Abstract
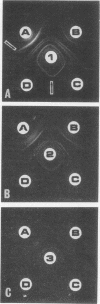
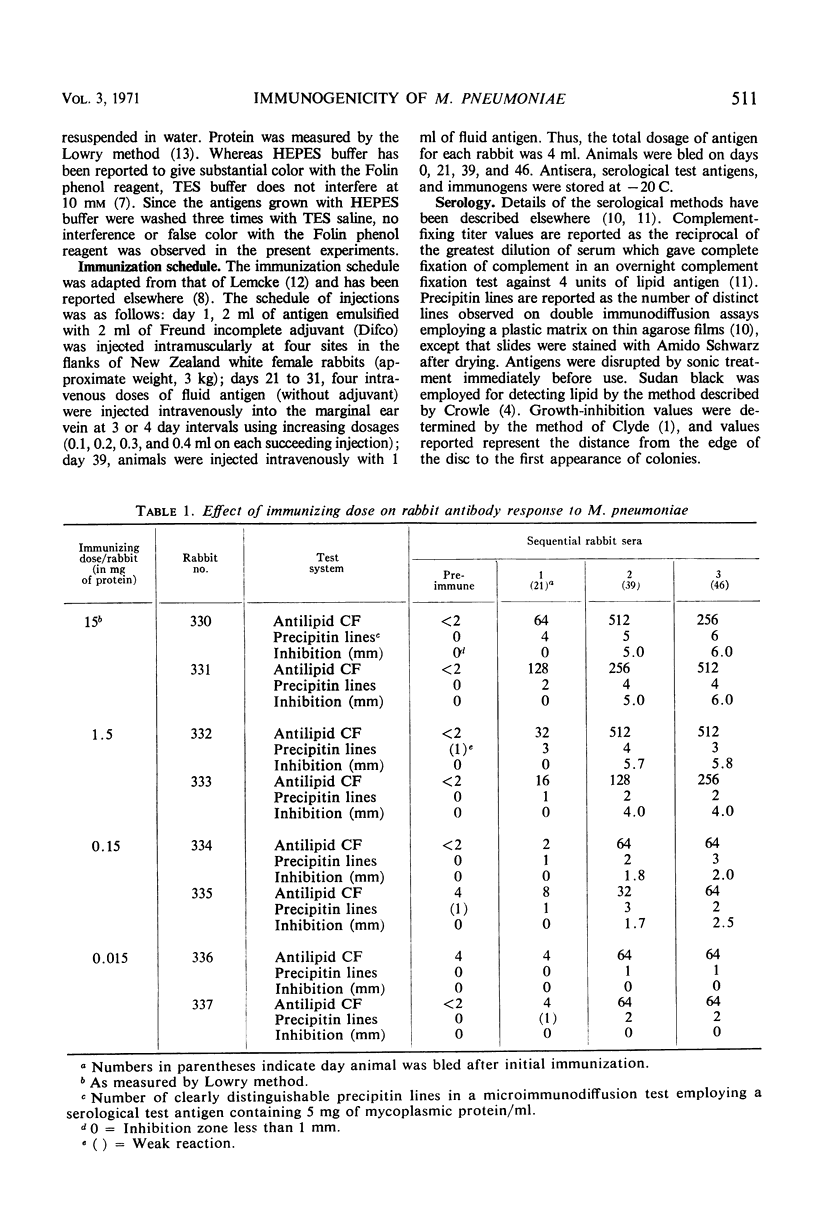
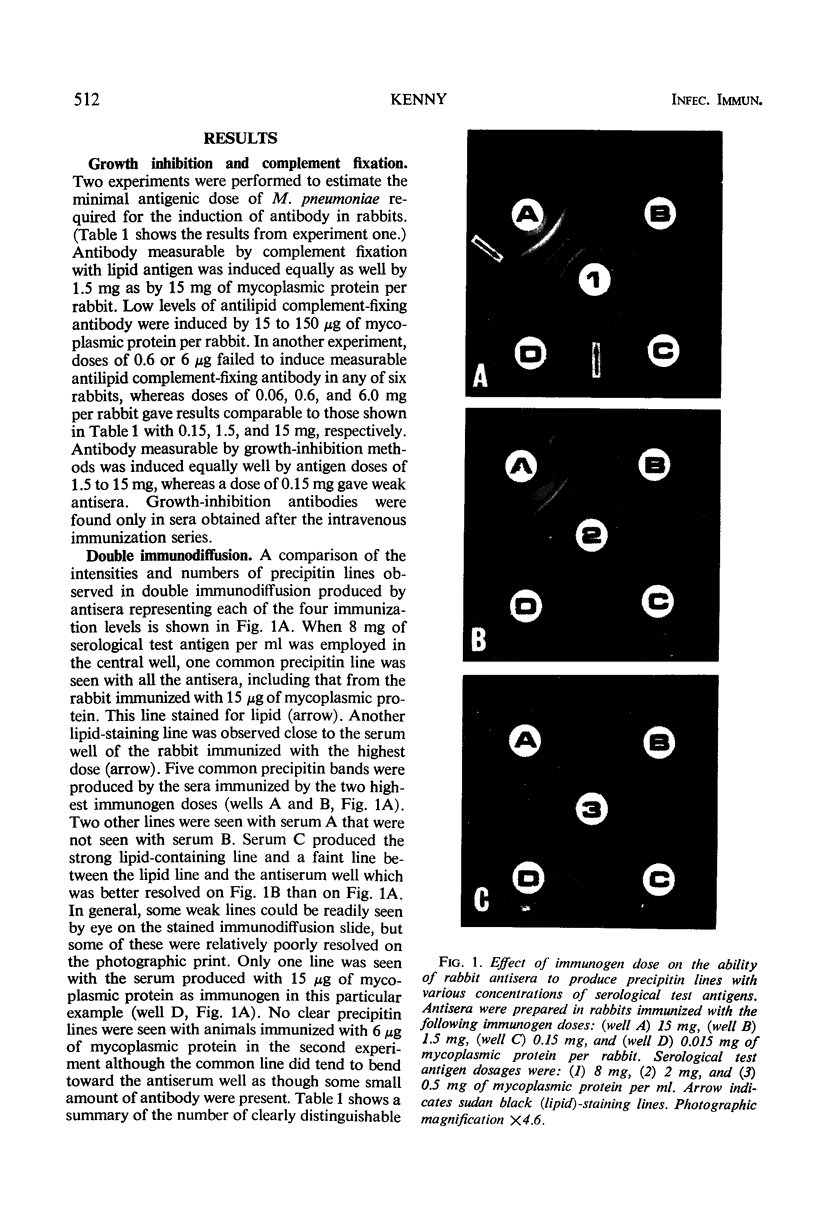
The immunogenicity of Mycoplasma pneumoniae for New Zealand white female rabbits was studied by using an immunization scheme which employed initial intramuscular immunization with vaccine in incomplete Freund adjuvant followed 3 weeks later by a series of five intravenous injections of fluid vaccine. Small doses of immunogen (15 to 150 μg of mycoplasmic protein per rabbit) gave rise to sera which contained antilipid complement-fixing antibody, produced one to three precipitin lines, but gave poor growth-inhibition on agar. Larger doses of immunogen (1.5 to 15 mg per rabbit) gave rise to sera which gave higher antilipid complement-fixing titers, four to eight precipitin lines, and good growth-inhibition. Doses smaller than 15 μg per rabbit failed to give rise to detectable antibody. Growth-inhibiting antibody was produced later than the other antibodies. The number of precipitin lines was controlled more critically by the quality of the antisera, as determined by the amount of immunogen, than by the quantity of serological test antigen. All sera which gave any precipitin lines produced a common precipitin line which stained for lipid.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CLYDE W. A., Jr MYCOPLASMA SPECIES IDENTIFICATION BASED UPON GROWTH INHIBITION BY SPECIFIC ANTISERA. J Immunol. 1964 Jun;92:958–965. [PubMed] [Google Scholar]
