Abstract
A dose-response strategy may not only allow investigation of the impact of foods and nutrients on human health but may also reveal differences in the response of individuals to food ingestion based on their metabolic health status. In a randomized crossover study, we challenged 19 normal-weight (BMI: 20–25 kg/m2) and 18 obese (BMI: >30 kg/m2) men with 500, 1000, and 1500 kcal of a high-fat (HF) meal (60.5% energy from fat). Blood was taken at baseline and up to 6 h postprandially and analyzed for a range of metabolic, inflammatory, and hormonal variables, including plasma glucose, lipids, and C-reactive protein and serum insulin, glucagon-like peptide-1, interleukin-6 (IL-6), and endotoxin. Insulin was the only variable that could differentiate the postprandial response of normal-weight and obese participants at each of the 3 caloric doses. A significant response of the inflammatory marker IL-6 was only observed in the obese group after ingestion of the HF meal containing 1500 kcal [net incremental AUC (iAUC) = 22.9 ± 6.8 pg/mL × 6 h, P = 0.002]. Furthermore, the net iAUC for triglycerides significantly increased from the 1000 to the 1500 kcal meal in the obese group (5.0 ± 0.5 mmol/L × 6 h vs. 6.0 ± 0.5 mmol/L × 6 h; P = 0.015) but not in the normal-weight group (4.3 ± 0.5 mmol/L × 6 h vs. 4.8 ± 0.5 mmol/L × 6 h; P = 0.31). We propose that caloric dose-response studies may contribute to a better understanding of the metabolic impact of food on the human organism. This study was registered at clinicaltrials.gov as NCT01446068.
See corresponding commentary on page 1493.
Introduction
The response of the organism to food ingestion evidently depends on the nutritional quality of the ingested food and on the health status of the person ingesting the food. The production of insulin in response to the ingestion of carbohydrates offers one of the most spectacular examples illustrating the importance of these 2 aspects because both the glycemic index of food (1) and the insulin resistance of humans (2) dramatically influence postprandial glucose concentrations.
The increased awareness that metabolic, hormonal, and inflammatory pathways intimately regulate each other (3, 4) led to a broadening of the spectrum of biologic pathways investigated in nutritional studies. The last decade has thus witnessed a steady increase in the number of nutritional biomarkers aimed at characterizing the health status of enrolled participants, their intake of nutrients, and the effect of these nutrients on their metabolism (5, 6). Still, for many of these biomarkers, demonstrating a differential impact of food quality or the health status of the investigated participants on postprandial metabolism and inflammation is challenging. For example, although high caloric intake was shown to trigger a postprandial inflammatory response in both healthy participants and participants with metabolic disorders (7–14), these studies failed to provide conclusions regarding appropriate experimental conditions (e.g., caloric dose, participants, inflammatory variables) likely to induce a significant postprandial inflammatory response. In particular, these studies do not address whether metabolic disorders are associated with an increased postprandial inflammatory response, or whether elevated postprandial concentrations of inflammatory markers, such as IL-6, are simply observed as a result of higher baseline values.
The nonpharmacologic nature of nutrition certainly contributes to the difficulty in demonstrating significant changes in nutritional biomarkers where only marginal changes are observed in response to nutritional interventions (4). In addition, endogenous phenomena such as the circadian rhythm are tightly connected to metabolic pathways and the immune system (15) and may thus contribute to the observed postprandial changes in the concentrations of the investigated variables. Finally, human biology is systemic and, as such, characterized by robustness (16). The robustness of a biologic system can, however, be challenged by integrating dose-response strategies into study designs (17). Surprisingly, although food quantity evidently influences the metabolic impact of nutrients on the organism, only a few nutritional studies have made use of a dose-response strategy to challenge the robustness of the organism in nutritional interventions. In this context, the Biomarkers of Nutrition for Development (BOND) Project, which was created to develop a consensus on the development of nutritional biomarkers, emphasizes the need for conducting dose-response studies (5, 6). In line with this policy, we propose that dose-response studies should allow for a better understanding of the impact of food on human metabolism.
The aim of this study was therefore to investigate the dose-dependent effect of a high-fat (HF)11 meal on postprandial metabolism and inflammation in 2 groups of participants differing in their metabolic health status (i.e., normal-weight and obese participants). In particular, this study investigated the following: 1) the caloric dose of the HF meal needed to induce a postprandial response; 2) the contribution of the dose-response strategy to differentiate the postprandial response of the 2 groups of participants; and 3) the impact of the caloric dosing on the identification of correlations between the fasting and postprandial concentrations of the investigated variables.
Biochemical analyses were undertaken to quantify metabolic and hormonal variables [glucose, insulin, blood lipids, glucagon-like peptide-1 (GLP-1)] as well as inflammatory mediators [C-reactive protein (CRP), IL-6, endotoxin]. Serum endotoxin was analyzed as an additional marker of inflammation being associated with metabolic disorders (18) and HF meal–induced inflammation (19–23).
Participants and Methods
Participants.
Normal-weight (n = 19, BMI: 20–25 kg/m2, waist circumference: <94 cm) and obese (n = 18, BMI: >30 kg/m2, waist circumference: >102 cm) men, between 25 and 55 y of age, were recruited from the region of Berne, Switzerland. The 2 groups were age-matched so that the mean age was not substantially different between the normal-weight and obese participants. The participants’ anthropometric measurements (weight, height, waist circumference) were measured on the first study day. The BMI was calculated as weight divided by the square of height (kg/m2). All participants signed an informed consent form after written and oral explanation of the procedures involved with this study. Participants were excluded if they had any past or present cardiovascular disease, diagnosed diabetes or inflammatory condition, or were taking any medications influencing the study outcome measurements (inflammation markers, lipids analyses). Further exclusion criteria were current smokers, food allergy, and impaired kidney or liver function. The participants were not allowed to take dietary supplements, such as vitamins, 2 wk prior to the start of the study until its end. In addition, blood donations were not allowed 3 mo before the start of the study until its end.
Study design.
