuli of babies during functional imaging – particularly with contrasts of own versus unknown babies - to activate brain regions that recognize and respond to the special salience to the parent of one’s own baby stimuli (Barrett and Fleming, 2011; Swain, 2011b; Swain et al., 2007).
In this review, we summarize the state-of-the art of human parental brain imaging in mothers and fathers. More specifically, we focus on the interpretation respectively of brain-responses to baby-cry and baby visual stimuli and consider attempts to integrate findings within the cognitive, affective, and social neurosciences. First we selectively review the psychological and imaging evidence of relevant executive, emotion response/regulation and reward mechanisms that may be activated in the service of parental responsiveness sensitivity. We then discuss these findings in the context of research on oxytocin, and review recent attempts to understand how parenting difficulties or even psychopathology may be understood as abnormalities or malfunctions of parental brain circuits. The importance of this mechanistic understanding of parenting is underscored by consideration of effects on offspring and possibilities to identify families at risk and optimize therapeutic interventions.
1.1 Parental Sensitivity and Child Attachment
Mary Ainsworth first defined maternal sensitivity as a mother’s ability to attend and respond to her child in ways that are contingent to the infant’s needs (Ainsworth et al., 1978). In the naturalistic context, maternal care behavior including sensitivity shows a great deal of variation despite being relatively stable within individual mothers across time and contexts (Behrens et al., 2012; Jaffari-Bimmel et al., 2006; Wan et al., 2013). Maternal sensitivity represents a pattern of behavior which provides the infant with its primary social experience. This suggests it is important for organizing and regulating the infant’s emotional, social and cognitive systems and is consistent with accumulating evidence that maternal sensitivity predicts a range of child outcomes - including the quality of their attachment relationships (Bakermans-Kranenburg et al., 2003; De Wolff and van Ijzendoorn, 1997), self-regulation (Eisenberg et al., 2001), social functioning (Kochanska, 2002; Van Zeijl et al., 2006), socio-emotional development (De Wolff and van Ijzendoorn, 1997), and cognitive and language competence (Bernier et al., 2010; Tamis-LeMonda et al., 2001). Furthermore, the absence of skills needed to respond sensitively to child signals has been linked to risk for maltreatment (Milner, 1993, 2003). Poor maternal sensitivity in infancy predicts later harsh parenting (Joosen et al., 2012) and attitudes towards punishment (Engfer and M, 1987). Frightening and anomalous maternal behavior confers profound risk to the parent-infant attachment relationship (Schuengel et al., 1999).
The concept of parent-infant attachment represents a landmark of contemporary developmental psychology (Bowlby, 1969b, 1973). In fact, John Bowlby formulated his attachment theory after studying associations between maternal deprivation and juvenile delinquency, postulating a universal human need to form close, affect-laden bonds, primarily between mother and infant. He strongly argued, from an evolutionary perspective, that attachment represents an innate biological system promoting proximity-seeking between an infant and a conspecific attachment figure. This proximity then increases the likelihood of survival to reproductive age.
Because of this powerful biological instinct for attachment, and in response to the patterns of attachment identified in the Ainsworth mother-infant studies (Ainsworth et al., 1978), Bowlby hypothesized that all human infants attach to their caregiver but that children manifest different patterns of attachment “security” depending on the quality of the care they receive (Bowlby, 1977). Indeed, a vast literature in the study of attachment over the last several decades has established that infants of caregivers who are available, responsive and sensitive to their emotional and physical needs tend to manifest patterns of “secure attachment.” Conversely, chaotic, unpredictable, rejecting or neglectful care in which non-contingent responses to the child occur frequently, result in insecure or disorganized patterns of attachment evolves (Shaver et al., 1987).
Understanding the neurobiology of attachment-formation through parental sensitivity may help to formulate and ameliorate pervasive and complex social problems such as child abuse and neglect. However, little is known about the cognitive or neurobiological mechanisms which underpin healthy sensitivity, let alone poor sensitivity in mothers with mental illness. This may explain why promising interventions targeted at improving parenting through improved sensitivity have shown inconsistent findings and generally small effect sizes (Bakermans-Kranenburg et al., 2003; Wan et al., 2008c).
1.2 Neurocognitive mechanisms underlying parental sensitivity
For sensitive caregiver responses to infant cues, complex brain systems must manage an array of complex thoughts and behaviors contingent on feedback from the baby. These include recognition and acknowledgment of child signals, attribution of salience to child cues, maintenance of visual contact, expression of positive affect, appropriate mirroring and vocal quality, resourcefulness in handling child’s distress or expanding the interaction, consistency of style, and display of an affective range that matches the infant’s readiness to interact. Such behaviors are likely to be the result of complex neural networks involved in generating and organizing emotional responses (Kober et al., 2008) as well as in attention & executive function, reward & motivation, and sensorimotor circuits – a model well supported by the literature (Swain and Lorberbaum, 2008). We update this model below incorporating the newest work on fathers and the effects of mental illness on parenting capacity. Indeed, mental health problems may manifest as impairment in any of these networks and play a significant role in poor parenting. Identifying these may provide an opportunity to predict which parents are likely to have which type of parenting problems and facilitate targeting of interventions. Here, we highlight brain processes, mechanistically related to certain cortico-limbic circuits, and relevant for healthy parenting sensitivity.
1.2a Executive Function
Executive functions, including attention control, working memory and flexible task-switching, are likely to be important to parental sensitivity. Deficits in executive function measures of attention set-shifting, spatial working memory and a sustained attention measure have been linked with poor maternal sensitivity to non-distress infant cues (Gonzalez et al., 2012). In closely related work, mothers with a classification of disorganized attachment responded more slowly to negative attachment words and the speed of response to such stimuli was correlated with disorganization, suggesting negative associations with attachment stimuli that may contribute to ongoing cognitive difficulties during mother-infant interactions (Atkinson et al., 2009). Greater attention bias to infant distress cues in late pregnancy has also been associated with better scores on a parental bonding questionnaire (Pearson et al., 2010), raising the question of how attention deficit disorder affects parenting – an issue yet to be studied. Attention bias to infant distress was also compared between breastfeeding and formula feeding mothers of 3–6 month old infants and observed to be greater in breastfeeding mothers (Pearson et al., 2011). It is plausible that reduced attention bias towards emotional stimuli (specifically infant distress) is associated with low maternal sensitivity in some mothers. However, other mothers with low sensitivity may show a selectively exaggerated attention bias to infant distress which results in the mother becoming overwhelmed by the stimuli; this may be particularly relevant for mothers and fathers with schizophrenia. In depressed patients, evidence suggests a tendency to pay more attention to negative emotional stimuli (Elliott et al., 2011); an effect mediated by enhanced response in the ventral anterior cingulate, which may contribute to the maintenance of low mood.
1.2b Emotion Regulation
Recognizing emotion in preverbal infants is more difficult than recognizing emotions in adults. In some parents, inability to recognize and distinguish the subtleties of infant emotion cues may underpin poor maternal sensitivity to infant cues. Consistent with this, depression is associated with decreased discrimination of facial emotion (Anderson et al., 2011). Response to distressing signals from the infant also requires a mother to distinguish positive from negative emotions; indeed, studies suggest that a mother’s sensitivity to distress may be a better predictor of child outcomes than her sensitivity to non-distress cues (Joosen et al., 2012; Leerkes, 2011; Leerkes et al., 2009; McElwain and Booth-Laforce, 2006). Thus, poor maternal care behavior could derive in part from reduced recognition, as well as reduced response to infant emotions generally, and/or specifically, to signals of infant distress. Conversely, some mothers may become overwhelmed by their infant’s distress. Notably, enhanced responsiveness to negative emotions, (mediated by enhanced amygdala response), has been observed in non-parent depression (Arnone et al., 2012). In depressed mothers, studies suggest women may avoid or limit exposure to distressing infant stimuli (Field, 2010; Murray et al., 1996; Pearson et al., 2012). In anxiety and depression, modulating stress and emotional responsiveness is an important target for treatment and is associated with clinical improvement (Harmer et al., 2011). The importance of emotion regulation in the responses to baby stimuli is also consistent with the ideas of postpartum preoccupations discussed below (1.2d).
1.2c Reward/Motivation
Extensive recent review of the animal literature (Numan and Woodside, 2010) suggests that response to infants forms a model motivational system employing dopamine and oxytocin-rich pathways such as the medial preoptic area (MPOA). Through such pathways, infant cues are thought to provide motivation for maternal care behavior. Reward processes include immediate hedonic responses (‘liking’) and approach motivation (‘wanting’) or learning (Berridge and Kringelbach, 2008). Frontostriatal brain regions are also critically implicated in reward, in particular the orbitofrontal cortex (OFC) (Rolls, 2004) and ventral striatum including nucleus accumbens (NAcc) (Born et al., 2011). Although the OFC generally codes hedonic signals, the medial OFC is particularly important for computing reward value while the lateral OFC makes stronger contributions to reward learning. In mothers, the initial experience of pleasure and activity in these brain circuits when exposed to their own infant’s cues may increase the salience of their infant’s stimuli and promote greater attention and bond-formation to ensure continuous engagement in sensitive caregiving. Indeed, reward/motivation pathways have been shown to be active in response to baby-stimuli (discussed below). In mothers with low sensitivity, the motivational or incentive salience of emotional and/or infant cues may be diminished through deficits in this and also in other OT-opioid reward pathways (Curley, 2011). Thus, in rhesus monkeys, mothers with greater attachment to infants possess the G allele of the OPRM1 μ-opioid receptor and have higher oxytocin responses to lactation and pregnancy (Higham et al., 2011). Strikingly, activity of opioids at the μ-opioid receptor is also central to the processing of reward in the context of drug dependence (Herz, 1997; Simmons and Self, 2009), and social behaviors (Higham et al., 2011). Very few studies, however, have examined neural substrates of reward-related processes among mothers who display disrupted caregiving behavior.
1.2d Parental Thoughts – Preoccupations/habits, empathy and positive thoughts
Parents experience dynamic change in their thoughts and behaviors oriented toward their new infants. Immediately after a child’s birth and during the first few months of the infant’s life, parents are particularly drawn to their infants’ vocalizations and physical attributes and focus their thoughts on the infants’ physical and psychological needs (Bowlby, 1969a; Winnicott, 1956). Such intense mental focus and behaviors were first referred to as ‘primary maternal preoccupation’ by Donald Winnicott (1956) and seem also consistent with the activation of motivational-reward pathways by infant cues reflecting an important evolutionary mechanism through which infants promote long-term emotional ties with their parents (Atkinson et al., 2009; Feldman et al., 1999; Gonzalez et al., 2012; Leckman and Mayes, 1999).
Leckman et al. (1999) reported that all domains of parental preoccupation peaked right after childbirth and then began to decrease over the course of the first three to four postpartum months in mothers and fathers, albeit less intensely for fathers. Since newborns have very limited ways of communicating their needs, parents must constantly check the infants in order to identify and attend to those needs. The intensity of parental preoccupations decreases as parents gain more experience in parenting and infants become more responsive. However, excessively and persistently high levels of parental preoccupation, particularly anxious and intrusive thoughts and harm-avoidant behaviors can be associated with postpartum psychopathology such as postpartum obsessive compulsive disorders (OCD). In one sample, levels of parental preoccupations were related to postpartum anxiety at the first month postpartum (Feldman et al., 1999) and lower maternal sensitivity at 3–4 months postpartum (Kim et al., 2013). Abnormally low levels of parental preoccupation, e.g. in postpartum depression also pose difficulties in developing emotional bonds with the infant, and risk inadequate parenting (Feldman et al., 2009; Feldman et al., 1999). Maternal schizophrenia also interferes with care giving behavior in ways that merit separate study and treatment (Abel et al., 2005; Wan et al., 2008a; Wan et al., 2008b; Wan et al., 2008c). For typically developing parents, the story for fathers will likely differ from mother. For example, unlike for mothers, paternal sensitivity was predicted by anxious as well as caregiving and positive thoughts (Kim et al., 2013). More nuanced assessments of mother and father thought, mood and behaviors is required to clarify how they are related over time and how they might inform the nascent study of paternal brain physiology (below).
Parental empathy, (appropriate perception, experience and response to another’s emotion) may be especially relevant for pre-verbal infants. Deficits of empathy in disorders such as schizophrenia and autism have been associated with abnormalities in certain brain networks (Gallese et al., 2004; Iacoboni, 2009; Uddin et al., 2007). These systems overlap significantly with brain responses of parents to infant stimuli reviewed here: i.e. cingulate and insular cortices (Bernhardt and Singer, 2012).
Experiencing pain personally and experiencing the pain of a loved one activates insula and anterior cingulate, with another’s pain activating more anterior regions and own pain also recruiting brainstem, cerebellum, and sensorimotor cortex. Such decoupled, yet parallel representations of empathy in cortical structures like insula and anterior cingulate are postulated as necessary for our ability to mentalize, that is, understand the thoughts, beliefs, and intentions of others in relation to ourselves (Frith and Frith, 2003; Hein and Singer, 2008). Humans may utilize separate circuitry to ‘decouple’ representations of external vs. internal information in order to understand physical properties and assess personal emotional values. In support of this, a brain network consistently activated during tasks that require mentalizing has emerged, including dorsomedial prefrontal cortex (DMPFC), medial prefrontal cortex (MPFC), precuneus/posterior cingulate cortex (PCC), temporoparietal junction (TPJ), and posterior superior temporal sulcus (pSTS) (Frith and Frith, 2006; Mitchell, 2009). Such a mentalizing framework promises to be very helpful in considering parent-infant dyads to support behavioral planning – with important implications for the function of mentalizing networks and health (Eisenberger and Cole, 2012).
By three to four months postpartum, infants are more socially interactive, and parents increasingly engage in reciprocal positive interactions. These positive interactions further help mothers to strengthen their attachment and heighten the experience of positive feelings toward their infants (Mercer, 1985). As they successfully feed, take care of, and build affectionate connections with their infants, mothers develop positive feelings and self-confidence about parenting (Benedek, 1954). Fathers may develop attachment to their infants more gradually (Anderson, 1996; Pruett, 1998). Positive feelings about their infants and parenting experience maybe critical in pathways engaging dopamine-oxytocin reward circuits described in animal models (MacDonald, 1992; Numan and Insel, 2003b; Shahrokh et al., 2010). Thus, interactions with the infant may enhance parental oxytocin and dopamine release and foster the maintenance of positive parental behaviors with associated attentiveness and sensitive caregiving – perhaps implicating future therapeutic potential (Macdonald et al., 2013). In contrast, if interactions with the infant are negative and stressful, parents may experience less brain activation in reward-motivation pathways, find the relationship less satisfying, harbor fewer positive parental thoughts and be less willing to maintain it.
Such reward pathways maybe relevant very early in the postpartum, as mother’s positive feelings towards her unborn fetus as well as her perception of her fetus have been associated with greater maternal sensitivity to the infant’s signals and more affectionate vocalizations and touch (Keller et al., 2003; Keren et al., 2003). It may be interesting in the future to examine prepartum thoughts as relevant for postpartum behaviors. Idealization of the infant and positive thoughts about parenting may positively reinforce both quality of parenting and feelings of reward. On the other hand, worrying about the infant – such as health and other concerns - may be associated with lower parental sensitivity and higher intrusiveness. Indeed, negative feelings about parenting may be linked to the parents’ difficulties in developing emotional bonds with their infants (Mercer, 1985; Nystrom and Ohrling, 2004) although the nature and direction of this link needs clarification.
The following sections review the current state of evidence from experiments designed to elucidate the brain basis of parental attachment by presenting infant stimuli – sometimes emotionally charged - during brain imaging. Such studies have incorporated psychological, behavioral and hormonal measures and a very few include parents with abnormalities in parenting or mental illness. The power and sensitivity of functional imaging deliver mechanistic understanding of abnormalities in brain associated with poor parenting and promise earlier detection of problems that may not be obvious from behavioral or self-report measures. In the future, brain-based understandings may allow refinement of tailored treatment approaches toward parenting and targeted augmentation of parenting resiliency (Rutter, 2013; Swain et al., 2012).
Table 1 and 2 summarizes experiments to date on human parents using baby sound and visual stimuli with brain fMRI.
Table 1 & 2. Human Parent Brain Responses to Infant Stimuli.
