SUMMARY
Measles virus (MV) is highly infectious and infects dendritic cells (DCs) for viral dissemination. DCs express RIG-I-like receptors (RLRs) RIG-I and Mda5 that sense MV to induce antiviral type I interferon (IFN) responses. Dephosphorylation of RIG-I and Mda5 by PP1 phosphatases is required for their activation. Here we demonstrate that MV suppresses RIG-I and Mda5 dephosphorylation via DC-SIGN signaling. MV binding to DC-SIGN leads to activation of kinase Raf-1, which induced association of the PP1 inhibitor I-1 with GADD34-PP1 holoenzymes, thereby inhibiting the phosphatase activity. As a result, GADD34-PP1 holoenzymes were unable to dephosphorylate RIG-I and Mda5, hence suppressing type I IFN responses and enhancing MV replication. Interference with DC-SIGN signaling allowed activation of RLRs and subsequently suppressed MV infection of DCs. Thus, MV subverts DC-SIGN signaling, leading to inhibition of PP1 phosphatases that control RIG-I and Mda5 activation, which might be used by other viruses to escape antiviral responses.
INTRODUCTION
Measles is a highly contagious airborne disease and remains a major cause of morbidity and mortality despite the availability of an effective vaccine (WHO, 2012). The causative agent, measles virus (MV) severely suppresses immune responses in the host, leading to secondary opportunistic infections (Moss and Griffin, 2012). Production of antiviral type I interferon (IFN) is important for the control of MV replication and hence disease progression. Therefore MV has evolved various strategies to suppress type I IFN responses, most of which rely on the nonstrucural MV-V protein that can antagonize activation of pattern recognition receptors (PRR) or signaling upstream of type I IFN responses (Fontana et al., 2008; Goodbourn and Randall, 2009).
Type I IFN responses induced by single-stranded (ss) RNA viruses, such as MV, are mediated by the cytoplasmic RIG-I-like receptors (RLRs) RIG-I and Mda5. RIG-I interacts with the 5’ leader of MV ssRNA to induce IFN-β (Plumet et al., 2007). The mechanisms leading to Mda5 activation by MV are still unknown (Ikegame et al., 2010). RLR triggering leads to activation of IkB kinase (IKK)-related kinases, IKKε and Tank-binding protein (TBK1), through the mitochondrial antiviral signaling (MAVS; also known as IPS-1) adaptor protein (Fitzgerald et al., 2003; Sharma et al., 2003). Both IKKε and TBK1 activate transcription factor IRF3, which induces expression of IFN-β (Kawai and Akira, 2008). Signaling by IFN-β via type I IFN-α/β receptor (IFNAR) on infected and neighbouring cells induces transcription of hundreds of interferon-stimulated genes (ISG), such as MxA and ISG15, that are paramount in defense against viruses (Fontana et al., 2008). RLR signaling pathways induce a very potent and rapid type I IFN response, and therefore activation of RLRs is tightly regulated by multiple consecutive processes, including dephosphorylation, ubiquitination and oligomerization of the RLR CARD domains (Gack et al., 2010; Gack et al., 2007; Jiang et al., 2012; Nistal-Villan et al., 2010; Wies et al., 2013; Zeng et al., 2010). Constitutive phosphorylation of CARD domain residues Ser8 and Thr170 of RIG-I and Ser88 of Mda5 keeps RLRs inactive (Gack et al., 2010; Nistal-Villan et al., 2010; Wies et al., 2013). RLR-induced type I IFN production requires RLR dephosphorylation by serine-threonine phosphatases PP1α and PP1γ (Wies et al., 2013). The exact regulation of these phosphatases is not yet understood, but dephosphorylation of RIG-I and Mda5 is crucial for activation of MAVS and subsequent downstream signaling, possibly through induction of oligomerization (Gack et al., 2010; Nistal-Villan et al., 2010; Wallach and Kovalenko, 2013; Wies et al., 2013).
Airborne infection of MV initiates in the lungs and disseminates to lymphocytes throughout the host within 2 weeks post infection (de Swart et al., 2007; Lemon et al., 2011). DC-SIGN+ dendritic cells (DCs) in the lungs are among the first cells that become infected (Lemon et al., 2011; Mesman et al., 2012) and express signaling lymphocyte activation molecule (SLAM, CD150), the entry receptor for wildtype MV (de Swart et al., 2007; Tatsuo et al., 2000). Interaction of MV with C-type lectin receptor DC-SIGN enhances infection of DCs and subsequent viral transmission to lymphocytes (de Witte et al., 2006; de Witte et al., 2008; Mesman et al., 2012). DCs also induce MV-specific adaptive immunity; DC-SIGN sensing of MV induces innate signaling mediated by serine-threonine kinase Raf-1, which modulates TLR-induced immune responses (Gringhuis et al., 2007). Raf-1 signaling induces phosphorylation and acetylation of TLR-induced NF-kB subunit p65, thereby increasing expression of proinflammatory cytokines affecting immune responses (Gringhuis et al., 2009a; Gringhuis et al., 2007). However, little is known about the role of innate signaling induced by MV on type I IFN responses in DCs.
Here, we show that MV efficiently infects primary human DCs by inhibiting RLR-induced type I IFN responses. We demonstrate that GADD34 is the regulatory subunit of the PP1 phosphatases that activate both RIG-I and Mda5, while activity of GADD34-PP1 holoenzymes is regulated by PP1 inhibitor 1 (I-1). Notably, MV binding to DC-SIGN at the cell-surface prevents dephosphorylation of both RIG-I and Mda5 by inhibiting GADD34-PP1 holoenzyme activation via Raf-1 signaling. Raf-1 activation induces phosphorylation of I-1, thereby inducing complex formation between I-1 and GADD34-PP1 holoenzymes, which inhibits its phosphatase activity. Inhibition of dephosphorylation of both RIG-I and Mda5 suppresses type I IFN responses in DCs upon MV infection. Interference with DC-SIGN signaling allows dephosphorylation of both RLRs, leading to strong antiviral type I IFN responses that suppress MV replication. Triggering of innate signaling by MV to circumvent cellular antiviral mechanisms precedes viral evasion tactics that rely on viral replication and de novo synthesis of viral proteins, hence acting earlier during infection. Thus, we have uncovered a novel regulatory mechanism that controls RLR activation and have identified the phosphatase inhibitor I-1 as a target for suppression of antiviral responses, which is exploited by MV to infect DCs. This evasion mechanism might be used by other viruses and targeted therapeutically for more efficient combating of viral infections.
RESULTS
MV infection of DCs requires both CD150 and DC-SIGN
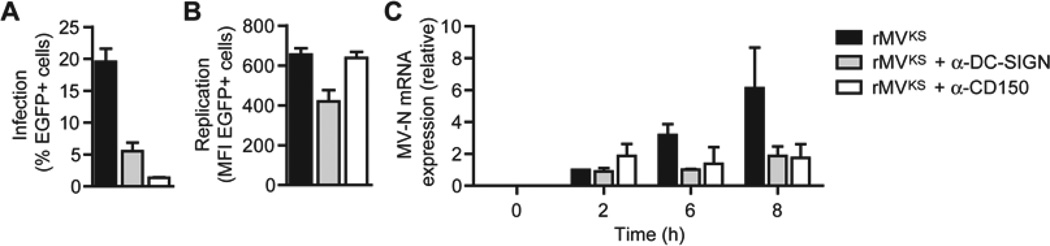
To investigate the function of viral receptors involved in replication of MV, human monocyte-derived DCs were infected with pathogenic strain rMVKSEGFP(3) (rMVKS) in the presence of blocking antibodies against CD150 and DC-SIGN. This virulent recombinant strain expresses enhanced GFP (EGFP) as a result of viral replication, without altering its pathogenicity (de Swart et al., 2007; de Vries et al., 2012; Lemon et al., 2011; Ludlow et al., 2013). DCs were efficiently infected with the virus as measured by flow cytometry 24 hours post infection (h.p.i.). CD150 was crucial for infection since blocking antibodies against CD150 almost completely abrogated infection, whereas blocking DC-SIGN binding decreased infection (Figure 1A). Analysis of GFP expression in infected cells, as a measure for replication, showed that CD150 blocking antibodies did not alter the replication level of MV in DCs, despite the large effect on the % infected cells (Figure 1B). Notably, blocking DC-SIGN binding decreased MV infection as well as replication in DCs (Figures 1A and 1B), suggesting that DC-SIGN signaling affects not only fusion but also the replication cycle of MV. We next determined viral transcription over time after infection of DCs. MV-N is the first transcribed gene of the unsegmented MV genome upon cellular entry (Rima and Duprex, 2009). We detected MV-N mRNA as early as 2 h.p.i., accumulating over time, while viral transcription was strongly decreased with blocking antibodies against both CD150 and DC-SIGN (Figure 1C). These data indicate that both CD150 and DC-SIGN are important for early transcription, however, whereas CD150 is required for infection, MV binding to DC-SIGN is critical for both infection and replication.
Figure 1. MV infection of DCs is dependent on CD150, while DC-SIGN enhances infection and replication.
(A,B) Infection and replication in DCs 24 h after infection with rMVKSEGFP(3) in the absence or presence of blocking DC-SIGN or CD150 antibodies, determined by flow cytometry by measuring % or mean fluorescence intensity (MFI) of EGFP+ cells. Data are presented as mean ± SD of duplicate samples.
