Abstract
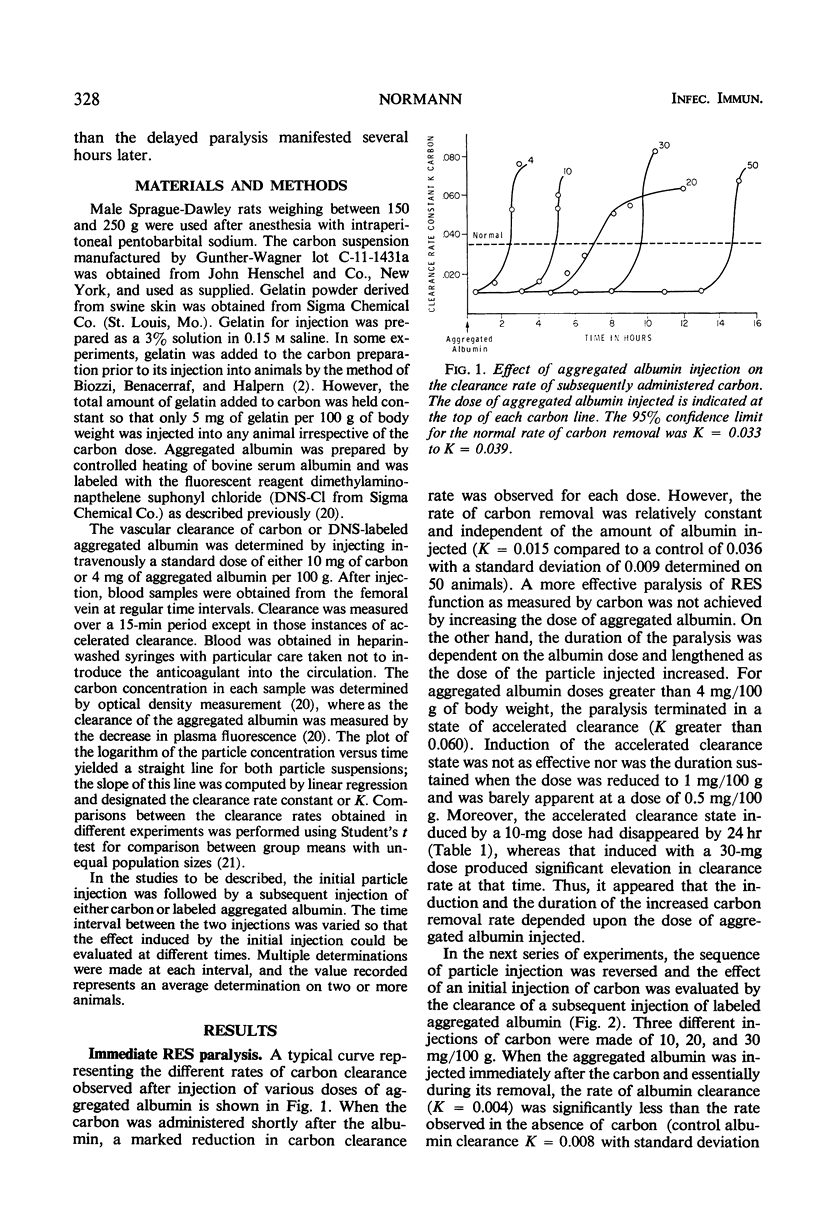
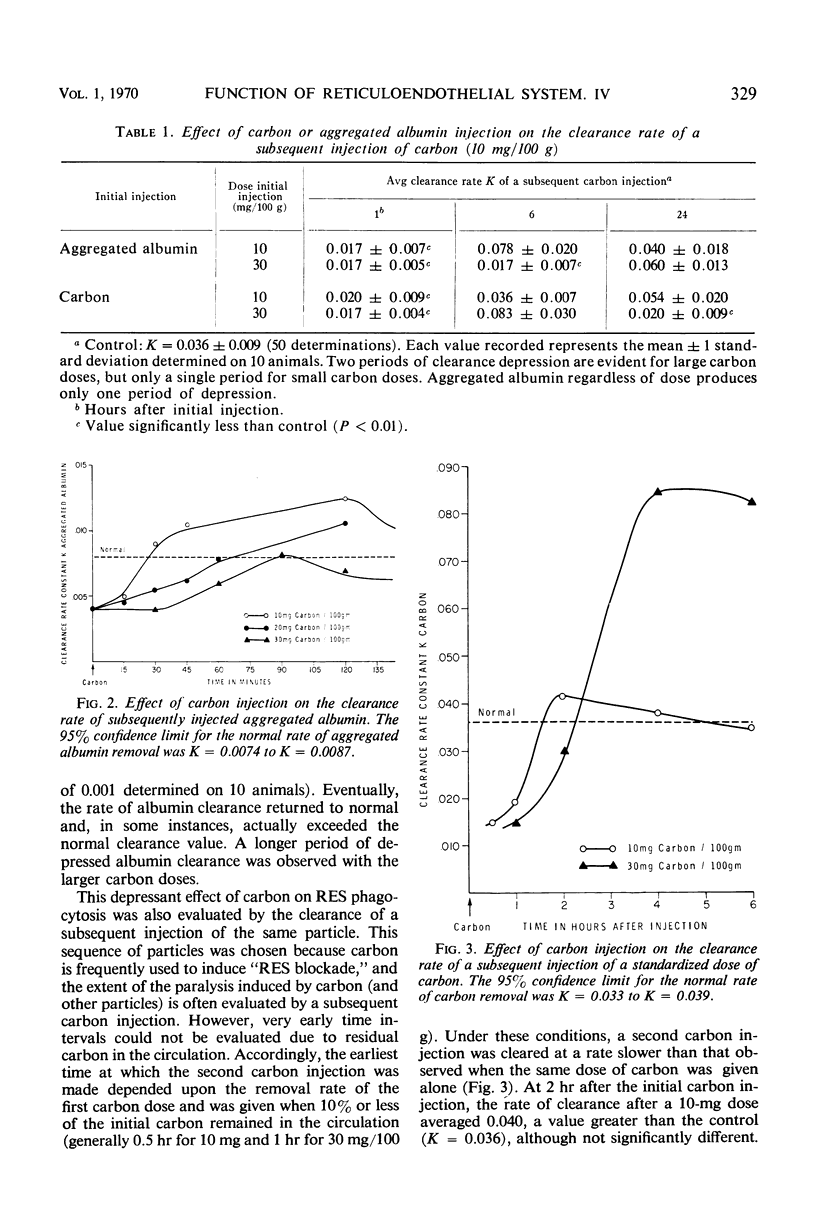
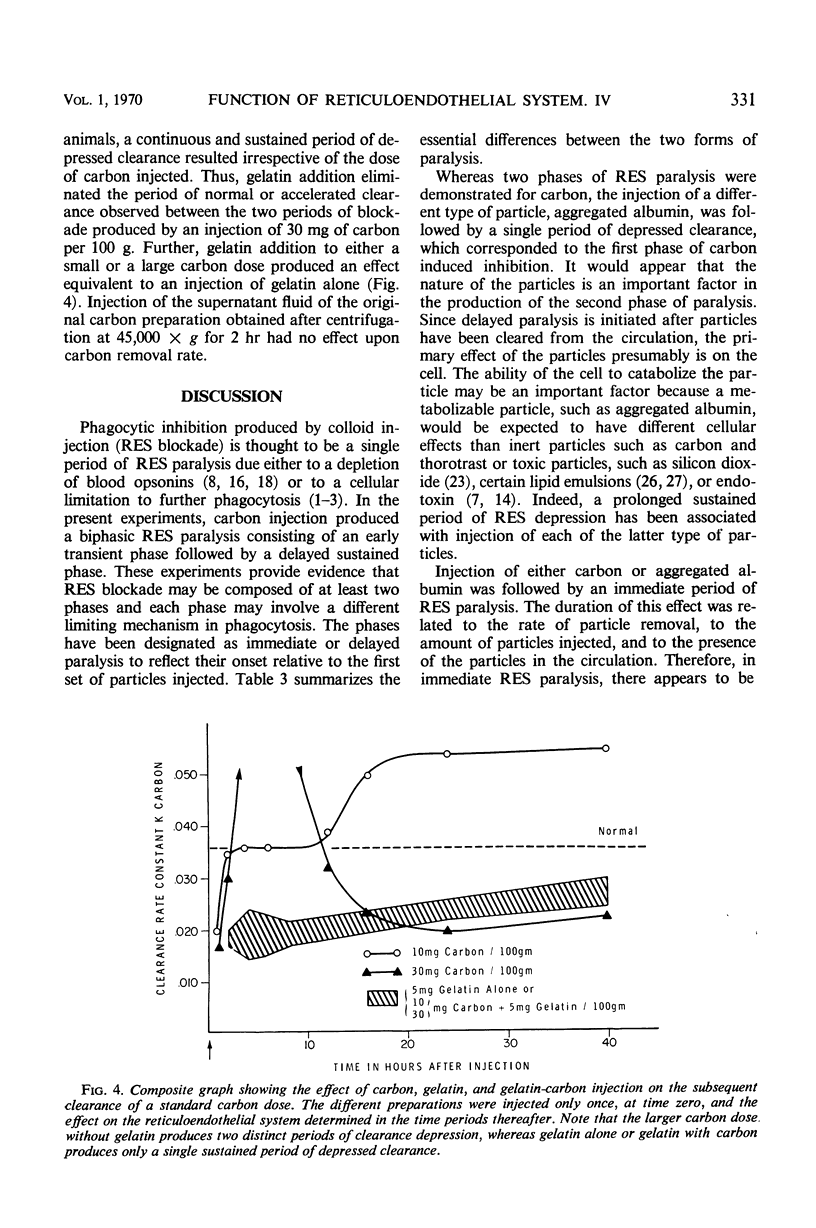
Reticuloendothelial system (RES) phagocytosis has been quantitated after intravenous injection of two different sets of particles by determining the clearance rate of subsequently injected identical or nonidentical particles. Injection of carbon produced a biphasic RES paralysis consisting of an early transient phase followed by a delayed sustained phase. The two phases were separated by a distinct interval of greatly augmented clearance rates. The injection of aggregated albumin was followed only by a single period of depressed clearance, which corresponded to the first phase of carbon-induced inhibition. This first phase, designated immediate RES paralysis, was initiated by particle injection and its duration was related to the rate of particle removal, to the dose of particles injected, and to the presence of the particles in the circulation. The second phase, designated delayed RES paralysis, began sometime after the particles had been engulfed by the cells, was independent of the rate of particle removal, and persisted without the presence of measurable particles in the circulation. The evidence indicates that the immediate paralysis arises from a competition between the particles in the circulation, whereas the delayed paralysis arises from a cellular derangement inhibitory to further phagocytosis. In contrast to the usual description of RES blockade as a single sustained period of depression, the present experiments indicate that the phenomenon has two phases which can be dissociated in time and mechanism.