Abstract
The circadian clock plays an integral role in the regulation of physiological processes, including the regulation of blood pressure. However, deregulation of the clock can lead to pathophysiological states including hypertension. Recent work has implicated the circadian clock genes in the regulation of processes in the heart, kidney, vasculature, and the metabolic organs, which are all critical in the regulation of the blood pressure. The goal of this review is to provide an introduction and general overview into the role of circadian clock genes in the regulation of blood pressure with a focus on their deregulation in the etiology of hypertension. This review will focus on the core circadian clock genes CLOCK, BMAL1, Per, and Cry.
Keywords: Blood Pressure, Kidney, Vasculature, Heart, Circadian
Introduction
The term “circadian” is derived from the Latin words circa and dies, meaning about a day. The existence of a biological clock, or circadian clock, that governs the rhythmic oscillations of a variety of physiological functions was first documented in the 18th century, when the French astronomer de Mairan noted that the heliotrope plant opens and closes its leaves and that this process occurs independently of sunlight [1]. From these basic observations, the field of chronobiology has expanded rapidly. Now it is well established that circadian rhythms exist in almost all life forms, from simple archaebacteria to complex humans [2]. In mammals, mounting evidence has demonstrated that the circadian clock regulates a multitude of physiological functions including but not limited to: metabolism, immune response, renal function, sleep-wake cycles, and blood pressure [3, 4]. Interestingly, it has been shown that deregulation of the circadian clock can also be attributed to certain pathological states, including hypertension [5]. The purpose of this review is to examine the role of the circadian clock genes in the regulation of blood pressure and their potential role in hypertension from insights gathered through the use of rodent models.
The circadian clock can be thought of in two parts: the central clock, located in the suprachiasmatic nucleus of the brain, and the peripheral clocks located throughout the rest of the body [6]. The central clock is entrained by a variety of signals; the best established are food and light. Once synchronized, the central clock in turn mediates the synchronization of the various peripheral clocks throughout the body (Reviewed in [4]).The molecular machinery of the circadian clock has been found in nearly every cell type and tissue tested.
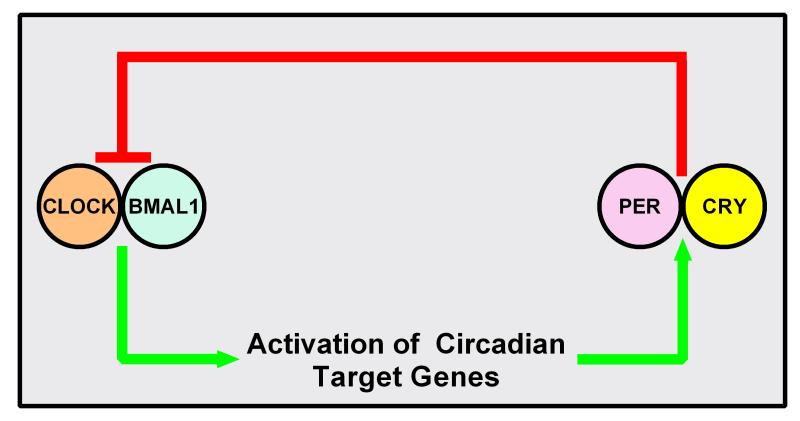
On the molecular level, the circadian clock is composed of a core group of transcription factors that act in a Transcription Translation Oscillating (TTO) loop (Figure 1) (Reviewed in [7]). The core proteins are: CLOCK, BMAL1, Period (Per), and Cryptochrome (Cry). In the TTO loop model, the circadian proteins CLOCK and BMAL1 heterodimerize and then bind to E-box response elements in the promoter regions of circadian target genes. These include the genes for Per (homologs: 1, 2, and 3) and Cry (homologs: 1 and 2). Once translated, Per and Cry presumably interact, translocate into the nucleus, and inhibit the activity of CLOCK and BMAL1. The TTO loop model consists of the activation of circadian target genes by CLOCK and BMAL1 with a negative feedback loop consisting of Per and Cry. There exists a plethora of accessory circadian proteins that play an important role in the regulation of biological rhythms, for more detail on these proteins please see this excellent review by Zhang and Kay [8]. Increasing evidence has also linked post-translational regulation of the circadian proteins to the control of circadian rhythms (reviewed in [3]). However, the focus of this review is on the four core circadian proteins, CLOCK, BMAL1, Per, and Cry and their homologs, on the regulation of blood pressure and their potential role in hypertension.
