Abstract
Arthropod-borne infectious diseases are responsible for nearly 1.5 million deaths annually across the globe, with malaria responsible for over 50% of these deaths. Recent efforts to enhance malaria control have focused on developing genetically modified Anopheles mosquitoes that are resistant to malaria parasite infection by manipulating proteins that are essential to the immune response. Though this approach has shown promise, the lack of efficient genetic tools in the mosquito makes it difficult to investigate innate immunity using reverse genetics. Current gene knockdown strategies based on small interfering RNAs (siRNA) are typically laborious, inefficient, and require extensive training. Here, we describe the use of morpholino anti-sense oligomers to knockdown MEK-ERK signaling in the midgut of Anopheles stephensi through a simple feeding protocol. Anti-MEK morpholino provided in a saline meal was readily ingested by female mosquitoes with minimal toxicity and resulted in knockdown of total MEK protein levels 3-4 days after morpholino feeding. Further, anti-MEK morpholino feeding attenuated inducible phosphorylation of the downstream kinase ERK and, as predicted by previous work, reduced parasite burden in mosquitoes infected with P. falciparum. To our knowledge, this is the first example of morpholino use for target protein knockdown via feeding in an insect vector. Our results suggest this method is not only efficient for studies of individual proteins, but also for studies of phenotypic control by complex cell signaling networks. As such, our protocol is an effective alternative to current methods for gene knockdown in arthropods.
Keywords: Mosquito, insect, morpholino, knockdown, malaria, mitogen-activated protein kinase, MAPK, Anopheles, Plasmodium
INTRODUCTION
The World Health Organization (WHO) now estimates that malaria causes over 200 million clinical episodes and over 650,000 deaths annually (WHO, 2012). While infection and mortality rates in much of the world have declined within the last decade, the failure of many common control methods has sparked the need for novel strategies to combat the disease. Among these are the development and improvement of anti-malarial drugs (Noedl, 2013), vaccine design (Birkett et al., 2013), and the enhancement of infrastructure in at-risk areas (Barclay et al., 2013; Klein, 2013). Additional efforts have focused on the development of genetically modified mosquitoes that are resistant to infection (Fuchs et al., 2013; Marshall & Taylor, 2009). Several groups, including our own, have now generated genetically modified mosquitoes that are refractory to Plasmodium infection (Corby-Harris et al., 2010; de Lara Capurro et al., 2000; Hauck et al., 2013; Isaacs et al., 2012; Kim et al., 2004) and similar methods are being used to combat other mosquito-borne infections such as dengue and yellow fever (Franz et al., 2006; Kokoza et al., 2000; Mathur et al., 2010; Travanty et al., 2004).
While the generation of stably transformed, pathogen-resistant mosquitoes has shown clear promise, the development and improvement of associated genetic techniques for use in the mosquito would greatly enhance research progress. Engineering pathogen resistance in a vector requires not only a detailed understanding of the complex mechanisms underlying natural immunity, but also the genetic tools to properly dissect these mechanisms in the lab. Readily available molecular methods to query the effects of mosquito immune genes and signaling pathways on pathogen infection include RNA interference (RNAi)-mediated knockdown (Boisson et al., 2006; Gulia-Nuss et al., 2011; Lamaccia et al., 2011), plasmid-based overexpression (Beumer et al., 2008; Peng et al., 2011), and provision of chemical inhibitors (Pakpour et al., 2012; Surachetpong et al., 2009). Virus-based expression has also been utilized with some success (de Lara Capurro et al., 2000). Although these techniques have been essential to ongoing progress in vector molecular biology, they each possess significant pitfalls. For example, large scale screens of chemical inhibitors against over 400 human kinases indicate that significant care must be taken to optimize inhibitor dose to minimize toxicity and off-target effects (Davis et al., 2011; Karaman et al., 2008). Further, studies using microinjection-based overexpression of gene or hairpin RNA-encoding sequences may result in higher mortality rates when compared to feeding based methods (Walshe et al., 2009) and may require multiple injections or rearing of transformed larvae to obtain adults with the desired genetic modification (Beumer et al., 2008; Peng et al., 2011). Efficient gene knockdown has been achieved through feeding of dsRNA in a variety of insects (Huvenne & Smagghe, 2010), including disease vectors such as the tsetse fly Glossina morsitans (Walshe et al., 2009), the triatomine bug Rhodnius prolixus (Araujo et al., 2006), and the deer tick Ixodes scapularis (Soares et al., 2005). Feeding of dsRNA to Anopheles mosquito larvae also yielded systemic target knockdown (Zhang et al., 2010). However, orally delivered dsRNA elicits a lower level of target knockdown when compared to injection in the tsetse fly (Walshe et al., 2009) and may be subject to degradation in the gut (Luo et al., 2013), suggesting that rapid methods for gene knockdown via feeding can be improved.
Anti-sense morpholino (MO) technology is an established method for gene knockdown that provides several key advantages over the aforementioned techniques (Heasman, 2012), including lower costs of materials and production (Summerton & Weller, 1997). Anti-sense MOs are small synthetic oligonucleotides, chemically modified to contain morpholine rings in place of a deoxyribose backbone for increased stability and can be conjugated to a cell-permeating moiety for in vivo uptake. MO oligomers reduce target protein levels by binding target transcript at the 5-prime untranslated region to prevent the initiation of translation (Summerton & Weller, 1997). Further, MOs are highly target specific due to their RNAse H-independent mechanism of action and inability to form small, transient RNA duplexes (Summerton, 2007). Previously, MOs have been used in a variety of vertebrate and invertebrate organisms to study gene function, though the method of delivery has been largely restricted to microinjection (Layden et al., 2013; McMahon et al., 2010; Melvin et al., 2013) or electroporation (Peng et al., 2012). However, MOs have also shown high bioavailability and efficiency for target knockdown when administered orally to adult rats (Arora et al., 2002).
The goal of this study, therefore, was to determine whether oral delivery of anti-sense MOs is a viable alternative for gene knockdown in mosquitoes using Anopheles stephensi as a model. In this study, we assayed the efficiency of inhibiting the mitogen-activated protein kinase (MAPK) MEK-ERK signaling pathway, a known regulator of immunity in the A. stephensi midgut (Surachetpong et al., 2009), via saline meal delivery of an anti-sense MO against MEK.
