Abstract
Aging tissues experience a progressive decline in homeostatic and regenerative capacities, which has been attributed to degenerative changes in tissue-specific stem cells, stem cell niches and systemic cues that regulate stem cell activity. Understanding the molecular pathways involved in this age-dependent deterioration of stem cell function will be critical for developing new therapies for diseases of aging that target the specific causes of age-related functional decline. Here we explore key molecular pathways that are commonly perturbed as tissues and stem cells age and degenerate. We further consider experimental evidence both supporting and refuting the notion that modulation of these pathways per se can reverse aging phenotypes. Finally, we ask whether stem cell aging establishes an epigenetic ‘memory’ that is indelibly written or one that can be reset.
Changes in tissue structure are nearly universal in aging animals. Such structural changes are evident at the microscopic and macroscopic levels and are almost invariably accompanied by impairment in normal tissue function and a deficient response to injury. In many tissues, homeostatic tissue maintenance and regenerative responsiveness to injury depend on tissue-specific stem cells—long-lived cells endowed with the capacity to both self-renew and differentiate to produce mature daughters. Stem cells in tissues typically display tissue-specific differentiation patterns, and their ability to balance quiescence with proliferative activity appears to be critical for their survival and maintenance of appropriate physiological and regenerative responses1. The life-long persistence of stem cells in the body makes them particularly susceptible to the accumulation of cellular damage, which ultimately can lead to cell death, senescence or loss of regenerative function. Indeed, stem cells in many tissues have been found to undergo profound changes with age, exhibiting blunted responsiveness to tissue injury, dysregulation of proliferative activities and declining functional capacities. These changes translate into reduced effectiveness of cell replacement and tissue regeneration in aged organisms.
Understanding the molecular processes controlling stem cell survival, self-renewal, quiescence, proliferative expansion and commitment to specific differentiated cell lineages is crucial to determining the drivers and effectors of age-associated stem cell dysfunction. Furthermore, such knowledge will be essential to inform development of therapeutic interventions that can slow, and perhaps reverse, age-related degenerative changes to enhance repair processes and maintain healthy function in aging tissues.
In this Review, we focus on recent discoveries that highlight the dynamic interplay between cell-intrinsic, environmental and systemic signals that have been reported to drive the loss of stem cell functionality during aging. We further discuss the potential reversibility of these processes as possible therapeutic avenues in age-related disease. Finally, we consider whether aging establishes a genetic or epigenetic ‘memory’ in tissue-specific stem cells or their differentiated daughters, and whether such a memory may be reversible, such that aged stem cells can be reset to a more youthful state. These issues are discussed in the context of conserved cellular processes—accumulation of toxic metabolites, DNA damage, proteostasis, mitochondrial dysfunction, proliferative exhaustion, extracellular signaling and epigenetic remodeling—that clearly affect the activity of both stem cells and non-stem cells with age and may be linked to mechanisms that determine organismal lifespan and healthspan (Fig. 1).
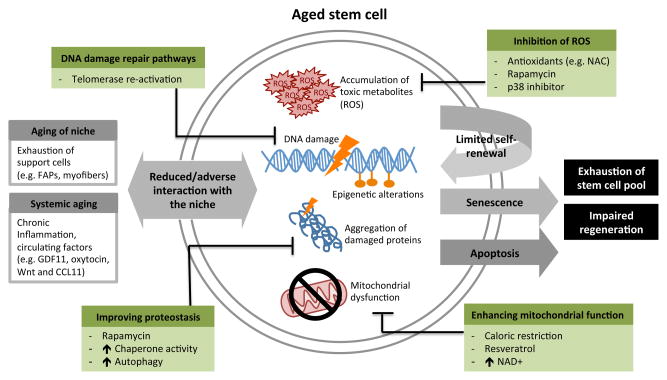
Figure 1.
Common pathways contributing to stem cell loss and dysfunction in the aging process. Common aging phenotypes within the stem cell are shown in orange, in the niche in pink, and the strategies by which to target and hopefully reverse these mechanisms in blue.
Age-related accumulation of toxic metabolites in stem cells
Reactive oxygen species and stem cell aging
To ensure continued function, tissue-resident stem cells, like many other cell types, must withstand potentially damaging modifications of cellular macromolecules that result from exposure to reactive molecules generated as a byproduct of normal metabolism or from extrinsic paracrine and endocrine mediators. Interestingly, analysis of aged stem cells in diverse tissues points to some common effectors and signaling pathways that contribute to stem cell dysfunction in response to toxic metabolites. Primary among these are pathways induced by reactive oxygen species (ROS), which are produced predominantly as a result of electron ‘leak’ during mitochondrial oxidative phosphorylation and appear to contribute to perturbed stem cell function and fate control in the context of aging2–5.
The notion that ROS may drive stem cell dysfunction with age draws precedence from the free radical theory of aging, described by Harman in 1972 (ref. 6). This theory proposes that accumulated cellular damage and declining mitochondrial integrity in aged cells leads to elevated ROS production, which in turn drives a vicious cycle that further damages cellular macromolecules and disrupts mitochondrial oxidative phosphorylation, leading to eventual cellular decomposition6. Yet the causal role of oxidative damage in the aging process remains controversial, in part because of the absence of a clear correlation between the efficacy of antioxidant defenses and extended cell function or longevity. ROS also have essential roles in cell signaling and homeostasis7,8, suggesting a dose-dependent, context-dependent and pleiotropic activity of these reactive mediators that may explain the complex relationship between ROS production, stem cell function and regulation of lifespan and healthspan.
In support of the hypothesis that ROS generation may promote stem cell aging, studies of aged human mesenchymal stem cells have found elevated ROS9, and the frequency of blood-forming hematopoietic stem cells (HSCs) with low ROS levels declines with age in mice10. In addition, in HSCs and neural stem cells (NSCs) of mice, excessive cellular ROS concentrations lead to abnormal proliferation, malignancy and compromised stem cell self-renewal11–14. Conditional ablation in the mouse hematopoietic system of the transcription factors FoxO1, FoxO3 and FoxO4, downstream effectors of the insulin and insulin-like growth factor 1 (IGF-1) signaling pathways, induces a marked increase in ROS accumulation in HSCs. ROS induction in these FoxO-deficient mice correlates with disruption of HSC quiescence, increases in HSC apoptosis and defects in hematopoietic repopulating abilities15. Similar results have been reported for NSCs in these FoxO-deficient mice14 and in HSCs and NSCs in mice lacking only FoxO3 (refs. 16–18). A decline in long-term regeneration capacity of HSCs in mice with conditional deletion of phosphatase and tensin homolog (PTEN) and double deficiency of protein kinase B (AKT) 1 and 2 also indicates that signaling through the PTEN–AKT–mammalian target of rapamycin (mTOR) pathway, upstream of FoxO, senses and controls ROS and regulates HSC self-renewal and survival19–21.
Potential associations of ROS with stem cell dysfunction also have been studied in animal models manipulated to have impaired cellular antioxidant mechanisms. Mice whose hematopoietic system was reconstituted with HSCs lacking the gene encoding superoxide dismutase 2 (Sod2), a mitochondrial antioxidant enzyme regulated by FoxO3, showed oxidative stress–mediated hematopoietic abnormalities22. A cytoprotective role of SOD2 was also observed in mouse NSCs, and its overexpression increased survival of NSCs both in vitro and in vivo23. Moreover, age-dependent alterations in regulation of SOD2 by FoxO3 and the DNA damage sensing serine/threonine protein kinase ATM (ataxia-telangiectasia mutated) have been shown to contribute to loss of HSC function with aging11,16, 24. Studies of Atm−/− mice indicate that HSC self-renewal capacity depends on ATM-mediated inhibition of oxidative stress25. Such studies have led some investigators to suggest that ROS accumulation may drive stem cell aging. However, in some situations, ROS can play an adaptive role in stem cell function. For example, in Drosophila, hematopoietic stem and progenitor cell differentiation is regulated by physiological ROS signaling26. Such observations highlight the need for further studies of the modulators of ROS generation and regulators of ROS activities in young and aged stem cells.
Regulation of oxidative metabolism has further been linked to stem cell aging through studies of the sirtuin family of NAD-dependent protein deacetylases, which are important regulators of oxidative stress, aging and stem cell function. For example, recent findings suggest a role for SIRT1 in maintaining mesenchymal stem cell growth and differentiation, which have been shown to decline with age27, 28. Another member of the sirtuin family, SIRT3, is critical to the maintenance of mitochondria and metabolism and controls ROS production during HSC aging29. In addition, ectopic induction of SIRT3 expression improves the function of aged HSCs by enhancing the antioxidant activity of SOD2 (ref. 30). Together, these studies suggest that aging of stem cells may be reversible by modulation of their metabolic and redox state, which in turn can influence intracellular accumulation of ROS.
Restoring aged stem cell function by targeting toxic metabolites
The antioxidant N-acetyl-L-cysteine (NAC), a precursor of glutathione and a direct ROS scavenger, has been used as a therapeutic agent to ameliorate the damaging effects of ROS31–33. NAC treatment restores the quiescence and reconstitution capacity of ATM-null HSCs24, and also improves survival of a distinct population of myogenic stem cells in skeletal muscle, both in vitro and in vivo34. In FoxO-deficient mice, defects in HSC quiescence, survival and repopulating capacity can be reversed by treatment with NAC15. As yet, however, it remains unclear whether NAC or other antioxidant treatments have a direct or indirect effect on age-dependent deficits in stem cells or stem cell function, and this remains an area of fertile research.
In aggregate, studies in many tissue systems suggest that aging phenotypes caused by uncontrolled accumulation of ROS can be reversed by reducing ROS levels; however, questions remain regarding the pleiotropic functions of ROS in different cell populations. In addition, as highlighted by a recent study reporting improvement of some aging-related phenotypes by expression of the mitochondria-targeted antioxidant skQ1 (ref. 35), a better understanding of the endogenous sources of ROS and the cellular compartments in which they act is also likely to be important for clarifying the stem cell regulatory actions and potential therapeutic value of ROS modulating agents.
DNA damage in aging stem cells
As discussed above, stem cells persist throughout life in a largely quiescent state. As a result, stem cells in aged tissues experience long-term exposure to genotoxic assaults, from both endogenous and exogenous sources, and an apparent accumulation of DNA damage in aged stem cells has been noted in several studies. For example, aged HSCs and muscle stem cells (also called satellite cells) show an increased number of nuclear foci that stain for the phosphorylated form of the variant histone H2A.X (γH2A.X)36–38, which serves as a marker of DNA double-strand breaks39. Accumulation of DNA damage in these stem cells can also be detected using single-cell gel electrophoresis assays38,40. Furthermore, telomeres—specialized chromatin structures that protect chromosome ends to prevent gene erosion and chromosome fusion—are shorter in aged hair follicle stem cells41. However, the level of DNA damage in stem cells must be interpreted with caution as regards its role in aging because such damage can be repaired40 or involved in normal cellular process such as differentiation42 or asymmetrically passed on to daughter cells43.
