Abstract
Introduction: The use of alternative therapies for the treatment of menopausal hot flashes has increased due to the serious risk of hormone therapy. Most alternative therapies have not been accepted by women. Therefore, conducting a study to find effective treatment, which has a low rate of complications and is more acceptable, is necessary. The aim of this study was to assess the effects of folic acid on menopausal hot flashes. Methods: In the present study 70 menopausal women were placed into two groups of 35 with random allocation, and were treated with folic acid 1 mg tablets and placebo tablets once a day during four weeks. Information was gathered by questionnaire, interviews, and hot flash diary during five stages. Comparisons of within-group Results were performed by ANOVA and between-group results were performed using ANCOVA. Data were analyzed by SPSS for Windows. Results: There was a significant difference between mean severity, duration, and frequency of hot flashes before and after treatment within both groups. In comparing the results between the groups, mean hot flash severity in second, third, and fourth weeks were significantly different. The mean hot flash frequency was significantly different in third and fourth weeks, and the mean hot flash duration was significantly different in the fourth week. Conclusion: The results of the present study indicated that folic acid was effective in reducing the severity, duration, and frequency of hot flashes during menopause. Therefore, it can be recommended as an affordable and accessible method for treating menopausal hot flash for women.
Keywords: Menopause, Hot flash, Folic acid
Introduction
Hot flash is the most common complaint of menopausal women.1 About 50 to 85% of menopausal women around the world (nearly 360 million) over 45 years of age experience it.2 Hot flash is more common in western countries, and it has its lowest prevalence among China and Asian nations, and it is strongly influenced by race and culture.3 In Iran hot flash is reported in 49 to 56% of menopausal women.4 Hot flash is temporary among the majority of women, who recover during 6 months to 2 years, but in 26% of women it lasts about 6 to 10 years, and in 10% of women more than 10 years.2,5
Hot flashes start by a sudden redness of skin on the face, neck and chest and is associated with a feeling of intense heat in the upper body; it sometimes ends with extreme sweating. This can be felt throughout the body and it may be combined with weakness, faintness, and dizziness. Hot flashes usually last about 1 to 5 minutes. In a small percentage of women it lasts about 15 minutes; there are also differences in its frequency for different people.6-8 Hot flash is not life threatening, but the experience can be unpleasant and painful, and it can result in insomnia, behavioral change, memory and concentration problems, fatigue, anxiety and depression,and decrease in libido.9-14 Overall it can decrease the quality of physical, emotional, and social life. Hot flash is the most common reason that women seek medical treatment during menopause.1 The most effective treatment is hormone therapy.15 In recent decades the rate of hormone therapy usage has decreased. This is due to the studies by the American Women's Health Initiative (WHI) which reported that hormone therapy is associated with increased risk of breast cancer, and cardiovascular diseases.16,17 Because of these serious concerns about hormone therapy women avoid these treatments, and health care providers are also reluctant to prescribe these medications; they prefer non-hormonal therapies to relieve these symptoms.18-20
Most previous studies have used alternative treatments like soy products, herbs (such as black cohosh), drugs (such as clonidine, and gabapentin), and antidepressants (such as fluoxetine, and venlafaxine). Although these treatments are non-hormonal therapies and do not increase the risk of breast cancer, they have some side effects, which may reduce the patients' tolerance, other drug interactions may limit their use, and they are not effective for all patients. These concerns prompted researchers to seek new alternatives.20-22
Despite the many studies that have been conducted on understanding the mechanisms involved in hot flash, its exact pathophysio- logy is not yet known. Estrogen plays an important role in the etiology of hot flash, but it is not the only reason for its occurrence. Decline in estrogen activity increases the activity of serotonin receptors (5-HT2A) in the hypothalamus and stimulates them. Activation of these receptors results in changes in the temperature regulation center, and raises autonomic responses such as increased body temperature and sweating. These actions conclude in shivering and cooling of the body, which are a result of hot flash.23 Therefore, any factor, which increases seroto- nin, estrogen, and endorphins, and reduces norepinephrine, is expected to decrease hot flashes. For example selective serotonin reup- take inhibitors (SSRIs), such as fluoxetine, and serotonin-norepinephrine reuptake inhi- bitors (SNRIs) such as venlafaxine increase serotonin in the synaptic gaps and result in hot flash relief.24
Folic acid is one of the B vitamins that when absorbed into its active form is converted to tetrahydrofolate. Folate is an essential cofactor for the biosynthesis of norepinephrine and serotonin. Studies have shown that taking folic acid has effects similar to that of antidepressants through interference with noradrenergic receptors (a1 and a2), and serotonergic receptors (5-HT1A and 5-HT2A/2c).25 They also reduce secretion of norepinephrine considerably, and increase the activity of serotonin.26-28 This drug has beneficial effects on aging. Studies show a relation between folic acid intake and decrease in depression, improved behavioral characteristics, decrease in serum homocysteine concentrations, improved macrocytic anemia, and reduce in breast cancer in menopausal women.25,29-32 Studies have also noted that folate, with a mechanism similar to hormone replacement therapy (HRT), can improve hot flash by interfering with monoamine neurotransmitters called norepinephrine and serotonin.28 Only one research has been conducted on the effects of folic acid therapy on menopausal hot flash. This study has been performed by Gaweesh and Ewies on 46 healthy menopausal women, who suffered from hot flash.28 The results of this study indicated that folic acid decreased hot flash; there was a 65% improvement of hot flash in the treatment group and 16% in the control group, this difference was significant (p = 0.002). In this survey folic acid (5 mg) was recommended as an affordable, safe, and acceptable method compared to HRT for women.28-33
