Abstract
Hydrogen sulfide (H2S) is a newly recognized signaling molecule with very potent cytoprotective actions. The fields of H2S physiology and pharmacology have been rapidly growing in recent years, but a number of fundamental issues must be addressed to advance our understanding of the biology and clinical potential of H2S in the future. Hydrogen sulfide releasing agents (also known as H2S donors) have been widely used in the field. These compounds are not only useful research tools, but also potential therapeutic agents. It is therefore important to study the chemistry and pharmacology of exogenous H2S and to be aware of the limitations associated with the choice of donors used to generate H2S in vitro and in vivo. In this review we summarized the developments and limitations of current available donors including H2S gas, sulfide salts, garlic-derived sulfur compounds, Lawesson’s reagent/analogs, 1,2-dithiole-3-thiones, thiol-activated donors, photo-caged donors, and thioamino acids. Some biological applications of these donors were also discussed.
1. INTRODUCTION
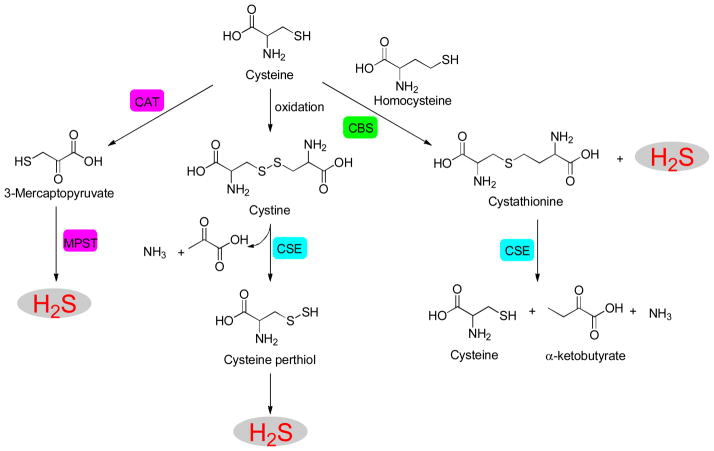
Hydrogen sulfide (H2S), first discovered in 1777 by Carl Wilhelm Scheele, has been traditionally known as a toxic air pollutant with the characteristic odor of rotten eggs. However, this gaseous molecule has been recently recognized as a member of the gasotransmitter family along with its congeners nitric oxide (NO) and carbon monoxide (CO).1–8 The production of H2S in mammalian systems has been attributed to at least three enzymes: cystathionine β-synthase (CBS), cystathionine γ-lyase (CSE), and 3-mercaptopyruvate sulfur-transferase (MPST) (Scheme 1).9–13 CBS is found predominantly in the brain, nervous system and liver. It converts cysteine and homocysteine to cystathionine and releases H2S. In comparison, CSE activity is higher than CBS in aorta, portal vein and other vascular tissue. CSE is responsible for H2S production in the vasculature and heart through a reaction involving the generation of L-cysteine, pyruvate, and ammonia from L-cystathionine and cysteine. MPST is mainly localized in mitochondria.14 Kimura and coworkers demonstrated that MPST, together with cysteine aminotransferase (CAT), produces H2S from cysteine in the presence of α–ketoglutarate.15 It has also been reported that MPST can convert D-cysteine to H2S in the presence of D-amino acid oxidase.16 Although the expression of these enzymes is tissue-specific, they all convert cysteine or cysteine derivatives to H2S. These enzymes work collectively and precisely regulate H2S levels in tissues, and therefore are crucial for H2S homeostasis.
Scheme 1.
Enzymatic synthesis of H2S.