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENACERRAF B., HALPERN B. N., BIOZZI G., BENOS S. A. Quantitative study of the granulopectic activity of the reticulo-endothelial system. III. The effect of cortisone and nitrogen mustard on the regenerative capacity of the R.E.S. after saturation with carbon. Br J Exp Pathol. 1954 Apr;35(2):97–106. [PMC free article] [PubMed] [Google Scholar]
- BIOZZI G., BENACERRAF B., HALPERN B. N. Quantitative study of the granulopectic activity of the reticulo-endothelial system. II. A study of the kinetics of the R. E. S. in relation to the dose of carbon injected; relationship between the weight of the organs and their activity. Br J Exp Pathol. 1953 Aug;34(4):441–457. [PMC free article] [PubMed] [Google Scholar]
- BIOZZI G., STIFFEL C., HALPERN B. N., MOUTON D. Lack of action of serum opsonins in phagocytosis of inert particles by cells of reticuloendothelial system. Proc Soc Exp Biol Med. 1963 Apr;112:1017–1020. doi: 10.3181/00379727-112-28239. [DOI] [PubMed] [Google Scholar]
- Filkins J. P., Smith J. J. Plasma factor influencing carbon phagocytosis in the isolated perfused rat liver. Proc Soc Exp Biol Med. 1965 Aug-Sep;119(4):1181–1184. doi: 10.3181/00379727-119-30408. [DOI] [PubMed] [Google Scholar]
- HEILMAN D. H. THE SELECTIVE TOXICITY OF ENDOTOXIN FOR PHAGOCYTIC CELLS OF THE RETICULOENDOTHELIAL SYSTEM. Int Arch Allergy Appl Immunol. 1965;26:63–79. doi: 10.1159/000229555. [DOI] [PubMed] [Google Scholar]
- JENKIN C. R., ROWLEY D. The role of opsonins in the clearance of living and inert particles by cells of the reticuloendothelial system. J Exp Med. 1961 Sep 1;114:363–374. doi: 10.1084/jem.114.3.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeunet F. S., Good R. A. Reticuloendothelial function in the isolated perfused liver. II. Phagocytosis of heat-aggregated bovine serum albumin. Demonstration of two components in the blockade of the reticuloendothelial system. J Reticuloendothel Soc. 1969 Feb;6(1):94–107. [PubMed] [Google Scholar]
