Background: The role of BMAL1 in lipogenesis in the liver is unknown.
Results: BMAL1 is necessary and required for driving lipogenic gene expression and AKT activation in vivo and in vitro in response to refeeding and insulin.
Conclusion: BMAL1 promotes lipogenesis through activation of AKT and stabilization of RICTOR.
Significance: BMAL1-dependent lipogenesis may be targeted for treating fatty liver disease.
Keywords: Akt, Circadian Clock, Lipid Metabolism, Lipogenesis, Liver, Insulin Signaling, Rictor-mTORC2
Abstract
The clock protein BMAL1 (brain and muscle Arnt-like protein 1) participates in circadian regulation of lipid metabolism, but its contribution to insulin AKT-regulated hepatic lipid synthesis is unclear. Here we used both Bmal1−/− and acute liver-specific Bmal1-depleted mice to study the role of BMAL1 in refeeding-induced de novo lipogenesis in the liver. Both global deficiency and acute hepatic depletion of Bmal1 reduced lipogenic gene expression in the liver upon refeeding. Conversely, Bmal1 overexpression in mouse liver by adenovirus was sufficient to elevate the levels of mRNA of lipogenic enzymes. Bmal1−/− primary mouse hepatocytes displayed decreased levels of de novo lipogenesis and lipogenic enzymes, supporting the notion that BMAL1 regulates lipid synthesis in hepatocytes in a cell-autonomous manner. Both refed mouse liver and insulin-treated primary mouse hepatocytes showed impaired AKT activation in the case of either Bmal1 deficiency or Bmal1 depletion by adenoviral shRNA. Restoring AKT activity by a constitutively active mutant of AKT nearly normalized de novo lipogenesis in Bmal1−/− hepatocytes. Finally, Bmal1 deficiency or knockdown decreased the protein abundance of RICTOR, the key component of the mTORC2 complex, without affecting the gene expression of key factors of insulin signaling. Thus, our study uncovered a novel metabolic function of hepatic BMAL1 that promotes de novo lipogenesis via the insulin-mTORC2-AKT signaling during refeeding.
Introduction
During normal cycles of feeding and fasting, the liver tightly regulates both catabolic and anabolic events based upon the body's energy demands (1–3). In the case of feeding the food-triggered insulin surge prompts the liver to switch on the expression of metabolic enzymes for anabolic events such as glycogenesis and de novo lipogenesis. Upon fasting, this lipogenic flux quickly returns to the basal level (1, 4). However, after chronic high fat diet feeding, de novo lipogenesis in the liver remains high even during fasting when insulin secretion is inhibited (5–7). It has been proposed that constantly elevated de novo lipogenesis contributes to the pathogenesis of non-alcoholic fatty liver diseases (5, 7). So far the mechanisms underlying the unchecked de novo lipogenesis in fatty liver diseases remain largely unknown. A deep understanding of this metabolic process is necessary to target the pathway for treatment of liver steatosis.
Genetic mouse models have demonstrated that hepatic AKT2-mTORC13 (mammalian target of rapamycin complex 1)-SREBP-1c (sterol regulatory element-binding protein-1c) pathway plays a critical role in promoting lipogenesis upon insulin stimulation (8–10). AKT2 activation is both necessary and sufficient for the induction of hepatic SREBP-1c and lipid accumulation. AKT2 activation of SREBP-1c requires a functional mTORC1 as rapamycin blocks AKT2 effects on SREBP-1c (10). Furthermore, the mTORC2 complex directly controls AKT2 phosphorylation and promotes lipogenesis in an AKT2-SREBP-1c-dependent manner (11, 12). Moreover, AKT2 activity is required for hepatic lipid accumulation in models of insulin resistance (13–15). Collectively, all the evidence points to the critical function of AKT2 in regulating hepatic lipid metabolism in both normal and diabetic conditions. However, during the postprandial phase, AKT2-mediated lipid metabolism is independent of transcription factor FOXO1 (forkhead box protein O1), FOXA2 (forkhead box protein A2), and SREBP-1c, suggesting that other transcription factors are required for this process (16).
BMAL1, a basic helix-loop-helix (bHLH) transcription factor, is the essential component of the mammalian circadian clock (17). BMAL1 forms a transcription complex with another bHLH protein CLOCK (circadian locomotor output cycles kaput) to activate the core circadian genes and a large array of circadian output genes (18–20). BMAL1 recognizes both E-box (AGGTCA) and E-box like elements present in the promoter region of its transcription targets (21, 22). The absence of Bmal1 or Clock results in not only abolishment of its circadian targets but also reduced expression in a great number of metabolic genes, providing direct evidence for BMAL1 being a novel metabolic regulator that couples circadian rhythms and metabolism (23–26). It has been observed that Bmal1 knock-out mice show insulin resistance (24, 27), hypo-insulinemia (28, 29), adipose dysfunction (30–32), and impaired lipid homeostasis (24, 32). Several studies have suggested a critical function of BMAL1 in regulating lipid synthesis and storage in white adipose tissue (30–32). It has been reported that Bmal1 mRNA was increased in high fat diet-treated mouse adipose tissue and in differentiating 3T3-L1 cells (31, 33), whereas adenoviral overexpression of Bmal1 enhanced peroxisome proliferator-activated receptor γ (PPARγ) expression and lipid synthesis in 3T3-L1 adipocytes (31). Bmal1 deficiency blocked the differentiation of embryonic fibroblast cells into adipocytes (31) and reduced the capacity of fat storage in adipose tissues (24, 30, 32). However, whether or not BMAL1 regulates de novo lipogenesis in the liver remains unclear.
In this report we demonstrate for the first time that BMAL1 is both necessary and sufficient to promote the expression of lipid synthesis enzymes in the mouse liver and de novo lipogenesis in mouse hepatocytes. Deletion or acute depletion of Bmal1 prevents refeeding- or insulin-induced lipogenic gene expression in the liver or hepatocytes. Mechanistically, BMAL1 is required for AKT activation upon refeeding in the liver or insulin stimulation in hepatocytes. Restoring AKT activity in Bmal1−/− primary mouse hepatocytes corrects the defect in de novo lipogenesis and restores the expression of lipid synthesis enzymes. Our findings highlight that in addition to its circadian role, BMAL1 functions as an important lipogenic regulator by maintaining the cellular mTORC2-AKT activity.
MATERIALS AND METHODS
Plasmids and Adenovirus Generation
The Bmal1 shRNA shuttle construct was made by ligating the targeting oligo nucleotide sequence into the pEntry/U6 vector (Invitrogen). The targeting sequence for mouse Bmal1 is 5′-CATCGATATGATAGATAACG-3′. Ad-shBmal1 plasmid was generated through Gateway LR recombination between pEntry/U6-shBmal1 and the pAdBlock-iT vector (Invitrogen). The adenoviral Bmal1 shuttle vector was made by cloning the full-length Bmal1 cDNA into the pShuttle-IRES-GFP (Agilent). pAdEasy Bmal1-FLAG-IRES-GFP viral DNA was made by in vitro recombination between pShuttle-Bmal1-FLAG-IRES-GFP and the pAdEasy-1 plasmid in BJ5183-AD-1 competent cells (Agilent) after electroporation. All the adenoviruses were produced in 293AD packaging cells (Agilent) after Lipofectamine-mediated transfection and concentrated after ultracentrifuge in cesium chloride gradient solutions according to the user's manual. The constitutively active AKT2 adenovirus was kindly provided by Dr. Morris Birnbaum at the University of Pennsylvania.
