Abstract
Small-interfering RNA (siRNA) is both a powerful tool in research and a promising therapeutic platform to modulate expression of disease-related genes. Malignant tumors are attractive disease targets for nucleic acid-based therapies. siRNA directed against oncogenes, and genes driving metastases or angiogenesis have been evaluated in animal models and in some cases, in humans. The outcomes of these studies indicate that drug delivery is a significant limiting factor. This review provides perspectives on in vivo validated nanoparticle-based siRNA delivery systems. Results of recent advances in liposomes and polymeric and inorganic formulations illustrate the need for mutually optimized attributes for performance in systemic circulation, tumor interstitial space, plasma membrane, and endosomes. Physiochemical properties conducive to efficient siRNA delivery are summarized and directions for future research are discussed.
Keywords: Gene silencing, siRNA delivery, Tumor accumulation and penetration, Cell uptake, Endosome escape, Liposomes, Polymeric nanoparticles, Inorganic nanoparticles
Introduction
First discovered in plants, RNA interference (RNAi) is a naturally occurring phenomenon. This form of post-transcriptional gene silencing results in either direct cleavage via small-interfering RNA (siRNA) or translational repression via microRNA [1]. Molecular mechanisms of RNAi have been reviewed elsewhere [2–4]. Cleavage of one target mRNA does not consume the guide strand. Hence, a few siRNA molecules introduced into cytoplasm can lead to complete or significant degradation of target mRNA. Fire et al. first demonstrated RNAi experimentally by introducing double-stranded RNA (dsRNA) into Caenorhabditis elegans in 1998 [5]. Shortly thereafter, Elbashir et al. demonstrated that synthetic siRNA could silence endogenous genes in mammalian cells [6]. The first successful RNAi in mice was conducted using siRNA-targeting the polymerase-encoding region of the hepatitis C viral genome [7]. These early studies established the foundation for subsequent rapid expansion of research in transforming RNAi as research tools to clinical therapeutics [8–10]. Unlike antisense oligodeoxynucleotides, siRNA operates exclusively in cytosol and nuclear import is not needed. The non-consuming feature of siRNA silencing should result in superior pharmacological potency.
Despite operating on enzymatic efficiency, development of siRNA into therapeutics is limited by several intrinsic properties. siRNA have been shown to cause cellular and physiological toxicities [11]. RNAi may interfere with endogenous microRNA pathways because both compete for the same RNAi machinery [12, 13]. siRNA may exert “microRNA-like” off target effects due to partial complementarity [14]. siRNA may induce innate immune responses in sequence dependent or independent manners [15, 16]. These biological limitations could be circumvented or minimized by careful screening of sequences or with chemical modifications. Advances in these efforts have been reviewed elsewhere [17].
A second hurdle is poor delivery efficiency in vivo. This can be addressed through chemical modifications and the use of carriers or delivery systems [18]. Soutschek et al. were the first to demonstrate silencing of an endogenous gene via systemic administration of chemically modified siRNA [19]. Cholesterol conjugated siRNA targeting apoB degraded the apoB mRNA effectively in mouse liver and jejunum. The accumulation of cholesterol-siRNA in liver was facilitated by binding to human serum albumin (HSA). Delivery systems have been designed to improve efficacy. apoB-specific siRNA encapsulated in stable nucleic acid-lipid particles (SNALP) reduced both apoB mRNA and protein in cynomolgus monkey by 80 % [20]. These early in vivo evidences raised expectations in ultimately transforming siRNA into drugs. Currently, more than 10 siRNA-based products are being tested in clinical trials, mainly in phases I and II (Table 1). These include treatments for age-related macular degeneration (AMD), kidney injury, and liver fibrosis, although most are directed at various forms of cancer.
Table 1.
siRNA therapeutics in clinical trials
| Sponsor | Disease | Target | Delivery system | Route | Status (identifier #) |
|---|---|---|---|---|---|
| Silenseed Ltd. | Pancreatic cancer; PDA | Kras oncogene G12D | Biodegradable polymeric matrix |
Intratumor injection |
Phase 1 NCT01188785 Phase 2 NCT01676259 |
| M.D. Anderson Cancer Center |
Advanced cancers | EphA2 | Neutral liposomes | IV | Phase 1 NCT01591356 |
| National Cancer Institute |
Hepatic metastases | PLK1 | Lipid nanoparticles | IV | Phase 1 NCT01437007 |
| Silence Therapeutics AG |
Pancreatic ductal carcinoma | PKN3 | Pegylated cationic liposomes |
IV | Phases 1 and 2 NCT01808638 |
| Silence Therapeutics AG |
Advanced solid tumors | PKN3 | Pegylated cationic liposomes |
IV | Phase 1 NCT00938574 |
| Duke University | Metastatic Melanoma | Immunoproteasome subunits beta type 9, 8, 10 |
Transfected dendritic cells |
Intradermal injection |
Phase 1 NCT00672542 |
| Hadassah Medical Organization |
Chronic Myeloid Leukemia | Bcl2 | Simian virus 40 | In vitro WBC from patients |
NA NCT00257647 |
| Quark Pharmaceuticals |
CNV Diabetic retinopathy DME |
RTP801 | Saline | Intravitreal injection |
Phase 2 NCT01445899 |
| Quark Pharmaceuticals |
Optic atrophy NAION |
Caspase-2 | Saline | Intravitreal injection |
Phase 1 NCT01064505 |
| Opko Health, Inc | DME Macular degeneration |
VEGF | Saline | Intravitreal injection |
Phase 2 NCT00306904 NCT00259753 |
| Opko Health, Inc | Macular degeneration | VEGF | Saline | Intravitreal injection |
Phase 2 NCT00259753 |
| Sylentis, S.A. | Ocular pain Dry eye syndrome |
Capsaicin receptor TrpV1 |
Ophthalmic drops | Topical | Phase 1 and 2 NCT01438281 NCT01776658 |
| Allergan | AMD, CNV | VEGFR1 | Saline | Intravitreal injection |
Phase 1 and 2 NCT00363714 |
| Sylentis, S.A. | Ocular hypertension Glaucoma |
β-2 adrenergic receptor | Ophthalmic drops | Topical | Phase 1 and 2 NCT01227291 NCT01739244 |
| Quark Pharmaceuticals |
Kidney injury Acute renal failure |
p53 | Saline | IV | Phase 1 NCT00554359 |
| Quark Pharmaceuticals |
Delayed graft function and complication of kidney transplant |
p53 | Saline | IV | Phase 1 and 2 NCT00802347 |
| Nitto Denko Corporation |
Liver fibroses | Hsp47 | Vitamin A-coupled lipid nanoparticles |
IV | Phase 1 NCT01858935 |
| Pachyonychia Congenita Project |
Pachyonychia congenita | Keratin K6a | Injection into a callus |
Phase 1 NCT00716014 |
Information is confirmed on March 6, 2014 from clinicaltrials.gov. Those listed as “terminated” are not included
PDA pancreatic ductal adenocarcinoma; CNV choroidal neovascularization; DME diabetic macular edema; NAION non-arteritic anterior ischemic optic neuropathy; AMD age-related macular degeneration; EphA2 ephrin type-A receptor 2; PLK1 polo-like kinase 1; PKN3 protein kinase N3; RRM2 ribonucleoside-diphosphate reductase subunit M2; Bcl-2 B cell lymphoma 2; RTP801 also known as Redd1; VEGF vascular endothelial growth factor; TrpV1 receptor potential cation channel subfamily V member 1; VEGFR1 vascular endothelial growth factor receptor 1; Hsp 47 heat shock protein 47; Tf transferrin; CDP cyclodextrin-containing polymer; IV intravenous injection; WBC white blood cells; NA not applicable
Cancer initiation, progression, and metastasis are associated with mutated and/or abnormally expressed genes [21]. Many of these genes encode proteins that are thought to be “undruggable” using small molecules and monoclonal antibodies [22]. Traditional small molecules have a limited range of chemical interactions in multi-domain kinases while monoclonal antibodies can only approach extracellular targets. Targeting upstream messenger RNA (mRNA) by siRNA appeared to be the next logical strategy. Indeed, effective in vivo gene silencing and tumor growth inhibition have been reported by delivering siRNA targeting mRNAs of oncogenes. Results gleaned from these studies indicate that pharmacokinetics is a critical attribute of efficacy. For advanced metastatic cancers, siRNA must circulate in the blood stream sufficiently long enough to allow partition into tumors.
Nanoparticles have been employed in cancer drug delivery for decades with several products approved by the FDA [23, 24]. Nanoparticles accumulate in tumor tissues selectively by leaky vasculature and impeded lymphatic drainage underpinning the enhanced permeation and retention (EPR) effect [25]. The circulating half-lives and tissue distribution of nanoparticles can be tuned by manipulating size and surface properties. Most commonly, this is accomplished by polyethylene glycol (PEG) coating and ligand incorporation [26]. This narrative highlights the technical challenges and focuses on systems with demonstrated efficacy in animals or humans.
Physiological Barriers to Particle-Based siRNA Delivery
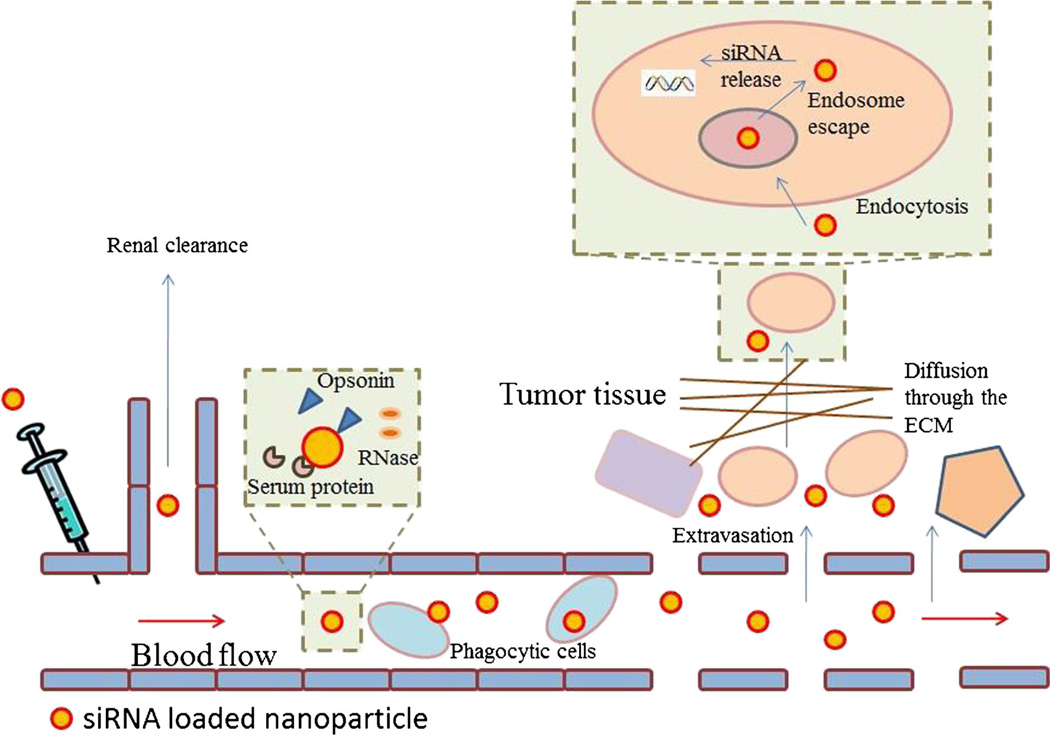
Nanoparticle formulations are meant to overcome several physiological barriers of siRNA trafficking (Fig. 1). In plasma, siRNA is rapidly removed by RNase and opsonization. siRNA must be protected from phagocytic cells residing in liver, spleen, and lung, collectively known as the mononuclear phagocyte system (MPS), which eliminates particulates from circulation [27]. Engineering a hydrophilic and surface with near-neutral zeta potentials [28, 29] and particle size between 10 to 150 nm appear to minimize MPS removal [29, 30]. Below 10 nm, the particles tend to be eliminated rapidly through renal clearance [29]. Long-circulating particulates have been developed to stay in blood circulation for an extended period of time. Therefore, the particles would cross the vascular endothelium and selectively accumulate in tumor via the EPR effect before they are cleared from circulation [18, 25].
Fig. 1.
