Abstract
Chondroprogenitor cells encapsulated in a chondrogenically supportive, three-dimensional hydrogel scaffold represents a promising, regenerative approach to articular cartilage repair. In this study, we have developed an injectable, biodegradable methacrylated gelatin (mGL)–based hydrogel capable of rapid gelation via visible light (VL)–activated crosslinking in air or aqueous solution. The mild photocrosslinking conditions permitted the incorporation of cells during the gelation process. Encapsulated human-bone-marrow-derived mesenchymal stem cells (hBMSCs) showed high, long-term viability (up to 90 days) throughout the scaffold. To assess the applicability of the mGL hydrogel for cartilage tissue engineering, we have evaluated the efficacy of chondrogenesis of the encapsulated hBMSCs, using hBMSCs seeded in agarose as control. The ability of hBMSC-laden mGL constructs to integrate with host tissues after implantation was further investigated utilizing an in vitro cartilage repair model. The results showed that the mGL hydrogel, which could be photopolymerized in air and aqueous solution, supports hBMSC growth and TGF-β3-induced chondrogenesis. Compared with agarose, mGL constructs laden with hBMSCs are mechanically stronger with time, and integrate well with native cartilage tissue upon implantation based on push-out mechanical testing. VL-photocrosslinked mGL scaffold thus represents a promising scaffold for cell-based repair and resurfacing of articular cartilage defects.
Introduction
Articular cartilage, found at the surface of the articular joint, has the principal mechanical function of allowing frictionless motion and absorbing and distributing loads.1 Exposures to forces outside the physiological range, combined with an intrinsic inability of the articular cartilage to manage normal loads, are sufficient to initiate cartilage degeneration and result in osteoarthritis (OA),2 a chronic degenerative disease of the articular joint that affects 27 million Americans, including about 60% of men and 70% of women above 65 years of age.3,4 Due to its avascular nature, cartilage has very limited capacity for self-repair.1 Currently, there is no effective therapy for OA, with only symptomatic relief until the need for surgical joint replacement.5,6 For small cartilage defects, the procedure of microfracture, which involves controlled drilling into the subchondral bone, is commonly employed by surgeons to introduce access to the bone marrow and its constituent cells for cartilage repair.7,8 However, the cartilage formed is usually of a fibrocartilaginous nature, and is structurally and mechanically inferior to the native hyaline articular cartilage, thus providing only relatively short-term benefits.
Regenerative medicine approaches that combine cells with scaffolds and signaling factors represent promising, alternative therapies for OA treatment.9 Chondrocytes and stem cells derived from different tissue sources have been tested as the starting cell population. Bone-marrow-derived mesenchymal stem cells (BMSCs) are considered one of the most promising candidate therapeutic cells for cartilage regeneration, owing to their self-renewal ability, chondrogenic potential, and other biological characteristics, such as anti-inflammatory activity.10 Because the articular cartilage is bathed in synovial fluid and is under constant movement, cells for cartilage repair or regeneration must be delivered to the tissue site encased in a suitable three-dimensional (3D) scaffold, such as a hydrogel, in order to limit diffusion of cells as well as to support site-specific proliferation and differentiation. In addition, because of the inherent irregular shape of the cartilage lesions, the cell-seeded construct must be able to fill the defect completely. To be used in minimally invasive procedures without drainage of the synovial fluid, the biomaterial needs to be injectable and able to cure under aqueous solution. It is also preferable that the scaffold biomaterial contains cell binding ligands, such as epitopes specific for cell surface integrins, to enhance cell binding and subsequent differentiation and tissue remodeling, as well as integration into the neighboring host tissue. Hydrogel materials are considered attractive, promising scaffolds because of their high water content, similar to the cartilage extracellular matrix, allowing load transfer from environment to chondrocytes as in native cartilage.10,11 To date, different types of natural and synthetic hydrogels have been tested in animal and even clinical trials, such as alginate/agarose,12 collagen,13 poly(vinyl alcohol),14 and poly(ethylene glycol) (PEG).15 Because no one hydrogel material has yet faithfully reproduced the tissue properties of cartilage, further investigations and optimization of hydrogel materials and preparation methods are needed.
Collagen is a major extracellular matrix component in cartilage,11 and is thus a natural biomaterial choice for the fabrication of cartilage tissue engineering scaffolds. However, collagen scaffolds have limitations in terms of concentration (usually <10 mg/mL) as well as stiffness. In addition, human BMSC (hBMSC)–seeded pure collagen scaffolds typically contract and lose their original structure, and are thus not suitable for tissue repair. Gelatin, a denatured form of collagen, has emerged to be an attractive alternative to native collagen for cartilage repair. It retains many of the native molecular epitopes for cell adhesion and signal transduction in collagen that are important for the maintenance of the chondrocyte phenotype, and its considerably higher saturation point in aqueous solution (up to 200 mg/mL) serves to increase the structural stability of a gelatin-based scaffold, compared with that formed using collagen.
To date, different forms of gelatin scaffolds have been developed. Among them, photocrosslinkable gelatin is especially attractive because photoactivation is fast and requires relatively mild chemical conditions.16 However, at present, the most commonly used photoinitiator I2959 utilized to initiate photocrosslinking is oxygen sensitive, and thus requires the use of air-tight protective barriers that are incompatible with clinical practice. In addition, I2959 activation requires ultraviolet (UV) light, which is potentially damaging to cellular DNA.
We have previously reported the use of the low-toxicity initiator lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP)17 to crosslink poly(ethylene glycol)-diacrylate (PEGDA) for visible light (VL)–based projection stereolithography.18 This crosslinking protocol is capable of rapid generation of cell-laden scaffolds with high cell viability. However, PEGDA scaffolds do not contain cell binding motifs and are not biodegradable, thus support limiting cell proliferation and tissue integration. Moreover, an elaborate instrument is required to perform the photocrosslinking. In this study, we demonstrate the application of VL photocrosslinking with a dental lamp for the production of a gelatin-based 3D hydrogel scaffold, using methacrylated gelatin (mGL)/LAP, which is injectable, biodegradable, and biocompatible, exhibits excellent in situ space-filling qualities in air or aqueous solution without the use of protective barriers, and is resistant to swelling and contraction. The applicability of the photocrosslinked gelatin scaffold for cartilage tissue engineering was assessed first by examining the viability, metabolic activity, and chondrogenic activity of hBMSCs encapsulated within the scaffold, as compared with agarose hydrogels. Second, the mechanical properties of both cell-seeded mGL and agarose constructs were tested immediately after polymerization/gelation and after 90 days of chondrogenic culture. Finally, the ability of the engineered construct using the mGL scaffold to integrate into host tissue, as a measure of space filling, was investigated employing an in vitro cartilage repair model.19 The results show that the mGL-based biomaterial scaffold developed in this study is a promising scaffold for cell-based repair and resurfacing of acute and/or osteoarthritic articular cartilage defects.