- KELLY L. S., BROWN B. A., DOBSON E. L. Cell division and phagocytic activity in liver reticulo-endothelial cells. Proc Soc Exp Biol Med. 1962 Jul;110:555–559. doi: 10.3181/00379727-110-27578. [DOI] [PubMed] [Google Scholar]
- KELLY L. S., DOBSON E. L., FINNEY C. R., HIRSCH J. D. Proliferation of the reticuloendothelial system in the liver. Am J Physiol. 1960 May;198:1134–1138. doi: 10.1152/ajplegacy.1960.198.5.1134. [DOI] [PubMed] [Google Scholar]
- KOENIG M. G., HEYSSEL R. M., MELLY M. A., ROGERS D. E. THE DYNAMICS OF RETICULOENDOTHELIAL BLOCKADE. J Exp Med. 1965 Jul 1;122:117–142. doi: 10.1084/jem.122.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampschmidt R. F., Upchurch H. F., Park A. Factors in the suspending media which alter the carbon clearance rate. J Reticuloendothel Soc. 1966 Sep;3(3):214–222. [PubMed] [Google Scholar]
- Levy E., Ruebner B. H. Hepatic changes produced by a single dose of endotoxin in the germfree mouse. Histochemistry, light microscopy, fluorescence microscopy, and electron microscopy. Am J Pathol. 1968 Jan;52(1):97–110. [PMC free article] [PubMed] [Google Scholar]
- MURRAY I. M. Clearance rate in relation to agglutinins for gelatin-stabilized colloid in the rat. Am J Physiol. 1963 Apr;204:655–659. doi: 10.1152/ajplegacy.1963.204.4.655. [DOI] [PubMed] [Google Scholar]
