Abstract
Protein folding is a complex, error-prone process that often results in an irreparable protein by-product. These by-products can be recognized by cellular quality control machineries and targeted for proteasome-dependent degradation. The folding of proteins in the secretory pathway adds another layer to the protein folding “problem,” as the endoplasmic reticulum maintains a unique chemical environment within the cell. In fact, a growing number of diseases are attributed to defects in secretory protein folding, and many of these by-products are targeted for a process known as endoplasmic reticulum-associated degradation (ERAD). Since its discovery, research on the mechanisms underlying the ERAD pathway has provided new insights into how ERAD contributes to human health during both normal and diseases states. Links between ERAD and disease are evidenced from the loss of protein function as a result of degradation, chronic cellular stress when ERAD fails to keep up with misfolded protein production, and the ability of some pathogens to coopt the ERAD pathway. The growing number of ERAD substrates has also illuminated the differences in the machineries used to recognize and degrade a vast array of potential clients for this pathway. Despite all that is known about ERAD, many questions remain, and new paradigms will likely emerge. Clearly, the key to successful disease treatment lies within defining the molecular details of the ERAD pathway and in understanding how this conserved pathway selects and degrades an innumerable cast of substrates.
I. INTRODUCTION
A. The Folding Problem: Inefficiency and Inaccuracy During Protein Folding
Accurate protein folding is key for biological function, but this process is hampered by the fact that folding is chemically complex. In eukaryotes, the translation of mRNAs occurs on 80S ribosomes, which are either free in the cytoplasm or tethered to the endoplasmic reticulum (ER), giving the organelle a studded or rough appearance by electron microscopy. As a nascent protein emerges from the ribosome exit tunnel, the polypeptide must adopt a functional conformation out of a huge number of possible conformations. It was originally thought that protein folding followed a direct pathway and was dictated by the sum of the interactions that can form between amino acids, including ion pairs, van der Waals forces, hydrophobic interactions, and hydrogen bonding. However, decades of biophysical and computational research have yielded the more sophisticated view that protein folding is instead a nonlinear process. Proteins can navigate through a number of different energy landscapes, which may adopt a funnel-like shape with the lowest energy (folded) state at the bottom of the funnel. Folding information is mainly present in the amino acid side chains, but involves both local and distant interactions and is driven largely by the need to isolate hydrophobic residues within the protein core (105). However, even this view may be naive, as protein folding must occur in a complex, highly crowded environment that has been estimated to reach concentrations as high as 300 mg/ml in the cytoplasm (118). Thus intra-molecular interactions impinge on the efficiency of protein folding in the cell.
To prevent illegitimate inter- and intramolecular interactions, and to protect hydrophobic amino acid side chains as they emerge from the ribosome, folding is aided by a class of proteins known as molecular chaperones. Based on their propensity to bind short peptides with hydrophobic character (47, 133), molecular chaperones guard against misfolding and aggregation. In most cases, molecular chaperones do not increase the rate of folding, but rather increase the number of productive interactions and prevent misguided interactions, thus augmenting folding efficiency (106).
Heat shock proteins (Hsp) of ~90, 70, and 40 kDa represent three important classes of chaperones (59, 60, 89, 274, 450, 573). Hsp70s have a substrate-binding pocket and a low ATPase activity, which is stimulated by the interaction with Hsp40 cochaperone partners that contain an ~70 amino acid motif known as the J-domain. Hsp70s are highly conserved and are critical for protein folding, protein translocation, protein degradation, and the assembly and disassembly of protein complexes. The Hsp40s also bind substrates and via their interaction with Hsp70 are thought to hand-off substrates to Hsp70s. Because Hsp40s enhance the basal ATPase activity of Hsp70s, and because Hsp70 in the ADP-bound state exhibits a higher affinity for peptide substrates, the transfer of substrate to Hsp70 results in a tight Hsp70-peptide complex. Ultimately, a member of a diverse class of nucleotide exchange factors helps release ADP from Hsp70, which in turn frees the peptide substrate. Thus the Hsp70-Hsp40 complex helps to control and maintain the solubility of proteins that may otherwise aggregate.
The interaction of Hsp90s with substrates is mediated through more than one domain in this dimeric chaperone (464, 520). In this case, the relationship between ATP hydrolysis and substrate binding and release is more complex, and a nucleotide-free state may be an important intermediate in the chaperone cycle. It is generally accepted, though, that Hsp90s usually function at later steps in the protein folding pathway than Hsp70s and Hsp40s.
Given the complexity of protein folding, it is not difficult to imagine that one of many problems may arise. For example, nascent polypeptides may aggregate due to unwanted interactions amongst hydrophobic amino acid side chains. It has been estimated that as many as one-third of all newly synthesized proteins may be degraded in some cell types, presumably due to problems during their synthesis or folding (438). Protein misfolding and aggregation are actually quite common and are responsible for a number of human pathologies, such as Alzheimer’s disease, Huntington’s disease, Parkinson’s disease, and α1-antitrypsin deficiency (22). Protein aggregation can lead to the formation of amyloid deposits in tissues throughout the body. Even though the proteins that result in amyloid fibril production are quite varied, the fibrils share many general structural features, suggesting a common fate amongst many aggregated products. However, the propensity of a given polypeptide to form amyloids is highly sensitive to the cellular environment (see, for example, Ref. 106).
B. The Trafficking Problem: Difficulty Transiting Throughout the Secretory Pathway
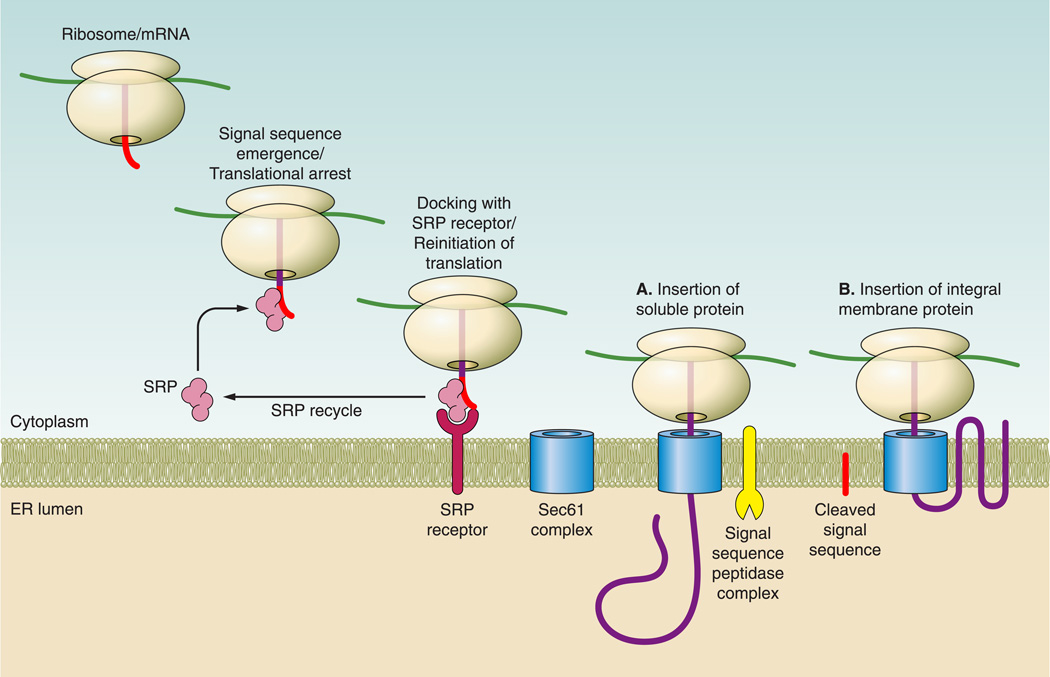
Nearly one-third of all newly synthesized proteins are targeted to the ER, which is the first step in the delivery of these proteins for trafficking to other organelles of the secretory pathway or to the extracellular space (157). As a nascent secreted protein emerges from the ribosome exit tunnel, it presents an NH2-terminal hydrophobic signal sequence to the aqueous environment of the cytoplasm. Signal sequences do not follow a strict consensus, but are generally ~20–30 residues in length and contain 3 common features: a basic motif, a hydrophobic core, and a short polar region (196). The emerging signal sequence is then captured by a ribonucleoprotein complex known as the signal recognition particle (SRP), which consists of six proteins and a single RNA (242, 422, 547). One of the SRP subunits binds GTP, which is required for the particle to function. SRP has two general roles: 1) translational arrest, which provides a window of opportunity for the ribosome-nascent chain complex to “find” the ER membrane, and 2) targeting and release of the nascent peptide to the ER membrane translocation complex (see FIG. 1). At the ER membrane, SRP associates with the heterodimeric SRP receptor, which stimulates SRP’s hydrolysis of GTP (389). SRP is then released from the ribosome-nascent chain complex and translation resumes, resulting in the insertion of the nascent peptide into the translocation pore, or “translocon.”
FIGURE 1. Protein targeting to the endoplasmic reticulum.
As a nascent secretory protein emerges from the ribosome exit tunnel, it presents a hydrophobic signal sequence that is recognized by the signal recognition particle (SRP). Binding of SRP to the signal sequence slows translation and targets the ribosome-nascent chain complex to the ER membrane via its interaction with the dimeric SRP receptor. Following release, SRP is recycled and translation resumes. A nascent soluble protein (A) is translocated into the ER lumen, and an integral membrane protein (B) is incorporated into the membrane by the Sec61 translocation complex, and for all soluble proteins and some membrane proteins the hydrophobic signal sequence is cleaved by the ER localized signal peptidase complex.
Protein translocation occurs through the Sec61 complex (365, 408). In mammalian cells, the three subunits of the Sec61 complex (alpha, beta, and gamma) provide an aqueous pore through which proteins can be inserted either into the ER lumen or, if hydrophobic membrane-spanning segments are encountered, directly into the lipid bilayer of the ER (FIG. 1, A AND B). Insertion of membrane-spanning domains is thought to occur through the opening of the translocon and lateral movement into the hydrophobic environment of the membrane. Several studies indicate that the translocon is quite flexible and may accommodate multiple transmembrane helicies at once during the insertion of polytopic membrane proteins (457). During translocation, the hydrophobic signal sequence is cleaved by the signal sequence peptidase complex, which is intimately associated with the ER membrane (473). Upon insertion into the ER space, translocating proteins also interact with chaperones to promote folding and undergo cotranslational modifications, as discussed below.
Protein targeting and translocation were originally thought to be highly efficient, but there is now evidence to the contrary. Given the fact that signal sequences are quite diverse, it is not surprising that they are sometimes interchangeable and can tolerate certain alterations (see, for example, Ref. 234). However, signal sequence diversity is now known to be important for targeting, translocation, and signal sequence cleavage efficiency (196). For example, the signal sequence of the ER lectin calreticulin is somewhat inefficient, resulting in a cytosolic population of this chaperone in addition to an abundant ER pool. Chimeric proteins containing the signal sequence of calreticulin or prolactin, another well-characterized secretory protein, were used to demonstrate inefficient translocation of chimeras containing the calreticulin signal sequence both in vitro and in vivo (447). In addition, the use of a translocation reporter that was appended onto a variety of signal sequences suggested that up to 50% of some other proteins that normally enter the secretory pathway are inefficiently targeted to and inserted into the ER (291). Whether this represents the need to retain a cytosolic pool of some secreted proteins or an inherently inefficient targeting system is not completely clear.
Signal sequence inefficiency has been proposed as the underlying mechanism for the pathophysiology of a neurodegenerative condition caused by prion protein (PrP), a glycoprotein of unknown function that resides on the cell surface. Prion diseases can be sporadic, genetic, or infectious and result in the accumulation of misfolded PrP and fatal neurodegeneration (392). During PrP synthesis, the protein is cotranslationally inserted into the ER and is modified with a glycophosphatidylinositol anchor that initially tethers PrP to the ER membrane and ultimately to the cellular plasma membrane. By examining the biogenesis of disease-causing mutations in PrP, it was suggested that PrP pathogenesis was the result of inefficient maturation in the ER, followed by “retrotranslocation” and aggregation in the cytosol (317). However, subsequent studies indicated that the cytoplasmic accumulation of PrP is not due to retrotranslocation (110, 401). Instead, the inefficiency of the PrP signal sequence results in the formation of either a cytoplasmic (cyPrP) form or a transmembrane (CtmPrP) form of the protein (71). Of note, CtmPrP accumulates as a stable species, and its expression correlates with neurodegeneration in mice and humans (197, 198). Consistent with these data, disease-causing mutations that result in the accumulation of CtmPrP can be rescued by increasing the efficiency of signal sequence insertion (400). These results highlight the sensitivity of protein homeostasis and disease to subtle differences in protein synthesis, targeting, and/or translocation efficiency. In further support of this view, silent mutations in a multidrug resistance transporter that simply change the protein’s translation rate, but leave the amino acid sequence unchanged, alter the transporter’s substrate specificity (249).
C. Protein Modification in the Unique Environment of the ER
Coincident with translocation, nascent proteins encounter the ER’s unique environment, which contrasts sharply with other organelles in its chemical properties and protein composition. Chemically, the ER is more oxidizing and is calcium-rich. The oxidizing potential favors the formation of disulfide bonds, and calcium serves as a necessary cofactor for many chaperones and is vital for cellular signaling. In addition, the ER is packed with proteins that aid the folding of other proteins, that catalyze ER-specific posttranslational modifications, and that form the protein quality control machinery. Unique posttranslational modifications also occur in the ER. After signal sequence cleavage, translocating proteins may be modified with sugar moieties on specific Asn side chains via a process known as N-linked glycosylation, and as noted above, they may acquire disulfide bonds. Each of these modifications contributes to a protein’s native fold (14, 56, 190, 256, 455).
Disulfide bonds are formed through the oxidation of pairs of free thiols on cysteine residues. Disulfide bonds help stabilize a protein, which may be especially important if a protein is to be secreted into the harsh extracellular milieu or into the lysosome, which is acidic. Sometimes disulfide bonds serve as the only link between proteolytically cleaved subunits, such as in cholera toxin (582) or insulin (see below) and help maintain the stability of oligomeric secreted proteins, such as immunoglobulins (80). The incorrect acquisition or maintenance of disulfide bonds can give rise to a form of the clotting disease, von Willebrand disease (435), and the connective tissue disorder, Marfan disease (543).
The enzymes that catalyze the formation of disulfide bonds are generally referred to as protein disulfide isomerases (PDIs). PDIs can also function during the isomerization of disulfide bonds. The mammalian ER contains 20 different PDI family members, which are characterized by the presence of one or more thioredoxin-like domains (190). During protein folding, disulfide bonds are formed and incorrectly formed bonds must be broken; therefore, cycles of PDI action are vital during protein folding. The family was first named for PDI, which was the first protein shown to have the ability to form disulfide bonds on ER proteins (61, 161, 511). Interestingly, PDI possesses both oxidoreductase activity as well as a chaperone-like activity, both of which are essential for the folding/secretion of many proteins, including procollagen trimers (20).
ER calcium concentrations range from 100 to 300 µM, which is in stark contrast to the cytoplasmic calcium concentration (10 to 100 nM; Refs. 65, 165). ER calcium release is mediated through inositol 1,4,5-trisphosphate receptors (IP3R), which open upon binding to the second messenger IP3. Plasma membrane excitation by various signaling proteins, including hormones, growth factors, and neurotransmitters, results in phospholipase C-mediated cleavage of a plasma membrane phospholipid to generate IP3 (42). The wave of released calcium is critical for numerous cellular events, such as muscle contraction, exocytosis, cell proliferation, the immune response, transcriptional activation, and apoptosis (43, 69). Of note, following activation by certain signals, IP3R levels are downregulated by ERAD as a mechanism to attenuate the response to IP3 signaling (551). Released calcium is reconcentrated by sarco/endoplasmic reticulum Ca2+-ATPases (SERCAs), which couple the free energy of ATP hydrolysis to pump calcium against its concentration gradient back into the ER (393). Many ER chaperones, including the PDIs and BiP (also known as Grp78), Grp94 (an ER lumenal Hsp90 homolog), calnexin, and calreticulin (see above), bind calcium and are thought to help buffer calcium levels, in addition to their roles in protein folding (85). The importance of ER calcium became even more evident when it was discovered that disturbing ER calcium stores altered the secretion of several proteins (306). Not surprising, many diseases, including spinocerebellar ataxia, heart disease, and Darier’s disease, have been directly linked to defects in IP3 receptor and SERCA function (54, 346, 355).
As introduced above, another posttranslational modification that occurs in the ER during protein translocation is the addition of sugar moieties guided by the consensus signal Asn-X-Ser/Thr. The sugar is transferred to the Asn side chain, and thus this event is termed N-linked glycosylation. N-glycans not only protect proteins and stabilize protein structure/interactions, but they mediate the interaction with quality control lectins in the ER, such as calnexin and calreticulin (also see below). Therefore, N-linked glycans are intimately involved in early protein folding events. A large number of diseases are also linked to the improper glycosylation of secreted proteins. Currently, there are 16 known genetic diseases that arise either from improper assembly or processing of N-glycans, and these are collectively known as congenital disorders of glycosylation (226, 265). For example, individuals with mutations in ALG6, which encodes glucosyltransferase I, have hyptonia, strabism, seizures, and low circulating levels of certain glycoproteins, including the coagulation inhibitors protein C and anti-thrombin.
The acquired N-glycan is composed of two N-acetylglucosamines, nine mannoses, and three glucose moieties, which are transferred by the oligosaccharyltranferase complex (OGT) en bloc from a precursor to the Asn side chain (14, 194, 372). Following enzymatic cleavage to remove two of the three glucose residues, the nascent glycoprotein can interact with the lectin-like chaperones calnexin and calreticulin. Protein interaction with calnexin and calreticulin is maintained until cleavage of the final glucose residue, following which a “decision” about folding status must be made. If still unfolded, glycoproteins can rebind calnexin/calreticulin after being reglucosylated by UDP-glucose:glycoprotein glycosyltransferase. This gives the protein more time to attain a folded conformation. However, if the protein fails to fold soon enough, the stochastic removal of mannose residues by ER mannosidases triggers the selection of the misfolded protein for degradation by the ERAD pathway. A particularly important player in these events is the ER degradation enhancing α-mannosidase-like protein I, EDEM1. EDEM1 has been proposed to interact with a specific glycan conformation produced by glycan trimming during repeated folding cycles, and/or functions as a “timer” for degradation by trimming the mannoses on the glycoprotein so that the substrate is committed for degradation (84, 87, 357, 395). However, recent studies have challenged this view, by demonstrating that EDEM1 can interact with misfolded substrates regardless of their glycosyation state (87, 169). A reconciling of these two contrasting views is provided by the observation that under low expression of EDEM1, the ERAD of a misfolded version of the asialoglycoprotein receptor (H2a) requires glycan trimming, whereas under high expression of EDEM1, mannose trimming is not required. Furthermore, overexpression of EDEM1 stimulates the ERAD of a nonglycosylated version of H2a, suggesting a dual role for EDEM1 that is perhaps regulated by cellular EDEM1 levels (411).
In addition to diseases that arise directly from defects in N-linked glycosylation, the importance of glycosylation in human health and disease is underscored by the fact that cellular stress results from defects in protein glycosylation. Experimentally, this is best evidenced by the fact that tunicamycin, a fungal metabolite, prevents N-glycosylation by inhibiting early steps in the assembly of the glycan chain (390). Treatment with tunicamycin results in a build-up of misfolded glycoproteins within the ER and the induction of the unfolded protein response (UPR). Diseases as diverse as diabetes, neurodegeneration, heart and kidney disease, and cancer have been linked to UPR induction (93, 207, 327, 478, 585). In at least some cases, disease progression arises from the fact that prolonged UPR activation triggers apoptosis (301, 323, 417, 581).
D. Strategies to Deal With Misfolded Proteins: The UPR
As discussed in the preceding sections, many problems can occur during protein targeting to the ER and subsequent folding events. Protein misfolding is detrimental as it results in a loss of native protein function and can lead to a toxic gain of function due to aggregation. Misfolded proteins in the ER also induce the UPR. Fortunately, cells evolved two main strategies to clear unwanted proteins from the secretory pathway: ERAD, which is the main focus of this review, and autophagy, which is beyond the scope of this article, but is discussed briefly in subsequent sections. If these degradative processes fail to effectively remove misfolded and aberrant proteins from the ER, then UPR induction becomes critical to maintain cellular homeostasis.
The UPR is a conserved cellular stress response that triggers the activation of three signal transduction pathways. The net effect of UPR activation is general translational repression coupled with an expansion of the ER’s folding and degradative capacity. First hints of the UPR’s existence arose from the observation that various stress conditions, including glucose starvation, inhibition of glycosylation, and treatment of mammalian cells with calcium ionophores, increased the expression of two ER-localized chaperones, BiP and Grp94 (270). Data from this study indicated that the presence of unfolded proteins in the ER was the primary trigger for the response. The identification of a stress response element in the promoter of the yeast BiP homolog (262, 339) and subsequent genetic approaches led to the identification of a conserved, ER-localized kinase, inositol-requiring protein 1 (Ire1; Refs. 88, 338). During ER stress, Ire1 activation initiates the cleavage of an intron from the message encoding a transcription factor, Hac1 (in yeast) or XBP1 (in mammals), which removes a translational inhibitory region. The protein product activates the transcription of genes that encode chaperones and enzymes required for ER protein folding, factors required for ERAD, lipid biosynthetic enzymes to enlarge the ER, and components that augment ER-to-Golgi protein trafficking (302, 358, 495). Subsequent work indicated that the mammalian UPR included the actions of two additional ER components, PERK and ATF6, which respectively activate down-stream responses that inhibit protein synthesis and activate stress-responsive and proapoptotic genes.
Two models have been proposed to describe how unfolded proteins activate the Ire1 branch of the UPR. First, Ire1 interacts with BiP, an ER luminal Hsp70 that participates in both protein folding and degradation processes (44). The Ire1-BiP interaction may act as a sensor of ER protein folding status. Accumulation of misfolded proteins would titrate BiP away from Ire1, thereby allowing for Ire1 dimerization, phosphorylation, and activation of its ribonuclease domain and splicing activity. In support of this model, it was demonstrated that diffusion of BiP within the ER lumen inversely correlates with the levels of misfolded proteins in the ER (278). In the second model, Ire1 directly binds to misfolded proteins via a peptide-binding pocket. These data, based on a crystal structure of yeast Ire1 and by the fact that mutations in the peptide-binding pocket compromise UPR induction, suggest that Ire1 and the UPR are activated directly by misfolded proteins (91). However, it is less obvious whether the mammalian Ire1 homolog also accommodates unfolded polypeptides (588). In the end, it is likely that UPR activation proceeds via some combination of the two models (248), and indeed, there is evidence that BiP modulates UPR efficiency, Ire1 localization, and signal duration (382).
E. Strategies to Deal With Misfolded Proteins: ERAD
Several of the UPR targets are genes encoding proteins that facilitate ERAD, which in principle provides the most rapid and direct means to clear the ER of potentially toxic proteins. In general, ERAD can be broken down into four steps: substrate recognition, ubiquitination, retrotranslocation to the cytosol, and proteasome-mediated degradation. In addition to clearing misfolded proteins from the ER due to cell stress and degrading proteins that are mutated and misfolded, the ERAD pathway is also used to regulate the levels of specific enzymes and lipid carriers. These include 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, which catalyzes the rate-determining step in cholesterol biosynthesis, and apolipoprotein B, which is required for the assembly of cholesterol-containing liposomes (131, 183). Defects in the metabolically-regulated destruction of these ER residents lead to a variety of diseases linked to lipid homeostasis and atherosclerosis. For all of these reasons, ERAD is vital for human health, and increasing evidence links this pathway to many diseases (TABLE 1). But, before embarking on a discussion of select ERAD-associated diseases, we will begin with a discussion of some of the key findings that have defined the ERAD pathway since its discovery. We will also provide an overview of the steps and describe the key players in the pathway that are highlighted in the remaining sections of this review.
Table 1.
Disease-related proteins degraded by ERAD or linked to ERAD
| Chromosomal Locus |
Gene | Protein | Disease | Reference Nos. | Class |
|---|---|---|---|---|---|
| Cardiovascular | |||||
| 2p24–23 | APOB | Apolipoprotein B (Apo B) | A-beta-lipoproteinemia | (62, 297, 298, 568) | S |
| Xq22 | GLA | Alpha-galactosidase A (alpha-Gal A) | Fabry disease | (179, 559) | S |
| 7q36.1 | KCNH2 | Human ether-a-go-go-related gene (HERG) voltage-gated potassium channel | Congenital long QT syndrome | (146, 163, 519, 589, 590) | S |
| 19p13.3 | LDLR | Low-density lipoprotein receptor (LDLR) | Familial hypercholesterolemia | (228, 286, 295) | S |
| 2q13–14 | PROC | Protein C | Protein C deficiency | (240, 241, 352, 494) | S |
| 3q11.2 | PROS1 | Protein S | Protein S deficiency | (502) | S |
| 1q23–25.1 | SERPINC1 | Antithrombin | Type I antithrombin deficiency | (492, 493) | S |
| 17p13 | SERPINF2 | Alpha-2-antiplasmin (A2AP) | Alpha 2-plasmin inhibitor deficiency | (81, 352) | S |
| 12p13.3 | VWF | von Willebrand factor (vWF) | von Willebrand’s disease type IIA | (17, 18, 48, 316) | S |
| Digestive | |||||
| 10q24 | ABCC2 | Multidrug resistance protein 2 (MRP2) | Dubin-Johnson syndrome | (187, 243) | S |
| 2q24 | ABCB11 | Bile salt export pump (BSEP) | Progressive familial intrahepatic cholestasis type II (PFIC II) | (192, 335, 524) | S |
| 13q14.3 | ATP7B | Copper transporting P-type ATPase | Wilson disease | (95, 185, 214, 506) | S |
| 18q21–22 | ATP8B1 | FIC1 aminophospholipid-transporting ATPase | Progressive familial intrahepatic cholestasis type II (PFIC I) | (134, 374, 506) | S |
| 4q28 | FGB | Fibrinogen | Hereditary hypofibrinogenemia | (53, 277, 555, 556) | S |
| 6p21.3 | HFE | Hemochromatosis | Hereditary hemochromatosis | (63, 517) | S |
| 10q11.2 | RET | RET tyrosine kinase | Hirschprung disease | (253, 254) | S |
| Endocrine | |||||
| 11p15.1 | ABCC8 | Sulfonylurea receptor 1 (SUR1) | Persistent hyperinsulinemic hypoglycemia of infancy (PHHI) | (481, 561, 562) | S |
| 3q13 | CASR | Calcium-sensing receptor (CasR) | Familial hypocalciuric hypercalcemia (FHH) in heterozygotes | (209, 210, 380) | S |
| 3q13 | CASR | Calcium-sensing receptor (CasR) | Neonatal severe hyperparathyroidism (NSHPT) in homozygotes | (209, 210, 380) | S |
| 11p15.5 | INS | Proinsulin | Neonatal diabetes (type 1) | (16, 186, 371) | S |
| 19p13.3–13.2 | INSR | Insulin receptor | Insulin resistance syndrome (type 2 diabetes) | (12, 219, 233, 399) | S |
| 3p22–21.1 | PTH1R | Parathyroid hormone receptor (PTHR) | Parathyroid hormone resistance | (19) | S |
| Immune | |||||
| 19p13.3 | Elane | Neutrophil elastase | Severe congenital neutropenia (SCN) | (347) | S |
| 17q23.1 | MPO | Myeloperoxidase (MPO) | Hereditary myeloperoxidase deficiency | (99) | S |
| Integumentary | |||||
| 4q21.21 | ANTXR2 | Anthrax toxin receptor 2 | Hyaline fibromatosis syndrome | (101) | S |
| 6p21.3 | HLA-A | Major histocompatibility complex I heavy chain (MHC1), viral E3 MIR1 | Kaposi’s sarcoma | (67) | C |
| 11q14–21 | TYR | Tyrosinase (TYR) | Oculocutaneous albinism | (27, 178, 475, 537) | S |
| Multisystemic | |||||
| 16p13.1 | ABCC1 | P-glycoprotein | Implications for cancer treatment | (312) | S |
| 4q22 | ABCG2 | Breast cancer resistance protein | Implications for cancer treatment | (145, 343, 344, 474) | S |
| 20p13 | AVP | Vasopressin precursor protein | Autosomal dominant neurohypophysial diabetes insipidus (ADNDI) | (140, 225, 349) | S |
| 2p12 | EIF2AK3 | Eukaryotic translation initiation factor 2-alpha kinase 3 (PERK) | Walcott-Rallison syndrome (WRS) | (172) | S/D |
| 15q21.1 | FBN1 | Fibrillin 1 | Marfan syndrome | (73, 543) | S |
| 6p21.3 | HLA-A | MHC1, viral proteins US2 and US11 | Cytomegalovirus (CMV) infection | (232, 300, 468, 544, 545) | C |
| 12q13.13 | RNF41 | Neuregulin receptor degradation protein 1 (Nrdp1) | Breast and other cancers | (141) | M |
| 14q32.1 | SERPINA1 | Alpha-1-antitrypsin (AT) | Alpha-1-antitrypsin deficiency | (394, 485) | S |
| 9p13.3 | VCP | Cdc48/p97/valosin-containing protein (VCP) | Inclusion body myopathy associated with Paget’s disease of bone (IBMFPD) | (72, 496, 539) | M |
| Musculoskeletal | |||||
| 17q21.33 | COL1A1 | Type I procollagen pro-alpha 1 chain | Osteogenesis imperfecta | (132, 221, 279) | S |
| 2p13.3 | DYSF | Dysferlin | Limb girdle muscular dystrophy type 2B/Miyoshi myopathy (LBMD2B/MM) | (78, 143) | S |
| 7q21 | SGCA | Sarcoglycan alpha subunit | Limb girdle muscular dystrophy type 2D(LBMD2D) | (143, 153) | S |
| 7q21.3 | SGCE | Sarcoglycan epsilon subunit | Myoclonus-dystonia syndrome (MDS) | (121) | S |
| 11q13.5 | SERPINH1 | Heat shock protein 47 (Hsp47) | Osteogenesis imperfecta | (78) | S |
| Nervous | |||||
| 1p36 | ATP13A2 | ATPase type 13A2 cation transporter | Kufor-Rakeb syndrome (KRS)/Parkinsons disease 9 | (504) | S |
| 14q21 | ATXN3 | Ataxin 3 | Machado-Joseph disease/spinocerebellar ataxia type 3 | (527, 570, 586) | S |
| 11q13 | BSCL2 | Seipin | Silver’s syndrome/distal hereditary motor neuropathy type V | (224) | S |
| 5q34 | GABRA1 | Gamma aminobutyric acid receptor (GABAR) alpha 1 | Juvenile myoclonic epilepsy | (52, 149) | S |
| 5q34 | GABRA1 | Gamma aminobutyric acid receptor (GABAR) alpha 1 | Childhood absence epilepsy (CAE) | (238) | S |
| 5q34 | GABRG2 | Gamma aminobutyric acid receptor (GABAR) gamma 2 | Generalized epilepsy with febrile seizures plus (GEFS+) | (237) | S |
| 1q21 | GBA | Glucocerebrosidase (GC) | Gaucher disease | (35, 341, 412) | S |
| Xq13.1 | GJB1 | Connexin 32 (Cx32) | X-linked Charcot-Marie-Tooth disease (CMTX) | (258, 420, 510) | S |
| 15q24.1 | HEXA | Beta-hexosaminidase alpha subunit | Tay-Sachs | (281, 318, 341) | S |
| 4p16.3 | HTT | Huntingtin | Huntington’s disease | (34, 114, 563) | D |
| 18q11–12 | NPC1 | Niemann-Pick type C 1 (NPC1), sterol transporting protein | Niemann-Pick type C disease | (155, 525) | S |
| 6q25.2–27 | PARK2 | Parkin | Autosomal recessive juvenile Parkinsonism (AR-JP) | (361, 368, 413) | M |
| Xq22 | PLP1 | Proteolipid protein (PLP) DM20 (splice isoform) | Pelizaeus-Merzbacher disease (PMD)/spastic paraplegia type 2 (SPG-2) | (166, 271) | S |
| 17p12 | PMP22 | Peripheral myelin protein 22 (PMP22) | Charcot-Marie-Tooth disease type 1A | (138, 139, 418) | S |
| 3q21–24 | RHO | Rhodopsin | Retinitis pigmentosa | (167, 168, 216, 266) | S |
| 3q26.1 | SERPINI1 | Neuroserpin | Familial encephalopathy with neuroserpin inclusion bodies (FENIB) | (273, 569) | S |
| 9q34 | TOR1A | Torsin A | Early-onset torsion dystonia | (160, 348) | S |
| Respiratory | |||||
| 7q31.2 | CFTR | Cystic fibrosis transmembrane conductance regulator (CFTR) | Cystic fibrosis (CF) | (229, 536) | S |
| 8q21 | SFTPC | Surfactant protein C (SP-C) | Interstitial lung disease | (108, 526) | S |
| Urinary | |||||
| 12q12–13 | AQP2 | Aquaporin 2 | Nephrogenic diabetes insipidus (NDI) | (202, 480) | S |
| Xq28 | AVPR2 | V2 vasopressin receptor 2 (V2R) | Nephrogenic diabetes insipidus (NDI) | (441) | S |
| 4q21.1 | PKD2 | Polycystin 2 | Autosomal dominant polycystic kidney disease type 2 (ADPKD) | (150, 296) | S |
| 17q21–22 | SLC4A1 | Kidney chloride/bicarbonate anion exchanger (kAE1) | Autosomal recessive renal tubular acidosis | (252) | S |
| Toxins | |||||
| Cholera toxin A1 subunit (Vibrio cholerae) | Cholera | (410, 488, 499) | C | ||
| Exotoxin A (Pseudomonas aeruginosa) | Respiratory/urinary tract infections | (13, 263) | C | ||
| Pertussis toxin (Bordetella pertussis) | Whooping cough | (552) | C | ||
| Ricin A chain (Ricinus communis) | Ricin poisoning | (294, 453, 460) | C | ||
| Shiga toxin (SHT) (Shigella dysenteriae) | Shigellosis | (575) | C | ||
| Shiga-like toxins (SLT-I and -II) (Escherichia coli) | Hemolytic uremic syndrome (HUS) | (280) | C | ||
The proteins listed in the table are either ERAD substrates (S), proteins that are part of the ERAD machinery (M), mutant proteins that result in a defect in ERAD (D), or proteins that coopt ERAD to subvert host immunity and cause disease (C). Inclusion in the table requires a clear demonstration of the involvement of ERAD. For ERAD substrates (S), a mutant form of the protein must have been shown to be degraded in a proteasome-dependent manner coupled with either ER retention and/or ubiquitination. Common abbreviations for each protein and disease are indicated in parentheses. In some cases, a single mutant protein is known to result in multiple forms of a disease, and this is denoted by a forward slash separating the two pathologies in the disease column.
Early studies on the assembly of the T-cell receptor (TCR) were the first to hint at the existence of what is now known as ERAD. Klausner and colleagues (303) discovered that unassembled alpha, beta, and delta subunits of the heptameric TCR were degraded in a nonlysosomal compartment. It was hypothesized that degradation either was occurring by an ER-resident protease or in another prelysosomal compartment. Further evidence for pre-Golgi degradation came from studies examining other mammalian proteins, including a subunit of the asialoglycoprotein receptor, HMG-CoA reductase, unassembled immunoglobulin light chains, and cytochrome P-450 (151, 333, 546). In addition to these reports using mammalian cell systems, parallel work in the budding yeast Saccharomyces cerevisiae also hinted at the existence of a novel, lysosome-independent degradation pathway. For example, translocation defects elicited by a temperature-sensitive mutant of Sec61, a translocon component, were found to be rescued by deletion of the gene encoding Ubc6; Ubc6 is a ubiquitin-conjugating enzyme that is integrated into the yeast ER membrane (461). These data suggested a role for the cytoplasmic proteasome in the turnover of a misfolded ER membrane protein, i.e., the Sec61 mutant. Soon after, the proteasome-dependent degradation of immature wild-type and mutant forms of the cystic fibrosis transmembrane conductance regulator (CFTR) was reported in mammalian cells (also see sect. IIA). The requirement for the delivery of an aberrant soluble protein in the ER to the cytoplasm emerged from studies on misfolded yeast secretory proteins, pro-alpha factor, and CPY*. Using both in vitro and genetic tools, these proteins were found to be retrotranslocated from the ER and destroyed by the proteasome (201, 330, 540). The retrotranslocation and degradation of soluble proteins from the mammalian ER was also observed (394). Ultimately, it quickly became clear that ERAD and most of the core requirements that underlie the mechanism of this pathway are conserved from yeast to humans.
