Abstract
The feline and canine transferrin receptors (TfRs) bind canine parvovirus to host cells and mediate rapid capsid uptake and infection. The TfR and its ligand transferrin have well-described pathways of endocytosis and recycling. Here we tested several receptor-dependent steps in infection for their role in virus infection of cells. Deletions of cytoplasmic sequences or mutations of the Tyr-Thr-Arg-Phe internalization motif reduced the rate of receptor uptake from the cell surface, while polar residues introduced into the transmembrane sequence resulted in increased degradation of transferrin. However, the mutant receptors still mediated efficient virus infection. In contrast, replacing the cytoplasmic and transmembrane sequences of the feline TfR with those of the influenza virus neuraminidase (NA) resulted in a receptor that bound and endocytosed the capsid but did not mediate viral infection. This chimeric receptor became localized to detergent-insoluble membrane domains. To test the effect of structural virus receptor interaction on infection, two chimeric receptors were prepared which contained antibody-variable domains that bound the capsid in place of the TfR ectodomain. These chimeric receptors bound CPV capsids and mediated uptake but did not result in cell infection. Adding soluble feline TfR ectodomain to the virus during that uptake did not allow infection.
The infection of cells by viruses and their replication and release are the result of a highly evolved series of interactions between the virus and its components and the cellular machinery. Cell entry and infection for many animal viruses initiate with receptor-mediated endocytosis, yet the importance of the specific receptor-capsid interactions or endocytic uptake mechanisms and the subsequent endosomal trafficking pathways appear to vary for different viruses and are often not well understood (56, 59, 71). Enveloped viruses that fuse their envelopes directly to the plasma membrane appear to use multiple receptors acting in concert or in series to allow the viral proteins to induce fusion (11, 74). Most nonenveloped viruses enter cells by endocytosis, which may be clathrin mediated, caveola mediated, or achieved through clathrin- and caveola-independent uptake mechanisms that include macropinocytosis and other less well defined processes (14, 43, 50, 56, 71). The receptor binding to the virus may simply tether it to the cell, allowing uptake, or there may be a more active process where the receptor induces structural changes in the virus (64). Receptor clustering and intracellular signaling may be required for infection, and some nonenveloped viruses engage multiple receptors which control different steps in the infection process (7, 47). For viruses that enter through endosomes, triggers for membrane penetration to allow infection can include structural changes in the viral proteins induced by receptor binding (64, 80), changes due to the low pH of the endosome (73), or cleavage of viral proteins by the activities of low-pH-dependent endosomal proteases (18).
After endocytosis the virions or their components may traffic through a variety of vesicular compartments before entering the cytoplasm, yet the importance of the endosomal uptake route and the subsequent trafficking of virions or their components are often not well understood. Variables could include the rate of uptake (whether the uptake is clathrin mediated, caveola mediated, or mediated through clathrin- and caveola-independent endosomes [71]), endosomal trafficking to recycling or degradative pathways, and the possible requirement of receptor signaling (24). The association of the receptor or virus with membrane structures of different compositions such as lipid rafts or microdomains may also change the rate and type of endocytosis, the receptor and ligand trafficking, and the membrane fusion or penetration (10, 56, 60). Infecting viruses or their components have been seen to become associated with early endosomes, caveolin-associated endosomes (caveosomes), the endoplasmic reticulum and Golgi compartments, the late endosome or multivesicular endosome, or the lysosome. However, the functional importance of that trafficking for viral infection is generally not well understood.
Canine parvovirus (CPV) and the closely related feline panleukopenia virus (FPV) are small nonenveloped viruses that replicate in the nuclei of cells and depend on cellular S phase for DNA replication. CPV and FPV both use the feline transferrin receptor (TfR) for the binding and infection of feline cells (54), and specific binding of CPV to the canine TfR is associated with the CPV infection of dogs and dog cells, since FPV does not bind that receptor (28). The TfR is a type II membrane protein which is expressed on the surface of cells as a homodimer (19). The structure of the human TfR ectodomain shows that each monomer is made up of a protease-like domain, an apical domain, and a helical domain which forms most of the dimer interface (8, 35). The TfR ectodomain is attached to a 32-residue stalk which holds the receptor about 30 Å above the plasma membrane (19), and the human TfR has a 28-residue transmembrane sequence and a 61-residue cytoplasmic domain (32). Tf binding is determined primarily by the TfR helical domain (9, 21, 87), while CPV and FPV binding to the feline or canine TfRs is controlled by interactions between the apical domain of the TfR and a raised region of the capsid surrounding the threefold axis of icosahedral symmetry (the threefold spike) (27, 52).
The TfR-Tf complex is a model for the endocytosis and recycling of receptor-ligand complexes; it is excluded from lipid raft domains in the plasma membrane and is taken up rapidly from the cell surface via clathrin-mediated endocytosis (44). The TfR is constitutively endocytosed although the rate of recycling is increased after Tf binding, and the TfR and Tf recycle efficiently back to the cell surface through vesicular pathways (20, 25, 40, 65, 69, 81, 82, 86). The TfR-Tf complex passes through the early or sorting endosome and then recycles back to the cell surface in a fast process dependent on Rab4 or more slowly through a perinuclear recycling compartment, which is dependent on Rab11 (25, 69). Each TfR may recycle up to 100 times, and the rate of recycling depends on the oligomeric state of the ligand: monomeric Tf recycles in 4 to 10 min, while 10-mer oligomeric Tf is retained in the endosomal system for 60 min or more, and a proportion of the oligomeric Tf-TfR complex is transported to the lysosome and degraded (39, 93). The uptake and recycling of the TfR depend on sequences in the cytoplasmic domain. The tyrosine in the sequence Tyr-Thr-Arg-Phe (YTRF) within the cytoplasmic sequence of the TfR binds adaptor protein 2 (AP-2) (13) via its μ2 subunit (51), leading to association with the clathrin endocytosis machinery and rapid uptake (29, 34). Mutation or deletion of the YTRF motif in the human TfR reduced the rate of Tf uptake by greater than 80% (13).
Several cell entry and infection steps of CPV capsids have been defined (53, 76-78, 83, 85). CPV capsids bind the TfR on the surface of cells, and in most cases that binding allows entry and infection (28, 54). However, strains of CPV show differences in binding to feline and canine cells, suggesting that some bind additional receptors on some host cells (28). Some combinations of mutant TfR or mutant virus can bind to each other without leading to infection, indicating that the success of the infection process requires specific TfR-capsid interactions (27, 52). The capsids are rapidly endocytosed in a process that can be inhibited by dominant-negative dynamin II mutants or by drugs inhibiting endosomal acidification (53). After cell entry the capsids remain in perinuclear vesicles for up to several hours (53, 78). The capsids also remain functionally associated with the TfR in the endosomes, as it has been shown that infection could be reduced by injecting antibodies binding the cytoplasmic sequences of the TfR 4 h after virus uptake (54). The capsids leave the endosomes by an unknown mechanism, then traffic through the cytoplasm to the nuclear pore, and enter the nucleus for replication (84). Transport from the cytoplasm into the nucleus may occur slowly and require the release of N-terminal sequences of VP1 proteins from within the capsid (77, 84, 85).
Here we use mutant and chimeric feline TfRs to examine their effects on CPV endosomal transport and cell infection. We also prepared chimeras between the binding domains of antiviral antibodies and the feline TfR sequences so as to form receptors with different binding sites that could still bind and endocytose the virus.
MATERIALS AND METHODS
Viruses and cells.
CPV and FPV were prepared from infectious plasmid clones, grown in feline NLFK cells, and titrated in those cells by using 50% tissue culture infective dose (TCID50) assays as previously described (55). Capsids were purified by precipitation with polyethylene glycol 8000 followed by sucrose gradient centrifugation; then they were dialyzed against either phosphate-buffered saline (PBS) or 20 mM Tris-HCl (pH 7.5) and stored at 4°C (1). Capsids and iron-loaded canine Tf (Sigma, St. Louis, Mo.) were labeled with Cy3.5 or Cy5 (Amersham Biosciences, Piscataway, N.J.) as described previously (28). Chinese hamster ovary-derived cells lacking the hamster TfR (TRVb cells) (41) were grown in Ham's F-12 medium containing 5% fetal bovine serum.
TfR clones, mutants, and chimeras.
