Abstract
Photoexcited acetophenone can catalyze the fluorination of unactivated C(sp3)–H groups. While acetophenone, a colorless oil, only has a trace amount of absorption in the visible light region, its photoexcitation can be achieved by irradiation with light generated by a household compact fluorescent lamp (CFL). This operational simple method provides improved substrate scope for the direct incorporation of a fluorine atom into simple organic molecules. CFL-irradiation can also be used to promote certain classic UV-promoted photoreactions of colorless monoarylketones and enones/enals.
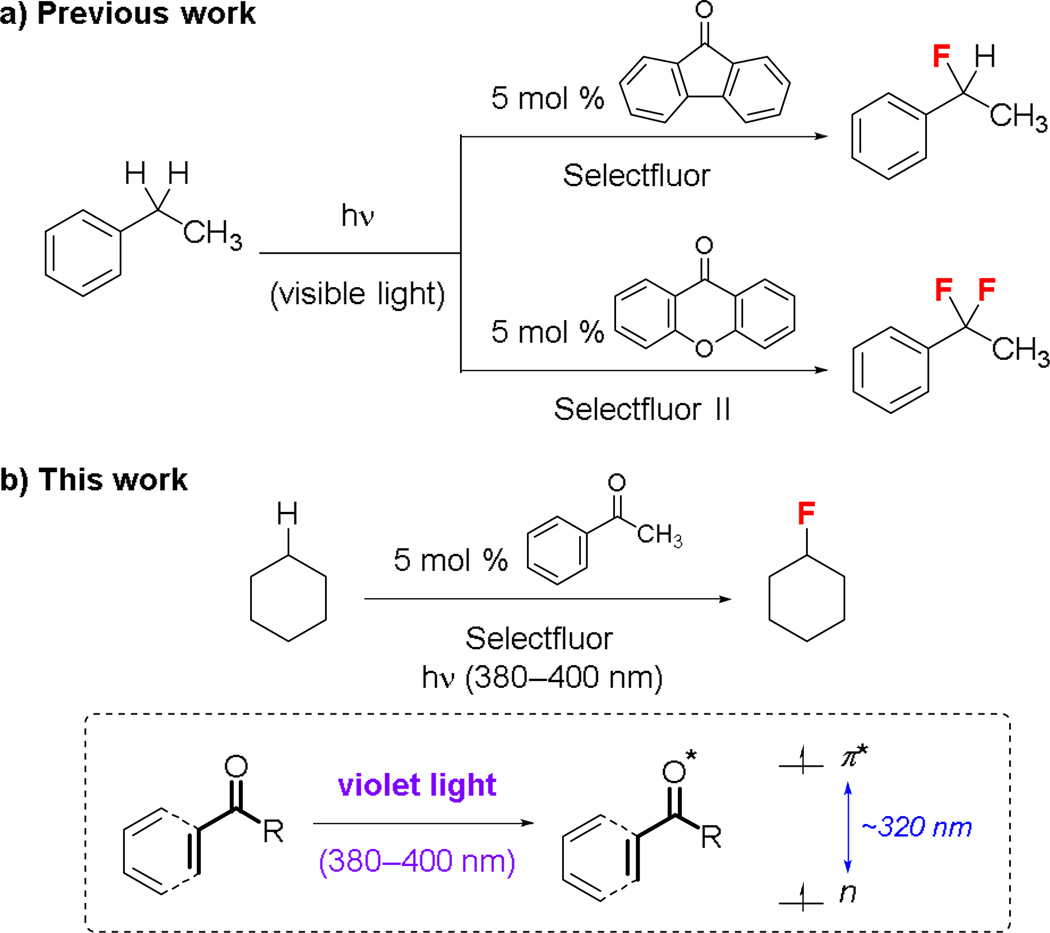
Direct C–H functionalization is a powerful new method for small-molecule synthesis.1,2 Recently, we have reported that visible light can activate diarylketones to catalyze C(sp3)–H fluorination at benzylic positions.3a 9-Fluorenone catalyzes benzylic C–H monofluorination while xanthone catalyzes benzylic C–H difluorination (Fig. 1a). We now show that the violet light (375–400 nm) generated by a household compact fluorescent lamp (CFL) can activate acetophenone, a monoarylketone, to catalyze the fluorination of unactivated C(sp3)–H groups (Fig. 1b). Acetophenone is a colorless oil that has only a trace amount of absorption above 375 nm (n→π* transition λmax ~325 nm). Herein, we demonstrate that CFL-irradation (>375 nm) can effectively promote its photoexcitation. We further show that certain classic UV-promoted photoreactions of monoarylketones and enones/enals can also be induced by CFL-irradiation.
Fig. 1.
Photolytic C–H fluorination reactions catalyzed by photoexcited arylketones. The reactivity and selectivity of the catalysts can be tuned by the ketone substituent groups.
