Abstract
Background
Inorganic arsenic exposure in water and food is a global public health problem. Chronic exposure to high levels of arsenicis consistently associated with increased risk of cardiovascular disease, whereas prospective data on low to moderate chronic arsenic exposure (<100μg/L in drinking water) are lacking.
Objective
To evaluate the association between chronic low to moderate arsenic exposure and incident cardiovascular disease.
Design
Prospective cohort study.
Setting
The Strong Heart Study baseline visit in 1989-1991, with follow-up through 2008.
Patients
3,575 American Indian men and women aged 45-74 years living in Arizona, Oklahoma, and North and South Dakota.
Measurements
The sum of inorganic and methylated arsenic species in urine at baseline was used as a biomarker of chronic arsenic exposure. Participants were followed for incident fatal and non-fatal cardiovascular disease, including coronary heart disease and stroke.
Results
1,184 participants developed fatal and non-fatal cardiovascular disease and 439 participants developed fatal cardiovascular disease. Comparing the highest to lowest quartile arsenic concentrations (>15.7 vs. <5.8 μg/g creatinine), the hazard ratios (95% confidence interval) for cardiovascular disease, coronary heart disease, and stroke mortality after adjustment for socio-demographic factors, smoking, body mass index, and lipids were 1.65 (1.20, 2.27; p-trend<0.001), 1.71 (1.19, 2.44; p-trend<0.001) and 3.03 (1.08, 8.50; p-trend=0.061), respectively. The corresponding hazard ratios for incident cardiovascular disease, coronary heart disease, and stroke were 1.32 (1.09, 1.59; p-trend=0.002), 1.30 (1.04, 1.62; p-trend=0.006), and 1.47 (0.97, 2.21; p-trend=0.032), respectively. These associations varied by study region and were attenuated following further adjustment for diabetes, hypertension, and measures of kidney disease.
Limitations
Direct measurement of individual arsenic in drinking water was unavailable. Residual confounding and differences in potential confounders across study regions may exist.
Conclusions
Low to moderate chronic arsenic exposure, as measured in urine, was prospectively associated with cardiovascular disease incidence and mortality.
Introduction
Inorganic arsenicin water and food (particularly rice and grain) is a major global health problem (1). High arsenic levels in drinking water (>100μg/L) increased the risk of peripheral artery disease, coronary heart disease, stroke, and carotid atherosclerosis in studies conducted in Taiwan (2-4), Bangladesh (5, 6), Chile (7), Inner Mongolia (8, 9), and Pakistan (10, 11). Less is known about the cardiovascular effects of low to moderate arsenic levels (<100μg/L in drinking water) that affect most populations around the world due to a lack of prospective studies, limitations in exposure and outcome assessment, and inadequate information on cardiovascular risk factors (12, 13). Indeed, the risk-benefit analysis that established the current United States (US) standard for arsenic in drinking water (10 μg/L) did not quantify the impact of arsenic on cardiovascular disease because of a lack of adequate data (14).
In the US, arsenic exposure in drinking water disproportionately affects small rural communities in the Southwest, Midwest, and Northeast (15). The Strong Heart Study is a population-based prospective cohort study of cardiovascular disease among three American Indians communities in rural Arizona, Oklahoma, and North Dakota and South Dakota (hereafter referred to as the Dakotas) (16). At the time of the study, drinking water arsenic in public water systems ranged from <10 to 61 μg/L in Arizona, <10 μg/L in Oklahoma, and from <1 to 21 μg/L in the Dakotas (17). In private wells, arsenic levels likely exceeded 10 and even 50 μg/L in Arizona and the Dakotas (15). In Arizona and the Dakotas, drinking water was likely the main source of inorganic arsenic in study participants. In Oklahoma, similar to other populations with low arsenic levels in drinking water (18, 19), diet was likely the main source of arsenic exposure. Potential dietary sources of inorganic arsenic in the Strong Heart Study communities include rice, flour, and other grains (especially common grain-based items are tacos, fry bread, and tortilla). Thus, the objective of this study was to examine the prospective association of chronic arsenic exposure with cardiovascular disease over almost 20 years of follow-up in the Strong Heart Study.
Methods
Study Population
The Strong Heart Study examined 4,549 men and women 45-75 years of age at baseline in 1989-1991. Participants were invited to subsequent clinical visits in 1993-1995 and 1998-1999 and were actively followed through 2008 (Appendix Figure 1). Every eligible person was invited to participate in Arizona and Oklahoma, whereas a cluster sampling technique was used in the Dakotas (20). The participation rate was 62% (21). Most participants were born in the communities and have lived there all their lives. Compared to non-participants, participants were similar in age, body mass index, and self-reported frequency of diabetes but were more likely to be female and to have self-reported hypertension (21). The Indian Health Service, institutional review boards, and the participating tribes approved the study protocol. All participants provided informed consent.
We used data from 3,973 Strong Heart Study participants with sufficient urine available for arsenic measurements. We then excluded 273 participants with self-reported or clinical cardiovascular disease at baseline, 3 participants missing urine creatinine, and 122 participants missing other variables of interest, leaving 3,575 participants for this analysis. Included participants were similar to those excluded because of missing data (not shown).
Data Collection
The clinical examinations consisted of a personal interview, physical examination, fasting blood draw, and spot urine sample collection (20). Trained and certified interviewers administered standardized questionnaires and centrally trained nurses and medical assistants measured height, weight, and systolic and diastolic blood pressure and collected blood and urine following standardized protocols (20). Methods to measure blood pressure, cholesterol, fasting glucose, oral glucose tolerance, hemoglobin A1c, and plasma creatinine have been described (20). We defined hypertension as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or antihypertensive medication use. We calculated low-density lipoprotein (LDL) cholesterol using the Friedewald equation for participants with triglycerides <4.52 mmol/L (400 mg/dL), with missing valuesre placed by measured LDL using the beta quantification method. We defined albuminuria as a urine albumin-creatinine ratio of ≥30 mg/g (22). We calculated estimated glomerular filtration rate (eGFR) from recalibrated plasma creatinine, age, and sex using the Chronic Kidney Disease – Epidemiology Collaboration formula (23). We defined diabetes as fasting glucose ≥126 mg/dL, 2-hour post-load plasma glucose ≥200 mg/dL, hemoglobin A1c ≥6.5%, or self-reported use of insulin or oral hypoglycemic agent.
Spot urine samples collected in the morning of the baseline clinical examination were frozen within 1-2 hours of collection and stored at -70°C or lower (20). Urine albumin and urine creatinine were measured by an automated nephelometric immunochemical procedure and an automated alkaline picrate methodology, respectively (20).
Urine Arsenic
To assess chronic arsenic exposure, we measured arsenic species concentrations in urine. The analytical methods and associated quality control criteria for arsenic analysis have been described in detail (24). Arsenic species concentrations were determined by high performance liquid chromatography coupled to inductively coupled plasma mass spectrometry that served as the arsenic selective detector (Agilent 1100 HPLC and Agilent 7700x ICP-MS, Agilent Technologies). Arsenic speciation distinguishes species directly related to inorganic arsenic exposure (arsenite, arsenate, monomethylarsonate [MMA], and dimethylarsinate [DMA]) from those related to organic arsenicals in seafood (arsenobetaine as an overall marker of seafood arsenicals), which are generally considered nontoxic (1). The limits of detection for inorganic arsenic (arsenite plus arsenate), MMA, DMA and arsenobetaine plus other cationic arsenic species were 0.1 μg/L (24). For participants with arsenic species below the limit of detection (5.1% for inorganic arsenic, 0.8% for MMA, 0.03% for DMA, and 2.1% for arsenobetaine), levels were imputed as the limit of detection divided by the square root of 2. The inter-assay coefficients of variation for inorganic arsenic, MMA, DMA and arsenobetaine for an in-house reference urine were 6.0%, 6.5%, 5.9%, and 6.5%, respectively (24).
