Abstract
The p10 fusion-associated small transmembrane protein of avian reovirus induces extensive syncytium formation in transfected cells. Here we show that p10-induced cell-cell fusion is restricted by rapid degradation of the majority of newly synthesized p10. The small ectodomain of p10 targets the protein for degradation following p10 insertion into an early membrane compartment. Paradoxically, conservative amino acid substitutions in the p10 ectodomain hydrophobic patch that eliminate fusion activity also increase p10 stability. The small amount of p10 that escapes intracellular degradation accumulates at the cell surface in a relatively stable form, where it mediates cell-cell fusion as a late-stage event in the virus replication cycle. The unusual relationship between a nonstructural viral membrane fusion protein and the replication cycle of a nonenveloped virus has apparently contributed to the evolution of a novel mechanism for restricting the extent of virus-induced cell-cell fusion.
Membrane fusion is essential to a wide array of biological processes (21, 25, 54, 56). Since inappropriate fusion of membranes would have severe adverse consequences, the process of membrane fusion must by tightly regulated. For vesicle transport within cells, the proteins involved in mediating vesicle fusion are ubiquitously and stably expressed, and the timing and the specificity of vesicle fusion are controlled by complex interactions among a diversity of soluble and membrane-bound proteins (4, 34, 38). Conversely, extracellular fusion of cell membranes, such as that which occurs during the formation of multinucleated myotubes, syncytiotrophoblasts, and osteoclasts (16, 50, 54), is regulated in large part by the differentiation- or tissue-specific expression of candidate cellular fusion proteins (24, 48, 35).
Enveloped virus entry into cells is also dependent on membrane fusion mediated by multimeric membrane fusion proteins embedded in the virus envelope (21, 55). The fusion activity of these viral fusion proteins must be regulated within cells during virus assembly to prevent aberrant fusion of cell membranes. In addition, the activity of the multimeric fusion proteins in the context of the mature, extracellular virus particle is regulated to ensure the correct timing and location of fusion of the virus envelope with a cell membrane during the entry process. Regulation is achieved through a series of complex conformational changes in the fusion proteins. Viral fusion proteins are frequently synthesized as precursors that must be proteolytically cleaved to allow assembly into fusion-incompetent, multimeric complexes during virus assembly (39). In addition, structural rearrangements of metastable, multimeric fusion proteins induced by environmental triggers, such as low endosomal pH and/or receptor binding, regulate fusion activity during virus entry (12, 19, 46, 49).
The fusogenic reoviruses are one of the few groups of nonenveloped viruses capable of inducing cell-cell fusion and polykaryon formation (10). The nonenveloped avian reovirus (ARV) and Nelson Bay reovirus (NBV) encode homologous 10-kDa proteins (p10) that induce syncytium formation when expressed in transfected or virus-infected cells (41). The p10 fusion-associated small transmembrane (FAST) protein is the smallest known viral membrane fusion protein and contains a central transmembrane (TM) domain that separates small, approximately equally sized (about 40 residues) ectodomains and endodomains (41). Since nonenveloped viruses lack a lipid membrane and do not enter cells through membrane fusion, p10 is not a component of virus particles; therefore, unlike that of enveloped viruses, the p10 membrane fusion protein has no role in reovirus entry. Rather, p10 is expressed in virus-infected cells, where it is trafficked to the cell surface in a type I topology to mediate cell-cell fusion and the induction of syncytium formation, a relatively late-stage event in the virus replication cycle (11, 41). In addition to providing direct cell-cell transmission of the virus, syncytium formation contributes to a more rapid lytic response and a more rapid rate of virus release, possibly due to an inherent membrane-destabilizing activity of p10 (2, 11).
In view of the toxic nature of p10-induced syncytium formation (i.e., the membrane integrity of syncytial cells is compromised), it seems clear that the rate and the extent of syncytium formation must be restricted in order to allow sufficient time for progeny virus assembly. However, p10 expression is not temporally regulated in virus-infected cells and follows the same general time course as the expression of other reovirus proteins (42). Furthermore, p10 is a promiscuous fusogen (i.e., it induces cell-cell fusion in a wide range of cell types) and functions at neutral pH; therefore, there is no evidence for the activation of p10 fusion activity by a specific trigger, such as low pH or receptor binding. Here we show that p10-induced syncytium formation is restricted by the rapid degradation of p10, thereby delaying the stable accumulation of p10 at the cell surface in quantities sufficient to initiate and propagate polykaryon formation. It would appear that the unusual role of p10 in the replication cycle of a nonenveloped virus has contributed to a novel means of restricting cell-cell fusion.
MATERIALS AND METHODS
Cells.
Continuous quail cell line QM5 was cultured as previously described (9). Mouse hybridoma cells producing 12CA5 monoclonal antibodies to hemagglutinin (HA) were grown in RPMI 1640 containing 10% fetal bovine serum and penicillin-streptomycin.
Materials.
The anti-HA immunoglobulin G2b kappa-chain [IgG2b(κ)] 12CA5 antibodies were produced in-house from mouse hybridoma cells, and the cell supernatant (approximately 0.12 μg of anti-HA immunoglobulin/μl) was used either directly for surface staining or following concentration (to approximately 1.6 μg/μl) by precipitation with 35% ammonium sulfate. Fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit IgG antibodies and protein G-agarose were obtained from Life Technologies Inc. Streptavidin-conjugated Texas red, biotin-conjugated rat anti-mouse IgG, and mouse anticalnexin antibodies were purchased from Sigma-Aldrich, St. Louis, Mo. A rabbit polyclonal anti-p10 antiserum specific for residues 19 to 34 of p10 was a kind gift from Richard Mulligan (Harvard University). A rabbit polyclonal antiserum raised against the C-terminal endodomain was previously described (41).
Cloning and transfection.
The eukaryotic expression vector pcDNA3 (Invitrogen) was used for the expression of p10 and its derivatives. The construction of the amino-terminal double-HA-tagged p10 expression construct (p10-2HAN) and the generation of all p10 substitutions by three-primer PCR were previously described (41). The p10 ectodomain construct (p10e) was amplified by a single “touchdown” PCR with a forward primer corresponding to the N terminus of HA-tagged p10. The primer contained the sequence encoding the signal peptide of influenza virus HA with a signal peptidase cleavage site (MLTIIALSYIFCLALGQ). The sequences of all constructs were confirmed. Plasmids were transfected into cells by using Lipofectamine (Life Technologies) as previously described (41).
Immunofluorescence staining.
For double labeling of p10-transfected cells with anti-p10 antiserum and anti-calnexin antibodies, cells were fixed with methanol and preblocked with normal goat serum and whole rat IgG before being incubated with rabbit anti-p10 antiserum (1:400) and mouse anti-calnexin antibodies (1:1,000). Bound antibodies were visualized by using FITC-conjugated goat anti-rabbit antibodies (1:400) and biotin-conjugated rat anti-mouse IgG (1:1,000) plus streptavidin-conjugated Texas red (1:2,000). Stained cells were washed extensively and visualized by using a Zeiss LSM510 scanning argon laser confocal microscope and a ×100 objective.