Several catalytic C–H fluorination reactions have been developed to introduce fluorine atoms directly without prior functionalization.4–13 For example, the Sanford,4 Yu,5 and Doyle6 groups have each reported an efficient palladium catalyst system while the Groves7 and the Lectka8 groups have disclosed a manganese and a copper catalyst system, respectively. The Inoue,9 Tang,10 and Hartwig11 groups have also shown that N-oxyl radical and silver salts can catalyze or promote C–H fluorination. More recently, photolytic activation methods have also been reported by Britton,12 Lectka,8c and Tan13 using decatungstate, 1,2,4,5-tetracyanobenzene (TCB), and anthraquinone (AQN) as the catalyst, respectively.
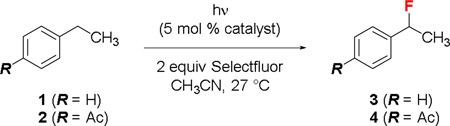
We have been interested in developing vanadium and ketone catalysts for C–H fluorination reactions.3 Previously, we showed that CFL-irradiation14 could activate 9-fluorenone, a yellow solid, to catalyze the benzylic fluorination of ethylbenzene (1) with Selectfluor to give 3 (Table 1, entries 1 and 2).3a Intriguingly, we recently found that the photolytic fluorination of 4-ethylacetophenone (2) did not require 9-fluorenone (entries 3 and 4). We suspected that CFL-irradiation led to the photoexcitation of 2. The resulting diradical-like 2* mediated this C–H fluorination reaction by abstracting a benzylic hydrogen atom from another molecule of 2. Subsequent fluorine atom transfer from Selectfluor gave 4.
Table 1.
Effects of the light source and the ketone catalyst on the benzylic fluorination of 1 and 2
 | |||||
|---|---|---|---|---|---|
| Entry | Substrate | Catalyst | Light source | Time | Yield[i] |
| 1 | 1 | 9-fluorenone | 1 × CFL[a] | 24 h | 89% (85%)[j] |
| 2 | 1 | – | 1 × CFL[a] | 24 h | 0% |
| 3 | 2 | 9-fluorenone | 1 × CFL[a] | 24 h | 80% (76%)[j] |
| 4 | 2 | – | 1 × CFL[a] | 30 h | 89% |
| 5 | 2 | – | 16 × RPR-3000Å[b] | 30 min | 46% |
| 6 | 2 | – | 16 × RPR-3500Å[c] | 1 h | 73% |
| 7 | 2 | – | 16 × RPR-4190Å[d] | 6 h | 96% |
| 8 | 1 | acetophenone | 16 × RPR-4190Å[d] | 6 h | 88% |
| 9 | 1 | acetophenone | 16 × RPR-4190Å[d,e] | 20 h | 70% |
| 10 | 1 | acetophenone | 16 × RPR-4190Å[d,f] | 20 h | 0% |
| 11 | 1 | acetophenone | 1 × violet LED[g] | 3 h | 85% |
| 12 | 1 | acetophenone | 1 × CFL[a] | 20 h | 61% |
| 13 | 1 | acetophenone | 1 × CFL[a,e] | 20 h | 50% |
| 14 | 1 | acetophenone | 1 × CFL[a,f] | 20 h | 0% |
| 15 | 1 | acetone | 1 × CFL[a} | 20 h | 0% |
| 16 | 1 | acetophenone | –[h] | 24 h | 0% |
19 W, household lamp.
21 W, 250–375 nm.
24 W, 300–420 nm.
375–465 nm.
With a 375 nm longpass filter.
With a 400 nm logpass fileter.
9 W, 370–405 nm.
50 °C.
Determined by 19F NMR using C6H5F as an external standard.
Isolated yield.
4-Ethylacetophenone (2) is a monoarylketone that has no apparent absorption above 375 nm (n→π* transition λmax ~325 nm). Its photoexcitation has been traditionally carried out by irradiation with <360 nm UV light. Indeed, UV-irradiation improved the quantum yield significantly but also promoted considerable decomposition (entries 5 and 6). In contrast, irradiation with violet light (375–465 nm, λmax 419 nm) resulted in a clean transformation (entry 7), suggestive of productive absorption at >375 nm.
Because acetophenone and 4-ethylacetophenone (2) have very similar UV absorption spectra, we hypothesized that violet light could also activate acetophenone to catalyze the benzylic fluorination of ethylbenzene (1). Indeed, a clean fluorination occurred to give 3 in 88% yield when irradiating a solution of 1 with violet light (375–465 nm) in the presence of 5 mol % acetophenone and 2 equiv Selectfluor (entry 8).