Health and menopausal care are one of the research priorities in Iran. Education and counseling about aging, health, menopause, and prevention of consequences of early menopause are among the duties of midwives. One of the most common distressing side effects of menopause is hot flash. Folic acid, with a therapeutic mechanism similar to HRT but with minimal side effects, is effective on hot flash.34,35 Folic acid also has beneficial effects during old age. Moreover, no studies have been conducted on this topic in Iran. Therefore, the present study investigated the effects of a low dose of this medication (1 mg tablets), to prevent its possible side effects, in this age group.
Materials and Methods
The present study was a randomized, double blind study with placebo. The subjects were 70 people determined by primary studies with confidence interval (Cl) of 0.95 and power of 0.8. Sampling was performed among menopausal women referring to AL-Zahra Hospital of Rasht, Iran, in 2010. The inclusion criteria of the study were as follows: being 45 to 65 years old, having hot flash during the day, being literate enough to answer the questions, more than 12 months since the last menstruation and 2 months from removal of both ovaries, not having the history of hormone use, depression, and anti-anxiety drugs over the past two months, not taking any kind of medication for hot flash treatment, lack of concomitant use of sulfonamide drugs, methotrexate, triamterene, sulfasalazine, estrogen, phenytoin, or any chemotherapy and daily multivitamins, non-malignant disease, pernicious anemia, aplastic and normocytic anemia, pathologic deficiency of vitamin B12, depression, and renal, liver, heart, and hypothyroidism disease. If any of the participants engaged in unusual physical activity, such as moving to another house or intense sports, or failed to complete the questionnaire over three days during the week or refused to complete it they were excluded from the study.
The data gathering tools consisted of a demographical information questionnaire, and a hot flash diary (HF diary). In this diary, according to the recommendation of the food and drug administration (FDA), the hot flash severity was categorized into mild (feeling heat without sweating), moderate (feeling heat with sweating, no disruption of daily activity), and severe (feeling too much heat and sweating with disruption of daily activity). This form was then completed by the participants during 24 hours, and the duration in minutes and the frequency of hot flashes was recorded daily.36-38
The checklists were given to ten academic members in order to check their validity, and their reliability was determined by its equivalent. Two parallel tools including HF diary and health-menopause information questionnaire was completed in two weeks by a single group of the participants (15 people). The correlation coefficient between them for the severity of hot flash was confirmed with a Spearman correlation coefficient of 0.96 and the duration and frequency, respectively, were also confirmed with a Pearson correlation of 0.88, and 0.99.37,39,40
The necessary permits were obtained from the ethics committee of Tabriz University of Medical Sciences and a referral letter was gained from the treatment deputy of Gilan University of Medical Sciences. The participants were chosen from the women who had complaints about their hot flashes and were willing to participate in the study. Then a gynecologist examined them. The aim of the study was explained to them and a written consent was obtained. The demographic questionnaire was completed through interview, and the instructions of the forms were given. They were asked to complete the HF diary according to the severity, duration, and frequency of hot flashes during one week previous to treatment. With random allocation the participants were placed into two groups of treatment with folic acid (35 people), and placebo (35 people) (figure 1). The medication instructions were then given to them. The folic acid 1 mg was manufactured by JalinousPharmaceutical Company. The placebo tablets had the same size and color of the folic acid but without the effective substance, and they were produced in the Department of Pharmacy of Tabriz University of Medical Sciences. Coding the tablets and placebo was done by the research assistant. The researcher, participants, and obstetrician were not aware of the codes until the end of the study. Both medications were taken daily at a certain time for four weeks. The HF diaries were then handed to the participants and they had to complete it every week. If the participants did not refer to return the forms then the researcher would collect it at their houses. The participants were followed by telephone calls to assure that they took the treatments and of their progress. If any side effects occurred the participants were asked to inform the researcher of the problem in the form. The data were analyzed by SPSS software version 13 (SPSS Inc., Chicago, IL, USA), and Mauchly'ssphericity test was conducted. Since there was no sphericity according to the results, the Greenhouse-Geisser test was performed. ANOVA was used to examine within the groups. Between-group comp-arisons were performed by using ANCOVA. P values of less than 0.05 were considered significant.