(C) MV-N mRNA expression by DCs 0, 2, 6, and 8 h after infection with rMVKS, measured by real-time PCR, normalized to GAPDH, and set at 1 in 2 h MV-infected cells. Data are presented as mean ± SD.
Data are representative of at least three (A,B) or two (C) independent experiments.
RIG-I and Mda5 mediate type I IFN responses to MV in DCs
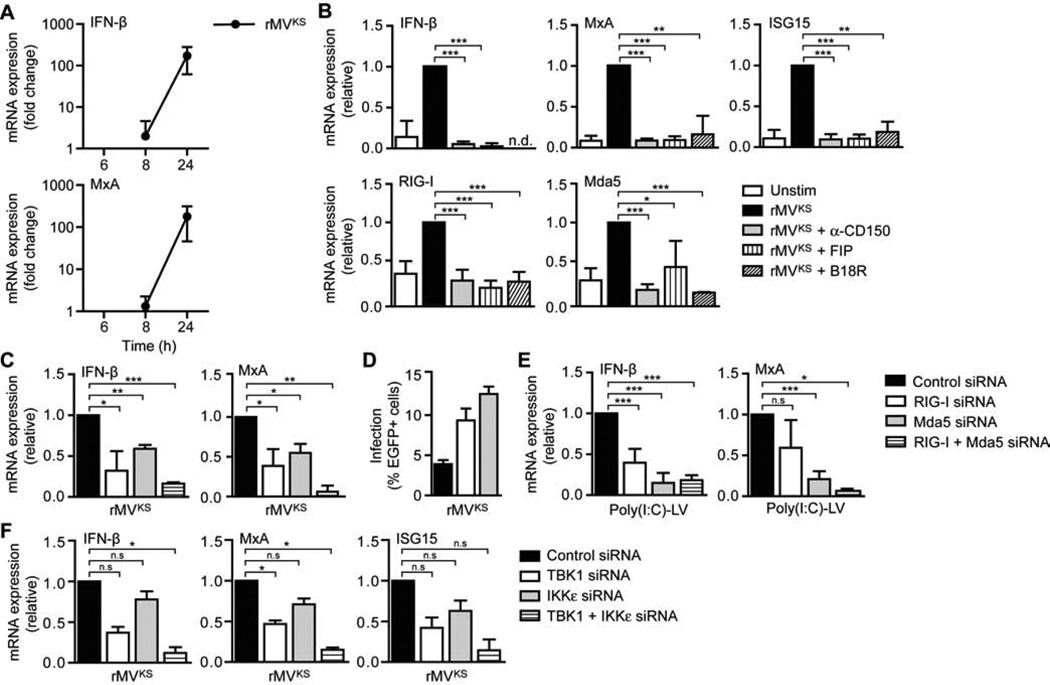
Since type I IFN responses affect viral replication (Fontana et al., 2008), we examined IFN-β expression as well as functional type I IFN responses by measuring induction of antiviral ISGs, such as MxA, in DCs infected with rMVKS. Infection of DCs with rMVKS induced IFN-β as well as MxA expression (Figures 2A and 2B). Viral transcription or replication of MV is required for induction of type I IFN responses (Duhen et al., 2010). Blocking viral entry or membrane fusion by preincubating DCs with blocking CD150 antibodies or fusion inhibitor protein (FIP) decreased mRNA expression of IFN-β and ISGs MxA, ISG15, RIG-I and Mda5 to steady-state levels (Figure 2B). Furthermore, MV-induced expression of ISGs was inhibited by soluble IFNAR, which scavenges IFN and prevents IFN-induced signaling (Figure 2B). Thus, MV infection induces type I IFN responses and enhances expression of antiviral proteins, including the sensors RIG-I and Mda5, in an IFNAR-dependent manner. We next studied whether RIG-I and Mda5 mediate MV-induced type I IFN responses in DCs. Silencing of either RIG-I or Mda5 by RNA interference (Figure S1) decreased expression of IFN-β and MxA in response to MV, whereas silencing of both receptors together almost completely abolished IFN-β and MxA expression (Figure 2C). Notably, DC infection was enhanced when either RIG-I or Mda5 was silenced (Figure 2D), strongly indicating that type I IFN responses to MV limit MV replication. Transfection of DCs with RLR ligand poly(I:C)-LyoVec (poly(I:C)-LV) induced type I IFN responses, which were abrogated by silencing of either receptor (Figure 2E), supporting specificity of RLR silencing. RLR signaling through adaptor protein MAVS induces activation of TBK1 and IKKε through phosphorylation at specific sites (Fitzgerald et al., 2003; Sharma et al., 2003). Silencing of either TBK1 or IKKε (Figure S1) reduced MV-induced IFN-β, MxA and ISG15 expression, whereas combined silencing of TBK1 and IKKε almost completely abrogated MV-induced type I IFN responses in DCs (Figure 2F). These data demonstrate that MV-induced antiviral type I IFN responses in DCs are triggered via both RIG-I and Mda5 and depend on TBK1-IKKε signaling.
Figure 2. MV-induced type I IFN responses in DCs are dependent on RLR signaling via TBK1 and IKKε.
(A–C,E–F) IFN-β, MxA, ISG15, RIG-I, and Mda5 mRNA expression by DCs at indicated times (A) or 24 h after infection with rMVKS (B,C,F), or 8 h after stimulation with poly(I:C)-LyoVec (poly(I:C)-LV) (E), in the absence or presence of blocking CD150 antibodies, fusion inhibitor protein (FIP), or neutralizing soluble IFNAR (B18R) (B), or after silencing of RIG-I and/or Mda5 (C,E), or TBK1 and/or IKKε (F) by RNA interference (siRNA), measured by real-time PCR, normalized to GAPDH, and set at 1 in MV- or poly(I:C)-LV-stimulated (control-silenced) cells. Data are presented as mean ± SD. N.d., not determined; n.s., not statistically significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001 (Student's t-test).
(D) Infection of DCs 24 h after infection with rMVKS after silencing of RIG-I or Mda5, determined by flow cytometry by measuring % of EGFP+ cells. Data are presented as mean ± SD of duplicate samples.
Data are representative of at least four (A), three (B–E) or two (F) independent experiments. See also Figure S1.
MV suppresses type I IFN responses via DC-SIGN
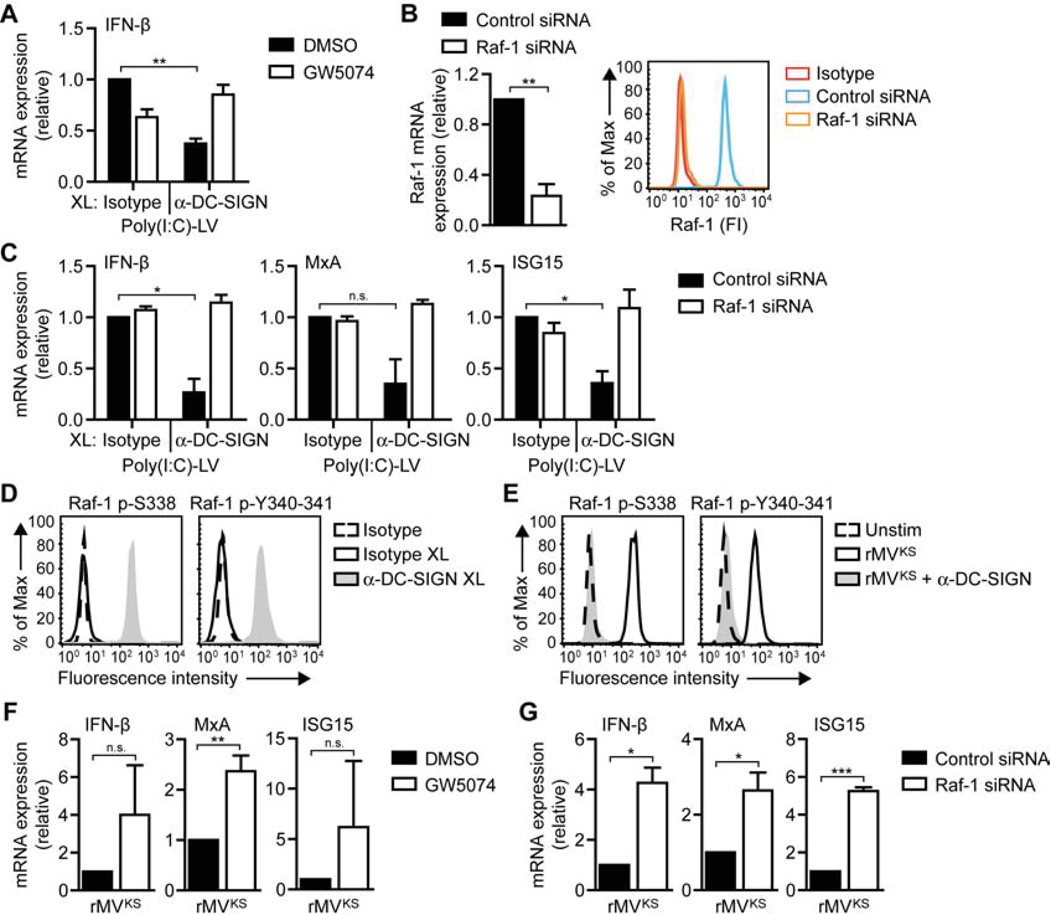
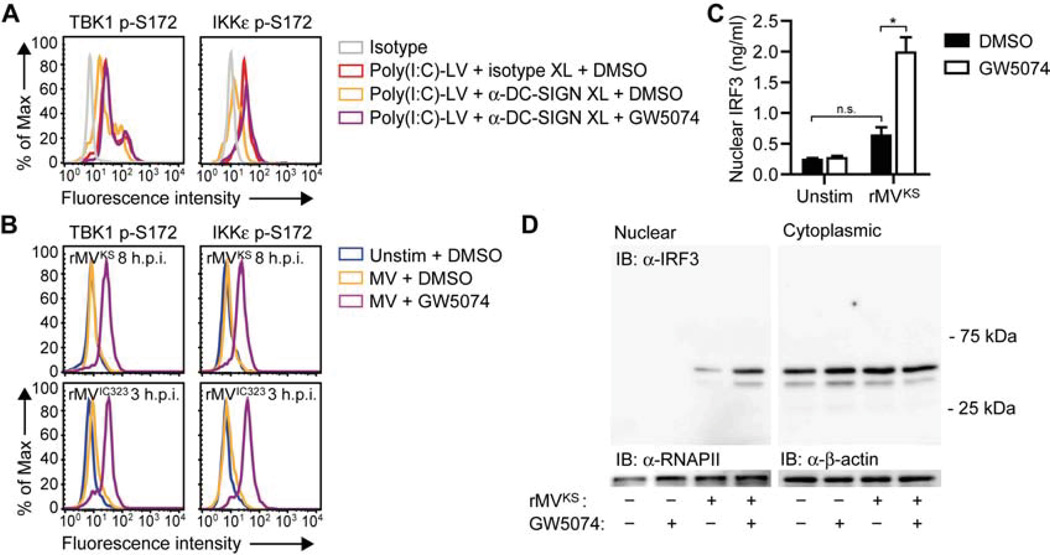
Efficient infection of DCs with MV suggests that MV escapes antiviral type I IFN responses. Since MV interacts with DC-SIGN and this interaction was crucial for MV replication (Figure 1), we next investigated whether DC-SIGN signaling affects RLR-induced type I IFN responses. Transfection of DCs with poly(I:C)-LV led to induction of type I IFN responses, which, notably, were strongly impaired by DC-SIGN triggering via antibody crosslinking (Figure 3A). To investigate whether DC-SIGN-mediated suppression of RLR-induced type I IFN responses was dependent on Raf-1 signaling (Gringhuis et al., 2007), we blocked Raf-1 activity via small molecule inhibitor GW5074 (Lackey et al., 2000) or Raf-1 silencing (Figure 3B). Blocking Raf-1 signaling reversed DC-SIGN-mediated suppression of poly(I:C)-LV-induced IFN-β, MxA and ISG15 mRNA expression (Figures 3A and 3C). Thus, DC-SIGN signaling suppresses RLR-induced type I IFN responses via Raf-1 activation.
Figure 3. Raf-1 activation via DC-SIGN decreases MV-induced type I IFN expression.