RESULTS
Efficiency of morpholino delivery
Female A. stephensi mosquitoes showed no significant aversion to feeding on a meal containing AsMEK-MO relative to feeding on a saline meal control. Of mosquitoes provided with a meal of saline alone, an average of 92% completely engorged within 30 minutes, while of mosquitoes provided a meal of 10μM AsMEK-MO, an average of 87.8% fed to completion within the same timeframe (Fig. 1A). Additionally, ingestion of AsMEK-MO had no significant effect on short-term mosquito survival relative to ingestion of the saline meal control. Of mosquitoes initially provided a control meal, an average of 93.9% survived to day 3 post-feeding, while of mosquitoes initially fed 10μM AsMEK-MO, 93.8% survived to the same time point (Fig. 1B).
Figure 1. Provision of AsMEK-MO in saline does not affect A. stephensi engorgement success or survivorship.
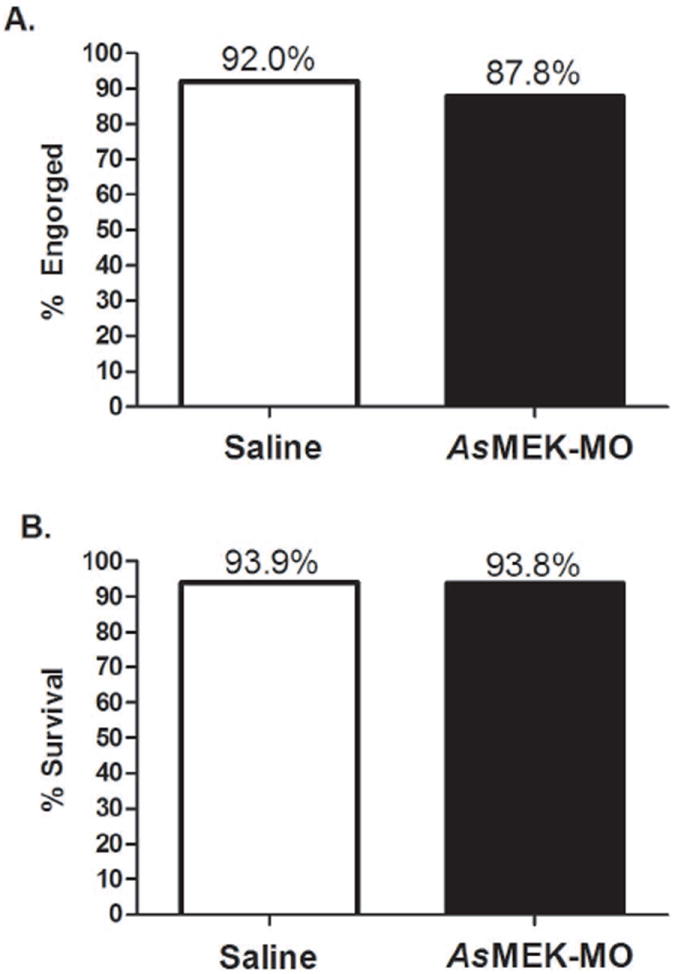
Mosquitoes were provided with a saline meal (white bar) or saline with AsMEK-MO (black bar) and monitored for (A) 30 minutes to determine engorgement success or (B) 3 days to assess survivorship. Bars represent the mean of three biological replicates comprised of 40-50 mosquitoes per saline and AsMEK-MO groups. Data were analyzed by goodness of fit (chi-square) test (p>0.1).
Knockdown of total MEK levels in the midgut
Changes in midgut total MEK protein levels following feeding of AsMEK-MO were observed in replicated assays (Fig. 2). As early as 2 days post-feeding, a modest (22%) reduction in total MEK was noted, although this was not significantly different from levels in saline-fed controls (p > 0.1). However, by day 3 post-feeding, total MEK levels were reduced by 59% relative to control (Fig. 2; p = 0.06); this knockdown was the largest achieved during the time course. MEK knockdown persisted through day 4 post-feeding, with a reduction of 43% relative to control (p = 0.03). By days 5 and 6 post-feeding, MEK levels were increased relative to control by 61% and 19%, respectively, although neither increase was different from control levels (p > 0.1) at the same time points.
Figure 2. Provision of As MEK-MO by feeding reduces total MEK levels in the midgut of A. stephensi on days 3-4 post-treatment relative to control.
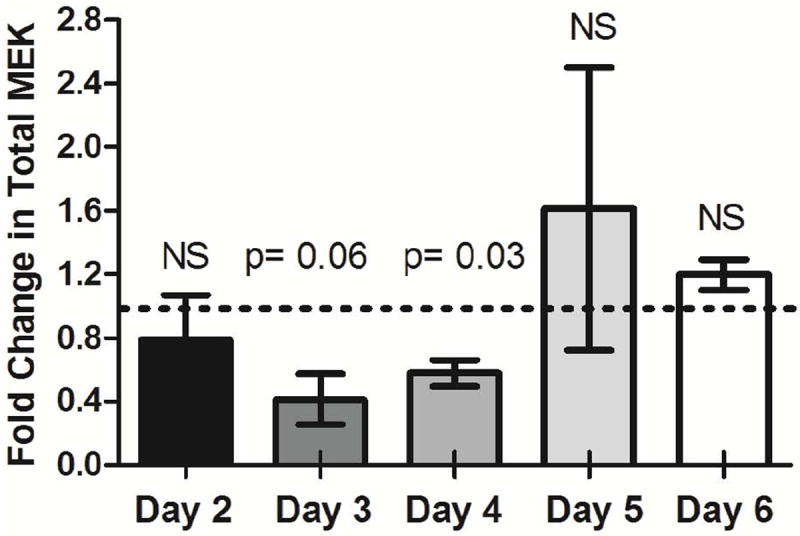
Mosquitoes were provided with a saline meal or saline with AsMEK-MO on day 0 and midgut tissues were dissected on days 2-6 post-feeding. Electrophoretically separated midgut proteins were probed for total MEK via western blot to determine knockdown efficiency relative to controls. Data are represented as mean fold inductions ± SEMs relative to day-matched controls (set at 1, dotted line) for three biological replicates of 12-15 midguts per timepoint. Densitometry data were normalized to total MEK in controls as well as to a GAPDH loading control and analyzed by Wilcoxon signed-rank test. NS = not significant, p>0.1.
