Abstract
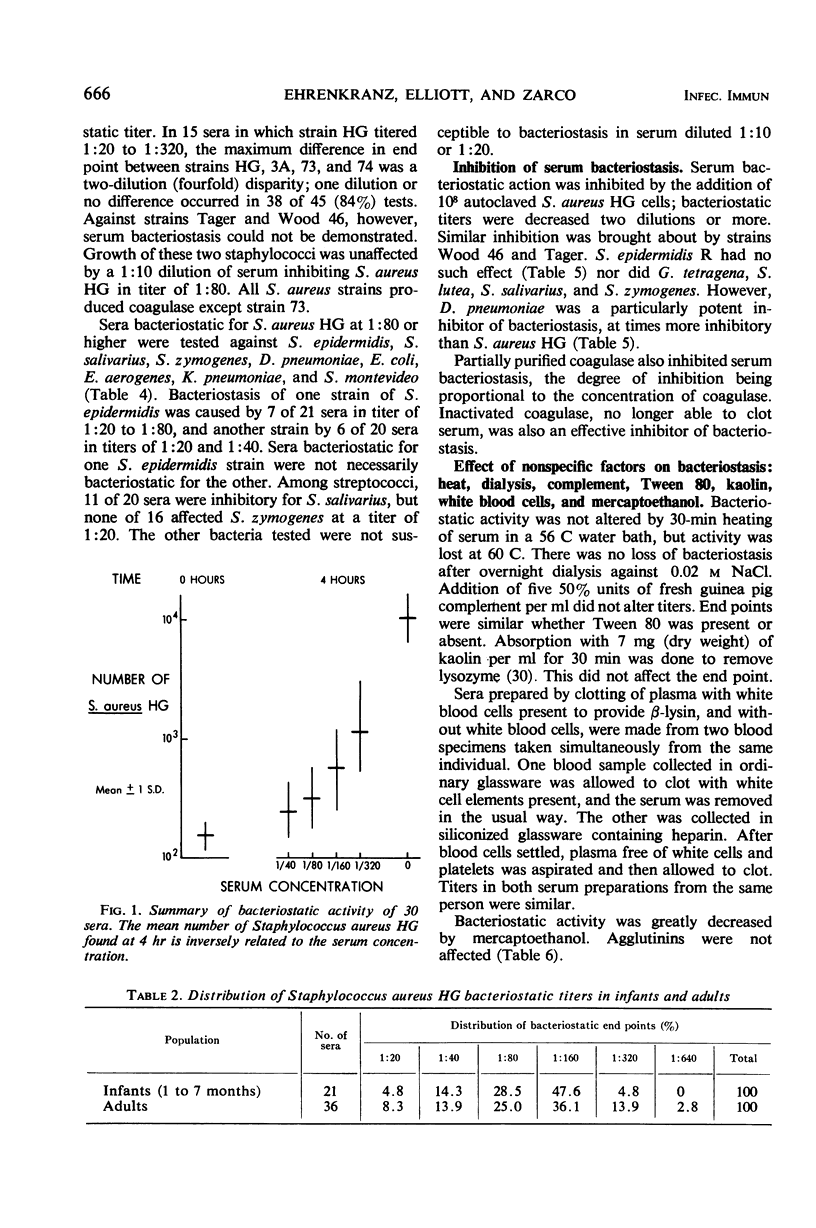
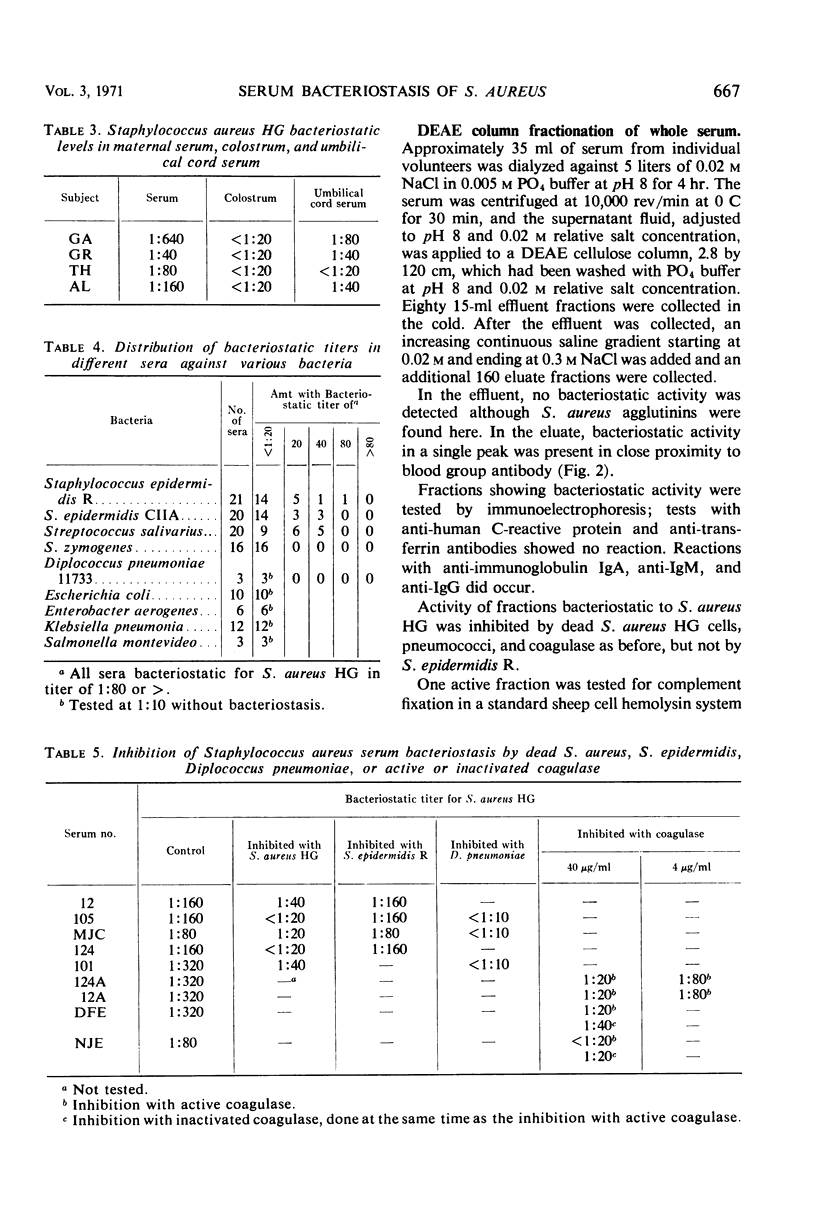
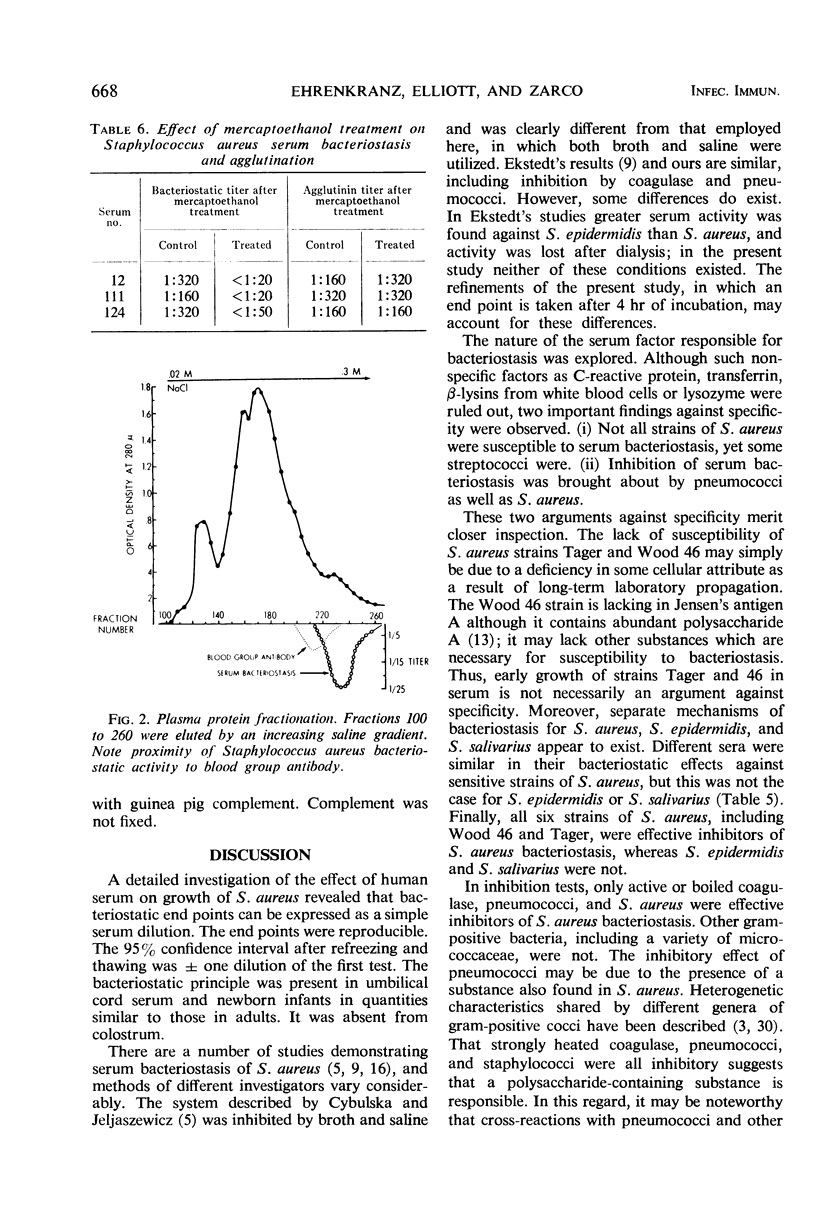
Serum bacteriostasis of Staphylococcus aureus was characterized quantitatively and quantitatively. Bacteriostasis was proportional to the concentration of serum. Reproducibility was good; freezing and thawing did not materially affect the end point. Four of six different strains, including the propagating S. aureus strain for phage 73 which does not produce coagulase, were susceptible to serum bacteriostasis in similar titers; two were not susceptible at all. All six strains were effective inhibitors of bacteriostasis. Active and inactive coagulase were also inhibitors. In contrast to sensitive S. aureus, S. epidermidis and Streptococcus salivarius were not uniformly susceptible to bacteriostasis by different serums. Escherichia coli, Enterobacter aerogenes, Klebsiella pneumoniae, Salmonella montevideo, S. zymogenes, and Diplococcus pneumoniae were not susceptible. Among gram-positive bacteria, only D. pneumoniae inhibited S. aureus bacteriostasis. Agglutinins of S. aureus and nonspecific substances such as lysozyme, β-lysin, C-reactive protein, and transferrin were not responsible for S. aureus serum bacteriostasis. After diethylaminoethyl column fractionation of serum, the bacteriostatic principle was eluted in proximity to blood group antibody; immunoglobulins A, G, and M appeared to be present in bacteriostatic fractions. It is suggested that S. aureus bacteriostasis by serum is due to natural antibody and that inhibitory reactions with pneumococci and coagulase are due to common antigens.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apsey M. E., Burston D., Maclagan N. F. The effects of tissue extracts and experimental granulomata on rat serum glycoproteins. Br J Exp Pathol. 