Animal Experiments
All mice in this study were maintained on 12 h/12 h light/dark cycles with ad libitum access to food and water. For the fasting and feeding study, C57BL6 wild-type or Bmal1−/− male mice (17) of 6–8 weeks (n = 5/group) were restricted from food overnight and fed for the indicated time points. For liver-specific knockdown, the Ad-shLacZ control or Ad-shBmal1 shRNA adenoviruses were injected into mice via tail vein injection at the dose of 1 × 1012 plaque-forming units. Bmal1flox/flox mice were generously provided by Dr. Jiandie Lin at the University of Michigan. Bmal1 liver-specific knock-out (Bmal1-LKO) mice were generated by crossing Bmal1flox/flox mice with an Albumin-Cre transgenic line from The Jackson Laboratory. The liver tissues were harvested and prepared for both mRNA and protein analysis. All animal experiments were approved by the Institutional Animal Care and Research Advisory Committee at the University of Michigan.
Primary Mouse Hepatocyte Isolation and Culture
Primary mouse hepatocytes (PMHs) were isolated from male mice (9–10 weeks in C57BL/6 background) using the protocol described previously (34). The liver was perfused with Earle's balanced salt solution (Invitrogen) with 0.5 mm EGTA for 5 min followed by perfusion with 100 units/ml type I collagenase (Worthington) via the inferior vena cava. After dissection, hepatocytes were released by scattering, passed through a 100-μm cell strainer, and then spun at 50 × g for 1 min. The pellet was resuspended in DMEM and then spun at 50 × g for 10 min in a Percoll gradient to remove dead hepatocytes. The viable cells were washed with DMEM at 50 × g for 10 min and checked by trypan blue staining. PMHs were cultured on collagen-coated plates in DMEM with 5% FBS. Adenoviral transduction was performed within 6 h after seeding.
cDNA Synthesis and QPCR
Total cellular RNA extraction was performed with TRIzol reagent (Invitrogen) and chloroform. cDNA was synthesized with the Verso cDNA kit (ThermoFisher Scientific, Surrey, UK) and subjected to QPCR using Absolute Blue SYBR Green ROX Mix (Thermo Fisher Scientific) on an ABI 7900 HT thermal cycler (Applied Biosystems, Foster City, CA). The value of each cDNA was calculated using the ΔCt method and normalized to the value of the housekeeping gene control, 18 S RNA. The data were plotted as -fold change. The primer sequences used in this study are available upon request.
Immunoblotting
The cells or primary hepatocytes were washed once in 1 × PBS buffer and lysed in lysis buffer supplemented with 1 × protease inhibitor (Roche Applied Science). After whole cell lysates were precleared at maximal speed at 4 °C in a microcentrifuge, the protein concentration of each supernatant was measured using the Bio-Rad reagent. Equal amounts of protein samples were separated in 10% SDS-polyacrylamide gels and transferred to PVDF membranes (Millipore). The membranes were incubated with antibodies against BMAL1 (sc-8550), FASN (sc-20140), ACC1 (sc-30212), AKT1/2 (sc-1619), phosphorylated AKT at Ser-473 (sc-7985-R), RICTOR (sc-271081) all from Santa Cruz Biotechnology, anti-mTor (2972) from Cell Signaling, and β-tubulin (T5201) from Sigma. HRP-conjugated secondary antibodies against mouse, rabbit, or goat IgG and Western Lightning ECL substrate were used to generate chemiluminescence signals on an HD2 AlphaImager (Cell Biosciences).
De Novo Lipogenesis Assay
The assay protocol was adopted from the protocol described previously (35). In brief, PMHs were seeded on 12-well plates (2 × 105 cells/well) and cultured overnight. The cells were switched to serum-free medium 199 (Invitrogen) the next day, and 6 h later they were incubated in medium 199 with 100 nm insulin and 25 mm glucose overnight to activate de novo lipogenesis. On the second day cells were incubated with culture medium containing both cold acetate and 1 μCi of 3H-labeled acetate (Moravek Biochemicals) for 4 h. After a 1 × PBS wash cells were lysed in 200 μl of 0.1 n HCl. Protein concentration was measured by using Bio-Rad reagent with 2 μl of lysate. Lipids were extracted by adding 800 μl of chloroform/methanol (2:1, v/v). The organic fraction was transferred to a fresh tube and let air-dry at room temperature overnight. The pellet was dissolved in hexane and 5% H2SO4 in methanol and heated at 100 °C for 30 min. The final radiolabeled lipids were extracted by using 500 μl of petroleum, and the 3H radioactivity was measured on a Beckman scintillation counter. De novo lipogenesis rate was normalized by protein amount for each sample.
RESULTS
BMAL1 Is Required for Refeeding- or Insulin-induced Expression of Clock Genes in the Mouse Liver and Primary Mouse Hepatocytes
During the transition from fasting to feeding, the liver activates the anabolic metabolic pathways to store excessive nutrients in the form of lipids and glycogen (1). Consistent with literature (9, 10), we observed a robust induction of lipogenic genes upon food intake, such as Fasn (fatty acid synthase), Gck (glucokinase), and L-pk (liver pyruvate kinase) (Fig. 1A). Interestingly, the expression of two circadian clock genes, Dbp (D-box binding protein) and Per2 (period 2), was also induced by refeeding (Fig. 1B). To determine whether the induction of Dbp and Per2 by refeeding requires the involvement of the clock system in the liver, we performed tail-vein injection of adenoviral shBmal1 or shLacZ control and then subjected mice to a fasting-refeeding regimen. Bmal1 knockdown was confirmed by the reduction of BMAL1 protein levels in the liver (Fig. 1C). In these animals, induction of Dbp, Per2, and Nr1d1 (nuclear receptor subfamily 1, group D, member 1) by refeeding was completely abolished (Fig. 1D). Of note, the BMAL1 protein levels in Ad-shLacZ-injected mice during fasting and refeeding were similar (data not shown), suggesting that refeeding can activate the BMAL1 function without impacting its protein abundance. Because insulin signaling has been reported to modulate the molecular clock in cells (36), we asked whether insulin could induce the expression of Dbp and Per2 in a cell-autonomous fashion in isolated PMHs. Consistent with our data on refed mice, insulin treatment induces both Dbp and Per2 mRNA in WT-PMHs but not in Bmal1−/− PMHs (Fig. 1E). In summary, our data indicate that both refeeding and insulin activate the BMAL1-mediated transcriptional activity in the liver and hepatocytes.
FIGURE 1.

Induction of hepatic lipogenic and circadian clock gene upon refeeding and insulin. C57BL6 WT mice (n = 3–4) were fasted for 16 h before refeeding for 2 or 8 h. Mice were then sacrificed for assessment of the mRNA levels of lipogenic genes (Fasn, Gck, and L-pk) (A) and circadian genes (Dbp and Per2) (B). C, adenoviral shRNA for Bmal1 reduces the protein level of BMAL1 in the mouse liver. WT mice were administrated with recombinant adenoviral control (Ad-shLacZ) (n = 3) or Bmal1-specific shRNA (Ad-shBmal1) (n = 3) via tail-vein injection. 2 weeks post-injection, mice were fasted for 16 h and then refed for 8 h before sacrifice for measurement of liver BMAL1 protein by immunoblotting. The loading was determined by the level of Lamin A/C. The protein expression was quantified and normalized by the levels of lamin A/C. D, adenoviral Bmal1 knockdown blocks clock gene induction by refeeding. Two weeks after Ad-shBmal1 or Ad-shLacZ control injection, WT mice (n = 3) were subjected to fasting for 16 h followed by refeeding for 8 h. The hepatic mRNA levels of Dbp, Nr1d1, and Per2 were determined by RT-QPCR. The data are plotted as the mean ± S.D. E, loss of Bmal1 impairs induction of circadian genes by insulin in PMHs. PMHs were isolated from WT and Bmal1−/− mice and treated with insulin at 10 nm for 6 h. RT-QPCR was performed to assess the induction of clock gene Dbp and Per2. Each condition was done in triplicate. *, p < 0.05. **, p < 0.01. Data are representative of 3–4 independent experiments.