Materials and Methods
All chemicals used in this study were purchased from Sigma-Aldrich (St. Louis, MO) unless stated otherwise.
Preparation of photoinitiator LAP
The VL-activated initiator LAP was synthesized as described by Fairbanks et al.17
Preparation of mGL
mGL was synthesized according to a procedure previously described with slight modification.20,21 Gelatin (15 g) was dissolved completely in 500 mL deionized H2O at 37°C, and then 15 mL of methacrylic anhydride was added dropwise. The mixture was placed in a 37°C shaker at 150 rpm for 24 h. The mGL solution was dialyzed for 4 days against H2O at room temperature to completely remove all low-molecular-weight byproducts using 2000 NMWCO dialysis tubing (Sigma-Aldrich). After lyophilization, the mGL product was stored in a desiccator for future use. The methacrylation efficiency of the process was ∼80%.21
Isolation of hBMSCs
hBMSCs were isolated from human bone marrow, obtained from femoral heads of patients undergoing total hip arthroplasty with Institutional Review Board approval (University of Washington and University of Pittsburgh). Trabecular bone was cored out using curette or rongeur and flushed using 18G hypodermic needles with rinsing medium (α-MEM and 1% Antibiotic-Antimycotic; Invitrogen, Carlsbad, CA). The flushed marrow was then passed through 40-μm strainers, and the cells in flow-through were pelleted by centrifugation for 5 min at 300 g. After the supernatant was discarded, cells were resuspended in hBMSC growth medium [GM; α-MEM containing 10% fetal bovine serum (Invitrogen), 1% Antibiotic-Antimycotic, and 1.5 ng/mL FGF-2 (RayBiotech, Norcross, GA)], and then plated into 150-cm2 tissue culture flasks. After 4 days of culture, nonadherent cells were washed with phosphate-buffered saline (PBS) and fresh GM was added. Medium was changed every 3–4 days. Once 70%–80% confluence was reached, cells were detached with 0.25% trypsin containing 1 mM EDTA (Invitrogen) and passaged. hBMSCs isolated from individual patients were validated as capable of osteogenic, adipogenic, and chondrogenic differentiation upon stimulation (data not shown). All experiments were performed with passage 3 (P3) hBMSCs. hBMSCs from three patients (54-year-old woman, 52-year-old woman, and 57-year-old man) were used in this study.
VL-activated photocrosslinking of mGL
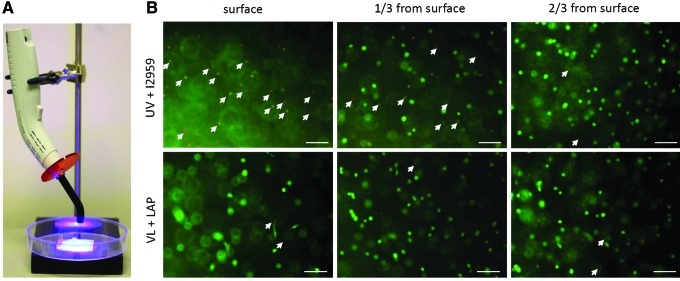
mGL was dissolved in Hank's balanced salt solution (HBSS) at 10% (w/v), and after adjustment of pH to 7.4 using 1 N NaOH, the photoinitiator LAP (0.15%, w/v) and 1% Antibiotic-Antimycotic were added. For scaffold fabrication, the mGL/LAP solution was poured into a 2-mm-height silicone mold. A dental curing light (Mega Light CL; DBI America, Lutz, FL), producing VL at 430–490 nm wavelength and 1400 mw/cm2 power, was applied directly over the mGL/LAP solution without any covering on top of the solution (Fig. 1A). The total exposure time was from 1.5 to 8 min. After exposure, the photopolymerized mGL hydrogel was removed from the mold and placed in HBSS for mechanical and degradation testing.
FIG. 1.
(A) Visible light (VL)–activated gelation using illumination from a dental lamp (wavelength 430–490 nm, power 1400 mw/cm2). (B) Live/dead analysis of human-bone-marrow-derived mesenchymal stem cells (hBMSCs) encapsulated into methacrylated gelatin (mGL) hydrogel at different depths (1/3 and 2/3 from surface), using commonly used ultraviolet (UV)/I2959 or VL/lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP). Green, live cells; red or orange, dead cells as indicated by arrows. Scale bar=100 μm. Color images available online at www.liebertpub.com/tea
Analysis of cell viability in different hydrogels
hBMSCs were suspended in the 10% mGL/0.15% LAP HBSS solution at a final density 4×106/mL and the suspension was poured into a mold with size 20×20×2 mm3 (L×W×H). After photocuring (4-min VL exposure as described earlier), the cell-laden mGL hydrogels were extracted and punched into 5-mm-diameter by 2-mm-height cylinders. This group was designated as “VL/LAP.”
For comparison, another type of hydrogel was produced using the photoinitiator Irgacure 2959 (I2959, 0.15% [w/v] final concentration; BASF, Ludwigshafen, Gr) instead of LAP. mGL solution concentration and cell density were identical to those used in the VL/LAP group. Without any covering to block air, mGL/I2959/hBMSC solution was subjected to 12-min exposure to long-wavelength UV light (5000 μW/cm2 UV-A, Spectroline lamp). The cell-laden mGL hydrogels were extracted and punched into 5-mm-diameter by 2-mm-height cylinders. This group was designated UV/I2959.
Cell viability was assessed after 1, 3, and 7 days of culture with the Live/Dead Viability/Cytotoxicity kit (Invitrogen) and observed by means of epifluorescence microscopy (CKX 41; Olympus, Center Valley, PA). Four microscopic fields (720×533 μm2) per sample were analyzed. Percentage of live cells was calculated based on the number of green-stained cells divided by the total number of cells (green- and red-stained cells, dual stained counted once as dead).