The study was conducted at the University Hospital, Berne, Switzerland. Approval for the study was obtained from the Ethics Committee of the Canton Berne (KEK number 006/11). The study is reported according to the checklist published in the Consolidated Standards of Reporting Trials Statement. In the crossover study design, each participant had to consume 3 different caloric doses of a HF meal (500, 1000, 1500 kcal) with at least 1 wk of a washout period between the test meals during which participants were requested to stick to their habitual diet. Randomization of the test meals was performed with the excel randomization function by allocating the 6 possible sequences of test meal administration (ABC, ACB, BAC, BCA, CAB, CBA) to the 2 blocks “normal-weight participants” (n = 19) and “obese participants” (n = 18). Blood was collected from participants who fasted overnight (10 h) prior to each test meal consumption between 0800 and 0900 (t = 0 h), as well as 1, 2, 4, and 6 h after the beginning of the test meal consumption by using an indwelling peripheral venous catheter. During the study days, the participants were not allowed to consume any additional foods or beverages except 1 L of water provided during the postprandial period.
Test meals composition.
The HF meals consisted of bread, palm fat, salami, and boiled eggs, obtained from Swiss supermarkets (Table 1). Palm fat was chosen as it is rich in the saturated palmitic acid, which was shown to induce inflammation in adipocytes as measured by mRNA expression of IL-6 and TNF-α (24). The 3 HF meals had the same macronutrient composition with 61% of the energy originating from fat, 21% from carbohydrates, and 18% from proteins. The HF meal composition was adapted from the study of Nappo et al. (12) showing that, compared with a high-carbohydrate meal, a high-fat meal significantly increased plasma concentrations of the inflammation markers IL-6 and TNF-α in healthy participants and showed a more sustained increase in diabetic patients. Table 1 also shows the macronutrient composition of the HF meal as calculated from the package labels of the individual foods. Total fatty acid composition was analyzed by gas chromatography after an extraction method developed for meat at our laboratory at Agroscope, Switzerland (25). The 3 HF meals only differed in their energy content: meal A contained 500 kcal, meal B contained 1000 kcal, and meal C contained 1500 kcal. To keep the ratio of liquid to solid food constant, participants had to drink 200 mL, 400 mL, and 600 mL of Vittel water during consumption of meal A, B, and C, respectively. During the 6 h postprandial period, the participants were provided with 1 L of Vittel water but were not asked to drink it.
TABLE 1.
Composition of the 500-kcal HF test meal A administered to the normal-weight and obese men participating in the study1
| Proteins | Carbohydrates | Fats2 | Energy | |
| g | g | g | kcal | |
| Bread (58 g) | 5.8 | 25.5 | 0.9 | 135 |
| Palm fat (13 g) | 0.0 | 0.0 | 13.0 | 117 |
| Sausage (52 g) | 13.5 | 0.5 | 17.2 | 210 |
| Boiled eggs (26 g) | 3.4 | 0.3 | 2.9 | 40 |
| Macronutrient | 22.7 | 26.3 | 34.0 | — |
| Macronutrient, kcal | 91 | 107 | 303 | — |
| Macronutrient, % | 18.2 | 21.3 | 60.5 | — |
Test meals B and C had the same composition but 2 and 3 times as many calories as meal A, i.e., 1000 and 1500 kcal, respectively. HF, high-fat.
The fatty acid composition of the test meals was as follows (g/kg test meal): SFAs = 89.1 g/kg, 16:0 = 62.4 g/kg, 18:0 = 18.0 g/kg, unsaturated fatty acids = 120 g/kg, MUFAs = 93.1 g/kg, 18:1n–9 = 83.3 g/kg, PUFAs = 26.6 g/kg, n–3 fatty acids = 1.7 g/kg, n–6 fatty acids = 24.7 g/kg.
Analyses of the routine variables insulin, glucose, CRP, and lipids (TGs, total cholesterol, and HDL cholesterol).
Blood was collected at fasting status (0 h) and 1, 2, 4, and 6 h postprandial via an indwelling peripheral venous catheter (Vasofix Safety; B. Braun Melsungen). Sarstedt tubes were used for blood collection for serum insulin (S-Monovette 4.7-mL Z-gel) and for plasma glucose, CRP, and lipids analyses (Li-Hep-gel). Samples were immediately sent for analyses to accredited laboratories of the University Hospital in Berne. Insulin was measured by an electro-chemiluminescent sandwich-immunoassay (Modular E170; Roche), glucose by enzymatic determination with hexokinase (Modular P800; Roche), CRP with a high-sensitivity immune turbidimetric test enhanced with latex particles (Modular P800; Roche), TGs with the glycerol-3-phosphate oxidase-phenol + aminophenazone-method (Modular P800; Roche), total cholesterol with the cholesterol oxidase- phenol + aminophenazone method (Modular P800; Roche), and HDL cholesterol with an enzymatic test for direct quantitative determination (Modular P800; Roche).
The HOMA-IR was calculated from baseline glucose and insulin concentrations as previously described (26). The visceral adiposity index (VAI) was calculated from the BMI, waist circumference, baseline TGs, and HDL cholesterol as described by Amato et al. (27).
Analyses of IL-6, endotoxin, and GLP-1.
For IL-6, endotoxin, and GLP-1 analyses, blood samples were collected at time 0 (fasting) and 1, 2, 4, and 6 h postprandial into S-Monovette (7.5-mL Z-gel) tubes and incubated for 25 min at room temperature. After centrifugation for 10 min at 1500 × g, serum was split into 1.5-mL aliquots and immediately snap-frozen in a mixture of dry ice and ethanol and stored at −80°C until analysis. IL-6 was measured with a high-sensitivity multiplex cytokine assay (Bio-Plex; Bio-Rad) by using the same kit lot for all samples to avoid lot-to-lot variability (28). The sensitivity of the kit, as provided by the manufacturer, was 0.2 pg/mL. The intra-assay CV was ≤5% and the inter-assay CV was ≤10%. Endotoxin was measured with commercially available Kinetic-Quantitative Chromogenic Limulus Amebocyte Lysate Assay (QCL-1000 LAL; Lonza). The assay was previously validated for human use with an intra-assay CV of 3.9 ± 0.5% and an inter-assay CV of 9.6 ± 0.8%, with the recovery rate for endotoxin noted at 82.0 ± 3.3% as previously detailed (29). Total GLP-1 was analyzed with the Multi Species GLP-1 Total ELISA kit (EZGLP1T-36K; Merck Millipore).