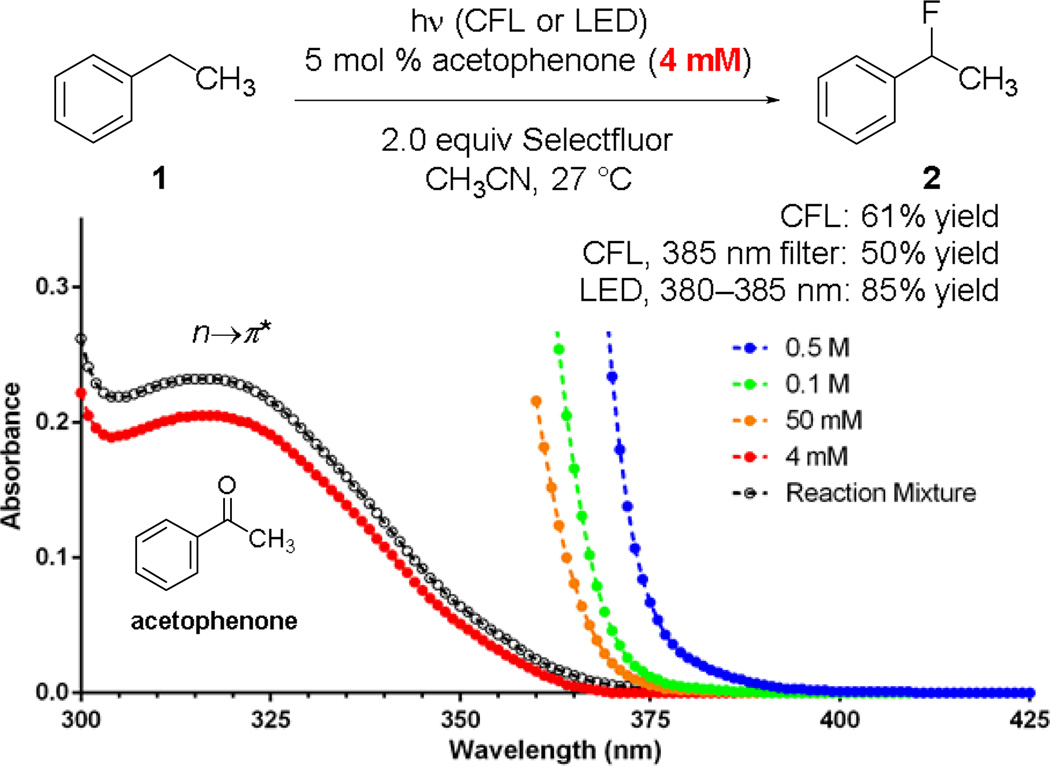
Similar to 2, acetophenone is a colorless oil that has no apparent absorption above 375 nm. To exclude the possibility of UV-contamination,15 we repeated the fluorination of 1 catalyzed by acetophenone (reagent grade, >98%; analytical grade, ≥99.5%; or freshly redistilled from calcium hydride) in the presence of a 375 nm longpass filter. The reaction proceeded slower but smoothly (entry 9). Consistently, using a 370–405 nm LED flashlight as the light source led to the formation of 3 in 85% yield in only 3 h (entry 11). No reaction occurred in the presence of a 400 nm longpass filter (entry 10). CFL-irradiation also promoted the acetophenone-catalyzed benzylic fluorination of 1 in the presence of a 375 nm filter but not a 400 nm filter (entries 12–14). No reaction occurred when using acetone (n→π* transition λmax ~280 nm) as the catalyst (entry 15) or heating the solution at 50 °C in dark (entry 16). Therefore, we believe that short violet light (375–400 nm)16 could effectively promote the photoexcitation of acetophenone under the regular reaction conditions.
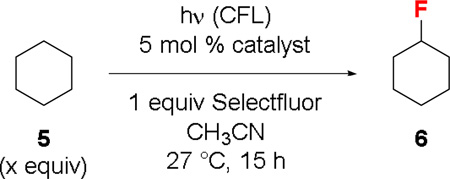
We envisioned that, with a larger n/π* energy gap, acetophenone would be more reactive than diarylketones toward unactivated C–H groups. To test this hypothesis, we examined the fluorination of cyclohexane (5) catalyzed by a series of different ketones (Table 2). Indeed, acetophenone was found to be the most effective catalyst (entry 1). Introducing a methoxy group to acetophenone led to a slightly reduced catalyst activity (entry 2) while adding a nitro group resulted in a low yield of 6 (entry 3). Trifluoroacetophenone and benzaldehyde are also less effective catalysts (entries 4 and 5). Consistent with our previous observations, benzaldehyde reacted with Selectfluor to give benzoyl fluoride under the reaction conditions.3a,17 Benzophenones and 9-fluorenone were less effective despite significantly better absorption (entry 6–9). Xanthone was nearly as good as acetophenone when a large excess of 5 was used (entry 10). However, acetophenone showed better catalyst activity when the amount of 5 was reduced to 5 equiv (entries 11 and 12). The substrate to Selectfluor ratio could be further reduced to ~1.5:1 for acetophenone (entries 13 and 14).