BMAL1 Is Required for Induction of Lipogenic Enzymes upon Refeeding
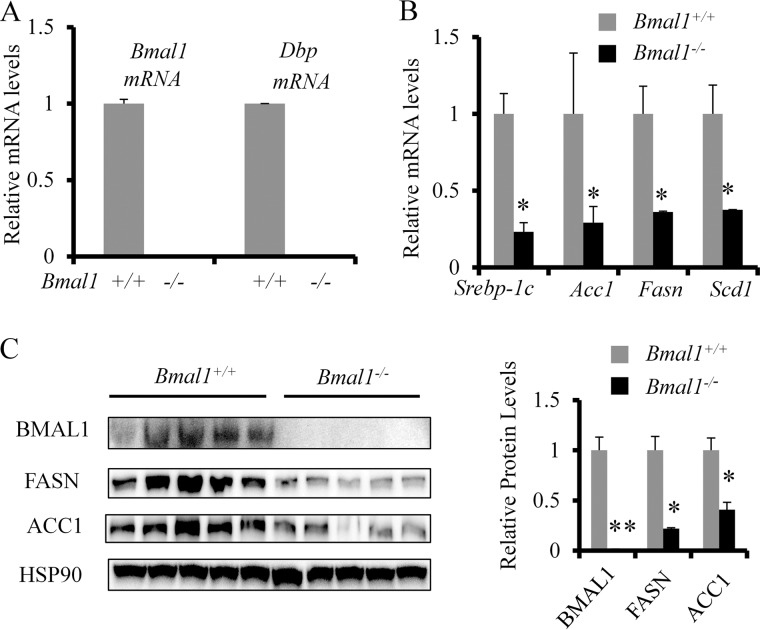
We have shown that BMAL1 is required for feeding- or insulin-induced activation of Dbp and Per2. To test whether BMAL1 is involved in the induction of lipogenic enzymes in the liver during refeeding, we fasted both Bmal1−/− mice and their wild-type littermates for 16 h and refed them for 8 h. The loss of Bmal1 expression in the liver was confirmed by the absence of the expression of Bmal1 and its target Dbp by RT-QPCR (Fig. 2A). Upon refeeding, the mRNA levels of key lipogenic enzymes such as acetyl-CoA carboxylase 1 (Acc1), Fasn, and stearoyl-CoA desaturase 1 (Scd1) dropped >50% of those of wild-type control (Fig. 2B). Interestingly, the mRNA level of the key lipogenic transcription factor Srebp-1c was also reduced. Bmal1−/− mice also showed significantly lowered protein levels of FASN and ACC1 in the liver (Fig. 2C). The severely down-regulated levels of lipogenic proteins in Bmal1−/− mice indicate that normal BMAL1 expression and function are required for full induction of lipogenic factors upon refeeding.
FIGURE 2.
Loss of Bmal1 impairs refeeding-induced lipogenic program in the liver. Both WT and Bmal1−/− (8-week male mice, n = 5) were fasted overnight and refed for 8 h. Mice were then sacrificed and harvested for analysis of levels of mRNA and protein in the liver. A, the liver mRNA levels of Bmal1 and Dbp determined by RT-QPCR. B and C, the liver mRNA and protein levels of major lipogenic enzymes or regulators in WT and Bmal1−/− mice determined by RT-QPCR and immunoblotting. The data are plotted as the mean ± S.D. (n = 5). * p < 0.05. **p < 0.01.
Liver-specific Knockdown of Bmal1 Leads to Impaired Expression of Lipogenic Factors
To further confirm the role of BMAL1 in refeeding-associated hepatic lipid metabolism, we acutely depleted Bmal1 expression in the mouse liver by tail vein injection of AdshBmal1. Two weeks after injection the mice were fasted overnight, and livers were collected before refeeding or 8 h after refeeding. In the AdshLacZ control group, refeeding robustly induced both mRNA and protein expression of major lipogenic enzymes (FASN and ACC1). However, these changes were largely abolished in the liver with Bmal1 depletion (Fig. 3, A and B). Together our data support that hepatic BMAL1 is required for refeeding-induced lipogenic program in the liver.
FIGURE 3.
Acute Bmal1 depletion in the liver impairs refeeding-induced expression of lipogenic enzymes. Three weeks after tail vein injection of Ad-shLacz or Ad-shBmal1, mice (n = 3) were fasted overnight and sacrificed either right after overnight fasting or after 8-h refeeding. Liver tissues were harvested for mRNA and protein analysis. A, protein levels of BMAL1 and the lipogenic enzyme, FASN, in the liver. Quantified protein band intensity is shown in the right panel. B, hepatic mRNA levels of lipogenic genes Acc1, Fasn, and Gck. The data were plotted as the mean ± S.D. (n = 3). *, p < 0.05.
Next, we sought to ask whether BMAL1 is sufficient to promote hepatic lipogenic program even in the absence of feeding input. Overexpression of BMAL1 in the liver was achieved by tail-vein injection of Ad-Bmal1. The expression of lipogenic enzymes was measured in the mouse liver after 16 h of fasting. The overexpression of Bmal1 was confirmed by Western blot for BMAL1 protein (Fig. 4A) and by RT-QPCR for Bmal1 targets Cry1, Dbp, and Nr1d1 (Fig. 4B). RT-QPCR analysis also revealed that the mRNA levels of lipogenic genes, such as Srebp-1c, Scd1, Fasn, and Acc1, were increased drastically in Ad-Bmal1-injected mouse livers (Fig. 4C). These data suggest that BMAL1 is both necessary and sufficient for the expression of lipogenic enzymes in the liver, highlighting the unrecognized critical function of BMAL1 in driving hepatic lipogenesis.
FIGURE 4.
Liver-specific overexpression of Bmal1 elevates the expression level of lipogenic genes. WT mice (n = 3) were injected with Ad-GFP (control virus) or Ad-Bmal1 through tail vein. One week after injection mouse livers were collected for confirmation of BMAL1 protein expression (A) and for measurement of mRNA levels by RT-QPCR for clock genes (B) and lipogenic enzymes (C). The data are plotted as the mean ± S.D. (n = 3). *, p < 0.05; **, p < 0.01.