- Arousal/Salience = amygdala, ventral striatum
- Reflex Care = hypothalamus
- Emotion Regulation = mPFC, ACC
- Cognition = dlPFC, insula, inferior frontal and orbitofrontal gyri, temporoparietal junction
| Author, Year | Parent Group N | Age of Infants | Paradigm, Variable | Study Design | Brain Network | |||
|---|---|---|---|---|---|---|---|---|
| Arousal Salience | Reflex Care | Emotion Regulation | Cognition | |||||
| Baby-Cry, Mothers | ||||||||
| Lorberbaum et al., 1999 | 4 | 3 weeks-3.5 years | Other vs. control | 30s blocks | Y | Y | Y | Y |
| Lorberbaum et al., 2002 | 10 | 1–2 months | Other vs. control | 30s blocks | Y | Y | Y | Y |
| Seifritz, 2003 | 10 | <3 years | Other vs. control, +ve/−ve | 6 s events | Y | N | Y | Y |
| Swain et al., 2003, 2004a, 2006 | 11–14 | 2–4 weeks & 3–4 months | Own vs. other, experience & thoughts | 30s blocks | Y | Y | Y | Y |
| Swain et al., 2008 | 12 | 2–4 weeks | Own vs. other, Delivery | 30s blocks | Y | Y | Y | Y |
| Kim et al., 2010a | 26 | 2–4 weeks | Own vs. control, Early-Life | 30s blocks | Y | N | Y | Y |
| Kim et al., 2011 | 20 | 2–4 weeks | Own vs. Other, Feeding | 30s blocks | Y | Y | Y | Y |
| Laurent & Ablow, 2012 | 22 | 18 months | Own vs. Other, Attachment | 21s blocks | ||||
| Laurent, Stevens, & Ablow, 2011 | 22 | 18 months | Own vs. Other, HPA axis | 21s blocks | N | Y | Y | Y |
| Venuti et al., 2012 | 9 (of 18) | >4years | Hunger cry vs. noise, atypical cry from infants with autism | 10s events | N | N | Y | Y |
| Baby Cry, Fathers | ||||||||
| Seifritz, 2003 | 10 | <3 years | Other vs. control, +ve/−ve | 6 s events | Y | Y | Y | Y |
| Swain et al., 2003, 2004a | 9 | 2–4 weeks & 3–4 months | Own vs. other, experience & thoughts | 21s blocks | Y | Y | Y | Y |
| De Pisapia et al, 2013 | 9M + 9F | 1 year | Hunger cry; males vs. females | 14s blocks | N | N | N | |
| Baby Cry, Maternal-pathology | ||||||||
| Laurent & Ablow, 2011 | 22 | 18 months | Own vs. Other, Postpartum Depression | 21s blocks | Y | Y | Y | Y |
| Musser et al., 2012 | 22 | 18 months | Own vs. Other, Maternal Sensitivity | 21s blocks | Y | Y | Y | Y |
| Landi et al., 2011 | 54 | 2 months | Other vs. noise, high & low distress cry, substance abuse | 2s, events | Y | Y | Y | Y |
| Author, Year | Parent Group N | Age of Infants | Paradigm, Variable | Study Design | Brain Network | |||
|---|---|---|---|---|---|---|---|---|
| Arousal Salience | Reflex Care | Emotion Regulation | Cognition | |||||
| Mothers | Baby Visuals | |||||||
| Swain et al., 2003/4/5, prep | 9–14 | 2–4 weeks & 3–4 months | Own vs. other, experience & thoughts | 30s blocks | Y | Y | Y | Y |
| Bartels and Zeki, 2004 | 19 | 9 months – 6 years | Own vs. other, comparison with romantic partner | 15s blocks | Y | Y | Y | Y |
| Leibenluft et al., 2004 | 7 | 5–12 years | Own vs. other | 1.5s event | Y | Y | Y | Y |
| Ranote et al., 2004 | 10 | 4–8 months | Own vs. other | 20–40s | Y | Y | N | N |
| Nitschke et al., 2004 | 6 | 2–4 months | Own vs. other, affect | 30s blocks | N | N | Y | Y |
| Strathearn et al., 2005,2008,2009 | 28–30 | 3–18 months | Own vs. other, affect, OT | 2s events | Y | Y | Y | Y |
| Noriuchi et al., 2008 | 13 | 15– 20months | Own vs. other, distressed | 32s video | Y | Y | Y | Y |
| Lenzi et al., 2009 | 16 | 6–12 months | Own vs. other, joy/distress | 2s events | Y | Y | Y | Y |
| Atzil et al., 2011 | 28 | 4–6 months | Own vs. other, parenting syn | 2min video | Y | Y | Y | Y |
| Barrett et al., 2011 | 22 | 3 months | Own vs. other, parenting | 3s events | Y | Y | Y | Y |
| Reim et al., 2011 | 21 (non-mom) | N/A | Cry (2 day) vs. control | 10s events | Y | Y | Y | Y |
| Atzil et al., 2012 | 15 | 4–6 months | Own videos, synchrony, OT | 2min video | Y | Y | Y | Y |
| Lahey et al., 2012 | 35 | 4–6years | Own vs. other, parenting | 13s video | N | N | Y | Y |
| Fathers | Baby Visuals | |||||||
| Swain et al., 2003,2004a,2004b,2005, subm | 9–14 | 2–4 weeks & 3–4 months | Own vs. other, experience & thoughts | 30s blocks | Y | Y | Y | Y |
| Atzil et al., 2012 | 15 | 4–6 months | Own videos, synchrony, OT | 2min video | Y | Y | Y | Y |
| Kuo et al., 2012 | 10 | 2–4 months | Own vs. other videos, Baby vs. Doll videos | 15s video | Y | Y | Y | Y |
| Mascaro et al. 2013 | 70 | 1–2 years | Own vs. other images, measure T, father-behav | 14s blocks, regional | Y | Y | Y | Y |
| Maternal Psycho-pathology | Baby Visuals | |||||||
| Moses-Kolko, et al., 2010 | 30 | 12 weeks | Emotion faces, depression | 4s blocks | Y | Y | Y | Y |
| Schechter et al 2012 | 20 | 12–42months | Own>Other, IPV-PTSD | 40s video | Y | Y | Y | Y |
| Moser et al., 2013 | 20 | 12–42months | Own>Other, IPV-PTSD | 40s video | Y | Y | Y | Y |
| Laurent & Ablow, 2013 | 22 | 18 months | Own vs. other - depression | 18s blocks | Y | Y | Y | Y |
Glossary for Table: Activations and deactivations, measured by functional magnetic resonance imaging, satisfied significance criteria of random effects analysis at p<0.05 or fixed effects analysis at p<0.001 at a minimum.
1.3 Human Brain Imaging Methods
The primary technique used to study the brain in this review is non-invasive blood-oxygen-dependent functional magnetic resonance imaging (fMRI). fMRI assays brain activity by indirectly measuring changes in regional blood oxygenation during different periods in which infant cues may be presented. The differences between a region’s oxygenated and deoxygenated hemoglobin, between states of action vs. inaction for instance, provide characteristic magnetic signals localized to millimeters that are detected by scanners positioned around each subject’s head. An important caveat throughout the interpretation of parenting fMRI studies, however, is that that brain activity measurements represent an integration of electrical brain activity that may be instantaneous yet the related blood flow change lags behind over seconds. Furthermore, experimental design captures brain activity over periods of a few seconds or 10’s of seconds. On the one hand, short blocks or events may capture briefly held mental states, but miss bigger changes such as sustained emotion; while on the other hand longer blocks may capture more complex brain responses, but also average them out making subtle responses more difficult to detect. Brain activity during these blocks may then be measured and compared between periods of attending to stimuli of interest and control stimuli to generate maps of the brain indicating differences in brain activity that may be important for one set of perceptions and thoughts versus another. With parents as subjects, infant cries and pictures have been stimuli and comparisons of brain activity measured during baby cry vs. control sound experience have been said to relate to the parental experience of a baby cry, and so the associated parenting thoughts and behaviors. Many of these studies make use of seed-based analyses that will require replication in different populations to permit generalization of findings and all results could relate to activity that is excitatory or inhibitory. Future imaging with connectivity analyses also promise to define more than just response regions, but circuit behavior involving coordinated activity between distant brain areas (Sripada et al., 2013). The important limitation that response to baby cues may not be directly related to parental care is being addressed in studies that correlate responses to parental care described below.
2. Parental Brain Responses to Infant Stimuli
2.1 What’s in a Baby-Cry?
In addition to communicating basic levels of discomfort, hunger, and pain (Soltis, 2004), baby cry also signals the need for physical proximity of caregivers leading to the dynamically interactive cry-care thoughts and behaviors that lead to attachment (Swain et al., 2004b), for which new parents appear to be primed (Bowlby, 1969b). Supportive studies have demonstrated that human mothers can recognize the cries of their own infants, and mothers and fathers activate brain in areas hypothesized to be involved in mammalian parenting behavior (Swain and Lorberbaum, 2008; Swain et al., 2007).
2.1a Brain Responses to Auditory Baby-Stimuli: Baby-Cry - 1st 10 years
Building on the thalamocingulate theory of maternal behavior in animals (MacLean, 1990), Lorberbaum and colleagues predicted and found that baby cries selectively activate cingulate and thalamus in mothers in the early postpartum months exposed to an audio taped 30 second standard baby cry using fMRI (Lorberbaum et al., 1999). Informed by the animal literature, they expanded their hypotheses to include the basal forebrain’s medial pre-optic area and ventral bed nucleus of the stria terminalis and its rich reciprocal connections as being critical to parental behaviors (Lorberbaum et al., 2002). These include the descending connections to modulate more basic reflexive caring behaviors such as nursing, licking, grooming and carrying reflexes in rodent studies, and ascending connections such as the mesolimbic and mesocortical dopamine systems for more general motivation and flexible responses to tend a crying infant or prepare for a threat. Even though these first studies involved cry stimuli that did not originate from the parent’s own infant, and the control sounds were emotionally negative (sounded like harsh static on the television), significant brain responses fit with existing knowledge about regulation of parenting behavior in animals (Numan and Insel, 2003a) and opened up the field.
Hypothesizing that gender and experience would also influence neural responses to baby sounds such as baby cry and laughter, Seifritz and colleagues (Seifritz et al., 2003) studied four groups: mothers and fathers of children under age 3, and non-parent males and females, with 10 subjects in each group. They used an event-related fMRI design, measuring brain responses to brief 6-s sounds. Over the entire sample, intensity-matched baby sounds of crying and laughing compared to “neutral” sounds (white noise pulsed at 5-Hz with an averaged frequency spectrum similar to the infant vocalizations) produced more brain activity in bilateral temporal regions. These regions might be important for hearing processes (Heshyl’s gyrus and temporal poles), processing human vocalizations, and empathic emotion processing including emotional memory. They also reported that women as a group including, parents and non-parents (but not men), showed decreased activity in response to both baby cry and laughter in the subgenual anterior cingulate cortex. This finding is contrary to the other studies (Lorberbaum et al., 1999; Lorberbaum et al., 2002; Swain et al., 2005; Swain and Lorberbaum, 2008) highlights how sample selection, choice of stimuli and precision of region of interest examined might affect findings. It is also the case that a 6 second vs. a 30 second stimulus time may have different meanings to new parents or there may be non-linear or multiphasic responses within anterior cingulate similar to the well-recognized phasic responses seen in amygdala. Their within-group analyses reported that parents activated more to infant crying than laughing in the right amygdala, while non-parent response was greater for infant laughing than crying (Seifritz et al., 2003). These within-group results suggest being a parent might be associated with changes in amygdala function, although there was no direct comparison of parents and non-parents. These data represent the first attempts to extend previous work and include gender and experience-dependent aspects of human parenting.
Taking this approach further, Swain and colleagues have been gathering data on groups of new parents across a range of experience, temperament and parent-infant interaction styles using each parent’s own baby cries and including comprehensive interviews and self-reports (Swain et al., 2003). In this design, parents underwent brain fMRI during 30 second blocks of infant cries generated by their own infant contrasted with a “standard” cry and control noises matched for pattern and intensity. In addition, they added a longitudinal component with scans and interviews at 2 time points: 2–4 weeks and 12–16 weeks postpartum to coincide with the transition to parenthood associated with peaks of parental preoccupation in the early postpartum (Leckman et al., 1999). They hypothesized that parental responses to own baby cries would include specific activations in thalamo-cortico-basal ganglia circuits believed to be involved in human ritualistic and obsessive-compulsive thoughts and behaviors (Baxter, 2003; Leckman et al., 2004). They also reasoned that emotional alarm, arousal and salience detection centers including amygdala, hippocampus and insula (Britton et al., 2006; LeDoux, 2003) would be particularly activated by own baby cry. The experimental block design was used in order to give parents a chance to reflect on their experience of parenting and, according to our hypothesis, become more preoccupied with their infants’ well-being and safety. In a group of first-time mothers (n=9) at 2–4 weeks postpartum, regions that were relatively more active with own versus unknown contrast included midbrain, basal ganglia, cingulate, amygdala and insula (Swain et al., 2003) which may reflect an increase in arousal, obsessive/anxiety circuits normally more active for new parents and persistently sensitive in some mental illnesses (Swain et al., 2007). Interview and self-report studies found that mothers were significantly more preoccupied than fathers, and consistent with the relatively greater activation of amygdala and basal ganglia in mothers compared with fathers (Swain et al., 2004a; Swain et al., 2014). In addition, grouping mothers and fathers together across experience and comparing activity at 2–4 weeks vs. 3–4 months postpartum for own baby cry showed that significant activity shifted from the amygdala and insula to medial prefrontal cortical and hypothalamic (including hormonal control) regions. This fits well with changes in parenting confidence/experience: in healthy parents, as a mother learns to associate her infant cries with more flexible social behaviors and more mature attachment, there is greater regulatory cortical brain activity and less alarm and anxiety-related activity (amygdala and insula).
2.1b Brain Responses to Auditory Baby-Stimuli: Baby-Cry - Last 5 years
Consistent with accumulating evidence of the importance of oxytocin (OT) in establishing and regulating parenting in humans (Galbally et al., 2011), neuroimaging in humans suggests brain circuits that respond to baby-stimuli are also associated with OT pathways. In one study, mothers experiencing vaginal vs. cesarean delivery – as a proxy for higher vs. lower OT - showed greater brain activity in response to own vs. other baby-cry at 2–4 weeks postpartum in emotion regulation and limbic regions, including the caudate, thalamus, hypothalamus, amygdala and pons (Swain et al., 2008). This fits with evidence for cesarean section being associated with increased risk of postpartum depression – a condition of aberrant emotion processing (Groenewold et al., 2012). At 3–4 months postpartum, the same mothers – all of whom remained healthy - no longer showed differing responses to own baby-cry (Swain, 2011a), suggesting that any potential early-postpartum abnormalities in oxytocin are reversible. However, the relationship between Cesarean section and PPD is strongly confounded by a range of factors including low maternal age, past depression and low social supports.
Recent studies have also used breastfeeding as a proxy for maternal OT levels Imaging studies using baby cry (Kim et al., 2011), find breastfeeding vs. formula feeding is associated with greater activations to own baby cries vs. other baby cries in anterior and posterior cingulate, thalamus, midbrain, hypothalamus, septal regions, dorsal and ventral striatum, medial prefrontal cortex, right orbitofrontal/insula/temporal polar cortex region, and right lateral temporal cortex and fusiform gyrus. Additionally, when cry response was compared with the inter-stimulus rest periods, instead of the control sound (which some mothers judged to be aversive), the amygdala was active. Furthermore, and for the first time, Kim and colleagues (2011) reported a correlation of brain activity to own vs. unknown baby-cry with independently-rated -behavioral measures of parenting in response (amygdala) and regulation (frontal cortex) regions, suggesting the importance of balanced responses for sensitive care giving. Such studies have led to the suggestion that, combined with behavioral strategies, acute or prolonged OT treatment may offer a safe and accessible intervention for women at risk of postpartum depression although no randomized controlled trials have emerged.
Among nulliparous women, acute administration of oxytocin has been reported to decrease amygdala responses and increase insula and inferior frontal gyrus responses to potentially adverse stimuli i.e. infant cries (Riem et al., 2011b). Similarly, acute testosterone was also found to increase insula response to infant cries (Bos et al., 2010) in nulliparous healthy women. Testosterone is metabolized to estradiol, which activates neural regions important for maternal motivation. Therefore, increasing oxytocin and testosterone availability in maternal brain may modulate the processing of distressing emotional information e.g. infant cries and facilitate more appropriate responses, especially in mothers with dysregulated responses to such stimuli (see above). By contrast, greater cortisol reactivity to stress has also been associated with reduced neural responses to own baby cries (vs. other baby cries) among primiparous mothers (Laurent et al., 2011). The reduced neural responses were detected in the regions important for emotion regulation (anterior cingulate cortex and medial PFC) and maternal motivation (limbic area and periaqueductal gray). Studies such as these implicitly address effects of early-life events on later parental brain function, translating to humans some aspects of well-established rodent and non-human primate models (Champagne, 2010; Kaffman and Meaney, 2007; Veenema, 2009) as well as long-term effects of early trauma on stress axis functioning in human studies (Lupien et al., 2009; McEwen, 2008). Thus, in mothers who report higher maternal care in their own experience of childhood, greater responses to infant cries may be seen in regulatory cortical regions, including emotion regulation areas of the middle and superior frontal gyri, whereas mothers reporting lower perceived maternal care may be more likely to show increased hippocampal activations (Kim et al., 2010b) This underlines the importance for parenting of coordinated responses of these cortical and subcortical regions, as well as the importance of early life experience in the functioning of these circuits.
Recent work has also examined whether variations in infant cries are associated with maternal neural response to the cries. One study exposed nulliparous women to two different (high and low) distress levels of infant cries (Montoya et al., 2012). Interestingly, women showed greater neural responses to low distress cries versus high distress cries in the superior and middle temporal gyri. The findings suggest that, compared to high distress cries, it was harder for women to interpret the possible causes and meaning of the low distress cries. Another study exposed a group of men and women (a half of the total participants were parents) to cries of typically developing infants and cries of infants who later were diagnosed with autism spectrum disorder (ASD) (Venuti et al., 2012). Cries of the ASD children showed abnormal features including high frequencies, which elicited more negative feelings in healthy adults and women, not men, showed greater deactivations in dorsal medial PFC and posterior cingulate cortex in response to cries of healthy infants, indicating that women recruited more attention to process the sound information than men. However, such gender difference was not detected in response to cries of ASD infants – perhaps because of abnormalities in ASD-cries that are easier to detect. Alternatively, high distress or atypical cries may inhibit maternal brain responses in some women particularly if they are rendered more sensitive to negative valence stimuli, such as with depression (Groenewold et al., 2012).