- MURRAY I. M. The mechanism of blockade of the reticuloendothelial system. J Exp Med. 1963 Jan 1;117:139–147. doi: 10.1084/jem.117.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normann S. J., Benditt E. P. Function of the reticuloendothelial system. I. A study on the phenomenon of carbon clearance inhibition. J Exp Med. 1965 Oct 1;122(4):693–707. doi: 10.1084/jem.122.4.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normann S. J., Benditt E. P. Function of the reticuloendothelial system. II. Participation of a serum factor in carbon clearance. J Exp Med. 1965 Oct 1;122(4):709–719. doi: 10.1084/jem.122.4.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normann S. J., Lagunoff D., Benditt E. P. Function of the reticuloendothelial system. 3. Simultaneous measurement of two particles during clearance inhibition. Lab Invest. 1968 Oct;19(4):353–357. [PubMed] [Google Scholar]
- PARKER H. G., FINNEY C. R. Latent period in the induction of reticuloendothelial blockade. Am J Physiol. 1960 Apr;198:916–920. doi: 10.1152/ajplegacy.1960.198.4.916. [DOI] [PubMed] [Google Scholar]
- Pearsall N. N., Weiser R. S. The macrophage in allograft immunity. I. Effects of silica as a specific macrophage toxin. J Reticuloendothel Soc. 1968 Apr;5(2):107–120. [PubMed] [Google Scholar]
- Pisano J. C., Patterson J. T., Di Luzio N. R. Reticuloendothelial blockade: effect of puromycin on opsonin-dependent recovery. Science. 1968 Nov 1;162(3853):565–567. doi: 10.1126/science.162.3853.565. [DOI] [PubMed] [Google Scholar]
- STUART A. E. Experimental necrosis of spleen. J Pathol Bacteriol. 1962 Jul;84:193–200. doi: 10.1002/path.1700840120. [DOI] [PubMed] [Google Scholar]
- Schapiro R. L., MacIntyre W. J., Schapiro D. I. The effect of homologous and heterologous carrier on the clearance of colloidal material by the reticuloendothelial system. J Lab Clin Med. 1966 Aug;68(2):286–299. [PubMed] [Google Scholar]


