ABSTRACT
The protozoan parasite Toxoplasma gondii resides within a nonfusogenic vacuole during intracellular replication. Although the limiting membrane of this vacuole provides a protective barrier to acidification and degradation by lysosomal hydrolases, it also physically segregates the parasite from the host cytosol. Accordingly, it has been suggested that T. gondii acquires material from the host via membrane channels or transporters. The ability of the parasite to internalize macromolecules via endocytosis during intracellular replication has not been tested. Here, we show that Toxoplasma ingests host cytosolic proteins and digests them using cathepsin L and other proteases within its endolysosomal system. Ingestion was reduced in mutant parasites lacking an intravacuolar network of tubular membranes, implicating this apparatus as a possible conduit for trafficking to the parasite. Genetic ablation of proteins involved in the pathway is associated with diminished parasite replication and virulence attenuation. We show that both virulent type I and avirulent type II strain parasites ingest and digest host-derived protein, indicating that the pathway is not restricted to highly virulent strains. The findings provide the first definitive evidence that T. gondii internalizes proteins from the host during intracellular residence and suggest that protein digestion within the endolysosomal system of the parasite contributes to toxoplasmosis.
IMPORTANCE
Toxoplasma gondii causes significant disease in individuals with weak immune systems. Treatment options for this infection have drawbacks, creating a need to understand how this parasite survives within the cells it infects as a prelude to interrupting its survival strategies. This study reveals that T. gondii internalizes proteins from the cytoplasm of the cells it infects and degrades such proteins within a digestive compartment within the parasite. Disruption of proteins involved in the pathway reduced parasite replication and lessened disease severity. The identification of a novel parasite ingestion pathway opens opportunities to interfere with this process and improve the outcome of infection.
INTRODUCTION
Nearly one-third of the global human population is infected with Toxoplasma gondii. Although immunocompetent individuals control tachyzoite-stage parasites during acute infection, the parasite survives indefinitely by forming dormant bradyzoites within tissue cysts residing in the brain and other tissues during chronic infection. When host immunity wanes, such as in patients receiving an organ transplant, chemotherapy, or those with untreated HIV/AIDS, the infection reactivates, causing life-threatening disease. Individuals infected congenitally also experience significant disease at birth or later in life (1).
Toxoplasma parasites are adept at invading and replicating within a wide variety of warm-blooded animal cells (2). Toxoplasma tachyzoites invaginate the host plasma membrane during invasion to create a safe intracellular niche, the parasitophorous vacuole (PV) (3). Parasites constitutively secrete dense granule (GRA) proteins into the PV, including some that help to form a network of tubule-like structures called the intravacuolar network (IVN) (4, 5). The IVN has been proposed to facilitate the uptake of resources from the host cell. A recent study implicated the IVN in parasite acquisition of lipids from the host (6), but this structure has not been linked to the acquisition of other host-derived resources, and its precise function remains unknown.
The nonfusogenic membrane of the PV renders it refractory to merging with the host endolysosomal system, thereby circumventing acidification of the PV and avoiding parasite exposure to destructive lysosomal enzymes (7). However, the PV membrane (PVM) is also a physical barrier between the parasite and the rich environment of the host cytoplasm. Previous studies using fluorescent dyes suggested the existence of small pores in the PVM that allow solutes of less than ~1,300 Da to cross the PVM (8). Hence, small nutrients (e.g., amino acids and nucleotides) were proposed to traverse such pores, supplementing the nutritional demands of parasites. However, the molecular basis of this hypothetical pore has not been reported, and its substrates remain unknown. The pore is also likely too small to acquire macromolecules from the host. Goldszmid et al. (9) reported that the endoplasmic reticulum (ER) membrane of mouse dendritic cells fuses with the PVM to deliver parasite antigens to the major histocompatibility complex class I (MHC-I) pathway, raising the converse possibility that Toxoplasma has access to host proteins derived from the ER. It remains unclear, however, if this phenomenon occurs in other cell types or whether it is used to acquire host resources.
A subpopulation of extracellular parasites was shown to internalize fluorescent heparin bound to the parasite surface (10, 11). Several studies have also reported markers for early and late endosomes within the parasite (12, 13). These findings suggest that Toxoplasma has the potential to endocytose host-derived macromolecules.
Toxoplasma encodes five cathepsin-like proteases (14), including two endopeptidases termed cathepsin protease L (CPL) and cathepsin protease B (CPB). CPL and CPB reside within a dynamic acidified organelle named the vacuolar compartment (VAC) (12, 15) that bears resemblance to a plant-like vacuole (PLV) (16). The VAC contains proteases classically associated with lysosomes, implicating it as the terminal compartment of the parasite endolysosomal system, where protein degradation occurs. Although CPL is capable of limited proteolysis for the maturation of at least two microneme proteins involved in parasite invasion, it can also degrade proteins under conditions optimal for enzymatic activity (12). Accordingly, we reasoned that in addition to its specialized role as a maturase for some microneme proteins, CPL functions as a classic degradative enzyme within the parasite endolysosomal system.
Here, we show that intracellular Toxoplasma tachyzoites ingest proteins derived from the host cytosol, but not the ER, and that these proteins accumulate in the endolysosomal system of parasites deficient in CPL expression or activity. The findings suggest a novel ingestion pathway to acquire macromolecules from host cells during intracellular replication.
RESULTS
Toxoplasma ingests host cytosolic proteins.
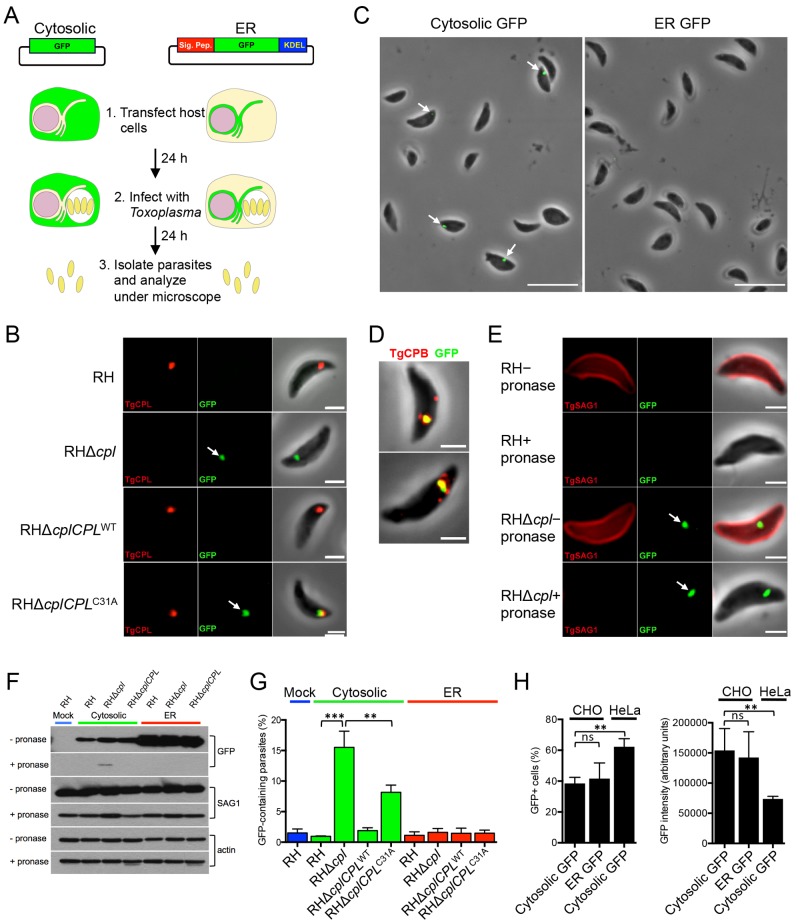
To assess T. gondii tachyzoite ingestion of host-derived proteins, we transiently transfected cytosolic and ER green fluorescent protein (GFP) constructs into Chinese hamster ovary (CHO) cells (Fig. 1A). A possible role for CPL in the degradation of host-derived proteins was tested by infecting the transfected CHO cells with virulent type I RH strain parasites of the wild type (WT), the RHΔcpl knockout strain, or one of the complemented strains RHΔcplCPLWT (complemented with active CPL) and RHΔcplCPLC31A (complemented with catalytically inactive CPL). Parasites were allowed to replicate overnight, mechanically liberated and purified from infected cells, fixed, and observed by fluorescence microscopy to quantify the percentage of GFP-containing parasites for each strain. Whereas host cytosolic GFP was not seen associated with RH parasites, it was found affiliated with RHΔcpl parasites (Fig. 1B), indicating an inverse correlation with CPL expression. GFP derived from host ER was not seen in either strain, implying that the parasite does lack access to proteins from the host ER (Fig. 1C). Moreover, cytosolic GFP was associated with RHΔcplCPLC31A parasites deficient in CPL activity but not with RHΔcplCPLWT parasites, consistent with GFP associating with the parasite in the absence of CPL activity (Fig. 1B). Host-derived GFP partially colocalized with CPL within the VAC (Fig. 1B) in 54% (15/28) of RHΔcplCPLC31A parasites displaying fluorescence, indicating that the acquired GFP enters the parasite endolysosomal system. GFP also colocalized with CPB (Fig. 1D) in a similar subset of parasites (49% [53/109]), further supporting entry into the parasite endolysosomal system. Together, these findings imply that parasites endocytose host cytosolic proteins, which are degraded in a CPL-dependent manner within the parasite endolysosomal system.