Figure 1. Transcription Translation Oscillating (TTO) loop.
Clock and Bmal1 heterodimerize to activate transcription of circadian target genes including the genes of Per (homologs: 1-3) and Cry(homologs:1-2). Per and Cry interact and inhibit Bmal1 and Clock.
BMAL1: Role of nitric oxide and the vasculature
The clock gene BMAL1, out of all the clock genes, is the most irreplaceable. BMAL1 knockout (KO) mice have lower blood pressure in the active phase [9]. This results in elimination of the circadian variation in blood pressure. A portion of this decreased blood pressure phenotype has been attributed to changes in the vasculature of these mice. BMAL1 KO mice have increased endothelial dysfunction, due to the uncoupling of nitric oxide signaling [10]; this uncoupling leads to an increase in super oxide production. One possible mechanism may involve deregulation of the biopterins, which are involved in the formation of nitric oxide. Later studies demonstrated that BMAL1 KO mice have increased expression of NADPH oxidase 4[11], providing another possible mechanism for increased superoxide production in these animals. BMAL1 KO mice also have increased arteriosclerotic disease [12]. Transplant of aortic grafts from BMAL1 KO mice into wild-type (WT) mice still led to severe arteriosclerotic disease, signifying the importance of vasculature in the generation of this phenotype. BMAL1 KO develop dilated cardiomyopathy with age [13]. If this is related to the arteriosclerotic disease remains to be seen.
Recent evidence has demonstrated that nitric oxide could play a critical role in the circadian control of blood pressure. It has been demonstrated that circadian oscillations decay with age [14], and this corresponds with a decrease in the circadian oscillation in blood pressure. Importantly, the corresponding decline in circadian oscillation was shown to be partially due to the role of nitric oxide [15]. When mice were administered a nitric oxide donor, this age-dependent decline in circadian oscillation was ameliorated.
It has been known since the mid-1900s that blood flow in humans decreases during the night and increases during the day [16]. Other vascular functions such as sympathetic and vascular tones, forearm vascular resistance, adrenergic receptor agonist response, and flow-mediated dilation have been shown to oscillate with a circadian pattern as well [17, 18]. The incidence of myocardial infarctions and strokes is higher during the morning surge of blood pressure (reviewed in[19]). Correspondingly, these effects were also linked to the expression of the plasminogen activator inhibitor-1 (PAI-1), which down regulates tissue plasminogen activator. Tissue plasminogen activator opens occluded vessels (reviewed in [19]). PAI-1 was shown to oscillate with a circadian pattern in both rodents and humans [20, 21]. A recent genome-wide study found an association between two single-nucleotide-polymorphisms (SNPs) in the intronic region of BMAL1between the third and fourth exon and circulating plasma concentrations of PAI-1 [22] These SNPs were associated with lower levels of both BMAL1 and PAI-1.
Increasing evidence has linked the clock gene BMAL1 with the regulation of insulin production, diabetes, and metabolic syndrome. BMAL1 KO mice exhibit a metabolic syndrome phenotype, including obesity and hyperlipidemia, and are diabetic [23, 24]. The diabetic phenotype was linked to a decrease in glucose-stimulated insulin secretion, due to up-regulation of mitochondrial uncoupling protein 2 [25]. This was shown to be a primarily pancreatic effect as pancreas-specific BMAL1 KO mice exhibit the full diabetic phenotype [24]. A recent human study demonstrated that two SNPs in the BMAL1 gene were associated with an increased risk for gestational diabetes mellitus in Greek women [26]. These SNPs were associated with lower mRNA expression of BMAL1 and were located within the intronic region of Bmal1 between the second and third exon and the fifth and sixth exon.
