Abstract
Asthma is a complex disease with sex-specific differences in prevalence. Candidate gene studies have suggested that genotype-by-sex interaction effects on asthma risk exist, but this has not yet been explored at a genome-wide level. We aimed to identify sex-specific asthma risk alleles by performing a genome-wide scan for genotype-by-sex interactions in the ethnically diverse participants in the EVE Asthma Genetics Consortium. We performed male- and female-specific genome-wide association studies in 2653 male asthma cases, 2566 female asthma cases and 3830 non-asthma controls from European American, African American, African Caribbean and Latino populations. Association tests were conducted in each study sample, and the results were combined in ancestry-specific and cross-ancestry meta-analyses. Six sex-specific asthma risk loci had P-values < 1 × 10−6, of which two were male specific and four were female specific; all were ancestry specific. The most significant sex-specific association in European Americans was at the interferon regulatory factor 1 (IRF1) locus on 5q31.1. We also identify a Latino female-specific association in RAP1GAP2. Both of these loci included single-nucleotide polymorphisms that are known expression quantitative trait loci and have been associated with asthma in independent studies. The IRF1 locus is a strong candidate region for male-specific asthma susceptibility due to the association and validation we demonstrate here, the known role of IRF1 in asthma-relevant immune pathways and prior reports of sex-specific differences in interferon responses.
INTRODUCTION
Many diverse human phenotypes are sexually dimorphic, including anatomical and morphological traits [e.g. height (1,2), fat distribution (3)], immune response (4), risk for complex diseases [e.g. asthma (5), autoimmune disease (6,7), psychiatric disorders (8), heart disease (9)] and gene expression traits (10). While X chromosome genes contribute to phenotypic differences between males and females, variation in autosomal genes can also have sex-specific phenotypic effects in humans (11,12). Because the sequence of the autosomal genome does not differ between males and females, it is likely that variation affecting the gene expression plays an important role in determining sex-specific phenotypes. Such variation influencing phenotypes differently in males and females would not necessarily be discovered in genome-wide association studies (GWASs), where typically sex effects are regressed out in the analyses. Direct studies of genotype-by-sex interaction effects on common phenotypes and complex diseases have been limited, although some notable examples have emerged from studies of morphological traits [e.g. height, weight, body mass index, fat distribution (3,13), bone mineral density (14)] and complex diseases [e.g. coronary artery disease and Crohn's disease (15)]. Thus, much of the regulatory variation influencing sexually dimorphic traits in humans remains largely unknown.
The objective of our study was to characterize the sex-specific genetic architecture of asthma. Asthma is a common, heterogeneous disease affecting nearly 300 million people worldwide (16). Asthma prevalence shows an intriguing sex-specific architecture that varies by age: males are more likely to develop asthma during early childhood and females are more likely to develop asthma around the time of and following puberty (5). As a result, more boys have asthma prepuberty and more women have persistent asthma throughout adult life. Furthermore, sex-specific differences in developmental patterns of immune responses associated with the development of childhood-onset asthma have been reported (4,17,18). For example, serum IgE levels are higher in boys than girls (17); among children who wheeze, boys have higher cytokine responses than do girls in the first 3 years of life (18). In addition, variation in the IFNG gene has shown genotype-by-sex interaction effects on both interferon (IFN)-γ response to lipopolysaccharide (endotoxin) in the first year of life and asthma risk at age 6 (19). Other candidate gene studies have also revealed genotype-by-sex interaction effects on asthma risk or asthma severity, including variation at the TSLP (20), ADRB2 (21) and KCNMB1 (22) loci. Suggestive evidence for genotype-by-sex interaction near the GRIA2 and TNFRSF11B loci for asthma risk by age 6 was found using a two-step genome-wide scanning method (23). To date, no genome-wide study of asthma has reported statistically significant evidence for genotype-by-sex interactions (www.genome.gov/26525384, 18 November 2013 last accessed).
Here we report the first meta-analysis of genome-wide genotype-by-sex interactions in asthma in ethnically diverse subjects from the EVE Consortium (24–26). We conducted meta-analyses of genome-wide genotype-by-sex associations in 2653 male cases, 2566 female asthma cases and 3830 controls from diverse North American populations and we identified variation associated with both sex-specific risks for asthma and sex-biased expression of nearby genes.
RESULTS
Power analysis
To investigate whether risk alleles interact with sex on asthma risk, we first evaluate the power to detect genotype-by-sex interactions in the EVE samples. We begin by considering three general additive models of genotype-by-sex interactions: male-driven, where males have increased risk compared with females, female-driven, where females have increased risk compared with males and flip-flop, where a given allele increases risk in one sex and is protective in the other sex. Given that the prevalence of asthma differs by sex, we hypothesize that asthma risk alleles follow either of the first two models and are sex specific, i.e. the risk allele is at higher frequency in the cases of one sex compared with all of the controls as well as the cases of the other sex (the pooled controls). To test this hypothesis, we constructed the cases versus pooled controls association test contrasting the cases of one sex to the combination of cases of the other sex and all controls. This design can be applied to both case control and trio design unlike other commonly used methods for testing genotype-by-environment interactions. We first explored the power of the cases versus pooled controls approach to detect a male-specific association in each of the three ancestry groups and the combined sample in the EVE Consortium (Supplementary Material, Fig. S1). Because the numbers of male and female cases in our study were similar, the power to detect male-specific associations should be similar to the power to detect female-specific associations. In each of the ancestry-specific analyses, we have >80% power to detect an odds ratio (OR) of ≥1.75 for single-nucleotide polymorphisms (SNPs) with minor allele frequency (MAF) ≥0.1. In the combined sample, we have >80% power to detect an OR ≥1.5 with MAF ≥0.1 (Supplementary Material, Fig. S1D).
Next, we compared the power of the cases versus pooled controls approach to two other commonly used methods for detecting additive genotype-by-sex interactions: (1) a case-only test directly comparing allele frequencies between male and female asthma cases and (2) including a genotype-by-sex interaction term in a logistic regression model to detect a male-specific effect with OR = 1.5. The cases versus pooled controls approach is optimal for detecting male-specific associations because the approach included the controls, which are excluded in a case-only test, and also included trio-design studies, to which logistic regression including the genotype-by-sex interaction term cannot be applied (Supplementary Material, Fig. S2A). Finally, we compared the power of the cases versus pooled controls approach and the two other interaction tests previously studied to detect associations under three different models: (1) a flip-flop effect (male OR = 1.5, female OR = 0.75), (2) an asthma main effect (OR = 1.5) and (3) a combination of an asthma main (OR = 1.5) and a male-specific effect (OR = 1.5, the sex effect is in the same direction as the main effect). The cases versus pooled controls approach had nearly ideal power to detect flip-flop effects, small but non-zero power to detect main effects and the highest power to detect the combination of main and sex-specific effects (Supplementary Material, Fig. S2B–D). The cases versus pooled controls approach is flexible and can be applied to both case–control and trio-study designs. Additionally, it is powerful for detecting sex-specific as well as other genotype-by-sex interaction models, thus validating our decision to use the cases versus pooled controls approach.