Biological barriers to siRNA-particles trafficking. Extracellular barriers RNase degradation, opsonization, macrophage uptake, renal clearance, extravasation, extracellular matrix (ECM) trapping, and internalization. Intracellular barriers endosome escape and siRNA release
Once extravasated to tumors, nanoparticles would have to diffuse through the extracellular matrix (ECM), a dense network consisting polysaccharides and fibrous proteins. ECM retards the diffusion of particulates via size-dependent entanglement and electrostatic interactions [31]. The hydrophilic (electro-negative) glycocalyx and hydrophobic lipid bilayer of cell membrane resist binding and entry of siRNA. Having a slightly positive surface and incorporation of a receptor ligand facilitates uptake of nanoparticles into cells [32]. But endocytosed substances are typically channeled for lyso-endosomal degradation [33]. Three major strategies have been proposed to facilitate endosome escape. Cationic lipids interacting with anionic endosome membrane electrostatically destabilize the enclosure by inducing the formation of inverted hexagonal phase [34, 35]. pH buffering materials such as polyethyleneimine (PEI) and polymers containing imidazole induce osmotic swelling and rupture of the endosome via influx of chloride ions, commonly referred to as the “proton sponge” effect [36, 37]. Synthetic fusogenic peptides, mimicking viral fusion domains, have also been utilized to enhance endosome escape [38]. Once escaped from endosomes, nanoparticles must release siRNA into the cytoplasm in order for RNA-induced silencing complex (RISC) assembly and subsequent hybridization with target mRNA to take place. Therefore, an ideal nanoparticle siRNA delivery system must be mutually optimized in siRNA complexation, accumulation and penetration in tumor, cellular uptake, endosome escape, and siRNA release. These can be achieved through the manipulation of material chemistry, hydrodynamic size, and surface properties. This review focused on examples of siRNA-nanoparticle systems that have been validated in animals or in some cases, humans.
Lipid-Based Systems
Various forms of lipid-based nanoparticles have been developed for systemic delivery of siRNA into tumors. These include liposomes, solid-lipid nanoparticles (SLN), and reconstituted high-density lipoprotein (rHDL) nanoparticles. Examples of each are discussed highlighting physiochemical composition and in vivo performance.
Cationic Liposomes
Liposomes are one of the first nucleic acid delivery systems characterized, dating back to the late 1980s [39, 40]. Cationic liposomes have been extensively investigated owing to their high encapsulation efficiency, effective transfection, and ease of surface modification [23]. Li et al. first developed a liposomal system with a solid core, termed lipid-protamine-DNA (LPD) [41]. Protamine and calf DNA were used to complex with siRNA. This complex is then encapsulated within cationic liposomes made of 1,2-dioleoyloxy-3-trimethylammonium propane (DOTAP) and cholesterol. The liposomes are further modified with two other lipids: 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-PEG (DSPE-PEG) and DSPE-PEG-anisamide (DSPE-PEG-AA). DSPE-PEG prevents aggregation and macrophage uptake, while DSPE-PEG-AA promotes cellular internalization via the sigma receptor over-expressed on tumor cells. Tissue distribution studies showed that tumor-bearing mice cleared the LPD nanoparticles from circulation more rapidly than tumor-free mice. More than 70 % of injected siRNA were found in tumors 4 h after a single-tail vein injection [41]. The impressive tumor accumulation is attributed to high surface coverage of DSPE-PEG which shields the particle charge effectively [42]. The compact protamine-nucleic acids core enhances structural integrity of the inner lipid layer, thereby allowing stable display of DSPE-PEG on the exterior. Coating the liposomes with anisamide has no effect on tumor extravasation but enhances cellular uptake; compared to 5 % for nanoparticles without the ligand, siRNA presented in 30 % of cells when delivered using the anisamide nanoparticles [41].
In a subsequent design, calf DNA in LPD was replaced with hyaluronic acid to reduce immunogenicity [43, 44]. Another change was replacing protamine with a recombinant tetrameric form of the histone protein H2A (TH) in order to improve de-complexation [45]. TH is degraded in endosomes by cathepsin D, an endogenous protease, resulting in release of siRNA. A single intravenous injection (0.25 mg/kg) reduced expression of a target gene (luciferase) by 66 % in the H460 human lung cancer model. The hyaluronic acid-TH liposomes are noted for significantly improved silencing efficiency compared to previous designs. Zhang et al. and Li et al. also developed a liposome formulation with calcium phosphate [46, 47]. In this format, siRNA is complexed with calcium phosphate into a cationic solid core before coating with the anionic dioleoylphosphatidic acid (DOPA). Other lipids, DOTAP, DSPE-PEG, and DSPE-PEG-AA, are added to the initial DOPA complex to form the final liposomes. Calcium phosphate dissolves immediately upon exposure to the acidic pH (5.5 to 6.5) in endosomes; this results in the swelling and bursting of the vesicles and release of siRNA (decomplex upon dissolution of calcium phosphate) into the cytoplasm [46].
Santel et al. have developed a liposomal siRNA formulation using a cationic lipid AtuFECT01, a neutral fusogenic helper lipid 1,2-diphytanoyl-sn-glycero-3-phosphoethanolamine (DPhyPE), and DSPE-PEG [48]. The enhanced uptake by vascular endothelial cells, attributed to the positive charges of cationic lipids, suggests a potential novel anti-angiogenic therapy [48]. Incorporation of siRNA-targeting protein kinase N3 (PKN3) into these liposomes resulted in inhibition of primary tumor growth and lymph node metastases in human prostate cancer and pancreatic cancer models in mice [49]. This formulation is currently in a phase I clinical trial.
Pirollo et al. have formulated a transferrin receptor (TfR)-targeted liposomal system, composed of DOTAP and 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) [50]. This design is unique in that DOPE is conjugated with a pH-sensitive histidinylated oligolysine (HoKC). The protonated lysine side chains render positive charges for siRNA complexation, while the imidazole groups enhance endosome release through pH buffering [51]. Efficient delivery of a fluorescent dye-labeled siRNA was demonstrated in both primary tumors and lung metastases. When administrated into mice carrying human pancreatic carcinoma (PANC-1), the cancer was found sensitized for apoptosis by gemcitabine [50].
Sato et al. synthesized a pH-sensitive cationic lipid, YSK05, for siRNA delivery [52, 53]. YSK05 contains an N-methyl piperidine ring, which becomes protonated only in acidic endosomes. In addition, the limited pegylation facilitates the interactions between fusogenic lipids and endosome membrane. The liposome formulation consists of YSK05, cholesterol, 1,2-distearoyl-sn-glycero-3-phosphatidylcholine (DSPC), PEG-dimyristoyl-sn-glycerol (PEG-DMG). When polo-like kinase 1 (PLK1) siRNA was delivered using the system, a single intravenous injection at 3 mg/kg resulted in 50 % reduction in target mRNA and proteins in a human renal cell cancer model [53].
Sonoke et al. used pegylated liposomes made of palmitoyl-oleoylphosphatidylcholine (POPC) and a cationic lipid CLZ-42 to deliver B cell lymphoma 2 (Bcl-2)-targeting siRNA [54]. Treated mice showed slower tumor growth and survived longer. Liver uptake was found dependent upon the length of the acyl chains in phosphatidylcholine (PC) conjugated to PEG (PEG-PC). Shorter (C-12 to C-16) and unsaturated acyl chains results in higher liver uptake compared to longer (C-17 and C-18) and saturated acyl chains. PC with longer acyl chain (C-18) have higher gel-to-liquid-crystalline temperature (Tc), resulting in rigid liposomes that might resist internalization by liver resident macrophages [54].
Li et al. reached similar conclusions regarding the effects of lipids projecting PEG. SNALP modified by PEG-lipids with different lipid chain lengths (C14, C16, or C18) showed different gene-silencing efficiencies in vitro and in vivo [55]. With a shorter lipid anchor, PEG dissociated rapidly from SNALP, resulting in stronger gene-silencing effect in vitro but short plasma half-life in vivo. Thus, increasing the length of the lipid anchor may prolong systemic circulation. On the other hand, pegylation can be counterproductive; the steric PEG brush can impede fusion between cationic lipids and anionic endosomal membrane [56]. This can be circumvented by using an acid-labile linker as a mechanism to shed PEG from in endosomes. Another method was proposed by Auguste et al. in using a block polymer (PEG-DMA) composed of PEG and cationic poly(2-(dimethylamino)ethyl methacrylate) (DMA) [57]. 1,2-dioleoyl-3-dimethylammonium-propane (DAP), which would be protonated only at acidic pH, is used to form liposomes with 1,2-dioleoyl-sn-glycero-3-phosphatidylcholine (DOPC). PEG-DMA is subsequently adsorbed onto the DOPC-DAP liposomes to extend circulation. In acidic endosomes, the repulsive forces between protonated DAP and DMA shed PEG-DMA from the liposomes. Superior performance of the PEG-DMA coated DOPC-DAP liposomes was evidenced by enhanced green fluorescence protein (GFP) gene knockdown in vitro [57]. In another study, Koren et al. conjugated PEG to 1,2-dipalmitoyl-sn-glycero-3-phosphatidylethanolamine (DPPE) with the pH-sensitive hydrazone bond. Desorption of PEG in acidic pH resulted in exposure of cell-penetrating peptide moieties [58]. Therapeutic benefits of these systems remain to be investigated in vivo.
Gene silencing may be decoupled from vesicle uptake into cells. In addition to endocytosis, siRNA can be directly released into the cytoplasm through fusion of the cell membrane and liposomal lipids [59]. Blocking clathrin, caveolin, and lipid raft-mediated endocytosis have no apparent effects on gene silencing efficiency. While depleting cholesterol does not affect cellular uptake, gene silencing can be compromised as a function of temperature [59]. Evidences are accumulating to suggest that endocytosis is responsible for cellular uptake of the majority of siRNA, but gene silencing may depend on other processes. Consequently, intracellular trafficking of siRNA-loaded lipid particles became the focus of several studies [60, 61]. Sahay et al. found out that exocytosis diminishes delivery efficiency [60]. Gilleron et al. have estimated that only 1–2 % of siRNA actually reached the cytosol [61]. Additional studies are undergoing to resolve the fate of siRNA after liposome internalization.
Cationic liposomes are associated with toxicities in humans. These include inflammation, hepatotoxicity, and hematologic toxicity [62, 63]. Recent studies also indicated that cationic lipids enhance siRNA-mediated innate immune responses. Ma et al. has demonstrated that intravenous injection of DOTAP-siRNA complex induces types 1 and 2 interferons in mice while DOTAP or siRNA injected separately were largely inert [64]. The authors hypothesized that macrophages are sensitized by the DOTAP lipids to respond to siRNA. On the other hand, toxicities associated with cationic lipids may be harnessed for cancer therapy. For instance, two novel lipids N,N-distearyl-N-methyl-N-2-(N0-arginyl) aminoethyl ammonium chloride (DSAA) and N,N-distearyl-N-methyl-N-2[N′-(N2-guanidino-L-lysinyl)] aminoethyl ammonium chloride (DSGLA) designed by Chen et al. contain guanidinium groups that generate reactive oxygen species (ROS). Synergistic antitumor effects with siRNA have been documented with these ROS-generating DSAA and DSGLA-containing liposomes [65, 66].
Neutral Liposomes
Neutral lipids have been used to avoid toxicities associated with cationic lipids. Efficient gene silencing has been shown by liposomes made of the neutral DOPC without surface modification. Delivery efficiency of this liposome has been demonstrated in ovarian carcinoma [67–72], colorectal tumor [73, 74] and melanoma tumor models [75]. In one study, DOPC-derived liposomes were shown to improve accumulation of siRNA in ovarian tumors 10-fold compared to cationic DOTAP-liposomes. DOPC liposomes loaded with EphA2-specific siRNA inhibited tumor growth by 67–82 % after 3 weeks of combination therapy with paclitaxel [67]. This formulation is currently in a phase I clinical trial for advanced cancers. The favorable tumor distribution and uptake is attributed to the near-neutral surface of the liposomes. Plasma circulation is enhanced because macrophages preferentially take up negatively charged particles [76]. A neutral surface enhances distribution within the tumor interstitium because positively charged particles bind to cells in the periphery, thus blocking further distribution [77]. Mechanisms of tumor cell uptake and siRNA release were not reported in these studies. Sustained delivery was demonstrated by combining DOPC liposomes with mesoporous silicon [78]. The electrostatic interactions between cationic mesoporous silicon (+8 mV) and anionic DOPC liposomes (−2.7 mV) provide a mechanism to control siRNA loading and release. A single intravenous injection of silicon-triple dose of EphA2-siRNA DOPC liposomes (15 µg) resulted in specific gene silencing for 3 weeks without significant increase in pro-inflammatory cytokines. This remains the first report of sustained siRNA gene knockdown in vivo.