ERAD begins with the recognition of a misfolded protein by molecular chaperones (FIG. 2). ERAD substrates can present a misfolded lesion either in the ER lumen, ER membrane, or cytoplasm, which will influence the types of chaperones with which they interact (55). BiP, which was introduced above, is an ER luminal Hsp70 that was discovered by virtue of its stable binding to immunoglobulin (Ig) heavy chains (174). As with other Hsp70s (see above), the binding of BiP to newly synthesized proteins occurs through exposed hydrophobic surfaces on the client protein, an event that obscures these aggregation-prone surfaces as the protein attempts to attain its natively folded state. However, if folding is delayed or if the protein is terminally misfolded, extended chaperone interaction may serve one of two functions: 1) the prolonged prevention of aggregation and maintenance of an unfolded substrate and/or 2) a more elaborate process in which the misfolded protein is shuttled to other chaperones and/or to the ERAD machinery. Evidence for the second function came from a study demonstrating that BiP and Grp94 bound to Ig light chains (Ig λ). BiP associated with both reduced and oxidized forms of Ig λ, whereas Grp94 bound primarily to the oxidized form, suggesting that the chaperones interact with Ig λ in a sequential fashion (334). BiP also bound more tightly to nonsecreted forms of Ig λ (259). Evidence for the first function came from a study examining the degradation of soluble yeast substrates. In yeast that have a temperature-sensitive allele in the gene encoding BiP, the substrates aggregated at high temperatures; the same phenomenon was observed when the Hsp40 cochaperone partners were mutated (351). Together, these studies highlight how chaperones work together during different stages of protein folding/degradation.
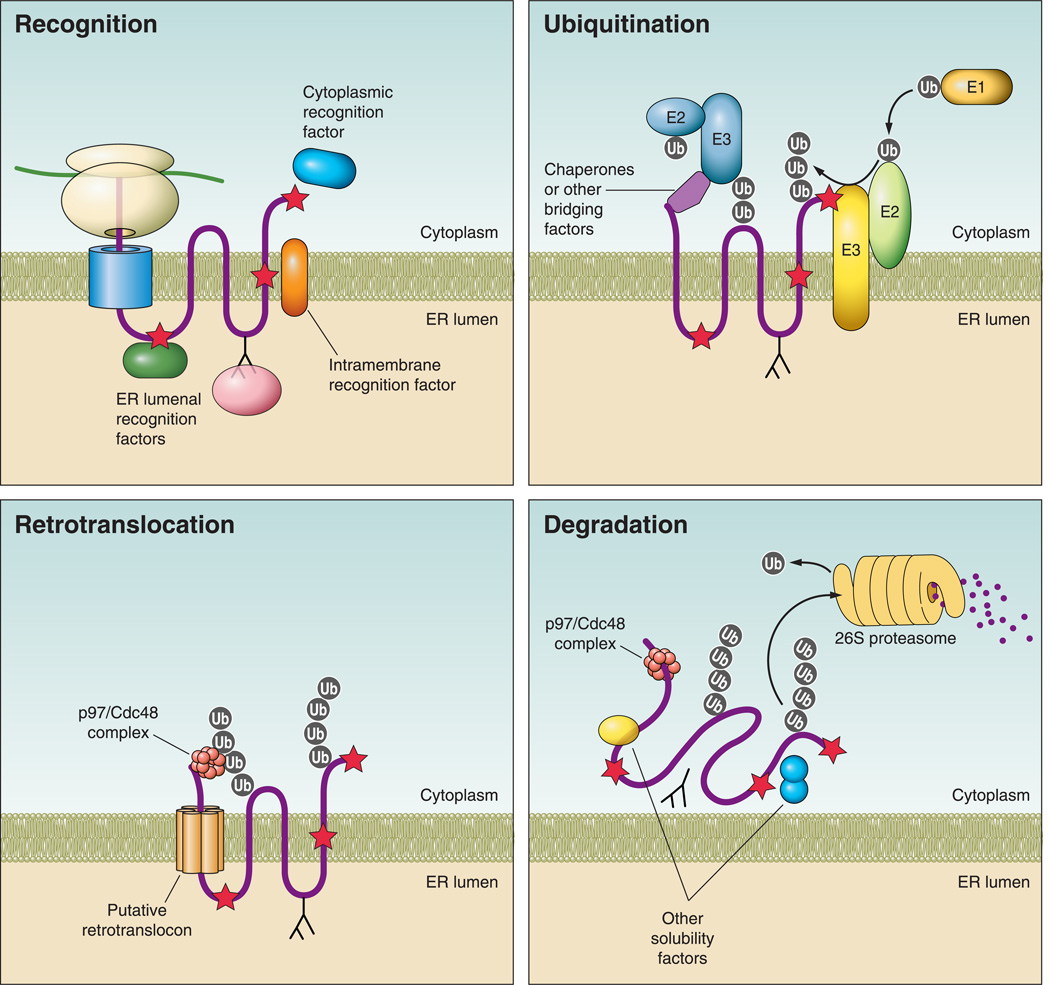
FIGURE 2. Steps in endoplasmic reticulum-associated degradation (ERAD).
Recognition: during protein synthesis and translocation, a misfolded region (red star) may reside in a protein’s cytoplasmic, ER luminal, or transmembrane domains. Recognition is mediated by ER luminal or cytoplasmic chaperones, as depicted, depending on the location of the folding lesion. For glycoproteins, lectins (pink) interact with N-glycans and in some cases they monitor the folding status of the protein. Ubiquitination: following recognition, the ubiquitination machinery is recruited to the misfolded substrate, either directly within the membrane or by interactions with cytoplasmic chaperones. A ubiquitin activating enzyme (E1) transfers ubiquitin (gray circle) to an active site cysteine in a ubiquitin conjugating enzyme (E2) in an ATP-dependent process. The ubiquitin is then transferred most commonly to a lysine residue on a client protein via a ubiquitin ligase (E3). Ubiquitination at the ER membrane can occur via cytoplasmic or ER-localized E3 ligases, both of which are shown. Retrotranslocation: for polytopic membrane proteins (pictured), retrotranslocation may occur by removal of the protein through a channel (retrotranslocon) and/or by removal of the protein and the surrounding membrane (not pictured). In either case, retrotranslocation almost always depends on the p97/Cdc48 complex, which includes Ufd1 and Npl4 and interacts with ubiquitin and misfolded regions on a substrate. p97/Cdc48 provides the mechanical force via ATP hydrolysis for substrate removal. Degradation: following retrotranslocation, misfolded proteins are ushered to the 26S proteasome and must be kept soluble to prevent aggregation. N-glycans are clipped by N-glycanse (not pictured), and ubiquitin moieties are removed by deubiquitinating enzymes either in the cytosol or in the proteasome cap. The proteasome contains three peptidase activities, trypsin-like, chymotrypsin-like, and caspase-like, which cleave proteins into short peptide fragments.
N-glycans, which bind to chaperone-like lectins in the ER, play an essential role in protein folding and in ERAD. The funneling of glucose-trimmed glycoproteins into the calnexin-calreticulin folding cycle, which is governed by OGT, is in competition with exit from the cycle and degradation. As discussed above, exit from the cycle is triggered by the action of ER mannosidases. In mammalian cells, ERManI and EDEM1 and their isozymes trim the mannose residues on specific branches of the glycan structure, preventing reglucosylation while simultaneously promoting substrate retrotranslocation and degradation (204). As expected, then, modulation of ER mannosidase levels has a direct effect on the efficiency of degrading specific substrates, such as a mutant form of the protein that causes anti-trypsin deficiency (205, 325, 357; see sect. IIE). Interestingly, the yeast homolog of EDEM1 is in a complex with PDI, and a similar interaction has been observed in mammalian cells between EDEM1 and the ER oxidoreductase ERdj5 (84, 154, 176, 421). These observations may indicate that substrate selection is linked to the acquisition of a protein’s proper disulfide bonds. In accordance with this view, a PDI homolog also associates with calnexin (386).
Protein ubiquitination is critical to target most substrates to the proteasome (129). The ubiquitin chain is usually attached onto a Lys residue on the misfolded protein, although recent data indicate that some ERAD substrates contain ubiquitin chains on Ser or Thr residues (223, 452, 530). Protein ubiquitination occurs via a three-step process that is mediated by a ubiquitin-activating enzyme (E1), a ubiquitin-conjugating enzyme (E2), and a ubiquitin ligase (E3), which catalyzes the final transfer of ubiquitin to the substrate. It is thought that the E3 ligase provides substrate specificity, and accordingly, E3s are the most abundant of the three classes, with over 500 putative ligases identified in mammalian cells (500). Key E3s involved in ERAD are Hrd1, gp78, RMA1, TEB4, and CHIP (29, 103, 123, 188, 236, 245, 272, 326, 574). On the basis of the chemical and structural diversity of ERAD substrates, it is likely that additional E3s that contribute to this pathway will emerge.
After initial E3-mediated ubiquitin attachment, ubiquitin chain extension (“polyubiquitination”) occurs by the covalent modification of additional ubiquitin monomers onto a Lys residue in a previously linked ubiquitin. This forms an extended isopeptide-linked polyubiquitin chain. In some cases, the cooperative extension of a polyubiquitin chain is mediated by another group of enzymes, the E4s, which augment ERAD efficiency (261, 345, 405).
A chain of four ubiquitins must be appended onto a substrate for efficient proteasome interaction (396, 491), and polyubiquitin chains are distinguished based on the residue upon which the chain is built. In other words, the type of linkage can determine the fate of the ubiquitinated substrate. Initially, a polyubiquitin chain built upon Lys-48 isopeptide linkages was assumed to be specific for proteasomal degradation, whereas a Lys-63 polyubiquitin chain served as a nonproteasomal signal for endocytosis and DNA repair (83). However, proteomic studies revealed that several other ubiquitin linkages (Lys-6, -11, -27, -29, and -33) exist in cells and that both Lys-48- and -11-linked chains are appended onto ERAD substrates (557). Future research will continue to unravel the functions of different linkages and mixed linkages that exist. Nevertheless, it is clear that proteins with extended polyubiquitin chains can be found in intracellular inclusions in neurodegenerative diseases, suggesting that inefficient proteasome degradation is linked to the disease phenotype (see Section IIB).
After or during polyubiquitination, the ERAD substrate must be delivered, or retrotranslocated to the proteasome. While the existence or identity of a retrotranslocation channel in the ER membrane remains contested, several putative channels have been considered. These include Sec61, which is the translocation channel, the Derlins, which are a family of polytopic membrane proteins intimately linked to some ERAD substrates and components of the ERAD machinery, and Hrd1, which is a polytopic E3 ubiquitin ligase. The use of Sec61 as a retrotranslocation channel initially seemed most logical. In yeast it was found that Sec61 mutants prevented the ERAD of some substrates (381, 385) and that an ERAD substrate formed disulfide bonds with Cys residues in Sec61 en route to its degradation (443). This result suggested that the protein was unfolded and retrotranslocating through Sec61. Another ERAD substrate, apolipoprotein B, clearly retrotranslocates through the Sec61 channel when it is cotranslationally targeted for ERAD (370). Cholera toxin also appears to utilize Sec61 to leave the mammalian ER during its journey from the plasma membrane, back through the secretory pathway, and ultimately into the cytoplasm (433; also see sect. IID). However, another yeast ERAD substrate depended on the functions of both Sec61 and a yeast Derlin homolog (548). The contribution of Derlin is consistent with findings that antibodies against this protein thwart the retrotranslocation of a model substrate from mammalian ER vesicles (518) and that a dominant negative form of Derlin disrupts the viral-induced retrotranslocation and degradation of MHC class I molecules (see sect. IIF). Moreover, Derlin recruits Cdc48/p97 (299, 567), which provides the energy required to drive ERAD substrates from the ER (see below). To add to an already complex situation, yeast Derlin interacts with the E3 ligase Hrd1, which has itself been implicated in the retrotranslocation of both ER luminal proteins and in the degradation of HMG-CoA reductase (70, 152). TEB4 and the yeast homolog Doa10 may act similarly (402, 477). Together, these studies highlight the enigmatic nature of the retro-translocation process and suggest that one of many proteins/complexes may provide the channel required for ERAD, or that the channel may be composed of several different proteins.
For most ERAD substrates, the mechanical force necessary to remove proteins from the ER lumen or membrane is provided by the cytoplasmic AAA+ ATPase p97 (also known as valosin-containing protein, VCP, or Cdc48 in yeast). p97/Cdc48 functions in several cellular processes, including cell division, membrane fusion, and the processing of membrane-associated transcription factors (26). In fact mutations in this protein give rise to the complex syndrome, inclusion body myopathy associated with Paget’s disease of bone and frontotemporal dementia (538). A role for p97/Cdc48 in proteasome-dependent degradation first emerged from a yeast genetic screen (158). Given its diverse activities, it makes sense that Cdc48 interacts with a wide range of partners that provide specificity for this ATPase’s energy-coupled functions (439). Thus, during ERAD, a complex containing p97/Cdc48, Npl4, and Ufd1 is required to extract soluble and integral membrane polyubiquitinated substrates from the ER for degradation (31, 227, 398, 566). p97/Cdc48 also interacts with E3 ligases, placing it in an ideal position to aid in the retrotranslocation of polyubiquitinated proteins (28, 587).
Following extraction, proteins must be ushered to the 26S proteasome, but with the exception of two cytosolic proteins in yeast (397), the identities of the factors that participate in this step are not clear. Membrane proteins pose a formidable challenge as some of these substrates can be retrotranslocated intact (152, 211, 287, 345, 545); therefore, the hydrophobic membrane-spanning domains are exposed to the aqueous environment of the cytoplasm and must be protected. A recent study suggests that a protein complex that participates in the recognition of tail-anchored proteins at the ribosome may help maintain membrane proteins in a soluble form en route to the proteasome; however, this complex does not seem to recognize membrane-spanning regions of its associated substrates (528), which are probably the regions most prone to aggregate.
ERAD substrate proteolysis occurs in the cytoplasm via the action of the 26S proteasome (129). The proteasome is a large (~2.5 MDa) protein complex that can be subdivided into two main assemblies: the 20S core particle and the 19S regulatory particle (also known as the 19S “cap” or PA700). The polyubiquitin tag on a substrate is recognized either by subunits in the proteasome cap and/or by proteasome-associated proteins (213, 436). After recognition, substrates must be deubiquitinated by deubiquitinating enzymes (DUBs). For example, the Rpn11 subunit in the 19S cap deubiquitinates proteasome substrates, which is a prerequisite for degradation and is important for maintaining high levels of free ubiquitin (328, 514, 565). DUBs have also been proposed to remove ubiquitin linkages from substrates as they are threaded through p97/Cdc48 (120) and a large-scale proteomic analysis identified a DUB, USP13, that associates with p97/Cdc48 and two p97/Cdc48 partners; USP13 knock-down stabilized an ERAD and a cytosolic ubiquitinated substrate, suggesting that this DUB regulates p97/Cdc48 function (465). In contrast, if DUBs interact with the substrate prior to proteasome association, they can trim ubiquitin chains and delay substrate degradation.
The 20S proteasome core contains three distinct proteolytic activities: trypsin-like, chymotrypsin-like, and caspase-like. The net result of proteasome-mediated degradation is the generation of small, ~2–30 amino acid peptides that can be further processed by other cellular proteases (251). Several proteasome inhibitors have been discovered, which target different activities of the proteasome and have proven useful in determining the contribution of the ubiquitin-proteasome system (UPS) (82, 513) in the regulation of cellular pathways, including ERAD. For example, acetyl-Leu-Leunorleucinal (ALLN) and Cbz-Leu-Leu-leucinal (MG-132) are reversible inhibitors of the chymotrypsin-like activity (147, 409) and laid the foundation for the discovery of proteasome inhibitors that are used clinically, i.e., Bortezomib (see sect. IIIA).
II. SPECIFIC EXAMPLES OF PATHOLOGIES LINKED TO ERAD
In this section, we focus on six examples of how ERAD is directly linked to human health and disease. We also provide a table (TABLE 1) that includes 67 diseases that have been connected to the ERAD pathway. Because the pace of research on newly discovered rare diseases is rapid, and because there are many disease-relevant proteins for which there are insufficient data to be classified as being ERAD-linked, the table is not exhaustive. Our goal is instead to illustrate the diverse nature of the diseases linked to this pathway. To be included in TABLE 1, there had to be a demonstration of a protein’s ER retention/impaired trafficking together with either proteasome-dependent degradation and/or polyubiquitination.
A. Cystic Fibrosis and Other Diseases of ATP-Binding Cassette Transporters
The first disease unambiguously linked to the ERAD pathway was cystic fibrosis (CF). CF is caused by defects in a chloride transporter, the CFTR. Mutations in CFTR alter salt and water balance across a variety of epithelia, and as a result, the main clinical features of CF are delayed growth due to digestive and nutrient-related disorders, severe constipation, and mucus build-up in the lungs, which results in recurring and prolonged infections. Over 1,000 disease-causing alleles have been reported in CFTR, and it is estimated that 1 in 29 Caucasian Americans carry a mutant CF allele (2, 4). Currently approved treatments are only supportive and focus on reducing the number and duration of lung infections and on providing diet supplementation to meet the patient’s nutritional needs. Unfortunately, the average life expectancy for a CF patient is ~35 years, a value that has increased significantly during the past three decades. Due to the devastating nature of this disease and its prevalence, a large research effort is devoted to uncovering the molecular mechanisms underlying CF. These efforts include studies on CFTR channel activity and regulation, CFTR folding in the early secretory pathway, and CFTR trafficking through the secretory pathway. Studies to identify modifiers of the disease phenotype have also been undertaken (see, for example, Ref. 30).
CFTR is a member of the ATP-binding cassette (ABC) family of transporters, which as their name implies couple ATP hydrolysis to solute transport. While CFTR is perhaps the most widely recognized member of the ABC family, other diseases are also attributed to mutated ABC family members (see TABLE 1). In general, ABC transporters are multipass integral membrane proteins that contain two membrane-spanning domains (MSD), each followed by a nucleotide-binding domain (NBD). However, CFTR is unique among ABC transporters in that it functions as an ion channel and possesses a regulatory (R) domain lying between NBD1 and the second MSD. CFTR channel opening is regulated in part by protein kinase A-mediated phosphorylation of the R domain and is tightly coupled to nucleotide binding to the NBDs (148, 250, 407).
Based on its size (1,480 amino acids) and complex domain organization, even the wild-type form of CFTR encounters a significant number of hurdles during synthesis. In fact, CFTR begins to fold cotranslationally, even though post-translational folding events must also take place before the protein achieves its native conformation (112, 113, 244, 257, 489, 490). To aid in folding, and to select misfolded species for ERAD, nascent CFTR engages both cytoplasmic and ER luminal chaperones (15, 309, 331, 472, 531, 564, 580). In addition, as portions of the protein enter the ER, nascent CFTR is N-glycosylated, producing an immature ER-localized form, termed “band B.” This species interacts with the lectin-like chaperones that reside in the ER, i.e., calnexin and calreticulin (124, 125, 162, 184, 360, 383, 414). After folding and upon ER exit, the core glycan structure is further elaborated in the Golgi apparatus, which decreases CFTR’s electrophoretic mobility on a polyacryl-amide gel and leads to the presence of “band C.” This species is most commonly designated as the mature form and can be found at the plasma membrane. Consistent with its many folding hurdles, as much as 45–80% of wild-type CFTR is degraded during or soon after synthesis (77, 229, 315, 535), although under some conditions and in some epithelial cells the wild-type protein may mature quite well (512).
The most common disease-causing mutant allele in CFTR is a deletion of a phenylalanine at position 508 (ΔF508). This mutation causes virtually all of the protein to misfold or prevents folding, which in either case leads to its degradation (77). However, the mutation only partially reduces channel function (94, 191). Computational and structural evidence suggest that the ΔF508 mutation disrupts the interaction between NBD1 and a cytoplasmic loop within MSD2 (446), and consistent with these data, earlier cross-linking experiments suggested that the ΔF508 mutation alters MSD packing (75). Interestingly, “rescued” forms of ΔF508-CFTR that reach the plasma membrane are also subject to protein quality control and are targeted for lysosomal degradation by some of the same chaperones and ubiquitin ligases that act during ERAD (see below; Ref. 359). Although ~90% of CF patients possess at least one ΔF508 allele, another disease-causing mutation in CFTR is G551D, which results in a channel gating defect rather than in a trafficking defect (111, 182).
Early studies revealed that ΔF508-CFTR was only present as band B (77). This result suggested that the protein had not been processed by Golgi-resident enzymes and that the disease was linked to an ER quality control phenomenon. Consistent with this hypothesis, Ward and Kopito (535) showed that both the immature wild-type form of CFTR and the ΔF508 mutant form of CFTR were degraded soon after translation, and thus before lysosomal delivery. Indeed, ΔF508-CFTR levels were unaffected by inhibitors of lysosomal proteases, and degradation was insensitive to treatment with the fungal toxin Brefeldin A (BFA) (315), which prevents ER to Golgi trafficking. Even though a role for the UPS in destroying secretory pathway proteins had not yet been observed, parallel studies by the Kopito and Riordan labs (229, 536) next demonstrated that the proteolysis of ER resident forms of CFTR required the activity of the cytoplasmic 26S proteasome. Together, these data established the first direct link between a disease caused by a mutated protein and ERAD.
As with most proteasome-targeted proteins, CFTR is polyubiquitinated once it is selected for degradation. By using a cell-free assay, it was found that ΔF508-CFTR polyubiquitination occurs cotranslationally while the nascent chains are associated with the ribosome (425). Moreover, polyubiquitinated ΔF508-CFTR can accumulate in an insoluble form when the activity of the proteasome is compromised (231). These data suggested that the amelioration of CF-linked maladies would require a more elaborate treatment than simply inhibiting ΔF508-CFTR degradation (see sect. IIIA). In fact, nonspecific “chemical chaperones,” osmolytes that improve the protein folding environment in the cell, partially repaired the ΔF508-CFTR folding defect such that band C and channel activity became evident (57, 208, 426, 583; also see sect. IIIA and TABLE 2). Incubating cells at low temperature similarly repaired the ΔF508-CFTR folding defect, consistent with ΔF508 exhibiting the features of a classical temperature-sensitive, folding-compromised mutant (100).
Table 2.
Select experimental compounds used to rescue disease-related ERAD substrates
| Protein | Compound | Reference Nos. |
|---|---|---|
| Alpha-1-antitrypsin (AT) | ||
| Carbamazepine (CBZ) | 200 | |
| 4-Phenylbutyric acid (PBA) | 64 | |
| Alpha-D-galactosidase (α-Gal A) | ||
| 1-Deoxy-galactonojirimycin (DGJ) | 36, 122, 179 | |
| Aquaporin 2 (APQ2) | ||
| Dimethyl sulfoxide (DMSO) | 479 | |
| Glycerol | 479 | |
| Trimethylamine-N-oxide (TMAO) | 479 | |
| Beta-hexosaminidase alpha subunit | ||
| Bisnaphthalimide | 497 | |
| Celastrol | 341 | |
| DMSO | 497 | |
| N-acetylglucosamine thiazoline | 318, 498 | |
| Nitro-indian-1-one | 497 | |
| Pyrrolo[3,4-d]pyridazin-1-one | 497 | |
| Pyrimethamine | 318 | |
| Cystic fibrosis transmembrane conductance regulator (CFTR) | ||
| Benzoquinolizinium compounds | 109, 471 | |
| Betaine | 583 | |
| Corrector-4a | 170, 171, 532 | |
| Curcumin | 38, 115, 529, 577 | |
| Glycerol | 57, 426 | |
| MG-132 | 229, 536 | |
| Myo-inositol | 208, 583 | |
| S-nitrosoglutathione | 208, 579 | |
| PBA | 415, 416, 454 | |
| Suberoylanilide hydroxaic acid (SAHA) | 215 | |
| Sorbitol | 208 | |
| Taurine | 208, 583 | |
| TMAO | 57, 130, 208 | |
| Glucocerebrosidase (GC) | ||
| Celastrol | 341 | |
| Dantrolene | 364, 521 | |
| Diltiazem | 364 | |
| N-(n-nonyl)deoxynojirimicin (DNJ) | 428, 429 | |
| MG-132 | 341, 521 | |
| Human ether-a-go-go-related gene (HERG) protein | ||
| Astemizole | 126, 127, 590 | |
| Cisapride | 127, 590 | |
| Methanesulfonanilide, E-4031 | 127, 590 | |
| P-glycoprotein (P-gp) | ||
| Capsaicin | 311 | |
| Corrector-4a | 533 | |
| Curcumin | 533 | |
| Cyclosporin | 310, 311, 533 | |
| Verapamil | 310, 311 | |
| Vinblastine | 310, 311 | |
| Rhodopsin | ||
| 11-cis−7-ring retinal | 354 | |
| Sulfonylurea receptor 1 (SUR1) | ||
| Diazoxide | 373 | |
| Transthyretin (TTR) | ||
| Benzoxazole, Tafimidis | 403 | |
| Diclofenac | 255 | |
| Flufenamic acid | 255 | |
| Resveratrol | 255 | |
| Tyrosinase | ||
| LLnL (proteasome inhibitor) | 177 | |
| V2 vasopressin receptor (V2R) | ||
| SR121463A (V2R antagonist) | 337 |
Several different classifications of small molecules have been used to rescue ERAD substrates, including pharmacological and chemical chaperones, and proteostatic regulators, such as heat-shock and stress-inducing agents, transcriptional regulators, ion channel inhibitors, and proteasome inhibitors. However, in some cases precise placement into these groups is difficult as the exact mechanism of action is unknown and/or the compound may have multiple effects (see text for additional details). The references included in the table are those that demonstrate a connection between the protein and ERAD and are not necessarily the first studies to describe the molecular basis of a particular disease.
Since then, much work has been devoted to characterizing the ubiquitination machinery that mediates ΔF508-CFTR quality control. Establishing a specific E3 ubiquitin ligase for ΔF508-CFTR was not trivial, as mammals encode up to 500 of these enzymes (see above), often with overlapping specificities and partially redundant functions (129). To date, three E3 ligases are the best candidates as being implicated directly in the ERAD of CFTR. These include the cytoplasmic protein CHIP and the integral ER membrane proteins RMA1 and gp78 (170, 332, 340). Since folding defects may occur at any one of several points during CFTR synthesis, it is not surprising that more than one E3 facilitates the ubiquitination of CFTR. However, it is unclear how these E3s coordinately target CFTR for degradation. One possibility is that different E3s work in a sequential manner to ubiquitinate CFTR, e.g., co- and posttranslationally. Evidence for this scenario comes from studies in which the overexpression of either RMA1 or CHIP decreased the levels of wild-type CFTR; however, RMA1 and CHIP showed different preferences for mutant forms of CFTR. Namely, CFTR containing mutations within NBD1 are more sensitive to RMA1, whereas CFTR with an NBD2 truncation is more sensitive to CHIP expression (574). These data suggest that RMA1 acts on CFTR cotranslationally, recognizing early folding defects with the assistance of select chaperones (see below), whereas CHIP and Hsp70 (332) recognize folding defects as CFTR translation is completed.
During protein quality control, chaperone and E3 function are tightly intertwined (329, 345; and see below). Therefore, the targeting of chaperones that are associated with E3 ligases may either facilitate the degradation of the misfolded protein, as suggested above, or prevent the premature degradation of CFTR. Thus chaperone modulation may provide a means to fold ΔF508-CFTR so that it can escape ERAD and ultimately function, albeit less efficiently, at the plasma membrane. In fact, an effective scheme to rescue ΔF508-CFTR was recently achieved by altering the activity of an Hsp40 cochaperone, which functions at the same step as RMA1. The chaperone, DNAJB12, is ER-associated, and decreasing DNAJB12 levels by siRNA increases CFTR folding efficiency (170, 560). When DNAJB12 was silenced in combination with a small molecule corrector, Corrector 4a (Corr-4a), which partially rescues ΔF508-CFTR maturation (375; also see sect. IIIA), a strong synergistic effect on CFTR maturation was evident (170). These data emphasize the importance of targeting both the degradation and folding machineries to achieve maximal, therapeutic effects.
Molecular chaperones act as ERAD gatekeepers, closely monitoring protein folding and regulating the decision between folding and degradation (59, 350). It remains mysterious how the same chaperone can engage in protein folding and degradation processes. One possibility is that the amount of time a client protein interacts with the chaperone is critical for this decision. In this model, as a protein folds, exposed chaperone-binding sites can go through several cycles of binding and release. But, as a protein attains its final conformation, these sites are obscured and the protein is released from the ER and traffics to the Golgi. If, however, binding sites remain accessible to chaperones for extended periods of time, then the bound chaperone may recruit the degradation machinery. Notably, chaperones have been suggested to act as bridging factors between misfolded proteins and E3 ligases (329, 345), and in support of this model, Hsp70 and Hsp40s regulate the degradation of ΔF508-CFTR together with the E3 ligases CHIP andRMA1 (also see above; Refs. 170, 332). In further support of this model, cytosolic Hsp70 interacts with ΔF508-CFTR longer than wild-type CFTR (564), and an enhanced interaction between ΔF508-CFTR and an Hsp70-Hsp40 chaperone pair was observed (331).
It is also mysterious how a distinct chaperone may exhibit a “profolding” versus “prodegradative” effect on different substrates. Studies in yeast show that Hsp70 acts in a prodegradative manner during CFTR quality control (584), but the cytoplasmic Hsp90 plays an important role in maintaining the solubility of NBD1 and facilitating CFTR folding (571). Specifically, perturbation of Hsp90 function, either by genetic ablation in yeast or chemical inhibition in mammalian cells, accelerates the degradation of CFTR or both CFTR and ΔF508-CFTR, respectively (309, 571). For other substrates, Hsp90 promotes ERAD (173). In mammals, the ATPase activity of Hsp90 is intrinsically low, but the cochaperone Aha1 binds to and stimulates Hsp90 ATPase activity (369). Since Hsp90 promotes CFTR folding, one might predict that increased levels of Aha1 might further promote CFTR maturation. Unexpectedly, an increase in Aha1 accelerated CFTR degradation, and decreasing Aha1 levels rescued ΔF508-CFTR folding/maturation (268, 531). One explanation for this result is that a slow rate of Hsp90 cycling benefits CFTR maturation, but by accelerating this cycle, CFTR maturation is compromised because the protein is delivered too soon to the degradation machinery. Ultimately, it is likely that additional chaperones and cochaperones, which might themselves become therapeutic targets to treat CF, will be discovered that augment ΔF508-CFTR maturation.
In addition to CFTR, other ABC transporters have been identified as substrates for ERAD, including the multidrug resistance proteins, P-glycoprotein, and the breast cancer resistance protein (BCRP) (see TABLE 1). P-glycoprotein and BCRP are members of the MDR/TAP and White subfamilies of ABC transporters, respectively (96). A key function of the multidrug resistance proteins is ATP-dependent export of xenobiotics, such as those administered as chemotherapeutics. Drug resistance is a major clinical problem, impacting the effective treatment of many diseases, especially cancer. In fact, sequencing studies have found that BCRP variants can have dramatic effects on protein levels and methotrexate transport, a measure of BCRP activity (222). This finding suggests that the variants affect either protein biosynthesis or trafficking. In particular, cells containing one of two alleles, F208S and S441N, produce comparable amounts of mRNA compared with those expressing wild-type BCRP, but have reduced protein levels. It was also shown that the mutant proteins produced by these variants remain sensitive to endoglycosidase H (endo H). In higher eukaryotic cells, as N-glycosylated proteins transit through the Golgi, their glycans are processed by Golgi α-mannosidase II, which renders glycoproteins insensitive to endo H. Therefore, prolonged sensitivity to endo H is indicative of ER retention. Furthermore, both the F208S and S441N mutants are ubiquitinated, and protein levels increase when cells are incubated with MG-132, a proteasome inhibitor that was noted above (343). These data strongly suggest that the BCRP variants are retained in the ER and targeted for ERAD. While no diseases are directly caused by mutant forms of these transporters, correlations between polymorphisms and patient responses to chemotherapeutics have been documented (222). Therefore, the importance of understanding the mechanisms that regulate the expression of multidrug transporters should not be underestimated. This knowledge may help predict how a patient will respond to chemotherapeutics. In addition, new strategies to combat drug-resistant tumors may be developed.
B. Neurodegenerative Disorders
Several disorders result from the accumulation of ERAD substrates in neuronal cells. Neuronal cells have a relatively low regenerative capacity and an age-dependent decrease in their ability to withstand cellular stress, rendering them particularly sensitive to toxic aggregates that result in apoptosis (516). In general, neurodegenerative diseases cause mental impairment and movement disorders, such as those seen in Alzheimer’s disease (AD). In AD, the accumulation of an incorrectly processed form of the amyloid precursor protein, termed amyloid beta (Aβ) is thought to be linked to the neurodegenerative phenotype in AD. Accumulation of Aβ has been proposed to result in neurodegeneration due to mitochondrial dysfunction, oxidative stress, disruption of synaptic transmission, disruption of axonal trafficking, and/or general membrane disruption (92, 445). While the specific molecular defect due to Aβ accumulation remains to be determined, the pathology of this disease highlights the sensitivity of neuronal cells to aggregating or aggregation-prone proteins. Although there are several examples of neurodegenerative diseases that follow the typical ERAD paradigm (protein misfolding, followed by proteasome-dependent degradation; TABLE 1), we focus our discussion here on two neurodegenerative diseases linked to the ERAD machinery in a somewhat atypical fashion.
Huntington’s disease (HD) results from abnormally high numbers (>35) of polyglutamine (polyQ) repeats in the huntingtin (Htt) protein. In HD, the length of the CAG codon repeats in HTT positively correlates with an increased propensity of Htt aggregate formation. In line with this observation, the length of the CAG repeat in HTT also directly correlates with the age of onset and disease severity in HD patients (469). The disease is inherited in an autosomal dominant pattern and afflicts nearly 1 in 10,000 Americans (10). In adult-onset HD, patients present with a combination of behavioral changes, movement disorders, and progressive dementia (8, 591). The native function of Htt is poorly understood, and the unusual nature of the link between HD and ERAD stems from the fact that Htt is a cytoplasmic protein, instead of a secretory protein.
An early hint that the UPS might be connected to the pathogenesis of HD came from a study demonstrating that HD inclusions in brain sections from affected individuals are immunoreactive for ubiquitin (104). A possible, functional link between HD and the UPS was provided by the Kopito lab, which showed that the UPS is less active in cells expressing polyQ Htt. Specifically, as the length of the polyQ repeat increased beyond a threshold that correlates with disease presentation, the ability of the proteasome to degrade a fluorescent reporter in cells was inhibited (34). These data provided one mechanistic explanation for why HD develops: the generation of Htt polyQ aggregates decreases UPS activity, which will then lead to a further accumulation of unprocessed UPS substrates and an escalation of aggregate toxicity. However, it is still debated whether the HD aggregates are a secondary effect of the disease or are the cause of neurodegeneration (24, 246, 427, 459). Thus misfolded Htt preaggregates might also accumulate, which may initiate disease onset.
Nevertheless, support for the UPS inhibition model comes from a study examining the levels of K48 ubiquitin-linked, proteasome-directed peptides present in an Htt polyQ-expressing cell line and in tissue taken from a mouse model for HD. In both experimental systems, there was a significant increase in the amount of K48 ubiquitinated peptides present in samples containing an Htt reporter with 150 polyQ repeats (37). Furthermore, K48 chain accumulation correlated strongly with proteasome inhibition. However, it was possible that the increase in K48 linked chains resulted from the accumulation of polyubiquitinated Htt aggregates, rather than from global impairment of the proteasome. To test for global impairment, the levels of other ubiquitin chain linkages, i.e., K11 and K63, were examined. Both the levels of K11 and K63 ubiquitin chains increased in the presence of either MG-132 or the 150 polyQ repeat form of Htt, which points toward wholesale dysregulation of the UPS (37).