Indeed in first study of baby cry in women who had a postpartum depression, mothers showed reduced responses to own vs. other baby in regions that process reward stimuli and promote maternal motivation -- nucleus accumbens, caudate and thalamus (Laurent and Ablow, 2012). More depressive symptoms were also associated with less activity in response to own vs. other baby cry in the OFC, dorsal ACC, and superior frontal gyrus - regions important for emotion information processing and regulation, including valence and salience. Similarly, mothers who used one or more teratogenic substances (e.g. tobacco, alcohol) showed decreased responses to low distress cries in prefrontal cortex, insula and amygdala (Landi et al., 2011). Although not causally, the findings demonstrate that maternal depression and addiction to substances are associated with disrupted mother-infant relationships and reduced neural activation to infants, including infant cries. Alternatively abnormalities in reward pathways that contributed to the substance use might also affect parenting in the absence of these substances. In any case, perhaps reduced activations in the PFC may be a biomarker associated with risk of harsh or neglectful and negative parenting, it would be an important predictor of such poor outcomes in high risk women such as mothers with depression or substance abuse (Goodman et al., 2011; Molitor and Mayes, 2010) and a target for therapies.
Parenting styles in humans are likely to be transmitted across generations (Belsky, 2005; Champagne, 2010) which explains in part why the neural and behavioral variations of mothering are associated with a mothers’ own parental care experiences. Quality of own parenting therefore acts as a specific early life environment that should perhaps be targeted for interventions for primiparous and multiparous mothers who report low quality of maternal care. These new mothers exhibit both reduced density and reduced activation to infant cries in frontal, orbital and temporal cortices compared to mothers who reported high quality maternal care (Kim et al., 2010b). Preliminary evidence also suggests that during the first few critical months postpartum, the same regions increase in size among healthy new mothers (Kim et al., 2010a) according to voxel based morphometry.
Parenting also influences the development of attachment style in children, which in turn affects the development of a child’s social information processing. For example, insecurely attached nulliparous women displayed greater amygdala reactivity to infant cry sounds than securely attached counterparts (Riem et al., 2012), suggesting infant cries represent more aversive stimuli for them. Amygdala responses to infant cry in new mothers has also been interpreted as heightened sensitivity (Barrett and Fleming, 2011). It seems that amygdala responses may be associated with negative and positive valence brain responses – perhaps relating to other maternal factors.
2.2 What’s in a Baby’s Face?
Not only is facial recognition of one’s infant and their emotional state critical to their survival, but evidence supports human preference for the exaggerated cute/infantile features of baby faces which appear to activate reward mechanisms within the caregiver and motivate/promote mothers to caregiving, bonding and attachment. In addition to activation of nucleus accumbens (NAcc) in response to baby schema (Glocker et al., 2009) and medial orbitofrontal cortex (OFC) response to infant faces relative to adults (Kringelbach et al., 2008), higher levels of infantile features are preferred (Parsons et al., 2011). Neuroimaging also suggests that the brain responds differentially to infant, relative to adult human and adult and infant animal faces, in non–parents, in regions including lateral premotor cortex, supplementary motor area, cingulate cortex, anterior insula and thalamus (Caria et al., 2012). Interestingly, activation in OFC and fusiform face area is disrupted when an alteration to the structure of an infant face (i.e. cleft lip) is perceived (Parsons et al., 2013). Once parenthood is established, data further indicate that greater specialization of the brain occurs with for example a right–sided lateralization in the prefrontal cortex (PFC) for emotional discrimination of infant relative to adult faces (Nishitani et al., 2011), perhaps as a result of attention processing (Thompson-Booth et al., 2014).
2.2a Brain Responses to Visual Baby Stimuli – 1st 10 years
Most early fMRI studies of parental responsiveness have very small samples and detail few characteristics of mothers or fathers making findings less reliable than some larger, newer studies. We shall outline the older literature first before considering more rigorous recent papers. One set of studies used photographs taken extremely early (i.e. 0–2 weeks postpartum), by the parents themselves. Using a block design, mothers and fathers 2–4 weeks postpartum saw 6 pictures continuously for 5 seconds each for blocks of 30 seconds (Swain et al., 2003; Swain et al., 2006) and own vs. other baby picture contrasts revealed activations in frontal and thalamo-cortical circuits. Correlations between activations to own vs. other contrast and parent -infant interactions also revealed significant activations in superior temporal lobe, OFC and ventral tegmental areas. These networks may be important for regulation of parental motivation and reward associated with baby-directed empathy, approach and caring behaviors, as well as social bonding
To examine whether parental love may make use of the same reward circuits as other forms of love, Bartels and Zeki used photographs of own, familiar and unfamiliar infants (9 months to 3.5 years of age) as stimuli for parent brains (Bartels and Zeki, 2004). In 20 healthy mothers viewing still face photographs of their own child compared to age-matched photographs of other children, they reported increased activity in midbrain periaqueductal gray and substantia nigra regions, dorsal and ventral striatum, thalamus, left insula, ORC, sub-, pre-, and supra-genual anterior cingulate, and superior medial prefrontal cortex. There were also increases in cerebellum, left fusiform, and left occipital cortex, but decreases in the left amygdala. They also compared maternal brain responses of own vs. familiar child to contrasts of best friend vs. familiar friend to control for familiarity and positive affect and argued that responses were unique to the ‘own child’s stimuli. They proposed that parent-infant attachment was regulated by a ‘push–pull’ mechanism involving selective activation of motivation and reward systems, with cortical regions suppressing critical social assessment and negative emotion systems (Bartels and Zeki, 2004); this they argued may be extended to orchestrate positive feelings and caring behaviors.
In another report, photograph stimuli of much older own vs. other children (5–12 years old) were used as stimuli and mothers were asked to focus on identity but not feelings during scanning (Leibenluft et al., 2004). Some social cognition regions which are important for empathy were significantly activated in this paradigm, including anterior paracingulate, posterior cingulate and superior temporal sulcus (Saxe, 2006). In another small fMRI experiment using visuals across familiarity, Nitschke and colleagues (2004) studied six healthy, primiparous mothers’ at 2–4 months postpartum as they viewed smiling pictures of their own and unfamiliar infants. They reported OFC activations that correlated positively with pleasant mood ratings to infant pictures. In contrast, areas of visual cortex that also discriminated between own and unfamiliar infants were unrelated to mood ratings (Nitschke et al., 2004). Perhaps, activity in the OFC – which may vary across individuals – is involved with high order dimensions of maternal attachment. The implication that complex aspects of parenting could be quantified using fMRI of frontal areas to predict risks of mood problems in parents is appealing but requires further detailed work. Ranote and colleagues conducted a similar small experiment (Ranote et al., 2004) with the innovative, and perhaps more ecological video (silent) infant stimuli in 10 healthy mothers viewing alternating 40 second blocks of own neutral, and an unknown infant. They reported significant activation in “own” versus “unknown” infant comparison in the left amygdala and temporal pole and interpreted involvement of circuitry regulating emotion and theory-of-mind (ability to attribute feelings and states of mind to others). This fits with fMRI experiments on biological motion, which activate similar temporal cortex regions (Morris et al., 2005).
Considering the contribution of the infant’s affect to maternal brain function prompted a study, again using silent video clips of own vs. other infants in play or separation situations (Noriuchi et al., 2008). First, these authors confirmed increased activation associated with own baby pictures, in cortical orbitofrontal, anterior insula and precuneus areas, as well as subcortical regions including periaqueductal gray and putamen. These areas activate in arousal and reward learning. Second, they found strong and specific differential responses of mother’s brain to her own-infant’s distress in substantia nigra, caudate nucleus, thalamus, posterior and superior temporal sulcus, anterior cingulate, dorsal regions of OFC, right inferior frontal gyrus and dorsomedial prefrontal cortex – regions involved in emotion regulation and habitual behavioral response systems that are active in a range of normal and abnormal emotion-control states including obsessive-compulsive disorder (Swain et al., 2007). They also found correlations in OFC with own baby-response and happiness as well as to their own distressed baby response in superior temporal regions. This is consistent with the emerging importance of these areas in social behaviors. Socially salient, personally tailored images and video-clips are now being combined with behavioral measures to understand better the functional architecture of parental brain. Taken together, fMRI experiments with parents, especially those using own vs other/unknown baby visual stimuli commonly activate emotion/motivation-reward areas along with cortical regulation areas, thus laying the groundwork to test hypotheses about how such brain structures regulate parental thoughts and behaviors.
For example, empathy may be conceived as a key parenting thought process and studied in mothers observing and imitating faces of their own and someone else’s child (Lenzi et al., 2009). In this study, regions believed to contain mirror neurons and connected limbic regions, including insula and amygdala respectively, were more active during emotional expressions from own child. Furthermore, the insula response correlated with the empathy-related measure of maternal reflective function. They also reported that baby joy expressions across identity evoked mostly right limbic and paralimbic areas important to emotional processing, whereas ambiguous expressions elicited responses in left-sided, high order cognitive and motor areas, logically reflecting associated cognitive effort and preparation to respond.
In addition to the capacity for complex empathic thoughts toward their infant, parents require motivation to undertake interactive behaviors and to derive reward from interacting with their infants. Integrating brain areas relevant for these actions with key hormones is likely to reinforce behaviors and provide for optimal parental sensitivity under a range of circumstances. Considering some of these complex factors, 28 healthy, first-time, singleton mother-infant dyads at 5–10 months postpartum were involved in one series of studies using visual infant facial cues of varying affect (smiling, neutral and crying) and scanning at 7–17 months postpartum. Notably, this is well past the ~3 month postpartum threshold for sophisticated social dyadic interactions (Strathearn et al., 2008; Strathearn et al., 2005). Dopamine-associated reward-processing regions were activated when mothers viewed their own vs. an unknown infant’s face, including ventral tegmental area/substantia nigra, and striatum. In addition, there were frontal lobe responses in emotion processing (medial prefrontal, anterior cingulate, and insula cortex), cognition (dorsolateral prefrontal cortex), and motor/behavioral outputs (primary motor area). Furthermore, happy, but not neutral or sad own-infant faces, activated nigrostriatal brain regions interconnected by dopaminergic neurons, including substantia nigra and dorsal putamen. Finally, a region-of-interest analysis revealed that activation in these regions was related to positive infant affect (happy > neutral > sad) for each own–unknown infant-face contrast.
2.2b Brain responses to Visual Baby Stimuli – last 5 years
In pursuit of the role of oxytocin in modulating the parental brain, Strathearn and colleagues (Strathearn et al., 2009) studied a total of 30 first-time mothers using a variety of affect-laden baby picture stimuli in combination with the Adult Attachment Interview and peripheral plasma oxytocin responses to infant play. In response to their own infant’s smiling and crying faces during fMRI, mothers with secure attachment showed greater activation of brain reward regions, including ventral striatum, and oxytocin-rich hypothalamic/pituitary regions. These results chime with effects of own parenting on fMRI activations to infant stimuli and suggesting that individual differences in maternal attachment experiences might also be crucial to measure in future imaging studies, and perhaps that they are linked with development and integration of dopaminergic and oxytocinergic neuroendocrine systems in striatum and hypothalamus.
In the last few years, parental brain circuitry has been studied in the context of variables associated with parental illness (such as depression, anxiety, substance misuse), and the work highlights regions-of-interest such as the amygdala, anterior cingulate cortex (ACC), PFC, insula and striatum. In a comparison of own-positive vs. unfamiliar-positive infant images, left amygdala response was reduced as a function of poorer concurrent maternal experience (measured by depression, anxiety, parental distress and attachment-related feelings about the infant) (Barrett et al., 2012). In a study employing similar task conditions, reduced dorsal (d)ACC activation to own–distress images was reported in depressed relative to non–depressed primiparous women (as measured by the Structured Clinical Interview for DSM-IV) (Laurent and Ablow, 2013). As a function of increased depressive symptomatology (Center for Epidemiological Studies Depression (CESD) measure, reduced response was observed in the OFC and insula to own-joy faces and in left PFC and insula/striatum to own–joy vs. own–distress faces. Though infant stimuli were not included, brain response to negative emotional faces (fear, anger) in depressed vs. healthy postpartum women has been examined, yielding observations such as reduced dorsomedial (dm)PFC activation in depressed vs. healthy women to faces; a negative correlation between left amgydala and depression severity in depressed women; a positive correlation between right amygdala and absence of infant–related hostility in depressed women; and negative functional connectivity between left dmPFC and left amygdala in healthy, but not depressed women (Moses-Kolko et al., 2010). Finally, in a study examining substance users in the postpartum period relative to non–substance users, reduced activation to infant faces was reported in dorsolateral (dl)PFC ventrolateral (vl)PFC, occipital regions, parahippocampus and amygdala (Landi et al., 2011). These studies suggest that maternal adversity and/or substance misuse may specifically be associated with reduced activation in reward and emotion circuits, with some indication that top–down connectivity between the PFC and limbic regions also may be compromised (Moses-Kolko et al., 2014).
2.2c Brain responses to mother-baby dyad movies
Recent studies have employed mother–child dyadic vignettes as visual stimuli in the study of the parental brain. In one small but intriguing study, parental adversity, as defined by interpersonal violence–related post-traumatic stress disorder (IPV–PTSD), was examined. Motivated by findings demonstrating atypical caregiving in PTSD mothers following separation, vignettes of own vs. unfamiliar toddlers during play and following separation were presented to healthy mothers and those with IPV–PTSD. (Schechter et al., 2012). IPV–PTSD mothers vs. healthy mothers (11 vs. 9) reported greater stress to viewing separation vignettes, greater limbic and reduced fronto-cortical activity was observed in IPV–PTSD mothers relative to healthy mothers in separation vs. play conditions, with reports of stress to viewing separation linking to the neural findings. These results are consistent with those in depression indicating reduced fronto-cortical activity in response to own vs. other baby-cry (Laurent and Ablow, 2012). A follow-up study of the same subjects reported correlation between limbic brain activations in response to own-child video vignettes and dissociative symptoms (Moser et al., 2013), suggesting dissociative symptoms as a mechanism for reduced maternal sensitivity among mothers affected by IPV. More work with mothers affected by high-risk circumstances, such as IPV, depression and anxiety is needed to clarify and substantiate findings and begin to inform treatments.
So far, however, a few other studies have assessed parental brain response to mother-infant dyadic vignettes as a function of optimal vs. non-optimal parenting. Atzil and colleagues categorized participants with a synchronous, relative to intrusive, parenting style and asked them to view vignettes of their own infants; of themselves interacting with their infants (Atzil et al., 2011) and those of unfamiliar infants and dyads. In another study, when own vs. other infant vignettes were compared, maternal synchrony was associated with significantly increased activity of left NAcc, while maternal intrusiveness was linked to activity of right amygdala (Atzil et al., 2012). However, in this experiment, it is unclear whether this pattern of brain response was a function of synchronous mothers or the viewing of a synchronous mother–infant interaction. The role of oxytocin was also been examined in this group, with positive correlations reported between plasma oxytocin and the left NAcc and right amygdala in synchronous mothers and left insula, left intraparietal lobule, left and right temporal cortices, left sgACC, and left NAcc in mothers. More studies with rigorous identification of maternal caregiving quality and parenting style with brain imaging are required (Wan et al., in press).
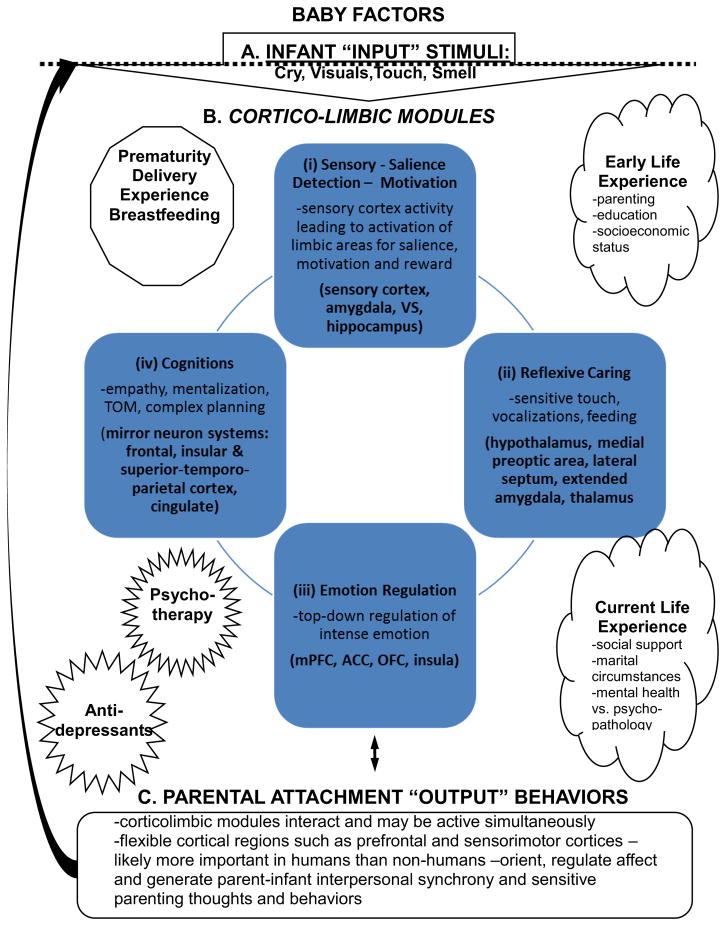
Accumulating research confirms what most people, and nearly all mothers know to be the case: infant stimuli occupy a privileged status for the adult human brain, which may be modeled as in figure 1. Auditory and visual signals from preverbal infants can enhance brain regions important to recognition, emotion and motivation (i), reflexive caring behaviors (ii), emotion regulation (iii), and empathy/theory of mind toward sensitive caregiving and attachment in order to increasing the likelihood that the vulnerable infant will survive into adulthood and successfully navigate the social world and find a mate.
