Abstract
Tuc2009 is a P335-type member of the tailed-phage supergroup Siphoviridae and was originally identified as a resident prophage of the gram-positive bacterium Lactococcus lactis UC509. A Tuc2009 gene designated tal2009 which is located within the morphogenic module was shown to specify a lytic activity within the 3′ portion of its coding region. Comparative sequence analysis indicated that the cell wall-degrading part of Tal2009 is a member of the M37 protein family and that Tal2009 lacks a cell-binding domain, a finding supported by binding studies. Tal2009 appears to undergo self-mediated posttranslational processing in both L. lactis and Escherichia coli. Antibodies directed against a purified C-terminal portion of Tal2009 were used for immunoelectron microscopy, which showed that Tal2009 is located at the tail tip of Tuc2009. Antibody neutralization studies demonstrated that Tal2009-directed antibodies inhibited the ability of phage to mediate host lysis by more than 100-fold. These data indicate that tal2009 encodes a tail-associated lysin involved in localized cell wall degradation, thus allowing the Tuc2009 DNA injection machinery access to the membrane of its bacterial host.
Lactic acid bacteria are economically important bacteria used in the production of fermented foods such as cheeses, yogurts, and sausages. Tuc2009 is a 38,347-bp lysogenic member of the P335 type of the Siphoviridae supergroup of non-contractile-tailed bacteriophages (GenBank accession no. NC_002703) and was originally identified in Lactococcus lactis subsp. cremoris UC509, a strain used in Cheddar cheese production, following mitomycin C induction (2, 42).
Muralytic enzymes or lysins degrade the peptidoglycan (PG) matrix and play essential roles for both phages and bacteria. “Autolysins” is the term used for lysins which are produced by bacteria and involved in cell division, while the term “endolysins” refers to lytic enzymes involved in phage release. Some bacteria also produce lysins which act as class III bacteriocins. Lysins fall into three categories, glycosidases, amidases, and endopeptidases, depending on the type of chemical bond they cleave within the PG. Glycosidases can be further subdivided into the muramidases, glucosaminidases, and transglycosylases (55). Progeny release for many double-stranded-DNA-tailed phages has been shown to employ a lysis system involving one or more holins in conjunction with an endolysin. The holins function by forming pores in the cytoplasmic membrane of the host, thereby abolishing membrane potential and allowing the endolysin to access the PG layer.
Lysins exhibit a modular design (16). While a portion (usually the N-terminal part in the case of endolysins) encodes bond cleavage, a second segment is involved in substrate binding. This is believed to help the enzymatic efficiency and specificity of such muralytic enzymes by locating the active motif directly at the site of the substrate and causing endolysins to lyse only those bacteria possessing both the specifically recognized binding region and the target bond of the cleaving domain. It is this specificity of target recognition that could make lysins attractive therapeutic agents. Indeed, studies have demonstrated the usefulness of lysins by specifically lysing streptococci which had colonized mice (38). The lysin is thus said to demonstrate independently functioning domains, as shown for the choline-binding motif of the majority of lysins of Streptococcus pneumoniae and its phages (16) and the endolysin of Tuc2009 (50). Furthermore, the level of homology between these modules from endolysins and autolysins is supportive of the modular theory of phage evolution, as it indicates that the genes encoding such enzymes have arisen as a result of genomic exchange and rearrangement (16).
While the cellular PG layer gives structural support to the bacterium, it also represents a formidable barrier across which the phage must transport its DNA during the infection process. Several proteins used by phages infecting gram-negative bacteria to perform this task of “hole punching” have been characterized (45). Phages T4, T7, PRD1, and φ6, all of which infect gram-negative hosts, have been shown to incorporate a lysozyme, two transglycosylases, and an endopeptidase, respectively, in the mature virion (9, 36, 37, 44). In addition, an endolysin was identified as a structural component of PRD1 (46). The entry-associated lysins of T4, T7, PRD1, and φ6 are located at the tail, within the phage head, in the internal membrane, and in the nucleocapsid, respectively. These structural positions appear to be optimal locations for the lysin to contact the PG layer given the distinctive methods of cell entry employed by each phage. In the cases of PRD1 and T7, mutations in the entry-associated lysins did not stop infection but merely delayed it. For gp16 of T7 this delay only applies under conditions in which the PG layer undergoes higher-than-normal levels of cross-linking.
The thickness of the PG layer in gram-negative bacteria is much less than that of their gram-positive counterparts, with estimated values ranging between approximately 2.5 and 7.5 nm and 20 and 50 nm, respectively (6, 26). In both cases the PG is expected to limit the size of diffusible molecules to about 50 kDa (14). Logically one would therefore expect phages infecting gram-positive bacteria to be accordingly equipped to passage their DNA across this obstacle, since this requirement would be more stringent for phages for which the hurdle is greater, as observed for gp16 of T7 (37). For Tuc2009, as for any other tailed phage infecting gram-positive bacteria, one would expect any lysin involved at the initiation of infection to be tail associated, although to our knowledge no data have been published to support this supposition.
In this study we describe a structural component of Tuc2009, ORF50, hereafter referred to as Tal2009 (for “tail-associated lysin”), which exhibits lytic activity within the C-terminal portion of the protein. This tail-associated lysin encoded by the bacteriophage Tuc2009 was shown to be located towards the end of the phage tail and to undergo autocatalytic posttranslational processing. Binding of antibodies (Abs) specific to the lysin reduced the ability of the phage to infect its host. To our knowledge this is the first time a protein with lytic activity has been found to be a structural component in a phage infecting a gram-positive bacterium.
MATERIALS AND METHODS
Bacterial strains, plasmids, bacteriophages, and growth media.
Bacteriophages, bacterial strains, and plasmids used in this study are listed in Table 1. L. lactis strains were grown in GM17 (Oxoid) broth or agar (1.4%) supplemented with 0.5% glucose at 30°C, while Escherichia coli strains were cultivated in Luria-Bertani (LB) broth or agar (1.4%) at 37°C (47). Bacteriophages Tuc2009 and c2 were propagated on L. lactis subsp. cremoris hosts UC509.9 and MG1614, respectively. E. coli M15 cells containing pQE60 or derivatives thereof were grown in the presence of 100 μg of ampicillin ml−1 and 25 μg of kanamycin ml−1. The pQE60 derivative containing the tetracycline resistance gene of pGhost8 was maintained in E. coli with LB broth containing tetracycline at a final concentration of 5 μg ml−1.
TABLE 1.
List of bacteriophage, bacterial strains, and plasmids used in this study
| Phage, strain or plasmid | Relevant feature(s) | Source or reference |
|---|---|---|
| Phage | ||
| Tuc2009 | Isolated from L. lactis subsp. cremoris UC509 following induction | 13 |
| c2 | Prolate-headed lytic phage | 34 |
| Strains | ||
| E. coli | ||
| M15 | Host for pQE60 plasmids; contains pREP4, Kanr | Qiagen |
| EC101 | E. coli JM101 with chromosomally encoded repA | 28 |
| L. lactis | ||
| UC509.9 | Prophage-cured derivative of L. lactis subsp cremoris, host for Tuc2009 | 13 |
| MG1614 | Host for c2 | 19 |
| MG1363 | Plasmid-free derivative of NCDO712 | 19 |
| NZ9800 | NZ9700 derivative; ΔnisA | 25 |
| S. thermophilus | ||
| CNRZ01205.3 | Prophage-cured derivative of S. thermophilus CNRZ01205 | 18 |
| S. aureus | ||
| ATCC 14458 | S. aureus subsp. aureus Rosenbach | ATCCa |
| Plasmids | ||
| pQE60 | E. coli expression vector, Ampr | Qiagen |
| pNZ8048 | L. lactis expression vector, Cmr | 15 |
| pTal2009-1 | pQE60 + tal2009 of Tuc2009 | This study |
| pTal2009-2ΔN | pQE60 + 1,617 bp of 3′ end of tal2009 of Tuc2009 | This study |
| pTal2009-3ΔN | pQE60 + 1,320 bp of 3′ end of tal2009 of Tuc2009 | This study |
| pTal2009-4ΔN | pQE60 + 852 bp of 3′ end of tal2009 of Tuc2009 | This study |
| pTal2009-5ΔN | pQE60 + 501 bp of 3′ end of tal2009 of Tuc2009 | This study |
| pTal2009-6ΔN | pQE60 + 381 bp of 3′ end of tal2009 of Tuc2009 | This study |
| pTal2009ΔC | pQE60 + 2,520 bp of 5′ end of tal2009 of Tuc2009 | This study |
| pTal2009mut-2ΔN | pQE60 + 1,617 bp of 3′ end of tal2009 of Tuc2009 with SOEing mutation | This study |
| pGhost8 | Mutagenising plasmid of lactococci | 33 |
| pTal2009-5ΔNtet | PTal2009-5ΔN + tetK from pGhost8 | This study |
| pLys | pQE60 + lys of Tuc2009 | This study |
| pNZTal2009-2ΔN | pNZ8048 + NcoI-HindIII region from pTal2009-2ΔN | This study |
ATCC, American Type Culture Collection.