Table 2.
Effects of the catalyst on the fluorination of 5[a]
 | ||||
|---|---|---|---|---|
| Entry | 5 (equiv) | ketone catalyst | Yield[b] | |
| 1 | 23 | 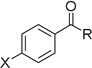 |
R = CH3, X = H | 90% |
| 2 | 23 | R = CH3, X = OMe | 82% | |
| 3 | 23 | R = CH3, X = NO2 | 18% | |
| 4 | 23 | R = CF3, X = H | 63% | |
| 5 | 23 | R = H, X = H | 59% | |
| 6 | 23 | 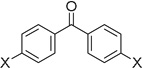 |
X = H | 78% |
| 7 | 23 | X = OMe | 76% | |
| 8 | 23 | X = NMe2 | 17% | |
| 9 | 23 | 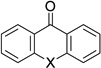 |
X = – | 75% |
| 10 | 23 | X = O | 87% | |
| 11 | 5 | X = O | 81% | |
| 12 | 5 |  |
88% | |
| 13 | 1.5 | 76% | ||
| 14 | 1.0 | 59% | ||
Reaction conditions: 0.01 mmol catalyst, 0.2 mmol Selectfluor, 2 mL CH3CN.
Determined by 19F NMR using C6H5F as an external standard.
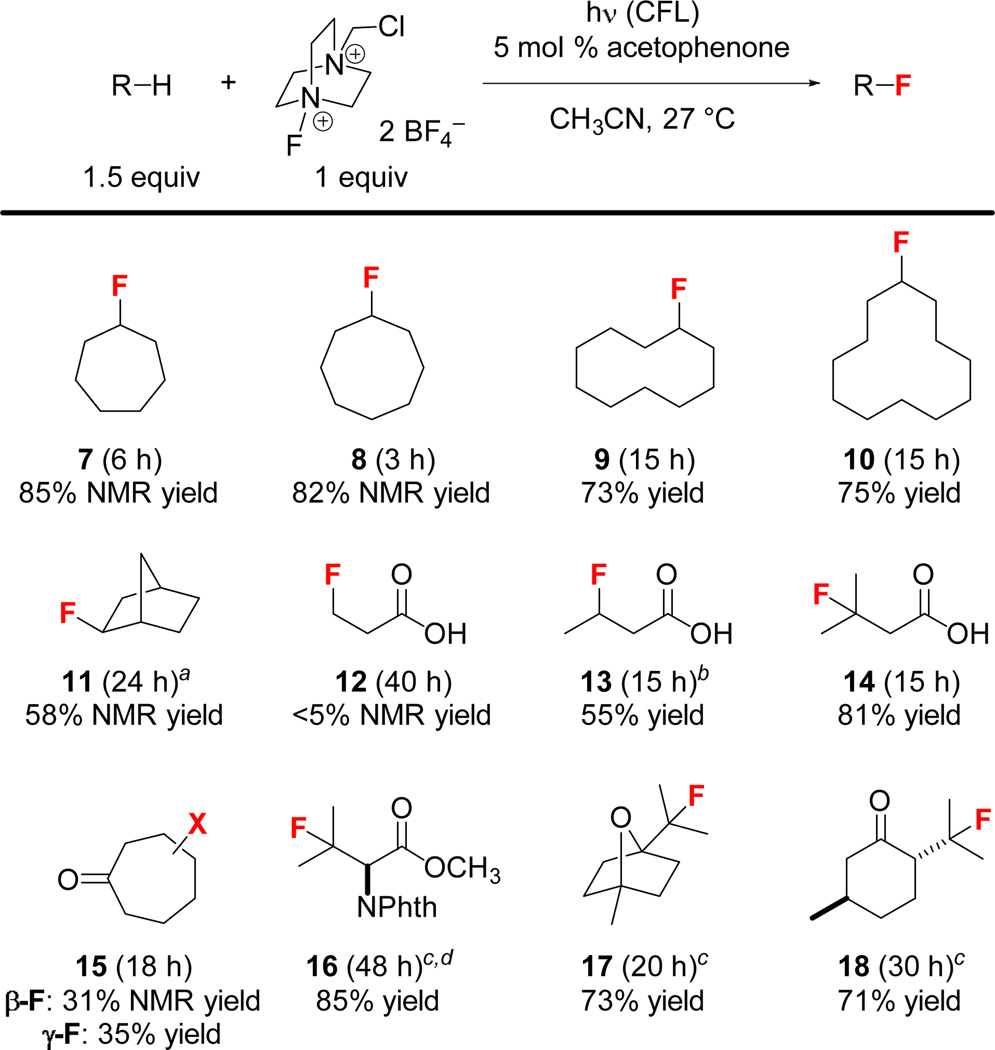
The scope of this acetophenone-catalyzed photolytic C–H fluorination reaction is shown in Fig. 2. The fluorination of cycloalkanes proceeded smoothly. Monofluorides 7–10 were obtained in good yields with only a small (~5%) amount of difluorination products as judged by the 19F NMR spectra of the crude reaction mixtures. The site selectivity of this reaction follows the general trend of innate C–H oxidation reactions (3°>2°>1°).18 For example, the fluorination of norbornane provided 11 with good regio- and stereoselectivity (exo/endo=15/1) because of hyperconjugation effects.18a The fluorination of propionic acid, butyric acid, and isovaleric acid gave 12, 13, and 14 in <5%, 55%, and 81% yield, respectively. The β- and γ-positions of cycloheptanone were fluorinated at comparable rates (β:γ=1:1). While attempts to isolate β-fluorocycloheptanone (15-β) resulted in elimination of the fluoride, γ-fluorocycloheptanone (15-γ) could be isolated in 35% yield. Fluorination of N-phthaloyl-l-valine methyl ester gave 16 in 85% yield. Monoterpenes 1,4-cineole and l-menthone reacted selectively at the tertiary positions to give 17 and 18 in 73% and 71% yield, respectively. We have also re-examined the fluorination of N-phthaloyl-l-valine and l-menthone under UV-irradiation (250–375 nm) conditions. The reactions proceeded significantly faster but the products degraded under the reaction conditions, leading to low yields (<40%) of 16 and 18.