(A,C,F,G) IFN-β, MxA, and ISG15 mRNA expression by DCs 8 h after stimulation with poly(I:C)-LV and/or receptor crosslinking with isotype or DC-SIGN-specific antibodies (A,C) or 24 h after infection with rMVKS (F,G), in the absence or presence of Raf inhibitor GW5074 (A,F) or after Raf-1 silencing (B,G), measured by real-time PCR, normalized to GAPDH, and set at 1 in MV- or poly(I:C)-LV-stimulated (control-silenced) cells. Data are presented as mean ± SD. N.s., not statistically significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001 (Student's t -test).
(B) Silencing of Raf-1 using specific SMARTpools and non-targeting siRNA as a control. Silencing was confirmed by real-time PCR (left panel) or flow cytometry (right panel; FI, fluorescence intensity). During real-time PCR analysis, mRNA expression was normalized to GAPDH and set at 1 in control-silenced cells. Data (real-time PCR) are presented as mean ± SD.
(D,E) Raf-1 phosphorylation at Ser338 or Tyr340-341 in DCs left unstimulated (dashed line), or 15 min after stimulation by receptor crosslinking with isotype (black line) or DC-SIGN-specific (filled) antibodies (D) or rMVKS infection in the absence (black line) or presence (filled) of blocking DC-SIGN antibodies (E).
Data are representative of at least three (A–G) independent experiments. See also Figure S3.
DC-SIGN triggering with various pathogen ligands activates Raf-1 through phosphorylation of Ser338 and Tyr340-Tyr341 (Gringhuis et al., 2009a; Gringhuis et al., 2007). Similarly, crosslinking of DC-SIGN with specific DC-SIGN antibodies, in contrast to isotype controls, led to Raf-1 phosphorylation at both Ser338 and Tyr340-Tyr341 (Figure 3D). Notably, DC infection with rMVKS also led to phosphorylation of Raf-1, in a DC-SIGN-dependent manner (Figure 3E). We next investigated whether Raf-1 signaling via DC-SIGN is involved in MV-mediated suppression of type I IFN responses. Notably, inhibition of Raf-1 by either GW5074 or Raf-1 silencing increased MV-mediated IFN-β, MxA and ISG15 expression in response to infection with rMVKS (Figures 3F and 3G). These data demonstrate that DC-SIGN signaling suppresses RLR-induced type I IFN responses via Raf-1, while MV uses this mechanism to impair type I IFN production in DCs.
DC-SIGN signaling via Raf-1 suppresses RLR activation
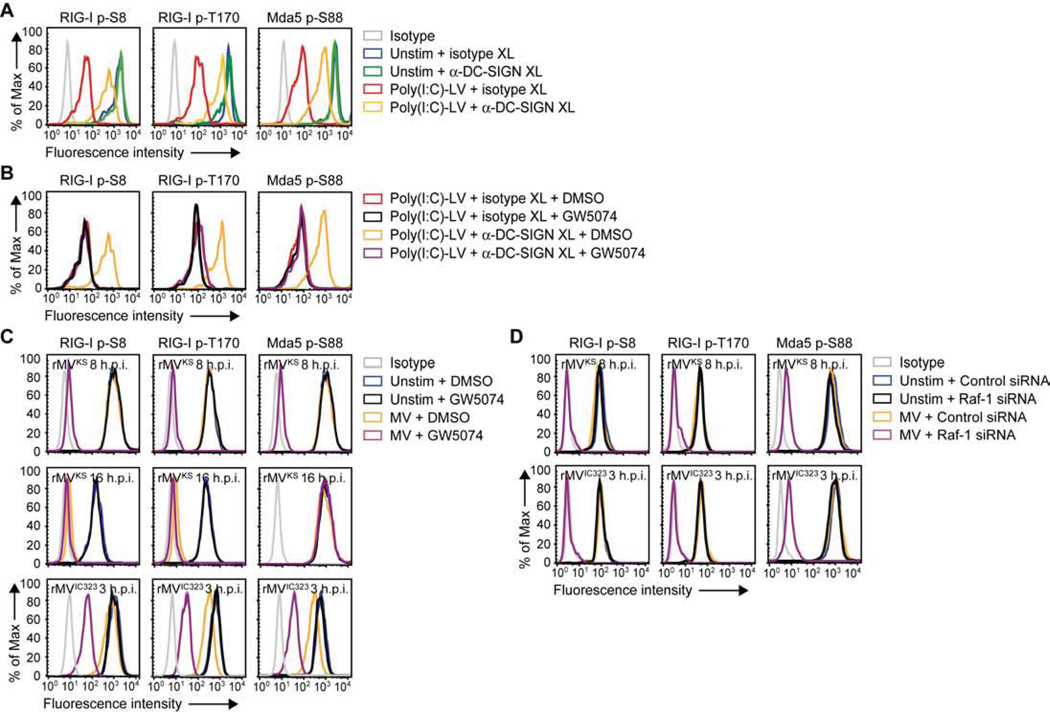
Constitutive phosphorylation of the CARD domains of RIG-I and Mda5 keeps the receptors in their inactive state. Therefore activation requires dephosphorylation of RIG-I at Ser8 and Thr170, and Mda5 at Ser88 (Gack et al., 2010; Nistal-Villan et al., 2010; Wies et al., 2013). We next investigated whether DC-SIGN/Raf-1 signaling affects phosphorylation of RIG-I and Mda5. First, we observed that DC-SIGN crosslinking prevented poly(I:C)-LV-induced dephosphorylation of RIG-I and Mda5 (Figure 4A). Moreover, Raf-1 inhibition restored dephosphorylation of both receptors (Figure 4B). These data indicate that DC-SIGN signaling suppresses RLR activation via Raf-1 by preventing dephosphorylation of their CARD domains. Strikingly, infection with rMVKS did not result in dephosphorylation of RIG-I and Mda5 at 8 h.p.i., whereas inhibition or silencing of Raf-1 dramatically increased the MV-induced dephosphorylation of both receptors (Figures 4C, 4D and S2). Similarly, dephosphorylation of RIG-I and Mda5 was blocked in a Raf-1-dependent manner after infection of DCs with another pathogenic MV strain, rMVIC323EGFP(1) (de Swart et al., 2007), that also required DC-SIGN for infection and replication (Figures 4C, 4D and S3). These data show that MV-induced Raf-1 signaling suppresses RIG-I and Mda5 activation by preventing dephosphorylation. Notably, at 16 h.p.i., rMVKS induced dephosphorylation of RIG-I at Ser8 and Thr170, but not Mda5 at Ser88 (Figure 4C), which could explain the increase in IFN-β and MxA levels between 8 and 24 h.p.i. (Figure 2A). Raf-1 inhibition did not restore Mda5 dephosphorylation 16 h after MV infection (Figure 4C), suggesting that, late during infection, Mda5 dephosphorylation is antagonized via another DC-SIGN-independent mechanism (Davis et al., submitted to CHM). We next investigated the effect of DC-SIGN triggering on downstream RLR signaling. We observed that phosphorylation of TBK1 and IKKε at Ser172 in response to poly(I:C)-LV stimulation was impaired by simultaneous DC-SIGN triggering, however was restored after Raf-1 inhibition (Figure 5A). Moreover, infection of DCs with either rMVKS or rMVIC323 induced a small increase in TBK1 and IKKε phosphorylation at Ser172, which was strongly enhanced after inhibition of Raf-1 signaling (Figure 5B). Activation of TBK1 and IKKε precedes phosphorylation and nuclear translocation of IRF3, which regulates IFN-β transcription (Kawai and Akira, 2008). We observed that rMVKS infection led to minor nuclear accumulation of IRF3, while, again, Raf-1 inhibition strongly increased nuclear translocation of IRF3, allowing IFN-β production (Figures 5C and 5D). These results demonstrate that DC-SIGN-induced Raf-1 activation suppresses downstream RLR signaling by blocking the dephosphorylation of both RIG-I and Mda5.