Statistical methods.
Statistical analyses of the study were performed with SYSTAT 13.0 (Systat Software) and R version 2.15.2 (http://www.R-project.org) with the R library nonparametric longitudinal data (nparLD) (30) and R library R Commander (31).
The number of participants per group was estimated by power analysis (power = 0.80; significance level α = 5%) with SYSTAT 13.0, based on the study by Nappo et al. (12) who investigated postprandial IL-6 in healthy participants and type 2 diabetic patients fed 760 kcal of HF and high-carbohydrate meals.
Differences between normal-weight and obese participants in the anthropometric and baseline blood variables were assessed by the Mann-Whitney U test and ANOVA-type statistics of the R library nparLD, respectively (α = 5%). Time-dependent changes in the concentrations of the investigated variables after the consumption of the 3 HF meals by the normal-weight and obese participants were analyzed by pairwise comparison of the postprandial time points (t = 1, 2, 4, and 6 h) with the baseline time point (t = 0 h), testing the zero hypothesis H0: Y(0) = Y(t) (ANOVA-type statistics of the R library nparLD). To investigate if there was an overall significant postprandial response in the variables, the following zero hypothesis was tested by using the Wilcoxon signed rank test H0: net incremental AUC (net iAUC) = 0. The net iAUC was calculated by subtracting the −iAUC from the +iAUC, which are the areas of excursion below and above the baseline values, respectively, by using the trapezoid method. The net iAUC was chosen to minimize biologically irrelevant deviations from the baseline values by solely calculating the +iAUC. This strategy particularly favors the postprandial analysis of variables such as IL-6 that only weakly change postprandially. The net iAUC was also used to evaluate the dose-dependent effect of the HF meal on the participants’ postprandial response. For this, the Wilcoxon test was used on the net iAUC values for pairwise comparison of the 3 test meals. Differences between normal-weight and obese participants in the respective dose-response to the HF meals were tested with the Mann-Whitney U test on the net iAUC values.
Spearman rank correlation coefficients were calculated to quantify relations among the variables (anthropometric variables, fasting values, and net iAUC data). No correction for multiple testing was applied because it would have been “family-wise“ testing in a situation where pairwise correlations are of interest and unduly conservative by the number of tests.
Results
Baseline characteristics.
The baseline characteristics of the study population are shown in Table 2. In both groups, the baseline values remained stable throughout the course of the study (data not shown). Age and height were not significantly different between the 2 groups. As expected, weight, BMI, and waist circumference were significantly higher in the obese than in the normal-weight participants (P < 0.001). Furthermore, fasting concentrations of insulin, glucose, TGs, CRP, and GLP-1, as well as the ratio of total cholesterol to HDL cholesterol, were significantly higher in the obese group (P < 0.05). HDL cholesterol was significantly higher in the normal-weight participants (P = 0.004). Fasting concentrations of total cholesterol and the inflammatory markers IL-6 and endotoxin were not significantly different between the 2 groups. The HOMA-IR and VAI, calculated from anthropometric and baseline blood variables, were significantly higher in the obese group. Taking 2.6 as the HOMA-IR cut-off value for insulin resistance (32), all obese participants except 1 (HOMA-IR = 2.1) were considered insulin resistant and all normal-weight participants except 1 (HOMA-IR = 3.2) were considered insulin sensitive. Removing these 2 participants from the analysis did not alter the significant differences in the baseline metabolic and inflammatory variables between the 2 groups (data not shown), and therefore the 2 participants were maintained in their respective group, i.e., normal-weight and obese, for all further analyses.
TABLE 2.
Baseline characteristics of the normal-weight and obese men participating in the study1
| Baseline variable | Normal-weight (n = 19) | Obese (n = 17) |
| Age, y | 40.6 ± 9.2 | 44.1 ± 8.0 |
| Height, m | 1.8 ± 0.1 | 1.8 ± 0.1 |
| Weight, kg | 77.2 ± 6.6* | 123 ± 17* |
| BMI, kg/m2 | 23.6 ± 1.4* | 38.8 ± 4.9* |
| Waist circumference, cm | 85.3 ± 4.4* | 127 ± 10* |
| Glucose (normal range: 3.3–5.5), mmol/L | 4.9 ± 0.5* | 5.3 ± 0.5* |
| Insulin (normal range: 2.6–24.9), mU/L | 6.0 ± 2.9* | 19.1 ± 7.4* |
| HOMA-IR (normal range: <2.6) | 1.3 ± 0.7* | 4.5 ± 1.8* |
| TGs (normal range: <2.3), mmol/L | 1.0 ± 0.5* | 1.5 ± 0.7* |
| Cholesterol (normal range: <5.2), mmol/L | 5.2 ± 0.8 | 5.5 ± 1.1 |
| HDL cholesterol (normal range: >1.0), mmol/L | 1.5 ± 0.3* | 1.2 ± 0.3* |
| Total:HDL cholesterol (normal range: <5.0) | 3.6 ± 1.1* | 4.7 ± 1.3* |
| VAI | 1.0 ± 0.7* | 2.0 ± 1.1* |
| CRP (normal range: <5.0), mg/L | 0.9 ± 0.9* | 3.0 ± 3.0* |
| IL-6, pg/mL | 20.4 ± 8.1 | 17.6 ± 7.3 |
| Endotoxin, EU/mL | 2.3 ± 0.5 | 2.5 ± 0.6 |
| GLP-1, pmol/L | 31.8 ± 15.8* | 48.0 ± 20.6* |
Values are means ± SDs. *Indicates a significant difference between the 2 groups, P < 0.05. Glucose, CRP, TGs, cholesterol, and HDL cholesterol were analyzed in plasma. Insulin, IL-6, endotoxin, and GLP-1 were analyzed in serum. One of the obese participants was excluded from the statistical analyses because his baseline glucose, insulin, and TG concentrations increased during the course of the study. CRP, C-reactive protein; EU, endotoxin units; GLP-1, glucagon-like peptide-1; VAI, visceral adiposity index.