Mechanical testing of mGL scaffolds with variable photocrosslinking
Acellular mGL scaffolds fabricated as described previously with different exposure times were tested under 10% uniaxial, unconfined compression in an electromechanical tester with a 250-g load cell (ElectroForce 3200; Bose, Eden Prairie, MN). The exact dimensions of cylinders were measured with a caliper, and the cylinders were then placed between stainless steel discs and preloaded to 0.06 g. The samples were subjected to 10% strain applied at room temperature using a constant rate (0.05%/s). Compressive moduli of mGL scaffolds were determined from the slope of force versus displacement plots.
In vitro degradation of scaffolds
The biodegradability of the mGL scaffolds with different exposure times (2, 4, and 8 min) was determined by incubating them with collagenase (1 scaffold in 4 mL 0.05% [w/v] collagenase in PBS [pH 7.4]). After determining the original wet weight (W1), the 8-mm-diameter/2-mm-height scaffold was completely immersed in the collagenase solution and incubated at 37°C with shaking at 50 rpm/min, and the wet weight (after blotting) at different time points (W2) was determined. The process was continued until all samples appeared to be completely degraded. The degradation percentage (Q) was calculated as follows: Q=(1−W2/W1)×100%.
hBMSC encapsulation into mGL scaffolds for chondrogenesis
hBMSC pellets were dissociated and the single cells were suspended within a 10% mGL solution (containing 0.15% LAP) to a final density of 4×106 cells/mL. After curing by 4-min VL exposure using dental curing lamp, the cell-laden mGL hydrogels were extracted and punched into 5-mm-diameter by 2-mm-height cylinders. Agarose (2%, w/v) scaffolds seeded with 4×106 cells/mL hBMSCs at identical densities and with identical dimensions served as controls [Note: In preliminary studies, cell-seeded agarose plugs retained a more stable shape during hBMSC chondrogenesis than those formed using alginate, collagen, or PuraMatrix™ peptide hydrogel (data not shown), and was thus chosen for this study.].
The constructs were cultured in chondrogenesis-inducing medium [DMEM with high glucose, 1% penicillin–streptomycin, 0.1 μM dexamethasone, 50 μg/mL ascorbate 2-phosphate, and 40 μg/mL L-proline (Sigma-Aldrich); 1×insulin–transferrin–selenium (Invitrogen); and 10 ng/mL TGF-β3 (R&D Systems, Minneapolis, MN)]. Cell viability was assessed after 1, 3, 7, and 90 days of culture with the Live/Dead Viability/Cytotoxicity kit as described previously.
Mechanical testing of live cell constructs
After 90 days of culture in chondrogenesis medium, the mechanical property of both mGL and agarose constructs was tested as described previously.
Quantitative real-time reverse transcription–polymerase chain reaction
Total RNA was extracted using TRIZOL (Invitrogen)–chloroform and purified using the RNeasy Plus mini kit (Qiagen, Hilden, Germany) with DNase I (Qiagen) treatment. SuperScript III kit (Invitrogen) was utilized to complete the reverse transcription. Real-time reverse transcription–polymerase chain reaction (RT-PCR) was performed using the StepOnePlus thermocycler (Applied Biosystems, Foster City, CA) and SYBR Green Reaction Mix (Applied Biosystems). Sox 9, aggrecan (ACN), and collagen type II (COL2A1) primer sequences are as follows: forward, 5′-AGCCTGCGCTCCAATGACT-3′ and reverse, 5′-TAATGGAACACGATGCCTTTCA-3′; forward, 5′-GGCAATAGCAGGTTCACGTACA-3′ and reverse, 5′-CGATAACAGTCTTGCCCCACTT-3′; and forward, 5′-TTCCGCGACGTGGACAT-3′ and reverse, 5′-TCAAACTCGTTGACATCGAAGGT-3′, respectively. Transcript level of ribosomal protein L13a (RPL13A) was used as endogenous control: forward, 5′-GACACAGGACCACTCATGAAGT-3′ and reverse, 5′-GTGCGGCTGCTTCCATAAG-3′. Gene expression folder changes were calculated using the comparative CT (ΔΔCT) method.
Glycosaminoglycan quantitation in constructs
Samples were papain-digested and analyzed for glycosaminoglycan (GAG) content using the dimethylmethylene blue dye binding assay (Blyscan, Biocolor, United Kingdom), with a chondroitin sulfate standard. Digested samples were also evaluated for total DNA content using the Picogreen assay (Invitrogen).
Histological analysis
Constructs were fixed in 4% paraformaldehyde, paraffin embedded, sectioned at 8-μm thickness, and stained with Alcian Blue/Fast Red using standard protocols for sulfated proteoglycans and cell distribution analysis. Color images were captured using a microscope equipped with digital camera (SZX 16; Olympus).
Integration testing of cell-laden constructs into cartilage
To test the ability of constructs to integrate into the surrounding host tissues, adhesive strength testing of the interface between constructs and native cartilage was employed as shown in Figure 8.22 Cartilage tissues (about 2-mm thickness) were harvested from the femoral condyles of adult calves and cultured in the chondrogenesis medium for 1 day. These tissues were then punched into 8-mm-diameter cylinders using biopsy punches (Skylar, West Chester, PA), and a cartilage ring with a central defect was further created by coring out the center using a 4-mm biopsy punch (Fig. 8A). The cartilage rings were then blot-dried, and hBMSC-laden agarose or mGL solutions (4×106/mL), prepared as described previously, were added slowly into the defects until completely filled, followed by gelation by cooling or photocrosslinking, respectively, and excess material outside the defect sites was removed carefully with a scalpel. The tissue composites were then cultured under chondrogenic conditions, with medium change twice weekly (Fig. 8B). After 6 weeks, mechanical push-out testing was employed to estimate the interface strength between scaffolds and native cartilage (Fig. 8D). Cartilage samples containing internal constructs (Fig. 8C) were placed on a chamber with a center hole (6-mm diameter, aligned to the defect of cartilage) as shown in Figure 8E and G. The constructs were pushed out with a hard plastic plunger (Fig. 8F) using the electromechanical tester at a rate of 0.025 mm/s, and the applied force was recorded until failure (Fig. 8D).
FIG. 8.