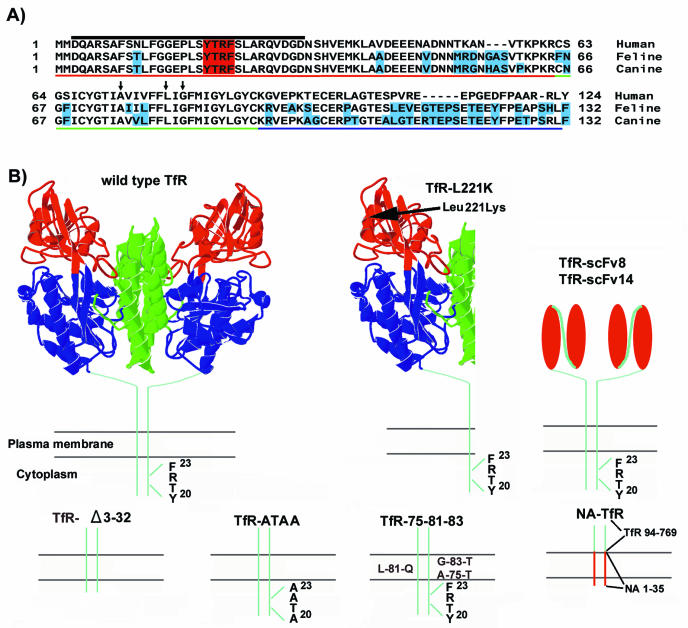
Clones of the cDNA of the feline TfR were prepared in the pcDNA3.1(−) vector (Invitrogen, Carlsbad, Calif.) and expressed under the control of the cytomegalovirus immediate early promoter (28, 54). The cytoplasmic domain sequence of the feline TfR shares features with the cytoplasmic and transmembrane sequences of the human TfR (Fig. 1A). We used the information derived for the human TfR to design mutants of the feline TfR that were likely to alter the receptor endocytosis and infection. The mutant, deleted, and chimeric receptors are diagrammed in Fig. 1B. Mutants or deletions of the feline TfR were prepared directly in the expression plasmid by using the Gene Editor system (Promega, Madison, Wis.). A control mutant feline TfR with residue Leu 221 replaced by Lys (feline TfR L221K) binds CPV but not FPV and does not mediate cell infection (52). Mutants constructed to examine specific receptor endocytosis (TfR-ATAA, TfR-Δ3-32, and TfR-75-81-83) were predicted to affect uptake and trafficking of the Tf and TfR complex (12, 13, 93). The mutant TfR-ATAA had three amino acids in the YTRF internalization motif that were replaced with alanines (Ala-Thr-Ala-Ala), while in the TfR-Δ3-32 mutant residues 3 to 32 were deleted by digesting the feline TfR expression plasmid with XhoI and PshaI and then joining the sites with a DNA adaptor linking codons 2 and 33. Mutant TfR-75-81-83 had three polar residues introduced into the transmembrane sequence in place of Ala 75, Leu 81, and Gly 85, similar to mutants of the human TfR that resulted in increased Tf degradation (93).
FIG. 1.
Mutant or chimeric receptors analyzed in this study and comparison of the TfR sequences mutated. (A) Aligned sequences of the cytoplasmic, transmembrane, and stalk domains of the human, feline, and canine TfRs. The YTRF internalization motif is shaded in red. Differences from the human TfR are shaded in blue for the feline and canine receptors. Red underlining, cytoplasmic sequence; green underlining, transmembrane sequences; blue underlining, stalk sequences. The arrows indicate residues mutated in TfR-75-81-83, and the black line above the cytoplasmic sequences indicates the deleted residues in TfR-Δ3-32. (B) A model of the human TfR ectodomain structure is shown, along with diagrams of the altered regions of the other receptors. In the TfR-Δ3-32 mutant, residues 3 to 32 of the cytoplasmic domain of the feline TfR were deleted. Three residues of the YTRF internalization motif were changed to Ala in TfR-ATAA. In the TfR-75-81-83 mutant, three nonpolar amino acids in the transmembrane domain were replaced by polar residues. Chimeric receptors prepared included an NA-TfR chimera, which had residues 1 to 35 of the influenza virus NA fused to residues 94 to 769 of the TfR, and TfR-scFv8 and TfR-scFv14, which contained residues 1 to 122 of the feline TfR fused to the variable domains derived from MAb 8 and MAb 14, respectively. In the previously described mutant TfR-L221K, a Leu at position 221 was replaced with a lycine; this mutant receptor binds capsids but does not mediate efficient infection (52). The colors in the TfR model indicate the different domains defined for the human receptor as follows: blue, protease-like domain; red, apical domain; and green, helical domain. For the scFv model, the red ovals represent the light and heavy variable domains of the antibodies, and the line connecting those domains indicates the linker peptide added during the production of the single chains.
The NA-TfR was a chimera between the N-terminal cytoplasmic and transmembrane sequences of the influenza WSN virus neuraminidase (NA) and the feline TfR ectodomain and stalk domain. The chimera was constructed by replacing the first 93 amino acids of the feline TfR with the first 35 amino acids of the NA sequence (31, 33).
Two chimeras fusing the feline TfR cytoplasmic tail, transmembrane, and stalk sequences with antibody single-chain variable fragments (scFv) were prepared by linking feline TfR residues 1 to 122 with the heavy and light chain variable domain sequences derived from antiviral monoclonal antibody (MAb) 8 or MAb 14 linked by a poly-Gly-Ser linker sequence (91). MAb 8 and scFv8 react with both CPV and FPV, while MAb 14 and scFv14 react only with CPV (91).
Baculovirus expression.
To prepare the soluble ectodomain of the feline TfR, an N-terminal sequence containing a six-His sequence and a baculovirus gp67 secretion sequence was fused to the feline TfR sequence after residue 120 and expressed in insect cells by using a baculovirus vector as described for the human TfR (36). After the infection of Trichoplusia ni (High Five) cells for 4 days, the culture supernatant was dialyzed against 50 mM Tris-HCl (pH 7.5) with 150 mM NaCl; then the TfR ectodomain was isolated on nickel-nitrilotriacetic acid (NTA) resin (QIAGEN, Valencia, Calif.) and eluted with 100 mM imidazole. The receptor-containing fractions were dialyzed against PBS and stored at 4°C.
Receptor expression and membrane localization.
Receptors were expressed in TRVb cells by the transfection of plasmids with Lipofectamine reagent (Invitrogen) (54). Cell lines stably expressing receptors were prepared by the selection of cells with 400 μg of G418/ml; then cell clones were picked. The surface expression binding of receptors containing the TfR ectodomain was determined by the incubation of the cells with both CPV capsids (10 μg/ml) and Cy5-labeled iron-loaded Tf in Dulbecco's modified Eagle's medium (DMEM) with 1% bovine serum albumin (BSA) for 1 h at 37°C, and then cell-associated capsids were detected by Cy2-labeled MAb 8 after cells were detached with EDTA and fixed with 4% paraformaldehyde (28). Cells expressing the TfR-scFv chimeric receptors were incubated with Cy5-labeled CPV capsids for 1 h at 37°C, and then after detachment and fixation the receptor was detected with MAb H68.4 against the cytoplasmic tail of the TfR (Zymed Laboratories Inc., South San Francisco, Calif.) (54, 88) in PBS containing 0.5% BSA with 0.1% Triton X-100. In all cases the cell-bound virus, Tf, and antibody binding to the TfR were quantified by using a FACScalibur flow cytometer, and the data were analyzed by using Cell Quest software (Becton Dickenson, San Jose, Calif.).
Uptake and intracellular transport of Tf and virus.
Canine Tf was radiolabeled with 125I (Dupont NEN, Boston, Mass.) by using Iodogen-coated beads (Pierce Biotechnology, Rockfort, Ill.), and the unbound iodine was separated from the labeled Tf with a PD10 column (Pharmacia Biotech, Uppsala, Sweden). The monomeric state of the labeled Tf was confirmed by chromatography through a Sephadex 200 column.
To examine the distribution of virus after binding and uptake, stable cell lines expressing the different receptors were incubated with 10 μg of CPV capsids per ml for 1 h at 37°C. After detachment with EDTA, the cells were fixed with 4% paraformaldehyde; then half of the cells were stained with Cy2-labeled MAb 8 in PBS-BSA with 0.5% Triton X-100 for 20 min, and the remainder were stained in PBS-BSA with no detergent. The percentage of virus internalized was estimated by comparing the total cell-associated virus in the Triton X-100-treated cells with that seen in the nonpermeabilized cells.
To examine the rate of Tf uptake from the cell surface, cells transiently transfected and expressing the different receptors were seeded on coverslips and then incubated with 125I-labeled canine Tf on ice for 30 min. The cells were washed two times with cold DMEM-BSA and then transferred to DMEM-BSA at 37°C. After 0, 3, 5, 10, 20, or 30 min, the coverslips were removed, the cells were washed twice with 0.2 M acetic acid in 0.5 M NaCl for 3 min on ice, and then the proportion of the radioactivity that remained cell associated (intracellular) was determined.
Association of the wild-type TfR or NA-TfR chimera with detergent-insoluble membranes.
