Abstract
Background
Chemotherapy with praziquantel (PZQ) has been the cornerstone of schistosomiasis control over the last two decades. Being the only available drug for the treatment of over 200 million people worldwide, continuous monitoring of PZQ efficacy under the pressure of widespread use is therefore advocated.
Methods
The efficacy of taking two doses of oral PZQ for the treatment of Schistosoma haematobium was examined among school children in Nigeria. Urine specimens were collected from 350 school children and examined using the filtration technique. Blood was collected for packed cell volume (PCV) estimation, and the weight and height of each child were estimated. S. haematobium egg positive pupils were treated with two oral doses of PZQ at 40 mg/kg with a four-week interval in between. Drug efficacy was determined based on the egg reduction rate (ERR).
Results
Among 350 school children, 245 (70.0%) – of which 132 were males and 113 were females, with an age range of 4 to 15 years – were diagnosed with S. haematobium. All the 245 infected children received a single oral dose of 40 mg/kg PZQ twice with a four-week interval in between and were followed up for 12 weeks. At four, eight and twelve weeks post treatment, the ERR was 57.1%, 77.6% and 100%, respectively. The ERR was significantly higher among the children with a light infection compared to those with a heavy infection. One hundred and twenty-one children were egg negative at four weeks post treatment, among which 1 (6.3) and 120 (52.4%) had heavy and light infections, respectively. Following the second round of treatment, the cure rate at eight weeks and twelve weeks was 85.3% and 100%, respectively.
Conclusion
This study demonstrated the efficacy of taking two doses of oral PZQ for the treatment of urinary schistosomiasis among school children in Nigeria.
Keywords: Urinary schistosomiasis, Praziquantel, Drug efficacy, School children, Nigeria
Multilingual abstracts
Please see Additional file 1 for translation of the abstract into the six official working languages of the United Nations.
Background
Schistosomiasis is responsible for significant health problems and is a socioeconomic burden in most of Sub-Saharan Africa and some other tropical countries [1,2]. It is endemic in 74 countries, with the bulk of the cases globally (90%) residing in Sub-Saharan Africa [3,4]. The significant health problems associated with schistosomiasis include impaired cognitive potential among primary school-age children, hepatosplenomegaly, anaemia, bladder cancer and stunted growth [5]. Also genital schistosomiasis, which manifests at reproductive age if schistosomiasis is not treated, is attributed as a risk factor for HIV transmission [6].
Currently the antischistosomal drug of choice for the treatment of schistosomiasis is praziquantel (PZQ). It is the mainstay of the current strategy against schistosomiasis morbidity control and is highly effective against the five schistosome species that infect humans [7]. Praziquantel is a pyrazinoquinoline derivative and its safety and efficacy have ensured its widespread usage. It is recommended that school-aged children and high-risk groups of adults in communities with a prevalence of 10% to 50% use it once every two years. In communities where the prevalence is above 50%, both children and adults are required to be treated once a year [8]. Praziquantel has been used widely successfully in many national control programmes. However, there is evidence of clinical relevant resistance developing [9].
The extensive use of PZQ and the problem of reduced therapeutic efficacy is on the increase globally, and this has led to the growing concern regarding the use of a single drug for the treatment of a disease affecting more than 200 million people [10]. Already countries such as Egypt [11], Zimbabwe [5] and Cameroon [12] have reported low cure rates of PZQ. There is therefore a need for constant and continuous monitoring of PZQ under the pressure of widespread use. A critical aspect in the assessment of PZQ efficacy is the drug’s activities during the different parasite development stages. Experimental laboratory studies have shown that activities of PZQ are stage dependent with the drug acting primarily against the adult worm stages, whereas immature schistosomes (two to four weeks old) are less susceptible [13]. All these observations point towards the need for the development of new potent drugs or combinations of drugs that will be effective against all stages of the parasite. While this is still being awaited, there is a need for constant monitoring of PZQ efficacy in endemic areas.
