Abstract
Animal models that express autism-related behavioral characteristics have been used to promote our understanding of factors that can influence specific behavioral aspects of the disorder. The BTBR T+tf/J (BTBR) mouse has been described as a mouse model of autism because it displays three core features of the disorder, including repetitive behavior patterns. The purpose of this study was to examine the effects of environmental enrichment on the quantity and quality of repetitive behaviors in the BTBR mouse model. Two types of repetitive behavior were examined: 1) repetitive grooming behaviors were investigated as a lower-order repetitive motor behavior and 2) repetitive object exploration was measured as a higher-order repetitive cognitive behavior. Baseline scores taken from mice at seven weeks of age confirmed that BTBR mice spend significantly more time grooming than control C57BL/6J mice and use a more rigid grooming sequence. After thirty days of enrichment housing, BTBR mice demonstrated a significant reduction in time spent grooming compared to BTBR mice placed in standard housing; no differences were found with regard to grooming sequence between enriched and standard housed BTBR mice. At baseline no differences were found between BTBR mice and control mice for the object exploration task. In addition, no differences were found in relation to sequential object exploration between BTBR mice housed in enriched vs. standard cages. The results suggest that environmental enrichment may be beneficial for reducing time spent engaging in lower-order repetitive behaviors, but may not change the overall quality of the behaviors when they do manifest.
Lower order and higher order repetitive behaviors have been documented in the BTBR T+tf/J (BTBR) mouse strain, a mouse model that exhibits all three core behavioral domains that define autism. The purpose of this study was to evaluate the effectiveness of environmental enrichment for reducing repetitive behaviors in BTBR mice. Lower order behaviors were captured by assaying the time and sequence of grooming, while higher order behaviors were measured using pattern analysis of an object exploration task from digital recordings. Baseline scores were established at seven weeks of age followed by 30 days of housing in either a standard or enriched cage. As expected, BTBR mice spent significantly more time grooming and had a more rigid grooming sequence than control C57BL/6J mice did at baseline. After 30 days of enrichment housing, BTBR mice demonstrated a significant reduction in time spent grooming, resulting in levels that were lower than those exhibited by BTBR mice in standard housing. However, no changes were noted in the rigidity of their grooming sequence. In contrast to previous findings, there was no difference in repetitive patterns of exploration at baseline between BTBR and C57BL/6J mice on the object exploration test. Subsequently, enrichment did not significantly alter the number of repetitive patterns at posttest. Overall the results suggest that environmental enrichment may be beneficial for reducing the time spent engaging in lower-order repetitive behaviors, but may not change the overall quality of the behaviors when they do manifest.
1. Introduction
Autism is a developmental disorder characterized by communication deficits, social impairments, and repetitive/stereotyped behaviors (American Psychiatric Association, 2000). Specifically, repetitive behaviors are defined as resistance to change and repeated sensory and motor actions with no functional purpose (Szatmari et al., 2006). Repetitive behaviors can be grouped in two broad categories, lower order and higher order behaviors (Lewis, Tanimura, Lee, & Bodfish, 2007; Lam, Bodfish, & Piven, 2008). Lower order behaviors include self-injury and motor stereotypy such as repetitive manipulations of an object, while higher order behaviors are described as cognitive rigidity that manifests as strict adherence to routines, rituals, obsessions, or narrow interests (Lam et al., 2008). These diverse manifestations of repetitive behavior are thought to have different developmental trajectories and etiologies, such as disruption of the basal ganglia pathways, but the exact cause and course of the behaviors are unknown, making them difficult to treat (Lam & Aman, 2007; Richler, Huerta, Bishop, & Lord, 2010).
To date, no specific pharmacological or behavioral therapies have proved to be fully effective in ameliorating repetitive behaviors in children with autism (Baranek, 2002; Wink, Erickson, & McDougle, 2010). Current clinical efforts focus on functional analysis and management of aberrant behaviors or early communication intervention to replace these behaviors with socially acceptable behaviors (Boyd, McDonough, Rupp, Khan, & Bodfish, 2011; Richman & Lindauer, 2005; Sigafoos & Meikle, 1996). Preliminary evidence also suggests that sensory-based therapy may be beneficial for reducing repetitive behaviors (Favell, McGimsey, & Schell, 1982; Smith, Press, Koenig, & Kinnealey, 2005). This type of sensory-based treatment involves providing individuals with varying sensory and motor experiences in a specialized, enriched environment with the goal of ameliorating atypical sensory responses and improving behavioral outcomes (Reynolds, Lane, Richards, 2010).