Genotype-by-sex interaction analysis
In each of the 11 EVE consortium samples (Table 1), we conducted two genome-wide genotype-by-sex interaction studies using the cases versus pooled controls approach. The results of these sample-specific analyses were combined and meta-analyzed in each of three ancestry groups (European American: Nmale cases = 1052, Nfemale cases = 1019 and Ncontrols = 1535; African American/African Caribbean: Nmale cases = 675, Nfemale cases = 837 and Ncontrols = 1503 and Latino: Nmale cases = 926, Nfemale cases = 710 and Ncontrols = 792) and in the combined sample (Nmale cases = 2653, Nfemale cases = 2566 and Ncontrols = 3830), for a total of eight meta-analyses. Each meta-analysis has ∼2.1 million imputed SNPs, which correspond to a genome-wide significance threshold of 2.3 × 10−8. The Q–Q plots of the distributions of P-values in each meta-analysis are shown in Supplementary Material, Figure S3 with the inflation factor lambda noted in each figure. In all eight analyses, the P-values closely follow the null (uniform) distribution, indicating there was little to no inflation.
Table 1.
Summary of the samples in EVE Consortium
| Ancestry | Study | No. male cases | No. female cases | No. male controls | No. female controls |
|---|---|---|---|---|---|
| European American | CAMPa | 244 | 143 | NA | NA |
| CAREa | 148 | 69 | NA | NA | |
| CHS | 348 | 295 | 498 | 461 | |
| CAG/CSGA/SARP | 312 | 512 | 211 | 365 | |
| European American total | 1052 | 1019 | 709 | 826 | |
| African Caribbean | BAGS | 188 | 194 | 219 | 242 |
| African American | GRAAD | 207 | 240 | 195 | 264 |
| SAPPHIRE | 60 | 90 | 0 | 133 | |
| CAG/CSGA/SARP | 220 | 313 | 163 | 287 | |
| African American/African Caribbean total | 675 | 837 | 577 | 926 | |
| Latino | CHS | 339 | 267 | 410 | 382 |
| GALAa | 298 | 240 | NA | NA | |
| MCCASa | 289 | 203 | NA | NA | |
| Latino total | 926 | 710 | 410 | 382 | |
| Combined sample | Total | 2653 | 2566 | 1696 | 2134 |
Studies: BAGS, Barbados Asthma Genetics Study; CAMP, Childhood Asthma Management Program; CARE, Childhood Asthma Research and Education; CHS, Children's Health Study; CAG, Chicago Asthma Genetics Study; CSGA, Collaborative Studies on the Genetics of Asthma; SARP, Study of Asthma Phenotypes and Pharmacogenetics; GRAAD, Genomic Research on Asthma in the African Diaspora; SAPPHIRE, Study of Asthma Phenotypes and Pharmacogenomic Interactions by Race-Ethnicity; GALA, Genetics of Asthma in Latino Americans; MCCAS, Mexico City Childhood Asthma Study.
aAsthma proband–parent trios.
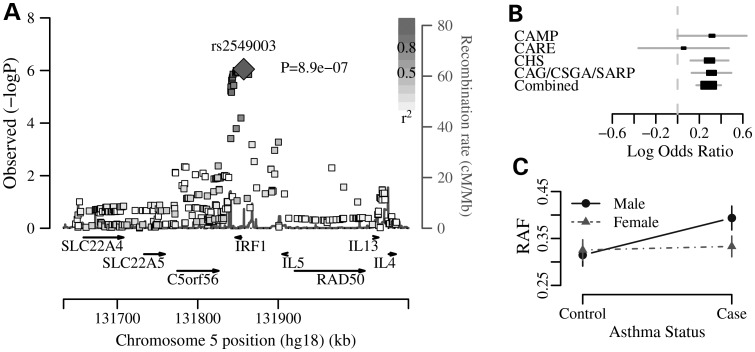
Although none of the associations reached the threshold for genome-wide significance, we observed six independent loci with at least one association P <1 × 10−6 (Table 2, Fig. 1, Supplementary Material, Figs S4 and S5). Two of the six associations were male specific (on chromosomes 5q31.1 and 10q26.1) and four were female specific (on chromosomes 2q23.3, 2q34, 6q27 and 17p13.3). The most significant associations in European Americans were among males with SNPs in and near the interferon regulatory factor 1 (IRF1) gene on chromosome 5q31.1. The association signal at the IRF1 locus is supported by rs2549003 and 19 additional SNPs with P < 1 × 10−5; the direction of association is the same in each of the four European American samples (Fig. 1A and B). The risk allele frequency (RAF) at this marker was higher in male cases compared both with female cases and with male and female controls (Fig. 1C). The most significant association in the African American/African Caribbean sample was also male specific, with an intergenic SNP, rs17642749, on chromosome 10q26.11 between the genes EMX2 (338 kb, encodes empty spiracles homeobox 2) and RAB11FIP2 (117 kb, encodes Rab11 family-interacting protein 2, Supplementary Material, Fig. S4). At this locus, the association was in the African Caribbean sample; this SNP was excluded in the other African American samples where the MAF of the associated SNP was <0.05. In the African Caribbean sample, the RAF was higher in male cases compared both with female cases and with male and female controls.
Table 2.
SNPs with sex-specific association P-values < 1.0 × 10−6
| Eth. | Sex | SNP | Location | Alleles (risk/alt) | RAFa | Nearest gene (distance/location within gene) | P-value | ORb (95% CI) |
|---|---|---|---|---|---|---|---|---|
| EA | M | rs2549003 | 5q31.1 | G/A | 0.39 | IRF1 (2.8 kb) | 8.90 × 10−7 | 1.33 (1.18, 1.49) |
| AA | M | rs17642749 | 10q26.11 | G/T | 0.086 | RAB11FIP2 (117 kb) | 3.57 × 10−7 | 2.97 (1.90, 4.63) |
| AA | F | rs1012307 | 2q23.3 | C/A | 0.20 | AK057517 (intronic) | 7.50 × 10−7 | 1.44 (1.25, 1.67) |
| Latino | F | rs4673659 | 2q34 | C/A | 0.72 | ERBB4 (intronic) | 9.29 × 10−7 | 1.42 (1.23, 1.64) |
| Latino | F | rs2675724 | 6q27 | A/C | 0.62 | C6orf118 (127 kb) | 1.58 × 10−7 | 1.44 (1.26, 1.66) |
| Latino | F | rs9895098 | 17p13.3 | C/T | 0.10 | RAP1GAP2 (3′ UTR) | 2.79 × 10−7 | 2.31 (1.90, 2.8) |
SNPs are ordered by sex, ancestry and genomic location. Allele frequency differences between male and female controls were non-significant (P-value > 0.05) for all six SNPs.
aRAF in the sex-specific cases.
bOR from the sex-specific cases versus pooled controls.