1966 Feb;47(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- BURKE J. F. IDENTIFICATION OF THE SOURCES OF STAPHYLOCOCCI CONTAMINATING THE SURGICAL WOUND DURING OPERATION. Ann Surg. 1963 Nov;158:898–904. doi: 10.1097/00000658-196311000-00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke J. F. The effective period of preventive antibiotic action in experimental incisions and dermal lesions. Surgery. 1961 Jul;50:161–168. [PubMed] [Google Scholar]
- COHEN J. O., COWART G. S., CHERRY W. B. Antibodies against Staphylococcus aureus in nonimmunized rabbits. J Bacteriol. 1961 Jul;82:110–114. doi: 10.1128/jb.82.1.110-114.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorpenning F. W., Dodd M. C. Heterogenetic antigens of gram-positive bacteria. J Bacteriol. 1966 Apr;91(4):1440–1445. doi: 10.1128/jb.91.4.1440-1445.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cybulska J., Jeljaszewicz J. Bacteriostatic activity of serum against staphylococci. J Bacteriol. 1966 Mar;91(3):953–962. doi: 10.1128/jb.91.3.953-962.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DINEEN P. A period of unusual microbial susceptibility in an experimental staphylococcal infection. J Infect Dis. 1961 Mar-Apr;108:174–180. doi: 10.1093/infdis/108.2.174. [DOI] [PubMed] [Google Scholar]
- EKSTEDT R. D. Further studies on the antibacterial activity of human serum on Micrococcus pyogenes and its inhibition by coagulase. J Bacteriol. 1956 Aug;72(2):157–161. doi: 10.1128/jb.72.2.157-161.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenkranz N. J. Nasal rejection of experimentally inoculated Staphylococcus aureus: evidence for an immune reaction in man. J Immunol. 1966 Mar;96(3):509–517. [PubMed] [Google Scholar]
- Ekstedt R. D. Mechanisms of resistance to staphylococcal infection: natural and acquired. Ann N Y Acad Sci. 1965 Jul 23;128(1):301–334. doi: 10.1111/j.1749-6632.1965.tb11646.x. [DOI] [PubMed] [Google Scholar]
