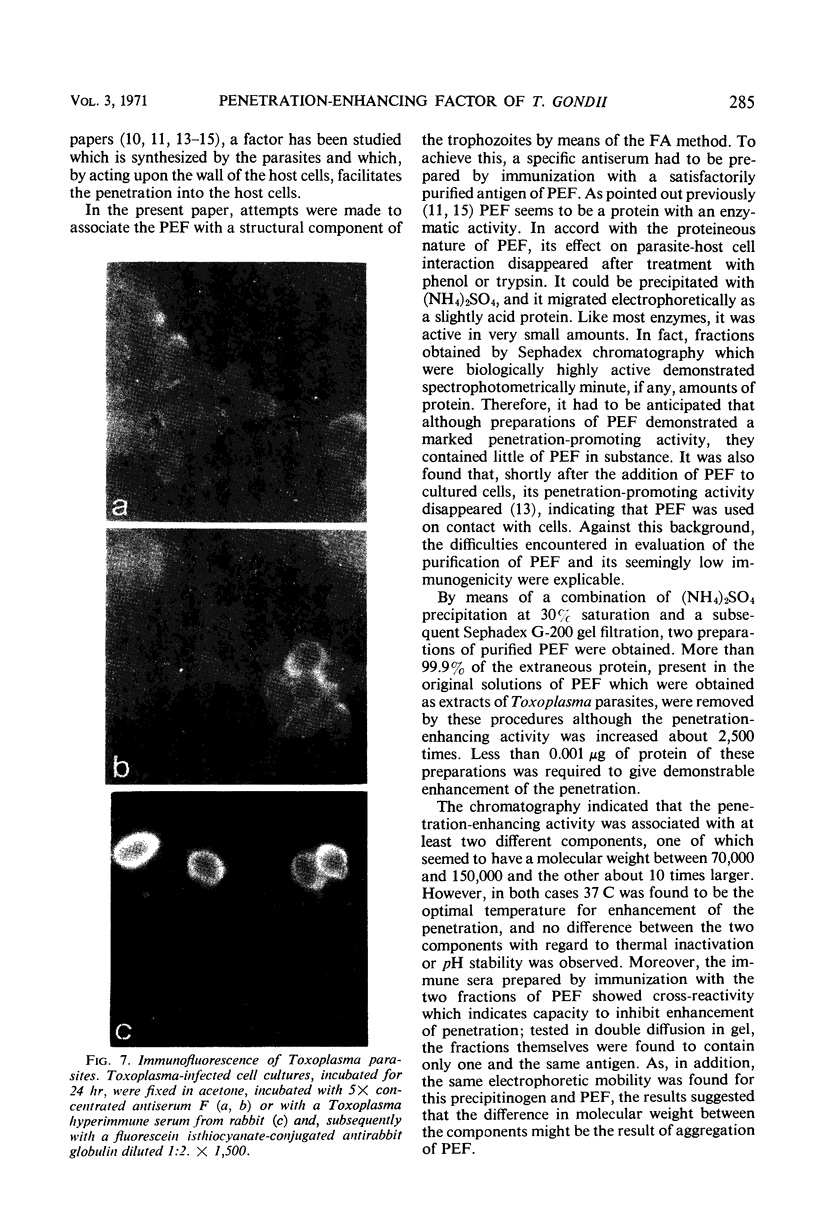
Abstract
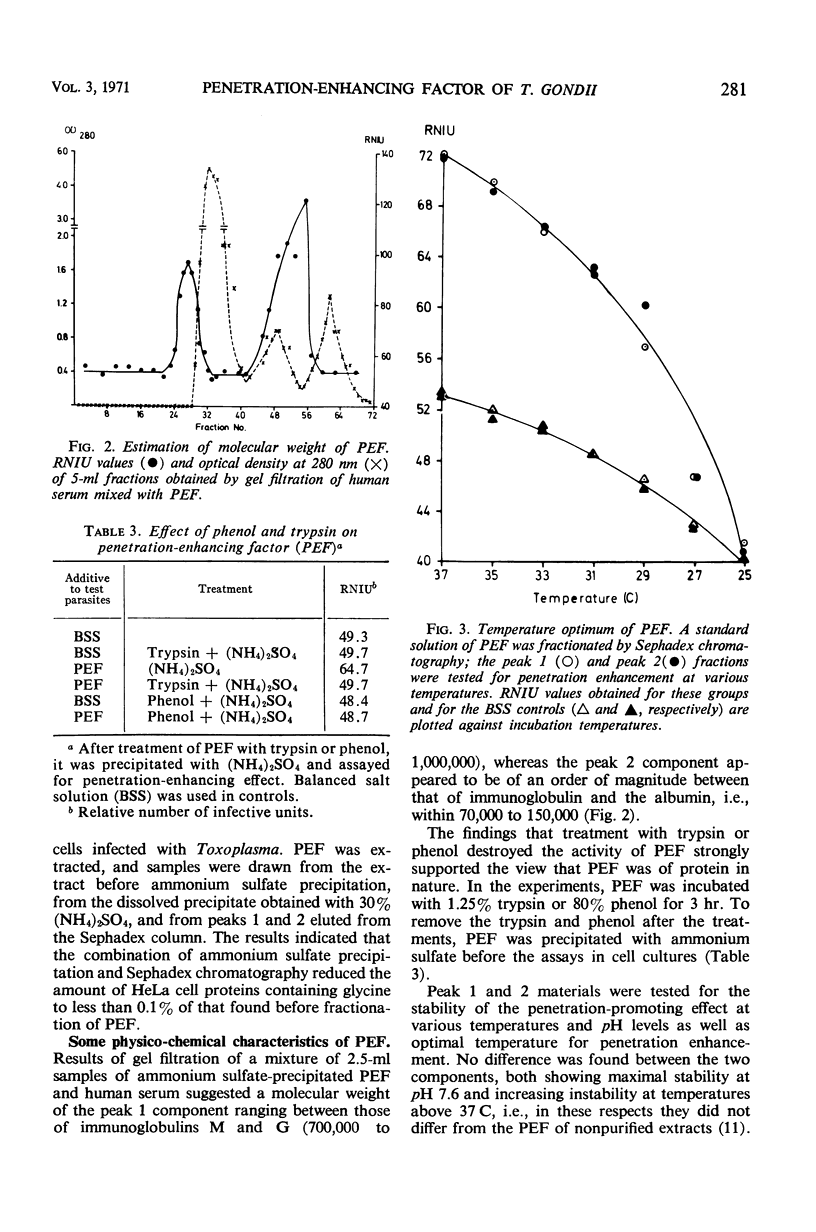
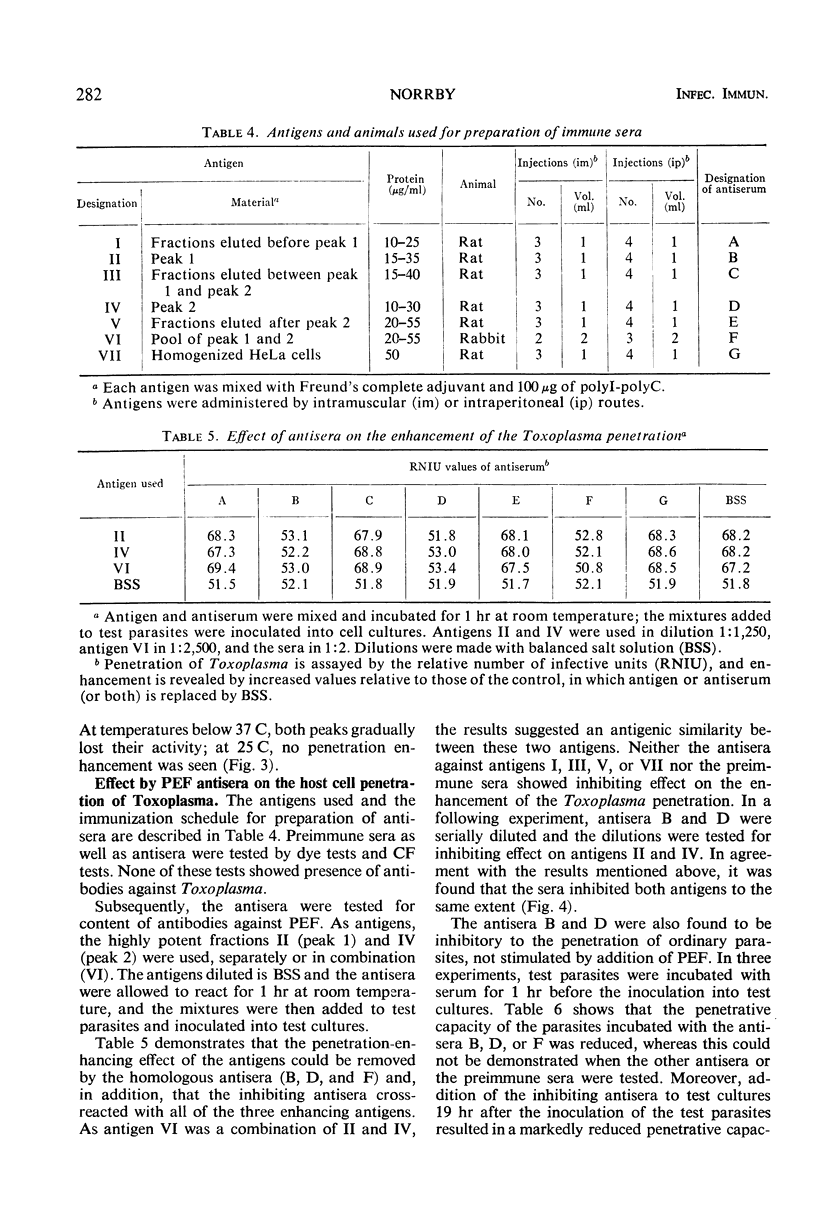
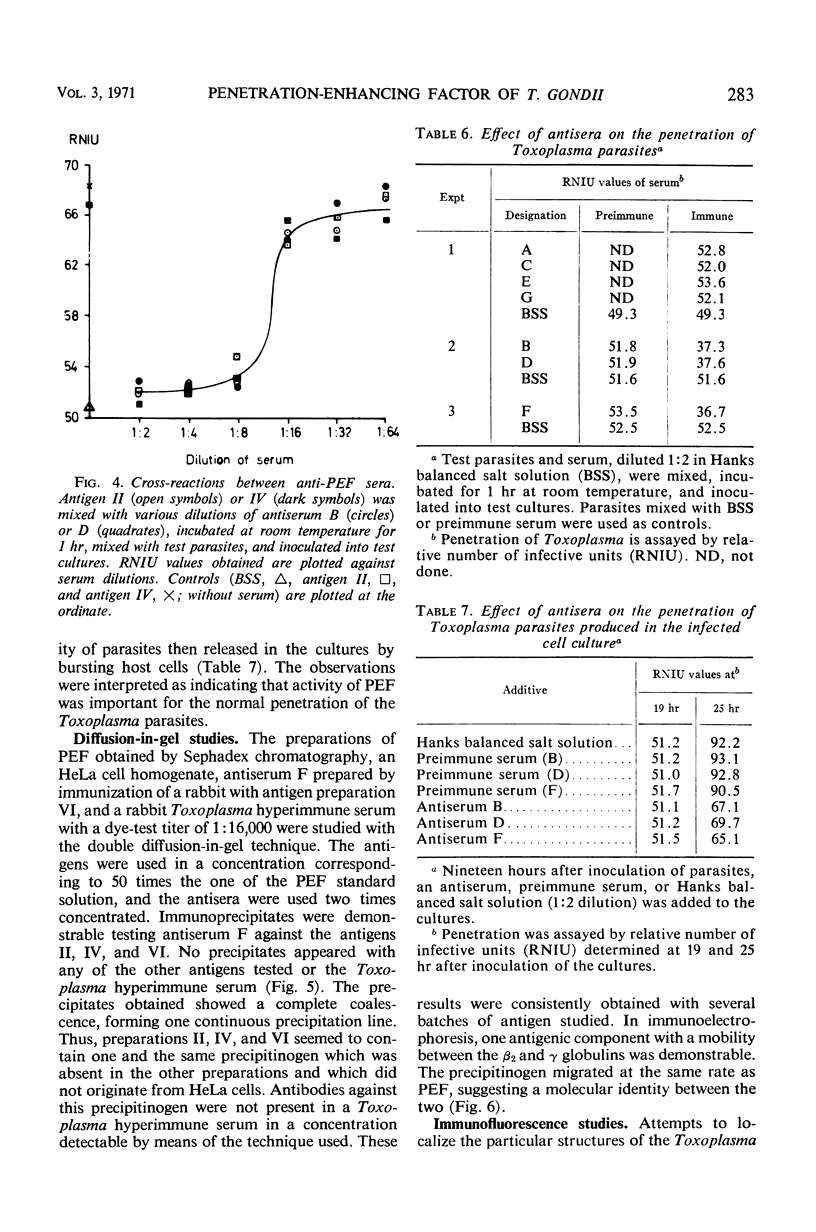
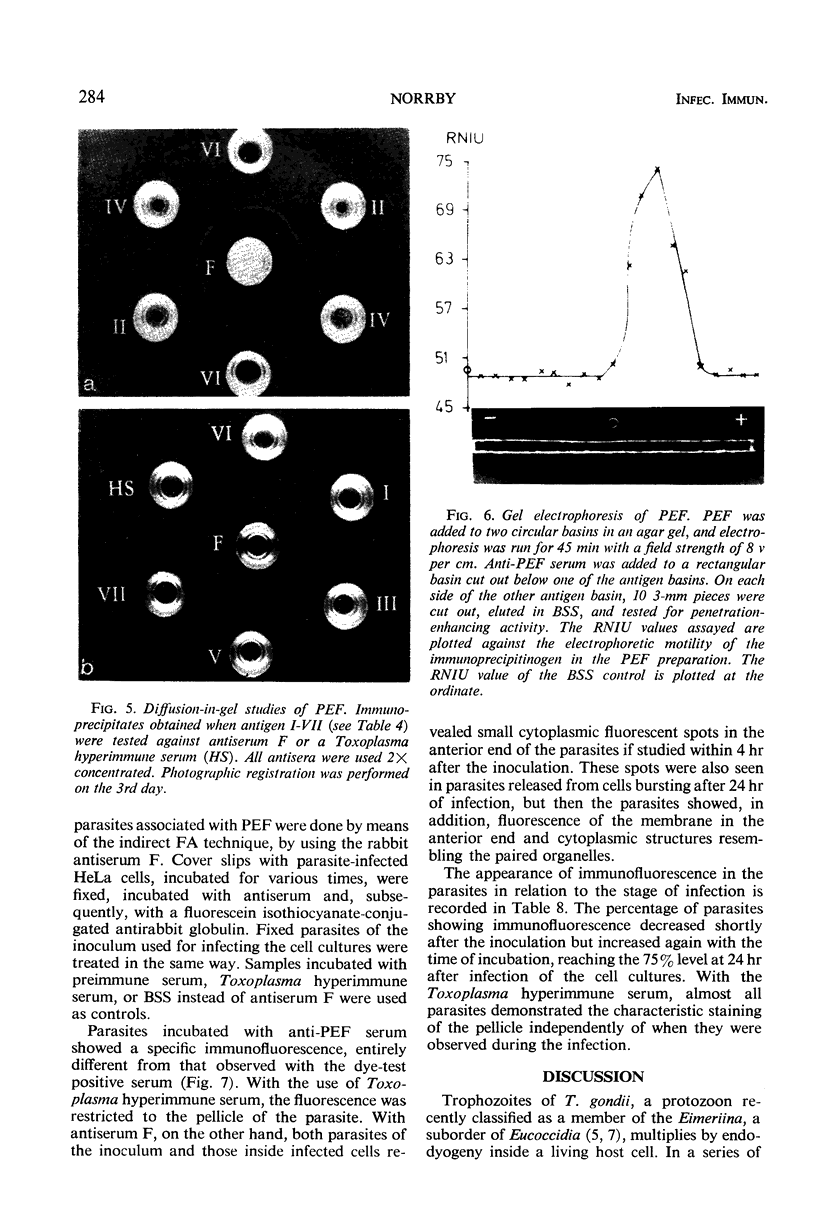
The host cell penetration factor (PEF) of Toxoplasma gondii was studied by biochemical and immunological techniques. Sephadex gel filtration of an ammonium sulfate-precipitated PEF yielded two components with different molecular weight, but both having penetration-enhancing activity. The methods for purification removed at least 99.9% of extraneous protein. For demonstration of a significant enhancement of penetration, 0.001 μg of protein was sufficient. Biochemically, they appeared to be acid proteins with the same electrophoretic mobility. Both components showed maximal enhancement of penetration at pH 7.6 and 37 C. PEF antisera reduced the penetrative capacity of Toxoplasma parasites. The penetration-enhancing effect of the two components of PEF was inhibited by antiserum against any of them. Moreover, immunologically identical immunoprecipitates were obtained when antiserum reacted with the two