Do ERAD substrates also accumulate under these conditions? The answer appears to be yes, as Duennwald and Lindquist (114) found that the concentration of several well-characterized ERAD substrates increases in the presence of aggregation-prone Htt. The accumulation of these ERAD substrates may arise from direct proteasome inhibition, as suggested above, and/or from the observed colocalization of p97/Cdc48 to the Htt inclusions. Also, a recent study reported an in vitro interaction between Htt polyQ aggregates and the ER-resident E3 ubiquitin ligase gp78, which is required for the ERAD of several substrates (230, 247, 297, 340); Htt aggregates block the association of gp78 with polyubiquitinated proteins and with p97/Cdc48 (563).
If ERAD substrates accumulate, one might predict that the UPR is induced in the presence of polyQ Htt. In fact, the connection between a long-term UPR induction and apoptosis (see sect. ID) might explain at least in part the neurodegenerative phenotype in HD. Indeed, expression of polyQ Htt in yeast activates the UPR and is synthetically lethal when combined with other inducers of ER stress, such as tunicamycin, the inhibitor of N-linked glycosylation (114). Studies in mammalian cells show that polyQ Htt induces cell stress, increases chaperone levels, and results in the conversion of IRE1 and PERK into their active, phosphorylated forms (114, 269, 353). Together, these data strongly suggest that polyQ Htt aggregates alter the activity of the ERAD machinery and induce the UPR, further contributing to cytotoxicity. This is not to say that Htt polyQ preaggregates don’t also contribute to the disease (427, 458, 459), and in fact, it has been proposed that the mechanism of pathogenesis of HD stems from disruption of other pathways, either through aberrant interactions or by loss of Htt function. For example, Htt localizes to the Golgi, endosomes, and vesicular carriers, among other locations in cells. If Htt function in axonal trafficking is blocked due to polyQ Htt, this could lead to decreased synaptic transmission and cell death (159). Together, future therapies for HD may need to focus on addressing the cytotoxicity associated with ER stress.
Another example of a neurological disease linked to the ERAD pathway is Parkinson’s disease (PD). PD is a member of the family of movement disorders and results from the loss of dopaminergic neurons. PD occurs in ~1–2% of the population, usually developing after 60 years of age. Patients with PD present with four main categories of symptoms: tremors, bradykinesia, rigidity, and postural instability (11). The probability of developing PD increases with age and either can be sporadic or arise from specific genetic mutations. For instance, mutations in PARK2, which encodes the E3 ubiquitin ligase Parkin, lead to an autosomalrecessive form of early-onset PD. Parkin is normally localized to the cytoplasm, which with the exception of CHIP (see above) is generally atypical for E3 ligases that participate in ERAD. However, it was demonstrated that Parkin is induced by the UPR (218). In addition, a candidate for a Parkin substrate is the Parkin-associated endothelin receptor-like receptor PaelR (217). PaelR, also known as GPR37, is a putative G protein-coupled receptor that is highly expressed in the central nervous system, but for which no endogenous ligands have been found (290). The connection between PaelR and PD is supported by the observation that PaelR accumulates in Lewy bodies, which are electrondense inclusions that serve as a histological indicator of PD (342). Furthermore, PaelR is polyubiquitinated in cells and can be ubiquitinated by Parkin in vitro. In addition, the proteasome-dependent degradation of PaelR is stimulated by Parkin overexpression (217). Finally, PaelR accumulates in a detergent-insoluble fraction in cells treated with a proteasome inhibitor or with ER stressors, indicating that PaelR has a natural propensity to misfold under stress conditions. These data indicate that Parkin mediates the quality control of PaelR.
Another connection between Parkin and ERAD was provided by the observation that Parkin function may be supplanted by another E3 required for the ERAD of select substrates in mammals, Hrd1 (41, 79, 206, 223). Modulation of Hrd1 levels changes PaelR levels, and Hrd1 expression can protect against PaelR-induced cell death (361). Furthermore, Hrd1 is expressed in dopaminergic neurons throughout the mouse brain, providing additional evidence that Hrd1 and Parkin function might be coordinated (362). However, Parkin has also been implicated in mitophagy, a process in which mitochondria are engulfed and destroyed via an autophagy-related pathway (239, 572). It was found that the mitochondrial serine/threonine kinase PINK1 accumulates on damaged mitochondria and in turn recruits Parkin. Parkin ubiquitinates the mitochondria fusion-promoting factor mitofusin. This results in the degradation of mitofusin via a p97/Cdc48-dependent ERAD-like process. Mitofusin degradation then triggers mitophagy (368). This pathway appears to be important for the pathogenesis of PD, as mutations in PINK1 can also cause inherited PD. However, the relationship and relative importance between the mitochondrial and ER functions of Parkin remain to be determined. But, in the past few years, communication between mitochondria and the ER has been established. Mitochondria directly interact with the ER, and this interaction is important for many processes, including lipid transfer, calcium signaling, and cell death (384). Also, some factors that comprise the ERAD machinery also function to recognize, extract, and destroy components that reside in the mitochondrial membrane (86, 199, 558). A recent study revealed that Parkin is not only upregulated by ER stress, but also by mitochondrial stress (50). Surprisingly, however, the cytoprotective effect associated with Parkin expression may occur independent of the proteasome. These collective data highlight the enigmatic nature of Parkin’s true function and the need to further examine Parkin’s role at the interface between ERAD and disease.
C. Metabolic Disease
Diabetes mellitus is a metabolic disease in which individuals either produce too little insulin or are unable to respond properly to insulin, resulting in hyperglycemia. In general, diabetes can be broken into two main classes based on the underlying cause of the disease and/or the conditions surrounding the onset of the symptoms. Type 1 diabetes accounts for ~5–10% of all cases and is usually diagnosed at a young age. Type 1 diabetes results from immune system-mediated destruction of pancreatic β-cells, thereby decreasing the levels of insulin. In contrast, type 2 diabetes accounts for the majority of cases (90–95%) and results primarily from insulin resistance due to a decreased ability of target cells to respond to insulin (25). Diabetic individuals can present with a range of both short-term and long-term symptoms, including blindness, heart disease, kidney failure, and the need for limb amputation due to poor circulation (6). Classification of patients into type 1 or type 2 is sometimes difficult due to a large number of disease modifiers (25). For the purpose of this review, we will focus on the genetic factors that can cause diabetes and have been directly linked to ERAD.
Normally, the peptide hormone insulin is produced by pancreatic β-cells and stimulates the clearance of blood glucose by muscle, fat, and liver cells (356). Clearance is achieved via interaction with insulin receptors on these cells, thereby initiating a signaling cascade. Pancreatic β-cells are specialized in that they have a highly elaborated secretory system that is designed to synthesize and export large amounts of insulin under hyperglycemic conditions. In fact, insulin production in these cells accounts for 50% of total protein synthesis under stimulated conditions (440, 470). Insulin biosynthesis begins with the cotranslational insertion of a 110 amino acid preproinsulin into the ER. Subsequent removal of the signal sequence from this precursor generates the 86 amino acid peptide proinsulin. In the oxidizing environment of the ER, proinsulin acquires three disulfide bonds. Following export to the Golgi complex, further proteolytic processing of proinsulin results in the production of mature insulin that is then packaged into secretory granules.
The demand placed on the biosynthetic and folding machinery by insulin production has been suggested to result in increased amounts of ER stress in β-cells (515). As discussed earlier, unresolved activation of the UPR can lead to apoptosis, and heterozygous mice that are unable to activate one leg of the UPR, which is mediated by PERK, become diabetic and glucose intolerant (432). And, XBP-1+/− mice, in which UPR signaling is only partially inhibited, develop insulin resistance (367). Thus, as anticipated, β-cell apoptosis is relevant for both type 1 and type 2 diabetes, and ER stress may play a significant role in cell death and insulin deficiency (135). These events suggest the presence of a positive-feedback loop: stressed β-cells are destroyed, which in turn potentiates the disease due to a decreased ability to produce insulin. Consistent with a link between insulin production, the UPR, and diabetes, ER stress is increased in the diabetic Akita mouse model. In this case, the disease arises from the expression of a single copy of an insulin mutant (Ins2C96Y) that is unable to form an intrachain disulfide bond (16, 21). Interestingly, the levels of both wild-type insulin and Ins2C96Y are increased in the presence of MG-132, which implies that even native insulin is subject to misfolding and proteasome-dependent degradation (16). Further support for a role for ER stress in the pathogenesis of diabetes comes from the finding that guanabenz, a small molecule, rescues cells from death resulting from exposure to ER stressors, including tunicamycin and expression of Ins2C96Y. Investigation into the mechanism of action of guanabenz revealed that rescue was due to an enhancement of UPR-mediated translational attenuation (501). This finding represents a novel approach to mitigate ER stress and may be useful in treating metabolic syndromes associated with misfolded insulin variants or other peptide hormones.
The data described above suggest that allelic variants of insulin in humans may contribute to the “normal” levels of ER stress in β-cells, a phenomenon that may push these cells over the edge and lead to apoptosis and diabetes. In support of this model, several point mutations in the insulin gene have been identified in patients with neonatal diabetes. The identified mutations result in the ER accumulation of proinsulin and an accompanying secretion defect (371). Interestingly, one of the naturally occurring mutations, C96Y, is the same one found in the Akita mouse (523). Other studies with the Akita model demonstrated robust Hrd1-dependent ubiquitination of Ins2C96Y, directly implicating the ERAD pathway in the pathogenesis of genetically linked diabetes (16). A mouse model with inducible expression of Ins2C96Y confirmed the relationship between ER stress, increased apoptosis, and diabetes. Of note, the apoptotic response was increased either by proteasome inhibition or by downregulation of a member of the Hrd1 complex (186). Therefore, ERAD plays an important role in mitigating the stress incurred upon expression of misfolded insulin.
Diabetes-causing mutations have also been identified in the insulin receptor (IR), which target the mutant receptor for ERAD. IR is synthesized as an immature form, which must then undergo posttranslational modifications that include N-glycosylation, dimerization, and proteolytic cleavage (534). Substitution of the highly conserved His at position 209 with Arg prevents receptor dimerization and reduces transport to the cell surface (233). A decrease in the levels of mutant IR precursors over time suggested that the protein was degraded. Further analysis of the H209R and another IR variant, F382V, revealed that these mutants exhibit an enhanced interaction with BiP, indicative of increased ER retention and perhaps recognition for degradation (12). Two additional IR variants, D1179E and L1193W, were identified in patients with insulin resistance and which result in decreased surface expression coupled with increased degradation of the pro-receptor form (220). Further study revealed that pro-receptor levels increased upon the addition of proteasome inhibitors (219). In addition, the mutant forms of IR associated with cytosolic Hsp90 to a greater extent than wild-type IR. To define the role of Hsp90 in IR degradation, anti-Hsp90 antibodies were microinjected into cells transfected with mutant IR. This treatment increased the amount of the mutant IR (219), suggesting that cytosolic Hsp90 enhances the degradation of mutant IR. In contrast, chemical inhibition of Hsp90 increased the proteasome-dependent degradation of wild-type immature forms of IR, suggesting that this chaperone also facilitates the formation of early biogenic intermediates of the receptor (399).
D. Bacterial Toxins and ERAD
Pathogenic bacteria, including Vibrio cholera, Bordetella pertussis, Shigella dysenteriae, and Shiga toxigenic Escherichia coli, infect hundreds of millions of people each year. Combined, these infections result in millions of deaths annually (391). For example, Vibrio cholera is a serious threat in developing nations and is estimated to infect 3–5 million persons each year, resulting in ~100,000 deaths (1). Infectious outbreaks occur primarily in these nations due to poor sanitation and inadequate medical care. Patients infected with pathogenic variants of Vibrio cholera present with abrupt-onset watery diarrhea and dangerous levels of fluid loss. If not properly treated, death can result within hours.
The key to Vibrio cholera’s virulence begins with the secretion of AB5 protein complexes, which alter host cellular homeostasis. The AB5 toxin family consists of four subfamilies based on the method of toxin entry into the host cell via the B subunit and upon the enzymatic activity of the A subunit. Cholera toxin (CTx) is composed of a B-homopentamer and disulfide-linked A1 (catalytic) and A2 (linking) domains. The AB5 holotoxin is internalized from the apical plasma membrane of enterocytes following cell surface interaction with gangliosides. After entry into endosomes, CTx is trafficked in a retrograde manner to the Golgi, possibly due to its association with the ganglioside. Further retrograde trafficking of CTx to the ER occurs via the KDEL receptor, which recognizes the KDEL ER retrieval signal in the A2 subunit. Once in the ER, the catalytic A1 subunit (CTA1) disassociates from the A2B5 subunits, due to the action of ER oxidoreductases (289, 466, 509). CTA1 is then retrotranslocated from the ER to the cytoplasm where it folds and catalyzes the addition of an ADP-ribosyl group onto the Gs subunit of adenylate cyclase. This results in constitutive activation of adenylate cyclase, increased cAMP production, and continuous activation of CFTR (509). Overactive CFTR causes diarrhea and the resulting water and electrolyte loss.
Retrotranslocation of CTA1 from the ER to the cytoplasm appears to follow an ERAD-like pathway. As observed during ERAD, the toxin must first be recognized prior to retrotranslocation. Interestingly, recognition involves the ERresident PDI. PDI does not function as an oxidoreductase in the CTA1 unfolding reaction, but rather as a “redox-dependent chaperone” (499). In one model, PDI directs CTA1 unfolding, which was found to depend on another ER oxidoreductase, Ero1α (136, 137, 336). Ero1α helps PDI cycle between the reduced (toxin binding) and oxidized (toxin releasing) states. In another model, CTA1 spontaneously unfolds once dissociated from PDI (482). Unlike other ERAD substrates, CTA1 retrotranslocation is p97/Cdc48-independent (267). What then is the driving force for CTA1’s retrotranslocation from the ER to the cytosol? One possibility is that CTA1 is extracted by the 19S cap of the proteasome, which is sufficient for the removal of pro α-factor from yeast (284) and mammalian (518) vesicles. However, data directly supporting a role for the 19S cap in CTA1 dislocation are currently lacking. Yet another hypothesis is that spontaneous refolding of CTA1 in the cytosol ratchets CTA1 through a retrotranslocon, while simultaneously helping CTA1 avoid recognition by the cytosolic quality control machinery. Evidence in support of yet another model derives from the fact that the cytosolic chaperone Hsp90 interacts with CTA1 in an ATP-dependent manner and is required for the ER dislocation of CTA1 (483). Finally, treatment of cells with the multifunctional drug 4-phenylbutyric acid (PBA) inhibits cholera intoxication (482). In contrast to its other modes of action (see sect. IIIA), PBA may stabilize the tertiary structure of CTA1 since the compound increases the thermal stability of CTA1, as assessed by circular dichroism and fluorescence spectroscopy. Cell lines treated with PBA also exhibited reduced translocation of CTA1 from the ER to the cytoplasm and were spared CTA1 intoxication (482).
There are three proteins that have been implicated as the retrotranslocon for CTA1. In 2000, Schmitz et al. (433) used a cell-free system to examine the role of Sec61 in CTA1 retrotranslocation. They showed that CTA1 can be retrotranslocated from CTA1-loaded microsomes, but this process was inhibited by the addition of a ribosome nascent chain complex to block Sec61 (433). In contrast, the retrotranslocation of CTA1 subunit was facilitated by Derlin-1 in human embryonic kidney cells. CTA1 interacted with Derlin-1, and reduced CTA1 retrotranslocation was evident in cells expressing a dominant-negative version of Derlin-1 (39). Derlin-1 also interacts with PDI, which could provide for a convenient mechanism to hand CTA1 from the holotoxin to the retrotranslocon. Recently, the interaction of CTA1 with the E3 ligases gp78 and Hrd1 were noted (40). Besides functioning as an E3, Hrd1 has been implicated in the retrotranslocation of HMG-CoA reductase (152, 424) and CPY* (70) in yeast (see sect. IE). Consistent with these data, downregulation of Hrd1 or the use of dominant-negative mutants of Hrd1 attenuated CTA1 retrotranslocation. Interestingly, the dominant-negative form of Derlin-1 also blocked the interaction of CTA1 with gp78 and Hrd1, suggesting that CTA1 interacts with Derlin-1 prior to the E3 ligases (40). These collective data support a role for multiple, putative channels in CTA1 retrotranslocation, although it is unclear if they act together, sequentially, or redundantly.
After retrotranslocation, it is unknown how CTA1 escapes polyubiquitination and proteasome-dependent degradation, which is the typical fate of ERAD substrates. It was hypothesized that CTA1 avoids polyubiquitination because it has only two lysines, which may be inaccessible to modification by the E3s (193, 410). Direct ubiquitination of CTA1 has not been observed, and one study demonstrated that CTA1 retrotranslocation continues in a lysine-less version of CTA1. However, as noted in section IE, ubiquitination has been noted on noncanonical sites such as Ser or Thr (67, 223, 452, 530). Therefore, the role of the E3 ligases and ubiquitination during CTA1 retrotranslocation remain uncertain. An alternate possibility is that polyubiquitinated CTA1 may be acted upon by a deubiquitinating enzyme that rescues proteins from being degraded. Or, the rapid refolding of CTA1 in the cytoplasm (see above) may obscure residues that might otherwise be ubiquitinated. This model is supported by data showing rapid acquisition of trypsin resistance upon release from PDI (410). Overall, these studies reveal that CTA1 intoxication requires a functional ERAD machinery and that CTA1 masquerades as an ERAD substrate, albeit one with unique features. But, because the AB5 toxins are structurally similar and seem to have evolved similar mechanisms to coopt the ERAD machinery, understanding the factors and processes required for CTx toxicity might provide useful targets for the treatment of symptoms resulting from other pathogenic bacteria harboring AB5 toxins. In fact, ricin (a plant AB5 toxin produced by Ricinus comminus) may spontaneously unfold once dissociated from PDI in the ER (33, 467) and also appears to forego ubiquitination after its retrotranslocation through either Sec61 or a Hrd1-based retrotranslocon (97, 293, 453). Since ricin is synthesized within eukaryotic cells, rather than in bacteria like CTx, it is glycosylated in the ER (102). This may explain some of the observed differences between the fates of ricin and CTA1, such as the interaction between ricin and EDEM1, and a measurable amount of degradation of ricin toxin in the cytoplasm, presumably due to its failure to efficiently translocate into the ER or completely refold after its retrotranslocation (453, 460, 541).
E. Serpinopathies
The family of serine protease inhibitors (serpins) is responsible for a class of diseases known as the serpinopathies (164). These diseases result from mutations in serpin family members, including α1-antitrypsin (AT), neuroserpin, antithrombin, and β2-antiplasmin. Mutant serpins can adopt altered, unstable conformations, leading to loss of native protein function and in some cases the acquisition of a toxic gain-of-function aggregate or polymer. The net outcomes are a group of pathologies that result in lung and liver disease, dementia, and blood clotting deficiencies.
Members of the serpin family share >30% sequence homology, as well as a conserved tertiary structure. Mutations in SERPINA1, which encodes the prototypical serpin α1-AT result in AT deficiency. Approximately 1 in 2,000 people of Northern European descent is homozygous for disease-causing alleles of AT (307). AT-deficient patients may present with chronic obstructive pulmonary disease (COPD) and can develop cirrhosis (3). Among individuals affected by AT deficiency, nearly 1–2% develop COPD symptoms, which is exacerbated by smoking. Liver disease is more common, with 10% of infants being born with jaundice and up to 50% of homozygous adults (over the age of 50) with some evidence of cirrhosis at death (116). This observation highlights the progressive nature of AT deficiency and the need for early diagnosis. Current treatment for AT-associated COPD relies on intravenous enzyme replacement therapy (3). It is much more difficult, to treat cirrhosis that results from polymer accumulation, and the only viable option is a liver transplant. Therefore, a significant body of research has been devoted to uncovering the pathways that degrade misfolded AT in hopes of developing therapies to reduce polymer formation and/or increase protein clearance in the liver.
AT is a 394-amino acid protein that is synthesized primarily in hepatocytes, and in the ER it becomes N-glycosylated. AT functions both in the bloodstream where it inhibits neutrophil-derived proteases and in lung tissue where it regulates elastase. The wild-type protein, known as the “M” allele, binds to and is cleaved by these proteases, thereby inducing a conformational change that inactivates them (117, 212). The most common disease-causing variant of AT is termed the Z variant (E342K), ATZ, based on its slower migration on an isoelectric focusing gel relative to ATM. Due to its inherent instability and the loss of an intramolecular salt bridge, ATZ self-associates and forms dimers and higher order polymers within the ER in hepatocytes. As a result, serum levels are only ~10–15% of wild-type levels (308, 378, 456). In essence, ATZ self-assembly underlies the two main pathologies associated with AT deficiency: 1) AT deficiency is a loss-of-function disease as a consequence of increased elastase activity, which results in the destruction of connective tissue in the lung; and 2) AT deficiency is a gain-of-function disease from the accumulation of toxic aggregates in the liver, which can lead to hepatitis, cirrhosis, and an increased risk for hepatocellular carcinoma (379).
Early work in mammalian cells and in a yeast model demonstrated that ATZ degradation requires the activity of the proteasome, pointing toward a role for ERAD in clearing soluble forms of ATZ from the secretory pathway. First, ATZ degradation was significantly delayed in a yeast strain lacking the chymotrypsin-like activity of the proteasome (540) and in a strain in which proteasome assembly was defective (442). Second, the half-life of ATZ expressed in human skin fibroblasts was increased threefold in the presence of a proteasome inhibitor (394). The proteasome is also required for the degradation of a naturally occurring truncated version of AT, null Hong Kong (NHK) (304). Surprisingly, it remains unclear whether mutant AT is ubiquitinated prior to degradation, although gp78 may be involved in ATZ turnover (451) and NHK resides in a Hrd1-containing complex (79). Moreover, a dominant-negative Hrd1 construct decreased the ubiquitination of NHK in HeLa cells (260), and ATZ solubility and degradation were enhanced by Hrd1 overexpression (522).
Other work demonstrated that ATM interacts with calnexin during its maturation (366); therefore, it is not surprising that lectins play an important role in the ERAD of misfolded forms of AT. ATZ was shown to interact with calnexin in patient fibroblasts transfected with the ATZ gene (554), and a similar interaction was demonstrated for NHK (282). Ongoing studies using NHK have yielded additional insights into the machinery required to degrade misfolded forms of AT. For example, calnexin-bound NHK appears to be transferred in a sequential manner to EDEM1 (see sect. IE) and then perhaps to the retrotranslocon (357). Another study suggested that EDEM1’s role during NHK degradation is cell-type specific, and ERManI may instead be the critical “timer” that choses NHK for degradation (553). Nevertheless, EDEM1 and the ERManI can act synergistically to trigger the degradation of NHK (205). NHK also binds two other lectins, XTP3-B and OS-9, which with BiP (66) act early in the pathway for ERAD substrate selection (79, 206).
Increasing evidence indicates that autophagy may play a complementary role to ERAD to destroy difficult-to-manage proteins (275). Because the autophagic pathway can remove large aggregates and even significant portions of damaged ER, one might imagine that autophagy could clear higher order ATZ polymers. Early evidence for this hypothesis came from studies examining ATZ polymer residence by immunoelectron microscopy. As predicted, ATZ was found not only in the ER but also in autophagosomes (486). Notably, chemical disruptors of autophagy stabilized ATZ, albeit rather modestly compared with proteasome inhibition (394, 486). This evidence was corroborated through the use of the yeast model, in which it was shown that autophagy became necessary to degrade ATZ but only when the protein was overexpressed and aggregated (276). Ultimately, the importance of the autophagic pathway and the fate of ATZ were demonstrated in mouse models (235). This model proved essential for the testing of small molecules that might one day improve the pathologies associated with AT deficiency (see sect. IIIA). Overall, the level of ATZ expression and components that regulate autophagy may represent genetic modifiers of AT deficiency. The identification of genetic modifiers for AT is vital given that <10% of ATZ homozygotes develop clinically relevant liver disease in childhood (476).
Surprisingly, there are some serpin family members that do not exhibit serine protease inhibitor activity but instead function as chaperones. Hsp47 resides in the ER and aids in the assembly of procollagen into a triple-helical form of procollagen (320). Mutations in Hsp47 compromise chaperone function, but at this point, the mutations do not appear to result in toxic polymerization (78). Instead, the loss of Hsp47 function manifests as osteogenesis imperfecta due to delayed secretion of procollagen trimers. Patient fibroblasts have virtually no detectable levels of Hsp47 protein; however, treatment with MG-132 increases Hsp47 levels, suggesting that the ERAD pathway plays a role in this form of the disease. In contrast, aggregates that arise in the ER directly from mutant forms of procollagen and that also cause osteogenesis imperfecta are destroyed by autophagy (221).
It should be mentioned that in addition to the serpinopathies described here, three other mutant forms of serpins exist that are ER retained (23, 503). Defects in these proteins lead to thyroxine binding globulin deficiency, alpha-1-antichimotrypsin deficiency, and type I hereditary angioedema. It remains unknown whether the mutant proteins are targeted for ERAD and/or autophagy, but based on the fates of other mutant serpins (273), it is likely that these pathways will contribute to their turnover.
F. Viruses and ERAD
In addition to bacteria, select viruses have also evolved ways to subvert host cells by capitalizing on ERAD-associated activities. Immune surveillance for virally infected cells requires the presentation of viral peptides by MHC-I molecules to cytotoxic T cells. The generation of viral peptides is accomplished by proteasome processing and cleavage by other proteases, which is followed by loading onto MHC-I in the ER and trafficking to the cell surface (98, 107). MHC-I biosynthesis is complex, requiring disulfide bond formation, N-linked glycosylation, heterodimerization, and peptide loading, and the failure to properly assemble these complexes can result in ERAD (74). In one example, the turnover of MHC-I molecules was accelerated by infection with human cytomegalovirus (HCMV) (32). HCMV is a herpes-type virus that can cause persistent, latent infections, but is usually only life-threatening in immunocompromised individuals. For those individuals, HCMV leads to mononucleosis-like symptoms, including fatigue, joint stiffness, and muscle pain, but the virus can also target specific organs, such as the eyes, lungs, gastrointestinal tract, and brain (5).
The study of MHC-I downregulation contributed significantly to early investigations of ERAD. Two viral genes were identified, US2 and US11, that direct newly synthesized MHC-I molecules to the ERAD pathway (232, 544, 545). Other early studies also demonstrated retrotranslocation of full-length MHC-I into a cytoplasmic pool in the presence of US11 (128, 449, 544). The retrotranslocated, or “dislocated” material, was deglycosylated, which may be important to feed the protein into the proteasome (203, 292). HCMV-induced degradation of MHC-I also required polyubiquitination, but interestingly ubiquitination does not occur on the cytoplasmic tail of MHC-I, which suggested that ubiquitination was occurring on the luminal domain of MHC-I (144). Thus the protein must form a hairpin across the ER membrane, and at least partial retrotranslocation is required for MHC-I ubiquitination. It is not surprising, then, that BiP plays a role in the US2- and US11-mediated degradation of MHC-I (195).
Interestingly, there are differences between the machinery used by US2 and US11, such as in the involvement of Derlin for US11 retrotranslocation, but not for US2 (299). In contrast, there is a requirement for signal peptide peptidase (SPP) for US2-mediated retrotranslocation (313), which at first glance is somewhat surprising given the fact that US2 is a type I membrane protein with a noncleavable signal sequence (156). However, a recent study found SPP in a complex together with PDI (285). These data suggest that substrate recruitment by SPP can occur independently of signal sequence cleavage. More generally, SPP may be important to clip integral membrane spans of select ERAD substrates, and consistent with this view a misfolded region in an integral membrane protein associated with SPP (90).
Other differences between US2 and US11 deserve mention. US2-induced ubiquitination of MHC-I has been attributed to the mammalian E3 ligase TRC-8 (468). By comparison, US11-dependent polyubiquitination of MHC-I appears to be facilitated by Hrd1 (189, 448). However, a recent study demonstrated that Hrd1 contributes to the physiological regulation of unfolded MHC-I but is dispensable for degradation induced by the presence of US11 (63). Together, the discovery of US2 and US11 has helped shed light on the molecular details underlying various facets of the ERAD pathway.
Another example in which the ERAD pathway has been co-opted by a virus is provided by the human immunodeficiency virus (HIV), which downregulates several aspects of host immunity, including CD4 function. HIV is spread through transmission of body fluids and results in a gradual decrease in CD4+ T cells. Ultimately, this can progress into acquired immune deficiency syndrome (AIDS) once the patient’s CD4 T-cell count has dropped below 200 cells/mm3. HIV-AIDS has reached epidemic proportions, affecting ~33 million individuals worldwide, and has caused over 25 million deaths (7). At first glance, the effect of HIV on MHC-I is unlike that of HCMV, since MHC-I is cleared from the cell surface and degraded through the action of the HIV protein Nef. However, the presence of CD4 at the cell surface, which is the primary cell surface receptor for the virus, also hinders HIV because it inhibits virus budding, interferes with assembly of the HIV virion, and can trigger a CD4+ T-cell immune response (314). To overcome these barriers, HIV expresses the Vpu protein. Vpu was first shown to induce the rapid degradation of CD4 in a prelysosomal compartment (549), and eventually Vpu-induced CD4 degradation was demonstrated to be dependent on a functional UPS (437). Interestingly, Vpu is a tail-anchored protein that interacts with the COOH terminus of the β-transducin repeat containing protein, β-TrCP (324). Through its F-box domain, an E3 Skp1-Cullin1-F-box (SCF) ligase is recruited to β-TrCP and therefore to CD4 (142). Normally, the SCFβ-TrCP complex does not participate in ERAD. Consistent with CD4 being “tricked” into becoming an ERAD substrate, recent data indicate that CD4 downregulation by Vpu also requires the p97/Cdc48 complex (46, 319).
Coronavirus and hepatitis C virus also interact with the ERAD machineries, but in very different ways from those discussed above. Coronaviurses (CoV) are RNA viruses that infect mammals, including humans, resulting in respiratory infections, i.e., severe acute respiratory syndrome (SARS) (376). Recently, it was shown that CoV replication occurs in virally induced membranous compartments that are enriched for EDEM1 and OS-9 (404). The importance of concentrating EDEM1 and OS-9 in these non-ER compartments is unknown, but among other possibilities, these data suggest a need to downregulate ERAD for efficient CoV replication. In line with this possibility, hepatitis C virus (HCV) replication was recently linked to the ERAD pathway. Specifically, ubiquitination of an HCV protein is increased by EDEM 1 or EDEM 3 overexpression, while viral replication is increased by treatment EDEM1 or EDEM3 siRNA (419).
III. CONCLUDING REMARKS
A. Current Experimental Treatments and Prospects for Future Therapies
As evidenced from the previous sections and TABLE 1, the ERAD pathway has been implicated in the underlying mechanisms of several diseases. But, because of the many factors that facilitate ERAD substrate selection and degradation, and because of diverse mechanisms by which different substrates may be destroyed, the treatment of ERAD-related diseases will be no simple task. In addition, any therapeutic treatments must take into account the proteostasis network within a cell, which comprises all of the factors that influence protein fate, such as synthesis, folding, degradation, and trafficking (363, 388). Even though these are still the “early days” for ERAD therapeutics, a growing number of approaches have been explored to correct ERAD-linked disease (TABLE 2). These approaches have had varying degrees of success for a number of reasons. For example, the proteostasis network is cell type specific, and cell toxicity is always problematic since modulation of ERAD may have secondary effects on the UPR and consequently lead to apoptosis. In addition, as described in the preceding sections, other degradative pathways, such as autophagy, may act complementary to ERAD. Therefore, autophagy may compensate for chemical inhibition of the ERAD pathway. In this section, we will discuss select examples of recent therapeutic strategies as they have applied to cystic fibrosis, a lysosomal storage disease, AT deficiency, and familial transthyretin amyloid disease.
Because the ΔF508-CFTR folding and trafficking defects were rescued by incubating cells at a lower temperature (94, 100, 111) (see sect. IIA), the destruction of the diseasecausing protein appeared to be a temperature-sensitive process. Consistent with this fact, temperature-dependent aggregation of wild-type CFTR can even be observed in vitro (162). Unfortunately, low temperature incubation of affected tissues in CF is not a viable therapeutic option. However, these data do suggest that modulating kinetic and/or thermodynamic aspects of the folding problem associated with ΔF508-CFTR may be sufficient to keep the protein from being selected for ERAD.
The most obvious approach to reduce the degradation of ΔF508-CFTR, thereby allowing the protein more time and perhaps a better environment in which to fold, is to inhibit the proteasome. Some of the most common proteasome inhibitors used are MG-132 (Cbz-Leu-Leu-leucinal), ALLN (acetyl-Leu-Leu-norleucinal), and lactacystin (a natural product isolated from a fungus; Refs. 49, 147). Refined proteasome inhibitors are currently used in the clinic and have shown efficacy in the treatment of late-stage multiple myeloma (434). These compounds acts as pseudosubstrates of the proteasome and inhibit the chymotryptic-like activity or both the chymotryptic- and tryptic-like activities (in the case of lactacystin; Ref. 283). Unfortunately, proteasome inhibition with ALLN or lactacystin shifted wild-type CFTR from a soluble form to a detergent-insoluble form (536). These results suggested that strategies to augment the folding and trafficking of ΔF508-CFTR must rescue the immature protein before it has been committed for degradation, since this pool is aggregation-prone once ubiquitinated.
Providing aid to the endogenous chaperone-assisted folding machinery in the cell has been considered as an attractive, alternate strategy to rescue ΔF508-CFTR and other conformationally challenged, disease-causing proteins. One means to achieve this outcome is via the administration of chemical chaperones. Chemical chaperones were first defined as osmolytes that provide a more favorable milieu in which a protein can fold, often by simply increasing the strength of the intramolecular hydrophobic bonds that are buried within a protein. Chemical chaperones may also more globally affect the cellular proteostasis network, thus leading to a more favorable folding/trafficking environment. Other chemical chaperones directly bind to the misfolded protein and have been termed “pharmacological chaperones” (51, 363, 505). These compounds lower the protein’s free energy state, which may help the protein fold, escape ERAD, and/or limit aggregation. For ΔF508-CFTR, one chemical chaperone that has been examined is curcumin. Curcumin is a component of turmeric and is derived from the plant Curcuma longa. Curcumin is something of a panacea, having been studied as a treatment for diseases from cancer to inflammatory bowel disease as a result of its in vitro effects as an anti-inflammatory, anti-oxidant, proapoptotic, and anti-cancer agent (119). Initial reports indicated that curcumin was able to rescue the ΔF508-CFTR trafficking defect and some disease-related phenotypes in a CF mouse model (115). However, the inability to reproduce some of these results casts doubt on curcumin’s efficacy for CF (321, 462, 533). These findings do not preclude the use of curcumin to treat CF, as the compound may function at different steps in CFTR biogenesis or work more effectively for other CFTR mutants (38, 529, 577).
More successful attempts to chemically correct ΔF508-CFTR were achieved by the outcomes of high-throughput screens for small molecules that increase ΔF508-CFTR activity at the cell surface. In one case, this led to the isolation of Corr-4a, which is a bisaminomethylbithiazole derivative (375). Although the mechanism of action of this compound is unknown, there is some evidence that Corr-4a binds directly to CFTR (532). More recent results suggest that Corr-4a rescues ΔF508-CFTR by repairing MSD2-related folding defects (171). Another ΔF508-CFTR corrector that was isolated through a high-throughput screen, Vertex-325 (508), may bind directly to NBD1 in CFTR (576). These combined data are consistent with evidence that the ΔF508 mutation disrupts interactions between NBD1 and MSD2 (446). Another compound, Vertex-770, rescues the gating defect in a less common CFTR mutant (G551D) that leads to disease (507). More likely than not, this potentiator also binds directly to CFTR to increase the channel’s open probability. Overall, there is continued interest in performing functional screens for small molecule modulators of ΔF508-CFTR, and in the end, the best therapeutic outcome may be through a combined application of drugs that restore both the folding/trafficking defect associated with the protein, as well as its reduced channel activity.