Phage preparations.
Phages were purified using CsCl density gradient centrifugation (47). Where indicated, Tuc2009 was further purified by means of isopycnic centrifugation using a sucrose gradient. The latter procedure was performed by loading CsCl-purified phage onto 20 to 70% sucrose in TBT (100 mM Tris-HCl [pH 7], 100 mM NaCl, 10 mM MgCl2) gradients, which had been preformed in clear SW41 tubes with a Hoefer SG gradient maker. The phage band was harvested following centrifugation at 35,000 rpm for 1.5 h at 4°C in a SW41 rotor with a Beckman L-60 ultracentrifuge. The virus particles were then suspended in TBT and pelleted by a further centrifugation at 35,000 rpm for 1 h at 4°C. The resultant phage pellet was resuspended in 100 μl of TBT. Plaque assays were performed as described by Lillehaug (31).
Sequence analysis.
Database searches and protein family (pfam) allocations were performed using BLASTN and BLASTP (1) and using conserved domain search programs, respectively, located at the following URL (http://www.ncbi.nlm.nih.gov/). Sequence alignments were performed using the Clustal alignment method and MEGALIGN 3.16 software from a DNASTAR 2002 version 5 software package.
DNA manipulations and sequencing.
PCR amplifications were carried out using an EXPAND long template PCR system (Roche) according to the manufacturer's instructions with a Gene Amp PCR system 2400 thermal cycler (Perkin-Elmer) and the primers described in Table 2. Similarly, restriction enzymes, shrimp alkaline phosphatase, and T4 DNA ligase were supplied by Roche and employed as recommended by the manufacturer. Oligonucleotides were manufactured by MWG (Ebersberg, Germany). Purification of plasmids from E. coli was performed using a Wizard Plus SV Miniprep kit (Promega). Plasmid DNA preparations from L. lactis were completed using the protocol of O'Sullivan and Klaenhammer (41). Sequence analysis was performed by MWG (Ebersberg, Germany).
TABLE 2.
List of oligonucleotides used in the construction of plasmids
| Oligonucleotide name | Oligonucleotide sequencea |
|---|---|
| Tal2009-1F | 5′ AAA CCA TGG GTA ATA TCT TAT TTT TAG ATA AG |
| Tal2009-2F | 5′ AAA CCA TGG GCA ATA TCT CTG ACC TTG |
| Tal2009-3F | 5′ AAA CCA TGG CAG ATT TTA TAA ATG CAG |
| Tal2009-4F | 5′ AAA CCA TGG TCT CTA AAC AAG CGG CG |
| Tal2009-5F | 5′ AAA CCA TGG CCT TAA ACG GAC ACC CTG AAC G |
| Tal2009-6F | 5′ AAA CCA TGG CAA GTG AAA TGG GTT GG |
| Tal2009mut-2ΔNF | 5′ GGG CGA AGC TCT CGC CGC G |
| Tal2009mut-2ΔNR | 5′ CGC GGC GAG AGC TTC GCC C |
| Tal2009-R | 5′ GGA AGA TCT AAA TTT GAT ATA ATC CCT TGG ATT C |
| Tal2009-RΔC | 5′ GGA AGA TCT CGC ATG CTT GAT GAC CGT G |
| Lys F | 5′ AAA CCA TGG AAA GAT TAA TCA AAA AAT C |
| Lys R | 5′ GGA AGA TCT ATA ATT TAG TGT TTG ACC AGC |
| TetKF | 5′ GGA ATT C[CA TAT G]GC TTC ACA GAA ATT CTA GAA CA |
| TetKR | 5′ GGA ATT C[CA TAT G]GT TAA TAC GTG AGC TCT GCG AGG C |
NcoI and BglII sites are underlined once and twice, respectively. NdeI sites are in brackets.
Plasmid construction.
Specific PCR-generated DNA fragments representing sections of tal2009 or the complete lys gene (2) from Tuc2009 were produced. NcoI and BglII sites were incorporated into the relevant synthetic oligonucleotides to insert these PCR products into the E. coli expression vector pQE60 (Table 1). The stop codon that defines the 3′ end of either tal2009 or lys was omitted from complementary oligonucleotides, allowing a translational fusion of the various Tal2009 sections or the Lys protein with the six-His tag encoded by pQE60. In the case in which the tetK gene was inserted into pTal2009-5ΔN to produce pTal2009-5ΔNtet, an NdeI restriction site was included at both ends of the tetK PCR product via incorporation of the NdeI recognition sequence into both oligonucleotides. Otherwise, they were identical to those used by Maguin et al. to amplify the tetK gene with pGhost8 as a template (33). For the construction of pTal2009mut-2ΔN, suitable primers were designed and site-directed mutagenesis was carried out using the PCR-based SOEing technique (22). To check expression of tal2009 in L. lactis, the DNA fragment corresponding to tal2009-2ΔN was restricted from pTal2009-2ΔN by the use of NcoI and HindIII and the smallest fragment generated by this digestion was ligated into the similarly restricted pNZ8048 to produce pNZTal2009-2ΔN. This allowed transfer of the tal2009-2ΔN fragment fused to the six-His codons from pQE60 in pTal2009-2ΔN to a position downstream of the nisin-inducible promoter of the lactococcal expression vector pNZ8048 (15). Electrotransformation of plasmid DNA into E. coli was performed as described by Sambrook et al. (47), while that of L. lactis NZ9800 was performed as described by Wells et al. (54). All DNA cloning steps were initially performed using E. coli as a host. The integrity of all clones was checked by restriction profiling and DNA sequencing.
Protein expression and purification.
Overexpression of target proteins was achieved using the E. coli expression plasmid pQE60 essentially as recommended by Qiagen. Induction was accomplished over a 4-h period and was initiated using isopropyl-β-d-thiogalactopyranoside (IPTG) (Sigma) at a final concentration of 1 mM when the culture had reached an optical density at 600 nm of 0.35 to 0.4. Protein expression in L. lactis from pNZ8048 was produced as outlined by de Ruyter et al. (15). Cultures of L. lactis or E. coli (30 ml) were used to express proteins for zymogram assays and autocleavage studies. The cells were harvested by centrifugation in a Hermle Z-323 centrifuge at 4,000 rpm for 20 min. Lysis was carried out by resuspending the pellet in 1 ml of lysis buffer (50 mM NaH2PO4 [pH 8.0], 1 M NaCl, 30 mM imidazole) and performing cell disruption in a mini-beadbeater-8 (Biospec products) for 10 min at 4°C.
