Abstract
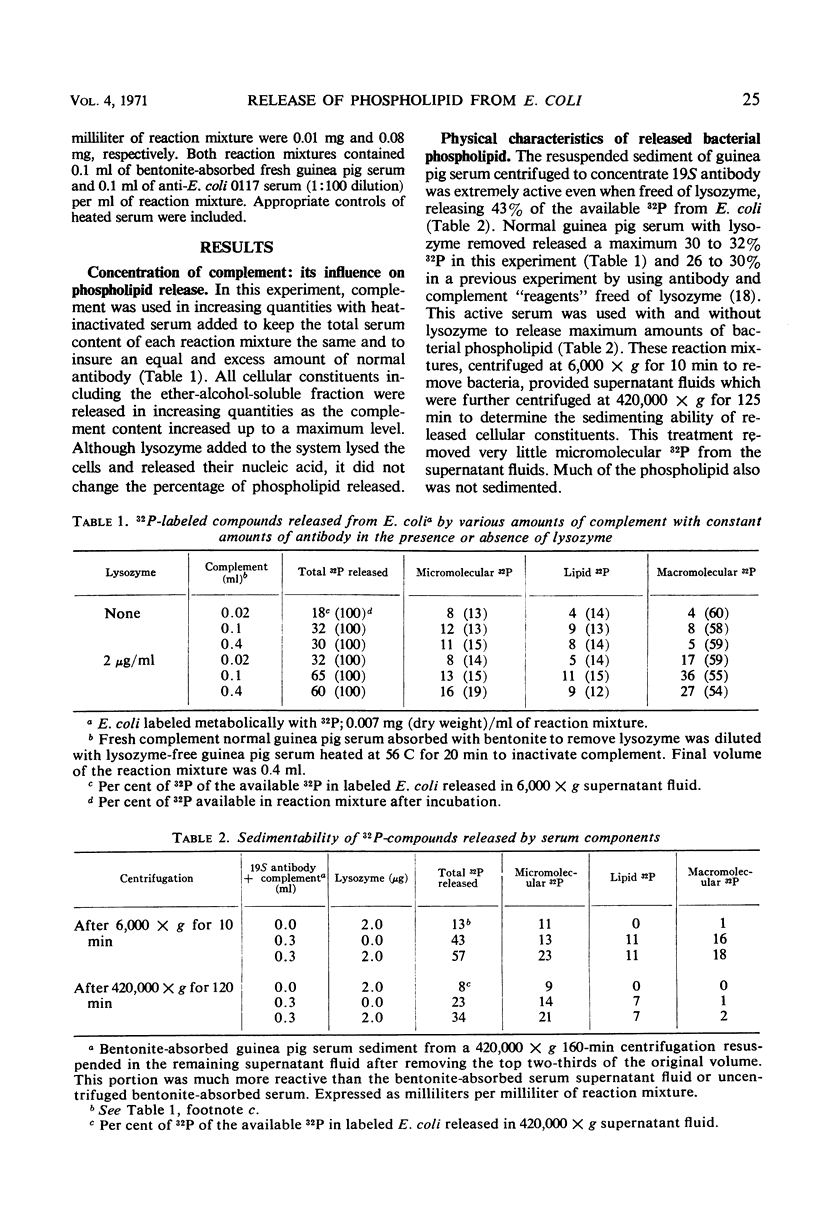
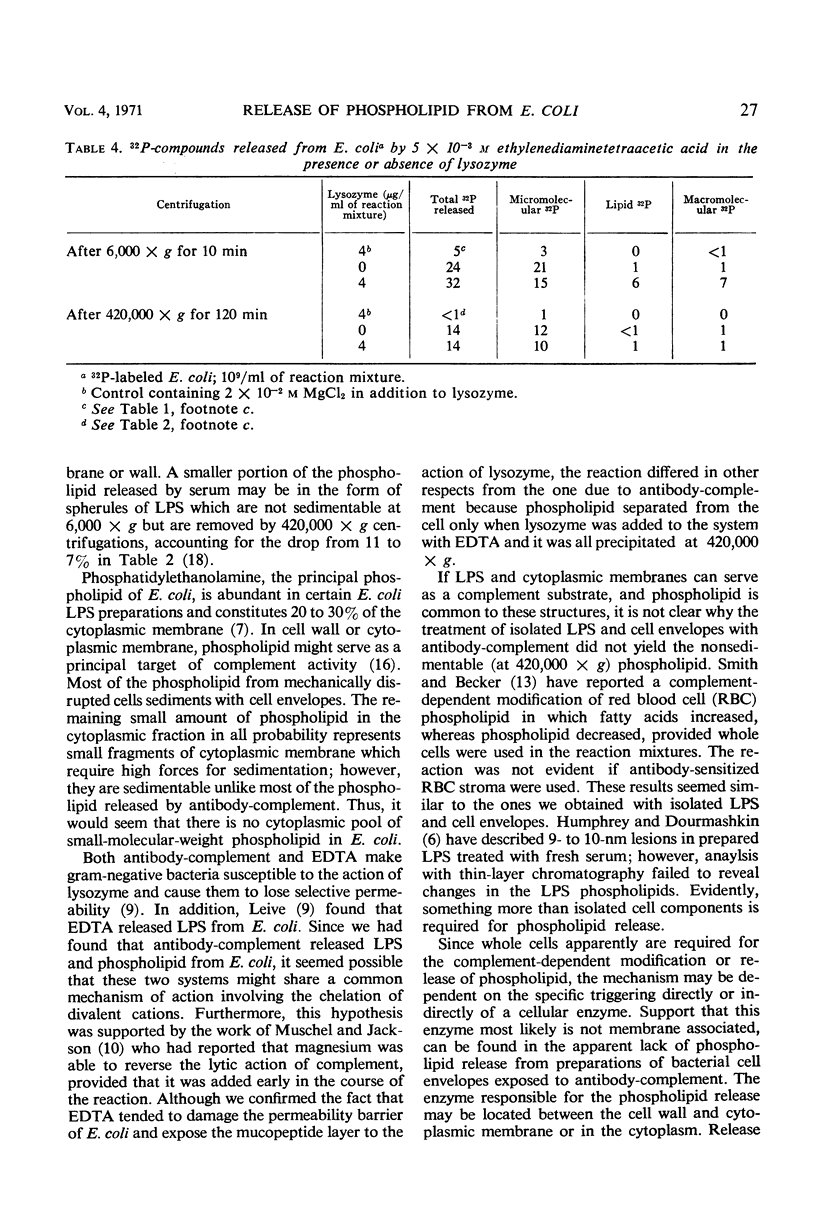
The release of 32P-labeled bacterial phospholipid from a smooth Escherichia coli by serum components depends on complement activated by antibody. Phospholipid release in excess antibody tends to be proportional to the concentration of complement as does the release of other cellular constituents. Phospholipids are not simply stripped off during cell lysis. Whereas 94% of the total phospholipid freed from E. coli by mechanical lysis sediments at centrifugal forces sufficient to sediment molecules of 106 molecular weight, similar centrifugation sediments only 50% of the phospholipid released by antibody-complement. In fact, after mechanical lysis more than 50% of the phospholipid sediments at velocities sufficient to bring down cell envelopes. Although the bulk of the bacterial phospholipid is located in the cell envelopes, isolated 32P-labeled cell envelopes and phenol-extracted lipopolysaccharide fails to release phospholipids in the presence of antibody-complement. Moreover, ethylenediaminetetraacetic acid, which like antibody-complement causes loss of cellular selective permeability and prepares E. coli cell walls for the action of lysozyme, releases only small amounts of phospholipid from E. coli and these are sedimentable. The most likely mechanism of phospholipid release caused by antibody-complement appears to be the activation directly or indirectly of an enzyme which is present only in the intact cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Lipids of Salmonella typhimurium and Escherichia coli: structure and metabolism. J Bacteriol. 1968 Mar;95(3):833–843. doi: 10.1128/jb.95.3.833-843.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bladen H. A., Gewurz H., Mergenhagen S. E. Interactions of the complement system with the surface and endotoxic lipopolysaccharide of Veillonella alcalescens. J Exp Med. 1967 May 1;125(5):767–786. doi: 10.1084/jem.125.5.