- Conant R. M., Somerson N. L., Senterfit L. B. Immunodiffusion reactions between human sera and Mycoplasma pneumoniae. Proc Soc Exp Biol Med. 1968 Nov;129(2):401–407. doi: 10.3181/00379727-129-33331. [DOI] [PubMed] [Google Scholar]
- Cooney M. K., Kenny G. E. Immunogenicity of rhinoviruses. Proc Soc Exp Biol Med. 1970 Feb;133(2):645–650. doi: 10.3181/00379727-133-34536. [DOI] [PubMed] [Google Scholar]
- ENNY G. E., GRAYSTON J. T. EATON PLEUROPNEUMONIA-LIKE ORGANISM (MYCOPLASMA PNEUMONIAE) COMPLEMENT-FIXING ANTIGEN: EXTRACTION WITH ORGANIC SOLVENTS. J Immunol. 1965 Jul;95:19–25. [PubMed] [Google Scholar]
- Fernald G. W., Clyde W. A., Jr, Denny F. W. Nature of the immune response to Mycoplasma pneumoniae. J Immunol. 1967 May;98(5):1028–1038. [PubMed] [Google Scholar]
- Gale J. L., Kenny G. E. Complement dependent killing of Mycoplasma pneumoniae by antibody: kinetics of the reaction. J Immunol. 1970 May;104(5):1175–1183. [PubMed] [Google Scholar]
- Gregory J. D., Sajdera S. W. Interference in the Lowry method for protein determination. Science. 1970 Jul 3;169(3940):97–98. doi: 10.1126/science.169.3940.97-a. [DOI] [PubMed] [Google Scholar]
- Kenny G. E. Heat-lability and organic solvent-solubility of mycoplasma antigens. Ann N Y Acad Sci. 1967 Jul 28;143(1):676–681. doi: 10.1111/j.1749-6632.1967.tb27713.x. [DOI] [PubMed] [Google Scholar]