Whole cells, cellular debris, and insoluble proteins were cleared by centrifugation at 14,000 rpm for 20 min in an Eppendorf benchtop centrifuge. Protein purification of Tal2009-5ΔN involved overexpressing the protein in 300 ml of LB broth. Cells were harvested in a Beckman J2-21 centrifuge at 4,000 rpm for 20 min. The pellet was resuspended in 10 ml of lysis buffer and disrupted in an alcohol ice bath by ultrasonication (Soniprep 150) with 30-s bursts at an amplitude of 10 μm followed by 15-s breaks. This sonication was continued for 10 min. Lysates were cleared as described above, and the overexpressed TalT-5ΔN was purified by immobilized-metal-affinity chromatography. Briefly, this involved passing the lysate through a column containing 4 ml of nickel-nitrilotriacetic acid matrix (Qiagen) which had been preequilibrated with 10 ml of the lysis buffer. Two 10-ml washes were carried out using wash buffer (50 mM NaH2PO4 [pH 8.0], 1 M NaCl, 30 mM imidazole, 0.5% Triton X-100, 5 mM β-mercaptoethanol). The protein was then eluted using 10 ml of the elution buffer (50 mM NaH2PO4 [pH 8.0], 1 M NaCl, 250 mM imidazole) in approximately 1-ml aliquots. Protein concentrations were determined using a Bio-Rad protein assay in conjunction with a bovine serum albumin standard curve.
SDS-PAGE and zymogram assays.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed as described by Laemmli (27) by the use of a 4% stacking gel and a 12% separating gel. Renaturing SDS-PAGE and zymogram analysis were carried out as outlined by Lepeuple et al. (29) except that whole-cell substrates were produced according to Sheehan et al. (51). When Micrococcus lysodeikticus ATCC4698 cells were employed, however, these were supplied in the form of a lyophilized powder (Sigma). Cells were incorporated into the gels at a final concentration of 0.4% (wt/vol). Protein sizes were compared to that of a prestained protein marker (New England Biolabs).
Western blotting and immunological detection.
Following SDS-PAGE, proteins were transferred onto polyvinylidine difluoride (PVDF) membranes (Immobilon-P; Millipore) with a 0.1 M CAPS (3-[cyclohexylamino]-1-propanesulfonic acid; pH 11)-10% methanol transfer buffer (Mini-protean II; Bio-Rad Laboratories). Tetra-His horseradish peroxidase-conjugated Abs (Qiagen) were used at a 1/1,000 dilution according to the manufacturer's instructions. Detection using polyclonal Abs involved washes (2 × 10 min) of the membranes in TBS (10 mM Tris-HCl [pH 7.5], 150 mM NaCl) followed by a 10-min wash in TBS-Tween-Triton (20 mM Tris-HCl [pH 7.5], 500 mM NaCl, 0.05% Tween 20, 0.2% Triton X-100). Membranes were then incubated for 1 h in blocking buffer (TBS, 5% skimmed milk powder, 0.1% Tween 20). The three washes were repeated, after which the primary Ab was allowed to interact with its antigen by incubating the membrane in blocking buffer with an appropriate dilution of the polyclonal rabbit serum. Following the repetition of the three wash steps, the secondary Ab, horse radish peroxidase-conjugated anti-rabbit donkey immunoglobulin G (IgG) (Amersham Biosciences), was incubated with the membrane in the same manner as described for the primary Ab. Further washes (4 × 10 min) in TBS-Tween-Triton were performed, and the antigen-Ab complexes were detected using an ECL Western blotting detection system (Amersham Biosciences) according to the manufacturer's instructions. Where Tetra-His-detecting Abs were employed, protein sizes were determined using a six-His protein marker (Qiagen). To monitor production of Tal2009 in vivo, 100 ml of a Tuc2009 lysate was added to 500 ml of an exponentially growing culture of UC509.9 at an optical density at 600 nm of 0.4 to give a multiplicity of infection (MOI) of approximately 1. Following this, 30-ml samples were taken at 20-min intervals. The cells were harvested and lysed using a bead-beater as described above. Equal concentrations of these protein samples were separated by SDS-PAGE, analyzed by Western blotting, and detected as described above with the polyclonal rabbit Abs directed against Tal2009-5ΔN.
N-terminal sequencing.
Specific protein bands of interest were transferred to PVDF membranes by Western blotting as described above and excised from the membrane following visualization with Coomassie blue R-250. These were N-terminally sequenced by Alta Biosciences, University of Birmingham, England, using Edman degradation.
Cell wall binding.
Assays to determine whether Tal2009 exhibited any cell wall binding capabilities were performed essentially as described by Buist (8). Briefly, 250 μl of exponentially growing UC509.9 cells was resuspended in an equal volume of a cell-free lysate of E. coli M15 cells expressing Tal2009 from pTal2009-1 and incubated for 30 min at 30°C. Cell-free lysates expressing lys (2) from pLys were similarly incubated with UC509.9 cells as a positive control. These mixtures were centrifuged, and the cell pellets and supernatants were collected. The cells were washed with 250 μl of 0.5× M17 and resuspended in 250 μl of denaturation buffer (3), while 100 μl of the supernatants was dialyzed against distilled water and dried by centrifugation under a vacuum prior to being dissolved in 50 μl of denaturation buffer. Lysin activity was detected by zymogram analysis as described above.
Ab production and neutralization studies.
Polyclonal Abs against purified TalT-5ΔN were raised in rabbits by CN Biosciences (UK) Ltd. An initial immunization with the protein of interest and Freund's complete adjuvant was followed by five subsequent boost injections of TalT-5ΔN. A final serum sample was acquired 11 weeks after the initial immunization. Specific test and control Ab preparations for immunoelectron microscopy were developed as described by Johnsen et al. (24). The glycine elution procedure from Harlow and Lane (21) was performed to specifically obtain only those Abs which bound to Tal2009-5ΔN with a high level of affinity.
To perform Ab neutralization studies, a 5% inoculum from a fresh overnight culture of UC509.9 was set up in 100 μl of double-strength GM17 and left at 30°C. Samples (2 μl) were taken at hourly intervals to count CFU ml−1. CsCl-prepared phage (2 μl) was added to render an MOI of 0.02 for c2 and MG1614 and for Tuc2009 and UC509.9. The cells were made up to a final volume of 200 μl with rabbit serum, which had been dialyzed against TBT and filter sterilized, and 2 μl of 1 M CaCl2. Further 2-μl samples were taken up to 9 h after the first inoculation. Cell counts at each time point were performed by diluting the cells in Ringers solution, spread plating suitable dilutions on GM17 agar, and incubating the plates at 30°C.
Electron microscopy.
A total of 5 μl of CsCl-purified Tuc2009 (∼1011 PFU ml−1) was dried onto a Formvar-coated copper grid (300 mesh) and stained with 2% (wt/vol) uranyl acetate. Immunoelectron microscopy was performed by initial incubation of 5 μl of the purified phages with 5 μl of the purified Ab preparations for 1 h at room temperature. These preparations were then dropped onto the Formvar-coated grids and allowed to attach for 10 min before being dried by the use of blotting paper. A total of 5 μl of the goat anti-rabbit IgG gold conjugate (Sigma) (5-nm-diameter grains) diluted 1/100 per the manufacturer's instructions (∼7.5 × 109 particles) was then added onto the grid for a further 1-h incubation at room temperature. The excess liquid was blotted clear, and the grids were fixed in 0.25% glutaraldehyde-0.1 M NaPO4 (pH 7.5), washed in H2O, and finally stained as described above. The samples were analyzed using a Hitachi H-7000 transmission electron microscope.
RESULTS
Sequence analysis of orf50 of Tuc2009 shows homology to a lytic enzyme.