BMAL1 Regulates Hepatocyte de Novo Lipogenesis in a Cell Autonomous Manner
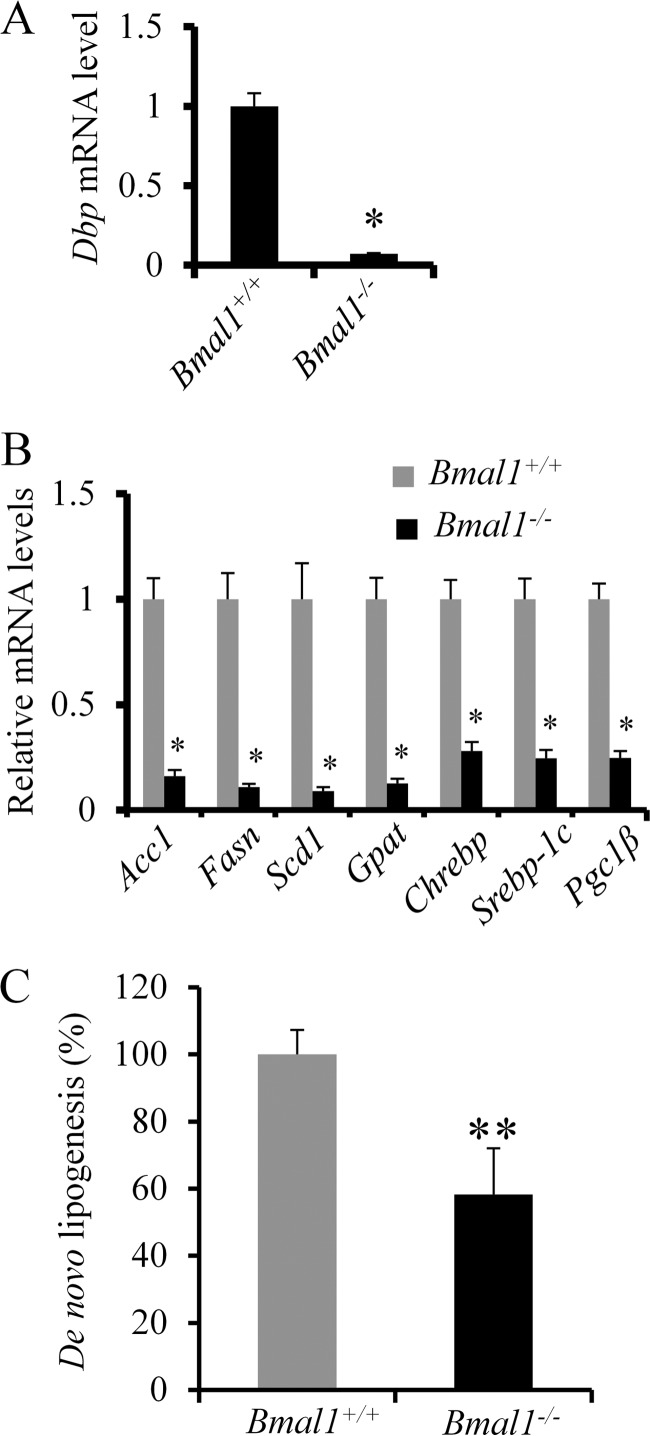
The liver is composed of hepatocytes, Kupffer cells, stellate cells, and sinusoidal endothelial cells. To confirm that BMAL1 regulation of lipogenesis occurs in hepatocytes, we isolated PMHs from both wild-type and Bmal1−/− mice and examined the rate of de novo lipogenesis by providing hepatocytes with [methyl-3H]acetate in vitro. We first confirmed that Bmal1−/− hepatocytes expressed very low levels of Dbp, the circadian target of BMAL1 (Fig. 5A). Consistent with our in vivo findings, PMHs with Bmal1 deficiency expressed significantly lower levels of lipogenic enzymes (Acc1, Fasn, Scd1, and Gpat (glycerol-3-phosphate acetyltransferase)) and lipogenic transcription factors such as Chrebp, Srebp1c, and Pgc-1β (Fig. 5B). More importantly, when challenged with high glucose and insulin, Bmal1−/− hepatocytes displayed an ∼50% reduction of de novo lipogenesis rate in comparison with WT control (Fig. 5C). Thus, we demonstrate that BMAL1 positively regulates de novo lipogenesis rate in hepatocytes.
FIGURE 5.
BMAL1 regulates de novo lipogenesis in a cell-autonomous manner. PMHs were isolated from either Bmal1−/− or WT mice and then used for measuring the levels of mRNA and protein of lipogenic enzymes and the rate of de novo lipogenesis. A, mRNA levels of Dbp in both WT and Bmal1−/− hepatocytes (n = 3). B, mRNA levels of lipogenic enzymes and regulators (n = 3). C, Bmal1−/− PMHs show decreased rate of de novo lipogenesis. PMHs from both WT and Bmal1−/− mice were treated with high glucose and insulin in six replicates before incubating with 3H-labeled acetate for 4 h. Radiolabeled lipids were extracted in petroleum, and the 3H radioactivity was normalized by protein amount for each sample. The data are plotted as the mean ± S.D. (n = 6). *, p < 0.05. **, p < 0.01. Data are representative of 3–4 independent experiments.
BMAL1 Deficiency or Knockdown Impairs Hepatic AKT Activation
In normal physiology, the feeding-triggered release of insulin activates lipogenesis through AKT in the liver. mTORC2-mediated AKT activation has been shown to be both necessary and sufficient for the induction of lipogenesis (9–12). mTORC2 inactivation through genetic deletion of its key component Rictor has been shown to inhibit the AKT pathway and lipogenesis in the liver, muscle, and adipose tissues (11, 37–39). Impaired circadian oscillations of AKT activities have been reported in Bmal1−/− mice (24, 27), prompting us to speculate that BMAL1 deficiency impairs de novo lipogenesis by blocking AKT activation. To test this hypothesis, we first examined hepatic AKT activation in the liver of Bmal1−/− mice after refeeding for 8 h. To validate insulin activation of AKT, we focused on phosphorylation at serine 473 of AKT (AKTSer(P)-473), which has been identified as a direct phosphorylation site of mTORC2 (40, 41). Indeed, AKTSer(P)-473 was reduced by ∼50% in the liver of Bmal1−/− mice (Fig. 6A). Furthermore, acute depletion of BMAL1 by adenoviral shRNA in the liver largely abolished AKTSer(P)-473 induction upon refeeding (Fig. 6B). We next asked whether BMAL1 regulates insulin-dependent AKT activation in hepatocytes. To this end, we examined the level of AKTSer(P)-473 after insulin treatment in PMHs isolated from either WT or Bmal1−/− mice. Although WT PMHs responded to insulin by increasing the levels of AKTSer(P)-473, Bmal1−/− PMHs displayed a resistance to insulin activation of AKTSer(P)-473 during the entire treatment (Fig. 6C). This effect could also be recapitulated in Hepa1 cells after acute depletion of Bmal1 using Ad-shBmal1 (Fig. 6D). To further test whether this is a BMAL1-dependent action, we acutely deleted Bmal1 by transducing Bmal1flox/flox PMHs with Ad-Cre virus and observed that Bmal1-deficient PMHs failed to show elevated levels of AKTSer(P)-473 in response to insulin treatment. However, restoring Bmal1 expression by Ad-Bmal1 completely rescued insulin-stimulated AKT phosphorylation in AdCre-transduced Bmal1flox/flox PMHs (Fig. 6E). In summary, we have demonstrated that BMAL1 is required for activation of AKT phosphorylation by refeeding or insulin in the liver and hepatocytes.
FIGURE 6.
BMAL1 is required for AKT activation by insulin in the liver and hepatocytes. A, Bmal1−/− mice show reduced AKT activity in the liver. Both WT and Bmal1−/− mice (n = 5) were fasted overnight and refed for 8 h. Liver tissues were used to examine the AKT activity (phosphorylation of AKTSer(P)-473) and the total AKT by immunoblotting. The levels of AKTSer(P)-473 were quantified and normalized to total AKT level. B, acute Bmal1 depletion in the liver reduces AKT phosphorylation induced by refeeding. Two weeks after tail vein injection of Ad-shLacZ or Ad-shBmal1, WT mice (n = 3) were fasted overnight and then refed for 8 h. After sacrifice, mouse liver lysates were prepared for Western blot analysis of AKTSer(P)-473 and total AKT. The levels of AKTSer(P)-473 were quantified and normalized to total AKT expression. C, loss of Bmal1 blocks insulin-induced AKT activation in PMHs. PMHs from WT or Bmal1−/− mice were treated with 0.2 nm insulin for the indicated time points before assessment of AKTSer(P)-473 by Western blot. D, acute depletion of Bmal1 expression attenuates insulin-induced AKT phosphorylation in Hepa1 cell. Hepa1 cells were transduced with Ad-shLacZ or Ad-shBmal1 for 36 h and then treated with 0.2 nm insulin for indicated time points. AKTSer(P)-473 levels were determined by immunoblotting. E, adenoviral overexpression of Bmal1 restores insulin-induced AKT phosphorylation in Bmal1-deleted PMHs. Bmal1flox/flox PMHs were transduced by AdGFP, or AdCre plus AdGFP, or AdCre plus AdBmal1 for 48 h before insulin treatment (0.2 nm) for the indicated time points. AKTSer(P)-473 and BMAL1 levels were measured by immunoblotting along with HSP90 as the loading control. The levels of AKT-P were quantified and normalized to total AKT expression.