DNA damage and stem cell function
Elevated levels of damaged DNA in aged stem cells could result from an accumulation of damage over time, an increase in the rate of damage, a decrease in the rate of repair in response to DNA damage, or a combination of these possibilities. Supporting a role for changes in the DNA damage response (DDR), aged human HSC show compromised capacity to repair experimentally introduced DNA damage, such as that produced by ionizing radiation37. These observations suggest that DDR pathways, evolved to safeguard genomic integrity and maximize survival of the organism13,44–46, may show reduced activity in stem cells with age. Indeed, such changes have been observed in other aging cell types47,48. Conversely, studies in yeast, flies and mammals have noted an age-associated increase in the activation of transposable elements, contributing to genomic instability49–51. Whether these shifts in cellular DNA repair pathways result from age-related dysfunction or represent an age-related strategy to respond to changing cellular physiology remains to be clarified.
The specific relevance for tissue stem cells of age-related alterations in DDR pathways is an area of ongoing interest and investigation. Notably, naturally occurring mutations that disrupt the ability of cells to detect, respond to and repair DNA damage frequently result in progeroid phenotypes, which show some features in common with chronological aging and may include perturbation of stem cell functions in various tissues (see Box 1). Likewise, experimentally induced genetic disruption of DDR pathways in mice also produces cell-autonomous defects in HSC function, as assayed by in vivo hematopoietic reconstitution and in vitro colony forming assays11,36,52,53. Yet recent data suggest that it may be unnecessary to posit a change in DDR mechanisms to explain the accumulation of DNA damage in aged stem cells. Analysis of DDR pathways in HSCs indicates that, independent of age, quiescent HSCs show reduced expression of DNA repair genes and accumulate DNA damage that is repaired only when the cells enter the cell cycle, such as during self-renewal or proliferation of HSC-derived progenitors40. Thus, as many HSCs remain quiescent for much of adult life54,55, the continuing accumulation of damage in these cells may eventually exceed a threshold that impairs stem cell function in aged animals.
Box 1. Progeroid Syndromes.
Progeroid syndromes are a group of human hereditary diseases with phenotypes resembling accelerated aging. However, progeroid syndromes are unimodal or segmental, mirroring one or multiple, but never all, of normal aging phenotypes. This distinction has raised some controversy regarding whether knowledge of the molecular bases of progeroid syndromes offers truly useful insights into the process of chronologic aging185. Yet studies indicate that, like chronologic aging, most progeroid syndromes are associated with an increased burden of DNA damage that may directly affect the tissue stem cell compartment186. The segmental nature of each disease may be partially explained by differences in the specific DNA damage repair strategies used by each tissue and cell type186. For example, quiescent HSCs and hair follicle stem cells rely more heavily on non-homologous end joining for repair of double-strand breaks, while actively proliferating intestinal stem cells rely on the homologous recombination pathway56,187,188. In Werner syndromes, Bloom syndrome and Rothmund-Thomson syndrome, patients have mutations in the RecQ helicase family of genes, which are important for the re-initiation of stalled replication forks and suppression of inappropriate homologous recombination. Mesenchymal stem cells, but not neural stem cells, reprogrammed from Werner syndrome iPSCs show premature cellular senescence189. In Hutchinson-Gilford progeria syndrome, cells accumulate an abnormally processed nuclear intermediate filament called progerin. Progerin is a product of the lamin A gene, and its accumulation in the nuclear envelope leads to increased DNA damage in reprogrammed mesenchymal stem cells derived from patients with this syndrome190. Finally, patients with dyskeratosis congenita have mutations in the DKC1 gene, an important component of the telomerase complex191. Defects in telomere maintainance lead to deprotection of telomeres and, in HSCs, cause failure to sustain bone marrow reconstituting activity192.
The mechanisms by which accumulation of DNA damage in aged stem cells may contribute to stem cell dysfunction are still being elucidated. Genotoxic lesions could cause stem cell senescence or apoptosis (Fig. 2) and might directly affect gene regulation, leading to alterations in stem cell self-renewal and differentiation. Some studies suggest that the ensuing loss of homeostasis in aging tissues might further create a microenvironment that favors the selection of stem cells with higher self-renewal but also neoplastic potentials46.
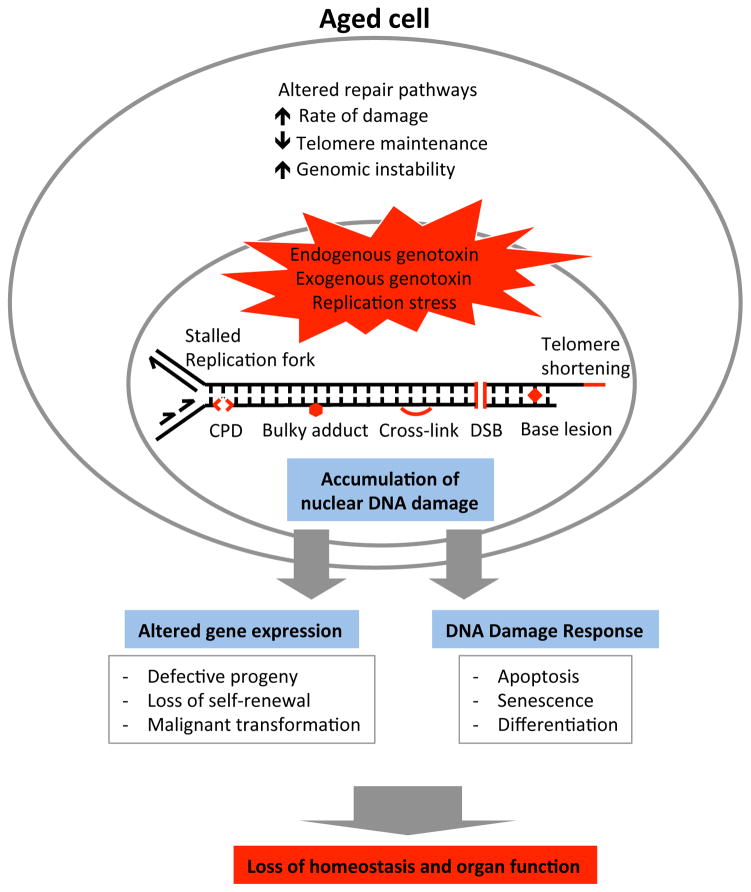
Figure 2.
Types of DNA damage in the aging genome that may affect stem cell function. Products of and effectors of DNA damage known to increase and accumulate during aging are shown, along with their effects on stem cell aging. CPD, cyclobutane pyrimidine dimer; DSB, double-strand break.
Interestingly, it has been suggested that the very mechanisms that some stem cells utilize in an attempt to maintain genomic stability could enhance their susceptibility to oncogenic mutations. In particular, because quiescent cells are restricted to using the error-prone non-homologous end joining pathway for repair of double-strand breaks, DNA repair, if it occurs in quiescent HSCs40, could introduce mutations and promote genomic instability. Consistent with this notion, mouse HSCs that were forced to proliferate showed fewer mutational events after exposure to DNA-damaging radiation, suggesting that in certain instances a break from quiescence that enables engagement of the high-fidelity homologous recombination pathway can help to maintain stem cell genomic integrity56.
To counter the potential for malignant transformation, an important downstream consequence of activation of the DNA damage checkpoint is induction of cellular senescence—an irreversible cell cycle arrest induced in response to cellular stresses, including telomere shortening57–59. An essential effector of senescence, p16INK4a, has been suggested to promote aging phenotypes in stem cells, whereas abolishing p16INK4a function can enhance the regenerative potential of stem cells from the brain and bone marrow (see below).
Targeting DNA damage repair pathways to restore stem cell function
Accumulation of DNA damage in stem cells may trigger the production of defective progeny, stem cell senescence or neoplastic transformation60,61, leading to age-dependent loss of organ function and homeostasis62 It is therefore reasonable to propose that an increase in the activity of DNA repair pathways might slow or prevent the accumulation of age-related defects in stem cells and thereby promote healthy functioning of aged tissues, particularly if such an intervention can take place before the establishment of DNA damage in the genome. To achieve such a globally enhanced DNA repair system would likely require the identification of master regulator genes that synthesize inputs from multiple DDR pathways to exert sufficient impact in the correct tissues and at the appropriate time and dosage, in conjunction with additional signaling pathways. This approach has been illustrated in the case of telomerase reactivation. In particular, studies in mice indicate that it is possible to reverse degenerative phenotypes in aged animals rendered genetically deficient in telomerase activity (through inactivation of the telomerase RNA component mTERC) by late life reactivation of mTERC63. Analysis of various cell types in these animals showed reduced foci of DNA damage, marked by staining with the DDR protein 53BP1, and decreased apoptosis. In addition, neural stem cells from mTERC-reactivated animals showed enhanced multipotency, suggesting recovered neurogenic function. Further examination of additional DNA damage repair/response pathways under analogous inducible settings will be informative to access the full rejuvenation potential of these pathways.
Yet while upregulation of the telomeric pathway may in theory reverse telomere attrition and rescue the function of aged stem cells and postmitotic somatic cells, this pathway is also used by cancer cells to overcome replicative senescence. Overexpression of the mouse telomerase reverse transcriptase catalytic subunit (mTERTcs) has been shown to induce a variety of malignancies64–66. Only when mTERTcs was overexpressed in a tumor-resistant background, produced by increasing gene dosage of p53 and p16/p19, could the balance be tipped in favor of function-promoting effects, resulting in prolonged median lifespan and improved neuromuscular coordination67. A recent parabiosis study further demonstrated the potential to correct DNA damage accumulated in aging mouse satellite cells via circulating systemic factors, with associated improvement of stem cell and tissue functions38. Whether it will be possible to translate these findings to design effective therapeutic strategies that exploit the beneficial effects of DDR and checkpoint regulation on aging-related phenotypes while mitigating risks of tumorigenesis remains to be seen.
Protein homeostasis and stem cell aging
Just as genome stability is critical for stem cell survival and function, maintenance of the stem cell proteome is equally important for assuring stem cell identity. The cellular processes responsible for the synthesis, folding and turnover of proteins are known collectively as protein homeostasis, or proteostasis, pathways68–70. Proteostasis is essential for development and for most cellular functions, including replication of genetic material, catalysis of metabolic reactions, maintenance of cellular architecture, signaling and immune responses69,70. Protein quality control is regulated by a complex protein network that monitors the concentration, subcellular location and folding of proteins and includes molecular chaperones and folding enzymes, as well as pathways that drive protein degradation when needed, through the proteasome, lysosome and autophagy pathways69–71.
