Abstract
Aims: Acquisition and detoxification of metal ions are vital biological processes. Given the requirement of metallochaperones in cellular copper distribution and metallation of cuproproteins, this study investigates whether the metallochaperones also deliver metal ions for transporters functioning in metal detoxification. Results: Resistance to excess cadmium and copper of the yeast Saccharomyces cerevisiae, which is conferred by PCA1 and CaCRP1 metal efflux P-type ATPases, respectively, does not rely on known metallochaperones, Atx1p, Ccs1p, and Cox17p. Copper deficiency induced by the expression of CaCRP1 encoding a copper exporter occurs in the absence of Atx1p. Intriguingly, CCS1 encoding the copper chaperone for superoxide dismutase 1 (Sod1p) is necessary for cadmium resistance that is mediated by Ycf1p, a vacuolar cadmium sequestration transporter. This is attributed to Ccs1p's role in the maturation of Sod1p rather than its direct interaction with Ycf1p for cadmium transfer. Functional defect in Ycf1p associated with the absence of Sod1p as well as another antioxidant enzyme Glr1p is rescued by anaerobic growth or substitutions of specific cysteine residues of Ycf1p to alanine or serine. This further supports oxidative inactivation of Ycf1p in the absence of Ccs1p, Sod1p, or Glr1p. Innovation: These results provide new insights into the mechanisms of metal metabolism, interaction among metal ions, and the roles for antioxidant systems in metal detoxification. Conclusion: Copper metabolism and antioxidant enzymes maintain the function of Ycf1p for cadmium defense. Antioxid. Redox Signal. 21, 1475–1489.
Introduction
Metallochaperones are important players in intracellular trafficking and insertion of copper into cuproproteins (23, 53, 56) (Fig. 1A). Atx1p (Atox1) transfers copper via a direct interaction with copper-transporting P1B-type ATPase(s) (e.g., ATP7A and ATP7B in mammals, Ccc2p in yeast Saccharomyces cerevisiae) at the trans-Golgi network where copper is loaded into copper-containing secretory proteins (23, 53, 56). Several molecular factors involved in copper incorporation into cytochrome c oxidase (Cco), including Cox17p, Sco1p, and Sco2p, have been characterized (56). Ccs1p (copper chaperone for superoxide dismutase 1) physically interacts with apo-superoxide dismutase 1 (Sod1p) for copper insertion (12, 53). Metallochaperones for other metal ions, including ArsD for ArsA arsenite-transporting ATPase in Escherichia coli (4), and a chaperone for iron (51) have also been identified.
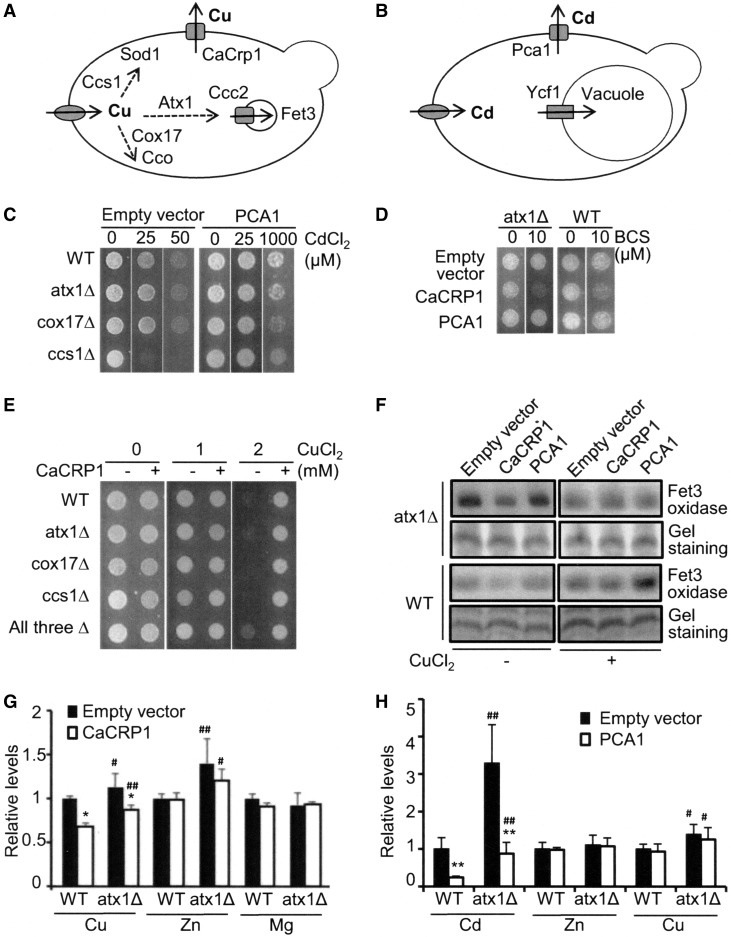
FIG. 1.
Metallochaperone-independent functions of Pca1p and CaCrp1p. (A) Copper ions that enter cells through transporters (e.g., Ctr1p) (32) are incorporated into copper-requiring proteins, including Sod1 superoxide dismutase, Fet3 ferroxidase, and Cco cytochrome c oxidase, with the assistance of target-specific metallochaperones (e.g., Ccs for Sod1, Atx1 for secretory pathway, and Cox17 for Cco). A copper-transporting P-type ATPase Ccc2p translocates copper into the lumen of secretory pathway. CaCrp1 is a copper-exporting P-type ATPase identified in C. albicans. When it is expressed in Saccharomyces cerevisiae by the transformation of an expression construct, it functions as a copper exporter. (B) Cadmium uptake in yeast occurs through transporters for nutritional divalent metals (e.g., zinc, iron, and calcium). Pca1p (P-type ATPase) and Ycf1p (ABC transporter) are known cadmium detoxification transporters. (C, D) Wild-type control (WT), atx1Δ, cox17Δ, ccs1Δ, and triple knockout (All three Δ) strains were individually transformed with empty vector, PCA1 and CaCRP1 expression plasmids. Cells were cultured in synthetic complete (SC) plasmid selection media and spotted on solid SC media that was supplemented with CdCl2 or CuCl2. Cell growth was monitored for 2 days. (E) Growth of atx1Δ and WT cells on copper-requiring media with and without the expression of CaCRP1 and PCA1. Cells were spotted on nonfermentable media that was supplemented with a copper chelator, bathocuproinedisulfonate (BCS). (F) Fet3p oxidase activities of WT and atx1Δ cells with and without expression of CaCRP1 and PCA1. Total protein extracts were subjected to in-gel oxidase assays. In vitro Cu(I) insertion into apo-Fet3p was induced by incubating protein extracts with reduced CuCl2. The intensity of the bands reflects Fet3p enzyme activities. After gel staining, a representative band was shown to indicate equal loading. (G, H) Steady-state metal levels in WT and atx1Δ cells with and without expression of CaCRP1 or PCA1 were measured. Cells were cultured in SC media at the mid-log phase (OD600=0.8–1) and cell-associated copper or cadmium levels were measured using inductively coupled plasma mass spectrometry (ICP-MS). To measure cellular cadmium accumulation, cells were co-cultured with cadmium (5 μM CdCl2) for 1 h. Each datum represents the average±SD of at least six samples. The results were normalized to cell numbers and presented as relative levels to those of wild-type control cells harboring empty vector. * and ** indicate p<0.05 and p<0.01, respectively, compared with empty vector expressing control cells by Student's t-test. # and ## indicate p<0.05 and p<0.01, respectively, compared with WT cells by Student's t-test. Experiments (C–F) were conducted at least twice with two different clones, and a representative figure is shown.
Innovation.
Cellular metabolism of metal ions in a manner preventing their toxicity represents an important process underlying health and disease. This study provides a new insight into the functional specificity of metallochaperones and the mode of action of transporters evolved for metal detoxification. Ycf1p-mediated cadmium tolerance, which relies on copper metabolism for superoxide dismutase and glutathione reductase, illustrates an intriguing interaction between metal ions and a new pathway that is affected by oxidative stress. Ycf1p is a useful model for further studies on redox-dependent regulation of this family of transporters that are associated with health concerns, such as cystic fibrosis, and multi-drug resistance.
Cadmium is a highly toxic environmental contaminant that is implicated with various disorders, such as kidney and bone damage, cancer, and endocrine disruption (25, 46). The pathways for nutritional metal acquisition provide a gateway for cadmium to enter the biological system, which is based on the broad substrate specificity of the transporters' and cadmium's similarity to other nutritional metals (41, 63). Cadmium's high affinity for thiols is considered the primary mechanism underlying cadmium toxicity. Various biochemical pathways, including redox homeostasis, DNA repair, signal transduction, and metabolic pathways, are the major known targets of cadmium (25, 46).
Organisms are equipped with cadmium detoxification mechanisms, such as chelation, compartmentalization, and efflux (33, 57, 71). Cadmium binding to metallothioneins (MTs) and glutathione (GSH) limits its interactions with other vital molecules. Cadmium ions are also sequestered into subcellular compartments (e.g., vacuole in yeast and plants) or exported out of cells through transporters. Yeast Cadmium Factor 1 (YCF1) confers cadmium resistance in yeast S. cerevisiae through vacuolar sequestration of GSH-conjugated cadmium, bis(glutathionato)cadmium (GS2-Cd) (38) (Fig. 1B). It belongs to the ABCC (MRP) subfamily of ATP-binding cassette (ABC) transporters (50). Ycf1p is regulated both positively and negatively by phosphorylation (16, 49). Several proteins, including Tus1p guanine nucleotide exchange factor and Rho1p, a Tus1p substrate, interact with Ycf1p and affect its activities (36, 48, 50).
Our previous study demonstrated that S. cerevisiae Pca1p is a P1B-type ATPase which extrudes cadmium out of the cell for cadmium detoxification (1) (Fig. 1B). This family of heavy metal transporters is widely conserved from bacteria to humans and mediates the ATP hydrolysis-driven transport of various metal ions (5, 20, 69). For instance, Candida albicans CRP1 (CaCRP1, CAD1) is involved in copper detoxification (55, 68), although a CaCrp1p-like copper efflux transporter does not exist in the S. cerevisiae genome.
It is intriguing to note that a bacterial cadmium-binding cytoplasmic protein replaces Atx1p function in yeast (45), and Atx1p metallochaperone can bind cadmium as well as copper (22). A copper chaperone CopZ in Bacillus subtilis is important for both copper and cadmium tolerance (60). These results suggest that known copper metallochaperones may be involved in subcellular trafficking of cadmium, and Pca1p and CaCrp1p may acquire cadmium and copper, respectively, via metallochaperone-dependent mechanisms.