Although the prevalence and incidence of S. haematobium has been largely reported in Nigeria [14-16], the efficacy of PZQ among school children has not been well documented in some highly endemic areas as the drugs are distributed on a large scale without monitoring or follow-up. Therefore, this study was conducted to evaluate the efficacy of taking two doses of oral PZQ for treatment, given as two single doses of 40 mg/kg with a four-week interval in between. Repeated doses of PZQ given two to eight weeks after the initial dose in endemic areas of Africa have been shown to have incremental benefits when compared to single doses [17,18].
Methods
Study site and subjects
This study was carried out among primary and secondary school children in Akala and Imala Odo (Oyan) in the Abeokuta North Local Government area of Ogun State between July 2011 and March 2012. The communities were located close to the bank of the Oyan Reservoir which has been shown to be endemic [19]. The communities had no health centre at the time of the study. Although there is a schistosomiasis co-ordinating unit at the local government council, its activity is hampered by the irregular supply of PZQ. The study enrolled 350 primary and secondary school children, aged four to fifteen years. The age of each child was determined based on school records, and the weight and height were measured using a ruler and a weighing scale, respectively.
Screening of urine
Universal bottles labelled with the corresponding identification number were given to each participating child. Each participants was asked to produce the urine specimen between 10:00 and 14:00, and submit it on the same day. The samples were transported to the laboratory for microscopic examination of S. haematobium eggs using the urine filtration method. Briefly, 10 ml of well-mixed urine was aspirated and slowly forced through a filter membrane. The filter was removed and placed on a slide, covered with a cover slip and examined under a light microscope [20]. The number of eggs on the entire filter was counted and recorded as the number of eggs per 10 ml urine (EP10ml). From the total slides, 10% were randomly selected and re-examined by an independent microscopist for quality control. A Combur-Test (Roche Diagnostics GmbH, Mannheim, Germany) reagent strip was used to detect the presence of blood in the urine. For those participants whose slides came out negative, urine samples were collected on two successive days to confirm that they were truly negative.
Determination of packed cell volume
For packed cell volume (PCV), microhematocrit tubes filled with blood were centrifuged in a microhematocrit rotor for five minutes at 10,000 g. Packed cell volumes values ≤ 31% were considered as anaemia, which was further classified as mild (21–30%), moderate (15–20%) or severe (≤15%).
Treatment and follow-up
All children who provided urine specimens in the pre-treatment survey were included in the analysis of infection patterns at baseline, but only the children positive for S. haematobium eggs were treated with two single oral doses (40 mg/kg) of PZQ, given with a four-week interval in between. The drug was administered with a glass of water following the confirmation that the child ate at home or ate food that was provided by the investigating team. Urine samples were taken on the fourth, eighth and twelfth week to monitor the cure and egg reduction rates.
Ethical consent
Approval for the study was obtained from the Ethical Committee of the Ogun State Ministry of Health in Abeokuta. Before the onset of the study, a meeting was held with the community heads and the parents of the school children where formal, oral informed consent was obtained from the parents and the children directly before the sample collection.
Data analysis
Data was entered into a Microsoft Excel spreadsheet and exported to SPSS (version 16) (SPSS Inc., Chicago, IL, USA) for analysis. The proportion of children infected with S. haematobium was expressed as prevalence. The chi-square test and one-way ANOVA were used to test for differences in the prevalence of infections and geometric mean egg counts, respectively. P-values < 0.05 were considered statistically significant. The intensity reduction rate was calculated as [1 - (GM egg counts per 10 mL of urine after treatment/GM egg counts per 10 mL before treatment)] × 100 [21]. The cure rate was calculated as [the number of children excreting no S. haematobium eggs/the number of children with confirmed infections before treatment] x 100.
Results
Of the 350 children (age range four to fifteen years; 186 male, 164 female) enrolled into the study, 245 (70.0%) – 132 males and 113 females – were positive for S. haematobium, determined using the urine filtration technique. The age group of four to nine years had the highest prevalence (70.4%), but the difference was not statistically significant. There was no significant difference in the intensity of infection according to sex or age in the study. The proportion of males (5.9%) that had a heavy infection was greater than females (3.1%), but the difference was not statistically significant. The age group of four to nine years had the highest proportion (5.4%) of those with a heavy infection but this difference was also not statistically significant (see Table 1).
