Abstract
Neural stem/progenitor cells (NSPCs) have the potential to differentiate into neurons, astrocytes, and/or oligodendrocytes. Because these cells can be expanded in culture, they represent a vast source of neural cells. With the recent discovery that patient fibroblasts can be reprogrammed directly into induced NSPCs, the regulation of NSPC fate and function, in the context of cell-based disease models and patient-specific cell-replacement therapies, warrants review.
Introduction
Neural stem/progenitor cells (NSPCs) exist at various locations and times throughout embryonic and adult development. For the sake of this review, we will define NSPCs to be any self-renewing neural cells capable of differentiation to neurons, astrocytes and/or oligodendrocytes. During embryogenesis, NSPCs are responsible for the development of the growing brain; in adults, NSPCs play a role in learning and memory but do not typically contribute to regenerative repair. Though the various subtypes of NSPCs can be described by their expression of unique markers, the extracellular signals and intracellular factors responsible for the regulation of NSPC fate and differentiation frequently overlap. Aberrations in NSPC regulation can lead to diseases ranging from psychiatric disorders to neurodegenerative disease to cancer. With the discovery that induced NSPCs (iNSPCs) can be generated from somatic cells of healthy and diseased individuals, the regulation of NSPC fate and function is increasingly important; iNSPCs have the potential to serve as a novel platform for cell-based replacement therapies and drug-based high-throughput screening for new therapeutics.
Spatial and temporal cues affect NPSC identity
NSPCs are responsible for both embryonic growth and adult neurogenesis. During embryonic development, NSPCs can be found in the neural crest (NC) and the cortex. Although the adult brain was thought to be post-mitotic, neurogenesis occurs in the subventricular zone (SVZ) of the lateral ventricles and the subgranular zone (SGZ) of the hippocampal dentate gyrus in the adult brain 1, 2. Though we will focus on markers that distinguish NSPC populations (Table 1), a number of genes broadly identify NSPCs, particularly SRY (sex-determining region)-box 2 (Sox2) 3, 4 and Nestin 5, 6, as well as Pax6, which is expressed in anterior NSPC populations 7, 8, including some, but not most, NC NSPCs 9, 10.
Table 1.
Partial list of NSPC markers helpful in distinguishing NSPC subpopulations
| NSPC Population |
Marker | References |
|---|---|---|
| Embryonic NC | MSX1/2, PAX3/7, ZIC1, SNAI1, FOXD3, SOX9/10, ID3, p75, AP2 | Hong et al, 2007 13; Light et al, 2005 14; Bellmeyer et al, 2003 15; Mori et al, 1990 16; Stemple et al, 1992 17; de Croze et al, 2011 18 |
| Embryonic Cortical | DLX1/2, NKX2.1, TBR2 | Basak et al, 2007 25; Mizutani et al, 2007 24; Englund et al, 2005 26 |
| Adult SVZ | Type B: GFAP Type A: DCX, PSA-NCAM, DLX2 Type C: MASH1 |
Doetsch et al, 1996 32; (Reviewed by Ming et al, 2011 1 and Zhao et al, 2008 2.) |
| Adult SGZ | RGC (type 1): GFAP, BLBP Progenitor/Neuroblast (type 2): TBR2, MCM2, DCX |
Garcia et al, 2004 39; Suh et al, 2007 40; (Reviewed by Ming et al, 2011 1 and Zhao et al, 2008 2.) |
Embryonic Neural Crest Cells
The NC is a multipotent migratory cell population that transiently exists during embryonic development. Unlike the other NPSCs discussed in this review, NC cells are unique in that they contribute to the peripheral nervous system (PNS). NC cells originate between the dorsal ectoderm and neural tube but migrate and differentiate to sensory neurons, Schwann cells, melanocytes and cells that make up the craniofacial structures such as bone and cartilage 11. WNT, bone morphogenetic proteins (BMPs) and fibroblast growth factors (FGFs) are responsible for NC induction, regulating expression of key NC genes including MSH homeobox 1 and 2 (MSX1/2), paired box 3 and 7 (PAX3/7) and zinc finger protein 1(ZIC1) 12. Together, PAX3 and ZIC1 act in a WNT dependent manner to increase snail (SNAI1), forkhead box D3 (FOXD3), SOX9 and SOX10 proteins 13. Inhibitor of DNA binding (ID) proteins are dominant negative antagonists of the basic helix-loop-helix transcription factors; of these, ID3, a downstream target of c-MYC, is required for the formation and maintenance of NC cells 14, 15. A few other well-established NC markers include p75 nerve growth factor receptor, which can be used to purify NC cells 16, 17, and AP2, a key regulator of NC specification and maintenance 18.
Embryonic stem cell (ESC)-derived NC cells have been reported by several independent groups 19–21. A single-step, highly efficient method generates NCs from ESCs by combining small molecule SMAD inhibition and WNT activation 22.
Embryonic Cortical NSPCs
Cortical development begins in the anterior neural tube and is specified by homeobox proteins such as DLX1, DLX2 and NKX2.1 23. Two types of NSPCs contribute to embryonic cortical development in vivo: radial glia cells (RGCs) and basal (intermediate) progenitors (BPs) 24. RGCs produce neurons and glia, and divide at the ventricular surface. BPs are derived from RGCs, produce only neurons, and divide away from the ventricular surface. In addition to SOX2 and NESTIN, RGCs express astroglial markers including glial fibrillary acidic protein (GFAP), glutamate aspartate transporter (GLAST) and brain lipid-binding protein (BLBP); BPs lack expression of transcription factors that maintain NSPC self-renewal, such as SOX2 and PAX6 that maintain NSPC self-renewal. RGCs are maintained by NOTCH signaling 24, 25, and their transition from RGC to BP is associated with upregulation of Tbr2, a T-domain transcription factor, and downregulation of Pax6 26.
ESCs are robustly differentiated into neuroectodermal precursors 26–28. An early report suggested that ESC-derived neural rosettes could be converted to a RGC-like population by treatment with mitogens such as FGF2 and EGF 29. New methods for the differentiation of ESCs have become increasingly sophisticated, claiming to recapitulate cortical neogenesis in vitro and form, via a NSPC intermediate, cortical neurons that can be transplanted in vivo to generate fully mature cortical neurons29–31. Following neural induction in the presence of two inhibitors of SMAD signaling, the addition of vitamin A efficiently induces a cortical progenitor population that can be expanded in the presence of FGF2 and differentiated into functional cortical neurons following an extended period of corticogenesis.
Adult SVZ Progenitors
SVZ neurogenesis leads to the generation of new neurons, astrocytes and oligodendrocytes in the olfactory bulb 32. The principal precursors in the SVZ are type B cells, a primarily quiescent RGC-like population. Type B cells produce type C cells, a type of transit-amplifying cell that divides rapidly to produce neuroblasts, also known as type A cells (B→C→A). Type A cells migrate along the rostral migratory stream to the olfactory bulb where they integrate with existing circuitry 33, 34. Type B cells are characterized by expression of GFAP, VIMENTIN and NESTIN; proliferating type C cells express Achaete-scute complex-like 1 (MASH1) and NESTIN; migrating and differentiating type A neuroblasts express doublecortin (DCX), PSA-NCAM and homeobox protein DLX2 (reviewed by 1, 2). Similar to RGCs, adult SVZ type B cells are maintained by NOTCH signaling 35. Just an in embryonic cortical development, the fate of adult SVZ progenitors in vivo is determined by positional information; populations of adult SVZ progenitors appear to be restricted and diverse in vivo 36, but much more plastic when cultured in vitro 37.
To our knowledge, SVZ progenitors have not yet been generated from ESCs. Primary SVZs, when cultured, form neurospheres in vitro and are propagated with FGF2 and EGF 28, 38.
Hippocampal SGZ NSPCs
As in the adult SVZ, the hippocampal SGZ is maintained by a population of quiescent RGC-like cells (reviewed by 1, 2). Often referred to as Type 1 cells, these progenitors have long radial processes, express GFAP, BLBP, NESTIN and SOX2 and are generally considered to be the primary progenitors of SGZ neurogenesis 39, 40. Once activated, these cells upregulate TBR2 and DNA replication licensing factor MCM2, and become a replicative cell population, sometimes referred to as Type 2 progenitors. These intermediate NSPCs express DCX and PSA-NCAM, but not GFAP, have only short processes, and in turn give rise to neuroblasts. Type 2 cells may arise from Type 1 cells through a SOX2 dependent reciprocal relationship between the two cell types 40. The multipotency of SGZ NSPCs remains unclear as under certain conditions, hippocampal SGZ NSPCs appear to display significant plasticity in their lineage choice, both in vivo and in vitro 41, 42.
Primary SGZ progenitors form neurospheres and monolayers in vitro, and like SVZ cells, are propagated with FGF2 and EGF 28.
Genetic regulation of NSPCs
Though embryonic and adult NSPCs have different characteristics, likely due to differences in the expression of key proteins described above, it should be noted that NSPCs retain significant plasticity and can robustly alter lineage choice as a consequence of altered environmental signals 41, 42. While the mechanism of plasticity remains unknown, it is well established that external signaling cues regulate many aspects of the replication, differentiation, migration, maturation and death of NSPCs. Despite their differences, many regulator pathways are shared between populations of NSPCs; for example, FGF can be used as a multifunctional growth factor to expand many types of NSPCs in vitro and in vivo 43–45. The differences between populations of NSPCs may not be inherently intrinsic but instead reflective of their different niche environments.