Cell wall-degrading enzymes involved in phage infection during the DNA injection process have been observed in gram-negative bacteria. To determine whether such proteins are also present in phage infecting gram-positive bacteria, a homology search of the proteins encoded by the region that was assumed to include the structural module of Tuc2009 was performed to identify any gene with sequence similarity to known lysins. The Tuc2009 region encompassing this structural module was deduced from the gene maps of previous comparative phage genome analyses (12, 42). One protein, the product of orf50, designated here as Tal2009 showed significant similarity (45% identity) to Zoocin A, a known lysin from Streptococcus equi subsp. zooepidemicus (52). This homology was confined to the region spanning amino acids (aa) 810 to 904 of Tal2009, a protein with a predicted length of 906 aa, and residues 56 to 157 of the 285-aa Zoocin A. Conserved-domain analysis of Tal2009 denoted the presence of an M23/M37 pfam domain at this C-terminal component of the protein. For the members of this family, homology with Tal2009 is generally confined to the specific conserved residues that associate the proteins with this pfam. Members of the M23/M37 family include zinc metalloendopeptidases, some of which are well characterized, mainly due to their lytic properties against known pathogens or because of their roles as virulence factors (4, 20, 30, 40, 43). Furthermore, the sequence of Tal2009 displays 94 and 89% identity to the entire expected protein sequence of ORF47 of TP901-1 (AF304433.1) and the complete structural protein from ul36 (AF349457.1), respectively. The 79% identity exhibited between protein 44 of the L. lactis IL1403 prophage bIL285 (AF323668.1) and Tal2009 is confined to approximately 550 aa at the C-terminal portion of both proteins. Thus, it appears that this C-terminal portion encodes a lysin as a conserved structural component of certain lactococcal phages.
Bacteriophage Tuc2009 contains a structural component with cell wall-degrading activity which can be assigned to Tal2009.
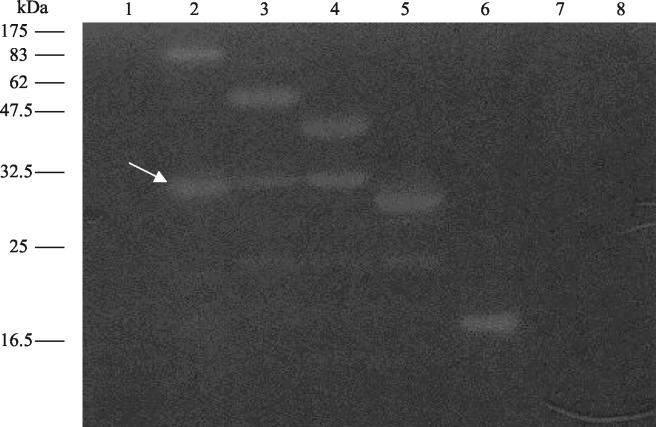
To detect whether Tuc2009 phage particles possess a structural component with lytic activity, zymogram assays were performed to visualize lytic functionality in CsCl-purified phage. A weakly visible lytic activity was observed, corresponding in size to that expected for the protein product of tal2009 (∼100 kDa). This result is not shown here due to the poor visibility of this lytic zone, which could be caused by weak enzymatic activity, low concentration, a low level of mobility, or refolding difficulties of this protein. To confirm the predicted lytic activity encoded by tal2009, the complete gene and five progressively smaller portions of the gene were amplified by PCR and individually inserted into the vector pQE60 to generate six different pQE60 derivatives (Table 1). The fragments were chosen to more precisely determine the specific region responsible for lytic activity within the protein. These proteins were overproduced in E. coli M15, and lysates of each of these strains were assayed for lytic abilities by zymogram analysis (Fig. 1). All of the fragments of the tal2009 gene, with the exception of the smallest one, were shown to specify a lytic activity. These results located the functional domain of apparent PG-degradative activity to within 166 C-terminal aa of Tal2009, in accordance with what was indicated by bioinformatic analysis. When the zymogram assay was used, Tal2009 was found to be active against cell walls of L. lactis UC509.9 and MG1363 but ineffective against those of Staphylococcus aureus ATCC14458, M. lysodeikticus ATCC4698 (Sigma), and Streptococcus thermophilus CNRZ01205.3 (results not shown).
FIG. 1.
Zymogram assay to determine lytic activity of Tal2009 in gels containing 0.4% (wt/vol) L. lactis UC509.9 autoclaved cells. Each well was loaded with E. coli M15 lysates with proteins expressed from the following plasmids: lane 1, pQE60; lane 2, pTal2009-1; lane 3, pTal2009-2ΔN; lane 4, pTal2009-3ΔN; lane 5, pTal2009-4ΔN; lane 6, pTal2009-5ΔN; lane 7, pTal2009-6ΔN; lane 8, pTal2009ΔC. Molecular masses are indicated to the left of the gel, and a white arrow denotes the ∼30-kDa processed proteins with lytic activity.
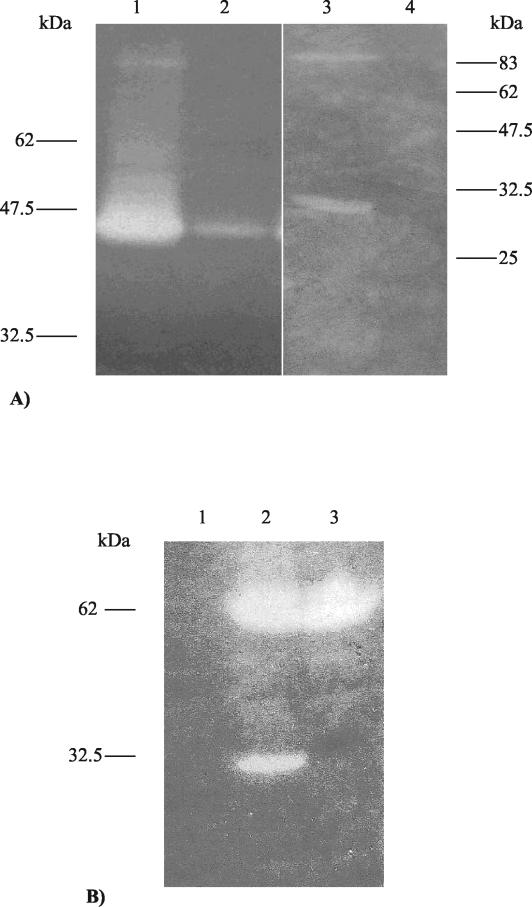
Comparative sequence analysis had failed to detect any cell-binding domain in Tal2009. To verify this observation a cell-binding assay was performed using the endolysin of Tuc2009 as a positive control. From work performed by Sheehan et al. (50) Lys, the Tuc2009 endolysin, is known to include a cell wall-recognizing component. As expected, Lys exhibited binding characteristics whereas Tal2009 did not appear to bind the cells of L. lactis UC509.9 (Fig. 2A).
FIG. 2.
(A) Zymogram assay to investigate any possible cell binding capabilities of Tal2009. Lanes 1 and 2 show a zone of lytic activity corresponding to the Tuc2009 endolysin in the supernatant and cell pellet, respectively, following incubation with 509.9 cells. Lane 3 shows that Tal2009 is in the supernatant, while lane 4 shows Tal2009 to be absent from the 509.9 cell pellet. The gels contained 0.4% (wt/vol) L. lactis UC509.9 autoclaved cells. Molecular masses are indicated. (B) Zymogram analysis of the E. coli M15 lysates containing pTal2009-2ΔN or the mutant pTal2009mut-2ΔN in gels containing 0.4% (wt/vol) L. lactis UC509.9 autoclaved cells. Lane 1 contains the negative control pQE60, and lanes 2 and 3 contain pTal2009-2ΔN and pTal2009mut-2ΔN, respectively. Molecular masses are indicated to the left of the gel.
Tal2009 undergoes autoproteolysis at a specific site.