Table 1.
Prevalence and mean egg count/10 ml urine, by sex and age, among 350 school children in the Abeokuta North Local Government area
| Sex | No Examined N = 350 | No. Positive (%) | No. Heavy (%) (≥50 egg/10 ml) | No. Light (%) (<50 egg/10 ml) | Geometric mean egg count/10 ml |
|---|---|---|---|---|---|
| Male |
186 |
132 (70.9) |
11 (5.9) |
121 (65.1) |
10.98 |
| Female |
164 |
113 (68.9) |
5 (3.1) |
108 (65.9) |
7.37 |
| p-value |
|
0.73 |
|
0.30 |
0.91 |
|
Age (years)
|
|
|
|
|
|
| 4–9 |
203 |
143 (70.4) |
11 (5.4) |
132 (65.0) |
7.93 |
| 10–15 |
147 |
102 (69.4) |
05 (3.4) |
97 (66.0) |
6.73 |
| p-value | 0.90 | 0.67 | 0.72 |
Figure 1 shows the operational results and PZQ treatment outcomes over a 12-week period. Two hundred and forty-five children were treated twice (with a four-week interval in between) over a 12-week period with follow-up. All 245 infected children received a single oral dose of 40 mg/kg of PZQ and another single dose of 40 mg/kg four weeks later under the supervision of their teachers and members of the study team. The overall ERR at four weeks, eight weeks and 12 weeks post treatment was 57.1%, 77.6% and 100%, respectively. The efficacy of PZQ in this study at 12 weeks post treatment is therefore considered satisfactory [21]. The cure rate at four weeks, eight weeks and twelve weeks was 49.4%, 85.5% and 100%, respectively.
Figure 1.
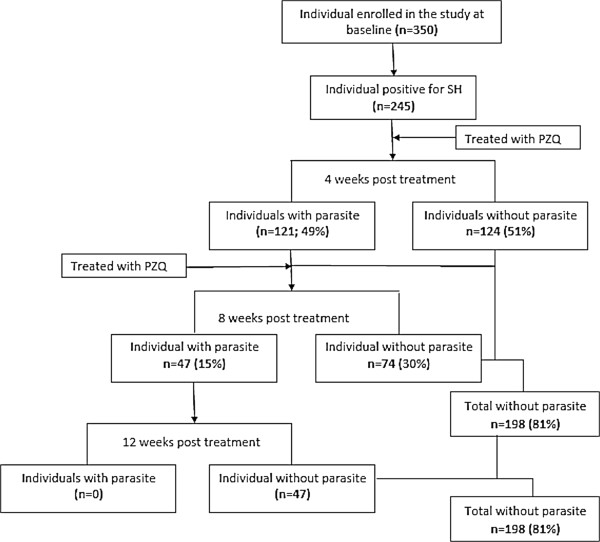
Study compliance for the efficacy of praziquantel to treat S. haematobium among school children in Abeokuta, Nigeria.
Before treatment, 6.5% (geometric mean egg 116.4 egg/gm) of the children were heavily infected (>50 eggs/10 ml), while 93% (geometric mean egg 8.2egg/gm) were lightly infected. At four weeks post treatment (before the second dose of PZQ), only 1 (6.3%) child was parasite free among those who were heavily infected. There was a significant reduction between day 0, and four, eight and twelve weeks in terms of the mean egg count (p = 0.0001). At eight weeks and twelve weeks (after the second dose of PZQ), 7 (43.8%; ERR 96.4%) and 16 (100%; ERR 100%) were parasite free, respectively, among those who were heavily infected. For those who were lightly infected, the ERR at four weeks, eight weeks and twelve weeks was 58.9%, 73.1% and 100%, respectively. The drug showed an overall cure rate of 49.4%, 85.3% and 100% at four weeks, eight weeks and twelve weeks, respectively (see Table 2).