Fig. 2.
Scope of the acetophenone-catalyzed photolytic C–H fluorination reaction. [a] Endo-2-fluoronorbornane was also formed in 4% NMR yield. [b] Isolated as the corresponding benzyl ester. [c] Substrate (1.0 equiv), Selectfluor (1.5 equiv). [d] Acetophenone (20 mol %).
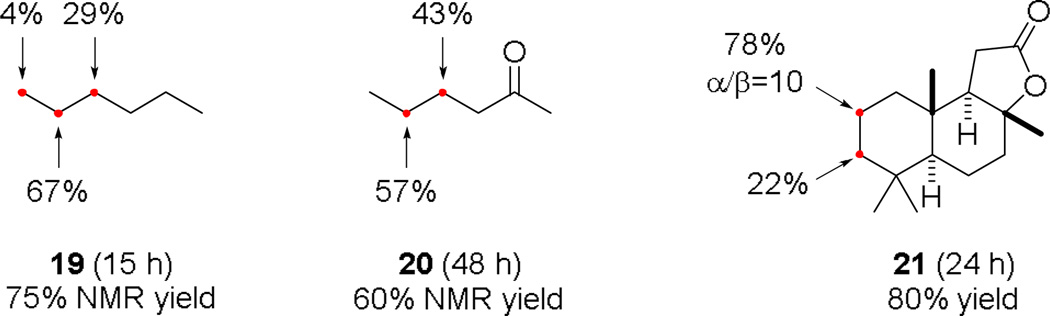
We have used hexane (19), 2-hexanone (20), and sclareolide (21) to further study the site selectivity of this acetophenone-catalyzed photolytic C–H fluorination reaction (Fig. 3). The fluorination of 19 occurred primarily on the methylene groups (C1:C2:C3=1:17:7). Introducing a carbonyl group deactivated the C3 methylene group, rendering the C4 and C5 methylenes the only detectable reaction sites of 20 (C4:C5=1:1.3). Similar to other innate radical C–H oxidation reactions,7a,18 sesquiterpenoid 21 reacted predominately at the C2 position (C2/C3 = 3.6/1).
Fig. 3.
Site selectivity of the acetophenone-catalyzed photolytic C–H fluorination reaction. Reaction conditions: CFL-irradiation, 19 (5.0 equiv), 20 and 21 (1.5 equiv), acetophenone (5 mol %), Selectfluor (1.0 equiv).
Mechanistically, it is rather intriguing that CFL-irradiation could promote the photoexcitation of acetophenone that is a colorless oil. Recently, the Melchiorre and Bach groups have demonstrated that Lewis base and Lewis acid can react with carbonyl groups to induce a bathochromic shift.19 However, we did not observe significant bathochromic shift when mixing acetophenone, benzophenone, or 9-fluorenone with Selectfluor and 1 in acetonitrile (Fig. 4 and ESI), indicating that the photoexcitation of acetophenone was not facilitated by Selectfluor through a fluoronium transfer. In contrast, we detected a trace amount of absorption between 375 and 400 nm with acetophenone at high concentrations. We thus believe that this weak absorption is responsible for driving this C–H fluorination reaction.20
Fig. 4.