To define the roles for known metallochaperones in metal detoxification, we characterized the functions of PCA1, CaCRP1, and YCF1 in atx1Δ, ccs1Δ, and cox17Δ strains, respectively. Our results indicate that cadmium and copper resistance conferred by Pca1p and CaCrp1p, respectively, does not depend on these metallochaperones. An unanticipated finding is that CCS1, but not ATX1 and COX17, is critical for cadmium tolerance conferred by YCF1. Several lines of evidence indicate that Ccs1p's role for Sod1p maturation is required for protecting Ycf1p from oxidative inactivation, which is further supported by the role of another antioxidant enzyme Glr1p in Ycf1p function. These results provide new insights into metal metabolism, interactions among metal ions, and the roles for antioxidant systems in cadmium tolerance.
Results
Pca1p- and CaCrp1p-mediated metal efflux in the absence of metallochaperones
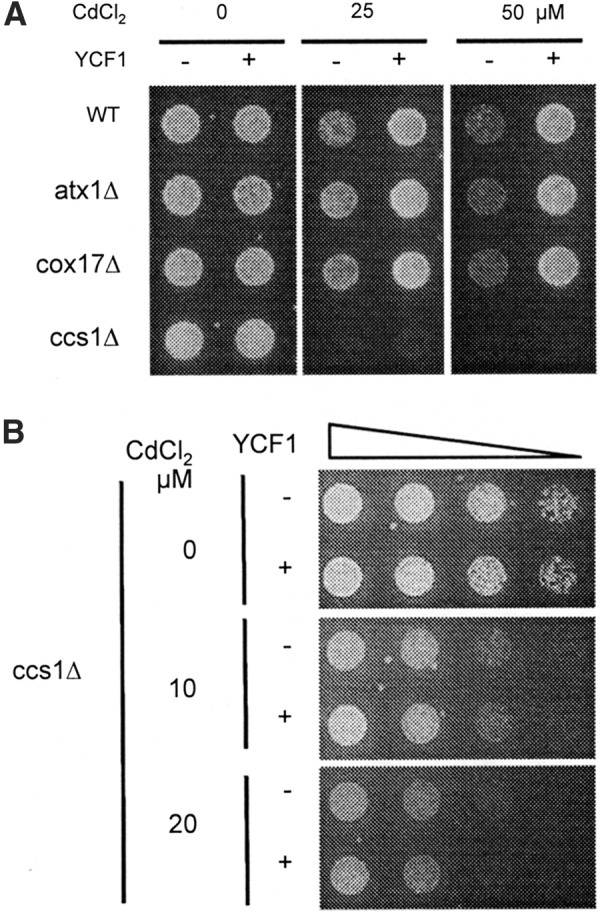
To gain a better understanding of metal metabolism, we sought to determine the roles for known metallochaperones in CaCrp1p and Pca1p-mediated metal tolerance (Fig. 1A, B). Due to a naturally occurring point mutation, chromosomal PCA1 in the S. cerevisiae strains used in this study and many other laboratory yeast strains are nonfunctional (1). The S. cerevisiae genome does not have an orthologue of CaCRP1, a copper-exporting P-type ATPase identified in C. albicans (55, 68). Atx1Δ, cox17Δ, and ccs1Δ yeast strains were transformed with an empty vector and expression construct of functional PCA1 or CaCRP1 (2, 3). Cadmium and copper resistance of the cells were determined by growth assays (Fig. 1C–H). Relative to empty vector transformation (Fig. 1C, left panel), PCA1 expression dramatically increases cadmium tolerance (more than 1 mM CdCl2) for wild-type (WT) control and each metallochaperone knockout strain to a similar degree (Fig. 1C, right panel). The deletion of individual or all three metallochaperones also did not affect CaCRP1-dependent copper tolerance (Fig. 1D). These results indicate that the known metallochaperones are not required for Pca1p- and CaCrp1p-mediated metal resistance.
Next, we examined whether CaCrp1p can efflux copper under copper limitation and Atx1p plays a role in this process. Atx1Δ cells expressing empty vector, CaCRP1, or PCA1 were cultured on copper-requiring media supplemented with bathocuproine disulphonate (BCS), a copper chelator. The observed growth inhibition of atx1Δ cells expressing CaCRP1 (Fig. 1E) suggests that CaCrp1p expression induces copper limitation. Fet3p is a copper-containing ferroxidase that forms a complex with Ftr1p for iron uptake (23, 53, 56). Fet3p oxidase activities are significantly reduced in the cells expressing CaCRP1 (Fig. 1F, middle line of left upper panel) but recovered by in vitro copper metallation of Fet3p (Fig. 1F, middle line of right upper panel), indicating the expression of apo-Fet3p. These results suggest that CaCRP1 extrudes copper under both copper excess and limited growth conditions in an Atx1-independent manner.
CaCRP1 expression reduces steady state-cellular levels of copper (Cu) but not zinc (Zn) and magnesium (Mg) in both WT and atx1Δ cells (Fig. 1G and Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/ars), confirming the Atx1p-independent function of CaCrp1p and its metal specificity. Cellular cadmium (Cd) accumulation is reduced by PCA1 expression, which is also independent of Atx1p (Fig. 1H and Supplementary Table S2). Higher Cu and Zn levels in the atx1Δ cells relative to those in WT cells (Fig. 1G) likely reflect the up-regulation of metal transporters displaying broad substrate specificity in response to iron deficiency in the atx1Δ cells (26). Cadmium co-culture significantly down-regulated zinc accumulation (Supplementary Tables S1, S2), reflecting the known competition between these metals for the uptake of transporters. While the mechanism(s) remain to be elucidated, cadmium elevated cellular copper levels (Supplementary Tables S1, S2).
YCF1-mediated cadmium resistance is dependent on CCS1
Ccs1Δ cells are sensitive to cadmium toxicity relative to isogenic wild type, atx1Δ, or cox17Δ cells (Figs. 1C, left panel and 2A), which is consistent with previous reports (58, 64). While Pca1p rescues the cadmium sensitivity of ccs1Δ cells to levels similar to those of wild-type cells (Fig. 1C, right panels), cadmium resistance by YCF1 encoding vacuolar cadmium importer is not evident in ccs1Δ cells (Fig. 2A), which is distinct from WT, atx1Δ, and cox17Δ cells (Fig. 2A). This unanticipated result indicates that Ccs1p is required for cadmium tolerance which is mediated by Ycf1p. We further confirmed this observation by examining the growth of ccs1Δ cells with and without expression of YCF1 by plating serially diluted cells on cadmium containing medium (Fig. 2B). These results suggest that Ccs1p might function as a cadmium chaperone for Ycf1p-mediated cadmium sequestration into the vacuole. However, given that Ccs1p is a critical factor for the maturation of cytoplasmic superoxide dismutase (Sod1p) (11), nonfunctionality of Ycf1p in ccs1Δ cells might be attributed to Sod1p defect.
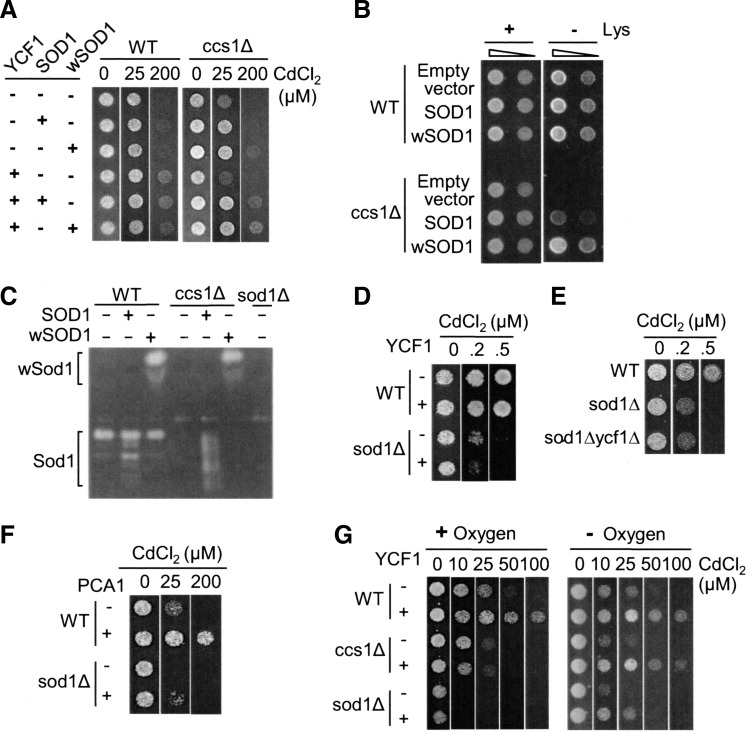
FIG. 2.
Ycf1p-mediated cadmium resistance relies on Ccs1p, the metallochaperone for Sod1p. (A) Wild-type control (WT), atx1Δ, cox17Δ, and ccs1Δ strains were transformed with empty vector and YCF1 expression plasmid. Cells were cultured in synthetic complete (SC) media and spotted on solid SC media that was supplemented with CdCl2 at the indicated concentrations. (B) Ccs1Δ cells with and without YCF1 expression plasmid were serially diluted. The triangle indicates the serial 1/5 dilution of cells from OD600 0.5. Cells (5 μl) were spotted on media containing indicated cadmium concentrations. Cell growth was photographed after 2 days. Experiments were conducted twice with two different clones, and a representative figure is shown.
Oxidative stress in ccs1Δ cells is involved in Ycf1p functional defect
While copper cofactor insertion into yeast Sod1p is dependent on Ccs1p (11), Sod1p in Caenorhabditis elegans (wSod1p) acquires copper in the absence of Ccs1p (37). We took advantage of this characteristic of wSod1p to define whether Ccs1p itself or Ccs1p-dependent Sod1p activation is required for Ycf1p-mediated cadmium resistance. YCF1 was co-expressed with either yeast SOD1 or wSOD1 in WT and ccs1Δ cells, and cell growth was examined on cadmium-containing media. SOD1 or wSOD1 expression does not confer significant additional cadmium resistance in WT cells, suggesting that Sod1p is not a limiting factor for cadmium defense in WT cells (Fig. 3A, left panel). However, the expression of wSOD1 or SOD1 rescues cadmium sensitivity of ccs1Δ cells (Fig. 3A, right panel upper three rows). Moreover, in the presence of Sod1p and wSod1p, YCF1 expression in ccs1Δ cells leads to cadmium hyper-resistance at similar levels to those of WT cells. These results clearly indicate that Ccs1p-dependent copper metallation of Sod1p is necessary for Ycf1p-mediated cadmium resistance. We also confirmed the activation of Sod1p and wSod1p in ccs1Δ cells by monitoring the rescue of lysine auxotrophy (11) (Fig. 3B) and Sod1p enzyme activity assays (Fig. 3C). These results also indicate that when yeast SOD1 is overexpressed, it acquires copper at least partially in the absence of Ccs1p.
FIG. 3.