We used the sum of urine inorganic arsenic (arsenite and arsenate) and methylated arsenic species (DMA and MMA) as a biomarker to integrate inorganic arsenic exposure from multiple sources (1, 25, 26). To account for urine dilution, urine arsenic concentrations were divided by urine creatinine and expressed as μg/g creatinine. Low urine concentrations of arsenobetaine (median 0.76, interquartile range 0.48-1.70 μg/g creatinine) confirmed that seafood intake was low in this population, indicating that measured methylated species reflect inorganic arsenic exposure. Inorganic arsenic and its methylated metabolites have estimated half-lives of 2, 9, and 38 days (27, 28). In a random sample stratified by study region of 380 participants with three repeated arsenic measures over 10 years, the intraclass correlation coefficient for the log-transformed sum of inorganic and methylated arsenic species was 0.64 (95% confidence interval 0.60, 0.69) and the average change in urine arsenic concentrations comparing visit 3 to visit 1 was -0.8 μg/g creatinine.
Cardiovascular Disease Incidence and Mortality Follow-up
Incident cardiovascular endpoints during follow-up were identified by annual contact, review of hospitalization and death records, and during two clinic visits conducted in 1993–1995 and 1998–1999. Follow-up through 2008 was 99.8% complete for mortality and 99.2% complete for non-fatal events. When possible cardiovascular events were identified, medical records were abstracted and mortality and morbidity review committees adjudicated cardiovascular events (20). Detailed definitions on the criteria used by the review committees have been described previously (20, 29) and are included in Appendix 1.
We defined incident coronary heart disease as the first occurrence of definite non-fatal coronary heart disease or definite and possible fatal coronary heart disease. We defined incident stroke as the first occurrence of a definite non-fatal stroke or a definite or possible fatal stroke. We defined incident cardiovascular disease as the first occurrence of coronary heart disease or stroke, as previously defined, definite non-fatal congestive heart failure, or other fatal cardiovascular disease.
Follow-up extended from the date of the baseline examination until the date of the cardiovascular event, the date of death, or December 31, 2008, whichever occurred first. The mean follow-up time among participants without a cardiovascular event was 15.0 years.
Statistical Analysis
We evaluated the prospective association of urine arsenic concentrations with incident cardiovascular disease using Cox proportional hazards models with age as time scale and individual entry times (age at baseline) treated as staggered entries. Urine arsenic concentrations were modeled as: (1) quartiles, (2) log-transformed concentrations to compare the 75th to the 25th percentile (interquartile range), and (3) log-transformed concentrations with restricted quadratic splines. We allowed the non-parametric underlying baseline hazards to differ by region as study locations differed by both urine arsenic concentrations (17) and cardiovascular risk factors (16). Models were progressively adjusted (see footnotes of Tables 2 and 3). P-values for linear and non-linear trend were obtained from Wald tests for log-transformed arsenic coefficients and restricted quadratic spline coefficients, respectively. We found no violations of the assumption of proportional hazards over time based on visual examinations of smoothed association between age and scaled Schoenfeld residuals over time and a test for a non-zero slope for this association (30). To estimate absolute rates, we modeled cardiovascular disease incidence and mortality using Poisson regression and then estimated the marginal response for each arsenic quartile given mean values of covariates. Poisson models were adjusted for Model 2 covariates, age, and study region.
Table 2. Hazard ratios (95% confidence interval) for cardiovascular mortality endpoints by urine arsenic* concentrations (μg/g creatinine) (N=3,575).
| Quartiles of inorganic plus methylated arsenic species (μg/g creatinine)** | 75th vs. 25th percentile** | p-trend** | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| <5.8 (4.2) | 5.8 - 9.7 (7.5) | 9.8 - 15.7 (12.4) | >15.7 (21.8) | |||
| Cardiovascular Disease Mortality | ||||||
| Cases | 86 | 95 | 115 | 143 | 439 | |
| Person-Years | 13,616 | 13,430 | 12,720 | 12,033 | 51,799 | |
| Model 1 | 1 (Referent) | 1.06 (0.78, 1.43) | 1.21 (0.89, 1.65) | 1.59 (1.17, 2.17) | 1.34 (1.15, 1.56) | <0.001 |
| Model 2 | 1 (Referent) | 1.12 (0.83, 1.52) | 1.26 (0.92, 1.73) | 1.65 (1.20, 2.27) | 1.36 (1.16, 1.58) | <0.001 |
| Model 3 | 1 (Referent) | 1.06 (0.78, 1.44) | 1.24 (0.90, 1.70) | 1.52 (1.10, 2.11) | 1.35 (1.15, 1.58) | <0.001 |
| Model 4 | 1 (Referent) | 1.02 (0.75, 1.39) | 1.15 (0.84, 1.58) | 1.29 (0.93, 1.79) | 1.25 (1.06, 1.47) | 0.007 |
| Coronary Heart Disease Mortality | ||||||
| Cases | 68 | 67 | 87 | 119 | 341 | |
| Person-Years | 13,616 | 13,430 | 12,720 | 12,033 | 51,799 | |
| Model 1 | 1 (Referent) | 0.91 (0.65, 1.30) | 1.11 (0.78, 1.57) | 1.59 (1.13, 2.25) | 1.37 (1.15, 1.63) | <0.001 |
| Model 2 | 1 (Referent) | 0.99 (0.70, 1.41) | 1.18 (0.83, 1.69) | 1.71 (1.19, 2.44) | 1.41 (1.18, 1.68) | <0.001 |
| Model 3 | 1 (Referent) | 0.93 (0.65, 1.32) | 1.15 (0.80, 1.66) | 1.57 (1.08, 2.27) | 1.40 (1.17, 1.68) | <0.001 |
| Model 4 | 1 (Referent) | 0.89 (0.62, 1.27) | 1.06 (0.74, 1.53) | 1.33 (0.92, 1.93) | 1.30 (1.08, 1.57) | 0.005 |
| Stroke Mortality | ||||||
| Cases | 6 | 17 | 13 | 18 | 54 | |
| Person-Years | 13,616 | 13,430 | 12,720 | 12,033 | 51,799 | |
| Model 1 | 1 (Referent) | 1.37 (0.53, 3.55) | 2.46 (0.88, 6.83) | 3.66 (1.34, 10.03) | 1.66 (1.11, 2.48) | 0.014 |
| Model 2 | 1 (Referent) | 1.41 (0.54, 3.67) | 2.16 (0.77, 6.09) | 3.03 (1.08, 8.50) | 1.51 (0.98, 2.32) | 0.061 |
| Model 3 | 1 (Referent) | 1.40 (0.54, 3.65) | 2.05 (0.72, 5.81) | 2.75 (0.97, 7.81) | 1.48 (0.95, 2.32) | 0.082 |
| Model 4 | 1 (Referent) | 1.30 (0.50, 3.39) | 1.97 (0.70, 5.55) | 2.35 (0.83, 6.69) | 1.37 (0.87, 2.14) | 0.175 |
Model 1: Stratified by study center and age-adjusted (age as time metric, age at baseline treated as staggered entries).
Model 2: Further adjusted for sex, education (no, some, or completed high school), smoking status (never, former, current), body mass index (kg/m2), and LDL cholesterol (mg/dL).
Model 3: Further adjusted for hypertension (yes, no), diabetes (yes, no), and estimated glomerular filtration rate (ml/min/1.73m2).
Model 4: Further adjusted for albuminuria (yes, no).
Adjustment for systolic blood pressure and hypertension medication instead of hypertension and hemoglobin A1c levels instead of diabetes resulted in similar results (data not shown).
Sum of inorganic and methylated arsenic (DMA and MMA).
Range (median) of each quartile.
Models comparing the 75th to the 25th percentile (interquartile range) of the sum of inorganic and methylated urine arsenic concentrations (15.7 vs. 5.8 μg/g creatinine). p-trend was obtained from Cox proportional hazards models with log-transformed arsenic as a continuous variable.