Ectopic Expression of AKT-CA Restores de Novo Lipogenesis in Bmal1−/− PMHs
Given our data that Bmal1 deficiency not only suppresses de novo lipogenesis but also abrogates AKT activation by either refeeding or insulin, we hypothesized that impaired de novo lipogenesis can be rescued in Bmal1−/− PMHs by ectopic expression of the E40K mutant of AKT (AKT-CA), which remains constitutively active due to an increased affinity of its pleckstrin homology domain for phospholipids (42). After isolating PMHs from both WT and Bmal1−/− mice and transducing with either Ad-GFP or Ad-Akt2-CA, we analyzed the expression of lipogenic enzymes and the rate of de novo lipogenesis in both groups. Indeed, ectopic expression of AKT-CA potently increases both the mRNA and protein levels of lipogenic enzymes in Bmal1−/− PMHs (Fig. 7, A and B). Moreover, overexpression of AKT-CA restored de novo lipogenesis in Bmal1−/− PMHs to the level comparable with that of WT PMHs (Fig. 7C). Taken together, our data support our hypothesis that BMAL1 promotes lipogenesis through activating AKT.
FIGURE 7.
Overexpression of constitutively active AKT2 restores de novo lipogenesis in primary hepatocytes with Bmal1 deficiency. Bmal1−/− mice (n = 3) were injected with Ad-Akt-CA or Ad-GFP adenovirus through tail vein. One week later PMHs were isolated for measuring the mRNA and protein levels of lipogenic enzymes (A and B) and the rate of de novo lipogenesis (C). The data are plotted as the mean ± S.D. (n = 3). *, p < 0.05. Data are representative of three independent experiments.
BMAL1 Promotes RICTOR Protein Stability
As a transcription activator, BMAL1 and its binding partner CLOCK activate the expression of a wide range of targets that is important for liver metabolism (43–45). It is plausible that BMAL1-CLOCK regulates the expression of the key components of insulin signaling that leads to enhanced AKT activation. We, therefore, systematically examined the mRNA levels of major insulin-mTORC2-AKT pathways in the PMHs harvested from Bmal1−/− and WT mice after fasting overnight and refeeding 8 h. To our surprise, none of the major players within this pathway was significantly affected, including positive regulators of AKT phosphorylation (Akt2, Irs (insulin receptor substrate)1/2, and Pik3 (phosphoinositide 3-kinase)ca/cb/cd/r1) (Fig. 8A), negative regulators of AKT phosphorylation (Pten (phosphatase and tensin homolog), Ptp1β (protein-tyrosine phosphatase 1β), Ship (SH2 domain-containing inositol phosphatase), and Shp2 (SH2 domain-containing phosphatase 2) (Fig. 8B), and the components of the mTORC2 complex (mTor (mammalian target of rapamycin), Tsc1 (tuberous sclerosis 1), Tsc2 (tuberous sclerosis 2), and Rictor (rapamycin-insensitive companion of mammalian target of rapamycin) (Fig. 8C). Thus, our data suggest that BMAL1 promotes AKT activation without altering the gene expression of known components and regulators of insulin signaling.
FIGURE 8.
BMAL1 regulates RICTOR protein expression in the mouse liver and cultured hepatocytes. A–C, loss of Bmal1 shows no effect on the gene expression of key factors of insulin signaling. After overnight fasting and refeeding for 8 h, the liver mRNA levels of key components of the insulin-signaling pathway were determined in both WT and Bmal1−/− mice (n = 5). The data are plotted as the mean ± S.D. (n = 5). D, liver-specific deletion of Bmal1 lowers the RICTOR protein levels in the liver of Bmal1-LKO mice. RICTOR, mTOR, BMAL1, and β-tubulin protein expression in the liver of Bmal1-LKO mice was detected by immunoblotting and quantified. E, acute deletion of Bmal1 reduces the abundance of RICTOR in PMHs. Bmal1 deletion was achieved by transducing Bmal1flox/flox PMHs with Ad-Cre versus Ad-GFP. 36 h later cells in triplicate were harvested for examining the protein expression of RICTOR and BMAL1. F, acute depletion of Bmal1 accelerates RICTOR protein degradation. Hepa1 or U2OS cells were transfected with either shLacZ control or shBmal1 construct for 48 h before treatment with protein synthesis inhibitor cycloheximide for 0, 2, 4, or 6 h. Cells were then harvested for examining the protein expression of RICTOR, BMAL1, and loading control β-tubulin. G, proteasome inhibitor MG-312 blocks RICTOR protein degradation after acute Bmal1 depletion. Hepa1 cells were transduced with either Ad-shLacZ control or Ad-shBmal1 virus for 48 h before treatment with MG-132 (10 μm) for 0, 4, and 8 h. Cells were then harvested to examine the protein abundance of RICTOR, BMAL1, and loading control β-tubulin. The protein level of RICTOR was quantified from three independent experiments and normalized to the expression of β-tubulin. **, p value <0.01 (n = 3). H, the working model of BMAL1 regulation of de novo lipogenesis in the liver in response to nutritional cues. Upon refeeding, insulin or other nutrients activate BMAL1-dependent transcription activity. BMAL1 is required for maintaining RICTOR protein stability and promoting AKT activation and subsequent de novo lipogenesis. In the case of Bmal1 deletion or depletion, destabilization of RICTOR is likely to attenuate AKT activation and suppress lipogenic pathway.
The knowledge that the mTORC2 complex activates AKT by phosphorylating Ser-473 prompted us to focus on whether BMAL1 regulation of AKT activity is mediated through modulating the protein expression of the mTORC2 complex. Indeed, we observed the reduced protein level of RICTOR, the key component of mTORC2 complex, in the liver of Bmal1-LKO mice during the refed state (Fig. 8D). We also observed a similar reduction of RICTOR protein in the liver of Bmal1−/− mice (data not shown). Moreover, acute deletion of Bmal1 eradicated the expression of RICTOR protein in Bmal1flox/flox PMHs after AdCre transduction, indicating that RICTOR protein expression is highly dependent on the presence of BMAL1 (Fig. 8E). All the data point to the possibility that BMAL1 regulates RICTOR protein abundance via a post-translational mechanism. So far, very little is known about the RICTOR protein degradation pathway. In a cycloheximide (CHX) chase experiment, we observed that RICTOR protein degraded more rapidly (>50% within 3 h) in Bmal1-depleted Hepa1 cells after protein synthesis was inhibited by cycloheximide (Fig. 8F, upper panel). A similar effect was also observed in U2OS cells, suggesting that regulation of RICTOR stability by BMAL1 is a more general mechanism (Fig. 8F, lower panel). Finally, inhibition of proteasome activity by MG132 restored RICTOR protein expression in the presence of Bmal1 knockdown (Fig. 8G), highlighting the critical role of BMAL1 during proteasome-dependent degradation of RICTOR protein. In summary, our data showed that BMAL1 is likely to contribute to insulin activation of AKT and lipogenesis by maintaining the RICTOR protein stability in hepatocytes.
DISCUSSION
In the present study we demonstrate that BMAL1 serves as an essential regulator of insulin-induced lipogenesis in the liver and hepatocytes. Bmal1 deficiency not only reduces hepatic lipogenic response in refed mice but also blunts the rate of de novo lipogenesis in insulin-stimulated PMHs. Loss or depletion of Bmal1 leads to resistance to AKT activation induced by refeeding or insulin treatment. Overexpression of constitutively active AKT restores both the expression of lipogenic enzymes and the rate of de novo lipogenesis in hepatocytes with BMAL1 deficiency. Although it remains unclear how BMAL1 promotes the AKT activity, our data so far suggest that BMAL1 directly affects the protein abundance of RICTOR, a key component of mTORC2 known to phosphorylate AKT2 at Ser-473. These findings highlighted BMAL1 as a novel insulin-responsive effector in maintaining mTORC2-AKT activation and the induction of hepatic lipogenesis (Fig. 8H).
