Abstract
Purpose of review
To provide an update on the latest clinical applications of serum antimüllerian hormone (AMH) testing with practical approaches to mitigate the impact of significant variability in AMH results.
Recent findings
Recent studies continue to demonstrate that AMH is the best single serum test for ovarian response management with, at most, a weak-to-moderate age-independent association with live-birth rate and time to conception. Data confirm serum AMH levels improve menopause prediction, monitoring of ovarian damage, and identification of women at risk for several ovary-related disorders such as polycystic ovary syndrome and premature or primary ovarian insufficiency. However, it is now recognized that serum AMH results can have dramatic variability due to common, biologic fluctuations within some individuals, use of hormonal contraceptives or other medications, certain surgical procedures, specimen treatment, assay changes, and laboratory calibration differences. Practical guidelines are provided to minimize the impact of variability in AMH results and maximize the accuracy of clinical decision-making.
Summary
AMH is an ovarian biomarker of central importance which improves the clinical management of women's health. However, with the simultaneous rapid expansion of AMH clinical applications and recognition of variability in AMH results, consensus regarding the clinical cutpoints is increasingly difficult. Therefore, a careful approach to AMH measurement and interpretation in clinical care is essential.
Keywords: AMH, menopause, ovarian reserve, polycystic ovarian syndrome, premature ovarian failure
INTRODUCTION: ANTIMÜLLERIAN HORMONE, OVARIAN RESERVE, AND WOMEN'S HEALTH
Appropriate assessment of ovarian reserve with serum antimüllerian hormone (AMH) testing has the potential to improve the medical care provided to women in a variety of ways [1▪,2,3]. However, ovarian reserve assessment, the quantitative and qualitative characterization of a woman's supply of oocytes, is exceptionally complicated to approach because of the lack of consensus in terminology, differences in clinical study designs, and variability in testing methodology in clinical research [4▪▪]. Ovarian reserve assessment has been difficult to obtain in routine clinical practice as no biomarker with sufficient clinical accuracy has been easily available. Rapidly increasing numbers of clinical publications confirm serum AMH as a clinically useful, widely available, primarily quantitative, measure of ovarian reserve that is more accurate than serum follicle-stimulating hormone (FSH) alone. AMH is a powerful clinical biomarker that helps improve the management of infertility treatment, planning of future pregnancy, menopause prediction, monitoring of ovarian damage from medications and procedures, and detection of ovary-related diseases such as polycystic ovary syndrome (PCOS) and premature or primary ovarian insufficiency (POI; Fig. 1 ).
FIGURE 1.
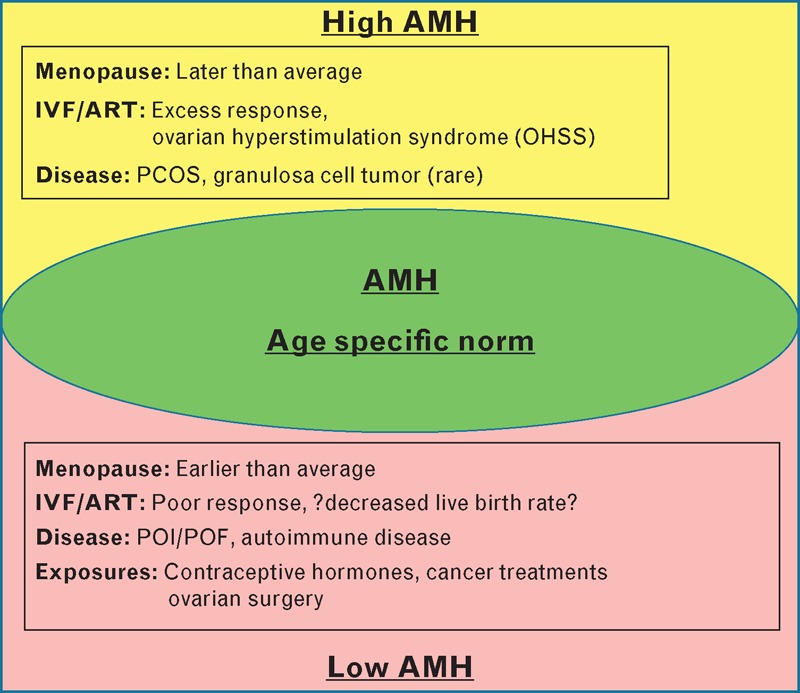
Age-specific AMH and associated conditions. Displayed at the top (yellow) are conditions associated with high age-specific AMH. At the bottom (pink), conditions associated with low age-specific AMH are listed. AMH, antimullerian hormone; PCOS, polycystic ovary syndrome; POI, premature/primary ovarian insufficiency; POF, premature ovarian failure.
Box 1.

no caption available
However, it is now clearly important for clinicians to understand that serum AMH results can be misleading if appropriate steps are not taken to account for sources of variability in serum AMH results in clinical practice and ensure patients understand that information from AMH testing is directional, not definitive. Key practical steps are provided here for consideration to help ensure the appropriate use of AMH testing in clinical practice.
ANTIMÜLLERIAN HORMONE BIOLOGY AND PHYSIOLOGY
At the cellular level, AMH is thought to restrict the growth of ovarian follicles in response to serum FSH and also inhibit estradiol (E2) secretion [1▪,2]. AMH is produced by the granulosa cells surrounding each oocyte in the developing follicles, with rapid reduction in both AMH gene and protein expression observed in follicles reaching 8 mm and above in diameter [5▪]. The decline in AMH expression in larger follicles appears to be because of, at least in part, a reduction in the AMH gene promoter activity through E2 receptor beta [6]. One study demonstrates that reduced AMH levels, averaged from large follicles retrieved during in-vitro fertilization (IVF), were associated with higher IVF success rates [7], suggesting this follicular AMH reduction is a desired physiologic process.
Using population average serum AMH levels (Fig. 2), the literature appears to agree that values decline yearly at a fairly consistent rate after 25 years of age until below the clinical detection limit by 50 years of age [8,9▪▪,10,11,12▪▪,13]. However, the pattern of rise prior to age 25 has conflicting representation in the literature, with one study showing peaks and troughs [14] and another showing a gradual rise from birth to 15 years of age then decline [15▪▪] (Fig. 2). Therefore, clinicians should currently be more cautious about interpreting the rates of change in serum AMH in women below 25 years of age. Additionally, there are few data available to determine whether the pattern of decline in AMH values within an individual woman matches the pattern in the population average.
FIGURE 2.
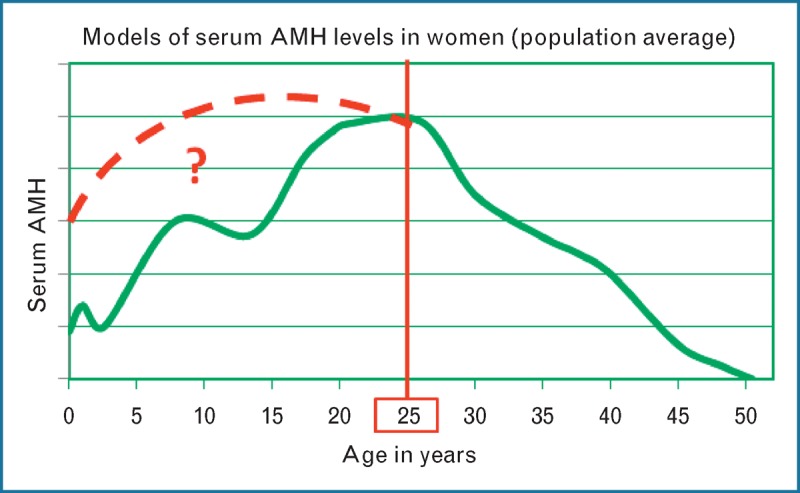
Population average serum AMH levels during a woman's lifetime. A model of a population average serum AMH level in women, plotted by year of life in green, demonstrates the rising serum AMH with two peaks and troughs prior to maximal value at 25 years of age followed by an almost linear decline (specific AMH values are not included on the Y-axis to avoid potential misapplication of laboratory-specific values). Although the literature generally agrees with the pattern of decline after 25 years of age, conflicting patterns of rise are described at younger ages (red dashed line and question mark). Caution is required with clinical interpretation of serum AMH in women below 25 years of age until further studies are reported. Further study is also required to determine how the pattern of decline in serum AMH within individuals relates to the decline of the population average display the same pattern of decline as demonstrated by the population average. AMH, antimüllerian hormone. Reproduced with permission [14].