A number of cell types, including endothelial cells, ependymal cells, astrocytes, microglia and NSPCs themselves, contribute to the neurogenic niche and help to regulate all aspects of neurogenesis. Endothelial cells secrete vascular endothelial growth factor (VEGF), which promotes the replication of both embryonic and adult NSPCs 46, 47. Conversely, spatial restriction of other growth factors can confer lineage restriction to specific populations of NSPCs. For example, unique to the SVZ, the ependymal cell layer of the lateral ventricles secretes NOGGIN, a protein that antagonizes BMP-mediated astrocyte differentiation 48–51. Astrocytes, both a product of NSPC differentiation and a component of the neurogenic niche, have been shown to regulate proliferation, fate specification, migration, maturation and synapse formation during neurogenesis, at least in part through modulating the effects of factors secreted from blood vessels and ependymal cells 52, 53. A multitude of signaling pathways regulate these diverse functions. Astrocyte derived WNT signaling influences NSPC replication and differentiation 53, 54. ROBO receptors regulate the rapid migration of SLIT1-expressing neuroblasts 55. Astrocyte-derived cholesterol supports synaptogenesis 56, while astrocyte-secreted extracellular matrix proteins, such as Thrombospondins and Sparc, modulate synapse formation 57, 58. Astrocyte released glutamate stimulates NMDRs and regulates the activity-dependent survival of newborn neurons during adult neurogenesis 59. Under basal conditions, microglia are responsible for phagocytosis of dead neurons 60, while under inflammatory conditions, reactivated microglia secrete both pro- and anti-inflammatory molecules 61. Even cell-cell interactions between NSPCs, through EGFR and Notch signaling, help to maintain the balance between NSCs and NPCs 24, 25, 62.
Although external factors regulate NSPC function, an intracellular network ultimately directs NSPC self-renewal and differentiation. Several common transcription factors are required to maintain many NSPC populations in an undifferentiated state; notable among these are SOX2, PAX6 and TLX. These transcription factors are key targets of cell cycle regulators, microRNAs and epigenetic factors that are major intracellular regulators of neurogenesis; many of these regulators act either cooperatively or in opposition.
Loss of cell-cycle inhibitors, including p16, p21, and p53, results in the activation and subsequent depletion of NSPCs 63–66. For example, p21 directly binds and represses the enhancer of SOX2 63. SOX1/2/3 have overlapping and redundant activity 67; the overexpression of any promotes NSPC proliferation, whereas their loss induces cell-cycle exit and onset of differentiation 3, 68. TLX also maintains NSPCs in the undifferentiated state 69 by recruiting histone deacetylase (HDAC) to repress the transcription of several cell cycle genes 70. MicroRNAs are short (~22 nucleotides) non-coding RNAs involved in gene silencing through translational repression and/or mRNA destabilization. miR-9 was one of the first microRNAs shown to regulate neurogenesis 71; it functions to decrease NSPC proliferation and increase neuronal differentiation. Known targets of miR-9 include TLX 72, FOXG1 73, and HES1 74, as well as key components of the FGF signaling pathway 75. Mitotic exit of NSPCs is accompanied by a subunit switch in the chromatin-remodeling SWI/SNF complex mediated by miR-9 and miR-124 76. Similarly, miR-137 decreases proliferation and promotes differentiation of NSPCs, via targets found primarily in the epigenetic machinery 77–79. Conversely, the epigenetic machinery also regulates microRNA expression. Methyl-CpG binding protein 1 (MBD1) directly represses miR-184; miR-184 promotes NSPC proliferation and inhibits neuronal differentiation 80. DNA methylation at CpG dinucleotides are bound by a family of methyl-CpG binding proteins (MBDs), including MBD1, MBD2, MBD3, MBD4, and MeCP2, leading to the recruitment of histone deacetylase (HDAC) repressor complexes and inactive chromatin structures. MBD1−/− NSPCs exhibit reduced neuronal differentiation and increased genomic instability 81, which may be mediated, at least in part, due to the ability of MBD1 to repress FGF2 expression 82.
Thus, we come full circle. Cell cycle regulators repress transcription factors. Transcription factors and microRNAs regulate the epigenetic machinery. Epigenetic modifiers regulate key growth factors essential in the in vivo and in vitro NSPC niche (Table 2).
Table 2.
Partial list of extrinsic and intracellular factors regulating NSPCs. (Adapted from and reprinted with permission from Zhao et al, 2008.)
| Factors | Proliferation | Differentiation | Survival | References |
|---|---|---|---|---|
| Extrinsic factors | ||||
| Growth factors & neurotrophins | ||||
| BDNF | + (SVZ) | +(SGZ) | Henry et al, 2007 83; Scharfman et al, 2005 84 | |
| EGF | + | Doetsch et al, 2002 37; Aguirre et al, 2010 62 | ||
| FGF | + | Westermann et al, 1990 43; Jin et al, 2003 44; Zhao et al, 2007 45 | ||
| PDGF | + | + (astrocyte) | Jackson et al, 2006 85 | |
| VEGF | + | + (neuron) | Leventhal et al, 1999 46; Cao et al, 2004 47 | |
| Morphogens | ||||
| BMP | + (astrocyte) | Nakashima et al, 1999 48; Yanagisawa et al, 2001 49 | ||
| Noggin | + (neuron) | Lim et al, 2000 50 | ||
| Notch | + | - (neuron) - (glia) |
Mizutani et al, 2007 24; Basak et al 2007 25; Aguirre et al, 2010 62 | |
| SHH | + | Ahn and Joyner, 2005 86; Banerjee et al, 2005 87; Machold et al, 2003 88; Palma et al, 2005 51 | ||
| WNT3 | + | Lie et al, 2005 54; Song et al, 2002 53 | ||
| Intracellular pathways | ||||
| Transcription factors | ||||
| SOX2 | + | Graham et al, 2003 3; Thomas et al, 2008 4 | ||
| TLX | + | Shi et al, 2004 69; Sun et al, 2007 70 | ||
| PAX6 | + (neuron) | Ericson et al, 1997 8; Zhang et al 2010 7 | ||
| Olig2 | + (oligodendrocytes) | Zhou et al, 2001 89 | ||
| Epigenetic regulators | ||||
| MBD1 | + (neuron) | Zhao et al, 2003 81; Li et al, 2008 82 | ||
| Histone deacetylase | + (with TLX) | Hsieh et al, 2004 90; Sun et al, 2007 70 | ||
| Cell cycle regulation | ||||
| p16INK4a | - | Molofsky et al, 2006 64 | ||
| p21 | - | Kippin et al, 2005 66; Marques-Torrejon et al, 2013 63 | ||
| p53 | - | Gil-Perotin et al, 2006 65 | ||
| microRNAs | ||||
| miR9 | - | + (neuron) | Krichevsky et al, 2006 71; Yoo et al, 2009 76 | |
| miR124 | - | Yoo et al, 2009 76 | ||
| miR137 | - | + | Sun et al, 2011 77; Smrt et al, 2010 78; Szulwach et al, 2010 79. | |
| miR184 | + | - (neuron) | Liu et al, 2010 80 |
Systems biology approaches to understanding NSPC regulation
Systems biology applies both experimental and computational approaches to explain how the numerous components of a cellular network interact to regulate molecular and cellular fate. Two approaches are generally employed. First, computational models simulate the intracellular interactions of key regulators, such as ligand-receptor dynamics, signal transduction pathways or transcription factor networks. Second, statistical analyses reduce large gene expression or protein datasets into principal components critical for regulating cell fate choice. While these analyses helped to explain ESC fate regulation 91, 92, their application to NSPCs to date has been limited.
Deterministic computational models, which always yield the same result given the same set of initial conditions93, have been used to mathematically model signaling pathways downstream of key growth factors and cytokines in NSPCs. Such work has already led to insights into both the threshold levels of FGF2 required for NSPC maintenance 94 as well as the neurotrophin-3 (NT-3) stimulation and downstream MAPK pathway activity required for neuronal differentiation 95. Because computational models require precise knowledge of the rate and binding constants of molecular interactions within the network, the lack of experimentally measured constants in NSPCs has somewhat restricted the application of these methods at this point in time.
One common outcome of deterministic models is network bistability, a situation where the continuous change in one input results in a transition between two steady state solutions, converting a graded input signal into an “all or nothing” biological response. For example, fluctuations in NOTCH signaling cause oscillations in Hes1 expression 96–98. In NSPCs, this results in a bistable switch regulating fate decisions between NSPC proliferation and differentiation; inhibition of NOTCH signaling leads to downregulation of Hes1, ultimately upregulating proneural genes such as Neurogenin2 (Ngn2) and the Notch ligand Delta-like1 (Dll1), and increasing neuronal differentiation 99. A subsequent study modeled the signaling cross-talk between the NOTCH, SHH, WNT and EGF signaling pathways in the regulation of Hes1 100.
The incorporation of stochastic statistical models, which include the effects of noise in intracellular signaling pathways 93, will greatly improve computational simulations. Similar to ESCs, NSPCs are heterogeneous, with cells moving between two or more metastable states, each defined by specific patterns of transcription factor expression, chromatin modifications and biases in their differentiation potential 101. For example, ESCs with high Nanog expression are less likely to differentiate than low Nanog expressing cells 92, an observation that might be explained by state transitions resulting from stochastic gene expression 102. Given that variable levels of SOX2, GFAP and HES1 can define distinct NSPC subpopulations in the SVG and SVZ, the application of stochastic models to NSPC behavior will be an important aspect of future computational models of NSPC regulation.
The increasing availability of gene expression and proteomic data sets from proliferating and differentiating NSPCs in vitro and in vivo should facilitate statistical analyses to elucidate regulatory networks. Gene expression changes during in vitro neural differentiation of ESCs were used to identifiy a principal component of approximately 4,000 genes that described degree of neural commitment 103. Subsequently, Bayesian network analysis of ESC neural differentiation found that GFAP upregulates genes in a neural gene set created through principal component analysis 104. Additionally, the gene expression profiles of NSPCs derived from human ESCs, human fetal NSPCs, oligodendrocyte precursor cells and astrocyte precursor cells were compared in order to identify common and unique characteristics of each examined NSPC population 105. Although ESC NSPC samples were generated through different methods in multiple labs, Shin et al identified a distinct ESC NSPC gene expression profile and concluded that ESC NSPCs had limited overall similarity to fetal NSPCs. They further speculated that the high expression of WNT molecules in ESC NSPCs may partially explain their broader differentiation potential relative to fetal NSPCs 105.
Large data sets can also be used to ask specific questions concerning individual signaling pathways or biological processes. For example, Wang et al superimposed those genes differentially expressed before and after NSPC differentiation 106 with the protein–protein interaction network, in order to identify a signaling network regulated by Rho-GDI-γ (guanine nucleotide dissociation inhibitor) during NSPC differentiation 107. Finally, Fietz et al identified genes differentially expressed between cortical zones by using mRNA sequencing of fetal human and embryonic mouse tissue from various cortical zones. Because the expression pattern also correlated to the relative abundance of RGCs, these genes, the majority of which involved cell adhesion and the cell-extracellular matrix, were predicted to promote the proliferation and self-renewal of NSPCs in the developing neocortex 108.
