Abstract
Oral mucosa is continuously exposed to environmental forces and has to be constantly renewed. Accordingly, the oral mucosa epithelium contains a large reservoir of epithelial stem cells necessary for tissue homeostasis. Despite considerable scientific advances in stem cell behavior in a number of tissues, fewer studies have been devoted to the stem cells in the oral epithelium. Most of oral mucosa stem cells studies are focused on identifying cancer stem cells (CSC) in oral squamous cell carcinomas (OSCCs) among other head and neck cancers. OSCCs are the most prevalent epithelial tumors of the head and neck region, marked by their aggressiveness and invasiveness. Due to their highly tumorigenic properties, it has been suggested that CSC may be the critical population of cancer cells in the development of OSCC metastasis. This review presents a brief overview of epithelium stem cells with implications in oral health, and the clinical implications of the CSC concept in OSCC metastatic dissemination.
Keywords: Oral squamous cell carcinoma, epithelial stem cells, invasion, metastasis, cancer stem cells, oral mucosa
Introduction
Oral mucosa has a remarkable regenerative potential [1]. Several stem cells markers are known to be expressed, mainly in the basal layers of oral mucosa. It has been proven that the expression of these markers is dysregulated in oral squamous cell carcinomas (OSCC), the most common cancer of the oral cavity [2]. There is a need for a better characterization of the oral stem cells in particularly of their cell behavior, tissue-specific regenerative potential and involvement in carcinogenesis. This review provides an overview of stem cells biological implications in oral mucosa with a special emphasis in OSCC.
Oral Mucosa
The epithelium on the inner surface of the lips, floor of the mouth, gingiva, cheeks and hard palate is derived from embryonic ectoderm, whereas the epithelium surrounding the tongue is derived from both endoderm and ectoderm [3–5]. The oral mucosa can be divided into: masticatory (hard palate and gingival), specialized (dorsal surface of the tongue) and lining (buccal mucosa, ventral surface of the tongue, soft palate, intra-oral surfaces of the lips and alveolar mucosa) [5]. Of the total surface of the oral lining, approximately 25% is keratinized resembling that of the epidermis covering the skin in regions subject to mechanical forces (masticatory mucosa of the gingiva and hard palate), 60% is the non-keratinized lining mucosa in the regions requiring flexibility to accommodate chewing, speech or swallowing (floor of the mouth, buccal regions, esophagus, etc), with the remaining 15% is the specialized mucosa (dorsum of the tongue) which can be represented as a mosaic of keratinized and non-keratinized epithelium [6]. Oral epithelium is a stratified squamous epithelium that consists in various layers: basal, spinous, granular and corneal layers for the keratinized area; basal, spinous, intermediate and superficial layers in the non-keratinized areas. The oral epithelium is in direct contact with an underlying, dense connective tissue (lamina propria) containing minor salivary glands, structural fibers, blood vessels, fibroblasts along with other cell types [6–11]. Its histological structure involves undulations of epithelium (rete ridges) protruding downwards into the lamina propria, with corresponding upward projections of lamina propria (dermal papillae) and thus provides increased surface contact, which prevent separation of the oral epithelium from the underlying lamina propria during mastication [12].
The squamous epithelium covering the oral mucosa relies on epithelial stem cells for tissue renewal [1]. It is unanimously accepted that normal tissue stem cells constitute a life-long reservoir of cells with active mechanisms for self-renewal. Cell division in oral mucosa epithelial cells takes place mainly in the basal layer which contains the stem cells compartment from which the oral mucosa is being regenerated [7]. After dividing, the committed cells undergo differentiation that leads to the expression of structural keratin proteins as cells move superficially, and eventually fall off the surface. In the oral epithelium, it takes 14–24 days for a stem cell to divide and the progeny to traverse through the entire thickness of the epithelium (turnover time) [8]. Expression of several stem cells markers including, CD44, Bmi1, Sox2, Keratin 14, have been described mainly in the basal layer (Fig. 1, Table 1), suggesting that it may contain a reservoir of stem cells [5, 9]. However, the mechanism of tissue maintenance and regeneration is still largely unknown for these cells. It is interesting to note that no many studies have focused on transient amplifying (TA) progenitor cells in the oral cavity and on their potential to provide a reservoir for would healing and homeostasis [2, 5, 8, 14]. TA cells are slightly more differentiated than stem cells yet highly proliferative; they are derived from stem cells and continue to divide several times before undergoing terminal differentiation/maturation into the functional cells of the tissue; the size of the dividing transit population differs dramatically from tissue to tissue, the number of generations defining the degree of amplification that the transit population provides for each stem cells division seems to be related inversely to the frequency that stem cells will be found within the proliferating compartment [2,13].
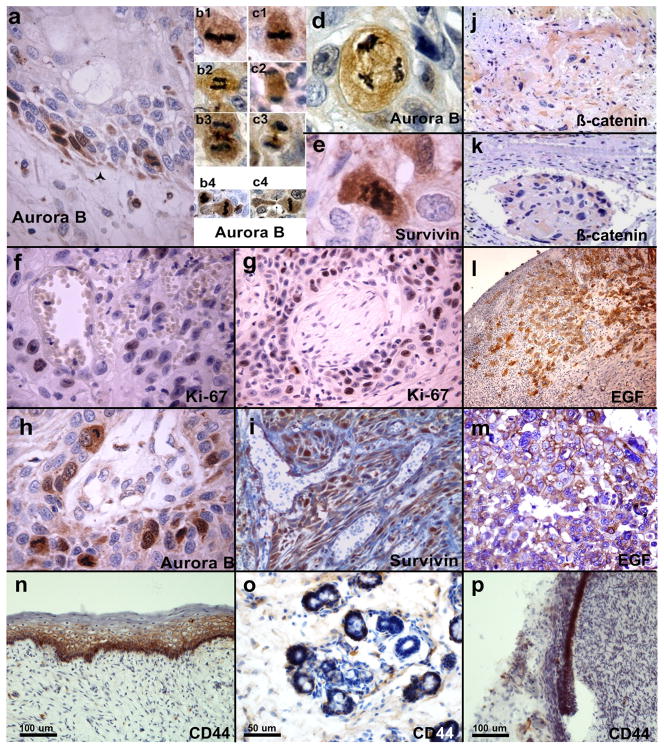
Figure 1. Markers of epithelial stem cells during normal development and in relation with poor clinical outcome in patients with OSCC.
Representative photomicrographs of primary human oral carcinomas immune-stained for markers that are associated with poor clinical outcome in patients with oral carcinomas: Aurora B (a–d, h), Survivin (e, i), beta-catenin (j–k), EGFR (l–m), Ki67 (f–g) and CD44 (n–p). a, Aurora B expression in mitotically active cells in the basal layer of severe oral dysplasia; note the limits with basal membrane outlined by star. b–c, increased and aberrant mitoses overexpressing Aurora B, in aggressive OSCC at invasive front; b1–c1, metaphases; b2, early anaphase; c2, late anaphase; b3–c3, late anaphase-cytokinesis; b4–c4, anomalous cytokineses. Aurora B overexpression is coupled with survivin up-regulation in the invasion fronts of OSCC giving raise to aberrant mitoses (d, Aurora B; e, survivin). High proliferative index of aggressive and invasive cells with perivascular (f) and perineural (g) localization positive for Ki-67, is coupled with Aurora B (h) and Survivin (i) over-expression. Beta-catenin expression in the invasive front (j) and a metastatic embolus (k) of OSCC. EGFR expression in the invasive cells front (l–m). CD44 expression in the basal layer of normal human oral mucosa (n), salivary gland (o) and dental root epithelia (p). All the samples were obtained with the signed informed consent of patients under approved protocols by the Ethical Committees of the Universities of Foggia and Marseille.
Table 1.
Markers for cancer stem cells (CSC) in head and neck squamous cell carcinomas (HNSCC).
CSC subpopulations of cells in HNCSS have been identified by the expression of specific markers (single or in combination) complemented by in vivo tumorigenic assay performed in immune-deficient mice and “sphere-forming” assays in vitro [92–117, 216–225].
| CD44 | Also known as phagocytic glycoprotein-1 (Pgp-1) and the receptor for hyaluronate. It is a cell surface glycoprotein involved in cell adhesion, cell proliferation, migration, and angiogenesis that exist as a large number of different isoforms resulting from alternative RNA splicing. CD44 also acts as a ligand for E-selectin. Measured by fluoresce activated cell sorting (FACS) can also be reported as CD44high (the 10 to 15% of cells with the highest CD44 expression) and CD44low (the 10 to 15% with the lowest or no expression). |
| Aldehyde dehydrogenase (ALDH) | ALDH gene superfamily encodes detoxifying enzymes; its activity is known to enrich cells with increased stem like properties in solid malignancies including HNSCC. Measured by FACS after staining of live cells with a non-immunologic enzymatic ALDEFLUOR kit. |
| EpCAM (ESA) | Epithelial cell adhesion glycosylated membrane protein involved in Wnt signaling and a cancer stem cell surface CD antigen (CD326) that can be measured by FACS. In HNSCC, two biologically distinct phenotypes of CSC have been reported based on ESA/CD44 expression: CD44(high)ESA(high) that are proliferative with epithelial characteristics (so called non–EMT CSC) versus CD44(high)ESA(low) that are migratory with mesenchymal traits (non-EMT CSC). EMT: epithelial-mesenchymal transition. |
| Side population (SP) | Defines cells able to efflux fluorescent DNA dye such as Hoechst 33342 and DyeCycle Violet; its phenotype depends on the concentration of ATP-binding cassette (ABC) transporters superfamily efflux pumps in the plasma membrane. In HNSCC, SP cells express high levels of Bmi-1, CD44, Oct-4 and are high in metastatic and aggressive HNSCC. |
| CD133 (protaminin-1) | A 120kDa glycoprotein with five transmembrane domains and two large extracellular loops with a role (through association with Src kinase) in the regulation of tumor initiating properties and the transition from an epithelial to a mesenchymal phenotype of head and neck carcinoma cells. In OSCC CD133+ stem-like cells possess higher clonogenicity, invasiveness and tumorigenesis as compared with CD133−; CD44+ cancer stem-like cells expressed higher CD133 levels than CD44− cells in HNSCC. |
| Bmi-1 | A polycomb protein and proto-oncogenic chromatin regulator known to promote stem cells self-renewal by negatively regulating the expression of Ink4a and Arf tumor suppressors. In HNSCC Bmi-1 is highly enriched in CD133+ cells, induces the proliferation of these cells, and prevents apoptosis. |
| Oct-4, Nanog and Sox2 | Transcription factors that play essential roles in the maintenance of pluripotency and self-renewal of embryonic stem cells. In human adult oral mucosa, stem cells were detected in the lamina propria based on their Oct4, Sox2 and Nanog expression. Triple positive Nanog/Oct-4/CD133 expression predicted worst survival in HNSCC patients. The usefulness of these factors for the sorting of CSC by FACS followed by culture and implantation in animals is hindered by the fact that they are not expressed in the cell membrane. |
| CD117 (c-kit, receptor of stem cell factor, SCF) | A transmembrane tyrosine-kinase receptor that is part of the platelet-derived growth-factor/colony stimulator factor 1 receptor subfamily involved in cell survival, differentiation, adhesion and chemotaxis. The co-expression of c-kit and SCF was observed in various solid tumors; this ligand/receptor system may have autocrine and paracrine effects on the regulation of tumor behavior (tumor growth and dissemination) in HNSCC. |
| CD24 | A cell adhesion molecule commonly used as a CSC marker with CD44 in breast cancer, or with CD44/ESA in pancreatic cancer. In HNSCC, CD24+/CD44+ cells possessed stemness characteristics of self-renewal and differentiation, showed higher in vitro invasiveness and made higher number of colonies in collagen gels and were more chemo-resistant compared to CD24−/CD44+ cells. In addition, CD24+/CD44+ HNSCC cells showed a tendency to generate larger tumors in nude mice than CD24−/CD44+ cell population. |
Recent findings derived from various solid malignancies models show that cancer progenitor cells have the capacity to dedifferentiate and acquire a stem-like phenotype in response to either genetic manipulation or environmental cues, via implication of various complex molecular circuitries. These findings highlight the need for a better understanding of the dynamic, contextually regulated, equilibrium between cancer stem cells (CSCs) and cancer progenitor cells as a critical step for the development of therapeutic strategies to deplete tumors of their tumor-propagating and treatment-resistant cell subpopulations [14–15]. Better characterization of the CSCs and progenitor cells will contribute to a better understanding of normal and abnormal epithelial growth and tissues regeneration in the oral cavity.
Challenges to the integrity of the oral mucosa
The mucosal lining of the oral cavity is an environment challenged by a large variety of insults, and functions to protect the underlying tissues and organs against mechanical and chemical insults, including microorganisms and toxins, or ingested antigens and carcinogens [10]. The oral epithelium is constantly replaced with a rapid clearance of surface cells, which acts as a protective mechanism against various insults and its structure constitutes an effective barrier [10].
The turnover of the oral mucosa is faster in the lining than in the masticatory regions, thus challenges to the integrity of the oral mucosa will affect in particular the more rapidly proliferating areas and the lining regions will suffer first [16–18]. The dorsal surface of the tongue is composed of many small filiform papillae that have a very uniform shape and size, based on early detailed histological investigations and cell kinetic studies [19–20]. Each papilla is composed of four columns of cells, two dominant (anterior and posterior, also so-called tongue proliferative units, the functional group of proliferative basal cells derived from a single stem cell, together with the distally arranged functional differentiated cells) and two buttressing columns. The lineage characterizing this epithelium is similar to that seen in the dorsal epidermis of the mouse, self-replacing asymmetrically dividing stem cells, occurring at a specific position in the tissue, and producing a cell lineage that has approximately three generations. The stem cells here have a particularly pronounced circadian rhythm [13, 17, 18–21] suggesting an involvement of the circadian clock in stem cell equilibrum. Of interest, disrupting this clock equilibrium in the skin, through deletion of Bmal1 (also known as Arntl) or Per1/2, resulted in a progressive accumulation or depletion of dormant stem cells, respectively [22]. Stem cell arrhythmia also led to premature epidermal ageing, and a reduction in the development of squamous tumors. Clinically, the squamous cell carcinoma of the tongue is considered one of the most aggressive tumors of the oral cavity. The potential effects of circadian disruption in tongue stem cells behavior and tongue cancer development remain largely unexplored.