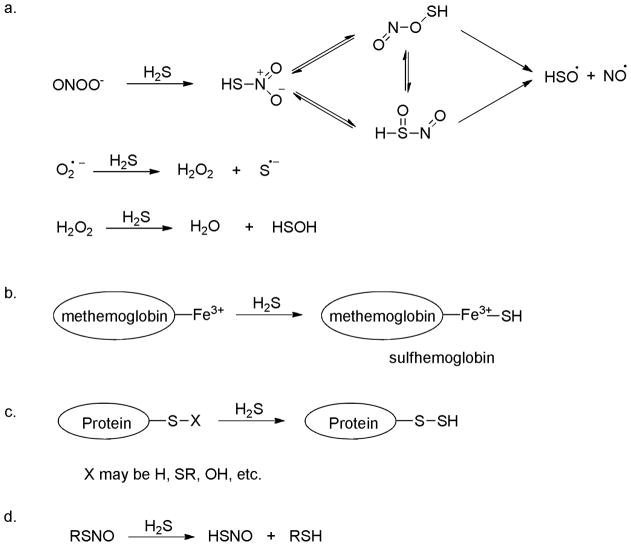
In 1996 Kimura demonstrated that endogenous H2S acts as a neuromodulator in the brain.17 Following his work, a number of studies have revealed various biological effects of H2S, which include the relaxation of blood vessels,18–20 protection against myocardial ischemia injury,21–23 and cytoprotection against oxidative stress.24–26 In addition, some chemical and biochemical catabolic reactions of H2S have also been disclosed, and these reactions may be responsible for the biological functions of H2S. For example, H2S is a powerful reducing agent and is likely to be consumed by endogenous oxidants such as peroxynitrite (ONOO−), superoxide (O2.−) and hydrogen peroxide (H2O2) (Scheme 2a).27–29 H2S reacts readily with methemoglobin to form sulfhemoglobin, which might act as a metabolic sink for H2S (Scheme 2b).30 It is reported that H2S can cause protein S-sulfhydration (i.e. to form -S-SH) (Scheme 2c),31–33 but the detailed mechanism is still unclear. Nevertheless this process is potentially significant as it provides a possible route by which H2S can alter the functions of a wide range of cellular proteins and enzymes.34–40 H2S can also interact with S-nitrosothiols to form thionitrous acid (HSNO), the smallest S-nitrosothiol, whose metabolites, such as NO, NO−, and NO+, have significant physiological functions (Scheme 2d).41 It is likely that many more important reactions of H2S remain to be discovered.
Scheme 2.
Some important biological reactions of H2S.
Although the endogenous formation of H2S and exogenous administration of H2S have been proved beneficial in some pathophysiological conditions, the molecular mechanisms of H2S action are still under investigation. It is therefore important to understand the chemistry and properties of H2S and to be aware of the problems associated with the choice of resources used to generate H2S in in vitro and in vivo experiments. H2S is a colorless gas under ambient temperature and pressure. The toxicity of H2S has been known for hundreds of years and is comparable to that of CO or hydrogen cyanide (HCN).42–44 Exposure to 300 ppm of H2S leads to pulmonary edema and 1000 ppm of H2S causes immediate death. Caution should therefore be taken when working with H2S. As a weak acid, H2S is very water soluble. Its solubility was reported to be ~80 mM at 37 °C as an equilibrium between molecular and ionic forms (H2Saq ⇌ HS− ⇌ S2−). The pKa values for the first and second dissociation steps are 7.0 and >12.0, respectively.45–47 Therefore, in aqueous state under the physiological pH of 7.4, the major form of hydrogen sulfide exists as HS− with a minor form of free H2S (the ratio of HS−/H2S is ~3:1). Very small amounts of sulfide anion (S2−) are also present. Since it has not been possible to determine which form of H2S (H2S, HS−, or S2−) is the active species in biological systems, the term of H2S is used to refer to the total sulfide present in the solution (i.e. H2S + HS− + S2−).
So far, one of the major challenges in the H2S field is precise measurement of H2S concentrations. Traditional methods such as methylene blue (MB) assay, ion selective electrodes (ISE), and gas chromatography, require complicated post-mortem processing and/or destruction of samples.48–50 Given the high reactivity of H2S under biological environments, these methods may yield inconsistent results.46, 51 Fluorescence based assays can be very useful due to high sensitivity and easy operation. Fluorescence methods are suitable for nondestructive detection of bio-targets in live cells or tissues with readily available instruments. In 2011 several groups reported the first reaction-based fluorescent probes for H2S detection in cell and blood samples.52–56 These works inspired researchers to develop new H2S fluorescent probes and a number of papers have been published in the past two years.57–62 All of these probes are based on reaction-based fluorescence turn-on strategies, i.e. using certain H2S specific reactions to convert non-fluorescent substrates to materials with strong fluorescence. So far three types of reactions have been employed for the probe design (Scheme 3): 1) H2S-mediated reductions, often using azide (N3) substrates; 2) H2S-mediated nucleophilic reactions, and 3) H2S-mediated metal-sulfide precipitations. However, although a number of probes have been reported, few can be applied to real biological detections due to slow reaction rate and/or low sensitivity of many probes. In addition, the selectivity of these probes for H2S vs other reactive sulfur species, especially the newly recognized persulfide species, is largely unaddressed. Due to these problems, further development of chemeospecific fluorescent probes for H2S remains critical.
Scheme 3.
