Abstract
Diagnosis of food allergy can be challenging. Given the limited specificity of available allergy tests, these need to be interpreted in light of pre-test probability that is determined by a careful history. Using likelihood ratios calculated from previous publication may allow a more individualized assessment. This approach is likely to be most useful in patients with low to moderate results, below the 95% positive predictive value for that food. This review covers the diagnostic approach of immunoglobulin E-mediated food allergy. We first focus on the pre-test clinical assessment of a patient with a suspected food allergy. We then compare currently available diagnostic tests and discuss their performance for frequent food allergens. Finally, we conclude with the interpretation of allergy tests in light of the pre-test assessment to determine final probability of food allergy and indications for referral to an allergy specialist for food challenge.
Diagnosis of food allergy is an imprecise science. Patient history is often unreliable and allergy test results can be difficult to interpret. Some patients will tolerate a food despite high levels of specific immunoglobulin E (IgE) against that food, whereas others will react with barely positive tests. In clinical practice, the physician needs to juggle with test probabilities and try to apply what has been reported in published cohorts to a patient sitting in his office. Positive predictive values (PPV) that have been published for different foods can be useful but are also fraught with inconveniences.1 For one, they are of limited value when evaluating a patient with low to moderate test results below the 95% PPV. Also, PPV for a given test will vary according to disease prevalence in the studied population and thus cannot easily be applied to an individual patient.
This review covers the diagnostic approach of IgE-mediated food allergy. We will first discuss the pre-test clinical assessment of a patient with a suspected food allergy. We will then review and compare the available diagnostic tests and discuss their performance for frequent food allergens. We conclude with the interpretation of allergy tests in light of the pre-test assessment to determine final probability of food allergy and indications for referral to an allergy specialist for food challenge. Specifically, we will focus on the use of likelihood ratios to better individualize probability of clinical reaction to the suspected food.
CLINICAL ASSESSMENT
Clinical history is more important than allergy testing when evaluating a patient with suspected food allergy. Tests should only be used to confirm clinical suspicions. The goal of the history is twofold: first, to evaluate the IgE-mediated nature of the reaction; and, second, to establish a relationship between the reaction and the suspected food.
IgE-mediated reactions can sometimes be difficult to differentiate from food intolerances, which do not involve an immune response to the food.2 IgE-mediated reactions are characterized by degranulation of mast cells upon IgE-mediated contact with the food allergen. Symptoms can occur with local contact and/or systemic distribution of the allergen after ingestion (anaphylaxis). Local symptoms include pruritus, erythema, swelling, and angioedema of the mouth and throat with potential airway compromise.
When the allergen reaches the stomach, it can produce severe abdominal pain and trigger protracted vomiting. If the allergen reaches the intestines, it may cause severe diarrhea. Once the allergen is absorbed, skin reactions can appear in areas that have not been in contact with the food, ranging from hives to severe erythrodermia. Concomitant rhinoconjunctivitis frequently occurs. Bronchospasm can also occur even in nonasthmatic individuals. Hypotension, loss of consciousness, and shock result from systemic vasodilation and cardiovascular collapse.
In most cases, intolerances can be easily differentiated from IgE-mediated allergy based on presentation (eg, reflux with spicy food). Some conditions, however, can mimic food allergy convincingly. Acute urticaria in children for example is caused by viral infection rather than allergy in more than 80% of cases.3
When an IgE-mediated reaction is suspected from symptoms, timing is often key to establish the relationship with food. Local symptoms can occur immediately upon contact with the food. Systemic symptoms can occur as soon as 5 minutes and up to 2 hours after ingestion.1 Reactions are systematic, occurring with each contact and usually with small amounts. Although the allergen may have been tolerated prior to the initial reaction, each subsequent contact should trigger a reaction. Useful questions for the diagnosis of food allergy are listed in Table 1.
TABLE 1.
