Abstract
Granzyme B (GrzB) is expressed by activated T cells and mediates cellular apoptosis. GrzB also acts as an extracellular protease involved in tissue degradation. We hypothesized that GrzB production from activated memory CD4 T cells may be associated with HIV pathogenesis. We found that stimulated memory CD4 T cells (via costimulation, cytokines, and TLR ligands) concomitantly produced GrzB and HIV. Both GrzB and HIV expression were mainly restricted to CCR5-expressing memory CD4+CD45RO+ T cells, including Th1 and Th17 subsets. Activated memory CD4 T cells also mediated tissue damage, such as disruption of intestinal epithelial monolayers. In non-human primates, CD4 T cells of rhesus macaques (pathogenic SIV hosts) expressed higher GrzB compared to African green monkeys (non-pathogenic SIV hosts). These results suggest that GrzB from CCR5+ memory CD4 T cells may have a role in cellular and tissue pathologies during HIV infection.
Keywords: CCR5, Granzyme B, HIV replication, Enteropathy, Memory CD4 T cells, SIV pathogenesis
Introduction
HIV infection is characterized by CD4 T cell dysfunction and death, chronic immune activation, and tissue pathologies including lymph node destruction, enteropathy, adipose wasting, and autoimmune diseases. The mechanisms and mediators by which HIV infection causes these problems are complex and unclear. HIV replicates most productively in memory CD4 T cells that are activated by stimulants such as CD3/TCR agonism, cytokines, or TLR ligands. These stimulants activate signaling pathways in infected CD4 T cells such as NFκB to induce HIV replication. In addition to activating HIV production, however, these stimulants also upregulate other mediators in CD4 T cells such as cytokines, chemokines, and enzymes such as granzyme B (GrzB), that mediate CD4 T cell function, and that might function in HIV pathogenesis. Because HIV production by memory CD4 T cells involves mechanisms that also regulate GrzB production, we explored the idea that HIV and GrzB may have a unique relationship in activated CD4 T cells that could influence HIV pathogenesis.
Granzymes are serine proteases that have intracellular and extracellular functions. Humans encode five granzymes (A, B, H, K, and M), with GrzB being the best characterized. Although better known as an important effector molecule of CD8 CTL’s and NK cells for eliminating infected or damaged cells, GrzB is important for CD4 T cell effector functions as well. Naïve CD4+CD45RA+ T cells do not express GrzB; CTL function and GrzB expression are acquired following CD4 T cell activation and differentiation into memory and effector subsets [Appay et al., 2002, Brown, 2010, Zaunders et al., 2004]. Antigen-specific CD4 CTL’s eliminate infected cells via GrzB/perforin and GrzA during infection with viruses such as HIV, CMV, HSV, RSV, and LCMV [Casazza et al., 2006, Hildemann et al., 2013, Loebbermann et al., 2012, Soghoian et al., 2012, Yanai et al., 2003]. CD4 CTL’s are also important for anti-tumor immunity by killing cancer cells via GrzB/perforin [Quezada et al., 2010]. Other effector CD4 T cell subsets including Th1, Th17, and Tregs also produce GrzB for death-inducing or suppressive functions [Ashley and Baecher-Allan, 2009, Cao et al., 2007, Gondek et al., 2005, Grossman et al., 2004, Loebbermann et al., 2012, Sharma et al., 2006]. We previously showed that despite higher constitutive intracellular protein expression of GrzB by resting memory CD8 T cells compared to resting memory CD4 T cells (purified from human peripheral blood), activated memory CD4 T cells secrete substantial amounts of GrzB at similar or higher levels than memory CD8 T cells [Medina et al., 2012]. GrzB from memory CD4 T cells is also biologically active because it cleaves a specific substrate, kills bystander T cell lines, and induces some disruption of Caco-2 epithelial monolayer integrity.
A key difference between natural (non-pathogenic) vs. non-natural (pathogenic) SIV host non-human primates (NHP) is that non-natural SIV hosts manifest AIDS-like complications similar to humans, such as enteropathy and chronic immune activation, whereas natural SIV hosts remain mostly pathogenesis-free without these effects. The reasons for these differences are unclear, but we found by immunohistochemical analysis of lamina propria from NHP intestinal biopsies that uninfected non-natural SIV hosts (rhesus macaques and pigtail macaques) contain more GrzB-expressing CD4 T cells than natural SIV hosts (African green monkeys and sooty mangabeys) [Hutchison et al., 2011]. This data suggested that GrzB from intestinal CD4 T cells could have a pathological role in pathogenic SIV hosts. HIV and GrzB are upregulated in memory CD4 T cells by similar stimulants and secretory mechanisms, but whether there is an interdependent relationship between HIV and GrzB in host cells, and if concomitant release of HIV and GrzB from CD4 T cells influence HIV pathogenesis is unknown.
The purpose of this study was to examine production of GrzB by memory CD4 T cells during HIV infection, as well as to determine the impact for induction of pathology. Within the pool of memory CD4+CD45RO+ T cells (purified from peripheral blood of healthy donors), we found that GrzB and HIV are produced mostly from CCR5+ memory CD4 T cells during in vitro HIV infection and stimulation. This association of GrzB and HIV by CCR5+ memory CD4 T cells may have important implications for HIV pathogenesis in vivo since CCR5 is highly expressed by memory CD4 T cells in lymphoid, mucosal, and intestinal compartments where substantial levels of HIV replication as well as tissue damage occur.
Results
Secretion of GrzB by memory CD4 T cells during HIV infection
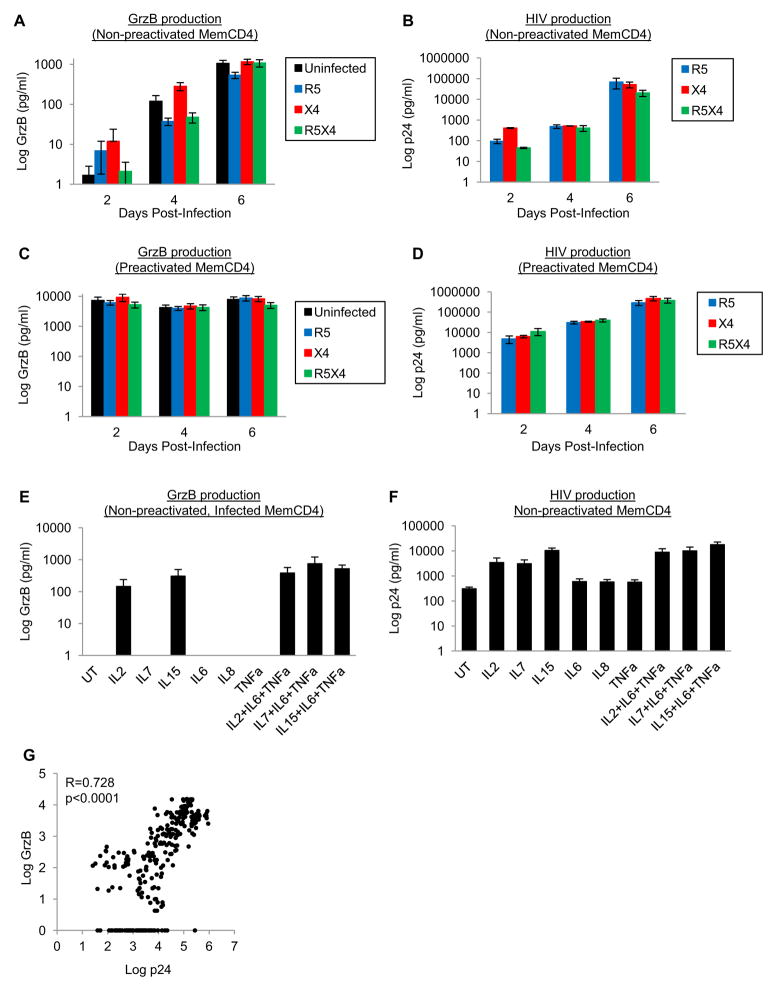
GrzB expressed by memory CD4 T cells, Th1, Th17, and Tregs is involved in target cell suppression and apoptosis, and also functions as an extracellular protease [Boivin et al., 2009, Hiebert and Granville, 2012, Hildemann et al., 2013, Soghoian et al., 2012]. GrzB and HIV are upregulated in CD4 T cells by stimulants that activate the NFκB pathway such as CD3/CD28 stimulation, and cytokines such as IL2, IL7, and IL15. To assess GrzB and HIV production from memory CD4 T cells, we first examined extracellular GrzB and HIV p24 from unactivated or activated (via CD3/CD28 costimulation) memory CD4+CD45RO+ T cells (purified from peripheral blood) after in vitro infection with HIV strains (R5-, X4-, and R5X4-tropic). Unactivated, uninfected and HIV-infected memory CD4 T cells released little to no GrzB after 2–4 days culture (<300pg/ml), but increased to ~1,000pg/ml by 6 days culture (perhaps driven by IL2 during the 24hrs infection period) (Fig. 1A). Unactivated, HIV-infected memory CD4 T cells also produced increasing amounts of p24 (from ~100pg/ml at 2 days to ~70,000pg/ml by 6 days post-infection) (Fig. 1B). There were no significant differences in GrzB production between uninfected and HIV-infected memory CD4 T cells.
Figure 1. Production of GrzB and HIV by memory CD4 T cells during HIV infection.
(A–B) Production of GrzB and HIV by non-preactivated (IL2 only stimulation) uninfected or HIV-infected (R5-, X4-, or R5X4-tropic) memory CD4 T cells 2–6 days post-infection (means±sem, n=5). (C–D) GrzB and HIV production by preactivated (CD3/CD28 costimulation) memory CD4 T cells 2–6 days post-infection (means±sem, n=5). (E–F) GrzB and HIV production by non-preactivated, HIV-infected memory CD4 T cells stimulated with common γ-chain and proinflammatory cytokines 6 days post-infection (n=3–4). (G) Pearson correlation of extracellular GrzB and p24 by memory CD4 T cells (n=301).
As expected, GrzB and HIV production in activated (CD3/CD28 costimulation) memory CD4 T cells was substantially higher compared to unactivated memory CD4 T cells (Fig. 1C–D). GrzB release from activated, uninfected memory CD4 T cells was near maximal by 2 days post-infection (~7,000pg/ml), and remained high at 4 (~4,000pg/ml) and 6 days post-infection (~8,000pg/ml) (Fig. 1C). HIV-infected memory CD4 T cells also released similar amounts of GrzB 2–6 days post-infection (~4,000–9,000pg/ml), and HIV production (Fig. 1D) was robust 2–6 days post-infection (~10,000pg/ml at 2 days to ~500,000pg/ml by 6 days).
We also examined if cytokines that enhance T cell activation and HIV replication such as IL2, IL7, IL15, IL6, IL8, and TNFα also promote GrzB production (Fig. 1E–F) [Chun et al., 1998, Unutmaz et al., 1999]. Unactivated memory CD4 T cells were infected in the presence of cytokines, washed, and cultured for 6 days with cytokines. IL2 and IL15 alone, and in combination with proinflammatory cytokines IL6, IL8, and TNFα, induced GrzB and HIV production (~100–700pg/ml GrzB and ~3,000–18,000pg/ml p24, n=3–4), whereas proinflammatory cytokines alone did not induce GrzB. IL7 in combination with proinflammatory cytokines also induced GrzB and HIV production. Thus, memory CD4 T cells secrete substantial amounts of both GrzB and HIV during acute infection, and extracellular GrzB strongly correlates with extracellular p24 (Fig. 1G, n=301). These results show that both GrzB and HIV production are functions of activated memory CD4 T cells during HIV infection.