Knockdown of downstream ERK signaling
To determine the functional effects of MEK knockdown, changes in phosphorylation of ERK, a MEK-dependent kinase, were quantified in response to uninfected blood meals (Fig. 3A) and to P. falciparum-infected blood meals (Fig. 3B). For these experiments, mosquitoes were fed either saline or AsMEK-MO in saline 3 days prior to blood-feeding; ERK phosphorylation in AsMEK-MO-fed mosquitoes was normalized to levels in saline-fed controls (white column, Fig. 3A; dotted line, Fig. 3B). At 1 hour following an uninfected blood meal, phospho-ERK levels in mosquitoes previously fed 10μM or 50μM AsMEK-MO were reduced relative to mosquitoes previously fed saline (p < 0.001; Fig. 3A). Inhibition of ERK phosphorylation was not dependent on AsMEK-MO concentration, as provision of 10μM and 50μM AsMEK-MO prior to blood-feeding resulted in 40% and 49% reductions, respectively, in ERK phosphorylation (Fig. 3A). Analyses of the effect of 10μM AsMEK-MO on ERK phosphorylation in the context of a P. falciparum-infected blood meal provided 3 days post-AsMEK-MO feeding included a 1, 6, and 24 hour post-infection timepoints. Midgut ERK phosphorylation levels at 1 hour post-infection in AsMEK-MO-fed and saline-fed controls were not different (p >0.1; Fig. 3B). At 6 hours post-infection, midgut phospho-ERK levels in AsMEK-MO-fed mosquitoes were reduced by 52% relative to levels in saline-fed controls (p = 0.09; Fig. 3B). By 24 hours post-infection, phospho-ERK levels in AsMEK-MO fed mosquitoes trended towards increased ERK phosphorylation, although these levels were not different from levels in mosquitoes previously fed saline (p > 0.1; Fig. 3B).
Figure 3. Provision of AsMEK-MO by feeding inhibits blood- and P. falciparum-induced phosphorylation of the downstream kinase ERK in the midgut for up to 6 hours relative to control.
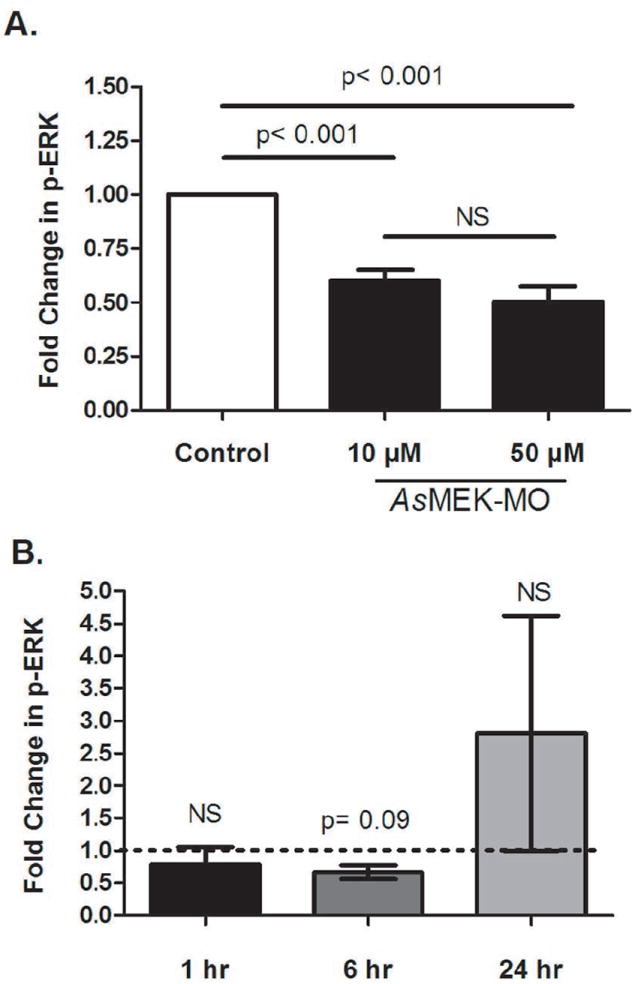
Female A. stephensi were provided a control saline meal or saline with AsMEK-MO (10μM and 50μM in A; 10μM in B) meal on day 0. On day 3, control and AsMEK-MO-treated mosquitoes were given a meal of either (A) uninfected red blood cells or (B) P. falciparum-infected red blood cells. Midguts were dissected at 1, 6, or 24 hours post-feeding and proteins were probed for phospho-ERK via western blot. Bars represent the mean fold changes ± SEMs relative to time-matched controls (set at 1, dotted line in B) for four (A) and three (B) biological replicates of 12-15 midguts. Densitometry data were normalized to ERK phosphorylation in controls as well as to a GAPDH loading control and analyzed ANOVA for overall significance followed by Tukey’s multiple comparison post-test for all pairwise comparisons of means from significant data sets (A) or Wilcoxon signed-rank test (B). NS = not significant, p>0.1.
Effects of MEK knockdown on P. falciparum infectivity
AsMEK-MO-mediated knockdown of MEK, a known regulator of malaria parasite development in the midgut of female A. stephensi (Surachetpong et al., 2009), reduced P. falciparum infectivity. Specifically, 65% of mosquitoes fed AsMEK-MO developed an infection (at least one oocyst per dissected midgut), whereas 75% of mosquitoes fed control-MO were infected (p = 0.08; Fig. 4A). In addition to a reduced prevalence of infection, treatment with AsMEK-MO was associated with a reduced intensity of infection in A. stephensi. Specifically, the mean intensity of infection in AsMEK-MO-fed A. stephensi was 2.37 oocysts per midgut compared to a mean of 3.19 oocysts per midgut in control-MO-fed mosquitoes (p = 0.04; Fig. 4B). This reduction was consistent with previous studies of chemical inhibition of MEK with PD98059. In particular, infection was reduced in two biological replicates by PD98059 treatment of A. stephensi from a mean of 4.75 oocysts per midgut in controls to mean of 0.20 oocysts per midgut and from a mean of 8.14 oocysts per midgut in controls to 4.56 oocysts per midgut (Surachetpong et al., 2009).
Figure 4. Provision of AsMEK-MO reduces P. falciparum infection in A. stephensi relative to control.