Figure 1. Human Parental Circuits.
Brain regions expected to be important to human parenting. This based on human and animal studies.
Based on brain imaging of parents to this point, the following model is presented to stimulate discourse on the brain basis of parenting behaviors. First, key parenting sensory signals, including cry, visuals as well as touch and smell from baby (A) activate in parallel a set of corticolimbic circuits (B) to (i) analyze the sensory input and update saliences toward motivation and reward and coordination of other modules for (ii) reflexive caring, (iii) emotion regulation, and (iv) complex cognitions, including mentalization, empathy and theory of mind. The output (C) of these modules forms the basis of parental sensitivity and influencing child development. This inclusive and general model may be dissected in future studies involving different stimuli and specific measures of behavior and cognition.
The early study of parental brain employed a variety of baby audiovisual stimuli to elucidate a putative neurocircuitry for parenting. More recently, studies have added correlations with psychometrics of parenting, parental adversity (e.g. poor maternal experience, attachment, perinatal depression and PTSD) as well as indices of parenting quality (e.g. synchronicity, maternal sensitivity) and finally neuroendocrine measures (oxytocin).
In spite of this encouraging scenario, there are important gaps in the evidence-base and significant inconsistencies between studies mean many questions remain to be answered. First, we need better understanding of how brain manages the presentation of static individual baby images relative to moving images involving the dyad. Does different response to a vignette reflect something specific to the participant or to the vignette itself? Second, we have to understand how findings from neuroimaging studies examining perception of infant stimuli relate to the existing affective cognition of individual mothers and fathers and whether neuroimaging studies of parental neurocognition and those that incorporate behavioral measures will change our view of a dedicated parental circuitry. The findings from extant parental brain studies that have employed reverse inference in interpretation would be strengthened by studies that select more constrained samples and perhaps more sophisticated paradigms. Third, only a few forms of parental adversity have been explored in imaging paradigms. It is difficult to design a study which can selectively examine or isolate the effects of, for example, the severe chronic stress of poverty, young maternal age and early life trauma, and no studies have yet focused on resilience or the rescuing of adversity following intervention. We still know far too little about how or whether behavioral or neural correlates of maternal sensitivity/maternal responsiveness are modifiable. However we have reason to be cautiously optimistic given recent possible brain imaging studies where biomarkers for depression and its treatment are becoming established (Harmer et al., 2011). Among possible medical treatments for low maternal sensitivity, oxytocin has been proposed. The following section examines points of intersection between the parental brain and oxytocin, and possible mechanisms that might inform studies which examine modulation of maternal responding in brain and behavioral paradigms through the use of oxytocin.
3. Parenting - Connections between Brain and Oxytocin (OT)
The posterior pituitary neuropeptide hormone, oxytocin (OT), is a well-recognized component of a complex bio-behavioral system crucial for the emergence of mother-infant bonding (Ross and Young, 2009). Research in rodents and other mammals has highlighted the importance of OT (on a background of changing levels of estrogen and progesterone during pregnancy and labor) to facilitate the onset and maintenance of maternal behavior (Champagne et al., 2001; Champagne, 2008; Champagne et al., 2003; Insel and Young, 2001; Rosenblatt and Ceus, 1998). This was first suggested from studies that reported display of ‘full maternal behavior’ in virgin female rats injected with OT (Pedersen and Prange, 1979). Conversely, inhibition of postpartum maternal behavior was affected in rats by injecting them with an OT-receptor antagonist (van Leengoed et al., 1987). Among high ‘licking and grooming’ (i.e. maternal caregiving) female dams, significantly higher levels of OT receptors were also seen in brain regions implicated in the expression of maternal behavior across species, during pregnancy, at parturition and when nursing pups, such as the central nucleus of the amygdala and the paraventricular nucleus of the hypothalamus (Champagne et al., 2001). Following birth, the female offspring of ‘low’ licking and grooming mothers, who are cross fostered and reared by ‘high’ licking and grooming mothers, express a higher density of brain OT receptors than non-cross fostered females, and similar levels to high licking and grooming mothers - suggesting that there is intergenerational transmission of the behavior sensitive to the rearing environment and likely dependent on epigenetic processes (Champagne, 2008).
Evidence for a similar ‘transition to maternity’ affected by hormonal exposure during pregnancy and childbirth in human mothers is notably less robust, but also supports a role for OT. Mothers with a rising pattern of plasma OT reported higher maternal fetal attachment in the postpartum (Levine et al., 2007), and higher plasma OT levels during pregnancy and the first postpartum month were correlated with higher levels of maternal postpartum behaviors toward infants, such as gaze, vocalizations and positive affect in new mothers (Feldman et al., 2007). Maternal synchrony (“episodes when mother and infant coordinate their positive social engagement”) is also reported to be positively correlated with maternal plasma OT level, while maternal intrusiveness (“inappropriate response from mother”) is not (Atzil et al., 2011). OT plasma levels have also been examined in relation to maternal own-attachment experience (Strathearn et al., 2009): higher levels of plasma OT were reported following mother-infant physical interaction among mothers reporting secure attachment patterns with their own mothers compared to those with insecure attachment patterns. Even among non-parents, plasma OT levels have been positively correlated with self-reported recall of parental care (maternal and paternal care) (Feldman et al., 2012; Gordon and Feldman, 2008).
The literature also points to a role for OT in the regulation of stress responses. Indeed, a well-established view has emerged that, in humans, OT is anxiolytic and reduces fear and stress (Ayers et al., 2011; Ishak et al., 2011). Studies imply this role is influenced by an individual’s previous experiences and difficulties in interpersonal relationships (Tabak et al., 2011): difficulties in relationships with primary partner (Taylor et al., 2006), or own infant (i.e. interactive stress) (Feldman et al., 2011), or romantic partner (Marazziti et al., 2006). These studies have all reported higher levels of plasma or urinary OT in relation to stress in social relationships. Some have concluded that OT appears to be an indicator of social affiliation, but it might also be a ‘signal’ for the need to affiliate with others (Taylor et al., 2010).
Overall, a large and robust animal literature exists on OT’s role in maternal care behavior and an accumulating human literature associates plasma OT with a wide range of social and emotional stimuli; scenarios, ranging from romantic love, or marital distress to psychopathology to changes in plasma OT. Interactions between central OT and dopamine systems have also been associated with individual differences in maternal behavior in rodents. For example, stable, individual differences in rat maternal licking/grooming of pups were abolished by OT receptor blockade mediated by the direct effect of OT on dopamine release within the mesocorticolimbic dopamine system (Shahrokh et al., 2010). While animal models of maternal behavior are compelling and have identified key regions in a putative mothering circuit, human behaviors are undertaken in a far more complex environment. As outlined above, processes such as cognitive flexibility, attention control, working memory, and the mother’s ability to understand the intentions and emotions of her child (maternal mind-mindedness or empathy) are fundamental components of human mothering and highly dependent on PFC. Additionally, several studies mentioned already above (Atzil et al., 2011; Atzil et al., 2012; Riem et al., 2011a; Strathearn et al., 2009) have attempted to assess neural circuitry related to parenting in subcortical reward/limbic regions as a function of oxytocin.
The clinical implications of these studies are yet to be fully explored. However, preliminary observations that expectant mothers at risk for post-partum depression have lower plasma OT during pregnancy (Skrundz et al., 2011) may suggest value in interventions which enhance availability of central OT during pregnancy and perinatally in order to reduce risk of postpartum depression. Therapeutic uses of oxytocin are currently being investigated in other neuropsychiatric disorders associated with poor social cognition, including ASD, OCD and schizophrenia e.g. (Bartz et al., 2011; Macdonald and Feifel, 2013). However, in order to understand the potential effects of exogenous oxytocin administration in humans, several important lessons from decades of animal research bear consideration. First, oxytocin effects are moderated by robust, hard-wired neurobiological mechanisms; thus, centrally administered oxytocin increases aggression to same-sex intruders after mating in monogamous Prairie voles, but not in non-monogamous Montane voles (Winslow et al., 1993). Similarly, pre-existing ‘primers’ are necessary to induce oxytocin-facilitated social bonding in monogamous Prairie voles, and, depending on the study designs, these primers can be gonadal hormones (e.g. estrogen), mating behaviors, or prolonged pre-exposure to a former stranger (for an early review, (Insel, 1997)). In other words, exposure to oxytocin alone is not sufficient to create de-novo social bonding. Perhaps oxytocin facilitates the development of social bonds when acting in the appropriate environment.
These findings have several critical implications for humans. It is unlikely that acute or chronic oxytocin administration alone can reverse genetically and/or environmentally determined affiliation/attachment styles. Oxytocin administration is likely to affect individuals according to their existing social cognition and attachment styles as well as their past experiences, much like with the monogamous Prairie vole and non-monogamous Montane vole. So, although performance on tasks highlighting empathy (such as trust games) can be enhanced by acute OT administration and empathic interactions can similarly increase plasma OT levels and subsequent generosity in humans (Barraza and Zak, 2009), cultivating empathy in an ‘uncaring’ individual is likely to be a more complex process; not least because its maintenance will surely rely on the accumulation of repeated positive experience. In support of this, oxytocin administration exerts no significant behavioral change in strangers who share no pre-existing bonding relationship (Barraza et al., 2011; De Dreu, 2012b; Kosfeld et al., 2005; Zak et al., 2007) and a recent report finds intranasal OT does not enhance approach/avoidance to social stimuli or exert a stronger effect on social vs. non-social stimuli in the context of processing emotional information but increases the salience of certain social stimuli and moderates salience of disgust stimuli (Theodoridou et al., 2013). Authors postulated that heightened responses to disgust may be particularly relevant for new mothers wishing to protect vulnerable young from contagion.
The positive effects of OT on social cognition may only be observed when the tasks demand a binding relationship in which reward contingencies are congruent between two players, where they form a partnership by becoming stake holders of common interest (Barraza et al., 2011; Kosfeld et al., 2005; Zak et al., 2007).Oxytocin administration also appears to increase polarized social behaviors in humans, e.g., in-group favoritism (De Dreu et al., 2011), by increasing within group cooperation and between group competition (De Dreu, 2012a) and its administration may increase the emotional reactivity to perceived positive and negative cues in a social context (Olff et al., 2013). When social cues in the environment are interpreted as secure or positive (“me among us”), oxytocin may promote prosocial behaviors. In situations of higher threat or higher social stress, when the social cues are interpreted as insecure or negative (“me among them”), oxytocin may promote “anti-social” behaviors. This suggests that OT may serve to sharpen in/out group distinction (Colonnello et al., 2013).
Interestingly, it was found that OT levels in mothers, assayed from urine, were higher after interaction with children, no matter whether these were their own or unknown children (Bick and Dozier, 2010). In fact, OT levels appeared to be higher if the child was unknown compared to when the child was their own – perhaps serving an anxiolytic or anti-stress function in this particular experiment (Heinrichs et al., 2003). It may also fit with the OT role in maternal protection and aggression towards intruders in several species including humans (Campbell, 2008; Neumann, 2008). Finally, new mothers with low sensitivity show higher plasma oxytocin before and after playing with their infants (Elmadih et al., 2013*-this issue) compared to mothers with higher than average maternal sensitivity. This suggests that OT may be released as part of a stress response related to poor ability to cope with infant demands and may be moderating maternal stress in an attempt to promote care and bonding.
The complex literature on the effects in humans of externally administered hormone argue for further refinements in our understanding of how, and in what direction OT (measured in the plasma or elsewhere) is, or is not, causally associated with behavioral or emotional manifestations in people. When someone inhales OT, evidence points to its effects being determined by past experiences and present circumstances. For example, we observe that intranasal oxytocin may increase startle responses to stressful stimuli in humans (Grillon et al., 2013; Striepens et al., 2012) and that less anxiously attached, healthy men remember their mother as more caring and close after OT (vs. placebo), while more anxiously attached men remember their mother as less caring and close after OT (vs. placebo) (Bartz et al., 2010). Finally, healthy men with low emotion regulation abilities show higher cortisol stress responses (as expected), but also benefit more from intranasal OT; whereas those with high emotion regulating abilities do not (Quirin et al., 2011). This chimes well with early studies (Light et al., 2000) that reported increased plasma OT in mothers following a social stress task only when the mother held her baby before the task, but not if she did not.
3.1 Controversies and Limits to the OT literature in parenting
The nature of the interaction between peripheral and central OT systems moment-to-moment remains elusive. Recent evidence in 41 non-psychiatric subjects concludes there is no correlation between baseline CSF and plasma OT levels measured using radioimmunoassay (Kagerbauer et al., 2013). These authors rigorously appraise past literature using basal measurements of peripheral OT to reflect central effects of the hormone in humans, particularly in studies proposing links between plasma/salivary OT and human social behaviors. Such evidence must caution future studies to acknowledge that OT occupies two physiologically distinct compartments (periphery and CSF) resulting in quite independent release patterns. This suggests that measuring plasma OT as a biomarker of a socio-emotional behavior assumed to be regulated by brain perhaps remains relevant only if a dynamic challenge paradigm is used with comparisons between distinct, well-characterized groups. Kargerbauer and colleagues (2013) acknowledge the relationship between gender, gonadal hormones and plasma OT; but they also point to a high quality study in pregnant women showing no correlation between plasma and CSF OT levels (Altemus et al., 2004).
Understanding how intranasal oxytocin affects human social behaviors clearly requires more thoughtful experimentation. These considerations do not foreclose the potential for exogenously/intranasally administered OT to reach brain and affect behavior relevant to parenting. Indeed, intranasal drug administration is Increasingly favored as an effective means of delivering molecules rapidly to brain (Grassin-Delyle et al., 2012). But it is likely that effects are not only sex-specific, but task specific (some suggest OT does not change the salience of social-emotional cues) and task-specific between sexes (Ditzen et al., 2012). Beyond pregnancy and infancy, we have little information on how the OT system functions in socio-emotional relationships during periods of rapid brain reorganization (e.g. puberty) vs. those of relative developmental stability and we know little in humans about brain OT-receptor distributions and densities and how they may be influenced by environmental effects and epigenetic regulation overtime and circumstance. To what extent central OT receptors are sensitive to epigenetic modification in humans and whether, like in the rodents from Champagne’s work (Champagne, 2008), they contribute to patterns of parental care that can be passed on (in a quasi-Lamarckian fashion) from generation to generation, are closely related questions. Other hormones and their receptor systems, including cortisol, estrogen, progesterone, endogenous opioids are also important in the regulation of parenting and interact with OT (Gordon et al., 2011; Lahey et al., 2012; Nowak et al., 2011; Swain et al., 2011)
In summary, the complex links between OT and parental brain physiology in humans requires further, more detailed examination, taking into account key individual differences and maternal experiences past and present into account, in addition to other hormones, in order to fully interpret the inferences that can be drawn from studies using basal peripheral measures of the hormone.
4. Advances on the Neurobiology of Father Brain
Studies of parent-infant interactions have historically targeted the mother-infant relationship. However, more recent research has demonstrated that fathering also plays a significant role for the child’s cognitive, emotional and social development (Lamb, 2004; Ramchandani and Psychogiou, 2009). For example, literature examining parental behaviors suggests gender differences in expressed emotion during interactions with children (Volling et al., 2002). Specifically, maternal sensitivity is typically expressed by emotional warmth and support whereas paternal sensitivity frequently manifests as the provision of stimulating interactions (Grossmann et al., 2008). This gender difference is supported by a study of a time series analysis of 100 first-time mothers and fathers interacting with their 5-month-old firstborn child (Feldman, 2003). Mother-child play was characterized by face-to-face exchange and included patterns of mutual gazing, co-vocalization, and affectionate touch. In contrast, during play with fathers, a pattern of interactive arousal was identified that contained several quick peaks of high positive emotionality, including joint laughter and open exuberance.
Behavioral data with fathers have demonstrated important differences in typical father versus mother-child interactions beginning in infancy (Crawley and Sherrod, 1984; Feldman, 2003). Fathers, for example, tend to exhibit increased physical interactions with their young children, often characterized as “rough and tumble” play (Carson et al., 1993). Furthermore, within father-child relationships, it is the quality of active, parent-child play interactions, and not sensitivity per se, that is related to positive social emotional outcomes in children such as interpersonal confidence and the development of positive peer relationships (MacDonald, 1987). These data suggest that there may be some shared brain mechanisms between mothers and fathers, and some differences. Recently, an interesting study with small groups showed that males respond differently to non-own hunger cries than mothers (De Pisapia et al., 2013). Studying 9 men and 9 women (half in each group non-parents), more activity was seen in the dorsal medial prefrontal and posterior cingulate areas of male brains regardless of parental status. Such sex differences were interpreted as relating to mind-wandering in men relative to women. Sex differences between mothers and fathers may relate to the establishment and maintenance of specific aspects of parent-infant relationship and warrant further study. A theoretical basis for sex differences may be found in differential roles of mothers and fathers in the development of exploratory systems in the infant and young child according to activation relationship theory (Paquette, 2004). This theory includes two dimensions of fathering that underlie the father-child relationship: 1) stimulation, wherein fathers encourage the child’s interaction with the outside world; and 2) discipline designed to provide children with limits that will maintain their safety (Paquette, 2004).