Preferential expression of GrzB in CCR5+ memory CD4 T cells, and relationship to CD4 CTL phenotype
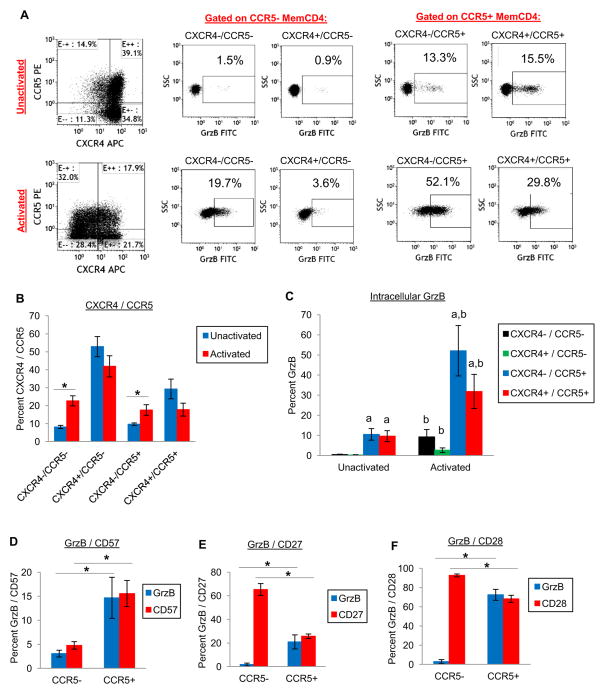
GrzB expression in CD4 T cells is associated with memory phenotype markers including CD45RO and CCR5 [Appay et al., 2002, Grossman et al., 2004, Zaunders et al., 2004]. CCR5 expression is also associated with HIV disease progression, CD4 T cell depletion, and tissue pathologies [Brenchley et al., 2004, Mattapallil et al., 2005, Yang et al., 2012]. To determine the relationship between GrzB and HIV coreceptors, we examined intracellular GrzB in conjunction with CCR5 and CXCR4 in uninfected memory CD4+CD45RO+ T cells. Resting memory CD4 T cells expressed high levels of CXCR4 and lower of levels of CCR5 (~80% total CXCR4 and ~40% total CCR5), which were both modestly reduced after 24hrs activation (Fig. 2B). In unactivated and activated memory CD4 T cells, GrzB was expressed most by CCR5+ memory CD4 T cells (Fig. 2C). Unactivated CXCR4−/CCR5− and CXCR4+/CCR5− cells did not express intracellular GrzB (<1% which was similar to isotype staining). However, GrzB expression was ~10% by CXCR4−/CCR5+ and CXCR4+/CCR5+ cells (p<0.05 compared to CXCR4−/CCR5− and CXCR4+/CCR5− cells, n=4). CD3/CD28 costimulation increased GrzB modestly in CXCR4−/CCR5− (9.3±3.6%) and CXCR4+/CCCR5− (2.7±1.2%) subsets, but robustly in CXCR4−/CCR5+ (52.1±12.5%), and CXCR4+/CCR5+ (31.9±8.5%) subsets. GrzB in activated CXCR4−/CCR5+ and CXCR4+/CCR5+ cells was also significantly higher than activated CXCR4−/CCR5− and CXCR4+/CCR5− cells (p<0.05). Although the overall expression levels of GrzB were higher by CCR5+ memory CD4 T cells, the relative induction levels of GrzB by costimulation were higher in CXCR4−/CCR5− and CXCR4+/CCR5− cells (~9-fold) compared to CXCR4−/CCR5+ and CXCR4+/CCR5+ cells (~4-fold). These data show that within the population of memory CD4+CD45RO+ T cells, CCR5+ cells harbor the most GrzB, although CCR5− negative memory CD4 T cells may produce GrzB as well after T cell activation.
Figure 2. Expression of GrzB by CCR5/CXCR4 memory CD4 T cells, and association with memory CD4 CTL phenotype.
(A–C) CXCR4 and CCR5 expression by memory CD4 T cells, and intracellular GrzB of unactivated or activated (CD3/CD28 costimulation) memory CD4 T cells after 24hrs culture. (A) Representative flow cytometry dotplots. (B) CXCR4/CCR5 expression (means±sem, *p<0.05, n=4), and (C) GrzB expression gated on either CXCR4−/CCR5−, CXCR4+/CCR5−, CXCR4−/CCR5+, or CXCR4+/CCR5+ cells (ap<0.05 compared to both CXCR4−/CCR5− and CXCR4+/CCR5− of unactivated or activated cells, bp<0.05 compared to CXCR4−/CCR5−, CXCR4+/CCR5−, CXCR4−/CCR5+ or CXCR4+/CCR5+ of unactivated cells). (D–F) Expression of GrzB, CD57, CD27, and CD28 by CCR5+ or CCR5− memory CD4 T cells (ap<0.05 compared to GrzB of CCR5− cells, bp<0.05 compared to CD57, CD27, or CD28 of CCR5− cells, n=3).
GrzB-expressing CD4 T cells are classified as CD4 CTL’s that are terminally-differentiated memory CD4+CD45RO+ T cells with increased CD57, and decreased CD27 and CD28 expression [Appay et al., 2002, Brown, 2010, Casazza et al., 2006]. To better understand GrzB expression in CCR5+ memory CD4 T cells in the context of CD4 CTL phenotypes, we examined expression of CD57, CD27, and CD28 in CCR5− and CCR5+ memory CD4 T cells. GrzB expression was <3% in CCR5− memory CD4 T cells, but significantly higher at >15% (p<0.05, n=3) in CCR5+ memory CD4 T cells (Fig. 2D–F). CD57 expression by CCR5− memory CD4 T cells was ~5%, and higher at ~15% (p<0.05) by CCR5+ memory CD4 T cells (Fig. 2D). CD27 expression by CCR5− memory CD4 T cells was ~65%, and lower at ~25% (p<0.05) by CCR5+ memory CD4 T cells (Fig. 2E). CD28 expression by CCR5− memory CD4 T cells was ~92%, and lower at ~68% (p<0.05) by CCR5+ memory CD4 T cells (Fig. 2F). These expression patterns indicate that CCR5+GrzB+ memory CD4 T cells are not strictly associated with markers of CD4 CTL’s since CCR5+ cells that express CD27, as well as CD28, also produce GrzB.
GrzB production by CCR5+ differentiated naïve CD4, Th1, and Th17 cells
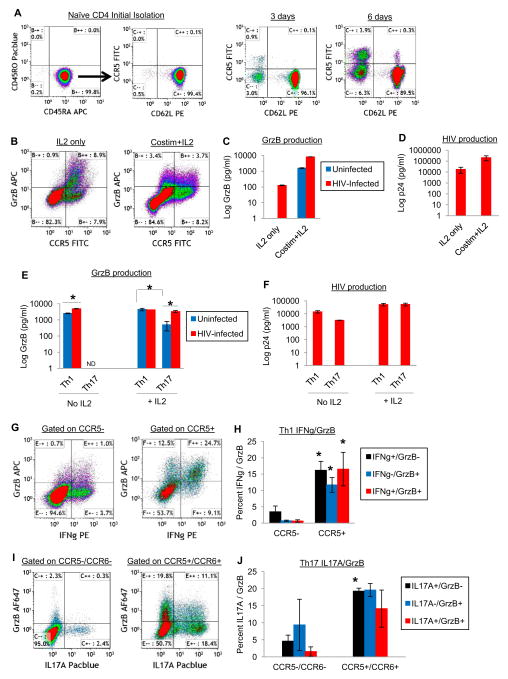
We additionally examined GrzB production by activated naïve CD4 T cells since GrzB expression is acquired during differentiation into memory and effector CD4 T cells (Fig. 3A–D). As expected, initially purified naive CD4+/CD45RA+/CD45RO− /CD62L+ T cells did not express CCR5 (Fig. 3A). However, this phenotype changed on a small fraction of cells during 6 days culture so that CD62L expression decreased and constitutive CCR5 expression was increased on CD62L− cells (with or without IL2). Longer-term experiments (~10 days) of uninfected and HIV-infected activated naïve CD4 T cell stimulated with IL2 alone or CD3/CD28 costimulation+IL2 (generally Th1-polarizing conditions) showed that differentiated naïve CD4 T cells coexpressed CCR5 and GrzB, in conjunction with release of GrzB and p24 (Fig. 3B–D). Interestingly, extracellular GrzB of differentiated naïve CD4 T cells was increased by HIV infection in 3 experiments when cultured in IL2 only, and increased by infection in 2 of 3 experiments if costimulated and cultured with IL2 (Fig. 3C shows a representative GrzB ELISA).
Figure 3. GrzB production by CCR5+ activated naïve CD4, Th1, and Th17 cells.
(A) Constitutive upregulation of CCR5 by naïve CD4 T cells. Naïve CD4 T cells were purified from peripheral blood and cultured in complete RPMI medium without IL2 for 3–6 days (dotplots are representative of 3–4 experiments). (B–D) GrzB and HIV production by activated naïve CD4 T cells. Purified naïve CD4 T cells were stimulated with IL2 only or CD3/CD28 costimulation+IL2 for 3 days. Cells were then uninfected or infected with HIV for 1 day, washed, and cultured with IL2 for 6 days. GrzB expression by uninfected CCR5+ differentiated naïve CD4 T cells (B), and extracellular GrzB (C, one representative ELISA) and p24 (D, means±sem, n=3) of differentiated naïve CD4 T cells after 6 days post-infection culture. (E–F) Extracellular GrzB and p24 of Th1 or Th17 cells 5 days post-infection +/− IL2 (means±sem, *p<0.01, n=4). (G–H) GrzB coexpression with IFNγ by CCR5+ Th1 cells. Th1 cells were cultured in IL2 medium for 5 days, then stimulated with PMA/IO+GolgiPlug for the final 5hrs and stained by flow cytometry (*p<0.05 compared to respective CCR5− IFNγ/GrzB cells, n=4). (I–J) GrzB coexpression with IL17A by CCR5+/CCR6+ Th17 cells. Th17 cells were cultured in IL2 medium for 5 days, then stimulated with PMA/IO+GolgiPlug for the final 5hrs and stained by flow cytometry (*p<0.05 compared to respective CCR5−/CCR6− IL17A/GrzB cells, n=3).