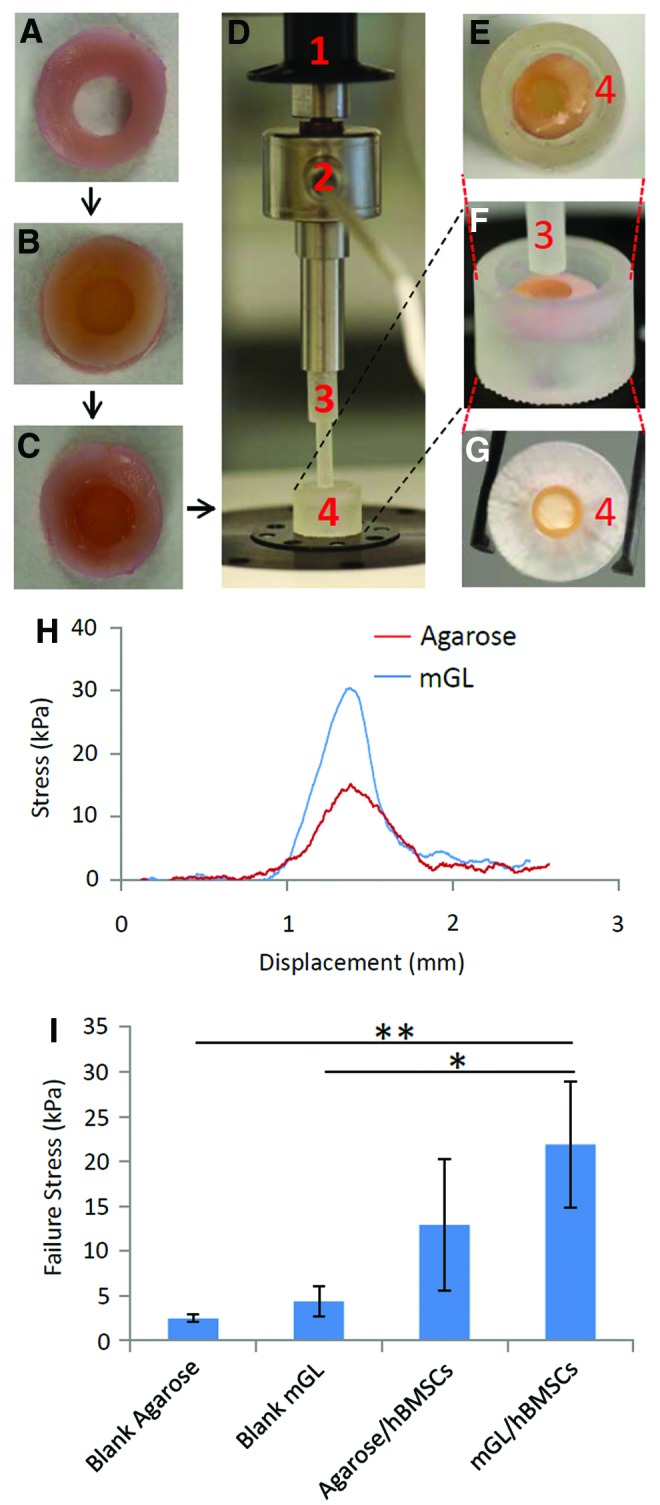
In vitro assessment of integration of hBMSC-laden mGL and agarose constructs into native cartilage. (A) Native bovine cartilage rings with 8-mm outer diameter and 4-mm inner diameter. (B) The inner gap in the cartilage explants was filled with hBMSC-laden mGL or agarose. (C) The composite constructs were cultured in chondrogenic medium for 6 weeks, and were then subjected to push-out mechanical testing. (D–G) Push-out test device. The composite constructs were placed in a custom-designed chamber (4), which held the cartilage in position, allowing push-out access to the inner-implanted component. The motor (1) controlled the movement of plunger (3), and the sensor (2) recorded the real-time force. (H) Typical stress–displacement curves of the agarose/hBMSC and mGL/hBMSC constructs from the push-out test. (I) Failure stress values for the different groups (*p<0.05, **p<0.01). Color images available online at www.liebertpub.com/tea
Statistical analysis
Each study was carried out with three experimental replicates and the results were expressed as the mean±SD. Significant differences among different groups were determined by two-tailed Student's t-test for two-group comparisons or ANOVA followed by post hoc analysis for multiple-group comparisons. Significance was considered at *p<0.05 and **p<0.01.
Results
VL-activated mGL scaffold fabrication
Prior to polymerization, the freshly made mGL/LAP solution had a viscous consistency, spontaneously forming a soft gel at 4°C that quickly melted at 37°C. The soft mGL gel was readily stabilized through covalent and irreversible LAP-mediated photocrosslinking in the presence of VL. These properties of mGL suggested that it could be well-suited for in situ tissue repair. As proof-of-concept, we performed a quick test of the VL-activated gelation capability of the mGL solution in the context of in situ repair of articular cartilage defects in a tissue specimen obtained as surgical waste following total joint arthroplasty. As shown in Supplementary Videos SV1 and SV2 (Supplementary Data are available online at www.liebertpub.com/tea), a portable VL dental lamp was able to successfully crosslink mGL, with LAP, in both air and aqueous environment in the absence of O2 barriers. The capability of crosslinking mGL under aqueous conditions was prospectively very desirable for tissue repair in clinical settings. We thus initiated the study of mGL for articular cartilage repair.
Uniform distribution and survival of cells encapsulated within scaffolds is critical to construct performance in tissue regeneration. Comparison of the viability and distribution of hBMSCs within mGL hydrogel scaffold produced using LAP (VL-mediated crosslinking) or the more commonly used photoinitiator I2959 (UV-light-mediated crosslinking) is shown in Figure 1B. With LAP, a 4-min VL exposure produced a construct with a compressive modulus of 19.8 kPa, whereas using I2959, without the use of a O2 barrier, a 12-min UV light exposure was required to produce a construct of comparable mechanical property (18.9 kPa). In constructs produced using LAP- and VL-mediated photocrosslinking, cell death was uniformly low (<6%) as shown by Live/Dead staining. In contrast, in constructs fabricated using UV crosslinking, cell death approached ∼100% at the surface (on the side of light exposure) and averaged 22% in regions 600–700 μm from the surface (∼1/3 the depth of the construct).