Useful Questions for Assessment of Food Allergy
| Question | Rationale |
|---|---|
| What was the timing of the reaction? | An IgE-mediated reaction occurs rapidly after food ingestion. Symptoms occurring more than 2 hours after ingestion are unlikely to be related to the food, except in the case of diarrhea, which can occur up to 6 hours later. Immediate symptoms may, however, be followed by a delayed phase reaction that occurs hours to several days later. It usually involves the skin, bronchi or GI tract. 2 |
| Were there reactions to this food in the past? | Although a reaction can present with a food that was previously tolerated, patients will often report previous minor symptoms with the food, such as mild pruritis, local hives or nausea. |
| Has your child eaten the food since? | Once sensitized, reaction should be systematic with every contact with the food. If the food was ingested and tolerated after the initial reaction, allergy to that food is basically ruled out. |
| How much food is needed to trigger the reaction? | Allergies usually require only a very small amount of the food to trigger a reaction. A patient reporting symptoms only to large quantities of a food suggests intolerance rather than allergy. |
| Does the reaction occur without exposure the food? | Children with chronic urticaria or dermographism often present local hives with spicy or acidic food (tomatoes, strawberries) that induce non-specific degranulation of mast cells. |
| Was the food cooked? | Symptoms to fresh food only suggest sensitization to a thermolabile allergen. |
| Did your child have fever (or other signs of viral infection)? | Viral infection is an important differential diagnosis of food allergy and should be sought. |
| Did anyone else have symptoms? | If others present with similar symptoms to the food, intoxication becomes more probable. |
| Does your child suffer from other allergies? | Atopic children are more likely to become sensitized to a food. 30% to 40% of children with atopic dermatitis will eventually develop a food allergy. If the patient has other food allergies, possibility of contamination or cross-reactivity should be considered.8,9 |
| Was there a concurrent use of alcohol, NSAIDs or exercise? | Alcohol intake, NSAIDs or exercise decrease reaction threshold. Some patients with oral allergy syndrome or wheat allergy will only present symptoms if they exercise before or after eating the food. 2 |
IgE = immunoglobulin E; GI = gastrointestinal; NSAID = nonsteroidal anti-inflammatory drug.
ESTABLISHING PRE-TEST PROBABILITY
There is no algorithm to determine pre-test probability. It depends on the physician’s assessment of how likely the reaction was caused by food allergy. For example, in a patient with 2 subsequent anaphylaxis episodes following the isolated ingestion of peanut, the diagnosis is almost obvious and history alone would confer a 98% pre-test probability.4 However, a less convincing history with a more probable alternative diagnosis would result in a lower pre-test probability. For example, the pre-test probability of allergy for a non-atopic child who presented with urticaria 1 hour after a meal during a viral infection could reasonably be estimated less than 20%. If the child had eaten and tolerated the same food since that episode, then food allergy would be unlikely, with pre-test probability of less than 1%.4
Establishing pre-test probability is more challenging when dealing with a patient who has never eaten the food and for which there is no reaction to assess. In some cases, the physician can rely on epidemiological data to determine pre-test probability.
Specific Populations
No History of Immediate Clinical Reaction
There are a number of situations that can motivate a parent to seek allergy evaluation for a food to which the child has never ingested or reacted. This is often the case with siblings of a first child with confirmed food allergy who has been avoiding the food either because it is banned from the house or for primary prevention. The actual risk of reaction upon introduction of a food in the sibling of food allergic child is unknown. However, the prevalence of food allergies in children who have a food-allergic sibling has been estimated at 17%.5 For the child with a peanut allergy, the risk of the sibling also developing peanut allergy is 7%.4,6
In children with a first food allergy, parents may inquire about the risk of allergy to other food that has not yet been introduced. A first food allergy is a risk factor for having multiple food allergies. The risk is more significant for closely related foods that may contain cross-reactive allergens (ie, 30% of patients allergic to cashew are also allergic to pistachio).7 Risk of cross-reaction for common food allergens is presented in Table 2.
TABLE 2.
Clinical cross-reactivity of common food allergens
| If allergic to: | Clinical reaction to : | Risk |
|---|---|---|
| Peanut | Another legume (soy) | 5% |
| A tree nut | Another tree nut | 12%–37% |
| A tree nut | Peanut or another legume | 33% |
| A fish | Another fish | 50% |
| Shrimp | Another crustacea | 38% |
| A mollusca | Another mollusca | 49% |
| A crustacea | A mollusca | 14% |
| Cow’s milk | Beef | 10% |
| Cow’s milk | Goat’s milk | 92% |
| Cow’s milk | Mare’s milk | 4% |
| Wheat | Another cereal | 22% |
| Hen’s egg | Chicken meat | 22%–32% |
| Peach | Another rosaceae (cherry, apple, plum) | 55% |
| Cantaloupe | Avocado, banana or melon | 92% |
| Latex | Kiwi, banana or avocado | 35% |
| Cashew | Pistachio | 30% |
In some children, food allergy does not manifest as an acute reaction upon exposure to the food but rather contributes to exacerbate chronic atopic dermatitis.2 This is a very tricky situation because the patient is presently tolerating regular intake of the food, which is probably offering some kind of protection against anaphylaxis. Therefore, in mild eczema that is controlled by topical medication, screening for underlying food allergy is inappropriate because stopping the food may lead to the loss of this protection.