Such specialization of father-infant interactions is consistent with play studies showing more object-oriented or physical play associated with smiles compared with mother-infant interactions (Dickson et al., 1997; Feldman, 2003; Lamb, 1977; Yogman, 1981). Thus, unique contributions from father-child interactions (Boyce et al., 2006; Grossmann et al., 2002) to evolutionarily favorable sex-specific emotional expressions of the developing child may significantly constitute the mechanism through which sex differences in the expression of emotion (Vigil, 2009) cross generations.
The differential nature of father- vs. mother-child relationship suggests that the underlying neurobiology of fathering behaviors may be at least in part distinct. For example, in the model presented here (Figure 1), paternal salience appraisal of infant stimuli may activate an emotional regulatory reaction in fathers that is stronger than that evoked for mothers. This kind of a response would underlie paternal capacity and motivation to effectively assess external contextual factors to determine whether situations are sufficiently safe to encourage the child’s engagement and interactions with the broader social environment. In contrast, hormonal changes corresponding to the perinatal period may result in a stronger reflexive caring response for mothers. Finally, empathic/mentalizing cognitive responses to infant cues may be similar for mothers and fathers, as it supports all types of parenting responses and behaviors – at least after an initial period of adaptation of some months. This not well studied but at least in keeping with the observation mother vs. father statistical equivalence of correct identification of own vs. other baby-cry (Swain et al., 2005).
Preliminary work on the brain physiology of parents is consistent with significantly overlapping neurobiological and behavioral parenting processes for mothers and fathers with some interesting differences. For example, using fMRI methodology with a sample of co-parent couples of 4- to 6-month old infants, Atzil and colleagues (Atzil et al., 2012) found greater activation in limbic areas in response to own-infant (vs. other infant) video clips for mothers (and not fathers) as well as correlations of these areas (e.g., NAcc, amygdala, ventral ACC) with increases in plasma oxytocin (OT) levels. In contrast, decreases in plasma OT for fathers was correlated in own- vs. other-infant comparisons, primarily with cognitive areas, including areas that are responsible for regulating and organizing behavioral responses to emotionally salient stimuli (e.g., dorso-lateral PFC, dorsal ACC, IPC, and pre-central gyrus). Further, in fathers (and not mothers), increases in plasma arginine vasopressin (AVP) was correlated with activations of the inferior frontal gyrus (IFG) and insula suggesting an increased social-cognitive response to infant cues for fathers.
Using similar methodology with a sample of young infants (8–19 weeks), the brain responses of fathers to own vs. other infant videos (Kuo et al., 2012). Whole brain analysis demonstrated increased activity in emotion regulation (iii) circuits, including bilateral inferior frontal gyrus, and the empathic/mentalizing (iv) module including supramarginal (parietal) gyrus and bilateral middle temporal gyrus among human fathers (N=10) in response to own (vs. other) baby stimuli. In addition, on the contrast of baby (both own and other babies) vs. doll video contrasts, fathers exhibited increased activity in all modules: Sensory/Salience (i), Reflexive Caring (ii), Emotion regulation (iii), and), Empathic/Mentalizing (iv), (iv) including bilateral caudate, orbitofrontal cortex, superior frontal gyrus, and superior parietal lobe. Activation of these systems plausibly supports a father’s ability to attend to the infant and the surrounding environment and react as necessary to promote infant-environment interactions or to protect the infant from environmental dangers. Interestingly, greater responses to own (vs. other) baby stimuli in the PFC was associated with less paternal sensitivity. The findings may reflect that the greater role of the OFC in interpretation of the unfamiliar infant cry among fathers.
Using infant photographs instead of video clips to stimulate father brains, Wittforth and colleagues (Wittfoth-Schardt et al., 2012) found activations for own child in the left GP, the left hippocampus, the right mOFC, as well as in the bilateral inferior frontal gyrus/anterior insula and activations for own child vs. other (unknown) child in the right GP, the left VTA, the left mOFC, and the left inferior frontal gyrus(IFG)/anterior insula. Furthermore, in recent analyses of father brain activity, using an own vs. other child vs. adult picture task 1–2 years postpartum (Mascaro et al., 2013b), there was a main effect for child vs. adult pictures in the fusiform gyrus, dorsal medial prefrontal cortex, thalamocingulate and mesolimbic areas – fitting with reflexive caring (i) parental brain circuits (Figure 1). VTA responses were associated with reduced testosterone levels, smaller testes volume as well as higher parental sensitivity. The findings support that fathers may gradually develop a strong attachment with their infants over the course of the first and second years, and the attachment building processes may be supported by neurobiological adaptations such as increased neural sensitivity to own infants and decreased testosterone levels. In response to 3–5 month-old infant cries, similar to the findings in mothers were reported for fathers 1–2 years postpartum (Mascaro et al., 2013a), including responses in bilateral IFG and GP. Furthermore, variations in androgen receptor gene were associated with activations in IFG and OFC – areas also involved in empathy and emotion regulation. Anterior insula activity had a non-linear relationship with paternal caregiving, such that fathers with intermediate activation were most involved. These results suggest that restrictive attitudes may be associated with decreased empathy and emotion regulation in response to a child in distress, and that moderate anterior insula activity reflects an optimal level of arousal that supports engaged fathering
Finally, father brain structure is also being examined based on animal models (Kinsley and Lambert, 2008), and one neuroimaging report on gray matter volume increases in a number of brain regions of human mothers - including the striatum, thalamocingulate, and PFC from the first month (T1) to the fourth postpartum month (T2) (Kim et al., 2010a). For new fathers, gray matter changes may be associated with depression symptoms and paternal behaviors (Kim et al., 2014). Ultimately, models that combine changes in structure and function may be required for comprehensive biological models of paternal behavior that may help identify biomarkers for risk, resilience and intervention.
In sum, brain imaging studies of the father brain over the last few years have yielded functional and structural results that fit with a picture of paternal brain-behavior adaptations coordinated with relevant hormones after having a child in support of adaptive parenting. Future work will no doubt explore similarities and differences to the maternal brain.
5. Conclusions and Future Directions
By mapping parental brain responses to infant stimuli, recent use of the non-invasive and highly sensitive functional magnetic resonance imaging (fMRI) in parenting research has added greatly to our knowledge of the brain basis of parental sensitivity. Furthermore, it has confirmed physiologically that observed maternal behavior reflects a composite of multiple behaviors, with discrete maternal brain activation likely to occur in relation to each aspect of this behavior. Such conceptualization presents the possibility of identifying distinct pathways to poor maternal sensitivity, and of using changes in brain activation in response to infant stimuli as potential biomarkers for the development and evaluation of new diagnostic and treatment strategies in at-risk parents.
Indeed, there are now many examples in which brain imaging can identify changes in neuronal activation that elude behavioural measures. For example amygdala activation and connectivity is significantly increased during subconscious processing of fearful faces (Pantazatos et al., 2012). fMRI might then provide insight into the neural basis of subconscious or ‘automatic’ parental responses (Papousek and Papousek, 2002). Similarly, behavioural measures of emotional function can be relatively insensitive, whereas using affective cognition challenges, fMRI has allowed the identification of important biomarkers for psychopathology and for treatment response in depression such as the amygdala and ACC (Groenewold et al., 2012; Harmer et al., 2011; Harmer et al., 2009). In this way, imaging data complements and enhances behavioural information. For example, observational measures of behaviour can address questions about whether a parent at risk of poor maternal care (or poor maternal sensitivity) has biases in attention systems, but only imaging can address questions about how such a parent attributes salience or valence to emotional images of her own as compared to an unknown infant, and how they might differentially engage brain reward systems. A combination of brain imaging plus behavioural paradigms seems more likely to result in enhanced mechanistic specificity. Thus, if we find impaired performance in a parent behaviourally when she is under stress, then the imaging may be able to indicate if this is because of over-activity in limbic stress systems (e.g. enhanced amygdala, insula activation to stressful cues) and/or because prefrontal control systems are underactive, making executive performance more readily disrupted. Having more information about which neural mechanisms drive which behavioural outcome has important implications for designing more effective interventions - e.g. depending on the parent, interventions may focus on reducing stress responses, or improving executive performance, or both
The interpretation of fMRI of parent brains responding to infant stimuli carry important caveats, most important of which include consideration of timing, averaging of signals and the limitation of the paradigms themselves as a set of circumstances that are still quite different from that actually experienced by a human parent in real-time with their own infant (Hari and Kujala, 2009; Schilbach et al., 2013). Lying in a magnet observing audiovisual stimuli is far from actual parenting and the field is working toward more ethologically accurate paradigms for use in brain imaging experiments of parental responsiveness.
In spite of these caveats, fMRI studies of parental brain are consistent with a set of cortico-limbic brain circuits that are activated in response to infant stimuli and which may correlate with parental cognitions and behaviors (Kim et al., 2011; Musser et al., 2012). Parental behavior is not fixed, and evidence suggests that there may be potentially modifiable aspects of such as past and current social experiences and supports which may also change or determine the neurophysiology of parenting. The effects of parenting interventions, including the use of intranasal oxytocin, are yet to be studied using functional imaging
Over the past decade, a number of clinical studies demonstrate the positive effect of intranasal OT (exogenous OT) on emotion recognition/social anxiety (e.g. (Guastella and MacLeod, 2012) and affiliative behavior between individuals (Riem et al., 2011a), including fathers (Weisman et al., 2012). These suggest that OT administration may be indicated for vulnerable parents with poor maternal sensitivity, although caution is still required (Tabak et al., 2011). If fMRI can discriminate different patterns of brain activation between parents at opposite ends of a spectrum of high and low maternal sensitivity, it prepares the way for future efficient hypothesis testing of the effects of novel interventions in small numbers of normal volunteers. In other words, a distinct neural profile of ‘higher’ sensitivity parents or parenting response means functional imaging may identify useful ‘biomarkers’ for future interventions; for example, monitoring the effects of intranasal OT or non-drug interventions aimed at enhancing parental responsiveness on aspects of neural circuitry.
We would argue that fMRI utility derives from the proposal that specific fMRI activation patterns could act as objective biomarkers for treatment development and in risk identification. We would suggest that future work using fMRI in new parents should assess affective cognition and how it relates to parental care behaviours before and after interventions in order to determine underlying neural pathways for what is, and what is not ‘working’ with respect to the parental care. such a model is already being developed for the mood disorders, in which fMRI studies have consistently demonstrated that limbic hyperactivity in response to emotional faces offers promise as a biomarker for response to antidepressant (Harmer et al., 2011; Victor et al., 2012). Similarly, abnormalities in response to rewarding stimuli are difficult to access in human behavioural studies, but numerous fMRI studies have mapped the different neural responses to different rewards. Brain imaging may be able to determine whether low sensitivity parents show reduced reward system responses to their infants – an extremely challenging task based on observed behaviours or self-report. In the same way, within-subject, placebo-controlled designs combined with the superior sensitivity of imaging are likely to be of particular value in answering whether acute oxytocin can modulate affective cognition and other neural responses to infants in low-sensitivity mothers.
Future fMRI investigation of parental sensitivity and its modulation should include examination of the relationship between deficits in key affective cognition pathways. Increasing amounts of money are likely to be spent on attempts to improve parenting or reduce the adverse effects of poor parenting. In our opinion, the success of such approaches will continue to be limited unless we understand in detail the neurobiology which underpins poor parental sensitivity. This means being able to distinguish differing pathways to, and components of, poor sensitivity. Brain imaging represents a highly efficient methodology to explore this in relatively small numbers compared to numbers needed even for pilot randomized control trials using exclusively behavioural outcomes. Significant differences in neural activation with intervention vs. placebo, (even if behavioural change is not recorded), would still support the value of comparing a combination of intervention plus behavioural vs. intervention alone in a future potential future randomized controlled trial.
Highlights.
Cortico-limbic brain networks respond to infants and regulate parental sensitivity
Parent brains are affected by early-life events, current mood/anxiety & environment
Parental brain circuits are modulated by oxytocin among other key hormones
Brain-based biomarkers may improve parenting risk and resilience identification
Acknowledgments
This authors of this paper are supported by grants from the National Alliance for Research on Schizophrenia and Depression/Brain and Behavior Foundation (JES), the Klingenstein Third Generation Foundation, NIMHD/NICHD RC2MD004767-01, the Michigan Institute for Clinical Health Research and the National Center for Advancing Translational Sciences, UL1TR000433 (JES, SSH, CJD), Robert Wood Johnson Health and Society Scholar Award (JES & SSH) and NIMH 5T32MH018264-28 (JS).