OOCYTE QUANTITY AND QUALITY, FERTILITY TREATMENT, AND NATURAL FERTILITY
It is well established that AMH testing effectively improves the management of ovarian stimulation in assisted reproductive technology (ART) therapy by identifying women likely to respond either poorly (with cycle cancelation/low oocyte yield) or excessively [with ovarian hyperstimulation syndrome (OHSS)/high oocyte yield] [1▪]. Recent research has targeted developing specific ovarian stimulation protocols individualized by AMH results [16▪▪,17▪]. Other recent studies focus on refining information such as demonstrating that an AMH value from a single point in time will remain predictive for approximately 12 months [18], how certain stimulation protocols affect different patient phenotypes such as PCOS vs. low responders [19▪▪,20], and prospectively demonstrating that application of AMH testing can improve expectation management and possibly treatment costs [21].
The value of serum AMH, independent of age, to predict live-birth rate or oocyte quality has remained controversial, with some studies continuing to show no association, whereas other studies demonstrate a small but useful association [22▪,23–28,29▪▪]. This controversy may be due in part to differences in AMH testing methodology and study design masking the association with serum AMH and oocyte quality. Ultimately, as a recent meta-analysis concludes, it is probable that AMH has an association with oocyte quality, but this association is likely weak to moderate at best independent of a woman's age [30▪▪]. AMH may best improve the oocyte quality prediction when incorporated in a multivariate, algorithmic approach [29▪▪]. However, as there continues to be evidence that live births are possible with very low AMH levels, AMH values alone should not be used to withhold care [31▪].
In terms of natural fertility or time to conception, recent reports demonstrate conflicting data [32,33,34▪], which perhaps may be because of small sample sizes or AMH only being helpful when below a certain threshold or in a certain population of patients. Currently, however, the available data suggest that serum AMH levels in isolation cannot be used to counsel a patient whether or not natural conception is possible.
MENOPAUSE
Menopause is complex and no single test currently can definitively predict the onset with a high degree of accuracy. However, independent studies from a variety of sources for over a decade demonstrate serum AMH improves the prediction of menopause [1▪]. Serum AMH was recently added as a marker for menopausal staging because it declines much earlier than other signs of menopause such as increasing serum FSH or irregular menses [35▪]. Recently, serum AMH was shown to improve the prediction of menopause onset more than maternal age [36▪]. Furthermore, different models using AMH to predict a higher chance of early or late onset of menopause relative to the expected average have now been cross-validated [37▪▪].
There are a number of variables which, if combined with serum AMH algorithmically, may increase the accuracy of menopause predictions. For example, one cross-sectional study of 2635 women demonstrated that for women with serum AMH in the lower 5th percentile for their age, expected age of menopause was 43.4 years of age for obese, nonsmokers versus 37.6 in thin women who smoked [38▪]. In women with serum AMH in the upper 95th percentile for age, predicted age of menopause increased considerably but was again affected by body weight and smoking status (55.1 years of age in obese, nonsmokers versus 52.4 years of age in underweight smokers). Ethnicity also may influence serum AMH [39]. Interestingly, a recent study in 44 Japanese women demonstrated after AMH became undetectable, menopause onset was within 3 years [40], instead of 5 years shown by a study of large U.S. and European populations [41], although the study design and assay performance may contribute to these observational differences.
Although the relationship of AMH to the timing of menopause onset is highly statistically significant in the studies cited, the confidence intervals related to the predicted age of menopause remain wide at present. Thus, currently the present clinical interpretation is qualitative: women with a serum AMH very low or very high for their age are more likely to go into menopause earlier or later than average, respectively. Notably, for women with very low age-specific serum AMH who are not ready to attempt conception naturally or with donor sperm, oocyte cryopreservation, no longer considered experimental [42▪▪], is now available as an alternative to help ensure the future ability to have children. Although serum AMH testing clearly provides helpful directional information in decision making, prior to ordering AMH testing, a clinician should consider verifying whether their patient considers qualitative information sufficiently meaningful to use.
POLYCYSTIC OVARY SYNDROME
The relationship of AMH with PCOS is complicated by the diagnosis itself being subject to a debate primarily centered, ironically, over whether polycystic ovaries are included (Rotterdam criteria) or are not included [National Institutes of Health (NIH) criteria] in the diagnosis of the disorder. Numerous studies demonstrate that PCOS is significantly associated with elevated serum AMH both with the NIH criteria [43▪,44▪] and with the Rotterdam criteria [44▪,45▪▪,46▪▪,47–49]. One study demonstrated that in women presenting for fertility evaluation with serum AMH elevated, high, and very high, the frequency of PCOS diagnosis was 51.6% (n = 84), 97% (n = 30), and 100% (n = 20), respectively [50▪]. As serum AMH correlates well with the polycystic appearance of ovaries on ultrasound, several studies are proposing to set thresholds by AMH as an alternative to ultrasound [44▪,46▪▪,48]. Recent evidence in 463 PCOS women suggests that serum AMH may provide insight into the subphenotypes of PCOS with higher serum AMH predicting longer menstrual cycle length, higher luteinizing hormone (LH) levels, and hirsuitism [51▪▪]. Clearly, markedly elevated age-specific AMH correlates with the clinical diagnosis and severity of PCOS and may eventually be adopted as a diagnostic criteria for PCOS.
DIMINISHED OVARIAN RESERVE, PREMATURE OR PRIMARY OVARIAN INSUFFICIENCY, AND PREMATURE OVARIAN FAILURE
Not surprisingly, numerous studies demonstrate serum AMH is dramatically lower in women symptomatic from ovarian insufficiency [47,52▪,53,54], although one study shows that serum LH levels may be better at identifying the onset of menstrual irregularities [55]. It seems unlikely that defects in the AMH molecule itself are a concern as a cause of POI as suggested by a recent study of 211 idiopathic POI women and 233 controls, which demonstrated no identifiable genetic differences in the AMH or the AMH receptor genes [56]. An area of current controversy is whether serum AMH has a clinically useful association with the number of CGG repeats in the fragile X gene, FMR1, which is known to have 55 or greater CGG repeats much more frequently in women with POI than normal controls [52▪,57]. An association of repeat length and serum AMH in women with fertility issues has been described by primarily one group [58–62], but this was not observed in another recent study of women not selected for fertility issues [63].
As AMH is clearly the earliest and most accurate serum biomarker reflecting decline in the quantitative aspects of ovarian reserve, it may be the earliest clinical means of detecting women likely to develop diminished ovarian reserve (DOR), POI, or premature ovarian failure (POF) prior to becoming symptomatic [52▪]. In fact, a recent U.S. study of 5354 women presenting to fertility centers from 30 different states demonstrated that in women with reassuring early follicular serum FSH, serum AMH values concerning for low ovarian reserve were identified in 20% of women under 35 years of age and in over 30% of women by 40 years of age (Fig. 3) [12▪▪]. Therefore, serum AMH testing can be an important means to identify many patients in routine practice at risk for an accelerated decline in ovarian reserve.
FIGURE 3.