Figure 1.
The male-specific asthma association on 5q31.1 in European Americans. (A) The −log 10(P-value) is shown on the y-axis and position along chromosome 5q31.1 is shown on the x-axis. SNPs are shaded according to LD with the most significant SNP, rs2549003. This figure was generated using SNAP (27,28). (B) The log OR and 95% confidence interval for each of the EVE samples and in the combined sample. The black boxes are scaled by the standard error (larger boxes correspond to smaller error). (C) The association model with RAF for rs2549003 plotted separately in cases and controls and in males (solid line) and females (dashed line); the vertical bars represent the 95% confidence interval of the RAF.
Four associations had P < 1 × 10−6 in the female-specific analyses, one in the African American/African Caribbean sample (Supplementary Material, Fig. S5A–C) and three in the Latino sample (Supplementary Material, Fig. S5D–L). All four of these associations showed increased RAFs among female cases compared with both male cases and all controls, and the same direction of effect was seen in all samples within each group. The African American/African Caribbean association is with rs1012307, an SNP located in an uncharacterized gene, which may be a large intergenic non-coding RNA (AK057517), on chromosome 2q23.3 (Supplementary Material, Fig. S5A). This association is supported by 16 additional SNPs with P < 10−5 (Supplementary Material, Fig. S5B). The most significant association in the Latino females is with rs2675724, located on chromosome 6p27, 127 kb from C6orf118; 15 additional SNPs have P < 1 × 10−5 in this region (Supplementary Material, Fig. S5G–I). The association at the 2q34 locus is with rs4673659, an intronic SNP in the ERBB4 gene encoding a cell surface receptor tyrosine-protein kinase (Supplementary Material, Fig. S5D–F). This association is supported by two additional SNPs with P < 1 × 10−5. Finally, the association with rs9895098 on 17p13.3 was supported by one additional SNP with P < 10−5. SNP rs9895098 is in the 3′ untranslated region (UTR) of the RAP1GAP2 gene that encodes a GTPase-activating protein regulating dense granule secretion in platelets.
Validation of associated SNPs in a published GWAS of asthma
We assume some of the sex-specific associations may also be detected as attenuated sex-averaged main effects in large GWAS of asthma. We therefore mined the results of the largest and independent asthma study, the GABRIEL Consortium meta-analysis of asthma in European subjects (29) to validate the six most significant male- and female-specific associations. Two sex-specific associations in our study had SNPs in perfect linkage disequilibrium (LD; measured in the 1000 Genomes Project CEU samples) with P < 0.05 in the GABRIEL study: the European American male-specific association with rs2549003 near IRF1 (5q31.1, P = 0.00863) and the Latino female-specific association with rs9895098 in RAP1GAP2 (17p13.3, P = 0.0464). The remaining sex-specific associations either had a P > 0.05 (rs1012307, rs4673659 and rs2675725) or were not well tagged in the GABRIEL study (rs17642749). Associations between a microsatellite maker in the IRF1 gene on chromosome 5q31.1 and asthma have also been reported in candidate gene studies in Taiwanese (30) and Japanese (31) populations. Furthermore, in a sex-stratified linkage analysis of asthma-related phenotypes in a European cohort, a male-specific linkage peak was detected on 5q31.1, including IRF1, for both lung function (FEV1/Height2) and allergen polysensitization (32). These results validated two of the six associations as asthma candidate loci. Our ability to validate sex-specific associations in the Latinos and African Americans is limited because nearly all GWAS of asthma in these populations are part of EVE and already included in this study. Nonetheless, validating two sex-specific associations in published GWAS provides additional independent evidence that these two loci contribute to asthma susceptibility.
Role of the associated SNPs in gene regulation
We hypothesized that SNPs with sex-specific effects on asthma risk do so by modulating gene expression. To test this hypothesis, we first used results of seven published expression quantitative trait locus (eQTL) mapping studies in four different tissues or cell types to determine if the six asthma-associated SNPs in our study, or SNPs in strong LD with asthma-associated SNPs, are also associated with transcript abundance of nearby genes (i.e. cis-eQTL). The same two SNPs validated in the GABRIEL study (rs2549003 on 5q31.1 and rs9895098 on 17p13.3) were also in high LD (r2 > 0.80) with reported cis-eQTLs (Table 3); SNPs at the other four loci were not associated with transcript abundance in any of the published studies. SNP rs2549003 on 5q31.1 is in perfect LD with a cis-eQTL for a specific IRF1 transcript (ENST00000245414) in lymphoblastoid cell lines (LCLs) from the European samples making up the 1000 Genomes Project (33). Additionally, rs2549003 was reported as a cis-eQTL and was in LD with six SNPs that were reported as cis-eQTLs for four of the eight genes at this locus, with the regulated gene varying by cell type and sample. Two SNPs (rs2070727 and rs9282761; both in perfect LD with rs2549003, r2 = 1.0) are eQTLs for IRF1 in LCLs from asthma probands and their siblings (36) and in sputum samples from subjects with chronic obstructive pulmonary disease (COPD) (34). A third SNP in perfect LD with rs2549003 (rs2548999) and rs2549003 are eQTLs for RAD50 in lung tissues (35) and in LCLs from healthy female twins (37), respectively, two SNPs in near perfect LD with rs2549003 (rs13165038 and rs10035166; r2 = 0.97 and 0.81, respectively) are eQTLs for SLC22A4 and SLC22A5 in monocytes in two different studies (38,39) and finally rs2548997 (r2 = 1.0) is an eQTL for SLC22A5 in LCLs from healthy female twins (37). None of these SNPs were reported as eQTLs for other genes at this region (C5orf56, IL5, IL13, IL4), but it is not possible to determine whether these genes were detected as expressed in the all of the published studies. One other SNP at a sex-specific asthma-associated locus (rs9895098) was in LD with a reported eQTL (rs4077990) on 17p13.3. This SNP is a cis-eQTL for the RAP1GAP2 gene in lung tissue (35). Thus, SNPs at two loci associated with sex-specific risks for asthma in our study and that show modest association with asthma as a main effect in the GABRIEL study are also in strong LD with functional regulatory variants in relevant tissues and cell types. However, the available data do not allow us to evaluate whether the possible eQTLs themselves have different effects in males and females.
Table 3.