FIG 1 .
Experimental design and discovery of the ingestion pathway. (A) Experimental design with GFP constructs expressing cytosolic or ER GFP. Constructs were transiently transfected into CHO cells, and expression was allowed to develop for 24 h before infection with the indicated strains. Parasites were mechanically liberated from host cells after 24 h of replication and examined by fluorescence microscopy. (B) Host cytosolic GFP is associated with RHΔcpl and RHΔcplCPLC31A strains lacking cathepsin L activity but not WT or genetically wild-type CPL-complemented parasites. Mechanically liberated parasites were fixed and stained with antibodies to CPL. Bar, 2 µm. (C) Fluorescent overlay images of RHΔcpl parasites harvested from host cells expressing the indicated GFP. Bars, 10 µm. (D) GFP colocalizes with CBP in the VAC of a subset of parasites. Shown are two examples of GFP-positive parasites stained with anti-CPB. Bars, 10 µm. (E) GFP is shielded from pronase treatment in RHΔcpl parasites. Parasites harvested from GFP-expressing host cells were treated with pronase to test for protease protection as an indicator of internalization. Parasites were stained with antibodies to the surface antigen SAG1, which was proteolysed on treated parasites. Bars, 2 µm. (F) Quantification of GFP in the indicated strains liberated from host cells transfected with constructs for expression of cytosolic or ER GFP. Statistical significance by unpaired Student’s t test: **, P < 0.01; ***, P < 0.001. (G) Immunoblot detection of ingested GFP after pronase treatment. RH, RHΔcpl, and RHΔcplCPLWT parasites were used to infect CHO cells transiently expressing cytosolic and ER GFP proteins, respectively. Twenty-four hours postinfection, parasites were harvested and treated with pronase and saponin. The lysates of treated parasites were probed with anti-GFP antibody. Lysates were also probed with polyclonal antibodies against actin and SAG1 as a loading control and by measurement of pronase digestion, respectively. (H) Transfection efficiency of cytosolic and ER-retained GFP in CHO and HeLa cells. DNA constructs were transfected into CHO and HeLa cells. Twenty-four hours posttransfection, cells were trypsinized and analyzed by flow cytometry to determine the percentage of GFP-positive cells (left) and GFP intensity (right). Values shown in panels A and B are means ± standard deviations (SD) from n = 3 experiments. Significance by unpaired Student’s t test: *, P < 0.05; **, P < 0.01. ns, not significant (i.e., P > 0.05).
To validate residence of host-derived GFP inside parasites, we performed protease protection experiments, wherein harvested parasites were treated with pronase to digest any externally exposed GFP. Digests were performed at 12°C to preclude endocytosis of pronase while retaining sufficient protease activity. Immunofluorescence (Fig. 1E) and immunoblotting (Fig. 1F) assays showed that host cytosolic GFP associated with RHΔcpl parasites was protected from protease treatment, suggesting that it is contained within parasites.
Quantification of GFP acquisition revealed that approximately 15% of RHΔcpl parasites displayed internalized host cytosolic GFP, whereas RH parasites showed negligible GFP fluorescence, similar to parasites derived from nontransfected CHO cells (mock) (Fig. 1G). Cytosolic and ER GFP showed comparable transfection efficiencies (~40%) and expression levels (~150,000 arbitrary units [AU]) (Fig. 1H), establishing that the exclusive detection of host-derived cytosolic GFP in parasites was not due to differences in GFP expression. Although infection with CHO cells stably expressing GFP resulted in a greater percentage of GFP-positive parasites (40 to 50%), GFP expression was lower than in transiently transfected cells, and thus enumeration was less accurate due to low signal (data not shown). Interestingly, RHΔcplCPLC31A parasites expressing catalytically inactive CPL showed a lower percentage of GFP-containing parasites than RHΔcpl parasites that lack CPL completely (Fig. 1G). We previously demonstrated that the CPLC31A mutant protein was inactive based on the complete loss of CPB maturation in the RHΔcplCPLC31A parasites (15). While the basis of the difference remains to be determined, this finding implies that CPL plays catalytic and noncatalytic roles in the digestion and/or ingestion of host-derived proteins.
Ingestion occurs during intracellular replication and is not restricted by parasite strain or host cell type.
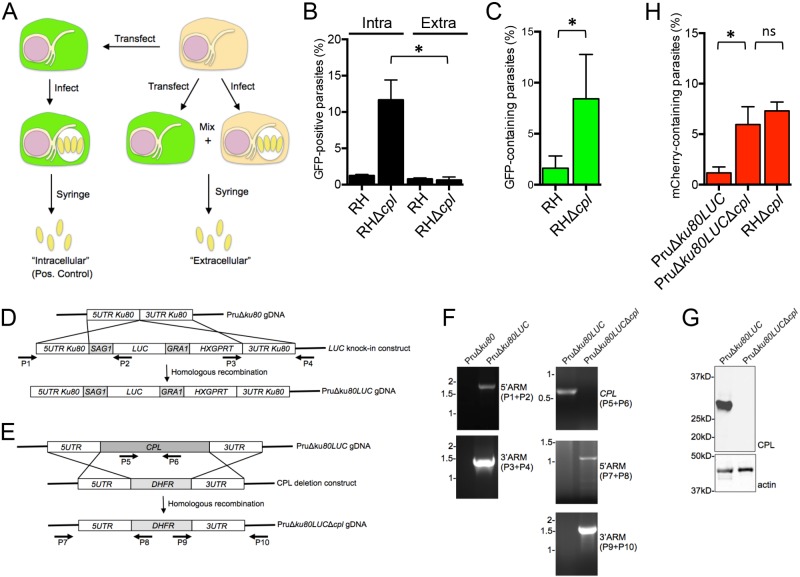
Botero-Kleiven et al. (11) reported that extracellular Toxoplasma parasites are capable of internalizing an endocytic tracer (heparin) when incubated at 37°C. Our harvest procedure was conducted on ice to prevent endocytosis from occurring during parasite isolation. Nonetheless, to confirm that the GFP was ingested during intracellular replication, we mixed infected, untransfected CHO cells with uninfected, transfected CHO cells prior to harvest (Fig. 2A). Thus, parasites were only exposed to GFP upon mechanical rupture of the mixed sample (i.e., when they were “extracellular”). The percentage of GFP-containing parasites for the “extracellular” RHΔcpl parasites was negligible, similar to that of RH parasites (Fig. 1B), suggesting that the ingestion of host proteins predominantly occurred during intracellular residence.
FIG 2 .
