Abstract
The identification of the hormones and other factors regulating Sertoli cell survival, proliferation, and maturation in neonatal, peripubertal, and pubertal life remains one of the most critical questions in testicular biology. The regulation of Sertoli cell proliferation and differentiation is thought to be controlled by cell–cell junctions and a set of circulating and local hormones and growth factors. In this review, we will focus on receptors and intracellular signaling pathways activated by androgen, follicle-stimulating hormone, thyroid hormone, activin, retinoids, insulin, insulin-like growth factor, relaxin, and estrogen, with special emphasis on estrogen receptors. Estrogen receptors activate intracellular signaling pathways that converge on cell cycle and transcription factors and play a role in the regulation of Sertoli cell proliferation and differentiation.
Keywords: estrogen receptor, androgen receptor, follicle-stimulating hormone receptor, thyroid hormone receptor, relaxin receptor
Introduction
Sertoli cells play a key role in spermatogenesis, and a better knowledge about the role of hormones and growth factors in the regulation of the homeostasis and functions of these cells will be important for the control of male fertility. Sertoli cell proliferation occurs during fetal and early neonatal life in rodents, and in the fetal and peripubertal period in higher primates (for a review, see refs. 1 and 2). Sertoli cells are known to divide in the fetal rat testis, proliferating maximally on day 20 post-conception, with a progressive decrease in division until postnatal day 21, when division ceases.3 In humans, Sertoli cells proliferate in fetal and neonatal period (12–18 mo), and then become quiescent for a period of 8 y. This is followed by a second wave of proliferation at puberty (10–14 y) (for a review, see refs. 1 and 2). The Sertoli cell differentiation (maturation) is associated with a cessation of proliferation, alterations in gene transcription and protein expression, establishment of the blood-testis barrier, and the capacity to sustain spermatogenesis (for a review, see refs. 1 and 2).
The Sertoli cell population remains relatively constant in adult mammals, and each Sertoli cell supports approximately 30–50 germ cells at different stages of development in the seminiferous epithelium.4 The final testis size, the number of germ cells in the adult testis, and sperm output are directly linked to the total number of Sertoli cells.5 Thus, the current dogma holds that Sertoli cells from post-pubertal testes are terminally differentiated, because they stop proliferating and mature when the males attain puberty. However, studies have shown that Sertoli cells are capable of dividing even in adult animals, including mice,6 hamster,2 and humans,6,7 under experimental and physiological conditions. A recent study has also shown return to proliferative abilities of non-dividing Sertoli cells after transplantation. This reinitiating of the proliferation is temporary, and the Sertoli cells stop proliferating within 2 wk after syngeneic or allogeneic transplantation.8 Overexpression of helix-loop-helix inhibitor of differentiation (ID) proteins in post-mitotic rat Sertoli cells allows reentrance in cell cycle.9 Moreover, proliferation and differentiation processes may overlap in time, as certain differentiation markers are expressed during Sertoli cell division.10 Nevertheless, Sertoli cell number is essentially defined during the prepubertal period; therefore, early perturbation of the proliferation and/or differentiation process can have long-term consequences on male reproduction. Failure of Sertoli cells to undergo complete differentiation leads to spermatogenic defects and infertility in transgenic mice.10,11 Furthermore, functionally immature Sertoli cells are observed in men with infertility.12,13 The identification of the hormones and other factors regulating Sertoli cell survival, proliferation, and maturation in neonatal, peripubertal, and pubertal life remains one of the most critical questions in testicular biology.
Morphological association between Sertoli cells and germ cells is clearly visible at different stages of their development in the seminiferous epithelium. Sertoli cells provide germ cells with a structural support and a preserved environment with the blood-testis barrier, assist their movement toward the seminiferous epithelium lumen, and sustain their development. Crucial to these cell–cell interactions in the seminiferous epithelium is the production of several molecules such as hormones, growth factors, proteases, protease inhibitors, and components of the extracellular matrix (ECM) by both Sertoli and germ cells, and there are several reports to support the direct role of both cell types in the maintenance of spermatogenesis.14,15 Furthermore, cell–cell junctions (gap junctions and tight junctions) are not only sites of attachment between cells but play a role in the regulation of cell proliferation and differentiation (for a review, see ref. 2). The Sertoli cell-specific knockout of the gap junction protein connexin-43 (GJA1) in mice showed that Sertoli cells continued to proliferate for 60 d compared with Sertoli cells from wild-type controls that stopped proliferating within 2 wk of age.16 It is important to emphasize that the loss of GJA1 from Sertoli cells was associated with dysregulation in the levels of tight junction and adherence junction proteins such as N-cadherin, β-catenin, occludin, and zonadhesin-1,17 further demonstrating that GJA1 plays a role in Sertoli cell development. In this review, we will focus on receptors and intracellular signaling pathways activated by some circulating and local hormones and growth factors, with special emphasis on estrogen receptors.
Androgen Receptor (AR)
Expression of the androgen receptor (AR) in the rat testis can be detected in Sertoli, Leydig, and peritubular myoid cells.18 AR is absent in Sertoli cells in fetal-neonatal testes, and its expression becomes progressively stronger during postnatal/peripubertal development in rodents,19-21 primates,22,23 and humans.24 Sertoli cells display nuclear-specific AR expression patterns that vary according to the stage of the seminiferous epithelium cycle in the adult rodent18,25 and human.24
Androgens do not appear to have a direct effect on Sertoli cell proliferation. Although androgens were initially shown to regulate Sertoli cell proliferation in Tfm mutant mice, which lack functional AR,26 mice models with Sertoli cell-specific genetic disruption of AR function (SCAR knockout, SCARKO) have shown that total testicular Sertoli cell number remained normal in these loss-of-function models,21,27,28 suggesting that the size of the Sertoli cell population is independent of local activation of AR in Sertoli cells.
The expression of four Sertoli cell maturation markers, anti-Müllerian hormone (AMH), cyclin-dependent kinases inhibitor p27Kip1 (CDKN1B), the zinc finger transcription factor GATA-1, and sulfated glycoprotein-2, does not change in SCARKO mice, but Sertoli cells from these mice are clearly unable to support late meiotic and post-meiotic germ cells, due to changes in expression of a number of other genes expressed by Sertoli cells.21 Furthermore, the integrity of junctional complexes that build the blood-testis barrier is not maintained in SCARKO mice.29
In contrast to SCARKO, a recent study using gain-of-function transgenic (Tg) mouse model (Tg Sertoli cell-specific AR [TgSCAR]), which presents premature postnatal AR immunolocalized in Sertoli cell nuclei, has shown a decrease of total Sertoli cell number in developing and mature TgSCAR testes, despite normal or higher follicle-stimulating hormone receptor (Fshr) mRNA and circulating FSH levels.30 TgSCAR induces age-related changes of Sertoli cell-specific transcripts associated with functional maturation, such as reproductive homeobox 5 (Rhox5), Spinlw1 serine peptidase inhibitor-like kunitz and WAP domains 1 (eppin), (Spinlw1) transcripts, and precocious of Sertoli cell function like early seminiferous tubular lumen formation and upregulation of expression of proteins involved with Sertoli cell tight-junction (Claudin 11 and tight junction protein 1, Tjp1) and phagocytic (engulfment and cell motility 1, Elmo1) transcripts. Thus, advanced AR expression in Sertoli cell can accelerate Sertoli cell maturation and spermatogenic development, which ultimately reduces Sertoli cell population and testis size, but does not have detectable effect on fertility.30
Follicle-Stimulating Hormone Receptor (FSHR)
Studies with hipogonadal (hpg) mice treated with recombinant FSH31 or with hpg mice expressing transgenic FSH32,33 have shown a major role for FSH in dictating Sertoli cell postnatal proliferation and final cell number in the rodent. It is well established that FSH is mitogenic for immature Sertoli cells, both in vitro and in vivo.31,34-36 Animals with genetic disruption of FSHR (FSHRKO) show reduced Sertoli and germ cell number, but remain fertile.37,38 The genetic disruption of the murine FSHR induces a small reduction in Sertoli cell number up to day 20 with more marked effects seen in the adult.28
FSH stimulates Sertoli cell division at day 9 in rats39 and differentiation in prepubertal animals, and these biological responses are correlated with qualitative as well as quantitative differences in FSHR signaling. The FSHR activates G protein- and β-arrestin-dependent signaling. Coupling of the FSHR to Gs induces adenylyl cyclase to produce cyclic AMP, whereas β-arrestins have been shown to stimulate the late phase of mitogen-activated protein kinase 3/1 (MAPK3/1, also known as ERK1/2) activation.40,41 In addition, a subtle interplay between cyclic AMP- and phosphoinositide-dependent signaling is required for p70 S6 kinase 1 (p70S6K) activation by FSH in Sertoli cells: both pathways are co-stimulatory in differentiating cells,42 but they exert opposite effects in proliferating cells.43 FSH stimulates MAPK3/1 phosphorylation and proliferation of Sertoli cells from immature rats, but inhibits MAPK pathway when the differentiation of Sertoli cells starts.44 The stimulatory actions of FSH on aromatase mRNA, aromatase protein, and estradiol production in Sertoli cells from immature rats were blocked by inhibition of phosphatidylinositol 3-kinase (PI3K)/AKT and augmented by inhibition of MAPK3/1 signaling pathway.45 PI3K/AKT/mammalian target of rapamycin complex 1 (mTORC1) pathway is also involved in FSH-induced stimulation of c-Myc expression and Sertoli cell proliferation. On the other hand, AMP-activated protein kinase (AMPK) activation may participate in the detention of Sertoli cell proliferation by, at least in part, a decrease in the activity of mTORC1 and an increase in cyclin-dependent kinase inhibitor (CDKI) expression.46 Thus, the specific effects of FSH on Sertoli cells in rodents are dependent on the developmental stage of the animal. In fact, Bhattacharya et al.47 found that production of cyclic AMP was higher in prepubertal compared with neonatal Sertoli cells, and suggest that the ability of FSHR to respond to FSH is significantly increased during prepubertal maturation of Sertoli cells. These authors suggest that a switch from FSH-resistant to FSH-responsive Sertoli cells is important for the robust initiation of germ cell differentiation.