The evidence from rodent models has consistently demonstrated that BMAL1 plays a critical role in the circadian regulation of blood pressure especially through regulation of both the vasculature and insulin production. Human studies demonstrate that SNPs in BMAL1 are associated with gestational diabetes and vessel occlusion. Further clinical studies are necessary to determine if SNPs in BMAL1 are associated with any other cardiovascular pathophysiology.
CLOCK: The heart and the kidney
CLOCK composes the other part of the activated heterodimer with BMAL1. Recent research has identified that CLOCK KO in mutant mice exhibit multiple cardiorenal and metabolic phenotypes. Multiple insights into the role of CLOCK in the circadian regulation of hypertension have come from the study of the cardiomyocyte-specific clock mutant (CCM) mice [27]. This mouse model has a cardiomyocyte specific mutation in the CLOCK protein. This mutation causes CLOCK to be unable to bind DNA and therefore cannot activate circadian target genes. Heart rate has been shown to exhibit circadian oscillations (reviewed in [28]). CCM mice exhibit reduced heart rate during the active phase, resulting in loss of the circadian oscillation in heart rate in these animals. However, the exact mechanism for CLOCK’s role in the circadian regulation of heart rate is unknown. Interestingly, recent evidence has demonstrated that myocardial repolarization appears to be controlled by the circadian clock, through a mechanism involving the regulation of Krüppel-like factor 15 (KLF15). KLF15 was shown to regulate the expression of several potassium channels, critical for repolarization of the heart [29].
Recent studies have demonstrated that CLOCK plays an important role in the regulation of kidney function and blood pressure. CLOCK KO mice are hypotensive compared to wild type (WT) mice, display mild diabetes insipidus, and excrete more sodium in their urine [30]. This phenotype was accompanied by significant changes in the rhythmic expression of several key transporters in the nephron, including the alpha subunit of the epithelial sodium channel (αENaC). Another possible mechanism was due to decreased levels of 20-HETE in CLOCK KO animals [31] ; 20-HETE is a vasoconstrictor of the preglomerular arteries and is involved in raising blood pressure.
The CLOCK protein has also been linked to diabetes and obesity. CLOCK (Δ19) mutant animals are obese and exhibit hyperlipidemia [32]. This is the same mutation as the CCM mouse, in which CLOCK is unable to bind DNA and therefore cannot activate circadian target genes. As was observed in BMAL1 KO mice, CLOCK KO mice are diabetic and have reduced plasma insulin levels [24]. However, the mechanism by which CLOCK regulates insulin production remains unknown. It is likely that CLOCK functions through a similar mechanism as BMAL1 since these proteins require one another for activation. In humans, overweight women with a SNP in the 3′ untranslated region of CLOCK have decreased activity, altered sleep patterns, and abnormal circadian rhythmicity [33]. Whether or not this SNP increased the propensity for obesity in these patients remains to be determined.
It is evident that CLOCK plays an important role in the heart, the kidney, and likely contributes to obesity and diabetes. CLOCK KO mice have reduced blood pressure, similar to the BMAL1 KO mice. Both are diabetic, with reduced plasma insulin levels. However, the CLOCK KO mice have been shown to have a renal phenotype, including mild diabetes insipidus and renal sodium wasting. Whether or not BMAL1 KO mice exhibit a renal phenotype has not been investigated.
Period 1: Regulation of sodium reabsorption in the kidney
Period 1 (Per1), is one of the three isoforms of Period, the assumed partner of Cry in the repression of CLOCK/BMAL1. However, recent evidence from our lab and others has demonstrated a novel role for Per1 in the regulation of renal sodium reabsorption and blood pressure. Our lab has shown that Per1 regulates the basal and aldosterone-induced regulation of the alpha subunit of ENaC in kidney collecting duct cells [34-37]. ENaC is a critical regulator of sodium reabsorption in the kidney collecting duct, and it is responsible for the fine-tuning of sodium reabsorption and subsequently contributes to control of blood volume. Importantly, we have shown that pharmacological inhibition of Per1 nuclear entry affected ENaC activity in amphibian and mouse kidney cells [36]. In addition to ENaC, we have also shown that Per1 coordinately regulates several genes involved in sodium reabsorption [38]. This regulation includes the positive modulation of Fxyd5, a positive regulator of Na,K-ATPase [39]. Fxyd5, a gamma like subunit of the Na,K-ATPase, increases Na,K- ATPase activity. Per1 was shown to negatively regulate genes involved in negative regulation of ENaC activity including Ube2e3, Caveolin-1, and Endothelin-1. Ube2e3 is an ubiquitin ligase that inhibits ENaC by ubiquitination, targeting ENaC to the proteasome [40]. Caveolin-1 is a lipid raft protein is involved in retrieval of ENaC from the plasma membrane [41]. Endothelin-1 (ET-1) is an inhibitor of ENaC activity via an Endothelin-B receptor and nitric oxide dependent pathway [42].