TRVb cells transiently expressing the feline TfR or NA-TfR were incubated on ice with 125I-labeled canine Tf in DMEM-BSA for 30 min. To disrupt lipid rafts, the cells were preincubated with 5 mM methyl-β-cyclodextrin for 1 h at 37°C and then were incubated with 125I-labeled canine Tf in the same medium. Cells were then washed three times with ice-cold DMEM-BSA and extracted for 10 min on ice with PBS containing 1% Triton X-100 and 2× complete protease inhibitor cocktail (Boehringer, Ingelheim, Germany). The cells were then lysed with 1 M NaOH, and the amount of 125I in the extracted and nonextracted fractions was counted.
Degradation of Tf after endocytosis.
The degradation of Tf bound to the feline TfR or TfR-75-81-83 mutant was measured in an assay similar to that described by Zaliauskiene et al. (93). TRVb cells transiently expressing those receptors were incubated with 125I-labeled canine Tf in DMEM-BSA for 1 h at 37°C, washed twice with cold DMEM-BSA, and then incubated for 2 h at 37°C in DMEM with 0.1% BSA containing 50 μg of unlabeled Tf per ml. The proteins in the medium were then precipitated in 10% trichloroacetic acid (TCA), and the proportion of the released radioactivity that became TCA soluble was determined as a measure of Tf degradation.
Microscopic analysis of virus localization.
TRVb cells were cotransfected with each of the plasmids expressing wild-type or mutant receptors and with a plasmid expressing Rab11 fused to green fluorescent protein (Rab11-GFP). The cells were seeded on coverslips the next day; 24 h later they were incubated with Cy3.5-labeled virus for 30, 60, or 120 min at 37°C, washed in cold PBS, fixed on ice with 4% paraformaldehyde in PBS, and examined by confocal microscopy. Confocal sections collected from the center of the cells were analyzed using the Olympus Fluoview software (Melville, N.Y.).
Infection assays.
TRVb cells transiently expressing the wild-type feline TfR or mutant TfRs or transfected with the empty pcDNA3.1(-) vector were seeded on coverslips and inoculated for 1 h at 37°C with between 0.001 and 1 TCID50 per cell of CPV and then incubated at 37°C for 24 h in growth medium. The cells were then fixed with 4% paraformaldehyde and stained with Cy2-labeled anti-NS1 antibody; the infection rate was determined as the percentage of infected cells expressing each receptor. The transfection rate was determined by the analysis of a parallel culture of the same transfection reaction tested for receptor expression by flow cytometry.
Effect of soluble TfR on infection mediated by nonfunctional receptors.
The TfR-scFv8, TfR-scFv14, and TfR-L221K receptors bound virus to cells but did not mediate infection. To determine whether exogenously added feline TfR might compensate for the lack of correct binding, cells expressing these receptors were inoculated with an excess (10 μg/ml) of virus in the presence of 15, 7.5, 3.7, or 1.25 μg of the baculovirus-expressed feline TfR ectodomain per ml, resulting in molar ratios of capsids to receptor of 1:60, 1:30, 1:15, and 1:5, respectively. Viruses were either mixed with the receptor and added to the cells and incubated at 37°C, or virus was incubated with the cells at 4°C for 30 min; then the soluble receptor was added, and cells were incubated for a further 30 min at 4°C. After 24 h at 37°C, the cells were fixed and stained for NS1 protein expression. To confirm that the soluble ectodomain bound the capsids, the TfR domain was bound to enzyme-linked immunosorbent assay plates in twofold dilutions, and each plate was blocked with PBS-BSA containing 0.05% Tween 20 and then incubated with dilutions of CPV capsids. The virus binding the TfR on the plate was detected with rabbit polyclonal anti-CPV antibody and anti-rabbit immunoglobulin G conjugated to horseradish peroxidase, and bound antibody was detected with 2,2′-azinobis(3-ethylbenzthiazoline sulfonic acid) substrate.
RESULTS
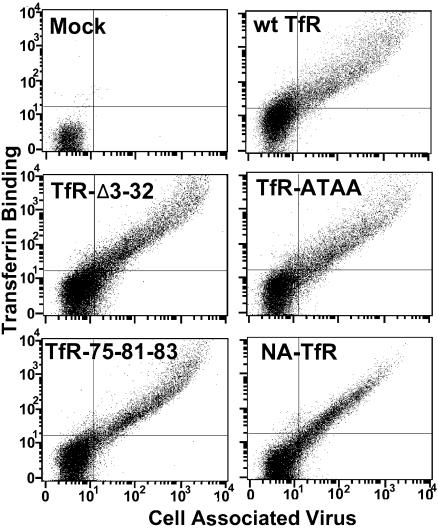
Cell infection by CPV initiates with uptake from the cell surface in association with the TfR, and the capsid then traffics within the endosomal systems of the cell before being released into the cytoplasm for transport to the nucleus. However, it is not known which of the receptor binding or endosomal transport steps are required for mediating viral infection. To start to define the important receptor-mediated events in cell infection, we prepared mutants of the feline TfR which would change three components in the infection process: the rate of endocytic uptake, the membrane domain association, and the extracellular capsid binding domain (Fig. 1). The mutant receptors which contained the TfR ectodomain were transported to the cell surface at levels similar to the level of the wild-type TfR, and all of the receptors bound both Tf and CPV capsids (Fig. 2). The level of expression of the NA-TfR chimera was slightly altered compared to the level of other receptors, as fewer cells showed the highest levels of binding to virus and Tf (Fig. 2), perhaps due to differences in the expression or trafficking of the receptor during synthesis or to altered recycling.
FIG. 2.
Flow cytometric analysis of the binding of CPV capsids and canine Tf to TRVb cells transiently expressing the feline TfR or receptors with altered transmembrane or cytoplasmic sequences. Cells were incubated with Cy5-labeled Tf and CPV together for 1 h at 37°C, and then after detachment from the tissue culture dishes and fixation, the cell-associated CPV was detected with Cy2-labeled MAb 8. y axis, Tf binding; x axis, capsid binding.
Effects of altered rate of uptake.
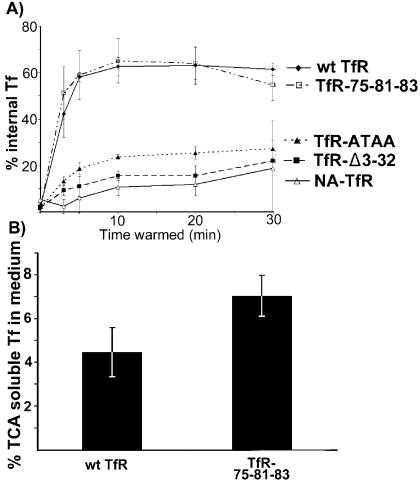
All mutations that changed or removed the tyrosine-based motif in the cytoplasmic domain (TfR-ATAA, TfR-Δ3-32, and NA-TfR) would prevent the association of the receptor with AP-2, and any uptake would be by nonclathrin-mediated uptake. Compared to clathrin-mediated uptake levels, these receptors all had their initial rate of uptake from the cell surface reduced by about 85% (Fig. 3A) and also showed lower levels of steady-state intracellular Tf after 30 min of incubation (Fig. 3B), as was seen for the human TfR with similar changes (13). The reduced internalization confirms the expected phenotypes of the receptors with a reduced endocytosis rate due to a lack of interaction between the receptor and AP-2. However, in the absence of clathrin-mediated uptake, receptors are endocytosed by other mechanisms (14), and these vesicles generally intersect with the same endocytic pathways (15, 30).
FIG. 3.
Distribution and uptake of Tf bound to TRVb cells expressing the wild-type or mutant feline TfRs. (A) Rate of 125I-labeled canine Tf uptake into TRVb cells expressing various mutant receptors. Tf was incubated with the cells on ice, and then after warming to 37°C for various times, the extracellular Tf was removed by acid wash. The data are shown for three independent experiments ±1 standard deviation. (B) Degradation of Tf in TRVb cells expressing TfR-75-81-83 or wild-type TfR. Cells were incubated with 125I-labeled Tf at 37°C for 1 h, washed on ice, and then incubated for a further 2 h at 37°C. The protein released into the supernatant was precipitated by using 10% TCA, and the amounts of TCA-soluble and insoluble radioactivity were counted. Data are for three independent experiments ±1 standard deviation. wt, wild type.
The human TfR with mutations equivalent to those in TfR-75-81-83 showed less efficient recycling (93). When the degradation of 125I-labeled canine Tf endocytosed by that receptor was examined by testing for release of TCA-soluble Tf fragments after 2 h, the cells expressing the TfR-75-81-83 mutant showed a modest increase in TCA-soluble released radioactivity to 7%, compared to 4.5% for the wild-type TfR, although that difference was not statistically significant (Fig. 3B).