Chemical chaperones have also been used to treat lysosomal storage diseases (LSDs), such as Gaucher disease (GD). GD results from a deficiency in glucocerebrosidase (GC), a lysosomal enzyme that normally breaks down the glycosphingolipid glucosylceramide. The inability to metabolize glucosylceramide affects many organs, resulting in hepatomegaly, splenomegaly, anemia, thrombocytopenia, bone lesions, and in some cases central nervous system (CNS) deficiencies (45, 211, 430). Some of the allelic variants that cause GD have been linked to ERAD, including N370S, G202R, and L444P (431). While enzyme replacement therapy has been successful for some LSDs, this strategy is ineffective for GD, presumably due to poor targeting of the exogenous enzyme to macrophages (430). This problem has been partially overcome by the development of a tagged version of GC that is recognized by a macrophage receptor; however, the modified enzyme is ineffective for patients with CNS symptoms due to its inability to cross the blood-brain barrier (578). Because reduced temperature also appears to rescue the G202R and L444P mutants (431), several chemical chaperones have been examined that increase the folding and trafficking efficiency of GC variants, some of which bind to GC’s active site as a pseudosubstrate (578). Unfortunately, one of the most common GC mutations, L444P, is largely unresponsive to chemical chaperones, as the mutation is not in the active site.
To circumvent the special problem associated with L444P, proteasome inhibitors and a proteostasis regulator were examined. The proteostasis regulator is celastrol, which is used in traditional Chinese medicine and induces the heat shock response and the UPR (341, 542). Surprisingly, both MG-132 and celastrol repaired the mutant GC, but lactacystin was insufficient to rescue L444P, in spite of the fact that the measured proteasome inhibition by MG-132 and lactacystin was indistinguishable (341). The explanation for this difference may stem from the finding that MG-132 and celastrol alter the expression of 400–500 genes, suggesting that the proteostatic network was affected. Among these genes were several factors regulated by the heat shock response and UPR, the latter of which is required for the effect of MG-123 and celastrol. Notably, upon treatment with celastrol, the L444P GC mutant now became receptive to treatment with chemical chaperones, and combining these treatments resulted in synergistic rescue (341). This study highlights the importance of considering the off-target, but potentially beneficial effects of a compound. In other observations, the accumulation of glucosylceramide has been noted to increase calcium efflux from the ER (264, 305, 377). As calcium is an important cofactor for components of the ER folding machinery (see above), increased efflux has been hypothesized to further impair the ER’s folding capacity. Indeed, altering ER calcium levels by inhibiting efflux, or by the overexpression of SERCAs, which increase ER calcium, increases the processing and activity of L444P GC (364, 521). In addition, it was noted that calcium modulation combined with proteostasis modulation yields synergistic rescue. These studies demonstrate the delicate nature of the ER folding environment and reinforce the benefit of combining therapeutic approaches.
Another compound for which a known and off-target, but desired, effect is evident is PBA. PBA is an FDA-approved drug for the treatment of urea cycle disorders due to its ability to function as a nitrogen scavenger (58). PBA has also been explored for the treatment of sickle cell anemia, β-thalassemia, and various malignancies (387). Thus it was something of a surprise that PBA administration led to measureable rescue of ΔF508-CFTR in several different cell types, including patient-derived epithelial cells (415). In a clinical trial examining the use of PBA to treat CF, a partial rescue of CFTR activity in nasal airway epithelium was observed, supporting continued research on the mechanism of action of this compound (416). In fact, a proteomic analysis of the outcome of PBA treatment revealed, among other changes, increases in BiP and Hsp70 and Hsp90, and a downregulation of the constitutively expressed Hsp70 (Hsc70) and a subunit of the 26S proteasome (454). Thus PBA may exert a desired effect on several key players in the protein folding and ERAD pathways. This effect is most likely mediated by the ability of PBA to inhibit histone deacetylases (HDAC) (76, 406). Histone acetylation and deacetylation are important regulators of gene expression (68, 175, 406). A second FDA-approved drug, suberoylanilide hydroxamic acid (SAHA), is also an HDAC inhibitor, and is currently used to treat cutaneous T-cell lymphoma and is in clinical trials for the treatment of other forms of lymphoma (288, 322). SAHA was recently shown to rescue ΔF508-CFTR channel activity to ~28% of wild-type levels in primary airway epithelial cells. The silencing of specific HDACs led to the identification of HDAC7 as a key mediator of ΔF508-CFTR rescue. Perhaps not surprisingly, HDAC7 silencing broadly altered the transcriptional profile in the epithelial cells and influenced the levels of many genes implicated in CFTR folding and trafficking (215).
The pleiotropic effects of HDAC inhibitors are likely responsible for their effectiveness in ameliorating disease phenotypes associated with other ERAD-targeted proteins, including ATZ. PBA increases the secretion of ATZ from patient-derived fibroblast cells expressing ATZ and in mice engineered to produce human ATZ (64). Unfortunately, PBA was ineffective in a clinical trial aimed at increasing serum AT levels, likely due to the difficulty in delivering an optimal dose to the affected tissues (484). However, another FDA-approved drug, carbamazepine (CBZ), was examined in a model for ATZ-associated liver disease. CBZ has been used to treat epilepsy and has applications as a mood stabilizer because of its effects on inositol (487, 550). Because of the effects on IP3 levels, CBZ also enhances autophagy and the clearance of polyQ HTT aggregates (423). Recently, CBZ was shown to enhance the degradation of both soluble and insoluble ATZ in a mouse model (200). These data make CBZ an attractive candidate to treat AT-associated liver disease.
In our last example, we discuss one of the rare cases in which knowledge about protein structure led to the design of an effective therapeutic that is being used in the clinic. Transthyretin (TTR) is a secreted protein that functions as a tetramer to transport thyroid hormone and retinol-binding protein in the bloodstream. Certain disease-causing variants of TTR, such as D18G, are secreted inefficiently because they are retained in the ER and degraded by ERAD (444). As might be expected, this variant binds to BiP to a much greater extent than wild-type TTR or to the variants that result in disease through an ERAD-independent mechanism (463). Other unstable TTR variants escape ERAD but form serum amyloid fibrils that can lead to cardiomyopathy, familial amyloid polyneuropathy, and CNS amyloidosis (180). On the basis of extensive biophysical studies, TTR amyloidosis was shown to arise due to a kinetic destabilization of secreted TTR tetramers into monomers, which then form fibrils (181). Therefore, molecules that stabilize the tetramer should have therapeutic potential. After X-ray crystallography was used to determine the key features by which amyloid inhibitors bind to TTR, computational analysis guided the design of more potent and specific small molecule inhibitors of TTR amyloidosis (255). Further refinement led to the discovery of a halogenated benzoxazole, compound 20, which bound tightly to TTR and prevented fibril formation (403). Compound 20, now referred to as Tafimidis, is currently used to treat familial transthyretin amyloid diseases (9).
B. Summary and Future Questions
The purpose of this review was to highlight key factors that direct ERAD substrates for degradation and to provide select examples on the number of unique ways in which ERAD can impact human physiology and disease. For more specific details and for a more inclusive list, we have provided TABLE 1. However, we are quite aware that this table is incomplete and that in the future many more diseaseassociated ERAD substrates will be added. In other cases, the data linking a particular disease-associated protein to the ERAD pathway are not definitive, but are only suggestive. Many proteins are only loosely associated with various aspects of ER quality control, but their residence in the ER and proteasome-dependent degradation have not been studied. We hope that our work will lead to a more in-depth investigation into potential connections to ERAD and stimulate future research efforts. Given the nearly infinite number of conformations that a secreted protein can adopt, especially when all possible mutant forms are considered, it is not surprising that the list is already relatively long. We suspect that the table will grow as additional secreted proteins are characterized and as ill-characterized genes encoding secreted proteins are better defined. We also hope that our work will raise important questions about how current and future research efforts can best be directed to treat these conditions. In the era of personal genomics, care must be taken to accurately define the underlying effects of disease-causing mutations that result in protein misfolding. And, as promising therapeutics are examined for their effects on a variety of disease models, secondary effects on the ERAD pathway will need to be examined. As TABLE 2 demonstrates, compounds that can control for effects on the fates of ERAD substrate are already in-hand for these efforts.
ACKNOWLEDGMENTS
C. J. Guerriero gives special thanks to Emily Guerriero for critical reading of the manuscript and support during its preparation as well as to the members of the Brodsky lab for many helpful discussions.
GRANTS
The study of ERAD in our laboratory is supported by grants from the National Institutes of Health (GM-75061) and via the Pittsburgh Center for Kidney Research (DK-79307) as well as from the Cystic Fibrosis Foundation (BRODSK08XX0) (to J. L. Brodsky). C. J. Guerriero is supported by a National Institutes of Health National Research Service Award F32 individual postdoctoral fellowship (GM-903642).
Footnotes
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1.Cholera Fact Sheet. http://www.who.int/mediacentre/factsheets/fs107/en/index.html.
- 2.Cystic Fibrosis Mutation Database. http://www.genet.sickkids.on.ca/app.
- 3.ADAM Medical Encyclopedia [Alpha-1 antitrypsin deficiency. A.D.A.M., Inc.; http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0001178/. [Google Scholar]
- 4.ADAM Medical Encyclopedia [Cystic fibrosis] A.D.A.M., Inc.; http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0001167/. [Google Scholar]
- 5.ADAM Medical Encyclopedia [Cytomegalovirus infection] A.D.A.M., Inc.; http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0001594/. [Google Scholar]
- 6.ADAM Medical Encyclopedia [Diabetes] A.D.A.M., Inc.; http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0002194/. [Google Scholar]
- 7.ADAM Medical Encyclopedia [HIV-AIDS] A.D.A.M., Inc.; http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0001620/. [Google Scholar]
- 8.ADAM Medical Encyclopedia [Huntington’s disease] A.D.A.M., Inc.; http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0001775/. [Google Scholar]
- 9.FoldRx Pharmeceuticals. www.foldrx.com.
- 10.Huntington’s Disease Society of America. www.hdsa.org.
- 11.National Institutes of Health Office of Rare Disease Reseach. www.rarediseaes.info.nih.gov.
- 12.Accili D, Kadowaki T, Kadowaki H, Mosthaf L, Ullrich A, Taylor SI. Immunoglobulin heavy chain-binding protein binds to misfolded mutant insulin receptors with mutations in the extracellular domain. J Biol Chem. 1992;267:586–590. [PubMed] [Google Scholar]
- 13.Ackerman AL, Giodini A, Cresswell P. A role for the endoplasmic reticulum protein retrotranslocation machinery during crosspresentation by dendritic cells. Immunity. 2006;25:607–617. doi: 10.1016/j.immuni.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 14.Aebi M, Bernasconi R, Clerc S, Molinari M. N-glycan structures: recognition and processing in the ER. Trends Biochem Sci. 2010;35:74–82. doi: 10.1016/j.tibs.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Alberti S, Bohse K, Arndt V, Schmitz A, Hohfeld J. The cochaperone HspBP1 inhibits the CHIP ubiquitin ligase and stimulates the maturation of the cystic fibrosis transmembrane conductance regulator. Mol Biol Cell. 2004;15:4003–4010. doi: 10.1091/mbc.E04-04-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allen JR, Nguyen LX, Sargent KEG, Lipson KL, Hackett A, Urano F. High ER stress in β-cells stimulates intracellular degradation of misfolded insulin. Biochem Biophys Res Commun. 2004;324:166–170. doi: 10.1016/j.bbrc.2004.09.035. [DOI] [PubMed] [Google Scholar]
- 17.Allen S, Abuzenadah AM, Hinks J, Blagg JL, Gursel T, Ingerslev J, Goodeve AC, Peake IR, Daly ME. A novel von Willebrand disease-causing mutation (Arg273Trp) in the von Willebrand factor propeptide that results in defective multimerization and secretion. Blood. 2000;96:560–568. [PubMed] [Google Scholar]
- 18.Allen S, Goodeve AC, Peake IR, Daly ME. Endoplasmic reticulum retention and prolonged association of a von Willebrand’s disease-causing von Willebrand factor variant with ERp57 and calnexin. Biochem Biophys Res Commun. 2001;280:448–453. doi: 10.1006/bbrc.2000.4139. [DOI] [PubMed] [Google Scholar]
- 19.Alonso V, Ardura JA, Wang B, Sneddon WB, Friedman PA. A naturally occurring isoform inhibits parathyroid hormone receptor trafficking and signaling. J Bone Miner Res. 2011;26:143–155. doi: 10.1002/jbmr.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Appenzeller-Herzog C, Ellgaard L. The human PDI family: versatility packed into a single fold. Biochim Biophys Acta. 2008;1783:535–548. doi: 10.1016/j.bbamcr.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 21.Araki E, Oyadomari S, Mori M. Impact of endoplasmic reticulum stress pathway on pancreatic β-cells and diabetes mellitus. Exp Biol Med. 2003;228:1213–1217. doi: 10.1177/153537020322801018. [DOI] [PubMed] [Google Scholar]
- 22.Aridor M. Visiting the ER: the endoplasmic reticulum as a target for therapeutics in traffic related diseases. Adv Drug Deliv Rev. 2007;59:759–781. doi: 10.1016/j.addr.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Aridor M, Hannan LA. Traffic jam: a compendium of human diseases that affect intracellular transport processes. Traffic. 2000;1:836–851. doi: 10.1034/j.1600-0854.2000.011104.x. [DOI] [PubMed] [Google Scholar]
- 24.Arrasate M, Mitra S, Schweitzer ES, Segal MR, Finkbeiner S. Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death. Nature. 2004;431:805–810. doi: 10.1038/nature02998. [DOI] [PubMed] [Google Scholar]
- 25.Association AD. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2009;32:S62–S67. doi: 10.2337/dc09-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bagola K, Mehnert M, Jarosch E, Sommer T. Protein dislocation from the ER. Biochim Biophys Acta. 2011;1808:925–936. doi: 10.1016/j.bbamem.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 27.Ballar P, Pabuccuoglu A, Kose FA. Different p97/VCP complexes function in retro-translocation step of mammalian ER-associated degradation (ERAD) Int J Biochem Cell Biol. 2011;43:613–621. doi: 10.1016/j.biocel.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 28.Ballar P, Shen Y, Yang H, Fang S. The role of a novel p97/valosin-containing protein-interacting motif of gp78 in endoplasmic reticulum-associated degradation. J Biol Chem. 2006;281:35359–35368. doi: 10.1074/jbc.M603355200. [DOI] [PubMed] [Google Scholar]
- 29.Ballinger CA, Connell P, Wu Y, Hu Z, Thompson LJ, Yin LY, Patterson C. Identification of CHIP, a novel tetratricopeptide repeat-containing protein that interacts with heat shock proteins and negatively regulates chaperone functions. Mol Cell Biol. 1999;19:4535–4545. doi: 10.1128/mcb.19.6.4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bartlett JR, Friedman KJ, Ling SC, Pace RG, Bell SC, Bourke B, Castaldo G, Castellani C, Cipolli M, Colombo C, Colombo JL, Debray D, Fernandez A, Lacaille F, Macek M, Rowland M, Salvatore F, Taylor CJ, Wainwright C, Wilschanski M, Zemková D, Hannah WB, Phillips MJ, Corey M, Zielenski J, Dorfman R, Wang Y, Zou F, Silverman LM, Drumm ML, Wright FA, Lange EM, Durie PR, Knowles MR. Genetic modifiers of liver disease in cystic fibrosis. JAMA. 2009;302:1076–1083. doi: 10.1001/jama.2009.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bays NW, Gardner RG, Seelig LP, Joazeiro CA, Hampton RY. Hrd1p/Der3p is a membrane-anchored ubiquitin ligase required for ER-associated degradation. Nat Cell Biol. 2001;3:24–29. doi: 10.1038/35050524. [DOI] [PubMed] [Google Scholar]
- 32.Beersma M, Bijlmakers M, Ploegh H. Human cytomegalovirus down-regulates HLA class I expression by reducing the stability of class I H chains. J Immunol. 1993;151:4455–4464. [PubMed] [Google Scholar]
- 33.Bellisola G, Fracasso G, Ippoliti R, Menestrina G, Rosén A, Soldà S, Udali S, Tomazzolli R, Tridente G, Colombatti M. Reductive activation of ricin and ricin A-chain immunotoxins by protein disulfide isomerase and thioredoxin reductase. Biochem Pharmacol. 2004;67:1721–1731. doi: 10.1016/j.bcp.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 34.Bence NF, Sampat RM, Kopito RR. Impairment of the ubiquitin-proteasome system by protein aggregation. Science. 2001;292:1552–1555. doi: 10.1126/science.292.5521.1552. [DOI] [PubMed] [Google Scholar]
- 35.Bendikov-Bar I, Ron I, Filocamo M, Horowitz M. Characterization of the ERAD process of the L444P mutant glucocerebrosidase variant. Blood Cells, Molecules, Diseases. 2011;46:4–10. doi: 10.1016/j.bcmd.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 36.Benjamin E, Flanagan J, Schilling A, Chang H, Agarwal L, Katz E, Wu X, Pine C, Wustman B, Desnick R, Lockhart D, Valenzano K. The pharmacological chaperone 1-deoxygalactonojirimycin increases α-galactosidase A levels in Fabry patient cell lines. J Inherited Metab Dis. 2009;32:424–440. doi: 10.1007/s10545-009-1077-0. [DOI] [PubMed] [Google Scholar]
- 37.Bennett EJ, Shaler TA, Woodman B, Ryu KY, Zaitseva TS, Becker CH, Bates GP, Schulman H, Kopito RR. Global changes to the ubiquitin system in Huntington’s disease. Nature. 2007;448:704–708. doi: 10.1038/nature06022. [DOI] [PubMed] [Google Scholar]
- 38.Berger AL, Randak CO, Ostedgaard LS, Karp PH, Vermeer DW, Welsh MJ. Curcumin stimulates cystic fibrosis transmembrane conductance regulator Cl− channel activity. J Biol Chem. 2005;280:5221–5226. doi: 10.1074/jbc.M412972200. [DOI] [PubMed] [Google Scholar]
- 39.Bernardi KM, Forster ML, Lencer WI, Tsai B. Derlin-1 facilitates the retro-translocation of cholera toxin. Mol Biol Cell. 2008;19:877–884. doi: 10.1091/mbc.E07-08-0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bernardi KM, Williams JM, Kikkert M, van Voorden S, Wiertz EJ, Ye Y, Tsai B. The E3 ubiquitin ligases Hrd1 and gp78 bind to and promote cholera toxin retro-translocation. Mol Biol Cell. 2010;21:140–151. doi: 10.1091/mbc.E09-07-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bernasconi R, Galli C, Calanca V, Nakajima T, Molinari M. Stringent requirement for HRD1, SEL1L, and OS-9/XTP3-B for disposal of ERAD-LS substrates. J Cell Biol. 2010;188:223–235. doi: 10.1083/jcb.200910042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berridge MJ. Inositol trisphosphate and calcium signalling mechanisms. Biochim Biophys-Acta. 2009;1793:933–940. doi: 10.1016/j.bbamcr.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 43.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 44.Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2:326–332. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- 45.Beutler E. Enzyme replacement in Gaucher Disease. PLoS Med. 2004;1:e21. doi: 10.1371/journal.pmed.0010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Binette J, Dube M, Mercier J, Halawani D, Latterich M, Cohen E. Requirements for the selective degradation of CD4 receptor molecules by the human immunodeficiency virus type 1 Vpu protein in the endoplasmic reticulum. Retrovirology. 2007;4:75. doi: 10.1186/1742-4690-4-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blond-Elguindi S, Cwirla SE, Dower WJ, Lipshutz RJ, Sprang SR, Sambrook JF, Gething MJ. Affinity panning of a library of peptides displayed on bacteriophages reveals the binding specificity of BiP. Cell. 1993;75:717–728. doi: 10.1016/0092-8674(93)90492-9. [DOI] [PubMed] [Google Scholar]
- 48.Bodó I, Katsumi A, Tuley EA, Eikenboom JCJ, Dong Z, Sadler JE. Type 1 von Wille-brand disease mutation Cys1149Arg causes intracellular retention and degradation of heterodimers: a possible general mechanism for dominant mutations of oligomeric proteins. Blood. 2001;98:2973–2979. doi: 10.1182/blood.v98.10.2973. [DOI] [PubMed] [Google Scholar]
- 49.Bogyo M, Gaczynska M, Ploegh HL. Proteasome inhibitors and antigen presentation. Peptide Sci. 1997;43:269–280. doi: 10.1002/(SICI)1097-0282(1997)43:4<269::AID-BIP2>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 50.Bouman L, Schlierf A, Lutz AK, Shan J, Deinlein A, Kast J, Galehdar Z, Palmisano V, Patenge N, Berg D, Gasser T, Augustin R, Trumbach D, Irrcher I, Park DS, Wurst W, Kilberg MS, Tatzelt J, Winklhofer KF. Parkin is transcriptionally regulated by ATF4: evidence for an interconnection between mitochondrial stress and ER stress. Cell Death Differ. 2011;18:769–782. doi: 10.1038/cdd.2010.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bouvier M. When an Inhibitor Promotes Activity. Chem Biol. 2007;14:241–242. doi: 10.1016/j.chembiol.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 52.Bradley CA, Taghibiglou C, Collingridge GL, Wang YT. Mechanisms involved in the reduction of GABAA receptor α1-subunit expression caused by the epilepsy mutation A322D in the trafficking-competent receptor. J Biol Chem. 2008;283:22043–22050. doi: 10.1074/jbc.M801708200. [DOI] [PubMed] [Google Scholar]
- 53.Brennan SO, Wyatt J, Medicina D, Callea F, George PM. Fibrinogen brescia: hepatic endoplasmic reticulum storage and hypofibrinogenemia because of a γ284 Gly→Arg mutation. Am J Pathol. 2000;157:189–196. doi: 10.1016/s0002-9440(10)64530-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brini M, Carafoli E. Calcium pumps in health and disease. Physiol Rev. 2009;89:1341–1378. doi: 10.1152/physrev.00032.2008. [DOI] [PubMed] [Google Scholar]
- 55.Brodsky JL. The protective and destructive roles played by molecular chaperones during ERAD (endoplasmic-reticulum-associated degradation) Biochem J. 2007;404:353–363. doi: 10.1042/BJ20061890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brodsky JL, Skach WR. Protein folding and quality control in the endoplasmic reticulum: recent lessons from yeast and mammalian cell systems. Curr Opin Cell Biol. 2011 doi: 10.1016/j.ceb.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brown CR, Hong-Brown LQ, Biwersi J, Verkman AS, Welch WJ. Chemical chaperones correct the mutant phenotype of the delta F508 cystic fibrosis transmembrane conductance regulator protein. Cell Stress Chaperones. 1996;1:117–125. doi: 10.1379/1466-1268(1996)001<0117:ccctmp>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brusilow SW, Horwich AL. Online Metabolic and Molecular Bases of Inherited Disease. New York: McGraw Hill; 2006. Urea cycle enzymes. [Google Scholar]
- 59.Buck TM, Wright CM, Brodsky JL. The activities and function of molecular chaperones in the endoplasmic reticulum. Semin Cell Dev Biol. 2007;18:751–761. doi: 10.1016/j.semcdb.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bukau B, Weissman J, Horwich A. Molecular chaperones and protein quality control. Cell. 2006;125:443–451. doi: 10.1016/j.cell.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 61.Bulleid NJ, Freedman RB. Defective co-translational formation of disulphide bonds in protein disulphide-isomerase-deficient microsomes. Nature. 1988;335:649–651. doi: 10.1038/335649a0. [DOI] [PubMed] [Google Scholar]
- 62.Burnett JR, Zhong S, Jiang ZG, Hooper AJ, Fisher EA, McLeod RS, Zhao Y, Barrett PHR, Hegele RA, van Bockxmeer FM, Zhang H, Vance DE, McKnight CJ, Yao Z. Missense mutations in APOB within the βα1 domain of human APOB-100 result in impaired secretion of ApoB and ApoB-containing lipoproteins in familial hypobetalipoproteinemia. J Biol Chem. 2007;282:24270–24283. doi: 10.1074/jbc.M702442200. [DOI] [PubMed] [Google Scholar]
- 63.Burr ML, Cano F, Svobodova S, Boyle LH, Boname JM, Lehner PJ. HRD1 and UBE2J1 target misfolded MHC class I heavy chains for endoplasmic reticulum-associated degradation. Proc Natl Acad Sci USA. 2011;108:2034–2039. doi: 10.1073/pnas.1016229108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Burrows JA, Willis LK, Perlmutter DH. Chemical chaperones mediate increased secretion of mutant alpha 1-antitrypsin (alpha 1-AT) Z: a potential pharmacological strategy for prevention of liver injury and emphysema in alpha 1-AT deficiency. Proc Natl Acad Sci USA. 2000;97:1796–1801. doi: 10.1073/pnas.97.4.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bygrave FL, Benedetti A. What is the concentration of calcium ions in the endoplasmic reticulum? Cell Calcium. 1996;19:547–551. doi: 10.1016/s0143-4160(96)90064-0. [DOI] [PubMed] [Google Scholar]
- 66.Cabral CM, Liu Y, Moremen KW, Sifers RN. Organizational diversity among distinct glycoprotein endoplasmic reticulum-associated degradation programs. Mol Biol Cell. 2002;13:2639–2650. doi: 10.1091/mbc.E02-02-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cadwell K, Coscoy L. Ubiquitination on nonlysine residues by a viral E3 ubiquitin ligase. Science. 2005;309:127–130. doi: 10.1126/science.1110340. [DOI] [PubMed] [Google Scholar]
- 68.Campos EI, Reinberg D. Histones: annotating chromatin. Annu Rev Genet. 2009;43:559–599. doi: 10.1146/annurev.genet.032608.103928. [DOI] [PubMed] [Google Scholar]
- 69.Carafoli E. Calcium signaling: a tale for all seasons. Proc Natl Acad Sci USA. 2002;99:1115–1122. doi: 10.1073/pnas.032427999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Carvalho P, Stanley AM, Rapoport TA. Retrotranslocation of a misfolded luminal ER protein by the ubiquitin-ligase Hrd1p. Cell. 2010;143:579–591. doi: 10.1016/j.cell.2010.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chakrabarti O, Ashok A, Hegde RS. Prion protein biosynthesis and its emerging role in neurodegeneration. Trends Biochem Sci. 2009;34:287–295. doi: 10.1016/j.tibs.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chang YC, Hung WT, Chang YC, Chang HC, Wu CL, Chiang AS, Jackson GR, Sang TK. Pathogenic VCP/TER94 alleles are dominant actives and contribute to neurode-generation by altering cellular ATP level in a Drosophila IBMPFD model. PLoS Genet. 2011;7:e1001288. doi: 10.1371/journal.pgen.1001288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chao SC, Chen JS, Tsai CH, Lin JM, Lin YJ, Sun HS. Novel exon nucleotide substitution at the splice junction causes a neonatal Marfan syndrome. Clin Genet. 2010;77:453–463. doi: 10.1111/j.1399-0004.2009.01337.x. [DOI] [PubMed] [Google Scholar]
- 74.Chapman DC, Williams DB. ER quality control in the biogenesis of MHC class I molecules. Semin Cell Dev Biol. 2010;21:512–519. doi: 10.1016/j.semcdb.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 75.Chen EY, Bartlett MC, Loo TW, Clarke DM. The DeltaF508 mutation disrupts packing of the transmembrane segments of the cystic fibrosis transmembrane conductance regulator. J Biol Chem. 2004;279:39620–39627. doi: 10.1074/jbc.M407887200. [DOI] [PubMed] [Google Scholar]
- 76.Chen WY, Bailey EC, McCune SL, Dong JY, Townes TM. Reactivation of silenced, virally transduced genes by inhibitors of histone deacetylase. Proc Natl Acad Sci USA. 1997;94:5798–5803. doi: 10.1073/pnas.94.11.5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cheng SH, Gregory RJ, Marshall J, Paul S, Souza DW, White GA, O’Riordan CR, Smith AE. Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell. 1990;63:827–834. doi: 10.1016/0092-8674(90)90148-8. [DOI] [PubMed] [Google Scholar]
- 78.Christiansen HE, Schwarze U, Pyott SM, AlSwaid A, Al Balwi M, Alrasheed S, Pepin MG, Weis MA, Eyre DR, Byers PH. Homozygosity for a missense mutation in SER-PINH1, which encodes the collagen chaperone protein HSP47, results in severe recessive osteogenesis imperfecta. Am J Hum Genet. 2010;86:389–398. doi: 10.1016/j.ajhg.2010.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Christianson JC, Shaler TA, Tyler RE, Kopito RR. OS-9 and GRP94 deliver mutant α1-antitrypsin to the Hrd1-SEL1L ubiquitin ligase complex for ERAD. Nat Cell Biol. 2008;10:272–282. doi: 10.1038/ncb1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Christis C, Lubsen NH, Braakman I. Protein folding includes oligomerization: examples from the endoplasmic reticulum and cytosol. FEBS J. 2008;275:4700–4727. doi: 10.1111/j.1742-4658.2008.06590.x. [DOI] [PubMed] [Google Scholar]
- 81.Chung DH, Ohashi K, Watanabe M, Miyasaka N, Hirosawa S. Mannose trimming targets mutant α2-plasmin inhibitor for degradation by the proteasome. J Biol Chem. 2000;275:4981–4987. doi: 10.1074/jbc.275.7.4981. [DOI] [PubMed] [Google Scholar]
- 82.Ciechanover A. The ubiquitin-proteasome pathway: on protein death and cell life. EMBO J. 1998;17:7151–7160. doi: 10.1093/emboj/17.24.7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Clague MJ, Urbé S. Ubiquitin: same molecule, different degradation pathways. Cell. 2010;143:682–685. doi: 10.1016/j.cell.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 84.Clerc S, Hirsch C, Oggier DM, Deprez P, Jakob C, Sommer T, Aebi M. Htm1 protein generates the N-glycan signal for glycoprotein degradation in the endoplasmic reticulum. J Cell Biol. 2009;184:159–172. doi: 10.1083/jcb.200809198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Coe H, Michalak M. Calcium binding chaperones of the endoplasmic reticulum. Gen Physiol Biophys. 2009;28:F96–F103. [PubMed] [Google Scholar]
- 86.Cohen MMJ, Leboucher GP, Livnat-Levanon N, Glickman MH, Weissman AM. Ubiquitin-proteasome-dependent degradation of a mitofusin, a critical regulator of mitochondrial fusion. Mol Biol Cell. 2008;19:2457–2464. doi: 10.1091/mbc.E08-02-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cormier JH, Tamura T, Sunryd JC, Hebert DN. EDEM1 recognition and delivery of misfolded proteins to the SEL1L-containing ERAD complex. Mol Cell. 2009;34:627–633. doi: 10.1016/j.molcel.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cox JS, Shamu CE, Walter P. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell. 1993;73:1197–1206. doi: 10.1016/0092-8674(93)90648-a. [DOI] [PubMed] [Google Scholar]
- 89.Craig EA, Huang P, Aron R, Andrew A. The diverse roles of J-proteins, the obligate Hsp70 co-chaperone. Rev Physiol Biochem Pharmacol. 2006;156:1–21. doi: 10.1007/s10254-005-0001-0. [DOI] [PubMed] [Google Scholar]
- 90.Crawshaw SG, Martoglio B, Meacock SL, High S. A misassembled transmembrane domain of a polytopic protein associates with signal peptide peptidase. Biochem J. 2004;384:9–17. doi: 10.1042/BJ20041216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Credle JJ, Finer-Moore JS, Papa FR, Stroud RM, Walter P. On the mechanism of sensing unfolded protein in the endoplasmic reticulum. Proc Natl Acad Sci USA. 2005;102:18773–18784. doi: 10.1073/pnas.0509487102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Crouch PJ, Harding SME, White AR, Camakaris J, Bush AI, Masters CL. Mechanisms of Aβ mediated neurodegeneration in Alzheimer’s disease. Int J Biochem Cell Biol. 2008;40:181–198. doi: 10.1016/j.biocel.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 93.Cunard R, Sharma K. The endoplasmic reticulum stress response and diabetic kidney disease. Am J Physiol Renal Physiol. 2011;300:F1054–F1061. doi: 10.1152/ajprenal.00021.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dalemans W, Barbry P, Champigny G, Jallat S, Dott K, Dreyer D, Crystal RG, Pavirani A, Lecocq JP, Lazdunski M. Altered chloride ion channel kinetics associated with the delta F508 cystic fibrosis mutation. Nature. 1991;354:526–528. doi: 10.1038/354526a0. [DOI] [PubMed] [Google Scholar]
- 95.De Bie P, van de Sluis B, Burstein E, van de Berghe PVE, Muller P, Berger R, Gitlin JD, Wijmenga C, Klomp LWJ. Distinct Wilson’s disease mutations in ATP7B are associated with enhanced binding to COMMD1 and reduced stability of ATP7B. Gastroenterology. 2007;133:1316–1326. doi: 10.1053/j.gastro.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dean MC. The Human ATP-Binding Cassette (ABC) Transporter Superfamily. Bethesda, MD: NCBI; 2002. [Google Scholar]
