Abstract
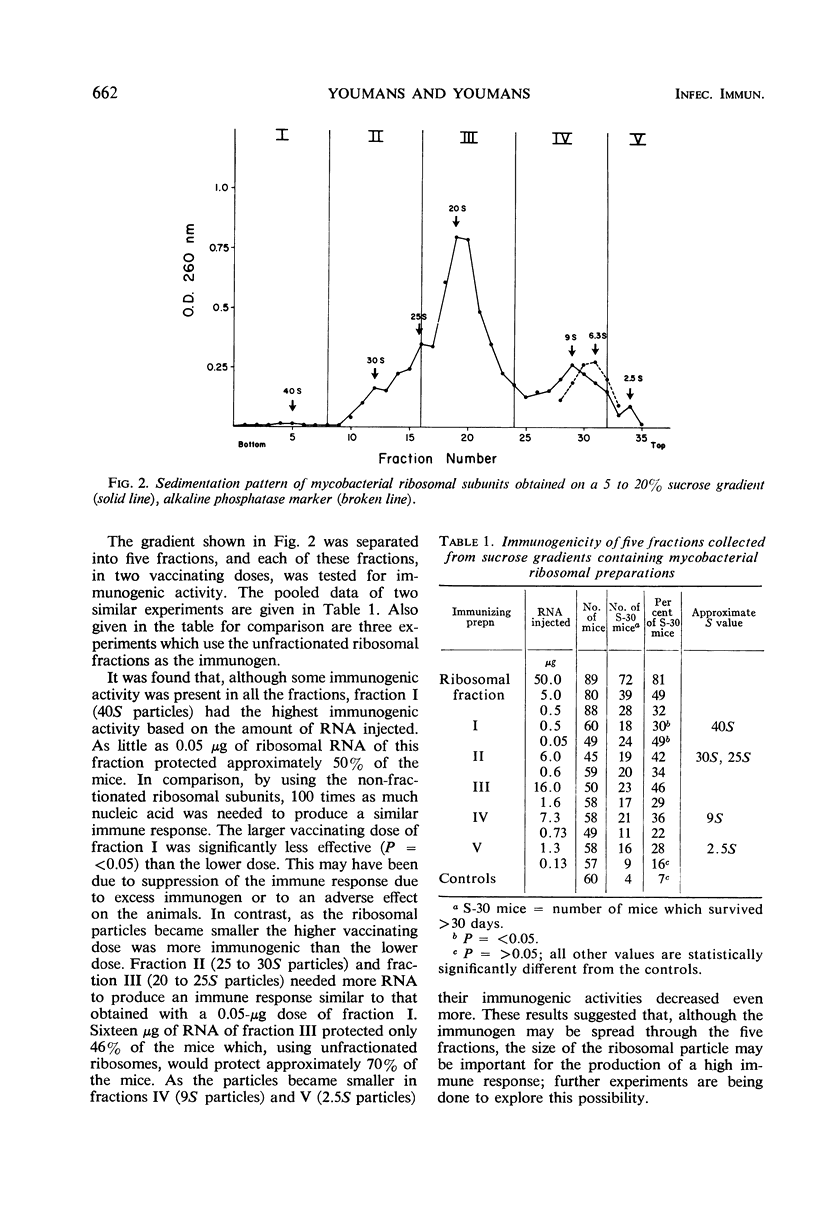
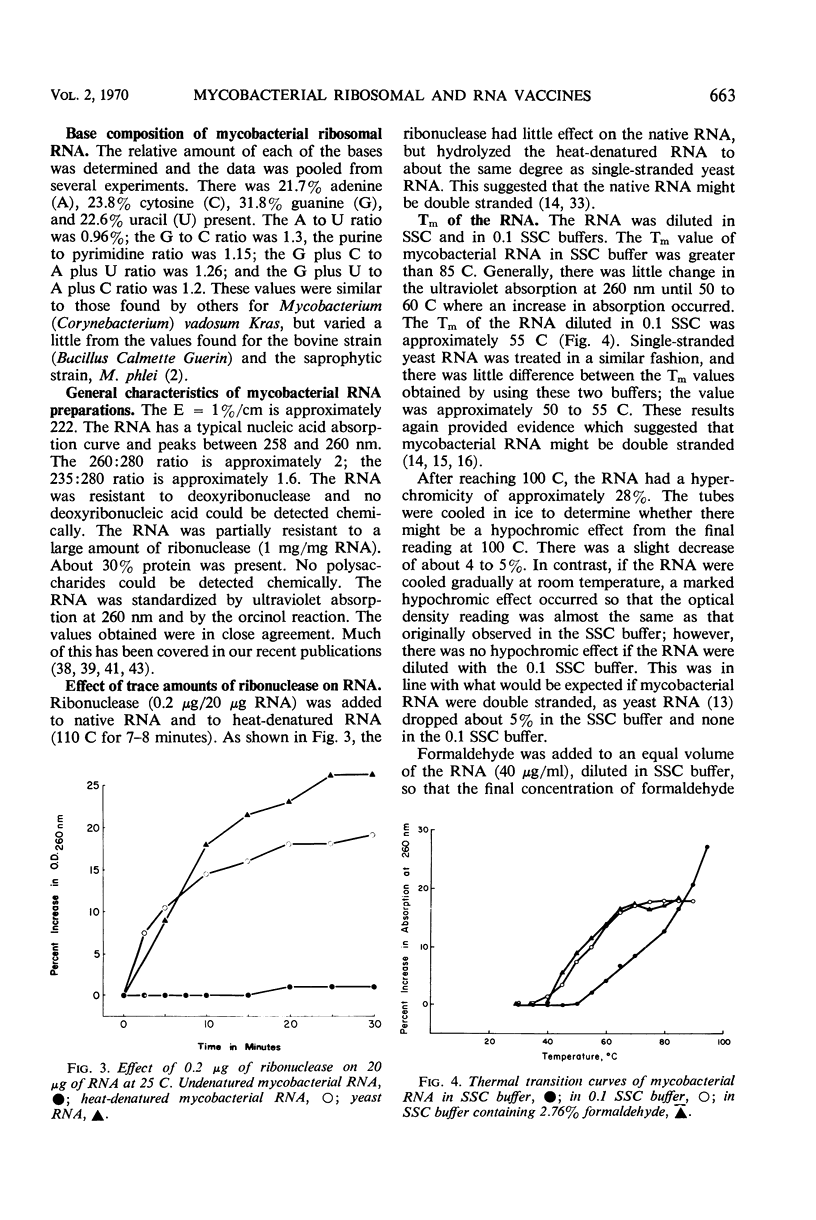
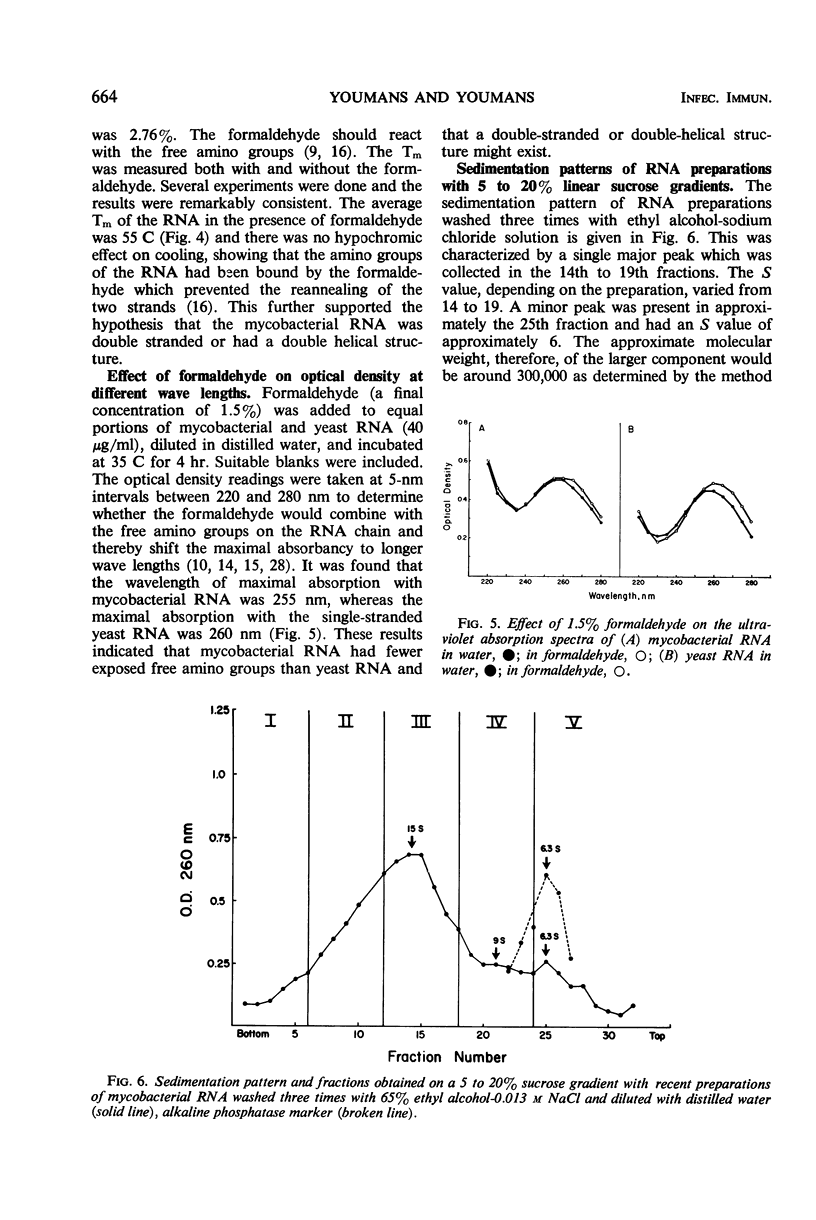
Five to 20% linear sucrose gradients were used to obtain sedimentation patterns of mycobacterial ribosomes, ribosomal subfractions, and ribonucleic acid (RNA) preparations. Classical 70S ribosomes were obtained when 10−1m magnesium chloride was used. These, when dialyzed against 10−4m MgCl2, yielded typical 50S, 30S, and smaller ribosomal subunits. The 30S subunits were the most immunogenic under these conditions. A ribosomal preparation containing subunits which varied from 2.5 to 40S was fractionated by collecting five fractions from a sucrose gradient; based upon the amount of nucleic acid present, the fraction containing the 40S particles was most immunogenic. Physical and chemical evidence suggested that mycobacterial RNA preparations extracted with 65% ethyl alcohol from the ribosomes and diluted in distilled water, were either double-stranded, or mostly double-helical, or had a highly organized secondary structure. This was based on the following observations. (i) Native RNA was resistant to trace amounts of ribonuclease. (ii) The approximate Tm value in SSC buffer (0.15 m NaCl plus 0.015 m sodium citrate) was greater than 85 C and in 0.1 SSC buffer was 55 C; the RNA diluted in SSC buffer produced a hypochromic effect on cooling at room temperature. (iii) Formaldehyde, in the presence of SSC buffer, decreased the Tm of the RNA to approximately 55 C, and there was no hypochromic effect on cooling. (iv) Formaldehyde did not increase the wavelength of maximal adsorption of the RNA. (v) The purine/pyrimidine ratio was close to one. (vi) The major peak of the RNA sedimented in the more dense zones of the sucrose gradients. There was a relationship between the sedimentation pattern obtained with the RNA-protein subunits on sucrose gradients and