Ccs1p-dependent maturation of Sod1p is necessary for Ycf1p-mediated cadmium resistance. (A) Expression of S. cerevisiae SOD1 or Caenorhabditis elegans wSOD1 rescues Ycf1p function for cadmium resistance in ccs1Δ cells. WT and ccs1Δ cells were co-transformed with YCF1 and SOD1 or wSOD1 expression plasmids and spotted on SC selection media containing indicated CdCl2 concentrations. (B) SOD1 or wSOD1 overexpression rescues lysine (Lys) auxotrophy of ccs1Δ cells. The ccs1Δ cells transformed with an empty vector, SOD1 or wSOD1 expression vector were diluted (OD600=0.5 and 0.1) and spotted on SC media prepared with (+) and without (−) amino acid lysine (Lys). The triangle indicates the 1/5 dilution of cells. (C) Equal amount of lysates prepared from WT, ccs1Δ, and sod1Δ strains with and without expression of SOD1 or wSOD1 were subjected to in-gel superoxide dismutase enzyme assays. Color changes to white on the gel reflect Sod1p activities. (D) YCF1 does not confer cadmium tolerance in sod1Δ cells. The WT and sod1Δ cells transformed with empty vector or YCF1 expression construct were spotted on SC selection media that was supplemented with the indicated CdCl2 concentration. (E) YCF1 knockout in sod1Δ cells does not lead to additional cadmium sensitivity. WT, SOD1 knockout (sod1Δ), and both SOD1 and YCF1 knockout cells (sod1Δycf1Δ) were spotted on SC selection media containing the indicated concentration of CdCl2. (F) Cadmium resistance of sod1Δ cells expressing PCA1. WT and sod1Δ cells transformed with an empty vector or PCA1 expression vector were spotted on SC selection media that was supplemented with CdCl2. (G) WT, ccs1Δ, and sod1Δ cells transformed with an empty vector, or YCF1 expression vector was spotted on SC selection plates that were supplemented with CdCl2. Cells were then cultured under normoxic and oxygen-limited conditions. Cell growth was photographed after 2 days except the right panel of (G), which was photographed in 4 days. Experiments were conducted at least twice with two different clones, and a representative figure is shown.
Consistent with the requirement of Sod1p activity in Ycf1p function, YCF1 expression in sod1Δ cells also cannot confer growth advantage on toxic cadmium media (Fig. 3D). Moreover, YCF1 deletion in sod1Δ cells does not further increase cadmium sensitivity (Fig. 3E). These phenotypes are not associated with the general sickness of sod1Δ cells, as the expression of PCA1 enables sod1Δ cells to grow to approximately 25 μM CdCl2 (Fig. 3F).
To address the role of superoxide stress in the functional defect of Ycf1p, we examined cadmium tolerance under oxygen-limited conditions. WT, ccs1Δ, and sod1Δ strains expressing empty vector or YCF1 were spotted on cadmium-supplemented media and then cultured in an anaerobic chamber and under normoxia. Indeed, YCF1 expression dramatically enhanced cadmium resistance in both ccs1Δ and sod1Δ strains under low oxygen conditions (Fig. 3G, right panel), which is distinct from the minimal functionality of Ycf1p in cadmium tolerance when these strains are cultured aerobically (Fig. 3G, left panel). These results strongly suggest that oxidative stress impairs Ycf1p function in ccs1Δ or sod1Δ cells.
No correlation between vacuolar morphology changes and cadmium sensitivity
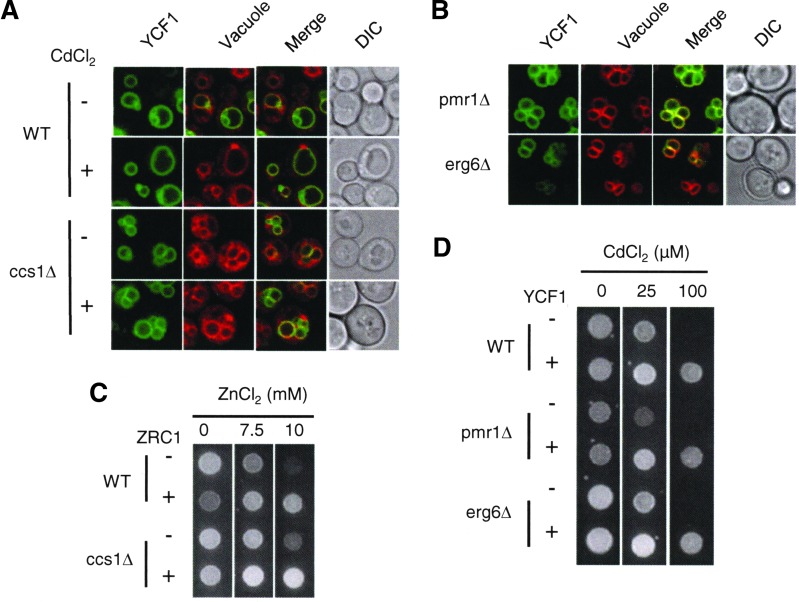
Sod1Δ yeast cells manifest vacuolar fragmentation (9). Consistent with the role of Ccs1p in Sod1p activation, ccs1Δ cells also display the same vacuolar defect, despite there being no further change in vacuolar morphology by cadmium co-culture (Fig. 4A). Given that Ycf1p is a vacuolar cadmium transporter, Ycf1p functional defect in ccs1Δ and sod1Δ strains might be associated with the vacuolar problem. To elaborate the possibility, we examined zinc resistance by the expression of ZRC1 gene encoding a vacuolar zinc importer (28). Overexpression of ZRC1 enables the cells to survive on toxic zinc media, and this protective effect of ZRC1 is displayed in both WT control and ccs1Δ strains (Fig. 4B), suggesting that despite vacuolar fragmentation Zrc1p remains functional. We also examined Ycf1p-dependent cadmium resistance in pmr1Δ and erg6Δ strains which manifest a similar vacuolar morphology to that of ccs1Δ (Fig. 4C). Cadmium resistance in these strains expressing Ycf1p is comparable to that of the WT control strain (Fig. 4D). Collectively, these results indicate that abnormal vacuole morphology is not a causal factor for the functional defect of Ycf1p observed in ccs1Δ or sod1Δ cells.
FIG. 4.
No significant correlation between vacuolar defects and the nonfunctionality of Ycf1p. (A) Confocal microscopy determining subcellular localization of GFP-fused functional Ycf1p in WT control and ccs1Δ cells. FM4-64 stained vacuolar membranes. Cells that were cultured with and without CdCl2 (15 μM for 9 h) in the media were subjected to confocal microscopy. (B) Zinc tolerance conferred by expression of ZRC1, a vacuolar zinc importer. WT and ccs1Δ cells transformed with empty vector or ZRC1 expression vector were spotted on SC selection media that was supplemented with ZnCl2 at the indicated concentrations. (C) Confocal microscopy of subcellular localization of GFP-fused functional Ycf1p in pmr1Δ and erg6Δ cells. Co-culture of cells with FM4-64 stained vacuolar membranes. (D) The pmr1Δ and erg6Δ cells were transformed with an empty vector or YCF1 expression vector and spotted on SC selection media containing CdCl2. Cell growth was photographed after 2 days. Experiments were conducted at least twice with two different clones, and a representative figure is shown. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Determination of Ycf1p function in yeast strains in which antioxidant systems are defective
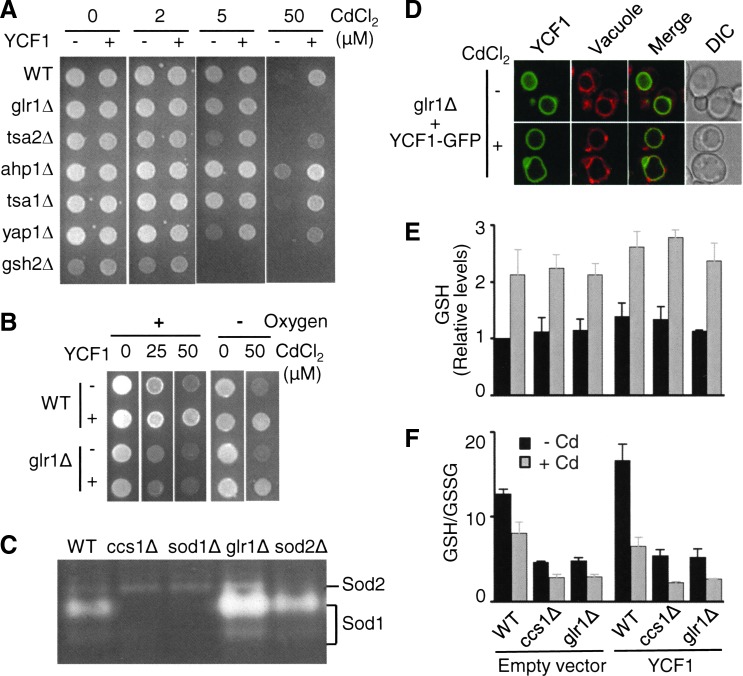
To further ascertain the oxidative stress-induced functional defect of Ycf1p, we next determined the roles of other antioxidant genes in Ycf1p-dependent cadmium tolerance. YCF1 was expressed in yeast strains in which major genes encoding antioxidant molecules, enzymes, or regulators (GLR1, TSA2, AHP1, PRB1, YAP1, GSH2, GTT2, ZWF1, SOD2, GRX1-8, TRX3, TRR2, PRX1, GPX1, GPX2, SKN7, CTA1, and TSA1) were deleted individually. Cadmium resistance of most of these strains is comparable to that of WT (e.g., tsa1Δ in Fig. 5A). The skn7Δ (35) and ahp1Δ (Fig. 5A) strains are intriguingly more resistant to cadmium relative to WT, although the underlying mechanism remains to be determined. The glr1Δ, tsa2Δ, yap1Δ, gsh2Δ, grx3Δ, trr2Δ, and zwf1Δ strains are more sensitive to cadmium (Fig. 5A). Nevertheless, most of these examined strains, including these cadmium-sensitive strains, manifested higher cadmium resistance by YCF1 expression (representative results in Fig. 5A), which indicates that Ycf1p is functional in these strains.
FIG. 5.