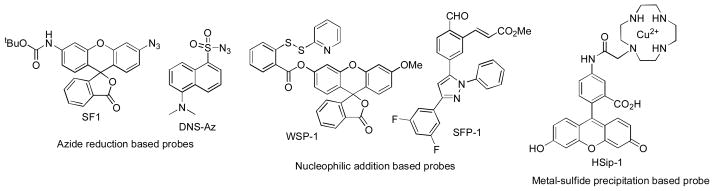
Representative fluorescent probes for H2S detection
In the study of H2S’s mechanisms and functions H2S releasing agents (i.e. donors) are important research tools. In the past several years the development of novel H2S donors has become a rapidly growing field, with several series of donors reported. 63, 64 These donors release H2S through different mechanisms. It should be noted that although H2S is biologically active, its functions sometimes appear inconsistent. For example, in addition to its anti-inflammatory effects, H2S has also been reported as a pro-inflammatory molecule.65, 66 These disparate results might be due to the use of different H2S donors in the research. H2S releasing capabilities of each donor category are quite different, which may lead to different results. Additionally, byproducts could form along with H2S generation and it is unclear whether these byproducts have biological effects. Therefore, the selection of suitable H2S donors is crucial. In this review we summarize the information about current available H2S donors with a focus on the chemistry of their development. Some biological applications are also discussed.
2. H2S DONORS
2.1. H2S gas
As the authentic resource, H2S gas has been directly used in this field. It has been reported that H2S gas promotes glucose uptake and provides amelioration in type II diabetes.67 In 2005 Roth found that H2S (g) could induce a suspended animation-like state in mice.68 The exposure of mice to 80 ppm of H2S (g) caused a significant drop in their oxygen (O2) consumption (by ~ 50%) and carbon dioxide (CO2) output (by ~ 60%) within first 5 minutes. A 6-hour H2S (g) exposure diminished mice’s metabolic rate (MR) by ~ 90%. The decrease in MR was followed by a drop in core body temperature (CBT) to as low as 15 °C. When mice were returned to normal air and room temperature after this H2S exposure, their MR and CBT returned to normal levels. It has been suggested that these effects were induced through reversible and competitive inhibition of the mitochondrial enzyme cytochrome c oxidase by H2S, which slowed respiration.69
The reversible H2S-induced hibernation state was later shown to protect mice from lethal hypoxia.70 C57BL/6J mice cannot survive in 5% O2 for more than 15 minutes. However, after inducing the suspended animation-like state by pretreating the animals with 150 ppm H2S (g) for 20 minutes, mice survived the duration of 1-h experiment under normally lethal hypoxia conditions. The longest exposure to 5% O2 was 6.5 hours and H2S-pretreated mice survived without notable damage.
Although these interesting experiments revealed promising effects of H2S gas in reducing mice metabolic rate, similar studies failed in large animals, such as sheep and piglets.71–74 From experimental operation perspective, it is hard to consider H2S gas as an ideal resource due to difficulties in obtaining precisely controlled concentrations and possible toxic impact of H2S excess. Because of this reason, H2S equivalents or releasing agents are often used in the field.
2.2. Inorganic sulfide salts
Inorganic sulfide salts such as sodium sulfide (Na2S) and sodium hydrogen sulfide (NaHS) have been used as H2S equivalents by many researchers. The treatments of cells, tissues, or animals with sulfide salts have shown protective effects against a number of disease states.75–78 By using Na2S as an exogenous H2S donor, Lefer et al. confirmed that long-term H2S therapy attenuates ischemia-induced heart failure.79 In this study heart failure was induced by subjecting C57BL6/J mice to 60 minutes of left coronary artery occlusion followed by reperfusion for up to 4 weeks. 100 μg/kg of Na2S was administered once at the time of reperfusion and then daily for the first 7 days of reperfusion. The results suggested that long-term H2S therapy leads to a decrease in left ventricular (LV) dilation, decrease in cardiac hypertrophy, and improvement in cardiac function. In addition, treatment with Na2S also reduced oxidative stress associated with heart failure. Although previous studies have provided solid evidence for the cardioprotective effects of short-term H2S therapy,80, 81 these results by Lefer showed for the first time that H2S therapy can provide long-term protection against myocardial injury and this effect is believed to be induced by the reduction of oxidative stress.
In addition to cardioprotection sulfide salts also showed protective effects against other diseases such as inflammation.82 Osteoarthritis (OA), a form of arthritis, is characterized by degenerative and inflammatory processes driven by the presence of enhanced levels of pro-inflammatory proteins such as IL-6 and IL-8. Kloesch et al demonstrated that short-term (15–30 minutes) treatment of human cells with NaHS is sufficient to down-regulate IL-6 and IL-8 expression, which may account for H2S anti-inflammatory effects against OA.82b However, care should be taken when NaHS is applied because it was also found that the anti-inflammatory effects of NaHS were altered to be pro-inflammatory when the NaHS incubation time was extended from 15 minutes to 1 hour.