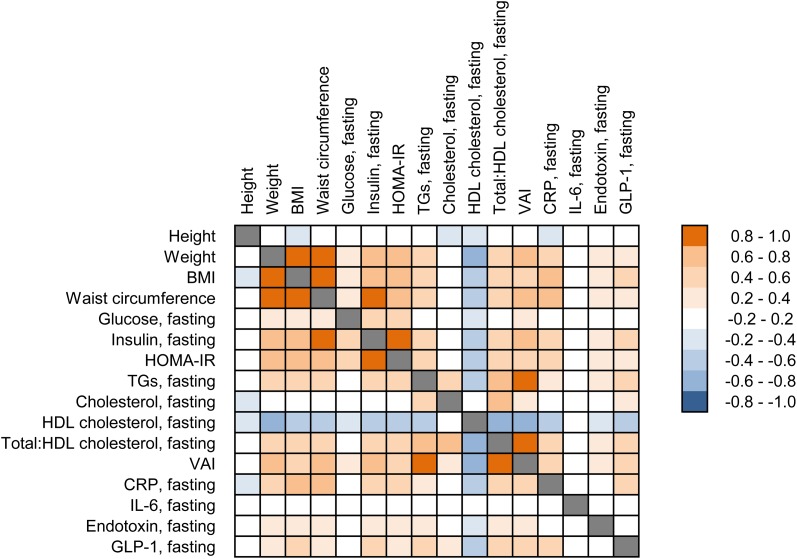
In a complementary approach, Spearman rank correlations between the baseline characteristics (anthropometric measurements, fasting values) were calculated for all participants and are illustrated in the form of a heat map in Figure 1. This analysis identified a range of positive correlations between anthropometric (body weight, BMI, waist circumference), metabolic (glucose, insulin, TGs), and inflammatory (CRP, endotoxin) variables. GLP-1 also correlated positively with various variables such as BMI, VAI, insulin, TGs, and CRP. HDL cholesterol, on the other hand, correlated negatively with all other variables except with total cholesterol and IL-6. Finally, no significant correlation involving IL-6 was identified.
FIGURE 1.
Spearman rank correlations between baseline characteristics (anthropometric measurements, fasting values) calculated for all participants. Positive correlations (≥0.2) are indicated with an orange color code, whereas negative correlations (≤ −0.2) are indicated with a blue color code. All color-coded correlations were significant (P < 0.05). Glucose, CRP, TGs, cholesterol, and HDL cholesterol were analyzed in plasma. Insulin, IL-6, endotoxin, and GLP-1 were analyzed in serum. CRP, C-reactive protein; GLP-1, glucagon-like peptide-1; VAI, visceral adiposity index.
Postprandial response to the 3 caloric doses of the HF test meal.
An integrated analysis of the postprandial data for the metabolic, hormonal, and inflammatory variables measured between 0 and 6 h following the ingestion of the HF meals by the normal-weight and obese participants is shown in the form of the net iAUC in Table 3 (Supplemental Table 1 gives the individual values at 0, 1, 2, 4, and 6 h and associated statistics for the same data set).
TABLE 3.
Postprandial response of metabolic, hormonal, and inflammatory variables in normal-weight and obese men after having consumed 500, 1000, and 1500 kcal of the HF meal1
| Normal-weight (n = 19) |
Obese (n = 17) |
|||||
| Net iAUC | 500 kcal | 1000 kcal | 1500 kcal | 500 kcal | 1000 kcal | 1500 kcal |
| Glucose, mmol/L × 6 h | −2.6 ± 0.4a,*,† | −1.8 ± 0.6a,b,#x2020 | −1.1 ± 0.5b,*,† | −1.2 ± 0.5b,*,† | −0.4 ± 0.6b | 1.8 ± 1.0a,* |
| Insulin, mU/L × 6 h | 12.9 ± 3.2c,*,† | 39.0 ± 5.3b,*,† | 63.9 ± 9.3a,*,† | 67 ± 11c,*,† | 186 ± 26b,*,† | 321 ± 45a,*,† |
| TGs, mmol/L × 6 h | 1.9 ± 0.3b,#x2020 | 4.3 ± 0.5a,#x2020 | 4.8 ± 0.5a,#x2020 | 2.4 ± 0.4c,#x2020 | 5.0 ± 0.5b,#x2020 | 6.0 ± 0.5a,#x2020 |
| Cholesterol, mmol/L × 6 h | −1.1 ± 0.3#x2020 | −1.2 ± 0.2*,† | −1.3 ± 0.2#x2020 | −0.4 ± 0.2a,b | −0.1 ± 0.2b,* | −0.8 ± 0.2a,#x2020 |
| HDL cholesterol, mmol/L × 6 h | −0.3 ± 0.1b,#x2020 | −0.7 ± 0.1a,#x2020 | −0.6 ± 0.1a,#x2020 | −0.2 ± 0.1b,#x2020 | −0.6 ± 0.2a,#x2020 | −0.5 ± 0.1a,#x2020 |
| Total:HDL cholesterol | 0.2 ± 0.2b | 1.2 ± 0.3a,#x2020 | 0.9 ± 0.3a,*,† | 0.4 ± 0.3b | 2.3 ± 0.6a,#x2020 | 1.8 ± 0.3a,*,† |
| CRP, mg/L × 6 h | 0.1 ± 0.2 | −0.2 ± 0.1#x2020 | −0.3 ± 0.1#x2020 | −0.3 ± 0.2 | −0.4 ± 0.3 | −0.7 ± 0.5 |
| IL-6, pg/mL × 6 h | −9.3 ± 4.9b | 5.8 ± 6.7a,b | 9.9 ± 5.6a | −2.1 ± 5.7b | 0.8 ± 6.3a,b | 22.9 ± 6.8a,#x2020 |
| Endotoxin, EU/mL × 6 h | 0.4 ± 0.4c | 1.8 ± 0.6b,#x2020 | 2.9 ± 0.5a,#x2020 | 0.4 ± 0.8b | 1.2 ± 1.1a,b | 2.7 ± 0.4a,#x2020 |
| GLP-1, pmol/L × 6 h | 46.6 ± 11.5#x2020 | 63.8 ± 15.7#x2020 | 88.1 ± 25.5#x2020 | 47.6 ± 8.2b,#x2020 | 70.6 ± 9.5a,#x2020 | 76.1 ± 12.8a,#x2020 |
Values are means ± SEMs. Within a group, labeled means in a row without a common letter differ, P < 0.05. *Indicates different from the corresponding normal-weight, P < 0.05. †Indicates a significant postprandial effect, P < 0.05, testing the zero hypothesis H0: net iAUC = 0. Glucose, CRP, TGs, cholesterol, and HDL cholesterol were analyzed in plasma. Insulin, IL-6, endotoxin, and GLP-1 were analyzed in serum. CRP, C-reactive protein; EU, endotoxin units; GLP-1, glucagon-like peptide-1; HF, high-fat; iAUC, incremental AUC.