Figure 4. DC-SIGN-Raf-1 signaling inhibits dephosphorylation of RIG-I and Mda5.
(A–D) RIG-I phosphorylation at Ser8 or Thr170 and Mda5 phosphorylation at Ser88 in DCs left unstimulated; or 3 h after stimulation by crosslinking with isotype or DC-SIGN-specific antibodies (A,B), in the absence or presence of Raf inhibitor GW5074 (B); or 8 or 16 h after rMVKS infection or 3 h after or rMVIC323EGFP(1) in the absence or presence of Raf-1 inhibition via GW5074 (C) or Raf-1 silencing (D), as determined by flow cytometry.
Data are representative of at least four (A,B,C (rMVIC323, 8 h rMVKS), two (C (16 h rMVKS)) or three (D) independent experiments. See also Figure S2.
Figure 5. DC-SIGN-Raf-1 signaling attenuates RLR-induced TBK1 and IKKε phosphorylation and IRF3 nuclear translocation.
(A,B) TBK1 and IKKε phosphorylation at Ser172 in DCs left unstimulated; or 3 h after stimulation by receptor crosslinking with isotype or DC-SIGN-specific antibodies, in the absence or presence of Raf inhibitor GW5074 (A); or 8 h after rMVKS infection or 3 h after or rMVIC323 in the absence or presence of Raf inhibitor GW5074 (B), as determined by flow cytometry.
(C,D) IRF3 nuclear translocation in DCs 8 h after rMVKS infection, in the absence or presence of GW5074, determined by ELISA in nuclear extracts (C) or immunoblotting (IB) of nuclear and cytoplasmic extracts (D). In (D), RNAPII and β-actin served as loading controls. Data in (C) are presented as mean ± SD. N.s., not statistically significant; *, P < 0.05 (Student's t-test).
Data are representative of at least four (A), three (B) or two (C) independent experiments.
Raf-1 activation blocks PP1 activity to attenuate RLR activation
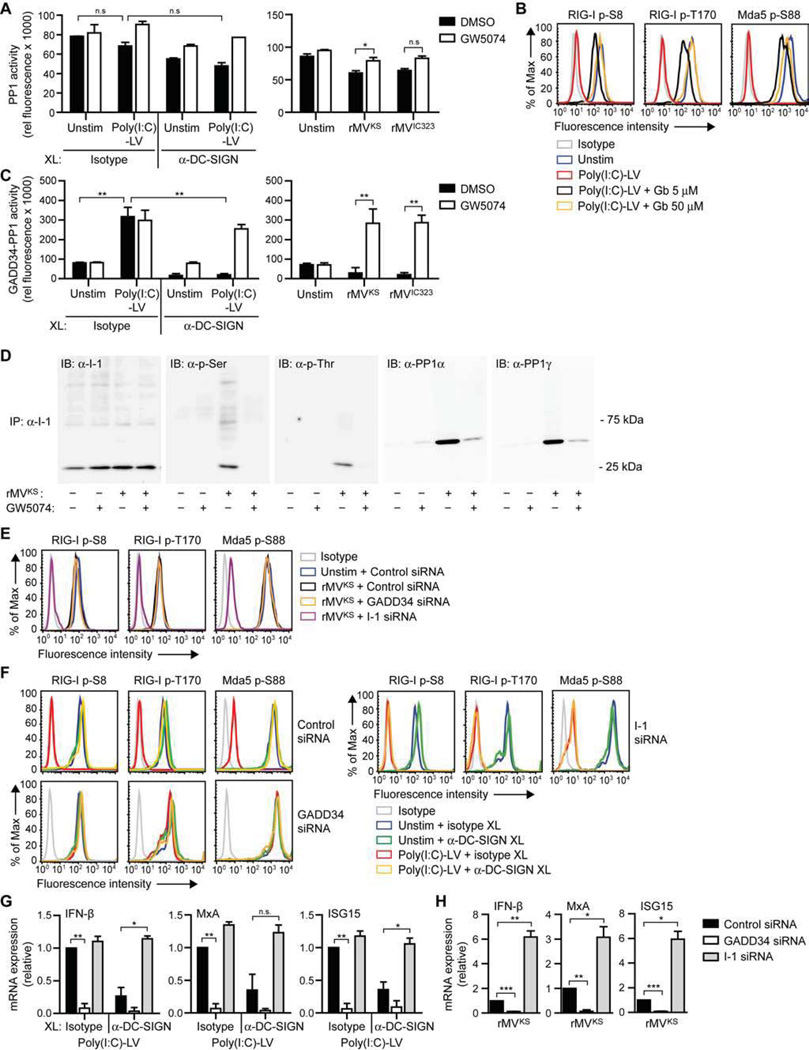
Dephosphorylation of the CARD domains of RIG-I and Mda5 is mediated by PP1α/γ phosphatases (Wies et al., 2013), however, little is known about the regulation of PP1 phosphatase activity. To investigate whether DC-SIGN signaling interferes with the activity of these phosphatases, we measured PP1 phosphatase activity in lysates of DCs stimulated with poly(I:C)-LV in combination with DC-SIGN crosslinking or after infection with MV. Although DC-SIGN activation slightly decreased overall PP1 activity, no statistical differences between unstimulated and stimulated cells were observed (Figure 6A). PP1 phosphatases are ubiquitously expressed in cells and PP1 holoenzymes consist of a catalytic and regulatory subunit, of which the latter determines substrate specificity (Cohen, 2002; Peti et al., 2013). To specifically examine whether Raf-1 influences PP1α/γ phosphatases, we set out to identify the regulatory subunit of the PP1 holoenzymes that control the dephosphorylation of RLRs. One of the possible PP1-binding partners, growth arrest and DNA damage protein (GADD34), has been reported to be required for IFN-β responses after viral infection in DCs (Clavarino et al., 2012). We therefore investigated whether GADD34 association with the catalytic PP1α/γ subunits is involved in dephosphorylation of RIG-I and Mda5. Pretreatment of DCs with the specific GADD34 inhibitor guanabenz (Tsaytler et al., 2011) blocked dephosphorylation of RIG-I at Ser8 and Thr170 and Mda5 at Ser88 in a concentration-dependent manner, indicative of the involvement of GADD34 in RLR activation (Figure 6B). We next assessed GADD34-PP1-specific activity in DCs following pulldown of GADD34 from whole cell lysates after poly(I:C)-LV stimulation or MV infection. We observed a strong increase in PP1 activity after poly(I:C)-LV stimulation, which was blocked by DC-SIGN triggering (Figure 6C). Inhibition of Raf-1 restored PP1 activity after poly(I:C)-LV plus DC-SIGN costimulation (Figure 6C). Notably, DC infection with either rMVKS or rMVIC323 did not induce GADD34-PP1 activity, however, inhibition of Raf-1 resulted in a strong increase in phosphatase activity (Figure 6C). These results strongly indicate that Raf-1 activation blocks PP1 activity in GADD34-PP1 complexes to prevent RLR activation. Activity of GADD34-PP1 complexes is specifically inhibited by I-1; association of I-1 with GADD34-PP1 blocks interactions between the holoenzymes and their substrates (Aggen et al., 2000). Since I-1 requires phosphorylation at Thr35 and Ser67 for the association with GADD34-PP1 complexes (Connor et al., 2001; Huang and Paudel, 2000), we examined whether Raf-1 modulates PP1 activity – and subsequent RLR activity – by phosphorylating I-1. First, we assessed phosphorylation of I-1 upon MV infection. I-1 became phosphorylated at both Ser and Thr residues in response to rMVKS, which coincided with its association with both PP1α and PP1γ holoenzymes as both catalytic subunits immunoprecipitated together with I-1 (Figure 6D). Both phosphorylation and association of I-1 with PP1α and PP1γ was dependent on Raf-1 activity (Figure 6D). Next, we measured RLR phosphorylation levels in DCs after silencing I-1 (Figure S1). Silencing of I-1 allowed dephosphorylation of both RIG-I and Mda5 after MV infection (Figure 6E). Similarly, I-1 silencing after costimulation with poly(I:C)-LV and DC-SIGN crosslinking allowed complete dephosphorylation of both RLRs to the same level as poly(I:C)-LV alone (Figure 6F). Together, these results show that Raf-1 signaling leads to phosphorylation of I-1 and subsequent association of I-1 with PP1 holoenzymes, which prevents RLR dephosphorylation and subsequent RLR activation. Moreover, silencing of GADD34 (Figure S1) completely abolished RLR dephosphorylation after MV infection or poly(I:C)-LV stimulation (Figures 6E and 6F), confirming the role of GADD34-PP1 holoenzymes in mediating RLR dephosphorylation. We next investigated the functional effects of this signaling pathway on type I IFN expression. GADD34 silencing suppressed RLR-induced IFN-β and ISG induction after poly(I:C)-LV stimulation (Figure 6G), whereas I-1 silencing completely abrogated DC-SIGN-mediated suppression of IFN-β and ISG mRNA expression (Figure 6G). Moreover, rMVKS-induced IFN-β and ISG mRNA levels, which were reduced via Raf-1 signaling, strongly increased after I-1 silencing (Figure 6H). Together, these results indicate that DC-SIGN-induced Raf-1-mediated signaling induces association of I-1 with GADD34-PP1 holoenzymes to block PP1 activity and subsequently RLR dephosphorylation and RLR-mediated type I IFN responses to MV.