As mentioned above, Per1 was first thought to behave primarily as a repressor of CLOCK and BMAL1 activity. However, we and others have shown that Per1 activates gene expression in a manner that appears to be gene and tissue-specific [34-36, 43-45]. Similarly, we proposed a mechanism for Per1 action involving repression of the circadian repressor Cry2 [45]. Since Per1 regulates multiple genes involved in the regulation of renal sodium reabsorption, it would predict that loss of Per1 should result in decreased renal sodium reabsorption, with subsequent decreased plasma volume and decreased blood pressure. Mice with reduced levels of Per1 do exhibit a renal sodium wasting phenotype [37]. Importantly, we have also demonstrated that Per1 KO mice have significantly lower blood pressure compared to WT controls [38]. Interestingly, a recent human study showed that Per1 mRNA expression was significantly increased in the renal medulla of kidneys from hypertensive patients compared to normotensive controls [46], suggesting a role for Per1 in the regulation of blood pressure by the kidney in humans.
Period 2: Potential role in the heart, vasculature, and in metabolism
Period 2 (Per2) has been shown to be the primary partner of Cry in the repression of CLOCK and BMAL1 [47]. However, the role of Per2 in hypertension is much less understood. Per2 mutant mice have decreased 24hr diastolic blood pressure, a mild increase in heart rate, and reduced diurnal dipping [48]. The mechanism behind this phenotype, however, has not been defined.
Aortic rings from Per2 KO mice exhibited impaired endothelium-dependent relaxation from acetylcholine [49], suggesting that Per1 may play a role in the regulation of vascular function. Another possible mechanism is in the heart, involving ischemic adaptation. It was recently shown that Per2 regulates hypoxia-inducible factor 1alpha [50]. Similarly, another study showed that Per2 KO mice have increased infarct sizes during myocardial ischemia [51]. In regards to metabolism, it is known that Per1/2/3 triple KO mice gain more weight under a high fat diet, compared to WT controls [52]. The role of Per2 in this phenotype is unknown, however. In humans, a recent study showed that multiple SNPs in Per2 were associated with high fasting blood glucose levels [53]. These SNPs were located throughout the Per2 gene body and untranslated regions. Another study showed that in patients with metabolic syndrome, SNPs in the Per2 coding region were associated with higher mean plasma serum fatty acids [54]. Thus, Per2 appears to play an integral role in the regulation of ischemic adaptation in the heart and may also contribute to regulation of vascular function and metabolism.
Cryptochrome: Regulation of aldosterone and salt-sensitivity
Cry1 and Cry2 have been shown to be the primary circadian repressors [47]. Cry1/2 KO mice exhibit salt sensitive hypertension that was associated with dramatically increased plasma aldosterone levels and higher levels of 3-beta dehydrogenase-isomerase, an adrenal gland-specific enzyme in the aldosterone synthesis pathway [55]. No changes were observed in the expression of the aldosterone synthase, which encodes the rate-limiting step of aldosterone production. A later study demonstrated that Cry1/2 KO mice have increased kidney damage on normal and high salt diets [56]. Interestingly, lowering the blood pressure, without affecting the high aldosterone levels, did not alleviate the kidney damage. Hence, it seems the high aldosterone levels in these mice could be responsible for the observed kidney damage independent of blood pressure.