- 97.Deeks ED, Cook JP, Day PJ, Smith DC, Roberts LM, Lord JM. The low lysine content of ricin A chain reduces the risk of proteolytic degradation after translocation from the endoplasmic reticulum to the cytosol. Biochemistry. 2002;41:3405–3413. doi: 10.1021/bi011580v. [DOI] [PubMed] [Google Scholar]
- 98.Del Val M, Iborra S, Ramos M, Lázaro S. Generation of MHC class I ligands in the secretory and vesicular pathways. Cell Mol Life Sci. 2011;68:1543–1552. doi: 10.1007/s00018-011-0661-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.DeLeo FR, Goedken M, McCormick SJ, Nauseef WM. A novel form of hereditary myeloperoxidase deficiency linked to endoplasmic reticulum/proteasome degradation. J Clin Invest. 1998;101:2900–2909. doi: 10.1172/JCI2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Denning GM, Anderson MP, Amara JF, Marshall J, Smith AE, Welsh MJ. Processing of mutant cystic fibrosis transmembrane conductance regulator is temperature-sensitive. Nature. 1992;358:761–764. doi: 10.1038/358761a0. [DOI] [PubMed] [Google Scholar]
- 101.Deuquet J, Lausch E, Guex N, Abrami L, Salvi S, Lakkaraju A, Ramirez MCM, Martignetti JA, Rokicki D, Bonafe L, Superti-Furga A, van der Goot FG. Hyaline Fibromatosis Syndrome inducing mutations in the ectodomain of anthrax toxin receptor 2 can be rescued by proteasome inhibitors. EMBO Mol Med. 2011;3:208–221. doi: 10.1002/emmm.201100124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Di Cola A, Frigerio L, Lord JM, Ceriotti A, Roberts LM. Ricin A chain without its partner B chain is degraded after retrotranslocation from the endoplasmic reticulum to the cytosol in plant cells. Proc Natl Acad Sci USA. 2001;98:14726–14731. doi: 10.1073/pnas.251386098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Didier C, Broday L, Bhoumik A, Israeli S, Takahashi S, Nakayama K, Thomas SM, Turner CE, Henderson S, Sabe H, Ronai ZE. RNF5, a RING finger protein that regulates cell motility by targeting paxillin ubiquitination and altered localization. Mol Cell Biol. 2003;23:5331–5345. doi: 10.1128/MCB.23.15.5331-5345.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.DiFiglia M, Sapp E, Chase KO, Davies SW, Bates GP, Vonsattel JP, Aronin N. Aggregation of Huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science. 1997;277:1990–1993. doi: 10.1126/science.277.5334.1990. [DOI] [PubMed] [Google Scholar]
- 105.Dill KA, Ozkan SB, Shell MS, Weikl TR. The protein folding problem. Annu Rev Biophys. 2008;37:289–316. doi: 10.1146/annurev.biophys.37.092707.153558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dobson CM. Protein folding and misfolding. Nature. 2003;426:884–890. doi: 10.1038/nature02261. [DOI] [PubMed] [Google Scholar]
- 107.Donaldson JG, Williams DB. Intracellular assembly and trafficking of MHC class I molecules. Traffic. 2009;10:1745–1752. doi: 10.1111/j.1600-0854.2009.00979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dong M, Bridges JP, Apsley K, Xu Y, Weaver TE. ERdj4 and ERdj5 are required for endoplasmic reticulum-associated protein degradation of misfolded surfactant protein C. Mol Biol Cell. 2008;19:2620–2630. doi: 10.1091/mbc.E07-07-0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dormer RL, Dérand R, McNeilly CM, Mettey Y, Bulteau-Pignoux L, Métayé T, Vierfond JM, Gray MA, Galietta LJV, Morris MR, Pereira MMC, Doull IJM, Becq F, McPherson MA. Correction of delF508-CFTR activity with benzo(c)quinolizinium compounds through facilitation of its processing in cystic fibrosis airway cells. J Cell Sci. 2001;114:4073–4081. doi: 10.1242/jcs.114.22.4073. [DOI] [PubMed] [Google Scholar]
- 110.Drisaldi B, Stewart RS, Adles C, Stewart LR, Quaglio E, Biasini E, Fioriti L, Chiesa R, Harris DA. Mutant PrP is delayed in its exit from the endoplasmic reticulum, but neither wild-type nor mutant PrP undergoes retrotranslocation prior to proteasomal degradation. J Biol Chem. 2003;278:21732–21743. doi: 10.1074/jbc.M213247200. [DOI] [PubMed] [Google Scholar]
- 111.Drumm ML, Wilkinson DJ, Smit LS, Worrell RT, Strong TV, Frizzell RA, Dawson DC, Collins FS. Chloride conductance expressed by delta F508 and other mutant CFTRs in Xenopus oocytes. Science. 1991;254:1797–1799. doi: 10.1126/science.1722350. [DOI] [PubMed] [Google Scholar]
- 112.Du K, Lukacs GL. Cooperative assembly and misfolding of CFTR domains in vivo. Mol Biol Cell. 2009;20:1903–1915. doi: 10.1091/mbc.E08-09-0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Du K, Sharma M, Lukacs GL. The DeltaF508 cystic fibrosis mutation impairs domain-domain interactions and arrests post-translational folding of CFTR. Nat Struct Mol Biol. 2005;12:17–25. doi: 10.1038/nsmb882. [DOI] [PubMed] [Google Scholar]
- 114.Duennwald ML, Lindquist S. Impaired ERAD and ER stress are early and specific events in polyglutamine toxicity. Genes Dev. 2008;22:3308–3319. doi: 10.1101/gad.1673408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Egan ME, Pearson M, Weiner SA, Rajendran V, Rubin D, Glockner-Pagel J, Canny S, Du K, Lukacs GL, Caplan MJ. Curcumin, a major constituent of turmeric, corrects cystic fibrosis defects. Science. 2004;304:600–602. doi: 10.1126/science.1093941. [DOI] [PubMed] [Google Scholar]
- 116.Ekeowa UI, Gooptu B, Belorgey D, Hagglof P, Karlsson-Li S, Miranda E, Perez J, MacLeod I, Kroger H, Marciniak SJ, Crowther DC, Lomas DA. alpha1-Antitrypsin deficiency, chronic obstructive pulmonary disease and the serpinopathies. Clin Sci. 2009;116:837–850. doi: 10.1042/CS20080484. [DOI] [PubMed] [Google Scholar]
- 117.Elliott PR, Lomas DA, Carrell RW, Abrahams JP. Inhibitory conformation of the reactive loop of alpha 1-antitrypsin. Nat Struct Biol. 1996;3:676–681. doi: 10.1038/nsb0896-676. [DOI] [PubMed] [Google Scholar]
- 118.Ellis RJ. Macromolecular crowding: obvious but underappreciated. Trends Biochem Sci. 2001;26:597–604. doi: 10.1016/s0968-0004(01)01938-7. [DOI] [PubMed] [Google Scholar]
- 119.Epstein J, Sanderson IR, Macdonald TT. Curcumin as a therapeutic agent: the evidence from in vitro, animal and human studies. Br J Nutr. 2010;103:1545–1557. doi: 10.1017/S0007114509993667. [DOI] [PubMed] [Google Scholar]
- 120.Ernst R, Claessen JHL, Mueller B, Sanyal S, Spooner E, van der Veen AG, Kirak O, Schlieker CD, Weihofen WA, Ploegh HL. Enzymatic blockade of the ubiquitin-proteasome pathway. PLoS Biol. 2011;8:e1000605. doi: 10.1371/journal.pbio.1000605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Esapa CT, Waite A, Locke M, Benson MA, Kraus M, McIlhinney RAJ, Sillitoe RV, Beesley PW, Blake DJ. SGCE missense mutations that cause myoclonus-dystonia syndrome impair ε-sarcoglycan trafficking to the plasma membrane: modulation by ubiquitination and torsinA. Hum Mol Genet. 2007;16:327–342. doi: 10.1093/hmg/ddl472. [DOI] [PubMed] [Google Scholar]
- 122.Fan JQ, Ishii S, Asano N, Suzuki Y. Accelerated transport and maturation of lysosomal alpha-galactosidase A in Fabry lymphoblasts by an enzyme inhibitor. Nat Med. 1999;5:112–115. doi: 10.1038/4801. [DOI] [PubMed] [Google Scholar]
- 123.Fang S, Ferrone M, Yang C, Jensen JP, Tiwari S, Weissman AM. The tumor autocrine motility factor receptor, gp78, is a ubiquitin protein ligase implicated in degradation from the endoplasmic reticulum. Proc Natl Acad Sci USA. 2001;98:14422–14427. doi: 10.1073/pnas.251401598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Farinha CM, Amaral MD. Most F508del-CFTR is targeted to degradation at an early folding checkpoint and independently of calnexin. Mol Cell Biol. 2005;25:5242–5252. doi: 10.1128/MCB.25.12.5242-5252.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Farinha CM, Nogueira P, Mendes F, Penque D, Amaral MD. The human DnaJ homologue (Hdj)-1/heat-shock protein (Hsp) 40 co-chaperone is required for the in vivo stabilization of the cystic fibrosis transmembrane conductance regulator by Hsp70. Biochem J. 2002;366:797–806. doi: 10.1042/BJ20011717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ficker E, Dennis AT, Wang L, Brown AM. Role of the cytosolic chaperones Hsp70 and Hsp90 in maturation of the cardiac potassium channel hERG. Circ Res. 2003;92:e87–e100. doi: 10.1161/01.RES.0000079028.31393.15. [DOI] [PubMed] [Google Scholar]
- 127.Ficker E, Obejero-Paz CA, Zhao S, Brown AM. The binding site for channel blockers that rescue misprocessed human long qt syndrome type 2 ether-a-gogo-related gene (HERG) mutations. J Biol Chem. 2002;277:4989–4998. doi: 10.1074/jbc.M107345200. [DOI] [PubMed] [Google Scholar]
- 128.Fiebiger E, Story C, Ploegh HL, Tortorella D. Visualization of the ER-to-cytosol dislocation reaction of a type I membrane protein. EMBO J. 2002;21:1041–1053. doi: 10.1093/emboj/21.5.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Finley D. Recognition and processing of ubiquitin-protein conjugates by the proteasome. Annu Rev Biochem. 2009;78:477–513. doi: 10.1146/annurev.biochem.78.081507.101607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fischer H, Fukuda N, Barbry P, Illek B, Sartori C, Matthay MA. Partial restoration of defective chloride conductance in ΔF508 CF mice by trimethylamine oxide. Am J Physiol Lung Cell Mol Physiol. 2001;281:L52–L57. doi: 10.1152/ajplung.2001.281.1.L52. [DOI] [PubMed] [Google Scholar]
- 131.Fisher EA, Ginsberg HN. Complexity in the secretory pathway: the assembly and secretion of apolipoprotein B-containing lipoproteins. J Biol Chem. 2002;277:17377–17380. doi: 10.1074/jbc.R100068200. [DOI] [PubMed] [Google Scholar]
- 132.Fitzgerald J, Lamandé SR, Bateman JF. Proteasomal degradation of unassembled mutant type I collagen Pro-α1(I) chains. J Biol Chem. 1999;274:27392–27398. doi: 10.1074/jbc.274.39.27392. [DOI] [PubMed] [Google Scholar]
- 133.Flynn GC, Pohl J, Flocco MT, Rothman JE. Peptide-binding specificity of the molecular chaperone BiP. Nature. 1991;353:726–730. doi: 10.1038/353726a0. [DOI] [PubMed] [Google Scholar]
- 134.Folmer DE, van der Mark VA, Ho-Mok KS, Oude Elferink RPJ, Paulusma CC. Differential effects of progressive familial intrahepatic cholestasis type 1 and benign recurrent intrahepatic cholestasis type 1 mutations on canalicular localization of ATP8B1. Hepatology. 2009;50:1597–1605. doi: 10.1002/hep.23158. [DOI] [PubMed] [Google Scholar]
- 135.Fonseca SG, Urano F, Burcin M, Gromada J. Stress hypER activation in the beta-cell. Islets. 2010;2:1–9. doi: 10.4161/isl.2.1.10456. [DOI] [PubMed] [Google Scholar]
- 136.Forster ML, Mahn JJ, Tsai B. Generating an unfoldase from thioredoxin-like domains. J Biol Chem. 2009;284:13045–13056. doi: 10.1074/jbc.M808352200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Forster ML, Sivick K, Park YN, Arvan P, Lencer WI, Tsai B. Protein disulfide isomerase-like proteins play opposing roles during retrotranslocation. J Cell Biol. 2006;173:853–859. doi: 10.1083/jcb.200602046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fortun J, Li J, Go J, Fenstermaker A, Fletcher BS, Notterpek L. Impaired proteasome activity and accumulation of ubiquitinated substrates in a hereditary neuropathy model. J Neurochem. 2005;92:1531–1541. doi: 10.1111/j.1471-4159.2004.02987.x. [DOI] [PubMed] [Google Scholar]
- 139.Fortun J, Verrier JD, Go JC, Madorsky I, Dunn WA, Notterpek L. The formation of peripheral myelin protein 22 aggregates is hindered by the enhancement of autophagy and expression of cytoplasmic chaperones. Neurobiol Dis. 2007;25:252–265. doi: 10.1016/j.nbd.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Friberg MA, Spiess M, Rutishauser J. Degradation of wild-type vasopressin precursor and pathogenic mutants by the proteasome. J Biol Chem. 2004;279:19441–19447. doi: 10.1074/jbc.M310249200. [DOI] [PubMed] [Google Scholar]
- 141.Fry WHD, Simion C, Sweeney C, Carraway KL., III Quantity control of the ErbB3 receptor tyrosine kinase at the endoplasmic reticulum. Mol Cell Biol. 2011;31:3009–3018. doi: 10.1128/MCB.05105-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fuchs SY, Spiegelman VS, Suresh Kumar KG. The many faces of β-TrCP E3 ubiquitin ligases: reflections in the magic mirror of cancer. Oncogene. 2004;23:2028–2036. doi: 10.1038/sj.onc.1207389. [DOI] [PubMed] [Google Scholar]
- 143.Fujita E, Kouroku Y, Isoai A, Kumagai H, Misutani A, Matsuda C, Hayashi YK, Momoi T. Two endoplasmic reticulum-associated degradation (ERAD) systems for the novel variant of the mutant dysferlin: ubiquitin/proteasome ERAD(I) and autophagy/lysosome ERAD(II) Hum Mol Genet. 2007;16:618–629. doi: 10.1093/hmg/ddm002. [DOI] [PubMed] [Google Scholar]
- 144.Furman MH, Ploegh HL, Tortorella D. Membrane-specific, host-derived factors are required for US2- and US11-mediated degradation of major histocompatibility complex class I molecules. J Biol Chem. 2002;277:3258–3267. doi: 10.1074/jbc.M109765200. [DOI] [PubMed] [Google Scholar]
- 145.Furukawa T, Wakabayashi K, Tamura A, Nakagawa H, Morishima Y, Osawa Y, Ishikawa T. Major SNP (Q141K) variant of human ABC transporter ABCG2 undergoes lysosomal and proteasomal degradations. Pharm Res. 2009;26:469–479. doi: 10.1007/s11095-008-9752-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Furutani M, Trudeau MC, Hagiwara N, Seki A, Gong Q, Zhou Z, Imamura Si Nagashima H, Kasanuki H, Takao A, Momma K, January CT, Robertson GA, Matsuoka R. Novel mechanism associated with an inherited cardiac arrhythmia: defective protein trafficking by the mutant HERG (G601S) potassium channel. Circulation. 1999;99:2290–2294. doi: 10.1161/01.cir.99.17.2290. [DOI] [PubMed] [Google Scholar]
- 147.Gaczynska M, Osmulski PA. Small-molecule inhibitors of proteasome activity. Methods Mol Biol. 2005;301:3–22. doi: 10.1385/1-59259-895-1:003. [DOI] [PubMed] [Google Scholar]
- 148.Gadsby DC, Vergani P, Csanady L. The ABC protein turned chloride channel whose failure causes cystic fibrosis. Nature. 2006;440:477–483. doi: 10.1038/nature04712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gallagher MJ, Ding L, Maheshwari A, Macdonald RL. The GABAA receptor α1 subunit epilepsy mutation A322D inhibits transmembrane helix formation and causes proteasomal degradation. Proc Natl Acad Sci USA. 2007;104:12999–13004. doi: 10.1073/pnas.0700163104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gao H, Wang Y, Wegierski T, Skouloudaki K, Pütz M, Fu X, Engel C, Boehlke C, Peng H, Kuehn EW, Kim E, Kramer-Zucker A, Walz G. PRKCSH/80K-H, the protein mutated in polycystic liver disease, protects polycystin-2/TRPP2 against HERP-mediated degradation. Hum Mol Genet. 2010;19:16–24. doi: 10.1093/hmg/ddp463. [DOI] [PubMed] [Google Scholar]
- 151.Gardner AM, Aviel S, Argon Y. Rapid degradation of an unassembled immunoglobulin light chain is mediated by a serine protease and occurs in a pre-Golgi compartment. J Biol Chem. 1993;268:25940–25947. [PubMed] [Google Scholar]
- 152.Garza RM, Sato BK, Hampton RY. In vitro analysis of Hrd1p-mediated retrotranslocation of its multispanning membrane substrate 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase. J Biol Chem. 2009;284:14710–14722. doi: 10.1074/jbc.M809607200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gastaldello S, D’Angelo S, Franzoso S, Fanin M, Angelini C, Betto R, Sandonà D. Inhibition of proteasome activity promotes the correct localization of disease-causing α-sarcoglycan mutants in HEK-293 cells constitutively expressing β-, γ-, and δ-sarcoglycan. Am J Pathol. 2008;173:170–181. doi: 10.2353/ajpath.2008.071146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gauss R, Kanehara K, Carvalho P, Ng Davis TW, Aebi M. A complex of Pdi1p and the mannosidase Htm1p initiates clearance of unfolded glycoproteins from the endoplasmic reticulum. Mol Cell. 2011;42:782–793. doi: 10.1016/j.molcel.2011.04.027. [DOI] [PubMed] [Google Scholar]
- 155.Gelsthorpe ME, Baumann N, Millard E, Gale SE, Langmade SJ, Schaffer JE, Ory DS. Niemann-Pick type C1 I1061T mutant encodes a functional protein that is selected for endoplasmic reticulum-associated degradation due to protein misfolding. J Biol Chem. 2008;283:8229–8236. doi: 10.1074/jbc.M708735200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gewurz BE, Ploegh HL, Tortorella D. US2, a human cytomegalovirus-encoded type I membrane protein, contains a non-cleavable amino-terminal signal peptide. J Biol Chem. 2002;277:11306–11313. doi: 10.1074/jbc.M107904200. [DOI] [PubMed] [Google Scholar]
- 157.Ghaemmaghami S, Huh WK, Bower K, Howson RW, Belle A, Dephoure N, O’Shea EK, Weissman JS. Global analysis of protein expression in yeast. Nature. 2003;425:737–741. doi: 10.1038/nature02046. [DOI] [PubMed] [Google Scholar]
- 158.Ghislain M, Dohmen RJ, Levy F, Varshavsky A. Cdc48p interacts with Ufd3p, a WD repeat protein required for ubiquitin-mediated proteolysis in Saccharomyces cerevisiae. EMBO J. 1996;15:4884–4899. [PMC free article] [PubMed] [Google Scholar]
- 159.Gil JM, Rego AC. Mechanisms of neurodegeneration in Huntington’s disease. Eur J Neurosci. 2008;27:2803–2820. doi: 10.1111/j.1460-9568.2008.06310.x. [DOI] [PubMed] [Google Scholar]
- 160.Giles LM, Chen J, Li L, Chin LS. Dystonia-associated mutations cause premature degradation of torsinA protein and cell-type-specific mislocalization to the nuclear envelope. Hum Mol Genet. 2008;17:2712–2722. doi: 10.1093/hmg/ddn173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Givol D, Goldberger RF, Anfinsen CB. Oxidation and disulfide interchange in the reactivation of reduced ribonuclease. J Biol Chem. 1964;239 PC3114-2712–PC3116. [PubMed] [Google Scholar]
- 162.Glozman R, Okiyoneda T, Mulvihill CM, Rini JM, Barriere H, Lukacs GL. N-glycans are direct determinants of CFTR folding and stability in secretory and endocytic membrane traffic. J Cell Biol. 2009;184:847–862. doi: 10.1083/jcb.200808124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gong Q, Keeney DR, Molinari M, Zhou Z. Degradation of trafficking-defective long QT syndrome type II mutant channels by the ubiquitin-proteasome pathway. J Biol Chem. 2005;280:19419–19425. doi: 10.1074/jbc.M502327200. [DOI] [PubMed] [Google Scholar]
- 164.Gooptu B, Lomas DA. Conformational pathology of the serpins: themes, variations, and therapeutic strategies. Annu Rev Biochem. 2009;78:147–176. doi: 10.1146/annurev.biochem.78.082107.133320. [DOI] [PubMed] [Google Scholar]
- 165.Gorlach A, Klappa P, Kietzmann T. The endoplasmic reticulum: folding, calcium homeostasis, signaling, and redox control. Antioxid Redox Signal. 2006;8:1391–1418. doi: 10.1089/ars.2006.8.1391. [DOI] [PubMed] [Google Scholar]
- 166.Gow A, Southwood CM, Lazzarini RA. Disrupted proteolipid protein trafficking results in oligodendrocyte apoptosis in an animal model of Pelizaeus-Merzbacher Disease. J Cell Biol. 1998;140:925–934. doi: 10.1083/jcb.140.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Griciuc A, Aron L, Piccoli G, Ueffing M. Clearance of RhodopsinP23H aggregates requires the ERAD effector VCP. Biochim Biophys Acta. 2010;1803:424–434. doi: 10.1016/j.bbamcr.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 168.Griciuc A, Aron L, Roux MJ, Klein R, Giangrande A, Ueffing M. Inactivation of VCP/ter94 suppresses retinal pathology caused by misfolded rhodopsin in Drosophila. PLoS Genet. 2010;6:e1001075. doi: 10.1371/journal.pgen.1001075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Groisman B, Shenkman M, Ron E, Lederkremer GZ. Mannose trimming is required for delivery of a glycoprotein from EDEM1 to XTP3-B and to late endoplasmic reticulum-associated degradation steps. J Biol Chem. 2011;286:1292–1300. doi: 10.1074/jbc.M110.154849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Grove DE, Fan CY, Ren HY, Cyr DM. The endoplasmic reticulum-associated Hsp40 DNAJB12 and Hsc70 cooperate to facilitate RMA1 E3-dependent degradation of nascent CFTRΔF508. Mol Biol Cell. 2011;22:301–314. doi: 10.1091/mbc.E10-09-0760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Grove DE, Rosser MFN, Ren HY, Naren AP, Cyr DM. Mechanisms for rescue of correctable folding defects in CFTRΔF508. Mol Biol Cell. 2009;20:4059–4069. doi: 10.1091/mbc.E08-09-0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gupta S, McGrath B, Cavener DR. PERK (EIF2AK3) regulates proinsulin trafficking and quality control in the secretory pathway. Diabetes. 2010;59:1937–1947. doi: 10.2337/db09-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gusarova V, Caplan AJ, Brodsky JL, Fisher EA. Apoprotein B degradation is promoted by the molecular chaperones hsp90 and hsp70. J Biol Chem. 2001;276:24891–24900. doi: 10.1074/jbc.M100633200. [DOI] [PubMed] [Google Scholar]
- 174.Haas IG, Wabl M. Immunoglobulin heavy chain binding protein. Nature. 1983;306:387–389. doi: 10.1038/306387a0. [DOI] [PubMed] [Google Scholar]
- 175.Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet. 2009;10:32–42. doi: 10.1038/nrg2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hagiwara M, Maegawa Ki Suzuki M, Ushioda R, Araki K, Matsumoto Y, Hoseki J, Nagata K, Inaba K. Structural basis of an ERAD pathway mediated by the ER-resident protein disulfide reductase ERdj5. Mol Cell. 2011;41:432–444. doi: 10.1016/j.molcel.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 177.Halaban R, Cheng E, Zhang Y, Moellmann G, Hanlon D, Michalak M, Setaluri V, Hebert DN. Aberrant retention of tyrosinase in the endoplasmic reticulum mediates accelerated degradation of the enzyme and contributes to the dedifferentiated phenotype of amelanotic melanoma cells. Proc Natl Acad Sci USA. 1997;94:6210–6215. doi: 10.1073/pnas.94.12.6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Halaban R, Svedine S, Cheng E, Smicun Y, Aron R, Hebert DN. Endoplasmic reticulum retention is a common defect associated with tyrosinase-negative albinism. Proc Natl Acad Sci USA. 2000;97:5889–5894. doi: 10.1073/pnas.97.11.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hamanaka R, Shinohara T, Yano S, Nakamura M, Yasuda A, Yokoyama S, Fan JQ, Kawasaki K, Watanabe M, Ishii S. Rescue of mutant α-galactosidase A in the endoplasmic reticulum by 1-deoxygalactonojirimycin leads to trafficking to lysosomes. Biochim Biophys Acta. 2008;1782:408–413. doi: 10.1016/j.bbadis.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 180.Hamilton JA, Benson MD. Transthyretin: a review from a structural perspective. Cell Mol Life Sci. 2001;58:1491–1521. doi: 10.1007/PL00000791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hammarström P, Jiang X, Hurshman AR, Powers ET, Kelly JW. Sequence-dependent denaturation energetics: a major determinant in amyloid disease diversity. Proc Natl Acad Sci USA. 2002;99:16427–16432. doi: 10.1073/pnas.202495199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hamosh A, King TM, Rosenstein BJ, Corey M, Levison H, Durie P, Tsui LC, McIntosh I, Keston M, Brock DJ. Cystic fibrosis patients bearing both the common missense mutation Gly—Asp at codon 551 and the delta F508 mutation are clinically indistinguishable from delta F508 homozygotes, except for decreased risk of meconium ileus. Am J Hum Genet. 1992;51:245–250. [PMC free article] [PubMed] [Google Scholar]
- 183.Hampton RY. ER-associated degradation in protein quality control and cellular regulation. Curr Opin Cell Biol. 2002;14:476–482. doi: 10.1016/s0955-0674(02)00358-7. [DOI] [PubMed] [Google Scholar]
- 184.Harada K, Okiyoneda T, Hashimoto Y, Ueno K, Nakamura K, Yamahira K, Sugahara T, Shuto T, Wada I, Suico MA, Kai H. Calreticulin negatively regulates the cell surface expression of cystic fibrosis transmembrane conductance regulator. J Biol Chem. 2006;281:12841–12848. doi: 10.1074/jbc.M512975200. [DOI] [PubMed] [Google Scholar]
- 185.Harada M, Sakisaka S, Terada K, Kimura R, Kawaguchi T, Koga H, Kim M, Taniguchi E, Hanada S, Suganuma T, Furuta K, Sugiyama T, Sata M. A mutation of the Wilson disease protein, ATP7B, is degraded in the proteasomes and forms protein aggregates. Gastroenterology. 2001;120:967–974. doi: 10.1053/gast.2001.22543. [DOI] [PubMed] [Google Scholar]
- 186.Hartley T, Siva M, Lai E, Teodoro T, Zhang L, Volchuk A. Endoplasmic reticulum stress response in an INS-1 pancreatic beta-cell line with inducible expression of a folding-deficient proinsulin. BMC Cell Biol. 2010;11:59. doi: 10.1186/1471-2121-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hashimoto K, Uchiumi T, Konno T, Ebihara T, Nakamura T, Wada M, Sakisaka S, Maniwa F, Amachi T, Ueda K, Kuwano M. Trafficking and functional defects by mutations of the ATP-binding domains in MRP2 in patients with Dubin-Johnson syndrome. Hepatology. 2002;36:1236–1245. doi: 10.1053/jhep.2002.36368. [DOI] [PubMed] [Google Scholar]
- 188.Hassink G, Kikkert M, van Voorden S, Lee SJ, Spaapen R, van Laar T, Coleman CS, Bartee E, Fruh K, Chau V, Wiertz E. TEB4 is a C4HC3 RING finger-containing ubiquitin ligase of the endoplasmic reticulum. Biochem J. 2005;388:647–655. doi: 10.1042/BJ20041241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hassink GC, Barel MT, Van Voorden SB, Kikkert M, Wiertz EJ. Ubiquitination of MHC class I heavy chains is essential for dislocation by human cytomegalovirus-encoded US2 but not US11. J Biol Chem. 2006;281:30063–30071. doi: 10.1074/jbc.M602248200. [DOI] [PubMed] [Google Scholar]
- 190.Hatahet F, Ruddock LW. Protein disulfide isomerase: a critical evaluation of its function in disulfide bond formation. Antioxidants & Redox Signaling. 2009;11:2807–2850. doi: 10.1089/ars.2009.2466. [DOI] [PubMed] [Google Scholar]
- 191.Haws CM, Nepomuceno IB, Krouse ME, Wakelee H, Law T, Xia Y, Nguyen H, Wine JJ. Delta F508-CFTR channels: kinetics, activation by forskolin, and potentiation by xanthines. Am J Physiol Cell Physiol. 1996;270:C1544–C1555. doi: 10.1152/ajpcell.1996.270.5.C1544. [DOI] [PubMed] [Google Scholar]
- 192.Hayashi H, Takada T, Suzuki H, Akita H, Sugiyama Y. Two common PFIC2 mutations are associated with the impaired membrane trafficking of BSEP/ABCB11. Hepatology. 2005;41:916–924. doi: 10.1002/hep.20627. [DOI] [PubMed] [Google Scholar]
- 193.Hazes B, Read RJ. Accumulating evidence suggests that several AB-toxins subvert the endoplasmic reticulum-associated protein degradation pathway to enter target cells. Biochemistry. 1997;36:11051–11054. doi: 10.1021/bi971383p. [DOI] [PubMed] [Google Scholar]
- 194.Hebert DN, Bernasconi R, Molinari M. ERAD substrates: Which way out? Semin Cell Dev Biol. 2010;21:526–532. doi: 10.1016/j.semcdb.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 195.Hegde NR, Chevalier MS, Wisner TW, Denton MC, Shire K, Frappier L, Johnson DC. The role of BiP in endoplasmic reticulum-associated degradation of major histocompatibility complex class I heavy chain induced by cytomegalovirus proteins. J Biol Chem. 2006;281:20910–20919. doi: 10.1074/jbc.M602989200. [DOI] [PubMed] [Google Scholar]
- 196.Hegde RS, Bernstein HD. The surprising complexity of signal sequences. Trends Biochem Sci. 2006;31:563–571. doi: 10.1016/j.tibs.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 197.Hegde RS, Mastrianni JA, Scott MR, DeFea KA, Tremblay P, Torchia M, DeArmond SJ, Prusiner SB, Lingappa VR. A transmembrane form of the prion protein in neurode-generative disease. Science. 1998;279:827–834. doi: 10.1126/science.279.5352.827. [DOI] [PubMed] [Google Scholar]
- 198.Hegde RS, Tremblay P, Groth D, DeArmond SJ, Prusiner SB, Lingappa VR. Transmissible and genetic prion diseases share a common pathway of neurodegeneration. Nature. 1999;402:822–826. doi: 10.1038/45574. [DOI] [PubMed] [Google Scholar]
- 199.Heo JM, Livnat-Levanon N, Taylor EB, Jones KT, Dephoure N, Ring J, Xie J, Brodsky JL, Madeo F, Gygi SP, Ashrafi K, Glickman MH, Rutter J. A stress-responsive system for mitochondrial protein degradation. Mol Cell. 2010;40:465–480. doi: 10.1016/j.molcel.2010.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Hidvegi T, Ewing M, Hale P, Dippold C, Beckett C, Kemp C, Maurice N, Mukherjee A, Goldbach C, Watkins S, Michalopoulos G, Perlmutter DH. An autophagy-enhancing drug promotes degradation of mutant α1-antitrypsin Z and reduces hepatic fibrosis. Science. 2010;329:229–232. doi: 10.1126/science.1190354. [DOI] [PubMed] [Google Scholar]
- 201.Hiller MM, Finger A, Schweiger M, Wolf DH. ER degradation of a misfolded luminal protein by the cytosolic ubiquitin-proteasome pathway. Science. 1996;273:1725–1728. doi: 10.1126/science.273.5282.1725. [DOI] [PubMed] [Google Scholar]
- 202.Hirano K, Zuber C, Roth J, Ziak M. The proteasome is involved in the degradation of different aquaporin-2 mutants causing nephrogenic diabetes insipidus. Am J Pathol. 2003;163:111–120. doi: 10.1016/S0002-9440(10)63635-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Hirsch C, Blom D, Ploegh HL. A role for N-glycanase in the cytosolic turnover of glycoproteins. EMBO J. 2003;22:1036–1046. doi: 10.1093/emboj/cdg107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Hirsch C, Gauss R, Horn SC, Neuber O, Sommer T. The ubiquitylation machinery of the endoplasmic reticulum. Nature. 2009;458:453–460. doi: 10.1038/nature07962. [DOI] [PubMed] [Google Scholar]
- 205.Hosokawa N, Tremblay LO, You Z, Herscovics A, Wada I, Nagata K. Enhancement of ER degradation of misfolded null Hong Kong alpha 1-antitrypsin by human ER mannosidase I. J Biol Chem. 2003;278:26287–26294. doi: 10.1074/jbc.M303395200. [DOI] [PubMed] [Google Scholar]
- 206.Hosokawa N, Wada I, Nagasawa K, Moriyama T, Okawa K, Nagata K. Human XTP3-B forms an endoplasmic reticulum quality control scaffold with the HRD1-SEL1L ubiquitin ligase complex and BiP. J Biol Chem. 2008;283:20914–20924. doi: 10.1074/jbc.M709336200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Howard M, Fischer H, Roux J, Santos BC, Gullans SR, Yancey PH, Welch WJ. Mammalian osmolytes and S-nitrosoglutathione promote Delta F508 cystic fibrosis transmembrane conductance regulator (CFTR) protein maturation and function. J Biol Chem. 2003;278:35159–35167. doi: 10.1074/jbc.M301924200. [DOI] [PubMed] [Google Scholar]
- 209.Huang Y, Breitwieser GE. Rescue of calcium-sensing receptor mutants by allosteric modulators reveals a conformational checkpoint in receptor biogenesis. J Biol Chem. 2007;282:9517–9525. doi: 10.1074/jbc.M609045200. [DOI] [PubMed] [Google Scholar]
- 210.Huang Y, Niwa Ji Sobue G, Breitwieser GE. Calcium-sensing receptor ubiquitination and degradation mediated by the E3 ubiquitin ligase dorfin. J Biol Chem. 2006;281:11610–11617. doi: 10.1074/jbc.M513552200. [DOI] [PubMed] [Google Scholar]
- 211.Hughes DA, Pastores GM. The pathophysiology of GD: current understanding and rationale for existing and emerging therapeutic approaches. WMW Wiener Medizinische Wochenschrift. 2010;160:594–599. doi: 10.1007/s10354-010-0864-4. [DOI] [PubMed] [Google Scholar]
- 212.Huntington JA, Read RJ, Carrell RW. Structure of a serpin-protease complex shows inhibition by deformation. Nature. 2000;407:923–926. doi: 10.1038/35038119. [DOI] [PubMed] [Google Scholar]
- 213.Husnjak K, Elsasser S, Zhang N, Chen X, Randles L, Shi Y, Hofmann K, Walters KJ, Finley D, Dikic I. Proteasome subunit Rpn13 is a novel ubiquitin receptor. Nature. 2008;453:481–488. doi: 10.1038/nature06926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Huster D, Hoppert M, Lutsenko S, Zinke J, Lehmann C, Mössner J, Berr F, Caca K. Defective cellular localization of mutant ATP7B in Wilson’s disease patients and hepatoma cell lines. Gastroenterology. 2003;124:335–345. doi: 10.1053/gast.2003.50066. [DOI] [PubMed] [Google Scholar]
- 215.Hutt DM, Herman D, Rodrigues APC, Noel S, Pilewski JM, Matteson J, Hoch B, Kellner W, Kelly JW, Schmidt A, Thomas PJ, Matsumura Y, Skach WR, Gentzsch M, Riordan JR, Sorscher EJ, Okiyoneda T, Yates JR, Lukacs GL, Frizzell RA, Manning G, Gottesfeld JM, Balch WE. Reduced histone deacetylase 7 activity restores function to misfolded CFTR in cystic fibrosis. Nat Chem Biol. 2010;6:25–33. doi: 10.1038/nchembio.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Illing ME, Rajan RS, Bence NF, Kopito RR. A rhodopsin mutant linked to autosomal dominant retinitis pigmentosa is prone to aggregate and interacts with the ubiquitin proteasome system. J Biol Chem. 2002;277:34150–34160. doi: 10.1074/jbc.M204955200. [DOI] [PubMed] [Google Scholar]
- 217.Imai Y, Soda M, Inoue H, Hattori N, Mizuno Y, Takahashi R. An unfolded putative transmembrane polypeptide, which can lead to endoplasmic reticulum stress, is a substrate of Parkin. Cell. 2001;105:891–902. doi: 10.1016/s0092-8674(01)00407-x. [DOI] [PubMed] [Google Scholar]
- 218.Imai Y, Soda M, Takahashi R. Parkin suppresses unfolded protein stress-induced cell death through its E3 ubiquitin-protein ligase activity. J Biol Chem. 2000;275:35661–35664. doi: 10.1074/jbc.C000447200. [DOI] [PubMed] [Google Scholar]