Figure 1.
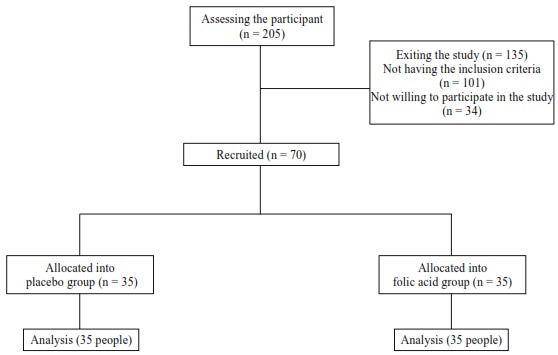
Clinical trial flowchart
Results
70 menopausal women, in two groups of 35, were studied and treated with folic acid and placebo. There was no significant difference regarding demographic information between the two groups (Table 1). There was no significant difference between the two groups based on the hot flash severity according to the t-test (p = 0.59). In comparison within the groups regarding hot flash severity before and after treatment there was a significant difference (p < 0.05). There was no significant difference between means of hot flash severity of the two groups in the first week after treatment; but, this difference was significant in the second, third, and fourth weeks after treatment (Table 2).
Table 1. Comparison of the demographic characteristics of the two study groups.
| Variable |
Folic acid
Mean (SD) |
Placebo
Mean (SD) |
Statistical index |
| Age (years) | 52.94 (3.37) | 53.05 (3.40) | t=-0.14, P=0.88,df= 68 |
| Gravidity | 4.88 (2.33) | 4.82 (2.09) | t=0.108, P=0.91, df=68 |
| Parity | 4.11 (1.92) | 4.05 (1.74) | t=0.130, P=0.89, df=68 |
| Duration of menopause (months) | 38.31 (27.01) | 38.48 (25.53) | t=0.184, P=0.85, df=68 |
| Systolic blood pressure | 110.57 (10.83) | 106.28 (10.59) | t=1.67, P=0.09, df=68 |
| Diastolic blood pressure | 69.71 (9.28) | 66 (10.05) | t=1.61, P=0.11, df=68 |
| BMI | 27.40 (4.74) | 26.54 (4.22) | t=0.805, P=0.42, df=68 |
| N(%) | N(%) | ||
| Menopause | |||
| Natural | 27 (77) | 29 (83) | χ 2 =0.357 P=0.55, df=1 |
| Induced | 8 (23) | 6 (17) | |
| Primary | 20 (57) | 22 (62) | |
| Education level | |||
| Secondary | 1 (3) | 2 (6) | Z=-0.459† P=0.64 |
| Collage | 3 (8) | 1 (3) | |
| University | 11 (32) | 10 (29) | |
| Occupation | |||
| Housewife | 23 (65) | 25 (72) | χ 2 =0.813 P=0.66, df=2 |
| Employee | 10 (29) | 7 (20) | |
| Retired | 2 (6) | 3 (8) | |
| Sufficient income | |||
| Yes | 18 (50) | 16 (44) | Z=-0.052† P=0.95 |
| No | 3 (9) | 7 (21) | |
| Somewhat | 14 (41) | 12 (35) | |
| Sports | |||
| Never | 19 (53) | 17 (47) | Z=-0.717 P=0.47 |
| Sometimes | 10 (29) | 9 (26) | |
| Often | 4 (12) | 5 (15) | |
| Always | 2 (6) | 4 (12) | |
| Marital status | |||
| Single | 1 (3) | 2 (6) | χ 2 =0.496 P=0.78, df=2 |
| Married | 29 (83) | 27 (77) | |
| Divorced | 0 | 0 | |
| Widow | 5 (14) | 6 (17) |
†For the considered variables U-Mann Whitney test was used
Table 2. Mean hot flush severity based on the follow up by time divisions in the treatment groups.