To examine the stability of surface-localized p10, cells transfected with p10-2HAN were incubated on ice for 30 min at 36 h posttransfection to slow cell metabolism and then were incubated for 30 min on ice with a 1:20 dilution of concentrated anti-HA antibodies. Cells were washed extensively with cold Hanks' balanced salt solution and then incubated at 37°C in complete medium 199 for various times (0, 45, or 90 min). Cells were reacted with FITC-labeled anti-mouse antibodies on ice for 30 min to detect the anti-HA antibodies that remained on the surface of cells. Cells were washed as described above and fixed with 4% paraformaldehyde prior to visualization and photography with appropriate filter sets. Low levels of surface-localized p10 confounded the quantification of p10 surface fluorescence by fluorescence-activated cell sorting analysis. Therefore, p10 stability at the cell surface was qualitatively assessed by fluorescence microscopy by capturing fluorescence images over time under identical capture parameters.
Immunoprecipitation.
Radiolabeled cells were lysed in 1× final lysis (radioimmunoprecipitation [RIPA]) buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 1 μg of aprotinin/ml, 1 μg of leupeptin/ml, 1 μg of pepstatin/ml). For immunoprecipitation of cell lysates under harsh lysis conditions (6), 2× final lysis buffer containing 0.1% sodium dodecyl sulfate (SDS) was used. For every 2 × 106 cells, 4 μl of concentrated anti-HA antibodies was preincubated with 15 μl of protein G-agarose for 1 h with shaking at room temperature. Antibody-protein G-agarose complexes were washed once with 1× lysis buffer and incubated with the cell lysates for 1 h at room temperature; these steps were followed by two stringent washes with each of the following: 1× lysis buffer, high-salt buffer (50 mM Tris-HCl [pH 7.5], 500 mM NaCl, 0.1% Nonidet P-40, 0.05% sodium deoxycholate), and low-salt buffer (50 mM Tris-HCl [pH 7.5], 0.1% Nonidet P-40, 0.05% sodium deoxycholate). Immune complexes were analyzed by SDS-polyacrylamide gel electrophoresis (PAGE) and fluorography with 15% acrylamide gels as previously described (8).
Pulse-chase analysis.
Transfected cells were prelabeled for 30 min with methionine-free Dulbecco minimal essential medium at 30 h posttransfection, pulse-labeled for 15 min with the same medium containing 75 μCi of [35S]methionine/ml, and chased for different times with complete medium 199 containing 10% fetal bovine serum. Samples were analyzed by immunoprecipitation, SDS-PAGE, and fluorography. Captured images were scanned, and pixels corresponding to p10 were quantified by using Scion software to determine the levels of p10 after various chase times relative to those in the pulse-labeled p10 sample. For assessment of the effects of proteasome or lysosome inhibitors, E64C, chloroquine, methylamine, and MG132 were added at final concentrations of 200 μM, 0.5 mM, 15 mM, and 100 μM, respectively, to all incubations. For assessment of the stability of p10 proteins resident in membranes, cells were pulse-chased as described above and then fractionated into membrane and soluble fractions. The membrane fraction was stripped of peripheral membrane proteins by treatment with sodium carbonate as previously described (41) prior to solubilization with 1× final lysis buffer and immunoprecipitation.
RESULTS
The ectodomain targets p10 for rapid and extensive degradation.
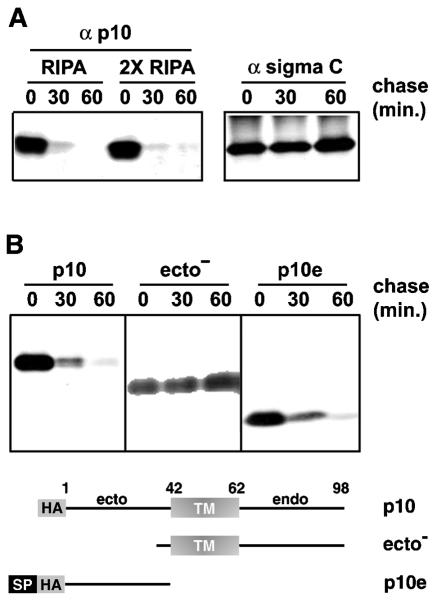
Nonsyncytial constructs of p10 were previously created by site-specific substitutions of the ectodomains or the endodomains (41). While determining whether the loss of fusion activity displayed by these modified p10 constructs reflected increased p10 instability, we made the surprising discovery that functional, HA-tagged p10 was rapidly degraded inside cells. Pulse-chase analysis demonstrated that the vast majority of newly synthesized p10 was degraded within 30 min of synthesis (Fig. 1A). Sigma C, a soluble structural protein of ARV, remained stable under identical conditions. The levels of p10 over time showed similar patterns under both mild and stringent solubilization conditions (Fig. 1A), suggesting that the loss of p10 reflected degradation and not aggresome formation (26). Rapid p10 degradation was not due to overexpression, since the levels of p10 expression attained in transfected cells were comparable to the levels attained in virus-infected cells (41). Since both N- and C-terminally tagged p10 constructs were functional and displayed the same degradation profiles (data not shown), rapid degradation was not attributable to the epitope tag but appeared to be an inherent feature of p10.
FIG. 1.
The ectodomain targets p10 for rapid degradation. (A) (Left) Transfected cells expressing full-length p10-2HAN were pulse-labeled and chased for the indicated times in minutes. Radiolabeled cells were solubilized in RIPA buffer or double-strength RIPA buffer, and p10 was immunoprecipitated with anti-p10 antiserum (α p10), resolved by SDS-PAGE, and visualized by fluorography. (Right) Same analysis with a control protein, the soluble sigma C protein of avian reovirus. (B) Full-length p10-2HAN (p10), p10ecto− (ecto−), or p10e, with two N-terminal HA epitope tags (HA) and the cleavable HA signal peptide (SP), were expressed in transfected cells. The stability of the various p10 constructs was assessed by pulse-chase analysis as described for panel A. The nature of the various constructs is diagrammed at the bottom.
The p10 protein is an integral membrane protein and resides exclusively in the membrane fraction of virus-infected and p10-transfected cells (41, 43, 44). Although p10 lacks an N-terminal signal peptide for membrane insertion, it utilizes a central TM domain as an internal signal-anchor sequence to direct an N exoplasmic/C cytoplasmic membrane topology (41). Accordingly, deletions in the p10 ectodomain do not alter p10 localization to the membrane fraction of cells (41). However, deletion of the majority of the p10 ectodomain (p10ecto−) stabilized the protein, eliminating the rapid degradation profile observed for the full-length protein (Fig. 1B). The p10ecto− protein still resided exclusively in the membrane fraction of cells (data not shown). These results suggested that the ectodomain contributes to p10 instability directly rather than indirectly by altering p10 membrane localization. To determine whether the ectodomain alone was responsible for the short half-life of p10, we expressed the soluble HA-tagged p10e construct in transfected cells. Since p10e lacked the natural p10 signal-anchor sequence (i.e., the TM domain), a cleavable signal sequence of influenza virus HA was added to the N terminus of p10e for translocation of the ectodomain into the lumen of the endoplasmic reticulum (ER), where it normally resides. Pulse-chase analysis revealed that the soluble, and presumably luminal, p10 ectodomain displayed the same rapid degradation profile as authentic p10 (Fig. 1B), further indicating that determinants in the p10 ectodomain target the protein for rapid degradation.