For all variables, except IL-6, at least 1 caloric dose of the test meal led to an overall significant postprandial response in the normal-weight participants. A dose-response could be demonstrated for glucose, insulin, TGs, HDL cholesterol, total:HDL cholesterol, IL-6, and endotoxin because the net iAUC after ingestion of the 1500 kcal meal was significantly different than after ingestion of the 500 kcal meal. For most of the aforementioned variables, except glucose and IL-6, a dose-response could already be demonstrated by increasing the caloric dose from 500 to 1000 kcal. No dose-response could be demonstrated for total cholesterol, CRP, and GLP-1.
In the obese group, a dose-response could be demonstrated for all variables except CRP. For insulin, TGs, HDL cholesterol, total:HDL cholesterol, and GLP-1, a dose-response could be identified by increasing the caloric dose from 500 to 1000 kcal. For insulin and TGs, a further significant increase in the net iAUC from the 1000 kcal meal to the 1500 kcal meal was noticed.
Differences in the postprandial response between normal-weight and obese participants.
For each variable investigated, Table 3 shows whether the postprandial response of normal-weight and obese participants can be differentiated by comparing their net iAUCs after ingestion of the same caloric dose of the HF meal. The insulin response of the normal-weight and obese participants was different for each of the 3 caloric doses tested. Of note, the net iAUC of insulin following the ingestion of the 500 kcal meal by the obese participants was at least as elevated as the net iAUC of the normal-weight participants after ingestion of the 1500 kcal meal. The glucose, cholesterol, and total:HDL cholesterol response was also different, albeit not at all 3 caloric doses investigated.
For TGs, HDL cholesterol, CRP, IL-6, endotoxin, and GLP-1, none of the caloric doses led to the identification of a significant difference in the postprandial response between normal-weight and obese participants. Looking at each group individually, however, we noted that the net iAUC of TGs significantly increased from the 1000 kcal meal to the 1500 kcal meal in the obese but not in the normal-weight group. Also, a significant postprandial effect in the net iAUC for IL-6 could only be demonstrated in the obese group after ingestion of the 1500 kcal meal. In this context, it should be mentioned that we also found a significant positive correlation between the HOMA-IR and the postprandial IL-6 response after the 1500 kcal meal (r = 0.34, P = 0.04). Finally, although no significant dose-response could be demonstrated for GLP-1 in the normal-weight group, a dose-response effect for GLP-1 was observed between the 500 and 1000 kcal meal in the obese group.
Correlations between fasting and postprandial values of the investigated variables.
Spearman rank correlations were calculated between the baseline characteristics (anthropometric measurements, fasting values) and the postprandial responses calculated by the net iAUC. Supplemental Figure 1 shows these correlations in the form of a heat map for the pooled dataset including both groups of participants separated by the caloric dose administered. Of note, the fasting and postprandial concentrations correlated significantly and positively at all 3 caloric doses investigated for insulin (meal A: r = 0.65, P < 0.01; meal B: r = 0.83, P < 0.01; meal C: r = 0.77, P < 0.01) and TGs (meal A: r = 0.53, P < 0.01; meal B: r = 0.64, P < 0.01; meal C: r = 0.51, P < 0.01). On the other hand, the baseline concentrations of GLP-1 correlated negatively with the corresponding postprandial GLP-1 response, the correlation coefficients increasing as the caloric dose of the HF meal increased (meal A: r = −0.23, P = 0.19; meal B: r = −0.46, P < 0.01; meal C: r = −0.61, P < 0.01).
Correlations between the postprandial values of the investigated variables.
The postprandial information, as evaluated by the net iAUC, was also used to identify the transient relation between the absorption of food and its metabolic and inflammatory postprandial effects. Supplemental Figure 2 shows these correlations in the form of a heat map for the pooled dataset comprising both groups of participants and all 3 caloric doses administered. In particular, besides the well-known relation between postprandial glucose and insulin (r = 0.51, P < 0.05), we also noted a significant positive correlation between postprandial TGs and endotoxin (r = 0.49, P < 0.05).
Discussion
In agreement with the literature, most of the baseline characteristics in our study differentiated the 2 groups composed of 19 normal-weight and 17 obese participants. Also, a majority of the correlations between the baseline characteristics of our study population was significant, highlighting the relation between metabolic, inflammatory, and hormonal processes (7, 14, 33, 34). Taken together, these baseline characteristics led us to conclude that the selection of participants and blood variables was appropriate to investigate the impact of increasing caloric doses of a HF meal on human metabolic functions.