Defects in proteostasis commonly lead to aberrant folding, toxic aggregation and accumulation of damaged proteins, which can in turn cause cellular damage and tissue dysfunction68. Indeed, age is one of the main risk factors for most diseases associated with protein misfolding, including neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, Huntington’s disease and amyotrophic lateral sclerosis72. This association suggests that aged cells may be more prone to form and accumulate misfolded protein aggregates71,73. Recent studies have reported age-dependent deficits in the activities of both the autophagy-lysosomal and the ubiquitin-proteasome systems, which represent the principal proteolytic systems implicated in protein quality control74,75. Yet other studies have challenged the view that proteolytic activity declines with age, showing instead that autophagic potential or proteasome activity remains intact in aged cells76,77 and attributing age-related disruptions in proteostasis mainly to increasing damage caused by metabolic stress that overwhelms the protective capacity of proteolytic systems76.
As regards stem cell function, proteostasis has been implicated as an important determinant of stem cell maintenance through studies in HSCs showing that deletion of autophagy-related gene 7 (Atg7) increases ROS levels and depletes HSCs78. This notion has been further supported by Warr et al., who reported autophagy induction by FoxO3A to be an essential mechanism that protects HSCs from metabolic stress in aging76. Also, mTOR, a potent activator of protein translation and inhibitor of autophagy, has been shown to regulate the self-renewal and differentiation of HSCs and intestinal stem cells79,80.
Proteostasis was also shown to be fundamental to maintenance of identity in human embryonic stem cells (hESCs). FOXO4 promotes proteasome activity in hESCs by regulating PSDM11 expression, thereby encouraging proteostasis and maintenance of pluripotency markers, such as POU5F1, OCT4, NANOG, SOX2, UTF1, DPPA4, DPPA2, ZFP42 and TERT81. In addition to its known roles in promoting longevity and stem cell function in tissues throughout the body, FoxO transcriptionally activates several protein-folding chaperone genes82–84 some of which may regulate aging84–87. However, a direct link between chaperone function and stem cell aging has not yet been demonstrated. It will be important to characterize the mechanisms that regulate proteostasis in stem cells to determine if they differ from those in other cells and to assess how they may influence changes in stem cell function during aging.
Reversing stem cell aging by improving protein homeostasis
Although no studies yet have directly addressed whether stimulation of autophagy or proteasome activity has particular beneficial effects for aged stem cells, pharmaceutical inhibition of mTOR by rapamycin treatment has been reported to restore the self-renewal and hematopoietic potential of aged HSCs88. Likewise, data from mice genetically manipulated to preserve chaperone-mediated autophagy (CMA), a form of autophagy involving chaperone-dependent selection of substrates, show improved cellular homeostasis, enhanced resistance to stressors and preservation of organ functions with age89. Overexpression of heat shock protein 70 (HSP70), a chaperone protein that recognizes damaged proteins in CMA, also protects against age- and disease-related tissue degeneration in brain, heart and skeletal muscle90–92. These studies provide intriguing insights into potential therapeutic interventions for age-related dysfunction, although the specific role, if any, of CMA and chaperone proteins in stem cell aging is a subject for future research.
Mitochondrial dysfunction and stem cell aging
A direct relationship between mitochondrial dysfunction and aging has been suggested by many studies (for review, see ref. 93), but dissecting the role of mitochondria in stem cell aging has been challenging, in part because the rarity of tissue stem cells renders traditional biochemical and bioenergetics analyses generally inapplicable in these cells. Mitochondria are often referred to as the cell’s powerhouse and contain their own small genome, which encodes key proteins required for their primary function of generating ATP by oxidative phosphorylation. Age-dependent reductions in mitochondrial function leading to respiratory chain dysfunction have been observed in several cell systems and have been thought to result largely from accumulation of mutations in mitochondrial DNA (mtDNA)94. The free radical theory of aging points to elevated ROS as a principal cause of mtDNA mutation, as mitochondria represent the primary sources of ROS in the cell5.
An alternative view on mtDNA mutagenesis suggests that replication errors mediated by mitochondrial DNA polymerase γ may instead drive mtDNA mutation95. Supporting this view, a genetically modified mtDNA mutator mouse, harboring a proofreading-defective mtDNA polymerase γ, displays severe respiratory chain deficiency and premature aging phenotypes96,97. Furthermore, hematopoietic progenitor cells of the mtDNA mutator mice are affected during fetal development, and their neural stem cells exhibit declining quiescence and decreased self-renewal capacity in response to mtDNA mutation accumulation98. Another study reported decreased proliferation and increased apoptosis of epithelial crypt cells in mtDNA mutator mice and impaired in vitro development of stem cell–derived organoids99. The abnormal phenotypes observed in somatic stem cells from mtDNA mutator mice can be rescued by supplementation with NAC, implying that mtDNA mutations alter the oxidative state of stem cells and that this impairs their functional capacity98. In addition to these animal studies, several studies have related mtDNA defects to human stem cell aging100–102. However, in adult mouse HSCs, rapidly accumulating mtDNA mutations induced by the presence of proofreading-defective mtDNA polymerase have little functional effect on the HSC pool and instead cause differentiation blocks and/or disappearance of downstream progenitors103. This discrepancy between HSC phenotypes observed in these animals and those observed in chronologically aged animals suggests that mtDNA mutations may not be a primary driver of tissue stem cell aging103. Still, the mechanisms by which mtDNA mutations do affect stem cell function and their role in stem cell aging in other tissues requires further investigation.
Aside from primary mitochondrial lesions such as mtDNA mutation, secondary alterations in mitochondrial function driven by age-related cellular and metabolic changes may also contribute to the aging process93. Nutrient sensing and energy homeostasis have been implicated as connecting mitochondrial function and longevity104,105. In HSCs, an age-dependent decrease in nutrient uptake capacity has been observed with age, implying that the nutrient sensing pathway is involved in stem cell aging76. In fact, several signaling pathways discussed above that have been reported to regulate stem cell activity during aging function in nutrient sensing as well. For instance, the liver kinase B1 (LKB1)-adenine monophosphate-activated protein kinase (AMPK) pathway senses cellular energy levels and activates glucose uptake when necessary by inhibiting mTOR in HSCs106. In addition, activation of the Phosphoinositide 3-kinase (PI3K)–AKT pathway by growth factors leads to inhibition of FoxO transcription factors107, which cross talk with AMPK to control the balance between oxidative phosphorylation and glycolysis in response to intracellular and environmental cues108. Finally, Peroxisome proliferator-activated receptor gamma coactivator 1 (PGC-1) a key regulator of mitochondrial energy metabolism, increases mitochondrial biogenesis and delays the age-dependent decline in intestinal stem cells and gut function in Drosophila109, although its role in mammalian stem cells has yet to be tested.
Improving mitochondrial function in aged stem cells
Enhanced mitochondrial function has been associated with improved stem cell function and tissue regeneration in mammals. Short-term calorie restriction (CR) increases the frequency and function of skeletal muscle stem cells in both young and old mice, potentially by increasing mitochondrial content and promoting oxidative metabolism110. CR has been widely employed as a dietary intervention to prevent or reduce the severity of aging phenotypes, and long-term CR can delay the onset of age-related pathologies and extend lifespan in many species, from yeast to primates111. CR can also enhance or maintain stem cell function during aging by increasing the expression of brain-derived neurotrophic factor in the brain and reducing the mTOR complex 1 (mTORC1) pathway in intestine79,112. Signaling pathways related to nutrient sensing and uptake, such as SIRT1, AMPK and FoxO, may represent molecular effectors of the benefits of CR on stem cell activity113,114, although a complete understanding of how CR affects stem cell function must await further studies. CR effects are not likely to be limited to enhanced mitochondrial function, however, as CR also modulates mitochondrial hormesis, autophagy induction, production and scavenging of ROS, and DNA damage115–117. However, resveratrol, a naturally occurring small molecule that induces metabolic benefits resembling those of CR, does increase mitochondrial biogenesis, in part through activation of SIRT1 and PGC-1α116.
A recent study reported that reduced levels of NAD+ contribute to the mitochondrial decay associated with skeletal muscle aging and that SIRT1 modulates this process118. The imbalance between nuclearly and mitochondrially encoded respiratory chain subunits caused by declining nuclear NAD+ was shown to disrupt oxidative phosphorylation in aged mice, whereas raising nuclear NAD+ levels restored mitochondrial function in old mice. These data indicate that age-related mitochondrial dysfunction is reversible and contributes to dysfunction in aged tissues. On the basis of this result, one may extrapolate that ectopic expression of the single-subunit yeast alternative NADH dehydrogenase in Drosophila intestinal stem cells delays the onset of intestinal aging and extends lifespan by regulating the NAD+/NADH ratio in aged cells119. It will be interesting to determine whether the roles of NAD+ and NADH dehydrogenase in age-dependent mitochondrial dysfunction are conserved in stem cells of different tissues and different species and whether similar strategies to manipulate the NAD+/NADH ratio in humans could have therapeutic benefit.
Stem cell depletion and senescence in aged tissues
Progressive loss of stem cell functionality caused by the age-related cellular permutations reviewed above typically leads to depletion of the functional stem cell pool in aged animals. Age-dependent reduction in stem cell number or perturbed cell-cycle activity has been reported in skeletal muscle stem cells, neural stem cells and germline stem cells110, 120–122. Depletion of the stem cell pool with age may occur because these cells lose self-renewal activity and terminally differentiate, thereby exiting the stem cell pool, or because they undergo apoptosis or senescence induced by exposure to cellular stress, although it is not exactly clear what mechanisms inform the choice between apoptosis and senescence. Regulation of cell number and cycling activity is different for aging HSCs, which actually show increases in cell number, possibly due to a reduced capacity for asymmetric division123, with no difference in cycling activity124. Yet aged animals still experience a functional depletion of the HSC pool, as aged HSCs show reduced hematopoietic reconstituting activity and skewed differentiation potential such that they overproduce myeloid lineage cells and underproduce lymphoid lineage cells.
Unlike apoptotic cells, senescent cells remain alive, despite “an essentially irreversible arrest of cell division”125. Generally, senescent cells upregulate cell cycle inhibitors like p53/p21 and p16INK4a (also called Cdkn2a)126. Furthermore, senescent cells secrete bioactive mediators, including degradative enzymes, inflammatory cytokines and growth factors, which may further drive stem cell dysfunction with aging127. One study reported that stem cells from geriatric skeletal muscle lose the capacity to enter reversible quiescence and become “pre-senescent” as a result of dysregulation of p16INK4a (ref. 120). Silencing p16INK4a in geriatric satellite cells restores their quiescence and regenerative potential, suggesting that this pre-senescent state is potentially escapable120. Old p16INK4a−/− HSCs also exhibit increased cell cycle activity and enhanced engraftment128, although these enhancements come with a price, as p16INK4a−/− animals show an increased incidence of malignancy. Whether the impact of p16INK4a deficiency reflects a direct role in HSCs themselves remains somewhat controversial, however, as some groups have failed to detect p16INK4a expression in HSCs freshly isolated from young or aged mice129. Given these data, it will be interesting to determine whether p16INK4a expression may be upregulated in vivo or in vitro in response to HSC activation.