The purpose of this study was to assess the effects of environmental enrichment (including sensory and motor features) on both lower order (self-grooming) and higher order (repetitive object exploration) repetitive behaviors in the BTBR T+tf/J (BTBR) mouse. BTBR mice have recently emerged as a well-validated mouse model of autism due to their manifestation of core phenotypic autism behaviors: impaired socialization, deficits in communication (measured by sniffing and social transmission of food behaviors) and repetitive behaviors (McFarlane, Kusek, Yang, Phoenix, Bolivar, & Crawley, 2008; Moy et al. 2007, Moy et al., 2008; Pearson et al., 2011). While it is unknown whether BTBR mice share a cause of these altered behaviors with autism in humans, the collective manifestation of these symptoms provides a model in which treatment options may be explored in phenotypic behaviors of interest.
Specifically, the BTBR mouse has been shown to exhibit both lower and higher order repetitive behaviors. Pearson et al. (2011) and McFarlane et al. (2008) found that BTBR mice engaged in repetitive self-grooming and bar-biting behaviors (lower-order behaviors) significantly more than control C57BL/6J mice, and spent significantly more time on specific objects in a test of a repetitive novel object contact task (higher-order behavior). Pearson et al. (2011) further analyzed grooming behaviors in BTBR mice by using a grooming analysis algorithm, a sequence of behaviors or steps that rodents tend to follow when grooming. The steps tend to progress in a cephalo-caudal fashion starting with wetting of the paws, a face wash, body grooming, leg wash, followed by a cleaning of the genital area or tail (Kalueff, Aldridge, LaPorte, Murphy, & Tuohimaa, 2007). Recording the individual behaviors within the grooming sequence evaluates the rigidity within the behavior. Pearson and colleagues found that BTBR mice exhibited significantly more sequentially-invariant transitions between their grooming stages suggesting that their grooming was not only more frequent but also more rigid than the C57BL/6J mice. The presence of both lower and higher order repetitive behaviors make the BTBR mouse a promising model for testing the effects of therapeutic interventions for the reduction of repetitive behaviors in autism.
Two previous studies have examined changes in autistic-like behaviors following forms of enrichment in the BTBR mouse model. MacPherson and colleagues (2008) examined the effect of continuous environmental enrichment presented in the animal’s home cage for eight days prior to memory training tasks. Results of this study suggested that even brief periods of enrichment may improve object recognition memory in BTBR mice. Yang and colleagues (2011) used a social enrichment paradigm, housing BTBR mice with a highly social C57BL/6J strain for up to 40 days. Compared to BTBR controls who were reared with other BTBR mice, the socially housed BTBR group showed significant improvements in social approach behaviors as young adults. Social housing did not, however, lead to any improvements in the high levels of self-grooming evidenced by this strain, suggesting specificity of the social intervention to social outcomes.
The first aim of this study was to replicate previous findings that BTBR mice exhibit significantly more repetitive behaviors compared with C57BL/6J mice at baseline. The second aim was to assess potential changes in repetitive behaviors after environmental enrichment by comparing the behaviors of mice housed in standard caging to those housed in an enriched condition. It was hypothesized that the BTBR group housed in the enriched condition would show less repetitive behavior at posttest compared to the BTBR standard-caged group. The third aim was to observe the changes in groups over time from pretest to posttest, and it was hypothesized that the BTBR mice housed in enrichment would show a significant decline in repetitive behavior and those housed in standard caging would show no significant change.
2. Methods
2.1 Animals and Housing
Sixteen male C57BL/6J and 15 male BTBR T+tf/J (BTBR) mice arrived from Jackson Laboratory at six weeks of age and were placed in standard caging for one week to acclimate to the new facility. The mice were tested at seven weeks of age during the first four hours of the light part of their light cycle. At the completion of baseline testing, the mice were placed in their assigned housing (standard, enriched) for 30 days. The four experimental groups were as follows: eight C57BL/6J mice in standard housing, eight C57BL/6J mice in an enrichment cage, seven BTBR mice in standard housing, and eight BTBR mice in an enrichment cage. Standard caged groups were housed in groups of three or four, while enriched caged groups were housed in groups of eight.
The enrichment cage was a dog kennel with two extra floors built in and several toys including running wheels and tunnels. The toys (not including the shelters and running wheel) were changed every five days to add an element of novelty. Harlan Teklad hardwood sani-chip bedding was used in both the enriched and standard cages. Following the 30 days in housing the animals were tested again at 12 weeks of age. All animals were maintained on a 12hr:12hr light:dark cycle with lights on at 7:00 a.m. (approximately 400 lux), and had constant access to food (Labdiet 5LOD rodent diet) and tap water. All procedures were pre-approved by the University of Florida’s Institutional Animal Care and Use Committee.
2.2. Behavioral Measures
Two aspects of grooming were analyzed in this study, which were thought to reflect lower order repetitive behavior: 1) time spent grooming and 2) the sequential invariance in cephalo-caudal grooming pattern. Individual mice were each tested in a clear plastic box (4 × 4 × 4 ¾) in a dimly lit room (145 lux) adjacent to the housing area. Following a 1 minute acclimation period, time spent grooming was recorded during a 10-minute observation session. The session was videotaped and then scored for elements of the grooming analysis algorithm (grooming stages: paw, face, body, leg, tail/genital) (Kalueff et al., 2007). The percentage of sequentially-invariant grooming transitions was derived by calculating the number of transitions that did not follow the grooming sequence divided by total number of transitions.