The postprandial changes observed in variables such as glucose, insulin, and TGs are unequivocally due to the ingestion of food. However, endogenous metabolic processes, in particular the circadian rhythm (15), may confound the contribution of food ingestion to postprandial changes observed in the concentrations of variables such as IL-6 and endotoxin. Except for CRP, for all investigated variables a dose-response effect could be demonstrated. We therefore conclude that the observed postprandial changes are directly related to the ingestion of the HF meal rather than mirroring endogenous phenomena.
Differences between the normal-weight and obese participants in the insulin response evidently reflected the insulin resistance of the obese group. In this case, a caloric dose-response strategy would not have been necessary to allow for a differentiation in the postprandial response between the 2 groups. Melanson et al. (35) advocated that meal size may strongly influence hormonal and metabolite responses and, therefore, should be considered in the design of nutritional intervention studies. In that context, we identified no significant difference in the postprandial TG response between the normal-weight and obese groups upon consumption of 500, 1000, and 1500 kcal of the HF test meal. However, pairwise comparisons of the test meals showed a significant increase in the net iAUC for TGs from the 1000 kcal meal to the 1500 kcal meal in the obese group but not in the normal-weight group. Interestingly, fasting TG concentrations, which were higher in the obese group, also correlated positively with the postprandial TG response. These findings may reflect differences in metabolic flexibility and saturation processes between the 2 groups in agreement with previous studies reporting an impaired TG clearance in obese participants (36, 37).
Obese participants may react stronger to meal-induced stress than do normal-weight participants (8, 9). The caloric dose needed to provoke a postprandial inflammatory response is, however, not known. Here we report a significant postprandial response for the inflammation marker IL-6 in the obese group after consumption of the 1500-kcal meal. These data are in agreement with previous studies reporting a more pronounced inflammatory response by insulin-resistant participants than by insulin-sensitive individuals (8). The data also highlights the importance of considering both the metabolic status of the participants and the caloric dose of the ingested meal while investigating the inflammatory response of the organism to food consumption.
One mechanism potentially linking nutrition to inflammation is the postprandial translocation of endotoxin from the gut into the circulation, a phenomenon that may be increased in patients with metabolic disorders (20–22). In addition, metabolic endotoxemia was identified as a causative factor in the onset of insulin resistance, obesity, and diabetes (38). In line with these studies, our participants had a postprandial increase in serum endotoxin concentration following the consumption of the HF meals. Interestingly, the net iAUC of endotoxin correlated positively, although modestly, with the postprandial TG response. The postprandial exposure to endotoxin may thus be linked to the absorption of TGs, supporting the hypothesis that fat may facilitate endotoxin absorption from the gut during the secretion of chylomicrons (39–41).
Several studies reported a reduced postprandial GLP-1 response in obese or insulin-resistant participants (42–45). By comparing the net iAUCs between the normal-weight and obese groups, we could not identify a significant difference in the GLP-1 response at any of the caloric doses investigated. However, we noted that fasting GLP-1 concentrations, which were higher in the obese group, correlated negatively with the postprandial GLP-1 response. Although not corroborated by the data presented in Supplemental Figure 1, this may suggest that participants with a higher BMI may indeed have an impaired GLP-1 response to the ingestion of food.
Altogether, our data provide novel insights into the relation between nutrition, metabolic health, and postprandial adaptations. However, the study suffers from certain limitations that need to be formally outlined here. First, the study was only conducted in male participants to avoid confounding factors related to reproductive hormone processes in females. Second, blood pressure was not assessed, and hypertensive participants may respond differently to meal-induced stress, e.g., in their TG response (46). Third, we did not standardize the caloric content of the HF meals to the body surface, age, or physical activity of the participants. Instead, we focused on a simple dose-response study design with a broad range of caloric content (500–1500 kcal) covering both suboptimal and excess calories in both the normal-weight and obese participants participating in our study.
The strength of our study lies in its dose-response design. By varying the caloric content of the HF meal we were able to better differentiate the postprandial response of participants with a different metabolic health status. Indeed, although insulin did not request a caloric dose-response to differentiate normal-weight from obese participants, other metabolic (TGs) and inflammatory (IL-6) markers could only be differentiated by challenging the participants with increasing caloric doses of a HF meal. Thus, we demonstrated that caloric dose-response challenges can reveal metabolic adaptations resulting from the immediate impact of food on the organism, which may be indicative of long-term effects ultimately shifting the balance between health and disease. We propose that the dose-response strategy presented in this study could be used to compare the nutritional quality of different types of foods by challenging a well-defined metabolic group of participants with increasing caloric doses of those foods.
Supplementary Material
Acknowledgments
The authors thank Marie-Jeanne Voirol (Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland) for measuring GLP-1 and IL-6, Dr. Werner Luginbühl (ChemStat, Berne, Switzerland) for statistical consulting, and Doreen Gille and Ueli Bütikofer (Agroscope, Berne, Switzerland) for stimulating discussions on the structure of the manuscript. G.V. conceived the research program with input from M.A.M.G.; F.S. and K.A.K.-B. designed the study with input from C.B., K.L., R.P., L.E., M.C., and G.V.; F.S. and K.A.K.-B. planned and conducted the study, including the randomization procedure, the enrollment of the participants, and their assignment to the interventions; K.L. provided the infrastructure for the intervention study; P.G.M. and M.K.P. designed and conducted the endotoxin analyses; N.V. and F.P. designed and conducted the GLP-1 and IL-6 analyses; and F.S. and G.V. analyzed the data and statistical reports, wrote the paper, and had primary responsibility for the final content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: CRP, C-reactive protein; GLP-1, glucagon-like peptide-1; HF, high-fat; iAUC, incremental AUC; nparLD, nonparametric longitudinal data; VAI, visceral adiposity index.