Hydrophobic and thiol residues in the ectodomain influence p10 degradation.
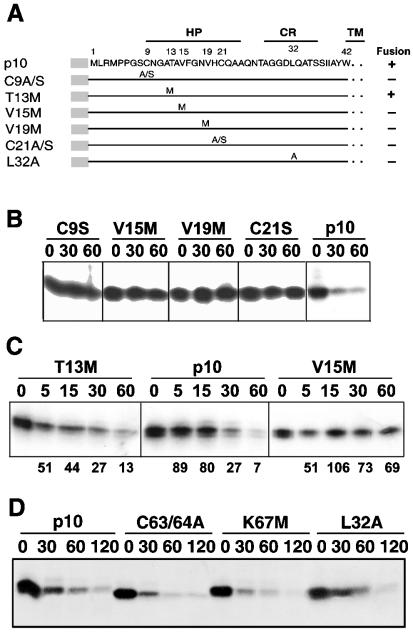
Shmulevitz and Duncan previously identified in the ectodomain of p10 a small, moderately hydrophobic patch (HP) that is flanked by two conserved cysteine residues (residues 9 to 24) (Fig. 2A) and that is important for p10-induced polykaryon formation (41). Since exposed hydrophobic or thiol residues can serve as recognition signals for the quality control degradation machinery (14, 15, 57), we examined the stability of p10 constructs containing point substitutions in the p10 HP (Fig. 2A).
FIG. 2.
Residues in the p10 HP influence p10 degradation. (A) Sequence and schematic representation of the p10 ectodomain. Numbers refer to amino acid residues. The locations of the HP, the CR in the ARV and NBV p10 proteins, and the start of the downstream TM are indicated. The locations of various site-specific substitutions in the ARV p10-2HAN construct are indicated. Constructs are named by using the single-letter amino acid code to indicate the identity of the authentic amino acid, its position, and the identity of the substitution. A/S indicates a substitution with either Ala or Ser. (B) Degradation rates for authentic p10-2HAN (p10) and constructs containing single amino acid substitutions, assessed by pulse-chase analysis (chase times are indicated in minutes) as described in the legend to Fig. 1. (C) Time course analysis of p10 degradation performed as described for panel B but with more precise time points (in minutes) to compare authentic p10-2HAN (p10) to two p10 constructs containing single residue substitutions; one of these constructs retains fusion activity (T13M), and one is nonfusogenic (V15M). Numbers below the gel lanes indicate the percentages of p10 detected after various chase times relative to that in the pulse-labeled sample with no chase, as determined by image analysis of the fluorogram. (D) Degradation rates for p10-2HAN (p10) and constructs containing single amino acid substitutions in the endodomain dicysteine (C63/64A) or polybasic (K67M) motifs or in the ectodomain conserved region (L32A), assessed as described for panel B.
Previous results indicated that both conserved ectodomain cysteine residues are essential for p10 fusion activity (41). Replacement of either cysteine residue with alanine or serine (C9A/S or C21A/S) eliminated both p10 fusion activity and rapid p10 degradation (Fig. 2A and B). To examine possible hydrophobic effects, both valine residues in the p10 HP were individually replaced with methionine residues (other than tryptophan, with its much bulkier side group, methionine introduced the smallest incremental decrease in the hydrophobicity of the p10 HP, from 0.285 to 0.256) (13). In spite of the subtle change in hydrophobicity and the relatively minor alteration in side group volumes imparted by these amino acid changes, the V15M and V19M substitutions had the same effect as the cysteine substitutions, eliminating p10-induced syncytium formation and rapid p10 degradation (Fig. 2A and B). The nonfusogenic property and increased stability of the p10 proteins containing the cysteine and valine substitutions did not reflect altered p10 membrane interactions, since all constructs assumed the correct surface-localized N exoplasmic/C cytoplasmic membrane topology (44).
A more detailed quantitative analysis indicated that less than 30% of authentic HA-tagged p10 remained 30 min after synthesis, and only 5 to 10% remained following a 60-min chase, yielding a p10 half-life of approximately 20 to 25 min (Fig. 2C). Longer chases indicated that the residual 5 to 10% of p10 that escaped rapid degradation persisted for an additional 2 h or more (Fig. 2D). In contrast, decreased degradation of the V15M construct resulted in approximately 70% of the protein persisting for more than 1 to 2 h (Fig. 2C). A functional, fusogenic threonine-methionine substitution construct, T13M, was also examined to probe the potential influence of altered hydrophobicity versus altered side chain volume on the degradation properties of the p10 HP. The degradation profile of T13M was generally the same as that of authentic p10, with the possible exception of a slightly more rapid initial rate of p10 degradation (Fig. 2C). Therefore, methionine substitutions of either of the two aliphatic residues in the p10 HP eliminated p10 degradation.
The ability of substitutions to alter p10 stability was restricted to the HP. As expected from the results obtained with the p10ecto− and p10e constructs, which suggested that the ectodomain alone is responsible for p10 degradation, alterations in the dicysteine motif (C63/64A) or the conserved basic region (K67M) present in the p10 endodomain had no effect on p10 stability (Fig. 2D). On the membrane-proximal side of the p10 HP resides a nine-residue region that is completely conserved between the ARV and the NBV p10 proteins (Fig. 2A, CR [conserved region]) The importance of this relatively polar region for p10 function has not been determined. A substitution (L32A) that significantly decreased the hydrophobicity of the CR (from 0.146 to 0.093) also decreased the rate of p10 degradation (Fig. 2D), although this effect was far less pronounced than that observed with the more subtle alterations in the aliphatic residues in the p10 HP. Therefore, changes in the structure and/or relative hydrophobicity of the p10 ectodomain have profound effects on p10 degradation.
Degradation of p10 occurs following p10 insertion into an early membrane compartment.