GFP is ingested during intracellular replication. (A) Experimental scheme of testing for the absence of GFP ingestion during parasite harvest. As a positive (Pos.) control (left side of the scheme), CHO cells were transfected with the cytosolic GFP expression construct, infected 24 h posttransfection with RH or RHΔcpl parasites, and mechanically liberated by syringing 24 h postinfection. These were termed “intracellular” parasites. To test for uptake of host cytosolic GFP during mechanical rupture and harvest (right side of the scheme), CHO cells were transiently transfected and mixed with untransfected and infected CHO cells, and parasites were mechanically liberated by syringing. These were termed “extracellular” parasites because internalization could only occur after the parasites were liberated. (B) GFP is not ingested during harvest, indicating ingestion during intracellular replication. Shown is the percentage of GFP-positive parasites harvested by the “intracellular” (intra) or “extracellular” (extra) scheme. Statistical significance by unpaired Student’s t test: *, P < 0.05. (C) GFP transiently expressed in HeLa cells is associated with CPL-deficient parasites, indicating that the parasites also ingest host cytosolic proteins from human cells. Statistical significance by unpaired Student’s t test: *, P < 0.05. (D) Schematic illustration of the strategy for creating PruΔku80LUC parasites. A luciferase expression cassette (LUC) was transfected into PruΔku80 parasites and selected with mycophenolic acid and xanthine for double crossover insertion via homologous recombination at the “empty” ku80 locus. (E) A CPL deletion construct was transfected into PruΔku80LUC parasites and selected with pyrimethamine for double crossover replacement of the CPL by homologous recombination. (F) Primers indicated in panels D and E were used to verify the insertion of LUC at the KU80 locus and CPL deletion by PCR and agarose gel electrophoresis. ARM, the end of the target gene. (G) Cell lysates were immunoblotted with antibodies against CPL to confirm the knockout and with antibodies for actin as a loading control. (H) PruΔku80LUCΔcpl (type II genotype) parasites also incorporate host cytosolic mCherry at approximately the same level as the RHΔcpl strain (type I genotype). Statistical significance by paired Student’s t test: *, P < 0.05. ns, not significant (i.e., P > 0.05). Values shown in panels E to H represent means ± SD from n ≥ 3 experiments.
To determine if the Toxoplasma ingestion pathway is independent of host cell type, we transfected human HeLa cells with the cytosolic GFP construct and infected them with RH or RHΔcpl parasites. Approximately 8% of RHΔcpl parasites harvested from HeLa cells were positive for GFP (Fig. 2C). This lower efficiency is probably due to reduced GFP expression in HeLa versus CHO cells (Fig. 1H). The findings indicate that parasite ingestion of host cytosolic protein is not limited to the type or origin of the infected host cell.
Since CPL is expressed in multiple strain types (http://toxodb.org), we tested the extent to which an avirulent genotype II strain also ingests host cytosolic protein. As shown in Fig. 2D, we began by introducing firefly luciferase under the control of the SAG1 promoter into PruΔku80, a strain that is more receptive to genetic manipulation than the parent Prugniaud strain (17), to generate PruΔku80LUC. Luciferase is a convenient reporter for parasite replication (18), which is assessed below. We subsequently deleted CPL in PruΔku80LUC parasites and validated the absence of the CPL gene and its expression (Fig. 2E to G). We transfected CHO cells with an mCherry expression construct since PruΔku80LUC parasites express GFP (17). PruΔku80LUCΔcpl parasites acquired host cytosolic mCherry to levels comparable to those of RHΔcpl in the same experiments (Fig. 2H). This finding establishes that the ingestion pathway is not limited to strain type and is functional both in highly virulent type I parasites and less virulent type II parasites. Although mCherry and GFP are similar sizes (~26 kDa), they are unrelated in amino acid sequence. The internalization of both reporter proteins suggests that this ingestion pathway is not selective for protein type. Collectively, our findings establish that Toxoplasma parasites ingest host cytosolic proteins during intracellular replication and that CPL is a key protease participating in digestion of internalized host proteins.
Residual digestion of GFP occurs in CPL-deficient extracellular parasites.
Host-derived cytosolic GFP was exclusively seen in CPL-deficient strains, suggesting that CPL contributes substantially to the digestion of host-derived protein. To test this further and to examine the extent to which other parasite proteases contribute to GFP degradation, we treated RH-infected host cells with the cathepsin inhibitor morpholine urea-leucyl-homophenyl-vinyl sulfone phenyl (LHVS), which is a selective and irreversible inhibitor of CPL (19). Parasites incubated with LHVS during intracellular replication and in medium as extracellular parasites (RH+LHVS) showed marginally less accumulation of host-derived cytosolic GFP than RHΔcpl parasites. This finding is consistent with those of the CPL catalytic mutant described above and suggests a principal requirement for CPL activity in the degradation of host-derived protein. As expected, nontreated RH parasites (RH-LHVS) failed to accumulate host-derived protein. Parasites were incubated subsequently in medium for 1, 2, 4, 8, and 12 h. The percentage of GFP-containing RHΔcpl parasites gradually decreased with time (Fig. 3), indicating that other hydrolases within parasites likely contribute to the residual digestion of the ingested host proteins. RH+LHVS parasites showed a similar trend of decreasing fluorescence, while untreated RH-LHVS parasites showed no change in residual fluorescence. Overall, these findings reaffirm that host-derived protein accumulates in parasites lacking CPL activity and other proteases in addition to CPL likely contribute to the degradation of ingested protein.
FIG 3 .
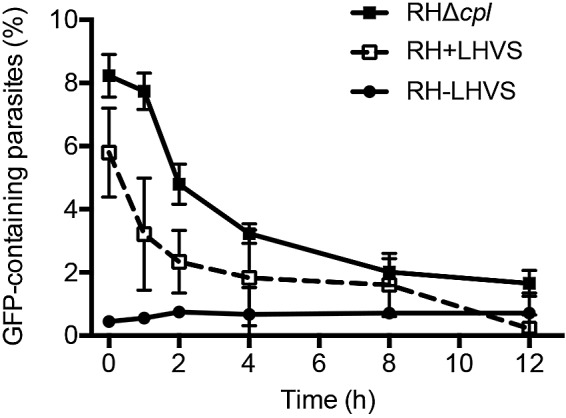
Residual digestion of GFP occurs in CPL-deficient extracellular parasites. CHO cells transiently expressing cytosolic GFP were infected with RHΔcpl parasites or RH parasites either treated with LHVS during replication and during extracellular incubation (RH+LHVS) or not treated with LHVS during either replication or extracellular incubation (RH-LHVS). The values shown represent means ± SD from n = 3 experiments.
An intact intravacuolar network is required for efficient ingestion of host protein.
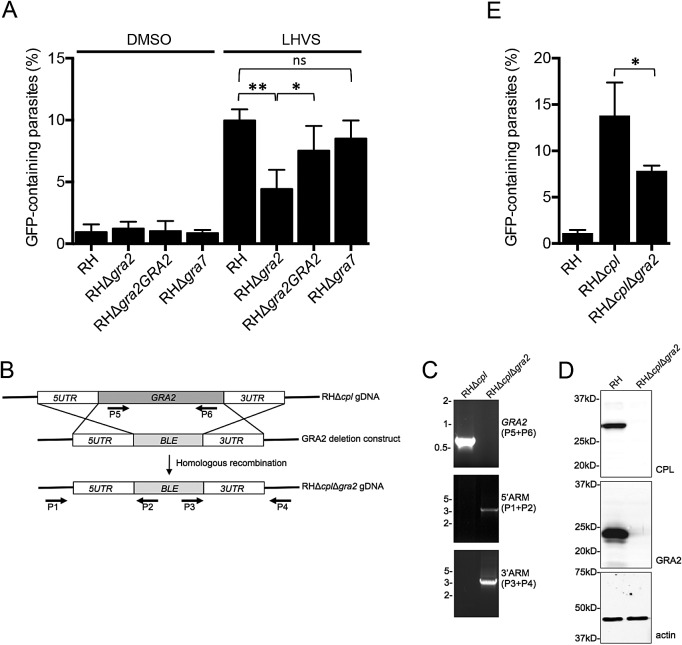
After entry into host cells, parasites modify the PV by secreting dense granule proteins, including GRA2, which is required for elaboration of the tubule-like IVN (4, 5). The IVN was proposed to facilitate nutrient acquisition (20, 21), but evidence supporting this conjecture is sparse. Separately, GRA7 has been suggested to help sequester host lysosomes within microtubule-based invaginations of the PVM as part of a pathway for host cholesterol acquisition (22). We reasoned that the IVN or the lysosome/microtubule invaginations might act as a conduit for parasite ingestion of host cytosolic proteins. To test this, we treated RH, RHΔgra2, RHΔgra2GRA2, and RHΔgra7 mutants with LHVS to inhibit CPL activity and facilitate the visualization of incorporated GFP. The RHΔgra7 strain displayed a similar percentage of GFP-containing parasites as RH parasites (Fig. 4A), rendering it unlikely that host cytosolic proteins traverse the PV via lysosome/microtubule invaginations. However, ablation of GRA2 resulted in an approximately 50% reduction of host protein acquisition, and reexpression of GRA2 in the RHΔgra2GRA2 complementation strain restored GFP accumulation.