Stem cell exhaustion may also be driven by an imbalance of stem cell quiescence and proliferation. Maintaining this balance is crucial to sustain the stem cell pool through multiple rounds of tissue regeneration, as demonstrated by studies in the Drosophila intestine, and in HSCs and neural stem cells of p21-null mice109,130,131. Excessive proliferation caused by loss of cell cycle regulation results in accelerated exhaustion of these stem cell pools. In a similar vein, increased fibroblast growth factor 2 (FGF2) signaling in the aged muscle stem cell niche also drives loss of quiescence in satellite cells, leading to impaired muscle regenerative capacity132. Changes in intracellular levels of ROS may also determine the balance between quiescence and proliferation of HSCs, and perhaps other adult stem cells133. Low levels of ROS are required for quiescence and stem cell maintenance, whereas ROS induction leads to proliferation and differentiation programs26. When combined with the prevailing notion that ROS increases with aging, this result provides a possible explanation for how stem cells lose quiescence and are depleted in aged tissues.
The balance between quiescence and proliferation in aged stem cells might also be disrupted by an increased demand for replacement of mature cells in aged tissues, as suggested by observations in disease models such as Duchenne muscular dystrophy (DMD). Repetitive damage, caused by continual degeneration of structurally unsound DMD fibers, elicits constant demand for regeneration, diminishing the regenerative capacity of muscle stem cells as a consequence of replicative aging134. By analogy, repeated demands on stem cells to generate replacement cells over an entire lifetime may cause an accumulation of cell-autonomous defects, including telomere erosion, mutations and epigenetic dysregulation, and eventual replicative exhaustion.
Replenishing the stem cell pool in aged tissues
Rescuing stem cells from senescence or activating them from a quiescent state could potentially enhance regenerative potential in aged tissues; however, such strategies must maintain the balance of stem cell self-renewal and differentiation. Stem cell transplantation also has been actively explored as a method for the replenishment of regenerative cells in the context of degenerative diseases such as bone marrow failure, muscular dystrophy, diabetes and neurological diseases135–138.
The therapeutic paradigm of stem cell transplantation is to infuse stem cells from an unaffected donor or gene-corrected autologous cells into a patient. For example, treatment of severe combined immunodeficiency, an X-linked immune cell disorder caused by mutations in the gene encoding the common γ-chain cytokine receptor subunit, via gene correction would seek to repair the gene encoding the common γ-chain in the patient’s own cells and then transfer these back to the patient to reconstitute a ‘corrected’ hematopoietic system. Currently, HSC transplantation is the only stem cell–based therapy widely accepted and used clinically, and it provides an effective cure for hematologic diseases such as leukemia and lymphoma135. Studies in mice suggest that stem cell transplantation may someday be possible for other tissues as well. Several groups have reported successful replenishment of the stem cell pool by satellite cell transplantation into dystrophic or irradiated/injured muscles in mice120,139,140, although the rarity of satellite cells in muscle tissue has been a challenge for translating muscle stem cell transplantation to clinical practice141 Importantly, given the many cell non-autonomous signals that contribute to stem cell aging (see below), it is unlikely that simple replacement of aged stem cells with younger ones will be sufficient therapeutically. Instead, strategies that attempt to restore the function of residual stem cells or that combine such strategies with stem cell transplant should be considered. For example, recent studies of aged muscle stem cells have shown that inhibition of the p38 mitogen-activated protein kinase signaling pathway in endogenous muscle stem cells can restore muscle regenerative potential in aged mice142 and can be combined with ex vivo culture on soft hydrogels to enhance the transplantation efficacy of these cells140.
Reprograming of somatic cells into a desired type of stem cell could provide an alternative source of cells for transplant. In addition to the advantage of being highly amenable to genetic correction143,144, this strategy has the advantage of generating potentially unlimited numbers of immune-compatible cells for transplant. Induced expression of PAX7 and exposure to defined chemical cocktails145–147 in human pluripotent stem cells has been shown to generate engraftable muscle progenitor cells that replenish the satellite cell compartment and sustain formation of dystrophin-positive myofibers in dystrophin-deficient mice145,147. Similarly, transplantation of neural stem or progenitor cells derived from human induced pluripotent stem cells (iPSCs) in a mouse of model of spinal cord injury was reported to stimulate continual motor function recovery148,149. However, there remain important obstacles that must be overcome to realize the full potential of stem cell–based therapy for these and other disorders, including the generation of sufficient numbers of cells for transplantation, the promotion of stem cell survival and engraftment in host tissues, and potential immunological barriers to long-term engraftment of allogeneic or even gene-corrected autologous cells after transplantation141.
Extracellular signals and stem cell aging
The stem cell niche
Stem cells reside in specialized microenvironments, called niches, that promote their maintenance and regulate their functions150–152. Aging of niche cells and age-dependent alterations in the acellular components of stem cell niches can cause maladaptive changes in stem cell function. In Drosophila, the number of cap cells and hub cells, which serve as support cells for germline stem cells (GSCs) in the testes and ovaries, respectively, decreases with age. This loss of the stem cell niche impairs bone morphogenetic protein (BMP) signaling from the niche that is necessary for GSC maintenance122,153. Reduced levels of E-cadherin and Unpaired also contribute to aging of GSC niche by weakening the association between GSCs and cap or hub cells; overexpression of these signaling molecules rescues the age-dependent decline in GSC frequency122,153. Similarly, expression of glial cell–derived neurotrophic factor, which is required for normal regulation of spermatogonial stem cell self-renewal and differentiation, is markedly reduced with age in Sertoli cells, the nurse cells of the testes154. Moreover, consecutive transplantation of spermatogonial stem cells to the testes of young male mice was sufficient to maintain stem cell function for extended periods of time, underscoring a major role of aging of the niche in GSC functional decline and suggesting the potential for reversing aging phenotypes by targeting the niche155.
The significance of niche cell support has been documented for other tissue stem cells as well. Paneth cells, found in close association with intestinal stem cells in humans and mice, are an important source of niche factors, including epidermal growth factor, Wnt3A and Notch ligand79,156, although the effect of aging on their activity is yet to be elucidated. In skeletal muscle, a complex network of interactions between satellite cells and their niche is emerging. For example, immune cells serve as critical regulators of satellite cell function during muscle regeneration, orchestrating inflammatory responses and ensuring appropriate proliferation of myogenic cells to prevent their premature differentiation157. Likewise, fibro/adipogenic progenitors (FAPs), stromal constituents of skeletal muscle, facilitate muscle regeneration and serve as a principal source of soluble extracellular matrix regulators158,159. The presence of FAPs in the muscle is essential for proper muscle repair after injury158, and this regulatory relationship is likely to involve direct communication between FAPs and satellite cells, as co-culturing FAPs with satellite cells enhances the rate of differentiation of primary myogenic progenitors159. Muscle fibers also serve as an important source of niche signals to regulate satellite cell function with age. Aged muscle fibers fail to adequately induce the Notch ligand delta, and they overproduce fibroblast growth factor 2 (ref. 132,160). Both of these alterations contribute to acquired loss of satellite cell responsiveness in older animals.
Systemic factors
In addition to locally produced signals, aging also causes changes in circulating factors that can profoundly impact tissue stem cells. In old mice, elevated transforming growth factor-β (TGF-β) that accumulates in aged muscle impedes regeneration and satellite cell proliferation161. Chronic elevation of inflammatory mediators in aged tissue also may contribute to age-related dysfunction162. Activation of the NF-κB pathway is a known transcriptional signature that mediates chronic inflammation in aged skin, skeletal muscle, bone and nervous system163, although the direct effects of NF-κB activation on stem function in these tissues is still under investigation. Accumulation of senescent cells in aging tissues can be another driver of chronic inflammation, as these cells secrete inflammatory factors, growth regulators, proteases and other signaling molecules127, affecting neighboring cells in the local environment and promoting senescence and inflammation.
Targeting extracellular signals to reverse stem cell aging
Strong evidence that aging of stem cells involves dominant signals from the local and systemic environment comes from studies in mice in which surgical and genetic systems have been used to modulate the aged tissue and systemic milieu. In particular, exposure of old skeletal muscle to a youthful systemic environment through heterochronic parabiosis, which creates a conjoined circulatory system between young and old animals, can promote efficient satellite cell activation in old muscle164. This surgical intervention also enhances rates of neurogenesis, remyelination and other measures of neural function in the old parabiont, suggesting that changes in stem cell function in the aging central nervous system are also affected by blood-borne factors165–167. Intriguingly, at least part of the ‘rejuvenating’ effect of a young systemic environment appears related to the higher levels of circulating growth differentiation factor 11 (GDF11) and oxytocin present in young blood as compared to aged38,166,168,169. Treatment of aged mice with either recombinant GDF11 or oxytocin reverses dysfunction of aged satellite cells and restores robust regenerative function in aged mice38,169. GDF11 supplementation in old mice further reverses age-related hypertrophy in cardiac muscle168, enhances neural stem cell and neuronal function166 and improves physical activity38. These studies raise the possibility that manipulation of blood-borne factors could provide an attractive strategy for the treatment of age-related muscle disease.
In addition to systemic factors, recent work suggests that targeting senescent cells and their products in aging tissues could also help restore stem cell function. Using an inducible genetic model for senescent cell ablation, one study demonstrated that life-long clearance of senescent cells from progeroid mouse tissues, via ablation of p16Ink4a-expressing cells, delays the onset of pathologies in multiple aging tissues, including the fat, muscle and eye170. Clearance of senescent cells only late in life did not improve age-related pathologies but did attenuate their progression170. These results have driven several groups to search for pharmacological strategies that could similarly induce the removal of senescent cells from aged tissues. As the benefits of senescent cell removal are apparent in multiple tissues, these so-called ‘senolytics’, if identified, could prove useful in a number of settings of age-associated pathology, including sarcopenia and metabolic disease.