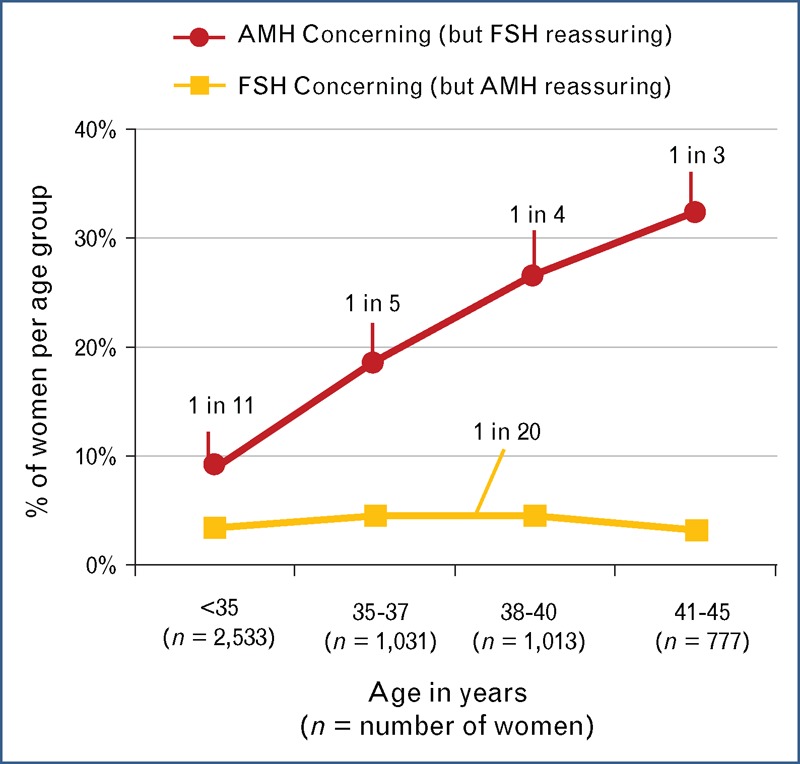
Frequency of discordance by age of serum FSH and AMH during estradiol-confirmed menstrual cycle days 2–4 in 5354 women from U.S. fertility centers. Discordance was defined when one test was ‘concerning’ and the other test was ‘reassuring’. Cutpoints for serum AMH were less than 0.8 ng/ml (concerning) and at least 0.8 ng/ml (reassuring), and for serum FSH were at least 10 IU/l (concerning) and less than 10 IU/l (reassuring). Y-axis displays the frequency of discordance of women in each of four age groups shown the X-axis. Note: AMH values are not standardized across laboratories. These data using cutpoints were calibrated to number of oocytes retrieved through ovarian stimulation and are specific to Ref. [12▪▪]. AMH, antimüllerian hormone; FSH, follicle-stimulating hormone. Modified with permission [12▪▪].
ASSOCIATION WITH OTHER DISORDERS: AUTOIMMUNITY, GRANULOSA CELL TUMORS, AND ENDOMETRIOSIS
Substantial evidence from a number of sources demonstrates serum AMH levels are associated with other diseases. For example, AMH is significantly lower in autoimmune disorders [64] such as lupus [65▪▪,66] and Crohn's disease [67▪]. Elevated AMH levels can also be useful in postmenopausal women as a strong indicator of granulosa cell tumors (GCTs) and for monitoring for the recurrence of GCTs, though in asymptomatic, premenopausal women, elevated AMH is too nonspecific for clinical utility as a screening test for these tumors [68,69]. The understanding of AMH and endometriosis continue to develop, with a recent report indicating AMH may actually play a role in endometriosis [70]. Although some consider endometrioma removal as important for improving fertility [71], clear evidence demonstrates serum AMH is lowered by ovarian surgeries such as removing cysts and endometriomas [72▪,73▪▪,74–76].
MONITORING OVARIAN DAMAGE WITH MEDICAL THERAPIES AND SURGICAL PROCEDURES
Medical and surgical treatments are now being assessed and monitored for ovarian damage by utilization of ovarian reserve markers such as AMH. Numerous cancer therapy publications have demonstrated the dramatic effects of various chemotherapeutics in reducing serum AMH levels. Some of these studies have demonstrated that AMH testing can improve treatment selection by identifying which therapies are most toxic to the ovary and which patients are most at risk for postchemotherapy ovarian insufficiency [77▪,78▪▪,79–84]. Furthermore, numerous reports are confirming that after some ovarian-related surgeries such as removal of benign cysts and endometriomas, significant reductions in AMH are observed which may persist [72▪,73▪▪,74–76] with a recent study demonstrating similar reductions with benign cysts and endometriomas and more severe reductions with bilateral procedures [85]. Interestingly, one report shows only transient reductions in AMH in 22 PCOS patients undergoing the more minor procedure of ovarian puncture [86]. Given this data, a clinician and patient must carefully consider the possible negative impact of common ovarian surgeries on ovarian reserve. The ability of serum AMH levels to indicate and predict damage to the ovary confirm it is important to measure serum AMH before and after medical and surgical treatments to help plan treatment approaches, counsel the patient, and monitor ovarian reserve status.
ALGORITHMS AND MULTIVARIATE COMBINATIONS OF TESTS WITH ANTIMÜLLERIAN HORMONE
Multivariate, algorithmic approaches allow optimization of combinations of clinical variables to predict outcome. The reality is clinicians, if not provided with a validated algorithm, are forced in daily practice to weigh AMH with the provided variables such as age, BMI, medical history, antral follicle count (AFC), and serum FSH to the best of their ability without the benefit of mathematical optimization and large datasets. A number of recent studies demonstrate benefit by combining AMH with other variables in predicting the outcomes such as menopause [37▪▪,38▪], live birth [33], or response to ovarian stimulation protocols in ART [29▪▪,87]. However, other studies have not found benefit in combining multiple tests, including a recent study combining 28 databases [88▪]. It should be noted that the differences in AMH assay materials and laboratory performance make combining different AMH datasets complex and likely would reduce the associations with AMH and outcome. Therefore, although a multivariate approach is likely to dramatically improve the accuracy of decision making, it will require minimization of variability in AMH assay, laboratory performance, patient population definition, and study design.
THE CHALLENGE OF VARIABILITY IN ANTIMÜLLERIAN HORMONE RESULTS: BIOLOGY, EXPOSURE, AND LABORATORY
Perhaps the most important clinical advance in the medical literature related to AMH is the recent recognition of the significant variability in AMH results which must be taken into account for appropriate interpretation in clinical care. As described below, there are now three recognized sources for this variability (Fig. 4): biological fluctuation within certain individuals; exposure to medications (such as contraceptive hormones) and certain ovarian surgeries; and laboratory-specific AMH values as each laboratory provides its own value ranges and calibration. That said, the variability should be contextualized with the fact that AMH is still the best available serum marker of quantitative aspects of ovarian reserve. The practical steps outlined below mitigate the issues of variability.
FIGURE 4.
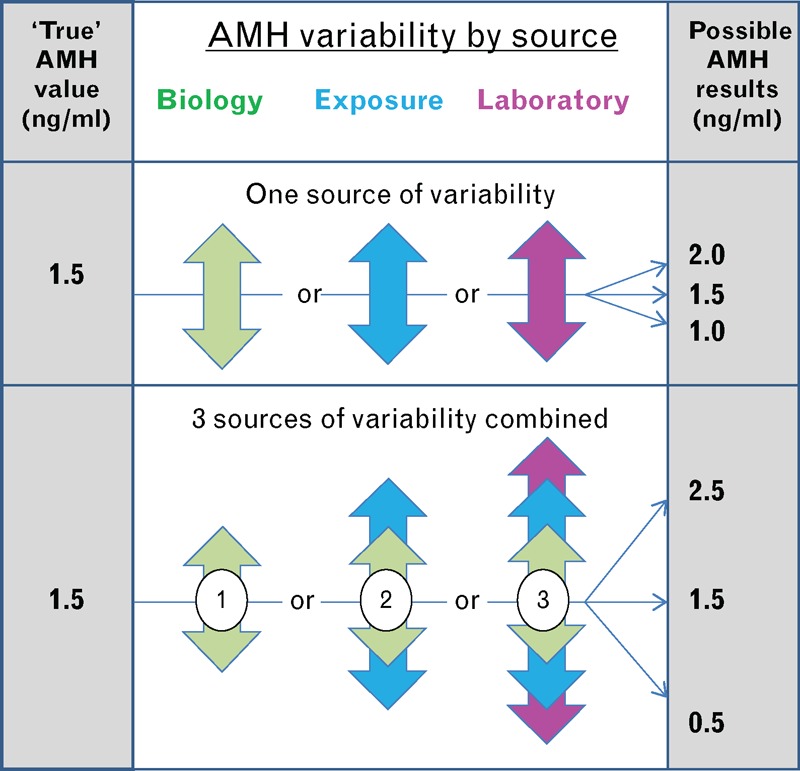
Variability by source possibly affecting a reported AMH result. Upper panel demonstrates the potential effect of a single source variable. Lower panel demonstrates how multiple sources of variability can be additive. AMH, antimüllerian hormone. Reproduced with permission from [3].