Advances in high throughput experimental techniques are rapidly creating large “omic” datasets. It is our hope that these will be fruitfully mined by systems biology approaches in order to improve our understanding of the complicated intracellular mechanisms regulating NSPC fate.
Discovery of induced NSPCs
The induction of iNSPCs from somatic cells provides a near limitless source of neural cells for cell-replacement therapies in vivo and cell-based in vitro models of neurological disease. iNSPC technology provides a fast and robust protocol to obtain proliferative neural precursors and generates more homogeneous populations than current induced Neuron (iNeuron) methods 109–112, all while bypassing time-consuming induced pluripotent stem cell (iPSC) generation 113, 114. This year, five groups reported the generation of iNSPCs from fibroblasts 115–118 and a sixth reported iNSPC generation from urine 119. Approaches for the iNSPC reprogramming generally follow one of two strategies: 1) incomplete iPSC reprogramming combined with neural growth conditions and 2) overexpression of neural transcription factors.
Incomplete sets of the original iPSC reprogramming cocktail (OCT4, SOX2, KLF4 and c-MYC) can reprogram iNSPCs from fibroblasts. Both constitutive expression of SOX2, KLF4 and c-MYC, when paired with transient OCT4 activity 115, as well as overexpression of OCT4, SOX2 and KLF4 in the presence of another pluripotency gene, ZIC3 116, are sufficient to generate iNSPCs. For reasons not yet understood, the former method generated CNS iNSPCs, while the latter generated PNS iNSPCs. iNSPCs were also generated from exfoliated renal epithelial cells present in urine by transfection with episomal vectors carrying the reprogramming factors OCT4, SOX2, SV40LT and KLF4, as well as the microRNA cluster MIR302–367 and a cocktail of small molecules 119. This last report generated integration-free iNSPCs, demonstrating that iNSPC multipotency can be maintained without persistent transgene expression.
Combinations of neural transcription factors also can be used to reprogram iNSPCs from fibroblasts. Overexpression of SOX2, BRN2, NR2E1, BMI1, HES1, HES5 and c-MYC produced iNSPCs 120, as did a smaller combination of just three factors, SOX2, BRN2 and FOXG1 117. Most recently, a third group used just SOX2 overexpression to generate tripotent iNSPCs 118, though this method is markedly less efficient, requiring several rounds of selection by neurosphere suspensions. Generating iNSPCs with just SOX2 lends itself to the possibility of patterning iNSPCs to specific identities by inducing with SOX2 in conjunction with other subtype-specific growth conditions or transcription factors.
To date, the combinations of factors responsible for iNSPC generation represent some of the most critical genes in the maintenance of NSPC populations in vivo. This is unlikely to be a coincidence. New methods to permit patterning of specific regional identities of iNSPCs are critical. We can imagine that at least two possible approaches are feasible: cellular patterning and environmental signaling. In the first, iNSPC cellular identity is further specified by overexpression of cell-type specific NSPC transcription factors or microRNAs unique to the desired identity. For example, genes such as FOXG1 might help to specify embryonic cortical NSPCs. Alternately, expansion of iNSPCs in growth conditions supportive of a particular fate may provide reinforcing patterning cures. For example, ESC-derived NSPCs are typically cultured in vitro with FGF2 to maintain forebrain identity and with SHH/FGF8/CHIR99021 (a WNT agonist) to maintain midbrain identity 121. By better considering what distinguishes extracellular niche signals and endogenous transcriptional, epigenetic and microRNA modulators of cellular identity, protocols for iNSPC generation will be refined.
Role of NSPCs in disease
While many neurological disorders are traditionally thought to be diseases of mature neurons, new lines of evidence now suggest that aberrant NSPC function may contribute to psychiatric diseases, such as schizophrenia and autism spectrum disorders (ASDs), neurodegenerative diseases, such as Parkinson’s disease (PD) and Alzheimer’s disease (AD) and certain brain tumors (Table 3).
Table 3.
Diseases implicated in aberrant regulation of NSPCs.
| Genes/ Pathways involved |
Induced mutation |
Cellular Phenotype | Behavioral phenotype | Reference | |
|---|---|---|---|---|---|
| Developmental Disorders: | |||||
| ASD/Rett Syndrome |
MEF2C | MEF2C Conditional knockout: Nestin-Cre |
Embryonic: neurons compacted Postnatal: disorganization of cortical plate, immature electrophysiology |
Abnormal anxiety-like behaviors, decreased cognitive function, and marked paw clasping stereotypy |
Li et al, 2008 134 |
| Schizophrenia | GABA- DISC1, AKT- mTOR |
Retroviral shRNA injection into postnatal DGh |
shRNA-DISC1: Accelerated dendritic growth, soma hypertrophy, ectopic primary dendrites; shRNA-NK1: Aberrant positioning, decreased dendritic length |
Neither alone show differences in behavior; synergistic effect in DISC1-KD when exposed to stress |
Kim et al, 2009 125; Kim et al, 2012 126 |
| DiGeorge Syndrome/ Schizophrenia |
22q11 genes: Ranbp1, Cdc451, Hira, Ufd1l, Sept5 |
Large deletion of chromosome 22q11 |
Embryonic: Reduced BP proliferation, aberrant neuronal connectivity |
N/A | Meechan et al, 2009 127 |
| Degenerative Disorders | |||||
| Parkinson's Disease |
α-synuclein, p53, Notch1 |
Lentiviral vectors, siRNA |
Reduced tubulin expression, neurite length, neuronal differentiation and survival, increased apoptosis. |
N/A | Desplats et al, 2012 135 |
| Alzheimer's Disease |
APOE4, PS1, APP |
ApoE Loss-of- function: Nestin-GFP |
Increased proliferation of early NPCs in DG |
Decreased water maze performance |
Mu and Gage, 2011 139 |
| Cancer: | |||||
| Medulloblastoma | miRNA | N/A | N/A | N/A | Genovesi et al, 2011 141 |
Mutations in Disrupted-in-Schizophrenia (DISC1) result in an extremely rare monogenic form of schizophrenia. It has long been known that dominant negative DISC1 expression during mouse embryonic cortical development leads to cellular and behavioral phenotypes consistent with schizophrenia 122–124. Now, it has also been shown that silencing of DISC1 specifically in adult hippocampal NSPCs leads to accelerated dendritic growth, soma hypertrophy and aberrant neural organization 125, mediated in part by depolarizing GABA signaling 126. This was an important proof of concept that loss of DISC1, specifically in adult neurogenesis, can lead to behavioral phenotypes even more severe than those observed by complete loss of neurogenesis.
A deletion at 22q11 (DiGeorge Syndrome) significantly increases genetic risk for schizophrenia and ASD. In a mouse model of this disease, significantly reduced expression of six cell cycle related genes located in the 22q11 region was observed during embryonic development, in conjunction with reduced NSPC proliferation, aberrant migration and altered connectivity 127. Notably, of the two populations of embryonic cortical NSPCs discussed earlier in the review (multipotent RGCs and transit-amplifying BPs), only BPs were observed to be affected in this 22q11 mouse model.
At onset, ASD is often characterized by excessive brain volume. MRI studies have found increased cortical white matter in 2- to 4-year-old autistic children, 128–130 and it has been hypothesized that the surplus of neurons in the prefrontal cortex in ASD131 may be due to excessive proliferation of NSPCs. Among other roles, Myocyte enhancer factor 2 (MEF2) regulates the NSPC differentiation and is a key regulator of signaling pathways that play a role in the pathogenesis of ASD 132, 133. When MEF2 was removed from NSPCs using a Nestin-Cre-floxed-MEF2C, though the conditional knockout mice had normal NSPC proliferation and survival, neurons were abnormally organized and showed immature electrophysiological properties 134. Additionally, these MEF2C null mice showed abnormal anxiety and decreased cognitive function, recapitulating those observed in the MECP2 mouse model of Rett Syndrome. This demonstrates that loss of an autism risk gene in NSPCs may contribute to cellular and behavioral phenotypes consistent with this disorder.
Evidence for abnormal adult neurogenesis has been accumulating in both PD and AD. While α-synuclein protein (α-SYN) accumulation is associated with the death of dopaminergic neurons in PD, in the SGZ NSPCs, it leads to down-regulation of NOTCH1 signaling, ultimately leading to increased NSPC proliferation and impaired neuronal differentiation and maturation 135. This phenotype can be rescued by knockdown of the cell cycle gene p53, suggesting that p53 moderates the effects of α-SYN on repression of NOTCH1 and disruption of neurogenesis 135. Similarly, aberrant expression of a number of candidate AD genes in NSPCs has been reported to cause aberrant NSPC replication and differentiation in the adult hippocampus. Apolipoprotein E (APOE) deficiency leads to increased proliferation of SGZ NSPCs, and ultimately to the depletion of the NSPC pool 136. Knockdown of Presenilin (PS1) promotes increased differentiation in the adult SGZ, reducing neurogenesis 137. Finally, over-expression of Amyloid precursor protein (APP) causes reduced survival and proliferation of SGZ NSPCs 138. These findings imply that dysregulation of neurogenesis may play a role in neurodegenerative disease pathology 139.
Medulloblastoma (MB) is the most frequent form of malignant brain tumor in children, and in a subset of cases, the cell type of origin is thought to be NSPCs 140. When microRNA expression profiles of MB patient samples were analyzed, the target genes of the down-regulated microRNAs in MB tissue included those involved in NSPC migration, cell adhesion and development, particularly reelin (RELN) and myelin transcription factor 1 (MYT1) 141. The target genes of the up-regulated microRNAs, conversely, included many associated with metastatic disease. In addition to regulating healthy NSPCs, described earlier in this review, abnormal microRNA-regulated networks may be associated with transformation of normal NSPCs into brain tumor stem cells. This is consistent with the stem cell hypothesis of cancer, which supposes that cancer can result from the dysregulation of growth and survival pathways in normal stem and progenitor cells.