Epigenetic memory of aging in stem cells
Many studies point to epigenetic regulation as important in determining stem cell function and further indicate that alterations in the epigenome that occur with aging can impinge on cellular processes in aged organisms and their stem cells. The acetylation and methylation status of DNA changes with age, while deletion or overexpression of enzymes responsible for these modifications can alter organismal longevity171. For example, the ASH-2 trithorax complex mediates trimethylation of histone H3 on Lys4 (H3K4me3), and its deletion increases Caenorhabditis elegans lifespan, consistent with excessive H3K4me3 being associated with an aging epigenome172,173. Interestingly, a ‘longevity memory’ of ASH-2 mutant parents is observed in homozygous wild-type offspring for up four generations174. This intriguing observation demonstrates that the aging epigenome functions like a heritable trait, but also can be modified. Pharmacologic treatment that alters histone modification such as acetylation status also shows an effect on longevity in aging and progeroid mouse models, suggesting that potential future therapeutic interventions targeting epigenetic regulators may be feasible175,176. Recent studies also have linked epigenetic variations with aging in stem cells. For example, aging HSCs show site-specific increases in DNA methylation that are particularly targeted to regions of the genome important for lineage-specific gene expression177, and perturbations of histone modifications (for example, H3K4me3) that may alter the expression of HSC self-renewal genes178.
Resetting epigenetic memory in aged stem cells
The development of adult frogs from the transfer of tadpole intestinal somatic nuclei into oocytes showed that cellular memory of somatic cells can be reversed and even erased179. Evidence suggests that a similar approach might be applied to reset the molecular memory of aging cells. Somatic cell nuclear transfer of senescent fibroblasts from aged bovine donors results in cloned calves with fibroblasts that have restored population doubling and increased telomere maintenance compared to age-matched control animals180. It has been further proposed that reprogramming aged somatic cells into iPSCs or iPSC-like cells followed by redifferentiation to the desired somatic cell types, may help reset the memory of aged somatic cells181,182. This possibility has been supported in part by studies in which aged hematopoietic progenitors were reprogrammed to produce iPSCs. These iPSCs were then used to create new HSCs in a chimeric embryo system and yielded complete rejuvenation of hematopoietic activity183. Thus, age-related epigenetic changes offer a model wherein ‘aging memory’ could potentially be altered or erased to restore stem cell function; however, it remains unclear whether more clinically viable rejuvenation strategies, such as modulating circulating factors or deleting senescent cells, which do not involve reprogramming to a fully pluripotent state, actually fully reset the aged epigenome. It may be that stem cells exposed to such restorative interventions adopt some functional features of younger cells but still retain an aging memory. Further analysis of shifts in epigenetic signatures of stem cells exposed to such interventions will help to answer this important question.
Conclusion
Stem cell aging is affected by many different cell-intrinsic and cell-extrinsic pathways, which often show cross-talk in the determination of stem cell function. This interdependence makes it difficult to place particular weight on any one pathway with respect to aging mechanisms; however, certain signals do appear more broadly involved than others, at least given available data, and this could imply that they are more important regulators of aging stem cells. For example, the mTOR pathway has been shown to be a major regulator of both ROS levels and autophagy in HSCs20,78,80, (Fig. 3). Likewise, mitochondrial function, largely regulated by sirtuins, not only affects stem cell functions directly but also causes secondary effects on ROS and nutrient-sensing activities, which further modulate stem cell phenotypes29,30,108,113,184.
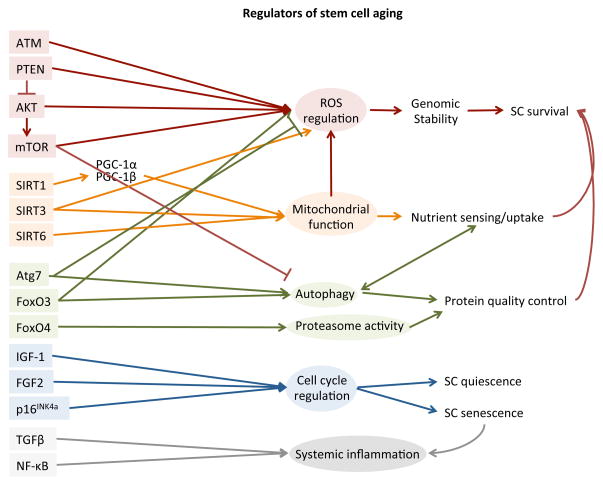
Figure 3.
Signaling pathways involved in aging of stem cells. Major signaling pathways related to aging of stem cells are listed in groups according to stem cell functions that they regulate.
Stem cell–autonomous phenotypes, such as DNA and protein damage caused by accumulated toxic metabolites, as well as non-autonomous stresses resulting from extracellular signals, contribute to a decline in stem cell function and depletion of the stem cell pool. However, in some cases at least, these aging phenotypes can be reversed to restore the regenerative function of stem cells. Such restorative interventions hold promise for the treatment of many diseases, including sarcopenia, heart failure and neurodegeneration, although whether these strategies truly restore stem cell function to a youthful state or instead induce a state of ‘pseudo-youth’ in which the reprogrammed cells retain an epigenetic memory of their true age remains an open and important question. Nonetheless, observations of the reversibility of stem cell aging have generated excitement about the development of ‘rejuvenating’ interventions that could extend the healthy years of life. Interventions that hold particular promise include those that target aging mechanisms that commonly affect tissues impaired by age-related diseases and dysfunction. For example, based on studies genetically targeting senescent cells for removal in progeroid mice170, the development of senolytics, compounds that induce (by direct targeting or mobilization of the immune system) the removal of tissue-ensconced senescent cells holds promise for improving stem cell and organ function across a range of tissue systems. Likewise, the demonstration that common blood-borne signals can influence stem cell behavior and aging phenotypes in the muscle, brain and heart, coupled with the accessibility of these circulating mediators for intervention, should encourage further preclinical and translational studies modulating the systemic microenvironment to enhance maintenance and repair in aged tissues. Finally, the availability of nutritional and pharmacological agents that can target derangements in metabolism of aged cells to improve stem cell function and regenerative potential provides another near-term opportunity for new therapeutic strategies. Ultimately, strategies to combat diseases of aging will likely achieve greater success by focusing on high-level integrators of tissue physiology that can evoke remodeling of aging tissue architecture at the cellular and molecular level.
Improving the healthspan of elders takes on increasing urgency as human lifespans continue to increase, and even small gains in healthspan could dramatically lessen the impact of an aging population on the health care system and economy. Although clearly there is much still to be discovered about stem cell function, aging and the pathways to therapy, what we know so far clearly encourages further studies. By continuing to clarify the fundamental mechanisms by which stem cells age, ongoing research will develop interventions that someday may change the way we age.
Acknowledgments
We thank all members of the Wagers laboratory for advice and comments during the preparation of this article. This work was funded in part by US National Cancer Institute grant T32CA-0216 from the Massachusetts General Hospital Department of Pathology (Y.D.L.), by US National Institutes of Health (NIH) grant T32DK007260 (J.O.), and by NIH grants 1R01 AG033053 and 5U01 HL100402 and the Paul F. Glenn Laboratories for the Biological Mechanisms of Aging (A.J.W.). A.J.W. is an Early Career Scientist of the Howard Hughes Medical Institute.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Reprints and permissions information is available online at http://www.nature.com/reprints/index.html.
Content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or other funding agencies.
References
- 1.Cheung TH, Rando TA. Molecular regulation of stem cell quiescence. Nat Rev Mol Cell Biol. 2013;14:329–340. doi: 10.1038/nrm3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takubo K, et al. Regulation of glycolysis by Pdk functions as a metabolic checkpoint for cell cycle quiescence in hematopoietic stem cells. Cell Stem Cell. 2013;12:49–61. doi: 10.1016/j.stem.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harris JM, et al. Glucose metabolism impacts the spatiotemporal onset and magnitude of HSC induction in vivo. Blood. 2013;121:2483–2493. doi: 10.1182/blood-2012-12-471201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu WM, et al. Metabolic regulation by the mitochondrial phosphatase PTPMT1 is required for hematopoietic stem cell differentiation. Cell Stem Cell. 2013;12:62–74. doi: 10.1016/j.stem.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pervaiz S, Taneja R, Ghaffari S. Oxidative stress regulation of stem and progenitor cells. Antioxid Redox Signal. 2009;11:2777–2789. doi: 10.1089/ars.2009.2804. [DOI] [PubMed] [Google Scholar]
- 6.Harman D. Free radical theory of aging: dietary implications. Am J Clin Nutr. 1972;25:839–843. doi: 10.1093/ajcn/25.8.839. [DOI] [PubMed] [Google Scholar]
- 7.Clément MV, Stamenkovic I. Superoxide anion is a natural inhibitor of FAS-mediated cell death. EMBO J. 1996;15:216–225. [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmad KA, Clement MVV, Pervaiz S. Pro-oxidant activity of low doses of resveratrol inhibits hydrogen peroxide-induced apoptosis. Ann NY Acad Sci. 2003;1010:365–373. doi: 10.1196/annals.1299.067. [DOI] [PubMed] [Google Scholar]
- 9.Stolzing A, Jones E, McGonagle D, Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev. 2008;129:163–173. doi: 10.1016/j.mad.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Jang YY, Sharkis S. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood. 2007;110:3056–3063. doi: 10.1182/blood-2007-05-087759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ito K, et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004;431:997–1002. doi: 10.1038/nature02989. [DOI] [PubMed] [Google Scholar]
- 12.Sahin E, Depinho RA. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature. 2010;464:520–528. doi: 10.1038/nature08982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rossi DJ, Jamieson CH, Weissman IL. Stems cells and the pathways to aging and cancer. Cell. 2008;132:681–696. doi: 10.1016/j.cell.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 14.Paik JH, et al. FoxOs cooperatively regulate diverse pathways governing neural stem cell homeostasis. Cell Stem Cell. 2009;5:540–553. doi: 10.1016/j.stem.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tothova Z, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Miyamoto K, et al. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell. 2007;1:101–112. doi: 10.1016/j.stem.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Yalcin S, et al. Foxo3 is essential for the regulation of ataxia telangiectasia mutated and oxidative stress-mediated homeostasis of hematopoietic stem cells. J Biol Chem. 2008;283:25692–25705. doi: 10.1074/jbc.M800517200. [DOI] [PubMed] [Google Scholar]