Abbreviations
- MPOA
medial preoptic area
- BNST
bed nucleus of the stria terminalis
- dlPFC
dorsolateral prefrontal cortex
- IPV–PTSD
interpersonal violence–related post-traumatic stress disorder
- nACC
nucleus accumbens
- ACC
anterior cingulate
- IFG
inferior frontal gyrus
- NAcc
nucleus accumbens
- OFC
orbitofrontal cortex
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel KM, Webb RT, Salmon MP, Wan MW, Appleby L. Prevalence and predictors of parenting outcomes in a cohort of mothers with schizophrenia admitted for joint mother and baby psychiatric care in England. J Clin Psychiatry. 2005;66:781–789. doi: 10.4088/jcp.v66n0618. quiz 808–789. [DOI] [PubMed] [Google Scholar]
- Ainsworth MS, Blehar MC, Waters E, Wall S. Patterns of attachment: A psychological study of the strange situation. Oxford, England: Erlbaum; 1978. [Google Scholar]
- Altemus M, Fong J, Yang R, Damast S, Luine V, Ferguson D. Changes in cerebrospinal fluid neurochemistry during pregnancy. Biol Psychiatry. 2004;56:386–392. doi: 10.1016/j.biopsych.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Anderson AM. Factors influencing the father–infant relationship. Journal of Family Nursing. 1996;2:306–324. [Google Scholar]
- Anderson IM, Shippen C, Juhasz G, Chase D, Thomas E, Downey D, Toth ZG, Lloyd-Williams K, Elliott R, Deakin JF. State-dependent alteration in face emotion recognition in depression. Br J Psychiatry. 2011;198:302–308. doi: 10.1192/bjp.bp.110.078139. [DOI] [PubMed] [Google Scholar]
- Arnone D, McKie S, Elliott R, Thomas EJ, Downey D, Juhasz G, Williams SR, Deakin JFW, Anderson IM. Increased Amygdala Responses to Sad But Not Fearful Faces in Major Depression: Relation to Mood State and Pharmacological Treatment. American Journal of Psychiatry. 2012;169:841–850. doi: 10.1176/appi.ajp.2012.11121774. [DOI] [PubMed] [Google Scholar]
- Atkinson L, Leung E, Goldberg S, Benoit D, Poulton L, Myhal N, Blokland K, Kerr S. Attachment and selective attention: disorganization and emotional Stroop reaction time. Dev Psychopathol. 2009;21:99–126. doi: 10.1017/S0954579409000078. [DOI] [PubMed] [Google Scholar]
- Atzil S, Hendler T, Feldman R. Specifying the neurobiological basis of human attachment: brain, hormones, and behavior in synchronous and intrusive mothers. Neuropsychopharmacology. 2011;36:2603–2615. doi: 10.1038/npp.2011.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atzil S, Hendler T, Zagoory-Sharon O, Winetraub Y, Feldman R. Synchrony and specificity in the maternal and the paternal brain: relations to oxytocin and vasopressin. J Am Acad Child Adolesc Psychiatry. 2012;51:798–811. doi: 10.1016/j.jaac.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Ayers LW, Missig G, Schulkin J, Rosen JB. Oxytocin reduces background anxiety in a fear-potentiated startle paradigm: peripheral vs central administration. Neuropsychopharmacology. 2011;36:2488–2497. doi: 10.1038/npp.2011.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van IMH, Juffer F. Less is more: meta-analyses of sensitivity and attachment interventions in early childhood. Psychol Bull. 2003;129:195–215. doi: 10.1037/0033-2909.129.2.195. [DOI] [PubMed] [Google Scholar]
- Barraza JA, McCullough ME, Ahmadi S, Zak PJ. Oxytocin infusion increases charitable donations regardless of monetary resources. Horm Behav. 2011;60:148–151. doi: 10.1016/j.yhbeh.2011.04.008. [DOI] [PubMed] [Google Scholar]
- Barraza JA, Zak PJ. Empathy toward strangers triggers oxytocin release and subsequent generosity. Ann N Y Acad Sci. 2009;1167:182–189. doi: 10.1111/j.1749-6632.2009.04504.x. [DOI] [PubMed] [Google Scholar]
- Barrett J, Fleming AS. Annual Research Review: All mothers are not created equal: neural and psychobiological perspectives on mothering and the importance of individual differences. J Child Psychol Psychiatry. 2011;52:368–397. doi: 10.1111/j.1469-7610.2010.02306.x. [DOI] [PubMed] [Google Scholar]
- Barrett J, Wonch KE, Gonzalez A, Ali N, Steiner M, Hall GB, Fleming AS. Maternal affect and quality of parenting experiences are related to amygdala response to infant faces. Social Neuroscience. 2012;7:252–268. doi: 10.1080/17470919.2011.609907. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The neural correlates of maternal and romantic love. Neuroimage. 2004;21:1155–1166. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: context and person matter. Trends Cogn Sci. 2011;15:301–309. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Ochsner KN, Bolger N, Kolevzon A, Ludwig N, Lydon JE. Effects of oxytocin on recollections of maternal care and closeness. Proc Natl Acad Sci U S A. 2010;107:21371–21375. doi: 10.1073/pnas.1012669107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter LR., Jr Basal ganglia systems in ritualistic social displays: reptiles and humans; function and illness. Physiol Behav. 2003;79:451–460. doi: 10.1016/s0031-9384(03)00164-1. [DOI] [PubMed] [Google Scholar]
- Behrens KY, Hart SL, Parker AC. Maternal Sensitivity: Evidence of Stability across Time, Contexts, and Measurement Instruments. Infant and Child Development. 2012;21:348–355. [Google Scholar]
- Belsky JJ, SR, Sligo J, Woodward L, Silva PA. Intergenerational transmission of warm-sensitive-stimulating parenting: a prospective study of mothers and fathers of 3-year-olds. Child Development. 2005;76:384–396. doi: 10.1111/j.1467-8624.2005.00852.x. [DOI] [PubMed] [Google Scholar]
- Benedek Parenthood as a developmental phase: A contribution to the libido theory. Journal of the American Psychoanalytic Association. 1954;7:379–417. doi: 10.1177/000306515900700301. [DOI] [PubMed] [Google Scholar]
- Bernhardt BC, Singer T. The neural basis of empathy. Annu Rev Neurosci. 2012;35:1–23. doi: 10.1146/annurev-neuro-062111-150536. [DOI] [PubMed] [Google Scholar]
- Bernier A, Carlson SM, Whipple N. From external regulation to self-regulation: early parenting precursors of young children’s executive functioning. Child Dev. 2010;81:326–339. doi: 10.1111/j.1467-8624.2009.01397.x. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Kringelbach ML. Affective neuroscience of pleasure: reward in humans and animals. Psychopharmacology (Berl) 2008;199:457–480. doi: 10.1007/s00213-008-1099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bick J, Dozier M. Mothers’ and Children’s Concentrations of Oxytocin Following Close, Physical Interactions with Biological and Non-biological Children. Dev Psychobiol. 2010;52:100–107. doi: 10.1002/dev.20411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born JM, Lemmens SG, Martens MJ, Formisano E, Goebel R, Westerterp-Plantenga MS. Differences between liking and wanting signals in the human brain and relations with cognitive dietary restraint and body mass index. The American journal of clinical nutrition. 2011;94:392–403. doi: 10.3945/ajcn.111.012161. [DOI] [PubMed] [Google Scholar]
- Bos PA, Hermans EJ, Montoya ER, Ramsey NF, van Honk J. Testosterone administration modulates neural responses to crying infants in young females. Psychoneuroendocrinology. 2010;35:114–121. doi: 10.1016/j.psyneuen.2009.09.013. [DOI] [PubMed] [Google Scholar]
- Bowlby J. Attachment. 2. Basic Books; New York: 1969a. [Google Scholar]
- Bowlby J. Attachment. Vol. 1. Hogarth Press; London: 1969b. Attachment and Loss. [Google Scholar]
- Bowlby J. Attachment and Loss, Vol 2. Separation: Anxiety and Anger. Basic Books; London: 1973. [Google Scholar]
- Bowlby J. The making and breaking of affectional bonds. I. Aetiology and psychopathology in the light of attachment theory. An expanded version of the Fiftieth Maudsley Lecture, delivered before the Royal College of Psychiatrists, 19 November 1976. Br J Psychiatry. 1977;130:201–210. doi: 10.1192/bjp.130.3.201. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Essex MJ, Alkon A, Goldsmith HH, Kraemer HC, Kupfer DJ. Early father involvement moderates biobehavioral susceptibility to mental health problems in middle childhood. J Am Acad Child Adolesc Psychiatry. 2006;45:1510–1520. doi: 10.1097/01.chi.0000237706.50884.8b. [DOI] [PubMed] [Google Scholar]
- Bridges RS. Neurobiology of the Parental Brain. Academic Press; Burlington, MA, USA: 2008. [Google Scholar]
- Britton JC, Phan KL, Taylor SF, Welsh RC, Berridge KC, Liberzon I. Neural correlates of social and nonsocial emotions: An fMRI study. Neuroimage. 2006 doi: 10.1016/j.neuroimage.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Campbell A. Attachment, aggression and affiliation: the role of oxytocin in female social behavior. Biol Psychol. 2008;77:1–10. doi: 10.1016/j.biopsycho.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Caria A, Falco S, Venuti P, Lee S, Esposito G, Rigo P, Birbaumer N, Bornstein MH. Species-specific response to human infant faces in the premotor cortex. Neuroimage. 2012;60:884–893. doi: 10.1016/j.neuroimage.2011.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson J, Burks V, Parke RD. Parent–child physical play: Determinants and consequences. In: MacDonald K, editor. Parent–child play: Descriptions and implications. State University of New York Press; Albany, NY US: 1993. pp. 197–220. [Google Scholar]
- Champagne F, Diorio J, Sharma S, Meaney MJ. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proc Natl Acad Sci U S A. 2001;98:12736–12741. doi: 10.1073/pnas.221224598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA. Epigenetic mechanisms and the transgenerational effects of maternal care. Front Neuroendocrinol. 2008;29:386–397. doi: 10.1016/j.yfrne.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA. Epigenetic influence of social experiences across the lifespan. Dev Psychobiol. 2010;52:299–311. doi: 10.1002/dev.20436. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Francis DD, Mar A, Meaney MJ. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol Behav. 2003;79:359–371. doi: 10.1016/s0031-9384(03)00149-5. [DOI] [PubMed] [Google Scholar]
- Clutton-Brock TH. The Evolution of Parental Care. Princeton University Press; 1991. [Google Scholar]
- Colonnello V, Chen FS, Panksepp J, Heinrichs M. Oxytocin sharpens self-other perceptual boundary. Psychoneuroendocrinology. 2013 doi: 10.1016/j.psyneuen.2013.08.010. [DOI] [PubMed] [Google Scholar]
- Crawley SB, Sherrod KB. Parent–infant play during the first year of life. Infant Behavior & Development. 1984;7:65–75. [Google Scholar]
- Curley JP. The mu-opioid receptor and the evolution of mother-infant attachment: theoretical comment on Higham et al. (2011) Behav Neurosci. 2011;125:273–278. doi: 10.1037/a0022939. [DOI] [PubMed] [Google Scholar]
- De Dreu CK. Oxytocin modulates cooperation within and competition between groups: an integrative review and research agenda. Horm Behav. 2012a;61:419–428. doi: 10.1016/j.yhbeh.2011.12.009. [DOI] [PubMed] [Google Scholar]
- De Dreu CK. Oxytocin modulates the link between adult attachment and cooperation through reduced betrayal aversion. Psychoneuroendocrinology. 2012b;37:871–880. doi: 10.1016/j.psyneuen.2011.10.003. [DOI] [PubMed] [Google Scholar]
- De Dreu CK, Greer LL, Van Kleef GA, Shalvi S, Handgraaf MJ. Oxytocin promotes human ethnocentrism. Proc Natl Acad Sci U S A. 2011;108:1262–1266. doi: 10.1073/pnas.1015316108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pisapia N, Bornstein MH, Rigo P, Esposito G, De Falco S, Venuti P. Sex differences in directional brain responses to infant hunger cries. Neuroreport. 2013;24:142–146. doi: 10.1097/WNR.0b013e32835df4fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wolff M, van Ijzendoorn MH. Sensitivity and attachment: A meta-analysis on parental antecedents of infant attachment. Child Development. 1997;68:571–591. [PubMed] [Google Scholar]
- Dickson KL, Walker H, Fogel A. The relationship between smile type and play type during parent-infant play. Dev Psychol. 1997;33:925–933. doi: 10.1037//0012-1649.33.6.925. [DOI] [PubMed] [Google Scholar]
- Ditzen B, Nater UM, Schaer M, La Marca R, Bodenmann G, Ehlert U, Heinrichs M. Sex-specific effects of intranasal oxytocin on autonomic nervous system and emotional responses to couple conflict. Soc Cogn Affect Neurosci. 2012 doi: 10.1093/scan/nss083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N, Cumberland A, Spinrad TL, Fabes RA, Shepard SA, Reiser M, Murphy BC, Losoya SH, Guthrie IK. The relations of regulation and emotionality to children’s externalizing and internalizing problem behavior. Child Dev. 2001;72:1112–1134. doi: 10.1111/1467-8624.00337. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Cole SW. Social neuroscience and health: neurophysiological mechanisms linking social ties with physical health. Nat Neurosci. 2012;15:669–674. doi: 10.1038/nn.3086. [DOI] [PubMed] [Google Scholar]
- Elliott R, Zahn R, Deakin JF, Anderson IM. Affective cognition and its disruption in mood disorders. Neuropsychopharmacology. 2011;36:153–182. doi: 10.1038/npp.2010.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engfer AMG. Antecedants and Consequences of Maternal Sensitivity: A Longitudinal Study. In: HRH-CS, editor. Psychobiology and Early Development. North Holland; 1987. pp. 71–99. [Google Scholar]
- Feldman R. Infant-mother and infant-father synchrony: The coregulation of positive arousal. Inf Mental Health Jounal. 2003;24:1–23. [Google Scholar]
- Feldman R, Gordon I, Zagoory-Sharon O. Maternal and paternal plasma, salivary, and urinary oxytocin and parent-infant synchrony: considering stress and affiliation components of human bonding. Dev Sci. 2011;14:752–761. doi: 10.1111/j.1467-7687.2010.01021.x. [DOI] [PubMed] [Google Scholar]
- Feldman R, Granat A, Pariente C, Kanety H, Kuint J, Gilboa-Schechtman E. Maternal depression and anxiety across the postpartum year and infant social engagement, fear regulation, and stress reactivity. J Am Acad Child Adolesc Psychiatry. 2009;48:919–927. doi: 10.1097/CHI.0b013e3181b21651. [DOI] [PubMed] [Google Scholar]
- Feldman R, Weller A, Leckman JF, Kuint J, Eidelman AI. The nature of the mother’s tie to her infant: maternal bonding under conditions of proximity, separation, and potential loss. J Child Psychol Psychiatry. 1999;40:929–939. [PubMed] [Google Scholar]
- Feldman R, Weller A, Zagoory-Sharon O, Levine A. Evidence for a neuroendocrinological foundation of human affiliation: plasma oxytocin levels across pregnancy and the postpartum period predict mother-infant bonding. Psychol Sci. 2007;18:965–970. doi: 10.1111/j.1467-9280.2007.02010.x. [DOI] [PubMed] [Google Scholar]
- Feldman R, Zagoory-Sharon O, Weisman O, Schneiderman I, Gordon I, Maoz R, Shalev I, Ebstein RP. Sensitive parenting is associated with plasma oxytocin and polymorphisms in the OXTR and CD38 genes. Biol Psychiatry. 2012;72:175–181. doi: 10.1016/j.biopsych.2011.12.025. [DOI] [PubMed] [Google Scholar]
- Field T. Postpartum depression effects on early interactions, parenting, and safety practices: a review. Infant Behav Dev. 2010;33:1–6. doi: 10.1016/j.infbeh.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD, Frith U. The neural basis of mentalizing. Neuron. 2006;50:531–534. doi: 10.1016/j.neuron.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Frith U, Frith CD. Development and neurophysiology of mentalizing. Philos Trans R Soc Lond B Biol Sci. 2003;358:459–473. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbally M, Lewis AJ, Ijzendoorn M, Permezel M. The role of oxytocin in mother-infant relations: a systematic review of human studies. Harv Rev Psychiatry. 2011;19:1–14. doi: 10.3109/10673229.2011.549771. [DOI] [PubMed] [Google Scholar]
- Gallese V, Keysers C, Rizzolatti G. A unifying view of the basis of social cognition. Trends Cogn Sci. 2004;8:396–403. doi: 10.1016/j.tics.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Glocker ML, Langleben DD, Ruparel K, Loughead JW, Valdez JN, Griffin MD, Sachser N, Gur RC. Baby schema modulates the brain reward system in nulliparous women. Proc Natl Acad Sci U S A. 2009;106:9115–9119. doi: 10.1073/pnas.0811620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez A, Jenkins JM, Steiner M, Fleming AS. Maternal early life experiences and parenting: the mediating role of cortisol and executive function. J Am Acad Child Adolesc Psychiatry. 2012;51:673–682. doi: 10.1016/j.jaac.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Goodman SH, Rouse MH, Connell AM, Broth MR, Hall CM, Heyward D. Maternal depression and child psychopathology: a meta-analytic review. Clin Child Fam Psychol Rev. 2011;14:1–27. doi: 10.1007/s10567-010-0080-1. [DOI] [PubMed] [Google Scholar]
- Gordon I, Feldman R. Synchrony in the triad: a microlevel process model of coparenting and parent-child interactions. Fam Process. 2008;47:465–479. doi: 10.1111/j.1545-5300.2008.00266.x. [DOI] [PubMed] [Google Scholar]
- Gordon I, Martin C, Feldman R, Leckman JF. Oxytocin and social motivation. Developmental cognitive neuroscience. 2011;1:471–493. doi: 10.1016/j.dcn.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassin-Delyle S, Buenestado A, Naline E, Faisy C, Blouquit-Laye S, Couderc LJ, Le Guen M, Fischler M, Devillier P. Intranasal drug delivery: an efficient and non-invasive route for systemic administration: focus on opioids. Pharmacol Ther. 2012;134:366–379. doi: 10.1016/j.pharmthera.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Grillon C, Krimsky M, Charney DR, Vytal K, Ernst M, Cornwell B. Oxytocin increases anxiety to unpredictable threat. Mol Psychiatry. 2013;18:958–960. doi: 10.1038/mp.2012.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewold NA, Opmeer EM, de Jonge P, Aleman A, Costafreda SG. Emotional valence modulates brain functional abnormalities in depression: Evidence from a meta-analysis of fMRI studies. Neurosci Biobehav Rev. 2012;37:152–163. doi: 10.1016/j.neubiorev.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Grossmann K, Grossmann KE, Fremmer-Bombik E, Kindler H, HSE, Zimmerman P. The uniqueness of the child-father attachment relationship: Fathers’ sensitive and challenging play as a pivotal variable in a 16-year longitudinal study. Social Development. 2002;11:307–331. [Google Scholar]