From the zymogram assays it appeared that the three largest fragments of Tal2009 were subjected to a specific cleavage event upon overexpression in E. coli, yielding a smaller lytic moiety of approximately 30 kDa (Fig. 1). To determine whether the observed processing was an E. coli-specific event, the second-largest fragment of tal2009, including the six-His tag-encoding region, was restricted from pQE60 and ligated into pNZ8048. Upon electrotransformation into L. lactis NZ9000, production of the resultant protein was induced by nisin and lysates were analyzed for lytic activity by zymogram assay. As seen with E. coli, Tal2009 appeared to undergo processing because the zymogram assays displayed a smaller protein of ∼30 kDa exhibiting lytic activity as well as a clear band of the expected size representing the entire protein being produced (data not shown). tal2009-2ΔN was overexpressed in both E. coli and L. lactis and transferred to PVDF membranes by Western blotting before being subjected to N-terminal sequencing. In both cases (expression in E. coli and L. lactis), the ∼30-kDa protein possessed the same N-terminal sequence, corresponding to cleavage of Tal2009 at a glycine-rich region GGSSG↓GG, where the arrow indicates the precise point where cleavage occurs. This finding is interesting, because the M37 protein family to which Tal2009 would seem to belong is known to include Gly-Gly endopeptidases. To ensure that this was indeed the cleavage site, gene SOEing was carried out which changed the naturally occurring GGSSGGG amino acid sequence to GRSSRRG by G-to-C transversions in the DNA sequence (mutated amino acid residues are underlined). The product of this gene was expressed in E. coli, and its lysis profile was compared to that of its nonmutated brethren. As expected, only one zone of clearing was observed, corresponding to the unprocessed version of Tal2009 (Fig. 2B).
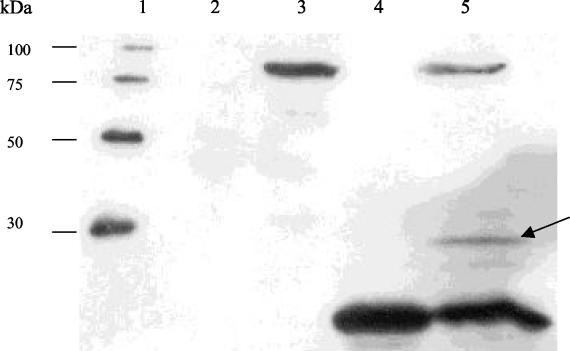
In an effort to discover whether autocatalysis was taking place, a deletion version of tal2009 was cloned into pQE60 to generate pTal2009ΔC. This deletion version of tal2009 is predicted to produce a truncated Tal2009 protein lacking the last C-terminal 66 aa and, as expected, was shown to be lytically inactive, failing to produce a zone of clearing in a zymogram assay (Fig. 1). The plasmid containing the smallest lytically active tal2009 fragment was modified by inserting the tetracycline resistance gene tetK into the NdeI restriction site of pQE60 to produce pTal2009-5ΔNtet. This plasmid was then introduced into cells harboring plasmid pTal2009ΔC and maintained by selecting for resistance to tetracycline. These two functional (Tal2009-5ΔN) and nonfunctional (Tal2009ΔC) proteins were then concomitantly expressed, and their interaction was analyzed by immunological detection. Interestingly, we found a discernible autoproteolytic mechanism whereby the larger inactive fragment was cleaved only in the presence of a lytically active segment of Tal2009. This is visualized by the presence of a third fragment reacting to the anti-Tetra-His Abs (Fig. 3). The size of this fragment is commensurate to that which would arise if the Tal2009ΔC protein were cleaved at the GGSSGGG region. The second protein fragment produced, corresponding to the N-terminal fraction of Tal2009ΔC, cannot be observed, since the Abs only bind to the C-terminal His-tagged portion of the Tal2009 proteins.
FIG. 3.
Western blot to detect possible interaction between Tal2009-Δ5N and Tal2009ΔC. The negative control is in lane 2, and the lysates of E. coli cultures overexpressing protein from pTal2009ΔC and pTal2009-5ΔNtet are in lanes 3 and 4, respectively. Lysates resulting from concomitant induction of expression from pTal2009-5ΔNtet and pTal2009ΔC can be observed in lane 5, with a band of ∼30 kDa denoted by an arrow. Lane 1 was loaded with a six-His protein ladder which reacts to the commercial Abs, and the molecular masses are indicated.
Identification of posttranslational processing of Tal2009 in vivo and its incorporation into the mature phage virion.
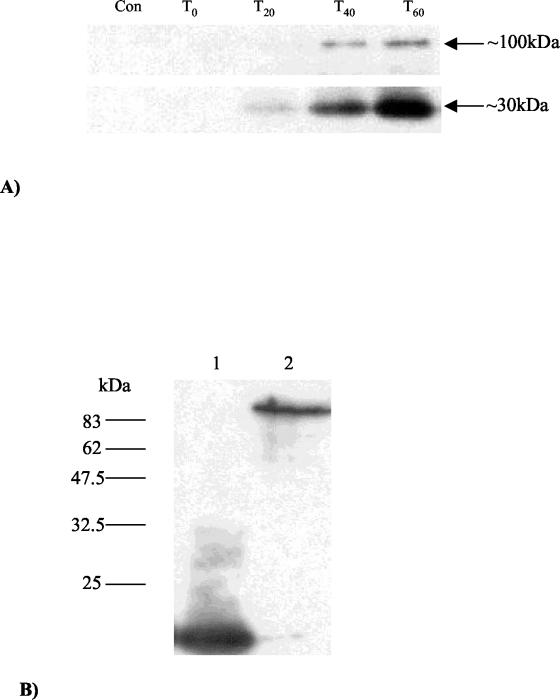
To elucidate the actual expression of Tal2009 in vivo, its production was monitored using polyclonal Abs raised against the purified C-terminal moiety of Tal2009 (Tal2009-5ΔN). As detailed in Materials and Methods, 30-ml samples of a Tuc2009-infected culture were taken at intervals of 20 min for an hour and the harvested cells were disrupted using glass beads. The reaction to the Abs showed the production of two bands, which tally in size to the lytic activities seen upon overexpression of Tal2009 in E. coli and L. lactis (Fig. 4A). These bands were first detected at 20 min following infection. To show that Tal2009 in its entirety is assimilated into the mature phage, Tuc2009 isolated by CsCl gradient was immunologically analyzed. In lanes containing ∼109 PFU of Tuc2009 phage particles, a single band was detected of the size expected for the full structural protein (Fig. 4B) and equal to that of the higher-molecular-weight band viewed upon zymogram analysis of the whole phage (results not shown). Determination of the N-terminal sequences of a number of structural proteins of Tuc2009 has indicated that unprocessed Tal2009 is indeed part of the phage structure and that an approximately 60-kDa portion of it inclusive of the N-terminal end is also incorporated into the mature virion (J. Seegers, D. van Sinderen, and G. F. Fitzgerald, unpublished results).
FIG. 4.
(A) Time point analysis of Tal2009 production in vivo including a lysate of uninfected UC509.9 cells to act as a negative control. The band sizes are indicated, with the lower band appearing slightly stronger than the larger band at 20 min (T20). (B) Western blots of the mature Tuc2009 virion exposed a single band analogous in size to the full Tal2009 protein. Tuc2009 is in the second lane, while a lysate containing Tal2009-5ΔN was run in lane 1 as a positive control. Molecular masses are indicated to the left of the gel.
Evidence for the localization of Tal2009 within the tail of Tuc2009 and for its involvement during infection.