immunogenicity; several examples are given. RNA-protein complexes of approximately 14 to 20S, and occasionally 23S in the major peak, appeared to produce the highest immune response. Smaller RNA-protein complexes such as 6S, which were obtained when the RNA preparation was diluted in certain buffers, were much less immunogenic. This was confirmed by collecting five fractions from sucrose gradients and finding the third fraction (containing RNA-protein complexes approximately 15 to 16S) the most immunogenic. Immunogenic activity was apparently related to the structure of the RNA since it was maximal when the RNA appeared to be either double stranded, double helical, or had a highly organized structure.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop J. M., Koch G. Purification and characterization of poliovirus-induced infectious double-stranded ribonucleic acid. J Biol Chem. 1967 Apr 25;242(8):1736–1743. [PubMed] [Google Scholar]
- CRESTFIELD A. M., SMITH K. C., ALLEN F. W. The preparation and characterization of ribonucleic acids from yeast. J Biol Chem. 1955 Sep;216(1):185–193. [PubMed] [Google Scholar]
- Eda T. [Studies on the ribosome of Mycobacteria. II. Stability of the ribosome of Mycobacterium tuberculosis]. Kekkaku. 1968 Oct;43(10):425–429. [PubMed] [Google Scholar]
- Eda T. [Studies on the ribosome of mycobacteria. I. Isolation and characterization of the ribosome of Mycobacterium tuberculosis H37Ra]. Kekkaku. 1968 Oct;43(10):419–423. [PubMed] [Google Scholar]
- FRAENKEL-CONRAT H. Reaction of nucleic acid with formaldehyde. Biochim Biophys Acta. 1954 Oct;15(2):307–309. doi: 10.1016/0006-3002(54)90083-9. [DOI] [PubMed] [Google Scholar]