In terms of a metabolic phenotype, Cry1/2 KO mice are hyperglycemic [57]. Overexpression of Cry1 in diabetic db/db mice caused lowering of blood glucose and increased insulin sensitivity [58]. Another recent study used a synthetic Cry1/2 agonist in mouse primary hepatocytes and showed that activation of Cry1/2 led to increased glucose production [59]. This result was likely due to increased synthesis of two rate-limiting gluconeogenic enzymes, Pck1 and G6pc. Evidence gained using KO, overexpression, or pharmacological mouse models showed that Cry1/2 has an important role in glucose control, diabetes, and salt-sensitive hypertension.
Conclusion
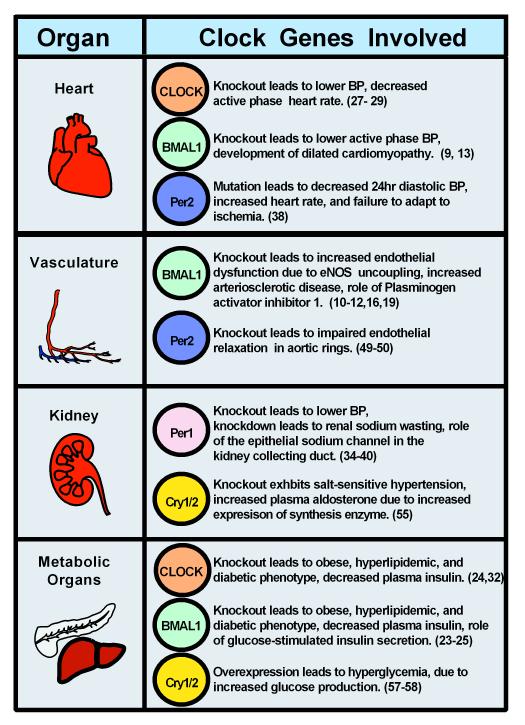
Rodent models have demonstrated that the circadian clock plays an integral role in the regulation of blood pressure, diabetes, and metabolism (Figure 2). Clock genes such as BMAL1, CLOCK, Per1, Per2, Cry1, and Cry2 have been implicated in many disease states such as hypertension, diabetes, and obesity. CLOCK, BMAL1, and Per2 exhibit an integral role in the heart and the vasculature. Per1 and CLOCK have a critical role in sodium reabsorption in the kidney. These rodent models provide great insight into the mechanism by which the circadian clock may contribute to pathophysiological states. However, clinical studies are needed to determine the therapeutic potential of targeting the circadian clock in diseases such as hypertension.
Figure 2. Role of Circadian Clock Genes in Blood pressure.
The role of each clock gene is organized by organ and its role in regulation of blood pressure. If a protein is not listed, it does not imply that it is not involved, but that it has not yet been tested. Numbers are indicative of respective references.
Acknowledgments
Conflict Of Interest and Source of Funding: None declared. This work was supported by NIH DK085193 and DK098460 and the ASN Foundation for Kidney Research to MLG, AHA Predoctoral fellowship 13PRE16910096 to JR.