- 18.Renault VM, et al. FoxO3 regulates neural stem cell homeostasis. Cell Stem Cell. 2009;5:527–539. doi: 10.1016/j.stem.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang J, et al. PTEN maintains haematopoietic stem cells and acts in lineage choice and leukaemia prevention. Nature. 2006;441:518–522. doi: 10.1038/nature04747. [DOI] [PubMed] [Google Scholar]
- 20.Chen C, et al. TSC-mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. J Exp Med. 2008;205:2397–2408. doi: 10.1084/jem.20081297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Juntilla MM, et al. AKT1 and AKT2 maintain hematopoietic stem cell function by regulating reactive oxygen species. Blood. 2010;115:4030–4038. doi: 10.1182/blood-2009-09-241000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Melov S, et al. Mitochondrial disease in superoxide dismutase 2 mutant mice. Proc Natl Acad Sci USA. 1999;96:846–851. doi: 10.1073/pnas.96.3.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakata H, et al. Interleukin 6-preconditioned neural stem cells reduce ischaemic injury in stroke mice. Brain. 2012;135:3298–3310. doi: 10.1093/brain/aws259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ito K, et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med. 2006;12:446–451. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- 25.Liang R, Ghaffari S. Stem cells, redox signaling, and stem cell aging. Antioxid Redox Signal. 2014;20:1902–1916. doi: 10.1089/ars.2013.5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Owusu-Ansah E, Banerjee U. Reactive oxygen species prime Drosophila haematopoietic progenitors for differentiation. Nature. 2009;461:537–541. doi: 10.1038/nature08313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuan HFF, et al. SIRT1 is required for long-term growth of human mesenchymal stem cells. J Mol Med. 2012;90:389–400. doi: 10.1007/s00109-011-0825-4. [DOI] [PubMed] [Google Scholar]
- 28.Chen H, et al. Role of SIRT1 and AMPK in mesenchymal stem cells differentiation. Ageing Res Rev. 2014;13:55–64. doi: 10.1016/j.arr.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Kim HSS, et al. SIRT3 is a mitochondria-localized tumor suppressor required for maintenance of mitochondrial integrity and metabolism during stress. Cancer Cell. 2010;17:41–52. doi: 10.1016/j.ccr.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown K, et al. SIRT3 reverses aging-associated degeneration. Cell Reports. 2013;3:319–327. doi: 10.1016/j.celrep.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sadowska AM, Manuel-y-Keenoy B, De Backer WA. Antioxidant and anti-inflammatory efficacy of NAC in the treatment of COPD: discordant in vitro and in vivo dose-effects: a review. Pulm Pharmacol Ther. 2007;20:9–22. doi: 10.1016/j.pupt.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 32.Abe M, Takiguchi Y, Ichimaru S, Tsuchiya K, Wada K. Comparison of the protective effect of N-acetylcysteine by different treatments on rat myocardial ischemia-reperfusion injury. J Pharmacol Sci. 2008;106:571–577. doi: 10.1254/jphs.fp0071664. [DOI] [PubMed] [Google Scholar]
- 33.Kondratov RV, Vykhovanets O, Kondratova AA, Antoch MP. Antioxidant N-acetyl-L-cysteine ameliorates symptoms of premature aging associated with the deficiency of the circadian protein BMAL1. Aging. 2009;1:979–987. doi: 10.18632/aging.100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drowley L, et al. Cellular antioxidant levels influence muscle stem cell therapy. Mol Ther. 2010;18:1865–1873. doi: 10.1038/mt.2010.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kolosova NG, Stefanova NA, Muraleva NA, Skulachev VP. The mitochondria-targeted antioxidant SkQ1 but not N-acetylcysteine reverses aging-related biomarkers in rats. Aging. 2012;4:686–94. doi: 10.18632/aging.100493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rossi DJ, et al. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 2007;447:725–730. doi: 10.1038/nature05862. [DOI] [PubMed] [Google Scholar]
- 37.Ru_be CE, et al. Accumulation of DNA damage in hematopoietic stem and progenitor cells during human aging. PLoS ONE. 2011;6:e17487. doi: 10.1371/journal.pone.0017487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sinha M, et al. Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science. 2014;344:649–652. doi: 10.1126/science.1251152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273:5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- 40.Beerman I, Seita J, Inlay MA, Weissman IL, Rossi DJ. Quiescent hematopoietic stem cells accumulate DNA damage during aging that is repaired upon entry into cell cycle. Cell Stem Cell. 2014 doi: 10.1016/j.stem.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Flores I, et al. The longest telomeres: a general signature of adult stem cell compartments. Genes Dev. 2008;22:654–667. doi: 10.1101/gad.451008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Larsen BD, et al. Caspase 3/caspase-activated DNase promote cell differentiation by inducing DNA strand breaks. Proc Natl Acad Sci USA. 2010;107:4230–4235. doi: 10.1073/pnas.0913089107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Charville GW, Rando TA. Stem cell ageing and non-random chromosome segregation. Phil Trans R Soc Lond B. 2011;366:85–93. doi: 10.1098/rstb.2010.0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ciccia A, Elledge S. The DNA damage response: making it safe to play with knives. Mol Cell. 2010;40:179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fortini P, Ferretti C, Dogliotti E. The response to DNA damage during differentiation: pathways and consequences. Mutat Res. 2013;743–744:160–168. doi: 10.1016/j.mrfmmm.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 46.Behrens A, van Deursen JM, Rudolph KL, Schumacher B. Impact of genomic damage and ageing on stem cell function. Nat Cell Biol. 2014;16:201–207. doi: 10.1038/ncb2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moskalev AA, et al. The role of DNA damage and repair in aging through the prism of Koch-like criteria. Ageing Res Rev. 2013;12:661–684. doi: 10.1016/j.arr.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 48.Bernardes de Jesus B, Blasco MA. Telomerase at the intersection of cancer and aging. Trends Genet. 2013;29:513–520. doi: 10.1016/j.tig.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dupressoir A, Puech A, Heidmann T. IAP retrotransposons in the mouse liver as reporters of ageing. Biochim Biophys Acta. 1995;1264:397–402. doi: 10.1016/0167-4781(95)00181-6. [DOI] [PubMed] [Google Scholar]
- 50.Li W, et al. Activation of transposable elements during aging and neuronal decline in Drosophila. Nat Neurosci. 2013;16:529–531. doi: 10.1038/nn.3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maxwell PH, Burhans WC, Curcio MJ. Retrotransposition is associated with genome instability during chronological aging. Proc Natl Acad Sci USA. 2011;108:20376–20381. doi: 10.1073/pnas.1100271108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wong KK, et al. Telomere dysfunction and Atm deficiency compromises organ homeostasis and accelerates ageing. Nature. 2003;421:643–648. doi: 10.1038/nature01385. [DOI] [PubMed] [Google Scholar]
- 53.Nijnik A, et al. DNA repair is limiting for haematopoietic stem cells during ageing. Nature. 2007;447:686–690. doi: 10.1038/nature05875. [DOI] [PubMed] [Google Scholar]
- 54.Wilson A, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135:1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 55.Foudi A, et al. Analysis of histone 2B-GFP retention reveals slowly cycling hematopoietic stem cells. Nat Biotechnol. 2009;27:84–90. doi: 10.1038/nbt.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mohrin M, et al. Hematopoietic stem cell quiescence promotes error-prone DNA repair and mutagenesis. Cell Stem Cell. 2010;7:174–185. doi: 10.1016/j.stem.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fumagalli M, et al. Telomeric DNA damage is irreparable and causes persistent DNA-damage-response activation. Nat Cell Biol. 2012;14:355–365. doi: 10.1038/ncb2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hewitt G, et al. Telomeres are favoured targets of a persistent DNA damage response in ageing and stress-induced senescence. Nat Commun. 2012;3:708. doi: 10.1038/ncomms1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Salama R, Sadaie M, Hoare M, Narita M. Cellular senescence and its effector programs. Genes Dev. 2014;28:99–114. doi: 10.1101/gad.235184.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Erol A. Deciphering the intricate regulatory mechanisms for the cellular choice between cell repair, apoptosis or senescence in response to damaging signals. Cell Signal. 2011;23:1076–1081. doi: 10.1016/j.cellsig.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 61.Mandal PK, Blanpain C, Rossi DJ. DNA damage response in adult stem cells: pathways and consequences. Nat Rev Mol Cell Biol. 2011;12:198–202. doi: 10.1038/nrm3060. [DOI] [PubMed] [Google Scholar]
- 62.Kirkwood TB. Understanding the odd science of aging. Cell. 2005;120:437–447. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 63.Jaskelioff M, et al. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature. 2011;469:102–106. doi: 10.1038/nature09603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Canela A, Martín-Caballero J, Flores JM, Blasco MA. Constitutive expression of tert in thymocytes leads to increased incidence and dissemination of T-cell lymphoma in Lck-Tert mice. Mol Cell Biol. 2004;24:4275–4293. doi: 10.1128/MCB.24.10.4275-4293.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Artandi SE, et al. Constitutive telomerase expression promotes mammary carcinomas in aging mice. Proc Natl Acad Sci USA. 2002;99:8191–8196. doi: 10.1073/pnas.112515399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.González-Suárez E, et al. Increased epidermal tumors and increased skin wound healing in transgenic mice overexpressing the catalytic subunit of telomerase, mTERT, in basal keratinocytes. EMBO J. 2001;20:2619–2630. doi: 10.1093/emboj/20.11.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tomás-Loba A, et al. Telomerase reverse transcriptase delays aging in cancer-resistant mice. Cell. 2008;135:609–622. doi: 10.1016/j.cell.2008.09.034. [DOI] [PubMed] [Google Scholar]
- 68.Bucciantini M, et al. Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature. 2002;416:507–511. doi: 10.1038/416507a. [DOI] [PubMed] [Google Scholar]
- 69.Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–919. doi: 10.1126/science.1141448. [DOI] [PubMed] [Google Scholar]
- 70.Powers ET, Morimoto RI, Dillin A, Kelly JW, Balch WE. Biological and chemical approaches to diseases of proteostasis deficiency. Annu Rev Biochem. 2009;78:959–991. doi: 10.1146/annurev.biochem.052308.114844. [DOI] [PubMed] [Google Scholar]
