Chicken anemia virus (CAV) is a small circular DNA virus that encodes three proteins: VP1, VP2, and VP3 (3, 6). VP1 and VP2 encode both structural and regulatory proteins (8), whereas VP3, also called apoptin (7), is a small 121-residue protein that is reported to be a tumor-selective, proapoptotic protein. Apoptin/VP3 has been shown to selectively reside in the cytosol of multiple normal cells and the nuclei of multiple transformed and tumorigenic cells (1, 9, 12). Rohn et al. (10) have recently reported that apoptin/VP3 contains a C-terminal, tumor-selective nuclear localization sequence (NLS) that requires activation by a tumor-selective phosphorylation at Thr108 (10). Several papers from 2003 have shown that assembly of a 30- to 40-VP3-unit apoptin/VP3 multimer in the cytoplasm is a prerequisite for nuclear import and induction of apoptosis (4, 5, 11).
We became interested in utilizing the apoptin/VP3 NLS to preferentially target chimeric proteins to the nuclei of tumor cells. Similar to results from Danen-Van Oorschot et al. (2), we mapped the nuclear export sequence to residues 33 to 46 and the NLS to residues 70 to 121 by genetic fusion to green fluorescent protein (GFP) and transfection of expression plasmids into ras-transformed 3T3 cells (3T3ras) (Fig. 1A). Phosphorylation of Thr108 has been reported to be required for nuclear import (10). However, mutation of the Thr108 phosphorylation site to Ala (T108A) did not alter GFP-VP370-121 nuclear accumulation in transformed 3T3ras cells (data not shown). In addition, we failed to detect any phosphorylation of this site in transfected tumorigenic cells in vivo by ortho-phosphate labeling or from transformed cell lysates in vitro (data not shown).
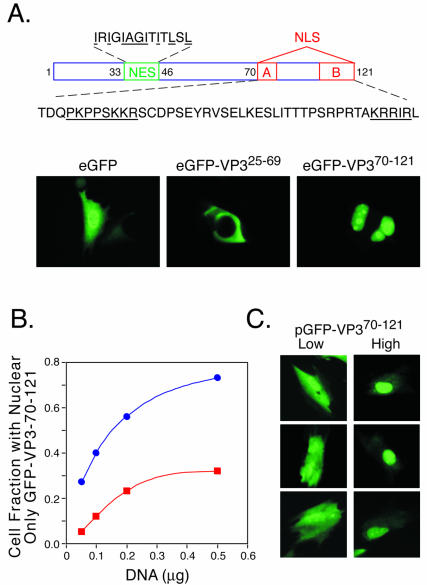
FIG. 1.
Apoptin/VP3 is imported into the nucleus due to high concentration levels, not tumorigenic status. (A) Structure-function diagram of apoptin/VP3 (top panel). NES, nuclear export sequence. Highlighted residues are key structural requirements. Indicated fragments of the apoptin/VP3 open reading frame were genetically fused to the C terminus of enhanced GFP (eGFP) and transfected into 3T3ras cells (bottom panel). (B) Decreasing the amount of GFP-VP370-121 expression plasmid transfected into transformed 3T3ras fibroblasts (blue circles) and primary human fibroblasts (red squares) from 0.5 to 0.05 μg/well dramatically altered the location of GFP-VP370-121. Plasmid DNA concentration was normalized to 0.5 μg/well by addition of corresponding amounts of empty expression vector. (C) Analysis of low- and high-level GFP-VP370-121-expressing transfected primary human fibroblasts from the same transfected population of cells showed nuclear import associated with high expression levels.
Recent reports of an aggregate assembly step prior to nuclear import (4, 5, 11) raised our concern that the putative tumor-selective nuclear accumulation might be due to high levels of apoptin/VP3 expression and not to tumorigenic status. Therefore, we transfected transformed 3T3ras cells with a limiting dilution of the GFP-VP370-121 expression vector while maintaining a constant total DNA concentration by adding empty plasmid vector. Decreasing the GFP-VP370-121 expression levels in either transformed 3T3ras fibroblasts or primary human foreskin fibroblasts resulted in a dramatic switch from a predominant nuclear accumulation at high concentrations to accumulation throughout the cell under low-level expression conditions (Fig. 1B), similar to GFP-only expression (Fig. 1A). Consistent with this observation, analysis of individual low- versus high-level-expressing GFP-VP370-121-transfected normal human fibroblasts also gave a similar result in that nuclear accumulation correlated with high expression levels and not tumorigenic status (Fig. 1C). Similar results were observed in multiple cell lines (normal and tumorigenic; data not shown).
