Abstract
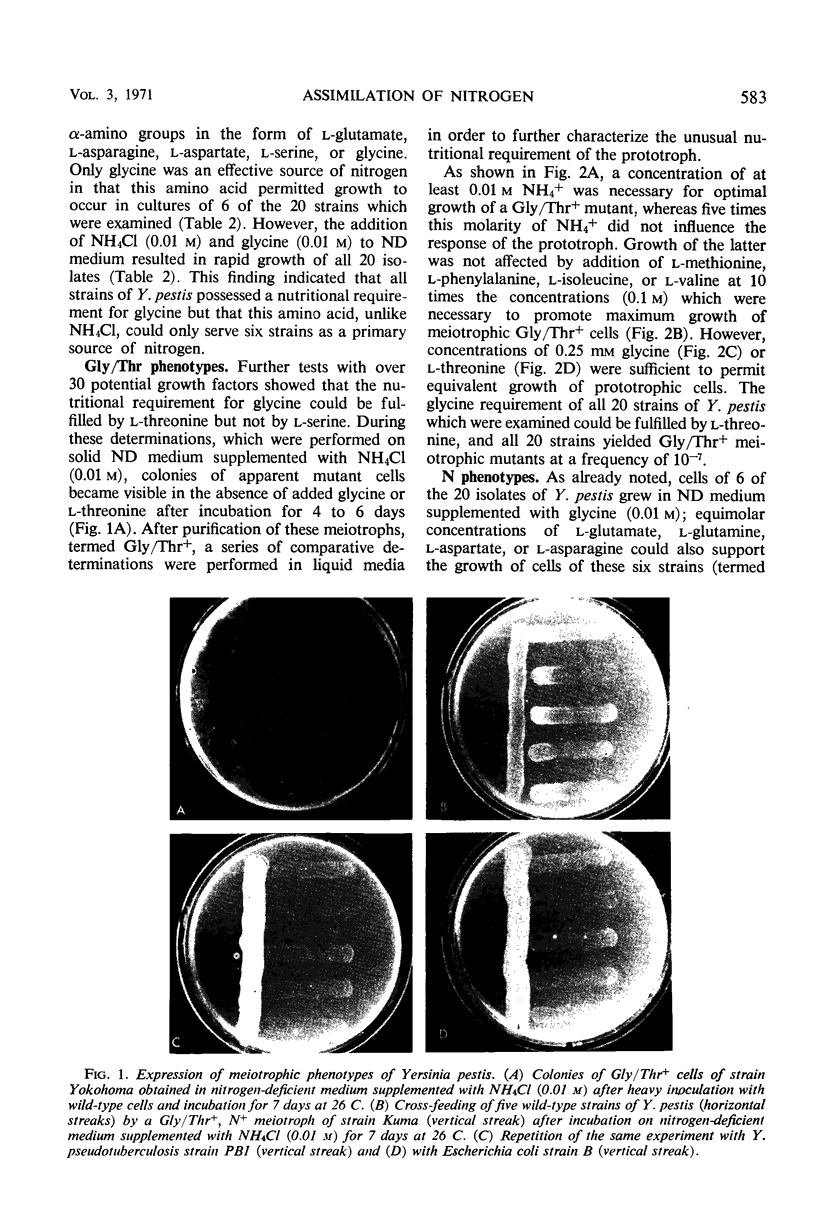
Cells of 20 isolates of Yersinia (Pasteurella) pestis exhibited an unusual nutritional requirement which could be fulfilled by glycine or l-threonine. Meiotrophic mutants which required neither of these amino acids (Gly/Thr+) were isolated from cultures of all 20 strains at a frequency of 10−7. Wild-type and Gly/Thr+ cells of 14 strains failed to utilize l-amino acids or urea (0.01 m) as primary sources of nitrogen and grew slowly in the presence of low concentrations of NH4+ (≦ 5 mm). Cells of six strains (termed N+) utilized certain l-amino acids and urea (0.01 m) as primary sources of nitrogen and grew rapidly in the presence of ≦ 5 mm NH4+. N+ but not N− organisms cultivated with NH4+ (0.01 m) as a primary source of nitrogen excreted a complete spectrum of naturally occurring amino acids; under this condition of growth the aspartase and particulate nicotinamide adenine dinucleotide phosphate transhydrogenase activities of N+ and N− cells were repressed. N+ meiotrophs arose at a frequency of 10−6 in cultures of all 14 N− isolates, and urease-positive meiotrophs could be selected at a frequency of 10−7 from N+ but not N− cells of all 20 strains on a medium containing urea (0.01 m) as a primary source of nitrogen. These findings illustrate a reversible loss of genetic potential which has occurred during the evolution of Y. pestis as an obligate parasite and suggest that this organism is unable to efficiently remove dispensable deoxyribonucleic acid from its chromosome.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOVARNICK M. R., MILLER J. C. Oxidation and transamination of glutamate by typhus rickettsiae. J Biol Chem. 1950 Jun;184(2):661–676. [PubMed] [Google Scholar]
- BROWNLOW W. J., WESSMAN G. E. Nutrition of Pasteurella pestis in chemically defined media at temperatures of 36 to 38 C. J Bacteriol. 1960 Feb;79:299–304. doi: 10.1128/jb.79.2.299-304.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURROWS T. W., JACKSON S. The pigmentation of Pasteurella pestis on a defined medium containing haemin. Br J Exp Pathol. 1956 Dec;37(6):570–576. [PMC free article] [PubMed] [Google Scholar]
- Beesley E. D., Brubaker R. R., Janssen W. A., Surgalla M. J. Pesticins. 3. Expression of coagulase and mechanism of fibrinolysis. J Bacteriol. 1967 Jul;94(1):19–26. doi: 10.1128/jb.94.1.19-26.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragg P. D., Hou C. Oxidative phosphorylation in Escherichia coli. Can J Biochem. 1968 Jul;46(7):631–641. doi: 10.1139/o68-099. [DOI] [PubMed] [Google Scholar]