A repetitive novel object contact test was conducted to measure higher order repetitive behaviors. A standard cage (11 × 7 × 5 in) filled with a one-inch layer of bedding was used with four novel objects (a miniature bowling pin, a die, a blue Lego, and a green marble) that were placed in each corner of the box approximately 1.5 inches from the edge. Each mouse was placed individually in the box for a total of 11 minutes: one minute of acclimation and 10 minutes of observation. Lighting in the room was set at 35 lux. A video camera was positioned above the box to record the mouse’s interactions with the objects. Interaction with an object was defined as sniffing, vibrissae contact, or burying of the object. Each object was assigned a number one through four and the mouse’s interactions with the objects were recorded to find patterns of repetition.
A MATLAB program was created to look for all possible three and four object patterns or permutations of the numbers one through four in the mouse’s interactions. A pattern had to repeat at least twice in the mouse’s activity to be counted. An individual pattern could not include consecutive repeating numbers (e.g. 3-3-1) but a pattern with an object appearing twice but separated by a visit to another object (e.g. 3-1-3) was acceptable. The total of each pattern’s appearance was summed to get the total number of each category (either three or four object patterns) for each individual mouse. For example, if pattern 3-1-3 appeared five times and pattern 1-2-3 appeared four times, the total number of three object patterns would be nine. This was consistent with the process used by Pearson and colleagues (2011), except that they did not include patterns that overlapped. It should be noted that they have included overlapping patterns in their more recent investigations (personal communication). Other differences include: the jack used in the original article was substituted for the marble and the mice were tested at seven weeks instead of 20-25 weeks.
2.3 Data Reduction and Analysis
Videos were de-identified so that research assistants were blinded to the subject’s housing condition and group. All videos were reviewed by an independent reviewer using the coding scheme described in previous sections. For the grooming task, time spent grooming was recorded separately from the grooming sequence (i.e. paw, face, body, leg, tail/genital, or not grooming). Object interaction (i.e. Lego, marble, die, bowling pin, or no interaction) was coded manually prior to entering the sequence into the MATLAB program for sequential analysis. An overall inter-rater reliability greater than 90% (time grooming: 99%, grooming stages: 92%, object interaction: 85%) was established for video coding on both behavioral tests; mean intra-rater reliability was greater than 95% (time grooming: 99%, grooming stages: 94%, object interaction: 95%). All data were entered into SPSS 18.0 software for statistical analysis.
All four experimental groups were treated identically prior to baseline testing; t-tests were used to confirm that there were no differences at baseline between the two C57BL/6J groups or between the two BTBR groups. Analysis of baseline scores for each of the four dependent variables (time spent grooming, sequentially invariant transitions, and number of three and four object repetitive patterns of exploration) was conducted between strains using standard t-tests. At posttest two-way analysis of variance models (ANOVA) with bonferonni post-hoc adjustments were used to examine differences by strain and by treatment condition. Effects of strain and treatment are reported separately, as well as the interaction effect which was used to assess whether the treatment affected the two strains differently. Paired t-tests were used to compare baseline (pretest)and posttest scores within each experimental group. Alpha level was set at 0.05 for all statistical analyses.