It seems likely that aberrant NSPC function may cause or exacerbate a number of neurological disorders. We hope that the study of NSPCs may lead to insights into the origins of these disorders and ultimately to therapies by which to correct these malfunctioning cells and ameliorate the disease phenotypes.
Future clinical uses of NSPCs
While laboratory use of iNSPCs could directly lead to insights into the genetic and cellular predisposition of a number of human diseases, iNSPCs can also be used as a source of cells for either cell-based human therapies or drug-based high throughput screens for novel therapeutics.
Neurodegenerative diseases result from the loss of neurons, either locally (such as the dopaminergic neurons of the substantia niagra in PD) or globally (throughout the cortex in AD). Though fetal ventral mesencephalic transplants into PD patients have demonstrated that transplanted cells survive and integrate into existing circuits 142, 143, these methods have yet to deliver clinical benefit. With their ability to expand in vitro and to differentiate into various neural lineages, iNSPCs serve as a promising source for genetically-matched neural cells for cell-replacement therapies for many neurodegenerative diseases. In 2011, midbrain dopaminergic neurons were derived from human iPSCs and successfully engrafted into three animal models of PD 121. Long-term transplantation in the mice and rat models demonstrated robust survival of the dopaminergic neurons and improvements in movement-based phenotypes, though subsequent grafts into monkey models demonstrated the scalability of the process but failed to show phenotypic improvements (Figure 1). A number of technical concerns need to be addressed, particularly the relatively inefficient integration after transplantation. Nonetheless, such progress suggests that iNSPCs are a viable cell source for regenerative medicine.
Figure 1.
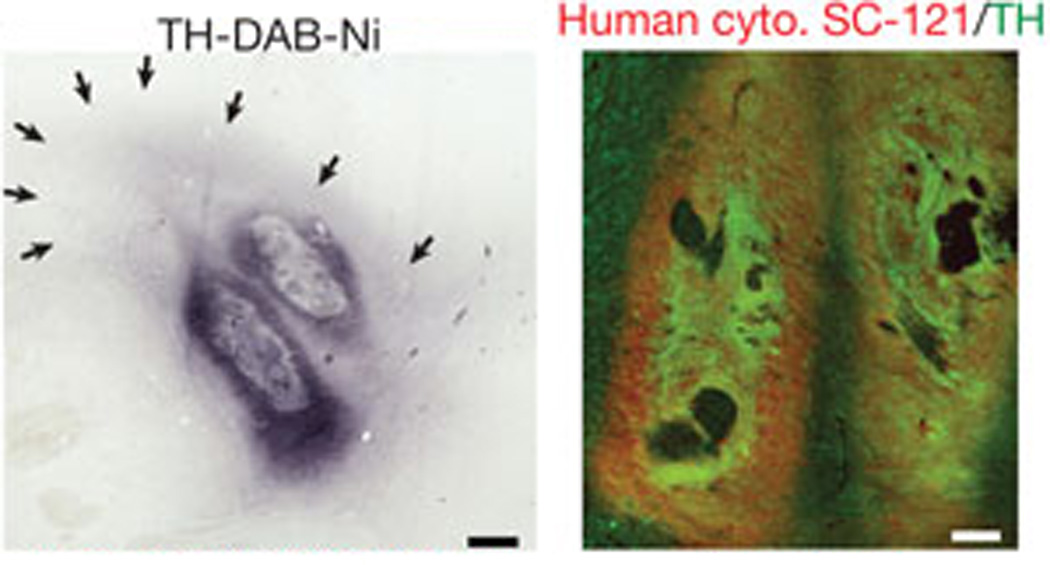
Successful cell transplantation of human ESC-derived dopaminergic neurons into a monkey model of PD (MPTP lesioned rhesus monkeys). Left, representative graft 1 month after transplantation, showing expression of the dopaminergic neuron marker Tyrosine Hydroxylase (TH), with surrounding TH+ fibers (arrows). Right, co-expression of human specific cytoplasm marker SC-121 (red) and TH (green) in graft.
In addition to cell-replacement therapies, NSPCs hold great potential as a platform for high-throughput screening to identify novel drug-based therapies. As a validation for future high-throughput screens, Marchetto et al demonstrated the ability of two compounds, IGF1 and genamicin, to ameliorate the neuronal phenotype of Rett syndrome human iPSC-derived neurons in culture 144. Furthermore, we demonstrated the ability of a clinically utilized antipsychotic, loxapine, to improve the neural connectivity of schizophrenia iPSC-derived neurons in culture 145 and Israel and colleagues demonstrated that treatment of AD iPSC neurons with β-secretase inhibitors led to significant reductions in phospho-Tau levels 146. A major technical issue is the scalability of both neural differentiation and phenotypic screening to permit testing of thousands of compounds. Though we are unaware of any high throughput screening using iNSPCs for neurological diseases to date, a human ESC-based phenotypic assay successfully screened for small molecules that inhibit neuronal degeneration induced by activated microglial cells 147. Their phenotypic screen of >10,000 compounds identified approximately 0.3% hits across a number of biological pathways, providing an excellent proof-of-principle that such screens are both feasible and can yield meaningful hits (Figure 2).
Figure 2.
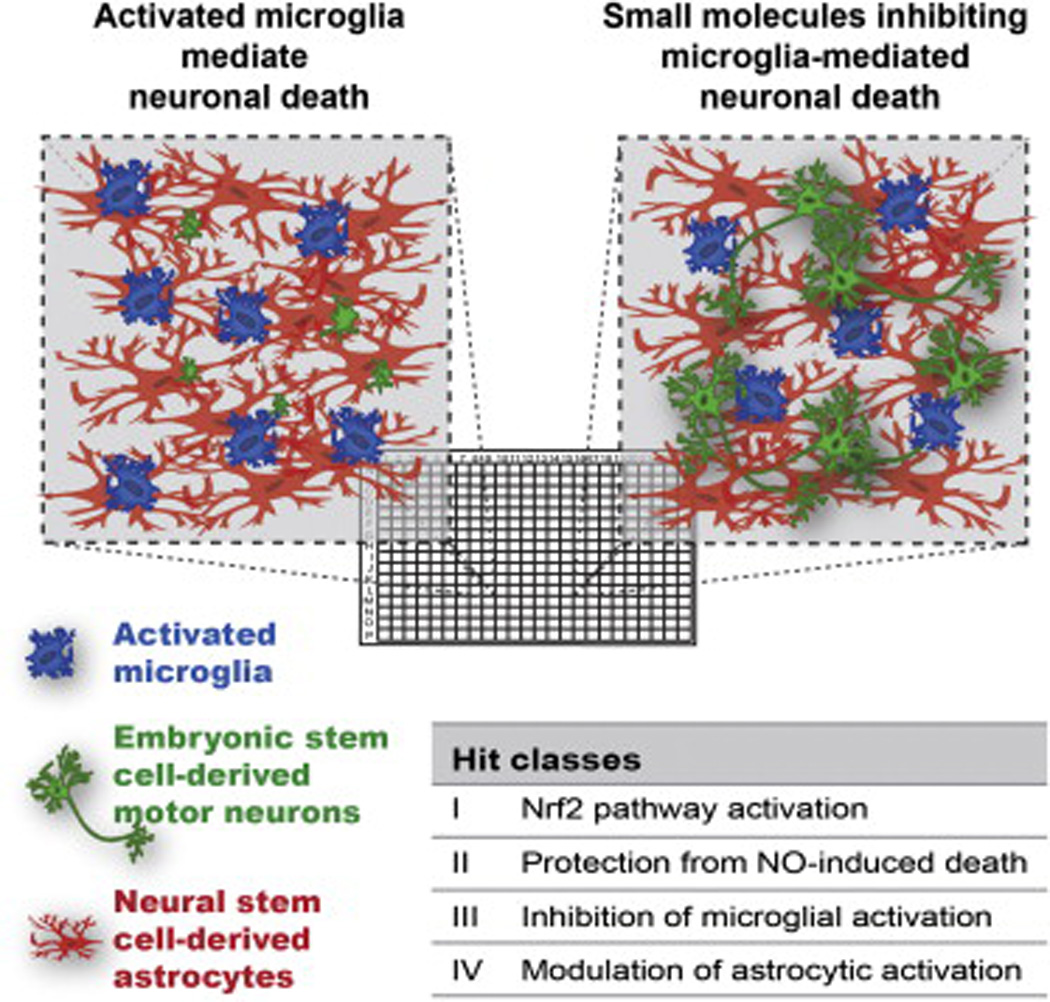
Paradigm of a high-throughput screen for small-molecules capable of neural protection of ESC-derived motor neurons and astrocytes in the presence of activated microglia. After screening more than 10,000 small molecules, the 0.3% hit rate included compounds acting in the activation of the Nrf2 pathway in microglia and astrocytes, direct protection of neurons from nitric-oxide-induced degeneration and inhibition of nitric oxide production by microglia. (Adapted from Hoing et al, 2011)
We predict that iNSPC-based high-throughput screens will soon be routinely performed to identify novel therapeutics across a range of neurological diseases. Furthermore, by screening complex genetic diseases using iNSPCs derived from an increasing number of individuals characterized by heterogeneous clinical outcomes and drug-responsiveness, iNSPC-based high-throughput screens will identify drugs suitable for individuals with known treatment resistance and should ultimately realize the potential system for personalized medicine.
Summary
Until recently, it was thought that adult humans could not generate new neurons. Now, it is widely accepted that neurogenesis continues throughout adult life, though the role for this process in health and disease is still being unraveled. Evidence continues to accumulate that aberrant replication, differentiation or migration of NSPCs can lead to a variety of neurological conditions, and we predict that NSPCs may one day be a therapeutic target in the treatment of psychiatric and neurodegenerative disorders.
Acknowledgements
The Brennand Laboratory is partially funded by a Brain and Behavior Young Investigator Grant.
Footnotes
The authors have declared that no competing interests exist.