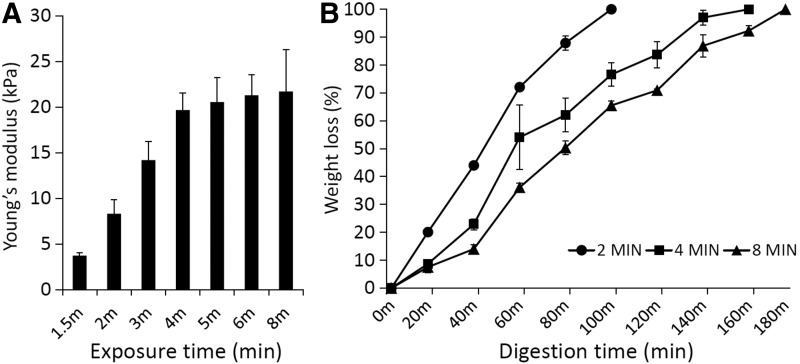
The stiffness of VL-crosslinked mGL scaffolds increased with exposure time, reaching a plateau at 19.8 kPa (compressive modulus) after 4 min of VL exposure. The plateau indicates maximum attainable crosslinking (Fig. 2A). This finding suggested that scaffold stiffness could be modulated by simply adjusting the time of VL exposure. Correspondingly, the time required for scaffold digestion with collagenase also increased with increased crosslinking (Fig. 2B). It is noteworthy that even at maximal photocrosslinking, the scaffolds were totally biodegraded within 3 hours.
FIG. 2.
(A) Compressive Young's moduli of mGL hydrogels prepared by different exposure times up to 8 min. (B) Collagenase digestion behavior of mGL hydrogels prepared by 2-, 4-, and 8-min light exposure. Wet weight loss of the scaffolds in collagenase solution was estimated at different times up to 180 min.
Chondrogenic differentiation of hBMSCs encapsulated within mGL scaffold
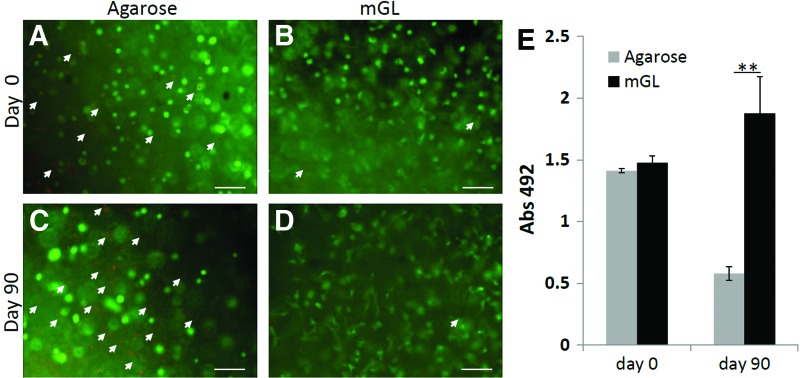
We next examined the ability of VL-crosslinked mGL to support chondrogenic differentiation of encapsulated hBMSCs. Because the I2959/UV-fabricated mGL scaffold resulted in low cell viability in mGL, we therefore employed agarose as the control (see Note in Materials and Methods section), as a representative of commonly used biomaterial scaffolds in chondrogenesis studies that also could be delivered via injection for gelation. All constructs (mGL and agarose) were subjected to up to 90 days of culture under chondrogenic stimulation. The high level of viability of the encapsulated cells in photocrosslinked mGL was maintained throughout long-term tissue culture, with viability averaging 92% after 90 days of culture, based on Live (green)/Dead (red) staining (Fig. 3B). In contrast, cell viability in agarose scaffolds decreased from 75% at day 3 to 40% at day 90 (Fig. 3A). In addition, this observation was supported by metabolic data (MTS assays), which showed that although cell numbers were identical in all constructs at the time of scaffold formation, cell viability was significantly higher in mGL constructs as compared with agarose constructs by day 90 (Fig. 3C).
FIG. 3.
Live/Dead assay of hBMSCs cultured in agarose at day 1 (A) or 90 (C), or in mGL hydrogel at day 1 (B) or 90 (D). Green, live cells; red or orange, dead cells as indicated by arrows. Scale bar=100 μm. (E) MTS assay of mGL or agarose constructs at day 0 and 90 (**p<0.01). Color images available online at www.liebertpub.com/tea
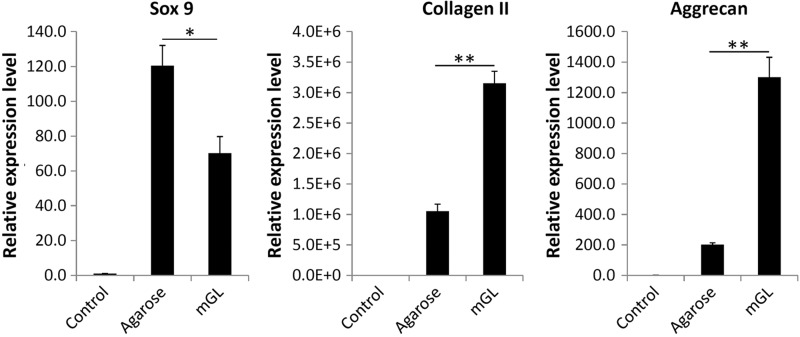
As shown in Figure 4, after chondrogenic culture for 90 days, substantially higher cartilage matrix gene expression (COL2A1 and ACN) was seen in mGL constructs versus agarose. This result was supported by biochemical data for GAG production that showed higher GAG levels in mGL scaffolds than agarose scaffolds, calculated on the basis of both per scaffold or per cell after 90 days of chondrogenic culture (Fig. 5). Interestingly, Sox 9 expression was lower in VL/LAP mGL constructs.
FIG. 4.
Real-time reverse transcription–polymerase chain reaction analysis of chondrogenic gene expression in hBMSC-laden agarose or mGL hydrogels at day 90 (*p<0.05, **p<0.01). Genes analyzed include Sox 9, collagen type II, and aggrecan. hBMSCs cultured on tissue culture plastic without chondrogenic induction served as control. All values are normalized to control.
FIG. 5.