However, in severe cases resistant to standard treatment, testing for food allergy may be warranted, as avoidance of the food may result in improvement of the dermatitis.2 In such cases, a positive result to allergy tests needs to be confirmed by exclusion diet of 6 weeks followed by oral food challenge and reintroduction, as the manifestations of the allergy may only be through the dermatitis. The prevalence of food allergy in individuals with moderate-to-severe atopic dermatitis is 30% to 40%.8,9
Infants and Young Children
Assessment also needs to be adjusted for the age of the patient. Infants and young children have an immature immune system and less reactive skin and usually have lower test results. This is reflected by different PPV in younger children (when available). For example, the 95% PPV for reacting to egg is 2 kU/L egg-specific IgE before age 2 years, whereas it is much higher (7 ku/L) after that age.1 The same is true with skin tests, which usually yield smaller wheals in young infants.10 The working parameters of an allergy test cannot be applied to young children unless they have been specifically calculated for that age.
Allergens can also be transmitted through maternal breast milk. More than 60% of mothers will excrete food allergens in their breast milk at variable concentrations that can trigger reactions or exacerbate atopic dermatitis in highly sensitized infants.11,12 Peak excretion usually occurs in the first 2 hours and is proportionate to the amount ingested. The physician thus must inquire about links between the reaction, breast-feeding, and maternal diet in the younger population.
Older Children and Adolescents
As atopic children grow up and become adolescents, most will develop inhalant allergen sensitizations as part of the atopic march.2 This can be relevant to the evaluation of a food allergy, as many pollen allergens cross-react with related food proteins in plant food (nuts, seeds, fruits, and vegetable). Inhalant sensitization needs to be evaluated so it can be taken into account when interpreting symptoms and allergy tests. Most cross-reactive plant-food allergens have conformational epitopes, meaning that with digestion the sites recognised by the specific IgE antibody will be denatured. The consequence for the clinician is that despite the presence of specific IgE to the food at high levels, it can be tolerated with minimal or no symptoms. For example, with birch pollen allergy, many patients will report local pruritis limited to the oral sphere with fresh fruits or nuts, referred to as oral allergy syndrome.13 These patients will generally tolerate baked food because the allergen is also denatured by cooking. Pollen sensitization must be taken into account when evaluating a patient with such a history.
Diagnostic Tests
Once pre-test probability has been determined, diagnostic tests can be a useful adjunct in the evaluation of a patient with suspected food allergy. The 2 currently validated and available methods are epicutaneaous testing and analysis of food-specific IgE.
Epicutaneous Testing
The principle of epicutanuous testing is to bring the allergen in contact with the patient’s mast cells in the skin by applying a drop of food extract on the skin and very lightly pricking or puncturing the skin. Mast cell degranulation will then produce a small wheal that can be measured. A wheal of 3 mm greater than the negative control is considered a positive response. Skin testing is usually considered highly sensitive ( at least 90%) but less specific.14 A larger wheal size carries a higher specificity for diagnosis, but does not correlate with greater reaction severity.10
The test can be performed with either needles or lancets. Both devices are valid and highly reliable when used by an experienced technician.15 The main advantage of skin testing is that it gives a rapid result (15 minutes) with minimal discomfort. However, the test can be affected by various variables. Children often have dermographism or atopic dermatitis, which may lead to false positives. An inexperienced technician may traumatize the skin and also cause false positives. Recent anaphylaxis, administration of antihistamine medication, or young age may lead to false negatives.
Skin testing can be valuable in the setting of oral allergy syndrome in which skin tests will be positive to fresh food but negative to food extracts, which have been heated and in which conformational epitopes have been denatured.
Specific IgE Testing
Specific IgE testing consists of dosing circulating IgE antibodies directed against the suspected food in vitro.1 It is not affected by skin conditions or by antihistamine administration. It is widely available without the need for a specialized technician. Specific IgE testing can be difficult to interpret, especially with low range values. The assay is also considered very sensitive but lacking specificity. Some patients with high levels of IgE directed against a food will tolerate it, probably due to sensitization to thermolabile allergens.16 This is why it is never appropriate to perform allergy tests to a food that is tolerated by the patient.
There is no cutoff value at which point the patient can be sure to react or not. Studies have shown that the positive predictive value increases with higher test results.1 Many studies refer to the 95% predictive value for a given food. Although useful in research, this concept has its limits in clinic because the predictive value of a test is bound to vary based on pre-test probability. Also, in the clinical setting, the cases that are the hardest to interpret are those with low to moderate test results that are below the 95% PPV. In these cases, using the positive (or negative) likelihood ratio may be found more useful, as discussed below.