FIG 4 .
An intact intravacuolar network is required for efficient ingestion of host protein. (A) Parasite strains were treated with LHVS during replication (or DMSO as vehicle control) in CHO cells transiently expressing cytosolic GFP. The results represent means ± SD from n = 3 independent determinations. Statistical analysis was by paired Student’s t test. (B) Schematic illustration for creation of the RHΔcplΔgra2 mutant. A PCR product carrying a phleomycin resistance cassette (BLE) flanked by GRA2 targeting sequences was transfected into RHΔcpl parasites for double crossover replacement of GRA2. (C) Primers indicated in panel A were used to verify the replacement of GRA2 with ble by PCR and agarose gel electrophoresis. (D) Cell lysates of RH and RHΔcplΔgra2 were immunoblotted with antibodies against CPL or GRA2 to confirm the absence of CPL and GRA2 expression in the RHΔcplΔgra2 mutant. Samples were immunoblotted also for actin as a loading control. (E) Ingestion is impaired in RHΔcplΔgra2 parasites. The values shown represent means ± SD from n = 3 independent experiments. Statistical significance by unpaired Student’s t test: *, P < 0.05; **, P < 0.01. ns, not significant (i.e., P > 0.05).
To validate these findings, we genetically ablated gra2 from RHΔcpl parasites, creating a Δcpl Δgra2 double knockout mutant, confirmed by PCR and immunoblotting (Fig. 4B to D). RHΔcplΔgra2 parasites similarly displayed an ~50% decrease in host protein ingestion compared to RHΔcpl parasites (Fig. 4E). Taken together, these findings implicate the IVN as a possible conduit for parasite ingestion of host cytosolic proteins.
CPL activity is required for normal replication.
We reasoned that the inefficient digestion of proteins in the Δcpl mutants might affect their replication. To test this, we grew RH, RHΔcpl, RHΔcplCPLWT, and RHΔcplCPLC31A parasites in human foreskin fibroblast (HFF) cells and quantified replication by enumerating parasites per PV. At 17 h postinfection, RHΔcpl parasites showed fewer four-parasite vacuoles than the RH strain (Fig. 5A). RHΔcpl parasites also showed fewer large vacuoles (≥16 parasites) than the RH strain at 26 h. Mirroring these results, RHΔcplCPLC31A parasites grew more slowly than RHΔcplCPLWT parasites, indicating a role for CPL proteolytic activity in replication. A wider kinetic analysis of replication suggested that RHΔcpl parasites are slow to initiate replication based on the shallow increase in divided parasites 12 to 20 h postinfection (Fig. 5B). CPL-deficient parasites appear to also replicate more slowly during logarithmic growth, as indicated by the lower slope 28 to 36 h postinfection. Conversely, the absence of GRA2 in RHΔgra2 parasites did not significantly affect parasite replication, a finding that was confirmed in the RHΔcplΔgra2 strain. Using a bioluminescence-based assay, we also found that PruΔku80LUCΔcpl replicated slower than its parental strain (Fig. 5C). The findings suggest that protein digestion within the parasite endolysosomal system contributes to parasite growth independent of strain type.
FIG 5 .
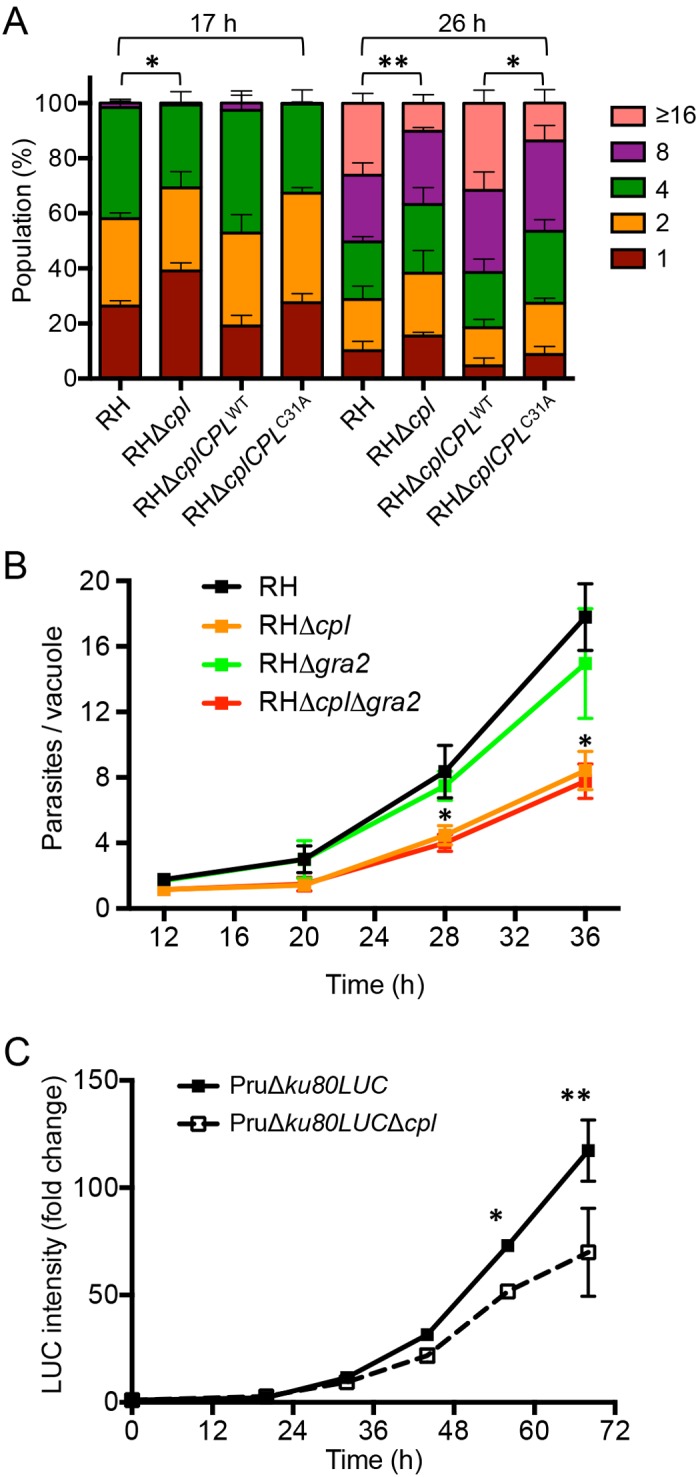
CPL activity is required for normal replication. (A) CPL-deficient parasites have a replication defect. Parasites were cultured in the monolayer HFF cells, and samples were collected at 17 and 26 h postinfection, fixed, stained with DAPI (4′,6-diamidino-2-phenylindole) and rabbit anti-SAG1 antibody, and quantified by fluorescence microscopy. At least 100 vacuoles were counted from 6 different fields of view. The percentages of different replication stages in the population for each strain were plotted. Results represent means ± SD from n = 3 experiments. Statistical significance by unpaired Student’s t test: *, P < 0.05; **, P < 0.01. (B) GRA2 does not significantly influence replication. Parasites were inoculated into HFF cells in chamber slides and allowed to replicate for the indicated times. Monolayers were fixed, stained with crystal violet, and enumerated by light microscopy. Parasitophorous vacuoles containing 1 parasite were not included in the data for 28 and 36 h to avoid skewing and to allow assessment of the logarithmic growth phase. Results represent means ± SD from n = 2 independent experiments, each with duplicate samples. Statistical significance by unpaired Student’s t test: *, P < 0.05. (C) Replication of PruΔku80LUC and PruΔku80LUCΔcpl assessed by luciferase activity. Results represent the mean ± standard error of the mean (SEM) fold change normalized to the respective 0-h value set at 1. n = 3 experiments. Statistical significance by paired Student’s t test: *, P < 0.05; **, P < 0.01.
Parasites deficient in CPL and GRA2 are virulence attenuated.