Th1 and Th17 effector CD4 T cells are HIV hosts that also express GrzB. Th1 and Th17 effector functions are also associated with numerous tissue pathologies. We purified Th1 and Th17 cells from peripheral blood, infected them in vitro, and examined GrzB and HIV production after 6 days culture +/− IL2 stimulation (Fig. 3E–J). Cells were stimulated with PMA/IO+GolgiPlug for the final 5hrs to examine GrzB coexpression with IFNγ (Th1) or IL17A (Th17) in CCR5+ cells by flow cytometry. Th1 and Th17 cells secreted GrzB and HIV with IL2 stimulation, with surprisingly high amounts of constitutive GrzB released by unstimulated Th1 cells (Fig. 3E). Unstimulated, uninfected Th1 cells released ~2,500pg/ml GrzB, whereas HIV-infected Th1 cells released ~5,000pg/ml (p<0.01 compared to uninfected Th1 cells, n=4). IL2-stimulated, uninfected and HIV-infected Th1 cells released similar amounts of GrzB (~4,000pg/ml). Unstimulated, uninfected or HIV-infected Th17 cells released no GrzB, whereas IL2-stimulated, uninfected Th17 cells released ~600pg/ml, and HIV-infected Th17 cells released ~3,000pg/ml GrzB (Fig. 3E, p<0.05 compared to uninfected Th17 cells, n=4). Stimulated, uninfected Th1 cells also released more GrzB than Th17 cells (p<0.01). HIV production by Th1 cells and Th17 cells was ~50,000pg/ml by 6 days post-infection (Fig. 3F). Lastly, after 6 days culture of Th1 and Th17 cells in IL2 medium, GrzB coexpression with IFNγ or IL17A in CCR5+ and CCR6+ (for Th17) cells was examined by intracellular cytokine assay (PMA/IO+GolgiPlug during the final 5hrs of culture). Expression of IFNγ and GrzB was higher in CCR5+ cells compared to CCR5− Th1 cells (Fig. 3G–H, p<0.05, n=4), and expression of IL17A and GrzB was higher in CCR5+/CCR6+ cells compared CCR5−/CCR6− Th17 cells (Fig. 3I–J, p<0.05, n=3). Thus, coordinate release of GrzB and HIV can occur from specific memory and effector CD4 T cell subsets, as well as from differentiating naïve CD4 T cells following activation.
GrzB and HIV production by CCR5+ memory CD4 T cells during HIV replication, and limited capacity of memory CD4 T cells to express GrzB and p24 protein simultaneously
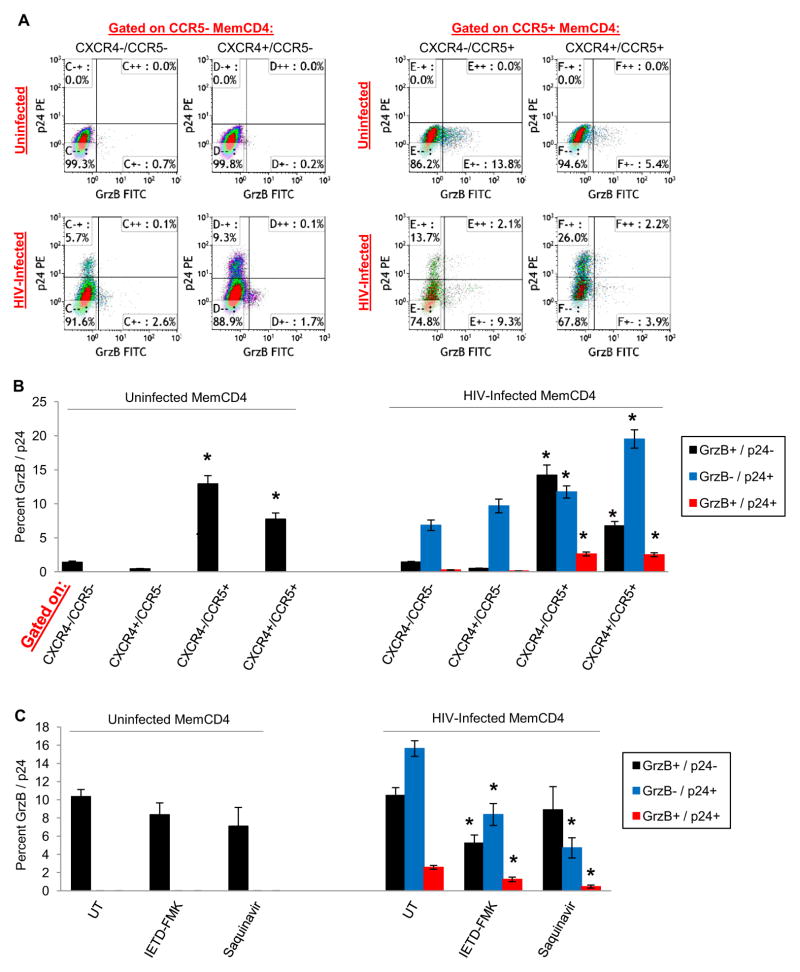
We next examined the relationship between intracellular GrzB and HIV p24 protein in memory CD4 T cells during HIV replication. We infected memory CD4 T cells and examined GrzB in conjunction with p24 in CXCR4/CCR5 populations after 6 days culture in IL2 medium (Fig. 4A–B). Uninfected CXCR4−/CCR5− and CXCR4+/CCR5− cells expressed no GrzB (~1%, similar to isotype staining), whereas CXCR4−/CCR5+ cells expressed ~13% and CXCR4+/CCR5+ cells expressed ~8% GrzB (p<0.05 compared to CXCR4−/CCR5− and CXCR4+/CCR5− cells, n>20) (Fig. 4B left). GrzB expression by uninfected memory CD4 T cells was mostly unaffected following infection with HIV. Regardless of the HIV strain used (R5-, X4-, or R5X4-tropic), we observed similar GrzB/p24 percentages between strains in CXCR4/CCR5 populations and therefore pooled all the infection data for analysis (Fig. 4B right). Intracellular p24 was found in all CXCR4/CCR5 populations, but was greatest in CXCR4−/CCR5+ and CXCR4+/CCR5+ cells (~10–20%, p<0.05 compared to CXCR4−/CCR5− and CXCR4+/CCR5− cells, n>20). GrzB without p24 expression was ~14% in CXCR4−/CCR5+ cells, and ~7% in CXCR4+/CCR5+ cells (similar to uninfected cells). p24 without GrzB expression was ~12% in CXCR4−/CCR5+ cells, and ~20% in CXCR4+/CCR5+ cells. However, a minor percentage of dual-expressing GrzB+/p24+ cells was observed in CXCR4−/CCR5+ and CXCR4+/CCR5+ cells (~3%), indicating that a small fraction of infected CCR5+ memory CD4 T cells is capable of simultaneous GrzB and p24 production. Thus, GrzB and HIV are produced mostly by CCR5+ memory CD4 T cells, mainly independently.
Figure 4. Intracellular HIV p24 and GrzB production by CCR5+ memory CD4 T cells.
(A) Representative flow cytometry dotplots of intracellular GrzB and p24 gated on either CXCR4−/CCR5−, CXCR4+/CCR5−, CXCR4−/CCR5+, or CXCR4+/CCR5+ uninfected or HIV-infected (R5-tropic) memory CD4 T cells after 5 days culture in IL2 medium (n=4). (B) Intracellular GrzB and p24 (means±sem, n>20) of uninfected or HIV-infected (pooled R5, X4, and R5X4-tropic) memory CD4 T cells (*p<0.05 compared to CXCR4−/CCR5− and CXCR4+/CCR5− of respective GrzB/p24 cells). (C) Reduction of GrzB and HIV production by CCR5+ memory CD4 T cells by pharmacological inhibitors of GrzB (IETD-FMK) or HIV (saquinavir) (means±sem, *p<0.05 compared to respective UT GrzB/p24 cells, n=7).
To further assess an interdependent relationship between GrzB and HIV in memory CD4 T cells, we examined GrzB and HIV expression during treatment of cells with pharmacological protein inhibitors specific for GrzB (IETD-FMK compound) or HIV (protease inhibitor saquinavir). GrzB and p24 percentages in CCR5+ cells were then examined (Fig. 4C). In uninfected cells, IETD-FMK or saquinavir did not affect GrzB expression compared to untreated cells. However, in HIV-infected cells, these compounds had suppressive effects on both GrzB and HIV production. In untreated (UT) cells: GrzB without p24 expression was 10.5±0.9%; p24 without GrzB was 15.6±0.9%; and dual-expressing GrzB+/p24+ cells was 2.6±0.2% (n>20). In IETD-FMK treated cells: GrzB without p24 fell to 5.2±0.9%; p24 without GrzB was reduced to 8.4±1.2%; and GrzB+/p24+ was also reduced to 1.2±0.3% (p<0.05 for each compared to respective UT cells, n>20). In saquinavir treated cells: GrzB+/p24− was 8.9±2.3%; GrzB−/p24+ was 4.7±1.1% (p<0.05 compared to UT cells); and GrzB+/p24+ was 0.5±0.2% (p<0.05 compared to UT cells, n=7). These data show that GrzB/caspase-specific inhibitors (IETD-FMK) also reduce intracellular HIV expression, and HIV-specific protease inhibitors (saquinavir) reduce intracellular GrzB, indicating a possible interdependent relationship between GrzB and HIV production in memory CD4 T cells.
We also examined the viability of each GrzB/p24 population (gated on CXCR4/CCR5 populations) to determine if GrzB+ cells were more prone to apoptosis. Viabilities of the GrzB+ cells were not significantly different from GrzB− cells, indicating that GrzB is not causing death of memory CD4 T cells (data not shown). The sum of these data show that both GrzB and HIV production occurs most in CCR5+ memory CD4 T cells, but infected cells have restricted ability to accommodate both GrzB and HIV at the single cell level. Additionally, there may be some interdependence of GrzB and HIV production in memory CD4 T cells that may involve enhanced spread of infection to new cells.