It is clear that extravasation of siRNA must be accompanied by tumor penetration for efficient gene silencing. Kostarelos et al. have studied liposomal surface charge, mean diameter, and lipid bilayer fluidity on penetration into prostate carcinoma spheroids [77]. They concluded that surface charge inversely correlated with liposome penetration and distribution in tumors. This explains in part the success of liposomes formulated with neutral lipids. Cationic lipids with low surface charge density and PEG coating may provide the same advantage.
Other Lipid-Based Nanoparticle Systems
In addition to liposomes, other lipid-containing particles have been explored. Cationic SLN has been tested for delivering c-Met specific siRNA in an orthotopic glioblastoma model [79]. The SLN consists of cholesteryl oleate, glyceryl trioleate, DOPE, cholesterol, and 3β-[N-(N′,N′-dimethylaminoethane)-carbamoyl]-cholesterol (DC-cholesterol). siRNA is conjugated to PEG through a disulfide bond and loaded onto the SLN via electrostatic interactions. These SLN crossed the blood brain barrier (BBB) and accumulated in tumors 48 h after intravenous injection. Downregulation of c-Met and tumor growth inhibition appeared to be dose-dependent [79]. rHDL nanoparticles represent another strategy. Tumors over-express type B1 scavenger receptors to capture HDL needed for hyper-proliferation. Shahzad et al. developed rHDL nanoparticles containing 4 mg of siRNA complexed with oligolysine [80]. These rHDL-siRNA nanoparticles, 10 nm in diameter and a charge-neutral surface, have been demonstrated effective in models of ovarian tumors colorectal metastases. While liver expresses high levels of scavenger receptors for HDL, hepatic toxicities were not observed [80].
Wolfrum et al. have investigated the delivery mechanism of a lipid-conjugated siRNA in mice [81]. They found that siRNA conjugated to cholesterol, bile acids, and long-chain fatty acids effectively knocked down liver apoB mRNA. The high transfection efficiency of the lipid-conjugated siRNA is attributed to spontaneous association with endogenous lipoproteins and subsequent internalization through hepatic receptors. The lag time between infusion and binding to endogenous lipoproteins makes siRNA vulnerable to elimination. Pre-assembled siRNA and lipoproteins (high- or low-density lipoproteins) may improve siRNA delivery [81].
Polymeric siRNA Delivery Systems
The use of non-lipidic cationic polymers for nucleic acid delivery dated back to the early 1990s [82]. Nano-sized particles are generated from electrostatic interactions between siRNA and synthetic polymers that include cyclodextrin derivatives, PEI, and polyamidoamine (PAMAM). In addition, naturally occurring polymers, proteins, and oligopeptides have been explored owing to their biodegradable and biocompatible properties. Molecular weight, charge density, and chemical structure of the polymer are known parameters affecting delivery efficiency [83].
Synthetic Polymers
Cyclodextrin-Containing Polymers
Davis and coworkers have constructed a siRNA delivery system around β-cyclodextrin (β-CD) [84]. The cyclodextrin-containing polymer (CDP) comprises four functional units: docking cavity (β-CD), pH buffering (imidazole), charge center (amidine), and spacer (methylene). A therapeutic formulation consisting of ribonucleoside-diphosphate reductase subunit M2 (RRM2) siRNA, CDP, adamantane-conjugated PEG (AD-PEG), and AD-PEG-transferrin (AD-PEG-Tf) appeared to be effective RNAi in patients [85]. However, the phase I safety study was terminated recently. Upon mixing, the components self-assemble into nanoparticles. siRNA is complexed via electrostatic interactions with protonated amidines while the imidazoles facilitate endosome escape AD-PEG and AD-PEG-Tf complex with β-CD through inclusion of AD in the cyclodextrin cavity [84]. These PEG form a thick brush layer (12.5 nm) on the surface that stabilizes the nanoparticles as colloids [84]. Structure-activity relationships of CDP siRNA systems have been thoroughly studied [86–89]. The average spacing between β-CD and amidine charge centers is important as the former may block interactions between the charged centers and siRNA. The distance between individual charge centers is also important. Increasing the distance decreases toxicity, but at the expense of siRNA-binding efficiency. Similar to liposomal systems, addition of targeting ligands does not alter tissue distribution but significantly improves cellular uptake and therapeutic efficacy in vivo [90, 91].
Polyethyleneimine
PEI is proven to be nucleic acid carriers owing to their high transfection efficiencies in vitro [92, 93]. The primary amines capture nucleic acids through electrostatic interactions while the tertiary amines mediate endosome escape. PEI nanoparticles are internalized by caveolae or clathrin-dependent endocytosis. Different routes of endocytosis lead to different intracellular trafficking of PEI-siRNA polyplexes [94]. The fraction internalized via caveolae circumvents lyso-endosomal degradation [94]. Those taken up by clathrin-mediated endocytosis might still escape endosomes through the “proton sponge” effect. Limitations of PEI include the propensity to aggregate, slow release of siRNA, immune cells activation, and cytotoxicity [95]. PEI has been modified chemically to improve delivery attributes. Internalization mechanism, transfection efficiency, and toxicities can be tuned by manipulating configuration (linear or branched) and molecule weight. For instance, in HUH-7 carcinoma cells, linear PEIs are internalized mainly by clathrin-mediated endocytosis while branched PEIs are endocytosed via both clathrin and caveolae [96]. Compared to linear PEIs, branched PEIs exhibit higher transfection efficiencies [93]. Both efficiency and toxicity increase with higher molecular weight [97]. PEIs employed in siRNA delivery typically have molecular weights between 5 to 25 kDa. Werth et al. have developed a 4–10 kDa branched PEI (PEI F25-LMW) [98]. PEI and vascular endothelial growth factor (VEGF) siRNA complexed at a high N/P ratio (33) were injected intraperitoneally. Synergistic therapeutic effects were observed when combined with bevacizumab in a prostate carcinoma model [99]. In addition, efficient knockdown of specific genes and tumor growth inhibition have been shown in glioblastoma [100] and colon carcinoma [101] models.
Optimization of PEI includes N-acylation [102], cross-linking low MW PEI with degradable bonds [103–105], ligand incorporation [106, 107], carbohydrate attachment [108], lipid conjugation [109–113], and pegylation [114]. Kim et al. modified a branched PEI with the integrin binding peptide Arg-Gly-Asp (RGD) through a PEG spacer [115]. At an N/P ratio of 10, the resulting nanoparticles loaded with vascular endothelial growth factor receptor 1 (VEGFR1) siRNA inhibited the growth of murine colon adenocarcinoma for 11 days. Schiffelers also reported on the inhibition of tumor angiogenesis and growth through intravenous administration of vascular endothelial growth factor receptor 2 (VEGFR2) siRNA PEI nanoparticles grafted with RGD via PEG [116]. Zheng et al. synthesized an amphiphilic PEI-polycaprolactone-PEG triblock copolymer. This polymer renders fourfold higher exposure of siRNA in mice [117]. In another design, pegylated siRNA form nano-sized micelles with PEI [118]. These micelles contain siRNA and PEI as the inner core stabilized by a PEG outer shell. Both intravenous and intratumoral injection resulted in VEGF knockdown and tumor growth inhibition in mice. Shim et al. designed acid-degradable ketalized linear PEI (KL-PEI) as siRNA delivery systems [119]. KL-PEI and siRNA self-assemble into nanoparticles, which are then coated with PEG through chemical conjugation to surface primary amines. The siRNA cargo is released when the ketal branches are hydrolyzed in endosomes. A single intravenous injection with 20-µg siRNA was able to silence the target gene in vivo. In another example, a matrix metalloproteinase 2 (MMP-2) sensitive copolymer (PEG-pp-PEI-PE) was synthesized [120]. This copolymer self-assembles into micelles and siRNA can be loaded via electrostatic interactions with PEI. Up-regulated MMP-2 in tumor tissue sheds PEG, which exposes PEI for enhanced cell uptake. The micelles were able to deliver paclitaxel and siRNA into 14.4 % of tumor cells after intravenous injection.
An important consideration for PEI-nucleic acid polyplexes is the excess PEI used in many formulations. Boeckle et al. found excess PEI as the major cause of toxicity as a function of dose [121]. Free PEI molecules compete for cellular association and diminish uptake of polyplexes. On the other hand, excess PEI may enhance endosome escape through acid buffering. Contribution by excess PEI content in PEI-siRNA polyplexes should be carefully examined.
Polyamidoamine
PAMAM is an important system in siRNA delivery owing to the ease with which molecule weight and charge density can be manipulated. Surface primary amines bind siRNA and interior tertiary amines mediate endosome escape. Freeman et al. constructed an endosome burst model for dendrimers using multiscale modeling with burst threshold set at reaching 5 % critical area strain within 15 min [122, 123]. The likelihood of endosome burst is found determined by the number and pKa of tertiary amines. The fourth-generation (G4) PAMAM, which has 128 tertiary amines with pKa estimated around 6.7, is predicted to induce burst at 1.8 mg/mL [123]. This is attainable with typical doses without intolerable toxicities given the small volume of endosomes [123].
The affinity between siRNA and PAMAM is determined by malleability of the polymer structure [124]. Flexibility facilitates siRNA binding and rigidity weakens the interactions with siRNA. The fifth-generation (G5) PAMAM is favored for siRNA delivery due to its pH-dependent flexibility-rigidity transition. G5 PAMAM can easily accommodate siRNA at neutral pH, but becomes rigid in acidic endosomes to release siRNA. Overhangs in siRNA play a role in G5 PAMAM-mediated gene silencing [125]. Complementary overhangs, (dA)n/(dT)n (n=5 or 7), in siRNA can facilitate formation of concatemers via cross-bridging. Sticky siRNA showed considerably higher gene silencing potency than non-complementary counterparts. Liu et al. synthesized a G5 PAMAM with a triethanolamine (TEA) core that forms stable nanoparticles with “sticky” heat shock protein 27 (Hsp27) siRNA. Potent Hsp27 knockdown was shown in a prostate cancer model after intratumoral injection [126]. Another version of PAMAM is to conjugate with an alkyl chain; this amphiphilic dendrimer has a critical micelle concentration as low as 15 µM. Hsp27 siRNA encapsulated in these PAMAM micelles reduced Hsp expression by 50 % after intratumoral injection. Transfection efficiency can be inhibited by the proton pump inhibitor, bafilimycin A, indicating that the buffering capacity of these PAMAM plays a role [127]. Patil et al. designed an internally cationic G4 PAMAM-OH dendrimer by quaternization of internal tertiary amines and hydroxylation of surface primary amines [128]. This modification enhances siRNA protection and decreases toxicity. The efficiency of delivery depends on degree of quaternization and targeting ligands; in this case, luteinizing hormone releasing hormone (LHRH). At 85 % quaternization, encapsulation and endosome escape appear mutually optimized.
Other Synthetic Polymers
Bioreducible conjugation has been incorporated in siRNA delivery systems. For instance, Chen et al. described polymerized N,N′-cystamine bisacrylamide and modified with N,N′-dimethyldipropylenetriamine (DMDPTA) and cholesterol (rPAA-CH) [129]. Cholesterol mediates endocytosis and DMDPTA facilitates endosome escape by the “proton sponge” effect. Cleavage of the double disulfide bonds results in release of siRNA cargo. In vivo VEGF silence was achieved with 57 % cholesterol grafted. Lin et al. synthesized a pegylated polycaprolactone (PCL)-grafted poly(2-dimethyl)-aminoethylmethacrylate (PDMAEMA) with disulfide linkages (PECssD) [130]. PECssD forms nanoparticles with PEG and PDMAEMA at the surface and PCL as the hydrophobic core. Once internalized, PDMAEMA-siRNA complexes dissociate from the nanoparticles and destabilize the anionic endosome membrane. Tumor growth inhibition of PLK1 siRNA-loaded particles was demonstrated in a Hela-luc xenograft mouse model. Pegylated PCL was conjugated to a cationic cytoplasm-responsive cell penetrating peptide (CHHRRRRHHC; C: Cys; H: His; R: Arg) [131]. The polymer adapts a V shape in forming micelles, which orients hydrophilic PEG and peptide on the surface. Cysteine residues cross-link through disulfide bonds. siRNA can be loaded via cationic arginines, and the cell perpetrating peptides facilitate cell uptake. Histidines mediate endosome escape. siRNA will be released in reductive cytoplasm in which disulfide bonds break. The efficacy of the VEGF siRNA in the micelles was evidenced in that tumor growth was completely suppressed for 8 days.