It is obvious that sulfide salts, as H2S donors, have the advantage of boosting H2S concentration rapidly. However, these compounds release H2S spontaneously at the time the solution is prepared, making it hard to precisely control H2S concentration. Modifications made between the time that a solution is prepared and the time that the biological effect is measured can dramatically affect results. This uncontrolled and rapid H2S release can cause severe damages in vivo. In addition, H2S can be quickly lost from solution due to volatilization under laboratory conditions. The effective residence time of sulfide salts in tissues is relatively short. Olson and co-workers conducted an experiment to test H2S loss from aqueous solutions.83 They found that H2S were lost from solutions with t1/2 values of about 5 min. After 12 hours, undetectable H2S was left from an original 10 μM of H2S Hepes solution. It should also be noted that commercial sulfide salts, especially NaHS, always contains significant amount of impurities. Recent study revealed that polysulfides rapidly form in NaHS solution.84 All of these problems should be kept in mind when using sulfide salts as H2S donors.
2.3. Garlic and related sulfur compounds
For hundreds of years garlic has been considered as a magic medicine. Recent studies suggest that at least some of the beneficial effects of garlic are due to H2S production. So far the best characterized compound from garlic is allicin (diallyl thiosulfinate). This compound is unstable in aqueous solutions and quickly decomposes to several compounds including diallyl sulfide (DAS), diallyl disulfide (DADS) and diallyl trisulfide (DATS) (Scheme 4).85 Kraus and coworkers demonstrated that human blood cells (RBCs) can convert garlic-derived organic polysulfides into H2S, which the vasoactivity of garlic is attributed to.86 Among all of the sulfur compounds, i.e. DAS, DADS, DATS, allyl methyl sulfide (AMS), and dipropyl disulfide (DPDS) (Scheme 4), DATS produced the highest amount of H2S in the presence of glutathione (GSH), followed by DADS. Apparently H2S production from these sulfur compounds is facilitated by allyl substituents and by increasing the numbers of tethering sulfur atoms.
Scheme 4.
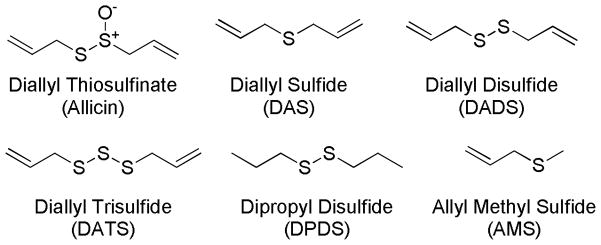
Garlic-related sulfur compounds.
From a reaction mechanism point of view, the regular thiol/disulfide exchange between DADS and GSH should not produce H2S. Instead, H2S generation is initiated by the nucleophilic substitution of GSH at the α-carbon of the allyl substituent, forming an allyl perthiol, which undergoes a thiol/disulfide exchange to release H2S. Trisulfides (R-SSS-R′) also undergo similar nucleophilic substitutions at the sulfur atom, yielding RSSH, and then H2S (Scheme 5).
Scheme 5.
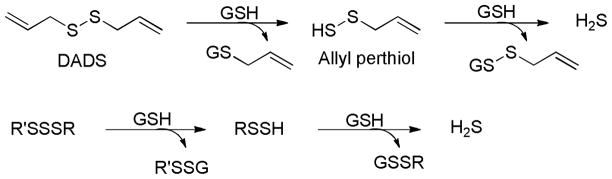
H2S generation from garlic-derived sulfur compounds.
The vasodilation effects caused by garlic and garlic-derived sulfur compounds were tested.86 In these experiments, phenylephrine-precontracted aorta rings were suspended in a 37 °C organ bath containing 1 mM GSH under physiological O2 conditions and treated with different doses of garlic (50, 200, and 500 μg/ml) or garlic-derived sulfur compounds. In the garlic-treated group, the aorta rings showed a concentration-dependent relaxation accompanied by H2S production. In the polysulfide-treated group, DATS and DADS exhibited the maximum relaxation of aorta rings. However, DPDS and AMS showed minimum effects. These results are paralleled with the compounds’ H2S yields, suggesting a link between bioactivity and H2S production.