3. Results
3.1 Lower Order Behaviors
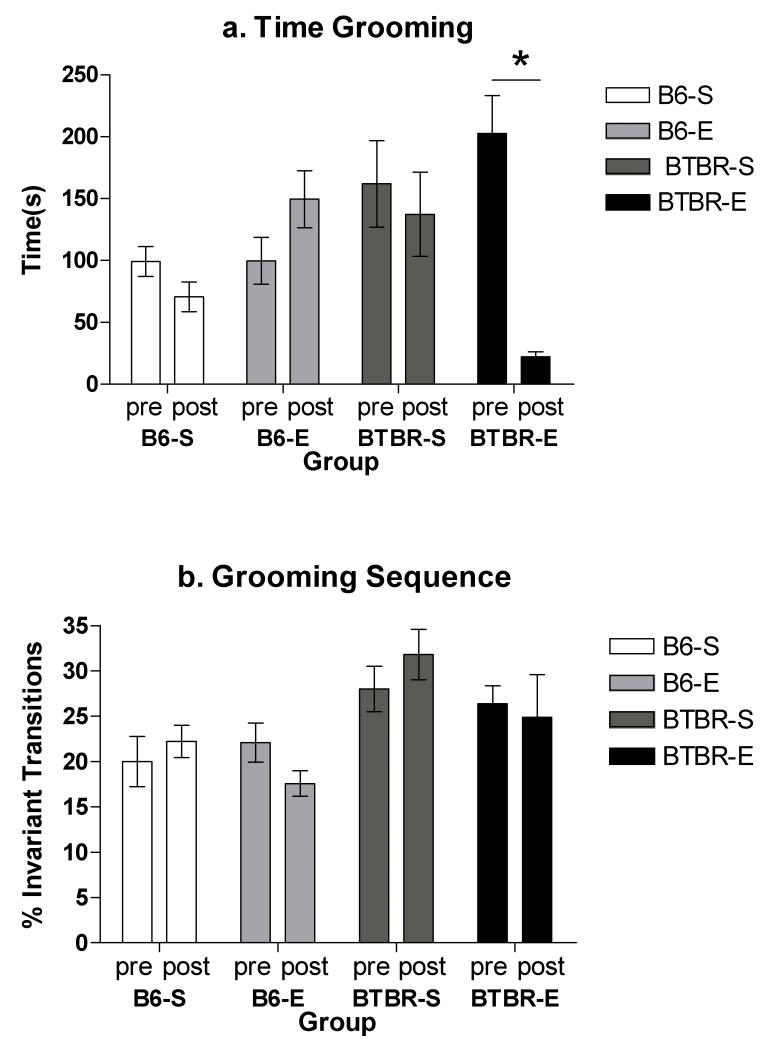
The BTBR mice groomed significantly more than the C57BL/6J mice (p<0.01) at baseline, prior to being placed in their experimental housing condition. Following the thirty days in housing, our two-way analysis of variance test found that housing condition (enriched, standard) did not have a significant effect (F(1,30)=.781,p=0.385) on time spent grooming though the enriched group averaged 15 seconds less grooming compared to standard housed mice. Mouse strain (C57BL/6J, BTBR) also did not have a significant effect (F(1,30)= 2.189, p=0.151). The interaction of mouse strain and housing condition was significant (F(1,30)=22.501, p<0.001). Post-hoc testing revealed that BTBR mice housed in the enriched condition groomed the least amount of time; significantly less than BTBR mice housed in standard conditions (p<0.01) and C57BL/6J mice housed in enrichment (p<0.01). For the BTBR mice housed in the enriched environment, there was a significant decrease in the amount of time spent grooming from pretest to posttest (p< 0.001); there was no significant change seen for the BTBR mice housed in standard caging (p=0.430). Similarly, no significant changes were seen from pretest to posttest for the C57BL/6J mice housed in standard (p=0.070) or enriched (p=0.160) conditions (Figure 1a).
Figure 1.
a. Pre and posttest grooming time (number of seconds grooming during a 10 minute observation) for C57BL/6J (B6) and BTBR T+tf/J (BTBR) mice housed in either standard (S) or enriched (E) conditions. * = p<.05
b. Pre and posttest scores are reported as the percent of sequentially invariant grooming transitions observed during a 10 minute grooming session for C57BL/6J (B6) and BTBR T+tf/J (BTBR) mice housed in either standard (S) or enriched (E) conditions.
The BTBR mice also exhibited a significantly higher percentage of sequentially invariant grooming transitions compared to the C57BL/6JJ mice at baseline, suggesting that they were more rigid in their grooming behaviors (p<0.05). At posttest, a two-way analysis of variance revealed that type of housing condition (enriched, standard) approached significance (F(1,30)=3.57, p=0.07); enriched mice averaged approximately 6% fewer invariant transitions at posttest compared to standard housed mice. Mouse strain (C57BL/6J, BTBR) had a significant effect (F(1,30)=7.629, p<0.05); BTBR mice averaged 9% more sequentially invariant transitions at posttest compared to C57BL/6J mice. The interaction for housing condition and mouse strain, however, was non-significant (F=(1,30)=.139, p=0.712). Post-hoc testing did reveal that BTBR mice housed in standard conditions demonstrated significantly more sequentially invariant grooming transitions compared to C57BL/6J mice housed in enrichment (p<0.05).
When changes from pretest to posttest were examined for each group, no significant differences were found with regards to percentage of sequentially invariant grooming transitions (C57BL/6J standard, p=0.479; C57BL/6J enriched, p=0.084; BTBR standard p=0.408; BTBR enriched p=0.735) (Figure 1b).