BIOLOGICAL FLUCTUATION IN SERUM ANTIMÜLLERIAN HORMONE VALUES
One of the most attractive aspects of serum AMH measurements, unlike serum FSH testing, is the lack of large changes in the average values at the population level during the menstrual cycle [89]. However, this led to the misconception that individuals do not show any fluctuation across the menstrual cycle. Fluctuation in serum AMH levels can be clearly observed by examining the graphs in previous studies which display individual patient data [89,90]. A recent study measuring AMH multiple times with the same menstrual cycle of 44 normally cycling healthy women demonstrated AMH values (ng/ml) within four individuals (9%) ranging approximately: 0.4–1.9, 1.9–4.2, 0.4–1.4, and 2.3–4.4 [91▪▪]. In another study, seven of 12 women re-measured during the same menstrual cycle were clinically reclassified [92▪]. Therefore, the clinician should avoid using AMH as the only marker of ovarian reserve, counsel patients that occasionally results can fluctuate, and consider re-testing if the AMH value is not consistent with the clinical picture.
EXPOSURE TO CONTRACEPTIVE MEDICATIONS, PREGNANCY, METHOTREXATE, AND DEHYDROEPIANDROSTERONE
Another attractive aspect of serum AMH measurements is that they are not affected by contraceptive medications to the same degree as serum FSH, which propagated the unfortunate misunderstanding that AMH was not affected by contraceptives. Numerous studies have now confirmed that hormonal contraceptives often lower serum AMH [93–96]. A well designed prospective study of 44 women demonstrated serum AMH was lowered by an average of approximately 30% within two menstrual cycles of starting the contraceptive regardless of the route [97▪▪]. This was expected as other scenarios which disrupt the hormonal milieu (such as pregnancy) affect serum AMH levels. One study of 554 women in pregnancy demonstrated an approximately 50% lowering of serum AMH with each trimester and recovery after delivery [98▪▪]. The limited recent data on methotrexate use do not demonstrate an effect on serum AMH [99], consistent with the prior observations [100]. A number of fertility centers administer dehydroepiandrosterone (DHEA) to poor responders to ovarian stimulation in order to improve AMH values and follicular responsiveness [101]; however, the number of treated patients is small and poorly controlled, and in sum, current data do not support a clear effect by this medication [102,103].
ANTIMÜLLERIAN HORMONE ASSAYS: VALUES ARE LABORATORY SPECIFIC
The major challenge for the clinician attempting to apply serum AMH values to clinical care is the fact that each laboratory provides their own value ranges which may be clinically significantly different. Currently, there is no international reference standard for AMH measurement. A major recent advance for improved patient care is simply the recognition in the clinical literature that AMH values are currently laboratory specific and generally cannot be ‘mixed and matched’ [9▪▪,104–110]. Previously, clinicians were misguided into thinking they could simply apply the values they received locally using reported cutpoints in AMH studies done elsewhere. This is largely because of the mistaken assumptions propagated in the clinical literature such as: high correlations between AMH assays meant results were interchangeable between laboratories with simple factoring; there is only one kit (i.e. the Beckman Gen II AMH assay) in use when in fact there are several kits in use; and laboratories using the same kit would supply the same numerical result. In fact, virtually every study comparing different AMH assay systems provides different conversion methods, none of which are simple factoring but usually involve linear equations [104–108,110]. Currently, no fewer than four kits are commercially available [111,112], with more on the way. Current publications describe the findings from seven different AMH kits used over the last 3–5 years. In addition, the Beckman Gen II assay itself has undergone two major methodology changes within 18 months (Beckman Product Notifications November 2012 and June 2013) [113], and not surprisingly, a systematic shift in calibration of the assay has just been reported in a cohort of 10 981 patients [9▪▪]. Furthermore, when 10 laboratories using the same Beckman Gen II assay were compared, there was a 40% variation in the values obtained [109]. In addition, specimen handling protocols affect the serum AMH values [107]. It is also critical to understand how a laboratory calibrates their clinical cutpoints by patient population and clinical outcome; otherwise, accurate interpretation of the result will be difficult.
A critical, additional mistake likely to be made in the future is concluding that FDA clearance of an AMH assay or production of an international standard will mean laboratories will report the same absolute value or calibrate clinical cutpoints the same way. FSH testing provides a recent history lesson demonstrating that this is not true. In fact, current FSH testing remains so controversial that no universally defined cutpoints are agreed upon despite multiple FDA cleared platforms and international reference standards [4▪▪]. Fortunately, to overcome this challenge, a clinician can follow a few practical steps outlined below.
PRACTICAL METHODS TO MINIMIZE THE VARIABILITY IN ANTIMÜLLERIAN HORMONE RESULTS AND MAXIMIZE THE CLINICAL UTILITY
Although the challenges facing the clinician applying laboratory results from ovarian testing such as AMH can seem daunting, by following a few practical steps in a checklist format, a clinician can overcome these challenges (Table 1). First and foremost, do not ‘mix and match’ the AMH values from different laboratories, but identify a reliable, single source of AMH testing which calibrates its testing to the clinical outcomes of interest and commits to updating the clinician should any changes occur in the calibration of the results. Medical insurance companies can present a challenge by restricting the testing services to a laboratory with which a clinician has no familiarity, but a clinician now has numerous publications as outlined in this review to demonstrate the medical risks this restrictive practice poses. Second, avoid using a serum AMH measurement alone to assess ovarian reserve, but incorporate other markers such as AFC and early follicular phase serum FSH. Third, identify whether the patient has a medical condition, has taken medications (e.g. hormonal contraceptive or chemotherapy), or had ovarian surgeries (e.g. cyst or endometrioma removal) that affect the AMH levels. Fourth, counsel the patient prior to testing about the qualitative nature of the information and that a single test result may change substantially in a certain subset of individuals because of biological fluctuations. Fifth, consider retesting if AMH results are clinically inconsistent, or if, based upon the testing results, life-changing decisions are to be made.
Table 1.
Checklist to maximize the clinical utility of serum AMH testing
| 1. Use one laboratory, calibrated to outcomes | Avoid ‘mixing and matching’ AMH values from different laboratories and identify a reliable, single source for testing which both calibrates the results to the clinical outcomes of interest and commits to updating the clinician if calibration of the results changes. |
| 2. Utilize more than one ovarian reserve test | Avoid using a single serum AMH measurement alone to assess ovarian reserve, and incorporate other markers such as antral follicle count (AFC) and/or early follicular phase serum FSH. |
| 3. Identify exposures | Identify whether the patient has taken medications (e.g. hormonal contraceptives and chemotherapy) or had surgery (ovarian cyst or endometrioma removal) that affects the AMH levels. |
| 4. Counsel patient | Prior to testing, verify the patient understands the directional nature of the information being provided by AMH testing, and that, in a subset of women, the test result may change substantially with biologic fluctuations. |
| 5. Consider retesting | If testing results lead to life-changing decisions or if the results are inconsistent with the clinical scenario, consider retesting. |
Many improvements to the management of women's health are possible through appropriate AMH testing. However, variability in AMH results can lead to clinically significant variability in AMH results, making careful approach to the interpretation essential. With the above simple steps, a clinician can rapidly minimize the risks for incorrect interpretation.
AMH, antimüllerian hormone; FSH, follicle stimulating hormone.
CONCLUSION
Hundreds of clinical studies confirm that adding serum AMH testing to a complete ovarian assessment provides a powerful tool to help provide better healthcare for women. The benefits of this testing can optimize fertility treatments; help lead to earlier diagnoses of PCOS, POI, POF, and certain autoimmune conditions; provide the opportunity for better planning for procreation and menopause; and allow for better medical decision-making by monitoring ovarian damage from exposures to medical or surgical therapies. Although challenges with variability in AMH results make the provided practical steps a prerequisite for appropriate interpretation of the testing, the clinical benefits of testing more than justify this additional effort.
Acknowledgements
The authors would like to thank Dr Eric Widra for a thorough and helpful review of this manuscript.
Funding for this review: None.
Conflicts of interest
B.L. – ReproSource, Inc; V.L.B. – no relevant conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1▪.Dewailly D, Andersen CY, Balen A, et al. The physiology and clinical utility of anti-mullerian hormone in women. Hum Reprod Update 2014; 20:370–385 [DOI] [PubMed] [Google Scholar]; A broad and thorough review of AMH, including biology and physiology.