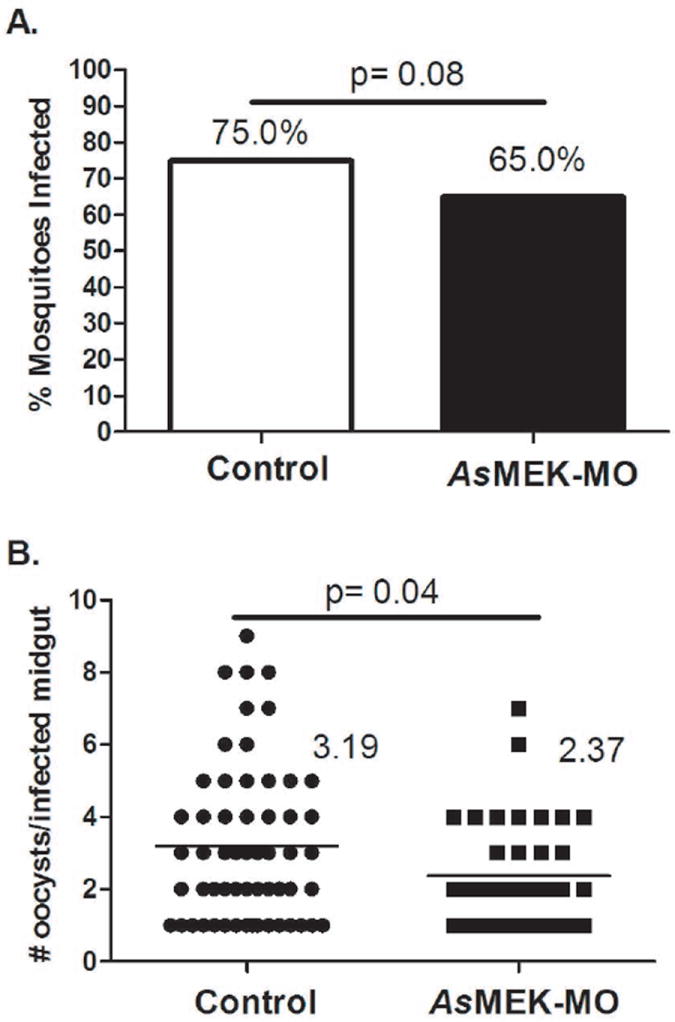
Female A. stephensi were fed AsMEK-MO or control-MO in saline on day 0 and infected with P. falciparum on day 3 post-MO feeding. On day 10, midguts were dissected and stained with mercurochrome to determine (A) infection prevalence (percentage of mosquitoes infected with at least one oocyst) and (B) infection intensity (average number of oocysts per infected midgut, indicated numerically to the right of the data). Data are represented as means for four biological replicates comprised of 20-30 mosquitoes per treatment group. Infection prevalence and intensity data were analyzed using goodness of fit (chi-square) and two-sample t-tests, respectively.
DISCUSSION
In the present study, we validated the use of anti-sense MOs for knockdown in A. stephensi using the MEK-ERK signaling pathway, which we had previously implicated in the control of P. falciparum development in this mosquito host (Surachetpong et al., 2009). To avoid potential confounding effects of blood digestion, we delivered MOs in a saline meal using ATP as a feeding stimulant. The feeding rates of mosquitoes provided MO in saline (Fig. 1A) were comparable to rates observed for mosquitoes fed on blood (Andreasen et al., 2004; unpublished data). Further, rates of mosquito survival following ingestion of MOs in saline (Fig. 1B) were comparable to those observed after dsRNA feeding (Walshe et al., 2009) and substantially higher than in studies using microinjection (Beumer et al., 2008; Peng et al., 2011). Together, these data demonstrate the suitability of direct feeding as a delivery method for anti-sense MOs.
Current gene knockdown strategies are based on small interfering RNA (siRNA) and plasmid microinjection. Overexpression of hairpin RNAs or zinc finger nucleases can provide stable, long-lasting knockdown; however, these strategies often require 1-2 generations of post-injection rearing to achieve significant knockdown of the target protein (Beumer et al., 2008; Peng et al., 2011). Feeding or injection of dsRNA typically requires 24-48 hours for maximal effect, but may only last for a period of several days (Araujo et al., 2006; Welshe et al., 2009). In comparison, knockdown of target protein levels in the A. stephensi midgut was first observed 3 days after feeding of AsMEK-MO, with the effect lasting through 4 days post-MO feeding (Fig. 2). Therefore, the use of MOs would be advisable when a strong and rapid, yet transient (2-3 days) effect is desired.
Interestingly, on days 5-6 post-AsMEK-MO feeding, total MEK levels in the midgut appeared to increase above control levels. Chemical inhibition of MEK is known to increase its phosphorylation, likely due to a decrease in negative feedback from activated ERK to upstream signaling proteins such as Son of Sevenless (SOS) and Rat Sarcoma (Ras) (Dougherty et al., 2005). MEK inhibition has also been shown to increase ERK gene expression (Gioeli et al., 2011). Thus, this pathway appears to be controlled by a complex balance of both positive and negative feedback signals that may account for increased MEK levels observed following prolonged knockdown by AsMEK-MO. The possibility of feedback regulation should be taken into consideration during MO assay design. For example, it may be possible to prolong knockdown with multiple, sequential MO treatments to block compensatory effects of knockdown as the MO effect declines over time.
In order to determine the effects of AsMEK-MO-mediated MEK knockdown on downstream signaling, we examined phosphorylation of ERK, the downstream target of MEK, following blood-feeding (Fig. 3). Ingestion of blood containing mammalian cytokines and growth factors (e.g., transforming growth factor-β1, insulin) or P. falciparum signaling factors (e.g., hemozoin, glycosylphosphatadylinositols) is known to activate MEK-ERK signaling in the A. stephensi midgut (Akman-Anderson et al., 2007; Lim et al., 2005; Pakpour et al., 2012; Surachetpong et al., 2009; Surachetpong et al., 2011). In our studies, when mosquitoes were provided AsMEK-MO 3 days prior to feeding on uninfected human RBCs, ERK phosphorylation was reduced by 40-49% relative to controls (Fig. 3). Intriguingly, AsMEK-MO-fed mosquitoes subsequently provided a P. falciparum-infected blood meal also showed a decrease in ERK signaling compared to controls (Fig. 3B), but signaling knockdown was delayed, perhaps due to alternative parasite stimuli that can also function to activate ERK signaling (Akman-Anderson et al., 2007; Lim et al., 2005). Although it may be difficult to achieve total knockdown of target protein levels, the level of knockdown we observed was comparable to that observed with RNAi in mosquitoes and other insects (Araujo et al., 2006; Gulia-Nuss et al., 2011; Lamacchia et al., 2013; Soares et al., 2005; Walshe et al., 2009) and impacted both signaling and parasite infection. These data suggest that because MOs directly inhibit mRNA translation, a lower level of efficiency may be sufficient to produce a biological effect when compared to RNAi-mediated knockdown of transcript levels. For example, Diekmann et al. (2009) reported that injection of brain-derived neurotrophic factor (BDNF)-MO produced only a 50% knockdown in BDNF protein levels in zebrafish, but this knockdown produced a more than 6-fold increase in caspase-3 activity that was associated with significant effects on zebrafish brain development. Another advantage to this methodology is that MOs can be combined with the use of small molecule inhibitors to target kinase enzymatic activity within the same pathway (Surachetpong et al., 2009).