- Kenny G. E. Serological comparison of ten glycolytic Mycoplasma species. J Bacteriol. 1969 Jun;98(3):1044–1055. doi: 10.1128/jb.98.3.1044-1055.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEMCKE R. M. THE SEROLOGICAL DIFFERENTIATION OF MYCOPLASMA STRAINS (PLEURO-PNEUMONIA-LIKE ORGANISMS) FROM VARIOUS SOURCES. J Hyg (Lond) 1964 Jun;62:199–219. doi: 10.1017/s0022172400039930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Morton H. E., Roberts R. J. Production of anti-Mycoplasma (PPLO) antibodies in rabbits. Proc Soc Exp Biol Med. 1967 Jun;125(2):538–543. doi: 10.3181/00379727-125-32140. [DOI] [PubMed] [Google Scholar]
- Plackett P., Marmion B. P., Shaw E. J., Lemcke R. M. Immunochemical analysis of Mycoplasma pneumoniae. 3. Separation and chemical identification of serologically active lipids. Aust J Exp Biol Med Sci. 1969 Apr;47(2):171–195. doi: 10.1038/icb.1969.19. [DOI] [PubMed] [Google Scholar]
- Pollack J. D., Somerson N. L., Senterfit L. B. Effect of pH on the immunogenicity of Mycoplasma pneumoniae. J Bacteriol. 1969 Feb;97(2):612–619. doi: 10.1128/jb.97.2.612-619.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack J. D., Somerson N. L., Senterfit L. B. Isolation, Characterization, and Immunogenicity of Mycoplasma pneumoniae Membranes. Infect Immun. 1970 Sep;2(3):326–339. doi: 10.1128/iai.2.3.326-339.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobeslavsky O., Prescott B., James W. D., Chanock R. M. Isolation and characterization of fractions of Mycoplasma pneumoniae. II. Antigenicity and immunogenicity. J Bacteriol. 1966 Jun;91(6):2126–2138. doi: 10.1128/jb.91.6.2126-2138.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]