- FAHEY F. L., LAWRENCE M. E. QUANTITATIVE DETERMINATION OF 6.6 S GAMMA-GLOBULINS, BETA-2A-GLOBULINS AND GAMMA-1-MACROGLOBULINS IN HUMAN SERUM. J Immunol. 1963 Nov;91:597–603. [PubMed] [Google Scholar]
- FISHER S. EXPERIMENTAL STAPHYLOCOCCAL INFECTION OF THE SUBCUTANEOUS TISSUE OF THE MOUSE. II. PROMOTION OF THE INFECTION WITH STAPHYLOCOCCAL CELLS AND PRODUCTS. J Infect Dis. 1963 Nov-Dec;113:213–218. doi: 10.1093/infdis/113.3.213. [DOI] [PubMed] [Google Scholar]
- FISHER S. The antistaphylococcal activity of human sera in vitro and its relationship to passive protective potency. Aust J Exp Biol Med Sci. 1960 Aug;38:339–346. doi: 10.1038/icb.1960.36. [DOI] [PubMed] [Google Scholar]
- HAUKENES G., OEDING P. On two new antigens in Staphylococcus aureus. Acta Pathol Microbiol Scand. 1960;49:237–248. doi: 10.1111/j.1699-0463.1960.tb01135.x. [DOI] [PubMed] [Google Scholar]
- HIRSCH J. G. Comparative bactericidal activities of blood serum and plasma serum. J Exp Med. 1960 Jul 1;112:15–22. doi: 10.1084/jem.112.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidelberger M., Slodki M. E. Predicted and unpredicted cross-reactions of an acetylphosphogalactan of Sporobolomyces yeast. J Exp Med. 1968 Jul 1;128(1):189–196. doi: 10.1084/jem.128.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAMES R. C., MACLEOD C. J. Induction of staphylococcal infections in mice with small inocula introduced on sutures. Br J Exp Pathol. 1961 Jun;42:266–277. [PMC free article] [PubMed] [Google Scholar]
- KERN R. A., KINGKADE M. J., KERN S. F., BEHRENS O. K. Characterization of the action of lysozyme on Staphylococcus aureus and on Micrococcus lysodeikticus. J Bacteriol. 1951 Feb;61(2):171–178. doi: 10.1128/jb.61.2.171-178.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa M. Antibacterial substance in human serum against non-pathogenic staphylococcus. Bibl Haematol. 1968;29:1176–1181. doi: 10.1159/000384760. [DOI] [PubMed] [Google Scholar]