Disruption of epithelial integrity by memory CD4 T cells, and upregulation of GrzB by microbial ligands
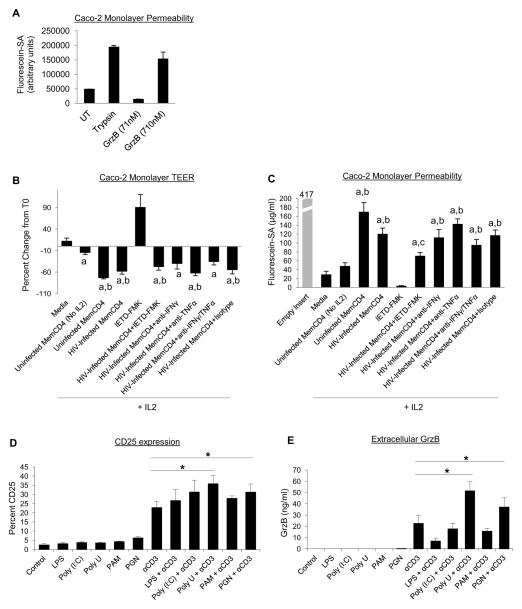
HIV infection is associated with gastrointestinal pathologies, such as memory CD4 T cell depletion, disruption of the epithelial monolayer and translocation of microbial products, and chronic immune activation [Brenchley and Douek, 2012, Epple et al., 2009]. We found that CCR5 is associated with GrzB production in memory CD4 T cells (purified from peripheral blood), but memory CD4 T cells within GALT express higher levels of CCR5 compared to peripheral blood memory CD4 T cells, suggesting that GrzB from CCR5+ memory CD4 T cells may have a role in intestinal pathologies [Gordon et al., 2010, Veazey et al., 2000]. To first determine the direct effect of GrzB on intestinal monolayer integrity, Caco-2 monolayers were treated apically with pure GrzB protein for 24hrs. Monolayer integrity was assessed by measuring transepithelial resistances (TEER), and transcellular flux of fluorescein-labeled sulfonic acid (Fluorescein-SA) markers at experimental end-point. GrzB protein (<100nM) inconsistently and transiently caused TEER reductions, although higher amounts of GrzB (~700nM) increased transcellular permeability to Fluorescein-SA after 24hrs treatment (Fig. 5A, n=2). To determine the influence of memory CD4 T cells and GrzB on intestinal epithelial integrity, uninfected or HIV-infected memory CD4 T cells were cocultured basolaterally with Caco-2 cells for 4–5 days. Disruption of Caco-2 TEER by IL2-stimulated memory CD4 T cells was observed after 3–4 days of coculture and is presented as TEER percent change from T0 (Fig. 5B). IL2 alone did not affect Caco-2 TEER, and Caco-2 in IL2 medium alone maintained stable resistances during culture in which the day 4 TEER percent change from T0 was approximately +12% (n=4). Additionally, uninfected memory CD4 T cells without IL2 did not significantly affect Caco-2 TEER (approximately −15%, p>0.05 compared to Caco-2+medium alone). However, IL2-stimulated uninfected and HIV-infected (R5-tropic) memory CD4 T cells decreased Caco-2 TEER. IL2-stimulated uninfected memory CD4 T cells decreased Caco-2 TEER by ~73%, and HIV-infected cells decreased TEER by ~58% (p<0.05 compared to either Caco-2+medium alone or Caco-2+uninfected memory CD4 T cells without IL2). Culture of Caco-2 alone with GrzB inhibitor IETD-FMK dramatically enhanced TEER, possibly due to oxidative stress, which made interpretation of Caco-2+memory CD4 T cell coculture data with IETD-FMK unclear. Attempts to knock down GrzB in memory CD4 T cells by nucleofection (Amaxa) prior to coculture were unsuccessful due to high toxicity of transfection. Activated memory CD4 T cells also produce inflammatory cytokines such as TNFα and IFNγ, and these cytokines are reported to disrupt epithelial monolayer integrity [Cao et al., 2013, Resta-Lenert and Barrett, 2006, Wang et al., 2005]. However, coculture of HIV-infected memory CD4 T cells with Caco-2 cells and blocking abs against TNFα and IFNγ did not consistently mitigate TEER decreases. Additionally, HIV proteins such as gp120 and Nef can impair epithelial integrity [Nazli et al., 2010, Quaranta et al., 2011], but we observed no differences in monolayer disruption between uninfected and HIV-infected cells, indicating that the effect was caused by factors from activated memory CD4 T cells.
Figure 5. Effects of GrzB and memory CD4 T cells on Caco-2 intestinal epithelial monolayers, and effect of microbial ligands on GrzB production.
(A) Increased transcellular permeability of Caco-2 monolayers to fluorescein-labeled sulfonic acid (Fluorescein-SA) markers after 24hrs treatment with pure GrzB protein (graph is representative of 2 replicate experiments). (B) Changes in Caco-2 monolayer transepithelial resistance (TEER, means±sem, n=4) after 4 days coculture with uninfected or HIV-infected (R5-tropic) memory CD4 T cells (basolaterally in lower wells) in IL2 medium (ap<0.05 compared to Caco-2 in medium alone; bp<0.05 compared to Caco-2+Uninfected MemCD4 without IL2 in coculture medium). (C) Caco-2 transcellular permeability to Fluorescein-SA markers (means±sem, n=4) after 4 days coculture with memory CD4 T cells (ap<0.05 compared to Caco-2 in medium alone; bp<0.05 compared to Caco-2+Uninfected MemCD4 without IL2 in coculture medium; cp<0.05 compared to Caco-2+HIV-Infected MemCD4). (D-E) Effects of TLR ligands on memory CD4 T cell activation (CD25) and GrzB production (means±sem, *p<0.05 compared to CD3 activation alone, n=5).
These TEER changes were also corroborated by end-point transcellular permeability of Fluorescein-SA markers (Fig. 5C). At the conclusion of experiments, 1mg/ml Fluorescein-SA was added apically to Caco-2 cells in inserts for 3hrs, and Fluorescein-SA concentration in lower wells was measured. Fluorescein-SA permeability of Caco-2 monolayers in IL2 medium alone was ~29μg/ml, and ~48μg/ml with unstimulated (No IL2) uninfected memory CD4 T cells. Fluorescein-SA permeability of Caco-2 cells with uninfected memory CD4 T cells in IL2 medium was ~170μg/ml, and ~120μg/ml with HIV-infected memory CD4 T cells (p<0.05 compared to Caco-2+medium alone and Caco-2+uninfected memory CD4 T cells without IL2, n=4). IETD-FMK alone reduced Fluorescein-SA permeability. Blocking abs against TNFα and IFNγ did not affect Fluorescein-SA permeability. Lastly, memory CD4 T cell activation (>10% CD69), as well as HIV (>10,000pg/ml) and GrzB production (>2,000pg/ml), by memory CD4 T cells was mostly unaffected after 4–5 days coculture with Caco-2 cells (data not shown). These data show that stimulated uninfected and HIV-infected memory CD4 T cells can mediate the breakdown of epithelial monolayers, which may involve GrzB.
During HIV infection, breakdown of the intestinal epithelial monolayer occurs that results in translocation of microbial products, and is associated with chronic immune activation. TLR ligands also promote activation of CD4 T cells and increase HIV replication [Brichacek et al., 2010, Funderburg et al., 2008]. To determine if microbial products increase GrzB production, we treated memory CD4 T cells with TLR ligands such as LPS (TLR4 agonist), Poly(I:C) (TLR3 agonist), Poly(U) (TLR8 agonist), peptidoglycan (PGN) (TLR2 agonist), and Pam3CSK4 (TLR1/2 agonist). TLR ligands alone did not affect T cell activation or GrzB production, but PGN and Poly(U) enhanced CD25 expression and GrzB secretion in conjunction with CD3/TCR activation after 2 days culture (Fig. 5D–E). CD25 expression by CD3-activated memory CD4 T cells was ~23%, and increased to ~36% with CD3+Poly(U) and to ~31% with CD3+PGN stimulation (p<0.05 compared to CD3 alone, n=5). Extracellular GrzB by CD3-activated memory CD4 T cells was ~22,000pg/ml, and increased to ~51,000pg/ml with CD3+Poly(U) and ~37,000pg/ml with CD3+PGN stimulation (p<0.05 compared to CD3 alone). PGN and FSL-1 (synthetic TLR2/6 agonist) also modestly increased HIV production by ~10–20% compared to CD3/TCR alone (data not shown). Thus, products of microbial translocation that stimulates TLR 2 or 8 on memory CD4 T cells may contribute to their activation and increased GrzB production.
Comparison of GrzB and CCR5 expression by CD4 T cells between pathogenic and non-pathogenic SIV host monkeys
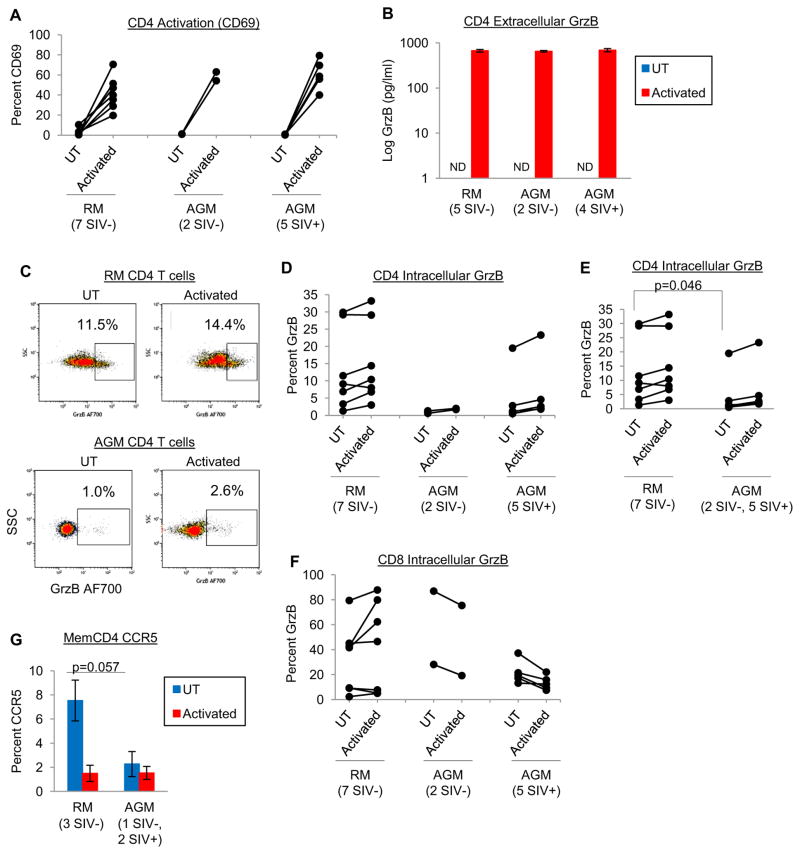
By contrast to natural/non-pathogenic SIV hosts (such as African green monkeys and sooty mangabeys) that usually does not develop simian AIDS and chronic immune activation, non-natural/pathogenic SIV hosts (rhesus macaques and pigtail macaques) experience these complications similarly to humans infected with HIV. To determine if pathogenic SIV hosts express more GrzB compared to non-pathogenic hosts, we examined peripheral blood CD4 and CD8 T cells of RM and AGM. We examined blood samples from 7 RM (all SIV−) and 7 AGM (2 SIV− and 5 SIV+). PBMC were isolated from blood, followed by positive selection for CD4 T cells (80–90% CD3+CD4+ purity). CD4 T cells were then activated by CD3/CD28 costimulation for 24hrs, and stained for GrzB. Activation increased CD4 T cell CD69 and extracellular GrzB similarly for RM and AGM (Fig. 6A–B). Interestingly, one difference between RM and AGM CD4 T cells was higher constitutive intracellular GrzB expression by RM CD4 T cells (Fig. 6C–E). Fig. 6C shows representative GrzB dotplots gated on CD4 T cells of uninfected RM and AGM, and Fig. 6D shows that CD4 T cells of most RM expressed GrzB, whereas most AGM CD4 T cells expressed little to no GrzB. When comparing RM to combined SIV− and SIV+ AGM, resting (UT) CD4 T cells of RM expressed significantly more GrzB compared to AGM (13.0±4.5% vs. 3.8±2.6%, p<0.05). By contrast to CD4 T cells, expression of GrzB by CD8 T cells was mostly similar between RM and AGM, which was also higher than GrzB expression by CD4 T cells as expected (Fig. 6F). Lastly, memory CD4+CD95+ cells of RM expressed modestly more CCR5 (7.5±1.7%) compared to AGM (2.3±1.0%, p=0.057, n=3), consistent with previous reports [Pandrea et al., 2007], and CCR5 expression was reduced by activation (Fig. 6G). These differences in GrzB and CCR5 expression by RM and AGM peripheral blood T cells were modest, but these differences could be more pronounced in GALT tissues where T cell activation and CCR5 expression is higher [Paiardini et al., 2011, Veazey et al., 2000, Wang et al., 2011].