Figure 6. DC-SIGN-Raf-1 signaling inhibits RLR activation and type I IFN responses via phosphorylation of GADD34-PP1 inhibitor I-1.
(A,C) Overall (A) or GADD34-specific (C) PP1 phosphatase activity in whole cell lysates of DCs 1 h after stimulation with poly(I:C)-LV and/or receptor crosslinking with isotype or DC-SIGN-specific antibodies (left panels) or 24 h after infection with rMVKS or rMVIC323 (right panels), in the absence or presence of Raf inhibitor GW5074. Data are presented as mean ± SD.
(B,E,F) RIG-I phosphorylation at Ser8 or Thr170 and Mda5 phosphorylation at Ser88 in DCs left unstimulated; or 3 h after stimulation by receptor crosslinking with isotype or DC-SIGN-specific (antibodies (B,F); or 8 h after rMVKS infection (E), in the absence or presence of GADD34 inhibitor guanabenz (Gb) (B) or after GADD34 or I-1 silencing (E,F), as determined by flow cytometry.
(D) I-1 phosphorylation at Ser or Thr residues, and association with PP1α or PP1γ after immunoprecipitation (IP) of I-1 from whole cell lysates of DCs left unstimulated or 3 h after infection with rMVKS, in the absence or presence of GW5074, determined by immunoblotting (IB).
(G,H) IFN-β, MxA, and ISG15 mRNA expression by DCs 8 h after stimulation with poly(I:C)-LV and/or receptor crosslinking with isotype or DC-SIGN-specific antibodies (G) or 24 h after infection with rMVKS (H), after GADD34 or I-1 silencing, measured by real-time PCR, normalized to GAPDH, and set at 1 in MV- or poly(I:C)-LV-stimulated control-silenced cells. Data are presented as mean ± SD. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (Student's t-test).
Data are representative of at least two (A,D,F), three (B,E,G,H) or four (C) independent experiments. See also Figure S1.
MV-induced Raf-1 activation enables DC infection
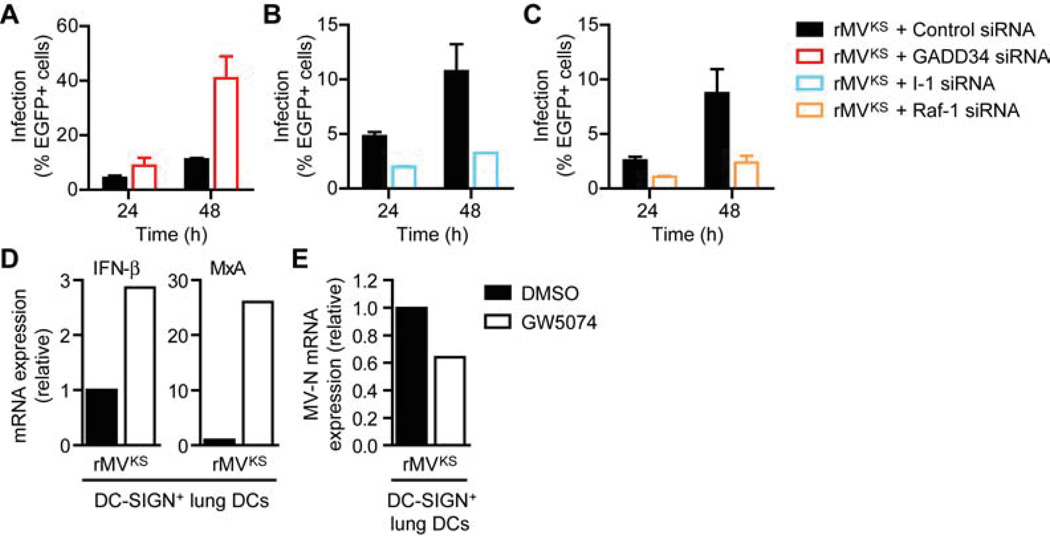
We next investigated whether DC-SIGN-induced inhibition of GADD34-PP1 holoenzyme activity was required for MV infection by silencing the regulatory subunit of PP1, GADD34, and the PP1 inhibitor I-1. Infection of DCs by MV was strongly increased by silencing of GADD34 (Figure 7A), suggesting that loss of type I IFN responses after GADD34 silencing (Figure 6H) allows efficient replication of MV in DCs. Notably, silencing of I-1, which is crucial in MV-induced type I IFN suppression, strongly decreased MV infection at both 24 and 48 h after infection (Figure 7B). Similarly, silencing of Raf-1 strongly decreased infection of MV in DCs (Figure 7C), which is in line with the observed increase of type I IFN responses (Figures 3E and 3F). We next examined whether MV infection of lung-resident DC-SIGN+ DCs isolated from Cynomolgus macaques was similarly dependent on Raf-1 activation. Inhibition of Raf-1 increased MV-mediated IFN-β and MxA expression in response to infection with rMVKS (Figure 7D), while replication was attenuated (Figure 7E). These data demonstrate that inhibition of phosphatase activity and subsequent activation of RIG-I and Mda5 is an important mechanism for MV to escape antiviral responses. Furthermore, our data suggest that viruses such as MV target DC-SIGN to suppress type I IFN responses and thereby infect DCs.
Figure 7. DC-SIGN-Raf-1 signaling induced by MV promotes infection of DCs.
(A–C) Infection of DCs 24 and 48 h after infection with rMVKS after silencing of GADD34 (A), I-1 (B) or Raf-1 (C), determined by flow cytometry by measuring % of EGFP+ cells. Data are presented as mean ± SD of duplicate samples.
(D,E) IFN-β, MxA (D) and MV-N (E) mRNA expression in lung-resident DC-SIGN+ DCs from Cynomolgus macaques 24 h after infection with rMVKS in the absence or presence of Raf inhibitor GW5074, measured by real-time PCR, normalized to GAPDH, and set at 1 in MV-infected cells.
Data are representative of at least three (A-C) or one (D,E) independent experiments. See also Figure S1.
DISCUSSION
Type I IFN responses are crucial in the elimination of viral infections and therefore viruses have developed several strategies to manipulate these antiviral responses to escape immune surveillance. DC-SIGN+ DCs are targeted by MV early in infection (Lemon et al., 2011; Mesman et al., 2012), which makes them an important target for suppression of antiviral responses by the virus. Here we have elucidated the mechanism by which MV suppresses type I IFN responses in DCs early after infection and we have identified DC-SIGN as an important suppressor of RLR activity. Although MV triggered type I IFN responses via RIG-I and Mda5, MV-induced DC-SIGN signaling via Raf-1 dampened RLR-elicited type I IFN expression. Raf-1 activation following DC-SIGN ligation resulted in phosphorylation of the inhibitory subunit I-1 at both Ser and Thr residues, thereby inducing association of I-1 to GADD34-PP1α/γ phosphatases. As a result, GADD34-PP1 holoenzymes were inhibited and unable to dephosphorylate the RIG-I and Mda5 CARD domains, preventing signaling via MAVS to downstream effectors TBK1 and IKKε, IRF3 and subsequent type I IFN expression, hence promoting DC infection. Activation of innate signaling by MV to circumvent cellular antiviral mechanisms precedes other viral evasion tactics that rely on viral replication. In vivo relevance of this DC-SIGN mechanism was emphasized in lung DCs from non-human primates.