Natural Polymers
Chitosan
Chitosan is deacetylated chitin, a linear polysaccharide found in exoskeletons of shellfish. Deacetylation generates primary amines that are protonated at physiological pH. Chitosan has a lower charge density and cytotoxicity than PEI [132]. Efficient siRNA delivery is associated with chitosan that weigh 40–150 KDa and are greater than 80 % deacetylated [133]. Han et al. have developed a RGD-tagged chitosan nanoparticles proved effective in a human ovarian carcinoma model [134]. Conjugation of RGD improves tumor localization after intravenous injection. Pille et al. utilized chitosan-coated polyisohexylcyanoacrylate (PIHCA) nanoparticles to deliver siRNA into MDA-MB-31 human breast tumors in nude mice [135]. The cationic surface was used to load a Ras homolog gene family member A (RhoA)-specific siRNA. The results indicated retarded tumor growth and angiogenesis without significant systemic toxicities. Comparable antitumor effects were obtained at a lower dose when the particles were injected intratumorally [136]. Yang et al. modified chitosan with the cell penetrating peptide, transactivator of transcription (TAT) [137]. The resultant TAT-g-CS survivin siRNA was able to inhibit tumor growth and metastasis in a mouse breast cancer model.
Proteins and Oligopeptides
Endogenous proteins and synthetic oligopeptides have been used for delivering siRNA. These include HSA [138], transferrin [139], atelocollagen [140], poly (Pro-Hyp-Gly) [141], cholesteryl oligoarginine [142, 143], and MPG-8, a 21-residue amphipathic peptide [144]. Son et al. introduced thiol groups to both VEGF siRNA and HSA to form disulfide-bonded complexes of the two. This strategy increases the amount of the siRNA in PC-3 prostate tumors by 1.6-fold, with evidence of retarded tumor angiogenesis [138]. The use of an endogenous plasma protein reduces the risk of toxicity. The same group also thiolated transferrin to form nanoparticles with 5′ thiol-modified siRNA. siRNA self-polymerizes through double-sulfide bonds (polysiRNA), and forms nanoparticle with thiolated transferrin. Nanoparticles were internalized via transferrin receptor mediated endocytosis, and were able to silence the target gene (green fluorescence protein) in a mouse melanoma model [139]. Similarly, gelatin was thiolated to deliver thiol-modified polysiRNA [145]. The mechanism of endosome escape was not examined in these studies. Atelocollagen is obtained from pepsin digestion of collagen from calf dermis. This purified type 1 collagen possesses positive charges and forms complexes with siRNA with diameters ranging from 100 to 300 nm [146]. Kawata et al. prepared nanoparticles by mixing PLK1 siRNA and atelocollagen solutions, which inhibited liver metastases effectively after multiple intravenous injections [140]. In another study, arginine-grafted bioreducible poly (disulfide amine) polymers were shown to form nano-sized complexes with siRNA [147]. Reduction of disulfide bonds in acidic endosomes leads to release of the siRNA. Size of these complexes is controlled by the N/P ratio. Efficacy has been tested with siRNAs targeting Bcl-2, VEGF, and Myc in B16-F10 melanoma. Injecting encapsulated siRNA at 0.3 mg/kg intravenously twice led to 50 % growth inhibition of the tumor compared to 15–25 % with naked siRNA treatments [147].
Inorganic siRNA Delivery Systems
Inorganic materials have wide-ranging applications in drug delivery and imaging [148]. These include nanostructures of gold, carbon (nanotubes), cadinum (quantum dots), iron oxide, and calcium phosphate. In general, inorganic substances are biologically inert and afford excellent controls over physical properties [148].
Gold Nanoparticles
Among the inorganic materials, gold nanoparticles (AuNP) are probably the most popular. Such metallic spheres can be fabricated into different sizes and can be thiolated [149]. siRNA can be loaded onto AuNP through gold-thiol chelation or electrostatically by precoating the particles with cationic materials [149]. Internalization of siRNA-loaded AuNP are mediated by scavenger receptors known to endocytose polyanionic molecules through a “quadruplex-like structure” [150]. As in the case with other forms of nanoparticles, pegylation and ligand incorporation improve plasma half-life and cellular uptake. Conde et al. reported tumor growth inhibition in a lung adenocarcinoma model by intratracheal instillation of c-myc siRNA/RGD AuNP [151]. In this design, siRNA is thiolated and loaded onto AuNP along with RGD attached through a thiolated PEG-COOH spacer. These AuNP also carry positive charged azide groups to enhance binding to cell membranes. Lu et al. achieved successful tumor-specific nuclear factor kappa-B (NF-κB) p65 knockdown by targeted hollow AuNP in a human HeLa cervical cancer model [152]. siRNA targeting NF-κB p65 is functionalized with a sulfhydryl group at the 5′ end of the sense chain. Folic acid is introduced via a thiolated PEG linker (F-PEG-TA) as a mean for targeted cellular uptake. After intravenous injection, near infrared (NIR) irradiation was applied to tumors to break the Au–S bond and release the siRNA. Specific silencing of NF-κB p65 in tumors was shown.
Carbon Nanotubes
Carbon nanotubes (CNT) are cylindrical structures composed of carbon atoms. The mechanism for CNT to penetrate cell membrane has not been fully revealed yet but they could mediate efficient cell uptake without apparent cell damage [153]. Pristine CNT are neutral and insoluble, therefore the application of CNT in siRNA delivery requires functionalization. Podesta et al. functionalized carbon nanotube with amines (f-CNT). A low dose of siRNA (4 µg) was complexed with f-CNT (32 µg), which suppressed growth of human epithelial lung carcinoma in nude mice after five intratumoral injections [154].
Calcium Phosphate
Pittella et al. developed a nanoparticle system for siRNA delivery based on calcium phosphate [155, 156]. A PEG-block-charge-conversional polymer (PEG-CCP), which undergoes anionic to cationic conversion in acidic endosomes, forms hybrid micelles with calcium phosphate and siRNA. The charge conversion leads to nanoparticle disassembly in endosome. The cationic PEG-CCP destabilizes endosome, and siRNA will be released into cytoplasm. This system was able to deliver about 40-ng siRNA per gram of tumor tissue, and the target gene was silenced in Hela-Luc tumors [156]. Besides those examples discussed above, iron oxide nanoparticles [157] and layered double hydroxide nanoparticles [158] have been employed for siRNA delivery. Like calcium phosphate, the rapid dissolution in acidic endosome compartment provides the advantage of desirable endosome escape and siRNA release.
Future Directions
The efficacy and safety of siRNA therapeutics have been examined in animal models and clinical trials. The hope is the launch of a new anticancer regimen that suppresses expression of proteins that drive malignant transformation and progression. siRNA lacks desired pharmaceutical attributes, but gaps can be bridged by concurrent optimization in multifunctional delivery systems. The review attempts to provide an account of the design and performance of siRNA nanoparticles (Table 2).
Table 2.
Representative examples of anticancer siRNA delivery systems
| Category | Delivery system | Core components | Size (nm) Zeta (mV) |
Target gene |
Animal model | Reference |
|---|---|---|---|---|---|---|
| Lipid-based particles |
Cationic liposome | DOTAP | 50 | Luciferase | Human H460 lung cancer | [45] |
| Cholesterol | +15 | |||||
| Hyaluronic acid | ||||||
| Recombinant tetra-H2A | ||||||
| DSPE-PEG | ||||||
| DSPE-PEG-AA | ||||||
| Cationic liposome | DOTAP | 20 | c-Myc | Human H460 lung cancer | [46] | |
| DOPA | +33 | |||||
| Cholesterol | ||||||
| Calcium phosphate | ||||||
| DSPE-PEG | ||||||
| DSPE-PEG-AA | ||||||
| Cationic liposome | AtuFECT01 | 118 | PKN3 | Human prostate and pancreatic cancer | [49, 48] | |
| DPhyPE | +46 | Clinical trial | ||||
| DSPE-PEG | ||||||
| Neutral liposome | DOPC | NA | EphA2 | Human ovarian cancer | [67] | |
| Tween 20 | −2.9 | Clinical trial | ||||
| SLN | DOPE | 117 | c-Met | Human glioblastoma | [79] | |
| DC-Cholesterol | +37 | |||||
| Cholesterol | ||||||
| Cholesterol oleate | ||||||
| Glyceryl trioleate | ||||||
| PEG-siRNA | ||||||
| rHDL | Apolipoprotein A-I | 10 | STAT3 | Human ovarian and colorectal cancer | [80] | |
| Cholesterol | −3.2 | FAK | ||||
| Cholesterol oleate | ||||||
| PC | ||||||
| Oligolysine | ||||||
| Polymeric particles |
CDP | CDP | 70 | RRM2 | Human melanoma | [84, 85] |
| AD-PEG | +10 | Clinical trial | ||||
| AD-PEG-Tf | ||||||
| PEI | F25 LMW branched PEI | 60–130 +35 to +40 |
VEGF | Human prostate carcinoma | [99] | |
| PEI | PEI-g-PEG-RGD | 161 +4.2 | VEGFR1 | Murine colon adenocarcinoma | [115] | |
| PA MAM | G5 PAMAM | 100 +40 | Hsp 27 | Human prostate cancer | [126] | |
| Chitosan | Chitosan-RGD | 200 +40 | PLXDC1 | Murine ovarian cancer | [134] | |
| Atellocollagen | Atellocollagen | NA NA | PLK1 | Human non-small cell lung cancer | [140] | |
| Protein | Thiolated human serum albumin | 170 NA | VEGF | Human prostate carcinoma | [138] | |
| Inorganic particles |
AuNP | Hollow AuNP | 40 | p65 | Human HeLa cervical cancer | [152] |
| Thiolated siRNA | NA | |||||
| F-PEG-TA | ||||||
| CNT | CNT-NH3+ | NA NA |
NA | Human epithelial lung cancer | [154] | |
| Calcium phosphate | Calcium phosphate | 38 | Luciferase | Human pancreatic cancer | [156] | |
| PEG-CPP | −2.2 |
Cationic components are in bold
SLN solid lipid nanoparticles; rHDL recombinant high-density lipoproteins; CDP cyclodextrin-containing polymer; PEI polyethyleneimine; PAMAM polyamidoamine; AuNP gold nanoparticles; CNT carbon nanotubes; DOTAP 1,2-dioleoyloxy-3-trimethylammonium propane; DSPE 1,2-distearoyl-sn-glycero-3-phosphoethanolamine; PEG polyethylene glycol; AA anisamide; DOPA dioleoylphosphatidic acid; DPhyPE 1,2-diphytanoyl-sn-glycero-3-phosphoethanolamine; DOPC 1,2-dioleoyl-sn-glycero-3-phosphatidylcholine; DOPE 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine; DC-Cholesterol 3β-[N-(N′,N′-dimethylaminoethane)-carbamoyl]-cholesterol; PC phosphatidylcholine; AD adamantane; Tf transferrin; RGD tri-peptide Arg-Gly-Asp; G5 PAMAM fifth-generation polyamidoamine; F-PEG-TA folic acid-polyethylene glycol-thiol; PEG-CPP PEG-block-charge-conversional polymer; NA not available; PKN3 protein kinase N3; EphA2 ephrin type-A receptor 2; STAT3 signal transducer and activator of transcription 3; FAK focal adhesion kinase; RRM2 ribonucleoside-diphosphate reductase subunit M2; VEGF vascular endothelial growth factor; VEGFR1 vascular endothelial growth factor receptor 1; Hsp27 heat shock protein 27; PLXDC1 Plexin domain-containing protein 1; PLK1 polo-like kinase 1
Studies of liposomes, polymeric nanoparticles, and inorganic nanoparticles in solid tumor models illustrated several essential attributes. A strong cationic component is needed for sufficient siRNA complexation. Pegylation allows prolonged circulation by minimizing adsorptive interactions with plasma proteins and eventual removal by phagocytes. Particles with sizes ranging between 10 and 100 nm tend to accumulate in tumors and avoid renal clearance. Slightly positive zeta potential can stabilize the particle colloids and facilitate interactions with cell membrane. Addition of targeting ligands tends to enhance cellular uptake. Gene silencing requires mechanisms for endosome escape. Charge density needs to be optimized to enable cargo release in cytoplasm but with limited premature disassembly. These factors parallel lessons learned from gene and antisense oligodeoxynucleotide delivery [159–164]. Alternatively, all of these functional domains can be incorporated into one polymer by chemical synthesis (e.g., Dynamic PolyConjugate) [165–167].