Table 3. Hazard ratios (95% confidence interval) for incident cardiovascular endpoints (fatal and non-fatal) by urine arsenic* concentrations (μg/g creatinine) (N=3,575).
| Quartiles of inorganic plus methylated arsenic species (μg/g creatinine)** | 75th vs. 25th percentile*** | p-trend*** | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| <5.8 (4.2) | 5.8 - 9.7 (7.5) | 9.8 - 15.7 (12.4) | >15.7 (21.8) | |||
| Cardiovascular Disease Incidence | ||||||
| Cases | 265 | 297 | 291 | 331 | 1,184 | |
| Person-Years | 12,146 | 11,701 | 11,305 | 10,586 | 45,738 | |
| Model 1 | 1 (Referent) | 1.11 (0.94, 1.32) | 1.04 (0.87, 1.25) | 1.30 (1.08, 1.56) | 1.15 (1.04, 1.26) | 0.004 |
| Model 2 | 1 (Referent) | 1.14 (0.95, 1.35) | 1.05 (0.87, 1.26) | 1.32 (1.09, 1.59) | 1.16 (1.05, 1.28) | 0.002 |
| Model 3 | 1 (Referent) | 1.13 (0.95, 1.34) | 1.02 (0.84, 1.23) | 1.24 (1.02, 1.50) | 1.14 (1.03, 1.26) | 0.008 |
| Model 4 | 1 (Referent) | 1.11 (0.93, 1.32) | 0.97 (0.80, 1.17) | 1.09 (0.90, 1.33) | 1.07 (0.97, 1.18) | 0.168 |
| Coronary Heart Disease Incidence | ||||||
| Cases | 202 | 206 | 197 | 241 | 846 | |
| Person-Years | 12,447 | 12,136 | 11,805 | 11,075 | 47,463 | |
| Model 1 | 1 (Referent) | 0.98 (0.81, 1.20) | 0.88 (0.71, 1.10) | 1.18 (0.95, 1.46) | 1.11 (1.00, 1.25) | 0.058 |
| Model 2 | 1 (Referent) | 1.05 (0.86, 1.28) | 0.95 (0.77, 1.19) | 1.30 (1.04, 1.62) | 1.17 (1.05, 1.32) | 0.006 |
| Model 3 | 1 (Referent) | 1.04 (0.85, 1.28) | 0.93 (0.74, 1.15) | 1.21 (0.97, 1.52) | 1.16 (1.03, 1.30) | 0.016 |
| Model 4 | 1 (Referent) | 1.03 (0.84, 1.26) | 0.88 (0.70, 1.10) | 1.08 (0.86, 1.35) | 1.09 (0.97, 1.23) | 0.155 |
| Stroke Incidence | ||||||
| Cases | 55 | 75 | 62 | 72 | 264 | |
| Person-Years | 13,375 | 13,117 | 12,435 | 11,741 | 50,667 | |
| Model 1 | 1 (Referent) | 1.11 (0.78, 1.59) | 1.25 (0.84, 1.85) | 1.64 (1.10, 2.44) | 1.32 (1.09, 1.60) | 0.005 |
| Model 2 | 1 (Referent) | 1.18 (0.82, 1.69) | 1.16 (0.77, 1.72) | 1.47 (0.97, 2.21) | 1.24 (1.02, 1.52) | 0.032 |
| Model 3 | 1 (Referent) | 1.16 (0.81, 1.66) | 1.11 (0.74, 1.66) | 1.32 (0.87, 2.00) | 1.21 (0.98, 1.48) | 0.074 |
| Model 4 | 1 (Referent) | 1.09 (0.76, 1.57) | 1.07 (0.72, 1.60) | 1.18 (0.77, 1.79) | 1.14 (0.93, 1.41) | 0.21 |
Model 1: Stratified by study center and age-adjusted (age as time metric, age at baseline treated as staggered entries).
Model 2: Further adjusted for sex, education (no, some, or completed high school), smoking status (never, former, current), body mass index (kg/m2), and LDL cholesterol (mg/dL).
Model 3: Further adjusted for hypertension (yes, no), diabetes (yes, no), and estimated glomerular filtration rate (ml/min/1.73m2).
Model 4: Further adjusted for albuminuria (yes, no).
Adjustment for systolic blood pressure and hypertension medication instead of hypertension and hemoglobin A1c levels instead of diabetes resulted in similar results (data not shown).
Sum of inorganic and methylated arsenic species (DMA and MMA).
Range (median) of each quartile.
Models comparing the 75th to the 25th percentile (interquartile range) of the sum of inorganic and methylated urine arsenic concentrations (15.7 vs. 5.8 μg/g creatinine). p-trend was obtained from Cox proportional hazards models with log-transformed arsenic as a continuous variable.
We conducted several sensitivity analyses. First, we evaluated the association of cardiovascular disease endpoints with arsenobetaine, a non-toxic seafood arsenical (1). We hypothesized that arsenobetaine would not be associated with cardiovascular disease. Second, we evaluated alternative methods to adjust spot urine samples for urine dilution. We measured urine arsenic concentrations in μg/L and adjusted the models for log-transformed urine creatinine concentrations. Among participants without diabetes or albuminuria (N=1,646), we also used urine arsenic concentrations adjusted to the mean specific gravity of 1.017 (31). Urine specific gravity is less dependent upon muscle mass and nutritional status than urine creatinine (31), but this adjustment is inadequate if albumin or glucose are present in urine (32). Both analyses resulted in similar findings (not shown).
We performed subgroup analyses to evaluate effect modification in adjusted models by including quantitative interaction terms for log-transformed urine arsenic with indicator variables for age groups, sex, smoking status, diabetes status, study region, and % inorganic arsenic, % MMA and % DMA in separate models. Based on prior evidence (6), we hypothesized that the association between arsenic and incident cardiovascular disease would be stronger in current smokers and in participants with higher % MMA and lower % DMA in urine. Other subgroup analyses were exploratory without a priori hypotheses. P-values for interactions were obtained using Wald tests for multiple coefficients.
Arsenic methylation patterns have been related to differences in cardiovascular endpoints in populations from Taiwan (4, 33). To evaluate the potential role of arsenic metabolism, we examined the relationship between the relative proportions of arsenic species in urine (log-transformed% inorganic arsenic, % MMA, and % DMA) with incident cardiovascular disease on the subset of participants with detectable inorganic arsenic, MMA, and DMA (N=3,381).
Statistical analyses were performed with R Version 2.5.1 (R foundation for Statistical Computing, http://www.r-project.org/) and Stata IC Version 12 (StataCorp).
Results
Over 45,738 person-years of follow-up, 439 participants died from cardiovascular disease (341 coronary heart disease deaths and 54 stroke deaths) and 1,184 participants developed fatal or non-fatal cardiovascular disease (846 incident coronary heart disease events and 264 incident strokes). Overall, the median (interquartile range; range) urine arsenic concentration was 9.7 (5.8, 15.7; 0.1, 183.4) μg/g creatinine. Urine arsenic concentrations varied by study region (medians of 14.2, 5.6, and 10.6 μg/g in Arizona, Oklahoma, and the Dakotas, respectively). Increasing baseline arsenic concentrations were associated with female sex, lower educational attainment, lower LDL cholesterol, higher eGFR, and an increased prevalence of never smoking and diabetes (Table 1).
Table 1. Baseline characteristics of study participants: overall and by urine arsenic* concentrations (μg/g creatinine) (N=3,575).