Literature-based eQTLs in LD with sex-specific asthma-associated SNPs
| eQTL | Locus | Sex-specific asthma-associated SNP | LD between associated SNP and eQTL (r2) | Regulated gene | eQTL P-value | Sample description |
|---|---|---|---|---|---|---|
| rs2549009 | 5q31.1 | rs2549003 | 1.0 | IRF1 | 6.24 × 10−10 | LCLs from the 1000 Genomes Project European populations (33) |
| rs2070727 | 5q31.1 | rs2549003 | 1.0 | IRF1 | 7.46 × 10−5 | Sputum from patients with COPD (34) |
| rs2548999a | 5q31.1 | rs2549003 | 1.0 | RAD50 | 3.50 × 10−5 | Lung tissue collected from patients during lung resectional surgery (35) |
| rs9282761a | 5q31.1 | rs2549003 | 1.0 | IRF1 | 7.30 × 10−9 | LCLs from asthmatic children and their siblings (36) |
| rs2548997a | 5q31.1 | rs2549003 | 1.0 | SLC22A5 | 5.15 × 10−8 | LCLs from healthy female twins in the MuTHER resource (37) |
| rs2549003a | 5q31.1 | rs2549003 | NA | RAD50 | 1.3 × 10−5 | LCLs from healthy female twins in the MuTHER resource (37) |
| rs13165038a | 5q31.1 | rs2549003 | 0.97 | SLC22A4 | 1.86 × 10−6 | Monocytes from volunteers (38) |
| rs10035166a | 5q31.1 | rs2549003 | 0.81 | SLC22A5 | 1.37 × 10−17 | Monocytes from subjects in the Gutenberg Heart Study (39) |
| rs4077990 | 17p13.3 | rs9895098 | 1.0 | RAP1GAP2 | 4.99 × 10−10 | Lung tissue collected from patients during lung resectional surgery (35) |
aThere exists an SNP with a smaller eQTL P-value for the same gene: top eQTLs for RAD50 in (a) lung tissue is rs11242103 (r2 = 0.21, P = 2.77 × 10−17) and (b) LCLs from MuTHER resource is rs11950562 (r2 = 0.18, P = 2.11 × 10−23); top eQTL for IRF1 in LCLs from asthmatic children and their siblings is rs2070729 (r2 = 0.74, P = 4.9 × 10−10); top eQTLs for SLC22A5 in (a) LCLs from the MuTHER resource is rs17772583 (r2 = 0.12, P = 1.19 × 10–35) and (b) monocytes from the GHS is rs2631360 (r2 = 0.18, P = 6.6 × 10−85) and the top eQTL for SLC22A4 in monocytes from healthy volunteers is rs274560 (r2 = 0.27 P = 3.19 × 10−13).
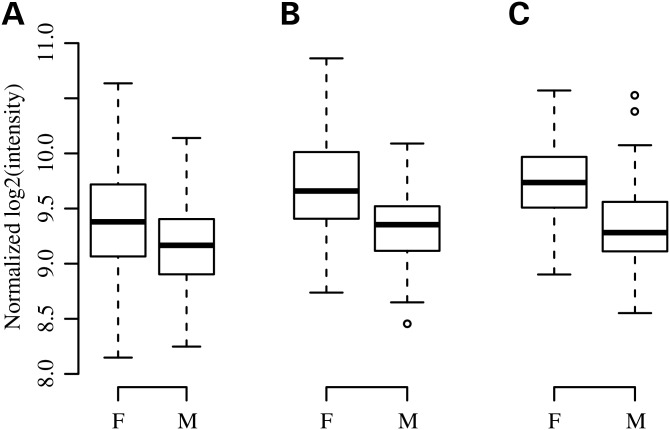
To further characterize sex effects on gene expression, we tested for differential expression by sex for genes listed in Table 3 in whole blood from EVE participants (Table 4). Three genes on 5q31.1 (SLC22A5, SLC22A4 and IRF1) were detected as expressed in whole blood and one of them, SCL22A4, was expressed at significantly higher levels in females compared with males in three different samples [P-value for male–female difference = 2.5 × 10−10, 4.0 × 10−9 and 7.7 × 10−8 in Childhood Asthma Management Program (CAMP), Children's Health Study (CHS) and Mexico City Childhood Asthma Study (MCCAS), respectively, Fig. 2]. Sex was not associated with expression of IRF1 in whole blood, suggesting the associated SNPs in IRF1 may be due to their sex-specific regulatory effects on expression of neighboring genes or of IRF1 in cell types not considered here (e.g. airway cells). RAP1GAP2 was also detected as expressed in whole blood, but expression of this gene did not differ between males and females.
Table 4.
Summary of samples with microarray expression data in whole blood RNA
| Study | Total (M : F) | European American (M : F) | African American (M : F) | Latino (M : F) | Othera (M : F) |
|---|---|---|---|---|---|
| CAMP | 292 : 186 | 206 : 128 | 40 : 32 | 33 : 14 | 13 : 12 |
| CHS | 79 : 122 | 30 : 29 | NA | 25 : 66 | 24 : 27 |
| MCCAS | 69 : 54 | NA | NA | 69 : 54 | NA |
CAMP, Childhood Asthma Management Program; CHS, Childhood Health Study; MCCAS, Mexico City Childhood Asthma Study.
aSelf-reported ancestries that were not one of the three North American populations, were mixed ancestry, or were missing ancestry information.
Figure 2.
SLC22A4 gene expression by sex. Gene expression of SLC22A4, in units of log2 (intensity), plotted by sex (F = female, M = male) in whole blood samples from (A) CAMP, (B) CHS and (C) MCCAS.
DISCUSSION
We report the first genome-wide meta-analysis of genotype-by-sex interaction study of asthma, a common disease with significant sex-specific architecture (5,17–22). We observed several intriguing biological candidate loci among the most significant associations, two of which were further supported as asthma risk loci at reduced levels of statistical significance, consistent with a true sex difference, and by results from published GWAS of asthma. These results are further supported by eQTL studies. The validated associations are for SNPs near the IRF1 gene on 5q31.1 in European American males and SNPs in the 3′ UTR of the RAP1GAP2 gene in Latino females. RAP1GAP2 (Rap1 GTPase-activating protein 2) is expressed in the lung (35), where it is involved in regulating the secretion of dense granules from platelets at sites of endothelial damage (40,41). Allergen exposure can result in recruitment of platelets to the airways (42), suggesting RAP1GAP2 might be specifically involved in response to allergens.
The most significant sex-specific association with asthma in the European American sample was with SNPs at the IRF1 locus. IRF1 encodes a transcription factor activating the transcription of the genes encoding IFN-α, β and γ, cytokines all of which have been implicated in asthma pathogenesis (18). Male-specific linkage results for asthma-related phenotypes (allergen polysensitization and lung function) have been reported at this locus (32). Furthermore, related genes, IRF5 and IRF7, were identified as hubs in a network module of gene expression responses to viral-induced exacerbation in nasal lavage samples from children with asthma (43). Another gene in the IRF1 activation pathway (INFG) showed sex-specific patterns of association with asthma and with early life wheezing illness in a candidate gene study (19), and IFN-γ responses show significant sex differences in early childhood, with increased response in boys compared with girls (18). Our study extends the sex-specific trends of genes in the IFN-γ pathway to include a key member of this important immune response, possibly providing a mechanism for these earlier observations.
