Abstract
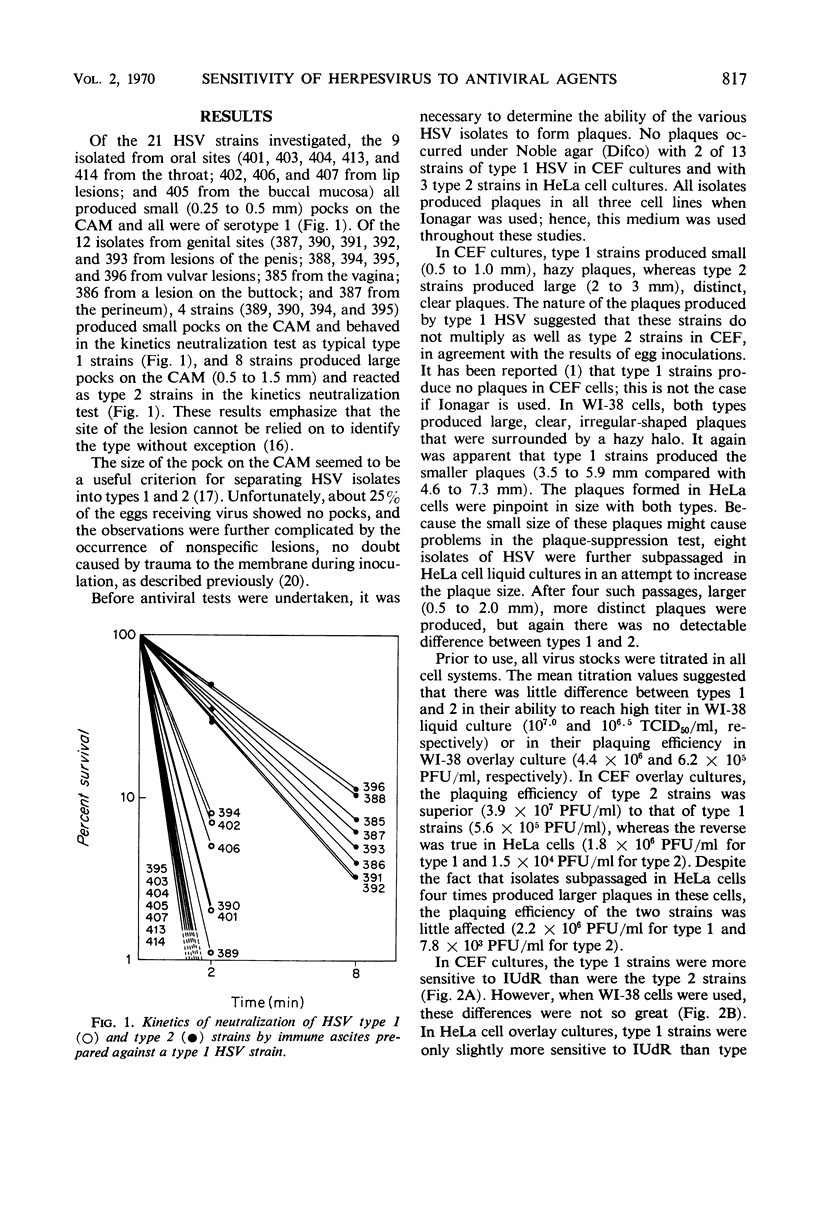
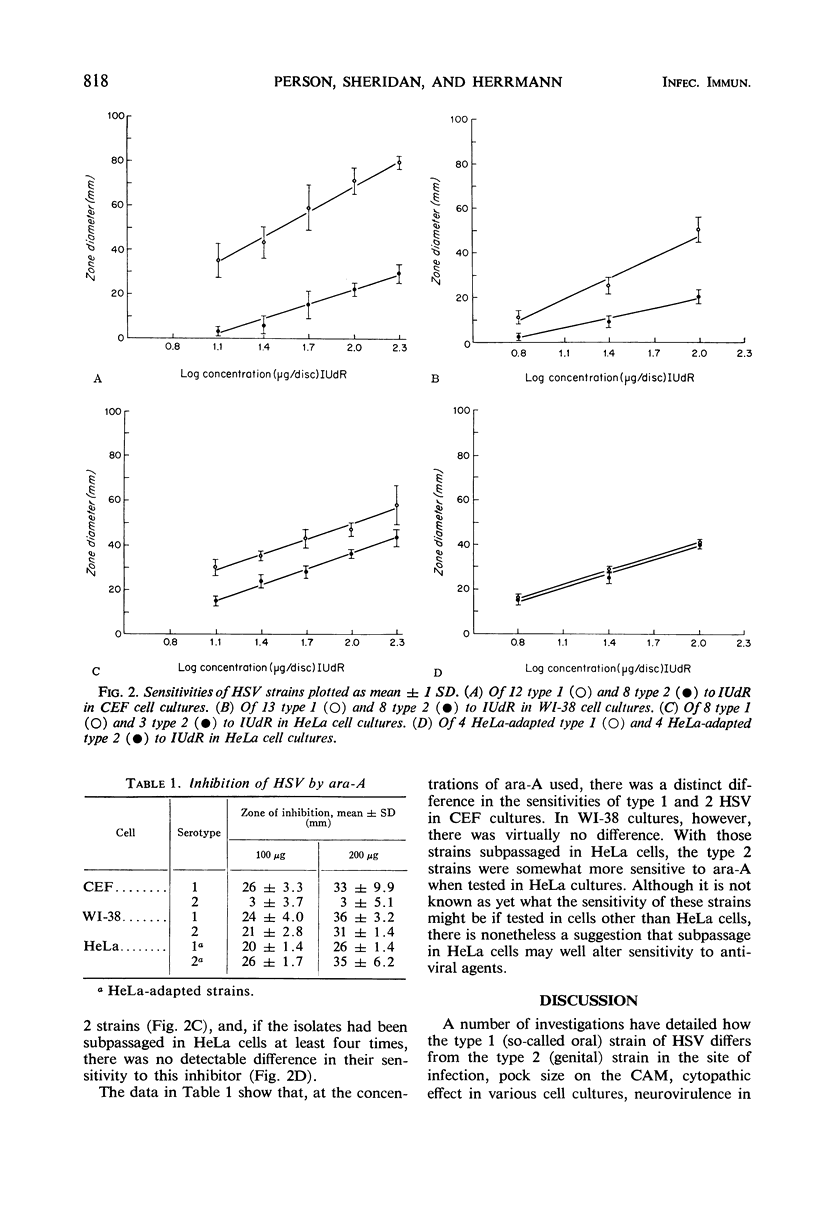
The sensitivities of 21 strains of herpes simplex virus (HSV), 13 type 1 strains and 8 type 2 strains, to 5-iodo-2′-deoxyuridine (IUdR) and 9-β-d-arabinofuranosyladenine (ara-A) were evaluated by the plaque-suppression test in chick embryo fibroblast (CEF), WI-38, and HeLa cell cultures. In CEF, type 1 strains were considerably more sensitive to the inhibitors than were the type 2 strains. In WI-38, the type 1 strains were more sensitive than the type 2 strains to IUdR; however, the two serotypes were equally sensitive to ara-A. In HeLa cells, the differences in sensitivity to IUdR between the two serotypes were less. Eight HeLa-adapted strains (four type 1 and four type 2) evaluated in HeLa cell cultures were equally sensitive to IUdR; the type 2 strains were slightly more sensitive than type 1 strains to ara-A. These results demonstrate the wide variation in sensitivity of HSV types 1 and 2 to antiviral agents which results from differences in the cell culture system and passage history of the strains.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Figueroa M. E., Rawls W. E. Biological markers for differentiation of herpes-virus strains of oral and genital origin. J Gen Virol. 1969 Mar;4(2):259–267. doi: 10.1099/0022-1317-4-2-259. [DOI] [PubMed] [Google Scholar]
- Garrod L. P., Waterworth P. M. Effect of medium composition on the apparent sensitivity of Pseudomonas aeruginosa to gentamicin. J Clin Pathol. 1969 Sep;22(5):534–538. doi: 10.1136/jcp.22.5.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYFLICK L., MOORHEAD P. S. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961 Dec;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- HERRMAN E. C., Jr Plaque inhibition test for detection of specific inhibitors of DNA containing viruses. Proc Soc Exp Biol Med. 1961 May;107:142–145. [PubMed] [Google Scholar]
- HERRMANN E. C., Jr, ENGLE C. Tumor cell-induced mouse ascites fluid as a source of viral antibodies. Proc Soc Exp Biol Med. 1958 Jun;98(2):257–259. doi: 10.3181/00379727-98-24009. [DOI] [PubMed] [Google Scholar]
- HERRMANN E. C., Jr, GABLIKS J., ENGLE C., PERLMAN P. L. Agar diffusion method for detection and bioassay of antiviral antibiotics. Proc Soc Exp Biol Med. 1960 Mar;103:625–628. doi: 10.3181/00379727-103-25617. [DOI] [PubMed] [Google Scholar]