|
Folic acid
Mean (SD) |
Placebo
Mean (SD) |
Statistical indicators (between-group) | |
| Before treatment | 2.23 (0.677) | 2.15 (0.673) | P = 0.59, df = 68, t = 0.531 |
| First week | 2.16 (0.789) | 2.14 (0.619) | P = 0.60, df = 1, F† = 0.270 |
| Second week | 1.86 (0.584) | 1.96 (0.624) | P = 0.03, df = 1, F† = 4.44 |
| Third week | 1.62 (0.621) | 1.95 (0.586) | P = 0.00, df = 1, F† = 16.09 |
| Fourth week | 1.42 (0.654) | 1.99 (0.609) | P = 0.00, df = 1, F† = 30.90 |
| ANOVA with repeated measure (within-group) | F = 26.13 df = 2.28 P < 0.001 |
F = 8.83 df = 1.93 P < 0.001 |
†ANCOVA
There was no significant difference between the two groups before treatment regarding the frequency of hot flashes (p = 0.47). There was a significant difference between the mean hot flash frequency of the groups before and after treatment (p < 0.05). The mean hot flash frequency of the two groups had no significant difference in the first and second weeks after treatment. However, there was a significant difference in the third and fourth weeks after treatment (Table 3). The results also indicated that there was no significant difference between the two groups regarding the duration of hot flash before the treatment (p = 0.46). Within-group comparison showed a significant difference regarding mean hot flash duration before and after the treatment (p < 0.05). There was no significant difference between the groups during the first, second, and third weeks after treatment based on the mean hot flash duration. However, in the fourth week after treatment there was a significant difference between the two groups (Table 4).
Table 3. Mean hot flash frequency based on the follow up by time divisions in the treatment groups.
|
Folic acid
Mean (SD) |
Placebo
Mean (SD) |
Statistical indicators (between-group) | |
| Before treatment | 7.31 (6.79) | 6.35 (3.98) | P = 0.47, df = 1, t = 0.72 |
| First week | 6.33 (4.58) | 6.77 (5.45) | P = 0.09, df = 1, F† = 2.93 |
| Second week | 5.17 (3.90) | 5.65 (3.67) | P = 0.12, df = 1, F† = 2.47 |
| Third week | 4.93 (3.99) | 5.51 (3.66) | P = 0.03, df = 1, F† = 4.65 |
| Fourth week | 4.48 (3.68) | 5.61 (3.59) | P = 0.00, df = 1, F† = 7.30 |
| ANOVA with repeated measure (within-group) | F = 9.16 df = 1.26 P <0.001 |
F = 4.57 df = 1.25 P = 0.03 |
†ANCOVA
Table 4. Mean hot flash duration based on the follow up by time divisions in the treatment groups.
|
Folic acid
Mean (SD) |
Placebo
Mean (SD) |
Statistical indicators (between-group) | |
| Before treatment | 4.83( 2.61) | 3.70 (2.24) | P = 0.46, df = 68, t = 0.729 |
| First week | 3.88 (2.79) | 3.83 (2.86) | P = 0.13, df = 1, F† = 2.35 |
| Second week | 3.28 (2.57) | 3.28 (2.16) | P = 0.7, df = 1, F† = 3.19 |
| Third week | 3.11 (2.69) | 3.22 (2.30) | P = 0.09, df = 1, F† = 2.91 |
| Fourth week | 2.87 (2.69) | 3.37 (2.35) | P = 0.00, df = 1, F† = 10.16 |
| ANOVA with repeated measure (within-group) | F = 14.36 df = 1.89 P < 0.001 |
F = 4.78 df = 2.03 P = 0.01 |
†ANCOVA
Discussion
The results indicated that folic acid and placebo were both effective in reducing the severity, frequency, and duration of hot flashes. Folic acid was significantly more effective on these three factors than placebo. This difference between the two groups regarding severity was observed from the second week after treatment. The differences in the mean frequency, and duration of hot flashes after treatment with folic acid and placebo were, respectively, observed in the third and fourth weeks. Folic acid reduced hot flash frequency in the third week more than placebo. Moreover, during the fourth week folic acid significantly affected hot flash duration.