Despite continuous challenges imposed on the oral mucosa, normal human oral epithelial cells undergo constant cell division leading to regeneration of tissue, provided that the cells retain their ability to limit their replicative life span through cellular senescence, induce cell cycle arrest upon DNA damaged and repair the damaged DNA before resuming the cell cycle. It is known that DNA is most vulnerable during mitosis and that mitotic activity can be affected by a number of factors, such as stress and inflammation, as well as circadian clock disruption [10, 17]. Preliminary findings from our laboratories have identified a strong expression of clock genes in areas rich in stem cells (basal layer) in oral tissues (unpublished observations). Furthermore, we also have been able to detect expression of clock genes and CD44 in the basal layer of oral mucosa surrounding the tooth root and salivary glands (Fig. 1; [23]). Of interest, expression of both stem cells markers and clock genes are found altered in oral squamous cell carcinomas [24], suggesting an implication of the circadian clock in stem cells behavior in carcinogenesis. Moreover, the expression of growth factors such as the Epithelial Growth Factor (EGF) may play a role in the oral epithelium differentiation and in CSC self-renewal in tumors originated in the head and neck area, particularly in the head and neck squamous cell carcinomas (HNSCC). EGF promotes acquisition of stem cell-like properties, increased cell proliferation and decreased sensitivity to cisplatin treatment in HNSCC [25].
CSCs and normal stem cells share similarities [8]. Normal adult stem cells have the ability to repopulate the cells that constitute the organ from which they are isolated and the capacity to propagate themselves; both processes are tightly regulated. Cancer stem cells reflect some of these same properties and are by definition able to sustain proliferation; however, the CSCs are not subject to the same genetic constraints to which normal stem cells are bound. The deregulation of pathways that control the self-renewal of normal stem cells (e.g. Wnt, Notch, Hedgehog, etc) leads to tumorigenesis in rodent models and also plays an important role in human carcinogenesis including HNSCC [2, 26–31]. Being long-lived, both CSCs and stem cells of normal tissues can be the targets of environmental carcinogens leading to the accumulation of consecutive genetic changes although several protective mechanisms have evolved to ensure the genetic integrity of the stem cell compartment in any given tissue [32]. Evolution of DNA methylation has allowed cells to respond to environmental cues in a flexible, yet stable manner, by properly regulating the response at the molecular level. However, dysregulation of DNA methylation can lead to hyper- or hypo-methylation of the promoters of critical genes, contributing to various diseases including HNSCC. Several reports have identified hypermethylation or hypomethylation in the promoters of key genes involved in oral, head and neck cancer [33–36], and have also demonstrated unequivocally that the vast majority of human HNSCC tumors contain multiple mutations [37–38]. It has been shown that variability in DNA methylation exists within subtypes of HNSCC, and is influenced by environmental factors such as diet [39–40].
Oral squamous cell carcinoma (OSCC)
OSCC is the most prevalent and aggressive epithelial tumor of the head and neck region with the poorest outcome; in the United States alone approximately 100 new cases are daily diagnosed, while one person dies from oral cancer every hour of every day [41–44] (oralcancerfoundation.com). Worldwide, the problem is far greater with new cases annually exceeding 640,000 (oralcancerfoundation.com). Traditionally a men’s illness, affecting six men for every woman, over the past 10 years that ratio has alarmingly become 2:1 also affecting younger patients [45]. Development of oral cancer proceeds through discrete molecular genetic changes that are acquired from the loss of genomic integrity after continued exposure to environmental risk factors. Predisposing risk factors such as tobacco use and alcohol consumption have a greater than multiplicative effect and account for the majority of the squamous cell carcinomas developed in the head and neck area [46]. However, these established risk factors do not account for about 40% of oral cavity cases. It has been suggested that yet unknown genetic, occupational, viral or nutritional factors could influence risk particularly in this younger patients group [47]. A growing body of evidence implicates human oral bacteria (over 700 bacterial species inhabit the oral cavity) in the etiology of oral cancers and epidemiological studies consistently report increased risks of these cancers in patients with periodontal disease or tooth loss; furthermore oral bacteria may activate alcohol and smoking-related carcinogens locally or systematically through chronic inflammation [48]. Human Papillomavirus (HPV) has recently emerged as the primary etiologic factor particularly for tumors developed in the tongue and oropharynx that are also associated with younger age at diagnosis [49–50]. Consequently, unique pathologic profiles have emerged that are consistent with the changing incidence of HNSCC [51–52]. Patients with HPV(+) head and neck cancer have a distinct risk profile, associated with a less remarkable history of tobacco and alcohol use [53–54], a more beneficial micronutrient profile [55], improved cellular immunity [56] and improved survival compared to those with HPV(−) tumors [57–58]. Notably, a significant subset (20–30%) of HPV(+) tumors fails to respond, recur locally, or spread distantly. Studies conducted at the University of Michigan have made significant contributions to the understanding of the impact of HPV infection on the pathobiology of HNSCC and response to therapy [58–60]. However, the mechanisms involved in these processes are not fully understood, and given the evolving epidemiology there is a growing controversy over the optimal strategy for oropharynx cancer treatment [59–60].
OSCC continues to be a disfiguring and deadly disease, which displays a wide range of metastatic behavior that cannot be predicted by tumor size, standard histology, or even individual gene or protein expression/activity [61, 62]. Despite advances in treatment, the survival of patients with advanced OSCC has not improved significantly over the last 30 years and remains one of the lowest among the major cancer types [42]. Metastatic tumor behavior is a critical factor in patient survival being responsible for more than 90% of cancer associated mortality in these patients; if distant disease, the 5-year survival rate is three times less than those with nodal metastases, while survival of patients with nodal metastases is half that of similar stage patients without metastases [61, 63]. Younger (<40 years) patients with oral cancer have higher rate of distant failure [64, 65]). Therapeutic options for metastatic OSCC are limited and unsuccessful [66, 67]. Accurate prediction of metastasis in OSCC would have an immediate clinical impact through avoidance of unnecessary treatment of patients at low risk with appropriate direction of resources toward aggressive treatment of patients at high risk of having metastatic disease.
Approximately two thirds of oral cancers occur in the oral cavity (lip, tongue, floor of mouth, palate, gingival, alveolar and buccal mucosa), while the remainder occurs in the oropharynx [61]. Evidence indicates infection of oral epithelial stem cells by high-risk types of human papillomavirus (HPV); clinical observations shown early lymphatic metastasis in HPV-related HNSCC [68, 69]. The microenvironment is increasingly recognized as relevant in the process of metastasis as it is the immunity [70–72]. Studies indicated that OSCC is associated with alterations in the immune system [73–77] and that CSC may be immunologically silent or at least compromised in OSCC [79].
Clinical relevance of cancer stem cells in oral tumorigenesis
The identification of cancer stem cells (CSC) has created a new area of research with promising applications in the prognosis and therapeutics of human cancer [69, 78–91]. Accumulating evidence indicates that the CSCs also play a role in the pathogenesis and progression of carcinomas developed in the oral cavity.
To date, two models of tumor heterogeneity are unanimously accepted: the hierarchical model that assumes that CSCs represents a biologically distinct subset within the total malignant cell population in contrast with the stochastic model that assumes that every cell within a tumor has the same potential to act as a CSC [7]. Work by Prince et al. in 2007 was the first to identify CSC in head and neck squamous cell carcinomas (HNSCC) based on their CD44 expression; these cells possessed the qualities of self-renewal, tumorigenesis and the ability to recapitulate a heterogeneous tumor [92]. Additional studies at the University of Michigan have identified various CSC markers in HNSCC (e.g. ALDH, CD44, Bmi-1; [93–96]. Other markers also have been proposed (e.g. CD133, Oct-4, Nanog, Sox2, CD24, Snail, Twist, etc, most of them in combination with CD44 or ALDH; [2, 5, 8, 69, 90, 97–102]). Currently, CD44 and ALDH are the most common markers used to identify CSC in HNSCC.
CD44 is a cell-surface glycoprotein involved in cell-cell interactions, cell migration, and adhesion with multiple isoforms (splice variants) known to be associated with cell transformation and tumor dissemination [103–106]; Clinically, overexpression of CD44 was associated with poor prognoses and decreased 5-year survival in HNSCC patients [107–110], although its clinical relevance seems dependent on the anatomical site where the cancer originates [106, 111–114]. The aldehyde dehydrogenase (ALDH) family of cytosolic enzymes catalyses the oxidation of aliphatic and aromatic aldehydes to carboxylic acids and ALDH1 is a family member that has a role in the conversion of retinol to retinoic acid, which is important for proliferation, differentiation and survival. ALDH1 activity seems to be responsible for the resistance of progenitor cells to chemotherapeutic agents [80, 90, 93, 115, 116]. Our group has shown that expression of ALDH and CD44 discriminates a highly tumorigenic cell subpopulation that can reconstitute the HNSCC heterogeneity; we performed ex vivo clonogenicity (“spheres-forming”) assays to measure the frequency with which these prospectively isolated cells form colonies (“orospheres”) when placed at clonal density in non-adherent conditions [117]. More recently, we reported sialyl Lewis X as a marker that associates with the metastatic abilities of CSC in OSCC [95]. We are currently investigating other putative markers to better characterize oral epithelial stem cells and their metastatic abilities in OSCC, particularly markers that we previously found associated with tumor progression and dissemination in OSCC: Aurora B [118], Survivin [119], beta-catenin [120] that are expressed in the basal layer and invasive front (Figs. 1–2). Survivin is a promising candidate for targeted anti-cancer therapy as its expression associates with poor clinical outcome, aggressive clinic-pathologic features, and resistance to radiation and chemotherapy in OSCC among other HNSCCs [83, 121–123].
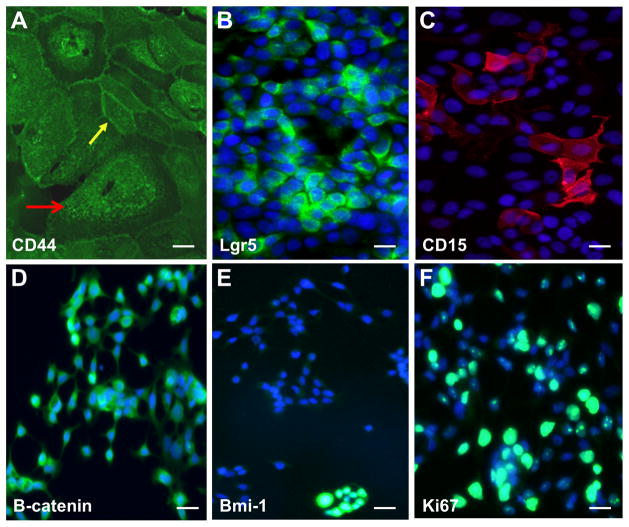
Figure 2. Putative cancer stem cells markers in oral carcinomas.
Representative photomicrographs (magnification 20x) of human derived oral squamous cell carcinomas cells immune-stained for CD44 (A), Lgr5 (B), CD15 (C), beta-catenin (D), Bmi-1 (E) and Ki67 (F). Larger size mesenchymal-like CSC co-exist with normal size CSC that retain the epithelial characteristics (yellow arrow). DAPI (blue) identified nuclei.
Implications of oral cancer stem cells in metastasis
Better purification of the stem-like cell population in oral carcinomas is necessary to clarify what metastatic characteristics are indeed unique to these cells. Such evidence would allow clinicians to exploit this particular set of attributes to target cancer stem cells that keep a tumor growing and allow it to spread. Our group has designed in vitro and in vivo models of metastasis to study the behavior of this unique tumor cell subpopulation in HNSCC. Our data showed that CSC possesses a greater capacity for tumor growth, increased mobility and invasive characteristics [85, 117]. Our data also has confirmed the greater metastatic potential of CSC compared to non-CSC, suggesting that CSC may be responsible for the development of metastasis in HNSCC [117]. Clinically, CSC enrichment is linked to treatment failure, tumor recurrence and metastasis in head and neck carcinomas [67, 124]. There is growing evidence that CSCs behavior is orchestrated in vivo in tissue-specific, “niche” microenvironments. Characterization of the microenvironment surrounding CSC suggest the existence of a perivascular niche that supports stem cells maintenance and resistance to anoikis, suggesting that targeting the crosstalk between CSCs and other cells of their supportive niche may provide effective way to abrogate the tumorigenic function of these cells [72, 125].
The mechanism underlying the invasion of carcinoma cells leading to tumor dissemination involves the epithelial-mesenchymal transition (EMT) of cells with high tumorigenic potential [126, 127]. It is also known that EMT endows epithelial cells with invasive and stem cell properties [128]. Normal stem cells and CSC may share a mesenchymal phenotype that enhances their ability to preserve stemness, to regain migratory properties, and to respond to different stimuli during the expansion and differentiation [69]. Cancer stem cells seem to localize at the invasive fronts of the HNSCC in close proximity with the blood vessels [129]. Of interest, emerging evidence including our findings reveal that the CSC cell populations in carcinomas originated in the oral cavity may be heterogeneous including various CSC subpopulations with distinct phenotypic and functional states: larger-size CSC with mesenchymal features and migratory abilities versus proliferative CSCs that retain epithelial characteristics (Fig. 2). Furthermore, in our spheres culture model, which is highly enriched in metastatic stem cells [95], we have observed that the majority of the cells are also highly enriched in epithelial markers, suggesting the existence of a predominant epithelial stem cells population (unpublished observations). It has been suggested that because the EMT-associated growth arrest, a re-differentiation into epithelial cells (so called Mesenchymal-Epithelial Transition, MET) may be necessary for the metastatic colonization [69, 130]. This evidence is suggestive of a new mechanism allowing metastatic colonization by uncoupling stemness from EMT and growth arrest, in favor of a parallel maintenance of stemness, proliferation phenotype and epithelial characteristics. However, it remains unclear how CSCs carry out the metastatic process in these carcinomas and how metastatic behavior of OSCC is modulated by CSC phenotypic characteristics.