References
- 1.Kraft M, Martin RJ. Chronobiology and chronotherapy in medicine. Dis Mon. 1995;41(8):506–75. doi: 10.1016/s0011-5029(95)90036-5. [DOI] [PubMed] [Google Scholar]
- 2.Loudon AS. Circadian biology: a 2.5 billion year old clock. Current biology : CB. 2012;22(14):R570–1. doi: 10.1016/j.cub.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 3.Richards J, Gumz ML. Mechanism of the circadian clock in physiology. Am J Physiol Regul Integr Comp Physiol. 2013;304(12):R1053–64. doi: 10.1152/ajpregu.00066.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richards J, Gumz ML. Advances in understanding the peripheral circadian clocks. FASEB J. 2012 doi: 10.1096/fj.12-203554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hermida RC, et al. Chronotherapy improves blood pressure control and reduces vascular risk in CKD. Nat Rev Nephrol. 2013;9(6):358–68. doi: 10.1038/nrneph.2013.79. [DOI] [PubMed] [Google Scholar]
- 6.Albrecht U, Eichele G. The mammalian circadian clock. Curr Opin Genet Dev. 2003;13(3):271–7. doi: 10.1016/s0959-437x(03)00055-8. [DOI] [PubMed] [Google Scholar]
- 7.Gekakis N, et al. Role of the CLOCK protein in the mammalian circadian mechanism. Science. 1998;280(5369):1564–9. doi: 10.1126/science.280.5369.1564. [DOI] [PubMed] [Google Scholar]
- 8.Zhang EE, Kay SA. Clocks not winding down: unravelling circadian networks. Nat Rev Mol Cell Biol. 2010;11(11):764–76. doi: 10.1038/nrm2995. [DOI] [PubMed] [Google Scholar]
- 9.Curtis AM, et al. Circadian variation of blood pressure and the vascular response to asynchronous stress. Proc Natl Acad Sci U S A. 2007;104(9):3450–5. doi: 10.1073/pnas.0611680104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anea CB, et al. Increased Superoxide and Endothelial NO Synthase Uncoupling in Blood Vessels of Bmal1-Knockout Mice. Circ Res. 2012;111(9):1157–65. doi: 10.1161/CIRCRESAHA.111.261750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anea CB, et al. Circadian clock control of nox4 and reactive oxygen species in the vasculature. PLoS One. 2013;8(10):e78626. doi: 10.1371/journal.pone.0078626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng B, et al. Tissue-intrinsic dysfunction of circadian clock confers transplant arteriosclerosis. Proc Natl Acad Sci U S A. 2011;108(41):17147–52. doi: 10.1073/pnas.1112998108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lefta M, et al. Development of Dilated Cardiomyopathy in Bmal1 Deficient Mice. Am J Physiol Heart Circ Physiol. 2012 doi: 10.1152/ajpheart.00238.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oster H, et al. Loss of circadian rhythmicity in aging mPer1−/−mCry2−/− mutant mice. Genes Dev. 2003;17(11):1366–79. doi: 10.1101/gad.256103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kunieda T, et al. Reduced nitric oxide causes age-associated impairment of circadian rhythmicity. Circ Res. 2008;102(5):607–14. doi: 10.1161/CIRCRESAHA.107.162230. [DOI] [PubMed] [Google Scholar]
- 16.Kaneko M, Zechman FW, Smith RE. Circadian variation in human peripheral blood flow levels and exercise responses. J Appl Physiol. 1968;25(2):109–14. doi: 10.1152/jappl.1968.25.2.109. [DOI] [PubMed] [Google Scholar]
- 17.Panza JA, Epstein SE, Quyyumi AA. Circadian variation in vascular tone and its relation to alpha-sympathetic vasoconstrictor activity. N Engl J Med. 1991;325(14):986–90. doi: 10.1056/NEJM199110033251402. [DOI] [PubMed] [Google Scholar]
- 18.Hossmann V, Fitzgerald GA, Dollery CT. Circadian rhythm of baroreflex reactivity and adrenergic vascular response. Cardiovasc Res. 1980;14(3):125–9. doi: 10.1093/cvr/14.3.125. [DOI] [PubMed] [Google Scholar]
- 19.Takeda N, Maemura K. Circadian clock and cardiovascular disease. J Cardiol. 2011;57(3):249–56. doi: 10.1016/j.jjcc.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Naito Y, et al. Circadian gene expression of clock genes and plasminogen activator inhibitor-1 in heart and aorta of spontaneously hypertensive and Wistar-Kyoto rats. J Hypertens. 2003;21(6):1107–15. doi: 10.1097/00004872-200306000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Schoenhard JA, et al. Regulation of the PAI-1 promoter by circadian clock components: differential activation by BMAL1 and BMAL2. J Mol Cell Cardiol. 2003;35(5):473–81. doi: 10.1016/s0022-2828(03)00051-8. [DOI] [PubMed] [Google Scholar]