Based on these observations, MO-mediated knockdown showed expected phenotypic effects. In particular, treatment with AsMEK-MO phenocopied chemical inhibition of MEK – that is, treatment with MO or with small molecule inhibitors (Surachetpong et al., 2009) reduced the intensity of P. falciparum infection to a similar degree (Fig. 4). These data support a highly relevant application of our protocol and prove its usefulness in studies of complex mechanisms such as innate immunity of insect-pathogen interactions.
CONCLUSIONS
The feeding protocol presented here provides several advantages over current methods for gene knockdown in insects. The method is easy to apply, requires no special skills for delivery, and is highly target specific. Provision of AsMEK-MO via feeding reduced MEK-ERK signaling by more than 50% relative to control treatment for a period of days with no notable toxicity. Furthermore, AsMEK-MO-mediated inhibition of the MEK-ERK pathway, a known regulator of anti-parasite immunity in A. stephensi, produced the expected decrease in P. falciparum infectivity in the mosquito (Surachetpong et al., 2009). Therefore, this protocol should be considered as an efficient alternative in studies requiring target-specific protein knockdown in mosquitoes and other arthropods.
EXPERIMENTAL PROCEDURES
Rearing and care of mosquitoes
The Indian wild type strain of A. stephensi was maintained in environmental chambers at 27°C and 80% relative humidity with a L12:D12 cycle. Mice were used as a blood source for colony maintenance in compliance with federal and institutional guidelines for Animal Use and Care. Larvae were provided with a 2% solution of 2:1 Sera Micron® powdered fish-fry food (Sera North America, Montgomeryville, PA) through day 4 post-hatching and then were reared on high protein, low fat Game Fish Chow pellets (Purina Mills, St. Louis, MO) until pupation. Emerged adults were maintained on cotton pads soaked in a 10% sucrose solution.
Preparation of morpholino solutions
Anti-AsMEK (5’-TTACAAGAAATGTGTCCTTGGGTGT-3’, Supplementary Fig.1) vivo-MO (AsMEK-MO; GeneTools LLC, Philomath, OR) at a stock concentration of 500 μM was diluted to 1:50 (10μM) or 1:10 (50μM) in saline (15 mmol l-1 NaCl, 10 mmol l-1 NaHCO3, pH 7.0). ATP (Sigma Aldrich, St. Louis, MO) was added to a final concentration 0.33mmol l-1as a feeding stimulant. Immediately prior to feeding, MO solutions were warmed in a water bath at 37°C for 10 minutes. Standard MO control (5-CCTCTTACCTCAGTTACAATTTATA-3) targeting a human beta-globin intron mutation (control-MO; GeneTools LLC) in saline at equal concentrations was used as a matched control meal for infection experiments while meals of saline were used as controls in all other assays (Supplementary Fig. 2).
Feeding of morpholino solutions
Four separate cohorts of newly emerged female A. stephensi were provided with water only (no sucrose) for 24 hours and then provided a meal of either control-MO or AsMEK-MO in saline via a Hemotek circulation system (Discovery Workshops, Accrington, UK; n=50-60 mosquitoes per control and treatment groups). After 30 minutes, any unfed mosquitoes were counted and discarded from experimental cohorts. Mosquitoes were maintained on cotton pads soaked in 10% sucrose through day 3 post-feeding and any dead mosquitoes were counted daily and removed.
Mosquito blood-feeding
Female A. stephensi previously fed MO meals on day 0 were starved starting on day 2 for 24 hours and then provided with a meal of washed human red blood cells (RBCs) in saline via a Hemotek circulation system (Discovery Workshops) on day 3. Mosquitoes were allowed to feed for 30 minutes and were then maintained on cotton pads soaked in 10% sucrose.
Plasmodium falciparum culture and A. stephensi infections
The NF54 strain of P. falciparum was initiated at 1% parasitemia in 10% heat-inactivated human serum and 6% washed RBCs in RPMI 1640 with HEPES (Gibco/Invitrogen, Carlsbad, CA) and hypoxanthine. At days 15-17, stage V gametocytes were evident and exflagellation was evaluated the day before and day of feeding by observation of blood smears before addition of fresh media at 200X magnification with phase-contrast or modified brightfield microscopy. Female A. stephensi previously fed MO meals on day 0 were starved starting on day 2 for 24 hours then provided an infected blood meal on day 3 and maintained on cotton pads soaked in 10% sucrose until day 10 post infection. To quantify infection levels, midguts were dissected on day 10 post-infection and stained with 1% mercurochrome for visualization of P. falciparum oocysts by microscopy. Infections were replicated with four separate cohorts and 20-30 mosquitoes per control and treatment groups.