Therapeutic relevance of stem cells research
Primitive stem cells capable of self-renewing proliferation and single or multiple cell lineage progeny generation have been identified in several human epithelial tissues. Although the biological characterization of various non-hematopoietic stem cells is still in its early stages in the laboratory, therapeutic experience with hematopoietic stem cells suggests that other stem cell types will likely have successful clinical applications. Better understanding of pluripotentiality, control of cellular differentiation and of epigenetic programming is critical to major future clinical applications. On the other hand, characterizing CSC subpopulations in oral, head and neck cancer will lead to a better understanding of cancer recurrence, metastasis, resistance to treatment, and should pave the way for more effective therapies for these types of cancer. In addition, the evaluation of the frequency of CSC, their molecular profiling and proliferative state, in a given tumor may be of prognostic value for the overall survival, response to therapy, risk of recurrence and metastasis. An overview of to date known anti-cancer therapies including those targeting CSC is presented in the Table 2. Current studies are conducted at the University of Michigan towards developing an autologous CSC-based therapeutic vaccine for clinical use in an adjuvant setting [131]. There are hopes that the near future will bring novel diagnostic and therapeutic approaches that will result in significant improvement of OSCC management and patient outcome.
Table 2.
Anti-cancer therapies targeting the cancer cells and cancer stem cells.
| Targets/Mechanisms | Drugs | Type of study | Results | References | |
|---|---|---|---|---|---|
| A | Pump targeting drugs | ||||
| 1. ATP-binding cassette (ABC) multidrug efflux pump (transporters) inhibitors: P- glycoprotein (PGP) inhibitors | 1st generation PGP inhibitors (calcium channel blockers: (e.g. verapamil), cyclosporine, tamoxifen | In vivo and in vitro | Enhanced the therapeutic effect of chemotherapeutic drugs (Vincristine, Adriamycin) circumventing tumor chemoresistance (leukemia); direct effects on cancer stem cells in head and neck cancers | [132–134] | |
| 2nd generation: valspodar (psc-833, a cyclosporine analog) | Clinical trial | Ability to modulate multidrug resistance (MDR) caused by expression of MDR1 gene, which encodes for the P-gp multidrug transporter, and is a determinant of both intrinsic and acquired drug resistance in many human cancers; decrease the clearance of several anticancer drugs. Usage in combination with anticancer cocktails showed limited benefits in patients with acute myeloid leukemia (AML) and metastatic cancer : non-small cell lung, gastro-intestinal, ovarian, mesothelioma, metastatic and recurrent head and neck | [133, 135–141] | ||
| 3rd generation: Zosuquidar (LY335979) | Clinical trial | Reverses P-glycoprotein-mediated multi-drug (MDR) resistance; Combined usage with Docetaxel to treat metastatic or locally recurrent carcinoma that have failed standard chemotherapy (breast cancer) or resistant malignancies (melanoma, ovary, lung, breast, sarcoma, head and neck cancer) | |||
| 4th generation: Natural products: curcumin, flavonoids: kaempferol, genistein, silymarin, quercetin etc | In vitro | MDR reversal; blocking of multiple pathways by which cancer cells can survive (breast cancer, head and neck cancer) | |||
| 2. Nanoparticle drug delivery | γ-secretase inhibitor- mesoporous silica nanoparticles (GSI-MSNPs) | In vivo and in vitro | Targeted inhibition of Notch signaling in cancer stem cells | [142] | |
| B | Targeting stemness | ||||
| 1. Targeting CSCs dormancy/quiescence | |||||
| 1.1. stimulate the cells to enter division cycle and to become sensitive to chemotherapies | Interferon (INF) α | In vivo and Clinical trial | Stimulate dormant cells to proliferate, used in combination with imatinib to treat chronic myelogenous leukemia (CML); inhibits tumor growth and metastasis, reduce intratumoral microvessel density, increased cell apoptosis and induced prolonged survival in head and neck cancer; enhance response to standard therapies and successful immunostimulation in patients with head and neck cancer | [143–151] | |
| Granulocyte colony-stimulating factor (G-CSF) | Clinical trial | Stimulate dormant cells to proliferate, used in combination with tyrosine kinase inhibitor (imatinib) to treat AML, CML; dendritic cells differentiation in head and neck cancer; administration of an oncolytic herpes simplex type-1 virus encoding human G-CSF in combination with standard therapies improve loco- regional control and survival in patients with head and neck; however, subcutaneous administration of G-CSF concurrently with radiation did not improve the quality of life as reported by patients with head and neck cancer | |||
| AMD3100 (plexixafor, a stromal cell- derived factor SDF-1/CXCL12- CXCR4 inhibitor) | Clinical trial; in vivo and in vitro | Mobilize stem cell in combination with G-CSF to treat non-Hodgkin’s lymphoma and multiple myeloma; potent anti-metastatic effect in head and neck cancer | |||
| 1.2. epigenetic therapy targeting chromatin acetylation and DNA replication | Histone deacetylase inhibitors (HDACi: suberoylanilide hydroxamic acid); valproic acid | Clinical trial | a/able to kill the cells in dormancy, eradicate the residual tumor by inducing apoptosis in nonproliferating cancer cell lines. Clinical trial in CML | [146] | |
| In Vivo and in vitro | b/able to induce replication-associated DNA damage without detectable alteration in cell cycle progression and unrelated to apoptosis; prevent aggressiveness of CSCs (colon cancer and breast cancer); induce apoptosis and cell cycle arrest, and alter the cancer stem cell phenotype in head and neck cancer; synergistic effects with standard chemotherapies in head and neck cancer; in combination with ribonuclease reductase inhibitor block efficiently tumor growth, induce tumor- cell apoptosis and induce EGFR downregulation in head and neck cancer | [147–154] | |||
| 1.3. differentiation therapy | Vitamin A derivative (retinoids and their naturally metabolized and synthetic products e.g. all-trans retinoic acid, 13- cis-retinoic acid, bexarotene) | Clinical trial | Successful treatment of various subtypes of leukemia harboring chromosomal translocations; limited success in the prevention and treatment of solid tumors may relate to the frequent epigenetic silencing of retinoic acid receptor beta (RARbeta); Limited effect to prevent progression and recurrence of head and neck (oral, pharyngeal) cancer | [155–156] | |
| 2. Targeting self-renewal and proliferation signaling pathways | |||||
| 2.1. Wnt (Wingless type) pathway | anti-Wnt antibody; anti-Wnt receptors: Frizzled antibody (OMP-18R5); LRP6 antibody (anti Wnt-1 and Wnt-3) | In vitro and in vivo | The WNT receptor Frizzled (FZD)7 is essential for maintenance of the pluripotent state in human embryonic stem cells; Decrease viability and proliferation of cancer cells; decrease growth and tumorigenicity of human tumors (hepatocellular carcinoma, breast, pancreatic, lung, colon, etc); exhibits synergistic activity with standard-of-care chemotherapeutic agents; induced extended delay in the re-growth of tumors following treatment with high-dose chemotherapy | [157–161] | |
| Wnt protein inhibitors (VS-507) | In vivo | Decrease population of breast cancer stem cell, reduce cancer grown and metastasis | [163] | ||
| anti-nuclear complexes; new compound (trinuclear ruthenium complex [RuIII3(TSA-H)2(TSA)4][NEt4] with the non-toxic 2-thiosalicylic acid (TSA-H2) ligand) | In vitro | Significantly attenuates the Wnt/beta- catenin signaling at both transcriptomic and proteomic levels; Induce apoptosis, and reduces transcription and expression of nuclear components; head and neck (nasopharyngeal) cancer | [164] | ||
| Phytochemicals: Resveratrol | In vitro and in vivo | Inhibition β-catenin/TCF- mediated transcriptional activity; effects are dose- dependent; reversal of epithelial- mesenchymal transition (EMT); inhibition of cancer cell invasiveness: colorectal, melanoma, breast cancer; decrease cancer stem cells (CSC) survival by blocking the lipogenic gene expression in CSC; impedes the stemness, EMT and metabolic reprogramming of cancer stem cells via p53 activation in head and neck (nasopharyngeal) cancer | [165–169] | ||
| Phytochemicals: selenium, green tea (Epigallo Catechin Gallate, EGCG), vitamin D | In vitro | Various mechanisms of inhibiting various signaling pathways (including Wnt) and targeting cancer stem cells; ability to cause growth arrest and cell death selectively in cancer cells; inhibit post-initiation cancer development, including self-renewal of cancer stem cells and epithelial-mesenchymal transition (various solid tumors: ovarian, breast, colon cancer, pancreas etc) | [170–171] | ||
| small-molecule drugs that antagonize Wnt/beta-catenin signaling pathway: ICG-001, PKF118-310 | In vivo and in vitro | Alter both proliferation and differentiation; loss of self-renewal capacity, decrease chemoresistance, down-regulation of survivin expression levels; reduce tumor growth and overcome tumor relapse; chromatic remodeling; eradicate tumor-initiating cells (breast cancer; acute lymphoblastic leukemia; head and neck cancer) | [172–174] | ||
| small-molecule drugs that inhibit ligand- induced Wnt/β-catenin signaling: Porcupine inhibitor LGK974 (secretion of Wnt protein requires Porcupine, a membrane bound O-acyltransferase dedicated to Wnt posttranslational acylation) | In vitro, in vivo, clinical trial | decrease cell and tumor growth ; decrease expression of Wnt target genes (Axin 2) (pancreatic adenocarcinoma, breast, and head and neck cancer) | [175–178] | ||
| 2.2. Shh pathway | Hedgehog pathway inhibitor: IPI-926 (saridegib); compounds and derivatives from natural products | Clinical trial | Eliminate tumors and delays regrowth (head and neck squamous carcinomas) | [179–180] | |
| 2.3. Notch pathway | γ secretase inhibitors (GSI; the GSIs synthesized to date are divided into three classes: peptide isosteres, azepines, and sulfonamides) | Clinical trial in vitro | Effectively block Notch activity by preventing its cleavage at the cell surface; prevent metastasis and recurrence (breast cancer); prevent cell proliferation and tumor necrosis factor (TNF-α)-dependent invasion of head and neck (oral) cancer cells; inhibits cell proliferation by inducing cell cycle arrest and apoptosis, inhibits the AKT and MEK signaling and enhance radio- sensitivity in head and neck (nasopharyngeal) cancer | [181–183] | |
| Phytochemicals (e.g. Resveratrol); natural products (e.g. curcumin, psoralidin) | In vitro | Reduce cell proliferation and induces apoptosis (T-cell acute lymphoblastic leukaemia, breast and head and neck (esophageal) cancer; induces growth arrest and EMT inhibition in cancer stem cells (breast cancer); down- regulate Notch activating gamma secretase complex proteins (e.g. presenilin) and specific microRNAs (miRNA-21 and -34a), and upregulates tumor suppressor (let-7a miRNA) in head and neck (esophageal) cancer | [184–186] | ||
| 2.4. Aldehyde dehydrogenase (ALDH) | ALDH inhibitors: AMPAL and its analogs, Benomyl, Chloral, Chlorpropamide analogs, Citral, Coprine, Cyanamide, Daidzin, Disulfiram, diethylaminobenzaldehyde (DEAB) | In vitro | An increasing body of evidence suggests relationships among the expression of ALDH enzymes, and their cooperation with ABC transporters in the development of drug resistance in various cancers, and their underlying mechanisms are being explored. Various mechanisms of inhibiting ALDH isoforms and ALDH activity; Reduce or completely reverse chemotherapy and radiation resistance of cancer stem cells; abolish cancer stem cells characters (breast cancer, hepatoma) | [187–190] | |
| ALDH 1A1-targeted siRNAs | In vivo and in vitro | Sensitize taxane- and platinum-resistant cell lines to chemotherapy; significantly reduce tumor growth; targeting cancer stem cells (ovarian cancer). Knockdown of ALDH1A1 and ALDH3A1 by siRNA decreases clonogenicity and motility, and increases sensitivity to 4- hydroperoxy-cyclophosphamide in non- small cell lung cancer cell lines | [190–191] | ||
| C | Epithelial mesenchymal transition (EMT)-targeting drugs | ||||
| 1. Targeting signaling pathways (Wnt, Shh, Notch) | See part B2. | ||||
| 2. Snail inhibitors | Small molecule SNAIL-inhibitor GN-25 | In vitro | Transcriptional reversal of the mesenchyal phenotype in cancer stem cells (breast cancer) | [192] | |
| 3. TGFβ inhibitors | TGFβ receptor inhibitors | In Vivo | Inhibit various components of TGFβ pathway (skin and oral squamous cell Carcinoma) | [193] | |
| 4. NF-kB inhibitors | Phytochemicals: Lupeol | In vivo | significant synergistic cytotoxic effect when combined with low-dose cisplatin (head and neck cancer) | [194] | |
| Phytochemicals: curcumin, resveratrol, ursolic acid, capsaicin, butein (a tetrahydroxychalcone plant polyphenol) | In vitro | Various mechanisms to inhibit activity of IKK, p65 phosphorylation, p65 translocation and DNA binding, or direct effect on cancer stem cells (by reducing ALDH and reducing their spheres-forming capacity through an inhibition of NK-kB signaling (multiple myeloma, prostate, breast, head and neck cancer) | [195, 215] | ||
| 5. miRNA therapy | miRNAs: miR200c | In vivo | Inhibit cancer stem cells by down- regulation of BMI1 and ZEB1; inhibits lung metastasis and prolongs survival rate (head and neck carcinoma) | [196] | |
| miRNA synergistic activators: curcumin | In vitro | Alter the expression miR-203, inhibits proliferation and increases apoptosis (bladder cancer) | [197] | ||
| D | Survival pathways targeting drugs | ||||
| 1. targeting growth factors | 1.1.1. receptor inhibitors | ||||
| anti-epithelial growth factor receptor (EGFR) monoclonal antibodies: Cetuximab | Clinical trials | In combination with chemo- or radiation therapy showed significant improvements but didn’t reduce their toxicity of chemo-radiation (squamous cell carcinoma from oropharynx, hypopharynx and larynx) | [198] | ||
| Vascular Endothelia Growth Factor Receptor (VEGFR) inhibitors: Bevaxizumab | Clinical trial | In addition to chemo-radiation showed delay in progression of the distant disease (nasopharyngeal carcinoma) | [199] | ||
| 1.1.2. kinase inhibitors: | |||||
| Tyrosine kinase inhibitors: Erlotinib; Cetiranib (VEGF tyrosine kinase inhibitor) | Clinical trial | Usage in combination with bevacizumab showed significant effect in treating metastatic and recurrent cancer (head and neck squamous cell carcinoma) | [200] | ||
| Dual kinase inhibitors: Lapatinib | Clinical trial | Inhibition of EGFR and EGFR-2 tyrosine kinase. Lapatinib monotherapy showed non-significant difference in locally advanced squamous cell carcinoma of the head and neck | [201] | ||
| Triple kinase [VEGFR, platelet-derived growth factor receptor (PDGFR), and fibroblast growth factor receptor (FGFR)] inhibitors: Nintedanib | In vitro | Monotherapy and chemotherapy combination reduced proliferation and enhanced apoptosis; antiangiogenic effects (lung and pancreatic cancer) | [202] | ||
| 1.1.3. targeting MEK/ERK (EGFR downstream signaling pathway) | |||||
| Raf inhibitors: sorafenib | Clinical trial | Single agent trials showed poor response, but well tolerated and favorable progression-free survival in recurred and metastatic squamous cell carcinoma of the head and neck | [203] | ||
| MEK inhibitors: Trametinib (GSK1120212) | In vitro, in vivo | Showed therapeutic potential for tumors with activating mutations in BRAF. Ongoing clinical trials in head and neck cancer | [204] | ||
| 1.2. Targeting PIK3 (Phosphatidylinositol- 3-kinase)/Akt (Protein Kinase B)/mTOR (MammalianTarget Of Rapamycin) pathways and promote PTEN (Phosphatase and tensin homolog) | AKT inhibitor: Perifosine | Clinical trial | Monotherapy showed poor response in incurable, recurrent or metastatic head and neck cancer | [205] | |
| PIK3 inhibitors LY294002 (reversible inhibitor), wortmannin (irreversible inhibitor) | In vivo | Inhibit tumor-induced angiogenesis and tumor growth (glioma, prostate cancer); preclinical evaluation in head and neck cancer | [206–207] | ||
| mTOR inhibitors: rapamycin, mTOR kinase inhibitors: Torin1, Torin 2 | Clinical trials and preclinical | Studies targeting various solid tumors and cancer stem cells (Lymphoma, glioma); decrease the capacity of sphere formation as well as ALDH activity, suppress the stimulation of stem-like cells by chemotherapy in colorectal cancer; preclinical evaluation in head and neck cancer | [208–209] | ||
| Dual PIK3/mTOR inhibitors: NVP- BEZ235 | In vivo and in vitro | Usage in combination with sorafenib inhibits tumor cell proliferation and increases tumor cell apoptosis, to overcome drug resistance in renal cell carcinoma; Phosphatidylinositol-3- phosphate kinase, AKT and dual PI3K- mTOR inhibitors caused marked in vitro enhancement of cytotoxicity induced by HDACIs in head and neck cancer | [209–211] | ||
| 1.3. Calcium influx inhibitors | Econazole, Ketotifen, Carboxyamidotriazole | In Vitro | loss of viability and clonogenicity of cancer stem cells (breast cancer); decrease neural stem cell differentiation; inhibition of cell proliferation, migration and chemoinvasion (head and neck cancer) | [212–214] | |
Acknowledgments
This work was made possible by funding from the NCI NIDCR P50 CA 97248 (University of Michigan Head and Neck Cancer Specialized Program of Excellence in Research, SPORE) and the University of Michigan Undergraduate Research Opportunity Program (UROP). Research reported in this publication was in part supported by the National Institute on Deafness and Other Communication Disorders of the National Institutes of Health under Award Number T32DC005356 (Dr. Nghia P.T. Nguyen was supported by the University of Michigan T32-DC005356). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding sources had no involvement in the study design, data collection and analysis, and writing of this review.