References
- 1.Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 3.Graham V, Khudyakov J, Ellis P, Pevny L. SOX2 functions to maintain neural progenitor identity. Neuron. 2003;39:749–765. doi: 10.1016/s0896-6273(03)00497-5. [DOI] [PubMed] [Google Scholar]
- 4.Thomas S, Thomas M, Wincker P, Babarit C, Xu P, Speer MC, Munnich A, Lyonnet S, Vekemans M, Etchevers HC. Human neural crest cells display molecular and phenotypic hallmarks of stem cells. Hum Mol Genet. 2008;17:3411–3425. doi: 10.1093/hmg/ddn235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- 6.Lothian C, Lendahl U. An evolutionarily conserved region in the second intron of the human nestin gene directs gene expression to CNS progenitor cells and to early neural crest cells. Eur J Neurosci. 1997;9:452–462. doi: 10.1111/j.1460-9568.1997.tb01622.x. [DOI] [PubMed] [Google Scholar]
- 7.Zhang X, Huang CT, Chen J, Pankratz MT, Xi J, Li J, Yang Y, Lavaute TM, Li XJ, Ayala M, et al. Pax6 is a human neuroectoderm cell fate determinant. Cell Stem Cell. 2010;7:90–100. doi: 10.1016/j.stem.2010.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ericson J, Rashbass P, Schedl A, Brenner-Morton S, Kawakami A, van Heyningen V, Jessell TM, Briscoe J. Pax6 controls progenitor cell identity and neuronal fate in response to graded Shh signaling. Cell. 1997;90:169–180. doi: 10.1016/s0092-8674(00)80323-2. [DOI] [PubMed] [Google Scholar]
- 9.Kanakubo S, Nomura T, Yamamura K, Miyazaki J, Tamai M, Osumi N. Abnormal migration and distribution of neural crest cells in Pax6 heterozygous mutant eye, a model for human eye diseases. Genes Cells. 2006;11:919–933. doi: 10.1111/j.1365-2443.2006.00992.x. [DOI] [PubMed] [Google Scholar]
- 10.Dottori M, Gross MK, Labosky P, Goulding M. The winged-helix transcription factor Foxd3 suppresses interneuron differentiation and promotes neural crest cell fate. Development. 2001;128:4127–4138. doi: 10.1242/dev.128.21.4127. [DOI] [PubMed] [Google Scholar]
- 11.Sauka-Spengler T, Bronner-Fraser M. A gene regulatory network orchestrates neural crest formation. Nat Rev Mol Cell Biol. 2008;9:557–568. doi: 10.1038/nrm2428. [DOI] [PubMed] [Google Scholar]
- 12.LaBonne C, Bronner-Fraser M. Neural crest induction in Xenopus: evidence for a two-signal model. Development. 1998;125:2403–2414. doi: 10.1242/dev.125.13.2403. [DOI] [PubMed] [Google Scholar]
- 13.Hong CS, Saint-Jeannet JP. The activity of Pax3 and Zic1 regulates three distinct cell fates at the neural plate border. Mol Biol Cell. 2007;18:2192–2202. doi: 10.1091/mbc.E06-11-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Light W, Vernon AE, Lasorella A, Iavarone A, LaBonne C. Xenopus Id3 is required downstream of Myc for the formation of multipotent neural crest progenitor cells. Development. 2005;132:1831–1841. doi: 10.1242/dev.01734. [DOI] [PubMed] [Google Scholar]
- 15.Bellmeyer A, Krase J, Lindgren J, LaBonne C. The protooncogene c-myc is an essential regulator of neural crest formation in xenopus. Dev Cell. 2003;4:827–839. doi: 10.1016/s1534-5807(03)00160-6. [DOI] [PubMed] [Google Scholar]
- 16.Mori N, Birren SJ, Stein R, Stemple D, Vandenbergh DJ, Wuenschell CW, Anderson DJ. Contributions of cell-extrinsic and cell-intrinsic factors to the differentiation of a neural-crest-derived neuroendocrine progenitor cell. Cold Spring Harb Symp Quant Biol. 1990;55:255–264. doi: 10.1101/sqb.1990.055.01.028. [DOI] [PubMed] [Google Scholar]
- 17.Stemple DL, Anderson DJ. Isolation of a stem cell for neurons and glia from the mammalian neural crest. Cell. 1992;71:973–985. doi: 10.1016/0092-8674(92)90393-q. [DOI] [PubMed] [Google Scholar]
- 18.de Croze N, Maczkowiak F, Monsoro-Burq AH. Reiterative AP2a activity controls sequential steps in the neural crest gene regulatory network. Proc Natl Acad Sci U S A. 2011;108:155–160. doi: 10.1073/pnas.1010740107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27:275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawaguchi J, Nichols J, Gierl MS, Faial T, Smith A. Isolation and propagation of enteric neural crest progenitor cells from mouse embryonic stem cells and embryos. Development. 2010;137:693–704. doi: 10.1242/dev.046896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koch P, Opitz T, Steinbeck JA, Ladewig J, Brustle O. A rosette-type, self-renewing human ES cell-derived neural stem cell with potential for in vitro instruction and synaptic integration. Proc Natl Acad Sci U S A. 2009;106:3225–3230. doi: 10.1073/pnas.0808387106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Menendez L, Kulik MJ, Page AT, Park SS, Lauderdale JD, Cunningham ML, Dalton S. Directed differentiation of human pluripotent cells to neural crest stem cells. Nat Protoc. 2013;8:203–212. doi: 10.1038/nprot.2012.156. [DOI] [PubMed] [Google Scholar]
- 23.Rubenstein JL, Rakic P. Genetic control of cortical development. Cereb Cortex. 1999;9:521–523. doi: 10.1093/cercor/9.6.521. [DOI] [PubMed] [Google Scholar]
- 24.Mizutani K, Yoon K, Dang L, Tokunaga A, Gaiano N. Differential Notch signalling distinguishes neural stem cells from intermediate progenitors. Nature. 2007;449:351–355. doi: 10.1038/nature06090. [DOI] [PubMed] [Google Scholar]
- 25.Basak O, Taylor V. Identification of self-replicating multipotent progenitors in the embryonic nervous system by high Notch activity and Hes5 expression. Eur J Neurosci. 2007;25:1006–1022. doi: 10.1111/j.1460-9568.2007.05370.x. [DOI] [PubMed] [Google Scholar]
- 26.Englund C, Fink A, Lau C, Pham D, Daza RA, Bulfone A, Kowalczyk T, Hevner RF. Pax6, Tbr2, and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex. J Neurosci. 2005;25:247–251. doi: 10.1523/JNEUROSCI.2899-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ying QL, Nichols J, Chambers I, Smith A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003;115:281–292. doi: 10.1016/s0092-8674(03)00847-x. [DOI] [PubMed] [Google Scholar]
- 28.Conti L, Cattaneo E. Neural stem cell systems: physiological players or in vitro entities? Nat Rev Neurosci. 2010;11:176–187. doi: 10.1038/nrn2761. [DOI] [PubMed] [Google Scholar]
- 29.Elkabetz Y, Panagiotakos G, Al Shamy G, Socci ND, Tabar V, Studer L. Human ES cell-derived neural rosettes reveal a functionally distinct early neural stem cell stage. Genes Dev. 2008;22:152–165. doi: 10.1101/gad.1616208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi Y, Kirwan P, Smith J, Robinson HP, Livesey FJ. Human cerebral cortex development from pluripotent stem cells to functional excitatory synapses. Nat Neurosci. 2012;15:477–486. doi: 10.1038/nn.3041. S471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mariani J, Simonini MV, Palejev D, Tomasini L, Coppola G, Szekely AM, Horvath TL, Vaccarino FM. Modeling human cortical development in vitro using induced pluripotent stem cells. Proc Natl Acad Sci U S A. 2012;109:12770–12775. doi: 10.1073/pnas.1202944109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doetsch F, Alvarez-Buylla A. Network of tangential pathways for neuronal migration in adult mammalian brain. Proc Natl Acad Sci U S A. 1996;93:14895–14900. doi: 10.1073/pnas.93.25.14895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 34.Lois C, Garcia-Verdugo JM, Alvarez-Buylla A. Chain migration of neuronal precursors. Science. 1996;271:978–981. doi: 10.1126/science.271.5251.978. [DOI] [PubMed] [Google Scholar]
- 35.Basak O, Giachino C, Fiorini E, Macdonald HR, Taylor V. Neurogenic subventricular zone stem/progenitor cells are Notch1-dependent in their active but not quiescent state. J Neurosci. 2012;32:5654–5666. doi: 10.1523/JNEUROSCI.0455-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Merkle FT, Mirzadeh Z, Alvarez-Buylla A. Mosaic organization of neural stem cells in the adult brain. Science. 2007;317:381–384. doi: 10.1126/science.1144914. [DOI] [PubMed] [Google Scholar]
- 37.Doetsch F, Petreanu L, Caille I, Garcia-Verdugo JM, Alvarez-Buylla A. EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron. 2002;36:1021–1034. doi: 10.1016/s0896-6273(02)01133-9. [DOI] [PubMed] [Google Scholar]
- 38.Hartfuss E, Galli R, Heins N, Gotz M. Characterization of CNS precursor subtypes and radial glia. Dev Biol. 2001;229:15–30. doi: 10.1006/dbio.2000.9962. [DOI] [PubMed] [Google Scholar]
- 39.Garcia AD, Doan NB, Imura T, Bush TG, Sofroniew MV. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat Neurosci. 2004;7:1233–1241. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- 40.Suh H, Consiglio A, Ray J, Sawai T, D'Amour KA, Gage FH. In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox2+ neural stem cells in the adult hippocampus. Cell Stem Cell. 2007;1:515–528. doi: 10.1016/j.stem.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jablonska B, Aguirre A, Raymond M, Szabo G, Kitabatake Y, Sailor KA, Ming GL, Song H, Gallo V. Chordin-induced lineage plasticity of adult SVZ neuroblasts after demyelination. Nat Neurosci. 2010;13:541–550. doi: 10.1038/nn.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jessberger S, Gage FH. Stem-cell-associated structural and functional plasticity in the aging hippocampus. Psychol Aging. 2008;23:684–691. doi: 10.1037/a0014188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Westermann R, Grothe C, Unsicker K. Basic fibroblast growth factor (bFGF), a multifunctional growth factor for neuroectodermal cells. J Cell Sci Suppl. 1990;13:97–117. doi: 10.1242/jcs.1990.supplement_13.10. [DOI] [PubMed] [Google Scholar]