- 2.Nelson SM. Biomarkers of ovarian response: current and future applications. Fertil Steril 2013; 99:963–969 [DOI] [PubMed] [Google Scholar]
- 3.Leader B, Baker V. Schlegel PN, Fauser BC, Carrell DT, Racowsky C. A practical approach to recent advances in ovarian reserve resting. Biennial reviews of infertility Springer Science + Business media, 3rd ed.New York:2013 [Google Scholar]
- 4▪▪.Practice Committee of the American Society for Reproductive Medicine Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril 2012; 98:1407–1415 [DOI] [PubMed] [Google Scholar]; This practice guideline describes no consensus for cutpoints with ovarian reserve tests and ‘site-specific’ nature of interpretation.
- 5▪.Jeppesen JV, Anderson RA, Kelsey TW, et al. Which follicles make the most anti-mullerian hormone in humans? Evidence for an abrupt decline in AMH production at the time of follicle selection. Mol Hum Reprod 2013; 19:519–527 [DOI] [PubMed] [Google Scholar]; This in-vivo modeling study using 3D ultrasound estimated that 5–8 mm size follicles account for 60% of the circulating concentration of serum AMH.
- 6.Grynberg M, Pierre A, Rey R, et al. Differential regulation of ovarian antimullerian hormone (AMH) by estradiol through alpha- and beta-estrogen receptors. J Clin Endocrinol Metab 2012; 97:E1649–E1657 [DOI] [PubMed] [Google Scholar]
- 7.Mehta BN, Chimote MN, Chimote NN, et al. Follicular-fluid anti-mullerian hormone (FF AMH) is a plausible biochemical indicator of functional viability of oocyte in conventional in vitro fertilization (IVF) cycles. J Hum Reprod Sci 2013; 6:99–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.La Marca A, Spada E, Grisendi V, et al. Normal serum anti-mullerian hormone levels in the general female population and the relationship with reproductive history. Eur J Obstet Gynecol Reprod Biol 2012; 163:180–184 [DOI] [PubMed] [Google Scholar]
- 9▪▪.Nelson SM, Iliodromiti S, Fleming R, et al. Reference range for the antimullerian hormone Generation II assay: a population study of 10 984 women, with comparison to the established Diagnostics Systems Laboratory nomogram. Fertil Steril 2014; 101:523–529 [DOI] [PubMed] [Google Scholar]; This study demonstrates a recent shift in results from the same Beckman Gen II AMH assay frequently used in the literature, requiring a recalibration of population reference ranges.
- 10.Nelson SM, Messow MC, Wallace AM, et al. Nomogram for the decline in serum antimullerian hormone: a population study of 9601 infertility patients. Fertil Steril 2011; 95:736–741e1–e3 [DOI] [PubMed] [Google Scholar]
- 11.Nelson SM, Messow MC, McConnachie A, et al. External validation of nomogram for the decline in serum anti-mullerian hormone in women: a population study of 15 834 infertility patients. Reprod Biomed Online 2011; 23:204–206 [DOI] [PubMed] [Google Scholar]
- 12▪▪.Leader B, Hegde A, Baca Q, et al. High frequency of discordance between antimullerian hormone and follicle-stimulating hormone levels in serum from estradiol-confirmed days 2 to 4 of the menstrual cycle from 5354 women in U.S. fertility centers. Fertil Steril 2012; 98:1037–1042 [DOI] [PubMed] [Google Scholar]; This study demonstrates that in women presenting for fertility evaluation during estradiol-confirmed menstrual cycle day 2-4, serum FSH is likely falsely reassuring in 20% under 35 years of age and in over 30% above 40 years of age.
- 13.Seifer DB, Baker VL, Leader B. Age-specific serum anti-mullerian hormone values for 17 120 women presenting to fertility centers within the United States. Fertil Steril 2011; 95:747–750 [DOI] [PubMed] [Google Scholar]
- 14.Kelsey TW, Wright P, Scott M, et al. A validated model of serum anti-mullerian hormone from conception to menopause. PLoS One 2011; 6:e22024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15▪▪.Lie Fong S, Visser JA, Welt CK, et al. Serum anti-mullerian hormone levels in healthy females: a nomogram ranging from infancy to adulthood. J Clin Endocrinol Metab 2012; 97:4650–4655 [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates average serum AMH values in 804 women with no history of hormonal contraception use and normal menstruation.
- 16▪▪.La Marca A, Sunkara SK. Individualization of controlled ovarian stimulation in IVF using ovarian reserve markers: from theory to practice. Hum Reprod Update 2014; 20:124–140 [DOI] [PubMed] [Google Scholar]; An extensive compilation of the studies applying serum AMH cutpoints to the clinical protocols of ovarian stimulation.
- 17▪.Lan VT, Linh NK, Tuong HM, et al. Anti-mullerian hormone versus antral follicle count for defining the starting dose of FSH. Reprod Biomed Online 2013; 27:390–399 [DOI] [PubMed] [Google Scholar]; This study demonstrated that in 386 women, dosing ovarian stimulation by serum AMH can be used with similar clinical results as when using AFC.
- 18.Polyzos NP, Nelson SM, Stoop D, et al. Does the time interval between antimullerian hormone serum sampling and initiation of ovarian stimulation affect its predictive ability in in vitro fertilization–intracytoplasmic sperm injection cycles with a gonadotropin-releasing hormone antagonist? A retrospective single-center study. Fertil Steril 2013; 100:438–444 [DOI] [PubMed] [Google Scholar]
- 19▪▪.Guzman L, Ortega-Hrepich C, Polyzos NP, et al. A prediction model to select PCOS patients suitable for IVM treatment based on anti-mullerian hormone and antral follicle count. Hum Reprod 2013; 28:1261–1266 [DOI] [PubMed] [Google Scholar]; This study demonstrates how multivariate incorporation of variables such as AMH, AFC, and testosterone can improve the prediction of clinical outcome in IVM treatment.
- 20.Weintraub A, Margalioth EJ, Chetrit AB, et al. The dynamics of serum anti-mullerian-hormone levels during controlled ovarian hyperstimulation with GnRH-antagonist short protocol in polycystic ovary syndrome and low responders. Eur J Obstet Gynecol Reprod Biol 2014; 176:163–167 [DOI] [PubMed] [Google Scholar]
- 21.Aboulghar M, Saber W, Amin Y, et al. Impact of antimullerian hormone assays on the outcomes of in vitro fertilization: a prospective controlled study. Fertil Steril 2014; 101:134–137 [DOI] [PubMed] [Google Scholar]
- 22▪.Brodin T, Hadziosmanovic N, Berglund L, et al. Antimullerian hormone levels are strongly associated with live-birth rates after assisted reproduction. J Clin Endocrinol Metab 2013; 98:1107–1114 [DOI] [PubMed] [Google Scholar]; In 892 consecutive IVF–ICSI couples, serum AMH strongly predicted live-birth rate independent of age.
- 23.Pup LD, Zanet E, Rupolo M, et al. Which tools may help physicians in female fertility prediction after autologous bone marrow transplantation for lymphoma? A pilot study. J Chemother 2014; 1973947813Y0000000162 [DOI] [PubMed] [Google Scholar]
- 24.Li HW, Lee VC, Lau EY, et al. Role of baseline antral follicle count and anti-mullerian hormone in the index stimulation cycle of IVF treatment in predicting outcome of subsequent frozen-thawed embryo transfers. Gynecol Endocrinol 2014; 30:490–493 [DOI] [PubMed] [Google Scholar]
- 25.Lehmann P, Velez MP, Saumet J, et al. Anti-mullerian hormone (AMH): a reliable biomarker of oocyte quality in IVF. J Assist Reprod Genet 2014; 31:493–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merhi Z, Zapantis A, Berger DS, Jindal SK. Determining an anti-mullerian hormone cutoff level to predict clinical pregnancy following in vitro fertilization in women with severely diminished ovarian reserve. J Assist Reprod Genet 2013; 30:1361–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin WQ, Yao LN, Zhang DX, et al. The predictive value of anti-mullerian hormone on embryo quality, blastocyst development, and pregnancy rate following in vitro fertilization-embryo transfer (IVF-ET). J Assist Reprod Genet 2013; 30:649–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Himabindu Y, Gopinathan KK, Pandey AK, Sriharibabu M. Correlation of age and antimullerian hormone in assisted reproductive technology program outcome. Indian J Physiol Pharmacol 2013; 57:9–15 [PubMed] [Google Scholar]
- 29▪▪.Khader A, Lloyd SM, McConnachie A, et al. External validation of anti-mullerian hormone based prediction of live birth in assisted conception. J Ovarian Res 2013; 6:3. [DOI] [PMC free article] [PubMed] [Google Scholar]; An external validation of an algorithmic combination of serum and AMH to predict live birth in 822 women between ages of 25 and 42.