Thyroid Hormone Receptor (TR)
Thyroid hormone T3 (T4 being the prohormone) acts, at least in large part, by binding to nuclear receptors TRα1, TRβ1, and TRβ2 (TRs). In humans, neonatal hypothyroidism leads to testicular enlargement in prepubertal boys (for a review, see ref. 48). Neonatal hypothyroidism in mice and rats leads to an increase in the weight of adult testes and epididymides due to an increase in daily sperm production.49-51 These findings are shown to be caused by the extension of the proliferative period of Sertoli cells and delay in their maturation50,52-54 (for a review, see ref. 55). The prolongation of Sertoli cell proliferation, as one of the major effects of transient neonatal hypothyroidism, leads to an increase of 157% in the number of Sertoli cells in the adult testis,53 increase of adult testis size, and sperm production. On the other hand, the neonatal hyperthyroidism results in a 50% reduction in the final Sertoli cell number at 23 d of age, due to direct suppression of Sertoli cell proliferation, while their maturation is promoted.50
In rodents, the arrest of Sertoli cell proliferation and entry into differentiation is marked by a peak in T3 blood levels.56 T3 can directly suppress proliferation and induce differentiation of cultured neonatal rat Sertoli cells,49 involving upregulation of cyclin-dependent kinase inhibitors.57 Furthermore, T3 increases the levels of Connexin 43, an important gap junctional protein that participates in the control of Sertoli cell differentiation/maturation in mammals.58 Male mice with a Sertoli cell-specific loss of Connexin 43 show an arrest of spermatogenesis at the level of spermatogonia or display a Sertoli cell-only syndrome.11
Mice with null deletion of the TRHA gene (TRαo/o) exhibit increased testicular weights and a tendency to higher daily sperm production and Sertoli cell number in comparison with the wild type in adults, which points to a predominant function of TRα1 in postnatal Sertoli cell development57 (for a review, see ref. 55). In fact, a recent study has shown that T3 directly limits postnatal Sertoli cell proliferation by activation of TRα1 present in these cells. Moreover, this study provided evidence that regulation of the cyclin-dependent kinase 4 (Cdk4)/JunD/c-Myc pathway is involved in this negative control.59
Activin Receptor (ACVR)
Activin A, a member of the TGF-β superfamily, is a key regulator of Sertoli cell proliferation and differentiation during embryogenesis and early postnatal life.60-62 During testis development, production of activin A decreases, with the most dramatic reduction occurring at puberty. This change contributes to the differentiation and maturation of Sertoli cells.63,64
Proliferation of Sertoli cells is maintained in mice deficient in the activin antagonist, inhibin. These mice develop testicular sex cord-stromal tumors with 100% penetrance from 4 wk of age.65 As these tumors progress, serum levels of activin A and B increase by as much as 500-fold and result in marked cachexia and death.66
Transgenic overexpression of activin A in prepubertal mice also disrupts normal fertility, with most males sterile by 10 wk of age.67 Activin production and signaling must be strictly controlled during embryonic development and for the terminal differentiation of adult Sertoli cells. Furthermore, the balance between activin and its endogenous antagonist, inhibin, performs an essential function in the process of Sertoli cell differentiation.
Isoforms of activin type II receptor have been shown to have a developmentally regulated expression pattern in Sertoli cells of the rat and may contribute to the switch in maturation of Sertoli cells around puberty.68 In activin type II receptor deficient mice, Sertoli cell numbers were only 40% of normal.69
Retinoid Receptor (RXR)
In the post-natal rodent testis, retinoids initiate spermatogonial differentiation,70-72 meiosis,72 and promote Sertoli cell maturation at puberty by suppressing proliferation,73 stimulating blood-testis barrier (BTB) integrity,74,75 and coordinating the adult spermatogenic cycle.76,77 In fact, mice with a Sertoli cell-specific knockout of retinoic acid receptor RARα show a delay in the final phase of Sertoli cell differentiation, loss of cell cycle gene expression, progressive spermatogenic degeneration, and infertility.74,77 Similarly, mice with a Sertoli cell-specific ablation of the retinoid receptor RXRβ exhibit spermiation failure, likely due to the loss of heterodimeric RARα/RXRβ signaling within Sertoli cells.78,79 These studies indicate that intact retinoid signaling pathways are necessary for Sertoli cell maturation and function. However, the mechanisms by which retinoids may simultaneously direct germ cell- and Sertoli cell-differentiation at puberty remain unclear.
A recent study has shown that rat Sertoli cells isolated during the pubertal proliferative and quiescent phases at postnatal days 10 and 20, respectively, and cultured with all-trans-retinoic acid suppress activin-induced proliferation by antagonizing G1 phase progression and entry into the cell cycle. Retinoid signaling was also found to initiate tight junction formation in primary Sertoli cells, consistent with a pro-differentiative role.80
Insulin Receptor (INSR) and Type I Insulin-Like Growth Factor Receptor (IGF1R)
The IGF-I-like immunoreactivity decreases in the Sertoli and Leydig cells during the third and fourth postnatal weeks. In adult rats, only spermatogenic cells show IGF-I-like immunoreactivity.81 A recent study has shown that transcripts coding for the growth factors Igf1 and Igf2 are present at levels approximately 10-fold higher in spermatogonia than in Sertoli cells isolated from mice.82 In contrast, transcripts for Insr and Igf1r are present at similar levels in immature Sertoli cells and spermatogonia-enriched fractions, suggesting that the insulin-like family of growth factors exerts autocrine-paracrine actions within the seminiferous epithelium.
The loss of IGF1R decreases the number of viable Sertoli cells as a consequence of a decrease in Sertoli cell proliferation and an increase in Sertoli cell death.83 In fact, in testes from mice lacking both Insr and Igf1r in Sertoli cells (SC-Insr;Igf1r mutant) is observed a progressive and cumulative reduction of Sertoli cell number during the late fetal and early neonatal stages of development. Adult testes of these mice display a 75% reduction in testis size and daily sperm production. Sertoli cell maturation is delayed in SC-Insr;Igf1r testes, resulting in a desynchronized progression of germ cells during the first wave of spermatogenesis.