Western blot analyses
Protein extracts for western blotting were prepared by collecting A. stephensi midguts in cell extraction buffer (Invitrogen, Carlsbad, CA). In brief, midguts were dissected into phosphate-buffered saline (PBS; Cellgro, Manassas, VA) with protease inhibitor (Sigma Aldrich), centrifuged at 10,000 g for 5 minutes, re-suspended in 200μL cell lysis buffer for 1 hour and vortexed periodically. Cell lysates were cleared at 16,000 g for 10 min and protein supernatants were mixed with sample loading buffer (125 mM Tris-HCl pH 6.8, 10% glycerol, 10% SDS,0.006% bromophenol blue, 130 mM dithiothreitol) and boiled for 5 minutes. Proteins were separated on 10% SDS-PAGE polyacrylamide gels and transferred to nitrocellulose membranes (BioRad, Hercules, CA). Membranes were blocked in 5% nonfat dry milk in Tris-buffered saline (TBS; pH 7.0) containing 0.1% Triton-100 (TBS-T) for 1 hour at room temperature. For total MEK detection, membranes were incubated at 4°C overnight with a 1:5,000 dilution of rabbit anti-MEK1/2 antibody (Cell Signaling, Danvers, MA) in 5% nonfat dry milk TBS-T. Membranes were washed three times for 5 minutes in TBS-T and incubated overnight with a 1:10000 dilution of HRP-conjugated goat anti-rabbit antibody. Detection of phospho-ERK and GAPDH followed the same protocol with a 1:10,000 dilution of mouse anti-phospho-ERK antibody (Cell Signaling) and 1:20,000 dilution of HRP-conjugated rabbit anti-mouse antibody or 1:10,000 dilution of rabbit anti-GAPDH and 1:20,000 dilution of HRP-conjugated goat anti-rabbit antibody, respectively. Proteins were visualized by incubating membranes with SuperSignal West Dura chemiluminescent reagent (Pierce, Rockford, IL) for 3 minutes and exposing on an Image Station 4000 Pro digital imager (Kodak, Rochester, NY) for 1-5 minutes. Analyses of total MEK levels (Fig. 2) were replicated three times with pooled samples of 12-15 midguts per replicate, while analyses of ERK phosphorylation were replicated three times (Fig. 3A) or four times (Fig. 3B) with pooled samples of 12-15 midguts per replicate. Representative western blots are shown for each experiment (Supplementary Fig. 3, Supplementary Fig. 4)
Data analysis and statistics
Densitometry of western blots was performed using the ImageJ (http://rsbweb.nih.gov/ij/) gel analysis tool. Densitometry data were normalized to appropriate control groups as well as a GAPDH loading control and analyzed by either Wilcoxon signed-ranked test or ANOVA for overall significance followed by Tukey’s multiple comparison post-test for all pairwise comparisons of means from significant data sets (GraphPad Prism version 5.02). Infection prevalence and intensity data for four replicates were combined (control intensities did not differ among replicates; ANOVA, p>0.1) and analyzed using goodness of fit (chi-square) and two-sample t-tests, respectively. Feeding and survival data were analyzed by goodness of fit (chi-square) test. For all analyses, confidence levels greater than 90% (p < 0.1) are reported.
Supplementary Material
Supplementary Figure 1. AsMEK-MO binds to the five-prime untranslated region of AsMEK. AsMEK-MO was designed to bind to a 25 base pair region (bolded and underlined) upstream of the translation start site (ATG, in parentheses). Untranslated sequence of the gene is underlined while the catalytic kinase domain is shown bolded in brackets.
Supplementary Figure 2. Provision of control-MO has no effect on total MEK levels in A. stephensi. Mosquitoes were provided a saline meal or control-MO in saline on day 0 and midguts were dissected on days 3-5 post-feeding. Midgut proteins were probed for total MEK via western blot to establish that control-MO feeding relative to saline alone had no effect on total MEK levels. Bars represent the mean fold changes ± SEMs relative to day-matched controls (set at 1, dotted line) from three biological replicates of 12-15 midguts per timepoint. NS= not significant, p>0.1.
Supplementary Figure 3. Representative western blots of total MEK. Densitometry of western blots from (A) Figure 2 and (B) Supplementary Figure 2 was performed. Data were normalized to appropriate control groups (set at 1.0) as well as a GAPDH loading control and normalized values are reported below blots. Blots are representative of three independently replicated experiments. For (A): MO= AsMEK-MO, C= control. At day 2, total MEK was not detectable in MO-treated A. stephensi (dash). For (B): S= saline, cMO= control-MO.
Supplementary Figure 4. Representative western blots of phospho-ERK. Densitometry of western blots from (A) Figure 3A and (B) Figure 3B was performed. Data were normalized to appropriate control groups (set at 1.0) as well as a GAPDH loading control and normalized values are reported below blots. Blots are representative of three (B) or four (A) independently replicated experiments. For (B): C= control, MO= AsMEK-MO.
Acknowledgments
We thank Molly Mulleague for assistance with P. falciparum culture and mosquito infection and Dr. Nazzy Pakpour for critical review and editing of the manuscript. Funding for these studies was provided by the United States National Institutes of Health National Institute of Allergy and Infectious Diseases (NIH NIAID) grants AI073745 and AI080799 as well as an F31-NRSA AI0058901 to JEP.
Footnotes
The authors declare no conflicts of interest.
Contributor Information
Jose E. Pietri, Email: jepietri@ucdavis.edu.
Kong W. Cheung, Email: kcheung@ucdavis.edu.
References
- Akman-Anderson L, Olivier M, Luckhart S. Induction of nitric oxide synthase and activation of signaling proteins in Anopheles mosquitoes by the malaria pigment, hemozoin. Infect Immun. 2007;75:4012–4019. doi: 10.1128/IAI.00645-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen MH, Birtles A, Curtis CF, Wood RJ. Enhanced blood feeding of Anopheles mosquitoes (Diptera: Culicidae) through membranes with applied host odour. Bull Entomol Res. 2004;94:291–295. doi: 10.1079/ber2004295. [DOI] [PubMed] [Google Scholar]
- Araujo RN, Santos A, Pinto FS, Gontijo NF, Lehane MJ, Pereira MH. RNA interference of the salivary gland nitrophorin 2 in the triatomine bug Rhodnius prolixus (Hemiptera: Reduviidae) by dsRNA ingestion or injection. Insect Biochem Mol Biol. 2006;36:683–693. doi: 10.1016/j.ibmb.2006.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora V, Knapp DC, Reddy MT, Weller DD, Iversen PL. Bioavailability and efficacy of antisense morpholino oligomers targeted to c-myc and cytochrome P-450 3A2 following oral administration in rats. J Pharm Sci. 2002;91:1009–1018. doi: 10.1002/jps.10088. [DOI] [PubMed] [Google Scholar]