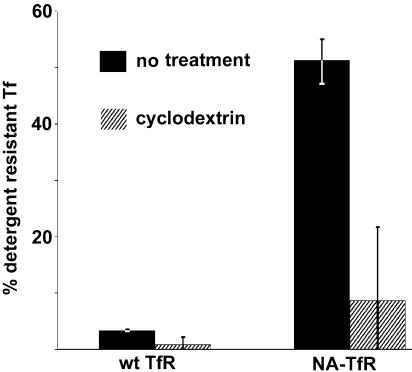
The human TfR is a classical non-raft domain-associated receptor, and feline TfR also proved to be non-raft-associated, as >95% of the 125I-labeled canine Tf bound to the feline TfR could be extracted by 1% Triton X-100 at 0°C, while only ∼50% of the Tf bound to the NA-TfR was extracted under the same conditions (Fig. 4) (4, 33). The resistance to extraction by cold Triton X-100 was largely eliminated by treatment of the cells with the cholesterol-depleting drug methyl-β-cyclodextrin, which reduced the Triton X-100-resistant Tf to around 10%, close to the levels of the wild-type TfR (Fig. 4).
FIG. 4.
Association of wild-type (wt) TfR and NA-TfR with detergent-insoluble lipid rafts. TRVb cells expressing either feline TfR or NA-TfR were incubated with 125I-labeled Tf for 30 min on ice and then extracted with PBS containing 1% Triton X-100. The radioactivity in the extracted supernatant and in the cells was measured. To disrupt lipid rafts the cells were pretreated with 5 mM methyl-β-cyclodextrin prior to incubation with the labeled Tf. The data are shown for three independent experiments. Error bar, ±1 standard deviation.
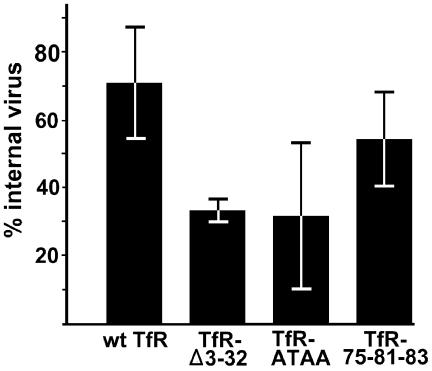
Examining the uptake and endocytosis of virus in TRVb-derived cell lines expressing mutants TfR-Δ3-32, TfR-ATAA, and TfR-75-81-83 showed that the internalization of the virus in 1 h was 70% for the wild-type TfR and 60% for the TfR-75-81-83 mutant, but that level was reduced to ∼35% for the mutants with changes which decreased the rate of Tf uptake (Fig. 5). Multiple attempts failed to generate stable cell lines expressing levels of the TfR-scFv chimeras or the NA-TfR fusion proteins high enough for detection; therefore, this assay could not be performed for these receptors. The reduction in the intracellular virus taken up with the TfR-Δ3-32 and TfR-ATAA mutants was not as great as that seen for the canine Tf (Fig. 3A). This result is likely to be due to the differences in receptor-ligand interaction and recycling in each case, since the Tf binds as a monomer and most of it recycles, while the capsid contains multiple TfR binding sites (possibly up to 60), resulting in receptor clustering that would both alter the endocytosis of the receptor and reduce any recycling to the cell surface (39).
FIG. 5.
Cell surface and intracellular distribution of capsids in TRVb cell lines stably expressing the wild-type (wt) or mutant feline TfR. The cells were incubated for 1 h at 37°C with CPV capsids and then fixed. Cell-associated virus was detected with Cy2-labeled MAb 8 in Triton X-100-permeabilized or nonpermeabilized cells. The ratio of total virus and surface-accessible virus was determined by flow cytometry. Data are for three independent experiments ±1 standard deviation.
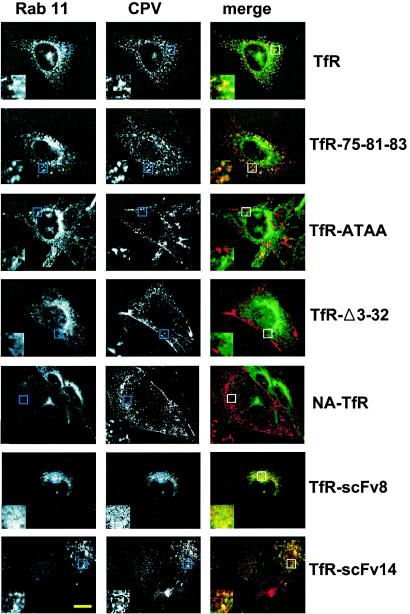
In addition, after incubation for 30 min at 37°C, there were differences in the cellular localization of CPV capsids. In cells expressing the feline TfR and TfR-75-81-83 along with Rab11-GFP, virus and Rab11 were colocalized in perinuclear vesicles (Fig. 6). In contrast, the TfR-Δ3-32, TfR-ATAA, and NA-TfR mutants showed little Rab11 colocalization at that time point, and much of the virus was on the cell surface or in the periphery (Fig. 6). At later times (60 and 120 min) after uptake, some virus endocytosed by TfR-Δ3-32, TfR-ATAA, and NA-TfR was seen to colocalize with Rab11, although most of the capsids were still not in the Rab11 compartment (data not shown).
FIG. 6.
Localization of CPV capsids in TRVb cells expressing the feline TfR or altered receptors and Rab11-GFP (green). Cells were incubated with Cy3.5-labeled capsids (red) for 30 min at 37°C and then fixed and examined by confocal microscopy. One section through the center of the cell body is shown. Scale bar, 10 μm. The inset boxes show threefold enlargements of a portion of each image.
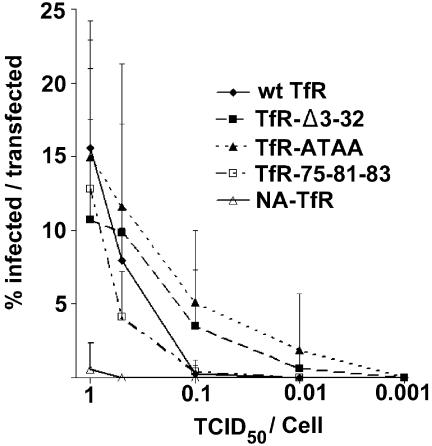
After transient expression in TRVb cells and inoculation with various titers of CPV, the TfR-Δ3-32, TfR-ATAA, and TfR-75-81-83 mutants all allowed CPV infection at levels to similar to the level for the wild-type TfR (Fig. 7). In contrast, cells expressing NA-TfR were only infected to very low levels, with only 4% as many cells infected compared to cells expressing the wild-type TfR (Fig. 7).
FIG. 7.
Infection of TRVb cells expressing altered receptors containing the feline TfR ectodomain. Cells were inoculated with between 0.001 and 1 TCID50 of CPV per cell for 1 h at 37°C and then incubated in growth medium for 24 h at 37°C. The proportion of cells expressing each TfR was determined by flow cytometry in parallel cultures. The data show the percentages of cells expressing the mutant TfR that became infected. Error bars represent data for at least five independent experiments ± standard deviations. wt, wild type.
Antibody binding domain-TfR chimera characterization.
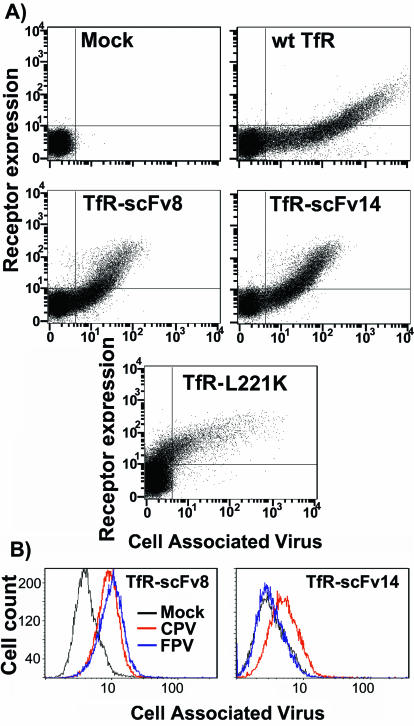
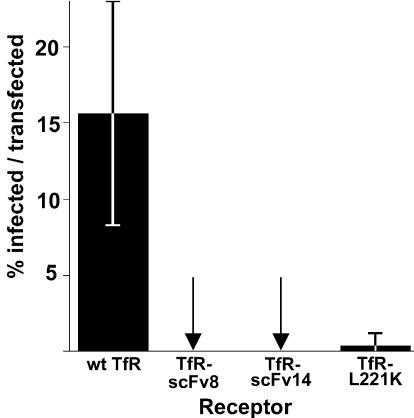
To examine the role of the ligand binding domain of the receptor, we prepared chimeras of the cytoplasmic, transmembrane, and stalk sequences of the feline TfR with the binding domains of two different MAbs, TfR-scFv8 and TfR-scFv14 (Fig. 1B). These MAbs were compared to the wild-type feline TfR and to a feline TfR with a mutation in its apical domain (TfR-L221K) which showed reduced binding to CPV and very low levels of cell infection (52). The TfR-scFv chimeric receptors did not bind Tf, and their expression was therefore determined by staining with an antibody against the TfR cytoplasmic sequence (Fig. 8A). These receptors were all expressed in cells to similar levels, but TfR-scFv8 and TfR-scFv14 both showed lower levels of virus binding compared to the level of the wild-type TfR, indicating that they did not bind with the same affinities. The apical domain mutant of the feline TfR (TfR-L221K) showed CPV binding only when the receptor was expressed at levels 10-fold higher than either the wild type or the TfR-scFvs (Fig. 8A). The antibody detection of the TfR cytoplasmic sequences was not sensitive to the lowest level of receptor, as some cells showed virus binding but did not show receptor expression. These cells must, however, express the receptors, as mock-transfected cells never showed any virus binding (Fig. 8A). MAb 8, which was used to prepare scFv8, bound to both CPV and FPV, while MAb 14, used to prepare scFv14, bound to only CPV and not FPV (91). We therefore tested for the specificity of virus binding, and the TfR-scFv8 bound both CPV and FPV, while TfR-scFv14 bound CPV but not FPV (Fig. 8B). No infections were detected in cells expressing TfR-scFv8 or TfR-scFv14, while only low levels of infection were seen in cells expressing TfR-L221K (Fig. 9); adding soluble feline TfR ectodomain to the inoculations did not change the result (data not shown).