Roles for genes encoding antioxidant in Ycf1p-mediated cadmium resistance. (A) WT and antioxidant gene deletion strains were transformed with an empty vector or a YCF1 expression plasmid. Cells were spotted on SC selection media containing indicated CdCl2 concentrations. (B) Limited oxygen rescues Ycf1p functions for cadmium resistance in glr1Δ cells. WT and glr1Δ cells transformed with empty vector or YCF1 expression vector were spotted on SC selection that was supplemented with CdCl2. Cells were then cultured under normal and limited oxygen conditions. (C) The glr1Δ cells display higher Sod1p enzyme activities. Sod1p activities in cell lysates of the indicated yeast strains were detected by in-gel assays. (D) The glr1Δ cells transformed with empty vector or an expression construct of GFP-fused functional Ycf1p were co-cultured with CdCl2 (15 μM, 9 h) and then subjected to confocal microscopy. Co-culture of cells with FM4-64 at the last 30 min of cadmium exposure stained the vacuolar membrane. (E) GSH levels and (F) GSH/GSSG ratio in WT control, ccs1Δ, and sod1Δ cells with and without YCF1 expression. GSH and GSSG levels were measured using deproteinated lysates of the cells that were cultured without (black bars) or with (gray bars) CdCl2 (15 μM CdCl2, 9 h). The results were normalized to protein concentrations of cell lysates before deproteination and presented as relative levels to those of WT expressing empty vector. Each datum represents the average±SD of four experiments. All other experiments were conducted at least twice with two different clones, and a representative figure is shown. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Among the strains examined, gsh2Δ and glr1Δ did not display significant cadmium resistance by YCF1 expression (Fig. 5A, B). Gsh2p is an enzyme that is involved in the synthesis of GSH, the key component of the bis(glutathionato)cadmium (GS2-Cd) complexes which serve as substrates of Ycf1p (38). Hence, no significant Ycf1p-mediated cadmium tolerance in the gsh2Δ cells likely reflects substrate unavailability for Ycf1p. The GLR1 gene encodes an enzyme that reduces oxidized GSH (GSSG) to GSH to maintain a high GSH/GSSG ratio, which is critical for cellular redox homeostasis (18). Similar to our observation in the ccs1Δ cells, when the glr1Δ cells are cultured under oxygen-limited conditions, YCF1 expression enhances cadmium resistance (Fig. 5B). Higher Sod1p activities in glr1Δ cells relative to WT cells are indicative of oxidative stress in glr1Δ cells (Fig. 5C). Nevertheless, no significant change in either cellular distribution of Ycf1p or vacuolar morphology was observed in these cells (Fig. 5D). Collectively, these results indicate that another major cytoplasmic antioxidant enzyme, Glr1p, is also required for Ycf1p-mediated cadmium resistance.
The ccs1Δ and glr1Δ strains may suffer GSH limitation akin to gsh2Δ strain, which, consequently, could compromise Ycf1p function. To address this possibility, we measured total GSH levels and the GSH/GSSG ratio in WT control, ccs1Δ, and glr1Δ cells co-cultured with cadmium. However, no significant change in the steady-state levels of reduced GSH and cadmium-induced up-regulation of GSH was observed (Fig. 5E). The GSH/GSSG ratio was approximately three-fold lower in ccs1Δ and glr1Δ cells relative to that in WT control cells (Fig. 5F). Cadmium co-culture also reduced GSH/GSSG ratios in a similar manner (Fig. 5F). These results support the conclusion that while ccs1Δ and glr1Δ strains suffer oxidative stress and cadmium perturbs cellular redox homeostasis, these cells are capable of maintaining the reduced GSH levels which are required for the formation of GSH-Cd complexes.
No significant change in Ycf1p expression, maturation, and interaction with other proteins
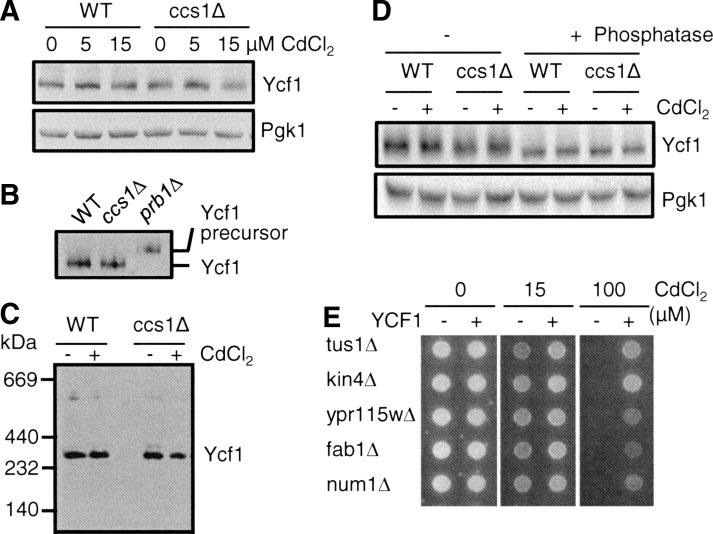
Oxidative stress results in reactive oxygen species (ROS)-induced damage of proteins, lipids, and nucleic acids (21); hence, Ycf1p might be considered a target of ROS in ccs1Δ, sod1Δ, and glr1Δ strains. The consequences of ROS-mediated protein damages include amino-acid (especially cysteine and methionine) oxidation, nonspecific intra- or inter-molecular disulfide bond formation, aggregation, glutathionylation, and changes in turnover rate. Western blotting of Ycf1p demonstrates no significant difference in Ycf1p levels between WT and ccs1Δ strains with and without cadmium co-culture (Fig. 6A). Ycf1p migration on reducing and nonreducing sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) is also unchanged, suggesting no disulfide bond(s) formation of Ycf1p (data not shown). Our several attempts for the detection of Ycf1p glutathionylation with anti-GSH antibodies did not reveal any evidence for such modification (data not shown). Mass spectrometry-based characterization of Ycf1p post-translational modifications faced technical difficulties due to the detection of only a small portion of the Ycf1p, which might be attributed to biophysical characteristics of Ycf1p (e.g., 17 trans-membrane helices). Hence, these lines of experiments failed to provide convincing evidence of any oxidative modification of Ycf1p.
FIG. 6.
Effects of CCS1 deletion and cadmium co-culture on post-translational events of Ycf1p. (A) WT and ccs1Δ cells expressing C-terminal HA-epitope-tagged functional Ycf1p were co-cultured with the indicated concentration of CdCl2 for 9 h. Total cell lysates prepared by vortexing the cells with glass beads were solubilized with 1% Triton X-100 and subjected to SDS-PAGE, and Ycf1p was detected by western blotting using anti-HA antibodies. To determine equal loading, each blot was also probed for 3-phosphoglycerate kinase (Pgk1p). (B) N-terminal proteolytic processing of Ycf1p. C-terminal HA-epitope-tagged functional Ycf1p was expressed in wild-type control (WT), ccs1Δ, and prb1Δ cells. Lysates extracted from the mid-log phase cells that were cultured in SC selection media were subjected to western blotting using anti-HA antibodies. (C) Ycf1p is detected as predicted homo-dimers. WT and ccs1Δ cells expressing HA epitope-tagged Ycf1p were co-cultured with CdCl2 (15 μM, 9 h). Ycf1-enriched fractions obtained by sucrose density gradient centrifugation of cell lysates were subjected to blue native polyacrylamide gel electrophoresis followed by western blotting using anti-HA antibodies. (D) Phosphorylation-dependent migration change of Ycf1p on SDS-polyacrylamide gel. WT and ccs1Δ cells expressing HA-epitope-tagged Ycf1p were cultured with or without CdCl2 (15 μM, 9 h) in the media. Cell lysates with or without phosphatase treatment were subjected to SDS-PAGE. Ycf1p was detected by western blotting using anti-HA antibodies. Dephosphorylated Ycf1p migrates fast. (E) YCF1-dependent cadmium resistance in the strains deleted for indicated genes encoding proteins that physically interact with Ycf1p. The strains expressing an empty vector or YCF1 expression construct were spotted on SC selection media that was supplemented with CdCl2. Cell growth was photographed after 2 days. Experiments were conducted at least twice with two different clones, and a representative figure is shown.
The N-terminal extension of Ycf1p is known to play a role in targeting Ycf1p to the vacuole, and Prb1p, vacuolar proteinase B, cleaves this domain in the maturation process (42). Therefore, changes in vacuolar physiochemical characteristics of ccs1Δ cells might alter the processing of Ycf1p; however, both WT and ccs1Δ strains express the N-terminal cleaved Ycf1p that is smaller than its precursor detected in the prb1Δ cells (Fig. 6B). Second, given that several ABCC (MRP)-type transporters are known to function as homo-dimers (44), we examined Ycf1 dimerization and its defect in ccs1Δ cells or by cadmium co-culture. Blue-native gel analysis of Ycf1p indicates that the majority of Ycf1p is detected as dimers in both WT and ccs1Δ cells independent of cadmium co-culture (Fig. 6C). Third, we correlated Ycf1p's phosphorylation status with its functional change (Fig. 6D). Ycf1p is phosphorylated under normal growth conditions as reflected by slower migration of phosphorylated Ycf1p in SDS-PAGE (16, 49). No significant migration difference of Ycf1p in response to CCS1 deletion or cadmium co-culture (Fig. 6D) indicates normal Ycf1p phosphorylation. Lastly, given that six proteins physically interact with Ycf1p (48), Ycf1p-mediated cadmium resistance might rely on the interaction, and oxidative stress may affect this. All of these genes except PSA1 are nonessential for cell growth, and YCF1 expression evidently increases cadmium tolerance in these gene knockout strains (Fig. 6E). Hence, impaired interaction(s) of Ycf1p with these proteins in ccs1Δ cells is unlikely to affect Ycf1p-mediated cadmium resistance. Collectively, these results suggest that previously known post-translational events and multimerization of Ycf1p are not altered by CCS1 gene deletion or cadmium co-culture.