Table 2.
Cure rates, geometric mean egg counts and intensity reduction rates over 12 weeks in 245 school children infected with Schistosoma haematobium after one treatment with praziquantel in Ogun State, Nigeria
| |
Before treatment
|
4 weeks after treatment
|
8 weeks after treatment
|
12 weeks after treatment
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Egg Intensity (egg/10 ml) | No. of subjects (%) | GM egg/ 10 ml | No. cured | Cure rate (%) | GM eggs/ 10 ml | Egg reduction rate (%) | No. cured | Cure rate (%) | GM eggs/ 10 ml | Egg reduction rate (%) | No. cured | Cure rate (%) | GM eggs/ 10 ml | Egg reduction rate (%) |
| < 50 |
229 (93.5) |
8.2 |
120 |
52.4 |
3.37 |
58.9 |
199 |
87.0 |
2.2 |
73.1 |
229 |
100.0 |
0.0 |
100.0 |
| ≥ 50 |
16 (6.5) |
116.4 |
1.00 |
6.3 |
13.4 |
88.5 |
7.0 |
43.8 |
4.2 |
96.4 |
16 |
100.00 |
0.0 |
100.0 |
| Total | 245 (100) | 9.8 | 121 | 49.4 | 4.2 | 57.1 | 206 | 85.3 | 2.2 | 77.6 | 244.0 | 100.00 | 0.0 | 100.0 |
Generally none of the pupils examined in the study population, either infected or non-infected with schistosomiasis, were anaemic. A significant difference was observed when the level of infection was compared with the mean PCV in the study population (p = 0.017). The mean weight and height of the pupils were also compared with respect to the infection level but the difference was not significant. A significant difference was observed when the presence of urine in the blood was compared with the different levels of infection (see Table 3). At the baseline cross-sectional survey, 228 of the 245 S. haematobium infected children had visible blood in their urine with a prevalence of 93.0%. At four weeks post treatment, only 2 (1.2%) children had blood detected, while none of the children had blood in their urine by the eighth and twelfth week.
Table 3.
Comparison between PCV, weight, height and blood in urine in relation to S. haematobium intensity of infections among school children
| Characteristics | No infection (0 EP10ml) N = 105 | Light infection (<50 EP10ml) N = 229 | Heavy infection (≥50 EP10ml) N = 16 | P-value |
|---|---|---|---|---|
|
PCV (geo mean) ± SD |
34.21 ± 2.7 |
33.86 ± 2.6 |
35.7 ± 2.5 |
0.017 |
|
Weight (mean) ± SD |
23.57 ± 7.5 |
23.42 ± 7.3 |
25.50 ± 5.2 |
0.97 |
|
Height (geo mean) ± SD |
116.73 ± 18.7 |
116.24 ± 20.5 |
114.94 ± 22.7 |
0.94 |
| Blood in urine (%) | 17 (16.2) | 212 (92.6) | 16 (100) | < 0.000 |
Discussion
Praziquantel remains the drug of choice for the treatment of schistosomiasis in spite of cases of low cure rates that have been reported in some areas [22,23]. As there have been reports of therapeutic failures [24,25], constant surveillance of PZQ efficacy is imperative while we wait for the discovery and development of more potent drugs. In this study, the performance of PZQ was considered satisfactory since the ERRs during the eighth and twelfth weeks were 77.6% and 100%, respectively [21]. Our results also demonstrated that there is a significant difference between the cure rates and infection intensities which is in agreement with previous studies where cure rates were shown to be consistently higher in individuals with light infections before treatment than in those with moderate or heavy infections [17,26]. In some studies, factors such as high pre-treatment egg intensities, poor drug absorption and a high rate of PZQ catabolism, rather than parasite resistance, have been attributed to the reduced PZQ cure rate in endemic areas [5]. The criteria of cure based on the absence of eggs after treatment with PZQ have shown a variation of 60% to 96% depending on the intensity of the infection. At four weeks post treatment before the administration of the second single dose of 40 mg/kg, only 49.3% of the study population treated were parasite free. At eight weeks post treatment, after the second dose of PZQ, 15% of the treated population were still egg positive. Most of the previous studies have reported a higher efficacy of PZQ when administered as two or three treatments spaced at certain time intervals [23,27]. It is assumed that patients in high transmission areas would harbour a high number of immature schistosomes that are less susceptible to PZQ [28,29]. These immature schistosomes have a high chance of surviving a single PZQ treatment. Another explanation is the suggestion that PZQ kill worms slowly or that dead worms still have the ability to release eggs [12]. The dead eggs may be present for months in the urine of treated patients infected with S. haematobium and will falsely reduce the egg reduction and cure rate results. Although we did not determine the viability of the excreted eggs, some studies have reported that dead eggs may be present for several months in the urine of S. haematobium infected individuals [12].