It should be noted that DADS and DATS are reactive sulfane sulfur species. Sulfane sulfur refers to a sulfur atom with six valence electrons but no charge (represented as S0).42 Sulfane sulfur compounds have unique reactivity and exhibit regulatory effects in diverse biological systems.87, 88 Biologically important sulfane sulfur compounds include perthiol (R–S–SH), polysulfides (R–S–Sn–S–R), and protein-bound elemental sulfur (S8).89 Sulfane sulfur and H2S usually coexist, and recent work even suggests that sulfane sulfur species, derived from H2S, may be the active signaling molecules and exhibit protection in mammals.90–93 Therefore, garlic derived and related sulfur compounds that have shown protective effects in biological systems must be further investigated as to whether the effects are derived from sulfane sulfurs, or hydrogen sulfide.
2.4. Lawesson’s reagent and analogs
2,4-Bis(4-methoxyphenyl)-1,3,2,4-dithiadiphosphetane-2,4-disulfide (Lawesson’s reagent) is a widely used sulfurization reagent in organic synthesis 94 that can also be used as an H2S donor. It can be easily synthesized by heating a mixture of anisole with phosphorus pentasulfide (P4S10) (Scheme 6a).95, 96
Scheme 6.
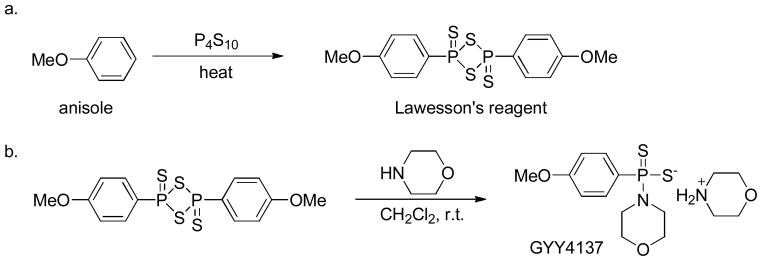
Chemical synthesis of Lawesson’s reagent (a) and GYY4137 (b).
As an H2S donor, Lawesson’s reagent showed some H2S-related bioactivities, such as ion channel regulation and anti-inflammation. 97, 98 In 2009 Wallace et al investigated the effects of H2S on inflammation and ulceration of the colon in a rat model of colitis.98 Treatment with Lawesson’s reagent dramatically reduced the severity of colitis. Additionally, Lawesson’s reagent also significantly attenuated the increase in colonic thickness that occurs in rats with colitis. The results are comparable with those obtained in NaHS-treated group, confirming the potency of Lawesson’s reagent as an H2S donor. However, this donor releases H2S upon spontaneous hydrolysis in aqueous solution. This uncontrollable release of H2S makes it difficult to mimic endogenous H2S formation. In addition, the poor solubility of Lawesson’s reagent in aqueous solutions limits its applications.
Morpholin-4-ium 4 methoxyphenyl(morpholino) phosphinodithioate (GYY4137), a derivative of Lawesson’s reagent, is water soluble. It can be synthesized by reacting Lawesson’s reagent with morpholine in methylene chloride at room temperature (Scheme 6b).99
Similar to Lawesson’s reagent, GYY4137 releases H2S upon hydrolysis. The in vitro H2S release was confirmed by colorimetric and amperometry assays.99 Compared to sulfide salts, H2S release from GYY4137 was much slower and the H2S concentration reached the maximum value within 6–10 minutes but at a very low level. 1 mM of GYY4137 released 40 μM of H2S within the first 10 minutes and another 50 μM of H2S in the following 90 minutes in aqueous solution (pH 3.0). H2S release from GYY4137 was pH- and temperature-dependent, with more release at acidic pH and less release at low temperatures. Under physiological conditions, H2S production from GYY4137 maintained at low level (less than 10 %) even after 7 days.106 For in vivo H2S production, GYY4137 (133 μmol/kg) was administrated (intravenous or intraperitoneal injections) to anesthetized male Sprague-Dawley rats. The plasma H2S concentration, measured by MB method, was increased at 30 minutes and remained elevated over 180 minutes.
In a report by Moore et al, no detectable cytotoxicity, cell cycle distribution change, or p53 expression induction was observed after treating rat vascular smooth muscle cells with GYY4137 (up to 100 μM) for up to 72 hours.99 Previous studies have shown that NaHS (at similar concentrations and time courses) promoted the apoptotic cell death of cultured fibroblasts and smooth muscle cells.100, 101 The very slow H2S release from GYY4137 may explain why GYY4137 did not cause apoptosis. In contrast to the rapid and reversible relaxation of precontracted aortic rings (~ 20 to 30 seconds) caused by NaHS, GYY4137 showed a slower onset (~ 10 minutes) and longer sustained effect (~ 40 minutes). NaHS (2.5 – 20 μmol/kg) caused fast (10 – 30 seconds) and dose-related decrease in blood pressure, but GYY4137 (26.6 to 133 μmol/kg) caused a slowly (apparent at 30 minutes) drop in blood pressure.