| Quartiles of inorganic plus methylated arsenic species (μg/g creatinine)** | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Overall | <5.8 (4.2) | 5.8- 9.7 (7.5) | 9.8 - 15.7 (12.4) | >15.7 (21.8) | p-trend** | |
| Age, mean (SD), years | 56.0 (8.0) | 56.3 (8.2) | 55.5 (7.9) | 56.2 (7.9) | 55.9 (7.9) | 0.77 |
| Female, % | 60.2 | 51.9 | 61.3 | 61.0 | 66.9 | <0.001 |
| Arizona, % | 34.3 | 7.5 | 25.7 | 44.3 | 59.9 | <0.001 |
| North/South Dakota, % | 32.5 | 22.7 | 35.2 | 38.6 | 33.6 | <0.001 |
| Oklahoma, % | 33.2 | 69.9 | 39.1 | 17.2 | 6.5 | <0.001 |
| < High School Education, % | 46.6 | 33.1 | 38.7 | 52.1 | 62.3 | <0.001 |
| BMI, mean (SD), kg/m2 | 30.8 (6.3) | 30.5 (5.7) | 31.4 (6.3) | 30.7 (6.2) | 30.7 (6.8) | 0.72 |
| Never Smoker, % | 32.8 | 30.5 | 32.1 | 33.2 | 35.3 | 0.026 |
| Former Smoker, % | 33.6 | 35.2 | 34.7 | 32.5 | 31.9 | 0.088 |
| Current Smoker, % | 33.7 | 34.4 | 33.1 | 34.3 | 32.8 | 0.61 |
| LDL Cholesterol, mean (SD), mmol/L | 3.0 (0.9) | 3.1 (0.8) | 3.1 (0.9) | 2.9 (0.9) | 2.9 (0.9) | <0.001 |
| LDL Cholesterol, mean (SD), mg/dL | 116.1 (33.7) | 121.6 (32.4) | 118.0 (33.4) | 113.0 (33.8) | 111.3 (34.4) | <0.001 |
| Hypertension, % | 37.3 | 36.9 | 35.4 | 38.5 | 38.4 | 0.31 |
| Diabetes, % | 48.5 | 36.9 | 45.0 | 49.6 | 62.4 | <0.001 |
| eGFR, mean (SD), ml/min/1.73m2 | 97.9 (17.8) | 94.9 (17.3) | 97.7 (16.7) | 98.1 (17.8) | 101.0 (18.9) | <0.001 |
| Albuminuria, % | 29.0 | 17.1 | 23.2 | 29.9 | 46.1 | <0.001 |
SD: Standard deviation; eGFR: estimated glomerular filtration rate; LDL: Low-density lipoprotein; BMI: Body mass index.
Sum of inorganic and methylated arsenic species (DMA and MMA).
Range (median) of each quartile.
p-trend obtained from linear or logistic models with arsenic quartiles entered as a continuous variable.
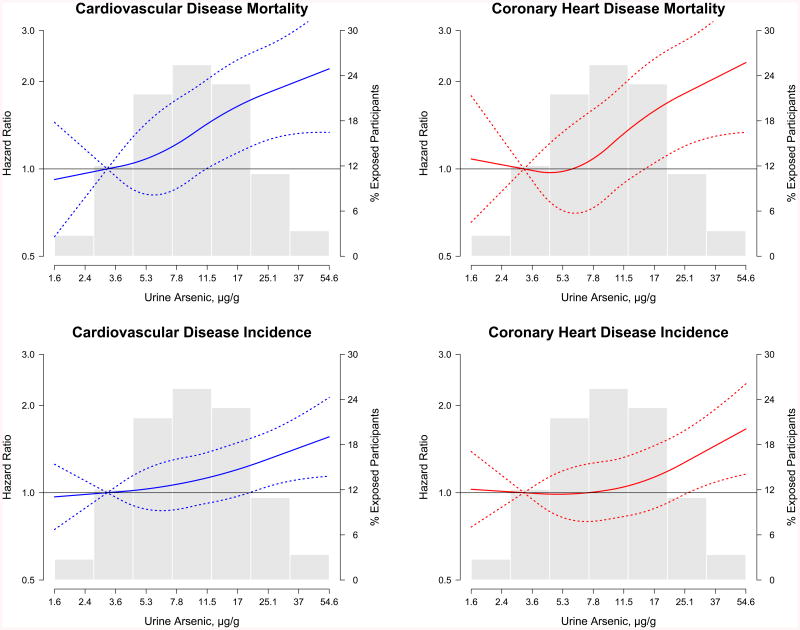
Baseline urine arsenic concentrations were prospectively associated with cardiovascular disease mortality and incidence (Tables 2 and 3, Model 2). The fully-adjusted hazard ratios (95% confidence interval [CI]) for cardiovascular disease, coronary heart disease, and stroke mortality comparing the highest to the lowest quartile in urine arsenic concentrations were 1.65 (1.20, 2.27; p-trend<0.001), 1.71 (1.19, 2.44; p-trend<0.001) and 3.03 (1.08, 8.50; p-trend=0.061), respectively (Table 2; Model 2). For incident cardiovascular disease, coronary heart disease, and stroke, the corresponding hazard ratios (95% CI) were 1.32 (1.09, 1.59; p-trend=0.002), 1.30 (1.04, 1.62; p-trend=0.006), and 1.47 (0.97, 2.21; p-trend=0.032) (Table 3; Model 2). Further adjustment for hypertension, diabetes, eGFR, and especially albuminuria (Tables 2 and 3, models 3 and 4) attenuated the associations. Based on Model 2, the incidence rates (95% CI) per 10,000 person-years for increasing arsenic quartiles were 49 (38, 61), 56 (44, 68), 62 (50, 75) and 82 (66, 97) for cardiovascular disease mortality and 189 (164, 214), 214 (189, 239), 198 (174, 223), and 238 (219, 279) for cardiovascular disease incidence. The dose-response relationships of arsenic concentrations with cardiovascular disease and coronary heart disease incidence and mortality were statistically significant (Tables 2 and 3), with no significant departures from linearity (Figure 1). For stroke incidence and mortality, the dose-response relationship was positive but not statistically significant (Tables 2 and 3, Appendix Figure 2).
Figure 1. Hazard ratios for cardiovascular disease and coronary heart disease incidence and mortality by urine arsenic concentrations (N=3,575).
Solid lines represent adjusted hazard ratios based on restricted quadratic splines for log-transformed sum of inorganic and methylated arsenic species with knots at the 10th, 50th, and 90th percentiles (3.8, 9.7, and 24.0 μg/g creatinine, respectively). Dotted lines represent upper and lower 95% confidence intervals. The reference was set at the 10th percentile of the arsenic distribution (3.8 μg/g creatinine). Adjustment factors were the same as for Model 2 in Table 2 and 3. Vertical bars represent a histogram of urine arsenic distribution among participants (the extreme tails of the histogram were truncated as only 1 and 31 participant had urine arsenic levels <1.6 and >54.6 μg/g creatinine, respectively).
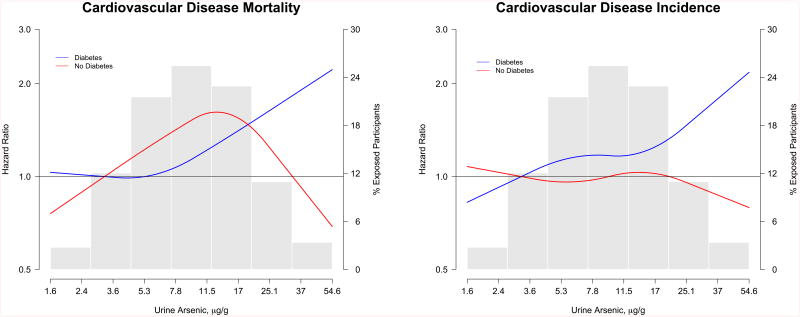
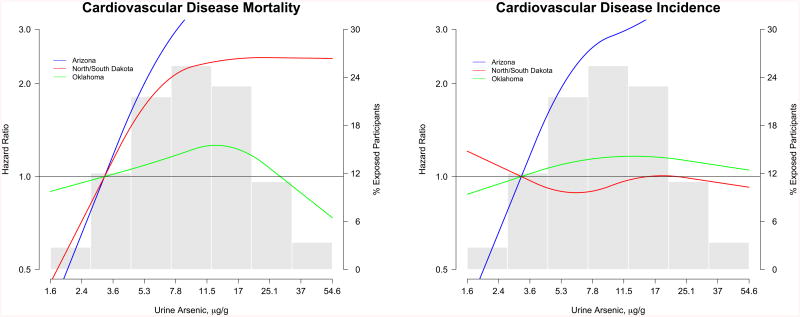
For cardiovascular and coronary heart disease mortality, the associations were positive for all subgroups but stronger among participants from Arizona, participants with diabetes and participants with %DMA above the median (Appendix Figure 3). For cardiovascular and coronary heart disease incidence, the associations with urine arsenic were stronger among women, never smokers, participants from Arizona, participants with diabetes, and participants with %DMA above the median (Appendix Figure 4). For stroke incidence, the associations were consistent across the subgroups evaluated (Appendix Figure 4). In dose-response analyses, the association of arsenic with cardiovascular disease mortality and incidence were stronger in participants from Arizona (Figure 2) and participants with diabetes (Figure 3).
Figure 2. Hazard ratios for cardiovascular disease incidence and mortality stratified by study region.