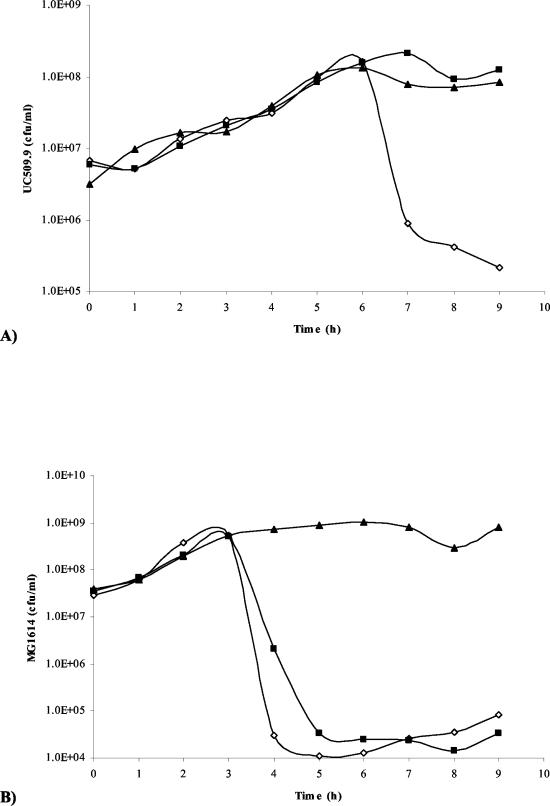
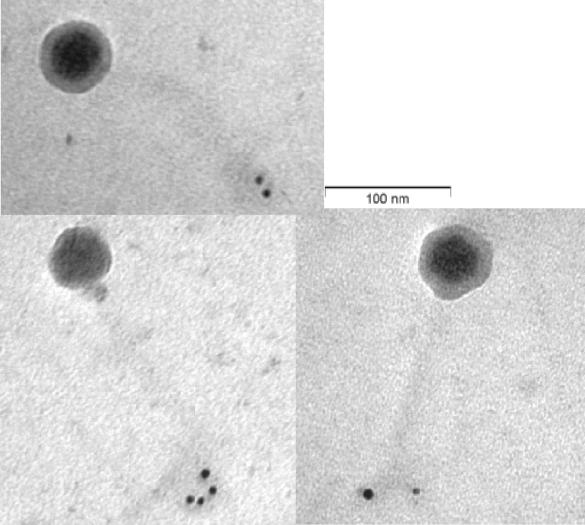
To determine the importance of Tal2009 in the phage multiplication process it was decided to perform an Ab neutralization experiment. When a range of MOI values from 0.02 to 20 was used, we observed a >2 log difference in viable counts between UC509.9 cells grown in the presence of terminal-bleed serum from the Tal2009-5ΔN-immunized rabbits and those grown in the presence of the preimmunization-bleed serum (Fig. 5A). No effect was seen on c2 infection of MG1614 (Fig. 5B). In addition, plaque assays were performed and PFU reductions of approximately 100-fold were observed for Tuc2009 compared to the results seen with a control (data not shown). c2 shows no homology to Tal2009 from Tuc2009. Since the only difference between these two UC509.9 cultures is the presence of Abs against Tal2009, these data suggest that Tal2009 is involved in Tuc2009 infection, although binding of anti-Tal2009 Abs may interfere with adjacent Tuc2009 structural proteins. To deduce the location of Tal2009 (including the C terminus) within Tuc2009 particles, immunoelectron microscopy was undertaken. The lytic module was found to be situated towards the end of the tail (Fig. 6). The control (described in the Materials and Methods section above) did not react with Tuc2009, thereby indicating a lack of anti-Tal2009 Abs in that preparation. Neither control nor test Ab preparations allowed the gold-conjugated anti-rabbit Abs to bind to c2 phage (data not shown). This is supportive of our findings obtained by homology searches and neutralization studies that c2 does not encode a homologue of Tal2009. The fact that secondary Abs did bind to the tail of Tuc2009 indicated the presence of Tal2009 at this location within the virion structure.
FIG. 5.
(A) Graph of numbers of viable L. lactis UC509.9 cells versus time (hours). This graph is representative of results for a number of MOI values and times of addition of Tuc2009. (B) Graph of numbers of viable L. lactis MG1614 cells versus time (hours) upon infection with c2 phage at an MOI of 0.02. In both cases, cultures without phage, with phage and preimmunization bleed, and with phage and bleeds postimmunization with Tal2009-5ΔN are denoted as closed triangles, open diamonds, and closed boxes, respectively.
FIG. 6.
Electron micrographs of Tuc2009 particles following incubation with rabbit anti-Tal2009-5Δ N Abs and gold (5-nm-diameter grains)-conjugated goat anti-rabbit IgG (magnification, ×200,000).
DISCUSSION
An increasing prevalence of multiple-drug-resistant pathogens has recently prompted interest in the methods employed by bacteriophage to lyse the host cell (32). Thus, work has been performed to determine the efficacy of lysins in killing colonizing pathogenic bacteria (38). In itself, this is an extension of bacteriophage therapy, a field undergoing a rejuvenation following a period spent loitering in the doldrums of Westernised medicine (35). One phage-encoded lysin has even been employed in the development of an in-field detection system for Bacillus anthracis (49). Much work has been performed in addition to elucidate the mechanisms by which phages penetrate the PG layer upon DNA injection during the infection of gram-negative bacteria, while to our knowledge no data on this topic is available for gram-positive bacteria. One would expect PG to be a greater barrier to phages infecting gram-positive bacteria, given that the thickness of this component of the gram-positive cell envelope is significantly greater than that of the gram-negative equivalent.
Using zymogram assays we identified a lytically active protein component of Tuc2009 particles of ∼100 kDa in size and identified the gene encoding this structural protein, Tal2009. Bioinformatic analysis denoted that the C-terminal portion of Tal2009 is a member of the M23/M37 pfam, which includes endopeptidases that target the interpeptide bridge of the PG layer. High levels of overall identity were recorded between Tal2009 and proteins of other phages infecting L. lactis. For the bacteriocin-like members of the M37 pfam, the similarities to Tal2009 and its closest phage-derived homologues are confined to those regions believed to be involved in enzymatic activity. Deletion analysis of Tal2009 did indeed show that the enzymatic activity is encoded by the 3′ portion of tal2009. This agrees with results from the site-directed mutagenesis of LasA from Pseudomonas aeruginosa (20), which demonstrated that a His residue (underlined) within the conserved sequence VTGPHLHF and also present in Tal2009 is essential for LasA activity. Upon expression of tal2009 in both E. coli and L. lactis, a strong and specific display of processing was exhibited which gave rise to a secondary lytic zone approximately 30 kDa in size. Processing of phage structural proteins is a common phenomenon (10). N-terminal sequencing of the ∼30-kDa Tal2009 fragment showed that cleavage occurred at a glycine (Gly)-rich region in both E. coli and L. lactis. In the case of the phage-encoded homologues of Tal2009 there are as many as eight glycine residues in a region of 10 aa. Glycine-rich regions within protein structures can act as flexible linkers between protein domains. It is possible, therefore, that this region in Tal2009 reflects a requirement of the phage to achieve mobility of the lytically active domain to effect hole formation in the PG layer while remaining incorporated in the phage structure. Interestingly, Gly-Gly regions are among the substrates targeted by members of the M23/M37 pfam, raising the possibility that this processing of Tal2009 is self-mediated. This was proven to be the case when a tertiary band of the expected size was produced upon coexpression of an inactive fragment of Tal2009 (Tal2009ΔC) with the smallest functional segment (Tal2009-5ΔN).
One major problem exists with marrying the theories of Tal2009 autocatalysis and lysis in that Tal2009 recognizes a Gly-rich region for autocatalytic cleavage but does not lyse S. aureus cells which contain an interpeptide bridge in the PG layer that encompasses a stretch of five glycines, while it is instead capable of lysis of L. lactis cells where no such Gly residues exist within their cell walls. Members of the M23/M37 pfam cut the interpeptide bridge between different amino acid residues (5, 30, 53). The limited spectrum of Tuc2009 could be partially explained by the blinkered lysis spectrum of Tal2009. Indeed, Rydman and Bamford (45) hypothesized that the incorporation of two lytic enzymes in the structure of PRD1 allowed for a broader host spectrum. There is virtually no homology between Tal2009 and LasA or β-lp except at the residues that group all three in the same pfam. One could also speculate that Tal2009 does recognize Gly-Gly in cell walls of S. aureus but that this bond is only weakly processed or perhaps that the flexibility of the Gly-rich region of Tal2009 predisposes it to autocleavage via increased exposure.