- Fenwick M. L. The effect of reaction with formaldehyde on the sedimentation rates of ribonucleic acids. Biochem J. 1968 May;107(6):851–859. doi: 10.1042/bj1070851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAREN A., LEVINTHAL C. A fine-structure genetic and chemical study of the enzyme alkaline phosphatase of E. coli. I. Purification and characterization of alkaline phosphatase. Biochim Biophys Acta. 1960 Mar 11;38:470–483. doi: 10.1016/0006-3002(60)91282-8. [DOI] [PubMed] [Google Scholar]
- GEIDUSCHEK E. P., MOOHR J. W., WEISS S. B. The secondary structure of complementary RNA. Proc Natl Acad Sci U S A. 1962 Jun 15;48:1078–1086. doi: 10.1073/pnas.48.6.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilova L. P., Ivanov D. A., Spirin A. S. Studies on the structure of ribosomes. 3. Stepwise unfolding of the 50 s particles without loss of ribosomal protein. J Mol Biol. 1966 Apr;16(2):473–489. doi: 10.1016/s0022-2836(66)80186-9. [DOI] [PubMed] [Google Scholar]
- Gomatos P. J., Tamm I. THE SECONDARY STRUCTURE OF REOVIRUS RNA. Proc Natl Acad Sci U S A. 1963 May;49(5):707–714. doi: 10.1073/pnas.49.5.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASELKORN R., DOTY P. The reaction of formaldehyde with polynucleotides. J Biol Chem. 1961 Oct;236:2738–2745. [PubMed] [Google Scholar]
- Hosokawa K., Fujimura R. K., Nomura M. Reconstitution of functionally active ribosomes from inactive subparticles and proteins. Proc Natl Acad Sci U S A. 1966 Jan;55(1):198–204. doi: 10.1073/pnas.55.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LITTAUER U. Z., EISENBERG H. Ribonucleic acid from Escherichia coli; preparation, characterization and physical properties. Biochim Biophys Acta. 1959 Apr;32:320–337. doi: 10.1016/0006-3002(59)90604-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lampson G. P., Tytell A. A., Field A. K., Nemes M. M., Hilleman M. R. Inducers of interferon and host resistance. I. Double-stranded RNA from extracts of Penicillium funiculosum. Proc Natl Acad Sci U S A. 1967 Aug;58(2):782–789. doi: 10.1073/pnas.58.2.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner M. P., Johnson T. C. Regulation of protein synthesis in developing mouse brain tissue. Alteration in ribosomal activity. J Biol Chem. 1970 Mar 25;245(6):1388–1393. [PubMed] [Google Scholar]