Solid lines represent adjusted hazard ratios based on restricted quadratic splines for log-transformed sum of inorganic and methylated arsenic species with knots at the 10th, 50th, and 90th percentiles (3.8, 9.7, and 24.0 μg/g creatinine, respectively). Blue lines indicate participants in Arizona, red lines indicate participants from North/South Dakota, and green lines indicate participants from Oklahoma. The reference was set at the 10th percentile of the arsenic distribution (3.8 μg/g creatinine). Adjustment factors were the same as for Model 2 in Table 2 and 3. Vertical bars represent a histogram of urine arsenic distribution among participants (the extreme tails of the histogram were truncated as only 1 and 31 participant had urine arsenic levels <1.6 and >54.6 μg/g creatinine, respectively).
Figure 3. Hazard ratios for cardiovascular disease incidence and mortality stratified by diabetes status.
Solid lines represent adjusted hazard ratios based on restricted quadratic splines for log-transformed sum of inorganic and methylated arsenic species with knots at the 10th, 50th, and 90th percentiles (3.8, 9.7, and 24.0 μg/g creatinine, respectively). Blue lines indicate participants with diabetes at baseline and red lines indicate participants without diabetes at baseline. The reference was set at the 10th percentile of the arsenic distribution (3.8 μg/g creatinine). Adjustment factors were the same as for Model 2 in Table 2 and 3. Vertical bars represent a histogram of urine arsenic distribution among participants (the extreme tails of the histogram were truncated as only 1 and 31 participant had urine arsenic levels <1.6 and >54.6 μg/g creatinine, respectively).
Urine arsenobetaine was not associated with any of the cardiovascular endpoints (data not shown in tables). The fully-adjusted hazard ratios (95% CI) for cardiovascular disease mortality and incidence comparing an interquartile range in arsenobetaine concentrations were 0.93 (0.83, 1.04) and 0.95 (0.88, 1.01), respectively.
In the subset of participants with detectable inorganic arsenic, MMA, and DMA (N=3,381), hazard ratios (95% CI) for incident cardiovascular disease comparing an interquartile range of % inorganic arsenic, % MMA, and % DMA were 0.91 (0.84,0.99), 0.98 (0.90, 1.07), and 1.04 (0.96,1.12), respectively, after adjustment for sex, education, smoking, body mass index, LDL cholesterol, and the sum of inorganic and methylated arsenic species (μg/g creatinine). We found no associations between any of the biomarkers of arsenic metabolism and other study endpoints (not shown).
Discussion
Low to moderate inorganic arsenic exposure, as measured in urine, was prospectively associated with fatal and non-fatal cardiovascular disease in a population of rural American Indians with a high burden of diabetes and cardiovascular disease. The associations persisted after adjustment for socio-demographic factors, smoking, and lipids and were attenuated with further adjustment for hypertension, diabetes, and measures of kidney disease, variables that could be in the causal pathway. The associations were largely similar for coronary heart disease and stroke and were stronger for cardiovascular mortality compared to cardiovascular incidence. Urine arsenobetaine, an organic arsenical found in seafood believed to be non-toxic, was not associated to cardiovascular disease. Overall, our findings support an association between chronic low to moderate inorganic arsenic exposure and incident cardiovascular disease.
The adverse cardiovascular effects of chronic exposure to high arsenic levels in drinking water (>100μg/L) have long been recognized (34). In early case reports, high arsenic exposure was associated with peripheral artery disease in Southwestern Taiwan and in German vintners (35), and with myocardial infarction in young adults from Chile (36). High-chronic arsenic exposure in drinking water was prospectively associated with coronary heart disease mortality in Southwestern Taiwan (3). Recently, prospective studies from Bangladesh found that urine and drinking water arsenic concentrations were associated with increased cardiovascular disease and coronary heart disease mortality (5, 6). Evidence from multiple countries with different ethnic backgrounds supports a causal association between high-chronic arsenic exposure and cardiovascular disease (12, 13).
Less is known about the cardiovascular effects of arsenic at levels <100 μg/L in drinking water. Some (37-44), but not all (45, 46) studies have found modestly increased cardiovascular risks, although most of these studies had important limitations including ecological designs, limited exposure and outcome assessment, or lack of adjustment for cardiovascular risk factors. In two recent prospective studies from Bangladesh, the hazard ratios for low-moderate arsenic exposure categories (arsenic in drinking water 12.1-62 vs. ≤12 μg/L (6) and 10-49 vs. <10 μg/L (5)) and cardiovascular mortality were supportive of an association but not statistically significant. Thus, our study provides important novel data in a Western population with high background cardiovascular risk.
Experimental studies also support the role of arsenic in cardiovascular disease. Arsenic-exposed animals were more likely to develop atherosclerotic plaque compared to unexposed animals (47-49). Potential mechanisms for arsenic-related atherosclerosis include endothelial dysfunction, smooth muscle proliferation, angiogenesis and apoptosis, vascular injury, and platelet aggregation (50, 51). In addition, arsenic up-regulates inflammation, disrupts lipid metabolism, and increases lipid oxidation (50, 51). Arsenic-related cardiovascular disease could also be mediated by other cardiovascular risk factors including hypertension (52), diabetes (53, 54), and kidney disease (55-57).
Our subgroup analyses need to be interpreted cautiously. Some studies have reported differences by smoking status (6), urine arsenic metabolic patterns (4, 33), and genetic factors (58). In our study, we found stronger associations among never smokers, contrary to a stronger association among current smokers in a prospective cohort study in Bangladesh (6). The susceptibility to arsenic toxicity may also differ by sex (59), although previous studies of arsenic and cardiovascular disease found no marked differences by sex (2, 10, 35, 40, 45). We found a stronger association among women, although arsenic was associated with an increased risk of cardiovascular disease in both men and women. In studies evaluating the health effects of arsenic metabolism conducted in Taiwan, individuals with a higher % MMA or lower % DMA in urine had a higher risk of peripheral artery disease (4), carotid atherosclerosis (33), and hypertension (60). In addition, the association between arsenic exposure and cardiovascular disease was stronger among participants with higher % MMA (4, 33). In our study, conducted at low-moderate arsenic levels, we found no association between arsenic metabolic patterns in urine and cardiovascular risk. The association between arsenic and cardiovascular disease, on the other hand, was stronger among participants with higher % DMA. Additional research is needed to evaluate effect modification of arsenic exposure by arsenic metabolism in populations exposed to low-moderate arsenic levels.
We also found differences in the association between arsenic and cardiovascular disease endpoints by study region and diabetes status, subgroups that have not been evaluated before. The stronger association in Arizona compared to other regions could be related to higher arsenic exposure, residual confounding, or effect-modification by other co-exposures, differences in access to care and surveillance methods across the three study regions, and gene-arsenic interactions. In a previous linkage study in the Strong Heart Family Study we found different peaks across the genome associated with arsenic metabolism measures across study regions (61). We found stronger associations among participants with diabetes compared to those without it. Arsenic has been consistently associated with diabetes in populations exposed to high levels in drinking water (54) and recent prospective studies from the US at low-moderate levels, including Southwestern American Indians, support arsenic as a diabetes risk factor (62, 63). Diabetes could potentially be in the causal pathway between arsenic exposure and cardiovascular disease. Alternatively, diabetes could confound the association between urine arsenic exposure, as measured in urine, and cardiovascular disease. In the Strong Heart Study, baseline urine arsenic levels were associated with poor diabetes control (53). Further adjustment for glycated hemoglobin in this analysis, however, produced similar results. Overall, the stronger association between arsenic and cardiovascular disease among participants with diabetes needs to be interpreted cautiously and requires replication in other populations. Hypertension (64) and kidney disease (55-57) could also be in the causal pathway. Including these variables in the models could have resulted in overadjustment.