- Brubaker R. R. Growth of Pasteurella pseudotuberculosis in simulated intracellular and extracellular environments. J Infect Dis. 1967 Dec;117(5):403–417. doi: 10.1093/infdis/117.5.403. [DOI] [PubMed] [Google Scholar]
- Brubaker R. R. Metabolism of carbohydrates by Pasteurella pseudotuberculosis. J Bacteriol. 1968 May;95(5):1698–1705. doi: 10.1128/jb.95.5.1698-1705.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows T. W., Gillett W. A. The nutritional requirements of some Pasteurella species. J Gen Microbiol. 1966 Nov;45(2):333–345. doi: 10.1099/00221287-45-2-333. [DOI] [PubMed] [Google Scholar]
- DEVIGNAT R. Variétés de l'espèce Pasteurella pestis; nouvelle hypothèse. Bull World Health Organ. 1951;4(2):247–263. [PMC free article] [PubMed] [Google Scholar]
- DOMARADSKII I. V., SEMENUSHKINA A. F. Nekotorye dannye ob usvoenii glikokola chumnym mikrobom. Vopr Med Khim. 1957 Jan-Feb;3(1):30–35. [PubMed] [Google Scholar]
- ENGLESBERG E. Mutation to rhamnose utilization in Pasteurella pestis. J Bacteriol. 1957 May;73(5):641–648. doi: 10.1128/jb.73.5.641-648.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENGLESBERG E. The irreversibility of methionine synthesis from cysteine in pasteurella pestis. J Bacteriol. 1952 May;63(5):675–680. doi: 10.1128/jb.63.5.675-680.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englesberg E., Ingraham L. MEIOTROPHIC MUTANTS OF Pasteurella Pestis AND THEIR USE IN THE ELUCIDATION OF NUTRITIONAL REQUIREMENTS. Proc Natl Acad Sci U S A. 1957 May 15;43(5):369–372. doi: 10.1073/pnas.43.5.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALPERN Y. S., UMBARGER H. E. Conversion of ammonia to amino groups in Escherichia coli. J Bacteriol. 1960 Sep;80:285–288. doi: 10.1128/jb.80.3.285-288.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALPERN Y. S., UMBARGER H. E. Utilization of L-glutamic and 2-oxoglutaric acid as sole sources of carbon by Escherichia coli. J Gen Microbiol. 1961 Oct;26:175–183. doi: 10.1099/00221287-26-2-175. [DOI] [PubMed] [Google Scholar]
- HIGUCHI K., CARLIN C. E. Studies on the nutrition and physiology of Pasteurella pestis. II. A defined medium for the growth of Pasteurella pestis. J Bacteriol. 1958 Apr;75(4):409–413. doi: 10.1128/jb.75.4.409-413.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIGUCHI K., SMITH J. L. Studies on the nutrition and physiology of Pasteurella pestis. VI. A differential plating medium for the estimation of the mutation rate to avirulence. J Bacteriol. 1961 Apr;81:605–608. doi: 10.1128/jb.81.4.605-608.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILLS G. M., SPURR E. D. The effect of temperature on the nutritional requirements of Pasteurella pestis. J Gen Microbiol. 1952 Feb;6(1-2):64–73. doi: 10.1099/00221287-6-1-2-64. [DOI] [PubMed] [Google Scholar]