- 71.Taylor RC, Dillin A. Aging as an event of proteostasis collapse. Cold Spring Harb Perspect Biol. 2011;3:a004440. doi: 10.1101/cshperspect.a004440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moreno-Gonzalez I, Soto C. Misfolded protein aggregates: mechanisms, structures and potential for disease transmission. Semin Cell Dev Biol. 2011;22:482–487. doi: 10.1016/j.semcdb.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Morimoto RI, Cuervo AM. Protein homeostasis and aging: taking care of proteins from the cradle to the grave. J Gerontol A Biol Sci Med Sci. 2009;64A:167–170. doi: 10.1093/gerona/gln071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rubinsztein DC, Mariño G, Kroemer G. Autophagy and aging. Cell. 2011;146:682–695. doi: 10.1016/j.cell.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 75.Tomaru U, et al. Decreased proteasomal activity causes age-related phenotypes and promotes the development of metabolic abnormalities. Am J Pathol. 2012;180:963–972. doi: 10.1016/j.ajpath.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 76.Warr MR, et al. FOXO3A directs a protective autophagy program in haematopoietic stem cells. Nature. 2013;494:323–327. doi: 10.1038/nature11895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cook C, et al. Aging is not associated with proteasome impairment in UPS reporter mice. PLoS ONE. 2009;4:e5888. doi: 10.1371/journal.pone.0005888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mortensen M, et al. The autophagy protein Atg7 is essential for hematopoietic stem cell maintenance. J Exp Med. 2011;208:455–467. doi: 10.1084/jem.20101145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yilmaz ÖH, et al. mTORC1 in the Paneth cell niche couples intestinal stem-cell function to calorie intake. Nature. 2012;486:490–495. doi: 10.1038/nature11163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vilchez D, et al. Increased proteasome activity in human embryonic stem cells is regulated by PSMD11. Nature. 2012;489:304–308. doi: 10.1038/nature11468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Murphy CT, et al. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature. 2003;424:277–283. doi: 10.1038/nature01789. [DOI] [PubMed] [Google Scholar]
- 83.Oh SW, et al. Identification of direct DAF-16 targets controlling longevity, metabolism and diapause by chromatin immunoprecipitation. Nat Genet. 2006;38:251–257. doi: 10.1038/ng1723. [DOI] [PubMed] [Google Scholar]
- 84.Demontis F, Perrimon N. FOXO/4E-BP signaling in Drosophila muscles regulates organism-wide proteostasis during aging. Cell. 2010;143:813–825. doi: 10.1016/j.cell.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tatar M, Khazaeli AA, Curtsinger JW. Chaperoning extended life. Nature. 1997;390:30. doi: 10.1038/36237. [DOI] [PubMed] [Google Scholar]
- 86.Walker GA, Lithgow GJ. Lifespan extension in C. elegans by a molecular chaperone dependent upon insulin-like signals. Aging Cell. 2003;2:131–139. doi: 10.1046/j.1474-9728.2003.00045.x. [DOI] [PubMed] [Google Scholar]
- 87.Morley JF, Morimoto RI. Regulation of longevity in Caenorhabditis elegans by heat shock factor and molecular chaperones. Mol Biol Cell. 2004;15:657–664. doi: 10.1091/mbc.E03-07-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen C, Liu Y, Liu Y, Zheng P. mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Sci Signal. 2009;2:ra75. doi: 10.1126/scisignal.2000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang C, Cuervo AM. Restoration of chaperone-mediated autophagy in aging liver improves cellular maintenance and hepatic function. Nat Med. 2008;14:959–965. doi: 10.1038/nm.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cummings CJ, et al. Over-expression of inducible HSP70 chaperone suppresses neuropathology and improves motor function in SCA1 mice. Hum Mol Genet. 2001;10:1511–1518. doi: 10.1093/hmg/10.14.1511. [DOI] [PubMed] [Google Scholar]
- 91.Feng Y, et al. Heat shock improves Sca-1+ stem cell survival and directs ischemic cardiomyocytes toward a prosurvival phenotype via exosomal transfer: a critical role for HSF1/miR-34a/HSP70 pathway. Stem Cells. 2014;32:462–472. doi: 10.1002/stem.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McArdle A, Dillmann WH, Mestril R, Faulkner JA, Jackson MJ. Overexpression of HSP70 in mouse skeletal muscle protects against muscle damage and age-related muscle dysfunction. FASEB J. 2004;18:355–357. doi: 10.1096/fj.03-0395fje. [DOI] [PubMed] [Google Scholar]
- 93.Bratic A, Larsson NGG. The role of mitochondria in aging. J Clin Invest. 2013;123:951–957. doi: 10.1172/JCI64125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Miquel J, Economos AC, Fleming J, Johnson JE. Mitochondrial role in cell aging. Exp Gerontol. 1980;15:575–591. doi: 10.1016/0531-5565(80)90010-8. [DOI] [PubMed] [Google Scholar]
- 95.Zheng W, Khrapko K, Coller HA, Thilly WG, Copeland WC. Origins of human mitochondrial point mutations as DNA polymerase gamma-mediated errors. Mutat Res. 2006;599:11–20. doi: 10.1016/j.mrfmmm.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 96.Trifunovic A, et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004;429:417–423. doi: 10.1038/nature02517. [DOI] [PubMed] [Google Scholar]
- 97.Kujoth GC, et al. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309:481–484. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- 98.Ahlqvist KJ, et al. Somatic progenitor cell vulnerability to mitochondrial DNA mutagenesis underlies progeroid phenotypes in Polg mutator mice. Cell Metab. 2012;15:100–109. doi: 10.1016/j.cmet.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 99.Fox RG, Magness S, Kujoth GC, Prolla TA, Maeda N. Mitochondrial DNA polymerase editing mutation, PolgD257A, disturbs stem-progenitor cell cycling in the small intestine and restricts excess fat absorption. Am J Physiol Gastrointest Liver Physiol. 2012;302:G914–G924. doi: 10.1152/ajpgi.00402.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Taylor RW, et al. Mitochondrial DNA mutations in human colonic crypt stem cells. J Clin Invest. 2003;112:1351–1360. doi: 10.1172/JCI19435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.McDonald SA, et al. Mechanisms of field cancerization in the human stomach: the expansion and spread of mutated gastric stem cells. Gastroenterology. 2008;134:500–510. doi: 10.1053/j.gastro.2007.11.035. [DOI] [PubMed] [Google Scholar]
- 102.Fellous TG, et al. Locating the stem cell niche and tracing hepatocyte lineages in human liver. Hepatology. 2009;49:1655–1663. doi: 10.1002/hep.22791. [DOI] [PubMed] [Google Scholar]
- 103.Norddahl GL, et al. Accumulating mitochondrial DNA mutations drive premature hematopoietic aging phenotypes distinct from physiological stem cell aging. Cell Stem Cell. 2011;8:499–510. doi: 10.1016/j.stem.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 104.Bonawitz ND, Chatenay-Lapointe M, Pan Y, Shadel GS. Reduced TOR signaling extends chronological life span via increased respiration and upregulation of mitochondrial gene expression. Cell Metab. 2007;5:265–277. doi: 10.1016/j.cmet.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Holzenberger M, et al. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- 106.Nakada D, Saunders TL, Morrison SJ. Lkb1 regulates cell cycle and energy metabolism in haematopoietic stem cells. Nature. 2010;468:653–658. doi: 10.1038/nature09571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gross DN, van den Heuvel AP, Birnbaum MJ. The role of FoxO in the regulation of metabolism. Oncogene. 2008;27:2320–2336. doi: 10.1038/onc.2008.25. [DOI] [PubMed] [Google Scholar]
- 108.Peserico A, et al. A novel AMPK-dependent FoxO3A-SIRT3 intramitochondrial complex sensing glucose levels. Cell Mol Life Sci. 2013;70:2015–2029. doi: 10.1007/s00018-012-1244-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rera M, et al. Modulation of longevity and tissue homeostasis by the Drosophila PGC-1 homolog. Cell Metab. 2011;14:623–634. doi: 10.1016/j.cmet.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cerletti M, Jang YC, Finley LW, Haigis MC, Wagers AJ. Short-term calorie restriction enhances skeletal muscle stem cell function. Cell Stem Cell. 2012;10:515–519. doi: 10.1016/j.stem.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Piper MD, Bartke A. Diet and aging. Cell Metab. 2008;8:99–104. doi: 10.1016/j.cmet.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 112.Lee J, Duan W, Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem. 2002;82:1367–1375. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- 113.Narala SR, et al. SIRT1 acts as a nutrient-sensitive growth suppressor and its loss is associated with increased AMPK and telomerase activity. Mol Biol Cell. 2008;19:1210–1219. doi: 10.1091/mbc.E07-09-0965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yang SAA, et al. FOXO/Fringe is necessary for maintenance of the germline stem cell niche in response to insulin insufficiency. Dev Biol. 2013;382:124–135. doi: 10.1016/j.ydbio.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 115.Schulz TJ, et al. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 2007;6:280–293. doi: 10.1016/j.cmet.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 116.Gredilla R, Sanz A, Lopez-Torres M, Barja G. Caloric restriction decreases mitochondrial free radical generation at complex I and lowers oxidative damage to mitochondrial DNA in the rat heart. FASEB J. 2001;15:1589–1591. doi: 10.1096/fj.00-0764fje. [DOI] [PubMed] [Google Scholar]
- 117.Sohal RS, Agarwal S, Candas M, Forster MJ, Lal H. Effect of age and caloric restriction on DNA oxidative damage in different tissues of C57BL/6 mice. Mech Ageing Dev. 1994;76:215–224. doi: 10.1016/0047-6374(94)91595-4. [DOI] [PubMed] [Google Scholar]
- 118.Gomes AP, et al. Declining NAD+ induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell. 2013;155:1624–1638. doi: 10.1016/j.cell.2013.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hur JH, et al. Increased longevity mediated by yeast NDI1 expression in Drosophila intestinal stem and progenitor cells. Aging. 2013;5:662–681. doi: 10.18632/aging.100595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sousa-Victor P, et al. Geriatric muscle stem cells switch reversible quiescence into senescence. Nature. 2014;506:316–321. doi: 10.1038/nature13013. [DOI] [PubMed] [Google Scholar]
- 121.Molofsky AV, et al. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature. 2006;443:448–452. doi: 10.1038/nature05091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Boyle M, Wong C, Rocha M, Jones DL. Decline in self-renewal factors contributes to aging of the stem cell niche in the Drosophila testis. Cell Stem Cell. 2007;1:470–478. doi: 10.1016/j.stem.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 123.Florian MC, et al. Cdc42 activity regulates hematopoietic stem cell aging and rejuvenation. Cell Stem Cell. 2012;10:520–530. doi: 10.1016/j.stem.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Geiger H, de Haan G, Florian MC. The ageing haematopoietic stem cell compartment. Nat Rev Immunol. 2013;13:376–389. doi: 10.1038/nri3433. [DOI] [PubMed] [Google Scholar]
- 125.Campisi J. Cancer, aging and cellular senescence. In Vivo. 2000;14:183–188. [PubMed] [Google Scholar]
- 126.Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 127.Coppé JPP, Desprez PYY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Janzen V, et al. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature. 2006;443:421–426. doi: 10.1038/nature05159. [DOI] [PubMed] [Google Scholar]
- 129.Attema JL, Pronk CJ, Norddahl GL, Nygren JM, Bryder D. Hematopoietic stem cell ageing is uncoupled from p16 INK4A-mediated senescence. Oncogene. 2009;28:2238–2243. doi: 10.1038/onc.2009.94. [DOI] [PubMed] [Google Scholar]
- 130.Cheng T, et al. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000;287:1804–1808. doi: 10.1126/science.287.5459.1804. [DOI] [PubMed] [Google Scholar]