3.2 Higher Order Behaviors
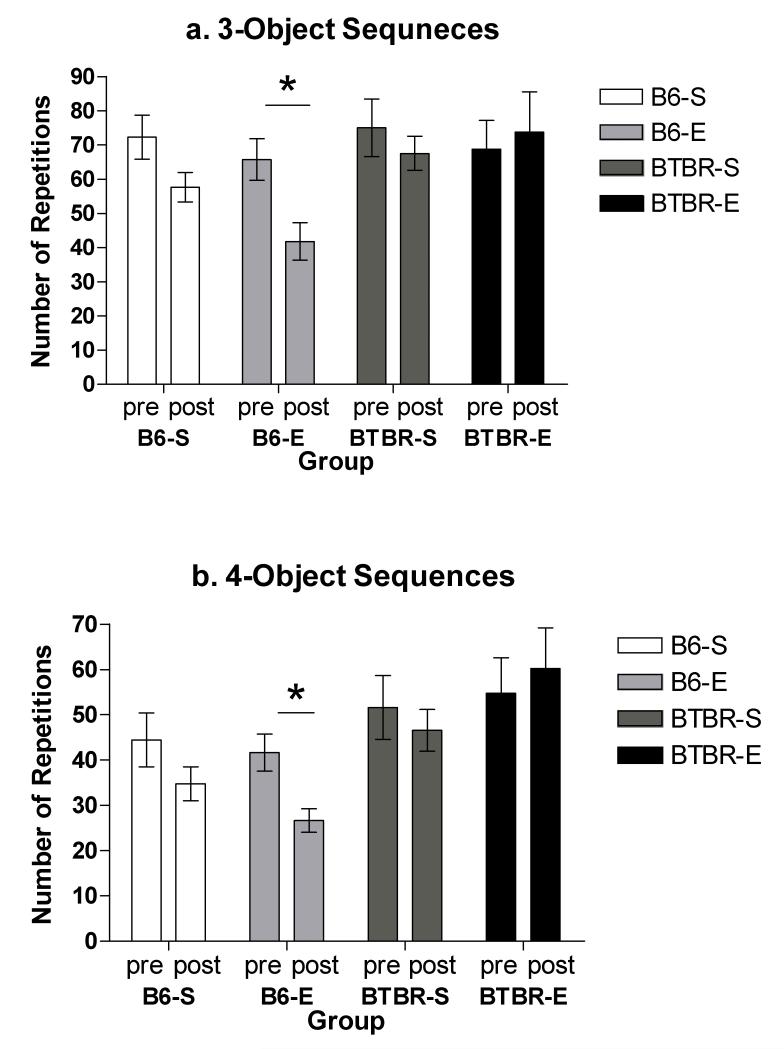
In contrast with previous findings, there was no significant difference in the amount of repetitive object investigation between BTBR and C57BL/6J mice at baseline. Specifically there was no significant difference in the number of three object (p=0.715) or four object (p=0.105) patterns. A two-way analysis of variance found that type of housing condition (enriched, standard) did not have a significant effect (F(1,30)=.424, p=0.520) on number of 3-object sequences at posttest, though the enriched group averaged 5 fewer 3-object sequences compared to standard housed mice. Mouse strain (C57BL/6J, BTBR) did have a significant effect (F(1,30)=7.941, p<0.01); BTBR mice had approximately 20 more 3-object exploration patterns at posttest compared to C57BL/6J mice. The interaction for housing condition and mouse strain was non-significant (F(1,30)=2.195, p=0.150). Post-hoc testing revealed differences between the two enriched groups at posttest, with the BTBR enriched group showing more sequentially invariant 3-object exploration patterns compared to the C57BL/6J enriched group (p<0.05).
Similar posttest results were found for the posttest variable of 4-object sequences. A two-way analysis of variance revealed that type of housing (enriched, standard) did not have a significant effect (F(1,30)=.245, p=0.625) on number of 4-object sequences. Mouse strain (C57BL/6J, BTBR) did have a significant effect (F(1,30)=16.409, p<0.001); BTBR mice had approximately 23 more 4-object sequences at posttest compared to C57BL/6J mice. The interaction effect for housing condition and mouse strain approached significance (F(1,30)=3.777, p=0.062). Post hoc testing revealed differences between the two enriched groups at posttest, with the BTBR enriched group showing more sequentially invariant 4-object exploration patterns compared to the C57BL/6J enriched group (p<0.01).
Paired t-tests were used to examine changes in the number of three and four object patterns from pre to posttest. There was no significant change from pretest to posttest in the number of three object (Figure 2a) or four object (Figure 2b) patterns for BTBR standard (p=0.239. p=0.470), BTBR enriched (p= 0.795, p=0.724), or C57BL/6J standard (p=0.153, p=0.292) groups. C57BL/6J mice housed in the enriched condition, however, significantly decreased in the number of three object (p<0.05) and four object patterns (p<0.05) from pretest to posttest.
Figure 2.