Given that Tal2009 in its entirety is included in the mature Tuc2009 phage, one would expect that too much processing to yield the smaller fragments could hinder virion construction or indeed functionality of the mature phage. In addition, immunological detection showed that the C terminus of Tal2009 is only present in mature Tuc2009 as part of the full protein (Fig. 4B). It is entirely possible, indeed probable, that the processing of Tal2009 allows the C-terminal portion of the protein to be released to the cell wall towards the end of the lytic cycle by the pore-forming holins to degrade the PG layer in conjunction with the Tuc2009 endolysin, resulting in a more efficient cell lysis. It is also possible that multiple molecules of both the complete and C-terminally truncated Tal2009 protein become incorporated into the phage upon assembly of the mature virion. Previous work (Seegers et al., unpublished) has shown that C-terminally truncated Tal2009 does indeed become incorporated into the phage. The purpose of this inclusion is as yet unknown, although it is probably a more likely reason for the autoproteolysis than the production of an auxiliary player in cell lysis. It may be that the stoichiometry or spatial arrangement of the tail tip proteins requires that some versions of Tal2009 incorporated into the phage do not have an additional 30 kDa on the C terminus of the protein.
Direction of Tal2009 to the target bond is probably caused by the spatial arrangement of the catalytic domain at the PG upon cell binding by the phage. In support of this theory, it was demonstrated that Tal2009 does not bind the cell wall. This omission of a binding domain probably decreases the lytic activity of Tal2009, which may be a deliberate property given the requirement for minimal disruption to the viability of the host cell upon infection initiation. The location of lytic activity in Tal2009 to one end of the protein promotes the idea of exposure of this zone at the end of the phage tail. The theory of phage evolution by exchange of functional modules has already been well articulated in terms of the whole genome (7) and the tail fibers (48) and down to the level of N- and C-terminus functionality of lysins (16). It has been suggested that homologous recombination can occur within DNA sequences specifying distinct protein domains (39). Gly-rich collagen-like repeats of phage structural proteins have been proposed as sites for gene reshuffling (17). Our results indicate that tail-associated lysins can follow the same mosaic pattern of functional modules of proteins. Indeed, preliminary work in this laboratory indicates that this is also true for tail-associated lysins of other phages which infect gram-positive bacteria.
To determine whether Tal2009 was necessary for phage infection we performed Ab neutralization experiments, an approach which has been used previously for lactococcal phages (11, 23). Initially tests were performed using an MOI of 0.02 for c2 and Tuc20009 with their respective hosts. Upon observing a >2 log difference in UC509.9 cell numbers in the presence or absence of Tal2009-specific Abs it was decided to increase the stringency of the test and drive cell lysis by using a MOI of 20 for Tuc2009 with UC509.9. Once again we observed a >2 log reduction of UC509.9 cell numbers when Tal2009-specific Abs were not present (Fig. 5A). No effect was observed for lysis of MG1614 by the bacteriophage c2 (Fig. 5B). However, we cannot exclude the possibility that binding of the Abs to Tal2009 elicits some sort of stearic hindrance to other phage-encoded proteins.
Our findings show that the tail lysin is located at the tip of the phage tail, which indicates that the Tuc2009 DNA infection process involves the action of a lysin, possibly to allow safe passage of a DNA injection device to the cell membrane. In an effort to more fully elucidate this mechanism and to explain the apparent disparity between the substrate spectrum and bond recognition seen in autoproteolysis, further studies of Tal2009 are in progress.
Acknowledgments
This work was funded by the Bioresearch Ireland Postgraduate Scheme, Enterprise Ireland (BR/2000/53), the Embark Initiative Postdoctoral Fellowship, and Science Foundation Ireland (02/IN1/B198).
We express our thanks to Horst Neve (Institut für Mikrobiologie, Kiel, Germany) for his advice concerning immunoelectron microscopy and the Centre for Microscopic Analysis (Trinity College, Dublin, Ireland).
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arendt, E. K., C. Daly, G. F. Fitzgerald, and M. van de Guchte. 1994. Molecular characterization of lactococcal bacteriophage Tuc2009 and identification and analysis of genes encoding lysin, a putative holin, and two structural proteins. Appl. Environ. Microbiol. 60:1875-1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beliveau, C., C. Potvin, J. Trudel, A. Asselin, and G. Bellemare. 1991. Cloning, sequencing, and expression in Escherichia coli of a Streptococcus faecalis autolysin. J. Bacteriol. 173:5619-5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beukes, M., G. Bierbaum, H. G. Sahl, and J. W. Hastings. 2000. Purification and partial characterization of a murein hydrolase, millericin B, produced by Streptococcus milleri NMSCC 061. Appl. Environ. Microbiol. 66:23-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beukes, M., and J. W. Hastings. 2001. Self-protection against cell wall hydrolysis in Streptococcus milleri NMSCC 061 and analysis of the millericin B operon. Appl. Environ. Microbiol. 67:3888-3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beveridge, T. J., and L. L. Graham. 1991. Surface layers of bacteria. Microbiol. Rev. 55:684-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Botstein, D. 1980. A theory of modular evolution for bacteriophages. Ann. N. Y. Acad. Sci. 354:484-490. [DOI] [PubMed] [Google Scholar]
- 8.Buist, G. 1997. AcmA of Lactococcus lactis, a cell-binding major autolysin. Ph.D. thesis. University of Groningen, Groningen, The Netherlands.
- 9.Caldentey, J., and D. H. Bamford. 1992. The lytic enzyme of the Pseudomonas phage phi 6. Purification and biochemical characterization. Biochim. Biophys. Acta 1159:44-50. [DOI] [PubMed] [Google Scholar]
- 10.Casjens, S., and R. Hendrix. 1988. Control mechanisms in dsDNA bacteriophage assembly, p. 15-91. In R. Calendar (ed.), The bacteriophages. Plenum, New York, N.Y.
- 11.Chibani, A., Sr., I. Fliss, R. E. Simard, and S. Moineau. 1998. Monoclonal antibodies raised against native major capsid proteins of lactococcal c2-like bacteriophages. Appl. Environ. Microbiol. 64:4255-4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chopin, A., A. Bolotin, A. Sorokin, S. D. Ehrlich, and M. Chopin. 2001. Analysis of six prophages in Lactococcus lactis IL1403: different genetic structure of temperate and virulent phage populations. Nucleic Acids Res. 29:644-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costello, V. A. 1988. Characterization of bacteriophage interactions in Streptococcus cremoris UC503 and related lactic streptococci. Ph.D. thesis. National University of Ireland, Cork, Ireland.