Rational design of siRNA delivery system requires multiscale pharmacokinetic optimization of systemic and cellular processes [168]. Future studies of siRNA delivery may focus on the following areas. Pegylation renders long-circulating vehicles. However, anti-PEG antibodies have been detected in mice [169]. Dextran, a hydrophilic carbohydrate, has been studied to minimize the interactions with plasma proteins [170]. Exploring polymers to shield the charges without immune stimulation can advance the design of siRNA delivery systems. As ligands of toll-like receptors (TLR) [15], dsRNA may act as adjuvant to generate high titers of anti-vehicle antibodies after repeated dosing [169]. This imposes limits on the dose and frequency of a given therapy. Another observation is that extravasation and penetration vary quantitatively among tumor types. Accumulation of siRNA-nanoparticle in tumor tissue is relatively low. Using physically guided targeting (i.e., magnetic) may improve cellular exposure. An example of this is iron oxide nanoparticles that designed to deliver siRNA [157]. Drug penetration depends on the extent of vascularization [171], with highly vascularized tumors being more sensitive [172]. Therefore, characteristics of each tumor type should guide the design. Intriguingly, intracellular studies have shown a disconnection between endocytosis and gene silencing [59], which may be resolved by careful studies of siRNA intracellular trafficking. Cytotoxicity of cationic lipids and polymers is an important limiting factor. Combinatorial synthesis and high throughput screening have been utilized to generate low-toxicity lipids for siRNA delivery to liver [173, 174]. Efficient target gene silence also requires the timely release of siRNA into cytoplasm. The acidic environment of endosome and the reductive potential of cytoplasm can be exploited to trigger the breakdown of nanoparticle [175, 176]. Taken together, system optimization is best achieved by using integrated and tailored approaches in characterizing complexation, distribution, penetration, cellular uptake, and trafficking in parallel in relevant models for specific tumor types [55, 177].
Acknowledgments
This work was supported in part by grants from the National Institutes of Health (A1081218), Juvenile Diabetes Research Foundation, and the Pennsylvania Department of Health through the Commonwealth Universal Research Enhancement Program.
Contributor Information
Yi Wen, Email: weny@duq.edu.
Wilson S. Meng, Email: meng@duq.edu.
References
- 1.Meister G, Tuschl T. Mechanisms of gene silencing by double-stranded RNA. Nature. 2004;431:343–349. doi: 10.1038/nature02873. [DOI] [PubMed] [Google Scholar]
- 2.Hannon GJ. RNA interference. Nature. 2002;418:244–251. doi: 10.1038/418244a. [DOI] [PubMed] [Google Scholar]
- 3.Rana TM. Illuminating the silence: understanding the structure and function of small RNAs. Nat Rev Mol Cell Biol. 2007;8(1):23–36. doi: 10.1038/nrm2085. [DOI] [PubMed] [Google Scholar]
- 4.Jinek M, Doudna JA. A three-dimensional view of the molecular machinery of RNA interference. Nature. 2009;457(7228):405–412. doi: 10.1038/nature07755. [DOI] [PubMed] [Google Scholar]
- 5.Fire A, Xu S, MM K, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 6.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 7.McCaffrey AP, Meuse L, Pham TT, Conklin DS, Hannon GJ, Kay MA. RNA interference in adult mice. Nature. 2002;418:38–39. doi: 10.1038/418038a. [DOI] [PubMed] [Google Scholar]
- 8.Hannon GJ, Rossi JJ. Unlocking the potential of the human genome with RNA interference. Nature. 2004;431:371–377. doi: 10.1038/nature02870. [DOI] [PubMed] [Google Scholar]
- 9.Bumcrot D, Manoharan M, Koteliansky V, Sah DWY. RNAi therapeutics: a potential new class of pharmaceutical drugs. Nat Chem Biol. 2006;2(12):711–719. doi: 10.1038/nchembio839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raemdonck K, Vandenbroucke RE, Demeester J, Sanders NN, De Smedt SC. Maintaining the silence: reflections on long-term RNAi. Drug Discov Today. 2008;13(21–22):917–931. doi: 10.1016/j.drudis.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aagaard L, Rossi JJ. RNAi therapeutics: principles, prospects and challenges. Adv Drug Deliv Rev. 2007;59(2–3):75–86. doi: 10.1016/j.addr.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim DH, Rossi JJ. Strategies for silencing human disease using RNA interference. Nat Rev Genet. 2007;8(3):173–184. doi: 10.1038/nrg2006. [DOI] [PubMed] [Google Scholar]
- 13.Khan AA, Betel D, Miller ML, Sander C, Leslie CS, Marks DS. Transfection of small RNAs globally perturbs gene regulation by endogenous microRNAs. Nat Biotechnol. 2009;27(6):549–555. doi: 10.1038/nbt.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jackson AL, Linsley PS. Recognizing and avoiding siRNA off-target effects for target identification and therapeutic application. Nat Rev Drug Discov. 2010;9(1):57–67. doi: 10.1038/nrd3010. [DOI] [PubMed] [Google Scholar]
- 15.Sioud M. Innate sensing of self and non-self RNAs by Toll-like receptors. Trends Mol Med. 2006;12(4):167–176. doi: 10.1016/j.molmed.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Sledz CA, Holko M, de Veer MJ, Silverman RH, Williams BRG. Activation of the interferon system by short-interfering RNAs. Nat Cell Biol. 2003;5(9):834–839. doi: 10.1038/ncb1038. [DOI] [PubMed] [Google Scholar]
- 17.Corey DR. Chemical modification: the key to clinical application of RNA interference? J Clin Invest. 2007;117(12):3615–3622. doi: 10.1172/JCI33483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whitehead KA, Langer R, Anderson DG. Knocking down barriers: advances in siRNA delivery. Nat Rev Drug Discov. 2009;8(2):129–138. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soutschek J, Akinc A, Bramlage B, Charisse K, Constien R, Donoghue M, et al. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature. 2004;432:173–178. doi: 10.1038/nature03121. [DOI] [PubMed] [Google Scholar]
- 20.Zimmermann TS, Lee ACH, Akinc A, Bramlage B, Bumcrot D, Fedoruk MN, et al. RNAi-mediated gene silencing in non-human primates. Nature. 2006;441(7089):111–114. doi: 10.1038/nature04688. [DOI] [PubMed] [Google Scholar]
- 21.Pai SI, Lin YY, Macaes B, Meneshian A, Hung CF, Wu TC. Prospects of RNA interference therapy for cancer. Gene Ther. 2005;13(6):464–477. doi: 10.1038/sj.gt.3302694. [DOI] [PubMed] [Google Scholar]
- 22.de Fougerolles A, Vornlocher HP, Maraganore J, Lieberman J. Interfering with disease: a progress report on siRNA-based therapeutics. Nat Rev Drug Discov. 2007;6(6):443–453. doi: 10.1038/nrd2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4(2):145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 24.Ferrari M. Frontiers in cancer nanomedicine: directing mass transport through biological barriers. Trends Biotechnol. 2010;28(4):181–188. doi: 10.1016/j.tibtech.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 26.Davis ME, Chen Z, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7(9):771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 27.Dobrovolskaia MA, Aggareal P, Hall JB, Mcneil SE. Preclinical studies to understand nanoparticle interaction with the immune system and its potential effects on nanoparticle biodistribution. Mol Pharm. 2008;5(4):487–495. doi: 10.1021/mp800032f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klibanov AL, Maruyama K, Torchilin VP, Huang L. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett. 1990;268(1):235–237. doi: 10.1016/0014-5793(90)81016-h. [DOI] [PubMed] [Google Scholar]
- 29.Alexis F, Pridgen E, Molnar LK, Farokhzad OC. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol Pharm. 2008;5(4):505–515. doi: 10.1021/mp800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schroeder A, Levins CG, Cortez C, Langer R, Anderson DG. Lipid-based nanotherapeutics for siRNA delivery. J Intern Med. 2010;267(1):9–21. doi: 10.1111/j.1365-2796.2009.02189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burke RS, Pun SH. Extracellular barriers to in vivo PEI and PEGylated PEI polyplex-mediated gene delivery to the liver. Bioconjug Chem. 2008;19:693–704. doi: 10.1021/bc700388u. [DOI] [PubMed] [Google Scholar]
- 32.Khalil IA. Uptake pathways and subsequent intracellular trafficking in nonviral gene delivery. Pharmacol Rev. 2006;58(1):32–45. doi: 10.1124/pr.58.1.8. [DOI] [PubMed] [Google Scholar]
- 33.Dominska M, Dykxhoorn DM. Breaking down the barriers: siRNA delivery and endosome escape. J Cell Sci. 2010;123(8):1183–1189. doi: 10.1242/jcs.066399. [DOI] [PubMed] [Google Scholar]
- 34.Xu Y, Szoka FC., Jr Mechanism of DNA release from cationic liposome-DNA complexes used in cell transfection. Biochemistry (Mosc) 1996;35:5616–5623. doi: 10.1021/bi9602019. [DOI] [PubMed] [Google Scholar]
- 35.Hafez IM, Maurer N, Cullis PR. On the mechanism whereby cationic lipids promote intracellular delivery of polynucleic acids. Gene Ther. 2001;8:1188–1196. doi: 10.1038/sj.gt.3301506. [DOI] [PubMed] [Google Scholar]
- 36.Akinc A, Thomas M, Klibanov AM, Langer R. Exploring polyethylenimine-mediated DNA transfection and the proton sponge hypothesis. J Gene Med. 2005;7(5):657–663. doi: 10.1002/jgm.696. [DOI] [PubMed] [Google Scholar]
- 37.Sonawane ND. Chloride accumulation and swelling in endosomes enhances DNA transfer by polyamine-DNA polyplexes. J Biol Chem. 2003;278(45):44826–44831. doi: 10.1074/jbc.M308643200. [DOI] [PubMed] [Google Scholar]
- 38.Stagmann T. Membrane fusion mechanisms the influenza hemagglutinin paradigm and its implications for intracellular fusion. Traffic. 2000;1:598–604. doi: 10.1034/j.1600-0854.2000.010803.x. [DOI] [PubMed] [Google Scholar]
- 39.Pinnaduwage P, Schmitt L, Huang L. Use of a quaternary ammonium detergent in liposome mediated DNA transfection of mouse L-cells. Biochim Biophys Acta. 1989;985(1):33–37. doi: 10.1016/0005-2736(89)90099-0. [DOI] [PubMed] [Google Scholar]
- 40.Wang CY, Huang L. Highly efficient DNA delivery mediated by pH-sensitive immunoliposomes. Biochemistry (Mosc) 1989;28(24):9508–9514. doi: 10.1021/bi00450a039. [DOI] [PubMed] [Google Scholar]
- 41.Li S-D, Chen Y-C, Hackett MJ, Huang L. Tumor-targeted delivery of siRNA by self-assembled nanoparticles. Mol Ther. 2008;16(1):163–169. doi: 10.1038/sj.mt.6300323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li S-D, Huang L. Nanoparticles evading the reticuloendothelial system: Role of the supported bilayer. Biochim Biophys Acta Biomembr. 2009;1788(10):2259–2266. doi: 10.1016/j.bbamem.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chono S, Li S-D, Conwell CC, Huang L. An efficient and low immunostimulatory nanoparticle formulation for systemic siRNA delivery to the tumor. J Control Release. 2008;131(1):64–69. doi: 10.1016/j.jconrel.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, Xu Z, Guo S, Zhang L, Sharma A, Robertson GP, et al. Intravenous delivery of siRNA targeting CD47 effectively inhibits melanoma tumor growth and lung metastasis. Mol Ther. 2013;21(10):1919–1929. doi: 10.1038/mt.2013.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y, Zhang L, Guo S, Hatefi A, Huang L. Incorporation of histone derived recombinant protein for enhanced disassembly of core-membrane structured liposomal nanoparticles for efficient siRNA delivery. J Control Release. 2013;172(1):179–189. doi: 10.1016/j.jconrel.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Y, Peng L, Mumper RJ, Huang L. Combinational delivery of c-myc siRNA and nucleoside analogs in a single, synthetic nanocarrier for targeted cancer therapy. Biomaterials. 2013;34(33):8459–8468. doi: 10.1016/j.biomaterials.2013.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li J, Yang Y, Huang L. Calcium phosphate nanoparticles with an asymmetric lipid bilayer coating for siRNA delivery to the tumor. J Control Release. 2012;158(1):108–114. doi: 10.1016/j.jconrel.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Santel A, Aleku M, Endruschat J. A novel siRNA-lipoplex technology for RNA interference in the mouse vascular endothelium. Gene Ther. 2006;13:1222–1234. doi: 10.1038/sj.gt.3302777. [DOI] [PubMed] [Google Scholar]