- 219.Imamura T, Haruta T, Takata Y, Usui I, Iwata M, Ishihara H, Ishiki M, Ishibashi O, Ueno E, Sasaoka T, Kobayashi M. Involvement of heat shock protein 90 in the degradation of mutant insulin receptors by the proteasome. J Biol Chem. 1998;273:11183–11188. doi: 10.1074/jbc.273.18.11183. [DOI] [PubMed] [Google Scholar]
- 220.Imamura T, Takata Y, Sasaoka T, Takada Y, Morioka H, Haruta T, Sawa T, Iwanishi M, Hu YG, Suzuki Y. Two naturally occurring mutations in the kinase domain of insulin receptor accelerate degradation of the insulin receptor and impair the kinase activity. J Biol Chem. 1994;269:31019–31027. [PubMed] [Google Scholar]
- 221.Ishida Y, Yamamoto A, Kitamura A, Lamande SR, Yoshimori T, Bateman JF, Kubota H, Nagata K. Autophagic elimination of misfolded procollagen aggregates in the endoplasmic reticulum as a means of cell protection. Mol Biol Cell. 2009;20:2744–2754. doi: 10.1091/mbc.E08-11-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Ishikawa T, Tamura A, Saito H, Wakabayashi K, Nakagawa H. Pharmacogenomics of the human ABC transporter ABCG2: from functional evaluation to drug molecular design. Naturwissenschaften. 2005;92:451–463. doi: 10.1007/s00114-005-0019-4. [DOI] [PubMed] [Google Scholar]
- 223.Ishikura S, Weissman AM, Bonifacino JS. Serine residues in the cytosolic tail of the T-cell antigen receptor α-chain mediate ubiquitination and endoplasmic reticulum-associated degradation of the unassembled protein. J Biol Chem. 2010;285:23916–23924. doi: 10.1074/jbc.M110.127936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ito D, Suzuki N. Molecular pathogenesis of seipin/BSCL2-related motor neuron diseases. Ann Neurol. 2007;61:237–250. doi: 10.1002/ana.21070. [DOI] [PubMed] [Google Scholar]
- 225.Ito M, Jameson JL. Molecular basis of autosomal dominant neurohypophysial diabetes insipidus. Cellular toxicity caused by the accumulation of mutant vasopressin precursors within the endoplasmic reticulum. J Clin Invest. 1997;99:1897–1905. doi: 10.1172/JCI119357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Jaeken J. Congenital disorders of glycosylation. Ann NY Acad Sci. 2010;1214:190–198. doi: 10.1111/j.1749-6632.2010.05840.x. [DOI] [PubMed] [Google Scholar]
- 227.Jarosch E, Taxis C, Volkwein C, Bordallo J, Finley D, Wolf DH, Sommer T. Protein dislocation from the ER requires polyubiquitination and the AAA-ATPase Cdc48. Nat Cell Biol. 2002;4:134–139. doi: 10.1038/ncb746. [DOI] [PubMed] [Google Scholar]
- 228.Jensen HK, Holst H, Jensen LG, Jørgensen MM, Andreasen PH, Jensen TG, Andresen BS, Heath F, Hansen PS, Neve S, Kristiansen K, Færgeman O, Kølvraa S, Bolund L, Gregersen N. A common W556S mutation in the LDL receptor gene of Danish patients with familial hypercholesterolemia encodes a transport-defective protein. Atherosclerosis. 1997;131:67–72. doi: 10.1016/s0021-9150(96)06059-5. [DOI] [PubMed] [Google Scholar]
- 229.Jensen TJ, Loo MA, Pind S, Williams DB, Goldberg AL, Riordan JR. Multiple proteolytic systems, including the proteasome, contribute to CFTR processing. Cell. 1995;83:129–135. doi: 10.1016/0092-8674(95)90241-4. [DOI] [PubMed] [Google Scholar]
- 230.Jo Y, Sguigna PV, DeBose-Boyd RA. Membrane-associated ubiquitin ligase complex containing gp78 mediates sterol-accelerated degradation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. J Biol Chem. 2011;286:15022–15031. doi: 10.1074/jbc.M110.211326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Johnston JA, Ward CL, Kopito RR. Aggresomes: a cellular response to misfolded proteins. J Cell Biol. 1998;143:1883–1898. doi: 10.1083/jcb.143.7.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Jones TR, Wiertz EJ, Sun L, Fish KN, Nelson JA, Ploegh HL. Human cytomegalovirus US3 impairs transport and maturation of major histocompatibility complex class I heavy chains. Proc Natl Acad Sci USA. 1996;93:11327–11333. doi: 10.1073/pnas.93.21.11327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kadowaki T, Kadowaki H, Accili D, Yazaki Y, Taylor SI. Substitution of arginine for histidine at position 209 in the alpha-subunit of the human insulin receptor. A mutation that impairs receptor dimerization and transport of receptors to the cell surface. J Biol Chem. 1991;266:21224–21231. [PubMed] [Google Scholar]
- 234.Kaiser CA, Preuss D, Grisafi P, Botstein D. Many random sequences functionally replace the secretion signal sequence of yeast invertase. Science. 1987;235:312–317. doi: 10.1126/science.3541205. [DOI] [PubMed] [Google Scholar]
- 235.Kamimoto T, Shoji S, Hidvegi T, Mizushima N, Umebayashi K, Perlmutter DH, Yoshimori T. Intracellular inclusions containing mutant α1-antitrypsin Z are propagated in the absence of autophagic activity. J Biol Chem. 2006;281:4467–4476. doi: 10.1074/jbc.M509409200. [DOI] [PubMed] [Google Scholar]
- 236.Kaneko M, Ishiguro M, Niinuma Y, Uesugi M, Nomura Y. Human HRD1 protects against ER stress-induced apoptosis through ER-associated degradation. FEBS Lett. 2002;532:147–152. doi: 10.1016/s0014-5793(02)03660-8. [DOI] [PubMed] [Google Scholar]
- 237.Kang JQ, Shen W, Macdonald RL. The GABRG2 mutation, Q351X, associated with generalized epilepsy with febrile seizures plus, has both loss of function and dominant-negative suppression. J Neurosci. 2009;29:2845–2856. doi: 10.1523/JNEUROSCI.4772-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Kang JQ, Shen W, Macdonald RL. Two molecular pathways (NMD and ERAD) contribute to a genetic epilepsy associated with the GABAA receptor GABRA1 PTC mutation, 975delC, S326fs328X. J Neurosci. 2009;29:2833–2844. doi: 10.1523/JNEUROSCI.4512-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Karbowski M, Youle RJ. Regulating mitochondrial outer membrane proteins by ubiquitination and proteasomal degradation. Curr Opin Cell Biol. 2011 doi: 10.1016/j.ceb.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Katsumi A, Kojima T, Senda T, Yamazaki T, Tsukamoto H, Sugiura I, Kobayashi S, Miyata T, Umeyama H, Saito H. The carboxyl-terminal region of protein C is essential for its secretion. Blood. 1998;91:3784–3791. [PubMed] [Google Scholar]
- 241.Katsumi A, Senda T, Yamashita Y, Yamazaki T, Hamaguchi M, Kojima T, Kobayashi S, Saito H. Protein C Nagoya, an elongated mutant of protein C, is retained within the endoplasmic reticulum and is associated with GRP78 and GRP94. Blood. 1996;87:4164–4175. [PubMed] [Google Scholar]
- 242.Keenan RJ, Freymann DM, Stroud RM, Walter P. The signal recognition particle. Annu Rev Biochem. 2001;70:755–775. doi: 10.1146/annurev.biochem.70.1.755. [DOI] [PubMed] [Google Scholar]
- 243.Keitel V, Nies AT, Brom M, Hummel-Eisenbeiss J, Spring H, Keppler D. A common Dubin-Johnson syndrome mutation impairs protein maturation and transport activity of MRP2 (ABCC2) Am J Physiol Gastrointest Liver Physiol. 2003;284:G165–G174. doi: 10.1152/ajpgi.00362.2002. [DOI] [PubMed] [Google Scholar]
- 244.Khushoo A, Yang Z, Johnson Arthur E, Skach William R. Ligand-driven vectorial folding of ribosome-bound human CFTR NBD1. Mol Cell. 2011;41:682–692. doi: 10.1016/j.molcel.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Kikkert M, Doolman R, Dai M, Avner R, Hassink G, van Voorden S, Thanedar S, Roitelman J, Chau V, Wiertz E. Human HRD1 is an E3 ubiquitin ligase involved in degradation of proteins from the endoplasmic reticulum. J Biol Chem. 2004;279:3525–3534. doi: 10.1074/jbc.M307453200. [DOI] [PubMed] [Google Scholar]
- 246.Kim M, Lee HS, LaForet G, McIntyre C, Martin EJ, Chang P, Kim TW, Williams M, Reddy PH, Tagle D, Boyce FM, Won L, Heller A, Aronin N, DiFiglia M. Mutant Huntingtin expression in clonal striatal cells: dissociation of inclusion formation and neuronal survival by caspase inhibition. J Neurosci. 1999;19:964–973. doi: 10.1523/JNEUROSCI.19-03-00964.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Kim SM, Acharya P, Engel JC, Correia MA. Liver cytochrome P450 3A ubiquitination in vivo by gp78/autocrine motility factor receptor and C terminus of Hsp70-interacting protein (CHIP) E3 ubiquitin ligases. J Biol Chem. 2010;285:35866–35877. doi: 10.1074/jbc.M110.167189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Kimata Y, Ishiwata-Kimata Y, Ito T, Hirata A, Suzuki T, Oikawa D, Takeuchi M, Kohno K. Two regulatory steps of ER-stress sensor Ire1 involving its cluster formation and interaction with unfolded proteins. J Cell Biol. 2007;179:75–86. doi: 10.1083/jcb.200704166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Kimchi-Sarfaty C, Oh JM, Kim IW, Sauna ZE, Calcagno AM, Ambudkar SV, Gottesman MM. A “silent” polymorphism in the MDR1 gene changes substrate specificity. Science. 2007;315:525–528. doi: 10.1126/science.1135308. [DOI] [PubMed] [Google Scholar]
- 250.Kirk KL, Wang W. A unified view of cystic fibrosis transmembrane conductance regulator (CFTR) gating: combining the allosterism of a ligand-gated channel with the enzymatic activity of an ATP-binding cassette (ABC) transporter. J Biol Chem. 2011;286:12813–12819. doi: 10.1074/jbc.R111.219634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Kisselev AF, Akopian TN, Woo KM, Goldberg AL. The sizes of peptides generated from protein by mammalian 26 and 20 S proteasomes. J Biol Chem. 1999;274:3363–3371. doi: 10.1074/jbc.274.6.3363. [DOI] [PubMed] [Google Scholar]
- 252.Kittanakom S, Cordat E, Akkarapatumwong V, Yenchitsomanus Pt, Reithmeier RAF. Trafficking defects of a novel autosomal recessive distal renal tubular acidosis mutant (S773P) of the human kidney anion exchanger (kAE1) J Biol Chem. 2004;279:40960–40971. doi: 10.1074/jbc.M405356200. [DOI] [PubMed] [Google Scholar]
- 253.Kjaer S, Hanrahan S, Totty N, McDonald NQ. Mammal-restricted elements predispose human RET to folding impairment by HSCR mutations. Nat Struct Mol Biol. 2010;17:726–731. doi: 10.1038/nsmb.1808. [DOI] [PubMed] [Google Scholar]
- 254.Kjaer S, Ibáñez CF. Intrinsic susceptibility to misfolding of a hot-spot for Hirschsprung disease mutations in the ectodomain of RET. Hum Mol Genet. 2003;12:2133–2144. doi: 10.1093/hmg/ddg227. [DOI] [PubMed] [Google Scholar]
- 255.Klabunde T, Petrassi HM, Oza VB, Raman P, Kelly JW, Sacchettini JC. Rational design of potent human transthyretin amyloid disease inhibitors. Nat Struct Biol. 2000;7:312–321. doi: 10.1038/74082. [DOI] [PubMed] [Google Scholar]
- 256.Kleizen B, Braakman I. Protein folding and quality control in the endoplasmic reticulum. Curr Opin Cell Biol. 2004;16:343–349. doi: 10.1016/j.ceb.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 257.Kleizen B, van Vlijmen T, de Jonge HR, Braakman I. Folding of CFTR is predominantly cotranslational. Mol Cell. 2005;20:277–287. doi: 10.1016/j.molcel.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 258.Kleopa KA, Yum SW, Scherer SS. Cellular mechanisms of connexin32 mutations associated with CNS manifestations. J Neurosci Res. 2002;68:522–534. doi: 10.1002/jnr.10255. [DOI] [PubMed] [Google Scholar]
- 259.Knittler MR, Dirks S, Haas IG. Molecular chaperones involved in protein degradation in the endoplasmic reticulum: quantitative interaction of the heat shock cognate protein BiP with partially folded immunoglobulin light chains that are degraded in the endoplasmic reticulum. Proc Natl Acad Sci USA. 1995;92:1764–1768. doi: 10.1073/pnas.92.5.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Kny M, Standera S, Hartmann-Petersen R, Kloetzel PM, Seeger M. Herp regulates Hrd1-mediated ubiquitylation in a ubiquitin-like domain-dependent manner. J Biol Chem. 2011;286:5151–5156. doi: 10.1074/jbc.M110.134551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Koegl M, Hoppe T, Schlenker S, Ulrich HD, Mayer TU, Jentsch S. A novel ubiquitination factor, E4, is involved in multiubiquitin chain assembly. Cell. 1999;96:635–644. doi: 10.1016/s0092-8674(00)80574-7. [DOI] [PubMed] [Google Scholar]
- 262.Kohno K, Normington K, Sambrook J, Gething MJ, Mori K. The promoter region of the yeast KAR2 (BiP) gene contains a regulatory domain that responds to the presence of unfolded proteins in the endoplasmic reticulum. Mol Cell Biol. 1993;13:877–890. doi: 10.1128/mcb.13.2.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Koopmann JO, Albring J, Hüter E, Bulbuc N, Spee P, Neefjes J, Hämmerling GJ, Momburg F. Export of antigenic peptides from the endoplasmic reticulum intersects with retrograde protein translocation through the Sec61p channel. Immunity. 2000;13:117–127. doi: 10.1016/s1074-7613(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 264.Korkotian E, Schwarz A, Pelled D, Schwarzmann G, Segal M, Futerman AH. Elevation of intracellular glucosylceramide levels results in an increase in endoplasmic reticulum density and in functional calcium stores in cultured neurons. J Biol Chem. 1999;274:21673–21678. doi: 10.1074/jbc.274.31.21673. [DOI] [PubMed] [Google Scholar]
- 265.Kornfeld S. Diseases of abnormal protein glycosylation: an emerging area. J Clin Invest. 1998;101:1293–1295. doi: 10.1172/JCI3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Kosmaoglou M, Kanuga N, Aguilà M, Garriga P, Cheetham ME. A dual role for EDEM1 in the processing of rod opsin. J Cell Sci. 2009;122:4465–4472. doi: 10.1242/jcs.055228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Kothe M, Ye Y, Wagner JS, De Luca HE, Kern E, Rapoport TA, Lencer WI. Role of p97 AAA-ATPase in the retrotranslocation of the cholera toxin A1 chain, a non-ubiquitinated substrate. J Biol Chem. 2005;280:28127–28132. doi: 10.1074/jbc.M503138200. [DOI] [PubMed] [Google Scholar]
- 268.Koulov AV, LaPointe P, Lu B, Razvi A, Coppinger J, Dong MQ, Matteson J, Laister R, Arrowsmith C, Yates JR, III, Balch WE. Biological and structural basis for Aha1 regulation of Hsp90 ATPase activity in maintaining proteostasis in the human disease cystic fibrosis. Mol Biol Cell. 2010;21:871–884. doi: 10.1091/mbc.E09-12-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Kouroku Y, Fujita E, Jimbo A, Kikuchi T, Yamagata T, Momoi MY, Kominami E, Kuida K, Sakamaki K, Yonehara S, Momoi T. Polyglutamine aggregates stimulate ER stress signals and caspase-12 activation. Hum Mol Genet. 2002;11:1505–1515. doi: 10.1093/hmg/11.13.1505. [DOI] [PubMed] [Google Scholar]
- 270.Kozutsumi Y, Segal M, Normington K, Gething MJ, Sambrook J. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature. 1988;332:462–464. doi: 10.1038/332462a0. [DOI] [PubMed] [Google Scholar]
- 271.Krämer-Albers EM, Gehrig-Burger K, Thiele C, Trotter J, Nave KA. Perturbed interactions of mutant proteolipid protein/DM20 with cholesterol and lipid rafts in oligodendroglia: implications for dysmyelination in spastic paraplegia. J Neurosci. 2006;26:11743–11752. doi: 10.1523/JNEUROSCI.3581-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Kreft SG, Wang L, Hochstrasser M. Membrane topology of the yeast endoplasmic reticulum-localized ubiquitin ligase Doa10 and comparison with its human ortholog TEB4 (MARCH-VI) J Biol Chem. 2006;281:4646–4653. doi: 10.1074/jbc.M512215200. [DOI] [PubMed] [Google Scholar]
- 273.Kroeger H, Miranda E, MacLeod I, Pérez J, Crowther DC, Marciniak SJ, Lomas DA. Endoplasmic reticulum-associated degradation (ERAD) and autophagy cooperate to degrade polymerogenic mutant serpins. J Biol Chem. 2009;284:22793–22802. doi: 10.1074/jbc.M109.027102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Krukenberg KA, Street TO, Lavery LA, Agard DA. Conformational dynamics of the molecular chaperone Hsp90. Q Rev Biophys. 2011;44:229–255. doi: 10.1017/S0033583510000314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Kruse KB, Brodsky JL, McCracken AA. Autophagy: an ER protein quality control process. Autophagy. 2006;2:135–137. doi: 10.4161/auto.2.2.2388. [DOI] [PubMed] [Google Scholar]
- 276.Kruse KB, Brodsky JL, McCracken AA. Characterization of an ERAD gene as VPS30/ATG6 reveals two alternative and functionally distinct protein quality control pathways: one for soluble Z variant of human alpha-1 proteinase inhibitor (A1PiZ) and another for aggregates of A1PiZ. Mol Biol Cell. 2006;17:203–212. doi: 10.1091/mbc.E04-09-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Kruse KB, Dear A, Kaltenbrun ER, Crum BE, George PM, Brennan SO, McCracken AA. Mutant fibrinogen cleared from the endoplasmic reticulum via endoplasmic reticulum-associated protein degradation and autophagy: an explanation for liver disease. Am J Pathol. 2006;168:1299–1308. doi: 10.2353/ajpath.2006.051097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Lai CW, Aronson DE, Snapp EL. BiP availability distinguishes states of homeostasis and stress in the endoplasmic reticulum of living cells. Mol Biol Cell. 2010;21:1909–1921. doi: 10.1091/mbc.E09-12-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Lamandé SR, Chessler SD, Golub SB, Byers PH, Chan C, Cole WG, Sillence DO, Bateman JF. Endoplasmic reticulum-mediated quality control of type I collagen production by cells from osteogenesis imperfecta patients with mutations in the proα1(I) chain carboxyl-terminal propeptide which impair subunit assembly. J Biol Chem. 1995;270:8642–8649. doi: 10.1074/jbc.270.15.8642. [DOI] [PubMed] [Google Scholar]
- 280.LaPointe P, Wei X, Gariépy J. A role for the protease-sensitive loop region of shiga-like toxin 1 in the retrotranslocation of its A1 domain from the endoplasmic reticulum lumen. J Biol Chem. 2005;280:23310–23318. doi: 10.1074/jbc.M414193200. [DOI] [PubMed] [Google Scholar]
- 281.Lau MM, Neufeld EF. A frameshift mutation in a patient with Tay-Sachs disease causes premature termination and defective intracellular transport of the alpha-subunit of beta-hexosaminidase. J Biol Chem. 1989;264:21376–21380. [PubMed] [Google Scholar]
- 282.Le A, Steiner JL, Ferrell GA, Shaker JC, Sifers RN. Association between calnexin and a secretion-incompetent variant of human alpha 1-antitrypsin. J Biol Chem. 1994;269:7514–7519. [PubMed] [Google Scholar]
- 283.Lee DH, Goldberg AL. Proteasome inhibitors: valuable new tools for cell biologists. Trends Cell Biol. 1998;8:397–403. doi: 10.1016/s0962-8924(98)01346-4. [DOI] [PubMed] [Google Scholar]
- 284.Lee RJ, Liu CW, Harty C, McCracken AA, Latterich M, Romisch K, DeMartino GN, Thomas PJ, Brodsky JL. Uncoupling retro-translocation and degradation in the ER-associated degradation of a soluble protein. EMBO J. 2004;23:2206–2215. doi: 10.1038/sj.emboj.7600232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Lee SO, Cho K, Cho S, Kim I, Oh C, Ahn K. Protein disulphide isomerase is required for signal peptide peptidase-mediated protein degradation. EMBO J. 2010;29:363–375. doi: 10.1038/emboj.2009.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Lehrman MA, Schneider WJ, Brown MS, Davis CG, Elhammer A, Russell DW, Goldstein JL. The Lebanese allele at the low density lipoprotein receptor locus. Nonsense mutation produces truncated receptor that is retained in endoplasmic reticulum. J Biol Chem. 1987;262:401–410. [PubMed] [Google Scholar]
- 287.Leichner GS, Avner R, Harats D, Roitelman J. Dislocation of HMG-CoA reductase and Insig-1, two polytopic endoplasmic reticulum proteins, en route to proteasomal degradation. Mol Biol Cell. 2009;20:3330–3341. doi: 10.1091/mbc.E08-09-0953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Lemoine M, Younes A. Histone deacetylase inhibitors in the treatment of lymphoma. Discov Med. 2010;10:462–470. [PubMed] [Google Scholar]
- 289.Lencer WI, Tsai B. The intracellular voyage of cholera toxin: going retro. Trends Biochem Sci. 2003;28:639–645. doi: 10.1016/j.tibs.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 290.Leng N, Gu G, Simerly RB, Spindel ER. Molecular cloning and characterization of two putative G protein-coupled receptors which are highly expressed in the central nervous system. Mol Brain Res. 1999;69:73–83. doi: 10.1016/s0169-328x(99)00092-3. [DOI] [PubMed] [Google Scholar]
- 291.Levine CG, Mitra D, Sharma A, Smith CL, Hegde RS. The efficiency of protein compartmentalization into the secretory pathway. Mol Biol Cell. 2005;16:279–291. doi: 10.1091/mbc.E04-06-0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Li G, Zhou X, Zhao G, Schindelin H, Lennarz WJ. Multiple modes of interaction of the deglycosylation enzyme, mouse peptide N-glycanase, with the proteasome. Proc Natl Acad Sci USA. 2005;102:15809–15814. doi: 10.1073/pnas.0507155102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Li S, Spooner RA, Allen SC, Guise CP, Ladds G, Schnoder T, Schmitt MJ, Lord JM, Roberts LM. Folding-competent and folding-defective forms of ricin A chain have different fates after retrotranslocation from the endoplasmic reticulum. Mol Biol Cell. 2010;21:2543–2554. doi: 10.1091/mbc.E09-08-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Li S, Spooner RA, Allen SCH, Guise CP, Ladds G, Schnoder T, Schmitt MJ, Lord JM, Roberts LM. Folding-competent and folding-defective forms of Ricin A chain have different fates after retrotranslocation from the endoplasmic reticulum. Mol Biol Cell. 2010;21:2543–2554. doi: 10.1091/mbc.E09-08-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Li Y, Lu W, Schwartz AL, Bu G. Degradation of the LDL receptor class 2 mutants is mediated by a proteasome-dependent pathway. J Lipid Res. 2004;45:1084–1091. doi: 10.1194/jlr.M300482-JLR200. [DOI] [PubMed] [Google Scholar]
- 296.Liang G, Li Q, Tang Y, Kokame K, Kikuchi T, Wu G, Chen XZ. Polycystin-2 is regulated by endoplasmic reticulum-associated degradation. Hum Mol Genet. 2008;17:1109–1119. doi: 10.1093/hmg/ddm383. [DOI] [PubMed] [Google Scholar]
- 297.Liang Js Kim T, Fang S, Yamaguchi J, Weissman AM, Fisher EA, Ginsberg HN. Overexpression of the tumor autocrine motility factor receptor Gp78, a ubiquitin protein ligase, results in increased ubiquitinylation and decreased secretion of apolipoprotein B100 in HepG2 cells. J Biol Chem. 2003;278:23984–23988. doi: 10.1074/jbc.M302683200. [DOI] [PubMed] [Google Scholar]
- 298.Liao W, Yeung SCJ, Chan L. Proteasome-mediated degradation of apolipoprotein B targets both nascent peptides cotranslationally before translocation and full-length apolipoprotein B after translocation into the endoplasmic reticulum. J Biol Chem. 1998;273:27225–27230. doi: 10.1074/jbc.273.42.27225. [DOI] [PubMed] [Google Scholar]
- 299.Lilley BN, Ploegh HL. A membrane protein required for dislocation of misfolded proteins from the ER. Nature. 2004;429:834–840. doi: 10.1038/nature02592. [DOI] [PubMed] [Google Scholar]
- 300.Lilley BN, Ploegh HL. Multiprotein complexes that link dislocation, ubiquitination, and extraction of misfolded proteins from the endoplasmic reticulum membrane. Proc Natl Acad Sci USA. 2005;102:14296–14301. doi: 10.1073/pnas.0505014102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Lin JH, Li H, Yasumura D, Cohen HR, Zhang C, Panning B, Shokat KM, Lavail MM, Walter P. IRE1 signaling affects cell fate during the unfolded protein response. Science. 2007;318:944–949. doi: 10.1126/science.1146361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Lin JH, Walter P, Yen TSB. Endoplasmic reticulum stress in disease pathogenesis. Annu Rev Pathol. 2008;3:399–425. doi: 10.1146/annurev.pathmechdis.3.121806.151434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Lippincott-Schwartz J, Bonifacino JS, Yuan LC, Klausner RD. Degradation from the endoplasmic reticulum: disposing of newly synthesized proteins. Cell. 1988;54:209–220. doi: 10.1016/0092-8674(88)90553-3. [DOI] [PubMed] [Google Scholar]
- 304.Liu Y, Choudhury P, Cabral CM, Sifers RN. Oligosaccharide modification in the early secretory pathway directs the selection of a misfolded glycoprotein for degradation by the proteasome. J Biol Chem. 1999;274:5861–5867. doi: 10.1074/jbc.274.9.5861. [DOI] [PubMed] [Google Scholar]
- 305.Lloyd-Evans E, Pelled D, Riebeling C, Bodennec J, de-Morgan A, Waller H, Schiffmann R, Futerman AH. Glucosylceramide and glucosylsphingosine modulate calcium mobilization from brain microsomes via different mechanisms. J Biol Chem. 2003;278:23594–23599. doi: 10.1074/jbc.M300212200. [DOI] [PubMed] [Google Scholar]
- 306.Lodish HF, Kong N. Perturbation of cellular calcium blocks exit of secretory proteins from the rough endoplasmic reticulum. J Biol Chem. 1990;265:10893–10899. [PubMed] [Google Scholar]
- 307.Lomas DA, Carrell RW. Serpinopathies and the conformational dementias. Nat Rev Genet. 2002;3:759–768. doi: 10.1038/nrg907. [DOI] [PubMed] [Google Scholar]
- 308.Lomas DA, Evans DL, Finch JT, Carrell RW. The mechanism of Z alpha 1-antitrypsin accumulation in the liver. Nature. 1992;357:605–607. doi: 10.1038/357605a0. [DOI] [PubMed] [Google Scholar]
- 309.Loo MA, Jensen TJ, Cui L, Hou Y, Chang XB, Riordan JR. Perturbation of Hsp90 interaction with nascent CFTR prevents its maturation and accelerates its degradation by the proteasome. EMBO J. 1998;17:6879–6887. doi: 10.1093/emboj/17.23.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Loo TW, Bartlett MC, Clarke DM. Processing mutations disrupt interactions between the nucleotide binding and transmembrane domains of P-glycoprotein and the cystic fibrosis transmembrane conductance regulator (CFTR) J Biol Chem. 2008;283:28190–28197. doi: 10.1074/jbc.M805834200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Loo TW, Clarke DM. Correction of defective protein kinesis of human P-glycoprotein mutants by substrates and modulators. J Biol Chem. 1997;272:709–712. doi: 10.1074/jbc.272.2.709. [DOI] [PubMed] [Google Scholar]
- 312.Loo TW, Clarke DM. Quality control by proteases in the endoplasmic reticulum. J Biol Chem. 1998;273:32373–32376. doi: 10.1074/jbc.273.49.32373. [DOI] [PubMed] [Google Scholar]
- 313.Loureiro J, Lilley BN, Spooner E, Noriega V, Tortorella D, Ploegh HL. Signal peptide peptidase is required for dislocation from the endoplasmic reticulum. Nature. 2006;441:894–897. doi: 10.1038/nature04830. [DOI] [PubMed] [Google Scholar]
- 314.Loureiro J, Ploegh HL. Antigen presentation and the ubiquitin-proteasome system in host-pathogen interactions. In: Frederick WA, editor. Advances in Immunology. New York: Academic; 2006. pp. 225–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Lukacs GL, Mohamed A, Kartner N, Chang XB, Riordan JR, Grinstein S. Conformational maturation of CFTR but not its mutant counterpart (delta F508) occurs in the endoplasmic reticulum and requires ATP. EMBO J. 1994;13:6076–6086. doi: 10.1002/j.1460-2075.1994.tb06954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Lyons SE, Bruck ME, Bowie EJ, Ginsburg D. Impaired intracellular transport produced by a subset of type IIA von Willebrand disease mutations. J Biol Chem. 1992;267:4424–4430. [PubMed] [Google Scholar]
- 317.Ma J, Wollmann R, Lindquist S. Neurotoxicity and neurodegeneration when PrP accumulates in the cytosol. Science. 2002;298:1781–1785. doi: 10.1126/science.1073725. [DOI] [PubMed] [Google Scholar]
- 318.Maegawa GHB, Tropak M, Buttner J, Stockley T, Kok F, Clarke JTR, Mahuran DJ. Pyrimethamine as a potential pharmacological chaperone for late-onset forms of GM2 gangliosidosis. J Biol Chem. 2007;282:9150–9161. doi: 10.1074/jbc.M609304200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Magadán JG, Pérez-Victoria FJ, Sougrat R, Ye Y, Strebel K, Bonifacino JS. Multilayered mechanism of CD4 downregulation by HIV-1 Vpu involving distinct ER retention and ERAD targeting steps. PLoS Pathog. 2010;6:e1000869. doi: 10.1371/journal.ppat.1000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Mala JGS, Rose C. Interactions of heat shock protein 47 with collagen and the stress response: an unconventional chaperone model? Life Sci. 2010;87:579–586. doi: 10.1016/j.lfs.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 321.Mall M, Kunzelmann K. Correction of the CF defect by curcumin: hypes and disappointments. Bioessays. 2005;27:9–13. doi: 10.1002/bies.20168. [DOI] [PubMed] [Google Scholar]
- 322.Mann BS, Johnson JR, Cohen MH, Justice R, Pazdur R. FDA approval summary: vorinostat for treatment of advanced primary cutaneous T-cell lymphoma. Oncologist. 2007;12:1247–1252. doi: 10.1634/theoncologist.12-10-1247. [DOI] [PubMed] [Google Scholar]
- 323.Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, Nagata K, Harding HP, Ron D. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18:3066–3077. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Margottin F, Bour SP, Durand H, Selig L, Benichou S, Richard V, Thomas D, Strebel K, Benarous R. A novel human WD protein, h-βTrCP, that interacts with HIV-1 Vpu connects CD4 to the ER degradation pathway through an F-box motif. Mol Cell. 1998;1:565–574. doi: 10.1016/s1097-2765(00)80056-8. [DOI] [PubMed] [Google Scholar]
- 325.Mast SW, Diekman K, Karaveg K, Davis A, Sifers RN, Moremen KW. Human EDEM2, a novel homolog of family 47 glycosidases, is involved in ER-associated degradation of glycoproteins. Glycobiology. 2005;15:421–436. doi: 10.1093/glycob/cwi014. [DOI] [PubMed] [Google Scholar]
- 326.Matsuda N, Suzuki T, Tanaka K, Nakano A. Rma1, a novel type of RING finger protein conserved from Arabidopsis to human, is a membrane-bound ubiquitin ligase. J Cell Sci. 2001;114:1949–1957. doi: 10.1242/jcs.114.10.1949. [DOI] [PubMed] [Google Scholar]
- 327.Matus S, Glimcher LH, Hetz C. Protein folding stress in neurodegenerative diseases: a glimpse into the ER. Curr Opin Cell Biol. 2011;23:239–252. doi: 10.1016/j.ceb.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 328.Maytal-Kivity V, Reis N, Hofmann K, Glickman M. MPN+, a putative catalytic motif found in a subset of MPN domain proteins from eukaryotes and prokaryotes, is critical for Rpn11 function. BMC Biochem. 2002;3:28. doi: 10.1186/1471-2091-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.McClellan AJ, Scott MD, Frydman J. Folding and quality control of the VHL tumor suppressor proceed through distinct chaperone pathways. Cell. 2005;121:739–748. doi: 10.1016/j.cell.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 330.McCracken AA, Brodsky JL. Assembly of ER-associated protein degradation in vitro: dependence on cytosol, calnexin, and ATP. J Cell Biol. 1996;132:291–298. doi: 10.1083/jcb.132.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Meacham GC, Lu Z, King S, Sorscher E, Tousson A, Cyr DM. The Hdj-2/Hsc70 chaperone pair facilitates early steps in CFTR biogenesis. EMBO J. 1999;18:1492–1505. doi: 10.1093/emboj/18.6.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Meacham GC, Patterson C, Zhang W, Younger JM, Cyr DM. The Hsc70 co-chaperone CHIP targets immature CFTR for proteasomal degradation. Nat Cell Biol. 2001;3:100–105. doi: 10.1038/35050509. [DOI] [PubMed] [Google Scholar]
- 333.Meigs TE, Simoni RD. Regulated degradation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase in permeabilized cells. J Biol Chem. 1992;267:13547–13552. [PubMed] [Google Scholar]
- 334.Melnick J, Dul JL, Argon Y. Sequential interaction of the chaperones BiP and GRP94 with immunoglobulin chains in the endoplasmic reticulum. Nature. 1994;370:373–375. doi: 10.1038/370373a0. [DOI] [PubMed] [Google Scholar]
- 335.Mochizuki K, Kagawa T, Numari A, Harris MJ, Itoh J, Watanabe N, Mine T, Arias IM. Two N-linked glycans are required to maintain the transport activity of the bile salt export pump (ABCB11) in MDCK II cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:G818–G828. doi: 10.1152/ajpgi.00415.2006. [DOI] [PubMed] [Google Scholar]
- 336.Moore P, Bernardi KM, Tsai B. The Ero1α-PDI redox cycle regulates retro-translocation of cholera toxin. Mol Biol Cell. 2010;21:1305–1313. doi: 10.1091/mbc.E09-09-0826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Morello JP, Salahpour A, Laperrière A, Bernier V, Arthus MF, Lonergan M, Petäjä-Repo U, Angers S, Morin D, Bichet DG, Bouvier M. Pharmacological chaperones rescue cell-surface expression and function of misfolded V2 vasopressin receptor mutants. J Clin Invest. 2000;105:887–895. doi: 10.1172/JCI8688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Mori K, Ma W, Gething MJ, Sambrook J. A transmembrane protein with a cdc2+/CDC28-related kinase activity is required for signaling from the ER to the nucleus. Cell. 1993;74:743–756. doi: 10.1016/0092-8674(93)90521-q. [DOI] [PubMed] [Google Scholar]
- 339.Mori K, Sant A, Kohno K, Normington K, Gething MJ, Sambrook JF. A 22 bp cis-acting element is necessary and sufficient for the induction of the yeast KAR2 (BiP) gene by unfolded proteins. EMBO J. 1992;11:2583–2593. doi: 10.1002/j.1460-2075.1992.tb05323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Morito D, Hirao K, Oda Y, Hosokawa N, Tokunaga F, Cyr DM, Tanaka K, Iwai K, Nagata AK. Gp78 cooperates with RMA1 in endoplasmic reticulum-associated degradation of CFTRDeltaF508. Mol Biol Cell. 2008;19:1328–1336. doi: 10.1091/mbc.E07-06-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Mu TW, Ong DST, Wang YJ, Balch WE, Yates HR, III, Segatori L, Kelly JW. Chemical and biological approaches synergize to ameliorate protein-folding diseases. Cell. 2008;134:769–781. doi: 10.1016/j.cell.2008.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Murakami T, Shoji M, Imai Y, Inoue H, Kawarabayashi T, Matsubara E, Harigaya Y, Sasaki A, Takahashi R, Abe K. Pael-R is accumulated in Lewy bodies of Parkinson’s disease. Ann Neurol. 2004;55:439–442. doi: 10.1002/ana.20064. [DOI] [PubMed] [Google Scholar]