Both alcoholic fatty liver disease and non-alcoholic fatty liver disease affect 50 million people in the United States (46, 47). One of the common pathological changes is constitutively elevated de novo lipogenesis in hepatocytes regardless of the feeding status (5, 7, 48, 49). In human studies using radioactive tracer, de novo lipogenesis was shown to be responsible for 26% of total liver triglycerides in non-alcoholic fatty liver disease patients but for 2–5% in normal subjects (5, 50). In the mouse models of non-alcoholic fatty liver disease, inhibition of de novo lipogenesis has been demonstrated to reduce lipid accumulation in hepatocytes along with enhanced insulin sensitivity and improved liver function, suggesting that targeting de novo lipogenesis may be a novel approach to treat non-alcoholic fatty liver disease (51–53). In our study we have focused on the role of BMAL1 in regulating de novo lipogenesis in hepatocytes in response to insulin and refeeding signal. In future studies, we will manipulate BMAL1 function in the liver and primary hepatocytes from animal models of liver steatosis to determine whether BMAL1-mediated de novo lipogenesis contributes to fat accumulation in fatty liver disease.
BMAL1 plays a critical role in maintaining systemic lipid homeostasis (24, 30, 32), although the molecular targets downstream of BMAL1 still remain to be identified. Even within the liver, BMAL1 could impact multiple pathways that contribute to lipid homeostasis (22, 23, 54). Our current work focuses on BMAL1 as a positive regulator for de novo lipogenesis in response to feeding signals. Although de novo lipogenesis is traditionally considered as a pathway, which stores extra energy in fatty acids, some recent findings suggest that de novo lipogenesis also plays an important role in generating various lipid-derived ligands that regulate other metabolic pathways within and outside hepatocytes. Semenkovich and co-workers (55) discovered that mice with hepatic ablation of Fasn exhibited hypoglycemia and fatty liver during fasting, which can be corrected by treatment with a selective PPARα agonist, providing evidence that FASN could be required for synthesizing endogenous ligands for PPARα. Consistent with this concept, a recent study from the Lee group suggested that PPARδ-mediated de novo lipogenesis plays a critical role in generating lipid messengers to regulate fatty acid uptake in skeletal muscle (56). In our study reduced FASN expression was observed in the Bmal1-deficient or -depleted mouse liver, whereas a robust FASN induction was found in the liver with BMAL1 overexpression. Our evidence strongly suggests a possibility that BMAL1 may activate the PPARα pathway via FASN in addition to directly regulating PPARα gene expression (57). Thus, we speculate that treatment with PPARα agonists can correct part of the dyslipidemia phenotype in Bmal1−/− mice.
One of the most interesting findings in our current work is about BMAL1 regulation of RICTOR protein expression. RICTOR is the essential component of mTORC2 complex that directly phosphorylates AKT at Ser-473 (58). Genetic deletion of Rictor in mouse hepatocytes nearly ablates AKT phosphorylation at Ser-473 during refeeding (11), indicating that RICTOR expression could be a rate-limiting factor for determining cellular activity of mTORC2. Transcription of Rictor is enhanced by FOXO1 and SIRT (sirtuin)-NRF1 (nuclear respiratory factor 1) transcription complex (59, 60). RICTOR is also heavily phosphorylated by S6K1 (S6 kinase 1), whereas rapamycin promotes dephosphorylation of RICTOR (61–64). However, whether phosphorylation of RICTOR is associated with the proteasome-mediated degradation remains unclear. In our study we observed that overall protein levels of RICTOR, but not its mRNA, are reduced in the liver of Bmal1 knockout mice. In cultured hepatocytes with acute Bmal1 knockdown, RICTOR protein degrades faster after cycloheximide treatment, indicating that BMAL1 may enhance the RICTOR protein stability by delaying its degradation by proteasome. Because target genes of BMAL1 include phosphatases and ubiquitin-specific proteases (22), we speculate that BMAL1 may inhibit the phosphorylation-dependent ubiquitination and degradation of RICTOR to promote its protein stability.
Various lifestyle choices such as shift work schedules, transcontinental travel, and chronic consumption of high fat diets have been linked with not only circadian dysfunction but also increased risk for metabolic diseases (65–67). Although it has been proposed that an impaired circadian clock in these situations may serve as one of causal effectors that promote onset and progression of obesity, diabetes, and fatty liver disease (68), it remains largely unknown about how this process works on the molecular levels. A better understanding of the metabolic actions of circadian components may shed light on the interplay between the circadian clock and metabolism in both physiological and pathological circumstances. Our findings revealed a critical function of BMAL1 protein in hepatic lipid metabolism during cycles of fasting and refeeding. In response to refeeding, BMAL1 promotes the efficient conversion of excessive acetyl-CoA into lipids by modulating AKT activity in hepatocytes. In this regard, BMAL1 integrates signaling inputs from both circadian and nutritional cues to coordinate the flux of metabolic intermediates. Our findings suggest that a defect in BMAL1 signaling could contribute to metabolic disorders in the context of circadian misalignment.
Acknowledgments
We thank Dr. Christopher Bradfield at the University of Wisconsin for generously sharing Bmal1 knock-out mice and Dr. Jiandie Lin (University of Michigan) for Bmal1flox/flox mice.
This work was supported, in whole or in part, by National Institutes of Health Grants K99/R00 DK077449 and R01 DK099593 (to L. Y.) and R01 Grant 094014 (to L. R.) from NIDDK, pilot grants from the Michigan Gastrointestinal Hormone Research Core Center (5P3ODKO34933) and from the University of Michigan Diabetes and Research Training Center (P60DK020572) (to L. Y.), and by a pilot grant from the University of Michigan Diabetes and Research Training Center P60DK020572 (to X. T.).
- mTORC1
- mammalian target of rapamycin complex 1
- SREBP-1c
- sterol regulatory element-binding protein-1c
- CLOCK
- circadian locomotor output cycles kaput
- PPARγ
- peroxisome proliferator-activated receptor γ
- PMH
- primary mouse hepatocyte
- QPCR
- quantitative PCR
- ACC1
- acetyl-CoA carboxylase 1
- Scd1
- stearoyl-CoA desaturase 1.