H2S plays disparate roles on inflammation, with both pro- and anti-inflammatory effects illustrated.65, 66, 102–104 In 2010, Moore and co-workers compared the effects of NaHS and GYY4137 on the release of pro- and anti-inflammatory mediators in lipopolysaccharide (LPS)-treated murine RAW 264.7 macrophages.105 The purpose of this study was to test whether the effects of H2S on inflammation were dependent on H2S generation rate. In this study GYY4137 significantly inhibited the LPS-induced release of pro-inflammatory mediators such as IL-1β, IL-6, TNF-α, nitric oxide, and PGE2, but increased the synthesis of the anti-inflammatory chemokine IL-10. In contrast the effects of NaHS were much less consistent. The results indicated that the effects of H2S on inflammation are complex and may depend not only on H2S concentration but also on the rate of H2S generation.
In addition to its roles in vasorelaxation and inflammation, anticancer effects of GYY4137 were recently reported.106 Proliferation of cancer cells, such as breast adenocarcinoma (MCF-7), acute promyelocytic leukemia (MV4-11), and myelomonocytic leukemia (HL-60), were significantly reduced by a 5-day treatment with GYY4137 (400 μM). NaHS and ZYJ1122 (a structural analog of GYY4137 lacking sulfur) remained inactive at the same concentration. GYY4137 (800 μM) killed 75–95% of these cells, but it did not affect the survival of human non-cancer diploid fibroblasts (WI-38 and IMR90). Mechanistic investigation revealed that the treatment of MCF-7 cells with GYY4137 led to cell cycle arrest in G2/M phase and promotion of apoptosis. The fact that the non-H2S-releasing compound ZYJ1122 did not show inhibitory effects on any cell lines may suggest the anticancer effects of GYY4137 are due to H2S release.
Although GYY4137 has been widely used, its fixed H2S release capability may not fulfill the requirements of different biological applications. In addition, the exact mechanism of H2S release from GYY4137 is still unclear as are the byproducts produced. As mentioned above, GYY4137 is proposed to exhibit anti-cancer effects due to H2S release since its non-H2S releasing analog, ZYJ1122, failed to show similar effects. This conclusion needs to be further clarified as it is unclear if ZYJ1122 is truly the byproduct of GYY4137. Therefore, control experiments should be conducted appropriately when using GYY4137 as an H2S donor. Otherwise, GYY4137-induced biological effects may not be concluded to be H2S-dependent.
Recently the Xian group developed a series of phosphorodithioate-based H2S donors by replacing the phosphorus-carbon bond in GYY4137 with phosphorus-oxygen bonds.107 It was expected that the structure modifications on phosphorodithioate core may result in H2S release capability change and in turn lead to biological activity changes. The synthesis of such phosphorodithioate-based H2S donors was achieved in 4 steps (Scheme 7): the starting material trichlorophosphine reacted with 1,2-dithioethane to yield 2-chloro-1,3,2-dithiaphospholane, which was then treated with aniline and followed by sulfurization with elemental sulfur to give the key intermediate 2-amine-1,3,2-dithiaphospholan-2-sulfide. Finally, this intermediate was condensed with different phenols and alcohols in the presence of 1,8-diazobicyclo[5.4.0]undec-7-ene (DBU) to afford the desired products.
Scheme 7.
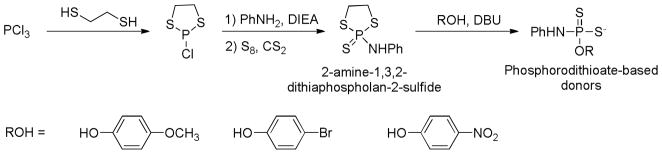
Chemical synthesis of phosphorodithioate-based H2S donors.