- 343.Nakagawa H, Tamura A, Wakabayashi K, Hoshijima K, Komada M, Yoshida T, Kometani S, Matsubara T, Mikuriya K, Ishikawa T. Ubiquitin-mediated proteasomal degradation of non-synonymous SNP variants of human ABC transporter ABCG2. Biochem J. 2008;411:623–631. doi: 10.1042/BJ20071229. [DOI] [PubMed] [Google Scholar]
- 344.Nakagawa H, Wakabayashi-Nakao K, Tamura A, Toyoda Y, Koshiba S, Ishikawa T. Disruption of N-linked glycosylation enhances ubiquitin-mediated proteasomal degradation of the human ATP-binding cassette transporter ABCG2. FEBS J. 2009;276:7237–7252. doi: 10.1111/j.1742-4658.2009.07423.x. [DOI] [PubMed] [Google Scholar]
- 345.Nakatsukasa K, Huyer G, Michaelis S, Brodsky JL. Dissecting the ER-associated degradation of a misfolded polytopic membrane protein. Cell. 2008;132:101–112. doi: 10.1016/j.cell.2007.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Nakazawa M, Uchida K, Aramaki M, Kodo K, Yamagishi C, Takahashi T, Mikoshiba K, Yamagishi H. Inositol 1,4,5-trisphosphate receptors are essential for the development of the second heart field. J Mol Cell Cardiol. 2011;51:58–66. doi: 10.1016/j.yjmcc.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 347.Nanua S, Murakami M, Xia J, Grenda DS, Woloszynek J, Strand M, Link DC. Activation of the unfolded protein response is associated with impaired granulopoiesis in transgenic mice expressing mutant Elane. Blood. 2011;2010:311704. doi: 10.1182/blood-2010-10-311704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Nery FC, Armata IA, Farley JE, Cho JA, Yaqub U, Chen P, da Hora CC, Wang Q, Tagaya M, Klein C, Tannous B, Caldwell KA, Caldwell GA, Lencer WI, Ye Y, Breake-field XO. TorsinA participates in endoplasmic reticulum-associated degradation. Nat Commun. 2011;2:393. doi: 10.1038/ncomms1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Nijenhuis M, Zalm R, Burbach JPH. Mutations in the vasopressin prohormone involved in diabetes insipidus impair endoplasmic reticulum export but not sorting. J Biol Chem. 1999;274:21200–21208. doi: 10.1074/jbc.274.30.21200. [DOI] [PubMed] [Google Scholar]
- 350.Nishikawa S, Brodsky JL, Nakatsukasa K. Roles of molecular chaperones in endoplasmic reticulum (ER) quality control and ER-associated degradation (ERAD) J Biochem. 2005;137:551–555. doi: 10.1093/jb/mvi068. [DOI] [PubMed] [Google Scholar]
- 351.Nishikawa SI, Fewell SW, Kato Y, Brodsky JL, Endo T. Molecular chaperones in the yeast endoplasmic reticulum maintain the solubility of proteins for retrotranslocation and degradation. J Cell Biol. 2001;153:1061–1070. doi: 10.1083/jcb.153.5.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Nishio M, Koyama T, Nakahara M, Egawa N, Hirosawa S. Proteasome degradation of protein C and plasmin inhibitor mutants. Thromb Haemost. 2008;100:405–412. [PubMed] [Google Scholar]
- 353.Nishitoh H, Matsuzawa A, Tobiume K, Saegusa K, Takeda K, Inoue K, Hori S, Kakizuka A, Ichijo H. ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev. 2002;16:1345–1355. doi: 10.1101/gad.992302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Noorwez SM, Kuksa V, Imanishi Y, Zhu L, Filipek S, Palczewski K, Kaushal S. Pharmacological chaperone-mediated in vivo folding and stabilization of the P23H-opsin mutant associated with autosomal dominant retinitis pigmentosa. J Biol Chem. 2003;278:14442–14450. doi: 10.1074/jbc.M300087200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Novak MJU, Sweeney MG, Li A, Treacy C, Chandrashekar HS, Giunti P, Goold RG, Davis MB, Houlden H, Tabrizi SJ. An ITPR1 gene deletion causes spinocerebellar ataxia 15/16: a genetic, clinical and radiological description. Movement Disorders. 2010;25:2176–2182. doi: 10.1002/mds.23223. [DOI] [PubMed] [Google Scholar]
- 356.O’Rahilly S. Human genetics illuminates the paths to metabolic disease. Nature. 2009;462:307–314. doi: 10.1038/nature08532. [DOI] [PubMed] [Google Scholar]
- 357.Oda Y, Hosokawa N, Wada I, Nagata K. EDEM as an acceptor of terminally misfolded glycoproteins released from calnexin. Science. 2003;299:1394–1397. doi: 10.1126/science.1079181. [DOI] [PubMed] [Google Scholar]
- 358.Okada T, Yoshida H, Akazawa R, Negishi M, Mori K. Distinct roles of activating transcription factor 6 (ATF6) and double-stranded RNA-activated protein kinase-like endoplasmic reticulum kinase (PERK) in transcription during the mammalian unfolded protein response. Biochem J. 2002;366:585–594. doi: 10.1042/BJ20020391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Okiyoneda T, Barrière H, Bagdány M, Rabeh WM, Du K, Höhfeld J, Young JC, Lukacs GL. Peripheral protein quality control removes unfolded CFTR from the plasma membrane. Science. 2010;329:805–810. doi: 10.1126/science.1191542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Okiyoneda T, Harada K, Takeya M, Yamahira K, Wada I, Shuto T, Suico MA, Hashimoto Y, Kai H. Delta F508 CFTR pool in the endoplasmic reticulum is increased by calnexin overexpression. Mol Biol Cell. 2004;15:563–574. doi: 10.1091/mbc.E03-06-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Omura T, Kaneko M, Okuma Y, Orba Y, Nagashima K, Takahashi R, Fujitani N, Matsumura S, Hata A, Kubota K, Murahashi K, Uehara T, Nomura Y. A ubiquitin ligase HRD1 promotes the degradation of Pael receptor, a substrate of Parkin. J Neurochem. 2006;99:1456–1469. doi: 10.1111/j.1471-4159.2006.04155.x. [DOI] [PubMed] [Google Scholar]
- 362.Omura T, Kaneko M, Tabei N, Okuma Y, Nomura Y. Immunohistochemical localization of a ubiquitin ligase HRD1 in murine brain. J Neurosci Res. 2008;86:1577–1587. doi: 10.1002/jnr.21616. [DOI] [PubMed] [Google Scholar]
- 363.Ong DST, Kelly JW. Chemical and/or biological therapeutic strategies to ameliorate protein misfolding diseases. Curr Opin Cell Biol. 2011;23:231–238. doi: 10.1016/j.ceb.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Ong DST, Mu TW, Palmer AE, Kelly JW. Endoplasmic reticulum Ca2+ increases enhance mutant glucocerebrosidase proteostasis. Nat Chem Biol. 2010;6:424–432. doi: 10.1038/nchembio.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Osborne AR, Rapoport TA, van den Berg B. Protein translocation by the Sec61/SecY channel. Annu Rev Cell Dev Biol. 2005;21:529–550. doi: 10.1146/annurev.cellbio.21.012704.133214. [DOI] [PubMed] [Google Scholar]
- 366.Ou WJ, Cameron PH, Thomas DY, Bergeron JJM. Association of folding intermediates of glycoproteins with calnexin during protein maturation. Nature. 1993;364:771–776. doi: 10.1038/364771a0. [DOI] [PubMed] [Google Scholar]
- 367.zcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Özdelen E, Tuncman G, Görgün C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 368.Pallanck LJ. Culling sick mitochondria from the herd. J Cell Biol. 2010;191:1225–1227. doi: 10.1083/jcb.201011068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Panaretou B, Siligardi G, Meyer P, Maloney A, Sullivan JK, Singh S, Millson SH, Clarke PA, Naaby-Hansen S, Stein R, Cramer R, Mollapour M, Workman P, Piper PW, Pearl LH, Prodromou C. Activation of the ATPase activity of Hsp90 by the stress-regulated cochaperone Aha1. Mol Cell. 2002;10:1307–1318. doi: 10.1016/s1097-2765(02)00785-2. [DOI] [PubMed] [Google Scholar]
- 370.Pariyarath R, Wang H, Aitchison JD, Ginsberg HN, Welch WJ, Johnson AE, Fisher EA. Co-translational interactions of apoprotein B with the ribosome and translocon during lipoprotein assembly or targeting to the proteasome. J Biol Chem. 2001;276:541–550. doi: 10.1074/jbc.M007944200. [DOI] [PubMed] [Google Scholar]
- 371.Park SY, Ye H, Steiner DF, Bell GI. Mutant proinsulin proteins associated with neonatal diabetes are retained in the endoplasmic reticulum and not efficiently secreted. Biochem Biophys Res Commun. 2010;391:1449–1454. doi: 10.1016/j.bbrc.2009.12.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Parodi AJ. Protein glucosylation and its role in protein folding. Annu Rev Biochem. 2000;69:69–93. doi: 10.1146/annurev.biochem.69.1.69. [DOI] [PubMed] [Google Scholar]
- 373.Partridge CJ, Beech DJ, Sivaprasadarao A. Identification and pharmacological correction of a membrane trafficking defect associated with a mutation in the sulfonylurea receptor causing familial hyperinsulinism. J Biol Chem. 2001;276:35947–35952. doi: 10.1074/jbc.M104762200. [DOI] [PubMed] [Google Scholar]
- 374.Paulusma CC, Folmer DE, Ho-Mok KS, de Waart DR, Hilarius PM, Verhoeven AJ, Oude Elferink RPJ. ATP8B1 requires an accessory protein for endoplasmic reticulum exit and plasma membrane lipid flippase activity. Hepatology. 2008;47:268–278. doi: 10.1002/hep.21950. [DOI] [PubMed] [Google Scholar]
- 375.Pedemonte N, Lukacs GL, Du K, Caci E, Zegarra-Moran O, Galietta LJV, Verkman AS. Small-molecule correctors of defective ΔF508-CFTR cellular processing identified by high-throughput screening. J Clin Invest. 2005;115:2564–2571. doi: 10.1172/JCI24898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Peiris JS, Guan Y, Yuen KY. Severe acute respiratory syndrome. Nat Med. 2004;10:S88–S97. doi: 10.1038/nm1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Pelled D, Trajkovic-Bodennec S, Lloyd-Evans E, Sidransky E, Schiffmann R, Futerman AH. Enhanced calcium release in the acute neuronopathic form of Gaucher disease. Neurobiol Dis. 2005;18:83–88. doi: 10.1016/j.nbd.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 378.Perlmutter DH. Liver injury in α1-antitrypsin deficiency: an aggregated protein induces mitochondrial injury. J Clin Invest. 2002;110:1579–1583. doi: 10.1172/JCI16787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Perlmutter DH. Pathogenesis of chronic liver injury and hepatocellular carcinoma in alpha-1-antitrypsin deficiency. Pediatr Res. 2006;60:233–238. doi: 10.1203/01.pdr.0000228350.61496.90. [DOI] [PubMed] [Google Scholar]
- 380.Pidasheva S, Grant M, Canaff L, Ercan O, Kumar U, Hendy GN. Calcium-sensing receptor dimerizes in the endoplasmic reticulum: biochemical and biophysical characterization of CASR mutants retained intracellularly. Hum Mol Genet. 2006;15:2200–2209. doi: 10.1093/hmg/ddl145. [DOI] [PubMed] [Google Scholar]
- 381.Pilon M, Schekman R, Romisch K. Sec61p mediates export of a misfolded secretory protein from the endoplasmic reticulum to the cytosol for degradation. EMBO J. 1997;16:4540–4548. doi: 10.1093/emboj/16.15.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Pincus D, Chevalier MW, Aragón T, van Anken E, Vidal SE, El-Samad H, Walter P. BiP Binding to the ER-stress sensor Ire1 tunes the homeostatic behavior of the unfolded protein response. PLoS Biol. 2010;8:e1000415. doi: 10.1371/journal.pbio.1000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Pind S, Riordan JR, Williams DB. Participation of the endoplasmic reticulum chaperone calnexin (p88, IP90) in the biogenesis of the cystic fibrosis transmembrane conductance regulator. J Biol Chem. 1994;269:12784–12788. [PubMed] [Google Scholar]
- 384.Pizzo P, Pozzan T. Mitochondria-endoplasmic reticulum choreography: structure and signaling dynamics. Trends Cell Biol. 2007;17:511–517. doi: 10.1016/j.tcb.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 385.Plemper RK, Bohmler S, Bordallo J, Sommer T, Wolf DH. Mutant analysis links the translocon and BiP to retrograde protein transport for ER degradation. Nature. 1997;388:891–895. doi: 10.1038/42276. [DOI] [PubMed] [Google Scholar]
- 386.Pollock S, Kozlov G, Pelletier MF, Trempe JF, Jansen G, Sitnikov D, Bergeron JJM, Gehring K, Ekiel I, Thomas DY. Specific interaction of ERp57 and calnexin determined by NMR spectroscopy and an ER two-hybrid system. EMBO J. 2004;23:1020–1029. doi: 10.1038/sj.emboj.7600119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Powell K, Zeitlin PL. Therapeutic approaches to repair defects in δF508 CFTR folding and cellular targeting. Adv Drug Delivery Rev. 2002;54:1395–1408. doi: 10.1016/s0169-409x(02)00148-5. [DOI] [PubMed] [Google Scholar]
- 388.Powers ET, Morimoto RI, Dillin A, Kelly JW, Balch WE. Biological and chemical approaches to diseases of proteostasis deficiency. Annu Rev Biochem. 2009;78:959–991. doi: 10.1146/annurev.biochem.052308.114844. [DOI] [PubMed] [Google Scholar]
- 389.Powers T, Walter P. A ribosome at the end of the tunnel. Science. 1997;278:2072–2073. doi: 10.1126/science.278.5346.2072. [DOI] [PubMed] [Google Scholar]
- 390.Prescher JA, Bertozzi CR. Chemical technologies for probing glycans. Cell. 2006;126:851–854. doi: 10.1016/j.cell.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 391.Proft T. Microbial Toxins: Current Research and Future Trends. Norfolk: Caister Academic; 2009. [Google Scholar]
- 392.Prusiner SB. Prions. Proc Natl Acad Sci USA. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Puzianowska-Kuznicka M, Kuznicki J. The ER and ageing II: Calcium homeostasis. Ageing Res Rev. 2009;8:160–172. doi: 10.1016/j.arr.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 394.Qu D, Teckman JH, Omura S, Perlmutter DH. Degradation of a mutant secretory protein, alpha1-antitrypsin Z, in the endoplasmic reticulum requires proteasome activity. J Biol Chem. 1996;271:22791–22795. doi: 10.1074/jbc.271.37.22791. [DOI] [PubMed] [Google Scholar]
- 395.Quan EM, Kamiya Y, Kamiya D, Denic V, Weibezahn J, Kato K, Weissman JS. Defining the glycan destruction signal for endoplasmic reticulum-associated degradation. Mol Cell. 2008;32:870–877. doi: 10.1016/j.molcel.2008.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Raasi S, Orlov I, Fleming KG, Pickart CM. Binding of polyubiquitin chains to ubiquitin-associated (UBA) domains of HHR23A. J Mol Biol. 2004;341:1367–1379. doi: 10.1016/j.jmb.2004.06.057. [DOI] [PubMed] [Google Scholar]
- 397.Raasi S, Wolf DH. Ubiquitin receptors and ERAD: a network of pathways to the proteasome. Semin Cell Dev Biol. 2007;18:780–791. doi: 10.1016/j.semcdb.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 398.Rabinovich E, Kerem A, Frohlich KU, Diamant N, Bar-Nun S. AAA-ATPase p97/Cdc48p, a cytosolic chaperone required for ndoplasmic reticulum-associated protein degradation. Mol Cell Biol. 2002;22:626–634. doi: 10.1128/MCB.22.2.626-634.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Ramos RR, Swanson AJ, Bass J. Calreticulin and Hsp90 stabilize the human insulin receptor and promote its mobility in the endoplasmic reticulum. Proc Natl Acad Sci USA. 2007;104:10470–10475. doi: 10.1073/pnas.0701114104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Rane NS, Chakrabarti O, Feigenbaum L, Hegde RS. Signal sequence insufficiency contributes to neurodegeneration caused by transmembrane prion protein. J Cell Biol. 2010;188:515–526. doi: 10.1083/jcb.200911115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Rane NS, Yonkovich JL, Hegde RS. Protection from cytosolic prion protein toxicity by modulation of protein translocation. EMBO J. 2004;23:4550–4559. doi: 10.1038/sj.emboj.7600462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Ravid T, Kreft SG, Hochstrasser M. Membrane and soluble substrates of the Doa10 ubiquitin ligase are degraded by distinct pathways. EMBO J. 2006;25:533–543. doi: 10.1038/sj.emboj.7600946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Razavi H, Palaninathan SK, Powers ET, Wiseman RL, Purkey HE, Mohamedmo-haideen NN, Deechongkit S, Chiang KP, Dendle MTA, Sacchettini JC, Kelly JW. Benzoxazoles as transthyretin amyloid fibril inhibitors: synthesis, evaluation, and mechanism of action. Angewandte Chemie Int Edition. 2003;42:2758–2761. doi: 10.1002/anie.200351179. [DOI] [PubMed] [Google Scholar]
- 404.Reggiori F, Monastyrska I, Verheije MH, Calµ T, Ulasli M, Bianchi S, Bernasconi R, de Haan CAM, Molinari M. Coronaviruses hijack the LC3-I-positive EDEMosomes, ER-derived vesicles exporting short-lived ERAD regulators, for replication. Cell Host Microbe. 2010;7:500–508. doi: 10.1016/j.chom.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Richly H, Rape M, Braun S, Rumpf S, Hoege C, Jentsch S. A series of ubiquitin binding factors connects CDC48/p97 to substrate multiubiquitylation and proteasomal targeting. Cell. 2005;120:73–84. doi: 10.1016/j.cell.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 406.Riggs MG, Whittaker RG, Neumann JR, Ingram VM. n-Butyrate causes histone modification in HeLa and Friend erythroleukaemia cells. Nature. 1977;268:462–464. doi: 10.1038/268462a0. [DOI] [PubMed] [Google Scholar]
- 407.Riordan JR. CFTR function and prospects for therapy. Annu Rev Biochem. 2008;77:701–726. doi: 10.1146/annurev.biochem.75.103004.142532. [DOI] [PubMed] [Google Scholar]
- 408.Robson A, Collinson I. The structure of the Sec complex and the problem of protein translocation. EMBO Rep. 2006;7:1099–1103. doi: 10.1038/sj.embor.7400832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Rock KL, Gramm C, Rothstein L, Clark K, Stein R, Dick L, Hwang D, Goldberg AL. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell. 1994;78:761–771. doi: 10.1016/s0092-8674(94)90462-6. [DOI] [PubMed] [Google Scholar]
- 410.Rodighiero C, Tsai B, Rapoport TA, Lencer WI. Role of ubiquitination in retro-translocation of cholera toxin and escape of cytosolic degradation. EMBO Rep. 2002;3:1222–1227. doi: 10.1093/embo-reports/kvf239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Ron E, Shenkman M, Groisman B, Izenshtein Y, Leitman J, Lederkremer GZ. Bypass of glycan-dependent glycoprotein delivery to ERAD by upregulated EDEM1. Mol Biol Cell. 2011;22:3945–3954. doi: 10.1091/mbc.E10-12-0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Ron I, Horowitz M. Intracellular cholesterol modifies the ERAD of glucocerebrosidase in Gaucher disease patients. Mol Genet Metab. 2008;93:426–436. doi: 10.1016/j.ymgme.2007.10.132. [DOI] [PubMed] [Google Scholar]
- 413.Ron I, Rapaport D, Horowitz M. Interaction between parkin and mutant glucocerebrosidase variants: a possible link between Parkinson disease and Gaucher disease. Hum Mol Genet. 2010;19:3771–3781. doi: 10.1093/hmg/ddq292. [DOI] [PubMed] [Google Scholar]
- 414.Rosser MFN, Grove DE, Chen L, Cyr DM. Assembly and misassembly of cystic fibrosis transmembrane conductance regulator: folding defects caused by deletion of F508 occur before and after the calnexin-dependent association of membrane spanning domain (MSD) 1 and MSD2. Mol Biol Cell. 2008;19:4570–4579. doi: 10.1091/mbc.E08-04-0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Rubenstein RC, Egan ME, Zeitlin PL. In vitro pharmacologic restoration of CFTRmediated chloride transport with sodium 4-phenylbutyrate in cystic fibrosis epithelial cells containing delta F508-CFTR. J Clin Invest. 1997;100:2457–2465. doi: 10.1172/JCI119788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Rubenstein Ronald C, Zeitlin PL. A pilot clinical trial of oral sodium 4-phenylbutyrate (Buphenyl) in Delta F508-homozygous cystic fibrosis patients partial restoration of nasal epithelial CFTR function. Am J Respir Crit Care Med. 1998;157:484–490. doi: 10.1164/ajrccm.157.2.9706088. [DOI] [PubMed] [Google Scholar]
- 417.Rutkowski DT, Kaufman RJ. That which does not kill me makes me stronger: adapting to chronic ER stress. Trends Biochem Sci. 2007;32:469–476. doi: 10.1016/j.tibs.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 418.Ryan MC, Shooter EM, Notterpek L. Aggresome formation in neuropathy models based on peripheral myelin protein 22 mutations. Neurobiol Dis. 2002;10:109–118. doi: 10.1006/nbdi.2002.0500. [DOI] [PubMed] [Google Scholar]
- 419.Saeed M, Suzuki R, Watanabe N, Masaki T, Tomonaga M, Muhammad A, Kato T, Matsuura Y, Watanabe H, Wakita T, Suzuki T. Role of the endoplasmic reticulum-associated degradation (ERAD) pathway in degradation of hepatitis C virus envelope proteins and production of virus particles. J Biol Chem. 2011;286:37264–37273. doi: 10.1074/jbc.M111.259085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Sakaguchi H, Yamashita S, Miura A, Hirahara T, Kimura E, Maeda Y, Terasaki T, Hirano T, Uchino M. A novel GJB1 frameshift mutation produces a transient CNS symptom of X-linked Charcot-Marie-Tooth disease. J Neurol. 2011;258:284–290. doi: 10.1007/s00415-010-5752-8. [DOI] [PubMed] [Google Scholar]
- 421.Sakoh-Nakatogawa M, Nishikawa SI, Endo T. Roles of protein-disulfide isomerase-mediated disulfide bond formation of yeast Mnl1p in endoplasmic reticulum-associated degradation. J Biol Chem. 2009;284:11815–11825. doi: 10.1074/jbc.M900813200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Saraogi I, Shan S. Molecular mechanism of co-translational protein targeting by the signal recognition particle. Traffic. 2011;12:535–542. doi: 10.1111/j.1600-0854.2011.01171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Sarkar S, Floto RA, Berger Z, Imarisio S, Cordenier A, Pasco M, Cook LJ, Rubinsztein DC. Lithium induces autophagy by inhibiting inositol monophosphatase. J Cell Biol. 2005;170:1101–1111. doi: 10.1083/jcb.200504035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Sato BK, Schulz D, Do PH, Hampton RY. Misfolded membrane proteins are specifically recognized by the transmembrane domain of the Hrd1p ubiquitin ligase. Mol Cell. 2009;34:212–222. doi: 10.1016/j.molcel.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Sato S, Ward CL, Kopito RR. Cotranslational ubiquitination of cystic fibrosis transmembrane conductance regulator in vitro. J Biol Chem. 1998;273:7189–7192. doi: 10.1074/jbc.273.13.7189. [DOI] [PubMed] [Google Scholar]
- 426.Sato S, Ward CL, Krouse ME, Wine JJ, Kopito RR. Glycerol reverses the misfolding phenotype of the most common cystic fibrosis mutation. J Biol Chem. 1996;271:635–638. doi: 10.1074/jbc.271.2.635. [DOI] [PubMed] [Google Scholar]
- 427.Saudou F, Finkbeiner S, Devys D, Greenberg ME. Huntingtin acts in the nucleus to induce apoptosis but death does not correlate with the formation of intranuclear inclusions. Cell. 1998;95:55–66. doi: 10.1016/s0092-8674(00)81782-1. [DOI] [PubMed] [Google Scholar]
- 428.Sawkar AR, Adamski-Werner SL, Cheng WC, Wong CH, Beutler E, Zimmer KP, Kelly JW. Gaucher disease-associated glucocerebrosidases show mutation-dependent chemical chaperoning profiles. Chem Biol. 2005;12:1235–1244. doi: 10.1016/j.chembiol.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 429.Sawkar AR, Cheng WC, Beutler E, Wong CH, Balch WE, Kelly JW. Chemical chaperones increase the cellular activity of N370S β-glucosidase: a therapeutic strategy for Gaucher disease. Proc Natl Acad Sci USA. 2002;99:15428–15433. doi: 10.1073/pnas.192582899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Sawkar AR, D’Haeze W, Kelly JW. Therapeutic strategies to ameliorate lysosomal storage disorders-a focus on Gaucher disease. Cell Mol Life Sci. 2006;63:1179–1192. doi: 10.1007/s00018-005-5437-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Sawkar AR, Schmitz M, Zimmer KP, Reczek D, Edmunds T, Balch WE, Kelly JW. Chemical chaperones and permissive temperatures alter localization of Gaucher disease associated glucocerebrosidase variants. ACS Chem Biol. 2006;1:235–251. doi: 10.1021/cb600187q. [DOI] [PubMed] [Google Scholar]
- 432.Scheuner D, Mierde DV, Song B, Flamez D, Creemers JWM, Tsukamoto K, Ribick M, Schuit FC, Kaufman RJ. Control of mRNA translation preserves endoplasmic reticulum function in beta cells and maintains glucose homeostasis. Nat Med. 2005;11:757–764. doi: 10.1038/nm1259. [DOI] [PubMed] [Google Scholar]
- 433.Schmitz A, Herrgen H, Winkeler A, Herzog V. Cholera toxin is exported from microsomes by the Sec61p complex. J Cell Biol. 2000;148:1203–1212. doi: 10.1083/jcb.148.6.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Schneekloth JS, Jr, Crews CM. Natural product inhibitors of the ubiquitin-proteasome pathway. Curr Drug Targets. 2011 doi: 10.2174/138945011798109491. [DOI] [PubMed] [Google Scholar]
- 435.Schneppenheim R, Brassard J, Krey S, Budde U, Kunicki TJ, Holmberg L, Ware J, Ruggeri ZM. Defective dimerization of von Willebrand factor subunits due to a Cys→Arg mutation in type IID von Willebrand disease. Proc Natl Acad Sci USA. 1996;93:3581–3586. doi: 10.1073/pnas.93.8.3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Schreiner P, Chen X, Husnjak K, Randles L, Zhang N, Elsasser S, Finley D, Dikic I, Walters KJ, Groll M. Ubiquitin docking at the proteasome through a novel pleckstrin-homology domain interaction. Nature. 2008;453:548–552. doi: 10.1038/nature06924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Schubert U, Anton LC, Cox JH, Bour S, Bennink JR, Orlowski M, Strebel K, Yewdell JW. CD4 glycoprotein degradation induced by human immunodeficiency virus type 1 Vpu protein requires the function of proteasomes and the ubiquitin-conjugating pathway. J Virol. 1998;72:2280–2288. doi: 10.1128/jvi.72.3.2280-2288.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Schubert U, Anton LC, Gibbs J, Norbury CC, Yewdell JW, Bennink JR. Rapid degradation of a large fraction of newly synthesized proteins by proteasomes. Nature. 2000;404:770–774. doi: 10.1038/35008096. [DOI] [PubMed] [Google Scholar]
- 439.Schuberth C, Buchberger A. UBX domain proteins: major regulators of the AAA ATPase Cdc48/p97. Cell Mol Life Sci. 2008;65:2360–2371. doi: 10.1007/s00018-008-8072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Schuit FC, In’t Veld PA, Pipeleers DG. Glucose stimulates proinsulin biosynthesis by a dose-dependent recruitment of pancreatic beta cells. Proc Natl Acad Sci USA. 1988;85:3865–3869. doi: 10.1073/pnas.85.11.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Schwieger I, Lautz K, Krause E, Rosenthal W, Wiesner B, Hermosilla R. Derlin-1 and p97/valosin-containing protein mediate the endoplasmic reticulum-associated degradation of human V2 vasopressin receptors. Mol Pharmacol. 2008;73:697–708. doi: 10.1124/mol.107.040931. [DOI] [PubMed] [Google Scholar]
- 442.Scott CM, Kruse KB, Schmidt BZ, Perlmutter DH, McCracken AA, Brodsky JL. ADD66, a gene involved in the endoplasmic reticulum-associated degradation of alpha-1-antitrypsin-Z in yeast, facilitates proteasome activity and assembly. Mol Biol Cell. 2007;18:3776–3787. doi: 10.1091/mbc.E07-01-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Scott DC, Schekman R. Role of Sec61p in the ER-associated degradation of short-lived transmembrane proteins. J Cell Biol. 2008;181:1095–1105. doi: 10.1083/jcb.200804053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Sekijima Y, Wiseman RL, Matteson J, Hammarstrom P, Miller SR, Sawkar AR, Balch WE, Kelly JW. The biological and chemical basis for tissue-selective amyloid disease. Cell. 2005;121:73–85. doi: 10.1016/j.cell.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 445.Selkoe DJ. Alzheimer’s disease. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a004457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Serohijos AWR, Hegedüs T, Aleksandrov AA, He L, Cui L, Dokholyan NV, Riordan JR. Phenylalanine-508 mediates a cytoplasmic-membrane domain contact in the CFTR 3D structure crucial to assembly and channel function. Proc Natl Acad Sci USA. 2008;105:3256–3261. doi: 10.1073/pnas.0800254105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Shaffer KL, Sharma A, Snapp EL, Hegde RS. Regulation of protein compartmentalization expands the diversity of protein function. Dev Cell. 2005;9:545–554. doi: 10.1016/j.devcel.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 448.Shamu CE, Flierman D, Ploegh HL, Rapoport TA, Chau V. Polyubiquitination is required for US11-dependent movement of MHC class I heavy chain from endoplasmic reticulum into cytosol. Mol Biol Cell. 2001;12:2546–2555. doi: 10.1091/mbc.12.8.2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Shamu CE, Story CM, Rapoport TA, Ploegh HL. The pathway of US11-dependent degradation ofMHCclass I heavy chains involves a ubiquitin-conjugated intermediate. J Cell Biol. 1999;147:45–58. doi: 10.1083/jcb.147.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Shaner L, Morano KA. All in the family: atypical Hsp70 chaperones are conserved modulators of Hsp70 activity. Cell Stress Chaperones. 2007;12:1–8. doi: 10.1379/CSC-245R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Shen Y, Ballar P, Fang S. Ubiquitin ligase gp78 increases solubility and facilitates degradation of the Z variant of α-1-antitrypsin. Biochem Biophys Res Commun. 2006;349:1285–1293. doi: 10.1016/j.bbrc.2006.08.173. [DOI] [PubMed] [Google Scholar]
- 452.Shimizu Y, Okuda-Shimizu Y, Hendershot LM. Ubiquitylation of an ERAD substrate occurs on multiple types of amino acids. Mol Cell. 2010;40:917–926. doi: 10.1016/j.molcel.2010.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Simpson JC, Roberts LM, Römisch K, Davey J, Wolf DH, Lord JM. Ricin A chain utilises the endoplasmic reticulum-associated protein degradation pathway to enter the cytosol of yeast. FEBS Lett. 1999;459:80–84. doi: 10.1016/s0014-5793(99)01222-3. [DOI] [PubMed] [Google Scholar]
- 454.Singh OV, Vij N, Mogayzel PJ, Jozwik C, Pollard HB, Zeitlin PL. Pharmacoproteomics of 4-phenylbutyrate-treated IB3–1 cystic fibrosis bronchial epithelial cells. J Proteome Res. 2006;5:562–571. doi: 10.1021/pr050319o. [DOI] [PubMed] [Google Scholar]
- 455.Sitia R, Braakman I. Quality control in the endoplasmic reticulum protein factory. Nature. 2003;426:891–894. doi: 10.1038/nature02262. [DOI] [PubMed] [Google Scholar]
- 456.Sivasothy P, Dafforn TR, Gettins PGW, Lomas DA. Pathogenic α1-antitrypsin polymers are formed by reactive loop-β-sheet A linkage. J Biol Chem. 2000;275:33663–33668. doi: 10.1074/jbc.M004054200. [DOI] [PubMed] [Google Scholar]
- 457.Skach WR. The expanding role of the ER translocon in membrane protein folding. J Cell Biol. 2007;179:1333–1335. doi: 10.1083/jcb.200711107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Slow EJ, Graham RK, Hayden MR. To be or not to be toxic: aggregations in Huntington and Alzheimer disease. Trends Genet. 2006;22:408–411. doi: 10.1016/j.tig.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 459.Slow EJ, Graham RK, Osmand AP, Devon RS, Lu G, Deng Y, Pearson J, Vaid K, Bissada N, Wetzel R, Leavitt BR, Hayden MR. Absence of behavioral abnormalities and neurodegeneration in vivo despite widespread neuronal huntingtin inclusions. Proc Natl Acad Sci USA. 2005;102:11402–11407. doi: 10.1073/pnas.0503634102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Sokolowska I, Walchli S, Wegrzyn G, Sandvig K, Slominska-Wojewodzka M. A single point mutation in ricin A-chain increases toxin degradation and inhibits EDEM1-dependent ER retrotranslocation. Biochem J. 2011;436:371–385. doi: 10.1042/BJ20101493. [DOI] [PubMed] [Google Scholar]
- 461.Sommer T, Jentsch S. A protein translocation defect linked to ubiquitin conjugation at the endoplasmic reticulum. Nature. 1993;365:176–179. doi: 10.1038/365176a0. [DOI] [PubMed] [Google Scholar]
- 462.Song Y, Sonawane ND, Salinas D, Qian L, Pedemonte N, Galietta LJ, Verkman AS. Evidence against the rescue of defective δF508-CFTR cellular processing by curcumin in cell culture and mouse models. J Biol Chem. 2004;279:40629–40633. doi: 10.1074/jbc.M407308200. [DOI] [PubMed] [Google Scholar]
- 463.Sörgjerd K, Ghafouri B, Jonsson BH, Kelly JW, Blond SY, Hammarström P. Retention of misfolded mutant transthyretin by the chaperone BiP/GRP78 mitigates amyloidogenesis. J Mol Biol. 2006;356:469–482. doi: 10.1016/j.jmb.2005.11.051. [DOI] [PubMed] [Google Scholar]
- 464.Southworth D, Agard D. Client-loading conformation of the Hsp90 molecular chaperone revealed in the cryo-EM structure of the human Hsp90:Hop complex. Mol Cell. 2011;42:771–781. doi: 10.1016/j.molcel.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Sowa ME, Bennett EJ, Gygi SP, Harper JW. Defining the human deubiquitinating enzyme interaction landscape. Cell. 2009;138:389–403. doi: 10.1016/j.cell.2009.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Spooner RA, Smith DC, Easton AJ, Roberts LM, Lord JM. Retrograde transport pathways utilised by viruses and protein toxins. Virol J. 2006;3:26. doi: 10.1186/1743-422X-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Spooner RA, Watson PD, Marsden CJ, Smith DC, Moore KA, Cook JP, Lord JM, Roberts LM. Protein disulphide-isomerase reduces ricin to its A and B chains in the endoplasmic reticulum. Biochem J. 2004;383:285–293. doi: 10.1042/BJ20040742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Stagg HR, Thomas M, van den Boomen D, Wiertz EJHJ, Drabkin HA, Gemmill RM, Lehner PJ. The TRC8 E3 ligase ubiquitinatesMHCclass I molecules before dislocation from the ER. J Cell Biol. 2009;186:685–692. doi: 10.1083/jcb.200906110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Stine OC, Pleasant N, Franz ML, Abbott MH, Folstein SE, Ross CA. Correlation between the onset age of Huntington’s disease and length of the trinucleotide repeat in IT-15. Hum Mol Genet. 1993;2:1547–1549. doi: 10.1093/hmg/2.10.1547. [DOI] [PubMed] [Google Scholar]