Improvement in severity, frequency, and duration of hot flash was also seen by placebo. This might be due to the psychological effects of using placebo. Moreover, the results of different studies showed responses to placebo for menopause symptoms especially hot flash.20 In a study conducted in Egypt by Gaweesh et al., 65% improvement in hot flash was reported with folic acid, and 16% improvement with placebo; this was consistent with the results of the present study.33 Since there was only one study on the folic acid effects on hot flash, other studies on medication which had the same mechanism as folic acid and were effective on reducing hot flash were used. Involvement of serotonergic and adrenergic systems in hot flash occurrence in addition to folic acid, result in the use of some neurotransmitters regulators such as selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), and vitamin E for treatment of hot flash. Loprinzi et al. investigated the effects of fluoxetine 20 mg tablets in treating the intensity of hot flashes in 81 women with breast cancer.41 The hot flash severity scores improved by 50% in the fluoxetine treatment group, and 36% in the placebo group. Cross-over analysis showed significantly higher improvement in hot flashes (24%) in the fluoxetine treatment group than the placebo group (p = 0.02). The hot flash frequency also reduced by 1.5 episodes per day.41 Yazdizadeh et al. also indicated that fluoxetine and placebo were both effective in reducing hot flashes but fluoxetine was significantly more effective in reducing hot flash severity than placebo.42 Ziaei et al. studied the effects of vitamin E on menopausal hot flashes.43 They reported that vitamin E and placebo both reduced hot flashes, but there was a significant difference between them in terms of reducing hot flash severity score and hot flash frequency. These results were consistent with the results of the present study.
Loprinzi et al. studied the effects of venlafaxine with different dosages during four weeks on 221 women with breast cancer.44 They reported a reduction in hot flash severity scores of 31% to 61% versus 27% in placebo, and a reduction in hot flash frequency of 30% to 58% versus 19% in placebo. The effects of Scitalopram compared with placebo in treating hot flash in 205 women was studied by Freeman et al.45 They discovered that Scitalopram was more effective in reducing hot flash severity after eight weeks compared to placebo. Stearns et al. Studied the effects of paroxetine in the treatment of hot flash.46 A 62.2% decrease was observed in hot flash with paroxetine with the dosage of 12.5 mg and a 64.4% decrease with the dosage of 25 mg after six weeks of treatment, and a 37.8% decrease by placebo. The mean daily hot flash frequency with a dosage of 12.5 mg decreased from 7.1 to 3.8, with a dosage of 25 mg it decreased from 6.4 to 3.3, and in placebo from 6.6 to 4.8. Another study on the effects of venlafaxine on hot flash of women caused by breast cancer was conducted by Carpenter et al.47 This study indicated that venlafaxine was more effective than placebo. Gordon et al. also reported that sertraline was more effective in reducing hot flashes in 102 women with menopause compared to placebo.48 The results of these studies are similar to the present research which is due to their similar mechanism. However, the results of the study by Grady et al. indicated that sertraline was not effective on the frequency and severity of perimenopausal and postmenopausal hot flashes.49Suvanto-Luukkonen et al. cond-ucted a randomized clinical trial during 9 months on the effects of citalopram and fluoxetine on hot flashes in 150 menopausal women. They reported that there was no significant difference between the groups regarding hot flash frequency.50 Bouchard et al. studied the effects of desvenlafaxine on vasomotor symptoms, in 35 regions of Europe, 2 regions of South Africa, and one region of Mexico.51 In week 12 no significant difference was observed in the reduction of mean frequency of daily hot flashes between the groups. In the present study folic acid had no side effects; therefore, it can be used for reducing hot flashes and improving the women's quality of life. Since there are a limited number of studies on the effect of folic acid on menopausal hot flash, further studies on this matter are recommended.
Conclusion
The results of the present study indicated that folic acid and placebo were both effective in the improvement of hot flash. However, folic acid was more effective. Folic acid is a safe, affordable, and acceptable medication for women; therefore, it can be considered as an alternative method for relieving menopausal hot flashes.
Ethical issues
None to be declared.
Conflict of Interest
The authors declare no conflict of interest in this study.
Acknowledgments
Our appreciation goes to the research deputy of Tabriz University of Medical Sciences for the financial support, Dr. Yousef Javadzadeh, an academic member of the Department of Pharmacy, Tabriz University of Medical Sciences, for producing the placebo, the staff of Al-Zahra teaching hospital of Rasht, and all the women participating in this study.