- 131.Kippin TE, Martens DJ, van der Kooy D. p21 loss compromises the relative quiescence of forebrain stem cell proliferation leading to exhaustion of their proliferation capacity. Genes Dev. 2005;19:756–767. doi: 10.1101/gad.1272305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chakkalakal JV, Jones KM, Basson MA, Brack AS. The aged niche disrupts muscle stem cell quiescence. Nature. 2012;490:355–360. doi: 10.1038/nature11438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hamanaka RB, Chandel NS. Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends Biochem Sci. 2010;35:505–513. doi: 10.1016/j.tibs.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sacco A, et al. Short telomeres and stem cell exhaustion model Duchenne muscular dystrophy in mdx/mTR mice. Cell. 2010;143:1059–1071. doi: 10.1016/j.cell.2010.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–1826. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 136.Salani S, et al. Generation of skeletal muscle cells from embryonic and induced pluripotent stem cells as an in vitro model and for therapy of muscular dystrophies. J Cell Mol Med. 2012;16:1353–1364. doi: 10.1111/j.1582-4934.2011.01498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chhabra P, Brayman KL. Stem cell therapy to cure type 1 diabetes: from hype to hope. Stem Cells Transl Med. 2013;2:328–336. doi: 10.5966/sctm.2012-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kim SU, de Vellis J. Stem cell-based cell therapy in neurological diseases: a review. J Neurosci Res. 2009;87:2183–2200. doi: 10.1002/jnr.22054. [DOI] [PubMed] [Google Scholar]
- 139.Cerletti M, et al. Highly efficient, functional engraftment of skeletal muscle stem cells in dystrophic muscles. Cell. 2008;134:37–47. doi: 10.1016/j.cell.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cosgrove BD, et al. Rejuvenation of the muscle stem cell population restores strength to injured aged muscles. Nat Med. 2014;20:255–264. doi: 10.1038/nm.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tabebordbar M, Wang ET, Wagers AJ. Skeletal muscle degenerative diseases and strategies for therapeutic muscle repair. Annu Rev Pathol. 2013;8:441–475. doi: 10.1146/annurev-pathol-011811-132450. [DOI] [PubMed] [Google Scholar]
- 142.Bernet JD, et al. p38 MAPK signaling underlies a cell-autonomous loss of stem cell self-renewal in skeletal muscle of aged mice. Nat Med. 2014;20:265–271. doi: 10.1038/nm.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ding Q, et al. Enhanced efficiency of human pluripotent stem cell genome editing through replacing TALENs with CRISPRs. Cell Stem Cell. 2013;12:393–394. doi: 10.1016/j.stem.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ding Q, et al. A TALEN genome-editing system for generating human stem cell-based disease models. Cell Stem Cell. 2013;12:238–251. doi: 10.1016/j.stem.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xu Y, et al. Integrating haplotypes and single genetic variability effects of the Pax7 gene on growth traits in two cattle breeds. Genome. 2013;56:9–15. doi: 10.1139/gen-2012-0124. [DOI] [PubMed] [Google Scholar]
- 146.Borchin B, Chen J, Barberi T. Derivation and FACS-mediated purification of PAX3+/PAX7+ skeletal muscle precursors from human pluripotent stem cells. Stem Cell Reports. 2013;1:620–631. doi: 10.1016/j.stemcr.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Darabi R, et al. Human ES- and iPS-derived myogenic progenitors restore DYSTROPHIN and improve contractility upon transplantation in dystrophic mice. Cell Stem Cell. 2012;10:610–619. doi: 10.1016/j.stem.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kobayashi Y, et al. Pre-evaluated safe human iPSC-derived neural stem cells promote functional recovery after spinal cord injury in common marmoset without tumorigenicity. PLoS ONE. 2012;7:e52787. doi: 10.1371/journal.pone.0052787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Nakamura M, Okano H. Cell transplantation therapies for spinal cord injury focusing on induced pluripotent stem cells. Cell Res. 2013;23:70–80. doi: 10.1038/cr.2012.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Maslov AY, Barone TA, Plunkett RJ, Pruitt SC. Neural stem cell detection, characterization, and age-related changes in the subventricular zone of mice. J Neurosci. 2004;24:1726–1733. doi: 10.1523/JNEUROSCI.4608-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132:598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pan L, et al. Stem cell aging is controlled both intrinsically and extrinsically in the Drosophila ovary. Cell Stem Cell. 2007;1:458–469. doi: 10.1016/j.stem.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 154.Ryu BYY, Orwig KE, Oatley JM, Avarbock MR, Brinster RL. Effects of aging and niche microenvironment on spermatogonial stem cell self-renewal. Stem Cells. 2006;24:1505–1511. doi: 10.1634/stemcells.2005-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sato T, et al. In vitro production of fertile sperm from murine spermatogonial stem cell lines. Nat Commun. 2011;2:472. doi: 10.1038/ncomms1478. [DOI] [PubMed] [Google Scholar]
- 156.Sato T, et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Schnoor M, et al. Production of type VI collagen by human macrophages: a new dimension in macrophage functional heterogeneity. J Immunol. 2008;180:5707–5719. doi: 10.4049/jimmunol.180.8.5707. [DOI] [PubMed] [Google Scholar]
- 158.Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development. 2011;138:3625–3637. doi: 10.1242/dev.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Joe AW, et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Conboy IM, Conboy MJ, Smythe GM, Rando TA. Notch-mediated restoration of regenerative potential to aged muscle. Science. 2003;302:1575–1577. doi: 10.1126/science.1087573. [DOI] [PubMed] [Google Scholar]
- 161.Carlson ME, et al. Relative roles of TGF-β1 and Wnt in the systemic regulation and aging of satellite cell responses. Aging Cell. 2009;8:676–689. doi: 10.1111/j.1474-9726.2009.00517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Franceschi C, et al. Inflamm-aging: an evolutionary perspective on immunosenescence. Ann NY Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 163.de Magalhães JP, Curado J, Church GM. Meta-analysis of age-related gene expression profiles identifies common signatures of aging. Bioinformatics. 2009;25:875–881. doi: 10.1093/bioinformatics/btp073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Conboy IM, et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 165.Villeda SA, et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477:90–94. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Katsimpardi L, et al. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science. 2014;344:630–634. doi: 10.1126/science.1251141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ruckh JM, et al. Rejuvenation of regeneration in the aging central nervous system. Cell Stem Cell. 2012;10:96–103. doi: 10.1016/j.stem.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Loffredo FS, et al. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell. 2013;153:828–839. doi: 10.1016/j.cell.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Elabd C, et al. Oxytocin is an age-specific circulating hormone that is necessary for muscle maintenance and regeneration. Nat Commun. 2014;5:4082. doi: 10.1038/ncomms5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Baker DJ, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Fraga MF, Esteller M. Epigenetics and aging: the targets and the marks. Trends Genet. 2007;23:413–418. doi: 10.1016/j.tig.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 172.Greer EL, et al. Members of the H3K4 trimethylation complex regulate lifespan in a germline-dependent manner in C. elegans. Nature. 2010;466:383–387. doi: 10.1038/nature09195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Han S, Brunet A. Histone methylation makes its mark on longevity. Trends Cell Biol. 2012;22:42–49. doi: 10.1016/j.tcb.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Greer EL, et al. Transgenerational epigenetic inheritance of longevity in Caenorhabditis elegans. Nature. 2011;479:365–371. doi: 10.1038/nature10572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Peleg S, et al. Altered histone acetylation is associated with age-dependent memory impairment in mice. Science. 2010;328:753–756. doi: 10.1126/science.1186088. [DOI] [PubMed] [Google Scholar]
- 176.Krishnan V, et al. Histone H4 lysine 16 hypoacetylation is associated with defective DNA repair and premature senescence in Zmpste24-deficient mice. Proc Natl Acad Sci USA. 2011;108:12325–12330. doi: 10.1073/pnas.1102789108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Beerman I, et al. Proliferation-dependent alterations of the DNA methylation landscape underlie hematopoietic stem cell aging. Cell Stem Cell. 2013;12:413–425. doi: 10.1016/j.stem.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 178.Sun D, et al. Epigenomic profiling of young and aged HSCs reveals concerted changes during aging that reinforce self-renewal. Cell Stem Cell. 2014;14:673–688. doi: 10.1016/j.stem.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Gurdon JB. The developmental capacity of nuclei taken from intestinal epithelium cells of feeding tadpoles. J Embryol Exp Morphol. 1962;10:622–640. [PubMed] [Google Scholar]
- 180.Lanza RP, et al. Extension of cell life-span and telomere length in animals cloned from senescent somatic cells. Science. 2000;288:665–669. doi: 10.1126/science.288.5466.665. [DOI] [PubMed] [Google Scholar]
- 181.Abramovich A, Muradian KK, Fraifeld VE. Have we reached the point for in vivo rejuvenation? Rejuvenation Res. 2008;11:489–492. doi: 10.1089/rej.2008.0658. [DOI] [PubMed] [Google Scholar]
- 182.Takahashi K, Yamanaka S. Induced pluripotent stem cells in medicine and biology. Development. 2013;140:2457–2461. doi: 10.1242/dev.092551. [DOI] [PubMed] [Google Scholar]
- 183.Wahlestedt M, et al. An epigenetic component of hematopoietic stem cell aging amenable to reprogramming into a young state. Blood. 2013;121:4257–4264. doi: 10.1182/blood-2012-11-469080. [DOI] [PubMed] [Google Scholar]
- 184.Price NL, et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012;15:675–690. doi: 10.1016/j.cmet.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Miller RA. Rebuttal to Hasty and Vijg: ‘Accelerating aging by mouse reverse genetics: a rational approach to understanding longevity’. Aging Cell. 2004;3:53–54. doi: 10.1111/j.1474-9728.2004.00084.x. [DOI] [PubMed] [Google Scholar]
- 186.Burtner CR, Kennedy BK. Progeria syndromes and ageing: what is the connection? Nat Rev Mol Cell Biol. 2010;11:567–578. doi: 10.1038/nrm2944. [DOI] [PubMed] [Google Scholar]
- 187.Hua G, et al. Crypt base columnar stem cells in small intestines of mice are radioresistant. Gastroenterology. 2012;143:1266–1276. doi: 10.1053/j.gastro.2012.07.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Sotiropoulou PA, et al. Bcl-2 and accelerated DNA repair mediates resistance of hair follicle bulge stem cells to DNA-damage-induced cell death. Nat Cell Biol. 2010;12:572–582. doi: 10.1038/ncb2059. [DOI] [PubMed] [Google Scholar]
- 189.Cheung HHH, et al. Telomerase protects Werner syndrome lineage-specific stem cells from premature aging. Stem Cell Reports. 2014;2:534–546. doi: 10.1016/j.stemcr.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhang J, et al. A human iPSC model of Hutchinson Gilford progeria reveals vascular smooth muscle and mesenchymal stem cell defects. Cell Stem Cell. 2011;8:31–45. doi: 10.1016/j.stem.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 191.Mitchell JR, Wood E, Collins K. A telomerase component is defective in the human disease dyskeratosis congenita. Nature. 1999;402:551–555. doi: 10.1038/990141. [DOI] [PubMed] [Google Scholar]
- 192.Jones M, et al. Hematopoietic stem cells are acutely sensitive to Acd shelterin gene inactivation. J Clin Invest. 2014;124:353–366. doi: 10.1172/JCI67871. [DOI] [PMC free article] [PubMed] [Google Scholar]