The high prevalence of S. haematobium reported in this study reflects the current transmission situation and ongoing control challenges in this area. Many epidemiological studies emanating from this region over the years have consistently revealed a high prevalence of urinary schistosomiasis [14,30,15]. Some of these reports show that the community members are aware that their constant continuous contact with endemic water from the river is responsible for their high infection rates, but because there is no alternative water source they have no choice but to continue to depend on the contaminated water for their daily domestic activities [31]. An alternative source of water and a well-laid-out control strategy by policy makers coupled with regular PZQ treatment are therefore needed in other to put an end to the menace of urinary schistosomiasis in this region.
No significant difference was observed in the prevalence between males (70.9%) and females (68.9%), as well as between the different ages in this study. This pattern of infection may be an indication of the equal exposure of both genders and the ages examined in the study due to significant water contact activities. The observed prevalence of microhematuria (70%) in the overall study population was similar to what was previously reported in this study area [19]. No significant difference was observed regarding microhematuria between ages and sexes, and this may be explained by the fact that microhematuria is a characteristic symptom of urinary schistosomiasis in endemic communities and its prevalence has been shown to correlate positively with urinary schistosomiasis infection. This prevalence decreased rapidly during the fourth and eighth week following PZQ treatment. This finding is in agreement with previous studies that suggest that urine reagent strips could potentially estimate the intensity of S. haematobium infection in endemic areas [31,20]. The current WHO guidelines for preventive chemotherapy recognize hematuria prevalence, in addition to egg count-based criteria, as an effective means to identify communities with high, moderate or low risk for schistosomiasis [32].
An unusual result observed in our study was that none of the students infected with either a light or heavy S. haematobium infection were anaemic. We observed that the mean PCV of pupils with heavy infection intensity was significantly higher than pupils with a light infection or those who were not infected. Many previous studies have demonstrated a high prevalence of anaemia among school children living in S. haematobium endemic areas [33,16]. While anaemia is well associated with schistosomiasis based on the pathogenesis of blood loss that results from S. haematobium infection, the blood loss could also be compensated with foods such as fish and green leafy vegetables which are abundantly available and consumed in these local communities. The unexpected observation in this study could raise important questions that need further research. In a similar manner, the S. haematobium infection status has no influence on the weight and height of the students in this endemic area.
Conclusion
This study demonstrated the efficacy of PZQ against S. haematobium in Nigeria. In view of the fact that the use of the drug will only increase as it is the only available widely-used drug, constant monitoring of drug resistance becomes imperative. It would also be beneficial to investigate the effects of repeated therapeutic doses of PZQ at different time intervals since repeated doses have been reported to produce a higher efficacy in comparison to single treatment in endemic areas. There is need to explore other alternative treatment regimens such the PZQ and artemisinin (ACT) derivatives combination as ACT derivatives have been shown to be active against immature schistosome worms [34]. There is need for a more co-ordinated effort by the government through the provision of alternative water sources that are cercariae free, through continuous and constant mass drug administration and through integrated vector control in order to bring an end to the ravaging scourge of this disease in this area.