Figure 6. Expression of GrzB and CCR5 by CD4 T cells of pathogenic (rhesus macaque) and non-pathogenic (African green monkey) SIV primate hosts.
(A–B) Activation (CD69 expression) and extracellular GrzB production (means±sem) by RM (7 SIV−) and AGM (2 SIV− and 4–5 SIV+) CD4 T cells after 24hrs activation (CD3/CD28 costimulation). (C) Representative flow cytometry dotplots of intracellular GrzB expression by CD4 T cells of uninfected RM and AGM monkeys after 24hrs culture. (D) Intracellular GrzB expression by CD4 T cells of RM and AGM monkeys after 24hrs culture, and (E) pooled analysis of RM (7 SIV−) and AGM (2 SIV− and 5 SIV+) monkeys. (F) Intracellular GrzB expression by CD8 T cells of RM and AGM monkeys after 24hrs culture. (G) CCR5 expression by memory CD4+CD95+ T cells of RM (3 SIV−) and AGM (1 SIV− and 2 SIV+) monkeys after 24hrs culture.
Discussion
HIV infection is characterized by memory CD4 T cell dysfunction and death, chronic immune activation, and tissue pathologies such as enteropathy. HIV replication occurs mostly in activated memory CD4 T cells, and is induced by CD3/TCR activation, cytokines such as IL2, IL7, or IL15, and other agents such as TLR ligands. In this report, we found that HIV production by stimulated memory CD4 T cells is also accompanied with GrzB release, mostly from CCR5+ memory CD4 T cells. Previous reports have shown that HIV production by CD4 T cells is associated with release of CD4 autocrine cytokines and chemokines, such as RANTES that affect HIV replication [Kinter et al., 1996]. Concomitant release of GrzB and HIV by memory CD4 T cells may be an important element of HIV pathogenesis because granzymes are mediators of cell death and tissue breakdown, and CCR5+ memory CD4 T cells are highly activated and infected with HIV, especially in lymphoid and intestinal tissues. Although CCR5 is best-known as a coreceptor for HIV attachment and entry into CD4 T cells, CCR5 expression levels are positively associated with intestinal tissue pathologies, increased immune activation, and disease progression [Pandrea et al., 2007, Portales et al., 2012, Taaffe et al., 2012, Yang et al., 2012]. Additionally, the initial phases of primary HIV infection are characterized by a predominance of HIV R5-tropic strains, increased memory CD4 T cell activation, and substantial amounts of viral production. The association of CCR5+ with both HIV and GrzB production by memory CD4 T cells may influence disease progression and AIDS.
Memory and effector CD4 T cells stimulated with CD3/CD28 costimulation, as well as by cytokines including IL2, IL7, IL15, IL6, IL8, and TNFα, produced high amounts of GrzB and HIV (Fig. 1 and 3). GrzB and HIV are induced through similar signaling pathways in CD4 T cells, such as NFκB or JNK activation [Huang et al., 2006, Medina et al., 2012, Wachstein et al., 2012]. However, concomitant production of HIV with host secreted factors (ie. p24 with granzymes or cytokines) is mostly unexplored in memory CD4 T cells. An interdependent effect between HIV and GrzB secretion was not consistently observed with memory CD4 T cells, although HIV infection increased GrzB production in Th1, Th17, and differentiated naïve CD4 T cells (Fig. 3). Direct treatment of CD4 T cells with recombinant GrzB or GrzA does not affect HIV replication [Mackewicz et al., 2000]. However, HIV replication in conjunction with excessive release of GrzB is likely detrimental to local tissues and bystander cells. GrzB functions extracellularly as a protease and mediates ECM remodeling by cleaving proteins such as fibronectin and laminins, promoting inflammation and impairing wound healing [Boivin et al., 2012, Buzza et al., 2005, Hiebert and Granville, 2012]. Extracellular GrzB in serum and tissues is associated with inflammation and disease severity, and plasma GrzB is increased in HIV-infected persons [Boivin et al., 2009].
In the context of the main HIV coreceptors, CCR5 and CXCR4, a distinct pattern of intracellular GrzB expression was observed in resting and activated memory CD4 T cells. GrzB expression was highest in CCR5+ (CXCR4−/CCR5+ and CXCR4+/CCR5+) memory CD4 T cells (Fig. 2A–C), although GrzB was also induced in CCR5− (CXCR4−/CCR5− and CXCR4+/CCR5−) memory CD4 T cells by T cell activation. Whether GrzB+ or GrzB− CCR5/CXCR4 cells represent independent memory CD4 subsets with unique functions is unclear. These are not naïve CD4 T cells since memory CD4 T cells were purified from PBMC for experiments (>95% CD4+CD45RO+CD45RA− purity). Additionally, these CCR5+GrzB+ memory CD4 T cells do not clearly fit within reported CD4 CTL phenotypes (ie. CD57+, CD27−, CD28−). GrzB/perforin expression and CTL functions are acquired by naïve CD4 T cells following activation and differentiation into memory CD4 T cells, which have increased CD57, and decreased CD27 and CD28 expression [Appay et al., 2002, Brown, 2010, Casazza et al., 2006, Zaunders et al., 2004]. Although CCR5+ memory CD4 T cells in the present study expressed more CD57, and less CD27 and CD28 compared to CCR5− cells, CD57 expression by CCR5+ cells was modest (~15%), and CD28 expression remained high (~70%) (Fig. 2D–F). Within the spectrum of memory CD4 T cell differentiation, CCR5−/GrzB− cells are likely at earlier stages of differentiation (ie. CCR7+, CD27+, CD28+), and CCR5+/GrzB+ cells are at later stages of differentiation (CD57+, CCR7−, CD27−, CD28−). Lastly, constitutive CCR5 expression by memory CD4 T cells purified from peripheral blood in this study was ~40%, but CCR5 expression by memory CD4 T cells is higher in mucosal and intestinal tissues (>60–70%) [Douek et al., 2003, Kunkel et al., 2002, Veazey et al., 2003]. Tissue-resident memory CD4+CD45RO+ T cells that are CCR5+/GrzB+/CXCR4± may represent pathogenic or inflammatory subsets of memory/effector CD4 T cells, whereas CXCR4+/CCR5−/GrzB− memory CD4 T cells may be benign or perhaps regulatory or anti-inflammatory subsets.
During HIV infection, we further observed another interesting pattern of intracellular GrzB and p24 protein expression by CCR5+ memory CD4 T cells in IL2 medium. HIV p24 and GrzB protein were highest in CCR5+ memory CD4 T cells, but memory CD4 T cells had limited capacity for producing both simultaneously (Fig. 4A–B). This supports the idea that GrzB and HIV secretion require similar pathways in CCR5+ memory CD4 T cells, consistent with a previous report showing HIV Env colocalization with secretory lysosomal proteins (CD63, CTLA-4, cathepsin D, and FasL) at virological synapses in primary CD4 T cells, although Env colocalization with GrzB or perforin was not examined [Jolly et al., 2011]. Also consistent with the idea that HIV secretion requires GrzB-related secretory pathways, we observed that treating memory CD4 T cells with the compound Z-IETD-FMK (a synthetic cell-permeable inhibitor of GrzB and caspase 8/10 activity) reduced intracellular GrzB and p24, whereas treatment with the HIV posttranslational protease inhibitor saquinavir reduced p24 but not GrzB (Fig. 4C). Whether functional activity of GrzB or caspases regulate HIV infection is unclear, although direct treatment of HIV-infected CD4 T cells with recombinant GrzA or GrzB does not affect HIV replication [Mackewicz et al., 2000]. At a single cell level, it is unclear if HIV virion assembly and secretion actually interferes with GrzB secretion by memory CD4 T cells because intracellular and extracellular GrzB production was similar between uninfected and HIV-infected cells. Secretion of GrzB and HIV virions is regulated by secretory mechanisms such as ESCRT I, II, and III pathways (endolysosomal and endosomal sorting complex required for transport). Although there is likely overlap or competition for host secretory components, it is unclear how much HIV and GrzB secretory pathways are intertwined. Additionally, temporal and kinetic aspects of HIV and GrzB secretion are likely important (ie. secretory pathways of activated CCR5+ memory CD4 T cells may initially be devoted mostly to granzyme or cytokine secretion, then gradually overcome by HIV virion secretion). These intracellular p24 vs. GrzB patterns may also differ compared to other secreted mediators that utilize different secretory mechanisms in CD4 T cells (ie. p24 vs. cytokines) [Huse et al., 2006]. These data support the well-known fact that HIV hijacks secretory pathways in CD4 T cells, but may further clarify the concept by showing that p24 secretion is limited in CCR5+ memory CD4 T cells in which the secretory pathway is actively involved in secretion of host mediators, such as GrzB or cytokines, prior to posttranscriptional steps of HIV replication. Alternatively, Fig. 4A–B may also suggest that HIV preferentially infects and replicates in CCR5+ memory CD4 T cells that do not express GrzB, as GrzB expression by IL2-stimulated CCR5+ memory CD4 T cells was usually <20%.
We observed that IL2- stimulated uninfected and HIV-infected memory CD4 T cells impaired Caco-2 monolayer integrity by decreasing TEER and increasing transcellular permeability. However, no differences in monolayer disruption were observed between uninfected and HIV-infected memory CD4 T cells, which are not surprising (Fig. 5B–C). A clear role for GrzB secreted by memory CD4 T cells was not established, as a GrzB pharmacological inhibitor (IETD-FMK) did not mitigate these monolayer effects, although transcellular permeability of monolayers was increased by direct treatment with pure GrzB (Fig. 5A). It is possible that extracellular GrzB activity may be transient or have a short half-life. Further confounding these results was the increased TEER caused by the IETD-FMK compound. Experiments with more specific inhibitors of GrzB are needed, but such inhibitors currently do not exist. A large proportion of lamina propria CD4 T cells are activated memory CD4 T cells that express CD45RO and CD69, and activated T cells are reported to disrupt epithelial barriers. For example, CD3-activated intraepithelial lymphocytes (IEL’s) induce death of intestinal epithelial cells and villus breakdown in perforin-knockout mice, although the specific IEL subsets (CD4 or CD8) involved were not determined [Ogata et al., 2013]. Activated memory CD4 T cells also express IFNγ and TNFα, and recombinant IFNγ or TNFα impair Caco-2 or T84 epithelial monolayers [Bruewer et al., 2003, Cui et al., 2010]. However, we did not observe a clear role for memory CD4 T cell IFNγ or TNFα in monolayer disruption by using blocking abs. It is likely that a variety of factors secreted by memory CD4 T cells, either alone or synergistically, mediate the breakdown of intestinal epithelial integrity.
Breach of the intestinal epithelium results in microbial translocation, which is associated with systemic CD4 T cell activation. We observed that TLR ligands alone generally do not affect memory CD4 T cell activation, consistent with other reports [Funderburg et al., 2008], but did find that peptidoglycan or poly(U) (TLR 2 and 8 agonists) enhanced GrzB production in concert with CD3/TCR activation (Fig. 4D–E). In addition to promoting chronic immune activation of memory CD4 T cells during HIV infection, persistent exposure to TLR ligands likely upregulates GrzB release and contributes to immune exhaustion. The clear conclusion from these in vitro experiments is that stimulated, GrzB-producing, uninfected and HIV-infected memory CD4 T cells can induce breakdown of an intestinal monolayer, and microbial products may further promote CD4 T cell activation and production of HIV and GrzB.