Component-Resolved Diagnosis
Specific IgE antibodies can be produced against many different proteins contained in a single food. Component-resolved diagnosis consists of dosing specific IgE response to these proteins separately, in order to establish the reactivity profile against that food.17 This is particularly useful to differentiate sensitization to thermo-labile conformational allergens such as those seen with oral allergy syndrome, from sensitization to allergens with sequential epitopes that are resistant to digestion and can cause systemic anaphylaxis. For example, ovalbumin is the major allergen contained in egg white but it is denatured by cooking. Ovomucoid, however, is very stable. Thus, a patient with positive-specific IgE to ovalbumin but negative to ovomucoid is more likely to tolerate a challenge to egg in baked goods.18
Component-resolved diagnosis is a useful new tool that adds precision to the food allergy assessment and could help better select patients for food challenge. However, it carries some of the same caveats as regular specific IgE testing with specific cut-off values being hard to individualize in the clinical setting.17 It is not widely available and component testing for some major food allergens are still lacking. The test also lacks sensitivity, so a negative result is insufficient to rule out an allergy.17 Food challenge therefore remains the gold standard and must be performed to exclude a food allergy.
ESTABLISHING POST-TEST PROBABILITY
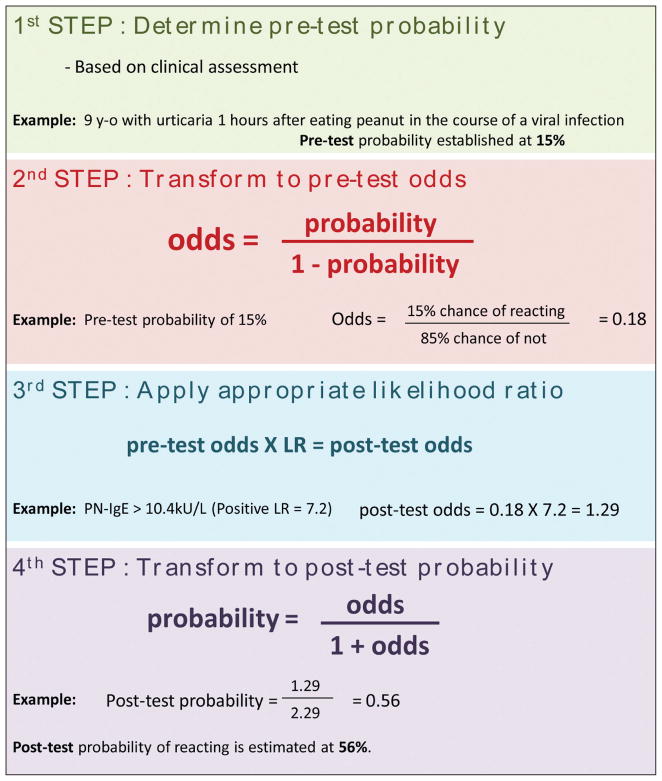
Likelihood ratios (LRs) are a reflection of a test sensitivity and specificity and are thus inherent to the test itself and, unlike positive predictive values, do not vary with sample prevalence. A positive or negative LR can be applied to the pre-test probability to determine an individual’s post-test probability of reacting upon exposure to the food. Figure 1 describes how this can be calculated manually. Free-to-use post-test probability calculators are also available online or as apps. The more traditional normogram can also be printed and used to calculate post-test probability rapidly (see Figure 2).
Figure 1.
Calculating post-test probability.
Figure 2.
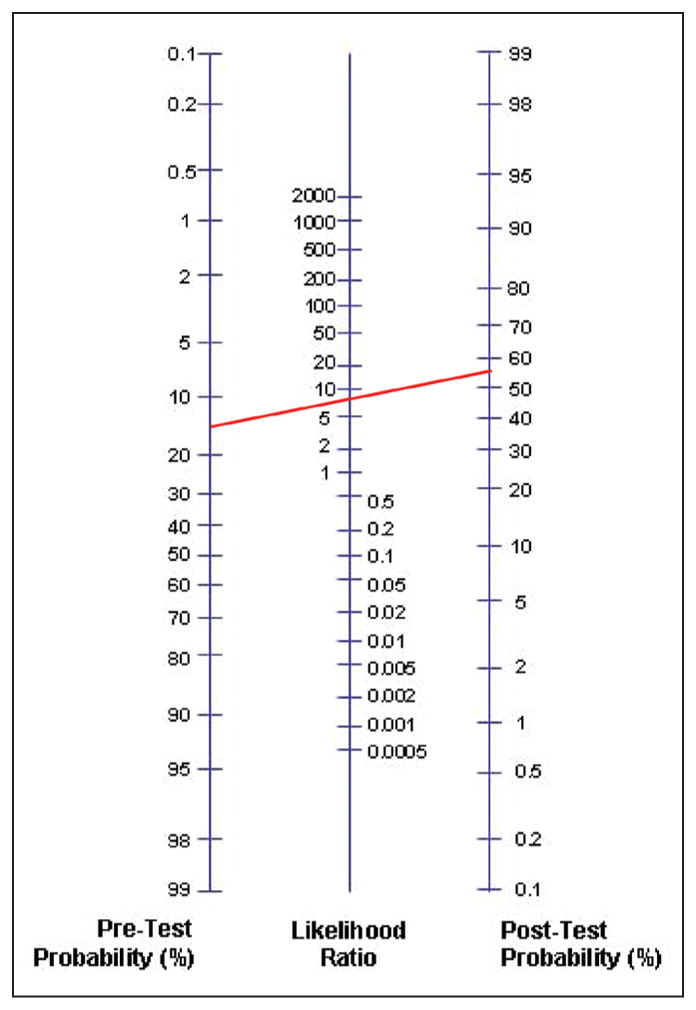
Diagnostic normogram. The red line corresponds to the case described in Figure 1. (Blank normograms are available for download at: www.cebm.net/?o=1043.)