- 44.Jin K, Sun Y, Xie L, Batteur S, Mao XO, Smelick C, Logvinova A, Greenberg DA. Neurogenesis and aging: FGF-2 and HB-EGF restore neurogenesis in hippocampus and subventricular zone of aged mice. Aging Cell. 2003;2:175–183. doi: 10.1046/j.1474-9728.2003.00046.x. [DOI] [PubMed] [Google Scholar]
- 45.Zhao M, Li D, Shimazu K, Zhou YX, Lu B, Deng CX. Fibroblast growth factor receptor-1 is required for long-term potentiation, memory consolidation, and neurogenesis. Biol Psychiatry. 2007;62:381–390. doi: 10.1016/j.biopsych.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 46.Leventhal C, Rafii S, Rafii D, Shahar A, Goldman SA. Endothelial trophic support of neuronal production and recruitment from the adult mammalian subependyma. Mol Cell Neurosci. 1999;13:450–464. doi: 10.1006/mcne.1999.0762. [DOI] [PubMed] [Google Scholar]
- 47.Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM, Young D, During MJ. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet. 2004;36:827–835. doi: 10.1038/ng1395. [DOI] [PubMed] [Google Scholar]
- 48.Nakashima K, Yanagisawa M, Arakawa H, Taga T. Astrocyte differentiation mediated by LIF in cooperation with BMP2. FEBS Lett. 1999;457:43–46. doi: 10.1016/s0014-5793(99)00997-7. [DOI] [PubMed] [Google Scholar]
- 49.Yanagisawa M, Nakashima K, Takizawa T, Ochiai W, Arakawa H, Taga T. Signaling crosstalk underlying synergistic induction of astrocyte differentiation by BMPs and IL-6 family of cytokines. FEBS Lett. 2001;489:139–143. doi: 10.1016/s0014-5793(01)02095-6. [DOI] [PubMed] [Google Scholar]
- 50.Lim DA, Tramontin AD, Trevejo JM, Herrera DG, Garcia-Verdugo JM, Alvarez-Buylla A. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron. 2000;28:713–726. doi: 10.1016/s0896-6273(00)00148-3. [DOI] [PubMed] [Google Scholar]
- 51.Palma V, Lim DA, Dahmane N, Sanchez P, Brionne TC, Herzberg CD, Gitton Y, Carleton A, Alvarez-Buylla A, Ruiz i Altaba A. Sonic hedgehog controls stem cell behavior in the postnatal and adult brain. Development. 2005;132:335–344. doi: 10.1242/dev.01567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barkho BZ, Song H, Aimone JB, Smrt RD, Kuwabara T, Nakashima K, Gage FH, Zhao X. Identification of astrocyte-expressed factors that modulate neural stem/progenitor cell differentiation. Stem Cells Dev. 2006;15:407–421. doi: 10.1089/scd.2006.15.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417:39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- 54.Lie DC, Colamarino SA, Song HJ, Desire L, Mira H, Consiglio A, Lein ES, Jessberger S, Lansford H, Dearie AR, et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- 55.Kaneko N, Marin O, Koike M, Hirota Y, Uchiyama Y, Wu JY, Lu Q, Tessier-Lavigne M, Alvarez-Buylla A, Okano H, et al. New neurons clear the path of astrocytic processes for their rapid migration in the adult brain. Neuron. 2010;67:213–223. doi: 10.1016/j.neuron.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goritz C, Mauch DH, Nagler K, Pfrieger FW. Role of glia-derived cholesterol in synaptogenesis: new revelations in the synapse-glia affair. J Physiol Paris. 2002;96:257–263. doi: 10.1016/s0928-4257(02)00014-1. [DOI] [PubMed] [Google Scholar]
- 57.Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, Lawler J, Mosher DF, Bornstein P, Barres BA. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120:421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 58.Eroglu C, Allen NJ, Susman MW, O'Rourke NA, Park CY, Ozkan E, Chakraborty C, Mulinyawe SB, Annis DS, Huberman AD, et al. Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell. 2009;139:380–392. doi: 10.1016/j.cell.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Platel JC, Dave KA, Gordon V, Lacar B, Rubio ME, Bordey A. NMDA receptors activated by subventricular zone astrocytic glutamate are critical for neuroblast survival prior to entering a synaptic network. Neuron. 2010;65:859–872. doi: 10.1016/j.neuron.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sierra A, Encinas JM, Deudero JJ, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, Tsirka SE, Maletic-Savatic M. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell. 2010;7:483–495. doi: 10.1016/j.stem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ekdahl CT, Kokaia Z, Lindvall O. Brain inflammation and adult neurogenesis: the dual role of microglia. Neuroscience. 2009;158:1021–1029. doi: 10.1016/j.neuroscience.2008.06.052. [DOI] [PubMed] [Google Scholar]
- 62.Aguirre A, Rubio ME, Gallo V. Notch and EGFR pathway interaction regulates neural stem cell number and self-renewal. Nature. 2010;467:323–327. doi: 10.1038/nature09347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marques-Torrejon MA, Porlan E, Banito A, Gomez-Ibarlucea E, Lopez-Contreras AJ, Fernandez-Capetillo O, Vidal A, Gil J, Torres J, Farinas I. Cyclin-Dependent Kinase Inhibitor p21 Controls Adult Neural Stem Cell Expansion by Regulating Sox2 Gene Expression. Cell Stem Cell. 2013;12:88–100. doi: 10.1016/j.stem.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Molofsky AV, Slutsky SG, Joseph NM, He S, Pardal R, Krishnamurthy J, Sharpless NE, Morrison SJ. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature. 2006;443:448–452. doi: 10.1038/nature05091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gil-Perotin S, Marin-Husstege M, Li J, Soriano-Navarro M, Zindy F, Roussel MF, Garcia-Verdugo JM, Casaccia-Bonnefil P. Loss of p53 induces changes in the behavior of subventricular zone cells: implication for the genesis of glial tumors. J Neurosci. 2006;26:1107–1116. doi: 10.1523/JNEUROSCI.3970-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kippin TE, Martens DJ, van der Kooy D. p21 loss compromises the relative quiescence of forebrain stem cell proliferation leading to exhaustion of their proliferation capacity. Genes Dev. 2005;19:756–767. doi: 10.1101/gad.1272305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wood HB, Episkopou V. Comparative expression of the mouse Sox1, Sox2 and Sox3 genes from pre-gastrulation to early somite stages. Mech Dev. 1999;86:197–201. doi: 10.1016/s0925-4773(99)00116-1. [DOI] [PubMed] [Google Scholar]
- 68.Bylund M, Andersson E, Novitch BG, Muhr J. Vertebrate neurogenesis is counteracted by Sox1-3 activity. Nat Neurosci. 2003;6:1162–1168. doi: 10.1038/nn1131. [DOI] [PubMed] [Google Scholar]
- 69.Shi Y, Chichung Lie D, Taupin P, Nakashima K, Ray J, Yu RT, Gage FH, Evans RM. Expression and function of orphan nuclear receptor TLX in adult neural stem cells. Nature. 2004;427:78–83. doi: 10.1038/nature02211. [DOI] [PubMed] [Google Scholar]
- 70.Sun G, Yu RT, Evans RM, Shi Y. Orphan nuclear receptor TLX recruits histone deacetylases to repress transcription and regulate neural stem cell proliferation. Proc Natl Acad Sci U S A. 2007;104:15282–15287. doi: 10.1073/pnas.0704089104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Krichevsky AM, Sonntag KC, Isacson O, Kosik KS. Specific microRNAs modulate embryonic stem cell-derived neurogenesis. Stem Cells. 2006;24:857–864. doi: 10.1634/stemcells.2005-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhao C, Sun G, Li S, Shi Y. A feedback regulatory loop involving microRNA-9 and nuclear receptor TLX in neural stem cell fate determination. Nat Struct Mol Biol. 2009;16:365–371. doi: 10.1038/nsmb.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shibata M, Kurokawa D, Nakao H, Ohmura T, Aizawa S. MicroRNA-9 modulates Cajal-Retzius cell differentiation by suppressing Foxg1 expression in mouse medial pallium. J Neurosci. 2008;28:10415–10421. doi: 10.1523/JNEUROSCI.3219-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tan SL, Ohtsuka T, Gonzalez A, Kageyama R. MicroRNA9 regulates neural stem cell differentiation by controlling Hes1 expression dynamics in the developing brain. Genes Cells. 2012;17:952–961. doi: 10.1111/gtc.12009. [DOI] [PubMed] [Google Scholar]
- 75.Leucht C, Stigloher C, Wizenmann A, Klafke R, Folchert A, Bally-Cuif L. MicroRNA-9 directs late organizer activity of the midbrain-hindbrain boundary. Nat Neurosci. 2008;11:641–648. doi: 10.1038/nn.2115. [DOI] [PubMed] [Google Scholar]