We hypothesized that GrzB expression by CD4 T cells of pathogenic, non-natural SIV host monkeys (RM) may be higher compared to non-pathogenic, natural SIV hosts (AGM), which may be a relevant difference for SIV pathogenesis (Fig. 6). By contrast to AGM’s, RM’s experience human AIDS-like diseases during SIV infection such as CD4 T cell depletion, enteropathies, and chronic immune activation, despite high viral loads in both species and similar degrees of antiviral responses [Brenchley and Pairadini 2011, Paiardini et al., 2009]. We previously reported that pathogenic SIV hosts (RM and pigtail macaques) contain more GrzB-expressing CD4 T cells in intestinal tissues compared to non-pathogenic SIV hosts (AGM and sooty mangabeys) [Hutchison et al., 2011]. In the present study of peripheral blood T cells, we further observed differences in GrzB and CCR5 expression, in which RM CD4 T cells expressed more intracellular GrzB than AGM (Fig. 6C–E). Although RM and AGM secreted comparable amounts of GrzB after strong CD3/CD28 activation in vitro, extracellular secretion may differ with more modest stimulants such as microbial ligands or cytokines in vivo. We also observed that AGM memory CD4+CD95+ T cells express little CCR5 (~2%), which is consistent with other reports showing minimal CCR5 expression by peripheral blood CD4 T cells of natural SIV hosts [Pandrea et al., 2007], whereas RM CD4+CD95+ T cells expressed higher levels of CCR5 (Fig. 6G). However, CCR5 expression by memory CD4 T cells is much higher in intestinal tissue compared to peripheral blood in pathogenic SIV hosts and humans. Expression of GrzB and CCR5 by memory CD4 T cells may be an important difference between SIV hosts because pathogenic SIV hosts experience higher immune activation, harbor more CD4 T cells in intestinal tissue, and express more CCR5 by CD4 T cells compared to non-pathogenic SIV hosts. However, it is also possible that decreased expression of CCR5 during primary HIV infection of normal individuals is also associated with decreased GrzB expression by memory CD4 T cells, suggesting a lesser role for CCR5+GrzB+ memory CD4 T cells during HIV-associated pathologies.
During HIV infection, CD4 T cells release mediators such as cytokines and chemokines that influence HIV pathogenesis, but the release of GrzB from CCR5+ memory CD4+ T cells may be an important aspect of infection as well, particularly for bystander cells. In addition to CD8 T cells and NK cells, higher levels of extracellular GrzB during infection and disease can come from CD4 T cells, regardless of their infection status.
Materials and Methods
CD4 T cell purifications
Human PBMC of healthy donors (Gulf Coast Regional Blood Center, Houston, TX USA) were isolated by Ficoll-Paque PLUS (GE Healthcare, Piscataway, NJ USA). Memory CD4+CD45RO+, naïve CD4+CD45RA+, Th1, and Th17 cells were then purified from PBMC by magnetic bead-based negative selection kits (Stemcell Technologies, Vancouver, BC Canada), and purities were assessed by flow cytometry. Memory CD4 T cell purity was at least 90–95% CD4+CD45RO+CD45RA−, and naïve CD4 T cell purity was at least 96% CD4+CD45RA+CD45RO−. Th1 purity (after depletion of CD8, CD14, CD16, CD19, CD56, CCR4, and glycophorin A cells from PBMC) was assessed by activating Th1 cells with PMA/IO for ~4hrs in the presence of GolgiPlug (BD Biosciences, San Jose, CA USA), followed by intracellular staining for IL2, IFNγ, or IL4, in which activated Th1’s expressed IL2 (~30%) and IFNγ (~20%), and no IL4. Th17 cells were purified from PBMC by negative selection for CD4+CD45RO+ T cells, followed by positive selection for CCR6+ (Stemcell Technologies), and purity was assessed by staining for CCR6 (>90%) and CXCR3 (<10%).
HIV infections
Memory CD4, Th1, Th17, or activated naïve CD4 T cells were infected with primary or lab-adapted HIV-1 strains. Virus stocks were produced by the Baylor/UT-Houston Center for AIDS Research (CFAR, Houston, TX USA), an NIH-funded program. R5-tropic strains used include NSN-SX, JR-CSF, and SF162. X4-tropic strains include 93BR019, ELI, and NL4.3. R5X4 dual-tropic strains were 89.6. For infection of resting memory CD4, Th1, and Th17 cells, cells were infected by culturing overnight in complete RPMI medium+20ng/ml rIL2 (Biolegend, San Diego, CA USA) with HIV stocks at MOI’s of 0.01–0.1, and uninfected cells were cultured in IL2 medium alone. After overnight infection, cells were washed 2x in medium and used for experiments. For infection of preactivated memory CD4 T cells, cells were first activated with 1μg/ml coated CD3 (clone UCHT1) + 1μg/ml soluble CD28 (clone CD28.2) mabs (BD Biosciences) for 2 days in IL2 medium. Cells were then washed and cultured in medium alone or with HIV stocks at 0.01–0.1 MOI overnight in IL2 medium. Cells were then washed and used for experiments. For infection of activated naïve CD4 T cells, purified naïve CD4 T cells were first activated with CD3/CD28 costimulation+IL2, or cultured with IL2 alone for 3 days. Cells were then washed and infected with HIV overnight (R5-tropic strains at 0.1 MOI) in IL2 medium. Uninfected and infected cells were then washed and cultured in IL2 medium for experiments.
Cell culture experiments, pharmacological inhibitors, and TLR ligands
Most experiments involved culture of uninfected or HIV-infected memory CD4 T cells in IL2 (20ng/ml) medium for up to 1 week, followed by data collection. Cells were cultured in 48-well plates (5x105 cells in 1ml medium) with appropriate reagents. The HIV antiviral inhibitor, Saquinavir, was acquired from NIHAIDS Reagent Program and used at 1–10μg/ml. The GrzB/caspase inhibitor, Z-IETD-FMK (Enzo Life Sciences, Farmingdale, NY USA), was used at 10μM. Experiments involving TLR stimulation of memory CD4 T cells utilized synthetic TLR ligands LPS (Sigma, St. Louis, MO USA), Pam3CSK4 (EMD Millipore, Billerica, MA USA), peptidoglycan, Poly(I:C), and Poly(U) (InvivoGen, San Diego, CA USA), and were used at 2–25μg/ml +/− CD3 activation (clone UCHT1 coated at 0.1μg/ml).
Flow cytometry
Flow cytometry studies of memory and naïve CD4, Th1, and Th17 cells involved surface staining for CD4 (PerCPCy5.5), CD45RO (Pacific blue), CD45RA (APC), CD62L (PE), CCR5 (FITC, PE, AF647, Pacific blue), CXCR4 (PerCPCy5.5, APC), CD69 (PECy7, APC, AF647, APCCy7), CD57 (FITC), CD27 (PerCPCy5.5), or CD28 (PE) (all from BD Biosciences or Biolegend, San Diego, CA USA). Intracellular mabs include GrzB (clone GB11- or GB12-PE, FITC, APC, AF700 from BD Biosciences, Biolegend, or Life Technologies, Grand Island, NY USA), p24 (clone KC57-PE from Beckman-Coulter, Miami, FL USA), and IFNγ (PE) or IL17A (Pacific blue) from Biolegend. Fixable Live/Dead viability dyes (Life Technologies) were also utilized. Cells were stained by first washing with PBS/2% FBS (wash buffer), then incubating 30mins with viability dye. Cells were washed, then incubated 30mins with surface mabs. Cells were then washed, and fixed 30mins with Cytofix/Cytoperm solution (BD Biosciences). Cells were then washed with Cytoperm buffer, and incubated 30mins with intracellular mabs. Cells were then washed with Cytoperm buffer and stored at 4°C until data acquisition. Unstained, isotype, or FMO controls were used to distinguish between negative and positive signals. Data was acquired with a Gallios Flow Cytometer and analyzed with Kaluza software (Beckman-Coulter).
For Th1 and Th17 intracellular cytokine assays (ICS), Th1 and Th17 cells were cultured for 6 days, then stimulated with PMA/IO+GolgiPlug (Leukocyte Activation Cocktail, BD Biosciences) for the final 5hrs. Cells were then stained for CXCR4, CCR5, CCR6, IFNγ, IL17A, p24, or GrzB via Cytofix/Cytoperm solutions.
ELISA
Extracellular GrzB and HIV p24 protein were measured in cell-free supernatants by ELISA kits. GrzB sandwich ELISA kits were from Mabtech (Mariemont, OH USA). High-binding 96-well plates (Nunc) were first coated overnight at 4°C with 2μg/ml GrzB mab (clone GB10) in PBS. After coating, plates were washed with PBS. Plates were then blocked with PBS/0.05% Tween-20/0.1% BSA. Plates were then washed and samples added for 2hrs at room temp. After sample incubation, plates were washed and GrzB-biotin (clone GB11) added for 1hr. Plates were then washed and streptavidin-HRP added for 1hr. Plates were then washed and TMB substrate added for 15mins, followed by addition of TMB stop solution. 450nm OD’s were then recorded with an absorbance plate reader (Tecan, San Jose, CA USA). Sample GrzB concentrations were determined from standard curve with a detection limit of 4pg/ml.
HIV p24 ELISA kits were from Advanced Bioscience Laboratories (Rockville, MD USA). Samples were incubated with disruption buffer in 96-well plates for 1hr at 37°C. Plates were then washed and conjugate solution added for 1hr. Plates were washed and TMB substrate added for 30mins, followed by addition of TMB stop solution. 450nm OD’s were recorded with an absorbance plate reader. Sample p24 concentrations were determined from standard curve with a detection limit of 3pg/ml.
Cocultures of Caco-2 epithelial cells with memory CD4 T cells
Caco-2 human colon epithelial cell lines (ATCC, Manassas, VA USA) were used to determine the effects of memory CD4 T cells on intestinal epithelial monolayer integrity. Prior to coculture with memory CD4 T cells, Caco-2 cells were first grown to confluency on collagen-coated transwell inserts (0.4μm pore size, Corning 3495) in 24-well plates and DMEM medium. Monolayer integrity was monitored by measuring transepithelial electrical resistances (TEER) with an EVOM2 epithelial voltohmmeter (World Precision Instruments, Sarasota, FL USA). Baseline TEER’s were ~800–1,000 ohms/cm2 before starting experiments. Following establishment of baseline resistances, inserts were then transferred to new 24-well plates containing pre-seeded (immediately prior to Caco-2 transfer) memory CD4 T cells in lower wells (5x105 cells in 1ml medium basolaterally). Caco-2 cells were cocultured with memory CD4 T cells and appropriate reagents for up to 5 days, and TEER’s were measured every 24hrs. For assessment of Caco-2 monolayer transcellular permeability at the conclusion of coculture experiments with memory CD4 T cells, inserts were transferred to new 24-well plates containing fresh DMEM medium. 1mg/ml fluorescein-labeled sulfonic acid (FL-SA, Life Technologies) was then added to inserts for 3hrs, and samples harvested from lower wells. Fluorescence intensities were measured with a fluorescence plate reader (BioTek, Winooski, VT USA) and concentrations determined by standard curve.