FIG. 8.
Flow cytometric analysis of the binding of virus to cells expressing receptors with altered ectodomains. (A) CPV binding to TRVb cells expressing TfR-scFv8 or TfR-scFv14 or to the control feline TfR-L221K which has a mutation in the apical domain that affects capsid binding and infection. Cells were incubated with Cy5-labeled CPV for 1 h at 37°C and then detached; TfR expression was detected with a MAb against the cytoplasmic tail of the TfR. y axis, receptor expression; x axis, CPV binding; wt, wild type. (B) Specificity of capsid binding to TfR-scFv chimeras. Cloned cell lines expressing TfR-scFv8 or TfR-scFv14 constructs were incubated with 10 μg of CPV or FPV capsids per ml for 1 h at 37°C; after detachment the cells were fixed and permeabilized, and the cell-associated virus was detected with Cy2-labeled MAb 8. Mock, mock-transfected cells.
FIG. 9.
Infection of TRVb cells expressing TfR-scFv8, TfR-scFv14, or TfR-L221K after inoculation with 1 TCID50 per cell of CPV, as described in the legend of Fig. 8. The data are shown for three independent experiments ±1 standard deviation.
DISCUSSION
The process of cell infection is a subject of investigation for many nonenveloped viruses. For the parvoviruses and adeno-associated viruses, many studies have shown that uptake and infection require receptor-mediated endocytosis, but the details of the later steps in the infection pathway and the roles played by the endosomal trafficking and processing of the capsids are less clearly defined. For CPV the infection pathway that uses TfR initiates with rapid uptake from the cell surface, and at later times the CPV capsids are seen in both recycling endosomes and late endosomes, where they colocalize with TfR and Tf or with late endosomal markers such as Lamp2 (53, 78, 83). The endosomal processing of CPV capsids leading to infection can be slow, and the virus is retained in the endosomal system for several hours prior to entering the cytoplasm; thus, antibodies binding the cytoplasmic sequence of the TfR could block many infections by CPV in feline cells even when they are injected 4 h after uptake of the virus (54).
Here we sought to define the cellular controls of cell infection by CPV by taking advantage of the properties of the TfR, anti-CPV antibodies, and CPV capsids to manipulate the receptor binding and endosomal entry steps of the infection pathway. The TfR and mutant receptors were expressed in the Chinese hamster ovary-derived TRVb cells, which normally do not bind any virus and completely resist infection. After transfection with plasmids expressing the TfR, over 20% of the transfected cells were infected by CPV, similar to the levels seen in nonsynchronized feline cells (28).
Here we showed that the rapid clathrin-mediated uptake of the TfR was not required for infection, since the TfR mutants with changes that would affect AP-2 binding still mediated infection to levels similar to the level of the wild-type TfR, despite the slow uptake of the receptors. The results parallel results reported for the uptake of other viruses where alternative modes of endocytosis can allow infection: influenza virus infection can occur via several different entry pathways (72), adenoviruses infect cells by using both clathrin-mediated uptake and macropinocytosis (42, 43), and Moloney murine leukemia virus infection is not affected when clathrin-mediated endocytosis is blocked (37).
The requirements for particular routes of endosomal trafficking of the viruses was not specifically examined in these studies. However, capsids entering through the wild-type TfR became colocalized with the Rab11-positive endosome within 30 min, while capsids entering with the TfR-ATAA and TfR-Δ3-32 mutants did not colocalize with Rab11 at 30 min, although some did colocalize by 60 or 120 min. However, entering the Rab11-positive endosome by itself was not sufficient to ensure cell infection, as both TfR-scFv8 and TfR-scFv14 transported capsids to that compartment but neither mediated infection (Fig. 6 and 9).
The TfR-75-81-83 mutant with additional polar residues in the transmembrane sequence was predicted to be diverted into the late endosomal and lysosomal compartment at a greater rate than the wild type, since for the human TfR with equivalent mutations 8% of the bound Tf became degraded in 1 h, compared to 2% for the wild type (93). We saw only small differences in the processing of TfR-75-81-83, as 7% of the Tf was degraded after uptake compared to 4.5% for the wild type (Fig. 3B). The level of uptake did not affect infection; the mutant receptor still allowed endocytosis of canine Tf and virus, the virus became colocalized with Rab11, and the cells were infected to levels similar to the level of the wild-type receptor.
In contrast to these findings, CPV infection mediated by the NA-TfR mutant was reduced to less than 5% of the level seen for the wild-type TfR (Fig. 9). While the wild-type TfR is a prototype non-raft-associated receptor, the NA sequences caused the TfR to associate with detergent-insoluble lipid rafts (Fig. 4), as predicted from the properties of the NA and from previous studies of the human TfR (4, 32). Lipid rafts are membrane domains enriched in cholesterol and sphingolipids (4, 26, 58). Various viruses can infect cells by using receptors associated with different membrane environments including those associated with lipids rafts, such as caveolae (38, 57). However, the effects of the alterations in membrane association have been examined in only a few studies. The avian sarcoma and leukosis virus can infect cells through either the transmembrane form of the Tva receptor or through a form that becomes glycosylphosphatidylinositol anchored. One study found that the glycosylphosphatidylinositol-anchored receptor became localized to raft domains in the membrane and that the rate of infection was slower but that the same number of infections occurred (46). Endocytic pathways initiating from lipid rafts may deliver their contents to the endoplasmic reticulum or Golgi complexes (45, 48, 49) or to a tubular-vesicular compartment that has been termed the caveosome (57), and some of these pathways intersect with those starting from clathrin-mediated endocytosis (68). The reasons for the lack of infection with the NA-TfR mutant could, therefore, include the possibility that the lipid environment of the receptor is unfavorable for virus penetration from the endosome, that the receptor-capsid complexes are transported to alternative endosomal compartments which do not provide the correct environment for infection, or that other unidentified factors required for CPV infection do not colocalize with the TfR-virus complex in the raft domains. In future studies we will examine these different possible effects to determine which one(s) changes the virus infection of the cell.
Other influences on the endosomal trafficking of CPV by the receptors with altered tails could include increased TfR transport after receptor clustering due to capsid binding, as is seen after oligomeric Tf binding (65), while the phospholipase A2 activity of the VP1-unique region may alter the structure and function of endosomal compartments (16, 92). For other parvoviruses and adeno-associated viruses, the capsids appear to spend various periods of time in late endosomes, recycling endosomes, the Golgi compartment, and perhaps other compartments, but little is known about either the specific endosomal requirements for infection or the ability of the viruses to exploit alternative pathways (2, 6, 17, 63, 66, 67).
Antibody-derived receptor ligands do not allow virus infection.