Footnotes
Disclosures: None.
References
- 1.Iglesias-Bartolome R, Callejas-Valera JL, Gutkind JS. Control of the epithelial stem cell epigenome: the shaping of epithelial stem cell identity. Current opinion in cell biology. 2013;25:162–169. doi: 10.1016/j.ceb.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Sinha N, Mukhopadhyay S, Das DN, Panda PK, Bhutia SK. Relevance of cancer initiating/stem cells in carcinogenesis and therapy resistance in oral cancer. Oral oncology. 2013;49:854–862. doi: 10.1016/j.oraloncology.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 3.Winning TA, Townsend GC. Oral mucosal embryology and histology. Clin Dermatol. 2000;18:499–511. doi: 10.1016/s0738-081x(00)00140-1. [DOI] [PubMed] [Google Scholar]
- 4.Rothova M, Thompson H, Lickert H, Tucker AS. Lineage tracing of the endoderm during oral development. Dev Dyn. 2012;241:1183–1191. doi: 10.1002/dvdy.23804. [DOI] [PubMed] [Google Scholar]
- 5.Jones KB, Klein OD. Oral epithelial stem cells in tissue maintenance and disease: the first steps in a long journey. Int J Oral Sci. 2013;5:121–129. doi: 10.1038/ijos.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins LM, Dawes C. The surface area of the adult human mouth and thickness of the salivary film covering the teeth and oral mucosa. Journal of dental research. 1987;66:1300–1302. doi: 10.1177/00220345870660080201. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen LV, Vanner R, Dirks P, Eaves CJ. Cancer stem cells: an evolving concept. Nature reviews. Cancer. 2012;12:133–143. doi: 10.1038/nrc3184. [DOI] [PubMed] [Google Scholar]
- 8.Richard V, Pillai MR. The stem cell code in oral epithelial tumorigenesis: ‘the cancer stem cell shift hypothesis’. Biochimica et biophysica acta. 2010;1806:146–162. doi: 10.1016/j.bbcan.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Abdulmajeed AA, Dalley AJ, Farah CS. Putative cancer stem cell marker expression in oral epithelial dysplasia and squamous cell carcinoma. Journal of oral pathology & medicine : official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology. 2013 doi: 10.1111/jop.12073. [DOI] [PubMed] [Google Scholar]
- 10.Squier CA, Kremer MJ. Biology of oral mucosa and esophagus. J Natl Cancer Inst Monogr. 2001:7–15. doi: 10.1093/oxfordjournals.jncimonographs.a003443. [DOI] [PubMed] [Google Scholar]
- 11.Squier CA, Johnson NW, Hopps RM. Human oral mucosa: development, structure and function. Blackwell Scientific; Oxford: 1976. [Google Scholar]
- 12.Wu T, Xiong X, Zhang W. Morphogenesis of rete ridges in human oral mucosa: a pioneering morphological and immunohistochemical study. Cell tissues organs. 2013;197:239–248. doi: 10.1159/000342926. [DOI] [PubMed] [Google Scholar]
- 13.Lanza R. In: Essential of stem cell bilogy. 2. Lanza R, Gearhart J, Hogan B, Melton D, Pedersen R, Thomas ED, Thomson J, Wilmut I, editors. Elsevier Inc. Academic press; [Google Scholar]
- 14.Jones PH, Simons BD, Watt FM. Sic transit gloria: farewell to the epidermal transit amplifying cells? Cell stem cell. 2007;1:371–381. doi: 10.1016/j.stem.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Laterra J. Cancer stem cells: distinct entities or dynamically regulated phenotypes? Cancer research. 2012;72:576–580. doi: 10.1158/0008-5472.CAN-11-3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Squier CA, Johnson NW, Hopps RM. Human oral mucosa: development, structure and function. Blackwell Scientific; Oxford: 1976. [Google Scholar]
- 17.Bjarnason GA, Jordan RC, Sothern RB. Circadian variation in the expression of cell-cycle proteins in human oral epithelium. The American journal of pathology. 1999;154:613–622. doi: 10.1016/S0002-9440(10)65306-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomson PJ, Potten CS, Appleton DR. Mapping dynamic epithelial cell proliferative activity within the oral cavity of man: a new insight into carcinogenesis? The British journal of oral & maxillofacial surgery. 1999;37:377–383. doi: 10.1054/bjom.1999.0130. [DOI] [PubMed] [Google Scholar]
- 19.Hume WJ, Potten CS. The ordered columnar structure of mouse filiform papillae. J cell science. 1976;22:149–160. doi: 10.1242/jcs.22.1.149. [DOI] [PubMed] [Google Scholar]
- 20.Hume WJ, Potten CS. Proliferative units in startified squamous epithelium. Clin experimental dermatology. 1983;8:95–106. doi: 10.1111/j.1365-2230.1983.tb01749.x. [DOI] [PubMed] [Google Scholar]
- 21.Kellett M, Hume WJ, Potten CS. A topographical study of the circadian rhythm in labelling index of mouse gingival and floor-of-mouth epithelium, including changes in labelling activity with individual cell position on the epithelial ridges. Arch oral biology. 1989;34:321–328. doi: 10.1016/0003-9969(89)90104-0. [DOI] [PubMed] [Google Scholar]
- 22.Janich P, Pascual G, Merlos-Suárez A, Batlle E, Ripperger J, Albrecht U, Cheng HY, Obrietan K, Di Croce L, Benitah SA. The circadian molecular clock creates epidermal stem cell heterogeneity. Nature. 2011;480:209–214. doi: 10.1038/nature10649. [DOI] [PubMed] [Google Scholar]
- 23.Zheng L, Papagerakis S, Schnell SD, Hoogerwerf WA, Papagerakis P. Expression of clock proteins in developing tooth. Gene expression patterns : GEP. 2011;11:202–206. doi: 10.1016/j.gep.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grimm M, Renz C, Munz A, Hoefert S, Krimmel M, Reinert S. Co-expression of CD44+/RANKL+ tumor cells in the carcinogenesis of oral squamous cell carcinoma. Odontology. 2013 doi: 10.1007/s10266-013-0133-2. [DOI] [PubMed] [Google Scholar]
- 25.Abhold EL, Kiang A, Rahimy E, Kuo SZ, Wang-Rodriguez J, Lopez JP, Blair KJ, Yu MA, Haas M, Brumund KT, Altuna X, Patel A, Weisman RA, Ongkeko WM. EGFR kinase promotes acquisition of stem cell-like properties: a potential therapeutic target in head and neck squamous cell carcinoma stem cells. PloS one. 2012;7:e32459. doi: 10.1371/journal.pone.0032459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Du J, Chen GG, et al. The nuclear localization of NFkappaB and p53 is positively correlated with HPV16 E7 level in laryngeal squamous cell carcinoma. J Histochem Cytochem. 2003;51:533–539. doi: 10.1177/002215540305100415. [DOI] [PubMed] [Google Scholar]
- 27.Nickoloff BJ, Osborne BA, et al. Notch signaling as a therapeutic target in cancer: a new approach to the development of cell fate modifying agents. Oncogene. 2003;22:6598–6608. doi: 10.1038/sj.onc.1206758. [DOI] [PubMed] [Google Scholar]
- 28.Al-Hajj M, Clarke MF. Self-renewal and solid tumor stem cells. Oncogene. 2004;23:7274–7282. doi: 10.1038/sj.onc.1207947. [DOI] [PubMed] [Google Scholar]
- 29.Karhadkar SS, Bova GS, et al. Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature. 2004;431:707–712. doi: 10.1038/nature02962. [DOI] [PubMed] [Google Scholar]
- 30.Dolled-Filhart M, McCabe A, et al. Quantitative in situ analysis of beta-catenin expression in breast cancer shows decreased expression is associated with poor outcome. Cancer Res. 2006;66:5487–5494. doi: 10.1158/0008-5472.CAN-06-0100. [DOI] [PubMed] [Google Scholar]
- 31.Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nat Rev Cancer. 2008;8:387–398. doi: 10.1038/nrc2389. [DOI] [PubMed] [Google Scholar]
- 32.Prise KM, Saran A. Concise review: stem cell effects in radiation risk. Stem Cells. 2011;29:1315–1321. doi: 10.1002/stem.690. [DOI] [PubMed] [Google Scholar]
- 33.Carvalho AL, Chuang A, et al. Deleted in colorectal cancer is a putative conditional tumor-suppressor gene inactivated by promoter hypermethylation in head and neck squamous cell carcinoma. Cancer Res. 2006;66:9401–9407. doi: 10.1158/0008-5472.CAN-06-1073. [DOI] [PubMed] [Google Scholar]
- 34.Tan HK, Saulnier P, et al. Quantitative methylation analyses of resection margins predict local recurrences and disease-specific deaths in patients with head and neck squamous cell carcinomas. Br J Cancer. 2008;99:357–363. doi: 10.1038/sj.bjc.6604478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun W, Zaboli D, et al. Detection of TIMP3 promoter hypermethylation in salivary rinse as an independent predictor of local recurrence-free survival in head and neck cancer. Clin Cancer Res. 2012;18:1082–1091. doi: 10.1158/1078-0432.CCR-11-2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Colacino JA, Dolinoy DC, et al. Comprehensive analysis of DNA methylation in head and neck squamous cell carcinoma indicates differences by survival and clinicopathologic characteristics. PLoS One. 2013;8:e54742. doi: 10.1371/journal.pone.0054742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Agrawal N, Frederick MJ, et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science. 2011;333:1154–1157. doi: 10.1126/science.1206923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stransky N, Egloff AM, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333:1157–1160. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sartor MA, Dolinoy DC, et al. Genome-wide methylation and expression differences in HPV(+) and HPV(−) squamous cell carcinoma cell lines are consistent with divergent mechanisms of carcinogenesis. Epigenetics. 2011;6:777–787. doi: 10.4161/epi.6.6.16216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Colacino JA, Arthur AE, et al. Pretreatment dietary intake is associated with tumor suppressor DNA methylation in head and neck squamous cell carcinomas. Epigenetics. 2012;7:883–891. doi: 10.4161/epi.21038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arbes SJ, Jr, Olshan AF, Caplan DJ, Schoenbach VJ, Slade GD, Symons MJ. Factors contributing to the poorer survival of black Americans diagnosed with oral cancer (United States) Cancer Causes Control. 1999;10:513–523. doi: 10.1023/a:1008911300100. [DOI] [PubMed] [Google Scholar]
- 42.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA: a cancer journal for clinicians. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 43.Canto MT, Drury TF, Horowitz AM. Oral cancer examinations among U.S. Hispanics in 1998. J Cancer Educ. 2003;18:48–52. doi: 10.1207/s15430154jce1801_15. [DOI] [PubMed] [Google Scholar]
- 44.Horowitz AM, Moon HS, Goodman HS, Yellowitz JA. Maryland adults’ knowledge of oral cancer and having oral cancer examinations. Journal of public health dentistry. 1998;58:281–287. doi: 10.1111/j.1752-7325.1998.tb03010.x. [DOI] [PubMed] [Google Scholar]
- 45.Eliassen AM, Hauff SJ, Tang AL, Thomas DH, McHugh JB, Walline HM, Stoerker J, Maxwell JH, Worden FP, Eisbruch A, Czerwinski MJ, Papagerakis SM, Chepeha DB, Bradford CR, Hanauer DA, Carey TE, Prince ME. Head and neck squamous cell carcinoma in pregnant women. Head & neck. 2013;35:335–342. doi: 10.1002/hed.22973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cruz GD, Le Geros RZ, et al. Oral cancer knowledge, risk factors and characteristics of subjects in a large oral cancer screening program. J Am Dent Assoc. 2002;133:1064–1071. doi: 10.14219/jada.archive.2002.0330. [DOI] [PubMed] [Google Scholar]
- 47.Hashibe M, Brennan P, et al. Interaction between tobacco and alcohol use and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Cancer Epidemiol Biomarkers Prev. 2009;18:541–550. doi: 10.1158/1055-9965.EPI-08-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ahn J, Chen CY, et al. Oral microbiome and oral and gastrointestinal cancer risk. Cancer Causes Control. 2012;23:399–404. doi: 10.1007/s10552-011-9892-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hausen H. Infections causing human cancer. Weinheim, Germany; Wiley-VCH Verlag: 2006. [Google Scholar]
- 50.Pannone G, Santoro A, et al. The role of human papillomavirus in the pathogenesis of head & neck squamous cell carcinoma: an overview. Infect Agent Cancer. 2011;6:4. doi: 10.1186/1750-9378-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vidal L, Gillison ML. Human papillomavirus in HNSCC: recognition of a distinct disease type. Hematol Oncol Clin North Am. 2008;22:1125–1142. doi: 10.1016/j.hoc.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 52.Chenevert J, Seethala RR, et al. Squamous cell carcinoma metastatic to neck from an unknown primary: the potential impact of modern pathologic evaluation on perceived incidence of human papillomavirus-positive oropharyngeal carcinoma prior to 1970. Laryngoscope. 2012;122:793–796. doi: 10.1002/lary.21899. [DOI] [PubMed] [Google Scholar]