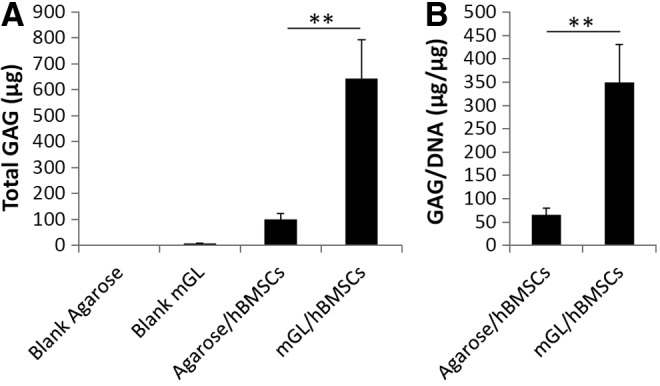
Quantitation of glycosaminoglycan (GAG) content deposited in hBMSC-laden constructs after 90 days of chondrogenic culture. (A) Total GAG in a construct. Blank agarose and mGL without cells served as control. (B) GAG production normalized to DNA content (**p<0.01).
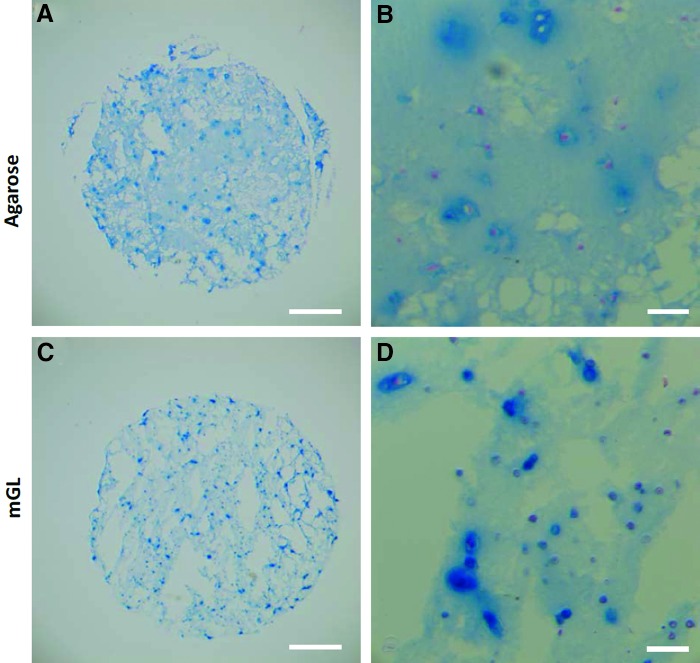
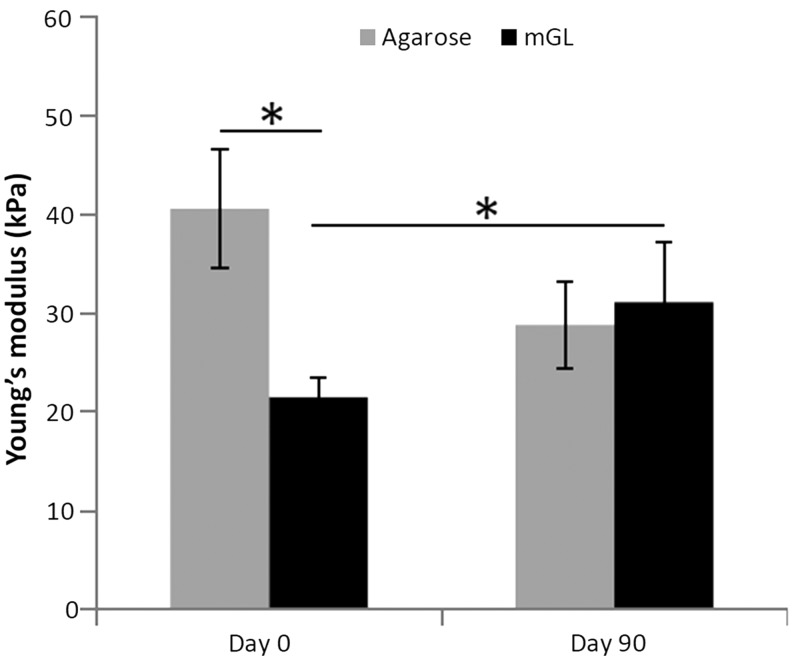
Histological examination revealed increased Alcian blue-stained GAG-rich matrix in the pericellular regions of mGL constructs as compared with agarose constructs (Fig. 6), although the overall staining intensity was rather low compared with that typically seen in hyaline cartilage tissue (not shown). We noted that both types of constructs maintained their shape and dimension over the 90-day period. Finally, mechanical testing revealed that immediately after curing, mGL scaffolds had a lower compressive modulus than agarose constructs (Fig. 7). However after 90 days of chondrogenic stimulation, the mGL scaffolds became stronger, attaining a compressive modulus of 31.1 kPa as compared with 21.5 kPa at the beginning of culture. In contrast, the stiffness of agarose constructs decreased to 28.8 kPa.
FIG. 6.
Alcian blue staining of sGAG in histological sections of the hBMSC-laden mGL and agarose constructs after 90 days of chondrogenic culture with Fast Red as nuclear counterstaining. Scale bar=1 mm (A, C) or 100 μm (B, D). Color images available online at www.liebertpub.com/tea
FIG. 7.
Mechanical properties of hBMSC-laden mGL and agarose constructs at day 0 and 90 culture in chondrogenic medium (*p<0.05).
Integration of mGL cartilage construct in an in vitro cartilage repair model
We noted that after 90 days of culture, hBMSC-laden mGL constructs were often bound to each other via neo-matrix (Supplementary Fig. S1). This phenomenon was not observed in the agarose constructs, suggesting that mGL could be specifically well-suited for enhanced tissue integration. To test the potential application of mGL and the process of LAP-initiated VL crosslinking for in situ articular cartilage repair, we undertook an in vitro testing of implant integration. In this study, the center 4-mm-diameter zone of an 8-mm-diameter disc of full-thickness osteochondral articular tissue, harvested from a bovine femoral condyle, was cored out and filled with hBMSC-laden constructs fabricated in situ. After culturing the composite in chondrogenic medium for a predetermined number of days, the extent of graft–host tissue integration was assessed by measuring the push-out force required to displace/remove the neo-tissue. Figure 8H shows the typical stress–displacement curves in hBMSC-laden mGL and agarose constructs. The force increased as the plunger pressed against the constructs. When the interface between the graft and host tissue failed (the peak force at failure), the curve quickly returned to baseline. All data were collected after 6 weeks of culture in chondro-stimulatory conditions. Without cells, blank mGL constructs placed within native osteochondral tissue had a greater peak force at failure than blank agarose (Fig. 8I), likely due to the formation of some crosslinking between the mGL and native matrix during polymerization. With cells encapsulated, both agarose and mGL had significantly increased peak force at failure stress compared with cell-free constructs, possibly reflecting the influence of the neo-matrix produced. However, although hBMSC-laden mGL constructs displayed, on average, higher push-out strength to failure than agarose constructs (Fig. 8I), the difference was not statistically significant.