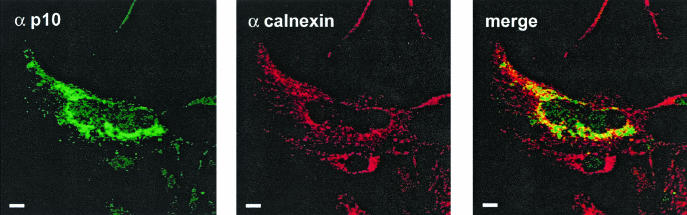
Proteins can be targeted for degradation by misfolding in the cytoplasm, by failure to properly translocate across the ER membrane, or following successful ER translocation (14, 15, 57). To determine which of these pathways might contribute to p10 degradation, confocal microscopy was used to examine the p10 subcellular distribution. Immunofluorescence staining with anti-p10 antisera revealed a punctate, reticular staining pattern (Fig. 3) similar to that observed when cells were stained with antisera specific for calnexin, an ER membrane-localized chaperone (51). An overlay of the two images revealed significant colocalization of p10 and calnexin (Fig. 3), indicating that the majority of p10 localizes, at least transiently, in the ER. Along with previous results indicating that p10-induced syncytium formation is inhibited by the vesicle transport inhibitor brefeldin A (11) and that p10 resides exclusively as an integral membrane protein (41), the colocalization data suggested that p10 is targeted to the ER membrane and trafficked through the secretory pathway to the cell surface.
FIG. 3.
p10 localizes to the ER. (Left) Cells transfected with a p10 expression plasmid were fixed and permeabilized, and the subcellular distribution of p10 was examined by confocal microscopy with anti-p10 polyclonal antiserum (α p10) and FITC-conjugated secondary antibody. (Middle) A similar analysis was performed with anticalnexin antibody (α calnexin) and Texas red-conjugated secondary antibody to reveal the distribution of an ER-localized protein. (Right) Merge of the p10 and calnexin panels, showing extensive colocalization of the two proteins. Scale bars, 10 μm.
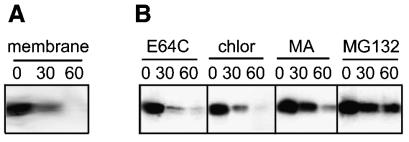
To determine whether p10 degradation reflected inefficient signal-anchor-mediated insertion of p10 into membranes, the stability of membrane-associated p10 was assessed by pulse-chase analysis. Following the pulse or each chase period, the membrane fraction was isolated and treated with high-pH carbonate buffer to removal peripheral membrane proteins. Subsequent gel analysis therefore would monitor the fate and relative stability of p10 molecules already present as integral membrane proteins. The p10 proteins resident in the membrane displayed the characteristic rapid degradation profile (Fig. 4A), indicating that p10 is degraded following insertion into the membrane.
FIG. 4.
Degradation of p10 occurs following membrane insertion and is altered by a proteasome inhibitor. (A) Pulse-chase analysis for the indicated times (in minutes) was performed with p10-transfected cells as described in the legend to Fig. 1. After the pulse or each chase, the membrane fraction was isolated, and the presence of membrane-associated p10 was determined by immunoprecipitation, SDS-PAGE, and fluorography. (B) Pulse-chase analysis for the indicated times (in minutes) was performed with p10-transfected cells as described in the legend to Fig. 1, except that cells were incubated during the pulse-chase in the presence of inhibitors of lysosomal proteases (E64C) or lysosome acidification (chloroquine [chlor] or methylamine [MA]) or a proteasome inhibitor (MG132).
Protein degradation following successful membrane translocation can occur through trafficking to the lysosome, retrieval from the ER, and targeting to the proteasome or through retrieval from the plasma membrane and targeting to the lysosome or proteasome (22, 47). The membrane-permeable proteasome inhibitor MG132 (31) significantly reduced the rate of p10 degradation (Fig. 4B). Since peptide aldehyde inhibitors of the proteasome may also inhibit certain lysosomal proteases (31, 37), we examined the effects of inhibitors of lysosome acidification (chloroquine and methylamine) or lysosomal proteases (E64C) on p10 degradation. None of these lysosome inhibitors reversed the rapid degradation of p10 (Fig. 4B), suggesting that p10 degradation might be proteasome mediated.
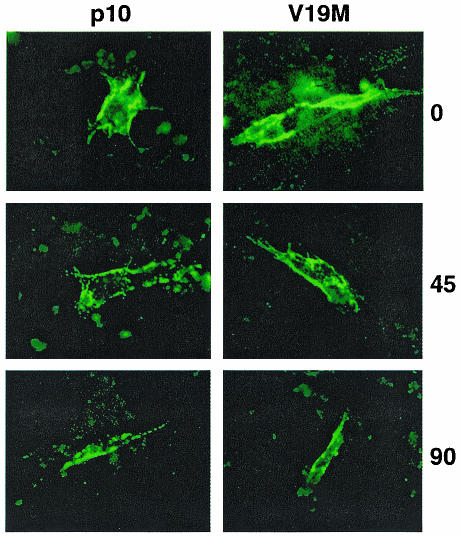
To determine whether p10 is retrieved for degradation before or after transport to the plasma membrane, the stability of surface-localized HA-tagged p10 was assessed. Relatively low levels of p10 surface expression confounded attempts to quantify p10 surface expression by flow cytometry (data not shown). Therefore, p10 surface expression was qualitatively assessed by immunofluorescence microscopy. HA-specific antiserum was added to live p10-transfected cells, which then were incubated for various times under optimal cell growth conditions prior to fixation as described in Materials and Methods. Fluorescence images were captured under identical parameters such that the extent of fluorescence was a relative indicator of p10 surface expression. In contrast to the extremely rapid degradation observed for membrane-associated p10 (Fig. 4A), the quantities of surface-localized, antibody-bound p10 remained relatively constant over the 90-min chase period following antibody binding (Fig. 5). Consequently, p10 appears to be relatively stable once expressed at the cell surface. In conjunction with the fact that p10 degradation begins within 5 min of its synthesis (Fig. 2C), these results were consistent with p10 degradation occurring following retrieval from an early membrane compartment.
FIG. 5.
Surface-localized p10 is relatively stable. The stabilities of surface-localized p10-2HAN (p10) and the V19M construct were assessed by surface staining of live transfected cells with anti-HA monoclonal antibody. Following antibody addition, the presence of surface-bound antibody specifically attached to the HA-tagged, surface-localized p10 ectodomain was monitored over time (indicated in minutes on the right). Fluorescence images were captured under identical parameters for comparison of the intensities of fluorescence.
Increased p10 stability leads to increased polykaryon formation.
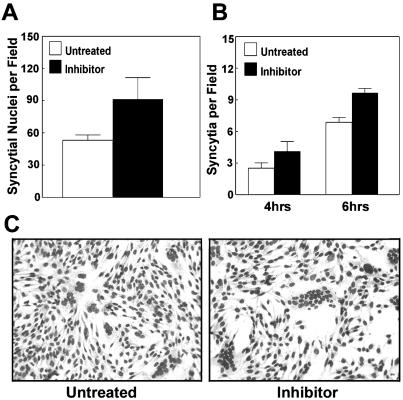
It seemed likely that the degradation of p10 would serve to restrict the rate of syncytium formation. If this hypothesis were correct, then increasing p10 stability should lead to an increased rate of syncytium formation. All of the substitutions that prolonged the p10 half-life also eliminated the fusogenic activity of the protein (Fig. 2A). We therefore used a proteasome inhibitor to decrease the extent of p10 degradation and monitored the effects on the rate and extent of cell-cell fusion over time by microscopic examination of Giemsa-stained monolayers (Fig. 6). The total number of nuclei present in syncytia in random microscopic fields was increased by approximately 70% following 6 h of treatment with the inhibitor (Fig. 6A). This effect was manifested by a modest increase in both the number of syncytial foci (Fig. 6B) and the relative size of individual foci (Fig. 6C). The correlation between decreased p10 degradation and increased syncytium formation suggested that rapid and extensive degradation of p10 might represent a significant factor regulating p10-induced cell-cell fusion.