MV has been shown to trigger both RIG-I and Mda5 in cell lines (Shingai et al., 2007) and here we show that MV triggers both RIG-I and Mda5 upon infection of DCs, however, their activation is counteracted by the interaction of MV with DC-SIGN allowing efficient infection of DCs. Strikingly, we found that MV infection of DCs almost completely inhibited dephosphorylation of RIG-I residues Ser8 and Thr170 and Mda5 residue Ser88. Dephosphorylation of RIG-I at these residues has recently been described as one of the steps preventing unnecessary signal transduction to MAVS following ligand binding, since it allows accessibility to the CARD domains enabling MAVS interactions (Kolakofsky et al., 2012). Dephosphorylation of these residues by serine-threonine phosphatases PP1α and PP1γ is therefore a prerequisite for RLR activation (Gack et al., 2010; Nistal-Villan et al., 2010; Wies et al., 2013). PP1 phosphatases are involved in many cellular processes, and their localization and actions are defined by specific regulatory and inhibitory subunits (Cohen, 2002; Peti et al., 2013). Here we demonstrated that GADD34 is the regulatory subunit of PP1α/γ holoenzymes that target RIG-I and Mda5 for dephosphorylation, and consequently crucial for subsequent type I IFN induction. Moreover, GADD34-PP1 complexes were specifically inhibited by I-1. Activity of GADD34-PP1 holoenzymes limited MV replication in DCs by inducing type I IFN expression. Identification of PP1α/γ regulatory subunit GADD34 and its specific inhibitor I-1 in the activation process of RIG-I and Mda5 in DCs further advances our knowledge of RLR receptor regulation.
Besides cytosolic RLRs, MV also triggered DC-SIGN signaling, leading to DC-SIGN-mediated Raf-1 activation. We found that MV-induced Raf-1 activation inhibited dephosphorylation of both RIG-I and Mda5, and thereby prevented RLR activation and subsequent antiviral responses. We identified the inhibitory subunit I-1 as a target for the kinase Raf-1. It remains to be established whether phosphorylation of I-1 occurs either directly by Raf-1 or via downstream effector(s). We observed that Raf-1-mediated phosphorylation of I-1 induced its association with GADD34-PP1-specific holoenzymes, which blocked the phosphatase activity of the GADD34-PP1 complexes and thereby prevented RLR activation. The inhibition of both RIG-I and Mda5 in DCs strongly suppressed type I IFN responses and allowed efficient replication of MV in DCs. Thus, these findings have identified PP1 phosphatases as targets for antiviral suppression. DC-SIGN-mediated suppression of RLR activation is crucial for MV to infect DCs, since Raf-1 inhibition by small molecule inhibitors or RNA interference led to a strong increase of IFN-β and ISG expression after MV infection, which inhibited viral replication, underscoring the importance of DC-SIGN-dependent RLR suppressive mechanism in establishing MV replication in DCs. DC-SIGN crosslinking with antibodies against DC-SIGN similarly induced Raf-1 activation and suppressed RLR dephosphorylation, strongly suggesting that antibody crosslinking induces mannose-specific signaling by DC-SIGN (Gringhuis et al., 2009a) and can be used to investigate the role of mannose signaling via DC-SIGN in modulation of immune responses.
Further investigation revealed the transient nature of the DC-SIGN-mediated modulation of RLR signaling. Two pathogenic MV strains inhibited RLR dephosphorylation early after infection in a Raf-1-dependent manner. Our data strongly suggest that the Raf-1-dependent suppressive mechanism is the major mechanism early in infection for MV to establish infection of DCs, since Raf-1 inhibition strongly increased type I IFN responses and decreased MV infection. Notably, at 16 h.p.i, we observed that Mda5 remained phosphorylated, whereas RIG-I was dephosphorylated. These data suggest that another mechanism later during infection prevents Mda5, but not RIG-I dephosphorylation. MV nonstructural P/V/C proteins have been described to interfere with type I IFN induction, e.g. by interacting with RLRs or downstream effector molecules in RLR or interferon signaling pathways (Andrejeva et al., 2004; Palosaari et al., 2003; Ramachandran and Horvath, 2009; Schuhmann et al., 2011; Devaux et al., 2013). The MV-V protein is known to specifically bind Mda5 (Andrejeva et al., 2004). In our accompanying paper, we show that MV-V protein, once synthesized, affects Mda5 dephosphorylation after MV infection (Davis et al., submitted to CHM). Thus, MV targets DC-SIGN early in infection to inhibit both RIG-I and Mda5 dephosphorylation, whereas later in infection, newly synthesized V protein prevents Mda5, but not RIG-I late during infection (Davis et al., submitted to CHM). Because of positive feedback in the RLR pathway (Hou et al., 2011), it is tempting to speculate that DC-SIGN-mediated antiviral suppression, which does not involve viral replication, is required to suppress both RIG-I and Mda5 to enable replication initiation in DCs, after which MV-V protein contributes further to antiviral suppression by blocking Mda5 activation. These data further indicate that PP1 phosphatases are important targets for MV to control RLRs and thereby type I IFN responses.
Crosstalk between signaling pathways of different PRRs, such as DC-SIGN and TLRs, is crucial for induction of pathogen-specific cytokine profiles (Gringhuis et al., 2009b; Gringhuis et al., 2007). We have previously shown that activation of Raf-1 via DC-SIGN modulates NF-kB activity, affecting adaptive responses (Gringhuis et al., 2007). Moreover, we have shown that DC-SIGN-Raf-1 signaling to NF-kB is exploited by HIV-1 for its replication in DCs (Gringhuis et al., 2010). Here we show that MV requires Raf-1 signaling to inhibit dephosphorylation of both RIG-I and Mda5 to escape antiviral responses. Although Raf-1 signaling also induces acetylation of NF-κB (Gringhuis et al., 2009b; Gringhuis et al., 2007), we did not observe a role for NF-κB acetylation in the inhibition of type I IFN responses by MV (data not shown).
Raf-1 has a central role in DC-SIGN-mediated suppression of antiviral responses; we observed both increased RLR activation and type I IFN expression as well as reduced infection of DCs after Raf-1 inhibition. Raf-1 has been implicated in type I IFN expression and viral replication previously (Battcock et al., 2006; Pleschka et al., 2001; Zhang et al., 2012), however, here we report a DC-SIGN-dependent effect of the kinase. A role for DC-SIGN in promotion of MV replication via attachment has been shown earlier (de Witte et al., 2008; Mesman et al., 2012). MV thus seems to target DC-SIGN to promote infection via multiple mechanisms, indicating a pivotal role for the receptor in viral infections.
DC-SIGN functions as an attachment receptor for many viruses such as HIV-1, hepatitis C virus and dengue virus (Geijtenbeek et al. 2009), and some of these viruses might also use DC-SIGN to target phosphatases to decrease virus-induced RLR activation and as such evade antiviral responses (Wies et al., 2013). Indeed, mannose-expressing viruses that interact with DC-SIGN induce Raf-1 activation (Gringhuis et al., 2007), suggesting that PP1 suppression is not restricted to MV. Moreover, since Raf-1 can be activated by other C-type lectin receptors (Gringhuis et al., 2009b), Raf-1 signaling to decrease type I IFN responses could be a more general evasion strategy, not limited to DC-SIGN+ DCs, but could also occur in other cells via other C-type lectin receptors.
These data strongly indicate that PP1 phosphatases are targets for viruses to evade antiviral immunity and preventing suppression of phosphatase activity by viruses might be a novel strategy to combat viral infections. Our study reveals that MV modulates antiviral responses at the level of RLR activation by blocking PP1 activity and highlights the role of DC-SIGN in viral infections.
EXPERIMENTAL PROCEDURES
Cells and ethics statement
Immature monocyte-derived human DCs were cultured for 6–7 days from monocytes obtained from buffy coats of healthy donors (Sanquin), as described in Supplemental Experimental Procedures. This study was done in accordance with the ethical guidelines of the Academic Medical Center.
Broncho alveolar lavage (BAL) cells from MV-seronegative cynomolgus macaques (Macaca fascicularis) had been collected as pre-vaccination control samples during a previous study (De Swart et al., 2006), and stored at −135°C. This study was approved by the independent animal experimentation ethical review committee Dier Experimenten Commissie in Driebergen, The Netherlands, and conducted in compliance with European guidelines (EU directive on animal testing 86/609/EEC) and Dutch legislation (Experiments on Animals Act, 1997). DC-SIGN+ DCs were isolated by positive selection from BAL cells using the CD209 MicroBead kit (Miltenyi) according to the manufacturer’s instructions. Cells from 4 different animals were pooled before experiments.
Cells were stimulated in the absence or presence of small molecule inhibitors or blocking antibodies as described in Supplemental Experimental Procedures. DCs were transfected with 25 nM siRNA using transfection reagent DF4 (Dharmacon) as described (Gringhuis et al., 2009b). Details on siRNAs used are in Supplemental Experimental Procedures. Silencing of expression was verified by real-time PCR and flow cytometry (Figures 3 and S1). Raf-1 expression was determined with anti-Raf-1 (9422; Cell Signaling).
Viruses and infection assay
rMVKSEGFP(3) and rMVIC323EGFP(1) (Hashimoto et al., 2002) were passaged maximally three times in human Epstein-Barr virus-transformed B-lymphoblastic cell lines (B-LCL) or VERO/hCD150 cells to prevent attenuation. The titer was determined by titration on VERO/hCD150 cells and the multiplicity of the infection (MOI) of each experiment was calculated based on this titer. DCs were infected with MV at a MOI of 1, unless stated otherwise. Infection was determined after 24 or 48 h; cells were fixed in 4% (w/v) para-formaldehyde and EGFP levels were determined by flow cytometry.