References
- 1.Umland EM. Treatment strategies for reducing the burden of menopause-associated vasomotor symptoms. J Manag Care Pharm. 2008;14(3 Suppl):14–9. doi: 10.18553/jmcp.2008.14.S6-A.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Utian WH. Psychosocial and socioeconomic burden of vasomotor symptoms in menopause: a comprehensive review. Health Qual Life Outcomes . 2005; 3:47. doi: 10.1186/1477-7525-3-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gold EB, Colvin A, Avis N, Bromberger J, Greendale GA, Powell L, Sternfeld B, Matthews K. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopausal transition: study of women's health across the nation. Am J Public Health. 2006;96(7):1226–35. doi: 10.2105/AJPH.2005.066936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alavi G, Hoshmand P. Survey of Knowledge, attitude and practice of Mashhad women toward menopause. Medical Journal of Mashhad University of Medical Sciences. 1998;14(59-60):13–22. (Persian) [Google Scholar]
- 5.National Institutes of Health State-of-the-Science Conference statement . management of menopause-related symptoms. Ann Intern Med. 2005;142(12 Pt 1):1003–13. [PubMed] [Google Scholar]
- 6.Freedman RR. Physiology of hot flashes. Am J Hum Biol. 2001;13(4):453–64. doi: 10.1002/ajhb.1077. [DOI] [PubMed] [Google Scholar]
- 7.Deecher DC, Dorries K. Understanding the pathophysiology of vasomotor symptoms (hot flushes and night sweats) that occur in perimenopause, menopause, and postmenopause life stages. Arch WomensMent Health. 2007;10(6):247–57. doi: 10.1007/s00737-007-0209-5. [DOI] [PubMed] [Google Scholar]
- 8.Rossmanith WG, Ruebberdt W. What causes hot flushes? The neuroendocrine origin of vasomotor symptoms in the menopause. GynecolEndocrinol. 2009;25(5):303–14. doi: 10.1080/09513590802632514. [DOI] [PubMed] [Google Scholar]
- 9.Ohayon MM. Severe hot flashes are associated with chronic insomnia. Arch Intern Med. 2006;166(12):1262–8. doi: 10.1001/archinte.166.12.1262. [DOI] [PubMed] [Google Scholar]
- 10.Tom SE, Kuh D, Guralnik JM, Mishra GD. Self-reported sleep difficulty during the menopausal transition: results from a prospective cohort study. Menopause. 2010;17(6):1128–35. doi: 10.1097/gme.0b013e3181dd55b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kravitz HM, Zhao X, Bromberger JT, Gold EB, Hall MH, Matthews KA, Sowers MR. Sleep disturbance during the menopausal transition in a multi-ethnic community sample of women. Sleep. 2008;31(7):979–90. [PMC free article] [PubMed] [Google Scholar]
- 12.Joffe H, Soares CN, Cohen LS. Assessment and treatment of hot flushes and menopausal mood disturbance. Psychiatr Clin North Am. 2003;26(3):563–80. doi: 10.1016/s0193-953x(03)00045-5. [DOI] [PubMed] [Google Scholar]
- 13.Juang KD, Wang SJ, Lu SR, Lee SJ, Fuh JL. Hot flashes are associated with psychological symptoms of anxiety and depression in peri- and post- but not premenopausal women. Maturitas. 2005;52(2):119–26. doi: 10.1016/j.maturitas.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Reed SD, Newton KM, LaCroix AZ, Grothaus LC, Ehrlich K. Night sweats, sleep disturbance, and depression associated with diminished libido in late menopausal transition and early postmenopause: baseline data from the Herbal Alternatives for Menopause Trial (HALT) Am J ObstetGynecol. 2007;196(6):593–7. doi: 10.1016/j.ajog.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacLennan A, Lester S, Moore V. Oral oestrogen replacement therapy versus placebo for hot flushes. Cochrane Database Syst Rev . 2001;( 1):CD002978. doi: 10.1002/14651858.CD002978. [DOI] [PubMed] [Google Scholar]
- 16.Bliss JM, Gray R. Breast cancer and hormone-replacement therapy: the Million Women Study. Lancet. 2003;362(9392):1328–9. doi: 10.1016/S0140-6736(03)14591-6. [DOI] [PubMed] [Google Scholar]
- 17.Manson JE, Hsia J, Johnson KC, Rossouw JE, Assaf AR, Lasser NL, Trevisan M, Black HR, HeckbertSR HeckbertSR, Detrano R. et al. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003 Aug 7;349(6):523–34. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- 18.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC. et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 19.Hersh AL, Stefanick ML, Stafford RS. National use of postmenopausal hormone therapy: annual trends and response to recent evidence. JAMA. 2004;291(1):47–53. doi: 10.1001/jama.291.1.47. [DOI] [PubMed] [Google Scholar]