Roles for cysteine residues in regulation of Ycf1p's function
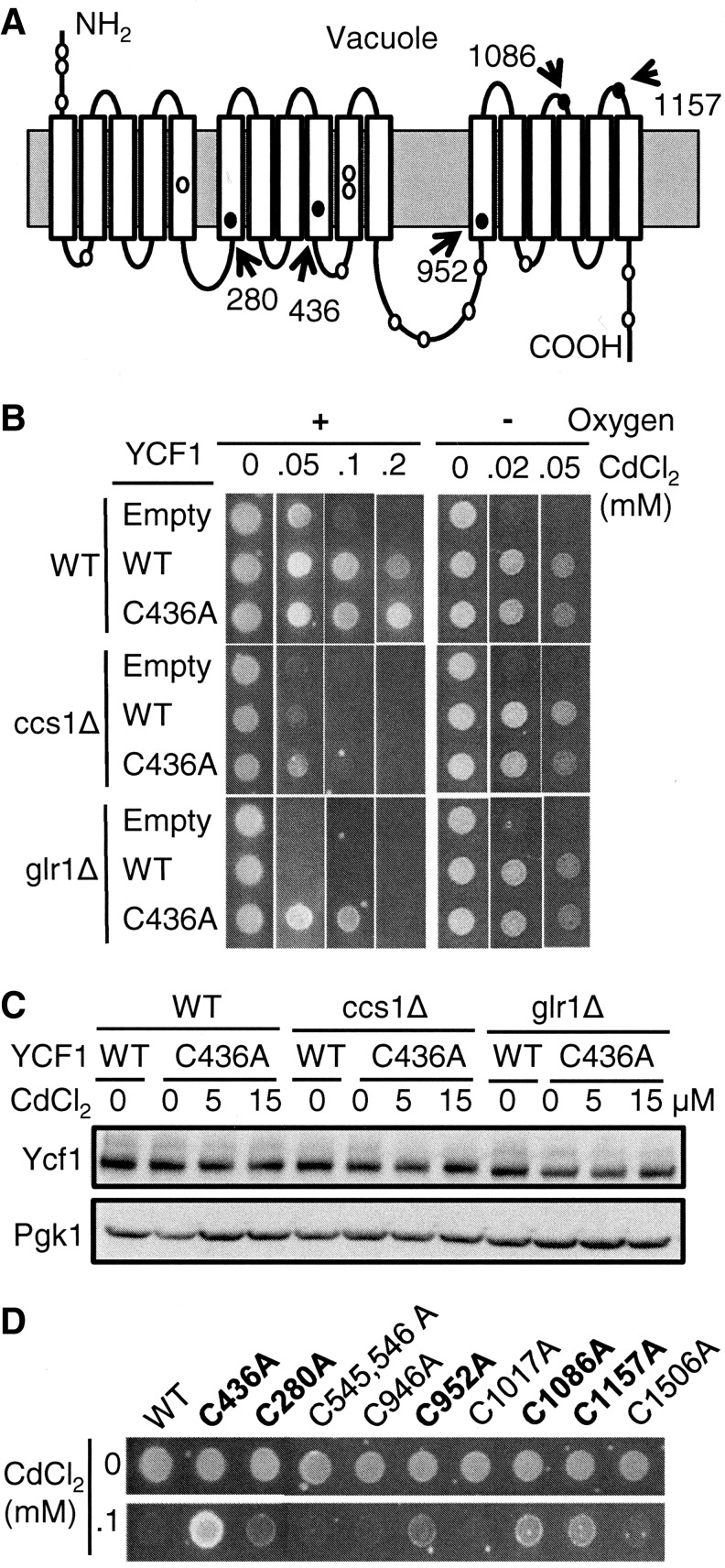
Cysteine residues are particularly sensitive to oxidation, which often leads to changes in protein function and activities (6, 43). Ycf1p contains 20 Cys residues (Fig. 7A). Sequence alignment of fungal Ycf1p-like proteins identified from the NCBI database, including XP_721319.1, XP_449053.1, XP_003672250.1, CCK71890.1, CCH40876.1, XP_001523679.1, XP_460066.2, and EFW95970.1, revealed that Cys436 is conserved among these transporters. To determine whether this Cys residue affects Ycf1p activity, YCF1 carrying a substitution of the Cys436 to Ala (C436A) was expressed in WT, ccs1Δ, and glr1Δ strains. While cadmium tolerance of WT cells expressing either Ycf1p or Ycf1(C436A)p was comparable (Fig. 7B upper panel), Ycf1(C436A)p conferred better cadmium resistance relative to Ycf1p when expressed in ccs1Δ strain (Fig. 7B middle panel). The C436S substitution showed similar results (data not shown). This effect was more pronounced when Ycf1p(C436A) was expressed in glr1Δ cells (Fig. 7B lower panel). However, no enhanced cadmium resistance is observed when the cells are cultured under oxygen limitation (Fig. 7B right panels), suggesting oxidative stress-dependent advantage of cadmium tolerance by Ycf1(C436A)p. Immunoblot analysis showed similar expression levels of Ycf1p and Ycf1(C436A)p (Fig. 7C). Green fluorescence protein (GFP)-fused functional Ycf1p and Ycf1(C436A)p displayed similar subcellular localization (data not shown).
FIG. 7.
Cysteine residues in Ycf1p affect its activities in antioxidant and oxygen-dependent manners. (A) Schematic depiction of the structural features of Ycf1p and location of the 20 Cys residues. Ycf1p contains 17 predicted transmembrane helices (white boxes). Cysteine residues are labeled as circles, and five of them that affect Ycf1p function in ccs1Δ or glr1Δ cells are filled with black. (B) YCF1(C436A) confers cadmium resistance when expressed in ccs1Δ and glr1Δ cells. WT control, ccs1Δ, or glr1Δ cells expressing an empty vector or YCF1(C436A) were cultured to the mid-log phase. Cells (∼5 μl of OD600=0.5) were spotted on SC selection media that was supplemented with indicated CdCl2 concentrations. Plates were cultured aerobically and anaerobically for 2 and 4 days, respectively, before photography. (C) Expression of Ycf1p determined by western blotting. WT, ccs1Δ, and glr1Δ cells expressing empty vector, YCF1, or YCF1(C436A) were co-cultured with CdCl2 (15 μM, 9 h). Cell lysates were prepared by vortexing cells with glass beads in the buffer containing 1% triton X-100. Samples were subjected to western blotting using anti-HA antibodies. To determine equal loading, each blot was probed for 3-phosphoglycerate kinase (Pgk1p). (D) Expression constructs of YCF1 possessing Ala substitution of indicated Cys residues were transformed into glr1Δ cells. Cell growth on cadmium-containing media was photographed in 2 days. YCF1 alleles that confer a better cadmium resistance relative to control YCF1 are highlighted in bold.
Given partial functional recovery by Cys436 to Ala or Ser substitutions, we determined whether other Cys residues are involved in the oxidative inactivation of Ycf1p. All other 19 Cys residues in Ycf1p (Fig. 7A) were substituted to Ala individually or in combination with a nearby Cys residue. The glr1Δ cells were used to examine cadmium resistance in response to the expression of these YCF1 alleles, because glr1Δ cells relative to ccs1Δ cells display better phenotypic rescue by Ycf1p(C436A) expression. C280A, C952A, C1086A, or C1157A substitution results in a higher cadmium tolerance (Fig. 7D). However, none of these YCF1 alleles were more effective than YCF1(C436A) in conferring cadmium resistance. All other remaining YCF1 alleles manifest cadmium tolerance similar to WT control YCF1 (representative results in Fig. 7D). Collectively, these results suggest that oxidative modification of particular Cys residues might cause the functional defect of Ycf1p in ccs1Δ, sod1Δ, and glr1Δ cells.
Conformational changes of Ycf1p in a manner dependent on cadmium, Glr1p, and Cys436
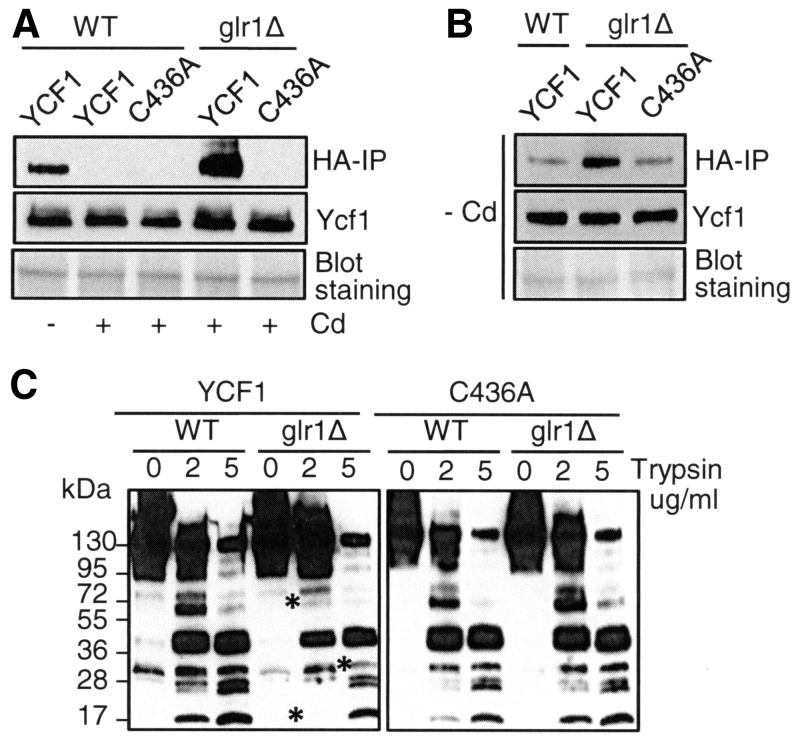
We attempted to enrich C-terminal triple hemagglutinin epitope (HA) epitope-tagged Ycf1p and Ycf1(C436A)p by immunoprecipitation (IP) using anti-HA antibodies. HA epitope-tagged functional YCF1 and YCF1(C436A) were expressed in WT and glr1Δ cells. Total protein extracts were obtained from the cells with and without co-culture of cadmium (15 μM, 9 h). While western blotting detected similar Ycf1p levels in those cell lysates (Fig. 8A, middle panel), an intriguing observation was that IP efficiency of Ycf1p and Ycf1(C436A)p was drastically different (Fig. 8A, upper panel). Distinct from Ycf1p in WT cells cultured without cadmium, IP of Ycf1p and Ycf1(C436A)p expressed in WT cells co-cultured with cadmium was not successful (Fig. 8A, upper panel, lines 2 and 3 vs. lane 1). Moreover, under the same experimental conditions, anti-HA antibodies could pull down Ycf1p but not Ycf1(C436A)p expressed in glr1Δ cells (Fig. 8A, upper panel, line 4 vs. 5). It is important to note that Ycf1p is nonfunctional in glr1Δ cells (Fig. 5B), but C436A substitution rescues Ycf1p function (Fig. 7B). Therefore, these data illustrate a clear correlation between Ycf1p's nonfunctionality and accessibility of the C-terminal HA epitope in Ycf1p. These results collectively suggest that cadmium induces changes in structural characteristics of Ycf1p, and this process is impaired in the glr1Δ cells in a Cys436-dependent manner.
FIG. 8.
Changes in biophysical characteristics of Ycf1p determined by immunoprecipitation (IP), sucrose density gradient fractionation, and limited trypsin digestion. WT and glr1Δ cells were transformed with an expression construct of YCF1 or YCF1(C436A) tagged with an HA epitope at the C terminus. Cells were cultured with and without CdCl2 supplementation (15 μM, 9 h) in the media. Cells lysates were prepared by vortexing cells with glass beads in a degassed buffer (50 mM HEPES, pH 7.4) containing iodoacetic acid (5 mM) to alkylate –SH groups (29). The samples were subjected to IP (A, B), limited trypsin digestion (C), and western blotting using anti-HA antibodies (A–C). Anti-Pgk1p immunoblotting and staining of the membranes determined equal loading. Representative figures of a minimum of two independent experiments are presented. Asterisks indicate the fragments displaying difference in glr1Δ cells.
Next, we conducted the same experiments without cadmium co-culture. Expression levels of Ycf1p in protein extracts were similar to each other (Fig. 8B, middle panel); nevertheless, IP efficiency of Ycf1p in glr1Δ cells was higher relative to Ycf1p in WT cells or Ycf1(C436A)p in glr1Δ cells (Fig. 8B, upper panel). Thus, the absence of Glr1p rather than cadmium is primarily attributed to the higher IP efficiency of Ycf1p expressed in grl1Δ cells.