The insulin/IGF family of growth factors acts mainly through INSR and IGF1R and activates two major signaling pathways, MAPK3/1 and PI3K/AKT, both of which are important mediators of proliferation and cell survival. MAPK and PI3K/AKT pathways are also activated upon FSH binding to its receptor (for a review, see ref. 84), suggesting that FSH and IGF actions may be interconnected and coordinated in Sertoli cells. A recent in vivo study demonstrated that both insulin receptor (INSR) and the type I insulin-like growth factor receptor (IGF1R) act independently of thyroid hormones but may be required for FSH-mediated Sertoli cell proliferation.82
Relaxin Receptor (RXFP1)
Relaxin (RLN) interacts with RXFP1, a member of the G-protein coupled receptor (GPCR) family, and stimulates several intracellular pathways (for a review, see ref. 85). Although most RLN production in the male reproductive system occurs in the prostate, testes are also a source of RLN.86-90 RLN and its receptor are co-expressed in testis, which suggests that RLN may be an autocrine or paracrine mediator of testicular function. The relaxin gene knockout (Rln−/−) mice have smaller testis, decreased sperm maturation, and increased apoptosis.91 It seems that the main site of relaxin expression in the testes varies depending on the species. While RLN in the rat is mainly found in the seminiferous epithelium and is absent in the interstitium,87 in the boar testis RLN expression is only found in Leydig cells.89
RLN mRNA levels are higher in testis of immature than adult rats,87 suggesting that the hormone may display more important roles in immature animals. Treatment with relaxin increased the incorporation of 3H-thymidine and the proliferating cell nuclear antigen (PCNA) on cultured Sertoli cells from 15-d-old rats.87,92 Relaxin-induced Sertoli cell proliferation involved activation of a Gi protein; it was blocked by inhibition of MAPK3/1 or PI3K/AKT pathways, but not by inhibition of protein kinase C (PKC) or epidermal growth factor receptor (EGFR) activity.92 RLN and FSH did not show an additive or synergistic effect on Sertoli cell proliferation.
The role of RLN receptors in the adult testis remains to be determined. Relaxin and Rxfp1 mRNA are also present in Sertoli cells from adult 120-d-old rats.87 Immunohistochemistry showed expression of RXFP1 in myoid, Sertoli, and post-meiotic germ cells (elongated spermatids), in testicular sections from adult animals.93
Estrogen Receptor (ESR)
Our laboratory has shown the presence of the classic estrogen receptors ESR1 (ERα) and ESR2 (ERβ),94 the intracellular signaling pathways,94,95 and the functions of these receptors95 in rat Sertoli cells. The expression of ESR1 is higher in cultured Sertoli cells obtained from 5- and 15-d-old rats than 20- and 30-d-old rats. Conversely, the expression of ESR2 is higher in cultured Sertoli cells from 20- and 30-d-old than 5- and 15-d-old rats. This differential expression of ESR1 and ESR2 is also present in freshly isolated Sertoli cells from these animals.95 G protein-coupled estrogen receptor 1 (GPER, or G protein-coupled receptor 30) is also present in Sertoli cells from 15-d-old rats96 (for a review, see ref. 97).
17β-estradiol (E2) at physiological concentration activates the translocation of ESRs to the plasma membrane mediated by SRC family of tyrosine kinases (SRC), which results in the phosphorylation of EGFR and MAPK3/1 in cultured Sertoli cells from 15-d-old rats.94 E2-ESRs also induce PI3K-AKT activation in these cells.98
In Sertoli cells from 15-d-old rats, E2 binds to ESR1, activates MAPK3/1 and PI3K pathways, which may induce phosphorylation of the inhibitor of nuclear transcription factor-kB (IkBα). The phospho-IkBα in turn dissociates from the nuclear transcription factor-kB (NFkB), and free NFkB rapidly translocates from the cytoplasm to the nucleus to regulate the target gene Ccnd1.95 In fact, E2 and ESR1-selective agonist PPT increase the expression of CCND1 (Cyclin D1), and these effects are blocked by the ESR1-selective antagonist MPP, indicating that ESR1 is the upstream receptor regulating this proliferation marker.95 Interestingly, mice with targeted disruption of estrogen receptor (Esr1−/−) exhibit Sertoli cells number similar to wild-type littermates at 11 wk postpartum.99 A possible explanation for this finding is that other hormones, such as testosterone, may play a compensatory role. In fact, the concentration of this hormone in the plasma and testes from Esr1−/− mice is increased.99
E2 also seems critical to determine the end of Sertoli cell proliferation and the start of cell differentiation, and ESR2 seems to be the major estrogen receptor involved in this effect. In fact, E2 or the ESR2-selective agonist DPN increases the expression of the cell cycle inhibitor CDKN1B (p27Kip1) in cultured Sertoli cells from 15-d-old rats, and ESR2-selective antagonist PHTPP blocks this effect.95 The phosphorylation of the transcription factor cyclic AMP response element-binding protein (CREB) induced by activation of the PI3K signaling pathway is essential for the regulation of ESR2-mediated increase of CDKN1B expression. Furthermore, E2/ESR2 inhibits the cell cycle not only because it increases CDKN1B expression but it favors the localization of CDKN1B in the nucleus, allowing the inhibitory effect of CDKN1B on the complex between CCND and CDKs, also localized in the nucleus.95
It is important to emphasize that the activation of ESR2/PI3K/CREB also increases the zinc finger transcription factors GATA1 and doublesex and mab-3-related transcription factor-1 (DMRT1).95 GATA121 (for a review, see ref. 100) and DMRT1101 are related with cell differentiation. GATA target genes are correlated with the onset of differentiation during pre-pubertal life and also with early differentiation genes (Dmrt1, Sry, and Sox9), synthesis of hormones, such as the inhibin subunits a and b, anti-Müllerian hormone, and junctional proteins, such as claudin 11 and Coxsackie-and adenovirus receptor-like membrane protein (CLMP) (for a review, see ref. 100). E2 may increase the expression of the early differentiation gene Dmrt1.95 A recent study has shown that DMRT1 directly regulates genes required for Sertoli cell differentiation, cell cycle control, tight-junction dynamics, germ cell differentiation, and pluripotency.102 Furthermore, DMRT1 in Sertoli cells may regulate tubule morphology, spermatogenesis, and sperm function via its effects on Sertoli cell maturation and polarity.103
In cultured Sertoli cells from 15-d-old rats, similar to other systems, the two classical estrogen receptors mediate effects in distinct directions, and the balance between both actions seems important to determine the end of cell proliferation and the start of cell differentiation. The ratio of ESR1/ESR2 expression in Sertoli cells from 15-d-old rats favors a predominant effect of ESR1. In fact, E2 and ESR1-selective agonist PPT enhance 45% and 88%, respectively, the [Methyl-3H]thymidine incorporation in these cells, confirming a proliferative effect of E2 at this stage of development.94,95
In cultured Sertoli cells from 20-d-old rats, the ratio of ESR1/ESR2 expression favors a predominant effect of ESR2.95 In fact, E2 modulates cell cycle exit and differentiation through ESR2/CREB-mediated increase of the proteins related with cell cycle exit CDKN1B, and differentiation GATA-1 and DMRT1 (unpublished data). A recent study has shown that E2/ESR2 binds to specific estrogen response elements (ERE2/3) in the promoter region of fatty acid amide hydrolase (FAAH) in Sertoli cells from 17-d-old mice. Moreover, the histone lysine demethylase 1 is recruited and associated with ERE2/3, and it plays a role in mediating E2 induction of FAAH expression. Finally, E2-stimulated FAAH expression protects Sertoli cells against apoptosis induced by anandamide, an endocannabinoid. FAAH gene expression is not regulated by E2/ESR2 in immature Sertoli cells.104
It is important also to emphasize that in Sertoli cells from 15-d-old rats E2 and GPER-selective agonist G1, through activation of EGFR/MAPK3/1 and PIK3 pathways, are involved in the upregulation of antiapoptotic proteins BCL2 and BCL2L298 (for a review, see ref. 97). E2 and G1, through activation of GPER/EGFR/MAPK3/1/phospho-CREB, also decrease the expression of the proapoptotic protein BAX98 (for a review, see ref. 97).