Tables 3 through 8 indicate LRs inferred from studies for frequent allergens. The 95% PPV is also indicated, if available.
TABLE 3.
Milk
TABLE 8.
Fish
| Test | Value | LR |
|---|---|---|
| SPT | < 3 mm25,26 | 0.16 |
| > 3 mm25,26 | 2.11 | |
| IgE* | < 0.35 kU/L26 | 0.10 |
| > 0.3526 | 2.69 | |
| > 1.8 kU/L26 | 7.08 |
CAP-FEIA system (Pharmacia/Pfizer, New York, NY).
95% PPV = 20 kU/L
IgE = immunoglobulin E; LR = likelihood ratio; PPV = positive predictive value; SPT = skin prick test.
Food Challenge
The food challenge is the final step in the diagnosis of a food allergy. It consists of administering the food under supervision, starting at a very low dose followed by progressive increments.1 It needs to be performed by a specialist in a setting where anaphylaxis can be treated, no matter how small the probability of reacting has been assessed to be.
The gold standard for food allergy diagnosis is the double-blind placebo-controlled food challenge (DBPCFC).2 This is performed on 2 separate visits in which neither the patient nor the physician knows whether the patient is receiving the food or placebo. The idea is to avoid subjective interpretation of symptoms either unrelated or triggered by anxiety, which could lead to a false positive.
In the clinic, the open food challenge is usually performed because it is much more convenient.2 However, when a patient is very anxious and symptoms are hard to differentiate, a single-blind placebo-controlled food challenge can also be done in which only the patient is masked.
WHEN TO REFER FOR FOOD CHALLENGE
Food challenge is the ultimate tool to confirm diagnosis. Depending on the parents’ and the treating physician’s preferences, the cutoff below which a challenge may be offered can vary between post-test probabilities of 50% to 95%. The reason for challenging patients with a moderate probability of reacting is to avoid putting them on an unnecessary avoidance diet, and giving an erroneous diagnosis of food allergy with all the psychosocial consequences it carries.2 In the right setting, food challenge is very safe. In research, challenges are also performed to confirm diagnosis in patients at high risk of reacting for inclusion in a study (generally DBPCFC). Threshold amounts of food triggering a reaction are also sometimes determined. However, this is inappropriate for clinical practice, as these thresholds may change over time, and thus may offer a false sense of safety.
A patient with a high post-test probability should also be referred to an allergist for follow-up. Depending on the evolution of allergy tests over the years, the patient may be offered a challenge to confirm resolution. In some foods, such as cow’s milk and hen’s eggs, a large proportion of children will tolerate a baked products challenge despite positive allergy tests.19,20 Although component-resolved diagnosis can help better identify candidates for baked food challenge, it is not a surrogate to a challenge. Determining tolerance to baked good is very important as it will have a major impact on the child’s diet and could even hasten resolution of allergy to the raw food.19,20
Unvalidated Methods
In addition to what has been discussed so far in this review, a number of new diagnostic tests are being developed and promoted. None of these have been validated and they should not be used in the clinical setting. While some are at a research stage and may yet be found to have a role in clinics, others are complete hoaxes and prey on the patients’ trust and their desire to help their child to make a profit. The only way to discuss these with patients is by knowing about them. Table 9 provides a non-extensive list of unvalidated allergy tests that may be encountered in practice.
TABLE 9.