Strengths of this study include high quality data collection methods and surveillance for cardiovascular disease outcomes over a long follow-up (20), and rigorous laboratory methods for measuring urine arsenic species (24). Urine arsenic measurements integrate all sources of exposure at the individual level, including water and food, and are an excellent biomarker of internal dose (1, 25, 26). This study also had several limitations. We measured urine arsenic in as ingle sample at baseline and individual levels of drinking water were unavailable. The temporal stability of arsenic levels in public and private drinking water and in urine has been shown in several studies in the US (17, 65-67). Several studies have also shown consistent associations with cardiovascular endpoints comparing arsenic measured in water and urine (4, 6). Other limitations include the possibility of residual confounding (e.g., access to care, geographical factors), overadjustment for variables that could be in the causal pathway (diabetes, hypertension, kidney disease), and exposure and outcome misclassification.
More than 100 million people worldwide are exposed to arsenic levels in drinking water above the World Health Organization standard of 10 μg/L (68, 69). In 2001, the US Environmental Protection Agency estimated that 13 million Americans were exposed to drinking water above 10 μg/L (14). Many more millions are exposed to arsenic through food, although currently no standards for inorganic arsenic in food exist. Given the large population exposed, even a modest increased risk of cardiovascular disease due to arsenic could have important public health implications. Cardiovascular disease is the leading cause of the death in the US, with rates among American Indians exceeding those of the US general population (70). Arsenic mitigation could potentially reduce the burden of cardiovascular disease. Discussions to revise the current US Environmental Protection Agency safety standard for arsenic in drinking water should quantitatively consider the evidence supporting the cardiovascular disease effects of low to moderate arsenic exposure.
In conclusion, low to moderate inorganic arsenic exposure, as measured in urine, was prospectively associated with increased fatal and non-fatal cardiovascular disease in a US population with a high burden of diabetes and cardiovascular disease. These findings support the importance of low to moderate arsenic exposure as a cardiovascular risk factor with no apparent threshold.
Supplementary Material
Acknowledgments
Support: Supported by grants from the National Heart Lung and Blood Institute (HL090863, 5T32HL007024, and Strong Heart Study grants HL41642, HL41652, HL41654 and HL65521) and from the National Institute of Environmental Health Sciences (P30ES03819, R01ES021367).
Role of the Sponsor: The funders played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Address for Reprint Requests: Dr. Ana Navas-Acien, Johns Hopkins Bloomberg School of Public Health, Department of Environmental Health Sciences, 615 N Wolfe Street, Room W7513D, Baltimore, MD 21205, anavas@jhsph.edu
Author Contributions: Katherine Moon and Ana Navas-Acien had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosures: The authors have no conflicts of interest to declare.
Reproducible Research Statement: Study protocol: The original study proposal funded by the National Institutes of Health and the manuscript proposal approved by the Strong Heart Study Publications and Presentations Committee are available from Dr. Navas-Acien (email: anavas@jhsph.edu).
Statistical code: The statistical code in is available from Dr. Navas-Acien (email: anavas@jhsph.edu).
Study data: Similar to other National Heart, Lung, and Blood Institute cohorts, the Strong Heart Study data are not publically available. Outside investigators may apply to use the data generated following the established protocols for Strong Heart Study Resource and Data sharing, including community approval, through formal application (http://strongheart.ouhsc.edu/datarequest.html).
Current Postal Addresses for All Authors: Katherine A. Moon, Johns Hopkins Bloomberg School of Public Health, Department of Environmental Health Sciences, 615 N Wolfe Street, Room W7604, Baltimore, MD 21205
Dr. Eliseo Guallar, Johns Hopkins Bloomberg School of Public Health, Department of Epidemiology, 2024 E Monument Street, Room 2-639, Baltimore, MD 21205
Dr. Jason G. Umans, MedStar Health Research Institute, 6525 Belcrest Road, Suite 700, Hyattsville, MD 20782
Dr. Richard B. Devereux, Weill Medical College of Cornell Medical Center, 520 East 70th Street, Room K-4, New York, NY 10021
Dr. Lyle G. Best, 1935 188th Ave NW, Watford City, ND 58854
Dr. Kevin A. Francesconi, Institute for Chemistry - Analytical Chemistry, Stremayrgasse 16 / 3. Stock, 8010 Graz, Austria
Dr. Walter Goessler, Institute for Chemistry - Analytical Chemistry, Stremayrgasse 16 / 3. Stock, 8010 Graz, Austria
Jonathan Pollak, Johns Hopkins Bloomberg School of Public Health, Department of Environmental Health Sciences, 615 N Wolfe Street, Room W7508A, Baltimore, MD 21205
Dr. Ellen K. Silbergeld, Johns Hopkins Bloomberg School of Public Health, Department of Environmental Health Sciences, 615 N Wolfe Street, Room E6644, Baltimore, MD 21205
Dr. Barbara V. Howard, MedStar Health Research Institute, 6525 Belcrest Road, Suite 700, Hyattsville, MD 20782
Dr. Ana Navas-Acien, Johns Hopkins Bloomberg School of Public Health, Department of Environmental Health Sciences, 615 N Wolfe Street, Room W7513D, Baltimore, MD 21205
References
- 1.National Research Council. Arsenic in Drinking Water Subcommittee on Arsenic in Drinking Water, National Research Council. Washington D.C.: The National Academy Press; 1999. [Google Scholar]
- 2.Cheng TJ, Ke DS, Guo HR. The association between arsenic exposure from drinking water and cerebrovascular disease mortality in Taiwan. Water Res. 2010;44(19):5770–6. doi: 10.1016/j.watres.2010.05.040. [DOI] [PubMed] [Google Scholar]
- 3.Chen CJ, Chiou HY, Chiang MH, Lin LJ, Tai TY. Dose-response relationship between ischemic heart disease mortality and long-term arsenic exposure. Arterioscler Thromb Vasc Biol. 1996;16(4):504–10. doi: 10.1161/01.atv.16.4.504. [DOI] [PubMed] [Google Scholar]
- 4.Tseng CH, Huang YK, Huang YL, Chung CJ, Yang MH, Chen CJ, et al. Arsenic exposure, urinary arsenic speciation, and peripheral vascular disease in blackfoot disease-hyperendemic villages in Taiwan. Toxicol Appl Pharmacol. 2005;206(3):299–308. doi: 10.1016/j.taap.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 5.Sohel N, Persson LA, Rahman M, Streatfield PK, Yunus M, Ekstrom EC, et al. Arsenic in drinking water and adult mortality: a population-based cohort study in rural Bangladesh. Epidemiology. 2009;20(6):824–30. doi: 10.1097/EDE.0b013e3181bb56ec. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Graziano JH, Parvez F, Liu M, Slavkovich V, Kalra T, et al. Arsenic exposure from drinking water and mortality from cardiovascular disease in Bangladesh: prospective cohort study. BMJ. 2011;342:d2431. doi: 10.1136/bmj.d2431. Epub 2011/05/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuan Y, Marshall G, Ferreccio C, Steinmaus C, Selvin S, Liaw J, et al. Acute myocardial infarction mortality in comparison with lung and bladder cancer mortality in arsenic-exposed region II of Chile from 1950 to 2000. Am J Epidemiol. 2007;166(12):1381–91. doi: 10.1093/aje/kwm238. [DOI] [PubMed] [Google Scholar]