- Barclay VC, Smith RA, Findeis JL. Surveillance considerations for malaria elimination. Malar J. 2012;11:304. doi: 10.1186/1475-2875-11-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beumer KJ, Trautman JK, Bozas A, Liu JL, Rutter J, Gall JG, et al. Efficient gene targeting in Drosophila by direct embryo injection with zinc-finger nucleases. Proc Natl Acad Sci U S A. 2008;105:19821–19826. doi: 10.1073/pnas.0810475105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkett AJ, Moorthy VS, Loucq C, Chitnis CE, Kaslow DC. Malaria vaccine R&D in the Decade of Vaccines: breakthroughs, challenges and opportunities. Vaccine. 2013;31(Suppl 2):B233–243. doi: 10.1016/j.vaccine.2013.02.040. [DOI] [PubMed] [Google Scholar]
- Boisson B, Jacques JC, Choumet V, Martin E, Xu J, Vernick K, et al. Gene silencing in mosquito salivary glands by RNAi. FEBS Lett. 2006;580:1988–1992. doi: 10.1016/j.febslet.2006.02.069. [DOI] [PubMed] [Google Scholar]
- Corby-Harris V, Drexler A, Watkins de Jong L, Antonova Y, Pakpour N, Ziegler R, et al. Activation of Akt signaling reduces the prevalence and intensity of malaria parasite infection and lifespan in Anopheles stephensi mosquitoes. PLoS Pathog. 2010;6:e1001003. doi: 10.1371/journal.ppat.1001003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MI, Hunt JP, Herrgard S, Ciceri P, Wodicka LM, Pallares G, et al. Comprehensive analysis of kinase inhibitor selectivity. Nat Biotechnol. 2011;29:1046–1051. doi: 10.1038/nbt.1990. [DOI] [PubMed] [Google Scholar]
- de Lara Capurro M, Coleman J, Beerntsen BT, Myles KM, Olson KE, Rocha E, et al. Virus-expressed, recombinant single-chain antibody blocks sporozoite infection of salivary glands in Plasmodium gallinaceum-infected Aedes aegypti. Am J Trop Med Hyg. 2000;62:427–433. doi: 10.4269/ajtmh.2000.62.427. [DOI] [PubMed] [Google Scholar]
- Diekmann H, Anichtchik O, Fleming A, Futter M, Goldsmith P, Roach A, et al. Decreased BDNF levels are a major contributor to the embryonic phenotype of huntingtin knockdown zebrafish. J Neurosci. 2009;29:1343–1349. doi: 10.1523/JNEUROSCI.6039-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty MK, Muller J, Ritt DA, Zhou M, Zhou XZ, Copeland TD, et al. Regulation of Raf-1 by direct feedback phosphorylation. Mol Cell. 2005;17:215–224. doi: 10.1016/j.molcel.2004.11.055. [DOI] [PubMed] [Google Scholar]
- Franz AW, Sanchez-Vargas I, Adelman ZN, Blair CD, Beaty BJ, James AA, et al. Engineering RNA interference-based resistance to dengue virus type 2 in genetically modified Aedes aegypti. Proc Natl Acad Sci U S A. 2006;103:4198–4203. doi: 10.1073/pnas.0600479103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs S, Nolan T, Crisanti A. Mosquito transgenic technologies to reduce Plasmodium transmission. Methods Mol Biol. 2013;923:601–622. doi: 10.1007/978-1-62703-026-7_41. [DOI] [PubMed] [Google Scholar]
- Gioeli D, Wunderlich W, Sebolt-Leopold J, Bekiranov S, Wulfkuhle JD, Petricoin EF, 3rd, et al. Compensatory pathways induced by MEK inhibition are effective drug targets for combination therapy against castration-resistant prostate cancer. Mol Cancer Ther. 2011;10:1581–1590. doi: 10.1158/1535-7163.MCT-10-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulia-Nuss M, Robertson AE, Brown MR, Strand MR. Insulin-like peptides and the target of rapamycin pathway coordinately regulate blood digestion and egg maturation in the mosquito Aedes aegypti. PLoS One. 2011;6:e20401. doi: 10.1371/journal.pone.0020401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauck ES, Antonova-Koch Y, Drexler A, Pietri J, Pakpour N, Liu D, et al. Overexpression of phosphatase and tensin homolog improves fitness and decreases Plasmodium falciparum development in Anopheles stephensi. Microbes Infect. 2013;15(12):775–787. doi: 10.1016/j.micinf.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heasman J. Morpholino oligos: making sense of antisense? Dev Biol. 2002;243:209–214. doi: 10.1006/dbio.2001.0565. [DOI] [PubMed] [Google Scholar]
- Huvenne H, Smagghe G. Mechanisms of dsRNA uptake in insects and potential of RNAi for pest control: a review. J Insect Physiol. 2010;56:227–235. doi: 10.1016/j.jinsphys.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Isaacs AT, Jasinskiene N, Tretiakov M, Thiery I, Zettor A, Bourgouin C, et al. Transgenic Anopheles stephensi coexpressing single-chain antibodies resist Plasmodium falciparum development. Proc Natl Acad Sci U S A. 2012;109:E1922–1930. doi: 10.1073/pnas.1207738109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaman MW, Herrgard S, Treiber DK, Gallant P, Atteridge CE, Campbell BT, et al. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008;26:127–132. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- Kim W, Koo H, Richman AM, Seeley D, Vizioli J, Klocko AD, et al. Ectopic expression of a cecropin transgene in the human malaria vector mosquito Anopheles gambiae (Diptera: Culicidae): effects on susceptibility to Plasmodium. J Med Entomol. 2004;41:447–455. doi: 10.1603/0022-2585-41.3.447. [DOI] [PubMed] [Google Scholar]
- Klein EY. Antimalarial drug resistance: a review of the biology and strategies to delay emergence and spread. Int J Antimicrob Agents. 2013;41:311–317. doi: 10.1016/j.ijantimicag.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokoza V, Ahmed A, Cho WL, Jasinskiene N, James AA, Raikhel A. Engineering blood meal-activated systemic immunity in the yellow fever mosquito, Aedes aegypti. Proc Natl Acad Sci U S A. 2000;97:9144–9149. doi: 10.1073/pnas.160258197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamacchia M, Clayton JR, Wang-Sattler R, Steinmetz LM, Levashina EA, Blandin SA. Silencing of genes and alleles by RNAi in Anopheles gambiae. Methods Mol Biol. 2013;923:161–176. doi: 10.1007/978-1-62703-026-7_11. [DOI] [PubMed] [Google Scholar]