FIG. 6.
Inhibition of proteasome activity leads to enhanced p10-mediated syncytium formation. Cell monolayers were transfected with a p10 expression plasmid. At 15 h posttransfection, as syncytia were beginning to appear, the culture medium was removed and replaced with medium lacking or containing the proteasome inhibitor MG132. Treated monolayers were incubated for a further 4 to 6 h prior to Giemsa staining to reveal syncytial foci. Microscopic examination was used to quantify the average number of syncytial nuclei per field at 6 h posttreatment (A) or the average number of syncytia per field at 4 and 6 h posttreatment (B). Results are presented as the mean and standard deviation of triplicate samples from a representative experiment. The numbers and sizes of the syncytia present in Giemsa-stained monolayers at 6 h posttreatment were captured by light microscopy at a magnification of ×194 (C).
DISCUSSION
Extensive degradation restricts p10-induced syncytium formation.
Previous studies indicated that the time course of p10 expression in ARV-infected cells follows a pattern similar to that of other reovirus proteins, suggesting little, if any, temporal control of syncytium formation (11, 41). However, syncytium formation is regulated, in part, by inefficient translation of p10 from a suboptimal translation start site (30, 42). Here we show that the majority of p10 is rapidly degraded shortly after synthesis and before reaching the plasma membrane. The net effect of extensive p10 degradation would be a delay in the rate of p10-induced syncytium formation. We assume that the small percentage of p10 that escapes degradation following membrane insertion successively transits the ER-Golgi apparatus transport pathway to slowly accumulate in a relatively stable state at the plasma membrane until some minimal threshold level is exceeded and cell-cell fusion ensues. Although protein degradation influences the activities of numerous cellular regulatory proteins (5, 27), as far as we are aware, this is the only example in which the cellular degradation machinery is exploited to regulate cell-cell fusion induced by a membrane fusion protein.
p10 degradation occurs following membrane insertion.
The ER-associated degradation (ERAD) pathway is an essential component of the cellular quality control machinery involved in monitoring protein folding and maturation (14, 15, 23, 32, 47, 57). Our present results suggest the possible involvement of the ERAD pathway in mediating p10 degradation. This speculation is supported by the following evidence: (i) degradation of p10 follows insertion of the protein into the membrane fraction of cells (Fig. 4A); (ii) colocalization studies indicated that p10 associates, at least transiently, with the ER (Fig. 3); (iii) targeting of the soluble p10 ectodomain to the lumen of the ER by using a cleavable HA signal sequence resulted in rapid degradation of this ectodomain construct (Fig. 1); (iv) pulse-chase analysis indicated that p10 degradation begins within 5 min of its synthesis (Fig. 2C); and (v) p10 molecules in the plasma membrane are relatively stable (Fig. 5). All of these results imply that p10 degradation involves an early membrane compartment, most likely the ER. According to this model, p10 degradation would reflect retrieval of p10 from the ER membrane and targeting to the proteasome, a hypothesis consistent with the ability of a proteasome inhibitor to decrease p10 degradation (Fig. 4B) and increase p10-induced syncytium formation (Fig. 6). Since the soluble, and presumably luminal, p10e construct was also rapidly degraded, retrieval of this polypeptide from the ER lumen would have to occur, possibly by retrotranslocation, as suggested for other luminal proteins degraded by the ERAD pathway (47). Additional studies are required to conclusively determine the roles, if any, of ER retrieval, ubiquitination, and the proteasome in p10 degradation.
Ectodomain signals in p10 target the protein for degradation.
Although many of the steps in the ERAD pathway have been elucidated, the specific signals that target proteins for rapid degradation are less clear. Two such signals are exposed hydrophobic or thiol residues, which may serve as general indicators of aberrant protein folding or failure to achieve a final native structure (15). Exposed hydrophobic or thiol residues are recognized by ER-resident molecular chaperones or protein disulfide isomerase (3, 17, 18, 20, 36). Interactions with these luminal ER proteins involved in facilitating protein folding can lead to protein retention in the ER and subsequent degradation.
Mutational analysis clearly revealed that the ectodomain of p10 serves as a signal for rapid degradation, somewhat analogous to a degron (53), specifically imparting a short half-life to the protein. The basis for this activity is still unclear, although there was a correlation between p10 degradation and the relative hydrophobicity of the ectodomain, primarily in the HP and to a lesser extent in the adjacent CR. For example, slight decreases in the relative hydrophobicity of the HP (e.g., V15M and V19M) decreased p10 degradation, while increasing hydrophobicity (T13M) had no such effect on preventing p10 degradation and, if anything, resulted in a slightly more rapid initial rate of p10 degradation. Since the methionine substitutions could exert their influence through means other than changes in hydrophobicity, more extensive mutagenic and structural analyses are required to more clearly define the roles of specific residues in p10 degradation.
The two ectodomain cysteine residues that are essential for p10 fusion activity are also important determinants of the p10 half-life. Similar to the situation with the valine substitutions, substitution of either C9 or C21 eliminates p10 fusion activity (41) while, as we have now shown, simultaneously extending the p10 half-life. These cysteines do not participate in the formation of an intermolecular disulfide bond, since p10 exhibits the same relative gel mobilities under reducing and nonreducing conditions (44). The cysteines may, however, contribute to the formation of a disulfide-stabilized loop structure through the formation of an intramolecular disulfide bond. If the p10 HP does exist as a cysteine noose structure, then substitution of either cysteine residue would be expected to significantly alter the structure of the p10 ectodomain, thereby affecting protein degradation.
How does the ectodomain contribute to p10 degradation?
Generally, mutations causing a loss of the native structure would be expected to increase degradation by the cellular quality control machinery (14, 15, 57). Paradoxically, ectodomain mutations that eliminate p10 fusion activity lead to a decrease in p10 degradation. What, then, might be the basis for p10 degradation and the effects of ectodomain substitutions on this process? Since misfolded proteins are targeted for degradation, the majority of p10 may fail to achieve the native structure and be targeted for degradation. More than 50% of proteins carrying N-terminal degrons are degraded cotranslationally, suggesting that nascent protein folding may occur in kinetic competition with cotranslational degradation pathways (52). This is not the case with p10, since full-length p10 is synthesized and inserted into the ER membrane prior to degradation, indicating that p10 degradation is a posttranslational event.