In summary, E2 induces proliferative effect via ESR1 and antiapoptotic effect via GPER in immature Sertoli cells, while activation of ESR2 plays a role to stop cell proliferation and the start of cell differentiation and may be also involved in regulation of apoptosis.
Whether E2 plays a role on dedifferentiation of the mature non-proliferative Sertoli cell to a functionally juvenile phenotype remains be explored. We are only now beginning to decipher the roles of ESR1, GPER, and ESR2 to regulate the proliferation, apoptosis, or differentiation of the Sertoli cells. A diagram summarizing our main findings and the proposed mechanism is in Figure 1.
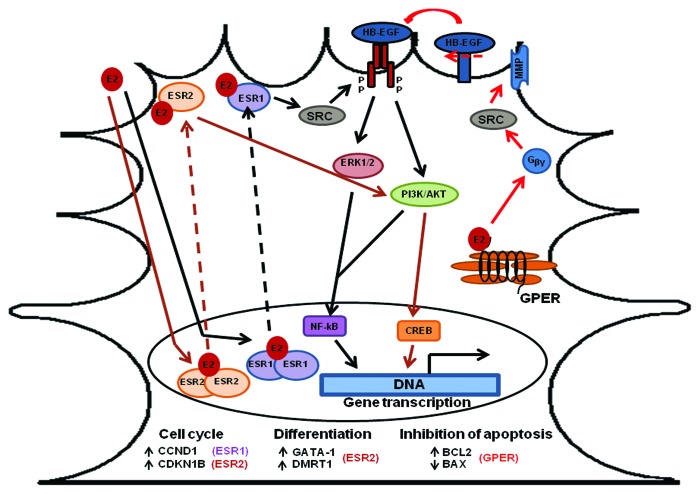
Figure 1. The proposed mechanism for the role of estrogen receptors on Sertoli cell proliferation and differentiation. E2 (or the selective ligand) interacts with ESR1 and induces activation of ERK1/2 and PI3K/AKT pathways, and translocation of the transcription factor NFKB to the nucleus, to activate expression of CCND1 (cyclin D1) and cell proliferation. Interaction of ESR2 with E2 (or the selective ligand) induces activation of PI3K/AKT pathway and phosphorylation of the transcription factor CREB, which induces expression of factors involved in inhibition of cell proliferation and/or start of cell differentiation: CDKN1B (p27kip1), GATA-1, and DMRT1.95 E2 (or the selective ligand) interacts with GPER and induces transactivation of EGFR via activation of SRC by G-protein βγ subunits that promotes a metalloprotease-dependent release of EGFR ligands, with a consequent activation of ERK1/2 and PIK3 pathways, which in turn triggers an increase in the expression of BCL2 and a decrease in the expression of BAX98 (for a review, see ref. 97). See text for more details.
Concluding Remarks and Future Perspectives
Sertoli cell fate is determined during embryonic period at the time of sex differentiation. This is followed by a phase of rapid cell proliferation and differentiation. These processes need to be tightly regulated to allow normal spermatogenesis and fertility in the adult life. The dynamics of Sertoli cell proliferation and differentiation seems to be controlled by a complex array of cell–cell junctions, hormones, and growth factors, which activate a complex net of intracellular signaling pathways that affect cell cycle gene expression, and/or transcription of functional markers of cell differentiation. Hormonal action is also regulated in an autocrine/paracrine way by other testicular factors. Overlapping and interconnected actions are observed with some of these hormones and growth factors. Furthermore, some of these hormones and growth factors increase proliferation of Sertoli cells obtained from neonatal or prepubertal testis, but promote inhibitory effects on the cell cycle in post-pubertal Sertoli cells. An imbalance of these mechanisms may result in infertility diseases, and much remains to be learned about the complex interplay of hormonal actions in Sertoli cells. Further experimental approaches using combination of these hormone and growth factors are necessary to address these issues on Sertoli cell biology.
The Sertoli cell population appears to be more dynamic than previously considered, and the identification of paracrine and autocrine factors capable of reprogramming Sertoli cells to immature dedifferentiated phenotype may be important for a complete understanding of spermatogenesis and pathologies of the testis and to develop new treatments for men with idiopathic infertility.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
Research in the authors’ laboratory is supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, grant numbers 2008/56564-1 and 2010/52306-8 to Porto CS, and 2008/57239-7 and 2010/10274-2 to Lazari MFM). We thank Espedita M.J. Silva Santos for technical assistance. Research fellowship (Porto CP and Lazari MFM) was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico, CNPq. Post-doctoral, doctoral, and master fellowships supported by FAPESP (Lucas TFG, Nascimento AR, Pimenta MT, Pisolato R).
References
- 1.Sharpe RM, McKinnell C, Kivlin C, Fisher JS. Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction. 2003;125:769–84. doi: 10.1530/rep.0.1250769. [DOI] [PubMed] [Google Scholar]
- 2.Tarulli GA, Stanton PG, Meachem SJ. Is the adult Sertoli cell terminally differentiated? Biol Reprod. 2012;87:13–, 1-11. doi: 10.1095/biolreprod.111.095091. [DOI] [PubMed] [Google Scholar]
- 3.Orth JM. Proliferation of Sertoli cells in fetal and postnatal rats: a quantitative autoradiographic study. Anat Rec. 1982;203:485–92. doi: 10.1002/ar.1092030408. [DOI] [PubMed] [Google Scholar]
- 4.Berndtson WE, Thompson TL. Changing relationships between testis size, Sertoli cell number and spermatogenesis in Sprague-Dawley rats. J Androl. 1990;11:429–35. [PubMed] [Google Scholar]
- 5.Orth JM, Gunsalus GL, Lamperti AA. Evidence from Sertoli cell-depleted rats indicates that spermatid number in adults depends on numbers of Sertoli cells produced during perinatal development. Endocrinology. 1988;122:787–94. doi: 10.1210/endo-122-3-787. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed EA, Barten-van Rijbroek AD, Kal HB, Sadri-Ardekani H, Mizrak SC, van Pelt AM, de Rooij DG. Proliferative activity in vitro and DNA repair indicate that adult mouse and human Sertoli cells are not terminally differentiated, quiescent cells. Biol Reprod. 2009;80:1084–91. doi: 10.1095/biolreprod.108.071662. [DOI] [PubMed] [Google Scholar]
- 7.Chui K, Trivedi A, Cheng CY, Cherbavaz DB, Dazin PF, Huynh AL, Mitchell JB, Rabinovich GA, Noble-Haeusslein LJ, John CM. Characterization and functionality of proliferative human Sertoli cells. Cell Transplant. 2011;20:619–35. doi: 10.3727/096368910X536563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mital P, Kaur G, Bowlin B, Paniagua NJ, Korbutt GS, Dufour JM. Nondividing, postpubertal rat sertoli cells resumed proliferation after transplantation. Biol Reprod. 2014;90:13. doi: 10.1095/biolreprod.113.110197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaudhary J, Sadler-Riggleman I, Ague JM, Skinner MK. The helix-loop-helix inhibitor of differentiation (ID) proteins induce post-mitotic terminally differentiated Sertoli cells to re-enter the cell cycle and proliferate. Biol Reprod. 2005;72:1205–17. doi: 10.1095/biolreprod.104.035717. [DOI] [PubMed] [Google Scholar]