- 53.Applebaum KM, Furniss CS, et al. Lack of association of alcohol and tobacco with HPV16-associated head and neck cancer. J Natl Cancer Inst. 2007;99:1801–1810. doi: 10.1093/jnci/djm233. [DOI] [PubMed] [Google Scholar]
- 54.Gillison ML, D’Souza G, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100:407–420. doi: 10.1093/jnci/djn025. [DOI] [PubMed] [Google Scholar]
- 55.Arthur AE, Duffy SA, et al. Higher micronutrient intake is associated with human papillomavirus-positive head and neck cancer: a case-only analysis. Nutr Cancer. 2011;63:734–742. doi: 10.1080/01635581.2011.570894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wansom DE, Light E, et al. Infiltrating lymphocytes and human papillomavirus-16-associated oropharyngeal cancer. Laryngoscope. 2012;122:121–127. doi: 10.1002/lary.22133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fakhry C, Westra WH, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 58.Maxwell JH, Kumar B, et al. Tobacco use in human papillomavirus-positive advanced oropharynx cancer patients related to increased risk of distant metastases and tumor recurrence. Clin Cancer Res. 2010;16:1226–1235. doi: 10.1158/1078-0432.CCR-09-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Worden FP, Kumar B, et al. Chemoselection as a strategy for organ preservation in advanced oropharynx cancer: response and survival positively associated with HPV16 copy number. J Clin Oncol. 2008;26:3138–3146. doi: 10.1200/JCO.2007.12.7597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kumar B, Cordell KG, et al. EGFR, p16, HPV Titer, Bcl-xL and p53, sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancer. J Clin Oncol. 2008;26:3128–3137. doi: 10.1200/JCO.2007.12.7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shah J, Johnson N, Batsakis J. Oral Cancer. Martin Dunitz Press; London: 2003. [Google Scholar]
- 62.Myers J. Oral cancer metastasis. Soringer Sciences, LLC; New York, NY: 2010. [Google Scholar]
- 63.de Bree R, Haigentz M, Jr, Silver CE, Paccagnella D, Hamoir M, Hartl DM, Machiels JP, Paleri V, Rinaldo A, Shaha AR, Takes RP, Leemans CR, Ferlito A. Distant metastases from head and neck squamous cell carcinoma. Part II. Diagnosis. Oral oncology. 2012;48:780–786. doi: 10.1016/j.oraloncology.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 64.Garavello W, Ciardo A, Spreafico R, Gaini RM. Risk factors for distant metastases in head and neck squamous cell carcinoma. Archives of otolaryngology--head & neck surgery. 2006;132:762–766. doi: 10.1001/archotol.132.7.762. [DOI] [PubMed] [Google Scholar]
- 65.Liao CT, Wang HM, Hsieh LL, Chang JT, Ng SH, Hsueh C, Lee LY, Lin CH, Chen IH, Kang CJ, Huang SF, Yen TC. Higher distant failure in young age tongue cancer patients. Oral oncology. 2006;42:718–725. doi: 10.1016/j.oraloncology.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 66.Haigentz M, Jr, Hartl DM, Silver CE, Langendijk JA, Strojan P, Paleri V, de Bree R, Machiels JP, Hamoir M, Rinaldo A, Paccagnella D, Shaha AR, Takes RP, Ferlito A. Distant metastases from head and neck squamous cell carcinoma. Part III. Treatment, Oral oncology. 2012;48:787–793. doi: 10.1016/j.oraloncology.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 67.Chinn SB, Darr OA, Peters RD, Prince ME. The role of head and neck squamous cell carcinoma cancer stem cells in tumorigenesis, metastasis, and treatment failure. Frontiers in endocrinology. 2012;3:90. doi: 10.3389/fendo.2012.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Desai PC, Jaglal MV, Gopal P, Ghim SJ, Miller DM, Farghaly H, Jenson AB. Human papillomavirus in metastatic squamous carcinoma from unknown primaries in the head and neck: a retrospective 7 year study. Experimental and molecular pathology. 2009;87:94–98. doi: 10.1016/j.yexmp.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 69.Albers AE, Chen C, Koberle B, Qian X, Klussmann JP, Wollenberg B, Kaufmann AM. Stem cells in squamous head and neck cancer. Critical reviews in oncology/hematology. 2012;81:224–240. doi: 10.1016/j.critrevonc.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 70.Ostrand-Rosenberg S. Immune surveillance: a balance between protumor and antitumor immunity. Current opinion in genetics & development. 2008;18:11–18. doi: 10.1016/j.gde.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rapidis AD, Wolf GT. Immunotherapy of head and neck cancer: current and future considerations. Journal of oncology. 2009;2009:346345. doi: 10.1155/2009/346345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904–5912. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Accomando WP, Wiencke JK, Houseman EA, Butler RA, Zheng S, Nelson HH, Kelsey KT. Decreased NK cells in patients with head and neck cancer determined in archival DNA. Clin Cancer Res. 2012;18:6147–6154. doi: 10.1158/1078-0432.CCR-12-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Duray A, Demoulin S, Hubert P, Delvenne P, Saussez S. Immune suppression in head and neck cancers: a review. Clinical & developmental immunology. 2010;2010:701657. doi: 10.1155/2010/701657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kuss I, Hathaway B, Ferris RL, Gooding W, Whiteside TL. Decreased absolute counts of T lymphocyte subsets and their relation to disease in squamous cell carcinoma of the head and neck. Clin Cancer Res. 2004;10:3755–3762. doi: 10.1158/1078-0432.CCR-04-0054. [DOI] [PubMed] [Google Scholar]
- 76.Kuss I, Hathaway B, Ferris RL, Gooding W, Whiteside TL. Imbalance in absolute counts of T lymphocyte subsets in patients with head and neck cancer and its relation to disease. Advances in oto-rhino-laryngology. 2005;62:161–172. doi: 10.1159/000082506. [DOI] [PubMed] [Google Scholar]
- 77.Wulff S, Pries R, Borngen K, Trenkle T, Wollenberg B. Decreased levels of circulating regulatory NK cells in patients with head and neck cancer throughout all tumor stages. Anticancer research. 2009;29:3053–3057. [PubMed] [Google Scholar]
- 78.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 79.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, Schott A, Hayes D, Birnbaum D, Wicha MS, Dontu G. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 82.Yin S, Li J, Hu C, Chen X, Yao M, Yan M, Jiang G, Ge C, Xie H, Wan D, Yang S, Zheng S, Gu J. CD133 positive hepatocellular carcinoma cells possess high capacity for tumorigenicity. Int J Cancer. 2007;120:1444–1450. doi: 10.1002/ijc.22476. [DOI] [PubMed] [Google Scholar]
- 83.Li C, Wu J-J, Hynes M, Dosch J, Sarkar B, Welling TH, Pasca di Magliano M, Simeone DM. c-Met is a marker of pancreatic cancer stem cells and therapeutic target. Gastroenterology. 2011;141:2218–2227. doi: 10.1053/j.gastro.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 84.Beier D, Hau P, Proescholdt M, Lohmeier A, Wischhusen J, Oefner PJ, Aigner L, Brawanski A, Bogdahn U, Beier CP. CD133(+) and CD133(−) glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer research. 2007;67:4010–4015. doi: 10.1158/0008-5472.CAN-06-4180. [DOI] [PubMed] [Google Scholar]
- 85.Dalerba P, Dylla SJ, Park I-K, Liu R, Wang X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM, Shelton AA, Parmiani G, Castelli C, Clarke MF. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci U S A. 2007;104:10158–10163. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ma S, Chan KW, Lee TK-W, Tang KH, Wo JY-H, Zheng B-J, Guan X-Y. Aldehyde dehydrogenase discriminates the CD133 liver cancer stem cell populations. Mol Cancer Res. 2008;6:1146–1153. doi: 10.1158/1541-7786.MCR-08-0035. [DOI] [PubMed] [Google Scholar]
- 87.Todaro M, Alea MP, Di Stefano AB, Cammareri P, Vermeulen L, Iovino F, Tripodo C, Russo A, Gulotta G, Medema JP, Stassi G. Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4. Cell Stem Cell. 2007;1:389–402. doi: 10.1016/j.stem.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 88.Yang ZF, Ngai P, Ho DW, Yu WC, Ng MNP, Lau CK, Li MLY, Tam KH, Lam CT, Poon RTP, Fan ST. Identification of local and circulating cancer stem cells in human liver cancer. Hepatology (Baltimore, Md) 2008;47:919–928. doi: 10.1002/hep.22082. [DOI] [PubMed] [Google Scholar]
- 89.Huang EH, Hynes MJ, Zhang T, Ginestier C, Dontu G, Appelman H, Fields JZ, Wicha MS, Boman BM. Aldehyde dehydrogenase 1 is a marker for normal and malignant human colonic stem cells (SC) and tracks SC overpopulation during colon tumorigenesis. Cancer research. 2009;69:3382–3389. doi: 10.1158/0008-5472.CAN-08-4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen Y-C, Chen Y-W, Hsu H-S, Tseng L-M, Huang P-I, Lu K-H, Chen D-T, Tai L-K, Yung M-C, Chang S-C, Ku H-H, Chiou S-H, Lo W-L. Aldehyde dehydrogenase 1 is a putative marker for cancer stem cells in head and neck squamous cancer. Biochem Biophys Res Commun. 2009;385:307–313. doi: 10.1016/j.bbrc.2009.05.048. [DOI] [PubMed] [Google Scholar]
- 91.Cheung PFY, Cheng CKC, Wong NCL, Ho JCY, Yip CW, Lui VCH, Cheung ANY, Fan ST, Cheung ST. Granulin-epithelin precursor is an oncofetal protein defining hepatic cancer stem cells. PloS one. 2011;6 doi: 10.1371/journal.pone.0028246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF, Ailles LE. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci U S A. 2007;104:973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Clay MR, Tabor M, Owen JH, Carey TE, Bradford CR, Wolf GT, Wicha MS, Prince ME. Single-marker identification of head and neck squamous cell carcinoma cancer stem cells with aldehyde dehydrogenase. Head & neck. 2010;32:1195–1201. doi: 10.1002/hed.21315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Krishnamurthy S, Dong Z, Vodopyanov D, Imai A, Helman JI, Prince ME, Wicha MS, Nor JE. Endothelial cell-initiated signaling promotes the survival and self-renewal of cancer stem cells. Cancer research. 2010;70:9969–9978. doi: 10.1158/0008-5472.CAN-10-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Czerwinski MJ, Desiderio V, Shkeir O, Papagerakis P, Lapadatescu MC, Owen JH, Athanassiou-Papaefthymiou M, Zheng L, Papaccio G, Prince ME, Papagerakis S. In vitro evaluation of sialyl Lewis X relationship with head and neck cancer stem cells. Otolaryngol Head Neck Surg. 2013;149:97–9104. doi: 10.1177/0194599813482879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Krishnamurthy S, Nor JE. Orosphere assay: a method for propagation of head and neck cancer stem cells. Head & neck. 2013;35:1015–1021. doi: 10.1002/hed.23076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rodda DJ, Chew J-L, Lim L-H, Loh Y-H, Wang B, Ng H-H, Robson P. Transcriptional regulation of nanog by OCT4 and SOX2. J Biol Chem. 2005;280:24731–24737. doi: 10.1074/jbc.M502573200. [DOI] [PubMed] [Google Scholar]
- 98.Yang J, Mani SA, Weinberg RA. Exploring a new twist on tumor metastasis. Cancer research. 2006;66:4549–4552. doi: 10.1158/0008-5472.CAN-05-3850. [DOI] [PubMed] [Google Scholar]
- 99.Chiou S-H, Yu C-C, Huang C-Y, Lin S-C, Liu C-J, Tsai T-H, Chou S-H, Chien C-S, Ku H-H, Lo J-F. Positive correlations of Oct-4 and Nanog in oral cancer stem-like cells and high-grade oral squamous cell carcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14:4085–4095. doi: 10.1158/1078-0432.CCR-07-4404. [DOI] [PubMed] [Google Scholar]
- 100.Wei XD, Zhou L, Cheng L, Tian J, Jiang JJ, Maccallum J. In vivo investigation of CD133 as a putative marker of cancer stem cells in Hep-2 cell line. Head & neck. 2009;31:94–9101. doi: 10.1002/hed.20935. [DOI] [PubMed] [Google Scholar]
- 101.St John MA, Dohadwala M, Luo J, Wang G, Lee G, Shih H, Heinrich E, Krysan K, Walser T, Hazra S, Zhu L, Lai C, Abemayor E, Fishbein M, Elashoff DA, Sharma S, Dubinett SM. Proinflammatory mediators upregulate snail in head and neck squamous cell carcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:6018–6027. doi: 10.1158/1078-0432.CCR-09-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vered M, Dayan D, Yahalom R, Dobriyan A, Barshack I, Bello IO, Kantola S, Salo T. Cancer-associated fibroblasts and epithelial-mesenchymal transition in metastatic oral tongue squamous cell carcinoma. Int J Cancer. 2010;127:1356–1362. doi: 10.1002/ijc.25358. [DOI] [PubMed] [Google Scholar]