- 30▪▪.Iliodromiti S, Kelsey TW, Wu O, et al. The predictive accuracy of anti-mullerian hormone for live birth after assisted conception: a systematic review and meta-analysis of the literature. Hum Reprod Update 2014; 20:560–570 [DOI] [PubMed] [Google Scholar]; A large and extensive review tabulating the studies evaluating the relationship between serum AMH and live-birth rate using ART.
- 31▪.Kedem A, Haas J, Geva LL, et al. Ongoing pregnancy rates in women with low and extremely low AMH levels. A multivariate analysis of 769 cycles. PLoS One 2013; 8:e81629. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrated in 70 women with serum AMH less than 0.2 ng/ml who underwent 249 IVF cycles, an ongoing pregnancy rate of 4.4% per cycle and 16% per patient.
- 32.Streuli I, de Mouzon J, Paccolat C, et al. AMH concentration is not related to effective time to pregnancy in women who conceive naturally. Reprod Biomed Online 2014; 28:216–224 [DOI] [PubMed] [Google Scholar]
- 33.Murto T, Bjuresten K, Landgren BM, Stavreus-Evers A. Predictive value of hormonal parameters for live birth in women with unexplained infertility and male infertility. Reprod Biol Endocrinol 2013; 11:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34▪.Morel N, Bachelot A, Chakhtoura Z, et al. Study of anti-mullerian hormone and its relation to the subsequent probability of pregnancy in 112 patients with systemic lupus erythematosus, exposed or not to cyclophosphamide. J Clin Endocrinol Metab 2013; 98:3785–3792 [DOI] [PubMed] [Google Scholar]; This study demonstrated cyclophosphamide treatment in 56 women with systemic lupus erythematosus (SLE) was associated with a significant reduction in serum AMH compared with 56 age-matched, nontreated SLE control women.
- 35▪.Harlow SD, Gass M, Hall JE, et al. Executive summary of the stages of Reproductive Aging Workshop +10 addressing the unfinished agenda of staging reproductive aging. Fertil Steril 2012; 97:843–851 [DOI] [PMC free article] [PubMed] [Google Scholar]; This consensus statement includes for the first time serum AMH as a biomarker in the staging menopause.
- 36▪.Dolleman M, Depmann M, Eijkemans MJ, et al. Anti-mullerian hormone is a more accurate predictor of individual time to menopause than mother's age at menopause. Hum Reprod 2014; 29:584–591 [DOI] [PubMed] [Google Scholar]; This study suggests a 46% improvement in the accuracy of multivariate-based menopause prediction by adding serum AMH level to age and mother's age of menopause.
- 37▪▪.Ramezani Tehrani F, Dolleman M, van Disseldorp J, et al. Predicting menopausal age with anti-mullerian hormone: a cross-validation study of two existing models. Climacteric 2014; 10:1–8 [DOI] [PubMed] [Google Scholar]; This study demonstrates two independently derived models using age and AMH to predict the onset of menopause with similar and useful clinical predictive properties when one model is applied to the raw data of the other.
- 38▪.La Marca A, Sighinolfi G, Papaleo E, et al. Prediction of age at menopause from assessment of ovarian reserve may be improved by using body mass index and smoking status. PLoS One 2013; 8:e57005. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates how smoking and low BMI are associated with earlier menopause within a given age-specific AMH percentile.
- 39.Tal R, Seifer DB. Potential mechanisms for racial and ethnic differences in antimullerian hormone and ovarian reserve. Int J Endocrinol 2013; 2013:818912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iino K, Tarakida A, Abe K, et al. Role of antimullerian hormone as a biomarker of the menopausal transition. Menopause 2013; 20:218–222 [DOI] [PubMed] [Google Scholar]
- 41.Dolleman M, Faddy MJ, van Disseldorp J, et al. The relationship between anti-mullerian hormone in women receiving fertility assessments and age at menopause in subfertile women: evidence from large population studies. J Clin Endocrinol Metab 2013; 98:1946–1953 [DOI] [PubMed] [Google Scholar]
- 42▪▪.Practice Committees of American Society for Reproductive Medicine; Society for Assisted Reproductive Technology Mature oocyte cryopreservation: a guideline. Fertil Steril 2013; 99:37–43 [DOI] [PubMed] [Google Scholar]; The guideline which cites the evidence demonstrating oocyte preservation should no longer be considered experimental.
- 43▪.Casadei L, Madrigale A, Puca F, et al. The role of serum anti-mullerian hormone (AMH) in the hormonal diagnosis of polycystic ovary syndrome. Gynecol Endocrinol 2013; 29:545–550 [DOI] [PubMed] [Google Scholar]; This study demonstrated greater than 90% sensitivity and specificity for diagnosing PCOS using NIH criteria.
- 44▪.Sahmay S, Aydin Y, Oncul M, Senturk LM. Diagnosis of polycystic ovary syndrome: AMH in combination with clinical symptoms. J Assist Reprod Genet 2014; 31:213–220 [DOI] [PMC free article] [PubMed] [Google Scholar]; In 150 women with PCOS treated with the same ovarian stimulation protocol, serum AMH did not predict the pregnancy rates.
- 45▪▪.Homburg R, Ray A, Bhide P, et al. The relationship of serum anti-mullerian hormone with polycystic ovarian morphology and polycystic ovary syndrome: a prospective cohort study. Hum Reprod 2013; 28:1077–1083 [DOI] [PubMed] [Google Scholar]; A prospective demonstration in 215 women that average serum AMH can differentiate PCOS (n = 90), PCOM (n = 35), and normal ovaries (n = 90).
- 46▪▪.Iliodromiti S, Kelsey TW, Anderson RA, Nelson SM. Can anti-mullerian hormone predict the diagnosis of polycystic ovary syndrome? A systematic review and meta-analysis of extracted data. J Clin Endocrinol Metab 2013; 98:3332–3340 [DOI] [PubMed] [Google Scholar]; An extensive compilation of the studies applying serum AMH cutpoints to PCOS diagnosis.
- 47.Chao KC, Ho CH, Shyong WY, et al. Anti-mullerian hormone serum level as a predictive marker of ovarian function in Taiwanese women. J Chin Med Assoc 2012; 75:70–74 [DOI] [PubMed] [Google Scholar]
- 48.Swellam M, Khaial A, Mosa T, et al. Antimullerian and androgens hormones in women with polycystic ovary syndrome undergoing IVF/ICSI. Iran J Reprod Med 2013; 11:883–890 [PMC free article] [PubMed] [Google Scholar]
- 49.Tian X, Ruan X, Mueck AO, et al. Anti-mullerian hormone levels in women with polycystic ovarian syndrome compared with normal women of reproductive age in China. Gynecol Endocrinol 2014; 30:126–129 [DOI] [PubMed] [Google Scholar]
- 50▪.Tal R, Seifer DB, Khanimov M, et al. Characterization of women with elevated antimullerian hormone levels (AMH): correlation of AMH with polycystic ovarian syndrome phenotypes and assisted reproductive technology outcomes. Am J Obstet Gynecol 2014 [DOI] [PubMed] [Google Scholar]; This study demonstrates percentage frequency of PCOS at various cutpoints of elevated AMH.
- 51▪▪.Sahmay S, Aydin Y, Atakul N, et al. Relation of antimullerian hormone with the clinical signs of hyperandrogenism and polycystic ovary morphology. Gynecol Endocrinol 2014; 30:130–134 [DOI] [PubMed] [Google Scholar]; A cross-sectional analysis of 463 PCOS patients demonstrating the relationship of serum AMH with a number of clinical signs, including menstrual cycle length.
- 52▪.Baker VL. Primary ovarian insufficiency in the adolescent. Curr Opin Obstet Gynecol 2013; 25:375–381 [DOI] [PubMed] [Google Scholar]; This publication provides a helpful overview and recent review of POI.