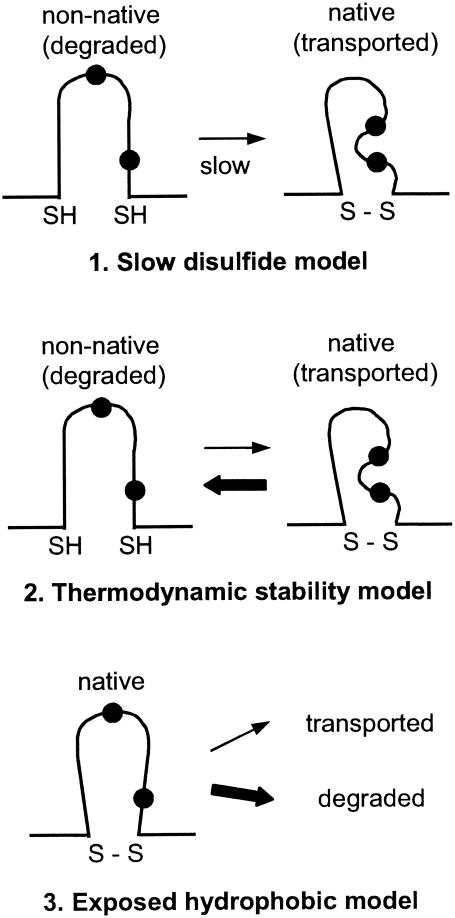
Inefficient formation of the predicted intramolecular disulfide bond is one conceivable cause of posttranslational misfolding of p10 (Fig. 7, model 1). Disulfide bond formation is known to be a slow process (40). Delayed formation of the predicted p10 cysteine noose structure would lead to exposed thiols and possibly hydrophobic residues in the nonnative structure, targeting p10 for ER retention and subsequent export to the cytoplasm for degradation (17). Inhibition of the proteasome would provide sufficient time for increased formation of the predicted cysteine noose and native p10 structure, a prediction in accord with the observed enhanced syncytium formation in the presence of proteasome inhibitors (Fig. 6). According to the slow disulfide bond formation model, the V15M and V19M substitutions should promote more efficient disulfide bond formation, thereby decreasing p10 degradation. Hydrophobic forces driving the valine residues to the interior of the loop structure could interfere with local residue packing and constrain disulfide bond formation (18). The less hydrophobic methionine residue more easily orients toward the solvent phase, a conformation which might allow tighter packing of the loop structure, increased disulfide bond formation, and decreased p10 degradation. However, the stabilizing effect of the cysteine substitutions appears to be at odds with this model, since the removal of one cysteine would guarantee the presence of a free thiol group. Possible explanations for this anomaly include sequestration of the free thiol within the nonnative structure or removal of the thiol by aberrant intermolecular disulfide bond formation between p10 molecules or with luminal ER proteins. However, such aberrant disulfide bond formation generally leads to increased protein degradation (7, 33, 36), which is not the case with the p10 cysteine substitutions.
FIG. 7.
Models of p10 degradation. Three possible models to explain the influence of the p10 ectodomain and the HP on p10 degradation by the ERAD pathway are shown. The first model implies that the slow formation of an intramolecular disulfide bond (S—S) leads to exposed thiol (SH) or hydrophobic residues (filled circles) in the nonnative structure which target p10 for degradation. The second model implies that it is not the rate of folding of the p10 ectodomain but rather a low thermodynamic stability of the native structure that leads to transient and repeated exposure of thiol or hydrophobic residues. The third model implies that hydrophobic residues are naturally surface exposed in the native p10 ectodomain structure, leading to recognition and degradation of the majority of p10.
A thermodynamic model of p10 degradation predicts that the p10 ectodomain may have a low free-energy barrier between native and nonnative conformations, with structural fluctuations in the p10 HP leading to reversible disulfide bond formation or exposure of hydrophobic residues and p10 degradation (Fig. 7, model 2). This model of protein degradation was derived from studies of bovine pancreatic trypsin inhibitor and single-chain T-cell receptor (28, 29, 45), where thermodynamic stability rather than the rate of protein folding correlates with protein secretion efficiency. The small size of the p10 ectodomain suggests that there may be minimal differences in free energy between the native and the nonnative states. In this scenario, the V15M and V19M substitutions would either increase the stability of the “native” ectodomain structure (while paradoxically eliminating the fusion activity of p10) or decrease the hydrophobic signal that targets p10 for degradation. Once again, one would have to explain how the cysteine substitutions promote a more stable “native” structure and decrease p10 degradation while eliminating p10-induced syncytium formation.
A third possibility is that native p10, rather than a misfolded population of p10, is targeted for degradation. While the exposure of hydrophobic residues may serve as a general indicator of aberrant protein folding, the relative surface hydrophobicity of the native protein correlates with the protein half-life and may be necessary for ubiquitination (1, 3). In this model, the stable native structure of the p10 ectodomain would contain solvent-exposed hydrophobic residues leading to recognition and degradation of the majority of p10 by the ERAD pathway (Fig. 7, model 3). The lack of complete fidelity of the quality control machinery or the extent of p10 surface hydrophobicity would allow a small percentage of p10 to escape degradation and slowly accumulate at the cell surface to induce cell-cell fusion. Methionine substitutions would reduce the surface hydrophobicity to below the level required for recognition by the cellular quality control machinery, while cysteine substitutions, by removing the predicted loop structure, could allow exposed hydrophobic residues to more easily bury themselves within a more compact or aggregated nonnative structure. As with the other models, how the free thiol created by a single cysteine substitution escapes recognition is not clear.
Further studies of p10 degradation and a structural determination of the p10 ectodomain are required to distinguish among these and other possible models of the basis for the ectodomain-mediated degradation of p10 and the relationship, if any, between p10 degradation and the mechanism of p10-induced membrane fusion.
Why would p10 evolve to be rapidly degraded?
The ARV p10 protein contributes to a loss of membrane integrity late in the virus replication cycle and is therefore toxic to cells (2). The p10 ectodomain may have evolved purely as a means to limit the rate of accumulation of p10, thereby delaying syncytium formation and membrane leakiness to allow sufficient time for progeny virus assembly.
A more intriguing possibility to explain the degradation properties of the p10 HP was derived from recent studies indicating that synthetic peptides based on the HP induce lipid mixing in liposome fusion assays (44). These results suggested that the p10 HP may function as a fusion peptide, analogous to the fusion peptides present in enveloped virus fusion proteins. Reversible or constitutive solvent exposure of hydrophobic residues may be a requirement for the fusion peptide activity of the p10 HP. Therefore, hydrophobic effects may serve a dual function in p10: as an essential feature of the membrane fusion reaction and as a means to delay syncytium formation by targeting the majority of p10 for degradation. The inherent or readily reversible exposure of hydrophobic residues in the predicted p10 fusion peptide is not observed with the enveloped virus fusion peptides that are stably sequestered within the tertiary structure of the multimeric fusion protein until triggered conformational changes lead to their exposure. Differences in the structure and solvent exposure properties of the enveloped and nonenveloped viral fusion peptides may reflect significant differences in the mechanisms by which these two groups of fusion proteins regulate and promote the fusion of biological membranes.