For coculture experiments using blocking abs, anti-IFNγ (Biolegend), anti-TNFα (R&D Systems, Minneapolis, MN USA), or isotype controls were used at 2–5μg/ml. Caco-2 monolayers were also directly treated with pure recombinant GrzB (ImmunoChemistry Technologies).
Studies of non-human primates
Studies of NHP peripheral blood cells involved rhesus macaques (RM) and African green monkeys (AGM). NHP’s were maintained at the New England Primate Research Center (Boston, MA USA). Whole blood of NHP’s were drawn and shipped to UT-Houston Medical School for experiments. PBMC were then isolated via ficoll, followed by positive selection of CD4 T cells using a primate-specific CD4 mab (clone L200 from BD Biosciences), and CD3+CD4+ purities were 80–90%. Cells were then cultured in complete RPMI medium for experiments. Cells were unstimulated or activated with 5μg/ml coated CD3 (clone SP34) + 2μg/ml soluble CD28 (clone CD28.2) mabs (BD Biosciences) for 24hrs. Cells were then stained for CD3 (Pacific blue), CD4 (PerCPCy5.5), CD8 (APCCy7), CCR5 (PE), CD95 (APC), CD69 (ECD), and GrzB (AF700) (BD Biosciences) using Cytofix/Cytoperm solutions. Extracellular GrzB was measured by ELISA kit (Cell Sciences, Canton, MA USA).
Statistics
The analyses were performed using SAS 9.3 or MS Excel. The means by experimental conditions were compared by two sample t-tests for independent samples and paired t-tests for paired samples respectively. For the highly skewed variables, the logarithm transformation was made for analysis. Pearson correlation coefficient was calculated to assess the correlation between two continuous variables. A two-sided p-value <0.05 was considered statistically significant.
Research Highlights.
CCR5+ memory CD4 T cells release GrzB and HIV during HIV replication
TCR activation, cytokines, and TLR agonists induce GrzB and HIV production
CD4 T cells mediate cellular monolayer tissue damage during HIV infection
Acknowledgments
This work was supported by NIH/NIAID grant AI R37 36682 and Baylor College of Medicine/UT-Health Center for AIDS Research (CFAR) grant AI RO1 36211 (JC, ATH, MAM, PM, DEL), NIH grants RO1AI0094001 and DK56338 (TCS, PU), NIH grant AI065335 (JES), NIH grant AI083095 (JTK), and Baylor Pediatric AIDS Initiative NIH training grant from the Fogarty International Center D43 TW001036 (CG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Appay V, Zaunders JJ, Papagno L, Sutton J, Jaramillo A, Waters A, Easterbrook P, Grey P, Smith D, McMichael AJ, Cooper DA, Rowland-Jones SL, Kelleher AD. Characterization of CD4+ CTLs ex vivo. J Immunol. 2002;168 (11):5954–5958. doi: 10.4049/jimmunol.168.11.5954. [DOI] [PubMed] [Google Scholar]
- Ashley CW, Baecher-Allan C. Cutting Edge: Responder T Cells Regulate Human DR+ Effector Regulatory T Cell Activity via Granzyme B. J Immunol. 2009;183(8):4843–4847. doi: 10.4049/jimmunol.0900845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin WA, Cooper DM, Hiebert PR, Granville DJ. Intracellular versus extracellular granzyme B in immunity and disease: challenging the dogma. Lab Invest. 2009;89 (11):1195–1220. doi: 10.1038/labinvest.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin WA, Shackleford M, Vanden Hoek A, Zhao H, Hackett TL, Knight DA, Granville DJ. Granzyme B Cleaves Decorin, Biglycan and Soluble Betaglycan, Releasing Active Transforming Growth Factor-β1. PLoS One. 2012;7 (3):e33163. doi: 10.1371/journal.pone.0033163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, Nguyen PL, Khoruts A, Larson M, Haase AT, Douek DC. CD4+ T Cell Depletion during all Stages of HIV Disease Occurs Predominantly in the Gastrointestinal Tract. J Exp Med. 2004;200 (6):749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchley JM, Paiardini M. Immunodeficiency lentiviral infections in natural and non-natural hosts. Blood. 2011;118 (4):847–854. doi: 10.1182/blood-2010-12-325936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchley JM, Douek DC. Microbial Translocation Across the GI Tract. Annu Rev Immunol. 2012;30:149–173. doi: 10.1146/annurev-immunol-020711-075001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brichacek B, Vanpouille C, Kiselyeva Y, Biancotto A, Merbah M, Hirsch I, Lisco A, Grivel JC, Margolis L. Contrasting Roles for TLR Ligands in HIV-1 Pathogenesis. PLoS One. 2010;5 (9):e12831. doi: 10.1371/journal.pone.0012831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DM. Cytolytic CD4 Cells: Direct Mediators in Infectious Disease and Malignancy. Cell Immunol. 2010;262 (2):89–95. doi: 10.1016/j.cellimm.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruewer M, Luegering A, Kucharzik T, Parkos CA, Madara JL, Hopkins AM, Nusrat A. Proinflammatory Cytokines Disrupt Epithelial Barrier Function by Apoptosis-Independent Mechanisms. J Immunol. 2003;171 (11):6164–6172. doi: 10.4049/jimmunol.171.11.6164. [DOI] [PubMed] [Google Scholar]
- Buzza MS, Zamurs L, Sun J, Bird CH, Smith AI, Trapani JA, Froelich CJ, Nice EC, Bird PI. Extracellular Matrix Remodeling by Human Granzyme B via Cleavage of Vitronectin, Fibronectin, and Laminin. J Biol Chem. 2005;280 (25):23549–23558. doi: 10.1074/jbc.M412001200. [DOI] [PubMed] [Google Scholar]
- Cao X, Cai SF, Fehniger TA, Song J, Collins LI, Piwnica-Worms DR, Ley TJ. Granzyme B and Perforin Are Important for Regulatory T Cell-Mediated Suppression of Tumor Clearance. Immunity. 2007;27 (4):635–646. doi: 10.1016/j.immuni.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Cao M, Wang P, Sun C, He W, Wang F. Amelioration of IFN-γ and TNF-α-Induced Intestinal Epithelial Barrier Dysfunction by Berberine via Suppression of MLCK-MLC Phosphorylation Signaling Pathway. PLoS One. 2013;8 (5):e61944. doi: 10.1371/journal.pone.0061944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casazza JP, Betts MR, Price DA, Precopio ML, Ruff LE, Brenchley JM, Hill BJ, Roederer M, Douek DC, Koup RA. Acquisition of direct antiviral effector functions by CMV-specific CD4+ T lymphocytes with cellular maturation. J Exp Med. 2006;203 (13):2865–2877. doi: 10.1084/jem.20052246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun TW, Engel D, Mizell SB, Ehler LA, Fauci AS. Induction of HIV-1 replication in latently infected CD4+ T cells using a combination of cytokines. J Exp Med. 1998;188 (1):83–91. doi: 10.1084/jem.188.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui W, Li LX, Sun CM, Wen Y, Zhou Y, Dong YL, Liu P. Tumor necrosis factor alpha increases epithelial barrier permeability by disrupting tight junctions in Caco-2 cells. Braz J Med Biol Res. 2010;43 (4):330–337. doi: 10.1590/S0100-879X2010007500020. [DOI] [PubMed] [Google Scholar]
- Devadas S, Das J, Liu C, Zhang L, Roberts AI, Pan Z, Moore PA, Das G, Shi Y. Granzyme B Is Critical for T Cell Receptor-Induced Cell Death of Type 2 Helper T Cells. Immunity. 2006;25 (2):237–247. doi: 10.1016/j.immuni.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Douek DC, Picker LJ, Koup RA. T Cell Dynamics in HIV-1 Infection. Annu Rev Immunol. 2003;21:265–304. doi: 10.1146/annurev.immunol.21.120601.141053. [DOI] [PubMed] [Google Scholar]
- Epple HJ, Schneider T, Troeger H, Kunkel D, Allers K, Moos V, Amasheh M, Loddenkemper C, Fromm M, Zeitz M, Schulzke JD. Impairment of the intestinal barrier is evident in untreated but absent in suppressively treated HIV-infected patients. Gut. 2009;58 (2):220–227. doi: 10.1136/gut.2008.150425. [DOI] [PubMed] [Google Scholar]
- Funderburg N, Luciano AA, Jiang W, Rodriguez B, Sieg SF, Lederman MM. Toll-Like Receptor Ligands Induce Human T Cell Activation and Death, a Model for HIV Pathogenesis. PLoS One. 2008;3 (4):e1915. doi: 10.1371/journal.pone.0001915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gondek DC, Lu LF, Quezada SA, Sakaguchi S, Noelle RJ. Cutting Edge: Contact-Mediated Suppression by CD4+CD25+ Regulatory Cells Involves a Granzyme B-Dependent, Perforin-Independent Mechanism. J Immunol. 2005;174 (4):1783–1786. doi: 10.4049/jimmunol.174.4.1783. [DOI] [PubMed] [Google Scholar]
- Gordon SN, Cervasi B, Odorizzi P, Silverman R, Aberra F, Ginsberg G, Estes JD, Paiardini M, Frank I, Silvestri G. Disruption of Intestinal CD4+ T Cell Homeostasis Is a Key Marker of Systemic CD4+ T Cell Activation in HIV-Infected Individuals. J Immunol. 2010;185 (9):5169–5179. doi: 10.4049/jimmunol.1001801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman WJ, Verbsky JW, Tollefsen BL, Kemper C, Atkinson JP, Ley TJ. Differential expression of granzymes A and B in human cytotoxic lymphocyte subsets and T regulatory cells. Blood. 2004;104 (9):2840–2848. doi: 10.1182/blood-2004-03-0859. [DOI] [PubMed] [Google Scholar]
- Hiebert PR, Granville DJ. Granzyme B in injury, inflammation, and repair. Trends Mol Med. 2012;18 (12):732–741. doi: 10.1016/j.molmed.2012.09.009. [DOI] [PubMed] [Google Scholar]
- Hildemann SK, Eberlein J, Davenport B, Nguyen TT, Victorino F, Homann D. High Efficiency of Antiviral CD4+ Killer T Cells. PLoS One. 2013;8 (4):e60420. doi: 10.1371/journal.pone.0060420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Bi E, Hu Y, Deng W, Tian Z, Dong C, Hu Y, Sun B. A Novel NF-κB Binding Site Controls Human Granzyme B Gene Transcription. J Immunol. 2006;176 (7):4173–4181. doi: 10.4049/jimmunol.176.7.4173. [DOI] [PubMed] [Google Scholar]
- Huse M, Lillemeier BF, Kuhns MS, Chen DS, Davis MM. T cells use two directionally distinct pathways for cytokine secretion. Nat Immunol. 2006;7 (3):247–255. doi: 10.1038/ni1304. [DOI] [PubMed] [Google Scholar]