REFERENCES
- 1. Leavens K. F., Birnbaum M. J. (2011) Insulin signaling to hepatic lipid metabolism in health and disease. Crit. Rev. Biochem. Mol. Biol. 46, 200–215 [DOI] [PubMed] [Google Scholar]
- 2. Postic C., Dentin R., Girard J. (2004) Role of the liver in the control of carbohydrate and lipid homeostasis. Diabetes Metab. 30, 398–408 [DOI] [PubMed] [Google Scholar]
- 3. Weickert M. O., Pfeiffer A. F. (2006) Signalling mechanisms linking hepatic glucose and lipid metabolism. Diabetologia 49, 1732–1741 [DOI] [PubMed] [Google Scholar]
- 4. Saltiel A. R., Kahn C. R. (2001) Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414, 799–806 [DOI] [PubMed] [Google Scholar]
- 5. Donnelly K. L., Smith C. I., Schwarzenberg S. J., Jessurun J., Boldt M. D., Parks E. J. (2005) Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Invest. 115, 1343–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ferré P., Foufelle F. (2010) Hepatic steatosis: a role for de novo lipogenesis and the transcription factor SREBP-1c. Diabetes Obes. Metab. 12, 83–92 [DOI] [PubMed] [Google Scholar]
- 7. Lambert J. E., Ramos-Roman M. A., Browning J. D., Parks E. J. (2014) Increased de novo Lipogenesis is a Distinct Characteristic of Individuals with Nonalcoholic Fatty Liver Disease. Gastroenterology 146, 726–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bakan I., Laplante M. (2012) Connecting mTORC1 signaling to SREBP-1 activation. Curr. Opin. Lipidol. 23, 226–234 [DOI] [PubMed] [Google Scholar]
- 9. Haas J. T., Miao J., Chanda D., Wang Y., Zhao E., Haas M. E., Hirschey M., Vaitheesvaran B., Farese R. V., Jr., Kurland I. J., Graham M., Crooke R., Foufelle F., Biddinger S. B. (2012) Hepatic insulin signaling is required for obesity-dependent expression of SREBP-1c mRNA but not for feeding-dependent expression. Cell Metab. 15, 873–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yecies J. L., Zhang H. H., Menon S., Liu S., Yecies D., Lipovsky A. I., Gorgun C., Kwiatkowski D. J., Hotamisligil G. S., Lee C.-H., Manning B. D. (2011) Akt stimulates hepatic SREBP1c and lipogenesis through parallel mTORC1-dependent and independent pathways. Cell Metab. 14, 21–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hagiwara A., Cornu M., Cybulski N., Polak P., Betz C., Trapani F., Terracciano L., Heim M. H., Rüegg M. A., Hall M. N. (2012) Hepatic mTORC2 activates glycolysis and lipogenesis through Akt, glucokinase, and SREBP1c. Cell Metab. 15, 725–738 [DOI] [PubMed] [Google Scholar]
- 12. Yuan M., Pino E., Wu L., Kacergis M., Soukas A. A. (2012) Identification of Akt-independent regulation of hepatic lipogenesis by mammalian target of rapamycin (mTOR) complex 2. J. Biol. Chem. 287, 29579–29588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. He L., Hou X., Kanel G., Zeng N., Galicia V., Wang Y., Yang J., Wu H., Birnbaum M. J., Stiles B. L. (2010) The critical role of AKT2 in hepatic steatosis induced by PTEN loss. Am. J. Pathol. 176, 2302–2308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Leavens K. F., Easton R. M., Shulman G. I., Previs S. F., Birnbaum M. J. (2009) Akt2 is required for hepatic lipid accumulation in models of insulin resistance. Cell Metab. 10, 405–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shearn C. T., Smathers R. L., Backos D. S., Reigan P., Orlicky D. J., Petersen D. R. (2013) Increased carbonylation of the lipid phosphatase PTEN contributes to Akt2 activation in a murine model of early alcohol-induced steatosis. Free Radic. Biol. Med. 65, 680–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wan M., Leavens K. F., Saleh D., Easton R. M., Guertin D. A., Peterson T. R., Kaestner K. H., Sabatini D. M., Birnbaum M. J. (2011) Postprandial hepatic lipid metabolism requires signaling through Akt2 independent of the transcription factors FoxA2, FoxO1, and SREBP1c. Cell Metab. 14, 516–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bunger M. K., Wilsbacher L. D., Moran S. M., Clendenin C., Radcliffe L. A., Hogenesch J. B., Simon M. C., Takahashi J. S., Bradfield C. A. (2000) Mop3 is an essential component of the master circadian pacemaker in mammals. Cell 103, 1009–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lowrey P. L., Takahashi J. S. (2004) Mammalian circadian biology: elucidating genome-wide levels of temporal organization. Annu. Rev. Genomics Hum. Genet. 5, 407–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Panda S., Hogenesch J. B., Kay S. A. (2002) Circadian rhythms from flies to human. Nature 417, 329–335 [DOI] [PubMed] [Google Scholar]
- 20. Reppert S. M., Weaver D. R. (2002) Coordination of circadian timing in mammals. Nature 418, 935–941 [DOI] [PubMed] [Google Scholar]
- 21. Hardin P. E. (2004) Transcription regulation within the circadian clock: the E-box and beyond. J. Biol. Rhythms 19, 348–360 [DOI] [PubMed] [Google Scholar]
- 22. Rey G., Cesbron F., Rougemont J., Reinke H., Brunner M., Naef F. (2011) Genome-wide and phase-specific DNA-binding rhythms of BMAL1 control circadian output functions in mouse liver. PLoS Biol. 9, e1000595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lamia K. A., Storch K.-F., Weitz C. J. (2008) Physiological significance of a peripheral tissue circadian clock. Proc. Natl. Acad. Sci. U.S.A. 105, 15172–15177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rudic R. D., McNamara P., Curtis A.-M., Boston R. C., Panda S., Hogenesch J. B., Fitzgerald G. A. (2004) BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2, e377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Takahashi J. S., Hong H.-K., Ko C. H., McDearmon E. L. (2008) The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat. Rev. Genet. 9, 764–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Turek F. W., Joshu C., Kohsaka A., Lin E., Ivanova G., McDearmon E., Laposky A., Losee-Olson S., Easton A., Jensen D. R. (2005) Obesity and metabolic syndrome in circadian clock mutant mice. Sci. Signal. 308, 1043–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shi S. Q., Ansari T. S., McGuinness O. P., Wasserman D. H., Johnson C. H. (2013) Circadian disruption leads to insulin resistance and obesity. Curr. Biol. 23, 372–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee J., Moulik M., Fang Z., Saha P., Zou F., Xu Y., Nelson D. L., Ma K., Moore D. D., Yechoor V. K. (2013) Bmal1 and β-cell clock are required for adaptation to circadian disruption, and their loss of function leads to oxidative stress-induced β-cell failure in mice. Mol. Cell. Biol. 33, 2327–2338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marcheva B., Ramsey K. M., Buhr E. D., Kobayashi Y., Su H., Ko C. H., Ivanova G., Omura C., Mo S., Vitaterna M. H. (2010) Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature 466, 627–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kennaway D. J., Varcoe T. J., Voultsios A., Boden M. J. (2013) Global loss of bmal1 expression alters adipose tissue hormones, gene expression, and glucose metabolism. PloS ONE 8, e65255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shimba S., Ishii N., Ohta Y., Ohno T., Watabe Y., Hayashi M., Wada T., Aoyagi T., Tezuka M. (2005) Brain and muscle Arnt-like protein-1 (BMAL1), a component of the molecular clock, regulates adipogenesis. Proc. Natl. Acad. Sci. U.S.A. 102, 12071–12076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shimba S., Ogawa T., Hitosugi S., Ichihashi Y., Nakadaira Y., Kobayashi M., Tezuka M., Kosuge Y., Ishige K., Ito Y. (2011) Deficient of a clock gene, brain and muscle Arnt-like protein-1 (BMAL1), induces dyslipidemia and ectopic fat formation. PloS ONE 6, e25231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zvonic S., Ptitsyn A. A., Conrad S. A., Scott L. K., Floyd Z. E., Kilroy G., Wu X., Goh B. C., Mynatt R. L., Gimble J. M. (2006) Characterization of peripheral circadian clocks in adipose tissues. Diabetes 55, 962–970 [DOI] [PubMed] [Google Scholar]