The R band (n→π* transition) of acetophenone extends to ~400 nm at high concentrations.
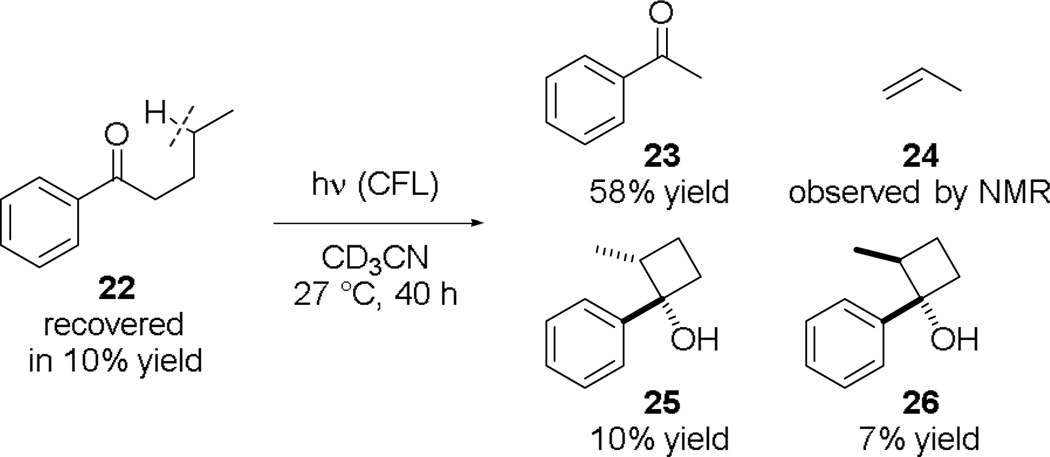
We believe that, similar to our previous fluorenone-catalyzed fluorination reactions,3a acetophenone functioned as a C–H abstraction catalyst instead of an electron- or energy-transfer catalyst.8c,13 To test this hypothesis and to further exclude the possibility that Selectfluor reacted with acetophenone to form a transient reactive species not detectable by UV, we examined if CFL-irradiation could also promote unimolecular C–H abstraction of monoarylketones. Indeed, CFL-irradiation induced the Norrish type II cleavage and Norrish–Yang cyclization of valerophenone (22) (n→π* transition λmax ~325 nm) to give 23–26 (Fig. 5). Addition of trifluoroacetic acid did not accelerate these reactions or cause a bathochromic shift of the R band of 22, indicating that the photoactivation of 22 was not promoted by adventitious acid protonating its carbonyl group. Furthermore, results of the light-dark cycle experiments suggest that acetophenone-catalyzed fluorination of cyclooctane is not a free radical chain reaction (ESI).21 The unimolecular photoreaction of 22 further suggest that acetophenone can function as a C–H abstraction catalyst instead of a photosensitizer in activating Selectfluor toward C–H abstraction.8d,13
Fig. 5.
CFL-irradiation promoted Norrish type II cleavage and Norrish–Yang cyclization of 22.
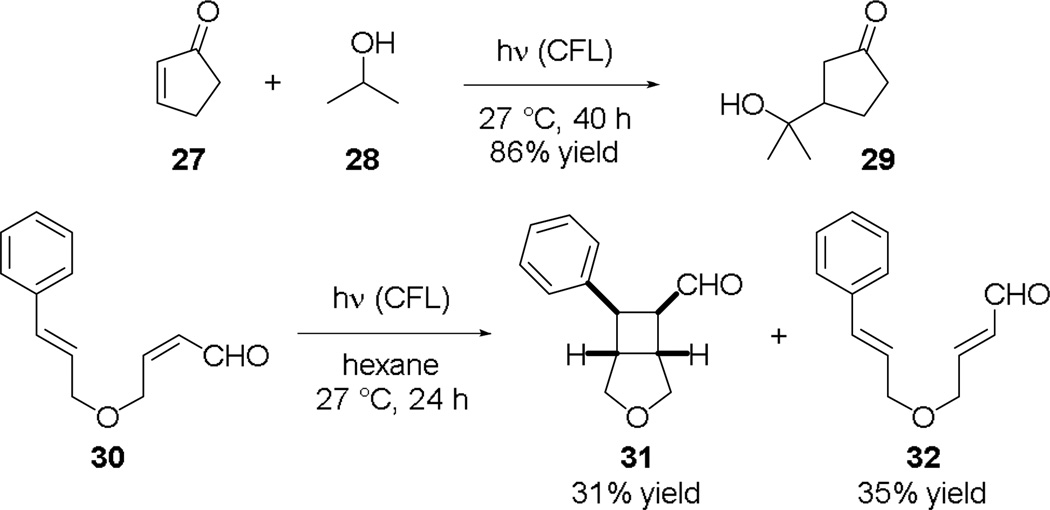
Further support for the hypothesis that CFL-irradiation can promote the photoexcitation of simple monoarylketones follows the observation that CFL-irradiation could also promote photoreactions of colorless enones/enals (n→π* transition λmax ~320 nm). For example, photolysis of cyclopentenone (27) in 2-propanol (28) gave 29 through an ethereal C–H abstraction by 27* (Fig. 6). Results of the light-dark cycle experiments also suggest this C–H abstraction/conjugate addition reaction is not a free radical chain reaction (ESI).21 Additionally, we found that [2+2] cycloaddition of (Z)-enal 30 could also be induced by CFL-irradiation to give 31 together with the E/Z isomerization product 32 that also cyclized to 31 very slowly.