The TfR-scFv receptors were expressed on cells at levels similar to the level of the wild type TfR, and they bound CPV capsids and transported them into cells where they became colocalized with Rab11-positive compartments, but no cell infection resulted. Both of the antibodies used to prepare the chimeric receptors were neutralizing, and each associated with a binding site on the surface of the capsid in regions that are involved in binding to the canine TfR and, most likely, also to the feline TfR (23, 27, 75). From cryoelectron microscopic studies, MAb 8 is known to bind to the shoulder of the threefold spike, while escape mutant analysis shows that MAb 14 binds near the top of that structure (27). The scFv of each antibody also neutralized CPV, although with an efficiency of 10% of that of the intact immunoglobulin G of the same antibody, but incubating the scFv with the capsids did not prevent CPV binding and uptake into feline cells (91). Changes in the interactions between the receptor and capsid that allowed binding to occur but that did not lead to infection have been seen previously for mutants of both the TfR and the virus (27, 52). For example, CPV and FPV can bind sialic acids on some host cells, but that binding is not required for infection. Indeed, passaging CPV type 2 in feline cells which express sialic acids that bind the capsid selects for viruses that no longer bind the sugar, suggesting that the sialic acid binding inhibits infection (3, 79). Differences in the TfR-scFv interaction with the capsid and that of the feline TfR ectodomain leading to the lack of infection could include binding at too high or low affinity to allow release at the appropriate point in the endocytic pathway or an intrinsic neutralization effect of the scFv, or the scFv may not induce the structural changes in the capsid needed for infection. Less virus was bound to cells expressing similar levels of TfR-scFv compared to cells expressing the wild-type TfR (Fig. 8), suggesting a lower binding affinity to the antibody domains compared to the TfR. Adding exogenous soluble feline TfR to the virus during binding to the cells did not rescue infection of the virus by the TfR-scFv chimeras or the control TfR-L221K, indicating that if any structural effects of the receptor on the capsids were needed for infection, they could not be readily supplied in trans.
Compared to some other viruses, CPV appears fastidious in its receptor requirements for infection. Adeno-associated virus type 2 can use a variety of carbohydrate or glycoprotein receptors or capsid-receptor cross-linking systems to mediate cell transduction (5, 22, 61, 70, 89, 90). Foot and mouth disease virus can infect through an antiviral scFv fused to intracellular adhesion molecule 1 (62). In contrast, poliovirus requires the poliovirus receptor for infection, and binding to the N-terminal domain of the receptor leads to the changes in the capsid structure required for infection, including the loss of the VP4 protein and exposure of hydrophobic portions of VP1 (80).
These studies provide new insights into the importance of receptor binding, endocytic uptake mechanisms, and the intracellular trafficking of CPV and into their roles in mediating infection. In future studies we will examine in detail the steps of viral infection revealed by these altered receptors, focusing on the structural role of the receptor on the capsid and its role in infection, the alternative trafficking of the capsids within the cells after capsid entry, and the effects of the NA-TfR chimera on infection through the localization of capsids in alternative compartments such as the caveosome or the endoplasmic reticulum.
Acknowledgments
Wendy Weichert and Gail Sullivan provided excellent technical support. We thank Craig Roy and Yoshihiro Kawaoka for providing plasmids.
This study was supported by grants AI 28385 and AI 33468 from the National Institutes of Health to C.R.P.
REFERENCES
- 1.Agbandje, M., R. McKenna, M. G. Rossmann, M. L. Strassheim, and C. R. Parrish. 1993. Structure determination of feline panleukopenia virus empty particles. Proteins 16:155-171. [DOI] [PubMed] [Google Scholar]
- 2.Bantel-Schaal, U., B. Hub, and J. Kartenbeck. 2002. Endocytosis of adeno-associated virus type 5 leads to accumulation of virus particles in the Golgi compartment. J. Virol. 76:2340-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbis, D. P., S.-F. Chang, and C. R. Parrish. 1992. Mutations adjacent to the dimple of canine parvovirus capsid structure affect sialic acid binding. Virology 191:301-308. [DOI] [PubMed] [Google Scholar]
- 4.Barman, S., and D. P. Nayak. 2000. Analysis of the transmembrane domain of influenza virus neuraminidase, a type II transmembrane glycoprotein, for apical sorting and raft association. J. Virol. 74:6538-6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartlett, J. S., J. Kleinschmidt, R. C. Boucher, and R. J. Samulski. 1999. Targeted adeno-associated virus vector transduction of nonpermissive cells mediated by a bispecific F(ab′gamma)2 antibody. Nat. Biotechnol. 17:181-186. [DOI] [PubMed] [Google Scholar]
- 6.Bartlett, J. S., R. Wilcher, and R. J. Samulski. 2000. Infectious entry pathway of adeno-associated virus and adeno-associated virus vectors. J. Virol. 74:2777-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barton, E. S., J. L. Connolly, J. C. Forrest, J. D. Chappell, and T. S. Dermody. 2001. Utilization of sialic acid as a coreceptor enhances reovirus attachment by multistep adhesion strengthening. J. Biol. Chem. 276:2200-2211. [DOI] [PubMed] [Google Scholar]
- 8.Bennett, M. J., J. A. Lebron, and P. J. Bjorkman. 2000. Crystal structure of the hereditary haemochromatosis protein HFE complexed with transferrin receptor. Nature 403:46-53. [DOI] [PubMed] [Google Scholar]
- 9.Buchegger, F., I. S. Trowbridge, L. F. Liu, S. White, and J. F. Collawn. 1996. Functional analysis of human/chicken transferrin receptor chimeras indicates that the carboxy-terminal region is important for ligand binding. Eur. J. Biochem. 235:9-17. [DOI] [PubMed] [Google Scholar]
- 10.Chazal, N., and D. Gerlier. 2003. Virus entry, assembly, budding, and membrane rafts. Microbiol. Mol. Biol. Rev. 67:226-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clapham, P. R., and A. McKnight. 2002. Cell surface receptors, virus entry and tropism of primate lentiviruses. J. Gen. Virol. 83:1809-1829. [DOI] [PubMed] [Google Scholar]
- 12.Collawn, J. F., A. Lai, D. Domingo, M. Fitch, S. Hatton, and I. S. Trowbridge. 1993. YTRF is the conserved internalization signal of the transferrin receptor, and a second YTRF signal at position 31-34 enhances endocytosis. J. Biol. Chem. 268:21686-21692. [PubMed] [Google Scholar]
- 13.Collawn, J. F., M. Stangel, L. A. Kuhn, V. Esekogwu, S. Q. Jing, I. S. Trowbridge, and J. A. Tainer. 1990. Transferrin receptor internalization sequence YXRF implicates a tight turn as the structural recognition motif for endocytosis. Cell 63:1061-1072. [DOI] [PubMed] [Google Scholar]
- 14.Conner, S. D., and S. L. Schmid. 2003. Regulated portals of entry into the cell. Nature 422:37-44. [DOI] [PubMed] [Google Scholar]
- 15.Dautry-Versat, A. 2001. Clathrin-independent endocytosis, p. 26-57. In M. Marsh (ed.), Endocytosis. Oxford University Press, New York, N.Y.