Acknowledgments
We thank Jingyun Shou for expert technical assistance and Richard Mulligan (Harvard University) for the donation of anti-p10 antiserum.
This research was funded by a grant from the Canadian Institutes of Health Research (CIHR). R.D. is the recipient of a CIHR-RPP investigator award. M.S. was funded by scholarships from the Natural Sciences and Engineering Research Council of Canada and from the Killam Foundation. J.S. was funded by a scholarship from Cancer Care Nova Scotia.
REFERENCES
- 1.Beal, R. E., D. Toscano-Cantaffa, P. Young, M. Rechsteiner, and C. M. Pickart. 1998. The hydrophobic effect contributes to polyubiquitin chain recognition. Biochemistry 37:2925-2934. [DOI] [PubMed] [Google Scholar]
- 2.Bodelon, G., L. Labrada, J. Martinez-Costas, and J. Benavente. 2002. Modification of late membrane permeability in avian reovirus-infected cells. J. Biol. Chem. 277:17789-17796. [DOI] [PubMed] [Google Scholar]
- 3.Bohley, P. 1996. Surface hydrophobicity and intracellular degradation of proteins. Biol. Chem. 377:425-435. [PubMed] [Google Scholar]
- 4.Chen, Y. A., and R. H. Scheller. 2001. SNARE-mediated membrane fusion. Nat. Rev. Mol. Cell. Biol. 2:98-106. [DOI] [PubMed] [Google Scholar]
- 5.Ciechanover, A., and A. L. Schwartz. 1994. The ubiquitin-mediated proteolytic pathway: mechanisms of recognition of the proteolytic substrate and involvement in the degradation of native cellular proteins. FASEB J. 8:182-191. [DOI] [PubMed] [Google Scholar]
- 6.Cuesta, R., G. Laroia, and R. J. Schneider. 2000. Chaperone Hsp27 inhibits translation during heat shock by binding eIF4G and facilitating dissociation of cap-initiation complexes. Genes Dev. 14:1460-1470. [PMC free article] [PubMed] [Google Scholar]
- 7.Dangoria, N. S., M. L. DeLay, D. J. Kingsbury, J. P. Mear, B. Uchanska-Ziegler, A. Ziegler, and R. A. Colbert. 2002. HLA-B27 misfolding is associated with aberrant intermolecular disulfide bond formation (dimerization) in the endoplasmic reticulum. J. Biol. Chem. 277:23459-23468. [DOI] [PubMed] [Google Scholar]
- 8.Dawe, S. J., and R. Duncan. 2002. The S4 genome segment of baboon reovirus is bicistronic and encodes a novel fusion-associated small transmembrane protein. J. Virol. 76:2131-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duncan, R., and K. Sullivan. 1998. Characterization of two avian reoviruses that exhibit strain-specific quantitative differences in their syncytium-inducing and pathogenic capabilities. Virology 250:263-272. [DOI] [PubMed] [Google Scholar]
- 10.Duncan, R. 1999. Extensive sequence divergence and phylogenetic relationships between the fusogenic and nonfusogenic orthoreoviruses: a species proposal. Virology 260:316-328. [DOI] [PubMed] [Google Scholar]
- 11.Duncan, R., Z. Chen, S. Walsh, and S. Wu. 1996. Avian reovirus-induced syncytium formation is independent of infectious progeny virus production and enhances the rate, but is not essential, for virus-induced cytopathology and virus egress. Virology 224:453-464. [DOI] [PubMed] [Google Scholar]
- 12.Eckert, D. M., and P. S. Kim. 2001. Mechanisms of viral membrane fusion and its inhibition. Annu. Rev. Biochem. 70:777-810. [DOI] [PubMed] [Google Scholar]
- 13.Eisenberg, D. 1984. Three-dimensional structure of membrane and surface proteins. Annu. Rev. Biochem. 53:595-623. [DOI] [PubMed] [Google Scholar]
- 14.Ellgaard, L., M. Molinari, and A. Helenius. 1999. Setting the standards: quality control in the secretory pathway. Science 286:1882-1887. [DOI] [PubMed] [Google Scholar]
- 15.Ellgaard, L., and A. Helenius. 2003. Quality control in the endoplasmic reticulum. Nat. Rev. Mol. Cell. Biol. 4:181-191. [DOI] [PubMed] [Google Scholar]
- 16.Fais, S., V. L. Burgio, M. R. Capobianchi, S. Gessani, F. Pallone, and F. Belardelli. 1997. The biological relevance of polykaryons in the immune response. Immunol. Today 18:522-527. [DOI] [PubMed] [Google Scholar]
- 17.Fra, A. M., C. Fagioli, D. Finazzi, R. Sitia, and C. M. Alberini. 1993. Quality control of ER synthesized proteins: an exposed thiol group as a three-way switch mediating assembly, retention and degradation. EMBO J. 12:4755-4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldenberg, D. P., L. S. Bekeart, D. A. Laheru, and J. D. Zhou. 1993. Probing the determinants of disulfide stability in native pancreatic trypsin inhibitor. Biochemistry 32:2835-2844. [DOI] [PubMed] [Google Scholar]
- 19.Han, X., J. H. Bushweller, D. S. Cafiso, and L. K. Tamm. 2001. Membrane structure and fusion-triggering conformational change of the fusion domain from influenza hemagglutinin. Nat. Struct. Biol. 8:715-720. [DOI] [PubMed] [Google Scholar]
- 20.Hartl, F. U. 1996. Molecular chaperones in cellular protein folding. Nature 381:571-579. [DOI] [PubMed] [Google Scholar]
- 21.Hernandez, L. D., L. R. Hoffman, T. G. Wolfsberg, and J. M. White. 1996. Virus-cell and cell-cell fusion. Annu. Rev. Cell Dev. Biol. 12:627-661. [DOI] [PubMed] [Google Scholar]
- 22.Hicke, L. 1997. Ubiquitin-dependent internalization and down-regulation of plasma membrane proteins. FASEB J. 11:1215-1226. [DOI] [PubMed] [Google Scholar]
- 23.Hirsch, C., and H. L. Ploegh. 2000. Intracellular targeting of the proteasome. Trends Cell Biol. 10:268-272. [DOI] [PubMed] [Google Scholar]
- 24.Huovila, A. P., E. A. Almeida, and J. M. White. 1996. ADAMs and cell fusion. Curr. Opin. Cell Biol. 8:692-699. [DOI] [PubMed] [Google Scholar]
- 25.Jahn, R., T. Lang, and T. C. Südhof. 2003. Membrane fusion. Cell 112:519-533. [DOI] [PubMed] [Google Scholar]
- 26.Johnston, J. A., C. L. Ward, and R. R. Kopito. 1998. Aggresomes: a cellular response to misfolded proteins. J. Cell Biol. 143:1883-1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.King, R. W., R. J. Deshaies, J. M. Peters, and M. W. Kirschner. 1996. How proteolysis drives the cell cycle. Science 274:1652-1659. [DOI] [PubMed] [Google Scholar]