References
- 1.Schwingshackl L, Hoffmann G. Long-term effects of low glycemic index/load vs. high glycemic index/load diets on parameters of obesity and obesity-associated risks: a systematic review and meta-analysis. Nutr Metab Cardiovasc Dis 2013;23:699–706. [DOI] [PubMed] [Google Scholar]
- 2.Borai A, Livingstone C, Ferns GA. The biochemical assessment of insulin resistance. Ann Clin Biochem 2007;44:324–42. [DOI] [PubMed] [Google Scholar]
- 3.Kersten S. Regulation of nutrient metabolism and inflammation. Results Probl Cell Differ 2010;52:13–25. [DOI] [PubMed] [Google Scholar]
- 4.Calder PC, Ahluwalia N, Brouns F, Buetler T, Clement K, Cunningham K, Esposito K, Jonsson LS, Kolb H, Lansink M, et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br J Nutr 2011;106(Suppl 3):S5–78. [DOI] [PubMed] [Google Scholar]
- 5.Combs GF, Jr, Trumbo PR, McKinley MC, Milner J, Studenski S, Kimura T, Watkins SM, Raiten DJ. Biomarkers in nutrition: new frontiers in research and application. Ann N Y Acad Sci 2013;1278:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raiten DJ, Namaste S, Brabin B, Combs G, Jr, L'Abbe MR, Wasantwisut E, Darnton-Hill I. Executive summary—biomarkers of nutrition for development: building a consensus. Am J Clin Nutr 2011;94:633S–50S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest 2005;115:1111–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blackburn P, Despres JP, Lamarche B, Tremblay A, Bergeron J, Lemieux I, Couillard C. Postprandial variations of plasma inflammatory markers in abdominally obese men. Obesity (Silver Spring) 2006;14:1747–54. [DOI] [PubMed] [Google Scholar]
- 9.Patel C, Ghanim H, Ravishankar S, Sia CL, Viswanathan P, Mohanty P, Dandona P. Prolonged reactive oxygen species generation and nuclear factor-kappaB activation after a high-fat, high-carbohydrate meal in the obese. J Clin Endocrinol Metab 2007;92:4476–9. [DOI] [PubMed] [Google Scholar]
- 10.Gregersen S, Samocha-Bonet D, Heilbronn LK, Campbell LV. Inflammatory and oxidative stress responses to high-carbohydrate and high-fat meals in healthy humans. J Nutr Metab 2012;2012:238056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lundman P, Boquist S, Samnegard A, Bennermo M, Held C, Ericsson CG, Silveira A, Hamsten A, Tornvall P. A high-fat meal is accompanied by increased plasma interleukin-6 concentrations. Nutr Metab Cardiovasc Dis 2007;17:195–202. [DOI] [PubMed] [Google Scholar]
- 12.Nappo F, Esposito K, Cioffi M, Giugliano G, Molinari AM, Paolisso G, Marfella R, Giugliano D. Postprandial endothelial activation in healthy subjects and in type 2 diabetic patients: role of fat and carbohydrate meals. J Am Coll Cardiol 2002;39:1145–50. [DOI] [PubMed] [Google Scholar]
- 13.Poppitt SD, Keogh GF, Lithander FE, Wang Y, Mulvey TB, Chan YK, McArdle BH, Cooper GJ. Postprandial response of adiponectin, interleukin-6, tumor necrosis factor-alpha, and C-reactive protein to a high-fat dietary load. Nutrition 2008;24:322–9. [DOI] [PubMed] [Google Scholar]
- 14.Hotamisligil GS. Inflammation and metabolic disorders. Nature 2006;444:860–7. [DOI] [PubMed] [Google Scholar]
- 15.Cagampang FR, Bruce KD. The role of the circadian clock system in nutrition and metabolism. Br J Nutr 2012;108:381–92. [DOI] [PubMed] [Google Scholar]
- 16.Kitano H. Biological robustness. Nat Rev Genet 2004;5:826–37. [DOI] [PubMed] [Google Scholar]
- 17.Stumpf WE. The dose makes the medicine. Drug Discov Today 2006;11:550–5. [DOI] [PubMed] [Google Scholar]
- 18.Lassenius MI, Pietilainen KH, Kaartinen K, Pussinen PJ, Syrjanen J, Forsblom C, Porsti I, Rissanen A, Kaprio J, Mustonen J, et al. Bacterial endotoxin activity in human serum is associated with dyslipidemia, insulin resistance, obesity, and chronic inflammation. Diabetes Care 2011;34:1809–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amar J, Burcelin R, Ruidavets JB, Cani PD, Fauvel J, Alessi MC, Chamontin B, Ferrieres J. Energy intake is associated with endotoxemia in apparently healthy men. Am J Clin Nutr 2008;87:1219–23. [DOI] [PubMed] [Google Scholar]
- 20.Clemente-Postigo M, Queipo-Ortuno MI, Murri M, Boto-Ordonez M, Perez-Martinez P, Andres-Lacueva C, Cardona F, Tinahones FJ. Endotoxin increase after fat overload is related to postprandial hypertriglyceridemia in morbidly obese patients. J Lipid Res 2012;53:973–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghanim H, Abuaysheh S, Sia CL, Korzeniewski K, Chaudhuri A, Fernandez-Real JM, Dandona P. Increase in plasma endotoxin concentrations and the expression of Toll-like receptors and suppressor of cytokine signaling-3 in mononuclear cells after a high-fat, high-carbohydrate meal: implications for insulin resistance. Diabetes Care 2009;32:2281–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harte AL, Varma MC, Tripathi G, McGee KC, Al-Daghri NM, Al-Attas OS, Sabico S, O'Hare JP, Ceriello A, Saravanan P, et al. High fat intake leads to acute postprandial exposure to circulating endotoxin in type 2 diabetic subjects. Diabetes Care 2012;35:375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erridge C, Attina T, Spickett CM, Webb DJ. A high-fat meal induces low-grade endotoxemia: evidence of a novel mechanism of postprandial inflammation. Am J Clin Nutr 2007;86:1286–92. [DOI] [PubMed] [Google Scholar]