- 49.Aleku M, Schulz P, Keil O, Santel A, Schaeper U, Dieckhoff B, et al. Atu027, a Liposomal Small Interfering RNA formulation targeting protein kinase N3, inhibits cancer progression. Cancer Res. 2008;68(23):9788–9798. doi: 10.1158/0008-5472.CAN-08-2428. [DOI] [PubMed] [Google Scholar]
- 50.Pirollo KF, Rait A, Zhou Q, Hwang SH, Dagata JA, Zon G, et al. Materializing the potential of small-interfering RNA via a tumor-targeting nanodelivery system. Cancer Res. 2007;67(7):2938–2943. doi: 10.1158/0008-5472.CAN-06-4535. [DOI] [PubMed] [Google Scholar]
- 51.Yu W, Pirollo KF, Yu B, Rait A, Xiang L, Huang W, et al. Enhanced transfection efficiency of a systemically delivered tumor-targeting immunolipoplex by inclusion of a pH-sensitive histidylated oligolysine peptide. Nucleic Acids Res. 2004;32(5):e48. doi: 10.1093/nar/gnh049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sato Y, Hatakeyama H, Sakurai Y, Hyodo M, Akita H, Harashima H. A pH-sensitive cationic lipid facilitates the delivery of liposomal siRNA and gene silencing activity in vitro and in vivo. J Control Release. 2012;163(3):267–276. doi: 10.1016/j.jconrel.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 53.Sakurai Y, Hatakeyama H, Sato Y, Hyodo M, Akita H, Harashima H. Gene silencing via RNAi and siRNA quantification in tumor tissue using MEND, a liposomal siRNA delivery system. Mol Ther. 2013;21(6):1195–1203. doi: 10.1038/mt.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sonoke S, Ueda T, Fujiwara K, Sato Y, Takagaki K, Hirabayashi K, et al. Tumor regression in mice by delivery of Bcl-2 small interfering RNA with pegylated cationic liposomes. Cancer Res. 2008;68(21):8843–8851. doi: 10.1158/0008-5472.CAN-08-0127. [DOI] [PubMed] [Google Scholar]
- 55.Li L, Wang R, Wilcox D, Sarthy A, Lin X, Huang X, et al. Developing lipid nanoparticle-based siRNA therapeutics for hepatocellular carcinoma using an integrated approach. Mol Cancer Ther. 2013;12(11):2308–2318. doi: 10.1158/1535-7163.MCT-12-0983-T. [DOI] [PubMed] [Google Scholar]
- 56.Tseng Y-C, Mozumdar S, Huang L. Lipid-based systemic delivery of siRNA. Adv Drug Deliv Rev. 2009;61(9):721–731. doi: 10.1016/j.addr.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Auguste D, Furman K, Wong A, Fuller J, Armes S, Deming T, et al. Triggered release of siRNA from poly(ethylene glycol)-protected, pH-dependent liposomes. J Control Release. 2008;130(3):266–274. doi: 10.1016/j.jconrel.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koren E, Apte A, Jani A, Torchilin VP. Multifunctional PEGylated 2C5-immunoliposomes containing pH-sensitive bonds and TAT peptide for enhanced tumor cell internalization and cytotoxicity. J Control Release. 2012;160(2):264–273. doi: 10.1016/j.jconrel.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lu JJ, Langer R, Chen J. A novel mechanism is involved in cationic lipid-mediated functional siRNA delivery. Mol Pharm. 2009;6(3):763–771. doi: 10.1021/mp900023v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sahay G, Querbes W, Alabi C, Eltoukhy A, Sarkar S, Zurenko C, et al. Efficiency of siRNA delivery by lipid nanoparticles is limited by endocytic recycling. Nat Biotechnol. 2013;31(7):653–658. doi: 10.1038/nbt.2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gilleron J, Querbes W, Zeigerer A, Borodovsky A, Marsico G, Schubert U, et al. Image-based analysis of lipid nanoparticle–mediated siRNA delivery, intracellular trafficking and endosomal escape. Nat Biotechnol. 2013;31(7):638–646. doi: 10.1038/nbt.2612. [DOI] [PubMed] [Google Scholar]
- 62.Zhang J, Liu F, Huang L. Implications of pharmacokinetic behavior of lipoplex for its inflammatory toxicity. Adv Drug Deliv Rev. 2005;57(5):689–698. doi: 10.1016/j.addr.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 63.Dokka S, Toledo D, Shi X, Castranova V, Rojanasakul Y. Oxygen radical-mediated pulmonary toxicity induced by some cationic liposomes. Pharm Res. 2000;17(5):521–525. doi: 10.1023/a:1007504613351. [DOI] [PubMed] [Google Scholar]
- 64.Ma Z, Li J, He F, Wilson A, Pitt B, Li S. Cationic lipids enhance siRNA-mediated interferon response in mice. Biochem Biophys Res Commun. 2005;330(3):755–759. doi: 10.1016/j.bbrc.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 65.Chen Y, Sen JS, Bathula SR, Yang Q, Fittipaldi R, Huang L. Novel cationic lipid that delivers siRNA and enhances therapeutic effect in lung cancer cells. Mol Pharm. 2009;6(3):696–705. doi: 10.1021/mp800136v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen Y, Bathula SR, Yang Q, Huang L. Targeted nanoparticles deliver siRNA to melanoma. J Invest Dermatol. 2010;130(12):2790–2798. doi: 10.1038/jid.2010.222. [DOI] [PubMed] [Google Scholar]
- 67.Landen CN. Therapeutic EphA2 gene targeting in vivo using neutral liposomal small interfering RNA delivery. Cancer Res. 2005;65(15):6910–6918. doi: 10.1158/0008-5472.CAN-05-0530. [DOI] [PubMed] [Google Scholar]
- 68.Halder J. Focal adhesion kinase targeting using in vivo short interfering RNA delivery in neutral liposomes for ovarian carcinoma therapy. Clin Cancer Res. 2006;12(16):4916–4924. doi: 10.1158/1078-0432.CCR-06-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mangala LS, Zuzel V, Schmandt R, Leshane ES, Halder JB, Armaiz-Pena GN, et al. Therapeutic targeting of ATP7B in ovarian carcinoma. Clin Cancer Res. 2009;15(11):3770–3780. doi: 10.1158/1078-0432.CCR-08-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Merritt WM, Lin YG, Spannuth WA, Fletcher MS, Kamat AA, Han LY, et al. Effect of interleukin-8 gene silencing with liposome-encapsulated small interfering RNA on ovarian cancer cell growth. J Natl Cancer Inst. 2008;100(5):359–372. doi: 10.1093/jnci/djn024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vivas-Mejia PE, Rodriguez-Aguayo C, Han HD, Shahzad MMK, Valiyeva F, Shibayama M, et al. Silencing survivin splice variant 2B leads to antitumor activity in taxane-resistant ovarian cancer. Clin Cancer Res. 2011;17(11):3716–3726. doi: 10.1158/1078-0432.CCR-11-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chakravarty D, Roy SS, Babu CR, Dandamudi R, Curiel TJ, Vivas-Mejia P, et al. Therapeutic targeting of PELP1 prevents ovarian cancer growth and metastasis. Clin Cancer Res. 2011;17(8):2250–2259. doi: 10.1158/1078-0432.CCR-10-2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gray MJ, Dallas NA, Van Buren G, Xia L, Yang AD, Somcio RJ, et al. Therapeutic targeting of Id2 reduces growth of human colorectal carcinoma in the murine liver. Oncogene. 2008;27(57):7192–7200. doi: 10.1038/onc.2008.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gray MJ, Van Buren G, Dallas NA, Xia L, Wang X, Yang AD, et al. Therapeutic targeting of neuropilin-2 on colorectal carcinoma cells implanted in the murine liver. J Natl Cancer Inst. 2008;100(2):109–120. doi: 10.1093/jnci/djm279. [DOI] [PubMed] [Google Scholar]
- 75.Villares GJ, Zigler M, Wang H, Melnikova VO, Wu H, Friedman R, et al. Targeting melanoma growth and metastasis with systemic delivery of liposome-incorporated protease-activated receptor-1 small interfering RNA. Cancer Res. 2008;68(21):9078–9086. doi: 10.1158/0008-5472.CAN-08-2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Miller CR, Bondurant B, McLean SD, McGovern KA, O'Brien DF. Liposome-cell interactions in vitro: effect of liposome surface charge on the binding and endocytosis of conventional and sterically stabilized liposomes. Biochemistry (Mosc) 1998;37(37):12875–12883. doi: 10.1021/bi980096y. [DOI] [PubMed] [Google Scholar]
- 77.Kostarelos K, Emfietzoglou D, Papakostas A, Yang W-H, Ballangrud Å, Sgouros G. Binding and interstitial penetration of liposomes within avascular tumor spheroids. Int J Cancer. 2004;112(4):713–721. doi: 10.1002/ijc.20457. [DOI] [PubMed] [Google Scholar]
- 78.Tanaka T, Mangala LS, Vivas-Mejia PE, Nieves-Alicea R, Mann AP, Mora E, et al. Sustained small interfering RNA delivery by mesoporous silicon particles. Cancer Res. 2010;70(9):3687–3696. doi: 10.1158/0008-5472.CAN-09-3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jin J, Bae KH, Yang H, Lee SJ, Kim H, Kim Y, et al. In vivo specific delivery of c-Met siRNA to glioblastoma using cationic solid lipid nanoparticles. Bioconjug Chem. 2011;22(12):2568–2572. doi: 10.1021/bc200406n. [DOI] [PubMed] [Google Scholar]
- 80.Shahzad MMK, Mangala LS, Han HD, Lu C, Bottsford-Miller J, Nishimura M, et al. Targeted delivery of small interfering RNA using reconstituted high density lipoprotein nanoparticles. Neoplasia. 2011;13(4):309–319. doi: 10.1593/neo.101372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wolfrum C, Shi S, Jayaprakash KN, Jayaraman M, Wang G, Pandey RK, et al. Mechanisms and optimization of in vivo delivery of lipophilic siRNAs. Nat Biotechnol. 2007;25(10):1149–1157. doi: 10.1038/nbt1339. [DOI] [PubMed] [Google Scholar]
- 82.Tomlinson E, Rolland AP. Controllable gene therapy pharmaceutics of non-viral gene delivery systems. J Control Release. 1996;39(2-3):357–372. [Google Scholar]
- 83.Howard KA. Delivery of RNA interference therapeutics using polycation-based nanoparticles. Adv Drug Deliv Rev. 2009;61(9):710–720. doi: 10.1016/j.addr.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 84.Bartlett DW, Davis ME. Physicochemical and biological characterization of targeted, nucleic acid-containing nanoparticles. Bioconjug Chem. 2007;18:456–468. doi: 10.1021/bc0603539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Davis ME, Zuckerman JE, Choi CHJ, Seligson D, Tolcher A, Alabi CA, et al. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464(7291):1067–1070. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hwang SH, Bellocq NC, Davis ME. Effects of structure of beta-cyclodextrin-containing polymers on gene delivery. Bioconjug Chem. 2001;12:280–290. doi: 10.1021/bc0001084. [DOI] [PubMed] [Google Scholar]
- 87.Reineke TM, Davis ME. Structural effects of carbohydrate-containing polycations on gene delivery. 1. Carbohydrate size and its distance from charge centers. Bioconjug Chem. 2003;14(247–254) doi: 10.1021/bc025592k. [DOI] [PubMed] [Google Scholar]
- 88.Reineke TM, Davis ME. Structural effects of carbohydrate-containing polycations on gene delivery. 2. Charge center type. Bioconjug Chem. 2003;14:255–261. doi: 10.1021/bc025593c. [DOI] [PubMed] [Google Scholar]
- 89.Popielarski SR, Mishra S, Davis ME. Structural effects of carbohydrate-containing polycations on gene delivery. 3. Cyclodextrin type and functionalization. Bioconjug Chem. 2003;14:672–678. doi: 10.1021/bc034010b. [DOI] [PubMed] [Google Scholar]
- 90.Bartlett DW, Su H, Hildebrandt IJ, Weber WA, Davis ME. Impact of tumor-specific targeting on the biodistribution and efficacy of siRNA nanoparticles measured by multimodality in vivo imaging. Proc Natl Acad Sci U S A. 2007;104(39):15549–15554. doi: 10.1073/pnas.0707461104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bartlett DW, Davis ME. Impact of tumor-specific targeting and dosing schedule on tumor growth inhibition after intravenous administration of siRNA-containing nanoparticles. Biotechnol Bioeng. 2008;99(4):975–985. doi: 10.1002/bit.21668. [DOI] [PubMed] [Google Scholar]