SNPs at the IRF1 locus were cis-eQTLs in previous studies of sputum from COPD patients and in LCLs from children with asthma and their siblings (34,36). Thus, it is possible that sex-specific effects on expression of IRF1 or eQTL-by-sex interaction effects are present in airway cells or variation at this locus has regulatory effects on 5q31.1 genes not well interrogated on the arrays. For example, in data from the 1000 Genomes Project, an SNP (rs2070724) in an IRF1 splice acceptor site is in perfect LD (r2 = 1.00) with rs2549003 and both the splice variant and the associated SNPs are in perfect LD with a cis-eQTL (rs2548997) for a specific IRF1 transcript isoform. Thus, the splice variant may affect mRNA splicing and cause sex-biased expression of alternative transcripts, a hypothesis that cannot be explored in the available datasets. Moreover, we observed sex-biased expression of another gene at this locus, SLC22A4, in whole blood samples, with increased expression in females. SLC22A4 encodes OCTN1, an organic cation transporter involved in eliminating environmental toxins and drugs, including the anticholinergic bronchodilator ipratropium, which is used to treat obstructive lung disease (44). Alternatively, sex-specific effects of this locus on asthma risk may be due to the regulation of expression of other genes, such as IL4, IL5 or IL13, which were either not detected as expressed or not assayed in whole blood RNA so could not be directly interrogated for sex effects.
Interestingly, the six most significant SNPs show population-specific patterns of association, even in instances where the MAF of the associated SNP is common in all populations. For example, the SNPs at the IRF1 locus are common in all ancestries (MAFs 0.32–0.44), yet the association is only observed in European Americans. Similarly, the associated SNP, rs9895098, on 17p13.3 has MAFs ranging from 0.065 to 0.28 in the different samples yet the association signal is present in only the Latinos. The exception to this is the female-specific association on 2q23.3 in African Americans, where the MAF is <0.05 in European Americans and Latinos but higher (0.15–0.18) in African Americans. These results exemplify the population-specific nature of asthma associations, which may not be surprising given the striking differences in asthma prevalence across populations with different ancestries.
The cases versus pooled controls approach used in this study was designed to detect male- and female-driven genotype-by-sex interactions in both case-control and trio-based study designs. This approach had optimal power to detect sex-specific associations, although other types of interactions could also be detected with good power (e.g. combinations of sex and main effects, and flip-flop interactions in which an allele increases risk in one sex and decreases risk in the other sex). In contrast, the cases versus pooled controls approach that we used here has low, but non-zero, power to detect main effects on asthma risk (Supplementary Material, Fig. S2C). As a result, some SNPs with true main effects could be identified as having sex-specific associations in our analysis. However, because we do not observe among our most significant results the associations reported in our previous meta-analysis of asthma GWAS that included these same subjects (e.g. ORMDL3, TSLP) (24), we think it is most likely that our results represent genotype-by-sex interactions and not main effects on asthma risk.
A limiting factor in this study is the power to detect genotype-by-sex interactions. Previous meta-analyses report ORs for asthma main effects on the order of 1.1–1.3 (24,29), and one asthma candidate gene study reported sex-specific ORs comparable in magnitude to the asthma main effects, ranging from 1.11 to 1.25 (20). Similarly, the GIANT Consortium meta-analysis of genotype-by-sex interaction effects on anthropomorphic traits revealed sex-specific effects in this same range as the main effects (3,13). Together, these studies suggest that the expectation for sex-specific effect sizes is an OR of ≤ 1.3 and that our study is underpowered (<10% to detect an OR = 1.25). Furthermore, sample and phenotype heterogeneity within and between studies can decrease the estimate of the effect sizes. A large-scale, international collaboration would be needed to obtain the sample sizes required to reliably detect modest sex-specific effects.
This study highlights the advantages and complexity of jointly considering the effects of both genotype and environment (i.e. sex in this example) on asthma susceptibility. By testing for genotype-by-sex interactions, we identified sex-specific associations with asthma for SNPs in IRF1 and RAP1GAP2, which are supported by two independent lines of evidence: GWAS of asthma and studies of gene regulation. These SNPs (or SNPs in strong LD with them) showed only nominally significant, not genome-wide significant, associations with asthma in an independent GWAS and these SNPs also showed evidence for a role in gene regulation in eQTL mapping studies. Thus, our study identified two candidate loci with sex-specific associations with asthma risk and implicated regulatory variation in these effects. Overall, these observations contribute toward our understanding of the sex-specific architecture of this very common and complex disease.
MATERIALS AND METHODS
The EVE Consortium
The EVE Consortium is comprising 10 centers, which contributed genotype and phenotype data from 11 studies. Sample ascertainment schemes, sample characteristics and information on genotyping platforms and quality control checks for each study have been previously described (24). Briefly, 3585 asthma cases, 3830 non-asthma controls and 1634 asthma case-parent trios were recruited from locations in the USA, Mexico and Barbados, representing European American, African American, African Caribbean and Latino populations (Table 1). The participating studies used Affymetrix and Illumina genotyping platforms. Therefore, to facilitate meta-analysis of all samples, we used MaCH (45) to impute all variants reported in the HapMap Phase 2, release 21, reference panel.
Statistical analysis
For each of the 11 samples, 2 genome-wide genotype-by-sex interaction studies (one for each sex) were performed to detect sex-specific associations. In the male-specific analysis, male cases were compared with a set of pooled controls composed of male controls, female cases and female controls. Similarly, in the female-specific association test, female cases were compared with female controls grouped with male cases and controls. The cases versus pooled controls approach was adapted to the trio-design studies by contrasting male and female affected probands, a case-only analysis. For both the case-control and trio studies, logistic regression was used to test for association between imputed allelic dosages and the sex-specific asthma status (e.g. male cases = 1, all other samples = 0), including either principal components or global ancestry estimates as population structure covariates. To account for relatedness in the extended families from Barbados, the MQLS test (46) was used as the association test. SNPs with either low MAF (<0.05) or low imputation accuracy (R2 < 0.3) were excluded from all analyses.
Meta-analyses were performed in the European Americans (Nmale cases = 1052, Nfemale cases = 1019 and Ncontrols = 1535), African Americans/African Caribbean (Nmale cases = 675, Nfemale cases = 837 and Ncontrols = 1503), Latinos (Nmale cases = 926, Nfemale cases = 710 and Ncontrols = 792) and the combined samples (i.e. across all ancestries, Nmale cases = 2653, Nfemale cases = 2566 and Ncontrols = 3830), as in our earlier studies (24,25). Test statistics from the sample-specific logistic regressions were combined using the weighted sums of z-score method. These weights (w) accounted for sample size (N), and the proportions of cases (v) in each study, as well as allele frequency (p) and the imputation accuracy (R2) of each SNP within each study (47). Significance was ascertained using standard normal approximations. The combined effect sizes were calculated as a linear combination of log ORs with weights proportional to the standard errors of each log OR. All statistical analyses were completed using R (www.r-project.org), and the R package meta was used to calculate the combined log OR.