Discussion
In this study, we have developed a VL-activated photocrosslinking procedure, using the photoinitiator LAP and a portable dental light (430–490 nm wavelength) as light source, to prepare injectable mGL hydrogel for cartilage repair. Compared with the commonly used I2959-initiated, UV-based method, our procedure is able to encapsulate hBMSCs into mGL scaffolds in both air and aqueous solution without the use of any oxygen barriers, and maintain substantially higher cell viability. The mechanical stiffness of the scaffolds is proportional to photocuring time until reaching a platform after 4-min light exposure. Full biodegradability of the mGL scaffolds is indicated by sensitivity to collagenase digestion. hBMSCs encapsulated in the mGL scaffolds show high viability and chondrogenesis upon stimulation. Compared with agarose, mGL promotes higher chondrogenic ECM gene expression in hBMSCs, as well as GAG deposition and mechanical property enhancement. Because these properties permit direct injection to deliver scaffolds containing cells and/or biofactors into tissue sites in situ using minimally invasive procedures, the mGL scaffold described here is a clinically promising scaffold for articular cartilage repair.
A wide variety of materials and composites have been studied for the production of bioscaffolds for the repair of articular cartilage lesions. Hydrogels are promising candidates for cartilage because the microenvironment they provide mimics some properties of native cartilage, including a highly hydrated structure that can transmit biological and mechanical cues, favorable capacity for uniform cell loading, and ready conformation to the recipient tissue site geometry.10,11 In addition, hydrogels composed of natural materials may also provide molecular signaling cues that may promote enhanced chondrogenic responses of the encapsulated cells. Most hydrogels, however, lack the required mechanical properties and geometric persistency to repair large articular defects. One solution to this problem is to increase covalent interactions between the hydrogel components through photocrosslinking. We have previously reported a method for VL-based photocrosslinking permitting live-cell encapsulation with PEG hydrogels in the process of projection stereolithography. The resulting cell-laden scaffolds were fabricated with greater than 95% cell viability. While PEG is biocompatible, it is relatively inert and does not support the induction of chondrogenesis as natural matrix macromolecules. Thus, the aim of this study was to assess the utility of the VL photocrosslinking method we have recently developed in producing constructs based on gelatin, a more bioactive material, for the purpose of articular cartilage wound repair.
The importance of uniformly encapsulating cells within prospective scaffolds for tissue repair has been demonstrated.23 Integral to this is the need to limit cytoxicity and cell stress during scaffold fabrication. Cells are easily mixed in hydrogels, but the commonly used means of crosslinking using UV light and electron donors, such as VA-086,24 I2959,17 Darocur TPO,25 and Eosin Y/TEA/VPL,26 are all cytotoxic to varying extents, and the stress experienced by the surviving cells may delay, alter, or inhibit resident cell differentiation. Moreover, these methods are very oxygen sensitive, and thus require a barrier, such as glass coverslip, to block exposure to air during the gelation process, thus presenting technical complications to in situ gelate and are incompatible with microinjection to deliver scaffolds with cells or bioactive factors to tissue sites. When the protective barrier is not employed, the curing time of these methods greatly increases, for example, up to 12 min as shown here, greatly impairing cell viability due to long UV exposure (Fig. 1B). In contrast, the VL-crosslinked mGL tested here quickly gelates without the use of a cover, maintains a high percentage of viable cells, and enhances hBMSC chondrogenesis compared with agarose encapsulation, a commonly employed hydrogel for the study of chondrogenesis.
The importance of space filling and geometric persistence is vital to implant's success. For example, collagen gels loaded with MSCs have been shown to contract dramatically during differentiation. Gelatin retains many native collagen epitopes that may support chondrogenesis while forming a stable gel. mGL crosslinked via LAP and VL for 4 min attains a compressive modulus comparable to that produced by I2959-initiated UV crosslinking of 10% mGL, indicating that a similar level of crosslinking has been achieved. Mechanically, constructs prepared using 10% mGL have a Young's modulus of 26 kPa, lower than that of 2% agarose constructs prepared in this study (40 kPa). It is noteworthy that these values are considerably lower than the 1–2 MPa modulus of native cartilage, severely limiting the utility of the scaffolds in their current formulation for application to articular cartilage repair. Interestingly, whereas the Young's modulus of agarose constructs decreases with time, mGL-based constructs increase their strength and retain their geometry, and upon placement into native osteochondral tissue show enhanced physical and possible biochemical interaction with the host tissue. Maintenance of construct geometry is indirectly tested here using a scaffold integration test employing an in vitro cartilage repair model, in which space filling of defects and subsequent integration of neo-tissue with host tissue can be measured as a function of the force required to push out the implant. In addition, in both in vitro and in situ articular-cartilage-simulated wound repair models, the same level of crosslinking is achieved when the process of gelation is performed when the process is carried out submerged in aqueous buffer (not shown), a feature that addresses an important requirement in developing an athroscopic application of this technique.
In summary, the VL-activated hydrogel described in this study has many of the properties desirable for an injectable scaffold suitable for articular cartilage repair. These include (i) uniform cell distribution at the time of gelation/polymerization and, in particular, delivery of sufficient numbers of viable cells undamaged by harsh chemistry or UV light to enable controlled, robust neo-tissue formation; (ii) biocompatibility and chondrogenic stimulatory capacity through its matrix epitope availability and/or growth factor delivery; (iii) controlled time and degree of gelation to facilitate defect filling in situ; and (iv) a stable, persistent construct geometry during long-term differentiation. These properties strongly suggest that the scaffold described here should be more suitable for in situ cartilage and even osteochondral repair than many of the hydrogels currently in use. We will further examine the applicability of this novel scaffold for cell-based articular cartilage repair in vivo. Technical optimization and material modifications to this formulation are also being explored to enhance the immediate mechanical properties of the scaffold while retaining its chondrogenic and injectable capacities.