a. Mean number of 3-object pattern repetitions seen at pretest and posttest for C57BL/6J (B6) and BTBR T+tf/J (BTBR) mice housed in either standard (S) or enriched (E) conditions. * = p<.05
b. Mean number of 4-object pattern repetitions seen at pretest and posttest for C57BL/6J (B6) and BTBR T+tf/J (BTBR) mice housed in either standard (S) or enriched (E) conditions. * = p<.05
4. Discussion
The best documented repetitive behavior in BTBR mice has been repetitive self-grooming. Grooming is natural and necessary for all rodents but is considered abnormal or excessive in the BTBR mouse because it contributes to abnormal hair loss (Pearson et al., 2011). The grooming behaviors observed in this study at baseline were consistent with previous research which found that BTBR mice groomed significantly more than the standard C57BL/6J mouse (McFarlane et al., 2008; Pearson et al., 2011). For the BTBR group, the time spent grooming decreased significantly for mice housed in enrichment, and not for those in standard housing. This finding suggests that changes in time spent grooming are not simply due to maturational effects, and that enrichment can significantly reduce time engaged in stereotyped grooming in the BTBR strain. Surprisingly, we saw a non-significant but apparent increase in grooming in the enriched C57BL/6J mice which we attribute to a possible increase in stress prior to post testing procedures. Extracting the C57BL/6J mice from the enrichment cage was challenging due to strong attempts by the mice to evade extraction (e.g. darting, hiding); this difficulty was not present during extraction of the BTBR mice from the enrichment cage or in removing mice from standard housing conditions. Overall, the time spent grooming was reduced by environmental enrichment in the BTBR mice, but not in the C57BL/6J mice. This suggests that environmental enrichment may interact specifically with the abnormal or excessive behavior of the BTBR mice. There are a variety of other mouse strains or genetic mutants in which excessive grooming is observed (e.g. C58, Sapap3 knockouts; Ryan et al., 2010, Welch et al., 2007), so it would be interesting to examine if enrichment has similar selective effects in those strains.
In this study, grooming patterns were also analyzed to determine the percentage of sequentially invariant grooming transitions, which was used as a measure of rigidity exhibited by the mice. At baseline the BTBR mice groomed with a higher percentage of invariance, suggesting more rigidity than the control C57BL/6J mice. While these baseline scores showed predicted differences in BTBR and C57BL/6J mice, no effects of enrichment were found at posttest; whereas the time spent grooming went down, the phenomenological expression of invariant grooming patterns was not significantly altered by enrichment in the BTBR mice.
Higher order repetitive behavior was evaluated using the novel object contact test. This test was designed to measure the repetitive patterns of exploration, and was first used with BTBR mice by Pearson and colleagues (2011). In that study, BTBR mice 20-25 weeks of age were found to engage in significantly more repetitive patterns of exploration compared to age-matched control C57BL/6J mice. In contrast, data from the current study revealed no significant difference in repetitive patterns of exploration at baseline between naïve C57BL/6J and BTBR mice that were tested between 7-8 weeks of age. The discrepancy in results may be explained by the age differences at the time of testing; it is possible that there is greater cognitive rigidity in older BTBR mice, at least with regards to exploration patterns. Supporting this hypothesis our posttest results conducted at 12 weeks of age found differences based on mouse strain for both 3-object and 4-object patterns; with BTBR mice demonstrating 57-70% more repetitive patterns of exploration compared to C57BL/6J mice. Interestingly it has been suggested that more complex repetitive behaviors like rituals and insistence on familiar objects and actions may be stronger or become more prevalent with age in individuals with autism (Lam & Aman, 2007; Militerni, Bravacccio, Falco, Fico, & Palermo, 2002; Richler et al.,2010). Age and many other factors, such as IQ and verbal functioning, are important indicators of repetitive behaviors in children (Esbensen, Seltzer, Lam, & Bodfish, 2009); this suggests that age may also need to be considered and analyzed within an animal model.
Despite the possible age component, this experiment showed that enrichment did not have an effect on the number of exploratory patterns for the BTBR mice while it did significantly reduce the number of patterns in the C57BL/6J mice. This phenomenon, where the control mice benefit from enrichment more than the experimental group, has been observed in several other studies of enrichment (Turner, Yang & Lewis, 2002; Turner & Lewis, 2003; Martínez-Cué et al., 2002). One recent study reports that BTBR mice exhibit greater learning about environmental stimuli if paired with a familiar C57Bl/6J mouse, than they do if they are paired with a familiar BTBR mouse (Lipina and Roder, 2013). In our experimental paradigm, since C57BL/6J mice were exposed to enrichment in one group, and BTBR mice were exposed to enrichment in a separate group, the C57BL/6J, may have had a greater response to the stimulation because of the facilitory effect of housing with other socially competent mice. This may suggest that exposure to a sensory enriched environment is not enough to produce a change in certain repetitive behaviors, and that some functional level of interaction with the environment, or with peers in the environment, is needed to maximize behavioral learning. There is also a question of dosage. Previous research with deer mice, which exhibit high levels of stereotypy and cognitive inflexibility, showed that 60 to 80 days of enrichment significantly improved cognitive-mediated behaviors such as reversal learning, procedural learning, and cognitive flexibility (Tanimura, Yang, & Lewis, 2008). Certain aspects of cognitive rigidity, therefore, may be affected by enrichment, but the subjects may need more time, a larger “dose” than what was provided in the current experiment. Finally, the timing of enrichment administration may also need to be considered. Many prior studies using the enrichment paradigm have initiated enrichment housing directly after the animals are weaned, around post-natal day 21 (Turner et al., 2002; Turner & Lewis, 2003). By keeping the mice in standard housing until completion of baseline testing at eight weeks of age, it is possible that the mice in the current study were not exposed to the enriched condition early enough in development to have a significant impact on neuroplasticity in the developing brain. These are important methodological issues to consider in future studies.