- 103.Goodison S, Urquidi V, Tarin D. CD44 cell adhesion molecules. Molecular pathology : MP. 1999;52:189–196. doi: 10.1136/mp.52.4.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Naor D, Wallach-Dayan SB, Zahalka MA, Sionov RV. Involvement of CD44, a molecule with a thousand faces, in cancer dissemination. Semin Cancer Biol. 2008;18:260–267. doi: 10.1016/j.semcancer.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 105.Wang SJ, Wong G, de Heer AM, Xia W, Bourguignon LY. CD44 variant isoforms in head and neck squamous cell carcinoma progression. The Laryngoscope. 2009;119:1518–1530. doi: 10.1002/lary.20506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.de Jong MC, Pramana J, van der Wal JE, Lacko M, Peutz-Kootstra CJ, de Jong JM, Takes RP, Kaanders JH, van der Laan BF, Wachters J, Jansen JC, Rasch CR, van Velthuysen M-LF, Grenman R, Hoebers FJ, Schuuring E, van den Brekel MW, Begg AC. CD44 expression predicts local recurrence after radiotherapy in larynx cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16:5329–5338. doi: 10.1158/1078-0432.CCR-10-0799. [DOI] [PubMed] [Google Scholar]
- 107.Maula S-M, Luukkaa M, Grenman R, Jackson D, Jalkanen S, Ristamaki R. Intratumoral lymphatics are essential for the metastatic spread and prognosis in squamous cell carcinomas of the head and neck region. Cancer research. 2003;63:1920–1926. [PubMed] [Google Scholar]
- 108.Han J, Kioi M, Chu WS, Kasperbauer JL, Strome SE, Puri RK. Identification of potential therapeutic targets in human head & neck squamous cell carcinoma. Head & neck oncology. 2009;1:27. doi: 10.1186/1758-3284-1-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lin J-T, Chang T-H, Chang C-S, Wang W-H, Su B-W, Lee K-D, Chang P-J. Prognostic value of pretreatment CD44 mRNA in peripheral blood of patients with locally advanced head and neck cancer. Oral oncology. 2010;46:29–33. doi: 10.1016/j.oraloncology.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 110.Kokko L-L, Hurme S, Maula S-M, Alanen K, Grenman R, Kinnunen I, Ventela S. Significance of site-specific prognosis of cancer stem cell marker CD44 in head and neck squamous-cell carcinoma. Oral oncology. 2011;47:510–516. doi: 10.1016/j.oraloncology.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 111.Stoll C, Baretton G, Soost F, Terpe HJ, Domide P, Lohrs U. Prognostic importance of the expression of CD44 splice variants in oral squamous cell carcinomas. Oral oncology. 1999;35:484–489. doi: 10.1016/s1368-8375(99)00021-4. [DOI] [PubMed] [Google Scholar]
- 112.Carinci F, Stabellini G, Calvitti M, Pelucchi S, Targa L, Farina A, Pezzetti F, Pastore A. CD44 as prognostic factor in oral and oropharyngeal squamous cell carcinoma. J Craniofac Surg. 2002;13:85–89. doi: 10.1097/00001665-200201000-00020. [DOI] [PubMed] [Google Scholar]
- 113.Gonzalez-Moles MA, Gil-Montoya JA, Ruiz-Avila I, Esteban F, Delgado-Rodriguez M, Bascones-Martinez A. Prognostic significance of p21WAF1/CIP1, p16INK4a and CD44s in tongue cancer. Oncol Rep. 2007;18:389–396. [PubMed] [Google Scholar]
- 114.Uwa N, Kataoka TR, Torii I, Sato A, Nishigami T, Song M, Daimon T, Saeki N, Sagawa K, Mouri T, Terada T, Sakagami M, Tsujimura T. CD44 expression is related to poor prognosis of hypopharyngeal squamous cell carcinoma. Acta otolaryngologica. 2011;131:323–329. doi: 10.3109/00016489.2010.528792. [DOI] [PubMed] [Google Scholar]
- 115.Magni M, Shammah S, Schiro R, Mellado W, Dalla-Favera R, Gianni AM. Induction of cyclophosphamide-resistance by aldehyde-dehydrogenase gene transfer. Blood. 1996;87:1097–1103. [PubMed] [Google Scholar]
- 116.Douville J, Beaulieu R, Balicki D. ALDH1 as a functional marker of cancer stem and progenitor cells. Stem cells and development. 2009;18:17–25. doi: 10.1089/scd.2008.0055. [DOI] [PubMed] [Google Scholar]
- 117.Davis SJ, Divi V, Owen JH, Bradford CR, Carey TE, Papagerakis S, Prince ME. Metastatic potential of cancer stem cells in head and neck squamous cell carcinoma. Archives of otolaryngology--head & neck surgery. 2010;136:1260–1266. doi: 10.1001/archoto.2010.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pannone G, Hindi SA, Santoro A, Sanguedolce F, Rubini C, Cincione RI, De Maria S, Tortorella S, Rocchetti R, Cagiano S, Pedicillo C, Serpico R, Lo Muzio L, Bufo P. Aurora B expression as a prognostic indicator and possible therapeutic target in oral squamous cell carcinoma. International journal of immunopathology and pharmacology. 2011;24:79–88. doi: 10.1177/039463201102400110. [DOI] [PubMed] [Google Scholar]
- 119.de Maria S, Lo Muzio L, Braca A, Rega P, Cassano A, Vinella A, Fumarulo R, Serpico R, Farina E, Metafora V, Pannone G, Ravagnan GP, Metafora S, Rubini C, Carteni M, Mariggio MA. Survivin promoter -31G/C polymorphism in oral cancer cell lines. Oncol Lett. 2011;2:935–939. doi: 10.3892/ol.2011.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Papagerakis P, Pannone G, Shabana AH, Depondt J, Santoro A, Ghirtis K, Berdal A, Papagerakis S. Aberrant beta-catenin and LEF1 expression may predict the clinical outcome for patients with oropharyngeal cancer. International journal of immunopathology and pharmacology. 2012;25:135–146. doi: 10.1177/039463201202500116. [DOI] [PubMed] [Google Scholar]
- 121.Necochea-Campion R, Chen CS, Mirshahidi S, Howard FD, Wall NR. Clinico-pathologic relevance of Survivin splice variant expression in cancer. Cancer letters. 2013;339:167–174. doi: 10.1016/j.canlet.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Guindalini RS, Mathias Machado MC, Garicochea B. Monitoring Survivin Expression in Cancer: Implications for Prognosis and Therapy. Molecular diagnosis & therapy. 2013 doi: 10.1007/s40291-013-0048-1. [DOI] [PubMed] [Google Scholar]
- 123.Li C, Wu JJ, Hynes M, Dosch J, Sarkar B, Welling TH, Pasca di Magliano M, Simeone DM. c-Met is a marker of pancreatic cancer stem cells and therapeutic target. Gastroenterology. 2011;141:2218–2227. e2215. doi: 10.1053/j.gastro.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 124.Prise KM, Saran A. Concise review: stem cell effects in radiation risk. Stem cells (Dayton, Ohio) 2011;29:1315–1321. doi: 10.1002/stem.690. [DOI] [PubMed] [Google Scholar]
- 125.Adams A, Warner K, Nor JE. Salivary gland cancer stem cells. Oral Oncology. 2013;49:845–853. doi: 10.1016/j.oraloncology.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Iwatsuki M, Mimori K, Yokobori T, Ishi H, Beppu T, Nakamori S, Baba H, Mori M. Epithelial-mesenchymal transition in cancer development and its clinical significance. Cancer science. 2010;101:293–299. doi: 10.1111/j.1349-7006.2009.01419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Papagerakis S, Pannone G. Epithelial-mesenchymal interactions in oral cancer metastasis. In: Ogbureke KU, editor. Oral Cancer. Tech; 2012. pp. 373–388. [Google Scholar]
- 128.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Krishnamurthy S, Nor JE. Head and neck cancer stem cells. Journal of dental research. 2012;91:334–340. doi: 10.1177/0022034511423393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Biddle A, Liang X, Gammon L, Fazil B, Harper LJ, Emich H, Costea DE, Mackenzie IC. Cancer stem cells in squamous cell carcinoma switch between two distinct phenotypes that are preferentially migratory or proliferative. Cancer Research. 2011;71:5317–5326. doi: 10.1158/0008-5472.CAN-11-1059. [DOI] [PubMed] [Google Scholar]
- 131.Li Q, Lu L, Tao H, Xue C, Teitz-Tennenbaum S, Owen JH, Moyer JS, Prince ME, Chang AE, Wicha MS. Generation of a novel dendritic-cell vaccine using melanoma and squamous cancer stem cells. Journal of Visualized Experiments. 2014;83:e50561. doi: 10.3791/50561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tsuruo T, Iida H, Nojiri M, Tsukagoshi S, Sakurai Y. Circumvention of Vincristine and Adriamycin Resistance in Vitro and in Vivo by Calcium Influx Blockers. Cancer Research. 1983;43:2905–2910. [PubMed] [Google Scholar]
- 133.Coley HM. Overcoming Multidrug Resistance in Cancer: Clinical Studies of P-Glycoprotein Inhibitors. Methods in Molecular Biology (Clifton, NJ) 2010;596:341–358. doi: 10.1007/978-1-60761-416-6_15. [DOI] [PubMed] [Google Scholar]
- 134.Zheng X, Cui D, Xu S, Brabant G, Derwahl M. Doxorubicin fails to eradicate cancer stem cells derived from anaplastic thyroid carcinoma cells: characterization of resistant cells. International Journal of Oncology. 2010;37:307–315. doi: 10.3892/ijo_00000679. [DOI] [PubMed] [Google Scholar]
- 135.Ruff P, Vorobiof DA, Jordaan JP, Demetriou GS, Moodley SD, Nosworthy AL, Werner ID, Raats J, Burgess LJ. A randomized, placebo-controlled, double-blind phase 2 study of docetaxel compared to docetaxel plus zosuquidar (LY335979) in women with metastatic or locally recurrent breast cancer who have received one prior chemotherapy regimen. Cancer Chemotherapy Pharmacolology. 2009;64:763–738. doi: 10.1007/s00280-009-0925-9. [DOI] [PubMed] [Google Scholar]
- 136.Bradshaw DM, Arceci RJ. Clinical relevance of transmembrane drug efflux as a mechanism of multidrug resistance. Journal of Clinical Oncology. 1998;16:3674–3690. doi: 10.1200/JCO.1998.16.11.3674. [DOI] [PubMed] [Google Scholar]
- 137.Advani R, Lum BL, Fisher GA, Halsey J, Chin DL, Jacobs CD, Sikic BI. A phase I trial of liposomal doxorubicin, paclitaxel and valspodar (PSC-833), an inhibitor of multidrug resistance. Ann Oncol. 2005 Dec;16(12):1968–73. doi: 10.1093/annonc/mdi396. Epub 2005 Aug 26. [DOI] [PubMed] [Google Scholar]
- 138.Fisher GA, Sikic BI. Clinical studies with modulators of multidrug resistance. Hematol Oncol Clin North Am. 1995;9:363–382. [PubMed] [Google Scholar]
- 139.Raaijmakers MH, de Grouw EP, van der Reijden BA, de Witte TJ, Jansen JH, Raymakers RA. ABCB1 modulation does not circumvent drug extrusion from primitive leukemic progenitor cells and may preferentially target residual normal cells in acute myelogenous leukemia. Clinical Cancer Research. 2006;12:3452–3458. doi: 10.1158/1078-0432.CCR-05-1945. [DOI] [PubMed] [Google Scholar]
- 140.Zhang S, Yang X, Morris ME. Flavonoids Are Inhibitors of Breast Cancer Resistance Protein (ABCG2)-Mediated Transport. Molecular Pharmacology. 2005;65:1208–1216. doi: 10.1124/mol.65.5.1208. [DOI] [PubMed] [Google Scholar]
- 141.Yuan JM. Cancer prevention by green tea: evidence from epidemiologic studies. American Journal of Clinical Nutrition. 2013;98:1676S–1681S. doi: 10.3945/ajcn.113.058271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mamaeva V, Rosenholm JM, Bate-Eya LT, Bergman L, Peuhu E, Duchanoy A, Fortelius LE. Mesoporous Silica Nanoparticles as Drug Delivery Systems for Targeted Inhibition of Notch Signaling in Cancer. Molecular Therapy : The Journal of the American Society of Gene Therapy. 2011;19:1538–1546. doi: 10.1038/mt.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mukherjee KK, Bose A, Ghosh D, Sarkar K, Goswami S, Pal S, Biswas J, Baral R. IFNα2b augments immune responses of cisplatin+5 fluorouracil treated tongue squamous cell carcinoma patients--a preliminary study. Indian Journal Medical Research. 2012;136:54–59. [PMC free article] [PubMed] [Google Scholar]
- 144.Liu X, Lu J, He ML, Li Z, Zhang B, Zhou LH, Li Q, Li G, Wang L, Tian WD, Peng Y, Li XP. Antitumor effects of interferon-alpha on cell growth and metastasis in human nasopharyngeal carcinoma. Curr Cancer Drug Targets. 2012;12:561–570. doi: 10.2174/156800912800673293. [DOI] [PubMed] [Google Scholar]
- 145.Young MR, Wright MA, Pandit R. Myeloid differentiation treatment to diminish the presence of immune-suppressive CD34+ cells within human head and neck squamous cell carcinomas. Journal of Immunology. 1997;159:990–996. [PubMed] [Google Scholar]
- 146.Ling L, Bhatia R. Stem Cell Quiescence. Clinical Cancer Research : An Official Journal of the American Association for Cancer Research. 2011;17:4936–4941. doi: 10.1158/1078-0432.CCR-10-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lathers DM, Lubbers E, Wright MA, Young MR. Dendritic cell differentiation pathways of CD34+ cells from the peripheral blood of head and neck cancer patients. Journal of Leukocyte Biology. 1999;65:623–628. doi: 10.1002/jlb.65.5.623. [DOI] [PubMed] [Google Scholar]