- Herrmann E. C., Jr Sensitivity of herpes simplex virus, vaccinia virus, and adenoviruses to deoxyribonucleic acid inhibitors and thiosemicarbazones in a plaque suppression test. Appl Microbiol. 1968 Aug;16(8):1151–1155. doi: 10.1128/am.16.8.1151-1155.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann E. C., Jr The usefullness of human fibroblast cell lines for the isolation of viruses. Am J Epidemiol. 1967 Mar;85(2):200–206. doi: 10.1093/oxfordjournals.aje.a120683. [DOI] [PubMed] [Google Scholar]
- Kucera L. S., Herrmann E. C., Jr Gradient plate technique applied to the study of antiviral substances. Proc Soc Exp Biol Med. 1966 May;122(1):258–262. doi: 10.3181/00379727-122-31104. [DOI] [PubMed] [Google Scholar]
- LIEBERMAN R., DOUGLAS J. O., HUMPHREY W., Jr Ascites induced in mice by Staphylococcus. Science. 1959 Mar 20;129(3351):775–775. doi: 10.1126/science.129.3351.775. [DOI] [PubMed] [Google Scholar]
- MacCallum F. O., Juel-Jensen B. E. Herpes simplex virus skin infection in man treated with idoxuridine in dimethyl sulphoxide. Results of double-blind controlled trial. Br Med J. 1966 Oct 1;2(5517):805–807. doi: 10.1136/bmj.2.5517.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBRIDE W. D. Antigenic analysis of polioviruses by kinetic studies of serum neutralization. Virology. 1959 Jan;7(1):45–58. doi: 10.1016/0042-6822(59)90176-x. [DOI] [PubMed] [Google Scholar]
- Miller F. A., Dixon G. J., Ehrlich J., Sloan B. J., McLean I. W., Jr Antiviral activity of 9-beta-D-arabinofuranosyladenine. I. Cell culture studies. Antimicrob Agents Chemother (Bethesda) 1968;8:136–147. [PubMed] [Google Scholar]
- Nahmias A. J., Dowdle W. R. Antigenic and biologic differences in herpesvirus hominis. Prog Med Virol. 1968;10:110–159. [PubMed] [Google Scholar]
- Nahmias A. J., Dowdle W. R., Naib Z. M., Highsmith A., Harwell R. W., Josey W. E. Relation of pock size on chorioallantoic membrane to antigenic type of herpesvirus hominis. Proc Soc Exp Biol Med. 1968 Apr;127(4):1022–1028. doi: 10.3181/00379727-127-32861. [DOI] [PubMed] [Google Scholar]
- Nolan D. C., Carruthers M. M., Lerner A. M. Herpesvirus hominis encephalitis in Michigan. Report of thirteen cases, including six treated with idoxuridine. N Engl J Med. 1970 Jan 1;282(1):10–13. doi: 10.1056/NEJM197001012820103. [DOI] [PubMed] [Google Scholar]