- 34. Tong X., Zhang D., Buelow K., Guha A., Arthurs B., Brady H. J., Yin L. (2013) Recruitment of histone methyltransferase G9a mediates transcriptional repression of Fgf21 gene by E4BP4 protein. J. Biol. Chem. 288, 5417–5425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sheng L., Cho K. W., Zhou Y., Shen H., Rui L. (2011) Lipocalin 13 protein protects against hepatic steatosis by both inhibiting lipogenesis and stimulating fatty acid β-oxidation. J. Biol. Chem. 286, 38128–38135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang E. E., Liu A. C., Hirota T., Miraglia L. J., Welch G., Pongsawakul P. Y., Liu X., Atwood A., Huss J. W., 3rd, Janes J., Su A. I., Hogenesch J. B., Kay S. A. (2009) A genome-wide RNAi screen for modifiers of the circadian clock in human cells. Cell 139, 199–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cybulski N., Polak P., Auwerx J., Rüegg M. A., Hall M. N. (2009) mTOR complex 2 in adipose tissue negatively controls whole-body growth. Proc. Natl. Acad. Sci. U.S.A. 106, 9902–9907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kumar A., Harris T. E., Keller S. R., Choi K. M., Magnuson M. A., Lawrence J. C. (2008) Muscle-specific deletion of rictor impairs insulin-stimulated glucose transport and enhances Basal glycogen synthase activity. Mol. Cell. Biol. 28, 61–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kumar A., Lawrence J. C., Jr., Jung D. Y., Ko H. J., Keller S. R., Kim J. K., Magnuson M. A., Harris T. E. (2010) Fat cell-specific ablation of rictor in mice impairs insulin-regulated fat cell and whole-body glucose and lipid metabolism. Diabetes 59, 1397–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hresko R. C., Mueckler M. (2005) mTOR.RICTOR is the Ser-473 kinase for Akt/protein kinase B in 3T3-L1 adipocytes. J. Biol. Chem. 280, 40406–40416 [DOI] [PubMed] [Google Scholar]
- 41. Sarbassov D. D., Guertin D. A., Ali S. M., Sabatini D. M. (2005) Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307, 1098–1101 [DOI] [PubMed] [Google Scholar]
- 42. Aoki M., Batista O., Bellacosa A., Tsichlis P., Vogt P. K. (1998) The akt kinase: molecular determinants of oncogenicity. Proc. Natl. Acad. Sci. U.S.A. 95, 14950–14955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dallmann R., Viola A. U., Tarokh L., Cajochen C., Brown S. A. (2012) The human circadian metabolome. Proc. Natl. Acad. Sci. U.S.A. 109, 2625–2629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Asher G., Schibler U. (2011) Crosstalk between components of circadian and metabolic cycles in mammals. Cell Metab. 13, 125–137 [DOI] [PubMed] [Google Scholar]
- 45. Bass J., Takahashi J. S. (2010) Circadian integration of metabolism and energetics. Science 330, 1349–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ahmadieh H., Azar S. T. (2014) Liver disease and diabetes: association, pathophysiology, and management. Diabetes Res. Clin. Pract. 104, 53–62 [DOI] [PubMed] [Google Scholar]
- 47. Bastien M., Poirier P., Lemieux I., Després J.-P. (2014) Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog. Cardiovasc. Dis. 56, 369–381 [DOI] [PubMed] [Google Scholar]
- 48. Cheung O., Sanyal A. J. (2010) Recent advances in nonalcoholic fatty liver disease. Curr. Opin. Gastroenterol. 26, 202–208 [DOI] [PubMed] [Google Scholar]
- 49. Postic C., Girard J. (2008) The role of the lipogenic pathway in the development of hepatic steatosis. Diabetes Metab. 34, 643–648 [DOI] [PubMed] [Google Scholar]
- 50. Diraison F., Moulin P., Beylot M. (2003) Contribution of hepatic de novo lipogenesis and reesterification of plasma non esterified fatty acids to plasma triglyceride synthesis during non-alcoholic fatty liver disease. Diabetes Metab. 29, 478–485 [DOI] [PubMed] [Google Scholar]
- 51. Abu-Elheiga L., Wu H., Gu Z., Bressler R., Wakil S. J. (2012) Acetyl-CoA carboxylase 2−/− mutant mice are protected against fatty liver under high fat, high carbohydrate dietary and de novo lipogenic conditions. J. Biol. Chem. 287, 12578–12588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dentin R., Benhamed F., Hainault I., Fauveau V., Foufelle F., Dyck J. R., Girard J., Postic C. (2006) Liver-specific inhibition of ChREBP improves hepatic steatosis and insulin resistance in ob/ob mice. Diabetes 55, 2159–2170 [DOI] [PubMed] [Google Scholar]
- 53. Knebel B., Haas J., Hartwig S., Jacob S., Köllmer C., Nitzgen U., Muller-Wieland D., Kotzka J. (2012) Liver-specific expression of transcriptionally active SREBP-1c is associated with fatty liver and increased visceral fat mass. PloS ONE 7, e31812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Inoue I., Shinoda Y., Ikeda M., Hayashi K., Kanazawa K., Nomura M., Matsunaga T., Xu H., Kawai S., Awata T. (2005) CLOCK/BMAL1 is involved in lipid metabolism via transactivation of the peroxisome proliferator-activated receptor (PPAR) response element. J. Atheroscler. Thromb. 12, 169–174 [DOI] [PubMed] [Google Scholar]
- 55. Chakravarthy M. V., Pan Z., Zhu Y., Tordjman K., Schneider J. G., Coleman T., Turk J., Semenkovich C. F. (2005) “New” hepatic fat activates PPARα to maintain glucose, lipid, and cholesterol homeostasis. Cell Metab. 1, 309–322 [DOI] [PubMed] [Google Scholar]
- 56. Liu S., Brown J. D., Stanya K. J., Homan E., Leidl M., Inouye K., Bhargava P., Gangl M. R., Dai L., Hatano B. (2013) A diurnal serum lipid integrates hepatic lipogenesis and peripheral fatty acid use. Nature 502, 550–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Canaple L., Rambaud J., Dkhissi-Benyahya O., Rayet B., Tan N. S., Michalik L., Delaunay F., Wahli W., Laudet V. (2006) Reciprocal regulation of brain and muscle Arnt-like protein 1 and peroxisome proliferator-activated receptor α defines a novel positive feedback loop in the rodent liver circadian clock. Mol. Endocrinol. 20, 1715–1727 [DOI] [PubMed] [Google Scholar]
- 58. Sarbassov D. D., Ali S. M., Kim D.-H., Guertin D. A., Latek R. R., Erdjument-Bromage H., Tempst P., Sabatini D. M. (2004) Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. 14, 1296–1302 [DOI] [PubMed] [Google Scholar]
- 59. Chen C.-C., Jeon S.-M., Bhaskar P. T., Nogueira V., Sundararajan D., Tonic I., Park Y., Hay N. (2010) FoxOs inhibit mTORC1 and activate Akt by inducing the expression of Sestrin3 and Rictor. Dev. Cell 18, 592–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wang R.-H., Kim H.-S., Xiao C., Xu X., Gavrilova O., Deng C.-X. (2011) Hepatic Sirt1 deficiency in mice impairs mTorc2/Akt signaling and results in hyperglycemia, oxidative damage, and insulin resistance. J. Clin. Invest. 121, 4477–4490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Akcakanat A., Singh G., Hung M. C., Meric-Bernstam F. (2007) Rapamycin regulates the phosphorylation of rictor. Biochem. Biophys. Res. Commun. 362, 330–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Dibble C. C., Asara J. M., Manning B. D. (2009) Characterization of Rictor phosphorylation sites reveals direct regulation of mTOR complex 2 by S6K1. Mol. Cell. Biol. 29, 5657–5670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Julien L.-A., Carriere A., Moreau J., Roux P. P. (2010) mTORC1-activated S6K1 phosphorylates Rictor on threonine 1135 and regulates mTORC2 signaling. Mol. Cell. Biol. 30, 908–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Treins C., Warne P. H., Magnuson M. A., Pende M., Downward J. (2010) Rictor is a novel target of p70 S6 kinase-1. Oncogene 29, 1003–1016 [DOI] [PubMed] [Google Scholar]
- 65. De Bacquer D., Van Risseghem M., Clays E., Kittel F., De Backer G., Braeckman L. (2009) Rotating shift work and the metabolic syndrome: a prospective study. Int. J. Epidemiol. 38, 848–854 [DOI] [PubMed] [Google Scholar]
- 66. Karlsson B., Knutsson A., Lindahl B. (2001) Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27,485 people. Occup. Environ. Med. 58, 747–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sookoian S., Gemma C., Fernández Gianotti T., Burgueño A., Alvarez A., González C. D., Pirola C. J. (2007) Effects of rotating shift work on biomarkers of metabolic syndrome and inflammation. J. Intern. Med. 261, 285–292 [DOI] [PubMed] [Google Scholar]
- 68. Scheer F. A., Hilton M. F., Mantzoros C. S., Shea S. A. (2009) Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci. U.S.A. 106, 4453–4458 [DOI] [PMC free article] [PubMed] [Google Scholar]