Quantitative real-time PCR
mRNA was isolated using mRNA capture kit (Roche). For detection of MV-N mRNA, cells were extensively washed before lysis. Detection of MV-N represents viral replication, as only mRNA, but no viral RNA, is isolated by mRNA capture kit. cDNA was synthesized with Reverse transcriptase kit (Promega). PCR amplification was performed in the presence of SYBR Green in an ABI 7500 Fast PCR detection system (Applied Biosystems). Expression of target genes was normalized for GAPDH transcription with Nt = 2Ct (GAPDH)-Ct (target). Primers (Table S1) were designed using Primer express (Applied Biosystems), except for MV-N primers.
RLR, TBK1, IKKε and Raf-1 phosphorylation
Phosphorylation of RIG-I, Mda5, TBK1, IKKε and Raf-1 was detected by flow cytometry and analyzed on a FACS Calibur. Details are described in Supplemental Experimental Procedures. Phosphorylated RIG-I and Mda5 were also detected by immunoblotting as described in Supplemental Experimental Procedures.
IRF3 cellular localization
Nuclear and cytoplasmic extracts were prepared using NucBuster protein extraction kit (Novagen). Proteins were resolved by SDS-PAGE and detected by immunoblotting as described in Supplemental Experimental Procedures. IRF3 was also detected by ELISA (USCN Life Sciences).
I-1 phosphorylation and association with PP1
I-1 was immunoprecipitated from 40 µg of extract with anti-PP1 inhibitor (ab40877; Abcam) on protein A/G-PLUS agarose beads (Santa Cruz), before immunocomplexes were resolved by SDS-PAGE and phosphorylation of I-1 or I-1-associated PP1 proteins were detected by immunoblotting as described in Supplemental Experimental Procedures.
PP1 phosphatase activity
Whole cell lysates were prepared using lysis buffer (50 mM HEPES [pH 7.4], 10% glycerol, 1% Triton X-100, supplemented with protease inhibitors). PP1 activity was measured using ProFluor Ser/Thr PPase assay (Promega); details are described in Supplemental Experimental Procedures.
Statistical analysis
Statistical analyses were performed using the Student’s t-test for paired observations. Statistical significance was set at P < 0.05.
Supplementary Material
HIGHLIGHTS.
MV suppresses antiviral type I IFN responses via DC-SIGN
DC-SIGN signaling inhibits RIG-I and Mda5 activation by blocking phosphatases
Raf-1 induces association of inhibitor I-1 with GADD34-PP1α/γ phosphatases
MV targets phosphatases through DC-SIGN to infect dendritic cells
ACKNOWLEDGEMENTS
Cor Verweij (VUmc, Amsterdam, The Netherlands) provided IFN-β, MxA and ISG15 primers. Yusuke Yanagi (Kyushu University, Fukuoka, Japan) provided Vero/hCD150 cells. This work is supported by the Dutch Organization for Scientific Research (NWO) (S.I.G.: NGI 40-41009-98-8057; T.B.H.G.: ZonMW 91208012, NWO 917-46-367, NWO 912-04-025, VICI 918.10.619) and National Institutes of Health (M.U.G.: R01 AI087846, R21 AI097699; W.P.D.: R01 AI105063).
REFERENCES
- Aggen JB, Nairn AC, Chamberlin R. Regulation of protein phosphatase-1. Chem. Biol. 2000;7:R13–R23. doi: 10.1016/s1074-5521(00)00069-7. [DOI] [PubMed] [Google Scholar]
- Andrejeva J, Childs KS, Young DF, Carlos TS, Stock N, Goodbourn S, Randall RE. The V proteins of paramyxoviruses bind the IFN-inducible RNA helicase, mda-5, and inhibit its activation of the IFN-beta promoter. Proc. Natl. Acad. Sci. U. S. A. 2004;101:17264–17269. doi: 10.1073/pnas.0407639101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JS, Alessi DR, Cohen P. The selectivity of protein kinase inhibitors: a further update. Biochem. J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battcock SM, Collier TW, Zu D, Hirasawa K. Negative regulation of the alpha interferon-induced antiviral response by the Ras/Raf/MEK pathway. J. Virol. 2006;80:4422–4430. doi: 10.1128/JVI.80.9.4422-4430.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavarino G, Claudio N, Couderc T, Dalet A, Judith D, Camosseto V, Schmidt EK, Wenger T, Lecuit M, Gatti E, Pierre P. Induction of GADD34 is necessary for dsRNA-dependent interferon-beta production and participates in the control of Chikungunya virus infection. PLoS Pathog. 2012;8:e1002708. doi: 10.1371/journal.ppat.1002708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen PT. Protein phosphatase 1--targeted in many directions. J. Cell Sci. 2002;115:241–256. doi: 10.1242/jcs.115.2.241. [DOI] [PubMed] [Google Scholar]
- Connor JH, Weiser DC, Li S, Hallenbeck JM, Shenolikar S. Growth arrest and DNA damage-inducible protein GADD34 assembles a novel signaling complex containing protein phosphatase 1 and inhibitor 1. Mol. Cell. Biol. 2001;21:6841–6850. doi: 10.1128/MCB.21.20.6841-6850.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Swart RL, Kuiken T, Fernandez-de Castro J, Papania MJ, Bennett JV, Valdespino JL, Minor P, Witham CL, Yüksel S, Vos H, van Amerongen G, Osterhaus AD. Aerosol measles vaccination in macaques: preclinical studies of immune responses and safety. Vaccine. 2006;24:6424–6436. doi: 10.1016/j.vaccine.2006.05.125. [DOI] [PubMed] [Google Scholar]
- de Swart RL, Ludlow M, de Witte L, Yanagi Y, van Amerongen G, McQuaid S, Yuksel S, Geijtenbeek TB, Duprex WP, Osterhaus AD. Predominant infection of CD150+ lymphocytes and dendritic cells during measles virus infection of macaques. PLoS Pathog. 2007;3:e178. doi: 10.1371/journal.ppat.0030178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries RD, McQuaid S, van Amerongen G, Yuksel S, Verburgh RJ, Osterhaus AD, Duprex WP, de Swart RL. Measles immune suppression: lessons from the macaque model. PLoS Pathog. 2012;8:e1002885. doi: 10.1371/journal.ppat.1002885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Witte L, Abt M, Schneider-Schaulies S, van Kooyk Y, Geijtenbeek TB. Measles virus targets DC-SIGN to enhance dendritic cell infection. J. Virol. 2006;80:3477–3486. doi: 10.1128/JVI.80.7.3477-3486.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Witte L, de Vries RD, van der Vlist M, Yuksel S, Litjens M, de Swart RL, Geijtenbeek TB. DC-SIGN and CD150 have distinct roles in transmission of measles virus from dendritic cells to T-lymphocytes. PLoS Pathog. 2008;4:e1000049. doi: 10.1371/journal.ppat.1000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux P, Priniski L, Cattaneo R. The measles virus phosphoprotein interacts with the linker domain of STAT1. Virology. 2013;444:250–256. doi: 10.1016/j.virol.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duhen T, Herschke F, Azocar O, Druelle J, Plumet S, Delprat C, Schicklin S, Wild TF, Rabourdin-Combe C, Gerlier D, Valentin H. Cellular receptors, differentiation and endocytosis requirements are key factors for type I IFN response by human epithelial, conventional and plasmacytoid dendritic infected cells by measles virus. Virus Res. 2010;152:115–125. doi: 10.1016/j.virusres.2010.06.013. [DOI] [PubMed] [Google Scholar]
- Fitzgerald KA, McWhirter SM, Faia KL, Rowe DC, Latz E, Golenbock DT, Coyle AJ, Liao SM, Maniatis T. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat. Immunol. 2003;4:491–496. doi: 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- Fontana JM, Bankamp B, Rota PA. Inhibition of interferon induction and signaling by paramyxoviruses. Immunol. Rev. 2008;225:46–67. doi: 10.1111/j.1600-065X.2008.00669.x. [DOI] [PubMed] [Google Scholar]
- Gack MU, Nistal-Villan E, Inn KS, Garcia-Sastre A, Jung JU. Phosphorylation-mediated negative regulation of RIG-I antiviral activity. J. Virol. 2010;84:3220–3229. doi: 10.1128/JVI.02241-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gack MU, Shin YC, Joo CH, Urano T, Liang C, Sun L, Takeuchi O, Akira S, Chen Z, Inoue S, Jung JU. TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature. 2007;446:916–920. doi: 10.1038/nature05732. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek TB, den Dunnen J, Gringhuis SI. Pathogen recognition by DC-SIGN shapes adaptive immunity. Future Microbiol. 2009;4:879–890. doi: 10.2217/fmb.09.51. [DOI] [PubMed] [Google Scholar]
- Goodbourn S, Randall RE. The regulation of type I interferon production by paramyxoviruses. J. Interferon Cytokine Res. 2009;29:539–547. doi: 10.1089/jir.2009.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gringhuis SI, den Dunnen J, Litjens M, van der Vlist M, Geijtenbeek TB. Carbohydrate-specific signaling through the DC-SIGN signalosome tailors immunity to Mycobacterium tuberculosis, HIV-1 and Helicobacter pylori. Nat. Immunol. 2009a;10:1081–1088. doi: 10.1038/ni.1778. [DOI] [PubMed] [Google Scholar]