- 20.Nelson HD, Vesco KK, Haney E, Fu R, Nedrow A, Miller J, Nicolaidis C, Walker M, Humphrey L. Nonhormonal therapies for menopausal hot flashes: systematic review and meta-analysis. JAMA . 2006;295(17):2057–71. doi: 10.1001/jama.295.17.2057. [DOI] [PubMed] [Google Scholar]
- 21.Sturdee DW. The menopausal hot flush-anything new? Maturitas. 2008;60(1):42–9. doi: 10.1016/j.maturitas.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Ayers B, Mann E, Hunter MS. A randomised controlled trial of cognitive-behavioural therapy for women with problematic menopausal hot flushes: MENOS 2 trial protocol. BMJ Open. 2011;1(1):e000047. doi: 10.1136/bmjopen-2010-000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berendsen HH. The role of serotonin in hot flushes. Maturitas. 2000;36(3):155–64. doi: 10.1016/s0378-5122(00)00151-1. [DOI] [PubMed] [Google Scholar]
- 24.Freedman RR. Treatment of menopausal hot flashes with 5-hydroxytryptophan. Maturitas. 2010;65(4):383–5. doi: 10.1016/j.maturitas.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brocardo PS, Budni J, Kaster MP, Santos AR, Rodrigues AL. Folic acid administration produces an antidepressant-like effect in mice: evidence for the involvement of the serotonergic and noradrenergic systems. Neuropharmacology. 2008;54(2):464–73. doi: 10.1016/j.neuropharm.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 26.Lucock MD, Green M, Levene MI. Methylfolate modulates potassium evoked neuro-secretion: evidence for a role at the pteridine cofactor level of tyrosine 3-hydroxylase. Neurochem Res. 1995;20(6):727–36. doi: 10.1007/BF01705542. [DOI] [PubMed] [Google Scholar]
- 27.Botez MI, Young SN, Bachevalier J, Gauthier S. Effect of folic acid and vitamin B12 deficiencies on 5-hydroxyindoleacetic acid in human cerebrospinal fluid. Ann Neurol. 1982;12(5):479–84. doi: 10.1002/ana.410120512. [DOI] [PubMed] [Google Scholar]
- 28.Gaweesh S, Ewies AA. Folic acid supplementation cures hot flushes in postmenopausal women. Med Hypotheses. 2010;74(2):286–8. doi: 10.1016/j.mehy.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 29.Reynolds EH. Folic acid, ageing, depression, and dementia. BMJ. 2002;324(7352):1512–5. doi: 10.1136/bmj.324.7352.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson CA, Jee SH, Charleston J, Narrett M, Appel LJ. Effects of folic acid supplementation on serum folate and plasma homocysteine concentrations in older adults: a dose-response trial. Am J Epidemiol. 2010;172(8):932–41. doi: 10.1093/aje/kwq197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morris MS, Jacques PF, Rosenberg IH, Selhub J. Folate and vitamin B-12 status in relation to anemia, macrocytosis, and cognitive impairment in older Americans in the age of folic acid fortification. Am J ClinNutr. 2007;85(1):193–200. doi: 10.1093/ajcn/85.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Inoue-Choi M, Ward MH, Cerhan JR, Weyer PJ, Anderson KE, Robien K. Interaction of nitrate and folate on the risk of breast cancer among postmenopausal women. Nutr Cancer. 2012;64(5):685–94. doi: 10.1080/01635581.2012.687427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gaweesh SS, Abdel-Gawad MM, Nagaty AM, Ewies AA. Folic acid supplementation may cure hot flushes in postmenopausal women: a prospective cohort study. Gynecol Endocrinol. 2010;26(9):658–62. doi: 10.3109/09513591003686288. [DOI] [PubMed] [Google Scholar]
- 34.Smith AD, Kim YI, Refsum H. Is folic acid good for everyone? Am J ClinNutr. 2008;87(3):517–33. doi: 10.1093/ajcn/87.3.517. [DOI] [PubMed] [Google Scholar]
- 35.Ansari Sh, Shhraz S, Ghaziani T. Iran farma (comprehensive text book of official agents of Iran) 2nd ed. Tehran, Iran: Teymourzadeh Publication; 2007. p. 307 [Google Scholar]
- 36.Tuomikoski P. Postmenopausal hot flushes vascular health and hormone therapy [dissertation]. Helsinki: Helsinki University; 2010.