- 76.Yoo AS, Staahl BT, Chen L, Crabtree GR. MicroRNA-mediated switching of chromatin-remodelling complexes in neural development. Nature. 2009;460:642–646. doi: 10.1038/nature08139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sun G, Ye P, Murai K, Lang MF, Li S, Zhang H, Li W, Fu C, Yin J, Wang A, et al. miR-137 forms a regulatory loop with nuclear receptor TLX and LSD1 in neural stem cells. Nat Commun. 2011;2:529. doi: 10.1038/ncomms1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Smrt RD, Szulwach KE, Pfeiffer RL, Li X, Guo W, Pathania M, Teng ZQ, Luo Y, Peng J, Bordey A, et al. MicroRNA miR-137 regulates neuronal maturation by targeting ubiquitin ligase mind bomb-1. Stem Cells. 2010;28:1060–1070. doi: 10.1002/stem.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Szulwach KE, Li X, Smrt RD, Li Y, Luo Y, Lin L, Santistevan NJ, Li W, Zhao X, Jin P. Cross talk between microRNA and epigenetic regulation in adult neurogenesis. J Cell Biol. 2010;189:127–141. doi: 10.1083/jcb.200908151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu C, Teng ZQ, Santistevan NJ, Szulwach KE, Guo W, Jin P, Zhao X. Epigenetic regulation of miR-184 by MBD1 governs neural stem cell proliferation and differentiation. Cell Stem Cell. 2010;6:433–444. doi: 10.1016/j.stem.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhao X, Ueba T, Christie BR, Barkho B, McConnell MJ, Nakashima K, Lein ES, Eadie BD, Willhoite AR, Muotri AR, et al. Mice lacking methyl-CpG binding protein 1 have deficits in adult neurogenesis and hippocampal function. Proc Natl Acad Sci U S A. 2003;100:6777–6782. doi: 10.1073/pnas.1131928100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li X, Barkho BZ, Luo Y, Smrt RD, Santistevan NJ, Liu C, Kuwabara T, Gage FH, Zhao X. Epigenetic regulation of the stem cell mitogen Fgf-2 by Mbd1 in adult neural stem/progenitor cells. J Biol Chem. 2008;283:27644–27652. doi: 10.1074/jbc.M804899200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Henry RA, Hughes SM, Connor B. AAV-mediated delivery of BDNF augments neurogenesis in the normal and quinolinic acid-lesioned adult rat brain. Eur J Neurosci. 2007;25:3513–3525. doi: 10.1111/j.1460-9568.2007.05625.x. [DOI] [PubMed] [Google Scholar]
- 84.Scharfman H, Goodman J, Macleod A, Phani S, Antonelli C, Croll S. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp Neurol. 2005;192:348–356. doi: 10.1016/j.expneurol.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 85.Jackson EL, Garcia-Verdugo JM, Gil-Perotin S, Roy M, Quinones-Hinojosa A, VandenBerg S, Alvarez-Buylla A. PDGFR alpha-positive B cells are neural stem cells in the adult SVZ that form glioma-like growths in response to increased PDGF signaling. Neuron. 2006;51:187–199. doi: 10.1016/j.neuron.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 86.Ahn S, Joyner AL. In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature. 2005;437:894–897. doi: 10.1038/nature03994. [DOI] [PubMed] [Google Scholar]
- 87.Banerjee SB, Rajendran R, Dias BG, Ladiwala U, Tole S, Vaidya VA. Recruitment of the Sonic hedgehog signalling cascade in electroconvulsive seizure-mediated regulation of adult rat hippocampal neurogenesis. Eur J Neurosci. 2005;22:1570–1580. doi: 10.1111/j.1460-9568.2005.04317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Machold R, Hayashi S, Rutlin M, Muzumdar MD, Nery S, Corbin JG, Gritli-Linde A, Dellovade T, Porter JA, Rubin LL, et al. Sonic hedgehog is required for progenitor cell maintenance in telencephalic stem cell niches. Neuron. 2003;39:937–950. doi: 10.1016/s0896-6273(03)00561-0. [DOI] [PubMed] [Google Scholar]
- 89.Zhou Q, Choi G, Anderson DJ. The bHLH transcription factor Olig2 promotes oligodendrocyte differentiation in collaboration with Nkx2.2. Neuron. 2001;31:791–807. doi: 10.1016/s0896-6273(01)00414-7. [DOI] [PubMed] [Google Scholar]
- 90.Hsieh J, Nakashima K, Kuwabara T, Mejia E, Gage FH. Histone deacetylase inhibition-mediated neuronal differentiation of multipotent adult neural progenitor cells. Proc Natl Acad Sci U S A. 2004;101:16659–16664. doi: 10.1073/pnas.0407643101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chickarmane V, Troein C, Nuber UA, Sauro HM, Peterson C. Transcriptional dynamics of the embryonic stem cell switch. PLoS Comput Biol. 2006;2:e123. doi: 10.1371/journal.pcbi.0020123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chambers I, Silva J, Colby D, Nichols J, Nijmeijer B, Robertson M, Vrana J, Jones K, Grotewold L, Smith A. Nanog safeguards pluripotency and mediates germline development. Nature. 2007;450:1230–1234. doi: 10.1038/nature06403. [DOI] [PubMed] [Google Scholar]
- 93.Peltier J, Schaffer DV. Systems biology approaches to understanding stem cell fate choice. IET Syst Biol. 2010;4:1–11. doi: 10.1049/iet-syb.2009.0011. [DOI] [PubMed] [Google Scholar]
- 94.Schaffer DV, O'Neill A, Hochrein L, McGranahan T. Quantitative analysis of signaling mechanisms controlling adult neural progenitor cell proliferation. Conf Proc IEEE Eng Med Biol Soc. 2004;7:4965. doi: 10.1109/IEMBS.2004.1404372. [DOI] [PubMed] [Google Scholar]
- 95.Willerth SM, Sakiyama-Elbert SE. Kinetic analysis of neurotrophin-3-mediated differentiation of embryonic stem cells into neurons. Tissue Eng Part A. 2009;15:307–318. doi: 10.1089/ten.tea.2008.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Monk NA. Oscillatory expression of Hes1, p53, and NF-kappaB driven by transcriptional time delays. Curr Biol. 2003;13:1409–1413. doi: 10.1016/s0960-9822(03)00494-9. [DOI] [PubMed] [Google Scholar]
- 97.Jensen MH, Sneppen K, Tiana G. Sustained oscillations and time delays in gene expression of protein Hes1. FEBS Lett. 2003;541:176–177. doi: 10.1016/s0014-5793(03)00279-5. [DOI] [PubMed] [Google Scholar]
- 98.Agrawal S, Archer C, Schaffer DV. Computational models of the Notch network elucidate mechanisms of context-dependent signaling. PLoS Comput Biol. 2009;5:e1000390. doi: 10.1371/journal.pcbi.1000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shimojo H, Ohtsuka T, Kageyama R. Oscillations in notch signaling regulate maintenance of neural progenitors. Neuron. 2008;58:52–64. doi: 10.1016/j.neuron.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 100.Sivakumar KC, Dhanesh SB, Shobana S, James J, Mundayoor S. A systems biology approach to model neural stem cell regulation by notch, shh, wnt, and EGF signaling pathways. OMICS. 2011;15:729–737. doi: 10.1089/omi.2011.0011. [DOI] [PubMed] [Google Scholar]
- 101.Graf T, Stadtfeld M. Heterogeneity of embryonic and adult stem cells. Cell Stem Cell. 2008;3:480–483. doi: 10.1016/j.stem.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 102.Chang HH, Hemberg M, Barahona M, Ingber DE, Huang S. Transcriptome-wide noise controls lineage choice in mammalian progenitor cells. Nature. 2008;453:544–547. doi: 10.1038/nature06965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Aiba K, Sharov AA, Carter MG, Foroni C, Vescovi AL, Ko MS. Defining a developmental path to neural fate by global expression profiling of mouse embryonic stem cells and adult neural stem/progenitor cells. Stem Cells. 2006;24:889–895. doi: 10.1634/stemcells.2005-0332. [DOI] [PubMed] [Google Scholar]
- 104.Nagano R, Akanuma H, Qin XY, Imanishi S, Toyoshiba H, Yoshinaga J, Ohsako S, Sone H. Multi-parametric profiling network based on gene expression and phenotype data: a novel approach to developmental neurotoxicity testing. Int J Mol Sci. 2012;13:187–207. doi: 10.3390/ijms13010187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shin S, Sun Y, Liu Y, Khaner H, Svant S, Cai J, Xu QX, Davidson BP, Stice SL, Smith AK, et al. Whole genome analysis of human neural stem cells derived from embryonic stem cells and stem and progenitor cells isolated from fetal tissue. Stem Cells. 2007;25:1298–1306. doi: 10.1634/stemcells.2006-0660. [DOI] [PubMed] [Google Scholar]
- 106.Lu W, Wang J, Wen T. Downregulation of Rho-GDI gamma promotes differentiation of neural stem cells. Mol Cell Biochem. 2008;311:233–240. doi: 10.1007/s11010-008-9713-9. [DOI] [PubMed] [Google Scholar]
- 107.Wang J, Hu F, Cheng H, Zhao XM, Wen T. A systems biology approach to identify the signalling network regulated by Rho-GDI-gamma during neural stem cell differentiation. Mol Biosyst. 2012;8:2916–2923. doi: 10.1039/c2mb25147g. [DOI] [PubMed] [Google Scholar]
- 108.Fietz SA, Lachmann R, Brandl H, Kircher M, Samusik N, Schroder R, Lakshmanaperumal N, Henry I, Vogt J, Riehn A, et al. Transcriptomes of germinal zones of human and mouse fetal neocortex suggest a role of extracellular matrix in progenitor self-renewal. Proc Natl Acad Sci U S A. 2012;109:11836–11841. doi: 10.1073/pnas.1209647109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pang ZP, Yang N, Vierbuchen T, Ostermeier A, Fuentes DR, Yang TQ, Citri A, Sebastiano V, Marro S, Sudhof TC, et al. Induction of human neuronal cells by defined transcription factors. Nature. 2011;476:220–223. doi: 10.1038/nature10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ambasudhan R, Talantova M, Coleman R, Yuan X, Zhu S, Lipton SA, Ding S. Direct Reprogramming of Adult Human Fibroblasts to Functional Neurons under Defined Conditions. Cell Stem Cell. 2011;9:113–118. doi: 10.1016/j.stem.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yoo AS, Sun AX, Li L, Shcheglovitov A, Portmann T, Li Y, Lee-Messer C, Dolmetsch RE, Tsien RW, Crabtree GR. MicroRNA-mediated conversion of human fibroblasts to neurons. Nature. 2011;476:228–231. doi: 10.1038/nature10323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Caiazzo M, Dell'Anno MT, Dvoretskova E, Lazarevic D, Taverna S, Leo D, Sotnikova TD, Menegon A, Roncaglia P, Colciago G, et al. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature. 2011;476:224–227. doi: 10.1038/nature10284. [DOI] [PubMed] [Google Scholar]
- 113.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 114.Takahashi K, Okita K, Nakagawa M, Yamanaka S. Induction of pluripotent stem cells from fibroblast cultures. Nat Protoc. 2007;2:3081–3089. doi: 10.1038/nprot.2007.418. [DOI] [PubMed] [Google Scholar]