Power analysis
The power of the cases versus pooled controls approach was determined in each of the four samples (European American, African American/African Caribbean, Latino and combined). We limited our analysis to identifying male-specific effects since the number of male and female cases is similar, and subsequently, the power to detect male- and female-specific effects should be similar. Phenotypes and genotypes were simulated under four additive models: (A) a male-specific effect, (B) a flip-flop effect (the same allele increases risk in males and decreases risk in females), (C) a main effect and (D) a combination of main and male-specific effects. We considered effect sizes ranging from an OR = 1 (no effect) to 2.0 (strong effect). Additionally, we assessed the power of three different genotype-by-sex interaction tests: (1) the cases versus pooled controls association test contrasting male cases to the pool of male controls and all female, (2) the case-only test comparing male-to-female cases and (3) the genotype-by-sex interaction term in a logistic model (case-control samples only), in the European Americans. For both power studies, the MAFs ranged from 0.05 to 0.5. The genome-wide significance threshold was 5 × 10−8 and 100 000 simulations were completed for each model. All power simulations and analyses were completed using R.
Gene expression and eQTL analysis
Gene expression data were available for whole blood samples from a subset of the EVE subjects, including 478 European American, African American and Latino asthmatic children from the CAMP, 123 Latino asthmatic children from MCCAS and 191 Latino and European American cases and controls from the CHS (Table 4). Gene expression was measured using the Illumina HumanHT-12v4 Expression BeadChip at the Channing Division of Network Medicine, using similar methods to those described previously (48). Samples with sex mismatches or low median pairwise rank correlation (r < 0.80) were removed. Probes were removed if they had poor mapping quality, mapped to the X or Y chromosome, contained an SNP with MAF > 0.01 (per the 1000 Genomes Project) in the probe sequence, or were detected in fewer than 20% of all subjects. Expression data were adjusted for background noise, log2 transformed and quantile normalized using the R Bioconductor package lumi (49). ComBat (50) was used to remove batch effects, and surrogate variable analysis (51) was used to detect and control for expression heterogeneity. Linear regression was used to test for differential expression by sex, adjusted for ancestry and asthma status.
Information on eQTLs was extracted from the eQTL browser [eqtl.uchicago.edu, specifically eQTLs in monocytes (39)] and reports from studies of a variety of cell types including lung tissues (35), B-cell and monocyte samples (38), sputum samples from patients with COPD (34), LCLs from probands with asthma and their siblings (36), LCLs from 462 samples in the 1000 Genomes Project (33), LCLs from healthy female twins in the MuTHER resource (37) and CD4+ lymphocytes from 200 cases of asthma (48).
SUPPLEMENTARY MATERIAL
FUNDING
This work was supported by grants from the Office of the Director, National Institutes of Health to C.O. and D.L.N.; the National Heart, Lung and Blood Institute (HL101651 to C.O. and D.L.N., HL085197 to C.O., HL087699 to K.C.B., HL075419, HL65899, HL083069 and HL066289 to S.T.W., HL101543 to B.A.R. and S.T.W., HL115606 and HL087680 to W.J.G., HL61768 and HL76647 to F.D.G., HL079055 and HL118267 to L.K.W., HL088133, HL078885, HL004464 and HL104608 to E.G.B.); the National Institute of Allergy and Infection Diseases (AI079139, AI061774 to L.K.W., AI095230 to C.O., AI077439 to E.G.B.); the National Institute of Environmental Health Sciences (ES022719 to W.J.G., ES011627 to F.D.G., ES007048 and ES009581 to F.D.G. and W.J.G., ES015794 to E.G.B.); the National Institute on Minority Health and Health Disparities (MD006902 to E.G.B.) the National Institute of General Medical Sciences (GM007546 to E.G.B.); Fundación Ramón Areces (M.P.Y.); American Asthma Foundation (L.K.W. and E.G.B.); the Fund for Henry Ford Hospital (L.K.W.); Hastings Foundation (F.D.G.); RWJF Amos Medical Faculty Development Award (E.G.B.); the Sandler Foundation (E.G.B.) and the Mary Beryl Patch Turnbull Scholar Program (K.C.B.). This research was supported in part by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Jose Rodriguez Santana, William Rodriguez Cintron, Rocio Chapela, Jean Ford, Shannon Thyne, Pedro C. Avila, Juan Jose Sienra Monge, Meher Boorgula, Chris Cheadle and Celeste S. Eng for data collection, management and analysis. We acknowledge support from J. Kiley, S. Banks-Schlegel and W. Gan at the National Heart, Lung and Blood Institute; all of the patients and families for their participation in these studies and the numerous healthcare providers and community clinics for their support.
Conflict of Interest Statement. None declared.
Contributor Information
Collaborators: Jose Rodriguez Santana, William Rodriguez Cintron, Rocio Chapela, Jean Ford, Shannon Thyne, Pedro C. Avila, Juan Jose Sienra Monge, Meher Boorgula, Chris Cheadle, Celeste S. Eng, J. Kiley, S. Banks-Schlegel, and W. Gan
REFERENCES
- 1.Weiss L.A., Pan L., Abney M., Ober C. The sex-specific genetic architecture of quantitative traits in humans. Nat. Genet. 2006;38:218–222. doi: 10.1038/ng1726. [DOI] [PubMed] [Google Scholar]
- 2.Gray J.P., Wolfe L.D. Height and sexual dimorphism of stature among human societies. Am. J. Phys. Anthropol. 1980;53:441–456. doi: 10.1002/ajpa.1330530314. [DOI] [PubMed] [Google Scholar]
- 3.Heid I.M., Jackson A.U., Randall J.C., Winkler T.W., Qi L., Steinthorsdottir V., Thorleifsson G., Zillikens M.C., Speliotes E.K., Auml Gi R., et al. Meta-analysis identifies 13 new loci associated with waist-hip ratio and reveals sexual dimorphism in the genetic basis of fat distribution. Nat. Genet. 2010;42:949–960. doi: 10.1038/ng.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grossman C. Possible underlying mechanisms of sexual dimorphism in the immune response, fact and hypothesis. J. Steroid Biochem. 1989;34:241–251. doi: 10.1016/0022-4731(89)90088-5. [DOI] [PubMed] [Google Scholar]