- 37.Sloan JA, Loprinzi CL, Novotny PJ, Barton DL, Lavasseur BI, Windschitl H. Methodologic lessons learned from hot flash studies. J Clin Oncol. 2001;19(23):4280–90. doi: 10.1200/JCO.2001.19.23.4280. [DOI] [PubMed] [Google Scholar]
- 38.Carpenter JS. State of the science: hot flashes and cancerPart 1: definition, scope, impact, physiology, and measurement. Oncol Nurs Forum. 2005;32(5):959–68. doi: 10.1188/05.ONF.959-968. [DOI] [PubMed] [Google Scholar]
- 39.Boorsma J. Hot flashes, Blood Glucose and Diabetic Postmenopausal Women [Master thesis]. Lethbridge: School of Graduate Studies, University of Lethbridge; 2008.
- 40.Nunnally JC, Bernstein IH. Psychometric theory. 3rd ed. New York: McGraw-Hill; 1994. [Google Scholar]
- 41.Loprinzi CL, Sloan JA, Perez EA, Quella SK, Stella PJ, Mailliard JA, Halyard MY, Pruthi S, Novotny PJ, Rummans TA. Phase III evaluation of fluoxetine for treatment of hot flashes. J Clin Oncol. 2002;20(6):1578–83. doi: 10.1200/JCO.2002.20.6.1578. [DOI] [PubMed] [Google Scholar]
- 42.Yazdizadeh V, Modares Gilani M, Kazemnejad A.. The Effect of Fluoxetin on Hot Flashes in Menopaused Women. Daneshvar Medicine. 2009;16(81):23–8. (Persian) [Google Scholar]
- 43.Ziaei S, Kazemnejad A, Zareai M. The effect of vitamin E on hot flashes in menopausal women. Gynecol Obstet Invest. 2007;64(4):204–7. doi: 10.1159/000106491. [DOI] [PubMed] [Google Scholar]
- 44.Loprinzi CL, Kugler JW, Sloan JA, Mailliard JA, Lavasseur BI, Barton DL, Novotny PJ, Dakhil SR, Rodger K, Rummans TA. et al. Venlafaxine in management of hot flashes in survivors of breast cancer: a randomised controlled trial. Lancet. 2000;356(9247):2059–63. doi: 10.1016/S0140-6736(00)03403-6. [DOI] [PubMed] [Google Scholar]
- 45.Freeman EW, Guthrie KA, Caan B, Sternfeld B, Cohen LS, Joffe H, Carpenter JS, Anderson GL, Larson JC, Ensrud KE. et al. Efficacy of escitalopram for hot flashes in healthy menopausal women: a randomized controlled trial. JAMA. 2011;305(3):267–74. doi: 10.1001/jama.2010.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stearns V, Beebe KL, Iyengar M, Dube E. Paroxetine controlled release in the treatment of menopausal hot flashes: a randomized controlled trial. JAMA. 2003;289(21):2827–34. doi: 10.1001/jama.289.21.2827. [DOI] [PubMed] [Google Scholar]
- 47.Carpenter JS, Storniolo AM, Johns S, Monahan PO, Azzouz F, Elam JL, Johnson CS, Shelton RC. Randomized, double-blind, placebo-controlled crossover trials of venlafaxine for hot flashes after breast cancer. Oncologist. 2007;12(1):124–35. doi: 10.1634/theoncologist.12-1-124. [DOI] [PubMed] [Google Scholar]
- 48.Gordon PR, Kerwin JP, Boesen KG, Senf J. Sertraline to treat hot flashes: a randomized controlled, double-blind, crossover trial in a general population. Menopause. 2006;13(4):568–75. doi: 10.1097/01.gme.0000196595.82452.ca. [DOI] [PubMed] [Google Scholar]
- 49.Grady D, Cohen B, Tice J, Kristof M, Olyaie A, Sawaya GF. Ineffectiveness of sertraline for treatment of menopausal hot flushes: a randomized controlled trial. Obstet Gynecol. 2007;109(4):823–30. doi: 10.1097/01.AOG.0000258278.73505.fa. [DOI] [PubMed] [Google Scholar]
- 50.Suvanto-Luukkonen E, Koivunen R, Sundstrom H, Bloigu R, Karjalainen E, Haiva-Mallinen L, Tapanainen JS. Citalopram and fluoxetine in the treatment of postmenopausal symptoms: a prospective, randomized, 9-month, placebo-controlled, double-blind study. Menopause. 2005;12(1):18–26. doi: 10.1097/00042192-200512010-00006. [DOI] [PubMed] [Google Scholar]
- 51.Bouchard P, Panay N, de Villiers TJ, Vincendon P, Bao W, Cheng RJ, Constantine G. Randomized placebo- and active-controlled study of desvenlafaxine for menopausal vasomotor symptoms. Climacteric. 2012;15(1):12–20. doi: 10.3109/13697137.2011.586445. [DOI] [PubMed] [Google Scholar]