Conformational change and/or interaction with other molecular factors in response to cadmium could explain the differential IP efficiency of Ycf1p and Ycf1(C436A)p under our experimental conditions. We tested this hypothesis by limited trypsin proteolysis of Ycf1p in the cell lysates obtained from cadmium co-cultured cells. The fragmentation patterns of Ycf1p in total protein extracts of WT cells (Fig. 8C, left panel, lines 1–3) and Ycf1(C436A)p expressed in WT (Fig. 8C, right panel, lines 1–3) and glr1Δ cells (Fig. 8C, right panel, lines 4–6) were similar to each other. This is consistent with the Ycf1p and Ycf1(C436A)p's functionality in these cells (Fig. 7B) and nonaccessibility of the C-terminal HA epitope for IP (Fig. 8A). However, Ycf1p expressed in glr1Δ cells, which is nonfunctional in cadmium resistance, manifested drastically different fragmentation patterns (Fig. 8C, left panel, asterisks on lines 5 and 6), suggesting distinct conformation of Ycf1p in the glr1Δ cells.
Discussion
Specificity of copper metallochaperones
Atx1p delivers copper to the secretory pathway via a copper-mediated physical interaction with the N-terminal metal-binding domains (N-MBDs) of copper-transporting P-type ATPases, such as Ccc2p in yeast (23, 53, 56). We previously showed that Pca1p, a cadmium ATPase, also has one conserved N-MBD, which is essential for Pca1p-mediated cadmium resistance (1). Given that Atx1p binds not only copper but also cadmium (22), Atx1p or other known metallochaperones (e.g., Ccs1p, Cox17p) might interact with Pca1p for cadmium extrusion. However, our results presented here indicate that these known metallochaperones are not required for Pca1p-dependent cadmium resistance. Copper resistance by CaCrp1p also occurs in the absence of these metallochaperones. Hence, these P-type ATPases that are evolved for metal detoxification could acquire metals without the assistance of metallochaperones. However, considering that chaperone-mediated trafficking of iron and arsenite has been recently characterized (4, 51), our results do not rule out the existence of yet unidentified metallochaperone(s) which work specifically for Pca1p or CaCrp1p.
It should be noted that copper chaperones appear to be required in organism- and tissue-specific manners. Distinct from S. cerevisiae Sod1p, other eukaryotic Sod1p acquire copper partially or fully in the absence of Ccs1p (37). The activation of human and C. elegans wSod1p expressed in ccs1Δ yeast cells and a site-directed mutagenesis study of wSOD1 revealed that structural characteristics rather than organism-specific cellular copper availability are attributed to Ccs1p-independent maturation of these enzymes (37). Certain feature(s) of Pca1p and CaCrp1p (Cad1p) might explain the Atx1p-independent metal acquisition. This argument is supported by the lack of ATX1-like genes in some organisms, such as E. coli and M. tuberculosis, despite P-type ATPases in their genome (27). Moreover, while the expression of both ATX1 (CUC-1) and copper P1B-type ATPase (CUA-1) of C. elegans is detected in the intestinal and hypodermal cells, CUC-1 is not co-expressed with CUA-1 in the pharyngeal muscle (65). This suggests Atx1p-independent activities of this copper transporter in certain tissue(s). The mechanisms and physiological significance of the organism and tissue-specific dependency of Sod1p and P1B-type ATPases on metallochaperones remain to be elucidated.
Contribution of Ccs1p and Sod1p in cadmium resistance
While Sod1p is not essential for survival under normal growth conditions, SOD1-deficient cells and organisms manifest diverse phenotypes that are linked to aerobic growth (7, 34, 39). Yeast cells lacking SOD1 are methionine and lysine auxotrophs because of the superoxide-induced damage of the enzymes that are involved in amino-acid biosynthesis (7, 39). Our results show that the Ycf1p-dependent cadmium detoxification pathway is another target of ROS accumulation in sod1Δ cells. This also suggests that cadmium sensitivity of ccs1Δ and sod1Δ strains can be attributed, at least in part, to Ycf1p's defect. It has been shown that SOD1 ablation results in altered iron homeostasis as indicated by the up-regulation of cell surface iron uptake transporters Fet3p and Fet4p (13, 26). Due to Fet4p's broad metal specificity, enhanced Fet4p-mediated cadmium uptake in response to sod1Δ could be considered another mechanism underlying the cadmium sensitivity of ccs1Δ and sod1Δ strains.
It was shown that yeast Sod1p strictly relies on Ccs1p for its copper cofactor acquisition (12). Surprisingly, the sod1Δ cells display significantly higher cadmium sensitivity relative to ccs1Δ cells (0.25 μM for sod1Δ cells vs. 25 μM CdCl2 for ccs1Δ cells). This is contradictory to the assumption that Sod1p is inactive in the absence of Ccs1p. Sod1p might acquire copper by Ccs1p-indepndent mechanism(s) to have activities that are not detectable by currently available methods. Nevertheless, residual Sod1p activities in ccs1Δ cells may not fully explain the difference in cadmium sensitivity between sod1Δ and ccs1Δ strains. It is possible that apo-Sod1p plays a certain role for cadmium tolerance independent of its enzyme activities. Apo-Sod1p might bind not only copper and zinc cofactors but also cadmium when cells are cultured in the media containing excess cadmium, which might result in cadmium sequestration to reduce its toxicity. Conversely, given that cadmium-bound Sod1p is likely inactive, competition of cadmium for copper or zinc sites on Sod1p might be one of the mechanisms underlying cadmium-induced oxidative stress in WT control cells. Of note, Sod1p confers copper tolerance in anaerobic growth conditions where it cannot acquire copper to form functional enzyme because of the requirement for oxygen in copper insertion (10, 17). This indicates that copper resistance under this condition does not rely on copper buffering or enzyme activity. Convincing evidence also indicates that Sod1p plays a role in zinc metabolism in addition to its superoxide dismutase activities (67). A recent report also showed that Sod1p is involved in oxygen and glucose signaling by a physical interaction with kinases (54). Sod1's contribution in cadmium resistance in the absence of Ccs1p could be attributed to its nonenzymatic function. Our ongoing experiments are addressing this hypothesis.
Roles for redox homeostasis in Ycf1p function
Dramatic rescue of Ycf1p-mediated cadmium tolerance when ccs1Δ, sod1Δ, and glr1Δ cells are cultured anaerobically supports the conclusion that oxidative stress leads to the functional defect of Ycf1p. However, it is unclear why Ycf1p inactivation occurs only in the ccs1Δ, sod1Δ, and glr1Δ strains among tested strains in which known antioxidant enzymes or molecules are individually deleted. Sod1p is the only known superoxide-scavenging enzyme in the cytosol, and due to the membrane impermeability of superoxide anion, mitochondrial Sodp (Sod2p) does not complement the SOD1 deficiency. Alternative translation initiation sites in GLR1 are responsible for mitochondrial and cytosolic isoforms of glutathione reductase (47), but no other enzyme carrying the activities has been identified so far. Hence, redundancy in other antioxidant defense systems that do not exist for Sod1p and Glr1p might explain such specificity.
GSH plays a vital role in normal growth and development, which is demonstrated by lethality of organisms (e.g., yeast and mice) by the knockout of GSH1 encoding the rate-limiting enzyme of GSH synthesis (59, 61). The gsh2Δ strain accumulates γ-Glu-Cys that is synthesized by Gsh1p and is viable (19). Both GSH and γ-Glu-Cys dipeptides bind cadmium and form complexes of GS2-Cd and (γ-Glu-Cys)2-Cd, respectively (14). However, the lack of Ycf1p-mediated cadmium resistance of gsh2Δ cells suggests that (γ-Glu-Cys)2-Cd is not a substrate for Ycf1p and/or Ycf1p is inactive in the gsh2Δ cells because of oxidative stress attributed to the absence of GSH.
Post-translational regulation of Ycf1p and other ABC transporters
Ycf1p is one of six transporters in the ABCC (MRP/CFTR) subfamily of transporters in S. cerevisiae (50, 52). Ycf1p and mammalian cystic fibrosis transmembrane conductance regulator (CFTR, ABCC7) and multidrug resistance-associated proteins (MRP1) manifest significant similarity in predicted structure and modes of action (50, 62). It is interesting to note that the cellular redox environment affects the maturation and function of WT and pathogenic human CFTR mutants. Infection and inflammation associated with cystic fibrosis could be considered a significant factor inducing oxidative stress followed by a functional change of CFTR (15). S-nitrosoglutathione (GSNO) has been proposed as a modulator of CFTR, which could explain the individual variations of clinical symptoms of cystic fibrosis patients bearing the same CFTR mutation (24, 72). Oxidized GSH, including GSSG and GSNO, inhibits CFTR channel activities by glutathionylation in a reversible manner (66). Since ccs1Δ and glr1Δ cells accumulate excess GSSG and induce oxidative stress (as indicated by increased GSH synthesis), Ycf1p, similar to CFTR, might be considered a target of redox-dependent glutathionylation. Moreover, given that Ycf1p and other ABCC members transport not only GSH conjugates but also GSH and GSSG (8), it is tempting to speculate that redox regulation of Ycf1p may be an active process which occurs in conjunction with cellular redox homeostasis. Resistance to chemotherapeutics due to enhanced efflux could be associated with not only higher expression but also elevated activities of ABC transporters. Further studies on redox regulation of Ycf1p might provide useful information for pharmacological control of this family of transporters for health benefits.
Materials and Methods
Yeast strains, media, and growth conditions
A haploid control yeast S. cerevisiae strain, BY4741, and other isogenic strains possessing single gene deletion (70) were purchased from the Open Biosystems. Strains carrying knockouts of more than one gene were created by a homologous recombination (40). Yeast cells were cultured in synthetic complete (SC) media (2% dextrose, 0.2% amino acid mixture, and 0.67% yeast nitrogen base) lacking specific amino acid(s) for plasmid selection, YPD media (1% yeast extract, 2% Bacto-peptone, and 2% dextrose), or nonfermentable media (2% Bacto-peptone, 1% yeast extract, 2% ethanol, and 3% glycerol) as specified in the figure legends. Solid media was prepared with the supplementation of 1.5% agar. Yeast cells were cultured at 30°C.
Cell growth assays
Cells cultured overnight in SC media were diluted into fresh media (OD600=0.2) and re-cultured to the mid-log phase (OD600=0.8–1.0). After dilution to OD600=0.5 in sterilized water, ∼5 μl of cells were spotted on selection media that was supplemented with indicated concentrations of metal. For specific experiments as indicated in figure legends, growth assays were conducted using cells of OD600=0.5 and 5×serial dilutions. Plates were incubated at 30°C for 2-4 days before photography. The GasPak™ EZ Anaerobe Container System was used for oxygen-limited culture. Each assay was repeated at least twice using two different colonies to confirm results.