- 28.Kowalski, J. M., R. N. Parekh, J. Mao, and K. D. Wittrup. 1998. Protein folding stability can determine the efficiency of escape from endoplasmic reticulum quality control. J. Biol. Chem. 273:19453-19458. [DOI] [PubMed] [Google Scholar]
- 29.Kowalski, J. M., R. N. Parekh, and K. D. Wittrup. 1998. Secretion efficiency in Saccharomyces cerevisiae of bovine pancreatic trypsin inhibitor mutants lacking disulfide bonds is correlated with thermodynamic stability. Biochemistry 37:1264-1273. [DOI] [PubMed] [Google Scholar]
- 30.Kozak, M. 1989. The scanning model for translation: an update. J. Cell Biol. 108:229-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee, D. H., and A. L. Goldberg. 1998. Proteasome inhibitors: valuable new tools for cell biologists. Trends Cell Biol. 8:397-403. [DOI] [PubMed] [Google Scholar]
- 32.Lord, J. M., J. Davey, L. Frigerio, and L. M. Roberts. 2000. Endoplasmic reticulum-associated protein degradation. Semin. Cell Dev. Biol. 11:159-164. [DOI] [PubMed] [Google Scholar]
- 33.Marquardt, T., and A. Helenius. 1992. Misfolding and aggregation of newly synthesized proteins in the endoplasmic reticulum. J. Cell Biol. 117:505-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mayer, A. 2001. What drives membrane fusion in eukaryotes? Trends Biochem. Sci. 26:717-723. [DOI] [PubMed] [Google Scholar]
- 35.Mi, S., X. Lee, X. Li, G. M. Veldman, H. Finnerty, L. Racie, E. LaVallie, X. Y. Tang, P. Edouard, S. Howes, J. C. Keith, Jr., and J. M. McCoy. 2000. Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature 403:785-789. [DOI] [PubMed] [Google Scholar]
- 36.Molinari, M., C. Galli, V. Piccaluga, M. Pieren, and P. Paganetti. 2002. Sequential assistance of molecular chaperones and transient formation of covalent complexes during protein degradation from the ER. J. Cell Biol. 158:247-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Myung, J., K. B. Kim, and C. M. Crews. 2001. The ubiquitin-proteasome pathway and proteasome inhibitors. Med. Res. Rev. 21:245-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pecheur, E. I., O. Maier, and D. Hoekstra. 2000. On the mechanism of intracellular membrane fusion: in search of the genuine fusion factor. Biosci. Rep. 20:613-631. [DOI] [PubMed] [Google Scholar]
- 39.Pettersson, R. F. 1991. Protein localization and virus assembly at intracellular membranes. Curr. Top. Microbiol. Immunol. 170:67-106. [DOI] [PubMed] [Google Scholar]
- 40.Raina, S., and D. Missiakas. 1997. Making and breaking disulfide bonds. Annu. Rev. Microbiol. 51:179-202. [DOI] [PubMed] [Google Scholar]
- 41.Shmulevitz, M., and R. Duncan. 2000. A new class of fusion-associated small transmembrane (FAST) proteins encoded by the nonenveloped fusogenic reoviruses. EMBO J. 19:902-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shmulevitz, M., Z. Yameen, S. Dawe, J. Shou, D. O'Hara, I. Holmes, and R. Duncan. 2002. Sequential partially overlapping gene arrangement in the tricistronic S1 genome segments of avian reovirus and Nelson Bay reovirus: implications for translation initiation. J. Virol. 76:609-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shmulevitz, M., J. Salsman, and R. Duncan. 2003. Palmitoylation, membrane-proximal basic residues, and transmembrane glycine residues in the reovirus p10 protein are essential for syncytium formation. J. Virol. 77:9769-9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shmulevitz, M., R. F. Epand, R. M. Epand, and R. Duncan. 2004. Structural and functional properties of an unusual internal fusion peptide in a nonenveloped virus membrane fusion protein. J. Virol. 78:2808-2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shusta, E. V., M. C. Kieke, E. Parke, D. M. Kranz, and K. D. Wittrup. 1999. Yeast polypeptide fusion surface display levels predict thermal stability and soluble secretion efficiency. J. Mol. Biol. 292:949-956. [DOI] [PubMed] [Google Scholar]
- 46.Skehel, J. J., and D. C. Wiley. 2000. Receptor binding and membrane fusion in virus entry: the influenza haemagglutinin. Annu. Rev. Biochem. 69:531-569. [DOI] [PubMed] [Google Scholar]
- 47.Sommer, T., and D. H. Wolf. 1997. Endoplasmic reticulum degradation: reverse protein flow of no return. FASEB J. 11:1227-1233. [DOI] [PubMed] [Google Scholar]
- 48.Takahashi, Y., D. Bigler, Y. Ito, and J. M. White. 2001. Sequence-specific interaction between the disintegrin domain of mouse ADAM 3 and murine eggs: role of beta1 integrin-associated proteins CD9, CD81, and CD98. Mol. Biol. Cell 12:809-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tamm, L. K., and X. Han. 2000. Viral fusion peptides: a tool set to disrupt and connect biological membranes. Biosci. Rep. 20:501-518. [DOI] [PubMed] [Google Scholar]
- 50.Taylor, M. V. 2002. Muscle differentiation: how two cells become one. Curr. Biol. 12:R224-R228. [DOI] [PubMed] [Google Scholar]
- 51.Trombetta, E. S., and A. Helenius. 1998. Lectins as chaperones in glycoprotein folding. Curr. Opin. Struct. Biol. 8:587-592. [DOI] [PubMed] [Google Scholar]
- 52.Turner, G. C., and A. Varshavsky. 2000. Detecting and measuring cotranslational protein degradation in vivo. Science 289:2117-2120. [DOI] [PubMed] [Google Scholar]
- 53.Varshavsky, A. 1996. The N-end rule: functions, mysteries, uses. Proc. Natl. Acad. Sci. USA 93:12142-12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vignery, A. 2000. Osteoclasts and giant cells: macrophage-macrophage fusion mechanism. Int. J. Exp. Pathol. 81:291-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.White, J. M. 1990. Viral and cellular membrane fusion proteins. Annu. Rev. Physiol. 52:675-679. [DOI] [PubMed] [Google Scholar]
- 56.White, J. M. 1992. Membrane fusion. Science 258:917-924. [DOI] [PubMed] [Google Scholar]
- 57.Wickner, S., M. R. Maurizi, and S. Gottesman. 1999. Posttranslational quality control: folding, refolding, and degrading proteins. Science 286:1888-1893. [DOI] [PubMed] [Google Scholar]