- 10.Mazaud-Guittot S, Meugnier E, Pesenti S, Wu X, Vidal H, Gow A, Le Magueresse-Battistoni B. Claudin 11 deficiency in mice results in loss of the Sertoli cell epithelial phenotype in the testis. Biol Reprod. 2010;82:202–13. doi: 10.1095/biolreprod.109.078907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brehm R, Zeiler M, Rüttinger C, Herde K, Kibschull M, Winterhager E, Willecke K, Guillou F, Lécureuil C, Steger K, et al. A sertoli cell-specific knockout of connexin43 prevents initiation of spermatogenesis. Am J Pathol. 2007;171:19–31. doi: 10.2353/ajpath.2007.061171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steger K, Rey R, Louis F, Kliesch S, Behre HM, Nieschlag E, Hoepffner W, Bailey D, Marks A, Bergmann M. Reversion of the differentiated phenotype and maturation block in Sertoli cells in pathological human testis. Hum Reprod. 1999;14:136–43. doi: 10.1093/humrep/14.1.136. [DOI] [PubMed] [Google Scholar]
- 13.Brehm R, Rey R, Kliesch S, Steger K, Marks A, Bergmann M. Mitotic activity of Sertoli cells in adult human testis: an immunohistochemical study to characterize Sertoli cells in testicular cords from patients showing testicular dysgenesis syndrome. Anat Embryol (Berl) 2006;211:223–36. doi: 10.1007/s00429-005-0075-8. [DOI] [PubMed] [Google Scholar]
- 14.Cheng CY, Mruk DD. Cell junction dynamics in the testis: Sertoli-germ cell interactions and male contraceptive development. Physiol Rev. 2002;82:825–74. doi: 10.1152/physrev.00009.2002. [DOI] [PubMed] [Google Scholar]
- 15.Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev. 2004;25:747–806. doi: 10.1210/er.2003-0022. [DOI] [PubMed] [Google Scholar]
- 16.Sridharan S, Simon L, Meling DD, Cyr DG, Gutstein DE, Fishman GI, Guillou F, Cooke PS. Proliferation of adult sertoli cells following conditional knockout of the Gap junctional protein GJA1 (connexin 43) in mice. Biol Reprod. 2007;76:804–12. doi: 10.1095/biolreprod.106.059212. [DOI] [PubMed] [Google Scholar]
- 17.Carette D, Weider K, Gilleron J, Giese S, Dompierre J, Bergmann M, Brehm R, Denizot JP, Segretain D, Pointis G. Major involvement of connexin 43 in seminiferous epithelial junction dynamics and male fertility. Dev Biol. 2010;346:54–67. doi: 10.1016/j.ydbio.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 18.Bremner WJ, Millar MR, Sharpe RM, Saunders PT. Immunohistochemical localization of androgen receptors in the rat testis: evidence for stage-dependent expression and regulation by androgens. Endocrinology. 1994;135:1227–34. doi: 10.1210/endo.135.3.8070367. [DOI] [PubMed] [Google Scholar]
- 19.You L, Sar M. Androgen receptor expression in the testes and epididymides of prenatal and postnatal Sprague-Dawley rats. Endocrine. 1998;9:253–61. doi: 10.1385/ENDO:9:3:253. [DOI] [PubMed] [Google Scholar]
- 20.Al-Attar L, Noël K, Dutertre M, Belville C, Forest MG, Burgoyne PS, Josso N, Rey R. Hormonal and cellular regulation of Sertoli cell anti-Müllerian hormone production in the postnatal mouse. J Clin Invest. 1997;100:1335–43. doi: 10.1172/JCI119653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan KA, De Gendt K, Atanassova N, Walker M, Sharpe RM, Saunders PT, Denolet E, Verhoeven G. The role of androgens in sertoli cell proliferation and functional maturation: studies in mice with total or Sertoli cell-selective ablation of the androgen receptor. Endocrinology. 2005;146:2674–83. doi: 10.1210/en.2004-1630. [DOI] [PubMed] [Google Scholar]
- 22.McKinnell C, Saunders PT, Fraser HM, Kelnar CJ, Kivlin C, Morris KD, Sharpe RM. Comparison of androgen receptor and oestrogen receptor beta immunoexpression in the testes of the common marmoset (Callithrix jacchus) from birth to adulthood: low androgen receptor immunoexpression in Sertoli cells during the neonatal increase in testosterone concentrations. Reproduction. 2001;122:419–29. doi: 10.1530/rep.0.1220419. [DOI] [PubMed] [Google Scholar]
- 23.Chemes HE, Rey RA, Nistal M, Regadera J, Musse M, González-Peramato P, Serrano A. Physiological androgen insensitivity of the fetal, neonatal, and early infantile testis is explained by the ontogeny of the androgen receptor expression in Sertoli cells. J Clin Endocrinol Metab. 2008;93:4408–12. doi: 10.1210/jc.2008-0915. [DOI] [PubMed] [Google Scholar]
- 24.Suárez-Quian CA, Martínez-García F, Nistal M, Regadera J. Androgen receptor distribution in adult human testis. J Clin Endocrinol Metab. 1999;84:350–8. doi: 10.1210/jcem.84.1.5410. [DOI] [PubMed] [Google Scholar]
- 25.Zhou Q, Nie R, Prins GS, Saunders PT, Katzenellenbogen BS, Hess RA. Localization of androgen and estrogen receptors in adult male mouse reproductive tract. J Androl. 2002;23:870–81. [PubMed] [Google Scholar]
- 26.Johnston H, Baker PJ, Abel M, Charlton HM, Jackson G, Fleming L, Kumar TR, O’Shaughnessy PJ. Regulation of Sertoli cell number and activity by follicle-stimulating hormone and androgen during postnatal development in the mouse. Endocrinology. 2004;145:318–29. doi: 10.1210/en.2003-1055. [DOI] [PubMed] [Google Scholar]
- 27.Lim P, Robson M, Spaliviero J, McTavish KJ, Jimenez M, Zajac JD, Handelsman DJ, Allan CM. Sertoli cell androgen receptor DNA binding domain is essential for the completion of spermatogenesis. Endocrinology. 2009;150:4755–65. doi: 10.1210/en.2009-0416. [DOI] [PubMed] [Google Scholar]
- 28.O’Shaughnessy PJ, Monteiro A, Abel M. Testicular development in mice lacking receptors for follicle stimulating hormone and androgen. PLoS One. 2012;7:e35136. doi: 10.1371/journal.pone.0035136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Willems A, Batlouni SR, Esnal A, Swinnen JV, Saunders PT, Sharpe RM, França LR, De Gendt K, Verhoeven G. Selective ablation of the androgen receptor in mouse sertoli cells affects sertoli cell maturation, barrier formation and cytoskeletal development. PLoS One. 2010;5:e14168. doi: 10.1371/journal.pone.0014168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hazra R, Corcoran L, Robson M, McTavish KJ, Upton D, Handelsman DJ, Allan CM. Temporal role of Sertoli cell androgen receptor expression in spermatogenic development. Mol Endocrinol. 2013;27:12–24. doi: 10.1210/me.2012-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh J, Handelsman DJ. Neonatal administration of FSH increases Sertoli cell numbers and spermatogenesis in gonadotropin-deficient (hpg) mice. J Endocrinol. 1996;151:37–48. doi: 10.1677/joe.0.1510037. [DOI] [PubMed] [Google Scholar]
- 32.Haywood M, Spaliviero J, Jimemez M, King NJ, Handelsman DJ, Allan CM. Sertoli and germ cell development in hypogonadal (hpg) mice expressing transgenic follicle-stimulating hormone alone or in combination with testosterone. Endocrinology. 2003;144:509–17. doi: 10.1210/en.2002-220710. [DOI] [PubMed] [Google Scholar]
- 33.Allan CM, Garcia A, Spaliviero J, Zhang FP, Jimenez M, Huhtaniemi I, Handelsman DJ. Complete Sertoli cell proliferation induced by follicle-stimulating hormone (FSH) independently of luteinizing hormone activity: evidence from genetic models of isolated FSH action. Endocrinology. 2004;145:1587–93. doi: 10.1210/en.2003-1164. [DOI] [PubMed] [Google Scholar]