Competing interests
The authors declare that there are no competing interests.
Authors’ contributions
OO designed, supervised and wrote the manuscript. S-AOR, BA and AAA participated in the collection of the sample, analysis of the sample and in drug administration and follow-up. OPN participated in manuscript preparation. OTA participated in the statistical analysis of the data. All authors read and approved the final manuscript.
Supplementary Material
Translation of the abstract into the six official working languages of the United Nations.
Contributor Information
Olusola Ojurongbe, Email: oojurongbe@lautech.edu.ng.
Olawunwi Risqat Sina-Agbaje, Email: olawunmi20@yahoo.com.
Abass Busari, Email: busbassi01@yahoo.com.
Patricia Nkem Okorie, Email: triciaokoye@yahoo.com.
Taiwo Adetola Ojurongbe, Email: taojurongbe@yahoo.com.
Akeem Abiodun Akindele, Email: aaakindele@lautech.edu.ng.
Acknowledgements
The authors are sincerely grateful to all consenting participants and parents of participants for their co-operation.
References
- Hotez PJ, Molyneux DH, Fenwick A, Kumaresan J, Sachs SE, Sachs JD, Savioli L. Control of neglected tropical diseases. N Engl J Med. 2007;357:1018–1027. doi: 10.1056/NEJMra064142. [DOI] [PubMed] [Google Scholar]
- Hodges MH, Dada N, Warmsley A, Paye J, Bangura MM, Nyorkor E, Sonnie M, Zhang Y. Mass drug administration significantly reduces infection of Schistosoma mansoni and hookworm in school children in the national control program in Sierra Leone. BMC Infect Dis. 2012;12:16. doi: 10.1186/1471-2334-12-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed AM, Abbas H, Mansour FA, Gasim GI, Adam I. Schistosoma haematobium infections among schoolchildren in central Sudan one year after treatment with praziquantel. Parasit Vectors. 2012;5:108. doi: 10.1186/1756-3305-5-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis. 2006;6:411–425. doi: 10.1016/S1473-3099(06)70521-7. [DOI] [PubMed] [Google Scholar]
- Midzi N, Sangweme D, Zinyowera S, Mapingure MP, Brouwer KC, Kumar N, Mutapi F, Woelk G, Mduluza T. Efficacy and side effects of praziquantel treatment against Schistosoma haematobium infection among primary school children in Zimbabwe. Trans R Soc Trop Med Hyg. 2008;102:759–766. doi: 10.1016/j.trstmh.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Feldmeier H, Krantz I, Poggensee G. Female genital schistosomiasis as a risk-factor for the transmission of HIV. Int J STD AIDS. 1994;5:368–372. doi: 10.1177/095646249400500517. [DOI] [PubMed] [Google Scholar]
- Seto EYW, Wong BK, Lu D, Zhong B. Human schistosomiasis resistance to praziquantel in China: should we be worried? Am J Trop Med Hyg. 2011;85:74–82. doi: 10.4269/ajtmh.2011.10-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Schistosomiasis and Soil Transmitted Helminth Infections – Preliminary Estimates of the Number of Children Treated with Albendazoleor Mebendazole. 2006. pp. 145–164. [PubMed]
- McManus DP, Loukas A. Current Status of Vaccines for Schistosomiasis. Clin Microbiol Rev. 2008;21:225–242. doi: 10.1128/CMR.00046-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai J-Y. Praziquantel Treatment in Trematode and Cestode Infections: An Update. Infect Chemother. 2013;45:32–43. doi: 10.3947/ic.2013.45.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barakat R, El Morshedy H. Efficacy of two praziquantel treatments among primary school children in an area of high Schistosoma mansoni endemicity, Nile Delta, Egypt. Parasitology. 2011;138:440–446. doi: 10.1017/S003118201000154X. [DOI] [PubMed] [Google Scholar]