- Gringhuis SI, den Dunnen J, Litjens M, van der Vlist M, Wevers B, Bruijns SC, Geijtenbeek TB. Dectin-1 directs T helper cell differentiation by controlling noncanonical NF-kappaB activation through Raf-1 and Syk. Nat. Immunol. 2009b;10:203–213. doi: 10.1038/ni.1692. [DOI] [PubMed] [Google Scholar]
- Gringhuis SI, den Dunnen J, Litjens M, van Het Hof B, van Kooyk Y, Geijtenbeek TB. C-type lectin DC-SIGN modulates Toll-like receptor signaling via Raf-1 kinase-dependent acetylation of transcription factor NF-kappaB. Immunity. 2007;26:605–616. doi: 10.1016/j.immuni.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Gringhuis SI, van der Vlist M, van den Berg LM, den Dunnen J, Litjens M, Geijtenbeek TB. HIV-1 exploits innate signaling by TLR8 and DC-SIGN for productive infection of dendritic cells. Nat. Immunol. 2010;11:419–426. doi: 10.1038/ni.1858. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Ono N, Tatsuo H, Minagawa H, Takeda M, Takeuchi K, Yanagi Y. SLAM (CD150)-independent measles virus entry as revealed by recombinant virus expressing green fluorescent protein. J. Virol. 2002;76:6743–6749. doi: 10.1128/JVI.76.13.6743-6749.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou F, Sun L, Zheng H, Skaug B, Jiang QX, Chen ZJ. MAVS forms functional prion-like aggregates to activate and propagate antiviral innate immune response. Cell. 2011;146:448–461. doi: 10.1016/j.cell.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang KX, Paudel HK. Ser67-phosphorylated inhibitor 1 is a potent protein phosphatase 1 inhibitor. Proc. Natl. Acad. Sci. U. S. A. 2000;97:5824–5829. doi: 10.1073/pnas.100460897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegame S, Takeda M, Ohno S, Nakatsu Y, Nakanishi Y, Yanagi Y. Both RIG-I and MDA5 RNA helicases contribute to the induction of alpha/beta interferon in measles virus-infected human cells. J. Virol. 2010;84:372–379. doi: 10.1128/JVI.01690-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Kinch LN, Brautigam CA, Chen X, Du F, Grishin NV, Chen ZJ. Ubiquitin-induced oligomerization of the RNA sensors RIG-I and MDA5 activates antiviral innate immune response. Immunity. 2012;36:959–973. doi: 10.1016/j.immuni.2012.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Akira S. Toll-like receptor and RIG-I-like receptor signaling. Ann. N. Y. Acad. Sci. 2008;1143:1–20. doi: 10.1196/annals.1443.020. [DOI] [PubMed] [Google Scholar]
- Kolakofsky D, Kowalinski E, Cusack S. A structure-based model of RIG-I activation. RNA. 2012;18:2118–2127. doi: 10.1261/rna.035949.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackey K, Cory M, Davis R, Frye SV, Harris PA, Hunter RN, Jung DK, McDonald OB, McNutt RW, Peel MR, et al. The discovery of potent cRaf1 kinase inhibitors. Bioorg. Med. Chem. Lett. 2000;10:223–226. doi: 10.1016/s0960-894x(99)00668-x. [DOI] [PubMed] [Google Scholar]
- Lemon K, de Vries RD, Mesman AW, McQuaid S, van Amerongen G, Yuksel S, Ludlow M, Rennick LJ, Kuiken T, Rima BK, et al. Early target cells of measles virus after aerosol infection of non-human primates. PLoS Pathog. 2011;7:e1001263. doi: 10.1371/journal.ppat.1001263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlow M, de Vries RD, Lemon K, McQuaid S, Millar E, van Amerongen G, Yuksel S, Verburgh RJ, Osterhaus AD, de Swart RL, Duprex WP. Infection of lymphoid tissues in the macaque upper respiratory tract contributes to the emergence of transmissible measles virus. J. Gen. Virol. 2013;94:1933–1944. doi: 10.1099/vir.0.054650-0. [DOI] [PubMed] [Google Scholar]
- Mesman AW, de Vries RD, McQuaid S, Duprex WP, de Swart RL, Geijtenbeek TB. A Prominent Role for DC-SIGN(+) Dendritic Cells in Initiation and Dissemination of Measles Virus Infection in Non-Human Primates. PLoS ONE. 2012;7:e49573. doi: 10.1371/journal.pone.0049573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss WJ, Griffin DE. Measles. Lancet. 2012;379:153–164. doi: 10.1016/S0140-6736(10)62352-5. [DOI] [PubMed] [Google Scholar]
- Nistal-Villan E, Gack MU, Martinez-Delgado G, Maharaj NP, Inn KS, Yang H, Wang R, Aggarwal AK, Jung JU, Garcia-Sastre A. Negative role of RIG-I serine 8 phosphorylation in the regulation of interferon-beta production. J. Biol. Chem. 2010;285:20252–20261. doi: 10.1074/jbc.M109.089912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palosaari H, Parisien JP, Rodriguez JJ, Ulane CM, Horvath CM. STAT protein interference and suppression of cytokine signal transduction by measles virus V protein. J. Virol. 2003;77:7635–7644. doi: 10.1128/JVI.77.13.7635-7644.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peti W, Nairn AC, Page R. Structural basis for protein phosphatase 1 regulation and specificity. FEBS J. 2013;280:596–611. doi: 10.1111/j.1742-4658.2012.08509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleschka S, Wolff T, Ehrhardt C, Hobom G, Planz O, Rapp UR, Ludwig S. Influenza virus propagation is impaired by inhibition of the Raf/MEK/ERK signalling cascade. Nat. Cell Biol. 2001;3:301–305. doi: 10.1038/35060098. [DOI] [PubMed] [Google Scholar]
- Plumet S, Herschke F, Bourhis JM, Valentin H, Longhi S, Gerlier D. Cytosolic 5’-triphosphate ended viral leader transcript of measles virus as activator of the RIG I-mediated interferon response. PLoS ONE. 2007;2:e279. doi: 10.1371/journal.pone.0000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran A, Horvath CM. Paramyxovirus disruption of interferon signal transduction: STATus report. J. Interferon Cytokine Res. 2009;29:531–537. doi: 10.1089/jir.2009.0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rima BK, Duprex WP. The measles virus replication cycle. Curr. Top. Microbiol. Immunol. 2009;329:77–102. doi: 10.1007/978-3-540-70523-9_5. [DOI] [PubMed] [Google Scholar]
- Schuhmann KM, Pfaller CK, Conzelmann KK. The measles virus V protein binds to p65 (RelA) to suppress NF-kappaB activity. J. Virol. 2011;85:3162–3171. doi: 10.1128/JVI.02342-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, tenOever BR, Grandvaux N, Zhou GP, Lin R, Hiscott J. Triggering the interferon antiviral response through an IKK-related pathway. Science. 2003;300:1148–1151. doi: 10.1126/science.1081315. [DOI] [PubMed] [Google Scholar]
- Shingai M, Ebihara T, Begum NA, Kato A, Honma T, Matsumoto K, Saito H, Ogura H, Matsumoto M, Seya T. Differential type I IFN-inducing abilities of wild-type versus vaccine strains of measles virus. J. Immunol. 2007;179:6123–6133. doi: 10.4049/jimmunol.179.9.6123. [DOI] [PubMed] [Google Scholar]
- Tatsuo H, Ono N, Tanaka K, Yanagi Y. SLAM (CDw150) is a cellular receptor for measles virus. Nature. 2000;406:893–897. doi: 10.1038/35022579. [DOI] [PubMed] [Google Scholar]
- Tsaytler P, Harding HP, Ron D, Bertolotti A. Selective inhibition of a regulatory subunit of protein phosphatase 1 restores proteostasis. Science. 2011;332:91–94. doi: 10.1126/science.1201396. [DOI] [PubMed] [Google Scholar]
- Wallach D, Kovalenko A. Phosphorylation and dephosphorylation of the RIG-I-like receptors: a safety latch on a fateful pathway. Immunity. 2013;38:402–403. doi: 10.1016/j.immuni.2013.02.014. [DOI] [PubMed] [Google Scholar]
- WHO. Progress in global measles control, 2000–2010. Wkly. Epidemiol. Rec. 2012;87:45–52. [PubMed] [Google Scholar]
- Wies E, Wang MK, Maharaj NP, Chen K, Zhou S, Finberg RW, Gack MU. Dephosphorylation of the RNA sensors RIG-I and MDA5 by the phosphatase PP1 is essential for innate immune signaling. Immunity. 2013;38:437–449. doi: 10.1016/j.immuni.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng W, Sun L, Jiang X, Chen X, Hou F, Adhikari A, Xu M, Chen ZJ. Reconstitution of the RIG-I pathway reveals a signaling role of unanchored polyubiquitin chains in innate immunity. Cell. 2010;141:315–330. doi: 10.1016/j.cell.2010.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Gong R, Qu J, Zhou Y, Liu W, Chen M, Liu Y, Zhu Y, Wu J. Activation of the Ras/Raf/MEK pathway facilitates hepatitis C virus replication via attenuation of the interferon-JAK-STAT pathway. J. Virol. 2012;86:1544–1554. doi: 10.1128/JVI.00688-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.