- Lessie T. G. The atypical ribosomal RNA complement of Rhodopseudomonas spheroides. J Gen Microbiol. 1965 Jun;39(3):311–320. doi: 10.1099/00221287-39-3-311. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- MONTAGNIER L., SANDERS F. K. REPLICATIVE FORM OF ENCEPHALOMYOCARDITIS VIRUS RIBONUCLEIC ACID. Nature. 1963 Aug 17;199:664–667. doi: 10.1038/199664a0. [DOI] [PubMed] [Google Scholar]
- Miall S. H., Walker I. O. Structural studies on ribosomes. II. Denaturation and sedimentation of ribosomal subunits unfolded in EDTA. Biochim Biophys Acta. 1969 Feb 18;174(2):551–560. doi: 10.1016/0005-2787(69)90284-6. [DOI] [PubMed] [Google Scholar]
- SARKAR N. K., DOUNCE A. L. A spectroscopic study of the reaction of formaldehyde with deoxyribonucleic and ribonucleic acids. Biochim Biophys Acta. 1961 Apr 29;49:160–169. doi: 10.1016/0006-3002(61)90879-4. [DOI] [PubMed] [Google Scholar]
- Venneman M. R., Bigley N. J., Berry L. J. Immunogenicity of Ribonucleic Acid Preparations Obtained from Salmonella typhimurium. Infect Immun. 1970 Jun;1(6):574–582. doi: 10.1128/iai.1.6.574-582.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venneman M. R., Bigley N. J. Isolation and partial characterization of an immunogenic moiety obtained from Salmonella typhimurium. J Bacteriol. 1969 Oct;100(1):140–148. doi: 10.1128/jb.100.1.140-148.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISSMANN C., BORST P., BURDON R. H., BILLETER M. A., OCHOA S. REPLICATION OF VIRAL RNA, III. DOUBLE-STRANDED REPLICATIVE FORM OF MSW PHAGE RNA. Proc Natl Acad Sci U S A. 1964 Apr;51:682–690. doi: 10.1073/pnas.51.4.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston S. H., Berry L. J. Antibacterial immunity induced by ribosomal vaccines. J Reticuloendothel Soc. 1970 Jul;8(1):13–24. [PubMed] [Google Scholar]