We also evaluated the virulence of RH, RHΔcpl, RHΔcplCPLWT, RHΔcplCPLC31A, RHΔgra2, and RHΔcplΔgra2 strains in vivo. One thousand parasites from each strain were injected subcutaneously into female CD-1 mice. Mice infected with RHΔcpl or RHΔgra2 showed a 4- to 5-day delay in lethality compared to mice infected with the RH strain (Fig. 6A), suggesting that proteolysis within the parasite endolysosomal system or the integrity of the IVN moderately contributes to virulence. Consistent with its faster in vitro replication, RHΔcplCPLWT parasites displayed slightly higher virulence than the RH strain, and RHΔcplCPLC31A showed slightly lower virulence. Interestingly, the RHΔcplΔgra2 double knockout parasites showed an apparent synergistic loss of virulence, with survival of 80% of the infected mice. The surviving mice were confirmed seropositive for anti-Toxoplasma antibodies. Although not definitive, the marked loss of virulence in the RHΔcplΔgra2 double knockout mutant is consistent with CPL and GRA2 functioning in the same or related pathway(s).
FIG 6 .
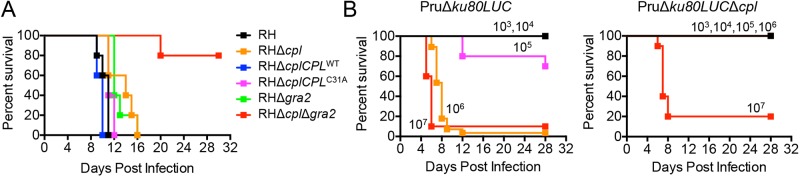
Parasites deficient in CPL and GRA2 are virulence attenuated. (A) CPL and GRA2 contribute synergistically to virulence. One thousand parasites of each strain were used to infect outbred CD-1 mice by subcutaneous injection. Data are combined from two independent experiments, each with 5 mice per group. (B) Pru strain parasites deficient in CPL are virulence attenuated. The indicated doses of PruΔku80LUC and PruΔku80LUCΔcpl parasites were injected into C57BL/6 mice intraperitoneally. Data are combined from two independent experiments, each with 5 mice per group.
We next analyzed the contribution of CPL to virulence in the PruΔku80LUC type II strain background. Mice infected with PruΔku80LUC parental strain parasites showed dose-dependent survival, with all mice surviving inoculation with 103 or 104 parasites, 70% of the mice surviving inoculation with 105 parasites, and 10% or fewer surviving inoculation with 106 or 107 parasites (Fig. 6B, left panel). Statistical analysis of the survival data estimated the 50% lethal dose (LD50) of this parental strain as 1.78 × 105 parasites. Mice infected with PruΔku80LUCΔcpl CPL-deficient parasites showed greater survival, with all mice surviving inoculation with 103, 104, 105, and 106 parasites and 20% of the mice surviving inoculation with 107 parasites (Fig. 6B, right panel). The LD50 for this strain was ~8.8 × 106, which is 47× higher than that of the parental strain. Collectively, these results establish that CPL contributes to parasite virulence irrespective of the strain type and that CPL and GRA2 together are crucial for virulence in a type I strain background.
CPL contributes to the course of infection.
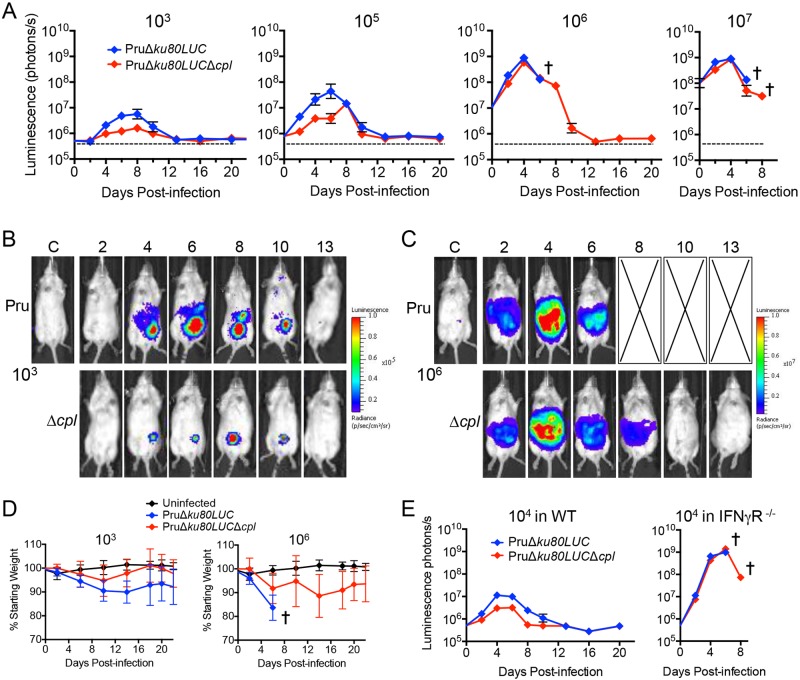
To investigate the relationship between virulence and parasite burden, we compared the in vivo replications of PruΔku80LUC parental and PruΔku80LUCΔcpl CPL-deficient parasites by measuring whole-body bioluminescence of infected mice. Mice infected with 103 or 105 CPL-deficient parasites showed a 5- to 10-fold reduction in total body bioluminescence compared to the parental strain (Fig. 7A and B), indicating a decrease and delay (for the 105 inoculum) in peak parasite burden. Interestingly, this differential pattern was not seen in higher-dose infections (106 and 107 parasites), where the kinetics of parasite burden by the two strains mirrored each other, with less than 2-fold differences observed (Fig. 7A and C). Consistent with the virulence findings above, mice infected with 106 parental parasites became moribund before day 8 postinfection, whereas CPL-deficient parasites survived the infection. Recording of body weight as a proxy for disease severity revealed a greater loss of weight by mice infected with 103 parental parasites than in those infected with the same dose of CPL-deficient parasites (Fig. 7D). Mice infected with 106 PruΔku80LUC parasites lost weight precipitously before becoming moribund. Mice infected with PruΔku80LUCΔcpl parasites also lost weight, but at a more moderate rate. These findings suggest that CPL deficiency results in decreased parasite burden at low to moderate doses and less severe disease during infection even at a higher dose.
FIG 7 .
CPL contributes to the course of infection. (A) In vivo replication of PruΔku80LUC and PruΔku80LUCΔcpl parasites assessed by bioluminescence imaging. Albino C57BL/6 mice (5 per group) were infected with the indicated doses of parasites by intraperitoneal injection. Mice were injected with d-luciferin, anesthetized, and imaged ventrally for 1 min. A cross indicates the point at which all mice became moribund and were humanely euthanized. (B) Pseudocolored images of bioluminescence from mice representative of PruΔku80LUC or PruΔku80LUCΔcpl groups infected with 103 parasites. (C) Pseudocolored images of bioluminescence from mice representative of PruΔku80LUC or PruΔku80LUCΔcpl groups infected with 106 parasites. (D) Mouse weight as an indicator of disease severity. Infected animals from the group inoculated with 103 or 106 parasites in panel A were weighed, and the values were plotted as the percentage of the initial weight over time. (E) Infection of IFN-γR−/− mice suggests a role for CPL in evasion of innate immunity. C57BL/6 WT or IFN-γR−/− mice (5 per group) were inoculated intraperitoneally with 104 PruΔku80LUC or PruΔku80LUCΔcpl parasites and monitored for bioluminescence.
The lower burden of CPL-deficient parasites at low to moderate doses could be due to slower replication of the parasite and/or a result of better clearance of parasites by the host immune response. If the lower burden is due to slower replication, then it is expected that the CPL-deficient parasite burden will remain lower than that of the parental strain in immunocompromised mice. On the other hand, if immune clearance contributes to the lower burden of CPL-deficient parasites, then differences in burden should be less evident in immunocompromised mice. Since interferon gamma (IFN-γ) is crucial for immunity to T. gondii, we infected WT and IFN-γ receptor knockout (IFN-γR−/−) mice with a moderate dose (104) of CPL-deficient and parental strains. As expected, both strains replicated rapidly and to a very high burden in the absence of IFN-γ signaling (Fig. 7E). The CPL-deficient parasite burden progressed initially at a lower rate, reaching 67% and 65% of parental strain levels at days 2 and 4, respectively, consistent with the findings for in vitro replication. Nevertheless, the differences in parasite burdens in IFN-γR−/− mice were much less pronounced than those of WT mice, suggesting that CPL-deficient parasites are vulnerable to clearance by the immune system.
DISCUSSION
Toxoplasma gondii forms a nonfusogenic PV to replicate within host cells. Although the PVM provides a protective barrier to cellular defenses, including acidification and lysosome fusion, it also partitions the parasites from the host cell. Herein, we show for the first time that Toxoplasma can acquire host cytosolic proteins, it does so in an IVN-dependent manner, and a cathepsin L protease residing in the parasite VAC plays a principal role in digesting the incorporated proteins.