- Grossmann K, Grossmann KE, Kindler H, Zimmermann P. A wider view of attachment and exploration: The influence of mothers and fathers on the development of psychological security from infancy to young adulthood. In: Cassidy J, Shaver PR, editors. Handbook of attachment: Theory, research, and clinical applications. Guilford Press; New York, NY: 2008. pp. 857–879. [Google Scholar]
- Guastella AJ, MacLeod C. A critical review of the influence of oxytocin nasal spray on social cognition in humans: evidence and future directions. Horm Behav. 2012;61:410–418. doi: 10.1016/j.yhbeh.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Hari R, Kujala MV. Brain basis of human social interaction: from concepts to brain imaging. Physiological reviews. 2009;89:453–479. doi: 10.1152/physrev.00041.2007. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Cowen PJ, Goodwin GM. Efficacy markers in depression. J Psychopharmacol. 2011;25:1148–1158. doi: 10.1177/0269881110367722. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, O’Sullivan U, Favaron E, Massey-Chase R, Ayres R, Reinecke A, Goodwin GM, Cowen PJ. Effect of acute antidepressant administration on negative affective bias in depressed patients. Am J Psychiatry. 2009;166:1178–1184. doi: 10.1176/appi.ajp.2009.09020149. [DOI] [PubMed] [Google Scholar]
- Hein G, Singer T. I feel how you feel but not always: the empathic brain and its modulation. Curr Opin Neurobiol. 2008;18:153–158. doi: 10.1016/j.conb.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry. 2003;54:1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Herz A. Endogenous opioid systems and alcohol addiction. Psychopharmacology (Berl) 1997;129:99–111. doi: 10.1007/s002130050169. [DOI] [PubMed] [Google Scholar]
- Higham JP, Barr CS, Hoffman CL, Mandalaywala TM, Parker KJ, Maestripieri D. Mu-opioid receptor (OPRM1) variation, oxytocin levels and maternal attachment in free-ranging rhesus macaques Macaca mulatta. Behav Neurosci. 2011;125:131–136. doi: 10.1037/a0022695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrdy SB. Mother Nature: Maternal Instincts and How They Shape the Human Species. Ballantine Books; 2000. [Google Scholar]
- Iacoboni M. Neurobiology of imitation. Curr Opin Neurobiol. 2009;19:661–665. doi: 10.1016/j.conb.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Insel TR. A neurobiological basis of social attachment. Am J Psychiatry. 1997;154:726–735. doi: 10.1176/ajp.154.6.726. [DOI] [PubMed] [Google Scholar]
- Insel TR, Young LJ. The neurobiology of attachment. Nat Rev Neurosci. 2001;2:129–136. doi: 10.1038/35053579. [DOI] [PubMed] [Google Scholar]
- Ishak WW, Kahloon M, Fakhry H. Oxytocin role in enhancing well-being: a literature review. J Affect Disord. 2011;130:1–9. doi: 10.1016/j.jad.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Jaffari-Bimmel N, Juffer F, van IJzendoorn MH, Bakermans-Kranenburg MJ, Mooijaart A. Social development from infancy to adolescence: Longitudinal and concurrent factors in an adoption sample. Developmental Psychology. 2006;42:1143–1153. doi: 10.1037/0012-1649.42.6.1143. [DOI] [PubMed] [Google Scholar]
- Joosen KJ, Mesman J, Bakermans-Kranenburg MJ, van IJzendoorn MH. Maternal sensitivity to infants in various settings predicts harsh discipline in toddlerhood. Attachment & Human Development. 2012;14:101–117. doi: 10.1080/14616734.2012.661217. [DOI] [PubMed] [Google Scholar]
- Kaffman A, Meaney MJ. Neurodevelopmental sequelae of postnatal maternal care in rodents: clinical and research implications of molecular insights. J Child Psychol Psychiatry. 2007;48:224–244. doi: 10.1111/j.1469-7610.2007.01730.x. [DOI] [PubMed] [Google Scholar]
- Kagerbauer SM, Martin J, Schuster T, Blobner M, Kochs EF, Landgraf R. Plasma oxytocin and vasopressin do not predict neuropeptide concentrations in human cerebrospinal fluid. J Neuroendocrinol. 2013;25:668–673. doi: 10.1111/jne.12038. [DOI] [PubMed] [Google Scholar]
- Keller H, Lohaus A, Völker S, Elben C, Ball J. Warmth and contingency and their relationship to maternal attitudes toward parenting. Journal of Genetic Psychology. 2003;164:275–292. doi: 10.1080/00221320309597984. [DOI] [PubMed] [Google Scholar]
- Keren M, Feldman R, Eidelman AI, Sirota L, Lester B. Clinical interview ofr high-risk parents of premature infants (CLIP): relations to mother-infant interaction. Infant Mental Health Journal. 2003;24:93–110. [Google Scholar]
- Kim P, Feldman R, Mayes LC, Eicher V, Thompson N, Leckman JF, Swain JE. Breastfeeding, brain activation to own infant cry, and maternal sensitivity. J Child Psychol Psychiatry. 2011;52:907–915. doi: 10.1111/j.1469-7610.2011.02406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Leckman JF, Mayes LC, Feldman R, Wang X, Swain JE. The plasticity of human maternal brain: longitudinal changes in brain anatomy during the early postpartum period. Behavioral Neuroscience. 2010a;124:695–700. doi: 10.1037/a0020884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Leckman JF, Mayes LC, Newman MA, Feldman R, Swain JE. Perceived quality of maternal care in childhood and structure and function of mothers’ brain. Developmental Science. 2010b;13:662–673. doi: 10.1111/j.1467-7687.2009.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Mayes L, Feldman R, Leckman JF, Swain JE. Early Postpartum Parental Preoccupation and Positive Parenting Thoughts: Relationship with ParentInfant Interaction. Infant Mental Health Journal. 2013;34:104–116. doi: 10.1002/imhj.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Rigo P, Mayes LC, Feldman R, Leckman JF, Swain JE. Neural Plasticity in Human Fathers’ Brains: Associations with Depressive Moods and Parenting. Social Neuroscience in submission. 2014 doi: 10.1080/17470919.2014.933713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsley CH, Lambert KG. Reproduction-induced neuroplasticity: natural behavioural and neuronal alterations associated with the production and care of offspring. J Neuroendocrinol. 2008;20:515–525. doi: 10.1111/j.1365-2826.2008.01667.x. [DOI] [PubMed] [Google Scholar]
- Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical-subcortical interactions in emotion: A meta-analysis of neuroimaging studies. Neuroimage. 2008;42:998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanska G. Mutually responsive orientation between mothers and their young children: A context for the early development of conscience. Current Directions in Psychological Science. 2002;11:191–195. [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Lehtonen A, Squire S, Harvey AG, Craske MG, Holliday IE, Green AL, Aziz TZ, Hansen PC, Cornelissen PL, Stein A. A specific and rapid neural signature for parental instinct. PLoS One. 2008;3:e1664. doi: 10.1371/journal.pone.0001664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo PX, Carp J, Light KC, Grewen KM. Neural responses to infants linked with behavioral interactions and testosterone in fathers. Biological Psychology. 2012;91:302–306. doi: 10.1016/j.biopsycho.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey BB, Michalska KJ, Liu C, Chen Q, Hipwell AE, Chronis-Tuscano A, Waldman ID, Decety J. Preliminary genetic imaging study of the association between estrogen receptor-alpha gene polymorphisms and harsh human maternal parenting. Neurosci Lett. 2012;525:17–22. doi: 10.1016/j.neulet.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb ME. A re-examination of the infant social world. Hum Dev. 1977;20:65–85. doi: 10.1159/000271548. [DOI] [PubMed] [Google Scholar]
- Lamb ME. The role of the father in child development. 4. Wiley and Sons; New Jersey: 2004. [Google Scholar]
- Landi N, Montoya J, Kober H, Rutherford HJ, Mencl WE, Worhunsky PD, Potenza MN, Mayes LC. Maternal neural responses to infant cries and faces: relationships with substance use. Front Psychiatry. 2011;2:32. doi: 10.3389/fpsyt.2011.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent HK, Ablow JC. A cry in the dark: depressed mothers show reduced neural activation to their own infant’s cry. Soc Cogn Affect Neurosci. 2012;7:125–134. doi: 10.1093/scan/nsq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent HK, Ablow JC. A face a mother could love: depression-related maternal neural responses to infant emotion faces. Soc Neurosci. 2013;8:228–239. doi: 10.1080/17470919.2012.762039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent HK, Stevens A, Ablow JC. Neural correlates of hypothalamic-pituitary-adrenal regulation of mothers with their infants. Biological Psychiatry. 2011;70:826–832. doi: 10.1016/j.biopsych.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leckman JF, Feldman R, Swain JE, Eicher V, Thompson N, Mayes LC. Primary parental preoccupation: circuits, genes, and the crucial role of the environment. J Neural Transm. 2004;111:753–771. doi: 10.1007/s00702-003-0067-x. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Mayes LC. Preoccupations and behaviors associated with romantic and parental love. Perspectives on the origin of obsessive-compulsive disorder. Child Adolesc Psychiatr Clin N Am. 1999;8:635–665. [PubMed] [Google Scholar]
- Leckman JF, Mayes LC, Feldman R, Evans DW, King RA, Cohen DJ. Early parental preoccupations and behaviors and their possible relationship to the symptoms of obsessive-compulsive disorder. Acta Psychiatr Scand Suppl. 1999;396:1–26. doi: 10.1111/j.1600-0447.1999.tb10951.x. [DOI] [PubMed] [Google Scholar]
- LeDoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. 2003;23:727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leerkes EM. Maternal sensitivity during distressing tasks: a unique predictor of attachment security. Infant Behav Dev. 2011;34:443–446. doi: 10.1016/j.infbeh.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leerkes EM, Nayena Blankson A, O’Brien M. Differential effects of maternal sensitivity to infant distress and nondistress on social-emotional functioning. Child Dev. 2009;80:762–775. doi: 10.1111/j.1467-8624.2009.01296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibenluft E, Gobbini MI, Harrison T, Haxby JV. Mothers’ neural activation in response to pictures of their children and other children. Biol Psychiatry. 2004;56:225–232. doi: 10.1016/j.biopsych.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Lenzi D, Trentini C, Pantano P, Macaluso E, Iacoboni M, Lenzi GL, Ammaniti M. Neural basis of maternal communication and emotional expression processing during infant preverbal stage. Cereb Cortex. 2009;19:1124–1133. doi: 10.1093/cercor/bhn153. [DOI] [PubMed] [Google Scholar]
- Levine A, Zagoory-Sharon O, Feldman R, Weller A. Oxytocin during pregnancy and early postpartum: individual patterns and maternal-fetal attachment. Peptides. 2007;28:1162–1169. doi: 10.1016/j.peptides.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Light KC, Smith TE, Johns JM, Brownley KA, Hofheimer JA, Amico JA. Oxytocin responsivity in mothers of infants: a preliminary study of relationships with blood pressure during laboratory stress and normal ambulatory activity. Health Psychol. 2000;19:560–567. doi: 10.1037//0278-6133.19.6.560. [DOI] [PubMed] [Google Scholar]
- Lorberbaum JP, Newman JD, Dubno JR, Horwitz AR, Nahas Z, Teneback CC, Bloomer CW, Bohning DE, Vincent D, Johnson MR, Emmanuel N, Brawman-Mintzer O, Book SW, Lydiard RB, Ballenger JC, George MS. Feasibility of using fMRI to study mothers responding to infant cries. Depress Anxiety. 1999;10:99–104. doi: 10.1002/(sici)1520-6394(1999)10:3<99::aid-da2>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Lorberbaum JP, Newman JD, Horwitz AR, Dubno JR, Lydiard RB, Hamner MB, Bohning DE, George MS. A potential role for thalamocingulate circuitry in human maternal behavior. Biol Psychiatry. 2002;51:431–445. doi: 10.1016/s0006-3223(01)01284-7. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- MacDonald K. Parent-child physical play with rejected, neglected, and popular boys. Developmental Psychology. 1987;23:705–711. [Google Scholar]
- MacDonald K. Warmth as a developmental construct: An evolutionary analysis. Child Development. 1992;63:753–773. [Google Scholar]
- Macdonald K, Feifel D. Helping oxytocin deliver: considerations in the development of oxytocin-based therapeutics for brain disorders. Front Neurosci. 2013;7:35. doi: 10.3389/fnins.2013.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald K, Macdonald TM, Brune M, Lamb K, Wilson MP, Golshan S, Feifel D. Oxytocin and psychotherapy: A pilot study of its physiological, behavioral and subjective effects in males with depression. Psychoneuroendocrinology. 2013 doi: 10.1016/j.psyneuen.2013.05.014. [DOI] [PubMed] [Google Scholar]
- MacLean PD. The triune brain in evolution: role in paleocerebral functions. Plenum Press; New York: 1990. [DOI] [PubMed] [Google Scholar]
- Marazziti D, Dell’osso B, Baroni S, Mungai F, Catena M, Rucci P, Albanese F, Giannaccini G, Betti L, Fabbrini L, Italiani P, Del Debbio A, Lucacchini A, Dell’osso L. A relationship between oxytocin and anxiety of romantic attachment. Clin Pract Epidemol Ment Health. 2006;2:28. doi: 10.1186/1745-0179-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascaro JS, Hackett PD, Gouzoules H, Lori A, Rilling JK. Behavioral and genetic correlates of the neural response to infant crying among human fathers. Soc Cogn Affect Neurosci. 2013a doi: 10.1093/scan/nst166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascaro JS, Hackett PD, Rilling JK. Testicular volume is inversely correlated with nurturing-related brain activity in human fathers. Proc Natl Acad Sci U S A. 2013b;110:15746–15751. doi: 10.1073/pnas.1305579110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElwain NL, Booth-Laforce C. Maternal sensitivity to infant distress and nondistress as predictors of infant-mother attachment security. J Fam Psychol. 2006;20:247–255. doi: 10.1037/0893-3200.20.2.247. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Understanding the potency of stressful early life experiences on brain and body function. Metabolism. 2008;57(Suppl 2):S11–15. doi: 10.1016/j.metabol.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer RT. The process of maternal role attainment over the first year. Nursing Research. 1985;34:198–204. [PubMed] [Google Scholar]
- Milner JS. Social Information-Processing and Physical Child-Abuse. Clinical Psychology Review. 1993;13:275–294. [Google Scholar]
- Milner JS. Social information processing in high-risk and physically abusive parents. Child Abuse Negl. 2003;27:7–20. doi: 10.1016/s0145-2134(02)00506-9. [DOI] [PubMed] [Google Scholar]
- Mitchell JP. Inferences about mental states. Philos Trans R Soc Lond B Biol Sci. 2009;364:1309–1316. doi: 10.1098/rstb.2008.0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molitor A, Mayes LC. Problematic Dyadic Interaction among Toddlers and Their Polydrug-Cocaine-Using Mothers. Infant Mental Health Journal. 2010;31:121–140. doi: 10.1002/imhj.20248. [DOI] [PubMed] [Google Scholar]
- Montoya JL, Landi N, Kober H, Worhunsky PD, Rutherford HJV, Mencl WE, Mayes LC, Potenza MN. Regional Brain Responses in Nulliparous Women to Emotional Infant Stimuli. PLoS One. 2012;7 doi: 10.1371/journal.pone.0036270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JP, Pelphrey KA, McCarthy G. Regional brain activation evoked when approaching a virtual human on a virtual walk. J Cogn Neurosci. 2005;17:1744–1752. doi: 10.1162/089892905774589253. [DOI] [PubMed] [Google Scholar]
- Moser DA, Aue T, Wang Z, Rusconi Serpa S, Favez N, Peterson BS, Schechter DS. Limbic brain responses in mothers with post-traumatic stress disorder and comorbid dissociation to video clips of their children. Stress. 2013;16:493–502. doi: 10.3109/10253890.2013.816280. [DOI] [PubMed] [Google Scholar]
- Moses-Kolko EL, Hipwell A, Swain JE. In search of neural endophenotypes of postpartum psychopathology and disrupted maternal caregiving. Journal of Neuroendocrinology. 2014 doi: 10.1111/jne.12183. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses-Kolko EL, Perlman SB, Wisner KL, James J, Saul AT, Phillips ML. Abnormally reduced dorsomedial prefrontal cortical activity and effective connectivity with amygdala in response to negative emotional faces in postpartum depression. Am J Psychiatry. 2010;167:1373–1380. doi: 10.1176/appi.ajp.2010.09081235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray L, Fiori-Cowley A, Hooper R, Cooper P. The impact of postnatal depression and associated adversity on early mother-infant interactions and later infant outcome. Child Dev. 1996;67:2512–2526. [PubMed] [Google Scholar]
- Musser ED, Kaiser-Laurent H, Ablow JC. The neural correlates of maternal sensitivity: An fMRI study. Developmental cognitive neuroscience. 2012;2:428–436. doi: 10.1016/j.dcn.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann ID. Brain oxytocin: a key regulator of emotional and social behaviours in both females and males. J Neuroendocrinol. 2008;20:858–865. doi: 10.1111/j.1365-2826.2008.01726.x. [DOI] [PubMed] [Google Scholar]
- Nishitani S, Doi H, Koyama A, Shinohara K. Differential prefrontal response to infant facial emotions in mothers compared with non-mothers. Neuroscience research. 2011;70:183–188. doi: 10.1016/j.neures.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Nelson EE, Rusch BD, Fox AS, Oakes TR, Davidson RJ. Orbitofrontal cortex tracks positive mood in mothers viewing pictures of their newborn infants. Neuroimage. 2004;21:583–592. doi: 10.1016/j.neuroimage.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Noriuchi M, Kikuchi Y, Senoo A. The functional neuroanatomy of maternal love: mother’s response to infant’s attachment behaviors. Biol Psychiatry. 2008;63:415–423. doi: 10.1016/j.biopsych.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Nowak R, Keller M, Levy F. Mother-young relationships in sheep: a model for a multidisciplinary approach of the study of attachment in mammals. J Neuroendocrinol. 2011;23:1042–1053. doi: 10.1111/j.1365-2826.2011.02205.x. [DOI] [PubMed] [Google Scholar]
- Numan M, Insel TR. The Neurobiology of Parental Behavior. Springer-Verlag; New York: 2003a. [Google Scholar]