- Tchuenté L-AT, Shaw DJ, Polla L, Cioli D, Vercruysse J. Efficacy of praziquantel against Schistosoma haematobium infection in children. Am J Trop Med Hyg. 2004;71:778–782. [PubMed] [Google Scholar]
- Sabah AA, Fletcher C, Webbe G, Doenhoff MJ. Schistosoma mansoni: chemotherapy of infections of different ages. Exp Parasitol. 1986;61:294–303. doi: 10.1016/0014-4894(86)90184-0. [DOI] [PubMed] [Google Scholar]
- Sam-Wobo SO, Ekpo UF, Ameh IG, Osileye OT. Continued high endemicity of urinary schistosomiasis in Ogun State, Nigeria. Niger J Parasitol. 2009;30:48–52. [Google Scholar]
- Ekpo UF, Alabi OM, Oluwole AS, Sam-Wobo SO. Schistosoma haematobium infections in preschool children from two rural communities in Ijebu East, south-western Nigeria. J Helminthol. 2012;86:323–328. doi: 10.1017/S0022149X11000459. [DOI] [PubMed] [Google Scholar]
- Tohon ZB, Mainassara HB, Garba A, Mahamane AE, Bosqué-Oliva E, Ibrahim M-L, Duchemin J-B, Chanteau S, Boisier P. Controlling schistosomiasis: significant decrease of anaemia prevalence one year after a single dose of praziquantel in Nigerian schoolchildren. PLoS Negl Trop Dis. 2008;2:e241. doi: 10.1371/journal.pntd.0000241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CH, Olbrych SK, Soon M, Singer ME, Carter J, Colley DG. Utility of Repeated Praziquantel Dosing in the Treatment of Schistosomiasis in High-Risk Communities in Africa: A Systematic Review. PLoS Negl Trop Dis. 2011;5:e1321. doi: 10.1371/journal.pntd.0001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Dong H-F, Guo Y, Zhao Q-P, Jiang M-S. Efficacy of praziquantel and artemisinin derivatives for the treatment and prevention of human schistosomiasis: a systematic review and meta-analysis. Parasit Vectors. 2011;4:201. doi: 10.1186/1756-3305-4-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinwale O, Ajayi M, Akande D, Gyang P, Adeleke M, Adeneye A, Adebayo M, Dike A. Urinary Schistosomiasis around Oyan Reservoir, Nigeria: Twenty Years after the First Outbreak. Iran J Public Heal. 2010;39:92–95. [PMC free article] [PubMed] [Google Scholar]
- Houmsou RS, Kela SL, Suleiman MM. Performance of microhaematuria and proteinuria as measured by urine reagent strips in estimating intensity and prevalence of Schistosoma haematobium infection in Nigeria. Asian Pac J Trop Med. 2011;4:997–1000. doi: 10.1016/S1995-7645(11)60233-2. [DOI] [PubMed] [Google Scholar]
- WHO. Assessing the Efficacy of Antihelminthic Drugs Against Schistosomiasis and Soil Transmitted Helminthiasis. Geneva: World health Organization; 2013. [Google Scholar]
- Stelma FF, Sall S, Daff B, Sow S, Niang M, Gryseels B. Oxamniquine cures Schistosoma mansoni infection in a focus in which cure rates with praziquantel are unusually low. J Infect Dis. 1997;176:304–307. doi: 10.1086/517273. [DOI] [PubMed] [Google Scholar]
- Guisse F, Polman K, Stelma FF, Mbaye A, Talla I, Niang M, Deelder AM, Ndir O, Gryseels B. Therapeutic evaluation of two different dose regimens of praziquantel in a recent Schistosoma mansoni focus in Northern Senegal. Am J Trop Med Hyg. 1997;56:511–514. doi: 10.4269/ajtmh.1997.56.511. [DOI] [PubMed] [Google Scholar]
- Liang YS, Coles GC, Doenhoff MJ. Short communication: Detection of praziquantel resistance in schistosomes. Trop Med Int Health. 2000;5:72–72. doi: 10.1046/j.1365-3156.2000.00514.x. [DOI] [PubMed] [Google Scholar]