We found that ER-derived GFP was not ingested by intracellular parasites. Although the host ER membrane was reported to fuse with the PVM in mouse dendritic cells (9), we did not see ER-derived GFP from CHO cells in the RHΔcpl mutant. The inability of parasites to ingest ER-derived GFP was not due to inefficient transfection of the ER GFP expression construct. Although we cannot exclude the possibility of host ER fusion with the PV in infected CHO cells, if this occurs, it does not appear to deliver substantial quantities of host-derived protein to the parasite. The extent to which Toxoplasma acquires proteins from other host organelles remains to be determined.
The ingested GFP is exclusively seen in CPL-deficient strains, suggesting that proteolytic activity of CPL is important for digesting incorporated proteins. However, fewer RHΔcplCPLC31A parasites showed host-derived GFP than RHΔcpl parasites, despite the absence of CPL activity in this catalytic mutant (15). While the basis for this finding remains to be determined, in addition to directly digesting host proteins, CPL might have an indirect role, such as acting as an escort to deliver other proteins to the VAC. In this scheme, the absence of catalytic activity would partially compromise protein digestion in the VAC, but CPL-dependent delivery of, e.g., another protease to the VAC would be unaltered, leading to better digestion than the CPL knockout. Regardless, that the accumulation of host-derived protein is only seen in CPL-deficient strains and not in WT parasites likely obscured the pathway until now. CPL is required for the maturation and activation of CPB in the VAC (15); thus, CPL-deficient parasites are also devoid of active CPB, potentially contributing further to the deficiency in protein digestion. The finding that residual degradation of GFP occurs in CPL-deficient parasites suggests that additional proteases occupy the VAC.
Our findings established that ingestion of host protein occurs in both virulent type I and avirulent type II strains. While additional studies on other strains will provide a more complete picture, the pathway is not restricted to highly virulent strains and thus could contribute to infection by a variety of strains, including those that commonly infect humans. We also show that ingestion is not limited to one particular type of host-derived protein. GFP and mCherry are foreign proteins and are likely internalized by a bulk flow mechanism that does not involve specific receptors for GFP or mCherry. Accordingly, the ingestion pathway is likely not selective for such proteins. It remains to be seen if the pathway involves an element of receptor-mediated uptake that targets specific host-derived proteins as a sensing mechanism or means of selectively destroying host proteins that threaten parasite survival.
Less efficient ingestion of host-derived GFP in RHΔgra2 parasites suggests that an intact IVN makes a significant contribution to the ingestion of host cytosol. Nonetheless, host cytosolic protein acquisition is not fully blocked in this mutant. Electron microscopic studies showed that most of the tubule-like structures of the IVN are not seen in the RHΔgra2 mutant; however, other structures, including abundant PV vesicles, are still present in this mutant (4). This putative vesiculation of the IVN could render it impaired but still partially functional, thus permitting residual transport of host resources. Alternatively, the IVN might contribute indirectly to protein ingestion since it is known to act as a mechanical support that helps organize parasites within the PV (23). In this scenario, the disorganization of parasites could reduce protein ingestion via a conduit other than the IVN. Additional work is necessary to distinguish between these possibilities.
We show that CPL deficiency results in slower parasite growth in vitro and virulence attenuation in vivo. It is unlikely that the contribution of CPL to parasite growth in vitro is linked to its role as a maturase for microneme proteins because growth was assessed within one intracellular replicative cycle, and micronemes are not required for intracellular growth (24). In vivo, the PruΔku80LUCΔcpl strain showed a lower parasite burden at low to moderate doses and less weight loss as a proxy of sickness. The lower parasite burden is likely multifaceted and due in part to the contributions of CPL to parasite invasion (12) and replication (this study). Notably though, the course of infection by CPL-deficient parasites in IFN-γR−/− mice was only modestly less than that of the parental strain, suggesting that the absence of CPL renders the parasite more susceptible to immune clearance. The extent to which CPL or the ingestion pathway interfaces with the host immune response, however, remains to be determined by additional studies.
Malaria parasites also ingest the cytosol of infected erythrocytes to obtain and digest hemoglobin in a prominent digestive vacuole. Hemoglobin is acquired via membrane vesicles that invaginate into the parasite cytostome (25), a portal that is ultrastructurally similar to the Toxoplasma micropore (10). Heme, liberated from hemoglobin digestion, is polymerized into a conspicuous crystal, hemozoin. Hemoglobin digestion is crucial to the parasite and is the target of many antimalarial drugs that directly or indirectly interfere with hemozoin formation in the digestive vacuole, resulting in heme toxicity. Toxoplasma does not replicate in erythrocytes; hence, antimalarial compounds that target the digestive vacuole are largely ineffective against Toxoplasma. Nevertheless, the malaria digestive vacuole contains cathepsin L proteases (falcipains 2, 2′, and 3 in Plasmodium falciparum) that contribute to hemoglobin digestion along with other proteases (26). Our findings suggest a similar model in which the Toxoplasma VAC harbors CPL and other proteases that degrade proteins ingested from the host cytosol. Whether underlying mechanisms are conserved between Toxoplasma and malaria parasites remains to be determined. Malaria parasites do not have an IVN, implying at least one distinction. Future studies should identify steps along the pathway in addition to distinguishing the roles of the IVN, parasite endocytic compartments, and the associated machinery for vesicular trafficking.
MATERIALS AND METHODS
Ethics statement.
This study was carried out in strict accordance with the Public Health Service Policy on Humane Care and Use of Laboratory Animals and Association for the Assessment and Accreditation of Laboratory Animal Care guidelines. The animal protocol was approved by the University of Michigan’s Committee on the Use and Care of Animals (Animal Welfare Assurance A3114-01, protocol 09482). All efforts were made to minimize pain and suffering.
Chemicals and reagents.
Morpholine urea-leucyl-homophenyl-vinyl sulfone phenyl (LHVS) was kindly provided by Matthew Bogyo, Stanford University. Other chemicals used in this work are analytical grade and were purchased from Sigma-Aldrich unless otherwise indicated.
Parasite culture.
Toxoplasma gondii tachyzoites were continuously cultured in human foreskin fibroblast (HFF) cells and harvested by membrane filtration as described previously (27).
Protease protection assay.
Harvested parasites were incubated with 0.01% saponin and 1 mg/ml pronase (Roche) at 12°C for 1 h before they were fixed and attached on the chamber slides. Parasites were stained with rabbit anti-SAG1 antibodies to control for digestion efficiency. Also, a lysate of purified pronase- and saponin-treated parasites was loaded onto an SDS-PAGE gel, transferred to polyvinylidene difluoride (PVDF) membrane, and probed with mouse anti-GFP monoclonal antibody (Clontech), rabbit anti-SAG1 antibody, and rabbit anti-actin antibody as a loading control.
Flow cytometric analysis of GFP expression.
CHO and HeLa cells were seeded in 6-well plates 1 day prior to transfection. Two micrograms of cytosolic or ER GFP constructs was transfected into CHO and HeLa cells using Fugene 6 transfection reagent (Roche) and Lipofectamine 2000 (Invitrogen), respectively. Twenty-four hours posttransfection, host cells transiently expressing GFP were trypsinized and resuspended with ice-cold culture medium. The cells were washed once with ice-cold phosphate-buffered saline (PBS) and fixed with 4% formaldehyde in PBS for 20 min. The fixed cells were washed with PBS three times prior to flow cytometry analysis. The percentage of GFP+ cells and intensities of expressed GFP were measured using a BD FACSCanto flow cytometer and recorded using BD FACSDiva software. Quantification was performed and plotted using FlowJO software version 9.1.
Intracellular GFP acquisition assay.