- 5.Osman M. Therapeutic implications of sex differences in asthma and atopy. Arch. Dis. Child. 2003;88:587–590. doi: 10.1136/adc.88.7.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moroni L., Bianchi I., Lleo A. Geoepidemiology, gender and autoimmune disease. Autoimmun. Rev. 2012;11:A386–A392. doi: 10.1016/j.autrev.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 7.McCombe P.A., Greer J.M., Mackay I.R. Sexual dimorphism in autoimmune disease. Curr. Mol. Med. 2009;9:1058–1079. doi: 10.2174/156652409789839116. [DOI] [PubMed] [Google Scholar]
- 8.Costello E.J., Mustillo S., Erkanli A., Keeler G., Angold A. Prevalence and development of psychiatric disorders in childhood and adolescence. Arch. Gen. Psychiatry. 2003;60:837–844. doi: 10.1001/archpsyc.60.8.837. [DOI] [PubMed] [Google Scholar]
- 9.Choi B.G., McLaughlin M.A. Why men's hearts break: cardiovascular effects of sex steroids. Endocrinol. Metab. Clin. North Am. 2007;36:365–377. doi: 10.1016/j.ecl.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 10.Rinn J.L., Snyder M. Sexual dimorphism in mammalian gene expression. Trends Genet. 2005;21:298–305. doi: 10.1016/j.tig.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Ober C., Loisel D.A., Gilad Y. Sex-specific genetic architecture of human disease. Nat. Rev. Genet. 2008;9:911–922. doi: 10.1038/nrg2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carrel L., Willard H.F. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature. 2005;434:400–404. doi: 10.1038/nature03479. [DOI] [PubMed] [Google Scholar]
- 13.Randall J.C., Winkler T.W., Kutalik Z., Berndt S.I., Jackson A.U., Monda K.L., Kilpelainen T.O., Esko T., Magi R., Li S., et al. Sex-stratified genome-wide association studies including 270,000 individuals show sexual dimorphism in genetic loci for anthropometric traits. PLoS Genet. 2013;9:e1003500. doi: 10.1371/journal.pgen.1003500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu C.T., Estrada K., Yerges-Armstrong L.M., Amin N., Evangelou E., Li G., Minster R.L., Carless M.A., Kammerer C.M., Oei L., et al. Assessment of gene-by-sex interaction effect on bone mineral density. J. Bone Miner. Res. 2012;27:2051–2064. doi: 10.1002/jbmr.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu L.Y., Schaub M.A., Sirota M., Butte A.J. Sex differences in disease risk from reported genome-wide association study findings. Hum. Genet. 2012;131:353–364. doi: 10.1007/s00439-011-1081-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masoli M., Fabian D., Holt S., Beasley R., Program G.I.F.A.G. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004;59:469–478. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 17.Kulig M., Tacke U., Forster J., Edenharter G., Bergmann R., Lau S., Wahn V., Zepp F., Wahn U. Serum IgE levels during the first 6 years of life. J. Pediatr. 1999;134:453–458. doi: 10.1016/s0022-3476(99)70203-9. [DOI] [PubMed] [Google Scholar]
- 18.Uekert S.J., Akan G., Evans M.D., Li Z., Roberg K., Tisler C., Dasilva D., Anderson E., Gangnon R., Allen D.B., et al. Sex-related differences in immune development and the expression of atopy in early childhood. J. Allergy Clin. Immunol. 2006;118:1375–1381. doi: 10.1016/j.jaci.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 19.Loisel D.A., Tan Z., Tisler C.J., Evans M.D., Gangnon R.E., Jackson D.J., Gern J.E., Lemanske R.F., Jr, Ober C. IFNG genotype and sex interact to influence the risk of childhood asthma. J. Allergy Clin. Immunol. 2011;128:524–531. doi: 10.1016/j.jaci.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hunninghake G.M., Soto-Quirós M.E., Avila L., Kim H.P., Lasky-Su J., Rafaels N., Ruczinski I., Beaty T.H., Mathias R.A., Barnes K.C., et al. TSLP polymorphisms are associated with asthma in a sex-specific fashion. Allergy. 2010;65:1566–1575. doi: 10.1111/j.1398-9995.2010.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Santillan A.A., Camargo C.A., Jr, Ramirez-Rivera A., Delgado-Enciso I., Rojas-Martinez A., Cantu-Diaz F., Barrera-Saldana H.A. Association between beta2-adrenoceptor polymorphisms and asthma diagnosis among Mexican adults. J. Allergy Clin. Immunol. 2003;112:1095–1100. doi: 10.1016/j.jaci.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 22.Seibold M.A., Wang B., Eng C., Kumar G., Beckman K.B., Sen S., Choudhry S., Meade K., Lenoir M., Watson H.G., et al. An African-specific functional polymorphism in KCNMB1 shows sex-specific association with asthma severity. Hum. Mol. Genet. 2008;17:2681–2690. doi: 10.1093/hmg/ddn168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gauderman W.J., Zhang P., Morrison J.L., Lewinger J.P. Finding novel genes by testing G×E interactions in a genome-wide association study. Genet. Epidemiol. 2013;37:603–613. doi: 10.1002/gepi.21748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Torgerson D.G., Ampleford E.J., Chiu G.Y., Gauderman W.J., Gignoux C.R., Graves P.E., Himes B.E., Levin A.M., Mathias R.A., Hancock D.B., et al. Meta-analysis of genome-wide association studies of asthma in ethnically diverse North American populations. Nat. Genet. 2011;43:887–892. doi: 10.1038/ng.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Myers R.A., Himes B.E., Gignoux C.R., Yang J.J., Gauderman W.J., Rebordosa C., Xie J., Torgerson D.G., Levin A.M., Baurley J., et al. Further replication studies of the EVE Consortium meta-analysis identifies 2 asthma risk loci in European Americans. J. Allergy Clin. Immunol. 2012;130:1294–1301. doi: 10.1016/j.jaci.2012.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levin A.M., Mathias R.A., Huang L., Roth L.A., Daley D., Myers R.A., Himes B.E., Romieu I., Yang M., Eng C., et al. A meta-analysis of genome-wide association studies for serum total IgE in diverse study populations. J. Allergy Clin. Immunol. 2013;131:1176–1184. doi: 10.1016/j.jaci.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saxena R., Voight B.F., Lyssenko V., Burtt N.P., de Bakker P.I., Chen H., Roix J.J., Kathiresan S., Hirschhorn J.N., Daly M.J., et al. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science. 2007;316:1331–1336. doi: 10.1126/science.1142358. [DOI] [PubMed] [Google Scholar]
- 28.Johnson A.D., Handsaker R.E., Pulit S.L., Nizzari M.M., O'Donnell C.J., de Bakker P.I.W. SNAP: a web-based tool for identification and annotation of proxy SNPs using HapMap. Bioinformatics. 2008;24:2938–2939. doi: 10.1093/bioinformatics/btn564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moffatt M.F., Gut I.G., Demenais F., Strachan D.P., Bouzigon E., Heath S., von Mutius E., Farrall M., Lathrop M., Cookson W.O.C.M., et al. A large-scale, consortium-based genomewide association study of asthma. N. Engl. J. Med. 2010;363:1211–1221. doi: 10.1056/NEJMoa0906312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang T.N., Chu Y.T., Chen W.Y., Feng W.W., Shih N.H., Hsiang C.H., Ko Y.C. Association of interferon-gamma and interferon regulatory factor 1 polymorphisms with asthma in a family-based association study in Taiwan. Clin. Exp. Allergy. 2006;36:1147–1152. doi: 10.1111/j.1365-2222.2006.02551.x. [DOI] [PubMed] [Google Scholar]