Conclusions
We have developed a VL-based crosslinking procedure for the preparation of mGL hydrogel. This method allows rapid gelation in air or aqueous solution without any protective barrier, and hBMSC encapsulation with high viability. The mGL scaffolds are injectable, biodegradable, and support efficient chondrogenic differentiation of hBMSCs. Compared with agarose, hBMSC-laden mGL constructs promote enhanced integration between grafts and native cartilage tissue. The mGL scaffold produced by means of VL-mediated crosslinking is thus a promising scaffold for clinical repair of cartilage defects.
Supplementary Material
Acknowledgments
The authors gratefully thank Dr. Jian Tan for preparation of the hBMSCs. This work is supported in part by the National Institutes of Health (U18TR000532), the Commonwealth of Pennsylvania Department of Health (SAP4100050913), and the U.S. Department of Defense (W81XWH-08-2-0032 and W81XWH-10-1-0850).
Disclosure Statement
No competing financial interests exist.
References
- 1.Tuli R., Li W.J., and Tuan R.S.Current state of cartilage tissue engineering. Arthritis Res Ther 5,235, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buckwalter J.A., Martin J.A., and Brown T.D.Perspectives on chondrocyte mechanobiology and osteoarthritis. Biorheology 43,603, 2006 [PubMed] [Google Scholar]
- 3.Goldring M.B.Update on the biology of the chondrocyte and new approaches to treating cartilage diseases. Best Pract Res Clin Rheumatol 20,1003, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Lethbridge-Cejku M., Helmick C.G., and Popovic J.R.Hospitalizations for arthritis and other rheumatic conditions—Data from the 1997 National Hospital Discharge Survey. Med Care 41,1367, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Anderson D.D., Chubinskaya S., Guilak F., Martin J.A., Oegema T.R., Olson S.A., and Buckwalter J.A.Post-traumatic osteoarthritis: improved understanding and opportunities for early intervention. J Orthop Res 29,802, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lotz M.K., and Kraus V.B.New developments in osteoarthritis. Posttraumatic osteoarthritis: pathogenesis and pharmacological treatment options. Arthritis Res Ther 12,211, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farr J., Cole B., Dhawan A., Kercher J., and Sherman S.Clinical cartilage restoration: evolution and overview. Clin Orthop Relat Res 469,2696, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noth U., Steinert A.F., and Tuan R.S.Technology insight: adult mesenchymal stem cells for osteoarthritis therapy. Nat Clin Pract Rheumatol 4,371, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Kuo C.K., Li W.J., Mauck R.L., and Tuan R.S.Cartilage tissue engineering: its potential and uses. Curr Opin Rheumatol 18,64, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Tuan R.S., Boland G., and Tuli R.Adult mesenchymal stem cells and cell-based tissue engineering. Arthritis Res Ther 5,32, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spiller K.L., Maher S.A., and Lowman A.M.Hydrogels for the repair of articular cartilage defects. Tissue Eng Part B Rev 17,281, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Selmi T.A., Verdonk P., Chambat P., Dubrana F., Potel J.F., Barnouin L., and Neyret P.Autologous chondrocyte implantation in a novel alginate-agarose hydrogel: outcome at two years. J Bone Joint Surg Br 90,597, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Ochi M., Uchio Y., Kawasaki K., Wakitani S., and Iwasa J.Transplantation of cartilage-like tissue made by tissue engineering in the treatment of cartilage defects of the knee. J Bone Joint Surg Br 84B,571, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Lange J., Follak N., Nowotny T., and Merk H.[Results of SaluCartilage implantation for stage IV chondral defects in the knee joint area]. Unfallchirurg 109,193, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Sharma B., Fermanian S., Gibson M., Unterman S., Herzka D.A., Cascio B., Coburn J., Hui A.Y., Marcus N., Gold G.E., and Elisseeff J.H.Human cartilage repair with a photoreactive adhesive-hydrogel composite. Sci Transl Med 5,167ra6, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leslie-Barbick J.E., Shen C., Chen C., and West J.L.Micron-scale spatially patterned, covalently immobilized vascular endothelial growth factor on hydrogels accelerates endothelial tubulogenesis and increases cellular angiogenic responses. Tissue Eng Part A 17,221, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fairbanks B.D., Schwartz M.P., Bowman C.N., and Anseth K.S.Photoinitiated polymerization of PEG-diacrylate with lithium phenyl-2,4,6-trimethylbenzoylphosphinate: polymerization rate and cytocompatibility. Biomaterials 30,6702, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin H., Zhang D.N., Alexander P.G., Yang G., Tan J., Cheng A.W.M., and Tuan R.S.Application of visible light-based projection stereolithography for live cell-scaffold fabrication with designed architecture. Biomaterials 34,331, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahsan T., and Sah R.L.Biomechanics of integrative cartilage repair. Osteoarthritis Cartilage 7,29, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Van den Bulcke A.I., Bogdanov B., De Rooze N., Schacht E.H., Cornelissen M., and Berghmans H.Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules 1,31, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Nichol J.W., Koshy S.T., Bae H., Hwang C.M., Yamanlar S., and Khademhosseini A.Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 31,5536, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Djouad F., Rackwitz L., Song Y., Janjanin S., and Tuan R.S.ERK1/2 activation induced by inflammatory cytokines compromises effective host tissue integration of engineered cartilage. Tissue Eng Part A 15,2825, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmidt J.J., Rowley J., and Kong H.J.Hydrogels used for cell-based drug delivery. J Biomed Mater Res A 87A,1113, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Rouillard A.D., Berglund C.M., Lee J.Y., Polacheck W.J., Tsui Y., Bonassar L.J., and Kirby B.J.Methods for photocrosslinking alginate hydrogel scaffolds with high cell viability. Tissue Eng Part C Methods 17,173, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Castelvetro V., Molesti M., and Rolla P.UV-curing of acrylic formulations by means of polymeric photoinitiators with the active 2,6-dimethylbenzoylphosphine oxide moieties pendant from a tetramethylene side chain. Macromol Chem Phys 203,1486, 2002 [Google Scholar]
- 26.Cruise G.M., Hegre O.D., Scharp D.S., and Hubbell J.A.A sensitivity study of the key parameters in the interfacial photopolymerization of poly(ethylene glycol) diacrylate upon porcine islets. Biotechnol Bioeng 57,655, 1998 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.