The gfp gene was cloned into the NotI and XhoI sites of pBudCE4.1 vector (Invitrogen) under the control of the human EF1α promoter to generate the cytosolic GFP expression construct. An ER signal peptide and a four-amino-acid ER retention motif (KDEL) were engineered at the N terminus and C terminus of GFP, respectively, by introducing the corresponding DNA sequences in the primers. CHO or HeLa cells were seeded in 6-well plates. CHO and HeLa cells (60 to 70% confluent) were transfected with 2 µg GFP constructs using Fugene 6 or Lipofectamine 2000, respectively. Transfected cells were allowed to express GFP transiently for 24 h before they were infected with parasites. Eighteen to 24 h postinfection, parasites were mechanically liberated by syringing and filter purified on ice. Harvested parasites were pelleted and washed with ice-cold PBS three times prior to deposition on Cell-Tak (Becton, Dickinson)-coated chamber slides. Parasites were fixed, permeabilized with 0.1% Triton X-100, stained with mouse anti-CPL or mouse anti-CPB antibodies, and imaged with an AxioCAM MRm camera-equipped Zeiss Axiovert Observer Z1 inverted fluorescence microscope. Due to the introduction of gfp in the PruΔku80LUC and PruΔku80LUCΔcpl strains, the gfp gene in the cytosolic GFP expression construct was swapped with the mCherry gene to test the efficiency of cytosolic protein incorporation in the PruΔku80LUCΔcpl strain. The same protocols for the GFP acquisition assay were applied to the mCherry acquisition assay for the PruΔku80LUC and PruΔku80LUCΔcpl strains.
Extracellular GFP acquisition assay.
Host cells were either transfected with the cytosolic GFP expression construct to transiently express GFP, or infected with RH or RHΔcpl parasites. After 24 h, host cells transiently expressing GFP and infected untransfected host cells were scraped, mixed, processed, and quantified using the same procedures described for the intracellular GFP acquisition assay.
Generation of mutant parasites.
To generate the PruΔku80LUC strain, the firefly luciferase gene (LUC) was flanked with 500 bp of 5′-untranscribed region (5′ UTR) of SAG1 and 3′ UTR of GRA1 to produce the luciferase expression cassette by fusion PCR. This cassette was cloned into pminiHX (28) carrying a hypoxanthine-xanthine-guanine phosphoribosyl transferase (HXGPRT) gene. One kilobase of 5′ and 3′ UTRs of ku80 gene was amplified from the genomic DNA of Prugniaud parasites and cloned at 5′- and 3′-flanking sites of the luciferase expression cassette, respectively. The final plasmid was introduced into the PruΔku80 strain, and transfectants were selected with mycophenolic acid and xanthine. Positive clones were identified by luciferase activity. To produce the CPL knockout construct, 1-kb 5′ and 3′ UTRs of CPL were amplified from PruΔku80 genomic DNA and cloned into 5′- and 3′-flanking sites of the pyrimethamine (DHFR) resistance gene. The resulting DNA segment was introduced into the PruΔku80LUC strain to replace the CPL with the DHFR gene by homologous recombination. Transfected parasites were cultured in HFF cells in the presence of pyrimethamine and cloned out when the population became drug resistant. The correct clones were confirmed by PCR and immunoblotting.
To create RHΔcplΔgra2, a linear PCR product was amplified from RHΔgra2 genomic DNA using two primers flanking the 5′- and 3′-UTR regions of gra2. The amplified DNA fragments carry a phleomycin resistance cassette (BLE) and were transfected into RHΔcpl by electroporation as described previously (29). The transfected parasites were introduced into HFF cells for one passage before being harvested and selected extracellularly in the presence of 50 µg/ml phleomycin in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10 mM HEPES, 1% fetal bovine serum (FBS), and 1 mM l-glutamine for 4 h. After two cycles of phleomycin selection, parasites were cloned by limiting dilution. The desired mutants were identified by PCR. Whole-cell lysates of PCR-positive clones were probed with rabbit anti-GRA2 and mouse anti-CPL to confirm the absence of expression. The sequences of the primers used in this study are available upon request.
Degradation kinetics in extracellular parasites.
RH parasites were grown in CHO cells transiently expressing cytosolic GFP in the presence of 10 µM LHVS. Twenty-four hours postinfection, parasites were harvested using the procedures mentioned above. Parasites were resuspended in DMEM supplemented with 10 mM HEPES, 10% FBS, and 1 mM l-glutamine. LHVS or dimethyl sulfoxide (DMSO) (vehicle) was added to parasite resuspensions at 10 µM or 1% (vol/vol), respectively. Nontreated RH and RHΔcpl parasites were also included as negative and positive controls, respectively. Parasite suspensions were incubated at 37°C. Samples were collected at 0, 1, 2, 4, 8, and 12 h, pelleted, and resuspended in ice-cold PBS. The resulting resuspensions were deposited on Cell-Tak-coated chamber slides as described above, fixed, and counted under the microscope.
Parasite growth assays.
For the data in Fig. 5A, infected HFF cells were fixed with 4% paraformaldehyde 18 and 27 h postinfection in chamber slides. Infected monolayers were permeabilized with 0.1% Triton X-100 and stained with rabbit anti-SAG1 antibody (1:1,000) followed by incubation with Alexa Fluor 594 goat anti-rabbit IgG antibody (Invitrogen) (1:1,000), mounting in Mowiol, and visualization by fluorescence microscopy. At least 100 vacuoles were counted from at least 6 fields of view for each experiment. For the data in Fig. 5B, chamber slides of infected HFF cells were fixed and stained (0.2% crystal violet in 70% ethanol for 7 min) at the indicated time points, washed three times for 7 min each in PBS, and mounted in Mowiol for light microscopy. At least 50 vacuoles were counted from at least 6 fields of view for each experiment. For bioluminescence analysis of replication, individual wells of HFF cells in a 96-well plate were inoculated with 1,000 parasites for 4 h before noninvaded parasites were washed away. The end of the invasion period was designated 0 h. At 0, 20, 32, 44, 56, and 68 h, the medium was removed, and infected cells were lysed with d-luciferin solution. Bioluminescence intensities were acquired by Bio-Tek Synergy HT microplate reader. Readings were normalized to the 0-h value set to 1 to account for interexperiment differences in initial parasite infectivity.
Mouse studies.
Six- to 8-week-old female outbred CD-1 mice (Charles River) were infected by subcutaneous injection of 1,000 RH or mutant tachyzoites diluted in PBS and monitored daily for survival. Mice that became moribund were humanely euthanized. The sera of surviving mice were tested for infection by enzyme-linked immunosorbent assay (ELISA). Six- to 8-week-old WT C57BL/6 or IFN-γR−/− C57BL/6 mice (Jackson Labs) were infected intraperitoneally with PruΔku80LUC strain parasites or derived mutants and monitored for morbidity and mortality. SPSS version 21 software (IBM, Armonk, NY) was used to calculate median LD50 using Logit analysis. In vivo growth of PruΔku80LUC and PruΔku80LUCΔcpl was evaluated by intraperitoneal injection of 103, 104, 105, 106, or 107 tachyzoites into WT C57BL/6 or IFN-γR−/− C57BL/6 mice. Bioluminescence was imaged at 2, 6, 8, 12, 16, and 22 days postinfection using a Xenogen IVIS200 system. Mice were intraperitoneally injected with 200 µl of 40 mg/ml d-luciferin in PBS and anesthetized with isoflurane. Imaging was started 10 min after the substrate was given. Mice were imaged ventrally for 1 min. The intensities of bioluminescence signals were quantified using the LiveImage software coupled with Xenogen IVIS200 and plotted by Prism 6. Infected mice subjected to the bioluminescence assay were also weighed on the imaging days, and the values were plotted as the percentage of the initial weight over time by Prism 6.
ACKNOWLEDGMENTS
Support for this research was provided by an American Heart Association Postdoctoral Fellowship to Z.D. and U.S. NIH grants R01AI063263 and R21AI097099 to V.B.C.
We thank L. David Sibley for providing RHΔgra2 and antibodies to GRA2 and actin, Corinne Mercier for RHΔgra2GRA2, John Boothroyd for RHΔgra7, David Bzik for PruΔku80, and Matthew Bogyo for LHVS. We also thank My-Hang Huynh and Beth Hayes for critically reading the manuscript prior to submission and assistance with some of the experiments.
Footnotes
Citation Dou Z, McGovern OL, Di Cristina M, Carruthers VB. 2014. Toxoplasma gondii ingests and digests host cytosolic proteins. mBio 5(4):e01188-14. doi:10.1128/mBio.01188-14.