- 22.Huang J, et al. Genome-wide association study for circulating levels of PAI-1 provides novel insights into its regulation. Blood. 2012;120(24):4873–81. doi: 10.1182/blood-2012-06-436188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo B, et al. The clock gene, brain and muscle Arnt-like 1, regulates adipogenesis via Wnt signaling pathway. FASEB J. 2012;26(8):3453–63. doi: 10.1096/fj.12-205781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marcheva B, et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466(7306):627–31. doi: 10.1038/nature09253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee J, et al. Loss of Bmal1 leads to uncoupling and impaired glucose-stimulated insulin secretion in beta-cells. Islets. 2011;3(6):381–8. doi: 10.4161/isl.3.6.18157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pappa KI, et al. The major circadian pacemaker ARNT-like protein-1 (BMAL1) is associated with susceptibility to gestational diabetes mellitus. Diabetes Res Clin Pract. 2012 doi: 10.1016/j.diabres.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 27.Durgan DJ, et al. The circadian clock within the cardiomyocyte is essential for responsiveness of the heart to fatty acids. J Biol Chem. 2006;281(34):24254–69. doi: 10.1074/jbc.M601704200. [DOI] [PubMed] [Google Scholar]
- 28.Durgan DJ, Young ME. The cardiomyocyte circadian clock: emerging roles in health and disease. Circ Res. 2010;106(4):647–58. doi: 10.1161/CIRCRESAHA.109.209957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeyaraj D, et al. Circadian rhythms govern cardiac repolarization and arrhythmogenesis. Nature. 2012;483(7387):96–9. doi: 10.1038/nature10852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zuber AM, et al. Molecular clock is involved in predictive circadian adjustment of renal function. Proc Natl Acad Sci U S A. 2009;106(38):16523–8. doi: 10.1073/pnas.0904890106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nikolaeva S, et al. The Circadian Clock Modulates Renal Sodium Handling. J Am Soc Nephrol. 2012 doi: 10.1681/ASN.2011080842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turek FW, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308(5724):1043–5. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bandin C, et al. Differences in circadian rhythmicity in CLOCK 3111T/C genetic variants in moderate obese women as assessed by thermometry, actimetry and body position. Int J Obes (Lond) 2012 doi: 10.1038/ijo.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gumz ML, et al. The circadian clock protein Period 1 regulates expression of the renal epithelial sodium channel in mice. J Clin Invest. 2009;119(8):2423–34. doi: 10.1172/JCI36908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gumz ML, et al. Regulation of alphaENaC expression by the circadian clock protein Period 1 in mpkCCD(c14) cells. Biochim Biophys Acta. 2010;1799(9):622–9. doi: 10.1016/j.bbagrm.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richards J, et al. Inhibition of alphaENaC expression and ENaC activity following blockade of the circadian clock-regulatory kinases CKIΔ/ε. Am J Physiol Renal Physiol. 2012 doi: 10.1152/ajprenal.00678.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richards J, et al. Role of Per1 and the mineralocorticoid receptor in the coordinate regulation of alphaENaC in renal cortical collecting duct cells. Front Physiol. 2013;4:253. doi: 10.3389/fphys.2013.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stow LR, et al. The circadian protein period 1 contributes to blood pressure control and coordinately regulates renal sodium transport genes. Hypertension. 2012;59(6):1151–6. doi: 10.1161/HYPERTENSIONAHA.112.190892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lubarski I, et al. Interaction with the Na,K-ATPase and tissue distribution of FXYD5 (related to ion channel) J Biol Chem. 2005;280(45):37717–24. doi: 10.1074/jbc.M506397200. [DOI] [PubMed] [Google Scholar]