- Layden MJ, Rottinger E, Wolenski FS, Gilmore TD, Martindale MQ. Microinjection of mRNA or morpholinos for reverse genetic analysis in the starlet sea anemone, Nematostella vectensis. Nat Protoc. 2013;8:924–934. doi: 10.1038/nprot.2013.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Gowda DC, Krishnegowda G, Luckhart S. Induction of nitric oxide synthase in Anopheles stephensi by Plasmodium falciparum: mechanism of signaling and the role of parasite glycosylphosphatidylinositols. Infect Immun. 2005;73:2778–2789. doi: 10.1128/IAI.73.5.2778-2789.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Wang X, Wang X, Yu D, Chen B, Kang L. Differential responses of migratory locusts to systemic RNA interference via double-stranded RNA injection and feeding. Insect Mol Biol. 2013;22:574–583. doi: 10.1111/imb.12046. [DOI] [PubMed] [Google Scholar]
- Marshall JM, Taylor CE. Malaria control with transgenic mosquitoes. PLoS Med. 2009;6:e20. doi: 10.1371/journal.pmed.1000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur G, Sanchez-Vargas I, Alvarez D, Olson KE, Marinotti O, James AA. Transgene-mediated suppression of dengue viruses in the salivary glands of the yellow fever mosquito, Aedes aegypti. Insect Mol Biol. 2010;19:753–763. doi: 10.1111/j.1365-2583.2010.01032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon A, Reeves GT, Supatto W, Stathopoulos A. Mesoderm migration in Drosophila is a multi-step process requiring FGF signaling and integrin activity. Development. 2010;137:2167–2175. doi: 10.1242/dev.051573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melvin VS, Feng W, Hernandez-Lagunas L, Artinger KB, Williams T. A morpholino-based screen to identify novel genes involved in craniofacial morphogenesis. Dev Dyn. 2013;242:817–831. doi: 10.1002/dvdy.23969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noedl H. The need for new antimalarial drugs less prone to resistance. Curr Pharm Des. 2013;19:266–269. [PubMed] [Google Scholar]
- Pakpour N, Corby-Harris V, Green GP, Smithers HM, Cheung KW, Riehle MA, et al. Ingested human insulin inhibits the mosquito NF-kappaB-dependent immune response to Plasmodium falciparum. Infect Immun. 2012;80:2141–2149. doi: 10.1128/IAI.00024-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H, Wu Y, Zhang Y. Efficient delivery of DNA and morpholinos into mouse preimplantation embryos by electroporation. PLoS One. 2012;7:e43748. doi: 10.1371/journal.pone.0043748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng R, Maklokova VI, Chandrashekhar JH, Lan Q. In vivo functional genomic studies of sterol carrier protein-2 gene in the yellow fever mosquito. PLoS One. 2011;6:e18030. doi: 10.1371/journal.pone.0018030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares CG, Lima CR, Dolan MC, Piesman J, Beard CB, Zeidner NS. Capillary feeding of specific dsRNA induces silencing of the isac gene in nymphal Ixodes scapularis ticks. Insect Mol Biol. 2005;14:443–452. doi: 10.1111/j.1365-2583.2005.00575.x. [DOI] [PubMed] [Google Scholar]
- Summerton J, Weller D. Morpholino antisense oligomers: design, preparation, and properties. Antisense Nucleic Acid Drug Dev. 1997;7:187–195. doi: 10.1089/oli.1.1997.7.187. [DOI] [PubMed] [Google Scholar]
- Summerton J. Morpholino, siRNA, and S-DNA compared: impact of structure and mechanism of action on off-target effects and sequence specificity. Curr Top Med Chem. 2007;7:651–650. doi: 10.2174/156802607780487740. [DOI] [PubMed] [Google Scholar]
- Surachetpong W, Pakpour N, Cheung KW, Luckhart S. Reactive oxygen species-dependent cell signaling regulates the mosquito immune response to Plasmodium falciparum. Antioxid Redox Signal. 2011;14:943–955. doi: 10.1089/ars.2010.3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surachetpong W, Singh N, Cheung KW, Luckhart S. MAPK ERK signaling regulates the TGF-beta1-dependent mosquito response to Plasmodium falciparum. PLoS Pathog. 2009;5:e1000366. doi: 10.1371/journal.ppat.1000366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travanty EA, Adelman ZN, Franz AW, Keene KM, Beaty BJ, Blair CD, et al. Using RNA interference to develop dengue virus resistance in genetically modified Aedes aegypti. Insect Biochem Mol Biol. 2004;34:607–613. doi: 10.1016/j.ibmb.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Walshe DP, Lehane SM, Lehane MJ, Haines LR. Prolonged gene knockdown in the tsetse fly Glossina by feeding double stranded RNA. Insect Mol Biol. 2009;18:11–19. doi: 10.1111/j.1365-2583.2008.00839.x. [DOI] [PubMed] [Google Scholar]
- World Health Organization. World Malaria Report 2011 [Google Scholar]
- Zhang X, Zhang J, Zhu KY. Chitosan/double-stranded RNA nanoparticle-mediated RNA interference to silence chitin synthase genes through larval feeding in the African malaria mosquito (Anopheles gambiae) Insect Mol Biol. 2010;19:683–693. doi: 10.1111/j.1365-2583.2010.01029.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. AsMEK-MO binds to the five-prime untranslated region of AsMEK. AsMEK-MO was designed to bind to a 25 base pair region (bolded and underlined) upstream of the translation start site (ATG, in parentheses). Untranslated sequence of the gene is underlined while the catalytic kinase domain is shown bolded in brackets.
Supplementary Figure 2. Provision of control-MO has no effect on total MEK levels in A. stephensi. Mosquitoes were provided a saline meal or control-MO in saline on day 0 and midguts were dissected on days 3-5 post-feeding. Midgut proteins were probed for total MEK via western blot to establish that control-MO feeding relative to saline alone had no effect on total MEK levels. Bars represent the mean fold changes ± SEMs relative to day-matched controls (set at 1, dotted line) from three biological replicates of 12-15 midguts per timepoint. NS= not significant, p>0.1.
Supplementary Figure 3. Representative western blots of total MEK. Densitometry of western blots from (A) Figure 2 and (B) Supplementary Figure 2 was performed. Data were normalized to appropriate control groups (set at 1.0) as well as a GAPDH loading control and normalized values are reported below blots. Blots are representative of three independently replicated experiments. For (A): MO= AsMEK-MO, C= control. At day 2, total MEK was not detectable in MO-treated A. stephensi (dash). For (B): S= saline, cMO= control-MO.
Supplementary Figure 4. Representative western blots of phospho-ERK. Densitometry of western blots from (A) Figure 3A and (B) Figure 3B was performed. Data were normalized to appropriate control groups (set at 1.0) as well as a GAPDH loading control and normalized values are reported below blots. Blots are representative of three (B) or four (A) independently replicated experiments. For (B): C= control, MO= AsMEK-MO.