- 34.Griswold M, Mably E, Fritz IB. Stimulation by follicle stimulating hormone and dibutyryl cyclic AMP of incorporation of 3H-thymidine into nuclear DNA of cultured Sertoli cell-enriched preparations from immature rats. Curr Top Mol Endocrinol. 1975;2:413–20. doi: 10.1007/978-1-4613-4440-7_29. [DOI] [PubMed] [Google Scholar]
- 35.Griswold MD, Solari A, Tung PS, Fritz IB. Stimulation by follicle-stimulating hormone of DNA synthesis and of mitosis in cultured Sertoli cells prepared from testes of immature rats. Mol Cell Endocrinol. 1977;7:151–65. doi: 10.1016/0303-7207(77)90064-8. [DOI] [PubMed] [Google Scholar]
- 36.Meachem SJ, McLachlan RI, de Kretser DM, Robertson DM, Wreford NG. Neonatal exposure of rats to recombinant follicle stimulating hormone increases adult Sertoli and spermatogenic cell numbers. Biol Reprod. 1996;54:36–44. doi: 10.1095/biolreprod54.1.36. [DOI] [PubMed] [Google Scholar]
- 37.Dierich A, Sairam MR, Monaco L, Fimia GM, Gansmuller A, LeMeur M, Sassone-Corsi P. Impairing follicle-stimulating hormone (FSH) signaling in vivo: targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proc Natl Acad Sci U S A. 1998;95:13612–7. doi: 10.1073/pnas.95.23.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abel MH, Wootton AN, Wilkins V, Huhtaniemi I, Knight PG, Charlton HM. The effect of a null mutation in the follicle-stimulating hormone receptor gene on mouse reproduction. Endocrinology. 2000;141:1795–803. doi: 10.1210/endo.141.5.7456. [DOI] [PubMed] [Google Scholar]
- 39.Meachem SJ, Ruwanpura SM, Ziolkowski J, Ague JM, Skinner MK, Loveland KL. Developmentally distinct in vivo effects of FSH on proliferation and apoptosis during testis maturation. J Endocrinol. 2005;186:429–46. doi: 10.1677/joe.1.06121. [DOI] [PubMed] [Google Scholar]
- 40.Kara E, Crépieux P, Gauthier C, Martinat N, Piketty V, Guillou F, Reiter E. A phosphorylation cluster of five serine and threonine residues in the C-terminus of the follicle-stimulating hormone receptor is important for desensitization but not for beta-arrestin-mediated ERK activation. Mol Endocrinol. 2006;20:3014–26. doi: 10.1210/me.2006-0098. [DOI] [PubMed] [Google Scholar]
- 41.Tranchant T, Durand G, Gauthier C, Crépieux P, Ulloa-Aguirre A, Royère D, Reiter E. Preferential β-arrestin signalling at low receptor density revealed by functional characterization of the human FSH receptor A189 V mutation. Mol Cell Endocrinol. 2011;331:109–18. doi: 10.1016/j.mce.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 42.Lécureuil C, Tesseraud S, Kara E, Martinat N, Sow A, Fontaine I, Gauthier C, Reiter E, Guillou F, Crépieux P. Follicle-stimulating hormone activates p70 ribosomal protein S6 kinase by protein kinase A-mediated dephosphorylation of Thr 421/Ser 424 in primary Sertoli cells. Mol Endocrinol. 2005;19:1812–20. doi: 10.1210/me.2004-0289. [DOI] [PubMed] [Google Scholar]
- 43.Musnier A, Heitzler D, Boulo T, Tesseraud S, Durand G, Lécureuil C, Guillou H, Poupon A, Reiter E, Crépieux P. Developmental regulation of p70 S6 kinase by a G protein-coupled receptor dynamically modelized in primary cells. Cell Mol Life Sci. 2009;66:3487–503. doi: 10.1007/s00018-009-0134-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crépieux P, Marion S, Martinat N, Fafeur V, Vern YL, Kerboeuf D, Guillou F, Reiter E. The ERK-dependent signalling is stage-specifically modulated by FSH, during primary Sertoli cell maturation. Oncogene. 2001;20:4696–709. doi: 10.1038/sj.onc.1204632. [DOI] [PubMed] [Google Scholar]
- 45.McDonald CA, Millena AC, Reddy S, Finlay S, Vizcarra J, Khan SA, Davis JS. Follicle-stimulating hormone-induced aromatase in immature rat Sertoli cells requires an active phosphatidylinositol 3-kinase pathway and is inhibited via the mitogen-activated protein kinase signaling pathway. Mol Endocrinol. 2006;20:608–18. doi: 10.1210/me.2005-0245. [DOI] [PubMed] [Google Scholar]
- 46.Riera MF, Regueira M, Galardo MN, Pellizzari EH, Meroni SB, Cigorraga SB. Signal transduction pathways in FSH regulation of rat Sertoli cell proliferation. Am J Physiol Endocrinol Metab. 2012;302:E914–23. doi: 10.1152/ajpendo.00477.2011. [DOI] [PubMed] [Google Scholar]
- 47.Bhattacharya I, Pradhan BS, Sarda K, Gautam M, Basu S, Majumdar SS. A switch in Sertoli cell responsiveness to FSH may be responsible for robust onset of germ cell differentiation during prepubartal testicular maturation in rats. Am J Physiol Endocrinol Metab. 2012;303:E886–98. doi: 10.1152/ajpendo.00293.2012. [DOI] [PubMed] [Google Scholar]
- 48.Jannini EA, Ulisse S, D’Armiento M. Thyroid hormone and male gonadal function. Endocr Rev. 1995;16:443–59. doi: 10.1210/edrv-16-4-443. [DOI] [PubMed] [Google Scholar]
- 49.Cooke PS, Zhao YD, Bunick D. Triiodothyronine inhibits proliferation and stimulates differentiation of cultured neonatal Sertoli cells: possible mechanism for increased adult testis weight and sperm production induced by neonatal goitrogen treatment. Biol Reprod. 1994;51:1000–5. doi: 10.1095/biolreprod51.5.1000. [DOI] [PubMed] [Google Scholar]
- 50.van Haaster LH, de Jong FH, Docter R, de Rooij DG. High neonatal triiodothyronine levels reduce the period of Sertoli cell proliferation and accelerate tubular lumen formation in the rat testis, and increase serum inhibin levels. Endocrinology. 1993;133:755–60. doi: 10.1210/endo.133.2.8344214. [DOI] [PubMed] [Google Scholar]
- 51.Palmero S, Prati M, Bolla F, Fugassa E. Tri-iodothyronine directly affects rat Sertoli cell proliferation and differentiation. J Endocrinol. 1995;145:355–62. doi: 10.1677/joe.0.1450355. [DOI] [PubMed] [Google Scholar]
- 52.De França LR, Hess RA, Cooke PS, Russell LD. Neonatal hypothyroidism causes delayed Sertoli cell maturation in rats treated with propylthiouracil: evidence that the Sertoli cell controls testis growth. Anat Rec. 1995;242:57–69. doi: 10.1002/ar.1092420108. [DOI] [PubMed] [Google Scholar]
- 53.Hess RA, Cooke PS, Bunick D, Kirby JD. Adult testicular enlargement induced by neonatal hypothyroidism is accompanied by increased Sertoli and germ cell numbers. Endocrinology. 1993;132:2607–13. doi: 10.1210/endo.132.6.8504761. [DOI] [PubMed] [Google Scholar]
- 54.Joyce KL, Porcelli J, Cooke PS. Neonatal goitrogen treatment increases adult testis size and sperm production in the mouse. J Androl. 1993;14:448–55. [PubMed] [Google Scholar]
- 55.Wagner MS, Wajner SM, Maia AL. Is there a role for thyroid hormone on spermatogenesis? Microsc Res Tech. 2009;72:796–808. doi: 10.1002/jemt.20759. [DOI] [PubMed] [Google Scholar]