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewurz H., Pickering R. J., Snyderman R., Lichtenstein L. M., Good R. A., Mergenhagen S. E. Interactions of the complement system with endotoxic lipopolysaccharides in immunoglobulin-deficient sera. J Exp Med. 1970 Apr 1;131(4):817–831. doi: 10.1084/jem.131.4.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray G. W., Wilkinson S. G. The effect of ethylenediaminetetra-acetic acid on the cell walls of some gram-negative bacteria. J Gen Microbiol. 1965 Jun;39(3):385–399. doi: 10.1099/00221287-39-3-385. [DOI] [PubMed] [Google Scholar]
- Kates M. Bacterial lipids. Adv Lipid Res. 1964;2:17–90. [PubMed] [Google Scholar]
- Koga Y., Kusaka I. Involvement of autolysis of cytoplasmic membranes in the process of autolysis of Bacillus cereus. J Gen Microbiol. 1968 Sep;53(2):253–258. doi: 10.1099/00221287-53-2-253. [DOI] [PubMed] [Google Scholar]
- Leive L. Release of lipopolysaccharide by EDTA treatment of E. coli. Biochem Biophys Res Commun. 1965 Nov 22;21(4):290–296. doi: 10.1016/0006-291x(65)90191-9. [DOI] [PubMed] [Google Scholar]
- Muschel L. H., Jackson J. E. Reversal of the bactericidal reaction of serum by magnesium ion. J Bacteriol. 1966 Apr;91(4):1399–1402. doi: 10.1128/jb.91.4.1399-1402.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. K., Becker E. L. Serum complement and the enzymatic degradation of erythrocyte phospholipid. J Immunol. 1968 Mar;100(3):459–474. [PubMed] [Google Scholar]
- Spitznagel J. K. Normal serum cytotoxicity for P32-labeled smooth Enterobacteriaceae. II. Fate of macromolecular and lipid phosphorus of damaged cells. J Bacteriol. 1966 Jan;91(1):148–152. doi: 10.1128/jb.91.1.148-152.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitznagel J. K., Wilson L. A. Normal serum cytotoxicity for P32-labeled smooth Enterobacteriaceae. I. Loss of label, death, and ultrastructural damage. J Bacteriol. 1966 Jan;91(1):393–400. doi: 10.1128/jb.91.1.393-400.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson L. A., Spitznagel J. K. Molecular and structural damage to Escherichia coli produced by antibody, complement, and lysozyme systems. J Bacteriol. 1968 Oct;96(4):1339–1348. doi: 10.1128/jb.96.4.1339-1348.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]