Fluorescence microscopy
Mid-log phase cells that were cultured with and without cadmium in the media were collected by centrifugation and washed once with fresh media. Vacuole membranes were stained by incubating cells with FM4-64 (8 μM) (Invitrogen) at 30°C in the dark for 1 h. Cells were washed once with phosphate-buffered saline by centrifugation, and GFP-fused Ycf1p and FM4-64 signals of the same cells were captured on a confocal microscope (Olympus FV500). Differential interference contrast (DIC) images were also captured to present cell morphology. Fluorescent signals and cell images were overlaid to determine subcellular distribution and co-localization.
Limited trypsin proteolysis of Ycf1p
WT and glr1Δ cells expressing YCF1 or YCF1(C436A) tagged with triple HA at the C-terminus were grown in SC media starting at OD600 0.2 for 9 h with 15μM CdCl2. Total protein extracts were prepared by glass bead vortexing of cells in a buffer containing 1% Triton X-100 and protease inhibitors. Trypsin (0, 2, and 5 μg/ml) (Bovine pancreas; Sigma) was added to the protein extracts (50 μg protein) for 10 min on ice. The reaction was stopped by the addition of 0.2 μg/ml of trypsin inhibitor form soybean (Fluka Biochemika) for 15 min on ice. The samples were then denatured in 1×SDS sample buffer containing 0.1 M DTT and denatured for 15 min at 37°C. Proteolysis patterns pf Ycf1 were visualized by SDS-PAGE followed by western blotting with anti-HA antibodies (Rockland, 600-401-384).
Blue native polyacrylamide gel electrophoresis
To obtain Ycf1p-enriched fractions, sucrose gradient fractionation was conducted as previously described (30). The fraction that was most highly enriched with Ycf1p was subjected to blue native polyacrylamide gel electrophoresis (31). Separated Ycf1p complexes were detected by immunoblotting.
Image quantification and statistical analysis
ImageJ software (http://rsbweb.nih.gov/ij/) was used for quantification. Data are presented as means±SD, and statistical analysis was conducted using paired and unpaired Student's t-tests for comparing data obtained with and without cadmium co-culture and strain differences, respectively. p<0.05 was considered significant. Other materials and methods are listed in the Supplementary Data.
Supplementary Material
Abbreviations Used
- ABC
ATP-binding cassette
- ABCC
ATP-binding cassette subfamily C
- Ala
alanine
- ATP
adenosine triphosphate
- ATPase
adenosine triphosphatase
- BCS
bathocuproine disulphonate
- Cco
cytochrome c oxidase
- CCS1
copper chaperone for superoxide dismutase 1
- Cd
cadmium
- CFTR
cystic fibrosis transmembrane conductance regulator
- Cu
copper
- Cys
cysteine
- Cys-OH
cysteine sulefenic acid
- DIC
differential interference contrast
- DNA
deoxyribonucleic acid
- DTT
dithiothreitol
- EDTA
ethylenediaminetetraacetic acid
- GFP
green fluorescence protein
- GLR1
glutathione reductase 1
- GSH
reduced glutathione
- GSNO
S-nitrosoglutathione
- GSSG
glutathione disulfide
- HA
hemagglutinin epitope
- HEPES
N-(2-Hydroxyethyl)piperazine-N'-2-ethanesulfonic Acid
- ICP-MS
inductively coupled plasma mass spectrometry
- IP
immunoprecipitation
- Mg
magnesium
- MRP
multidrug resistance protein
- MTs
metallothioneins
- NCBI
National Center for Biotechnology Information
- N-MBDs
N-terminal metal-binding domains
- OD
optical density
- PMSF
phenylmethylsulfonyl fluoride
- ROS
reactive oxygen species
- SC
synthetic complete
- SOD1
superoxide dismutase 1
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- wSOD1
C. elegans superoxide dismutase 1
- WT
wild type
- YCF1
yeast cadmium resistance factor 1
- YPD
1% yeast extract, 2% Bacto-peptone, 2% dextrose
- Zn
zinc
Acknowledgments
The authors thank D. Kornitzer and V. Culotta for providing cDNA plasmids of C. albicans CaCRP1 (CAD1) and C. elegans wSOD1, respectively. They also thank Lee lab members, including M. Hessel, E. Shuman, E. Bender, and S. Swenson, for technical assistance and helpful discussions. This work was supported by National Institutes of Health grants, ES16337 (to J. L.), DK79209 (to J. L.) (the portion addressing copper metabolism), and P30RM103335 (The Nebraska Redox Biology Center) (metal measurement using inductively coupled plasma mass spectrometry).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Adle DJ, Sinani D, Kim H, and Lee J. A cadmium-transporting P1B-type ATPase in yeast Saccharomyces cerevisiae. J Biol Chem 282: 947–955, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adle DJ. and Lee J. Expressional control of a cadmium-transporting P1B-type ATPase by a metal sensing degradation signal. J Biol Chem 283: 31460–31468, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adle DJ, Wei W, Smith N, Bies JJ, and Lee J. Cadmium-mediated rescue from ER-associated degradation induces expression of its exporter. Proc Natl Acad Sci U S A 106: 10189–10194, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ajees AA, Yang J, and Rosen BP. The ArsD As(III) metallochaperone. Biometals 24: 391–399, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Argüello JM, Eren E, and González-Guerrero M. The structure and function of heavy metal transport P1B-ATPases. Biometals 20: 233–248, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Brandes N, Schmitt S, and Jakob U. Thiol-based redox switches in eukaryotic proteins. Antioxid Redox Signal 11: 997–1014, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang EC, Crawford BF, Hong Z, Bilinski T, and Kosman DJ. Genetic and biochemical characterization of Cu,Zn superoxide dismutase mutants in Saccharomyces cerevisiae. J Biol Chem 266: 4417–4424, 1991 [PubMed] [Google Scholar]
- 8.Cole SP. and Deeley RG. Transport of glutathione and glutathione conjugates by MRP1. Trends Pharmacol Sci 27: 438–446, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Corson LB, Folmer J, Strain JJ, Culotta VC, and Cleveland DW. Oxidative stress and iron are implicated in fragmenting vacuoles of Saccharomyces cerevisiae lacking Cu,Zn-superoxide dismutase. J Biol Chem 274: 27590–25696, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Culotta VC, Joh HD, Lin SJ, Slekar KH, and Strain J. A physiological role for Saccharomyces cerevisiae copper/zinc superoxide dismutase in copper buffering. J Biol Chem 270: 29991–29997, 1995 [DOI] [PubMed] [Google Scholar]
- 11.Culotta VC, Klomp LW, Strain J, Casareno RL, Krems B, and Gitlin JD. The copper chaperone for superoxide dismutase. J Biol Chem 272: 23469–23472, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Culotta VC, Yang M, and O'Halloran TV. Activation of superoxide dismutases: putting the metal to the pedal. Biochim Biophys Acta 1763: 747–758, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Freitas JM, Liba A, Meneghini R, Valentine JS, and Gralla EB. Yeast lacking Cu-Zn superoxide dismutase show altered iron homeostasis. Role of oxidative stress in iron metabolism. J Biol Chem 275: 11645–11649, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Delalande O, Desvaux H, Godat E, Valleix A, Junot C, Labarre J, and Boulard Y. Cadmium-glutathione solution structures provide new insights into heavy metal detoxification. FEBS J 277: 5086–5096, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Drumm ML, Ziady AG, and Davis PB. Genetic variation and clinical heterogeneity in cystic fibrosis. Annu Rev Pathol 7: 267–282, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eraso P, Martínez-Burgos M, Falcón-Pérez JM, Portillo F, and Mazón MJ. Ycf1-dependent cadmium detoxification by yeast requires phosphorylation of residues Ser908 and Thr911. FEBS Lett 577: 322–326, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Furukawa Y, Torres AS, and O'Halloran TV. Oxygen-induced maturation of SOD1: a key role for disulfide formation by the copper chaperone CCS. EMBO J 23: 2872–2881, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grant CM, Collinson LP, Roe JH, and Dawes IW. Yeast glutathione reductase is required for protection against oxidative stress and is a target gene for yAP-1 transcriptional regulation. Mol Microbiol 21: 171–179, 1996 [DOI] [PubMed] [Google Scholar]
- 19.Grant CM, MacIver FH, and Dawes IW. Glutathione synthetase is dispensable for growth under both normal and oxidative stress conditions in the yeast Saccharomyces cerevisiae due to an accumulation of the dipeptide gamma-glutamylcysteine. Mol Biol Cell 8: 1699–1707, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta A. and Lutsenko S. Evolution of copper transporting ATPases in eukaryotic organisms. Curr Genomics 13: 124–133, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halliwell B. and Gutteridge JM. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol 186: 1–85, 1990 [DOI] [PubMed] [Google Scholar]
- 22.Heo DH, Baek IJ, Kang HJ, Kim JH, Chang M, Kang CM, and Yun CW. Cd2+ binds to Atx1 and affects the physical interaction between Atx1 and Ccc2 in Saccharomyces cerevisiae. Biotechnol Lett 34: 303–307, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Huffman DL. and O'Halloran TV. Function, structure, and mechanism of intracellular copper trafficking proteins. Annu Rev Biochem 70: 677–701, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Innis SM. and Davidson AG. Cystic fibrosis and nutrition: linking phospholipids and essential fatty acids with thiol metabolism. Annu Rev Nutr 28: 55–72, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Järup L. and Akesson A. Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol 238: 201–208, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Jensen LT. and Culotta VC. Regulation of Saccharomyces cerevisiae FET4 by oxygen and iron. J Mol Biol 318: 251–260, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Jordan IK, Natale DA, and Galperin MY. Copper chaperones in bacteria: association with copper-transporting ATPases. Trends Biochem Sci 25: 480–481, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Kamizono A, Nishizawa M, Teranishi Y, Murata K, and Kimura A. Identification of a gene conferring resistance to zinc and cadmium ions in the yeast Saccharomyces cerevisiae. Mol Gen Genet 219: 161–167, 1989 [DOI] [PubMed] [Google Scholar]