- Hutchison AT, Schmitz JE, Miller CJ, Sastry KJ, Nehete PN, Major AM, Ansari AA, Tatevian N, Lewis DE. Increased inherent intestinal granzyme B expression may be associated with SIV pathogenesis in Asian non-human primates. J Med Primatol. 2011;40 (6):414–426. doi: 10.1111/j.1600-0684.2011.00482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly C, Welsch S, Michor S, Sattentau QJ. The Regulated Secretory Pathway in CD4+ T cells Contributes to Human Immunodeficiency Virus Type-1 Cell-to-Cell Spread at the Virological Synapse. PLoS Pathog. 2011;7 (9):e1002226. doi: 10.1371/journal.ppat.1002226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N, Becher B, Prat A. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13 (10):1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinter AL, Ostrowski M, Goletti D, Oliva A, Weissman D, Gantt K, Hardy E, Jackson R, Ehler L, Fauci AS. HIV replication in CD41 T cells of HIV-infected individuals is regulated by a balance between the viral suppressive effects of endogenous β-chemokines and the viral inductive effects of other endogenous cytokines. Proc Natl Acad Sci U S A. 1996;93 (24):14076–14081. doi: 10.1073/pnas.93.24.14076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel EJ, Boisvert J, Murphy K, Vierra MA, Genovese MC, Wardlaw AJ, Greenberg HB, Hodge MR, Wu L, Butcher EC, Campbell JJ. Expression of the Chemokine Receptors CCR4, CCR5, and CXCR3 by Human Tissue-Infiltrating Lymphocytes. Am J Pathol. 2002;160 (1):347–355. doi: 10.1016/S0002-9440(10)64378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Huang L, Li J, Zhou X, Zhang H, Zhang T, Lei Y, Wang K, Xie N, Zheng Y, Wang F, Nice EC, Rong L, Huang C, Wei Y. HIV Infection in Gastric Epithelial Cells. J Infect Dis. 2013;208 (8):1221–1230. doi: 10.1093/infdis/jit314. [DOI] [PubMed] [Google Scholar]
- Loebbermann J, Thornton H, Durant L, Sparwasser T, Webster KE, Sprent J, Culley FJ, Johansson C, Openshaw PJ. Regulatory T cells expressing granzyme B play a critical role in controlling lung inflammation during acute viral infection. Mucosal Immunol. 2012;5 (2):161–172. doi: 10.1038/mi.2011.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackewicz CE, Lieberman J, Froelich C, Levy JA. HIV Virions and HIV Infection in Vitro Are Unaffected by Human Granzymes A and B. AIDS Res. Hum Retroviruses. 2000;16 (4):367–372. doi: 10.1089/088922200309241. [DOI] [PubMed] [Google Scholar]
- Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434 (7037):1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- Medina MA, Couturier J, Feske ML, Mahne AE, Turner M, Yu X, Kozinetz CA, Orozco AF, Hutchison AT, Savidge TC, Rodgers JR, Lewis DE. Granzyme B- and Fas ligand-mediated cytotoxic function induced by mitogenic CD28 stimulation of human memory CD4+ T cells. J Leukoc Biol. 2012;91 (5):759–771. doi: 10.1189/jlb.0511264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazli A, Chan O, Dobson-Belaire WN, Ouellet M, Tremblay MJ, Gray-Owen SD, Arsenault AL, Kaushic C. Exposure to HIV-1 Directly Impairs Mucosal Epithelial Barrier Integrity Allowing Microbial Translocation. PLoS Pathog. 2010;6 (4):e1000852. doi: 10.1371/journal.ppat.1000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata M, Ota Y, Matsutani T, Nanno M, Suzuki R, Itoh T. Granzyme B-dependent and perforin-independent DNA fragmentation in intestinal epithelial cells induced by anti-CD3 mAb-activated intra-epithelial lymphocytes. Cell Tissue Res. 2013;352 (2):287–300. doi: 10.1007/s00441-012-1549-7. [DOI] [PubMed] [Google Scholar]
- Paiardini M, Pandrea I, Apetrei C, Silvestri G. Lessons Learned from the Natural Hosts of HIV-Related Viruses. Annu Rev Med. 2009;60:485–495. doi: 10.1146/annurev.med.60.041807.123753. [DOI] [PubMed] [Google Scholar]
- Paiardini M, Cervasi B, Reyes-Aviles E, Micci L, Ortiz AM, Chahroudi A, Vinton C, Gordon SN, Bosinger SE, Francella N, Hallberg PL, Cramer E, Schlub T, Chan ML, Riddick NE, Collman RG, Apetrei C, Pandrea I, Else J, Munch J, Kirchhoff F, Davenport MP, Brenchley JM, Silvestri G. Low levels of SIV infection in sooty mangabey central memory CD4+ T cells are associated with limited CCR5 expression. Nat Med. 2011;17 (7):830–836. doi: 10.1038/nm.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandrea I, Apetrei C, Gordon S, Barbercheck J, Dufour J, Bohm R, Sumpter B, Roques P, Marx PA, Hirsch VM, Kaur A, Lackner AA, Veazey RS, Silvestri G. Paucity of CD4+CCR5+ T cells is a typical feature of natural SIV hosts. Blood. 2007;109 (3):1069–1076. doi: 10.1182/blood-2006-05-024364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portales P, Psomas KC, Tuaillon E, Mura T, Vendrell JP, Eliaou JF, Reynes J, Corbeau P. The intensity of immune activation is linked to the level of CCR5 expression in human immunodeficiency virus type 1-infected persons. Immunology. 2012;137 (1):89–97. doi: 10.1111/j.1365-2567.2012.03609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaranta MG, Vincentini O, Felli C, Spadaro F, Silano M, Moricoli D, Giordani L, Viora M. Exogenous HIV-1 Nef Upsets the IFN-γ-Induced Impairment of Human Intestinal Epithelial Integrity. PLoS One. 2011;6 (8):e23442. doi: 10.1371/journal.pone.0023442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quezada SA, Simpson TR, Peggs KS, Merghoub T, Vider J, Fan X, Blasberg R, Yagita H, Muranski P, Antony PA, Restifo NP, Allison JP. Tumor-reactive CD4+ T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med. 2010;207 (3):637–650. doi: 10.1084/jem.20091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resta-Lenert S, Barrett KE. Probiotics and Commensals Reverse TNF-α– and IFN-γ–Induced Dysfunction in Human Intestinal Epithelial Cells. Gastroenterology. 2006;130 (3):731–746. doi: 10.1053/j.gastro.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Sharma V, Delgado M, Ganea D. Granzyme B, a New Player in Activation-Induced Cell Death, Is Down-Regulated by Vasoactive Intestinal Peptide in Th2 but Not Th1 Effectors. J Immunol. 2006;176 (1):97–110. doi: 10.4049/jimmunol.176.1.97. [DOI] [PubMed] [Google Scholar]
- Soghoian DZ, Jessen H, Flanders M, Sierra-Davidson K, Cutler S, Pertel T, Ranasinghe S, Lindqvist M, Davis I, Lane K, Rychert J, Rosenberg ES, Piechocka-Trocha A, Brass AL, Brenchley JM, Walker BD, Streeck H. HIV-Specific Cytolytic CD4 T Cell Responses During Acute HIV Infection Predict Disease Outcome. Sci Transl Med. 2012;4 (123):123ra25. doi: 10.1126/scitranslmed.3003165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taaffe JE, Bosinger SE, Del Prete GQ, Else JG, Ratcliffe S, Ward CD, Migone T, Paiardini M, Silvestri G. CCR5 blockade is well tolerated and induces changes in the tissue distribution of CCR5+ and CD25+ T cells in healthy, SIV-uninfected rhesus macaques. J Med Primatol. 2012;41 (1):24–42. doi: 10.1111/j.1600-0684.2011.00521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unutmaz D, KewalRamani VN, Marmon S, Littman DR. Cytokine signals are sufficient for HIV-1 infection of resting human T lymphocytes. J Exp Med. 1999;189 (11):1735–1746. doi: 10.1084/jem.189.11.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veazey RS, Mansfield KG, Tham IC, Carville AC, Shvetz DE, Forand AE, Lackner AA. Dynamics of CCR5 Expression by CD4+ T Cells in Lymphoid Tissues during Simian Immunodeficiency Virus Infection. J Virol. 2000;74 (23):11001–11007. doi: 10.1128/jvi.74.23.11001-11007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veazey RS, Marx PA, Lackner AA. Vaginal CD4+ T Cells Express High Levels of CCR5 and Are Rapidly Depleted in Simian Immunodeficiency Virus Infection. J Infect Dis. 2003;187 (5):769–776. doi: 10.1086/368386. [DOI] [PubMed] [Google Scholar]
- Wachstein J, Tischer S, Figueiredo C, Limbourg A, Falk C, Immenschuh S, Blasczyk R, Eiz-Vesper B. HSP70 Enhances Immunosuppressive Function of CD4+CD25+FoxP3+ T Regulatory Cells and Cytotoxicity in CD4+CD25− T Cells. PLoS One. 2012;7 (12):e51747. doi: 10.1371/journal.pone.0051747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Graham WV, Wang Y, Witkowski ED, Schwarz BT, Turner JR. Interferon-γ and Tumor Necrosis Factor-α Synergize to Induce Intestinal Epithelial Barrier Dysfunction by Up-Regulating Myosin Light Chain Kinase Expression. Am J Pathol. 2005;166 (2):409–419. doi: 10.1016/s0002-9440(10)62264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Xu H, Alvarez X, Pahar B, Moroney-Rasmussen T, Lackner AA, Veazey RS. Distinct Expression Patterns of CD69 in Mucosal and Systemic Lymphoid Tissues in Primary SIV Infection of Rhesus Macaques. PLoS One. 2011;6 (11):e27207. doi: 10.1371/journal.pone.0027207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanai F, Ishii E, Kojima K, Hasegawa A, Azuma T, Hirose S, Suga N, Mitsudome A, Zaitsu M, Ishida Y, Shirakata Y, Sayama K, Hashimoto K, Yasukawa M. Essential Roles of Perforin in Antigen-Specific Cytotoxicity Mediated by Human CD4+ T Lymphocytes: Analysis Using the Combination of Hereditary Perforin-Deficient Effector Cells and Fas-Deficient Target Cells. J Immunol. 2003;170 (4):2205–2213. doi: 10.4049/jimmunol.170.4.2205. [DOI] [PubMed] [Google Scholar]
- Yang X, Jiao YM, Wang R, Ji YX, Zhang HW, Zhang YH, Chen DX, Zhang T, Wu H. High CCR5 Density on Central Memory CD4+ T Cells in Acute HIV-1 Infection Is Mostly Associated with Rapid Disease Progression. PLoS One. 2012;7 (11):e49526. doi: 10.1371/journal.pone.0049526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaunders JJ, Dyer WB, Wang B, Munier ML, Miranda-Saksena M, Newton R, Moore J, Mackay CR, Cooper DA, Saksena NK, Kelleher AD. Identification of circulating antigen-specific CD4+ T lymphocytes with a CCR5+ cytotoxic phenotype in an HIV-1 long-term nonprogressor and in CMV infection. Blood. 2004;103 (6):2238–2247. doi: 10.1182/blood-2003-08-2765. [DOI] [PubMed] [Google Scholar]