- 470.Stoy J, Steiner D, Park SY, Ye H, Philipson L, Bell G. Clinical and molecular genetics of neonatal diabetes due to mutations in the insulin gene. Rev Endocr Metab Disorders. 2010;11:205–215. doi: 10.1007/s11154-010-9151-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Stratford FLL, Pereira MMC, Becq F, McPherson MA, Dormer RL. Benzo(c)quinolizinium drugs inhibit degradation of ΔF508-CFTR cytoplasmic domain. Biochem Biophys Res Commun. 2003;300:524–530. doi: 10.1016/s0006-291x(02)02883-8. [DOI] [PubMed] [Google Scholar]
- 472.Strickland E, Qu BH, Millen L, Thomas PJ. The molecular chaperone Hsc70 assists the in vitro folding of the N-terminal nucleotide-binding domain of the cystic fibrosis transmembrane conductance regulator. J Biol Chem. 1997;272:25421–25424. doi: 10.1074/jbc.272.41.25421. [DOI] [PubMed] [Google Scholar]
- 473.Stroud RM, Walter P. Signal sequence recognition and protein targeting. Curr Opin Struct Biol. 1999;9:754–759. doi: 10.1016/s0959-440x(99)00040-8. [DOI] [PubMed] [Google Scholar]
- 474.Sugiyama T, Shuto T, Suzuki S, Sato T, Koga T, Suico MA, Kusuhara H, Sugiyama Y, Cyr DM, Kai H. Posttranslational negative regulation of glycosylated and non-glycosylated BCRP expression by Derlin-1. Biochem Biophys Res Commun. 2011;404:853–858. doi: 10.1016/j.bbrc.2010.12.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Svedine S, Wang T, Halaban R, Hebert DN. Carbohydrates act as sorting determinants in ER-associated degradation of tyrosinase. J Cell Sci. 2004;117:2937–2949. doi: 10.1242/jcs.01154. [DOI] [PubMed] [Google Scholar]
- 476.Sveger T, Eriksson S. The liver in adolescents with alpha 1-antitrypsin deficiency. Hepatology. 1995;22:514–517. doi: 10.1002/hep.1840220221. [DOI] [PubMed] [Google Scholar]
- 477.Swanson R, Locher M, Hochstrasser M. A conserved ubiquitin ligase of the nuclear envelope/endoplasmic reticulum that functions in both ER-associated and Matalpha2 repressor degradation. Genes Dev. 2001;15:2660–2674. doi: 10.1101/gad.933301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Tabas I. The role of endoplasmic reticulum stress in the progression of atherosclerosis. Circ Res. 2010;107:839–850. doi: 10.1161/CIRCRESAHA.110.224766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Tamarappoo BK, Verkman AS. Defective aquaporin-2 trafficking in nephrogenic diabetes insipidus and correction by chemical chaperones. J Clin Invest. 1998;101:2257–2267. doi: 10.1172/JCI2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Tamarappoo BK, Yang B, Verkman AS. Misfolding of mutant aquaporin-2 water channels in nephrogenic diabetes insipidus. J Biol Chem. 1999;274:34825–34831. doi: 10.1074/jbc.274.49.34825. [DOI] [PubMed] [Google Scholar]
- 481.Taschenberger G, Mougey A, Shen S, Lester LB, LaFranchi S, Shyng SL. Identification of a familial hyperinsulinism-causing mutation in the sulfonylurea receptor 1 that prevents normal trafficking and function of KATP channels. J Biol Chem. 2002;277:17139–17146. doi: 10.1074/jbc.M200363200. [DOI] [PubMed] [Google Scholar]
- 482.Taylor M, Banerjee T, Navarro-Garcia F, Huerta J, Massey S, Burlingame M, Pande AH, Tatulian SA, Teter K. A therapeutic chemical chaperone inhibits cholera intoxication and unfolding/translocation of the cholera toxin A1 subunit. PLoS ONE. 2011;6:e18825. doi: 10.1371/journal.pone.0018825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Taylor M, Navarro-Garcia F, Huerta J, Burress H, Massey S, Ireton K, Teter K. Hsp90 is required for transfer of the cholera toxin a1 subunit from the endoplasmic reticulum to the cytosol. J Biol Chem. 2010;285:31261–31267. doi: 10.1074/jbc.M110.148981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Teckman JH. Lack of effect of oral 4-phenylbutyrate on serum alpha-1-antitrypsin in patients with alpha-1-antitrypsin deficiency: a preliminary study. J Pediatr Gastroenterol Nutr. 2004;39:34–37. doi: 10.1097/00005176-200407000-00007. [DOI] [PubMed] [Google Scholar]
- 485.Teckman JH, Burrows J, Hidvegi T, Schmidt B, Hale PD, Perlmutter DH. The proteasome participates in degradation of mutant α1-antitrypsin Z in the endoplasmic reticulum of hepatoma-derived hepatocytes. J Biol Chem. 2001;276:44865–44872. doi: 10.1074/jbc.M103703200. [DOI] [PubMed] [Google Scholar]
- 486.Teckman JH, Gilmore R, Perlmutter DH. Role of ubiquitin in proteasomal degradation of mutant alpha(1)-antitrypsin Z in the endoplasmic reticulum. Am J Physiol Gastrointest Liver Physiol. 2000;278:G39–G48. doi: 10.1152/ajpgi.2000.278.1.G39. [DOI] [PubMed] [Google Scholar]
- 487.Teo R, King J, Dalton E, Ryves J, Williams RSB, Harwood AJ. PtdIns(3,4,5)P-3 and inositol depletion as a cellular target of mood stabilizers. Biochem Soc Trans. 2009;37:1110–1114. doi: 10.1042/BST0371110. [DOI] [PubMed] [Google Scholar]
- 488.Teter K, Allyn RL, Jobling MG, Holmes RK. Transfer of the cholera toxin A1 polypeptide from the endoplasmic reticulum to the cytosol is a rapid process facilitated by the endoplasmic reticulum-associated degradation pathway. Infect Immun. 2002;70:6166–6171. doi: 10.1128/IAI.70.11.6166-6171.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Thibodeau PH, Brautigam CA, Machius M, Thomas PJ. Side chain and backbone contributions of Phe508 to CFTR folding. Nat Struct Mol Biol. 2005;12:10–16. doi: 10.1038/nsmb881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Thibodeau PH, Richardson JM, Wang W, Millen L, Watson J, Mendoza JL, Du K, Fischman S, Senderowitz H, Lukacs GL, Kirk K, Thomas PJ. The cystic fibrosis-causing mutation ΔF508 affects multiple steps in cystic fibrosis transmembrane conductance regulator biogenesis. J Biol Chem. 2010;285:35825–35835. doi: 10.1074/jbc.M110.131623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Thrower JS, Hoffman L, Rechsteiner M, Pickart CM. Recognition of the polyubiquitin proteolytic signal. EMBO J. 2000;19:94–102. doi: 10.1093/emboj/19.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Tokunaga F, Hara K, Koide T. N-linked oligosaccharide processing, but not association with calnexin/calreticulin is highly correlated with endoplasmic reticulum-associated degradation of antithrombin Glu313-deleted mutant. Arch Biochem Biophys. 2003;411:235–242. doi: 10.1016/s0003-9861(02)00717-8. [DOI] [PubMed] [Google Scholar]
- 493.Tokunaga F, Shirotani H, Hara K, Kozuki D, Omura S, Koide T. Intracellular degradation of secretion defect-type mutants of antithrombin is inhibited by proteasomal inhibitors. FEBS Lett. 1997;412:65–69. doi: 10.1016/s0014-5793(97)00745-x. [DOI] [PubMed] [Google Scholar]
- 494.Tokunaga F, Tsukamoto T, Koide T. Cellular basis for protein C deficiency caused by a single amino acid substitution at Argl5 in the γ-carboxyglutamic acid domain. J Biochem. 1996;120:360–368. doi: 10.1093/oxfordjournals.jbchem.a021421. [DOI] [PubMed] [Google Scholar]
- 495.Travers KJ, Patil CK, Wodicka L, Lockhart DJ, Weissman JS, Walter P. Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell. 2000;101:249–258. doi: 10.1016/s0092-8674(00)80835-1. [DOI] [PubMed] [Google Scholar]
- 496.Tresse E, Salomons FA, Vesa J, Bott LC, Kimonis V, Yao TP, Dantuma NP, Taylor JP. VCP/p97 is essential for maturation of ubiquitin-containing autophagosomes and this function is impaired by mutations that cause IBMPFD. Autophagy. 2010;6:217–227. doi: 10.4161/auto.6.2.11014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Tropak MB, Blanchard JE, Withers SG, Brown ED, Mahuran D. High-throughput screening for human lysosomal β-N-acetyl hexosaminidase inhibitors acting as pharmacological chaperones. Chem Biol. 2007;14:153–164. doi: 10.1016/j.chembiol.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Tropak MB, Reid SP, Guiral M, Withers SG, Mahuran D. Pharmacological enhancement of β-hexosaminidase activity in fibroblasts from adult Tay-Sachs and Sandhoff patients. J Biol Chem. 2004;279:13478–13487. doi: 10.1074/jbc.M308523200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Tsai B, Rodighiero C, Lencer WI, Rapoport TA. Protein disulfide isomerase acts as a redox-dependent chaperone to unfold cholera toxin. Cell. 2001;104:937–948. doi: 10.1016/s0092-8674(01)00289-6. [DOI] [PubMed] [Google Scholar]
- 500.Tsai YC, Weissman AM. Ubiquitylation in ERAD: reversing to go forward? PLoS Biol. 2011;9:e1001038. doi: 10.1371/journal.pbio.1001038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Tsaytler P, Harding HP, Ron D, Bertolotti A. Selective inhibition of a regulatory subunit of protein phosphatase 1 restores proteostasis. Science. 2011;332:91–94. doi: 10.1126/science.1201396. [DOI] [PubMed] [Google Scholar]
- 502.Tsuda H, Tokunaga F, Nagamitsu H, Koide T. Characterization of endoplasmic reticulum-associated degradation of a protein S mutant identified in a family of quantitative protein S deficiency. Thromb Res. 2006;117:323–331. doi: 10.1016/j.thromres.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 503.Tsuda M, Sei Y, Ohkubo T, Yamamura M, Kamiguchi H, Akatsuka A, Tsuda T, Tachikawa H, Yamamoto M, Shinohara Y. The defective secretion of a naturally occurring α-1-antichymotrypsin variant with a frameshift mutation. Eur J Biochem. 1996;235:821–827. doi: 10.1111/j.1432-1033.1996.00821.x. [DOI] [PubMed] [Google Scholar]
- 504.Ugolino J, Fang S, Kubisch C, Monteiro MJ. Mutant Atp13a2 proteins involved in parkinsonism are degraded by ER-associated degradation and sensitize cells to ER-stress induced cell death. Hum Mol Genet. 2011;20:3565–3577. doi: 10.1093/hmg/ddr274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Ulloa-Aguirre A, Janovick JA, Brothers SP, Conn PM. Pharmacologic rescue of conformationally-defective proteins: implications for the treatment of human disease. Traffic. 2004;5:821–837. doi: 10.1111/j.1600-0854.2004.00232.x. [DOI] [PubMed] [Google Scholar]
- 506.Van der Velden LM, Stapelbroek JM, Krieger E, van den Berghe PVE, Berger R, Verhulst PM, Holthuis JCM, Houwen RHJ, Klomp LWJ, van de Graaf SFJ. Folding defects in P-type ATP 8B1 associated with hereditary cholestasis are ameliorated by 4-phenylbutyrate. Hepatology. 2010;51:286–296. doi: 10.1002/hep.23268. [DOI] [PubMed] [Google Scholar]
- 507.Van Goor F, Hadida S, Grootenhuis PDJ, Burton B, Cao D, Neuberger T, Turnbull A, Singh A, Joubran J, Hazlewood A, Zhou J, McCartney J, Arumugam V, Decker C, Yang J, Young C, Olson ER, Wine JJ, Frizzell RA, Ashlock M, Negulescu P. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci USA. 2009;106:18825–18830. doi: 10.1073/pnas.0904709106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Van Goor F, Straley KS, Cao D, González J, Hadida S, Hazlewood A, Joubran J, Knapp T, Makings LR, Miller M, Neuberger T, Olson E, Panchenko V, Rader J, Singh A, Stack JH, Tung R, Grootenhuis PDJ, Negulescu P. Rescue of δF508-CFTR trafficking and gating in human cystic fibrosis airway primary cultures by small molecules. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1117–L1130. doi: 10.1152/ajplung.00169.2005. [DOI] [PubMed] [Google Scholar]
- 509.Vanden Broeck D, Horvath C, De Wolf MJS. Vibrio cholerae: cholera toxin. Int J Biochem Cell Biol. 2007;39:1771–1775. doi: 10.1016/j.biocel.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 510.VanSlyke JK, Deschenes SM, Musil LS. Intracellular transport, assembly, and degradation of wild-type and disease-linked mutant gap junction proteins. Mol Biol Cell. 2000;11:1933–1946. doi: 10.1091/mbc.11.6.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Varandani PT. Acceleration of regeneration of insulin activity from its inactive reduced A and B chains by pancreatic glutathioneinsulin transhydrogenase. Biochim Biophysica Acta. 1967;132:10–14. doi: 10.1016/0005-2744(67)90186-6. [DOI] [PubMed] [Google Scholar]
- 512.Varga K, Jurkuvenaite A, Wakefield J, Hong JS, Guimbellot JS, Venglarik CJ, Niraj A, Mazur M, Sorscher EJ, Collawn JF, Bebok Z. Efficient intracellular processing of the endogenous cystic fibrosis transmembrane conductance regulator in epithelial cell lines. J Biol Chem. 2004;279:22578–22584. doi: 10.1074/jbc.M401522200. [DOI] [PubMed] [Google Scholar]
- 513.Varshavsky A. Discovery of cellular regulation by protein degradation. J Biol Chem. 2008;283:34469–34489. doi: 10.1074/jbc.X800009200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Verma R, Aravind L, Oania R, McDonald WH, Yates JR, 3rd, Koonin EV, Deshaies RJ. Role of Rpn11 metalloprotease in deubiquitination and degradation by the 26S proteasome. Science. 2002;298:611–615. doi: 10.1126/science.1075898. [DOI] [PubMed] [Google Scholar]
- 515.Volchuk A, Ron D. The endoplasmic reticulum stress response in the pancreatic β-cell. Diabetes Obesity Metab. 2010;12:48–57. doi: 10.1111/j.1463-1326.2010.01271.x. [DOI] [PubMed] [Google Scholar]
- 516.Von Bernhardi R, Tichauer JE, Eugenín J. Aging-dependent changes of microglial cells and their relevance for neurodegenerative disorders. J Neurochem. 2010;112:1099–1114. doi: 10.1111/j.1471-4159.2009.06537.x. [DOI] [PubMed] [Google Scholar]
- 517.Waheed A, Parkkila S, Zhou XY, Tomatsu S, Tsuchihashi Z, Feder JN, Schatzman RC, Britton RS, Bacon BR, Sly WS. Hereditary hemochromatosis: effects of C282Y and H63D mutations on association with β2-microglobulin, intracellular processing, and cell surface expression of the HFE protein in COS-7 cells. Proc Natl Acad Sci USA. 1997;94:12384–12389. doi: 10.1073/pnas.94.23.12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Wahlman J, DeMartino GN, Skach WR, Bulleid NJ, Brodsky JL, Johnson AE. Real-time fluorescence detection of ERAD substrate retrotranslocation in a mammalian in vitro system. Cell. 2007;129:943–955. doi: 10.1016/j.cell.2007.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Walker VE, Wong MJH, Atanasiu R, Hantouche C, Young JC, Shrier A. Hsp40 chaperones promote degradation of the hERG potassium channel. J Biol Chem. 2010;285:3319–3329. doi: 10.1074/jbc.M109.024000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.Wandinger SK, Richter K, Buchner J. The Hsp90 chaperone machinery. J Biol Chem. 2008;283:18473–18477. doi: 10.1074/jbc.R800007200. [DOI] [PubMed] [Google Scholar]
- 521.Wang F, Agnello G, Sotolongo N, Segatori L. Ca2+ homeostasis modulation enhances the amenability of L444P glucosylcerebrosidase to proteostasis regulation in patient-derived fibroblasts. ACS Chem Biol. 2010;6:158–168. doi: 10.1021/cb100321m. [DOI] [PubMed] [Google Scholar]
- 522.Wang H, Li Q, Shen Y, Sun A, Zhu X, Fang S, Shen Y. The ubiquitin ligase Hrd1 promotes degradation of the Z variant alpha 1-antitrypsin and increases its solubility. Mol Cell Biochem. 2011;346:137–145. doi: 10.1007/s11010-010-0600-9. [DOI] [PubMed] [Google Scholar]
- 523.Wang J, Takeuchi T, Tanaka S, Kubo SK, Kayo T, Lu D, Takata K, Koizumi A, Izumi T. A mutation in the insulin 2 gene induces diabetes with severe pancreatic β-cell dysfunction in the Mody mouse. J Clin Invest. 1999;103:27–37. doi: 10.1172/JCI4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Wang L, Dong H, Soroka CJ, Wei N, Boyer JL, Hochstrasser M. Degradation of the bile salt export pump at endoplasmic reticulum in progressive familial intrahepatic cholestasis type II. Hepatology. 2008;48:1558–1569. doi: 10.1002/hep.22499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Wang L, Wang J, Li N, Ge L, Li B, Song B. Molecular characterization of the NPC1L1 variants identified from cholesterol low absorbers. J Biol Chem. 2011;286:7397–7408. doi: 10.1074/jbc.M110.178368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.Wang M, Bridges JP, Na CL, Xu Y, Weaver TE. Meckel-Gruber Syndrome protein MKS3 is required for endoplasmic reticulum-associated degradation of surfactant protein C. J Biol Chem. 2009;284:33377–33383. doi: 10.1074/jbc.M109.034371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.Wang Q, Li L, Ye Y. Regulation of retrotranslocation by p97-associated deubiquitinating enzyme ataxin-3. J Cell Biol. 2006;174:963–971. doi: 10.1083/jcb.200605100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Wang Q, Liu Y, Soetandyo N, Baek K, Hegde R, Ye Y. A ubiquitin ligase-associated chaperone holdase maintains polypeptides in soluble states for proteasome degradation. Mol Cell. 2011;42:758–770. doi: 10.1016/j.molcel.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.Wang W, Bernard K, Li G, Kirk KL. Curcumin opens cystic fibrosis transmembrane conductance regulator channels by a novel mechanism that requires neither ATP binding nor dimerization of the nucleotide-binding domains. J Biol Chem. 2007;282:4533–4544. doi: 10.1074/jbc.M609942200. [DOI] [PubMed] [Google Scholar]
- 530.Wang X, Herr RA, Rabelink M, Hoeben RC, Wiertz EJHJ, Hansen TH. Ube2j2 ubiquitinates hydroxylated amino acids on ER-associated degradation substrates. J Cell Biol. 2009;187:655–668. doi: 10.1083/jcb.200908036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531.Wang X, Venable J, LaPointe P, Hutt DM, Koulov AV, Coppinger J, Gurkan C, Kellner W, Matteson J, Plutner H, Riordan JR, Kelly JW, Yates JR, 3rd, Balch WE. Hsp90 cochaperone Aha1 downregulation rescues misfolding of CFTR in cystic fibrosis. Cell. 2006;127:803–815. doi: 10.1016/j.cell.2006.09.043. [DOI] [PubMed] [Google Scholar]
- 532.Wang Y, Loo TW, Bartlett MC, Clarke DM. Correctors promote maturation of cystic fibrosis transmembrane conductance regulator (CFTR)-processing mutants by binding to the protein. J Biol Chem. 2007;282:33247–33251. doi: 10.1074/jbc.C700175200. [DOI] [PubMed] [Google Scholar]
- 533.Wang Y, Loo TW, Bartlett MC, Clarke DM. Modulating the folding of P-Glycoprotein and cystic fibrosis transmembrane conductance regulator truncation mutants with pharmacological chaperones. Mol Pharmacol. 2007;71:751–758. doi: 10.1124/mol.106.029926. [DOI] [PubMed] [Google Scholar]
- 534.Ward C, Lawrence M, Streltsov V, Garrett T, McKern N, Lou MZ, Lovrecz G, Adams T. Structural insights into ligand-induced activation of the insulin receptor. Acta Physiol. 2008;192:3–9. doi: 10.1111/j.1748-1716.2007.01781.x. [DOI] [PubMed] [Google Scholar]
- 535.Ward CL, Kopito RR. Intracellular turnover of cystic fibrosis transmembrane conductance regulator. Inefficient processing and rapid degradation of wild-type and mutant proteins. J Biol Chem. 1994;269:25710–25718. [PubMed] [Google Scholar]
- 536.Ward CL, Omura S, Kopito RR. Degradation of CFTR by the ubiquitin-proteasome pathway. Cell. 1995;83:121–127. doi: 10.1016/0092-8674(95)90240-6. [DOI] [PubMed] [Google Scholar]
- 537.Watabe H, Valencia JC, Yasumoto Ki Kushimoto T, Ando H, Muller J, Vieira WD, Mizoguchi M, Appella E, Hearing VJ. Regulation of tyrosinase processing and trafficking by organellar pH and by proteasome activity. J Biol Chem. 2004;279:7971–7981. doi: 10.1074/jbc.M309714200. [DOI] [PubMed] [Google Scholar]
- 538.Watts GD, Wymer J, Kovach MJ, Mehta SG, Mumm S, Darvish D, Pestronk A, Whyte MP, Kimonis VE. Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nat Genet. 2004;36:377–381. doi: 10.1038/ng1332. [DOI] [PubMed] [Google Scholar]
- 539.Weihl CC, Dalal S, Pestronk A, Hanson PI. Inclusion body myopathy-associated mutations in p97/VCP impair endoplasmic reticulum-associated degradation. Hum Mol Genet. 2006;15:189–199. doi: 10.1093/hmg/ddi426. [DOI] [PubMed] [Google Scholar]
- 540.Werner ED, Brodsky JL, McCracken AA. Proteasome-dependent endoplasmic reticulum-associated protein degradation: an unconventional route to a familiar fate. Proc Natl Acad Sci USA. 1996;93:13797–13801. doi: 10.1073/pnas.93.24.13797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541.Wesche J, Rapak A, Olsnes S. Dependence of ricin toxicity on translocation of the toxin A-chain from the endoplasmic reticulum to the cytosol. J Biol Chem. 1999;274:34443–34449. doi: 10.1074/jbc.274.48.34443. [DOI] [PubMed] [Google Scholar]
- 542.Westerheide SD, Bosman JD, Mbadugha BN, Kawahara TL, Matsumoto G, Kim S, Gu W, Devlin JP, Silverman RB, Morimoto RI. Celastrols as inducers of the heat shock response and cytoprotection. J Biol Chem. 2004;279:56053–56060. doi: 10.1074/jbc.M409267200. [DOI] [PubMed] [Google Scholar]
- 543.Whiteman P, Handford PA. Defective secretion of recombinant fragments of fibrillin-1: implications of protein misfolding for the pathogenesis of Marfan syndrome and related disorders. Hum Mol Genet. 2003;12:727–737. doi: 10.1093/hmg/ddg081. [DOI] [PubMed] [Google Scholar]
- 544.Wiertz EJ, Jones TR, Sun L, Bogyo M, Geuze HJ, Ploegh HL. The human cytomegalovirus US11 gene product dislocates MHC class I heavy chains from the endoplasmic reticulum to the cytosol. Cell. 1996;84:769–779. doi: 10.1016/s0092-8674(00)81054-5. [DOI] [PubMed] [Google Scholar]
- 545.Wiertz EJ, Tortorella D, Bogyo M, Yu J, Mothes W, Jones TR, Rapoport TA, Ploegh HL. Sec61-mediated transfer of a membrane protein from the endoplasmic reticulum to the proteasome for destruction. Nature. 1996;384:432–438. doi: 10.1038/384432a0. [DOI] [PubMed] [Google Scholar]
- 546.Wikstrom L, Lodish HF. Endoplasmic reticulum degradation of a subunit of the asialoglycoprotein receptor in vitro. Vesicular transport from endoplasmic reticulum is unnecessary. J Biol Chem. 1992;267:5–8. [PubMed] [Google Scholar]
- 547.Wild K, Rosendal KR, Sinning I. A structural step into the SRP cycle. Mol Microbiol. 2004;53:357–363. doi: 10.1111/j.1365-2958.2004.04139.x. [DOI] [PubMed] [Google Scholar]
- 548.Willer M, Forte GMA, Stirling CJ. Sec61p is required for ERAD-L. J Biol Chem. 2008;283:33883–33888. doi: 10.1074/jbc.M803054200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549.Willey RL, Maldarelli F, Martin MA, Strebel K. Human immunodeficiency virus type 1 Vpu protein induces rapid degradation of CD4. J Virol. 1992;66:7193–7200. doi: 10.1128/jvi.66.12.7193-7200.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Williams RSB, Cheng L, Mudge AW, Harwood AJ. A common mechanism of action for three mood-stabilizing drugs. Nature. 2002;417:292–295. doi: 10.1038/417292a. [DOI] [PubMed] [Google Scholar]
- 551.Wojcikiewicz RJH, Pearce MMP, Sliter DA, Wang Y. When worlds collide: IP3 receptors and the ERAD pathway. Cell Calcium. 2009;46:147–153. doi: 10.1016/j.ceca.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 552.Worthington ZEV, Carbonetti NH. Evading the proteasome: absence of lysine residues contributes to pertussis toxin activity by evasion of proteasome degradation. Infect Immun. 2007;75:2946–2953. doi: 10.1128/IAI.02011-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553.Wu Y, Swulius MT, Moremen KW, Sifers RN. Elucidation of the molecular logic by which misfolded α1-antitrypsin is preferentially selected for degradation. Proc Natl Acad Sci USA. 2003 doi: 10.1073/pnas.1430537100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Wu Y, Whitman I, Molmenti E, Moore K, Hippenmeyer P, Perlmutter DH. A lag in intracellular degradation of mutant alpha 1-antitrypsin correlates with the liver disease phenotype in homozygous PiZZ alpha 1-antitrypsin deficiency. Proc Natl Acad Sci USA. 1994;91:9014–9018. doi: 10.1073/pnas.91.19.9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 555.Xia H, Redman C. The degradation of nascent fibrinogen chains is mediated by the ubiquitin proteasome pathway. Biochem Biophys Res Commun. 1999;261:590–597. doi: 10.1006/bbrc.1999.1081. [DOI] [PubMed] [Google Scholar]
- 556.Xia H, Redman CM. Differential degradation of the three fibrinogen chains by proteasomes: involvement of Sec61p and cytosolic Hsp70. Arch Biochem Biophys. 2001;390:137–145. doi: 10.1006/abbi.2001.2374. [DOI] [PubMed] [Google Scholar]
- 557.Xu P, Duong DM, Seyfried NT, Cheng D, Xie Y, Robert J, Rush J, Hochstrasser M, Finley D, Peng J. Quantitative proteomics reveals the function of unconventional ubiquitin chains in proteasomal degradation. Cell. 2009;137:133–145. doi: 10.1016/j.cell.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 558.Xu S, Peng G, Wang Y, Fang S, Karbowski M. The AAA-ATPase p97 is essential for outer mitochondrial membrane protein turnover. Mol Biol Cell. 2011;22:291–300. doi: 10.1091/mbc.E10-09-0748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 559.Yam GHF, Bosshard N, Zuber C, Steinmann B, Roth J. Pharmacological chaperone corrects lysosomal storage in Fabry disease caused by trafficking-incompetent variants. Am J Physiol Cell Physiol. 2006;290:C1076–C1082. doi: 10.1152/ajpcell.00426.2005. [DOI] [PubMed] [Google Scholar]
- 560.Yamamoto YH, Kimura T, Momohara S, Takeuchi M, Tani T, Kimata Y, Kadokura H, Kohno K. A novel ER J-protein DNAJB12 accelerates ER-associated degradation of membrane proteins including CFTR. Cell Struct Funct. 2010;35:107–116. doi: 10.1247/csf.10023. [DOI] [PubMed] [Google Scholar]
- 561.Yan FF, Lin CW, Cartier EA, Shyng SL. Role of ubiquitin-proteasome degradation pathway in biogenesis efficiency of β-cell ATP-sensitive potassium channels. Am J Physiol Cell Physiol. 2005;289:C1351–C1359. doi: 10.1152/ajpcell.00240.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562.Yan FF, Pratt EB, Chen PC, Wang F, Skach WR, David LL, Shyng SL. Role of Hsp90 in Biogenesis of the β-cell ATP-sensitive potassium channel complex. Mol Biol Cell. 2010;21:1945–1954. doi: 10.1091/mbc.E10-02-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 563.Yang H, Liu C, Zhong Y, Luo S, Monteiro MJ, Fang S. Huntingtin interacts with the cue domain of gp78 and inhibits gp78 binding to ubiquitin and p97/VCP. PLoS One. 2010;5:e8905. doi: 10.1371/journal.pone.0008905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564.Yang Y, Janich S, Cohn JA, Wilson JM. The common variant of cystic fibrosis transmembrane conductance regulator is recognized by hsp70 and degraded in a pre-Golgi nonlysosomal compartment. Proc Natl Acad Sci USA. 1993;90:9480–9484. doi: 10.1073/pnas.90.20.9480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 565.Yao T, Cohen RE. A cryptic protease couples deubiquitination and degradation by the proteasome. Nature. 2002;419:403–407. doi: 10.1038/nature01071. [DOI] [PubMed] [Google Scholar]
- 566.Ye Y, Meyer HH, Rapoport TA. The AAA ATPase Cdc48/p97 and its partners transport proteins from the ER into the cytosol. Nature. 2001;414:652–656. doi: 10.1038/414652a. [DOI] [PubMed] [Google Scholar]
- 567.Ye Y, Shibata Y, Yun C, Ron D, Rapoport TA. A membrane protein complex mediates retro-translocation from the ER lumen into the cytosol. Nature. 2004;429:841–847. doi: 10.1038/nature02656. [DOI] [PubMed] [Google Scholar]
- 568.Yeung SJ, Chen SH, Chan L. Ubiquitin-proteasome pathway mediates intracellular degradation of apolipoprotein B. Biochemistry. 1996;35:13843–13848. doi: 10.1021/bi9618777. [DOI] [PubMed] [Google Scholar]
- 569.Ying Z, Wang H, Fan H, Wang G. The ER-associated degradation system regulates aggregation and degradation of mutant neuroserpin. J Biol Chem. 2011;286:20835–20844. doi: 10.1074/jbc.M110.200808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570.Ying Z, Wang H, Fan H, Zhu X, Zhou J, Fei E, Wang G. Gp78, an ER associated E3, promotes SOD1 and ataxin-3 degradation. Hum Mol Genet. 2009;18:4268–4281. doi: 10.1093/hmg/ddp380. [DOI] [PubMed] [Google Scholar]
- 571.Youker RT, Walsh P, Beilharz T, Lithgow T, Brodsky JL. Distinct roles for the Hsp40 and Hsp90 molecular chaperones during cystic fibrosis transmembrane conductance regulator degradation in yeast. Mol Biol Cell. 2004;15:4787–4797. doi: 10.1091/mbc.E04-07-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572.Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol. 2011;12:9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 573.Young JC, Agashe VR, Siegers K, Hartl FU. Pathways of chaperone-mediated protein folding in the cytosol. Nat Rev Mol Cell Biol. 2004;5:781–791. doi: 10.1038/nrm1492. [DOI] [PubMed] [Google Scholar]
- 574.Younger JM, Chen L, Ren HY, Rosser MF, Turnbull EL, Fan CY, Patterson C, Cyr DM. Sequential quality-control checkpoints triage misfolded cystic fibrosis transmembrane conductance regulator. Cell. 2006;126:571–582. doi: 10.1016/j.cell.2006.06.041. [DOI] [PubMed] [Google Scholar]
- 575.Yu M, Haslam DB. Shiga toxin is transported from the endoplasmic reticulum following interaction with the luminal chaperone HEDJ/ERdj3. Infect Immun. 2005;73:2524–2532. doi: 10.1128/IAI.73.4.2524-2532.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 576.Yu W, Chiaw PK, Bear CE. Probing conformational rescue induced by a chemical corrector of F508del-cystic fibrosis transmembrane conductance regulator (CFTR) mutant. J Biol Chem. 2011;286:24714–24725. doi: 10.1074/jbc.M111.239699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 577.Yu YC, Miki H, Nakamura Y, Hanyuda A, Matsuzaki Y, Abe Y, Yasui M, Tanaka K, Hwang TC, Bompadre SG, Sohma Y. Curcumin and genistein additively potentiate G551D-CFTR. J Cystic Fibrosis. 2011;10:243–252. doi: 10.1016/j.jcf.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 578.Yu Z, Sawkar AR, Kelly JW. Pharmacologic chaperoning as a strategy to treat Gaucher disease. FEBS J. 2007;274:4944–4950. doi: 10.1111/j.1742-4658.2007.06042.x. [DOI] [PubMed] [Google Scholar]
- 579.Zaman K, Carraro S, Doherty J, Henderson EM, Lendermon E, Liu L, Verghese G, Zigler M, Ross M, Park E, Palmer LA, Doctor A, Stamler JS, Gaston B. S-nitrosylating agents: a novel class of compounds that increase cystic fibrosis transmembrane conductance regulator expression and maturation in epithelial cells. Mol Pharmacol. 2006;70:1435–1442. doi: 10.1124/mol.106.023242. [DOI] [PubMed] [Google Scholar]
- 580.Zhang H, Schmidt BZ, Sun F, Condliffe SB, Butterworth MB, Youker RT, Brodsky JL, Aridor M, Frizzell RA. Cysteine string protein monitors late steps in cystic fibrosis transmembrane conductance regulator biogenesis. J Biol Chem. 2006;281:11312–11321. doi: 10.1074/jbc.M512013200. [DOI] [PubMed] [Google Scholar]
- 581.Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454:455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 582.Zhang RG, Scott DL, Westbrook ML, Nance S, Spangler BD, Shipley GG, Westbrook EM. The three-dimensional crystal structure of cholera toxin. J Mol Biol. 1995;251:563–573. doi: 10.1006/jmbi.1995.0456. [DOI] [PubMed] [Google Scholar]
- 583.Zhang XM, Wang XT, Yue H, Leung SW, Thibodeau PH, Thomas PJ, Guggino SE. Organic solutes rescue the functional defect in ΔF508 cystic fibrosis transmembrane conductance regulator. J Biol Chem. 2003;278:51232–51242. doi: 10.1074/jbc.M309076200. [DOI] [PubMed] [Google Scholar]
- 584.Zhang Y, Nijbroek G, Sullivan ML, McCracken AA, Watkins SC, Michaelis S, Brodsky JL. Hsp70 molecular chaperone facilitates endoplasmic reticulum-associated protein degradation of cystic fibrosis transmembrane conductance regulator in yeast. Mol Biol Cell. 2001;12:1303–1314. doi: 10.1091/mbc.12.5.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 585.Zhao L, Ackerman SL. Endoplasmic reticulum stress in health and disease. Curr Opin Cell Biol. 2006;18:444–452. doi: 10.1016/j.ceb.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 586.Zhong X, Pittman RN. Ataxin-3 binds VCP/p97 and regulates retrotranslocation of ERAD substrates. Hum Mol Genet. 2006;15:2409–2420. doi: 10.1093/hmg/ddl164. [DOI] [PubMed] [Google Scholar]
- 587.Zhong X, Shen Y, Ballar P, Apostolou A, Agami R, Fang S. AAA ATPase p97/valosin-containing protein interacts with gp78, a ubiquitin ligase for endoplasmic reticulum-associated degradation. J Biol Chem. 2004;279:45676–45684. doi: 10.1074/jbc.M409034200. [DOI] [PubMed] [Google Scholar]
- 588.Zhou J, Liu CY, Back SH, Clark RL, Peisach D, Xu Z, Kaufman RJ. The crystal structure of human IRE1 luminal domain reveals a conserved dimerization interface required for activation of the unfolded protein response. Proc Natl Acad Sci USA. 2006;103:14343–14348. doi: 10.1073/pnas.0606480103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 589.Zhou Z, Gong Q, Epstein ML, January CT. HERG channel dysfunction in human long QT syndrome. J Biol Chem. 1998;273:21061–21066. doi: 10.1074/jbc.273.33.21061. [DOI] [PubMed] [Google Scholar]
- 590.Zhou Z, Gong Q, January CT. Correction of defective protein trafficking of a mutant HERG potassium channel in human long QT syndrome. J Biol Chem. 1999;274:31123–31126. doi: 10.1074/jbc.274.44.31123. [DOI] [PubMed] [Google Scholar]
- 591.Zoghbi HY, Orr HT. Glutamine rpeats and neurodegeneration. Annu Rev Neurosci. 2000;23:217–247. doi: 10.1146/annurev.neuro.23.1.217. [DOI] [PubMed] [Google Scholar]