- MACLEOD C. M., HALL C. A., Jr, FROHMAN L. A. RELATIONSHIP OF ABSCESS FORMATION IN MICE, GUINEA-PIGS AND RABBITS TO ANTISTAPHYLOCOCCAL ACTIVITY OF THEIR TISSUES AND BLOOD SERUM. Br J Exp Pathol. 1963 Dec;44:612–620. [PMC free article] [PubMed] [Google Scholar]
- Patterson L. T., Higginbotham R. D. Mouse C-reactive protein and endotoxin-induced resistance. J Bacteriol. 1965 Dec;90(6):1520–1524. doi: 10.1128/jb.90.6.1520-1524.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHADE A. L. SIGNIFICANCE OF SERUM IRON FOR THE GROWTH, BIOLOGICAL CHARACTERISTICS, AND METABOLISM OF STAPHYLOCOCCUS AUREUS. Biochem Z. 1963;338:140–148. [PubMed] [Google Scholar]
- SKARNES R. C., WATSON D. W. Antimicrobial factors of normal tissues and fluids. Bacteriol Rev. 1957 Dec;21(4):273–294. doi: 10.1128/br.21.4.273-294.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAUBLER J. H., MUDD S., SALL T. Partial protection of mice by human gamma-globulin against Staphylococcus aureus on subcutaneous sutures. Proc Soc Exp Biol Med. 1962 Jan;109:20–23. doi: 10.3181/00379727-109-27090. [DOI] [PubMed] [Google Scholar]
- Tomasi T. B., Jr, Bienenstock J. Secretory immunoglobulins. Adv Immunol. 1968;9:1–96. doi: 10.1016/s0065-2776(08)60441-1. [DOI] [PubMed] [Google Scholar]
- WARDLAW A. C. The complement-dependent bacteriolytic activity of normal human serum. I. The effect of pH and ionic strength and the role of lysozyme. J Exp Med. 1962 Jun 1;115:1231–1249. doi: 10.1084/jem.115.6.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOTIS W. W. Effect of the antibacterial serum factor on staphylococcal infections. J Bacteriol. 1962 Jan;83:137–143. doi: 10.1128/jb.83.1.137-143.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yotis W., Stanke R. Bacteriostatic action of progesterone on staphylococci and other microorganisms. J Bacteriol. 1966 Nov;92(5):1285–1289. doi: 10.1128/jb.92.5.1285-1289.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]