Fig. 6.
CFL-irradiation promoted photoreactions of 27 and 30.
In conclusion, we have shown that, despite low quantum yields, short violet light (375–400 nm) generated by a low-energy household CFL can promote photoreactions of monoarylketones and enones/enals that have an R band λmax ~320 nm. By avoiding the harmful high-energy UV light, these photoreactions can be performed without using specialized photochemical equipment and give fewer side-reactions. Using this mild photolysis method, photoexcited acetephenone can be readily generated to catalyze the fluorination of unactivated C(sp3)–H groups. This new fluorination reaction is operationally simple and utilizes cheap, readily available catalyst. Further investigation of the utility of this photolytic fluorination method is underway.
Supplementary Material
Acknowledgements
Financial Support was provided by NIH (NIGMS R01-GM079554), the Welch Foundation (I-1596) and UT Southwestern. We thank Mr. Wenhan Zhang of Prof. Joeseph Ready’s lab (UT Southwestern) for repeating and confirming the reactions in Figures 4 and 5.
Notes and references
- 1.(a) Ishihara Y, Baran PS. Synlett. 2010:1733. [Google Scholar]; (b) Roizen JL, Harvey ME, Du Bois J. Acc. Chem. Res. 2011;45:911. doi: 10.1021/ar200318q. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Engle KM, Mei T-S, Wasa M, Yu J-Q. Acc. Chem. Res. 2012;45:788. doi: 10.1021/ar200185g. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Neufeldt SR, Sanford MS. Acc. Chem. Res. 2011;45:936. doi: 10.1021/ar300014f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(a) Lu J, Tan X, Chen C. J. Am. Chem. Soc. 2007;129:7768. doi: 10.1021/ja072844p. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Xia J-B, Cormier KW, Chen C. Chem. Sci. 2012;3:2240. doi: 10.1039/C2SC20178J. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Zhu C, Xia J-B, Chen C. Tetrahedron Lett. 2014;55:232. doi: 10.1016/j.tetlet.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Xia J-B, Zhu C, Chen C. J. Am. Chem. Soc. 2013;135:17494. doi: 10.1021/ja410815u. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Xia J-B, Ma Y, Chen C. Org. Chem. Front. 2014;1:468. doi: 10.1039/C4QO00057A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Hull KL, Anani WQ, Sanford MS. J. Am. Chem. Soc. 2006;128:7134. doi: 10.1021/ja061943k. [DOI] [PubMed] [Google Scholar]; (b) McMurtrey KB, Racowski JM, Sanford MS. Org. Lett. 2012;14:4094. doi: 10.1021/ol301739f. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Racowski JM, Gary JB, Sanford MS. Angew. Chem. Int. Ed. 2012;51:3414. doi: 10.1002/anie.201107816. [DOI] [PubMed] [Google Scholar]
- 5.(a) Wang X, Mei T-S, Yu J-Q. J. Am. Chem. Soc. 2009;131:7520. doi: 10.1021/ja901352k. [DOI] [PubMed] [Google Scholar]; (b) Chan KSL, Wasa M, Wang X, Yu J-Q. Angew. Chem. Int. Ed. 2011;50:9081. doi: 10.1002/anie.201102985. [DOI] [PubMed] [Google Scholar]
- 6.Braun M-G, Doyle AG. J. Am. Chem. Soc. 2013;135:12990. doi: 10.1021/ja407223g. [DOI] [PubMed] [Google Scholar]
- 7.(a) Liu W, Huang X, Cheng M-J, Nielsen RJ, Goddard WA, III, Groves JT. Science. 2012;337:1322. doi: 10.1126/science.1222327. [DOI] [PubMed] [Google Scholar]; (b) Liu W, Groves JT. Angew. Chem. Int. Ed. 2013;52:6024. doi: 10.1002/anie.201301097. [DOI] [PubMed] [Google Scholar]; (c) Huang X, Liu W, Ren H, Neelamegam R, Hooker JM, Groves JT. J. Am. Chem. Soc. 2014;136:6842. doi: 10.1021/ja5039819. [DOI] [PubMed] [Google Scholar]