- 148.Hoffman KE, Pugh SL, James JL, Scarantino C, Movsas B, Valicenti RK, Fortin A, Pollock J, Kim H, Brachman DG, Berk LB, Bruner DW, Kachnic LA. The impact of concurrent granulocyte-macrophage colony-stimulating factor on quality of life in head and neck cancer patients: results of the randomized, placebo-controlled Radiation Therapy Oncology Group 9901 trial. Quality Life Research. 2014 Feb 4; doi: 10.1007/s11136-014-0628-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Harrington KJ, Hingorani M, Tanay MA, Hickey J, Bhide SA, Clarke PM, Renouf LC, Thway K, Sibtain A, McNeish IA, Newbold KL, Goldsweig H, Coffin R, Nutting CM. Phase I/II study of oncolytic HSV GM-CSF in combination with radiotherapy and cisplatin in untreated stage III/IV squamous cell cancer of the head and neck. Clinical Cancer Research. 2010;16:4005–4015. doi: 10.1158/1078-0432.CCR-10-0196. [DOI] [PubMed] [Google Scholar]
- 150.Gros SJ, Kurschat N, Drenckhan A, Dohrmann T, Forberich E, Effenberger K, Reichelt U, Hoffman RM, Pantel K, Kaifi JT, Izbicki JR. Involvement of CXCR4 chemokine receptor in metastastic HER2-positive esophageal cancer. PLoS One. 2012;7:e47287. doi: 10.1371/journal.pone.0047287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Uchida D, Onoue T, Kuribayashi N, Tomizuka Y, Tamatani T, Nagai H, Miyamoto Y. Blockade of CXCR4 in oral squamous cell carcinoma inhibits lymph node metastases. European Journal of Cancer. 2011;47:452–459. doi: 10.1016/j.ejca.2010.09.028. [DOI] [PubMed] [Google Scholar]
- 152.Stauber RH, Knauer SK, Habtemichael N, Bier C, Unruhe B, Weisheit S, Spange S, Nonnenmacher F, Fetz V, Ginter T, Reichardt S, Liebmann C, Schneider G, Krämer OH. A combination of a ribonucleotide reductase inhibitor and histone deacetylase inhibitors downregulates EGFR and triggers BIM-dependent apoptosis in head and neck cancer. Oncotarget. 2012;3:31–43. doi: 10.18632/oncotarget.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chikamatsu K, Ishii H, Murata T, Sakakura K, Shino M, Toyoda M, Takahashi K, Masuyama K. Alteration of cancer stem cell-like phenotype by histone deacetylase inhibitors in squamous cell carcinoma of the head and neck. Cancer Science. 2013;104:1468–1475. doi: 10.1111/cas.12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Conti C, Leo E, Eichler GS, Sordet O, Martin MM, Fan A, Aladjem MI, Pommier Y. Inhibition of Histone Deacetylase in Cancer Cells Slows Down Replication Forks, Activates Dormant Origins, and Induces DNA Damage. Cancer Research. 2010;70:4470–4480. doi: 10.1158/0008-5472.CAN-09-3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Connolly RM, Nguyen NK, Sukumar S. Molecular pathways: current role and future directions of the retinoic acid pathway in cancer prevention and treatment. Clin Cancer Res. 2013;19:1651–1659. doi: 10.1158/1078-0432.CCR-12-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Khuri FR, Lee JJ, Lippman SM, Kim ES, Cooper JS, Benner SE, et al. Randomized phase III trial of low-dose isotretinoin for prevention of second primary tumors in stage I and II head and neck cancer patients. Journal Natl Cancer Institute. 2006;98:441–450. doi: 10.1093/jnci/djj091. [DOI] [PubMed] [Google Scholar]
- 157.Wei W, Chua M-S, Grepper S, So SK. Blockade of Wnt-1 Signaling Leads to Anti-Tumor Effects in Hepatocellular Carcinoma Cells. Molecular Cancer. 2009;8:76. doi: 10.1186/1476-4598-8-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gurney A, Axelrod F, Bond CJ, Cain J, Chartier C, Donigan L, Fischer M, Chaudhari A, Ji M, Kapoun AM, Lam A, Lazetic S, Ma S, Mitra S, Park IK, Pickell K, Sato A, Satyal S, Stroud M, Tran H, Yen WC, Lewicki J, Hoey T. Wnt pathway inhibition via the targeting of Frizzled receptors results in decreased growth and tumorigenicity of human tumors. Proc Natl Acad Sci U S A. 2012;109:11717–11722. doi: 10.1073/pnas.1120068109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ettenberg SA, Charlat O, Daley MP, Liu S, Vincent KJ, Stuart DD, Schuller AG, Yuan J, Ospina B, Green J, Yu Q, Walsh R, Li S, Schmitz R, Heine H, Bilic S, Ostrom L, Mosher R, Hartlepp KF, Zhu Z, Fawell S, Yao YM, Stover D, Finan PM, Porter JA, Sellers WR, Klagge IM, Cong F. Inhibition of tumorigenesis driven by different Wnt proteins requires blockade of distinct ligand-binding regions by LRP6 antibodies. Proc Natl Acad Sci U S A. 2010;107:15473–15478. doi: 10.1073/pnas.1007428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gong Y, Bourhis E, Chiu C, Stawicki S, DeAlmeida VI, Liu BY, Phamluong K, Cao TC, Carano RA, Ernst JA, Solloway M, Rubinfeld B, Hannoush RN, Wu Y, Polakis P, Costa M. Wnt isoform-specific interactions with coreceptor specify inhibition or potentiation of signaling by LRP6 antibodies. PLoS One. 2010;5:e12682. doi: 10.1371/journal.pone.0012682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.King TD, Zhang W, Suto MJ, Li Y. Frizzled7 as an emerging target for cancer therapy. Cell Signaling. 2012;24:846–851. doi: 10.1016/j.cellsig.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fernandez A, Huggins IJ, Perna L, Brafman D, Lu D, Yao S, Gaasterland T, Carson DA, Willert K. The WNT Receptor FZD7 Is Required for Maintenance of the Pluripotent State in Human Embryonic Stem Cells. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:1409–14. doi: 10.1073/pnas.1323697111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ring JE, Kolev VN, Padval MV, Keegan M, Vidal CM, Neill AA, Shapiro IM, Pachter JA, Xu The Cancer Stem Cell-Targeting Wnt Inhibitor VS-507 Reduces Breast Cancer Growth and Metastasis. Cancer Research. 2012;72:1024. [Google Scholar]
- 164.Chan L-F, Sharon, Sun RW-Y, Choi M-Y, Zeng Y, Shek L, Sin-Yin Chui S, Che C-M. An Anti-Cancer Trinuclear Ruthenium(iii) Complex with 2-Thiosalicylate Ligands Attenuates Wnt-B-Catenin Signaling. Chemical Science. 2011;2:1788. [Google Scholar]
- 165.Chen H-J, Hsu L-S, Shia Y-T, Lin M-W, Lin C-M. The B-catenin/TCF Complex as a Novel Target of Resveratrol in the Wnt/β-Catenin Signaling Pathway. Biochemical Pharmacology. 2012;84:1143–1153. doi: 10.1016/j.bcp.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 166.Tsai JH, Hsu LS, Lin CL, Hong HM, Pan MH, Way TD, Chen WJ. 3,5,4′-Trimethoxystilbene, a natural methoxylated analog of resveratrol, inhibits breast cancer cell invasiveness by downregulation of PI3K/Akt and Wnt/β-catenin signaling cascades and reversal of epithelial-mesenchymal transition. Toxicol Appl Pharmacol. 2013;272:746–756. doi: 10.1016/j.taap.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 167.Chen YJ, Chen YY, Lin YF, Hu HY, Liao HF. Resveratrol inhibits alpha-melanocyte-stimulating hormone signaling, viability, and invasiveness in melanoma cells. Evid Based Complement Alternat Med. 2013;2013:632121. doi: 10.1155/2013/632121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Shen YA, Lin CH, Chi WH, Wang CY, Hsieh YT, Wei YH, Chen YJ. Resveratrol Impedes the Stemness, Epithelial-Mesenchymal Transition, and Metabolic Reprogramming of Cancer Stem Cells in Nasopharyngeal Carcinoma through p53 Activation. Evid Based Complement Alternat Med. 2013;2013:590393. doi: 10.1155/2013/590393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Pandey PR, Xing F, Sharma S, Watabe M, Pai SK, Iiizumi-Gairani M, Fukuda K, Hirota S, Mo YY, Watabe K. Elevated lipogenesis in epithelial stem-like cell confers survival advantage in ductal carcinoma in situ of breast cancer. Oncogene. 2013;32:5111–5122. doi: 10.1038/onc.2012.519. [DOI] [PubMed] [Google Scholar]
- 170.Kawasaki BT, Hurt EM, Mistree T, Farrar WL. Targeting cancer stem cells with phytochemicals. Mol Interv. 2008;8:174–184. doi: 10.1124/mi.8.4.9. [DOI] [PubMed] [Google Scholar]
- 171.Singh SV, Singh K. Cancer chemoprevention with dietary isothiocyanates mature for clinical translational research. Carcinogenesis. 2012;33:1833–1842. doi: 10.1093/carcin/bgs216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gang EJ, Hsieh YT, Pham J, Zhao Y, Nguyen C, Huantes S, Park E, Naing K, Klemm L, Swaminathan S, Conway EM, Pelus LM, Crispino J, Mullighan CG, McMillan M, Müschen M, Kahn M, Kim YM. Small-molecule inhibition of CBP/catenin interactions eliminates drug-resistant clones in acute lymphoblastic leukemia. Oncogene. 2013 Jun 3; doi: 10.1038/onc.2013.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wend P, Fang L, Zhu Q, Schipper JH, Loddenkemper C, Kosel F, Brinkmann V, Eckert K, Hindersin S, Holland JD, Lehr S, Kahn M, Ziebold U, Birchmeier W. Wnt/β-catenin signalling induces MLL to create epigenetic changes in salivary gland tumours. EMBO J. 2013;32:1977–1989. doi: 10.1038/emboj.2013.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hallett RM, Kondratyev MK, Giacomelli AO, Nixon AM, Girgis-Gabardo A, Ilieva D, Hassell JA. Small molecule antagonists of the Wnt/β-catenin signaling pathway target breast tumor-initiating cells in a Her2/Neu mouse model of breast cancer. PLoS One. 2012;7:e33976. doi: 10.1371/journal.pone.0033976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan C-W, Wei S. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nature Chemical Biology. 2009;5:100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Jiang X, Hao H-X, Growney JD, Woolfenden S, Bottiglio C, Ng N, Lu B. Inactivating Mutations of RNF43 Confer Wnt Dependency in Pancreatic Ductal Adenocarcinoma. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:12649–12654. doi: 10.1073/pnas.1307218110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Proffitt KD, Madan B, Ke Z, Pendharkar V, Ding L, Lee MA, Hannoush RN, Virshup DM. Pharmacological inhibition of the Wnt acyltransferase PORCN prevents growth of WNT-driven mammary cancer. Cancer Research. 2013;73:502–507. doi: 10.1158/0008-5472.CAN-12-2258. [DOI] [PubMed] [Google Scholar]
- 178.Liu J, Pan S, Hsieh MH, Ng N, Sun F, Wang T, Kasibhatla S, Schuller AG, Li AG, Cheng D, Li J, Tompkins C, Pferdekamper A, Steffy A, Cheng J, Kowal C, Phung V, Guo G, Wang Y, Graham MP, Flynn S, Brenner JC, Li C, Villarroel MC, Schultz PG, Wu X, McNamara P, Sellers WR, Petruzzelli L, Boral AL, Seidel HM, McLaughlin ME, Che J, Carey TE, Vanasse G, Harris JL. Targeting Wnt-driven cancer through the inhibition of Porcupine by LGK974. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:20224–20229. doi: 10.1073/pnas.1314239110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Huang YC, Chao KS, Liao HF, Chen YJ. Targeting sonic hedgehog signaling by compounds and derivatives from natural products. Evid Based Complement Alternative Medicine. 2013;2013:748587. doi: 10.1155/2013/748587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Keysar SB, Le PN, Anderson RT, Morton JJ, Bowles DW, Paylor JJ, Vogler BW. Hedgehog signaling alters reliance on EGF receptor signaling and mediates anti-EGFR therapeutic resistance in head and neck cancer. Cancer Research. 2013;73:3381–3392. doi: 10.1158/0008-5472.CAN-12-4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Olsauskas-Kuprys R, Zlobin A, Osipo C. Gamma secretase inhibitors of Notch signaling. OncoTargets and Therapy. 2013;6:943–955. doi: 10.2147/OTT.S33766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yoshida R, Nagata M, Nakayama H, Niimori-Kita K, Hassan W, Tanaka T, Shinohara M, Ito T. The pathological significance of Notch1 in oral squamous cell carcinoma. Lab Invest. 2013;93:1068–1081. doi: 10.1038/labinvest.2013.95. [DOI] [PubMed] [Google Scholar]
- 182.Chen SM, Liu JP, Zhou JX, Chen C, Deng YQ, Wang Y, Tao ZZ. Suppression of the notch signaling pathway by γ-secretase inhibitor GSI inhibits human nasopharyngeal carcinoma cell proliferation. Cancer Letters. 2011;306:76–84. doi: 10.1016/j.canlet.2011.02.034. [DOI] [PubMed] [Google Scholar]
- 183.Yu S, Zhang R, Liu F, Hu H, Yu S, Wang H. Down-regulation of Notch signaling by a γ-secretase inhibitor enhances the radiosensitivity of nasopharyngeal carcinoma cells. Oncology Reports. 2011;26:1323–1328. doi: 10.3892/or.2011.1402. [DOI] [PubMed] [Google Scholar]