- 56.Hadj-Sahraoui N, Seugnet I, Ghorbel MT, Demeneix B. Hypothyroidism prolongs mitotic activity in the post-natal mouse brain. Neurosci Lett. 2000;280:79–82. doi: 10.1016/S0304-3940(00)00768-0. [DOI] [PubMed] [Google Scholar]
- 57.Holsberger DR, Buchold GM, Leal MC, Kiesewetter SE, O’Brien DA, Hess RA, França LR, Kiyokawa H, Cooke PS. Cell-cycle inhibitors p27Kip1 and p21Cip1 regulate murine Sertoli cell proliferation. Biol Reprod. 2005;72:1429–36. doi: 10.1095/biolreprod.105.040386. [DOI] [PubMed] [Google Scholar]
- 58.Gilleron J, Nebout M, Scarabelli L, Senegas-Balas F, Palmero S, Segretain D, Pointis G. A potential novel mechanism involving connexin 43 gap junction for control of sertoli cell proliferation by thyroid hormones. J Cell Physiol. 2006;209:153–61. doi: 10.1002/jcp.20716. [DOI] [PubMed] [Google Scholar]
- 59.Fumel B, Guerquin MJ, Livera G, Staub C, Magistrini M, Gauthier C, Flamant F, Guillou F, Fouchécourt S. Thyroid hormone limits postnatal Sertoli cell proliferation in vivo by activation of its alpha1 isoform receptor (TRalpha1) present in these cells and by regulation of Cdk4/JunD/c-myc mRNA levels in mice. Biol Reprod. 2012;87:16–, 1-9. doi: 10.1095/biolreprod.111.098418. [DOI] [PubMed] [Google Scholar]
- 60.Buzzard JJ, Wreford NG, Morrison JR. Marked extension of proliferation of rat Sertoli cells in culture using recombinant human FSH. Reproduction. 2002;124:633–41. doi: 10.1530/rep.0.1240633. [DOI] [PubMed] [Google Scholar]
- 61.Archambeault DR, Yao HH. Activin A, a product of fetal Leydig cells, is a unique paracrine regulator of Sertoli cell proliferation and fetal testis cord expansion. Proc Natl Acad Sci U S A. 2010;107:10526–31. doi: 10.1073/pnas.1000318107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mendis SH, Meachem SJ, Sarraj MA, Loveland KL. Activin A balances Sertoli and germ cell proliferation in the fetal mouse testis. Biol Reprod. 2011;84:379–91. doi: 10.1095/biolreprod.110.086231. [DOI] [PubMed] [Google Scholar]
- 63.Barakat B, O’Connor AE, Gold E, de Kretser DM, Loveland KL. Inhibin, activin, follistatin and FSH serum levels and testicular production are highly modulated during the first spermatogenic wave in mice. Reproduction. 2008;136:345–59. doi: 10.1530/REP-08-0140. [DOI] [PubMed] [Google Scholar]
- 64.Mithraprabhu S, Mendis S, Meachem SJ, Tubino L, Matzuk MM, Brown CW, Loveland KL. Activin bioactivity affects germ cell differentiation in the postnatal mouse testis in vivo. Biol Reprod. 2010;82:980–90. doi: 10.1095/biolreprod.109.079855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Matzuk MM, Finegold MJ, Su JG, Hsueh AJ, Bradley A. Alpha-inhibin is a tumour-suppressor gene with gonadal specificity in mice. Nature. 1992;360:313–9. doi: 10.1038/360313a0. [DOI] [PubMed] [Google Scholar]
- 66.Li Q, Kumar R, Underwood K, O’Connor AE, Loveland KL, Seehra JS, Matzuk MM. Prevention of cachexia-like syndrome development and reduction of tumor progression in inhibin-deficient mice following administration of a chimeric activin receptor type II-murine Fc protein. Mol Hum Reprod. 2007;13:675–83. doi: 10.1093/molehr/gam055. [DOI] [PubMed] [Google Scholar]
- 67.Tanimoto Y, Tanimoto K, Sugiyama F, Horiguchi H, Murakami K, Yagami K, Fukamizu A. Male sterility in transgenic mice expressing activin betaA subunit gene in testis. Biochem Biophys Res Commun. 1999;259:699–705. doi: 10.1006/bbrc.1999.0833. [DOI] [PubMed] [Google Scholar]
- 68.Fragale A, Puglisi R, Morena AR, Stefanini M, Boitani C. Age-dependent activin receptor expression pinpoints activin A as a physiological regulator of rat Sertoli cell proliferation. Mol Hum Reprod. 2001;7:1107–14. doi: 10.1093/molehr/7.12.1107. [DOI] [PubMed] [Google Scholar]
- 69.Wreford NG, Rajendra Kumar T, Matzuk MM, de Kretser DM. Analysis of the testicular phenotype of the follicle-stimulating hormone beta-subunit knockout and the activin type II receptor knockout mice by stereological analysis. Endocrinology. 2001;142:2916–20. doi: 10.1210/endo.142.7.8230. [DOI] [PubMed] [Google Scholar]
- 70.Gely-Pernot A, Raverdeau M, Célébi C, Dennefeld C, Feret B, Klopfenstein M, Yoshida S, Ghyselinck NB, Mark M. Spermatogonia differentiation requires retinoic acid receptor γ. Endocrinology. 2012;153:438–49. doi: 10.1210/en.2011-1102. [DOI] [PubMed] [Google Scholar]
- 71.Griswold MD, Bishop PD, Kim KH, Ping R, Siiteri JE, Morales C. Function of vitamin A in normal and synchronized seminiferous tubules. Ann N Y Acad Sci. 1989;564:154–72. doi: 10.1111/j.1749-6632.1989.tb25895.x. [DOI] [PubMed] [Google Scholar]
- 72.Raverdeau M, Gely-Pernot A, Féret B, Dennefeld C, Benoit G, Davidson I, Chambon P, Mark M, Ghyselinck NB. Retinoic acid induces Sertoli cell paracrine signals for spermatogonia differentiation but cell autonomously drives spermatocyte meiosis. Proc Natl Acad Sci U S A. 2012;109:16582–7. doi: 10.1073/pnas.1214936109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Buzzard JJ, Wreford NG, Morrison JR. Thyroid hormone, retinoic acid, and testosterone suppress proliferation and induce markers of differentiation in cultured rat sertoli cells. Endocrinology. 2003;144:3722–31. doi: 10.1210/en.2003-0379. [DOI] [PubMed] [Google Scholar]
- 74.Chung SS, Choi C, Wang X, Hallock L, Wolgemuth DJ. Aberrant distribution of junctional complex components in retinoic acid receptor alpha-deficient mice. Microsc Res Tech. 2010;73:583–96. doi: 10.1002/jemt.20797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hasegawa K, Saga Y. Retinoic acid signaling in Sertoli cells regulates organization of the blood-testis barrier through cyclical changes in gene expression. Development. 2012;139:4347–55. doi: 10.1242/dev.080119. [DOI] [PubMed] [Google Scholar]
- 76.Sugimoto R, Nabeshima Y, Yoshida S. Retinoic acid metabolism links the periodical differentiation of germ cells with the cycle of Sertoli cells in mouse seminiferous epithelium. Mech Dev. 2012;128:610–24. doi: 10.1016/j.mod.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 77.Vernet N, Dennefeld C, Guillou F, Chambon P, Ghyselinck NB, Mark M. Prepubertal testis development relies on retinoic acid but not rexinoid receptors in Sertoli cells. EMBO J. 2006;25:5816–25. doi: 10.1038/sj.emboj.7601447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kastner P, Mark M, Leid M, Gansmuller A, Chin W, Grondona JM, Décimo D, Krezel W, Dierich A, Chambon P. Abnormal spermatogenesis in RXR beta mutant mice. Genes Dev. 1996;10:80–92. doi: 10.1101/gad.10.1.80. [DOI] [PubMed] [Google Scholar]
- 79.Vernet N, Dennefeld C, Klopfenstein M, Ruiz A, Bok D, Ghyselinck NB, Mark M. Retinoid X receptor beta (RXRB) expression in Sertoli cells controls cholesterol homeostasis and spermiation. Reproduction. 2008;136:619–26. doi: 10.1530/REP-08-0235. [DOI] [PubMed] [Google Scholar]