- 115.Thier M, Worsdorfer P, Lakes YB, Gorris R, Herms S, Opitz T, Seiferling D, Quandel T, Hoffmann P, Nothen MM, et al. Direct conversion of fibroblasts into stably expandable neural stem cells. Cell Stem Cell. 2012;10:473–479. doi: 10.1016/j.stem.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 116.Kumar A, Declercq J, Eggermont K, Agirre X, Prosper F, Verfaillie CM. Zic3 induces conversion of human fibroblasts to stable neural progenitor-like cells. J Mol Cell Biol. 2012;4:252–255. doi: 10.1093/jmcb/mjs015. [DOI] [PubMed] [Google Scholar]
- 117.Lujan E, Chanda S, Ahlenius H, Sudhof TC, Wernig M. Direct conversion of mouse fibroblasts to self-renewing, tripotent neural precursor cells. Proc Natl Acad Sci U S A. 2012;109:2527–2532. doi: 10.1073/pnas.1121003109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ring KL, Tong LM, Balestra ME, Javier R, Andrews-Zwilling Y, Li G, Walker D, Zhang WR, Kreitzer AC, Huang Y. Direct reprogramming of mouse and human fibroblasts into multipotent neural stem cells with a single factor. Cell Stem Cell. 2012;11:100–109. doi: 10.1016/j.stem.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang L, Huang W, Su H, Xue Y, Su Z, Liao B, Wang H, Bao X, Qin D, He J, et al. Generation of integration-free neural progenitor cells from cells in human urine. Nat Methods. 2012;10:84–89. doi: 10.1038/nmeth.2283. [DOI] [PubMed] [Google Scholar]
- 120.Tian C, Ambroz RJ, Sun L, Wang Y, Ma K, Chen Q, Zhu B, Zheng JC. Direct conversion of dermal fibroblasts into neural progenitor cells by a novel cocktail of defined factors. Curr Mol Med. 2012;12:126–137. doi: 10.2174/156652412798889018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kriks S, Shim JW, Piao J, Ganat YM, Wakeman DR, Xie Z, Carrillo-Reid L, Auyeung G, Antonacci C, Buch A, et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson's disease. Nature. 2011;480:547–551. doi: 10.1038/nature10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kamiya A, Kubo K, Tomoda T, Takaki M, Youn R, Ozeki Y, Sawamura N, Park U, Kudo C, Okawa M, et al. A schizophrenia-associated mutation of DISC1 perturbs cerebral cortex development. Nat Cell Biol. 2005;7:1167–1178. doi: 10.1038/ncb1328. [DOI] [PubMed] [Google Scholar]
- 123.Kamiya A, Tomoda T, Chang J, Takaki M, Zhan C, Morita M, Cascio MB, Elashvili S, Koizumi H, Takanezawa Y, et al. DISC1-NDEL1/NUDEL protein interaction, an essential component for neurite outgrowth, is modulated by genetic variations of DISC1. Hum Mol Genet. 2006;15:3313–3323. doi: 10.1093/hmg/ddl407. [DOI] [PubMed] [Google Scholar]
- 124.Clapcote SJ, Lipina TV, Millar JK, Mackie S, Christie S, Ogawa F, Lerch JP, Trimble K, Uchiyama M, Sakuraba Y, et al. Behavioral phenotypes of Disc1 missense mutations in mice. Neuron. 2007;54:387–402. doi: 10.1016/j.neuron.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 125.Kim JY, Duan X, Liu CY, Jang MH, Guo JU, Pow-anpongkul N, Kang E, Song H, Ming GL. DISC1 regulates new neuron development in the adult brain via modulation of AKT-mTOR signaling through KIAA1212. Neuron. 2009;63:761–773. doi: 10.1016/j.neuron.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kim JY, Liu CY, Zhang F, Duan X, Wen Z, Song J, Feighery E, Lu B, Rujescu D, St Clair D, et al. Interplay between DISC1 and GABA signaling regulates neurogenesis in mice and risk for schizophrenia. Cell. 2012;148:1051–1064. doi: 10.1016/j.cell.2011.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Meechan DW, Tucker ES, Maynard TM, LaMantia AS. Diminished dosage of 22q11 genes disrupts neurogenesis and cortical development in a mouse model of 22q11 deletion/DiGeorge syndrome. Proc Natl Acad Sci U S A. 2009;106:16434–16445. doi: 10.1073/pnas.0905696106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Carper RA, Moses P, Tigue ZD, Courchesne E. Cerebral lobes in autism: early hyperplasia and abnormal age effects. Neuroimage. 2002;16:1038–1051. doi: 10.1006/nimg.2002.1099. [DOI] [PubMed] [Google Scholar]
- 129.Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, Tigue ZD, Chisum HJ, Moses P, Pierce K, Lord C, et al. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology. 2001;57:245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- 130.Hazlett HC, Poe M, Gerig G, Smith RG, Provenzale J, Ross A, Gilmore J, Piven J. Magnetic resonance imaging and head circumference study of brain size in autism: birth through age 2 years. Arch Gen Psychiatry. 2005;62:1366–1376. doi: 10.1001/archpsyc.62.12.1366. [DOI] [PubMed] [Google Scholar]
- 131.Courchesne E, Mouton P, Calhoun M, Semendeferi K, Ahrens-Barbeau C, Hallet M, Carter Barnes C, Pierce K. Neuron Number and Size in Prefrontal Cortex of Children With Autism. JAMA. 2011;206:2001–2010. doi: 10.1001/jama.2011.1638. [DOI] [PubMed] [Google Scholar]
- 132.Chen RZ, Akbarian S, Tudor M, Jaenisch R. Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat Genet. 2001;27:327–331. doi: 10.1038/85906. [DOI] [PubMed] [Google Scholar]
- 133.Zhou Z, Hong EJ, Cohen S, Zhao WN, Ho HY, Schmidt L, Chen WG, Lin Y, Savner E, Griffith EC, et al. Brain-specific phosphorylation of MeCP2 regulates activity-dependent Bdnf transcription, dendritic growth, and spine maturation. Neuron. 2006;52:255–269. doi: 10.1016/j.neuron.2006.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li H, Radford JC, Ragusa MJ, Shea KL, McKercher SR, Zaremba JD, Soussou W, Nie Z, Kang YJ, Nakanishi N, et al. Transcription factor MEF2C influences neural stem/progenitor cell differentiation and maturation in vivo. Proc Natl Acad Sci U S A. 2008;105:9397–9402. doi: 10.1073/pnas.0802876105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Desplats P, Spencer B, Crews L, Pathel P, Morvinski-Friedmann D, Kosberg K, Roberts S, Patrick C, Winner B, Winkler J, et al. alpha-Synuclein induces alterations in adult neurogenesis in Parkinson disease models via p53-mediated repression of Notch1. J Biol Chem. 2012;287:31691–31702. doi: 10.1074/jbc.M112.354522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yang CP, Gilley JA, Zhang G, Kernie SG. ApoE is required for maintenance of the dentate gyrus neural progenitor pool. Development. 2011;138:4351–4362. doi: 10.1242/dev.065540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gadadhar A, Marr R, Lazarov O. Presenilin-1 regulates neural progenitor cell differentiation in the adult brain. J Neurosci. 2011;31:2615–2623. doi: 10.1523/JNEUROSCI.4767-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Haughey NJ, Nath A, Chan SL, Borchard AC, Rao MS, Mattson MP. Disruption of neurogenesis by amyloid beta-peptide, and perturbed neural progenitor cell homeostasis, in models of Alzheimer's disease. J Neurochem. 2002;83:1509–1524. doi: 10.1046/j.1471-4159.2002.01267.x. [DOI] [PubMed] [Google Scholar]
- 139.Mu Y, Gage FH. Adult hippocampal neurogenesis and its role in Alzheimer's disease. Mol Neurodegener. 2011;6:85. doi: 10.1186/1750-1326-6-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gibson P, Tong Y, Robinson G, Thompson MC, Currle DS, Eden C, Kranenburg TA, Hogg T, Poppleton H, Martin J, et al. Subtypes of medulloblastoma have distinct developmental origins. Nature. 2010;468:1095–1099. doi: 10.1038/nature09587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Genovesi LA, Carter KW, Gottardo NG, Giles KM, Dallas PB. Integrated analysis of miRNA and mRNA expression in childhood medulloblastoma compared with neural stem cells. PLoS One. 2011;6:e23935. doi: 10.1371/journal.pone.0023935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Olanow CW, Schapira AH, Agid Y. Neuroprotection for Parkinson's disease: prospects and promises. Ann Neurol. 2003;53(Suppl 3):S1–S2. doi: 10.1002/ana.10566. [DOI] [PubMed] [Google Scholar]
- 143.Freed CR, Greene PE, Breeze RE, Tsai WY, DuMouchel W, Kao R, Dillon S, Winfield H, Culver S, Trojanowski JQ, et al. Transplantation of embryonic dopamine neurons for severe Parkinson's disease. N Engl J Med. 2001;344:710–719. doi: 10.1056/NEJM200103083441002. [DOI] [PubMed] [Google Scholar]
- 144.Marchetto MC, Carromeu C, Acab A, Yu D, Yeo GW, Mu Y, Chen G, Gage FH, Muotri AR. A model for neural development and treatment of rett syndrome using human induced pluripotent stem cells. Cell. 2010;143:527–539. doi: 10.1016/j.cell.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Brennand KJ, Simone A, Jou J, Gelboin-Burkhart C, Tran N, Sangar S, Li Y, Mu Y, Chen G, Yu D, et al. Modelling schizophrenia using human induced pluripotent stem cells. Nature. 2011 doi: 10.1038/nature09915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Israel MA, Goldstein LS. Capturing Alzheimer's disease genomes with induced pluripotent stem cells: prospects and challenges. Genome Med. 2011;3:49. doi: 10.1186/gm265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hoing S, Rudhard Y, Reinhardt P, Glatza M, Stehling M, Wu G, Peiker C, Bocker A, Parga JA, Bunk E, et al. Discovery of inhibitors of microglial neurotoxicity acting through multiple mechanisms using a stem-cell-based phenotypic assay. Cell Stem Cell. 2012;11:620–632. doi: 10.1016/j.stem.2012.07.005. [DOI] [PubMed] [Google Scholar]