- 24.Ajuwon KM, Spurlock ME. Palmitate activates the NF-kappaB transcription factor and induces IL-6 and TNFalpha expression in 3T3–L1 adipocytes. J Nutr 2005;135:1841–6. [DOI] [PubMed] [Google Scholar]
- 25.Collomb M, Bühler T. Analyse de la composition en acide gras de la graisse de lait. Trav Chim Alim Hyg. 2000;91:306–32. [Google Scholar]
- 26.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–9. [DOI] [PubMed] [Google Scholar]
- 27.Amato MC, Giordano C, Galia M, Criscimanna A, Vitabile S, Midiri M, Galluzzo A. Visceral Adiposity Index: a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care 2010;33:920–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Breen EC, Reynolds SM, Cox C, Jacobson LP, Magpantay L, Mulder CB, Dibben O, Margolick JB, Bream JH, Sambrano E, et al. Multisite comparison of high-sensitivity multiplex cytokine assays. Clin Vaccine Immunol 2011;18:1229–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Creely SJ, McTernan PG, Kusminski CM, Fisher FM, Da Silva NF, Khanolkar M, Evans M, Harte AL, Kumar S. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am J Physiol Endocrinol Metab 2007;292:E740–7. [DOI] [PubMed] [Google Scholar]
- 30.Noguchi K, Gel YR, Brunner E, Konietschke F. nparLD: an R software package for the nonparametric analysis of longitudinal data in factorial experiments. J Stat Softw 2012;50:1–23.25317082 [Google Scholar]
- 31.Fox JA. The R commander: a basic statistics graphical user interface to R. J Stat Softw 2005;14:1–42. [Google Scholar]
- 32.Ascaso JF, Pardo S, Real JT, Lorente RI, Priego A, Carmena R. Diagnosing insulin resistance by simple quantitative methods in subjects with normal glucose metabolism. Diabetes Care 2003;26:3320–5. [DOI] [PubMed] [Google Scholar]
- 33.Aronson D, Bartha P, Zinder O, Kerner A, Markiewicz W, Avizohar O, Brook GJ, Levy Y. Obesity is the major determinant of elevated C-reactive protein in subjects with the metabolic syndrome. Int J Obes Relat Metab Disord 2004;28:674–9. [DOI] [PubMed] [Google Scholar]
- 34.Pannacciulli N, Cantatore FP, Minenna A, Bellacicco M, Giorgino R, De PG. C-reactive protein is independently associated with total body fat, central fat, and insulin resistance in adult women. Int J Obes Relat Metab Disord 2001;25:1416–20. [DOI] [PubMed] [Google Scholar]
- 35.Melanson KJ, Greenberg AS, Ludwig DS, Saltzman E, Dallal GE, Roberts SB. Blood glucose and hormonal responses to small and large meals in healthy young and older women. J Gerontol A Biol Sci Med Sci 1998;53:B299–305. [DOI] [PubMed] [Google Scholar]
- 36.Blackburn P, Lamarche B, Couillard C, Pascot A, Bergeron N, Prud'homme D, Tremblay A, Bergeron J, Lemieux I, Despres JP. Postprandial hyperlipidemia: another correlate of the “hypertriglyceridemic waist” phenotype in men. Atherosclerosis 2003;171:327–36. [DOI] [PubMed] [Google Scholar]
- 37.Couillard C, Bergeron N, Prud'homme D, Bergeron J, Tremblay A, Bouchard C, Mauriege P, Despres JP. Postprandial triglyceride response in visceral obesity in men. Diabetes 1998;47:953–60. [DOI] [PubMed] [Google Scholar]
- 38.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007;56:1761–72. [DOI] [PubMed] [Google Scholar]
- 39.Ghoshal S, Witta J, Zhong J, de VW, Eckhardt E. Chylomicrons promote intestinal absorption of lipopolysaccharides. J Lipid Res 2009;50:90–7. [DOI] [PubMed] [Google Scholar]
- 40.Laugerette F, Vors C, Geloen A, Chauvin MA, Soulage C, Lambert-Porcheron S, Peretti N, Alligier M, Burcelin R, Laville M, et al. Emulsified lipids increase endotoxemia: possible role in early postprandial low-grade inflammation. J Nutr Biochem 2011;22:53–9. [DOI] [PubMed] [Google Scholar]
- 41.Moreira AP, Texeira TF, Ferreira AB, Peluzio MC, Alfenas RC. Influence of a high-fat diet on gut microbiota, intestinal permeability and metabolic endotoxaemia. Br J Nutr 2012;108:801–9. [DOI] [PubMed] [Google Scholar]
- 42.Carr RD, Larsen MO, Jelic K, Lindgren O, Vikman J, Holst JJ, Deacon CF, Ahren B. Secretion and dipeptidyl peptidase-4-mediated metabolism of incretin hormones after a mixed meal or glucose ingestion in obese compared to lean, nondiabetic men. J Clin Endocrinol Metab 2010;95:872–8. [DOI] [PubMed] [Google Scholar]
- 43.Ranganath LR, Beety JM, Morgan LM, Wright JW, Howland R, Marks V. Attenuated GLP-1 secretion in obesity: cause or consequence? Gut 1996;38:916–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verdich C, Toubro S, Buemann B, Lysgard MJ, Juul HJ, Astrup A. The role of postprandial releases of insulin and incretin hormones in meal-induced satiety—effect of obesity and weight reduction. Int J Obes Relat Metab Disord 2001;25:1206–14. [DOI] [PubMed] [Google Scholar]
- 45.Fernández-Garcia JC, Murri M, Coin-Araguez L, Alcaide J, El BR, Tinahones FJ. GLP-1 and peptide YY secretory response after fat load is impaired by insulin resistance, impaired fasting glucose and type 2 diabetes in morbidly obese subjects. Clin Endocrinol (Oxf) 2014;80:671–6. [DOI] [PubMed] [Google Scholar]
- 46.Kolovou GD, Daskalova DC, Iraklianou SA, Adamopoulou EN, Pilatis ND, Hatzigeorgiou GC, Cokkinos DV. Postprandial lipemia in hypertension. J Am Coll Nutr 2003;22:80–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.