We conclude that the previously reported tumor-selective nuclear accumulation and differential killing of tumor cells by apoptin/VP3 is based not on tumorigenic status but on higher expression levels achieved in transfection of tumorigenic cells versus lower expression levels obtained in transfection of normal cells. However, these observations also suggest that by reaching a critical threshold level to trigger cytoplasmic multimerization and subsequent nuclear import, apoptin/VP3 levels may serve as a sensor for viral host cell killing during the CAV life cycle.
REFERENCES
- 1.Danen-Van Oorschot, A. A., D. F. Fischer, J. M. Grimbergen, B. Klein, S. Zhuang, J. H. Falkenburg, C. Backendorf, P. H. Quax, A. J. van der Eb, and M. H. Noteborn. 1997. Apoptin induces apoptosis in human transformed and malignant cells but not in normal cells. Proc. Natl. Acad. Sci. USA 94:5843-5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Danen-Van Oorschot, A. A., Y. H. Zhang, S. R. Leliveld, J. L. Rohn, M. C. Seelen, M. W. Bolk, A. Van Zon, S. J. Erkeland, J. P. Abrahams, D. Mumberg, and M. H. Noteborn. 2003. Importance of nuclear localization of apoptin for tumor-specific induction of apoptosis. J. Biol. Chem. 278:27729-27736. [DOI] [PubMed] [Google Scholar]
- 3.Douglas, A. J., K. Phenix, K. A. Mawhinney, D. Todd, D. P. Mackie, and W. L. Curran. 1995. Identification of a 24 kDa protein expressed by chicken anaemia virus. J. Gen. Virol. 76:1557-1562. [DOI] [PubMed] [Google Scholar]
- 4.Leliveld, S. R., R. T. Dame, M. A. Mommaas, H. K. Koerten, C. Wyman, A. A. Danen-Van Oorschot, J. L. Rohn, M. H. Noteborn, and J. P. Abrahams. 2003. Apoptin protein multimers form distinct higher-order nucleoprotein complexes with DNA. Nucleic Acids Res. 31:4805-4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leliveld, S. R., Y. H. Zhang, J. L. Rohn, M. H. Noteborn, and J. P. Abrahams. 2003. Apoptin induces tumor-specific apoptosis as a globular multimer. J. Biol. Chem. 278:9042-9051. [DOI] [PubMed] [Google Scholar]
- 6.Noteborn, M. H., and G. Koch. 1995. Chicken anaemia virus infection: molecular basis of pathogenicity. Avian Pathol. 24:11-31. [DOI] [PubMed] [Google Scholar]
- 7.Noteborn, M. H., D. Todd, C. A. Verschueren, H. W. de Gauw, W. L. Curran, S. Veldkamp, A. J. Douglas, M. S. McNulty, A. J. van der Eb, and G. Koch. 1994. A single chicken anemia virus protein induces apoptosis. J. Virol. 68:346-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noteborn, M. H., C. A. Verschueren, G. Koch, and A. J. van der Eb. 1998. Simultaneous expression of recombinant baculovirus-encoded chicken anaemia virus (CAV) proteins VP1 and VP2 is required for formation of the CAV-specific neutralizing epitope. J. Gen. Virol. 79:3073-3077. [DOI] [PubMed] [Google Scholar]
- 9.Pietersen, A. M., M. M. van der Eb, H. J. Rademaker, D. J. van den Wollenberg, M. J. Rabelink, P. J. Kuppen, J. H. van Dierendonck, H. van Ormondt, D. Masman, C. J. van de Velde, A. J. van der Eb, R. C. Hoeben, and M. H. Noteborn. 1999. Specific tumor-cell killing with adenovirus vectors containing the apoptin gene. Gene Ther. 6:882-892. [DOI] [PubMed] [Google Scholar]
- 10.Rohn, J. L., Y. H. Zhang, R. I. Aalbers, N. Otto, J. Den Hertog, N. V. Henriquez, C. J. van de Velde, P. J. Kuppen, D. Mumberg, P. Donner, and M. H. Noteborn. 2002. A tumor-specific kinase activity regulates the viral death protein apoptin. J. Biol. Chem. 277:50820-50827. [DOI] [PubMed] [Google Scholar]
- 11.Zhang, Y. H., S. R. Leliveld, K. Kooistra, C. Molenaar, J. L. Rohn, H. J. Tanke, J. P. Abrahams, and M. H. Noteborn. 2003. Recombinant apoptin multimers kill tumor cells but are nontoxic and epitope-shielded in a normal-cell-specific fashion. Exp. Cell Res. 289:36-46. [DOI] [PubMed] [Google Scholar]
- 12.Zhuang, S. M., J. E. Landegent, C. A. Verschueren, J. H. Falkenburg, H. van Ormondt, A. J. van der Eb, and M. H. Noteborn. 1995. Apoptin, a protein encoded by chicken anemia virus, induces cell death in various human hematologic malignant cells in vitro. Leukemia 9(Suppl. 1):S118-S120. [PubMed] [Google Scholar]