- KANN E. E., MILLS R. C. Oxidation of glutamic acid by Pasteurella tularensis. J Bacteriol. 1955 Jun;69(6):659–664. doi: 10.1128/jb.69.6.659-664.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARR A. G., OLSEN C. B., UNGER H. S., WILSON J. B. The oxidation of glutamic acid by Brucella abortus. J Bacteriol. 1953 Nov;66(5):606–610. doi: 10.1128/jb.66.5.606-610.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOLLARET H. H., NGUYENVAN B. A., VANDEKERKOVE M., KARIMI Y., EFTEKHARI M. SUR L'UR'EASE DU BACILLE DE YERSIN. Ann Inst Pasteur (Paris) 1964 Sep;107:424–429. [PubMed] [Google Scholar]
- Mallavia L. P., Weiss E. Catabolic activities of Neisseria meningitidis: utilization of glutamate. J Bacteriol. 1970 Jan;101(1):127–132. doi: 10.1128/jb.101.1.127-132.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olenicheva L. S., Atarova G. T. O dezaminirovanii aminokislot chumnym mikrobom. Ukr Biokhim Zh. 1968;40(2):213–216. [PubMed] [Google Scholar]
- RENDINA G., MILLS R. C. Reduced triphosphopyridine nucleotide oxidase of Pasteurella tularensis. J Bacteriol. 1957 Nov;74(5):572–576. doi: 10.1128/jb.74.5.572-576.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surgalla M. J., Beesley E. D. Congo red-agar plating medium for detecting pigmentation in Pasteurella pestis. Appl Microbiol. 1969 Nov;18(5):834–837. doi: 10.1128/am.18.5.834-837.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TONHAZY N. E., PELCZAR M. J., Jr Oxidation of amino acids and compounds associated with the tricarboxylic acid cycle by Neisseria gonorrhoeae. J Bacteriol. 1953 Apr;65(4):368–377. doi: 10.1128/jb.65.4.368-377.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VANLENTEN E. J., SIMMONDS S. METABOLIC RELATIONS BETWEEN L-THREONINE AND GLYCINE IN ESCHERICHIA COLI. J Biol Chem. 1965 Aug;240:3361–3371. [PubMed] [Google Scholar]
- Vender J., Jayaraman K., Rickenberg H. V. Metabolism of glutamic acid in a mutant of Escherichia coli. J Bacteriol. 1965 Nov;90(5):1304–1307. doi: 10.1128/jb.90.5.1304-1307.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WESSMAN G. E., MILLER D. J., SURGALLA M. J. Toxic effect of glucose on virulent Pasteurella pestis in chemically defined media. J Bacteriol. 1958 Oct;76(4):368–375. doi: 10.1128/jb.76.4.368-375.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]