components. The results thus indicated that the two components of PEF were immunogenically identical and that the difference in molecular weights might result from aggregation. Immunofluorescence indicated that PEF was related to cytoplasmic structures located in the anterior end of Toxoplasma. A possible relation between these structures and the paired organelle or the convoluted tubes was discussed. The number of parasites with immunofluorescence was low shortly after host cell penetration and increased during the intracellular life of the parasites after kinetics, previously observed for synthesis of PEF as well as for lysosomal activity of Toxoplasma.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aikawa M. The fine structure of the erythrocytic stages of three avian malarial parasites, Plasmodium fallax, P. lophurae, and P. cathemerium. Am J Trop Med Hyg. 1966 Jul;15(4):449–471. doi: 10.4269/ajtmh.1966.15.449. [DOI] [PubMed] [Google Scholar]
- Field A. K., Tytell A. A., Lampson G. P., Hilleman M. R. Inducers of interferon and host resistance. II. Multistranded synthetic polynucleotide complexes. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1004–1010. doi: 10.1073/pnas.58.3.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel J. K., Dubey J. P., Miller N. L. Toxoplasma gondii in cats: fecal stages identified as coccidian oocysts. Science. 1970 Feb 6;167(3919):893–896. doi: 10.1126/science.167.3919.893. [DOI] [PubMed] [Google Scholar]
- GARNHAM P. C., BAKER J. R., BIRD R. G. Fine structure of cystic form of Toxoplasma gondii. Br Med J. 1962 Jan 13;1(5271):83–84. doi: 10.1136/bmj.1.5271.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepler P. K., Huff C. G., Sprinz H. The fine structure of the exoerythrocytic stages of Plasmodium fallax. J Cell Biol. 1966 Aug;30(2):333–358. doi: 10.1083/jcb.30.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison W. M., Dunachie J. F., Siim J. C., Work K. Coccidian-like nature of Toxoplasma gondii. Br Med J. 1970 Jan 17;1(5689):142–144. doi: 10.1136/bmj.1.5689.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LYCKE E., LUND E. A TISSUE CULTURE METHOD FOR TITRATION OF INFECTIVITY AND DETERMINATION OF GROWTH RATE OF TOXOPLASMA GONDII. 2. Acta Pathol Microbiol Scand. 1964;60:221–233. doi: 10.1111/apm.1964.60.2.221. [DOI] [PubMed] [Google Scholar]