- 8.(a) Bloom S, Pitts CR, Miller DC, Haselton N, Holl MG, Urheim E, Lectka T. Angew. Chem. Int. Ed. 2012;51:10580. doi: 10.1002/anie.201203642. [DOI] [PubMed] [Google Scholar]; (b) Bloom S, Pitts CR, Woltornist R, Griswold A, Holl MG, Lectka T. Org. Lett. 2013;15:1722. doi: 10.1021/ol400424s. [DOI] [PubMed] [Google Scholar]; (c) Bloom S, Knippela JL, Lectka T. Chem. Sci. 2014;5:1175. [Google Scholar]; (d) Pitts CR, Bloom S, Woltornist R, Auvenshine DJ, Ryzhkov LR, Siegler MA, Lectka T. J. Am. Chem. Soc. 2014;136:9780. doi: 10.1021/ja505136j. [DOI] [PubMed] [Google Scholar]; (e) Bloom S, Knippel JL, Holl MG, Barber R, Lectka T. Tetrahedron Lett. 2014;55:4576. [Google Scholar]
- 9.Amaoka Y, Nagatomo M, Inoue M. Org. Lett. 2013;15:2160. doi: 10.1021/ol4006757. [DOI] [PubMed] [Google Scholar]
- 10.Xu P, Guo S, Wang L, Tang P. Angew. Chem. Int. Ed. 2014;53:5955. doi: 10.1002/anie.201400225. [DOI] [PubMed] [Google Scholar]
- 11.Fier PS, Hartwig JF. Science. 2013;342:956. doi: 10.1126/science.1243759. [DOI] [PubMed] [Google Scholar]
- 12.Halperin SD, Fan H, Chang S, Martin RE, Britton R. Angew. Chem. Int. Ed. 2014;53:4690. doi: 10.1002/anie.201400420. [DOI] [PubMed] [Google Scholar]
- 13.Kee CW, Chin KF, Wong MW, Tan C-H. Chem. Commun. 2014;50:8211. doi: 10.1039/c4cc01848f. [DOI] [PubMed] [Google Scholar]
- 14.For examples of CFL/LED-promoted photoreactions, see: Yoon TP. ACS Catal. 2013;3:895. doi: 10.1021/cs400088e. Prier CK, Rankic DA, MacMillan DWC. Chem. Rev. 2013;113:5322. doi: 10.1021/cr300503r. Narayanam JMR, Stephenson CRJ. Chem. Soc. Rev. 2011;40:102. doi: 10.1039/b913880n. Xun J, Xiao W-J. Angew. Chem. Int. Ed. 2012;51:6828. doi: 10.1002/anie.201200223.
- 15.(a) Khazova M, O’Hagan JB. Radiat. Prot. Dosimetry. 2008;131:521. doi: 10.1093/rpd/ncn234. [DOI] [PubMed] [Google Scholar]; (b) Mironava T, Hadjiargyrou M, Simon M, Rafailovich MH. Photochem. Photobiol. 2012;88:1497. doi: 10.1111/j.1751-1097.2012.01192.x. [DOI] [PubMed] [Google Scholar]
- 16.Light in the range of 375–400 nm has been referred to as both UV and visible light.
- 17.Banks RE, Lawrence NJ, Popplewell AL. Synlett. 1994:831. [Google Scholar]
- 18.(a) Newhouse T, Baran PS. Angew. Chem. Int. Ed. 2011;50:3362. doi: 10.1002/anie.201006368. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Chen MS, White MC. Science. 2010;327:566. doi: 10.1126/science.1183602. [DOI] [PubMed] [Google Scholar]
- 19.(a) Arceo E, Jurberg ID, Álvarez-Fernández A, Melchiorre P. Nat. Chem. 2013;5:750. doi: 10.1038/nchem.1727. [DOI] [PubMed] [Google Scholar]; (b) Brimioulle R, Bach T. Science. 2013;342:840. doi: 10.1126/science.1244809. [DOI] [PubMed] [Google Scholar]
- 20.(a) Walker DL, Fraser-Reid B. J. Am. Chem. Soc. 1975;97:6251. doi: 10.1021/ja00854a055. [DOI] [PubMed] [Google Scholar]; (b) Benko Z, Fraser-Reid B. J. Org. Chem. 1988;53:2066. [Google Scholar]; (c) Fagnoni M, Dondi D, Ravelli D, Albini A. Chem. Rev. 2007;107:2725. doi: 10.1021/cr068352x. [DOI] [PubMed] [Google Scholar]
- 21.These experiments do not exclude that possibility that a radical chain reaction with non-negligible rates of chain termination is operating.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.