H2S release capabilities of these compounds were tested by spectroscopic methods and compared with GYY4137. In previous studies H2S release from GYY4137 was mainly measured by MB assay, a standard H2S detection method involving strong acidic conditions. It is known that the hydrolysis of phosphorodithioates is pH dependent and hydrolysis is much faster under acidic conditions than under neutral pH. Therefore, MB may not be a viable way to evaluate phosphorodithioate-based donors like GYY4137. Dansyl azide (DNS-Az), a fluorescent probe for H2S,55 was used to measure H2S generation from phosphorodithioate-based H2S donors under neutral pH. Similar to GYY4137, O-aryl substituted phosphorodithioate-based donors showed slow and low H2S release. Up to 1 μM of H2S was detected from 100 μM of donor in a mixed acetonitrile/phosphate buffer solution (1:1 v/v, pH 7.4) within 3 hours of experiment period. However O-alkyl substituted donors showed almost un-detectable H2S release. Presumably the O-alkyl substitutions led to increased stability of phosphorodithioates and therefore decreased the rate of hydrolysis to generate H2S. O-Aryl substituted phosphorodithioate-based donors were also shown to release H2S in cells. The cell imaging experiments were conducted by incubating H9c2 cardiac myocytes with the donor at various concentrations (0, 100, and 200 μM) for 24 hours. Then WSP-1, an H2S-specific fluorescent probe,53 was applied to the cells to monitor H2S production. Compared to vehicle-treated group, enhanced fluorescence in donor-treated group demonstrated a sustain H2S generation.
Since H2S has been known to exhibit cellular protection against oxidative injury,108 it was hypothesized that phosphorodithioate-based H2S donors may have similar effects due to H2S release. Two O-aryl substituted donors were selected to evaluate their protective effects against H2O2-induced oxidative damage in H9c2 cells. In these experiments, H9c2 cells were incubated with each donor (50, 100 and 200 μM) for 24 hours, followed by 5-hour incubation with H2O2 (150 μM). Cell viability results showed that 35% of cells were killed if H2S donors were absent. In comparison, cell viability increased significantly in the presence of donors, suggesting that H2S donors may have some protective effects against oxidative injury.
It should be noted that although phosphorodithioate-based donors have shown H2S-like biological activities, it is still premature to attribute those activities to H2S production. H2S production from those phosphorodithioate-based donors is very slow and at very low levels. The major species in media is still the donor molecule itself. It is known that phosphorodithioate core structures are biologically active. For example, phosphorodithioate DNA is resistant to nuclease degradation and has been reported as a potential therapeutic drug.109, 110 In addition, phosphorodithioate oligodeoxycytidine has also shown inhibitive activities against human immunodeficiency virus.111 Therefore whether phosphorodithioate-induced effects are due to H2S release needs further confirmation.
2.5. 1, 2-Dithiole-3-thiones and H2S-hybrid nonsteroidal anti-inflammatory drugs
1, 2-Dithiole-3-thiones (DTTs) are known to release H2S in aqueous solutions. Although the detailed mechanism is still unclear, it has been demonstrated that DTTs decompose to the corresponding 1, 2-dithiole-3-one upon heating to 120 °C in a DMSO-aqueous phosphate buffer system. This observation implies that hydrolysis might be the mechanism of H2S generation from DTTs (Scheme 8).112–114
Scheme 8.
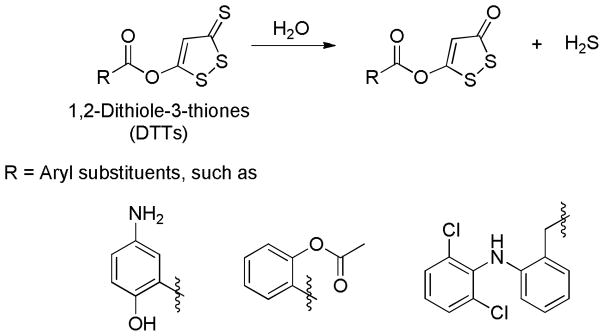
Proposed mechanism for H2S release from DTTs.
Several different methods have been applied to synthesize DTTs. In most cases, elemental sulfur or phosphorus pentasulfide is used to dehydrogenate and sulfurize an allylic methyl group to afford the desired products (Scheme 9a).115–117 In an alternative method dithioic aicds, obtained from a reaction between ketones and carbon disulfide (CS2), react with hexamethyldisilathiane (HMDT, a sulfur resource) and N-chlorosuccinimide (NCS, an oxidizing agent) to give substituted DTTs (Scheme 9b).118, 119 In addition, β-ketoesters were also reported to react with Lawesson’s reagent to form desired DTTs (Scheme 9c).120
Scheme 9.
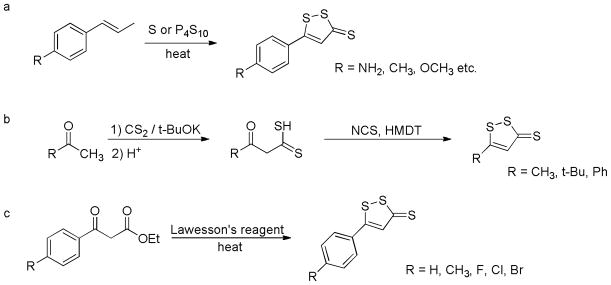
Chemical synthesis of DTTs.