- 16.de Figueiredo, P., A. Doody, R. S. Polizotto, D. Drecktrah, S. Wood, M. Banta, M. S. Strang, and W. J. Brown. 2001. Inhibition of transferrin recycling and endosome tubulation by phospholipase A2 antagonists. J. Biol. Chem. 276:47361-47370. [DOI] [PubMed] [Google Scholar]
- 17.Douar, A. M., K. Poulard, D. Stockholm, and O. Danos. 2001. Intracellular trafficking of adeno-associated virus vectors: routing to the late endosomal compartment and proteasome degradation. J. Virol. 75:1824-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ebert, D. H., J. Deussing, C. Peters, and T. S. Dermody. 2002. Cathepsin L and cathepsin B mediate reovirus disassembly in murine fibroblast cells. J. Biol. Chem. 277:24609-24617. [DOI] [PubMed] [Google Scholar]
- 19.Fuchs, H., U. Lucken, R. Tauber, A. Engel, and R. Gessner. 1998. Structural model of phospholipid-reconstituted human transferrin receptor derived by electron microscopy. Structure 6:1235-1243. [DOI] [PubMed] [Google Scholar]
- 20.Gagescu, R., N. Demaurex, R. G. Parton, W. Hunziker, L. A. Huber, and J. Gruenberg. 2000. The recycling endosome of Madin-Darby canine kidney cells is a mildly acidic compartment rich in raft components. Mol. Biol. Cell 11:2775-2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giannetti, A. M., P. M. Snow, O. Zak, and P. J. Bjorkman. 2003. Mechanism for multiple ligand recognition by the human transferrin receptor. Public Lib. Sci. Biol. 1:E51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Girod, A., M. Ried, C. Wobus, H. Lahm, K. Leike, J. Kleinschmidt, G. Deleage, and M. Hallek. 1999. Genetic capsid modifications allow efficient re-targeting of adeno-associated virus type 2. Nat. Med. 5:1052-1056. [DOI] [PubMed] [Google Scholar]
- 23.Govindasamy, L., K. Hueffer, C. R. Parrish, and M. Agbandje-McKenna. 2003. Structures of host range-controlling regions of the capsids of canine and feline parvoviruses and mutants. J. Virol. 77:12211-12221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greber, U. F. 2002. Signalling in viral entry. Cell. Mol. Life Sci. 59:608-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hao, M., and F. R. Maxfield. 2000. Characterization of rapid membrane internalization and recycling. J. Biol. Chem. 275:15279-15286. [DOI] [PubMed] [Google Scholar]
- 26.Harder, T. 2003. Formation of functional cell membrane domains: the interplay of lipid- and protein-mediated interactions. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 358:863-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hueffer, K., L. Govindasamy, M. Agbandje-McKenna, and C. R. Parrish. 2003. Combinations of two capsid regions controlling canine host range determine canine transferrin receptor binding by canine and feline parvoviruses. J. Virol. 77:10099-10105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hueffer, K., J. S. Parker, W. S. Weichert, R. E. Geisel, J. Y. Sgro, and C. R. Parrish. 2003. The natural host range shift and subsequent evolution of canine parvovirus resulted from virus-specific binding to the canine transferrin receptor. J. Virol. 77:1718-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jing, S. Q., T. Spencer, K. Miller, C. Hopkins, and I. S. Trowbridge. 1990. Role of the human transferrin receptor cytoplasmic domain in endocytosis: localization of a specific signal sequence for internalization. J. Cell Biol. 110:283-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johannes, L., and C. Lamaze. 2002. Clathrin-dependent or not: is it still the question? Traffic 3:443-451. [DOI] [PubMed] [Google Scholar]
- 31.Kundu, A., R. T. Avalos, C. M. Sanderson, and D. P. Nayak. 1996. Transmembrane domain of influenza virus neuraminidase, a type II protein, possesses an apical sorting signal in polarized MDCK cells. J. Virol. 70:6508-6515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kundu, A., M. A. Jabbar, and D. P. Nayak. 1991. Cell surface transport, oligomerization, and endocytosis of chimeric type II glycoproteins: role of cytoplasmic and anchor domains. Mol. Cell. Biol. 11:2675-2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kundu, A., and D. P. Nayak. 1994. Analysis of the signals for polarized transport of influenza virus (A/WSN/33) neuraminidase and human transferrin receptor, type II transmembrane proteins. J. Virol. 68:1812-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lafer, E. M. 2002. Clathrin-protein interactions. Traffic 3:513-520. [DOI] [PubMed] [Google Scholar]
- 35.Lawrence, C. M., S. Ray, M. Babyonyshev, R. Galluser, D. W. Borhani, and S. C. Harrison. 1999. Crystal structure of the ectodomain of human transferrin receptor. Science 286:779-782. [DOI] [PubMed] [Google Scholar]
- 36.Lebron, J. A., M. J. Bennett, D. E. Vaughn, A. J. Chirino, P. M. Snow, G. A. Mintier, J. N. Feder, and P. J. Bjorkman. 1998. Crystal structure of the hemochromatosis protein HFE and characterization of its interaction with transferrin receptor. Cell 93:111-123. [DOI] [PubMed] [Google Scholar]
- 37.Lee, S., Y. Zhao, and W. F. Anderson. 1999. Receptor-mediated Moloney murine leukemia virus entry can occur independently of the clathrin-coated-pit-mediated endocytic pathway. J. Virol. 73:5994-6005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marjomaki, V., V. Pietiainen, H. Matilainen, P. Upla, J. Ivaska, L. Nissinen, H. Reunanen, P. Huttunen, T. Hyypia, and J. Heino. 2002. Internalization of echovirus 1 in caveolae. J. Virol. 76:1856-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marsh, E. W., P. L. Leopold, N. L. Jones, and F. R. Maxfield. 1995. Oligomerized transferrin receptors are selectively retained by a lumenal sorting signal in a long-lived endocytic recycling compartment. J. Cell Biol. 129:1509-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCaffrey, M. W., A. Bielli, G. Cantalupo, S. Mora, V. Roberti, M. Santillo, F. Drummond, and C. Bucci. 2001. Rab4 affects both recycling and degradative endosomal trafficking. FEBS Lett. 495:21-30. [DOI] [PubMed] [Google Scholar]
- 41.McGraw, T. E., L. Greenfield, and F. R. Maxfield. 1987. Functional expression of the human transferrin receptor cDNA in Chinese hamster ovary cells deficient in endogenous transferrin receptor. J. Cell Biol. 105:207-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meier, O., K. Boucke, S. V. Hammer, S. Keller, R. P. Stidwill, S. Hemmi, and U. F. Greber. 2002. Adenovirus triggers macropinocytosis and endosomal leakage together with its clathrin-mediated uptake. J. Cell Biol. 158:1119-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meier, O., and U. F. Greber. 2003. Adenovirus endocytosis. J. Gene Med. 5:451-462. [DOI] [PubMed] [Google Scholar]
- 44.Mellman, I. 1996. Endocytosis and molecular sorting. Annu. Rev. Cell Dev. Biol. 12:575-625. [DOI] [PubMed] [Google Scholar]
- 45.Nabi, I. R., and P. U. Le. 2003. Caveolae/raft-dependent endocytosis. J. Cell Biol. 161:673-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Narayan, S., R. J. Barnard, and J. A. Young. 2003. Two retroviral entry pathways distinguished by lipid raft association of the viral receptor and differences in viral infectivity. J. Virol. 77:1977-1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nemerow, G. R. 2000. Cell receptors involved in adenovirus entry. Virology 274:1-4. [DOI] [PubMed] [Google Scholar]
- 48.Nichols, B. J. 2002. A distinct class of endosome mediates clathrin-independent endocytosis to the Golgi complex. Nat. Cell Biol. 4:374-378. [DOI] [PubMed] [Google Scholar]
- 49.Nichols, B. J., A. K. Kenworthy, R. S. Polishchuk, R. Lodge, T. H. Roberts, K. Hirschberg, R. D. Phair, and J. Lippincott-Schwartz. 2001. Rapid cycling of lipid raft markers between the cell surface and Golgi complex. J. Cell Biol. 153:529-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nichols, B. J., and J. Lippincott-Schwartz. 2001. Endocytosis without clathrin coats. Trends Cell Biol. 11:406-412. [DOI] [PubMed] [Google Scholar]
- 51.Owen, D. J., and P. R. Evans. 1998. A structural explanation for the recognition of tyrosine-based endocytotic signals. Science 282:1327-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Palermo, L. M., K. Hueffer, and C. R. Parrish. 2003. Residues in the apical domain of the feline and canine transferrin receptors control host-specific binding and cell infection of canine and feline parvoviruses. J. Virol. 77:8915-8923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parker, J. S., and C. R. Parrish. 2000. Cellular uptake and infection by canine parvovirus involves rapid dynamin-regulated clathrin-mediated endocytosis, followed by slower intracellular trafficking. J. Virol. 74:1919-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Parker, J. S. L., W. J. Murphy, D. Wang, S. J. O'Brien, and C. R. Parrish. 2001. Canine and feline parvoviruses can use human or feline transferrin receptors to bind, enter, and infect cells. J. Virol. 75:3896-3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parker, J. S. L., and C. R. Parrish. 1997. Canine parvovirus host range is determined by the specific conformation of an additional region of the capsid. J. Virol. 71:9214-9222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pelkmans, L., and A. Helenius. 2003. Insider information: what viruses tell us about endocytosis. Curr. Opin. Cell Biol. 15:414-422. [DOI] [PubMed] [Google Scholar]