- 8.Wade TJ, Xia Y, Wu K, Li Y, Ning Z, Le XC, et al. Increased mortality associated with well-water arsenic exposure in Inner Mongolia, China. Int J Environ Res Public Health. 2009;6(3):1107–23. doi: 10.3390/ijerph6031107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xia Y, Wade TJ, Wu K, Li Y, Ning Z, Le XC, et al. Well water arsenic exposure, arsenic induced skin-lesions and self-reported morbidity in Inner Mongolia. Int J Environ Res Public Health. 2009;6(3):1010–25. doi: 10.3390/ijerph6031010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Afridi HI, Kazi TG, Kazi N, Kandhro GA, Baig JA, Jamali MK, et al. Association of environmental toxic elements in biological samples of myocardial infarction patients at different stages. Biol Trace Elem Res. 2011;141(1-3):26–40. doi: 10.1007/s12011-010-8713-2. [DOI] [PubMed] [Google Scholar]
- 11.Afridi HI, Kazi TG, Kazi N, Kandhro GA, Baig JA, Shah AQ, et al. Evaluation of toxic elements in scalp hair samples of myocardial infarction patients at different stages as related to controls. Biol Trace Elem Res. 2010;134(1):1–12. doi: 10.1007/s12011-009-8450-6. [DOI] [PubMed] [Google Scholar]
- 12.Navas-Acien A, Sharrett AR, Silbergeld EK, Schwartz BS, Nachman KE, Burke TA, et al. Arsenic exposure and cardiovascular disease: a systematic review of the epidemiologic evidence. American Journal of Epidemiology. 2005;162(11):1037–49. doi: 10.1093/aje/kwi330. [DOI] [PubMed] [Google Scholar]
- 13.Moon K, Guallar E, Navas-Acien A. Arsenic exposure and cardiovascular disease:an updated systematic review. Curr Atheroscler Rep. 2012;14(6):542–55. doi: 10.1007/s11883-012-0280-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.U.S. Environmental Protection Agency. National primary drinking water regulations; arsenic and clarifications to compliance and new source contaminants monitoring; final rule. Federal Register. 2001;(66):6976–7066. [Google Scholar]
- 15.Focazio MJ, Welch AH, Watkins SA, Helsel DR, Horn MA. A Retrospective Analysis on the Occurrence of Arsenic in Ground-Water Resources of the United States and Limitations in Drinking-Water-Supply Characterizations. Reston, VA: U.S. Geological Survey; 2000. Water-Resources Investigations Report 99-4279. [Google Scholar]
- 16.Howard BV, Welty TK, Fabsitz RR, Cowan LD, Oopik AJ, Le NA, et al. Risk factors for coronary heart disease in diabetic and nondiabetic Native Americans. The Strong Heart Study. Diabetes. 1992;41(Suppl 2):4–11. doi: 10.2337/diab.41.2.s4. [DOI] [PubMed] [Google Scholar]
- 17.Navas-Acien A, Umans JG, Howard BV, Goessler W, Francesconi KA, Crainiceanu CM, et al. Urine arsenic concentrations and species excretion patterns in American Indian communities over a 10-year period: the Strong Heart Study. Environmental Health Perspectives. 2009;117(9):1428–33. doi: 10.1289/ehp.0800509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurzius-Spencer M, O'Rourke MK, Hsu CH, Hartz V, Harris RB, Burgess JL. Measured versus modeled dietary arsenic and relation to urinary arsenic excretion and total exposure. J Expo Sci Environ Epidemiol. 2013 doi: 10.1038/jes.2012.120. Epub 2013/01/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Navas-Acien A, Nachman KE. Public Health Responses to Arsenic in Rice and Other Foods. JAMA internal medicine. 2013:1–2. doi: 10.1001/jamainternmed.2013.6405. Epub 2013/05/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee ET, Welty TK, Fabsitz R, Cowan LD, Le NA, Oopik AJ, et al. The Strong Heart Study. A study of cardiovascular disease in American Indians: design and methods. Am J Epidemiol. 1990;132(6):1141–55. doi: 10.1093/oxfordjournals.aje.a115757. [DOI] [PubMed] [Google Scholar]
- 21.Stoddart ML, Jarvis B, Blake B, Fabsitz RR, Howard BV, Lee ET, et al. Recruitment of American Indians in epidemiologic research: the Strong Heart Study. Am Indian AlskNative MentHealth Res. 2000;9(3):20–37. doi: 10.5820/aian.0903.2000.20. [DOI] [PubMed] [Google Scholar]
- 22.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–266. [PubMed] [Google Scholar]
- 23.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scheer J, Findenig S, Goessler W, Francesconi KA, Howard B, Umans JG, et al. Arsenic species and selected metals in human urine: validation of HPLC/ICPMS and ICPMS procedures for a long-term population-based epidemiological study. Analytical methods: advancing methods and applications. 2012;4(2):406–13. doi: 10.1039/c2ay05638k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marchiset-Ferlay N, Savanovitch C, Sauvant-Rochat MP. What is the best biomarker to assess arsenic exposure via drinking water? Environ Int. 2012;39(1):150–71. doi: 10.1016/j.envint.2011.07.015. Epub 2012/01/03. [DOI] [PubMed] [Google Scholar]
- 26.Hughes MF. Biomarkers of exposure: a case study with inorganic arsenic. Environ Health Perspect. 2006;114(11):1790–6. doi: 10.1289/ehp.9058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pomroy C, Charbonneau SM, McCullough RS, Tam GK. Human retention studies with 74As. Toxicol Appl Pharmacol. 1980;53(3):550–6. doi: 10.1016/0041-008x(80)90368-3. [DOI] [PubMed] [Google Scholar]
- 28.Cullen WR, Reimer KJ. Arsenic Speciation in the Environment. Chemical Reviews. 1989;89(4):713–64. [Google Scholar]
- 29.Strong Heart Study. Strong Heart Study Operations Manual. Phase IV. Volume II: Morbidity and Mortality Surveillance Procedures. 2001 [cited July 2013]. Available from: http://strongheart.ouhsc.edu/manual/PhaseIV/Volume2.html.
- 30.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515–26. doi: 10.1093/biomet/81.3.515. [DOI] [Google Scholar]
- 31.Nermell B, Lindberg AL, Rahman M, Berglund M, Persson LA, El Arifeen S, et al. Urinary arsenic concentration adjustment factors and malnutrition. Environ Res. 2008;106(2):212–8. doi: 10.1016/j.envres.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 32.Voinescu GC, Shoemaker M, Moore H, Khanna R, Nolph KD. The relationship between urine osmolality and specific gravity. American Journal of the Medical Sciences. 2002;323(1):39–42. doi: 10.1097/00000441-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Huang YL, Hsueh YM, Huang YK, Yip PK, Yang MH, Chen CJ. Urinary arsenic methylation capability and carotid atherosclerosis risk in subjects living in arsenicosis-hyperendemic areas in southwestern Taiwan. Sci Total Environ. 2009;407(8):2608–14. doi: 10.1016/j.scitotenv.2008.12.061. [DOI] [PubMed] [Google Scholar]
- 34.Tseng CH. Blackfoot disease and arsenic: a never-ending story. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2005;23(1):55–74. doi: 10.1081/gnc-200051860. [DOI] [PubMed] [Google Scholar]
- 35.Engel RR, Smith AH. Arsenic in drinking water and mortality from vascular disease: an ecologic analysis in 30 counties in the United States. ArchEnvironHealth. 1994;49(5):418–27. doi: 10.1080/00039896.1994.9954996. [DOI] [PubMed] [Google Scholar]
- 36.Zaldivar R. A morbid condition involving cardio-vascular, broncho-pulmonary, digestive and neural lesions in children and young adults after dietary arsenic exposure. ZentralblBakteriol[B] 1980;170(1-2):44–56. [PubMed] [Google Scholar]
- 37.Yoshikawa M, Aoki K, Ebine N, Kusunoki M, Okamoto A. Correlation between the arsenic concentrations in the air and the SMR of lung cancer. Environ Health Prev Med. 2008;13(4):207–18. doi: 10.1007/s12199-008-0032-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rapant S, Cvecková V, Dietzová Z, Khun M, Letkovicová M. Medical geochemistry research in Spissko-Gemerské rudohorie Mts., Slovakia. Environmental geochemistry and health. 2009;31(1):11–25. doi: 10.1007/s10653-008-9152-2. [DOI] [PubMed] [Google Scholar]
- 39.Varsányi I, Fodré Z, Bartha A. Arsenic in drinking water and mortality in the Southern Great Plain, Hungary. Environmental Geochemistry and Health. 1991;13(1):14–22. doi: 10.1007/BF01783491. [DOI] [PubMed] [Google Scholar]
- 40.Medrano MAJ, Boix R, Pastor-Barriuso R, Palau M, Damián J, Ramis R, et al. Arsenic in public water supplies and cardiovascular mortality in Spain. Environ Res. 2010;110(5):448–54. doi: 10.1016/j.envres.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 41.Lisabeth LD, Ahn HJ, Chen JJ, Sealy-Jefferson S, Burke JF, Meliker JR. Arsenic in drinking water and stroke hospitalizations in Michigan. Stroke. 2010;41(11):2499–504. doi: 10.1161/strokeaha.110.585281. [DOI] [PubMed] [Google Scholar]