- 53.Kallio S, Aittomaki K, Piltonen T, et al. Anti-mullerian hormone as a predictor of follicular reserve in ovarian insufficiency: special emphasis on FSH-resistant ovaries. Hum Reprod 2012; 27:854–860 [DOI] [PubMed] [Google Scholar]
- 54.Visser JA, Schipper I, Laven JS, Themmen AP. Anti-mullerian hormone: an ovarian reserve marker in primary ovarian insufficiency. Nat Rev Endocrinol 2012; 8:331–341 [DOI] [PubMed] [Google Scholar]
- 55.Sahmay S, Usta TA, Erel T, et al. Elevated LH levels draw a stronger distinction than AMH in premature ovarian insufficiency. Climacteric 2014; 17:197–203 [DOI] [PubMed] [Google Scholar]
- 56.Yoon SH, Choi YM, Hong MA, et al. Association study of anti-mullerian hormone and anti-mullerian hormone type II receptor polymorphisms with idiopathic primary ovarian insufficiency. Hum Reprod 2013; 28:3301–3305 [DOI] [PubMed] [Google Scholar]
- 57.Spath MA, Feuth TB, Allen EG, et al. Intra-individual stability over time of standardized anti-mullerian hormone in FMR1 premutation carriers. Hum Reprod 2011; 26:2185–2191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gleicher N, Kim A, Barad DH, et al. FMR1-dependent variability of ovarian aging patterns is already apparent in young oocyte donors. Reprod Biol Endocrinol 2013; 11:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gleicher N, Weghofer A, Kim A, Barad DH. The impact in older women of ovarian FMR1 genotypes and sub-genotypes on ovarian reserve. PLoS One 2012; 7:e33638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gleicher N, Kim A, Weghofer A, Barad DH. Differences in ovarian aging patterns between races are associated with ovarian genotypes and sub-genotypes of the FMR1 gene. Reprod Biol Endocrinol 2012; 10:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gleicher N, Weghofer A, Oktay K, Barad DH. Correlation of triple repeats on the FMR1 (fragile X) gene to ovarian reserve: a new infertility test? Acta Obstet Gynecol Scand 2009; 88:1024–1030 [DOI] [PubMed] [Google Scholar]
- 62.Choe SA, Kim KC, Lee JY, et al. The relationship between the number of CGG repeats and serum level of anti-mullerian hormone in women without FMR1 premutation. Eur J Obstet Gynecol Reprod Biol 2013; 169:275–278 [DOI] [PubMed] [Google Scholar]
- 63.Kline JK, Kinney AM, Levin B, et al. Intermediate CGG repeat length at the FMR1 locus is not associated with hormonal indicators of ovarian age. Menopause 2014; 21:740–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.La Marca A, Brozzetti A, Sighinolfi G, et al. Primary ovarian insufficiency: autoimmune causes. Curr Opin Obstet Gynecol 2010; 22:277–282 [DOI] [PubMed] [Google Scholar]
- 65▪▪.Ma W, Zhan Z, Liang X, et al. Subclinical impairment of ovarian reserve in systemic lupus erythematosus patients with normal menstruation not using alkylating therapy. J Women's Health 2013; 22:1023–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrated with (n = 19) or without (n = 23) treatment, SLE patients exhibit the signs of diminished ovarian reserve compared with healthy age-matched controls (n = 21).
- 66.Lawrenz B, Henes J, Henes M, et al. Impact of systemic lupus erythematosus on ovarian reserve in premenopausal women: evaluation by using anti-mullerian hormone. Lupus 2011; 20:1193–1197 [DOI] [PubMed] [Google Scholar]
- 67▪.Senates E, Colak Y, Erdem ED, et al. Serum anti-mullerian hormone levels are lower in reproductive-age women with Crohn's disease compared to healthy control women. J Crohn's Colitis 2013; 7:e29–e34 [DOI] [PubMed] [Google Scholar]; This study shows in 35 patients with Crohn's disease and 35 age-matched controls that AMH was almost half in Crohn's disease patients compared with controls.
- 68.Chong YH, Campbell AJ, Farrand S, McLennan IS. Anti-mullerian hormone level in older women: detection of granulosa cell tumor recurrence. Int J Gynecol Cancer 2012; 22:1497–1499 [DOI] [PubMed] [Google Scholar]
- 69.Mancaux A, Grardel Chambenoit E, Gagneur O, et al. [Granulosa cell tumor: difficulty of diagnosis and contribution of imaging]. Gynecol Obstet Fertil 2013; 41:439–445 [DOI] [PubMed] [Google Scholar]
- 70.Carrarelli P, Rocha AL, Belmonte G, et al. Increased expression of antimullerian hormone and its receptor in endometriosis. Fertil Steril 2014; 101:1353–1358 [DOI] [PubMed] [Google Scholar]
- 71.Muzii L, Luciano AA, Zupi E, Panici PB. Effect of surgery for endometrioma on ovarian function: a different point of view. J Minim Invasive Gynecol 2014 [DOI] [PubMed] [Google Scholar]
- 72▪.Urman B, Alper E, Yakin K, et al. Removal of unilateral endometriomas is associated with immediate and sustained reduction in ovarian reserve. Reprod Biomed Online 2013; 27:212–216 [DOI] [PubMed] [Google Scholar]; This study demonstrated in 25 women undergoing laparoscopic unilateral endometrioma removal, the average serum AMH was 24% lower at 1 and 6 months postoperatively than observed preoperatively.
- 73▪▪.Alborzi S, Keramati P, Younesi M, et al. The impact of laparoscopic cystectomy on ovarian reserve in patients with unilateral and bilateral endometriomas. Fertil Steril 2014; 101:427–434 [DOI] [PubMed] [Google Scholar]; This study demonstrates unilateral ovarian surgery reduces AMH and bilateral surgery reduces serum AMH more than unilateral.
- 74.Arthur R, Kachura J, Liu G, et al. Laparoscopic myomectomy versus uterine artery embolization: long-term impact on markers of ovarian reserve. J Obstet Gynaecol 2014; 36:240–247 [DOI] [PubMed] [Google Scholar]
- 75.Jang WK, Lim SY, Park JC, et al. Surgical impact on serum anti-mullerian hormone in women with benign ovarian cyst: a prospective study. Obstet Gynecol Sci 2014; 57:121–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pacchiarotti A, Frati P, Milazzo GN, et al. Evaluation of serum anti-mullerian hormone levels to assess the ovarian reserve in women with severe endometriosis. Eur J Obstet Gynecol Reprod Biol 2014; 172:62–64 [DOI] [PubMed] [Google Scholar]
- 77▪.Dillon KE, Sammel MD, Prewitt M, et al. Pretreatment antimullerian hormone levels determine rate of posttherapy ovarian reserve recovery: acute changes in ovarian reserve during and after chemotherapy. Fertil Steril 2013; 99:477.e1–483.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]; In a study of 46 young and adolescent women from four centers with a new cancer diagnosis, serum AMH levels were demonstrated to be reduced subsequent to chemotherapy, with recovery dependent upon pretreatment levels and alkylating agent.
- 78▪▪.Peigne M, Decanter C. Serum AMH level as a marker of acute and long-term effects of chemotherapy on the ovarian follicular content: a systematic review. Reprod Biol Endocrinol 2014; 12:26. [DOI] [PMC free article] [PubMed] [Google Scholar]; A helpful compilation of the studies evaluating the utility of serum AMH in chemotherapy treatment.