- 31.Nakao F., Ihara K., Kusuhara K., Sasaki Y., Kinukawa N., Takabayashi A., Nishima S., Hara T. Association of IFN-gamma and IFN regulatory factor 1 polymorphisms with childhood atopic asthma. J. Allergy Clin. Immunol. 2001;107:499–504. doi: 10.1067/mai.2001.113051. [DOI] [PubMed] [Google Scholar]
- 32.Aschard H., Bouzigon E., Corda E., Ulgen A., Dizier M.H., Gormand F., Lathrop M., Kauffmann F., Demenais F. Sex-specific effect of IL9 polymorphisms on lung function and polysensitization. Genes Immun. 2009;10:559–565. doi: 10.1038/gene.2009.46. [DOI] [PubMed] [Google Scholar]
- 33.Lappalainen T., Sammeth M., Friedlander M.R., t Hoen P.A., Monlong J., Rivas M.A., Gonzalez-Porta M., Kurbatova N., Griebel T., Ferreira P.G., et al. Transcriptome and genome sequencing uncovers functional variation in humans. Nature. 2013;501:506–511. doi: 10.1038/nature12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qiu W., Cho M.H., Riley J.H., Anderson W.H., Singh D., Bakke P., Gulsvik A., Litonjua A.A., Lomas D.A., Crapo J.D., et al. Genetics of sputum gene expression in chronic obstructive pulmonary disease. PLoS One. 2011;6:e24395. doi: 10.1371/journal.pone.0024395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hao K., Bosse Y., Nickle D.C., Pare P.D., Postma D.S., Laviolette M., Sandford A., Hackett T.L., Daley D., Hogg J.C., et al. Lung eQTLs to help reveal the molecular underpinnings of asthma. PLoS Genet. 2012;8:e1003029. doi: 10.1371/journal.pgen.1003029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dixon A.L., Liang L., Moffatt M.F., Chen W., Heath S., Wong K.C., Taylor J., Burnett E., Gut I., Farrall M., et al. A genome-wide association study of global gene expression. Nat. Genet. 2007;39:1202–1207. doi: 10.1038/ng2109. [DOI] [PubMed] [Google Scholar]
- 37.Grundberg E., Meduri E., Sandling J.K., Hedman A.K., Keildson S., Buil A., Busche S., Yuan W., Nisbet J., Sekowska M., et al. Global analysis of DNA methylation variation in adipose tissue from twins reveals links to disease-associated variants in distal regulatory elements. Am. J. Hum. Genet. 2013;93:876–890. doi: 10.1016/j.ajhg.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fairfax B.P., Makino S., Radhakrishnan J., Plant K., Leslie S., Dilthey A., Ellis P., Langford C., Vannberg F.O., Knight J.C. Genetics of gene expression in primary immune cells identifies cell type-specific master regulators and roles of HLA alleles. Nat. Genet. 2012;44:502–510. doi: 10.1038/ng.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeller T., Wild P., Szymczak S., Rotival M., Schillert A., Castagne R., Maouche S., Germain M., Lackner K., Rossmann H., et al. Genetics and beyond —the transcriptome of human monocytes and disease susceptibility. PLoS One. 2010;5:e10693. doi: 10.1371/journal.pone.0010693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schultess J., Danielewski O., Smolenski A.P. Rap1GAP2 is a new GTPase-activating protein of Rap1 expressed in human platelets. Blood. 2005;105:3185–3192. doi: 10.1182/blood-2004-09-3605. [DOI] [PubMed] [Google Scholar]
- 41.Neumuller O., Hoffmeister M., Babica J., Prelle C., Gegenbauer K., Smolenski A.P. Synaptotagmin-like protein 1 interacts with the GTPase-activating protein Rap1GAP2 and regulates dense granule secretion in platelets. Blood. 2009;114:1396–1404. doi: 10.1182/blood-2008-05-155234. [DOI] [PubMed] [Google Scholar]
- 42.Pitchford S.C., Momi S., Baglioni S., Casali L., Giannini S., Rossi R., Page C.P., Gresele P. Allergen induces the migration of platelets to lung tissue in allergic asthma. Am. J. Respir. Crit. Care Med. 2008;177:604–612. doi: 10.1164/rccm.200702-214OC. [DOI] [PubMed] [Google Scholar]
- 43.Bosco A., Ehteshami S., Panyala S., Martinez F.D. Interferon regulatory factor 7 is a major hub connecting interferon-mediated responses in virus-induced asthma exacerbations in vivo. J. Allergy Clin. Immunol. 2012;129:88–94. doi: 10.1016/j.jaci.2011.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakamura T., Nakanishi T., Haruta T., Shirasaka Y., Keogh J.P., Tamai I. Transport of ipratropium, an anti-chronic obstructive pulmonary disease drug, is mediated by organic cation/carnitine transporters in human bronchial epithelial cells: implications for carrier-mediated pulmonary absorption. Mol. Pharm. 2010;7:187–195. doi: 10.1021/mp900206j. [DOI] [PubMed] [Google Scholar]
- 45.Li Y., Willer C.J., Ding J., Scheet P., Abecasis G.R. MaCH: using sequence and genotype data to estimate haplotypes and unobserved genotypes. Genet. Epidemiol. 2010;34:816–834. doi: 10.1002/gepi.20533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thornton T., McPeek M.S. Case-control association testing with related individuals: a more powerful quasi-likelihood score test. Am. J. Hum. Genet. 2007;81:321–337. doi: 10.1086/519497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zaitlen N., Eskin E. Imputation aware meta-analysis of genome-wide association studies. Genet. Epidemiol. 2010;34:537–542. doi: 10.1002/gepi.20507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murphy A., Chu J.H., Xu M., Carey V.J., Lazarus R., Liu A., Szefler S.J., Strunk R., Demuth K., Castro M., et al. Mapping of numerous disease-associated expression polymorphisms in primary peripheral blood CD4+ lymphocytes. Hum. Mol. Genet. 2010;19:4745–4757. doi: 10.1093/hmg/ddq392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Du P., Kibbe W.A., Lin S.M. Lumi: a pipeline for processing Illumina microarray. Bioinformatics. 2008;24:1547–1548. doi: 10.1093/bioinformatics/btn224. [DOI] [PubMed] [Google Scholar]
- 50.Johnson W.E., Li C., Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics. 2007;8:118–127. doi: 10.1093/biostatistics/kxj037. [DOI] [PubMed] [Google Scholar]
- 51.Leek J.T., Storey J.D. Capturing heterogeneity in gene expression studies by surrogate variable analysis. PLoS Genet. 2007;3:1724–1735. doi: 10.1371/journal.pgen.0030161. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.