Unvalidated Methods for Diagnosing Food Allergy
| Method | Rationale |
|---|---|
| Muscle testing (or applied kinesiology) | Muscle testing consists of having the patient hold the allergen in his hand with an extended arm. A positive test is found if the arm drops when pressure is applied. The principle is that a “bad” substance will make the muscle weaker. Other versions have the patient chew the food and measure salivation.34 |
| Hair analysis | This test consists in examining the patient’s hair for its mineral content. If a harmful substance has been ingested, it is claimed that it will show in hair composition. This is false. Hair grows slowly (1 cm/month), so even hair closest to the scalp is several weeks old and thus may not reflect current body conditions for purposes of food allergy diagnosis.35 |
| Specific Immuno- globulin G (IgG) | Some companies offer dose-specific immunoglobulin G IgG against a large food panel for a large sum of money. They claim that IgG anti- bodies cause inflammation and that avoiding these foods could cure Crohn’s disease, diabetes, depression, and obesity. Specific IgG against food has not been shown to have any physiological significance. If any, the IgG4 subtype could be a marker of food tolerance, although it has not been validated for clinical use. In addition to the cost, IgG testing may lead to inappropriate diet restrictions.36 |
| Vega testing (electroacupuncture) | This method is a mix of acupuncture and homeopathic medicine. The patient is asked to hold the food in one hand and electric current is measured on a related meridian. Again, this method has no scientific basis and has been shown to be ineffective. In 2001, it was estimated that more than 500 Vega machines were being used in the United States to diagnose allergies.37 |
CONCLUSION
Clinical assessment remains the most important element of food allergy diagnosis and should be completed before ordering allergy tests. Given the limited specificity of available allergy tests, the results should be interpreted in light of clinical probability. Using likelihood ratios calculated from previous publication may allow a more individualized assessment. They are likely to be most useful in patients with low to moderate results, below the 95% PPV. Patients should be referred to an allergy specialist for food challenge when post-test probability of allergic reaction is low to moderate. Those with higher post-test probability should also see an allergist for follow-up. Appropriate diagnosis of food allergy can sometimes be challenging, but it is worth the effort because it will have a major impact on patient safety and quality of life.
TABLE 4.
Peanut
TABLE 5.
Egg
TABLE 6.
Soy
| Test | Value | LR |
|---|---|---|
| SPT | < 3 mm22,24–26,30 | 0.66 |
| > 3 mm22,24–26,30 | 1.71 | |
| IgE* | < 0.35 kU/L24,26 | 0.57 |
| > 0.35 kU/L24,26 | 1.25 | |
| > 5 kU/L26 | 1.83 |
CAP-FEIA system (Pharmacia/Pfizer, New York, NY).
IgE = immunoglobulin E; LR = likelihood ratio; SPT = skin prick test.
TABLE 7.
Wheat
| Test | Value | LR |
|---|---|---|
| SPT | < 3 mm22,24–26,30 | 0.37 |
| > 3 mm22,24–26,30 | 2.72 | |
| IgE* | < 0.35 kU/L 24,26,27,30 | 0.55 |
| > 0.35 kU/L 24,26,27,30 | 1.17 | |
| > 8.1 kU/L26 | 2.63 |
CAP-FEIA system (Pharmacia/Pfizer, New York, NY).
IgE = immunoglobulin E; LR = likelihood ratio; SPT = skin prick test.
Footnotes
Disclosure: The authors have no relevant financial relationships to disclose.
Contributor Information
Philippe Bégin, An Allergist-Immunologist at the University of Montreal Health Center; and Visiting Research Fellow at Stanford University.
Kari C. Nadeau, An Allergist-Immunologist, and Assistant Professor of Pediatrics, Stanford University.