- 79.Beneventi F, Locatelli E, Giorgiani G, et al. Gonadal and uterine function in female survivors treated by chemotherapy, radiotherapy, and/or bone marrow transplantation for childhood malignant and nonmalignant diseases. BJOG 2014; 121:856–865 [DOI] [PubMed] [Google Scholar]
- 80.Ruddy KJ, O’Neill A, Miller KD, et al. Biomarker prediction of chemotherapy-related amenorrhea in premenopausal women with breast cancer participating in E5103. Breast Cancer Res Treat 2014; 144:591–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hadji P, Kauka A, Ziller M, et al. Effect of adjuvant endocrine therapy on hormonal levels in premenopausal women with breast cancer: the ProBONE II study. Breast Cancer Res Treat 2014; 144:343–351 [DOI] [PubMed] [Google Scholar]
- 82.Henry NL, Xia R, Schott AF, et al. Prediction of postchemotherapy ovarian function using markers of ovarian reserve. Oncologist 2014; 19:68–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Miyoshi Y, Ohta H, Namba N, et al. Low serum concentrations of anti-mullerian hormone are common in 53 female childhood cancer survivors. Hormone Res Paediatr 2013; 79:17–21 [DOI] [PubMed] [Google Scholar]
- 84.Mok CC, Chan PT, To CH. Antimullerian hormone and ovarian reserve in systemic lupus erythematosus. Arthritis Rheum 2013; 65:206–210 [DOI] [PubMed] [Google Scholar]
- 85.Kwon SK, Kim SH, Yun SC, et al. Decline of serum antimullerian hormone levels after laparoscopic ovarian cystectomy in endometrioma and other benign cysts: a prospective cohort study. Fertil Steril 2014; 101:435–441 [DOI] [PubMed] [Google Scholar]
- 86.Ortega-Hrepich C, Polyzos NP, Anckaert E, et al. The effect of ovarian puncture on the endocrine profile of PCOS patients who undergo IVM. Reprod Biol Endocrinol 2014; 12:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gleicher N, Kim A, Kushnir V, et al. Clinical relevance of combined FSH and AMH observations in infertile women. J Clin Endocrinol Metab 2013; 98:2136–2145 [DOI] [PubMed] [Google Scholar]
- 88▪.Broer SL, van Disseldorp J, Broeze KA, et al. Added value of ovarian reserve testing on patient characteristics in the prediction of ovarian response and ongoing pregnancy: an individual patient data approach. Hum Reprod Update 2013; 19:26–36 [DOI] [PubMed] [Google Scholar]; This study pooled 28 different study databases with various AMH testing methods, and demonstrated the pooled serum AMH values combined with other variables did not show statistically significant improvement in predicting poor response and no ability of ovarian reserve tests to predict live delivery rate.
- 89.Sowers M, McConnell D, Gast K, et al. Anti-mullerian hormone and inhibin B variability during normal menstrual cycles. Fertil Steril 2010; 94:1482–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dorgan JF, Spittle CS, Egleston BL, et al. Assay reproducibility and within-person variation of mullerian inhibiting substance. Fertil Steril 2010; 94:301–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91▪▪.Overbeek A, Broekmans FJ, Hehenkamp WJ, et al. Intra-cycle fluctuations of anti-mullerian hormone in normal women with a regular cycle: a re-analysis. Reprod Biomed Online 2012; 24:664–669 [DOI] [PubMed] [Google Scholar]; This study clearly demonstrated individual patient serum AMH levels throughout the menstrual cycle and revealed a subset of patients in which serum AMH clinical values change significantly.
- 92▪.Hadlow N, Longhurst K, McClements A, et al. Variation in antimullerian hormone concentration during the menstrual cycle may change the clinical classification of the ovarian response. Fertil Steril 2013; 99:1791–1797 [DOI] [PubMed] [Google Scholar]; This study showed in 12 women each measured at least five times for serum AMH during a single menstrual cycle that up to one-third could have a different clinical assessment using the lowest and highest AMH values obtained.
- 93.Van den Berg MH, van Dulmen-den Broeder E, Overbeek A, et al. Comparison of ovarian function markers in users of hormonal contraceptives during the hormone-free interval and subsequent natural early follicular phases. Hum Reprod 2010; 25:1520–1527 [DOI] [PubMed] [Google Scholar]
- 94.Fabregues F, Castelo-Branco C, Carmona F, et al. The effect of different hormone therapies on antimullerian hormone serum levels in anovulatory women of reproductive age. Gynecol Endocrinol 2011; 27:216–224 [DOI] [PubMed] [Google Scholar]
- 95.Deb S, Pincott-Allen CB, Clewes C, et al. Quantifying effect of combined oral contraceptive pill on functional ovarian reserve as measured by serum anti-müllerian hormone and small antral follicle count using three-dimensional ultrasound. Ultrasound Obstet Gynecol 2012; 39:574–580 [DOI] [PubMed] [Google Scholar]
- 96.Mes-Krowinkel MG, Louwers YV, Mulders AG, et al. Influence of oral contraceptives on anthropomorphometric, endocrine, and metabolic profiles of anovulatory polycystic ovary syndrome patients. Fertil Steril 2014; 101:1757–1765 [DOI] [PubMed] [Google Scholar]
- 97▪▪.Kallio S, Puurunen J, Ruokonen A, et al. Antimullerian hormone levels decrease in women using combined contraception independently of administration route. Fertil Steril 2013; 99:1305–1310 [DOI] [PubMed] [Google Scholar]; A prospective demonstration that hormonal contraceptive medication delivered by oral, patch, or vaginal ring lowered the serum AMH within 9 weeks.
- 98▪▪.Koninger A, Kauth A, Schmidt B, et al. Anti-mullerian-hormone levels during pregnancy and postpartum. Reprod Biol Endocrinol 2013; 11:60. [DOI] [PMC free article] [PubMed] [Google Scholar]; An extensive analysis of serum AMH during pregnancy in 554 healthy women from 16 to 47 years of age utilizing a mixture of cross-sectional and longitudinal approaches.
- 99.Brouwer J, Laven JS, Hazes JM, et al. Levels of serum anti-mullerian hormone, a marker for ovarian reserve, in women with rheumatoid arthritis. Arthritis Care Res 2013; 65:1534–1538 [DOI] [PubMed] [Google Scholar]
- 100.Oriol B, Barrio A, Pacheco A, et al. Systemic methotrexate to treat ectopic pregnancy does not affect ovarian reserve. Fertil Steril 2008; 90:1579–1582 [DOI] [PubMed] [Google Scholar]
- 101.Fouany MR, Sharara FI. Is there a role for DHEA supplementation in women with diminished ovarian reserve? J Assist Reprod Genet 2013; 30:1239–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hyman JH, Margalioth EJ, Rabinowitz R, et al. DHEA supplementation may improve IVF outcome in poor responders: a proposed mechanism. Eur J Obstet Gynecol Reprod Biol 2013; 168:49–53 [DOI] [PubMed] [Google Scholar]
- 103.Narkwichean A, Maalouf W, Campbell BK, Jayaprakasan K. Efficacy of dehydroepiandrosterone to improve ovarian response in women with diminished ovarian reserve: a meta-analysis. Reprod Biol Endocrinol 2013; 11:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Naasan MN, Harrity C, Pentony L, Mocanu E. Anti-mullerian hormone nomogram in an Irish subfertile population. Ir J Med Sci 2014; 23: [DOI] [PubMed] [Google Scholar]
- 105.Nelson SM, La Marca A. The journey from the old to the new AMH assay: how to avoid getting lost in the values. Reprod Biomed Online 2011; 23:411–420 [DOI] [PubMed] [Google Scholar]
- 106.Li HW, Ng EH, Wong BP, et al. Correlation between three assay systems for anti-mullerian hormone (AMH) determination. J Assist Reprod Genet 2012; 29:1443–1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rustamov O, Smith A, Roberts SA, et al. The measurement of anti-mullerian hormone: a critical appraisal. J Clin Endocrinol Metab 2014; 99:723–732 10.1210/jc.2013-3476 [DOI] [PubMed] [Google Scholar]
- 108.Rustamov O, Smith A, Roberts SA, et al. Anti-mullerian hormone: poor assay reproducibility in a large cohort of subjects suggests sample instability. Human Reprod 2012; 27:3085–3091 [DOI] [PubMed] [Google Scholar]
- 109.Zuvela E, Walls M, Matson P. Within-laboratory and between-laboratory variability in the measurement of antimullerian hormone determined within an external quality assurance scheme. Reprod Biol 2013; 13:255–257 [DOI] [PubMed] [Google Scholar]
- 110.Lukaszuk K, Ludwikowska B, Liss J, et al. Decreasing quality of the new generations of antimullerian hormone assays. BioMed Res Int 2014; 2014:165352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gassner D, Jung R. First fully automated immunoassay for anti-mullerian hormone. Clin Chem Lab Med 2014 [DOI] [PubMed] [Google Scholar]
- 112.Welsh P, Smith K, Nelson SM. A single-centre evaluation of two new anti-mullerian hormone assays and comparison with the current clinical standard assay. Hum Reprod 2014; 29:1035–1041 [DOI] [PubMed] [Google Scholar]
- 113.Han X, McShane M, Sahertian R, et al. Premixing serum samples with assay buffer is a prerequisite for reproducible anti-mullerian hormone measurement using the Beckman Coulter Gen II assay. Hum Reprod 2014; 29:1042–1048 [DOI] [PubMed] [Google Scholar]