- 80.Nicholls PK, Harrison CA, Rainczuk KE, Wayne Vogl A, Stanton PG. Retinoic acid promotes Sertoli cell differentiation and antagonises activin-induced proliferation. Mol Cell Endocrinol. 2013;377:33–43. doi: 10.1016/j.mce.2013.06.034. [DOI] [PubMed] [Google Scholar]
- 81.Hansson HA, Billig H, Isgaard J. Insulin-like growth factor I in the developing and mature rat testis: immunohistochemical aspects. Biol Reprod. 1989;40:1321–8. doi: 10.1095/biolreprod40.6.1321. [DOI] [PubMed] [Google Scholar]
- 82.Pitetti JL, Calvel P, Zimmermann C, Conne B, Papaioannou MD, Aubry F, Cederroth CR, Urner F, Fumel B, Crausaz M, et al. An essential role for insulin and IGF1 receptors in regulating sertoli cell proliferation, testis size, and FSH action in mice. Mol Endocrinol. 2013;27:814–27. doi: 10.1210/me.2012-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Froment P, Vigier M, Nègre D, Fontaine I, Beghelli J, Cosset FL, Holzenberger M, Durand P. Inactivation of the IGF-I receptor gene in primary Sertoli cells highlights the autocrine effects of IGF-I. J Endocrinol. 2007;194:557–68. doi: 10.1677/JOE-07-0258. [DOI] [PubMed] [Google Scholar]
- 84.Walker WH, Cheng J. FSH and testosterone signaling in Sertoli cells. Reproduction. 2005;130:15–28. doi: 10.1530/rep.1.00358. [DOI] [PubMed] [Google Scholar]
- 85.Bathgate RA, Halls ML, van der Westhuizen ET, Callander GE, Kocan M, Summers RJ. Relaxin family peptides and their receptors. Physiol Rev. 2013;93:405–80. doi: 10.1152/physrev.00001.2012. [DOI] [PubMed] [Google Scholar]
- 86.Gunnersen JM, Crawford RJ, Tregear GW. Expression of the relaxin gene in rat tissues. Mol Cell Endocrinol. 1995;110:55–64. doi: 10.1016/0303-7207(95)03516-A. [DOI] [PubMed] [Google Scholar]
- 87.Cardoso LC, Nascimento AR, Royer C, Porto CS, Lazari MF. Locally produced relaxin may affect testis and vas deferens function in rats. Reproduction. 2010;139:185–96. doi: 10.1530/REP-09-0146. [DOI] [PubMed] [Google Scholar]
- 88.Kohsaka T, Kato S, Qin S, Minagawa I, Yogo K, Kawarasaki T, Sasada H. Identification of boar testis as a source and target tissue of relaxin. Ann N Y Acad Sci. 2009;1160:194–6. doi: 10.1111/j.1749-6632.2008.03809.x. [DOI] [PubMed] [Google Scholar]
- 89.Kato S, Siqin, Minagawa I, Aoshima T, Sagata D, Konishi H, Yogo K, Kawarasaki T, Sasada H, Tomogane H, et al. Evidence for expression of relaxin hormone-receptor system in the boar testis. J Endocrinol. 2010;207:135–49. doi: 10.1677/JOE-10-0149. [DOI] [PubMed] [Google Scholar]
- 90.Samuel CS, Tian H, Zhao L, Amento EP. Relaxin is a key mediator of prostate growth and male reproductive tract development. Lab Invest. 2003;83:1055–67. doi: 10.1097/01.LAB.0000079784.81186.B9. [DOI] [PubMed] [Google Scholar]
- 91.Samuel CS, Zhao C, Bathgate RA, Du XJ, Summers RJ, Amento EP, Walker LL, McBurnie M, Zhao L, Tregear GW. The relaxin gene-knockout mouse: a model of progressive fibrosis. Ann N Y Acad Sci. 2005;1041:173–81. doi: 10.1196/annals.1282.025. [DOI] [PubMed] [Google Scholar]
- 92.Nascimento AR, Pimenta MT, Lucas TF, Royer C, Porto CS, Lazari MF. Intracellular signaling pathways involved in the relaxin-induced proliferation of rat Sertoli cells. Eur J Pharmacol. 2012;691:283–91. doi: 10.1016/j.ejphar.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 93.Filonzi M, Cardoso LC, Pimenta MT, Queiróz DB, Avellar MC, Porto CS, Lazari MF. Relaxin family peptide receptors Rxfp1 and Rxfp2: mapping of the mRNA and protein distribution in the reproductive tract of the male rat. Reprod Biol Endocrinol. 2007;5:29. doi: 10.1186/1477-7827-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lucas TF, Siu ER, Esteves CA, Monteiro HP, Oliveira CA, Porto CS, Lazari MF. 17beta-estradiol induces the translocation of the estrogen receptors ESR1 and ESR2 to the cell membrane, MAPK3/1 phosphorylation and proliferation of cultured immature rat Sertoli cells. Biol Reprod. 2008;78:101–14. doi: 10.1095/biolreprod.107.063909. [DOI] [PubMed] [Google Scholar]
- 95.Lucas TF, Lazari MF, Porto CS. Differential role of the estrogen receptors ESR1 and ESR2 on the regulation of proteins involved with proliferation and differentiation of Sertoli cells from 15-day-old rats. Mol Cell Endocrinol. 2014;382:84–96. doi: 10.1016/j.mce.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 96.Lucas TF, Royer C, Siu ER, Lazari MF, Porto CS. Expression and signaling of G protein-coupled estrogen receptor 1 (GPER) in rat sertoli cells. Biol Reprod. 2010;83:307–17. doi: 10.1095/biolreprod.110.084160. [DOI] [PubMed] [Google Scholar]
- 97.Lucas TF, Pimenta MT, Pisolato R, Lazari MF, Porto CS. 17β-estradiol signaling and regulation of Sertoli cell function. Spermatogenesis. 2011;1:318–24. doi: 10.4161/spmg.1.4.18903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Royer C, Lucas TF, Lazari MF, Porto CS. 17Beta-estradiol signaling and regulation of proliferation and apoptosis of rat Sertoli cells. Biol Reprod. 2012;86:108. doi: 10.1095/biolreprod.111.096891. [DOI] [PubMed] [Google Scholar]
- 99.Gould ML, Hurst PR, Nicholson HD. The effects of oestrogen receptors alpha and beta on testicular cell number and steroidogenesis in mice. Reproduction. 2007;134:271–9. doi: 10.1530/REP-07-0025. [DOI] [PubMed] [Google Scholar]
- 100.Viger RS, Guittot SM, Anttonen M, Wilson DB, Heikinheimo M. Role of the GATA family of transcription factors in endocrine development, function, and disease. Mol Endocrinol. 2008;22:781–98. doi: 10.1210/me.2007-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen JK, Heckert LL. Dmrt1 expression is regulated by follicle-stimulating hormone and phorbol esters in postnatal Sertoli cells. Endocrinology. 2001;142:1167–78. doi: 10.1210/endo.142.3.8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Murphy MW, Sarver AL, Rice D, Hatzi K, Ye K, Melnick A, Heckert LL, Zarkower D, Bardwell VJ. Genome-wide analysis of DNA binding and transcriptional regulation by the mammalian Doublesex homolog DMRT1 in the juvenile testis. Proc Natl Acad Sci U S A. 2010;107:13360–5. doi: 10.1073/pnas.1006243107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Agbor VA, Tao S, Lei N, Heckert LLA. A Wt1-Dmrt1 transgene restores DMRT1 to sertoli cells of Dmrt1(-/-) testes: a novel model of DMRT1-deficient germ cells. Biol Reprod. 2013;88:51. doi: 10.1095/biolreprod.112.103135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Grimaldi P, Pucci M, Di Siena S, Di Giacomo D, Pirazzi V, Geremia R, Maccarrone M. The faah gene is the first direct target of estrogen in the testis: role of histone demethylase LSD1. Cell Mol Life Sci. 2012;69:4177–90. doi: 10.1007/s00018-012-1074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]