References
- 1.Sampson HA. Update on food allergy. J Allergy Clin Immunol. 2004;113(5):805–819. doi: 10.1016/j.jaci.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 2.Boyce JA, Assa’ad A, Burks AW, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126(6 Suppl):S1–58. doi: 10.1016/j.jaci.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sackesen C, Sekerel BE, Orhan F, Kocabas CN, Tuncer A, Adalioglu G. The etiology of different forms of urticaria in childhood. Pediatr Dermatol. 2004;21(2):102–108. doi: 10.1111/j.0736-8046.2004.21202.x. [DOI] [PubMed] [Google Scholar]
- 4.Sicherer SH, Wood RA. Advances in diagnosing peanut allergy. J Allergy Clin Immunol Pract. 2013;1(1):1–13. doi: 10.1016/j.jaip.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Tsai HJ, Kumar R, Pongracic J, et al. Familial aggregation of food allergy and sensitization to food allergens: a family-based study. Clin Exp Allergy. 2009;39(1):101–109. doi: 10.1111/j.1365-2222.2008.03111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hourihane JO, Dean TP, Warner JO. Peanut allergy in relation to heredity, maternal diet, and other atopic diseases: results of a questionnaire survey, skin prick testing, and food challenges. BMJ. 1996;313(7056):518–521. doi: 10.1136/bmj.313.7056.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rance F, Bidat E, Bourrier T, Sabouraud D. Cashew allergy: observations of 42 children without associated peanut allergy. Allergy. 2003;58(12):1311–1314. doi: 10.1046/j.1398-9995.2003.00342.x. [DOI] [PubMed] [Google Scholar]
- 8.Eigenmann PA, Sicherer SH, Borkowski TA, Cohen BA, Sampson HA. Prevalence of IgE-mediated food allergy among children with atopic dermatitis. Pediatrics. 1998;101(3):E8. doi: 10.1542/peds.101.3.e8. [DOI] [PubMed] [Google Scholar]
- 9.Thompson MM, Tofte SJ, Simpson EL, Hanifin JM. Patterns of care and referral in children with atopic dermatitis and concern for food allergy. Dermatol Ther. 2006;19(2):91–96. doi: 10.1111/j.1529-8019.2006.00062.x. [DOI] [PubMed] [Google Scholar]
- 10.Hill DJ, Heine RG, Hosking CS. The diagnostic value of skin prick testing in children with food allergy. Pediatr Allergy Immunol. 2004;15(5):435–441. doi: 10.1111/j.1399-3038.2004.00188.x. [DOI] [PubMed] [Google Scholar]
- 11.Jarvinen KM, Makinen-Kiljunen S, Suomalainen H. Cow’s milk challenge through human milk evokes immune responses in infants with cow’s milk allergy. J Pediatr. 1999;135(4):506–512. doi: 10.1016/s0022-3476(99)70175-7. [DOI] [PubMed] [Google Scholar]
- 12.Palmer DJ, Gold MS, Makrides M. Effect of maternal egg consumption on breast milk ovalbumin concentration. Clin Exp Allergy. 2008;38(7):1186–1191. doi: 10.1111/j.1365-2222.2008.03014.x. [DOI] [PubMed] [Google Scholar]
- 13.Webber CM, England RW. Oral allergy syndrome: a clinical, diagnostic, and therapeutic challenge. Ann Allergy Asthma Immunol. 2010;104(2):101–108. doi: 10.1016/j.anai.2009.11.007. quiz 109–110, 117. [DOI] [PubMed] [Google Scholar]
- 14.American College of Allergy A, Immunology. Food allergy: a practice parameter. Ann Allergy Asthma Immunol. 2006;96(3 Suppl 2):S1–68. [PubMed] [Google Scholar]
- 15.Demoly P, Bousquet J, AR . In vivo methods for the study of allergy. In: Adkinson NF Jr, Busse WW, Bochner BS, Lemanske RF Jr, Holgate ST, Simons FE, editors. Middleton’s Allergy: Principles and Practice. St. Louis, MO: Mosby; 2008. pp. 1267–1278. [Google Scholar]
- 16.Sicherer SH, Sampson HA. Food allergy. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S116–125. doi: 10.1016/j.jaci.2009.08.028. [DOI] [PubMed] [Google Scholar]
- 17.Kattan JD, Wang J. Allergen component testing for food allergy: ready for prime time? Curr Allergy Asthma Rep. 2013;13(1):58–63. doi: 10.1007/s11882-012-0311-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caubet JC, Bencharitiwong R, Moshier E, Godbold JH, Sampson HA, Nowak-Wegrzyn A. Significance of ovomucoid- and ovalbumin-specific IgE/IgG(4) ratios in egg allergy. J Allergy Clin Immunol. 2012;129(3):739–747. doi: 10.1016/j.jaci.2011.11.053. [DOI] [PubMed] [Google Scholar]
- 19.Kim JS, Nowak-Wegrzyn A, Sicherer SH, Noone S, Moshier EL, Sampson HA. Dietary baked milk accelerates the resolution of cow’s milk allergy in children. J Allergy Clin Immunol. 2011;128(1):125–131. doi: 10.1016/j.jaci.2011.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leonard SA, Sampson HA, Sicherer SH, et al. Dietary baked egg accelerates resolution of egg allergy in children. J Allergy Clin Immunol. 2012;130(2):473–480. doi: 10.1016/j.jaci.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sicherer SH. Clinical implications of cross-reactive food allergens. J Allergy Clin Immunol. 2001;108(6):881–890. doi: 10.1067/mai.2001.118515. [DOI] [PubMed] [Google Scholar]