- 40.Debonneville C, Staub O. Participation of the ubiquitin-conjugating enzyme UBE2E3 in Nedd4-2-dependent regulation of the epithelial Na+ channel. Mol Cell Biol. 2004;24(6):2397–409. doi: 10.1128/MCB.24.6.2397-2409.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee IH, et al. The activity of the epithelial sodium channels is regulated by caveolin-1 via a Nedd4-2-dependent mechanism. J Biol Chem. 2009;284(19):12663–9. doi: 10.1074/jbc.M809737200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bugaj V, et al. Collecting duct-specific endothelin B receptor knockout increases ENaC activity. Am J Physiol Cell Physiol. 2012;302(1):C188–94. doi: 10.1152/ajpcell.00301.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bose S, Boockfor FR. Episodes of prolactin gene expression in GH3 cells are dependent on selective promoter binding of multiple circadian elements. Endocrinology. 2010;151(5):2287–96. doi: 10.1210/en.2009-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oster H, van der Horst GT, Albrecht U. Daily variation of clock output gene activation in behaviorally arrhythmic mPer/mCry triple mutant mice. Chronobiol Int. 2003;20(4):683–95. doi: 10.1081/cbi-120022408. [DOI] [PubMed] [Google Scholar]
- 45.Richards J, et al. Opposing actions of Per1 and Cry2 in the Regulation of Per1 Target Gene Expression in the Liver and Kidney. Am J Physiol Regul Integr Comp Physiol. 2013 doi: 10.1152/ajpregu.00195.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marques FZ, et al. Gene expression profiling reveals renin mRNA overexpression in human hypertensive kidneys and a role for microRNAs. Hypertension. 2011;58(6):1093–8. doi: 10.1161/HYPERTENSIONAHA.111.180729. [DOI] [PubMed] [Google Scholar]
- 47.Chen R, et al. Rhythmic PER abundance defines a critical nodal point for negative feedback within the circadian clock mechanism. Mol Cell. 2009;36(3):417–30. doi: 10.1016/j.molcel.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vukolic A, et al. Role of mutation of the circadian clock gene Per2 in cardiovascular circadian rhythms. Am J Physiol Regul Integr Comp Physiol. 2010;298(3):R627–34. doi: 10.1152/ajpregu.00404.2009. [DOI] [PubMed] [Google Scholar]
- 49.Viswambharan H, et al. Mutation of the circadian clock gene Per2 alters vascular endothelial function. Circulation. 2007;115(16):2188–95. doi: 10.1161/CIRCULATIONAHA.106.653303. [DOI] [PubMed] [Google Scholar]
- 50.Eckle T, et al. Adora2b-elicited Per2 stabilization promotes a HIF-dependent metabolic switch crucial for myocardial adaptation to ischemia. Nat Med. 2012 doi: 10.1038/nm.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bonney S, et al. Cardiac Per2 functions as novel link between fatty acid metabolism and myocardial inflammation during ischemia and reperfusion injury of the heart. PLoS One. 2013;8(8):e71493. doi: 10.1371/journal.pone.0071493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dallmann R, Weaver DR. Altered body mass regulation in male mPeriod mutant mice on high-fat diet. Chronobiol Int. 2010;27(6):1317–28. doi: 10.3109/07420528.2010.489166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Englund A, et al. NPAS2 and PER2 are linked to risk factors of the metabolic syndrome. J Circadian Rhythms. 2009;7:5. doi: 10.1186/1740-3391-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garcia-Rios A, et al. A Period 2 genetic variant interacts with plasma SFA to modify plasma lipid concentrations in adults with metabolic syndrome. J Nutr. 2012;142(7):1213–8. doi: 10.3945/jn.111.156968. [DOI] [PubMed] [Google Scholar]
- 55.Doi M, et al. Salt-sensitive hypertension in circadian clock-deficient Cry-null mice involves dysregulated adrenal Hsd3b6. Nat Med. 2010;16(1):67–74. doi: 10.1038/nm.2061. [DOI] [PubMed] [Google Scholar]
- 56.Nugrahaningsih DA, et al. Chronic hyperaldosteronism in Cryptochrome-null mice induces high-salt- and blood pressure-independent kidney damage in mice. Hypertens Res. 2013 doi: 10.1038/hr.2013.143. [DOI] [PubMed] [Google Scholar]
- 57.Tanida M, et al. Autonomic and cardiovascular responses to scent stimulation are altered in cry KO mice. Neurosci Lett. 2007;413(2):177–82. doi: 10.1016/j.neulet.2006.11.050. [DOI] [PubMed] [Google Scholar]
- 58.Zhang EE, et al. Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Nat Med. 2010;16(10):1152–6. doi: 10.1038/nm.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hirota T, et al. Identification of small molecule activators of cryptochrome. Science. 2012;337(6098):1094–7. doi: 10.1126/science.1223710. [DOI] [PMC free article] [PubMed] [Google Scholar]