- 92.Günther M, Lipka J, Malek A, Gutsch D, Kreyling W, Aigner A. Polyethylenimines for RNAi-mediated gene targeting in vivo and siRNA delivery to the lung. Eur J Pharm Biopharm. 2011;77(3):438–449. doi: 10.1016/j.ejpb.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 93.Neu M, Fischer D, Kissel T. Recent advances in rational gene transfer vector design based on poly(ethylene imine) and its derivatives. J Gene Med. 2005;7(8):992–1009. doi: 10.1002/jgm.773. [DOI] [PubMed] [Google Scholar]
- 94.Rejman J, Bragonzi A, Conese M. Role of clathrin- and caveolae-mediated endocytosis in gene transfer mediated by lipo- and polyplexes. Mol Ther. 2005;12(3):468–474. doi: 10.1016/j.ymthe.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 95.Hunter A. Molecular hurdles in polyfectin design and mechanistic background to polycation induced cytotoxicity. Adv Drug Deliv Rev. 2006;58(14):1523–1531. doi: 10.1016/j.addr.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 96.Vongersdorff K, Sanders N, Vandenbroucke R, Desmedt S, Wagner E, Ogris M. The internalization route resulting in successful gene expression depends on both cell line and polyethylenimine polyplex type. Mol Ther. 2006;14(5):745–753. doi: 10.1016/j.ymthe.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 97.Godbey WT, Wu KK, Mikos AG. Size matters: molecular weight affects the efficiency of poly(ethylenimine) as a gene delivery vehicle. Biochim Biophys Acta Gen Subj. 1999;1725(3):377–384. doi: 10.1002/(sici)1097-4636(19990605)45:3<268::aid-jbm15>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 98.Werth S, Urban-Klein B, Dai L, Höbel S, Grzelinski M, Bakowsky U, et al. A low molecular weight fraction of polyethylenimine (PEI) displays increased transfection efficiency of DNA and siRNA in fresh or lyophilized complexes. J Control Release. 2006;112(2):257–270. doi: 10.1016/j.jconrel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 99.Hobel S, Koburger I, John M, Czubayko F, Hadwiger P, Vornlocher H-P, et al. Polyethylenimine/small interfering RNA-mediated knockdown of vascular endothelial growth factorin vivoexerts anti-tumor effects synergistically with Bevacizumab. J Gene Med. 2010;12:287–300. doi: 10.1002/jgm.1431. [DOI] [PubMed] [Google Scholar]
- 100.Hendruschk S, Wiedemuth R, Aigner A, Topfer K, Cartellieri M, Martin D, et al. RNA interference targeting survivin exerts antitumoral effects in vitro and in established glioma xenografts in vivo. Neuro-oncol. 2011;13(10):1074–1089. doi: 10.1093/neuonc/nor098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kaestner P, Aigner A, Bastians H. Therapeutic targeting of the mitotic spindle checkpoint through nanoparticle-mediated siRNA delivery inhibits tumor growth in vivo. Cancer Lett. 2011;304(2):128–136. doi: 10.1016/j.canlet.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 102.Thomas M. Enhancing polyethylenimine's delivery of plasmid DNA into mammalian cells. Proc Natl Acad Sci U S A. 2002;99(23):14640–14645. doi: 10.1073/pnas.192581499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tarcha PJ, Pelisek J, Merdan T, Waters J, Cheung K, von Gersdorff K, et al. Synthesis and characterization of chemically condensed oligoethylenimine containing beta-aminopropionamide linkages for siRNA delivery. Biomaterials. 2007;28(25):3731–3740. doi: 10.1016/j.biomaterials.2007.04.037. [DOI] [PubMed] [Google Scholar]
- 104.Breunig M, Lungwitz U, Liebl R, Goepferich A. Breaking up the correlation between efficacy and toxicity for nonviral gene delivery. Proc Natl Acad Sci U S A. 2007;104(36):14454–14459. doi: 10.1073/pnas.0703882104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Breunig M, Hozsa C, Lungwitz U, Watanabe K, Umeda I, Kato H, et al. Mechanistic investigation of poly(ethylene imine)-based siRNA delivery: Disulfide bonds boost intracellular release of the cargo. J Control Release. 2008;130(1):57–63. doi: 10.1016/j.jconrel.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 106.Jiang G, Park K, Kim J, Kim KS, Hahn SK. Target specific intracellular delivery of siRNA-PEI-HA complex by receptor mediated endocytosis. Mol Pharm. 2009;6(3):727–737. doi: 10.1021/mp800176t. [DOI] [PubMed] [Google Scholar]
- 107.Ganesh S, Iyer AK, Morrissey DV, Amiji MM. Hyaluronic acid based self-assembling nanosystems for CD44 target mediated siRNA delivery to solid tumors. Biomaterials. 2013;34(13):3489–3502. doi: 10.1016/j.biomaterials.2013.01.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Höbel S, Loos A, Appelhans D, Schwarz S, Seidel J, Voit B, et al. Maltose- and maltotriose-modified, hyperbranched poly(ethylene imine)s (OM-PEIs): physicochemical and biological properties of DNA and siRNA complexes. J Control Release. 2011;149(2):146–158. doi: 10.1016/j.jconrel.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 109.Zintchenko A, Philipp A, Dehshahri A, Wagner E. Simple modifications of branched PEI lead to highly efficient siRNA carriers with low toxicity. Bioconjug Chem. 2008;19:1448–1455. doi: 10.1021/bc800065f. [DOI] [PubMed] [Google Scholar]
- 110.Schroeder A, Dahlman JE, Sahay G, Love KT, Jiang S, Eltoukhy AA, et al. Alkane-modified short polyethyleneimine for siRNA delivery. J Control Release. 2012;160(2):172–176. doi: 10.1016/j.jconrel.2011.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Navarro G, Sawant RR, Biswas S, Conchita SE, Ilarduya T, Torchilin VP. P-glycoprotein silencing with siRNA delivered by DOPE-modified PEI overcomes doxorubicin resistance in breast cancer cells. Nanomedicine. 2012;7(1):65–78. doi: 10.2217/nnm.11.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Alshamsan A, Hamdy S, Samuel J, El-Kadi AOS, Lavasanifar A, Uludağ H. The induction of tumor apoptosis in B16 melanoma following STAT3 siRNA delivery with a lipid-substituted polyethylenimine. Biomaterials. 2010;31(6):1420–1428. doi: 10.1016/j.biomaterials.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 113.Kim WJ, Chang C-W, Lee M, Kim SW. Efficient siRNA delivery using water soluble lipopolymer for anti-angiogenic gene therapy. J Control Release. 2007;118(3):357–363. doi: 10.1016/j.jconrel.2006.12.026. [DOI] [PubMed] [Google Scholar]
- 114.Malek A, Merkel O, Fink L, Czubayko F, Kissel T, Aigner A. In vivo pharmacokinetics, tissue distribution and underlying mechanisms of various PEI(-PEG)/siRNA complexes. Toxicol Appl Pharmacol. 2009;236(1):97–108. doi: 10.1016/j.taap.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 115.Kim J, Kim SW, Kim WJ. PEI-g-PEG-RGD/small interference RNA polyplex-mediated silencing of vascular endothelial growth factor receptor and its potential as an anti-angiogenic tumor therapeutic strategy. Oligonucleotides. 2011;21(2):101–107. doi: 10.1089/oli.2011.0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schiffelers RM. Cancer siRNA therapy by tumor selective delivery with ligand-targeted sterically stabilized nanoparticle. Nucleic Acids Res. 2004;32(19):e149. doi: 10.1093/nar/gnh140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zheng M, Librizzi D, Kılıç A, Liu Y, Renz H, Merkel OM, et al. Enhancing in vivo circulation and siRNA delivery with biodegradable polyethylenimine-graft-polycaprolactone-block-poly(ethylene glycol) copolymers. Biomaterials. 2012;33(27):6551–6558. doi: 10.1016/j.biomaterials.2012.05.055. [DOI] [PubMed] [Google Scholar]
- 118.Kim SH, Jeong JH, Lee SH, Kim SW, Park TG. Local and systemic delivery of VEGF siRNA using polyelectrolyte complex micelles for effective treatment of cancer. J Control Release. 2008;129(2):107–116. doi: 10.1016/j.jconrel.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 119.Shim MS, Chang S-S, Kwon YJ. Stimuli-responsive siRNA carriers for efficient gene silencing in tumors via systemic delivery. Biomater Sci. 2014;2(1):35. doi: 10.1039/c3bm60187k. [DOI] [PubMed] [Google Scholar]
- 120.Zhu L, Perche F, Wang T, Torchilin VP. Matrix metalloproteinase 2-sensitive multifunctional polymeric micelles for tumor-specific co-delivery of siRNA and hydrophobic drugs. Biomaterials. 2014;35(13):4213–4222. doi: 10.1016/j.biomaterials.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Boeckle S, von Gersdorff K, van der Piepen S, Culmsee C, Wagner E, Ogris M. Purification of polyethylenimine polyplexes highlights the role of free polycations in gene transfer. J Gene Med. 2004;6(10):1102–1111. doi: 10.1002/jgm.598. [DOI] [PubMed] [Google Scholar]
- 122.Freeman E, Weiland LM, Meng WS. Application of proteins in burst delivery systems. Smart Mater Struct. 2010;19(9):094015. [Google Scholar]
- 123.Freeman E, Weiland LM, Meng WS. Modeling the proton sponge hypothesis: examining proton sponge effectiveness for enhancing intracellular gene delivery through multiscale modeling. J Biomater Sci Polym Ed. 2013;24(4):398–416. doi: 10.1080/09205063.2012.690282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pavan GM, Albertazzi L, Danani A. Ability to adapt- different generations of PAMAM dendrimers show different behaviors in binding siRNA. J Phys Chem B. 2010;114:2667–2675. doi: 10.1021/jp100271w. [DOI] [PubMed] [Google Scholar]
- 125.Posocco P, Liu X, Laurini E, Marson D, Chen C, Liu C, et al. Impact of siRNA overhangs for dendrimer-mediated siRNA delivery and gene silencing. Mol Pharm. 2013;10(8):3262–3273. doi: 10.1021/mp400329g. [DOI] [PubMed] [Google Scholar]
- 126.Liu X, Liu C, Laurini E, Posocco P, Pricl S, Qu F, et al. Efficient delivery of sticky siRNA and potent gene silencing in a prostate cancer model using a generation 5 triethanolamine-core PAMAM dendrimer. Mol Pharm. 2012;9(3):470–481. doi: 10.1021/mp2006104. [DOI] [PubMed] [Google Scholar]
- 127.Yu T, Liu X, Bolcato-Bellemin A-L, Wang Y, Liu C, Erbacher P, et al. An amphiphilic dendrimer for effective delivery of small interfering RNA and gene silencing in vitro and in vivo. Angew Chem Int Ed. 2012;51(34):8478–8484. doi: 10.1002/anie.201203920. [DOI] [PubMed] [Google Scholar]
- 128.Patil ML, Zhang M, Taratula O, Garbuzenko OB, He H, Minko T. Internally cationic polyamidoamine PAMAM-OH dendrimers for siRNA delivery- effect of the degree of quaternization and cancer targeting. Biomacromolecules. 2009;10:258–266. doi: 10.1021/bm8009973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chen C-J, Wang J-C, Zhao E-Y, Gao L-Y, Feng Q, Liu X-Y, et al. Self-assembly cationic nanoparticles based on cholesterol-grafted bioreducible poly(amidoamine) for siRNA delivery. Biomaterials. 2013;34(21):5303–5316. doi: 10.1016/j.biomaterials.2013.03.056. [DOI] [PubMed] [Google Scholar]
- 130.Lin D, Jiang Q, Cheng Q, Huang Y, Huang P, Han S, et al. Polycation-detachable nanoparticles self-assembled from mPEG-PCL-g-SS-PDMAEMA for in vitro and in vivo siRNA delivery. Acta Biomater. 2013;9(8):7746–7757. doi: 10.1016/j.actbio.2013.04.031. [DOI] [PubMed] [Google Scholar]
- 131.Tanaka K, Kanazawa T, Horiuchi S, Ando T, Sugawara K, Takashima Y, et al. Cytoplasm-responsive nanocarriers conjugated with a functional cell-penetrating peptide for systemic siRNA delivery. Int J Pharm. 2013;455(1–2):40–47. doi: 10.1016/j.ijpharm.2013.07.069. [DOI] [PubMed] [Google Scholar]
- 132.Borchard G. Chitosans for gene delivery. Adv Drug Deliv Rev. 2001;52:145–150. doi: 10.1016/s0169-409x(01)00198-3. [DOI] [PubMed] [Google Scholar]