- Ladda R., Aikawa M., Sprinz H. Penetration of erythrocytes by merozoites of mammalian and avian malarial parasites. J Parasitol. 1969 Jun;55(3):633–644. [PubMed] [Google Scholar]
- Lycke E., Norrby R., Remington J. Penetration-enhancing factor extracted from Toxoplasma gondii which increases its virulence for mice. J Bacteriol. 1968 Sep;96(3):785–788. doi: 10.1128/jb.96.3.785-788.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrby R. Host Cell Penetration of Toxoplasma gondii. Infect Immun. 1970 Sep;2(3):250–255. doi: 10.1128/iai.2.3.250-255.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrby R., Lindholm L., Lycke E. Lysosomes of Toxoplasma gondii and their possible relation to the host-cell penetration of toxoplasma parasites. J Bacteriol. 1968 Oct;96(4):916–919. doi: 10.1128/jb.96.4.916-919.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrby R., Lycke E. Factors enhancing the host-cell penetration of Toxoplasma gondii. J Bacteriol. 1967 Jan;93(1):53–58. doi: 10.1128/jb.93.1.53-58.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabin A. B., Feldman H. A. Dyes as Microchemical Indicators of a New Immunity Phenomenon Affecting a Protozoon Parasite (Toxoplasma). Science. 1948 Dec 10;108(2815):660–663. doi: 10.1126/science.108.2815.660. [DOI] [PubMed] [Google Scholar]
- Strannegård O., Lycke E. Properdin and the antibody-effect on Toxoplasma gondii. Acta Pathol Microbiol Scand. 1966;66(2):227–238. doi: 10.1111/apm.1966.66.2.227. [DOI] [PubMed] [Google Scholar]
- WADSWORTH C. A slide microtechnique for the analysis of immune precipitates in gel. Int Arch Allergy Appl Immunol. 1957;10(6):355–360. doi: 10.1159/000228394. [DOI] [PubMed] [Google Scholar]
- WADSWORTH C. COMPARATIVE TESTING OF A NEW PHOTOGRAPHIC MATERIAL FOR RAPID REGISTRATION OF IMMUNOPRECIPITATES. Int Arch Allergy Appl Immunol. 1963;23:103–114. doi: 10.1159/000229408. [DOI] [PubMed] [Google Scholar]
- WADSWORTH C., HANSON L. A. Comparative analysis of immune electrophoretic precipitates employing a modified immune electrophoretic technique. Int Arch Allergy Appl Immunol. 1960;17:165–177. doi: 10.1159/000229122. [DOI] [PubMed] [Google Scholar]