- da Silva IM, Thiengo R, Conceição MJ, Rey L, Lenzi HL, Pereira Filho E, Ribeiro PC. Therapeutic failure of praziquantel in the treatment of Schistosoma haematobium infection in Brazilians returning from Africa. Memórias Inst Oswaldo Cruz. 2005;100:445–449. doi: 10.1590/s0074-02762005000400018. [DOI] [PubMed] [Google Scholar]
- Olds GR, King C, Hewlett J, Olveda R, Wu G, Ouma J, Peters P, McGarvey S, Odhiambo O, Koech D, Liu CY, Aligui G, Gachihi G, Kombe Y, Parraga I, Ramirez B, Whalen C, Horton RJ, Reeve P. Double-Blind Placebo-Controlled Study of Concurrent Administration of Albendazole and Praziquantel in Schoolchildren with Schistosomiasis and Geohelminths. J Infect Dis. 1999;179:996–1003. doi: 10.1086/314686. [DOI] [PubMed] [Google Scholar]
- Kabatereine NB, Kemijumbi J, Ouma JH, Sturrock RF, Butterworth AE, Madsen H, Ornbjerg N, Dunne DW, Vennnervald BJ. Efficacy and side effects of praziquantel treatment in a highly endemic Schistosoma mansoni focus at Lake Albert, Uganda. Trans R Soc Trop Med Hyg. 2003;97:599–603. doi: 10.1016/S0035-9203(03)80044-5. [DOI] [PubMed] [Google Scholar]
- Keiser J, Chollet J, Xiao S-H, Mei J-Y, Jiao P-Y, Utzinger J, Tanner M. Mefloquine—An Aminoalcohol with Promising Antischistosomal Properties in Mice. PLoS Negl Trop Dis. 2009;3:e350. doi: 10.1371/journal.pntd.0000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erko B, Degarege A, Tadesse K, Mathiwos A, Legesse M. Efficacy and side effects of praziquantel in the treatment of Schistosomiasis mansoni in schoolchildren in Shesha Kekele Elementary School, Wondo Genet, Southern Ethiopia. Asian Pac J Trop Biomed. 2012;2:235–239. doi: 10.1016/S2221-1691(12)60049-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouf EA, Ojurongbe O, Akindele AA, Sina-Agbaje OR, Van Tong H, Adeyeba AO, Kremsner PG, Kun JFJ, Velavan T. Ficolin-2 levels and FCN2 genetic polymorphisms as a susceptibility factor in schistosomiasis. J Infect Dis. 2012;206:562–570. doi: 10.1093/infdis/jis396. [DOI] [PubMed] [Google Scholar]
- Ekpo UF, Laja-Deile A, Oluwole AS, Sam-Wobo SO, Mafiana CF. Urinary schistosomiasis among preschool children in a rural community near Abeokuta, Nigeria. Parasit Vectors. 2010;3:58. doi: 10.1186/1756-3305-3-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CH, Bertsch D. Meta-analysis of Urine Heme Dipstick Diagnosis of Schistosoma haematobium Infection. Including Low-Prevalence and Previously-Treated Populations. PLoS Negl Trop Dis. 2013;7:e2431. doi: 10.1371/journal.pntd.0002431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacko M, Magnussen P, Keita AD, Traoré MS, Landouré A, Doucouré A, Madsen H, Vennervald BJ. Impact of Schistosoma haematobium infection on urinary tract pathology, nutritional status and anaemia in school-aged children in two different endemic areas of the Niger River Basin, Mali. Acta Trop. 2011;120(Suppl 1):S142–150. doi: 10.1016/j.actatropica.2010.12.009. [DOI] [PubMed] [Google Scholar]
- Utzinger J, Keiser J, Shuhua X, Tanner M, Singer BH. Combination chemotherapy of schistosomiasis in laboratory studies and clinical trials. Antimicrob Agents Chemother. 2003;47:1487–1495. doi: 10.1128/AAC.47.5.1487-1495.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Translation of the abstract into the six official working languages of the United Nations.