- 133.Liu X, Howard KA, Dong M, Andersen MØ, Rahbek UL, Johnsen MG, et al. The influence of polymeric properties on chitosan/siRNA nanoparticle formulation and gene silencing. Biomaterials. 2007;28(6):1280–1288. doi: 10.1016/j.biomaterials.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 134.Han HD, Mangala LS, Lee JW, Shahzad MMK, Kim HS, Shen D, et al. Targeted gene silencing using RGD-labeled chitosan nanoparticles. Clin Cancer Res. 2010;16(15):3910–3922. doi: 10.1158/1078-0432.CCR-10-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pille J, Li H, Blot E, Bertrand J, Pritchard L, Opolon P, et al. Intravenous delivery of anti-RhoA small interfering RNA loaded in nanoparticles of chitosan in mice safety and efficacy in xenografted aggressive breast cancer. Hum Gene Ther. 2006;17:1019–1026. doi: 10.1089/hum.2006.17.1019. [DOI] [PubMed] [Google Scholar]
- 136.de Martimprey H, Bertrand JR, Fusco A, Santoro M, Couvreur P, Vauthier C, et al. siRNA nanoformulation against the Ret/PTC1 junction oncogene is efficient in an in vivo model of papillary thyroid carcinoma. Nucleic Acids Res. 2007;36(1):e2. doi: 10.1093/nar/gkm1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yang F, Huang W, Li Y, Liu S, Jin M, Wang Y, et al. Anti-tumor effects in mice induced by survivin-targeted siRNA delivered through polysaccharide nanoparticles. Biomaterials. 2013;34(22):5689–5699. doi: 10.1016/j.biomaterials.2013.03.047. [DOI] [PubMed] [Google Scholar]
- 138.Son S, Song S, Lee SJ, Min S, Kim SA, Yhee JY, et al. Self-crosslinked human serum albumin nanocarriers for systemic delivery of polymerized siRNA to tumors. Biomaterials. 2013;34(37):9475–9485. doi: 10.1016/j.biomaterials.2013.08.085. [DOI] [PubMed] [Google Scholar]
- 139.Yhee JY, Lee SJ, Lee S, Song S, Min HS, Kang S-W, et al. Tumor-targeting transferrin nanoparticles for systemic polymerized siRNA delivery in tumor-bearing mice. Bioconjug Chem. 2013;24(11):1850–1860. doi: 10.1021/bc400226b. [DOI] [PubMed] [Google Scholar]
- 140.Kawata E, Ashihara E, Kimura S, Takenaka K, Sato K, Tanaka R, et al. Administration of PLK-1 small interfering RNA with atelocollagen prevents the growth of liver metastases of lung cancer. Mol Cancer Ther. 2008;7(9):2904–2912. doi: 10.1158/1535-7163.MCT-08-0473. [DOI] [PubMed] [Google Scholar]
- 141.Adachi T, Kawakami E, Ishimaru N, Ochiya T, Hayashi Y, Ohuchi H, et al. Delivery of small interfering RNAwith a synthetic collagen poly(Pro-Hyp-Gly) for gene silencing in vitro and in vivo. Dev Growth Differ. 2010;52(8):693–699. doi: 10.1111/j.1440-169X.2010.01206.x. [DOI] [PubMed] [Google Scholar]
- 142.Kim SW, Kim NY, Choi YB, Park SH, Yang JM, Shin S. RNA interference in vitro and in vivo using an arginine peptide/siRNA complex system. J Control Release. 2010;143(3):335–343. doi: 10.1016/j.jconrel.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 143.Kim W, Christensen L, Jo S, Yockman J, Jeong J, Kim Y, et al. Cholesteryl oligoarginine delivering vascular endothelial growth factor siRNA effectively inhibits tumor growth in colon adenocarcinoma. mol ther. 2006;14(3):343–350. doi: 10.1016/j.ymthe.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 144.Crombez L, Morris MC, Dufort S, Aldrian-Herrada G, Nguyen Q, Mc Master G, et al. Targeting cyclin B1 through peptide-based delivery of siRNA prevents tumour growth. Nucleic Acids Res. 2009;37(14):4559–4569. doi: 10.1093/nar/gkp451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lee SJ, Yhee JY, Kim SH, Kwon IC, Kim K. Biocompatible gelatin nanoparticles for tumor-targeted delivery of polymerized siRNA in tumor-bearing mice. J Control Release. 2013;172(1):358–366. doi: 10.1016/j.jconrel.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 146.Minakuchi Y. Atelocollagen-mediated synthetic small interfering RNA delivery for effective gene silencing in vitro and in vivo. Nucleic Acids Res. 2004;32(13):e109. doi: 10.1093/nar/gnh093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Beloor J, Choi CS, Nam HY, Park M, Kim SH, Jackson A, et al. Arginine-engrafted biodegradable polymer for the systemic delivery of therapeutic siRNA. Biomaterials. 2012;33(5):1640–1650. doi: 10.1016/j.biomaterials.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 148.Wang Z, Liu G, Zheng H, Chen X. Rigid nanoparticle-based delivery of anti-cancer siRNA: challenges and opportunities. Biotech Adv. 2013 doi: 10.1016/j.biotechadv.2013.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lytton-Jean AKR, Langer R, Anderson DG. Five years of siRNA delivery: spotlight on gold nanoparticles. Small. 2011;7(14):1932–1937. doi: 10.1002/smll.201100761. [DOI] [PubMed] [Google Scholar]
- 150.Patel PC, Giljohann DA, Daniel WL, Zheng D, Prigodich AE, Mirkin CA. Scavenger receptors mediate cellular uptake of polyvalent oligonucleotide-functionalized gold nanoparticles. Bioconjug Chem. 2010;21:2250–2256. doi: 10.1021/bc1002423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Conde J, Tian F, Hernández Y, Bao C, Cui D, Janssen K-P, et al. In vivo tumor targeting via nanoparticle-mediated therapeutic siRNA coupled to inflammatory response in lung cancer mouse models. Biomaterials. 2013;34(31):7744–7753. doi: 10.1016/j.biomaterials.2013.06.041. [DOI] [PubMed] [Google Scholar]
- 152.Lu W, Zhang G, Zhang R, Flores LG, Huang Q, Gelovani JG, et al. Tumor site-specific silencing of NF- B p65 by targeted hollow gold nanosphere-mediated photothermal transfection. Cancer Res. 2010;70(8):3177–3188. doi: 10.1158/0008-5472.CAN-09-3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Elhissi AMA, Ahmed W, Hassan IU, Dhanak VR, D'Emanuele A. Carbon nanotubes in cancer therapy and drug delivery. J Drug Deliv. 2012;2012:1–10. doi: 10.1155/2012/837327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Podesta JE, Al-Jamal KT, Herrero MA, Tian B, Ali-Boucetta H, Hegde V, et al. Antitumor activity and prolonged survival by carbon-nanotube-mediated therapeutic siRNA silencing in a human lung xenograft model. Small. 2009;5(10):1176–1185. doi: 10.1002/smll.200801572. [DOI] [PubMed] [Google Scholar]
- 155.Pittella F, Zhang M, Lee Y, Kim HJ, Tockary T, Osada K, et al. Enhanced endosomal escape of siRNA-incorporating hybrid nanoparticles from calcium phosphate and PEG-block charge-conversional polymer for efficient gene knockdown with negligible cytotoxicity. Biomaterials. 2011;32(11):3106–3114. doi: 10.1016/j.biomaterials.2010.12.057. [DOI] [PubMed] [Google Scholar]
- 156.Pittella F, Cabral H, Maeda Y, Mi P, Watanabe S, Takemoto H, et al. Systemic siRNA delivery to a spontaneous pancreatic tumor model in transgenic mice by PEGylated calcium phosphate hybrid micelles. J Control Release. 2014;178:18–24. doi: 10.1016/j.jconrel.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 157.Jiang S, Eltoukhy AA, Love KT, Langer R, Anderson DG. Lipidoid-coated iron oxide nanoparticles for efficient DNA and siRNA delivery. Nano Lett. 2013;13(3):1059–1064. doi: 10.1021/nl304287a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Li L, Gu W, Chen J, Chen W, Xu ZP. Co-delivery of siRNAs and anti-cancer drugs using layered double hydroxide nanoparticles. Biomaterials. 2014;35(10):3331–3339. doi: 10.1016/j.biomaterials.2013.12.095. [DOI] [PubMed] [Google Scholar]
- 159.Chamarthy SP, Kovacs JR, McClelland E, Gattens D, Meng WS. A cationic peptide consists of ornithine and histidine repeats augments gene transfer in dendritic cells. Mol Immunol. 2003;40(8):483–490. doi: 10.1016/j.molimm.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 160.Kovacs JR, Zheng Y, Shen H, Meng WS. Polymeric microspheres as stabilizing anchors for oligonucleotide delivery to dendritic cells. Biomaterials. 2005;26(33):6754–6761. doi: 10.1016/j.biomaterials.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 161.Meng WS, Butterfield LH. Activation of antigen-presenting cells by DNA delivery vectors. Expert Opin Biol Ther. 2005;5(8):1019–1028. doi: 10.1517/14712598.5.8.1019. [DOI] [PubMed] [Google Scholar]
- 162.Pack DW, Hoffman AS, Pun S, Stayton PS. Design and development of polymers for gene delivery. Nat Rev Drug Discov. 2005;4(7):581–593. doi: 10.1038/nrd1775. [DOI] [PubMed] [Google Scholar]
- 163.Kullberg M, McCarthy R, Anchordoquy TJ. Systemic tumor-specific gene delivery. J Control Release. 2013;172(3):730–736. doi: 10.1016/j.jconrel.2013.08.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Rojanasakul Y. Antisense oligonucleotide therapeutics drug delivery and targeting. Adv Drug Deliv Rev. 1996;18:115–131. [Google Scholar]
- 165.Rozema DB, Lewis DL, Wakefield DH, Wong SC, Klein JJ, Roesch PL, et al. Dynamic PolyConjugates for targeted in vivo delivery of siRNA to hepatocytes. Proc Natl Acad Sci U S A. 2007;104(32):12982–12987. doi: 10.1073/pnas.0703778104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wooddell CI, Rozema DB, Hossbach M, John M, Hamilton HL, Chu Q, et al. Hepatocyte-targeted RNAi therapeutics for the treatment of chronic hepatitis B virus infection. Mol Ther. 2013;21(5):973–985. doi: 10.1038/mt.2013.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wong SC, Klein JJ, Hamilton HL, Chu Q, Frey CL, Trubetskoy VS, et al. Co-injection of a targeted, reversibly masked endosomolytic polymer dramatically improves the efficacy of cholesterol-conjugated small interfering RNAs in vivo. Nucleic Acid Ther. 2012;22(6):380–390. doi: 10.1089/nat.2012.0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Grodzinski P, Farrell D. Future opportunities in cancer nanotechnology—NCI strategic workshop report. Cancer Res. 2014;74(5):1307–1310. doi: 10.1158/0008-5472.CAN-13-2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Judge A, McClintock K, Phelps J, Maclachlan I. Hypersensitivity and loss of disease site targeting caused by antibody responses to PEGylated liposomes. Mol Ther. 2006;13(2):328–337. doi: 10.1016/j.ymthe.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 170.Kim JS, Oh MH, Park JY, Park TG, Nam YS. Protein-resistant, reductively dissociable polyplexes for in vivo systemic delivery and tumor-targeting of siRNA. Biomaterials. 2013;34(9):2370–2379. doi: 10.1016/j.biomaterials.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 171.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 172.Li L, Wang R, Wilcox D, Zhao X, Song J, Lin X, et al. Tumor vasculature is a key determinant for the efficiency of nanoparticle-mediated siRNA delivery. Gene Ther. 2011;19(7):775–780. doi: 10.1038/gt.2011.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Semple SC, Akinc A, Chen J, Sandhu AP, Mui BL, Cho CK, et al. Rational design of cationic lipids for siRNA delivery. Nat Biotechnol. 2010;28(2):172–176. doi: 10.1038/nbt.1602. [DOI] [PubMed] [Google Scholar]
- 174.Zhang Y, Pelet JM, Heller DA, Dong Y, Chen D, Gu Z, et al. Lipid-modified aminoglycoside derivatives for in vivo siRNA delivery. Adv Mater. 2013;25(33):4641–4645. doi: 10.1002/adma.201301917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Guk K, Lim H, Kim B, Hong M, Khang G, Lee D. Acid-cleavable ketal containing poly(β-amino ester) for enhanced siRNA delivery. Int J Pharm. 2013;453(2):541–550. doi: 10.1016/j.ijpharm.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 176.Hong BJ, Chipre AJ, Nguyen ST. Acid-degradable polymer-caged lipoplex (PCL) platform for siRNA delivery: facile cellular triggered release of siRNA. J Am Chem Soc. 2013;135(47):17655–17658. doi: 10.1021/ja404491r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Abeylath SC, Ganta S, Iyer AK, Amiji M. Combinatorial-designed multifunctional polymeric nanosystems for tumor-targeted therapeutic delivery. Acc Chem Res. 2011;44(10):1009–1017. doi: 10.1021/ar2000106. [DOI] [PubMed] [Google Scholar]