- 14.Demchick, P., and A. L. Koch. 1996. The permeability of the wall fabric of Escherichia coli and Bacillus subtilis. J. Bacteriol. 178:768-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Ruyter, P. G., O. P. Kuipers, W. C. Meijer, and W. M. De Vos. 1997. Food-grade controlled lysis of Lactococcus lactis for accelerated cheese ripening. Nat. Biotechnol. 15:976-979. [DOI] [PubMed] [Google Scholar]
- 16.Diaz, E., R. Lopez, and J. L. Garcia. 1990. Chimeric phage-bacterial enzymes: a clue to the modular evolution of genes. Proc. Natl. Acad. Sci. USA 87:8125-8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duplessis, M., and S. Moineau. 2001. Identification of a genetic determinant responsible for host specificity in Streptococcus thermophilus bacteriophages. Mol. Microbiol. 41:325-336. [DOI] [PubMed] [Google Scholar]
- 18.Fayard, B., M. Haefliger, and J. P. Accolas. 1993. Interactions of temperate bacteriophages of Streptococcus salivarius subsp. thermophilis with lysogenic indicators affect phage DNA restriction patterns and host ranges. J. Dairy Res. 60:385-399. [Google Scholar]
- 19.Gasson, M. 1983. Plasmid complement of Streptococcus lactis NCDO712 and other lactic streptococci after protoplast-induced curing. J. Bacteriol. 154:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gustin, J. K., E. Kessler, and D. E. Ohman. 1996. A substitution at His-120 in the LasA protease of Pseudomonas aeruginosa blocks enzymatic activity without affecting propeptide processing or extracellular secretion. J. Bacteriol. 178:6608-6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harlow, E., and D. Lane. 1988. Purifying antibodies from immunoblots, p. 471-510. In E. Harlow and D. Lane (ed.), Antibodies: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 22.Horton, R. M., Z. L. Cai, S. N. Ho, and L. R. Pease. 1990. Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. BioTechniques 8:528-535. [PubMed] [Google Scholar]
- 23.Jarvis, A. W. 1978. Serological studies of a host range mutant of a lactic streptococcal bacteriophage. Appl. Environ. Microbiol. 36:785-789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnsen, M. G., H. Neve, F. K. Vogensen, and K. Hammer. 1995. Virion positions and relationships of lactococcal temperate bacteriophage TP901-1 proteins. Virology 212:595-606. [DOI] [PubMed] [Google Scholar]
- 25.Kuipers, O. P., M. M. Beerthuyzen, R. J. Siezen, and W. M. De Vos. 1993. Characterization of the nisin gene cluster nisABTCIPR of Lactococcus lactis. Requirement of expression of the nisA and nisI genes for development of immunity. Eur. J. Biochem. 216:281-291. [DOI] [PubMed] [Google Scholar]
- 26.Labischinski, H., E. W. Goodell, A. Goodell, and M. L. Hochberg. 1991. Direct proof of a “more-than-single-layered” peptidoglycan architecture of Escherichia coli W7: a neutron small-angle scattering study. J. Bacteriol. 173:751-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 28.Law, J., G. Buist, A. Haandrikman, J. Kok, G. Venema, and K. Leenhouts. 1995. A system to generate chromosomal mutations in Lactococcus lactis which allows fast analysis of targeted genes. J. Bacteriol. 177:7011-7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lepeuple, A. S., E. Van Gemert, and M. P. Chapot-Chartier. 1998. Analysis of the bacteriolytic enzymes of the autolytic Lactococcus lactis subsp. cremoris strain AM2 by renaturing polyacrylamide gel electrophoresis: identification of a prophage-encoded enzyme. Appl. Environ. Microbiol. 64:4142-4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li, S., S. Norioka, and F. Sakiyama. 1998. Bacteriolytic activity and specificity of Achromobacter beta-lytic protease. J. Biochem. (Tokyo) 124:332-339. [DOI] [PubMed] [Google Scholar]
- 31.Lillehaug, D. 1997. An improved plaque assay for poor plaque-producing temperate lactococcal bacteriophages. J. Appl. Microbiol. 83:85-90. [DOI] [PubMed] [Google Scholar]
- 32.Loeffler, J. M., and V. A. Fischetti. 2003. Synergistic lethal effect of a combination of phage lytic enzymes with different activities on penicillin-sensitive and -resistant Streptococcus pneumoniae strains. Antimicrob. Agents Chemother. 47:375-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maguin, E., H. Prevost, S. D. Ehrlich, and A. Gruss. 1996. Efficient insertional mutagenesis in lactococci and other gram-positive bacteria. J. Bacteriol. 178:931-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McKay, L. L., and K. A. Baldwin. 1973. Induction of prophage in Streptococcus lactis C2 by ultraviolet irradiation. Appl. Microbiol. 25:682-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Merril, C. R., D. Scholl, and S. L. Adhya. 2003. The prospect for bacteriophage therapy in Western medicine. Nat. Rev. Drug Discovery 2:489-497. [DOI] [PubMed] [Google Scholar]
- 36.Miller, E. S., E. Kutter, G. Mosig, F. Arisaka, T. Kunisawa, and W. Ruger. 2003. Bacteriophage T4 genome. Microbiol. Mol. Biol. Rev. 67:86-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moak, M., and I. J. Molineux. 2000. Role of the Gp16 lytic transglycosylase motif in bacteriophage T7 virions at the initiation of infection. Mol. Microbiol. 37:345-355. [DOI] [PubMed] [Google Scholar]
- 38.Nelson, D., L. Loomis, and V. A. Fischetti. 2001. Prevention and elimination of upper respiratory colonization of mice by group A streptococci by using a bacteriophage lytic enzyme. Proc. Natl. Acad. Sci. USA 98:4107-4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neve, H., K. I. Zenz, F. Desiere, A. Koch, K. J. Heller, and H. Brüssow. 1998. Comparison of the lysogeny modules from the temperate Streptococcus thermophilus bacteriophages TP-J34 and Sfi21: implications for the modular theory of phage evolution. Virology 241:61-72. [DOI] [PubMed] [Google Scholar]
- 40.Nilsen, T., I. F. Nes, and H. Holo. 2003. Enterolysin A, a cell wall-degrading bacteriocin from Enterococcus faecalis LMG 2333. Appl. Environ. Microbiol. 69:2975-2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O'Sullivan, D. J., and T. R. Klaenhammer. 1993. High- and low-copy-number Lactococcus shuttle cloning vectors with features for clone screening. Gene 137:227-231. [DOI] [PubMed] [Google Scholar]
- 42.Proux, C., D. van Sinderen, J. Suarez, P. Garcia, V. Ladero, G. F. Fitzgerald, F. Desiere, and H. Brüssow. 2002. The dilemma of phage taxonomy illustrated by comparative genomics of Sfi21-like Siphoviridae in lactic acid bacteria. J. Bacteriol. 184:6026-6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramadurai, L., K. J. Lockwood, M. J. Nadakavukaren, and R. K. Jayaswal. 1999. Characterization of a chromosomally encoded glycylglycine endopeptidase of Staphylococcus aureus. Microbiology 145(Pt. 4):801-808. [DOI] [PubMed] [Google Scholar]
- 44.Rydman, P. S., and D. H. Bamford. 2000. Bacteriophage PRD1 DNA entry uses a viral membrane-associated transglycosylase activity. Mol. Microbiol. 37:356-363. [DOI] [PubMed] [Google Scholar]
- 45.Rydman, P. S., and D. H. Bamford. 2002. Phage enzymes digest peptidoglycan to deliver DNA. ASM News 68:330-335. [Google Scholar]
- 46.Rydman, P. S., and D. H. Bamford. 2002. The lytic enzyme of bacteriophage PRD1 is associated with the viral membrane. J. Bacteriol. 184:104-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sambrook, J., A. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 48.Sandmeier, H. 1994. Acquisition and rearrangement of sequence motifs in the evolution of bacteriophage tail fibres. Mol. Microbiol. 12:343-350. [DOI] [PubMed] [Google Scholar]
- 49.Schuch, R., D. Nelson, and V. A. Fischetti. 2002. A bacteriolytic agent that detects and kills Bacillus anthracis. Nature 418:884-889. [DOI] [PubMed] [Google Scholar]
- 50.Sheehan, M. M., J. L. Garcia, R. Lopez, and P. Garcia. 1996. Analysis of the catalytic domain of the lysin of the lactococcal bacteriophage Tuc2009 by chimeric gene assembling. FEMS Microbiol. Lett. 140:23-28. [DOI] [PubMed] [Google Scholar]
- 51.Sheehan, M. M., E. Stanley, G. F. Fitzgerald, and D. van Sinderen. 1999. Identification and characterization of a lysis module present in a large proportion of bacteriophages infecting Streptococcus thermophilus. Appl. Environ. Microbiol. 65:569-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simmonds, R. S., W. J. Simpson, and J. R. Tagg. 1997. Cloning and sequence analysis of zooA, a Streptococcus zooepidemicus gene encoding a bacteriocin-like inhibitory substance having a domain structure similar to that of lysostaphin. Gene 189:255-261. [DOI] [PubMed] [Google Scholar]
- 53.Sugai, M., T. Fujiwara, T. Akiyama, M. Ohara, H. Komatsuzawa, S. Inoue, and H. Suginaka. 1997. Purification and molecular characterization of glycylglycine endopeptidase produced by Staphylococcus capitis EPK1. J. Bacteriol. 179:1193-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wells, J. M., P. W. Wilson, and R. W. Le Page. 1993. Improved cloning vectors and transformation procedure for Lactococcus lactis. J. Appl. Bacteriol. 74:629-636. [DOI] [PubMed] [Google Scholar]
- 55.Young, R. 1992. Bacteriophage lysis: mechanism and regulation. Microbiol. Rev. 56:430-481. [DOI] [PMC free article] [PubMed] [Google Scholar]