- 57.Pelkmans, L., J. Kartenbeck, and A. Helenius. 2001. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat. Cell Biol. 3:473-483. [DOI] [PubMed] [Google Scholar]
- 58.Pike, L. J. 2003. Lipid rafts: bringing order to chaos. J. Lipid Res. 44:655-667. [DOI] [PubMed] [Google Scholar]
- 59.Poranen, M. M., R. Daugelavicius, and D. H. Bamford. 2002. Common principles in viral entry. Annu. Rev. Microbiol. 56:521-538. [DOI] [PubMed] [Google Scholar]
- 60.Rawat, S. S., M. Viard, S. A. Gallo, A. Rein, R. Blumenthal, and A. Puri. 2003. Modulation of entry of enveloped viruses by cholesterol and sphingolipids. Mol. Membr. Biol. 20:243-254. [DOI] [PubMed] [Google Scholar]
- 61.Ried, M. U., A. Girod, K. Leike, H. Buning, and M. Hallek. 2002. Adeno-associated virus capsids displaying immunoglobulin-binding domains permit antibody-mediated vector retargeting to specific cell surface receptors. J. Virol. 76:4559-4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rieder, E., A. Berinstein, B. Baxt, A. Kang, and P. W. Mason. 1996. Propagation of an attenuated virus by design: engineering a novel receptor for a noninfectious foot-and-mouth disease virus. Proc. Natl. Acad. Sci. USA 93:10428-10433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ros, C., C. J. Burckhardt, and C. Kempf. 2002. Cytoplasmic trafficking of minute virus of mice: low-pH requirement, routing to late endosomes, and proteasome interaction. J. Virol. 76:12634-12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rossmann, M. G., J. Bella, P. R. Kolatkar, Y. He, E. Wimmer, R. J. Kuhn, and T. S. Baker. 2000. Cell recognition and entry by rhino- and enteroviruses. Virology 269:239-247. [DOI] [PubMed] [Google Scholar]
- 65.Sainte-Marie, J., V. Lafont, E. I. Pecheur, J. Favero, J. R. Philippot, and A. Bienvenue. 1997. Transferrin receptor functions as a signal-transduction molecule for its own recycling via increases in the internal Ca2+ concentration. Eur. J. Biochem. 250:689-697. [DOI] [PubMed] [Google Scholar]
- 66.Sanlioglu, S., P. K. Benson, J. Yang, E. M. Atkinson, T. Reynolds, and J. F. Engelhardt. 2000. Endocytosis and nuclear trafficking of adeno-associated virus type 2 are controlled by Rac1 and phosphatidylinositol-3 kinase activation. J. Virol. 74:9184-9196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Seisenberger, G., M. U. Ried, T. Endress, H. Buning, M. Hallek, and C. Brauchle. 2001. Real-time single-molecule imaging of the infection pathway of an adeno-associated virus. Science 294:1929-1932. [DOI] [PubMed] [Google Scholar]
- 68.Sharma, D. K., A. Choudhury, R. D. Singh, C. L. Wheatley, D. L. Marks, and R. E. Pagano. 2003. Glycosphingolipids internalized via caveolar-related endocytosis rapidly merge with the clathrin pathway in early endosomes and form microdomains for recycling. J. Biol. Chem. 278:7564-7572. [DOI] [PubMed] [Google Scholar]
- 69.Sheff, D. R., E. A. Daro, M. Hull, and I. Mellman. 1999. The receptor recycling pathway contains two distinct populations of early endosomes with different sorting functions. J. Cell Biol. 145:123-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shi, W., and J. S. Bartlett. 2003. RGD inclusion in VP3 provides adeno-associated virus type 2 (AAV2)-based vectors with a heparan sulfate-independent cell entry mechanism. Mol. Ther. 7:515-525. [DOI] [PubMed] [Google Scholar]
- 71.Sieczkarski, S. B., and G. R. Whittaker. 2002. Dissecting virus entry via endocytosis. J. Gen. Virol. 83:1535-1545. [DOI] [PubMed] [Google Scholar]
- 72.Sieczkarski, S. B., and G. R. Whittaker. 2002. Influenza virus can enter and infect cells in the absence of clathrin-mediated endocytosis. J. Virol. 76:10455-10464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Skehel, J. J., T. Bizebard, P. A. Bullough, F. M. Hughson, M. Knossow, D. A. Steinhauer, S. A. Wharton, and D. C. Wiley. 1995. Membrane fusion by influenza hemagglutinin. Cold Spring Harbor Symp. Quant. Biol. 60:573-580. [DOI] [PubMed] [Google Scholar]
- 74.Spear, P. G., R. J. Eisenberg, and G. H. Cohen. 2000. Three classes of cell surface receptors for alphaherpesvirus entry. Virology 275:1-8. [DOI] [PubMed] [Google Scholar]
- 75.Strassheim, L. S., A. Gruenberg, P. Veijalainen, J.-Y. Sgro, and C. R. Parrish. 1994. Two dominant neutralizing antigenic determinants of canine parvovirus are found on the threefold spike of the virus capsid. Virology 198:175-184. [DOI] [PubMed] [Google Scholar]
- 76.Suikkanen, S., T. Aaltonen, M. Nevalainen, O. Valilehto, L. Lindholm, M. Vuento, and M. Vihinen-Ranta. 2003. Exploitation of microtubule cytoskeleton and dynein during parvoviral traffic toward the nucleus. J. Virol. 77:10270-10279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Suikkanen, S., M. Antila, A. Jaatinen, M. Vihinen-Ranta, and M. Vuento. 2003. Release of canine parvovirus from endocytic vesicles. Virology 316:267-280. [DOI] [PubMed] [Google Scholar]
- 78.Suikkanen, S., K. Saajarvi, J. Hirsimaki, O. Valilehto, H. Reunanen, M. Vihinen-Ranta, and M. Vuento. 2002. Role of recycling endosomes and lysosomes in dynein-dependent entry of canine parvovirus. J. Virol. 76:4401-4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tresnan, D. B., L. Southard, W. Weichert, J. Y. Sgro, and C. R. Parrish. 1995. Analysis of the cell and erythrocyte binding activities of the dimple and canyon regions of the canine parvovirus capsid. Virology 211:123-132. [DOI] [PubMed] [Google Scholar]
- 80.Tsang, S. K., B. M. McDermott, V. R. Racaniello, and J. M. Hogle. 2001. Kinetic analysis of the effect of poliovirus receptor on viral uncoating: the receptor as a catalyst. J. Virol. 75:4984-4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.van Dam, E. M., and W. Stoorvogel. 2002. Dynamin-dependent transferrin receptor recycling by endosome-derived clathrin-coated vesicles. Mol. Biol. Cell 13:169-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.van Dam, E. M., T. Ten Broeke, K. Jansen, P. Spijkers, and W. Stoorvogel. 2002. Endocytosed transferrin receptors recycle via distinct dynamin and phosphatidylinositol 3-kinase-dependent pathways. J. Biol. Chem. 277:48876-48883. [DOI] [PubMed] [Google Scholar]
- 83.Vihinen-Ranta, M., A. Kalela, P. Makinen, L. Kakkola, V. Marjomaki, and M. Vuento. 1998. Intracellular route of canine parvovirus entry. J. Virol. 72:802-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vihinen-Ranta, M., D. Wang, W. S. Weichert, and C. R. Parrish. 2002. The VP1 N-terminal sequence of canine parvovirus affects nuclear transport of capsids and efficient cell infection. J. Virol. 76:1884-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vihinen-Ranta, M., W. Yuan, and C. R. Parrish. 2000. Cytoplasmic trafficking of the canine parvovirus capsid and its role in infection and nuclear transport. J. Virol. 74:4853-4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang, X., R. Kumar, J. Navarre, J. E. Casanova, and J. R. Goldenring. 2000. Regulation of vesicle trafficking in Madin-Darby canine kidney cells by Rab11a and Rab25. J. Biol. Chem. 275:29138-29146. [DOI] [PubMed] [Google Scholar]
- 87.West, A. P., Jr., A. M. Giannetti, A. B. Herr, M. J. Bennett, J. S. Nangiana, J. R. Pierce, L. P. Weiner, P. M. Snow, and P. J. Bjorkman. 2001. Mutational analysis of the transferrin receptor reveals overlapping HFE and transferrin binding sites. J. Mol. Biol. 313:385-397. [DOI] [PubMed] [Google Scholar]
- 88.White, S., K. Miller, C. Hopkins, and I. S. Trowbridge. 1992. Monoclonal antibodies against defined epitopes of the human transferrin receptor cytoplasmic tail. Biochim. Biophys. Acta 1136:28-34. [DOI] [PubMed] [Google Scholar]
- 89.Wu, P., W. Xiao, T. Conlon, J. Hughes, M. Agbandje-McKenna, T. Ferkol, T. Flotte, and N. Muzyczka. 2000. Mutational analysis of the adeno-associated virus type 2 (AAV2) capsid gene and construction of AAV2 vectors with altered tropism. J. Virol. 74:8635-8647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang, Q., M. Mamounas, G. Yu, S. Kennedy, B. Leaker, J. Merson, F. Wong-Staal, M. Yu, and J. R. Barber. 1998. Development of novel cell surface CD34-targeted recombinant adenoassociated virus vectors for gene therapy. Hum. Gene Ther. 9:1929-1937. [DOI] [PubMed] [Google Scholar]
- 91.Yuan, W., and C. R. Parrish. 2000. Comparison of two single-chain antibodies that neutralize canine parvovirus: analysis of an antibody-combining site and mechanisms of neutralization. Virology 269:471-480. [DOI] [PubMed] [Google Scholar]
- 92.Zadori, Z., J. Szelei, M.-C. Lacoste, P. Raymond, M. Allaire, I. R. Nabi, and P. Tijssen. 2001. A viral phospholipase A2 is required for parvovirus infectivity. Dev. Cell 1:291-302. [DOI] [PubMed] [Google Scholar]
- 93.Zaliauskiene, L., S. Kang, C. G. Brouillette, J. Lebowitz, R. B. Arani, and J. F. Collawn. 2000. Down-regulation of cell surface receptors is modulated by polar residues within the transmembrane domain. Mol. Biol. Cell 11:2643-2655. [DOI] [PMC free article] [PubMed] [Google Scholar]