- 29.Kaplan N, Urao N, Furuta E, Kim SJ, Razvi M, Nakamura Y, McKinney RD, Poole LB, Fukai T, and Ushio-Fukai M. Localized cysteine sulfenic acid formation by vascular endothelial growth factor: role in endothelial cell migration and angiogenesis. Free Radic Res 45: 1124–1135, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katzmann DJ, Epping EA, and Moye-Rowley WS. Mutational disruption of plasma membrane trafficking of Saccharomyces cerevisiae Yor1p, a homologue of mammalian multidrug resistance protein. Mol Cell Biol 19: 2998–3009, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khalimonchuk O, Bestwick M, Meunier B, Watts TC, and Winge DR. Formation of the redox cofactor centers during Cox1 maturation in yeast cytochrome oxidase. Mol Cell Biol 30: 1004–1017, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim H, Wu X, and Lee J. SLC31 (CTR) family of copper transporters in health and disease. Mol Aspects Med 34: 561–570, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klaassen CD, Liu J, and Diwan BA. Metallothionein protection of cadmium toxicity. Toxicol Appl Pharmacol 238: 215–220, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kojima T, Wakamatsu TH, Dogru M, Ogawa Y, Igarashi A, Ibrahim OM, Inaba T, Shimizu T, Noda S, Obata H, Nakamura S, Wakamatsu A, Shirasawa T, Shimazaki J, Negishi K, and Tsubota K. Age-related dysfunction of the lacrimal gland and oxidative stress: evidence from the Cu,Zn-superoxide dismutase-1 (Sod1) knockout mice. Am J Pathol 2180: 1879–1896, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Lee J, Godon C, Lagniel G, Spector D, Garin J, Labarre J, and Toledano MB. Yap1 and Skn7 control two specialized oxidative stress response regulons in yeast. J Biol Chem 274: 16040–16046, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Lee ME, Singh K, Snider J, Shenoy A, Paumi CM, Stagljar I, and Park HO. The Rho1 GTPase acts together with a vacuolar glutathione S-conjugate transporter to protect yeast cells from oxidative stress. Genetics 188: 859–870, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leitch JM, Jensen LT, Bouldin SD, Outten CE, Hart PJ, and Culotta VC. Activation of Cu,Zn-superoxide dismutase in the absence of oxygen and the copper chaperone CCS. J Biol Chem 284: 21863–21871, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li ZS, Lu YP, Zhen RG, Szczypka M, Thiele DJ, and Rea PA. A new pathway for vacuolar cadmium sequestration in Saccharomyces cerevisiae: YCF1-catalyzed transport of bis(glutathionato)cadmium. Proc Natl Acad Sci U S A 94: 42–47, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu XF, Elashvili I, Gralla EB, Valentine JS, Lapinskas P, and Culotta VC. Yeast lacking superoxide dismutase. Isolation of genetic suppressors. J Biol Chem 267: 18298–18302, 1992 [PubMed] [Google Scholar]
- 40.Longtine MS, McKenzie A, III, Demarini DJ, Shah NG, Wach A, Brachat A, Philippsen P, and Pringle JR. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14: 953–961, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Martelli A, Rousselet E, Dycke C, Bouron A, and Moulis JM. Cadmium toxicity in animal cells by interference with essential metals. Biochimie 88: 1807–1814, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Mason DL. and Michaelis S. Requirement of the N-terminal extension for vacuolar trafficking and transport activity of yeast Ycf1p, an ATP-binding cassette transporter. Mol Biol Cell 13: 4443–4455, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mieyal JJ. and Chock PB. Posttranslational modification of cysteine in redox signaling and oxidative stress: Focus on s-glutathionylation. Antioxid Redox Signal 16: 471–475, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mo W. and Zhang JT. Oligomerization of human ATP-binding cassette transporters and its potential significance in human disease. Expert Opin Drug Metab Toxicol 5: 1049–1063, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morin I, Cuillel M, Lowe J, Crouzy S, Guillain F, and Mintz E. Cd2+- or Hg2+-binding proteins can replace the Cu+-chaperone Atx1 in delivering Cu+ to the secretory pathway in yeast. FEBS Lett 579: 1117–1123, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Moulis JM. and Thévenod F. New perspectives in cadmium toxicity: an introduction. Biometals 23: 763–768, 2010 [DOI] [PubMed] [Google Scholar]
- 47.Outten CE. and Culotta VC. Alternative start sites in the Saccharomyces cerevisiae GLR1 gene are responsible for mitochondrial and cytosolic isoforms of glutathione reductase. J Biol Chem 279: 7785–7791, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paumi CM, Menendez J, Arnoldo A, Engels K, Iyer KR, Thaminy S, Georgiev O, Barral Y, Michaelis S, and Stagljar I. Mapping protein-protein interactions for the yeast ABC transporter Ycf1p by integrated split-ubiquitin membrane yeast two-hybrid analysis. Mol Cell 26: 15–25, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Paumi CM, Chuk M, Chevelev I, Stagljar I, and Michaelis S. Negative regulation of the yeast ABC transporter Ycf1p by phosphorylation within its N-terminal extension. J Biol Chem 283: 27079–27088, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paumi CM, Chuk M, Snider J, Stagljar I, and Michaelis S. ABC transporters in Saccharomyces cerevisiae and their interactors: new technology advances the biology of the ABCC (MRP) subfamily. Microbiol Mol Biol Rev 73: 577–593, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Philpott CC. Coming into view: eukaryotic iron chaperones and intracellular iron delivery. J Biol Chem 287: 13518–13523, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prasad R, and Goffeau A. Yeast ATP-binding cassette transporters conferring multidrug resistance. Annu Rev Microbiol 66: 39–63, 2012 [DOI] [PubMed] [Google Scholar]
- 53.Pufahl RA, Singer CP, Peariso KL, Lin SJ, Schmidt PJ, Fahrni CJ, Culotta VC, Penner-Hahn JE, and O'Halloran TV. Metal ion chaperone function of the soluble Cu(I) receptor Atx1. Science 278: 853–856, 1997 [DOI] [PubMed] [Google Scholar]
- 54.Reddi AR. and Culotta VC. SOD1 integrates signals from oxygen and glucose to repress respiration. Cell 152: 224–235, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Riggle PJ. and Kumamoto CA. Role of a Candida albicans P1-type ATPase in resistance to copper and silver ion toxicity. J Bacteriol 182: 4899–4905, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Robinson NJ. and Winge DR. Copper metallochaperones. Annu Rev Biochem 79: 537–562, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rosen BP. Transport and detoxification systems for transition metals, heavy metals and metalloids in eukaryotic and prokaryotic microbes. Comp Biochem Physiol A Mol Integr Physiol 133: 689–693, 2002 [DOI] [PubMed] [Google Scholar]
- 58.Serero A, Lopes J, Nicolas A, and Boiteux S. Yeast genes involved in cadmium tolerance: Identification of DNA replication as a target of cadmium toxicity. DNA Repair (Amst) 7: 1262–1275, 2008 [DOI] [PubMed] [Google Scholar]
- 59.Shi ZZ, Osei-Frimpong J, Kala G, Kala SV, Barrios RJ, Habib GM, Lukin DJ, Danney CM, Matzuk MM, and Lieberman MW. Glutathione synthesis is essential for mouse development but not for cell growth in culture. Proc Natl Acad Sci U S A 97: 5101–5106, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Solovieva IM. and Entian KD. Metalloregulation in Bacillus subtilis: the copZ chromosomal gene is involved in cadmium resistance. FEMS Microbiol Lett 236: 115–122, 2004 [DOI] [PubMed] [Google Scholar]
- 61.Spector D, Labarre J, and Toledano MB. A genetic investigation of the essential role of glutathione: mutations in the proline biosynthesis pathway are the only suppressors of glutathione auxotrophy in yeast. J Biol Chem 276: 7011–7016, 2001 [DOI] [PubMed] [Google Scholar]
- 62.Szczypka MS, Wemmie JA, Moye-Rowley WS, and Thiele DJ. A yeast metal resistance protein similar to human cystic fibrosis transmembrane conductance regulator (CFTR) and multidrug resistance-associated protein. J Biol Chem 269: 22853–22857, 1994 [PubMed] [Google Scholar]
- 63.Thévenod F. Catch me if you can! Novel aspects of cadmium transport in mammalian cells. Biometals 23: 857–875, 2010 [DOI] [PubMed] [Google Scholar]
- 64.Thorsen M, Perrone GG, Kristiansson E, Traini M, Ye T, Dawes IW, Nerman O, and Tamás MJ. Genetic basis of arsenite and cadmium tolerance in Saccharomyces cerevisiae. BMC Genomics 10: 105, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wakabayashi T, Nakamura N, Sambongi Y, Wada Y, Oka T, and Futai M. Identification of the copper chaperone, CUC-1, in Caenorhabditis elegans: tissue specific co-expression with the copper transporting ATPase, CUA-1. FEBS Lett 440: 141–146, 1998 [DOI] [PubMed] [Google Scholar]
- 66.Wang W, Oliva C, Li G, Holmgren A, Lillig CH, and Kirk KL. Reversible silencing of CFTR chloride channels by glutathionylation. J Gen Physiol 125: 127–141, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wei JP, Srinivasan C, Han H, Valentine JS, and Gralla EB. Evidence for a novel role of copper-zinc superoxide dismutase in zinc metabolism. J Biol Chem 276: 44798–44803, 2001 [DOI] [PubMed] [Google Scholar]
- 68.Weissman Z, Berdicevsky I, Cavari BZ, and Kornitzer D. The high copper tolerance of Candida albicans is mediated by a P-type ATPase. Proc Natl Acad Sci U S A 97: 3520–3525, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Williams LE. and Mills RF. P(1B)-ATPases—an ancient family of transition metal pumps with diverse functions in plants. Trends Plant Sci 10: 491–502, 2005 [DOI] [PubMed] [Google Scholar]
- 70.Winzeler EA, Shoemaker DD, Astromoff A, Liang H, Anderson K, Andre B, Bangham R, Benito R, Boeke JD, Bussey H, Chu AM, Connelly C, Davis K, Dietrich F, Dow SW, El Bakkoury M, Foury F, Friend SH, Gentalen E, Giaever G, Hegemann JH, Jones T, Laub M, Liao H, Liebundguth N, Lockhart DJ, Lucau-Danila A, Lussier M, M'Rabet N, Menard P, Mittmann M, Pai C, Rebischung C, Revuelta JL, Riles L, Roberts CJ, Ross-MacDonald P, Scherens B, Snyder M, Sookhai-Mahadeo S, Storms RK, Véronneau S, Voet M, Volckaert G, Ward TR, Wysocki R, Yen GS, Yu K, Zimmermann K, Philippsen P, Johnston M, and Davis RW. Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science 285: 901–906, 1999 [DOI] [PubMed] [Google Scholar]
- 71.Wysocki R. and Tamás MJ. How Saccharomyces cerevisiae copes with toxic metals and metalloids. FEMS Microbiol Rev 34: 925–951, 2010 [DOI] [PubMed] [Google Scholar]
- 72.Zaman K, Carraro S, Doherty J, Henderson EM, Lendermon E, Liu L, Verghese G, Zigler M, Ross M, Park E, Palmer LA, Doctor A, Stamler JS, and Gaston B. S-nitrosylating agents: a novel class of compounds that increase cystic fibrosis transmembrane conductance regulator expression and maturation in epithelial cells. Mol Pharmacol 70: 1435–1442, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.