5. Limitations
The primary limitation of this study was the relatively small number of animals included per group, which reduces the overall power of the study. Despite the small sample, however, significant effects were seen in response to our intervention; suggesting very large treatment effects. It must also be considered however, that a few of our statistical p-values were between 0.05-.10. While not significant, these trends could be related to meaningful changes or group differences. Future research could include a larger sample to explore these issues.
6. Conclusion
The behavioral and neurological benefits of enrichment have been noted since the 1950’s and the idea of experience driven developmental changes in the brain is not new. Although further research is required, this study suggests that enrichment may significantly reduce some aspects of repetitive behavior, mainly lower order or motor stereotypy, in an animal model of autism. Future research should consider how these findings can be extrapolated into designing effective treatments for reducing repetitive behavior in children with autism spectrum disorders.
Acknowledgements
This project was funded by the National Center for Medical Rehabilitation Research and the National Institute of Neurological Disorders and Stroke (K12 HD055929) Rehabilitation Research Career Development Program. The authors would like to acknowledge Dr. Mark Lewis and his staff for their assistance on this project, as well as Stephanie Cameron, Corinne Mackiewicz, and Alexandre Millette who served as part of the project team.
Grant Sponsor: National Center for Medical Rehabilitation Research and the National Institute of Neurological Disorders and Stroke, Rehabilitation Research Career Development Program
Grant Number: K12 HD055929
Contributor Information
Stacey Reynolds, Virginia Commonwealth University Box 980008 Richmond, VA 23298.
Meagan Urruela, University of Florida Box 100164 Gainesville, FL 32610 Phone: 352-273-6098 murruela333@ufl.edu.
Darragh P. Devine, University of Florida Box 112250 Gainesville, FL 32611 Phone: 352-273-2174 dpdevine@ufl.edu.
References
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders DSM-IV-TR. Fourth Edition (Text Revision) American Psychiatric Publishing, Inc; Washington, DC: 2000. [Google Scholar]
- Baranek GT. Efficacy of Sensory and Motor Interventions for Children with Autism. Journal Autism Developmental Disorders. 2002;32:397–422. doi: 10.1023/a:1020541906063. [DOI] [PubMed] [Google Scholar]
- Boyd BA, McDonough SG, Rupp B, Khan F, Bodfish JW. Effects of a family-implemented treatment on the repetitive behaviors of children with autism. Journal of Autism and Developmental Disorders. 2011;41:1330–1341. doi: 10.1007/s10803-010-1156-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esbensen AJ, Seltzer MM, Lam K, Bodfish JW. Age-Related Differences in Restricted Repetitive Behaviors in Autism Spectrum Disorders. Journal of Autism and Developmental Disorders. 2009;39:57–66. doi: 10.1007/s10803-008-0599-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favell JE, McGimsey JF, Schell RM. Treatment of self-injury by providing alternate sensory activities. Analysis & Intervention Dev. Disab. 1982;2:83–104. [Google Scholar]
- Kalueff AV, Aldridge JW, LaPorte JL, Murphy DL, Tuohimaa P. Analyzing grooming microstructure in neurobehavioral experiments. Nature Protocols. 2007;2:2538–2544. doi: 10.1038/nprot.2007.367. [DOI] [PubMed] [Google Scholar]
- Lam KS, Aman MG. The Repetitive Behavior Scale-Revised: Independent Validation in Individuals with Autism Spectrum Disorders. Journal of Autism and Developmental Disorders. 2007;37:855–866. doi: 10.1007/s10803-006-0213-z. [DOI] [PubMed] [Google Scholar]
- Lam KS, Bodfish JW, Piven J. Evidence for three subtypes of repetitive behavior in autism that differ in familiality and association with other symptoms. Journal of Child Psychology and Psychiatry. 2008;49:1193–1200. doi: 10.1111/j.1469-7610.2008.01944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MH, Tanimura Y, Lee WL, Bodfish JW. Animal models of restricted repetitive behavior in autism. Behavioural Brain Research. 2007;176:66–74. doi: 10.1016/j.bbr.2006.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipina TV, Roder JC. Co-learning facilitates memory in mice: A new avenue in social neuroscience. Neuropharmacology. 2013;64:283–293. doi: 10.1016/j.neuropharm.2012.06.054. [DOI] [PubMed] [Google Scholar]
- MacPherson P, McGaffigan R, Wahlsten D, Nguyen PV. Impaired fear memory, altered object memory and modified hippocampal synaptic plasticity in split-brain mice. Brain Research. 2008;1210:179–188. doi: 10.1016/j.brainres.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Martínez-Cué C, Baamonde C, Lumbreras M, Paz J, Davisson MT, Schmidt C, Flórez J. Differential effects of environmental enrichment on behavior and learning of male and female Ts65Dn mice, a model for Down syndrome. Behavioral Brain Research. 2002;134:185–200. doi: 10.1016/s0166-4328(02)00026-8. [DOI] [PubMed] [Google Scholar]
- McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism-like behavioral phenotypes in BTBR T1tf/J mice. Genes, Brain and Behavior. 2008;7:152–163. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- Militerni R, Bravaccio C, Falco C, Fico C, Palermo MT. Repetitive behaviors in autistic disorder. European Child Adolescent Psychiatry. 2002;11:210–218. doi: 10.1007/s00787-002-0279-x. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Poe MD, Nonneman RJ, Young NB, Koller BH, Bodfish JW. Development of a mouse test for repetitive, restricted behaviors: Relevance to autism. Behavioural Brain Research. 2008;188:178–194. doi: 10.1016/j.bbr.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, Crawley JN. Mouse behavioral tasks relevant to autism: Phenotypes of 10 inbred strains. Behavioural Brain Research. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson BL, Pobbe RL, Defensor EB, Oasay L, Bolivar VJ, Blanchard DC, Blanchard RJ. Motor and cognitive stereotypies in the BTBR T+tf/J mouse model of autism. Genes, Brain and Behavior. 2011;10:228–235. doi: 10.1111/j.1601-183X.2010.00659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds S, Lane SJ, Richards L. Using animal models of enriched environments to inform research on sensory integration intervention for the rehabilitation of neurodevelopmental disorders. Journal of Neurodevelopmental Disorders. 2010;2:120–132. doi: 10.1007/s11689-010-9053-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richler J, Huerta M, Bishop S, Lord C. Developmental Trajectories of Restricted and Repetitive Behaviors and Interests in Children with Autism Spectrum Disorders. Developmental Psychopathology. 2010;22:55–69. doi: 10.1017/S0954579409990265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman DM, Lindauer SE. Longitudinal assessment of stereotypic, proto-injurious, and self-injurious behavior exhibited by young children with developmental delays. American Journal of Mental Retardation. 2005;110:439–450. doi: 10.1352/0895-8017(2005)110[439:LAOSPA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Ryan BC, Young NB, Crawley JN, Bodfish JW, Moy SS. Social deficits, stereotypy and early emergence of repetitive behavior in the C58/J inbred mouse strain. Behavioural Brain Research. 2010;208:178–188. doi: 10.1016/j.bbr.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigafoos J, Meikle B. Functional communication training for the treatment of multiply determined challenging behavior in two boys with autism. Behavior Modification. 1996;20:60–84. doi: 10.1177/01454455960201003. [DOI] [PubMed] [Google Scholar]
- Smith SA, Press B, Koenig KP, Kinnealey M. Effects of sensory integration intervention on self-stimulating and self-injurious behaviors. American Journal of Occupational Therapy. 2005;59:418–425. doi: 10.5014/ajot.59.4.418. [DOI] [PubMed] [Google Scholar]
- Szatmari P, Georgiades S, Bryson S, Zwaigenbaum L, Roberts W, Mahoney W, Tuff L. Investigating the structure of the restricted, repetitive behaviours and interests domain of autism. Journal of Child Psychology and Psychiatry. 2006;47:582–590. doi: 10.1111/j.1469-7610.2005.01537.x. [DOI] [PubMed] [Google Scholar]
- Tanimura Y, Yang MC, Lewis MH. Procedural learning and cognitive flexibility in a mouse model of restricted, repetitive behaviour. Behavioral Brain Research. 2008;189:250–256. doi: 10.1016/j.bbr.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Turner CA, Lewis MH. Environmental enrichment: effects on stereotyped behavior and neurotrophin levels. Physiology and Behavior. 2003;80:259–266. doi: 10.1016/j.physbeh.2003.07.008. [DOI] [PubMed] [Google Scholar]
- Turner CA, Yang MC, Lewis MH. Environmental enrichment: effects on stereotyped behavior and regional neuronal metabolic activity. Brain Research. 2002;938:15–21. doi: 10.1016/s0006-8993(02)02472-1. [DOI] [PubMed] [Google Scholar]
- Welch JM, Lu J, Rodriguiz RM, Trotta NC, Peca J, Ding JD, Feliciano C, Chen M, Adams JP, Luo J, Dudek SM, Weinberg RJ, Calakos N, Wetsel WC, Feng G. Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice. Nature. 2007;448:894–900. doi: 10.1038/nature06104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wink LK, Erickson CA, McDougle CJ. Pharmacologic treatment of behavioral symptoms associated with autism and other pervasive developmental disorders. Curr Treat Options Neurol. 2010;12:529–538. doi: 10.1007/s11940-010-0091-8. [DOI] [PubMed] [Google Scholar]
- Yang M, Perry K, Weber MD, Katz AM, Crawley JN. Social peers rescue autism-relevant sociability deficits in adolescent mice. Autism Research. 2011;4:17–27. doi: 10.1002/aur.163. [DOI] [PMC free article] [PubMed] [Google Scholar]