- 42.Gong G, O'Bryant SE. Low-level arsenic exposure, AS3MT gene polymorphism and cardiovascular diseases in rural Texas counties. Environ Res. 2012;113:52–7. doi: 10.1016/j.envres.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 43.Meliker JR, Wahl RL, Cameron LL, Nriagu JO. Arsenic in drinking water and cerebrovascular disease, diabetes mellitus, and kidney disease in Michigan: a standardized mortality ratio analysis. Environ Health. 2007;6:4. doi: 10.1186/1476-069X-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zierold KM, Knobeloch L, Anderson H. Prevalence of chronic diseases in adults exposed to arsenic-contaminated drinking water. Am J Public Health. 2004;94(11):1936–7. doi: 10.2105/ajph.94.11.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lewis DR, Southwick JW, Ouellet-Hellstrom R, Rench J, Calderon RL. Drinking water arsenic in Utah: A cohort mortality study. Environ Health Perspect. 1999;107(5):359–65. doi: 10.1289/ehp.99107359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruiz-Navarro ML, Navarro-Alarcon M, Lopez Gonzalez-de la S, Perez-Valero V, Lopez-Martinez MC. Urine arsenic concentrations in healthy adults as indicators of environmental contamination: relation with some pathologies. Sci Total Environ. 1998;216(1-2):55–61. doi: 10.1016/s0048-9697(98)00136-3. [DOI] [PubMed] [Google Scholar]
- 47.Srivastava S, Vladykovskaya EN, Haberzettl P, Sithu SD, D'Souza SE, States JC. Arsenic exacerbates atherosclerotic lesion formation and inflammation in ApoE-/- mice. Toxicol Appl Pharmacol. 2009;241(1):90–100. doi: 10.1016/j.taap.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simeonova PP, Hulderman T, Harki D, Luster MI. Arsenic exposure accelerates atherogenesis in apolipoprotein E(-/-) mice. Environmental health perspectives. 2003;111(14):1744–8. doi: 10.1289/ehp.6332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bunderson M, Brooks DM, Walker DL, Rosenfeld ME, Coffin JD, Beall HD. Arsenic exposure exacerbates atherosclerotic plaque formation and increases nitrotyrosine and leukotriene biosynthesis. Toxicol Appl Pharmacol. 2004;201(1):32–9. doi: 10.1016/j.taap.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 50.States JC, Srivastava S, Chen Y, Barchowsky A. Arsenic and cardiovascular disease. Toxicol Sci. 2009;107(2):312–23. doi: 10.1093/toxsci/kfn236. Epub 2008/11/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Balakumar P, Kaur J. Arsenic exposure and cardiovascular disorders: an overview. Cardiovasc Toxicol. 2009;9(4):169–76. doi: 10.1007/s12012-009-9050-6. [DOI] [PubMed] [Google Scholar]
- 52.Abhyankar LN, Jones MR, Guallar E, Navas-Acien A. Arsenic exposure and hypertension: a systematic review. Environmental health perspectives. 2012;120(4):494–500. doi: 10.1289/ehp.1103988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gribble MO, Howard BV, Umans JG, Shara NM, Francesconi KA, Goessler W, et al. Arsenic exposure, diabetes prevalence, and diabetes control in the strong heart study. American journal of epidemiology. 2012;176(10):865–74. doi: 10.1093/aje/kws153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maull EA, Ahsan H, Edwards J, Longnecker MP, Navas-Acien A, Pi J, et al. Evaluation of the Association between Arsenic and Diabetes: A National Toxicology Program Workshop Review. Environ Health Perspect. 2012;120(12):1658–70. doi: 10.1289/ehp.1104579. Epub 2012/08/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zheng LY, Umans JG, Tellez-Plaza M, Yeh F, Francesconi KA, Goessler W, et al. Urine arsenic and prevalent albuminuria: evidence from a population-based study. Am J Kidney Dis. 2013;61(3):385–94. doi: 10.1053/j.ajkd.2012.09.011. Epub 2012/11/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen Y, Parvez F, Liu M, Pesola GR, Gamble MV, Slavkovich V, et al. Association between arsenic exposure from drinking water and proteinuria: results from the Health Effects of Arsenic Longitudinal Study. Int J Epidemiol. 2011;40(3):828–35. doi: 10.1093/ije/dyr022. Epub 2011/02/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.James K, Meliker JR. Is arsenic a contributor to CKD? Am J Kidney Dis. 2013;61(3):364–5. doi: 10.1053/j.ajkd.2012.12.004. Epub 2013/02/19. [DOI] [PubMed] [Google Scholar]
- 58.Hsieh YC, Lien LM, Chung WT, Hsieh FI, Hsieh PF, Wu MM, et al. Significantly increased risk of carotid atherosclerosis with arsenic exposure and polymorphisms in arsenic metabolism genes. Environ Res. 2011;111(6):804–10. doi: 10.1016/j.envres.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 59.Vahter M, Akesson A, Lidén C, Ceccatelli S, Berglund M. Gender differences in the disposition and toxicity of metals. Environ Res. 2007;104(1):85–95. doi: 10.1016/j.envres.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 60.Huang YK, Tseng CH, Huang YL, Yang MH, Chen CJ, Hsueh YM. Arsenic methylation capability and hypertension risk in subjects living in arseniasis-hyperendemic areas in southwestern Taiwan. Toxicol Appl Pharmacol. 2007;218(2):135–42. doi: 10.1016/j.taap.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 61.Tellez-Plaza M, Gribble MO, Voruganti VS, Francesconi KA, Goessler W, Umans JG, et al. Heritability and Preliminary Genome-Wide Linkage Analysis of Arsenic Metabolites in Urine. Environ Health Perspect. 2013 doi: 10.1289/ehp.1205305. Epub 2013/01/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.James KA, Marshall JA, Hokanson JE, Meliker JR, Zerbe GO, Byers TE. A case-cohort study examining lifetime exposure to inorganic arsenic in drinking water and diabetes mellitus. Environ Res. 2013;123:33–8. doi: 10.1016/j.envres.2013.02.005. Epub 2013/03/20. [DOI] [PubMed] [Google Scholar]
- 63.Kim NH, Mason CC, Nelson RG, Afton SE, Essader AS, Medlin JE, et al. Arsenic Exposure and Incidence of Type 2 Diabetes in Southwestern American Indians. Am J Epidemiol. 2013 doi: 10.1093/aje/kws329. Epub 2013/03/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Abhyankar LN, Jones MR, Guallar E, Navas-Acien A. Arsenic exposure and hypertension: a systematic review. Environ Health Perspect. 2012;120(4):494–500. doi: 10.1289/ehp.1103988. Epub 2011/12/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Karagas MR, Le CX, Morris S, Blum J, Lu X, Spate V, et al. Markers of low level arsenic exposure for evaluating human cancer risks in a US population. Int J Occup Med Environ Health. 2001;14(2):171–5. [PubMed] [Google Scholar]
- 66.Ryan PB, Huet N, MacIntosh DL. Longitudinal investigation of exposure to arsenic, cadmium, and lead in drinking water. Environ Health Perspect. 2000;108(8):731–5. doi: 10.1289/ehp.00108731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Steinmaus CM, Yuan Y, Smith AH. The temporal stability of arsenic concentrations in well water in western Nevada. Environ Res. 2005;99(2):164–8. doi: 10.1016/j.envres.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 68.Alaerts GJ, Khouri N, Kabir B. Arsenic in Drinking Water United Nations Synthesis Report on Arsenic in Drinking Water. Washington, DC, USA: The World Bank; 2001. Strategies to mitigate arsenic contamination of water supply. [Google Scholar]
- 69.Parvez F, Chen Y, Argos M, Hussain AZMI, Momotaj H, Dhar R, et al. Prevalence of arsenic exposure from drinking water and awareness of its health risks in a Bangladeshi population: results from a large population-based study. Environmental health perspectives. 2006;114(3):355–9. doi: 10.1289/ehp.7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Howard BV, Lee ET, Cowan LD, Devereux RB, Galloway JM, Go OT, et al. Rising tide of cardiovascular disease in American Indians. The Strong Heart Study. Circulation. 1999;99(18):2389–95. doi: 10.1161/01.cir.99.18.2389. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.