- Winston S., Berry L. J. Immunity induced by ribosomal extracts from Staphylococcus aureus. J Reticuloendothel Soc. 1970 Jul;8(1):66–73. [PubMed] [Google Scholar]
- Worcel A., Goldman D. S., Sachs I. B. Properties and fine structure of the ribosomes from Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 1968 Sep;61(1):122–129. doi: 10.1073/pnas.61.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUMANS G. P., YOUMANS A. S. The measurement of the response of immunized mice to infection with Mycobacterium tuberculosis va. hominis. J Immunol. 1957 May;78(5):318–329. [PubMed] [Google Scholar]
- Youmans A. S., Youmans G. P. Effect of trypsin and ribonuclease on the immunogenic activity of ribosomes and ribonucleic acid isolated from Mycobacterium tuberculosis. J Bacteriol. 1966 Jun;91(6):2146–2154. doi: 10.1128/jb.91.6.2146-2154.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youmans A. S., Youmans G. P. Factors affecting immunogenic activity of mycobacterial ribosomal and ribonucleic acid preparations. J Bacteriol. 1969 Jul;99(1):42–50. doi: 10.1128/jb.99.1.42-50.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youmans A. S., Youmans G. P. Preparation and effect of different adjuvants on the immunogenic activity of mycobacterial ribosomal fraction. J Bacteriol. 1967 Oct;94(4):836–843. doi: 10.1128/jb.94.4.836-843.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youmans A. S., Youmans G. P. Preparation of highly immunogenic ribosomal fractions of Mycobacterium tuberculosis by use of sodium dodecyl sulfate. J Bacteriol. 1966 Jun;91(6):2139–2145. doi: 10.1128/jb.91.6.2139-2145.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youmans G. P., Youmans A. S. Recent studies on acquired immunity in tuberculosis. Curr Top Microbiol Immunol. 1969;48:129–178. doi: 10.1007/978-3-642-46163-7_6. [DOI] [PubMed] [Google Scholar]