- OVERMAN J. R., TAMM I. Quantitative titration of vaccinia virus on the chorioallantoic membrane. J Immunol. 1956 Mar;76(3):228–236. [PubMed] [Google Scholar]
- Plummer G., Waner J. L., Bowling C. P. Comparative studies of type 1 and type 2 & 'herpes simplex' viruses. Br J Exp Pathol. 1968 Apr;49(2):202–208. [PMC free article] [PubMed] [Google Scholar]
- Plummer G., Waner J. L., Phuangsab A., Goodheart C. R. Type 1 and type 2 herpes simplex viruses: serological and biological differences. J Virol. 1970 Jan;5(1):51–59. doi: 10.1128/jvi.5.1.51-59.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls W. E., Laurel D., Melnick J. L., Glicksman J. M., Kaufman R. H. A search for viruses in smegma, premalignant and early malignant cervical tissues. The isolation of Herpesviruses with distinct antigenic properties. Am J Epidemiol. 1968 May;87(3):647–655. doi: 10.1093/oxfordjournals.aje.a120855. [DOI] [PubMed] [Google Scholar]
- Renis H. E., Buthala D. A. Development of resistance to antiviral drugs. Ann N Y Acad Sci. 1965 Jul 30;130(1):343–354. doi: 10.1111/j.1749-6632.1965.tb12568.x. [DOI] [PubMed] [Google Scholar]
- SCHNEWEIS K. E. [Serological studies on the type differentiation of Herpesvirus hominis]. Z Immun exp ther. 1962 Sep;124:24–48. [PubMed] [Google Scholar]
- Schardein J. L., Sidwel R. W. Antiviral activity of 9-beta-D-arabinofuranosyladenine. 3. Reduction in evidence of encephalitis in treated herpes Simplex-infected hamsters. Antimicrob Agents Chemother (Bethesda) 1968;8:155–160. [PubMed] [Google Scholar]
- Sidwell R. W., Dixon G. J., Schabel F. M., Jr, Kaump D. H. Antiviral activity of 9-beta-D-arabinofuranosyladenine. II. Activity against Herpes simplex keratitis in hamsters. Antimicrob Agents Chemother (Bethesda) 1968;8:148–154. [PubMed] [Google Scholar]
- Sloan B. J., Miller F. A., Ehrlich J., McLean I. W., Machamer H. E. Antiviral activity of 0-beta-D-arabinofuranosyladenine. IV. Activity against intracerebral herpes simplex virus infections in mice. Antimicrob Agents Chemother (Bethesda) 1968;8:161–171. [PubMed] [Google Scholar]
- Tuffi G. A., Nahmias A. J. Neonatal herpetic infection. Report of two premature infants treated with systemic use of idoxuridine. Am J Dis Child. 1969 Dec;118(6):909–914. [PubMed] [Google Scholar]
- WEINBERG E. D. The mutual effects of antimicrobial compounds and metallic cations. Bacteriol Rev. 1957 Mar;21(1):46–68. doi: 10.1128/br.21.1.46-68.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]