- 184.Subramaniam D, Ponnurangam S, Ramamoorthy P, Standing D, Battafarano RJ, Anant S, Sharma P. Curcumin induces cell death in esophageal cancer cells through modulating Notch signaling. PLoS One. 2012;7:e30590. doi: 10.1371/journal.pone.0030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Cecchinato V, Chiaramonte R, Nizzardo M, Cristofaro B, Basile A, Sherbet GV, Comi P. Resveratrol-induced apoptosis in human T-Cell acute lymphoblastic leukaemia MOLT-4 cells. Biochemical Pharmacology. 2007;74:1568–1574. doi: 10.1016/j.bcp.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 186.Suman S, Das TP, Damodaran C. Silencing NOTCH signaling causes growth arrest in both breast cancer stem cells and breast cancer cells. British Journal of Cancer. 2013;109:2587–2596. doi: 10.1038/bjc.2013.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Koppaka V, Thompson DC, Chen Y, Ellermann M, Nicolaou KC, Juvonen RO, Petersen D, Deitrich RA, Hurley TD, Vasiliou V. Aldehyde dehydrogenase inhibitors: a Comprehensive review of the pharmacology, mechanism of action, substrate specificity, and clinical application. Pharmacological Reviews. 2012;64:520–539. doi: 10.1124/pr.111.005538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Croker AK, Allan AL. Inhibition of aldehyde dehydrogenase (ALDH) activity reduces chemotherapy and radiation resistance of stem-like ALDHhiCD44-human breast cancer cells. Breast Cancer Research Treatment. 2012;133:75–87. doi: 10.1007/s10549-011-1692-y. [DOI] [PubMed] [Google Scholar]
- 189.Liu P, Kumar IS, Brown S, Kannappan V, Tawari PE, Tang JZ, Jiang W, Armesilla AL, Darling JL, Wang W. Disulfiram targets cancer stem-like cells and reverses resistance and cross-resistance in acquired paclitaxel-resistant triple-negative breast cancer cells. British Journal of Cancer. 2013;109:1876–1885. doi: 10.1038/bjc.2013.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Januchowski R, Wojtowicz K, Zabel M. The role of aldehyde dehydrogenase (ALDH) in cancer drug resistance. Biomedical Pharmacotherapy. 2013;67:669–680. doi: 10.1016/j.biopha.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 191.Landen CN, Goodman B, Katre AA, Steg AD, Nick AM, Stone RL, Miller LD. Targeting Aldehyde Dehydrogenase Cancer Stem Cells in Ovarian Cancer. Molecular Cancer Therapeutics. 2010;9:3186–3199. doi: 10.1158/1535-7163.MCT-10-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Azmi AS, Bollig-Fischer A, Bao B, Park B-J, Lee S-H, Yong-Song G, Dyson G, Reddy C, Sarkar FH, Mohammad RM. Systems analysis reveals a transcriptional reversal of the mesenchymal phenotype induced by SNAIL-inhibitor GN-25. BMC Systems Biology. 2013;7:85. doi: 10.1186/1752-0509-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Glick AB. The Role of TGFβ Signaling in Squamous Cell Cancer: Lessons from Mouse Models. Journal of Skin Cancer. 2012:249063. doi: 10.1155/2012/249063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Lee TK, Poon RTP, Wo JY, Ma S, Guan X-Y, Myers JN, Altevogt P, Yuen APW. Lupeol Suppresses Cisplatin-Induced Nuclear Factor-kappaB Activation in Head and Neck Squamous Cell Carcinoma and Inhibits Local Invasion and Nodal Metastasis in an Orthotopic Nude Mouse Model. Cancer Research. 2007;67:8800–8809. doi: 10.1158/0008-5472.CAN-07-0801. [DOI] [PubMed] [Google Scholar]
- 195.Prasad S, Ravindran J, Aggarwal BB. NF-kappaB and Cancer: How Intimate Is This Relationship. Molecular and Cellular Biochemistry. 2010;336:25–37. doi: 10.1007/s11010-009-0267-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Lo W-L, Yu Cheng-Chia, Chiou Guang-Yuh, Chen Yi-Wei, Huang Pin-I, Chien Chian-Shiu, Tseng Ling-Ming, et al. MicroRNA-200c Attenuates Tumour Growth and Metastasis of Presumptive Head and Neck Squamous Cell Carcinoma Stem Cells. The Journal of Pathology. 2011;223:482–95. doi: 10.1002/path.2826. [DOI] [PubMed] [Google Scholar]
- 197.Saini S, Arora Sumit, Majid Shahana, Shahryari Varahram, Chen Yi, Deng Guoren, Yamamura Soichiro, Ueno Koji, Dahiya Rajvir. Curcumin Modulates microRNA-203-Mediated Regulation of the Src-Akt Axis in Bladder Cancer. Cancer Prevention Research (Philadelphia, Pa) 2011;4:1698–1709. doi: 10.1158/1940-6207.CAPR-11-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Merlano M, Occelli M. Review of Cetuximab in the Treatment of Squamous Cell Carcinoma of the Head and Neck. Therapeutics and Clinical Risk Management. 2007;3:871–876. [PMC free article] [PubMed] [Google Scholar]
- 199.Lee NY, Zhang Qiang, Pfister David G, Kim John, Garden Adam S, Mechalakos James, Hu Kenneth, et al. Addition of Bevacizumab to Standard Chemoradiation for Locoregionally Advanced Nasopharyngeal Carcinoma (RTOG 0615): A Phase 2 Multi-Institutional Trial. The Lancet Oncology. 2012;13:172–180. doi: 10.1016/S1470-2045(11)70303-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Cohen E, Davis Darren W, Karrison Theodore G, Seiwert Tanguy Y, Wong Stuart J, Nattam Sreenivasa, Kozloff Mark F, et al. Erlotinib and Bevacizumab in Patients with Recurrent or Metastatic Squamous-Cell Carcinoma of the Head and Neck: A Phase I/II Study. The Lancet Oncology. 2009;10:247–257. doi: 10.1016/S1470-2045(09)70002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Del Campo JM, Hitt R, Sebastian P, Carracedo C, Lokanatha D, Bourhis J, Temam S, et al. Effects of Lapatinib Monotherapy: Results of a Randomised Phase II Study in Therapy-Naive Patients with Locally Advanced Squamous Cell Carcinoma of the Head and Neck. British Journal of Cancer. 2011;105:618–627. doi: 10.1038/bjc.2011.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kutluk Cenik B, Ostapoff Katherine T, Gerber David E, Brekken Rolf A. BIBF 1120 (nintedanib), a Triple Angiokinase Inhibitor, Induces Hypoxia but Not EMT and Blocks Progression of Preclinical Models of Lung and Pancreatic Cancer. Molecular Cancer Therapeutics. 2013;12:992–1001. doi: 10.1158/1535-7163.MCT-12-0995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Williamson SK, Moon James, Huang Chao H, Guaglianone Perry P, LeBlanc Michael, Wolf Gregory T, Urba Susan G. Phase II Evaluation of Sorafenib in Advanced and Metastatic Squamous Cell Carcinoma of the Head and Neck: Southwest Oncology Group Study S0420. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology. 2010;28:3330–3335. doi: 10.1200/JCO.2009.25.6834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Gilmartin AG, Bleam Maureen R, Groy Arthur, Moss Katherine G, Minthorn Elisabeth A, Kulkarni Swarupa G, Rominger Cynthia M, et al. GSK1120212 (JTP-74057) Is an Inhibitor of MEK Activity and Activation with Favorable Pharmacokinetic Properties for Sustained in Vivo Pathway Inhibition. Clinical Cancer Research : An Official Journal of the American Association for Cancer Research. 2011;17:989–1000. doi: 10.1158/1078-0432.CCR-10-2200. [DOI] [PubMed] [Google Scholar]
- 205.Argiris A, Cohen Ezra, Karrison Theodore, Esparaz Benjamin, Mauer Ann, Ansari Rafat, Wong Stuart, et al. A Phase II Trial of Perifosine, an Oral Alkylphospholipid, in Recurrent or Metastatic Head and Neck Cancer. Cancer Biology and Therapy. 2006;5:766–770. doi: 10.4161/cbt.5.7.2874. [DOI] [PubMed] [Google Scholar]
- 206.Garlich JR, De Pradip, Dey Nandini, Su Jing Dong, Peng Xiaodong, Miller Antoinette, Murali Ravoori, et al. A Vascular Targeted Pan Phosphoinositide 3-Kinase Inhibitor Prodrug, SF1126, with Antitumor and Antiangiogenic Activity. Cancer Research. 2008;68:206–215. doi: 10.1158/0008-5472.CAN-07-0669. [DOI] [PubMed] [Google Scholar]
- 207.Freudlsperger C, Burnett Jeffrey R, Friedman Jay A, Kannabiran Vishnu R, Chen Zhong, Van Waes Carter. EGFR-PI3K-AKT-mTOR Signaling in Head and Neck Squamous Cell Carcinomas: Attractive Targets for Molecular-Oriented Therapy. Expert Opinion on Therapeutic Targets. 2011;15:63–74. doi: 10.1517/14728222.2011.541440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Cai Z, Ke J, He X, Yuan R, Chen Y, Wu X, Wang L, Wang J, Lan P, Wu X. Significance of mTOR signaling and its inhibitor against cancer stem-like cells in colorectal cancer. Ann Surg Oncol. 2014;21:179–88. doi: 10.1245/s10434-013-3146-8. [DOI] [PubMed] [Google Scholar]
- 209.Zaytseva YY, Valentino Joseph D, Gulhati Pat, Mark Evers B. mTOR Inhibitors in Cancer Therapy. Cancer Letters. 2012;319:1–7. doi: 10.1016/j.canlet.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 210.Erlich RB, Kherrouche Z, Rickwood D, Endo-Munoz L, Cameron S, Dahler A, Hazar-Rethinam M, de Long LM, Wooley K, Guminski A, Saunders NA. Preclinical evaluation of dual PI3K-mTOR inhibitors and histone deacetylase inhibitors in head and neck squamous cell carcinoma. Br J Cancer. 2012;106:107–115. doi: 10.1038/bjc.2011.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Roulin D, Waselle Laurent, Dormond-Meuwly Anne, Dufour Marc, Demartines Nicolas, Dormond Olivier. Targeting Renal Cell Carcinoma with NVP-BEZ235, a Dual PI3K/mTOR Inhibitor, in Combination with Sorafenib. Molecular Cancer. 2011;10:90. doi: 10.1186/1476-4598-10-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.D’Ascenzo M, Piacentini R, Casalbore P, Budoni M, Pallini R, Azzena GB, Grassi C. Role of L-type Ca2+ channels in neural stem/progenitor cell differentiation. Eur J Neurosci. 2006;23:935–944. doi: 10.1111/j.1460-9568.2006.04628.x. [DOI] [PubMed] [Google Scholar]
- 213.Zhang Y, Crump M, Berge SA. Purging of contaminating breast cancer cells from hematopoietic progenitor cell preparations using activation enhanced cell death. Breast Cancer Res Treat. 2002;72:265–278. doi: 10.1023/a:1014965726663. [DOI] [PubMed] [Google Scholar]
- 214.Wu Y, Palad AJ, Wasilenko WJ, Blackmore PF, Pincus WA, Schechter GL, Spoonster JR, Kohn EC, Somers KD. Inhibition of head and neck squamous cell carcinoma growth and invasion by the calcium influx inhibitor carboxyamidotriazole. Clin Cancer Res. 1997;3:1915–1921. [PubMed] [Google Scholar]
- 215.Cioce M, Gherardi S, Viglietto G, Strano S, Blandino G, Muti P, et al. Mammosphere-forming cells from breast cancer cell lines as a tool for the identification of CSC-like- and early progenitor-targeting drugs. Cell Cycle. 2010;9:2878–2887. [PubMed] [Google Scholar]
- 216.Chiou SH, Yu CC, Huang CY, Lin SC, Liu CJ, Tsai TH, Chou SH, Chien CS, Ku HH, Lo JF. Positive correlations of Oct-4 and Nanog in oral cancer stem-like cells and high-grade oral squamous cell carcinoma. Clin Cancer Res. 2008;14:4085–4095. doi: 10.1158/1078-0432.CCR-07-4404. [DOI] [PubMed] [Google Scholar]
- 217.Tabor MH, Clay MR, Owen JH, Bradford CR, Carey TE, Wolf GT, Prince ME. Head and neck cancer stem cells: the side population. The Laryngoscope. 2011;12:527–533. doi: 10.1002/lary.21032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Chen H, Zhou L, Dou T, Wan G, Tang H, Tian J. Bmi-1’s maintenance of the proliferative capacity of laryngeal cancer stem cells. Head Neck. 2011;33:1115–1125. doi: 10.1002/hed.21576. [DOI] [PubMed] [Google Scholar]
- 219.Huang CF, Xu X-R, Wu T-F, Sun Z-J, Zhang WF. Correlation of ALDH1, CD44, OCT4 and SOX2 in tongue squamous cell carcinoma and their association with disease progression and prognosis. Journal of Oral Pathology and Medicine. 2014 doi: 10.1111/jop.12159. [DOI] [PubMed] [Google Scholar]
- 220.Laimer K, Fong D, Gasti G, Obrist P, Kloss F, Tuli T, Spizzo G. EpCam expression in squamous cell carcinoma of the oral cavity: frequency and relationship to clinicopathologic features. Oral Oncology. 2008;44:72–77. doi: 10.1016/j.oraloncology.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 221.Qiao B, He B, Cai J, Yang W. The xpresion profile of Oct4 and Sox 2 in the carcinogenesis of oral mucosa. International Journal of Clinical and Experimental Pathology. 2014;7:28–37. [PMC free article] [PubMed] [Google Scholar]
- 222.Han J, Fujisawa T, Husain SR, Puri RK. Identification and characterization of cancer stem cells in human head and neck squamous cell carcinoma. BioMed Central Cancer. 2014;11:1471–2407. doi: 10.1186/1471-2407-14-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Marynka-kalmani K, treves S, Yafee M, Rachima H, Gafni Y, Cohen MA, et al. The lamina propria of adult human oral mucosa harbors a novel stem cell population. Stem Cell. 2010;28:984–995. doi: 10.1002/stem.425. [DOI] [PubMed] [Google Scholar]
- 224.Sheu L-F, Lee W-C, Lee H-S, Kao W-Y, Chen A. Co-expression of c-kit and stem cell factor in primary and metastatic nasopharyngeal carcinomas and nasopharyngeal epithelium. The Journal of Pathology. 2005;207:216–223. doi: 10.1002/path.1822. [DOI] [PubMed] [Google Scholar]
- 225.Biddle A, Liang X, Gammon L, Fazil B, Harper LJ, Emich H, Costea DE, Mackenzie IC. Cancer stem cells in squamous carcinoma switch between two distinct phenotypes that are preferentially migratory or proliferative. Cancer Research. 2011;71:5317–5326. doi: 10.1158/0008-5472.CAN-11-1059. [DOI] [PubMed] [Google Scholar]