- 22.Eigenmann PA, Sampson HA. Interpreting skin prick tests in the evaluation of food allergy in children. Pediatr Allergy Immunology. 1998;9(4):186–191. doi: 10.1111/j.1399-3038.1998.tb00371.x. [DOI] [PubMed] [Google Scholar]
- 23.Keskin O, Tuncer A, Adalioglu G, Sekerel BE, Sackesen C, Kalayci O. Evaluation of the utility of atopy patch testing, skin prick testing, and total and specific IgE assays in the diagnosis of cow’s milk allergy. Ann Allergy Asthma Immunology. 2005;94(5):553–560. doi: 10.1016/S1081-1206(10)61133-7. [DOI] [PubMed] [Google Scholar]
- 24.Mehl A, Rolinck-Werninghaus C, Staden U, et al. The atopy patch test in the diagnostic workup of suspected food-related symptoms in children. J Allergy Clin Immunol. 2006;118(4):923–929. doi: 10.1016/j.jaci.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Sampson HA, Albergo R. Comparison of results of skin tests, RAST, and double-blind, placebo-controlled food challenges in children with atopic dermatitis. J Allergy Clin Immunol. 1984;74(1):26–33. doi: 10.1016/0091-6749(84)90083-6. [DOI] [PubMed] [Google Scholar]
- 26.Sampson HA, Ho DG. Relationship between food-specific IgE concentrations and the risk of positive food challenges in children and adolescents. J Allergy Clin Immunol. 1997 Oct;100(4):444–451. doi: 10.1016/s0091-6749(97)70133-7. [DOI] [PubMed] [Google Scholar]
- 27.Breuer K, Heratizadeh A, Wulf A, et al. Late eczematous reactions to food in children with atopic dermatitis. Clin Exp Allergy. 2004;34(5):817–824. doi: 10.1111/j.1365-2222.2004.1953.x. [DOI] [PubMed] [Google Scholar]
- 28.van den Berg ME, Flokstra-de Blok BM, Vlieg-Boerstra BJ, et al. Parental eczema increases the risk of double-blind, placebo-controlled reactions to milk but not to egg, peanut or hazelnut. Int Arch Allergy Immunol. 2012;158(1):77–83. doi: 10.1159/000330645. [DOI] [PubMed] [Google Scholar]
- 29.Caffarelli C, Cavagni G, Giordano S, Stapane I, Rossi C. Relationship between oral challenges with previously uningested egg and egg-specific IgE antibodies and skin prick tests in infants with food allergy. J Allergy Clin Immunol. 1995;95(6):1215–1220. doi: 10.1016/s0091-6749(95)70078-1. [DOI] [PubMed] [Google Scholar]
- 30.Scibilia J, Pastorello EA, Zisa G, et al. Wheat allergy: a double-blind, placebo-controlled study in adults. J Allergy Clin Immunol. 2006;117(2):433–439. doi: 10.1016/j.jaci.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 31.Rance F, Abbal M, Lauwers-Cances V. Improved screening for peanut allergy by the combined use of skin prick tests and specific IgE assays. J Allergy Clin Immunol. 2002;109(6):1027–1033. doi: 10.1067/mai.2002.124775. [DOI] [PubMed] [Google Scholar]
- 32.Flinterman AE, Pasmans SG, Hoekstra MO, et al. Determination of no-observed-adverse-effect levels and eliciting doses in a representative group of peanut-sensitized children. J Allergy Clin Immunol. 2006;117(2):448–454. doi: 10.1016/j.jaci.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 33.van Nieuwaal NH, Lasfar W, Meijer Y, et al. Utility of peanut-specific IgE levels in predicting the outcome of double-blind, placebo-controlled food challenges. J Allergy Clin Immunol. 2010;125(6):1391–1392. doi: 10.1016/j.jaci.2010.01.057. [DOI] [PubMed] [Google Scholar]
- 34.Kenney JJ, Clemens R, Forsythe KD. Applied kinesiology unreliable for assessing nutrient status. J Am Diet Assoc. 1988;88(6):698–704. [PubMed] [Google Scholar]
- 35.Hambidge KM. Hair analyses: worthless for vitamins, limited for minerals. Am J Clin Nutr. 1982;36(5):943–949. doi: 10.1093/ajcn/36.5.943. [DOI] [PubMed] [Google Scholar]
- 36.Carr S, Chan E, Lavine E, Moote W. CSACI Position statement on the testing of food-specific IgG. Allergy Asthma Clin Immunol. 2012;8(1):12. doi: 10.1186/1710-1492-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lewith GT, Kenyon JN, Broomfield J, Prescott P, Goddard J, Holgate ST. Is electrodermal testing as effective as skin prick tests for diagnosing allergies? A double blind, randomised block design study. BMJ. 2001;322(7279):131–134. doi: 10.1136/bmj.322.7279.131. [DOI] [PMC free article] [PubMed] [Google Scholar]