- Numan M, Insel TR. The neurobiology of parental behavior. Springer; New York: 2003b. [Google Scholar]
- Numan M, Woodside B. Maternity: neural mechanisms, motivational processes, and physiological adaptations. Behav Neurosci. 2010;124:715–741. doi: 10.1037/a0021548. [DOI] [PubMed] [Google Scholar]
- Nystrom K, Ohrling K. Parenthood experiences during the child’s first year: literature review. Journal of Advanced Nursing. 2004;46:319–330. doi: 10.1111/j.1365-2648.2004.02991.x. [DOI] [PubMed] [Google Scholar]
- Olff M, Frijling JL, Kubzansky LD, Bradley B, Ellenbogen MA, Cardoso C, Bartz JA, Yee JR, van Zuiden M. The role of oxytocin in social bonding, stress regulation and mental health: An update on the moderating effects of context and interindividual differences. Psychoneuroendocrinology. 2013;38:1883–1894. doi: 10.1016/j.psyneuen.2013.06.019. [DOI] [PubMed] [Google Scholar]
- Pantazatos SP, Talati A, Pavlidis P, Hirsch J. Decoding unattended fearful faces with whole-brain correlations: an approach to identify condition-dependent large-scale functional connectivity. PLoS Comput Biol. 2012;8:e1002441. doi: 10.1371/journal.pcbi.1002441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papousek H, Papousek M. Intuitive parenting. In: Bornstein MH, editor. Handbook of Parenting. Erlbaum; Mahwah, N.J: 2002. pp. 183–203. [Google Scholar]
- Paquette D. Theorizing the Father-Child Relationship: Mechanisms and Developmental Outcomes. Human Development. 2004;47:193–219. [Google Scholar]
- Parsons CE, Young KS, Kumari N, Stein A, Kringelbach ML. The motivational salience of infant faces is similar for men and women. PLoS One. 2011;6:e20632. doi: 10.1371/journal.pone.0020632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons CE, Young KS, Mohseni H, Woolrich MW, Thomsen KR, Joensson M, Murray L, Goodacre T, Stein A, Kringelbach ML. Minor structural abnormalities in the infant face disrupt neural processing: a unique window into early caregiving responses. Soc Neurosci. 2013;8:268–274. doi: 10.1080/17470919.2013.795189. [DOI] [PubMed] [Google Scholar]
- Pearson RM, Cooper RM, Penton-Voak IS, Lightman SL, Evans J. Depressive symptoms in early pregnancy disrupt attentional processing of infant emotion. Psychol Med. 2010;40:621–631. doi: 10.1017/S0033291709990961. [DOI] [PubMed] [Google Scholar]
- Pearson RM, Lightman SL, Evans J. The impact of breastfeeding on mothers’ attentional sensitivity towards infant distress. Infant Behav Dev. 2011;34:200–205. doi: 10.1016/j.infbeh.2010.12.009. [DOI] [PubMed] [Google Scholar]
- Pearson RM, Lightman SL, Evans J. Symptoms of depression during pregnancy are associated with increased systolic blood pressure responses towards infant distress. Arch Womens Ment Health. 2012;15:95–105. doi: 10.1007/s00737-012-0269-z. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Prange AJ., Jr Induction of maternal behavior in virgin rats after intracerebroventricular administration of oxytocin. Proc Natl Acad Sci U S A. 1979;76:6661–6665. doi: 10.1073/pnas.76.12.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruett KD. Role of the father. Pediatrics. 1998;102:1253–1261. [PubMed] [Google Scholar]
- Quirin M, Kuhl J, Dusing R. Oxytocin buffers cortisol responses to stress in individuals with impaired emotion regulation abilities. Psychoneuroendocrinology. 2011;36:898–904. doi: 10.1016/j.psyneuen.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Ramchandani P, Psychogiou L. Paternal psychiatric disorders and children’s psychosocial development. Lancet. 2009;374:646–653. doi: 10.1016/S0140-6736(09)60238-5. [DOI] [PubMed] [Google Scholar]
- Ranote S, Elliott R, Abel KM, Mitchell R, Deakin JF, Appleby L. The neural basis of maternal responsiveness to infants: an fMRI study. Neuroreport. 2004;15:1825–1829. doi: 10.1097/01.wnr.0000137078.64128.6a. [DOI] [PubMed] [Google Scholar]
- Riem MM, Bakermans-Kranenburg MJ, Pieper S, Tops M, Boksem MA, Vermeiren RR, van Ijzendoorn MH, Rombouts SA. Oxytocin Modulates Amygdala, Insula, and Inferior Frontal Gyrus Responses to Infant Crying: A Randomized Controlled Trial. Biol Psychiatry. 2011a doi: 10.1016/j.biopsych.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Riem MM, Bakermans-Kranenburg MJ, Pieper S, Tops M, Boksem MA, Vermeiren RR, van Ijzendoorn MH, Rombouts SA. Oxytocin modulates amygdala, insula, and inferior frontal gyrus responses to infant crying: a randomized controlled trial. Biological Psychiatry. 2011b;70:291–297. doi: 10.1016/j.biopsych.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Riem MME, Bakermans-Kranenburg MJ, van Ijzendoorn MH, Out D, Rombouts S. Attachment in the brain: adult attachment representations predict amygdala and behavioral responses to infant crying. Attachment & Human Development. 2012;14:533–551. doi: 10.1080/14616734.2012.727252. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The functions of the orbitofrontal cortex. Brain Cogn. 2004;55:11–29. doi: 10.1016/S0278-2626(03)00277-X. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JS, Ceus K. Estrogen implants in the medial preoptic area stimulate maternal behavior in male rats. Hormones and Behavior. 1998;33:23–30. doi: 10.1006/hbeh.1997.1430. [DOI] [PubMed] [Google Scholar]
- Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocrinol. 2009;30:534–547. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M. Annual Research Review: Resilience--clinical implications. J Child Psychol Psychiatry. 2013;54:474–487. doi: 10.1111/j.1469-7610.2012.02615.x. [DOI] [PubMed] [Google Scholar]
- Saxe R. Uniquely human social cognition. Curr Opin Neurobiol. 2006;16:235–239. doi: 10.1016/j.conb.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Schechter DS, Moser DA, Wang Z, Marsh R, Hao X, Duan Y, Yu S, Gunter B, Murphy D, McCaw J, Kangarlu A, Willheim E, Myers MM, Hofer MA, Peterson BS. An fMRI study of the brain responses of traumatized mothers to viewing their toddlers during separation and play. Soc Cogn Affect Neurosci. 2012;7:969–979. doi: 10.1093/scan/nsr069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilbach L, Timmermans B, Reddy V, Costall A, Bente G, Schlicht T, Vogeley K. Toward a second-person neuroscience. Behav Brain Sci. 2013;36:393–414. doi: 10.1017/S0140525X12000660. [DOI] [PubMed] [Google Scholar]
- Schuengel C, Bakermans-Kranenburg MJ, Van IMH. Frightening maternal behavior linking unresolved loss and disorganized infant attachment. J Consult Clin Psychol. 1999;67:54–63. doi: 10.1037//0022-006x.67.1.54. [DOI] [PubMed] [Google Scholar]
- Seifritz E, Esposito F, Neuhoff JG, Luthi A, Mustovic H, Dammann G, von Bardeleben U, Radue EW, Cirillo S, Tedeschi G, Di Salle F. Differential sex-independent amygdala response to infant crying and laughing in parents versus nonparents. Biol Psychiatry. 2003;54:1367–1375. doi: 10.1016/s0006-3223(03)00697-8. [DOI] [PubMed] [Google Scholar]
- Shahrokh DK, Zhang TY, Diorio J, Gratton A, Meaney MJ. Oxytocin-dopamine interactions mediate variations in maternal behavior in the rat. Endocrinology. 2010;151:2276–2286. doi: 10.1210/en.2009-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaver P, Schwartz J, Kirson D, O’Connor C. Emotion knowledge: further exploration of a prototype approach. J Pers Soc Psychol. 1987;52:1061–1086. doi: 10.1037//0022-3514.52.6.1061. [DOI] [PubMed] [Google Scholar]
- Simmons D, Self DW. Role of mu- and delta-opioid receptors in the nucleus accumbens in cocaine-seeking behavior. Neuropsychopharmacology. 2009;34:1946–1957. doi: 10.1038/npp.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrundz M, Bolten M, Nast I, Hellhammer DH, Meinlschmidt G. Plasma oxytocin concentration during pregnancy is associated with development of postpartum depression. Neuropsychopharmacology. 2011;36:1886–1893. doi: 10.1038/npp.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltis J. The signal functions of early infant crying. Behav Brain Sci. 2004;27:443–458. discussion 459–490. [PubMed] [Google Scholar]
- Sripada C, Angstadt M, Kessler D, Phan KL, Liberzon I, Evans GW, Welsh RC, Kim P, Swain JE. Volitional regulation of emotions produces distributed alterations in connectivity between visual, attention control, and default networks. Neuroimage. 2013;89C:110–121. doi: 10.1016/j.neuroimage.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathearn L, Fonagy P, Amico J, Montague PR. Adult attachment predicts maternal brain and oxytocin response to infant cues. Neuropsychopharmacology. 2009;34:2655–2666. doi: 10.1038/npp.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathearn L, Li J, Fonagy P, Montague PR. What’s in a smile? Maternal brain responses to infant facial cues. Pediatrics. 2008;122:40–51. doi: 10.1542/peds.2007-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathearn L, Li J, Montague PR. An fMRI Study of Maternal Mentalization: Having the Baby’s Mind in Mind. Neuroimage. 2005;26:S25. [Google Scholar]
- Striepens N, Scheele D, Kendrick KM, Becker B, Schafer L, Schwalba K, Reul J, Maier W, Hurlemann R. Oxytocin facilitates protective responses to aversive social stimuli in males. Proc Natl Acad Sci U S A. 2012;109:18144–18149. doi: 10.1073/pnas.1208852109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE. Brain Imaging of Human Parent-Infant Relationships Archives of Women’s Mental Health. 2011a;14:s93–94. [Google Scholar]
- Swain JE. The human parental brain: in vivo neuroimaging. Prog Neuropsychopharmacol Biol Psychiatry. 2011b;35:1242–1254. doi: 10.1016/j.pnpbp.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Kim P, Ho SS. Neuroendocrinology of parental response to baby-cry. Journal of Neuroendocrinology. 2011;23:1036–1041. doi: 10.1111/j.1365-2826.2011.02212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Konrath S, Brown SL, Finegood ED, Akce LB, Dayton CJ, Ho SS. Parenting and Beyond: Common Neurocircuits Underlying Parental and Altruistic Caregiving. Parenting, Science and Practice. 2012;12:115–123. doi: 10.1080/15295192.2012.680409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Leckman JF, Mayes LC, Feldman R, Constable RT, Schultz RT. The neural circuitry of parent-infant attachment in the early postpartum, American College of Neuropsychopharmacology. Puerto Rico 2003 [Google Scholar]
- Swain JE, Leckman JF, Mayes LC, Feldman R, Constable RT, Schultz RT. Neural substrates of human parent-infant attachment in the postpartum. Biological Psychiatry. 2004a;55:153s–153s. [Google Scholar]
- Swain JE, Leckman JF, Mayes LC, Feldman R, Hoyt E, Kang H, Kim P, David D, Nguyen S, Constable RT, Schultz RT. Functional Brain Activations of Parents Listening to Their Own Baby-Cry Change over the Early Postpartum. Developmental Psychobiology in submission 2014 [Google Scholar]
- Swain JE, Leckman JF, Mayes LC, Feldman R, Schultz RT. Early human parent-infant bond development: fMRI, thoughts and behaviors. Biological Psychiatry. 2005;57:112s–112s. [Google Scholar]
- Swain JE, Leckman JF, Mayes LC, Feldman R, Schultz RT. Own baby pictures induce parental brain activations according to psychology, experience and postpartum timing. Biological Psychiatry. 2006;59:126s–126s. [Google Scholar]
- Swain JE, Lorberbaum JP. Imaging the Human Parental Brain. Neurobiology of the Parental Brain. 2008:83–100. [Google Scholar]
- Swain JE, Lorberbaum JP, Kose S, Strathearn L. Brain basis of early parent-infant interactions: psychology, physiology, and in vivo functional neuroimaging studies. Journal of Child Psychology and Psychiatry. 2007;48:262–287. doi: 10.1111/j.1469-7610.2007.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Mayes LC, Leckman JF. The development of parent-infant attachment through dynamic and interactive signaling loops of care and cry. Behavioral and Brain Sciences. 2004b;27:472–473. [Google Scholar]
- Swain JE, Tasgin E, Mayes LC, Feldman R, Constable RT, Leckman JF. Maternal brain response to own baby-cry is affected by cesarean section delivery. Journal of Child Psychology and Psychiatry. 2008;49:1042–1052. doi: 10.1111/j.1469-7610.2008.01963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabak BA, McCullough ME, Szeto A, Mendez AJ, McCabe PM. Oxytocin indexes relational distress following interpersonal harms in women. Psychoneuroendocrinology. 2011;36:115–122. doi: 10.1016/j.psyneuen.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamis-LeMonda CS, Bornstein MH, Baumwell L. Maternal responsiveness and children’s achievement of language milestones. Child Dev. 2001;72:748–767. doi: 10.1111/1467-8624.00313. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Gonzaga GC, Klein LC, Hu P, Greendale GA, Seeman TE. Relation of oxytocin to psychological stress responses and hypothalamic-pituitary-adrenocortical axis activity in older women. Psychosom Med. 2006;68:238–245. doi: 10.1097/01.psy.0000203242.95990.74. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Seeman TE, Eisenberger NI, Kozanian TA, Moore AN, Moons WG. Effects of a supportive or an unsupportive audience on biological and psychological responses to stress. Journal of Personality and Social Psychology. 2010;98:47–56. doi: 10.1037/a0016563. [DOI] [PubMed] [Google Scholar]
- Theodoridou A, Penton-Voak IS, Rowe AC. A direct examination of the effect of intranasal administration of oxytocin on approach-avoidance motor responses to emotional stimuli. PLoS One. 2013;8:e58113. doi: 10.1371/journal.pone.0058113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Booth C, Viding E, Mayes LC, Rutherford HJ, Hodsoll S, McCrory EJ. Here’s looking at you, kid: attention to infant emotional faces in mothers and non-mothers. Dev Sci. 2014;17:35–46. doi: 10.1111/desc.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Iacoboni M, Lange C, Keenan JP. The self and social cognition: the role of cortical midline structures and mirror neurons. Trends Cogn Sci. 2007;11:153–157. doi: 10.1016/j.tics.2007.01.001. [DOI] [PubMed] [Google Scholar]
- van Leengoed E, Kerker E, Swanson HH. Inhibition of post-partum maternal behaviour in the rat by injecting an oxytocin antagonist into the cerebral ventricles. J Endocrinol. 1987;112:275–282. doi: 10.1677/joe.0.1120275. [DOI] [PubMed] [Google Scholar]
- Van Zeijl J, Mesman J, Van IJzendoorn MH, Bakermans-Kranenburg MJ, Juffer F, Stolk MN, Koot HM, Alink LRA. Attachment-based intervention for enhancing sensitive discipline in mothers of 1-to 3-year-old children at risk for externalizing behavior problems: A randomized controlled trial. Journal of Consulting and Clinical Psychology. 2006;74:994–1005. doi: 10.1037/0022-006X.74.6.994. [DOI] [PubMed] [Google Scholar]
- Veenema AH. Early life stress, the development of aggression and neuroendocrine and neurobiological correlates: what can we learn from animal models? Front Neuroendocrinol. 2009;30:497–518. doi: 10.1016/j.yfrne.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Venuti P, Caria A, Esposito G, De Pisapia N, Bornstein MH, de Falco S. Differential brain responses to cries of infants with autistic disorder and typical development: an fMRI study. Res Dev Disabil. 2012;33:2255–2264. doi: 10.1016/j.ridd.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor TA, Furey ML, Fromm SJ, Bellgowan PS, Ohman A, Drevets WC. The extended functional neuroanatomy of emotional processing biases for masked faces in major depressive disorder. PLoS One. 2012;7:e46439. doi: 10.1371/journal.pone.0046439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigil JM. A socio-relational framework of sex differences in the expression of emotion. Behav Brain Sci. 2009;32:375–390. doi: 10.1017/S0140525X09991075. discussion 391–428. [DOI] [PubMed] [Google Scholar]
- Volling BL, McElwain NL, Notaro PC, Herrera C. Parents’ emotional availability and infant emotional competence: predictors of parent-infant attachment and emerging self-regulation. J Fam Psychol. 2002;16:447–465. doi: 10.1037//0893-3200.16.4.447. [DOI] [PubMed] [Google Scholar]
- Wan MW, Abel KM, Green J. The transmission of risk to children from mothers with schizophrenia: a developmental psychopathology model. Clin Psychol Rev. 2008a;28:613–637. doi: 10.1016/j.cpr.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Wan MW, Green J, Elsabbagh M, Johnson M, Charman T, Plummer F, Team B. Quality of interaction between at-risk infants and caregiver at 12–15months is associated with 3-year autism outcome. Journal of Child Psychology and Psychiatry. 2013;54:763–771. doi: 10.1111/jcpp.12032. [DOI] [PubMed] [Google Scholar]
- Wan MW, Penketh V, Salmon MP, Abel KM. Content and style of speech from mothers with schizophrenia towards their infants. Psychiatry Res. 2008b;159:109–114. doi: 10.1016/j.psychres.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Wan MW, Warren K, Salmon MP, Abel KM. Patterns of maternal responding in postpartum mothers with schizophrenia. Infant Behav Dev. 2008c;31:532–538. doi: 10.1016/j.infbeh.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Weisman O, Zagoory-Sharon O, Feldman R. Oxytocin administration to parent enhances infant physiological and behavioral readiness for social engagement. Biol Psychiatry. 2012;72:982–989. doi: 10.1016/j.biopsych.2012.06.011. [DOI] [PubMed] [Google Scholar]
- Winnicott DW. Primary Maternal Preoccupation. In: Winnicott DW, editor. Through Paediatrics to Psycho-analysis. Hogarth; London: 1956. [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365:545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- Wittfoth-Schardt D, Gründing J, Wittfoth M, Lanfermann H, Heinrichs M, Domes G, Buchheim A, Gündel H, Waller C. Oxytocin modulates neural reactivity to children’s faces as a function of social salience. Neuropsychopharmacology. 2012;37:1799–1807. doi: 10.1038/npp.2012.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogman MW. Games fathers and mothers play with their infants. infant Mental Health Journal. 1981;2:241–248. [Google Scholar]
- Zak PJ, Stanton AA, Ahmadi S. Oxytocin increases generosity in humans. PLoS One. 2007;2:e1128. doi: 10.1371/journal.pone.0001128. [DOI] [PMC free article] [PubMed] [Google Scholar]