REFERENCES
- 1. Lindsay DS, Dubey JP. 2011. Toxoplasma gondii: the changing paradigm of congenital toxoplasmosis. Parasitology 138:1–3. 10.1017/S003118201000096X [DOI] [PubMed] [Google Scholar]
- 2. Black MW, Boothroyd JC. 2000. Lytic cycle of Toxoplasma gondii. Microbiol. Mol. Biol. Rev. 64:607–623. 10.1128/MMBR.64.3.607-623.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carruthers VB. 2002. Host cell invasion by the opportunistic pathogen Toxoplasma gondii. Acta Trop. 81:111–122. 10.1016/S0001-706X(01)00201-7 [DOI] [PubMed] [Google Scholar]
- 4. Mercier C, Dubremetz JF, Rauscher B, Lecordier L, Sibley LD, Cesbron-Delauw MF. 2002. Biogenesis of nanotubular network in Toxoplasma parasitophorous vacuole induced by parasite proteins. Mol. Biol. Cell 13:2397–2409. 10.1091/mbc.E02-01-0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mercier C, Adjogble KD, Däubener W, Delauw MF. 2005. Dense granules: are they key organelles to help understand the parasitophorous vacuole of all Apicomplexa parasites? Int. J. Parasitol. 35:829–849. 10.1016/j.ijpara.2005.03.011 [DOI] [PubMed] [Google Scholar]
- 6. Caffaro CE, Boothroyd JC. 2011. Evidence for host cells as the major contributor of lipids in the intravacuolar network of Toxoplasma-infected cells. Eukaryot. Cell 10:1095–1099. 10.1128/EC.00002-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sibley LD. 2004. Intracellular parasite invasion strategies. Science 304:248–253. 10.1126/science.1094717 [DOI] [PubMed] [Google Scholar]
- 8. Schwab JC, Beckers CJ, Joiner KA. 1994. The parasitophorous vacuole membrane surrounding intracellular Toxoplasma gondii functions as a molecular sieve. Proc. Natl. Acad. Sci. U. S. A. 91:509–513. 10.1073/pnas.91.2.509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goldszmid RS, Coppens I, Lev A, Caspar P, Mellman I, Sher A. 2009. Host ER-parasitophorous vacuole interaction provides a route of entry for antigen cross-presentation in Toxoplasma gondii-infected dendritic cells. J. Exp. Med. 206:399–410. 10.1084/jem.20082108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nichols BA, Chiappino ML, Pavesio CE. 1994. Endocytosis at the micropore of Toxoplasma gondii. Parasitol. Res. 80:91–98. 10.1007/BF00933773 [DOI] [PubMed] [Google Scholar]
- 11. Botero-Kleiven S, Fernández V, Lindh J, Richter-Dahlfors A, von Euler A, Wahlgren M. 2001. Receptor-mediated endocytosis in an apicomplexan parasite (Toxoplasma gondii). Exp. Parasitol. 98:134–144. 10.1006/expr.2001.4624 [DOI] [PubMed] [Google Scholar]
- 12. Parussini F, Coppens I, Shah PP, Diamond SL, Carruthers VB. 2010. Cathepsin L occupies a vacuolar compartment and is a protein maturase within the endo/exocytic system of Toxoplasma gondii. Mol. Microbiol. 76:1340–1357. 10.1111/j.1365-2958.2010.07181.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Robibaro B, Stedman TT, Coppens I, Ngô HM, Pypaert M, Bivona T, Nam HW, Joiner KA. 2002. Toxoplasma gondii Rab5 enhances cholesterol acquisition from host cells. Cell. Microbiol. 4:139–152. 10.1046/j.1462-5822.2002.00178.x [DOI] [PubMed] [Google Scholar]
- 14. Dou Z, Carruthers VB. 2011. Cathepsin proteases in Toxoplasma gondii. Adv. Exp. Med. Biol. 712:49–61. 10.1007/978-1-4419-8414-2_4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dou Z, Coppens I, Carruthers VB. 2013. Non-canonical maturation of two papain-family proteases in Toxoplasma gondii. J. Biol. Chem. 288:3523–3534. 10.1074/jbc.M112.443697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Miranda K, Pace DA, Cintron R, Rodrigues JC, Fang J, Smith A, Rohloff P, Coelho E, de Haas F, de Souza W, Coppens I, Sibley LD, Moreno SN. 2010. Characterization of a novel organelle in Toxoplasma gondii with similar composition and function to the plant vacuole. Mol. Microbiol. 76:1358–1375. 10.1111/j.1365-2958.2010.07165.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fox BA, Falla A, Rommereim LM, Tomita T, Gigley JP, Mercier C, Cesbron-Delauw MF, Weiss LM, Bzik DJ. 2011. Type II Toxoplasma gondii KU80 knockout strains enable functional analysis of genes required for cyst development and latent infection. Eukaryot. Cell 10:1193–1206. 10.1128/EC.00297-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Franke-Fayard B, Djokovic D, Dooren MW, Ramesar J, Waters AP, Falade MO, Kranendonk M, Martinelli A, Cravo P, Janse CJ. 2008. Simple and sensitive antimalarial drug screening in vitro and in vivo using transgenic luciferase expressing Plasmodium berghei parasites. Int. J. Parasitol. 38:1651–1662. 10.1016/j.ijpara.2008.05.012 [DOI] [PubMed] [Google Scholar]
- 19. Larson ET, Parussini F, Huynh MH, Giebel JD, Kelley AM, Zhang L, Bogyo M, Merritt EA, Carruthers VB. 2009. Toxoplasma gondii cathepsin L is the primary target of the invasion-inhibitory compound morpholinurea-leucyl-homophenyl-vinyl sulfone phenyl. J. Biol. Chem. 284:26839–26850. 10.1074/jbc.M109.003780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sibley LD, Krahenbuhl JL, Adams GM, Weidner E. 1986. Toxoplasma modifies macrophage phagosomes by secretion of a vesicular network rich in surface proteins. J. Cell Biol. 103:867–874. 10.1083/jcb.103.3.867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Labruyere E, Lingnau M, Mercier C, Sibley LD. 1999. Differential membrane targeting of the secretory proteins GRA4 and GRA6 within the parasitophorous vacuole formed by Toxoplasma gondii. Mol. Biochem. Parasitol. 102:311–324. 10.1016/S0166-6851(99)00092-4 [DOI] [PubMed] [Google Scholar]
- 22. Coppens I, Dunn JD, Romano JD, Pypaert M, Zhang H, Boothroyd JC, Joiner KA. 2006. Toxoplasma gondii sequesters lysosomes from mammalian hosts in the vacuolar space. Cell 125:261–274. 10.1016/j.cell.2006.01.056 [DOI] [PubMed] [Google Scholar]
- 23. Magno RC, Lemgruber L, Vommaro RC, De Souza W, Attias M. 2005. Intravacuolar network may act as a mechanical support for Toxoplasma gondii inside the parasitophorous vacuole. Microsc. Res. Tech. 67:45–52. 10.1002/jemt.20182 [DOI] [PubMed] [Google Scholar]
- 24. Breinich MS, Ferguson DJ, Foth BJ, van Dooren GG, Lebrun M, Quon DV, Striepen B, Bradley PJ, Frischknecht F, Carruthers VB, Meissner M. 2009. A dynamin is required for the biogenesis of secretory organelles in Toxoplasma gondii. Curr. Biol. 19:277–286. 10.1016/j.cub.2009.01.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Slomianny C. 1990. Three-dimensional reconstruction of the feeding process of the malaria parasite. Blood Cells 16:369–378 [PubMed] [Google Scholar]
- 26. Rosenthal PJ. 2011. Falcipains and other cysteine proteases of malaria parasites. Adv. Exp. Med. Biol. 712:30–48. 10.1007/978-1-4419-8414-2_3 [DOI] [PubMed] [Google Scholar]
- 27. Harper JM, Huynh MH, Coppens I, Parussini F, Moreno S, Carruthers VB. 2006. A cleavable propeptide influences Toxoplasma infection by facilitating the trafficking and secretion of the TgMIC2-M2AP invasion complex. Mol. Biol. Cell 17:4551–4563. 10.1091/mbc.E06-01-0064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Donald RG, Roos DS. 1998. Gene knock-outs and allelic replacements in Toxoplasma gondii: HXGPRT as a selectable marker for hit-and-run mutagenesis. Mol. Biochem. Parasitol. 91:295–305. 10.1016/S0166-6851(97)00210-7 [DOI] [PubMed] [Google Scholar]
- 29. Brydges SD, Carruthers VB. 2003. Mutation of an unusual mitochondrial targeting sequence of SODB2 produces multiple targeting fates in Toxoplasma gondii. J. Cell Sci. 116:4675–4685. 10.1242/jcs.00750 [DOI] [PubMed] [Google Scholar]