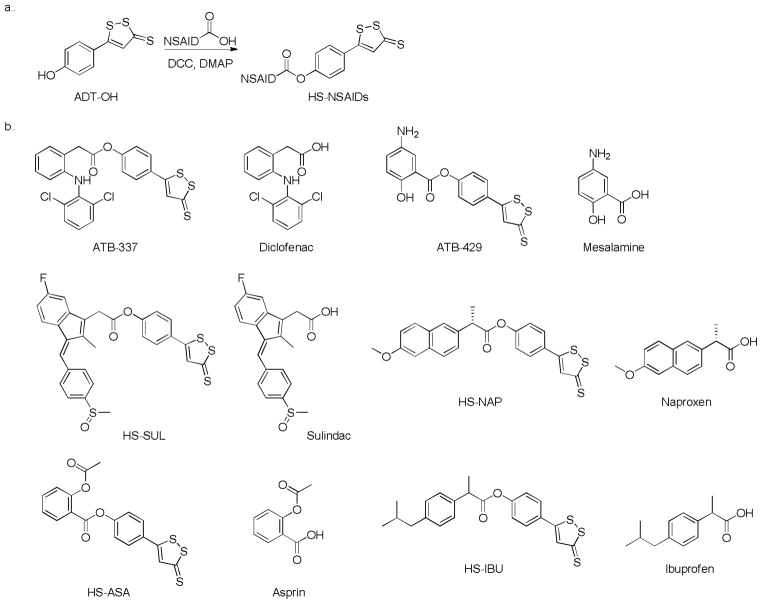
DTTs have been widely used in studying H2S-related biological effects in the alimentary system. The use of nonsteroidal anti-inflammatory drugs (NSAIDs) is associated with an unacceptable risk for gastrointestinal ulceration and bleeding.121–123 DTTs have been coupled with NSAIDs and the resultant HS-hybrid NSAIDs (HS-NSAIDs) showed significant reduction of gastrointestinal damage compared to the parent NSAIDs.114, 124–126 HS-NSAIDs are usually synthesized by coupling NSAID counterparts with 5-(4-hydroxyphenyl)-3H-1,2-dithiole-3-thione (ADT-OH), an H2S releasing molecule, in the presence of dicyclohexylcarbodiimide (DCC) and 4-dimethylaminopyridine (DMAP) (Scheme 10a).127 Some representative HS-NSAIDs and their parent NSAIDs are listed in Scheme 10b.
Scheme 10.
Chemical synthesis of HS-NSAIDs (a) and structures of representative HS-NSAIDs and corresponding NSAIDs (b).
Wallace and co-workers evaluated the anti-inflammatory effects of ATB-337 in rats.103 H2S release from ATB-337 and ADT-OH was measured by an in vitro system. Briefly, ATB-337 or ADT-OH (10 μM in polyethylene glycol) were incubated in a potassium phosphate buffer (100 mM, pH 7.4) alone or in the presence of rat liver homogenate (10% wt/vol) and pyridoxal 5′-phosphate (2 mM) for 30 minutes. The generation of H2S was detected by a sulfide-sensitive electrode. Results suggested that when incubated in buffer, ADT-OH released negligible amounts of H2S, while ~ 12 nmol/min of H2S was released from ATB-337. On the other hand, incubation of both compounds in liver homogenate caused 3-fold greater levels of H2S generated from ATB-337 (~ 43 nmol/min) than those from ADT-OH (~ 14 nmol/min). In order to measure plasma H2S concentrations, male Wistar rats were fasted overnight and then orally treated with diclofenac, ATB-337 (both drugs at 50 μmol/kg), or vehicle. Results showed that plasma H2S levels were significantly increased (by ~ 40%) after the administration of ATB-337 but unchanged in rats treated with diclofenac. These findings suggest that ATB-337 indeed releases H2S both in vitro and in vivo.
Further investigations of the gastrointestinal damages caused by diclofenac and ATB-337 showed that oral administration of diclofenac led to hemorrhagic erosions in rat stomach. In comparison, the same dose of ATB-337 did not produce this damage. In order to determine whether the separate but concomitant administration of the NSAID moiety (diclofenac) and H2S donor moiety (ADT-OH) of ATB-337 would induce the same degree of gastric damages as the intact compound, rats were treated with diclofenac alone or together with ADT-OH. Results showed that gastric damages caused by the co-administration of these two moieties were similar to that observed in diclofenac-treated group, indicating ADT-OH alone did not protect the stomach against the damages caused by NSAIDs. Considering ATB-337 released 3 times amount of H2S more than ADT-OH in liver homogenate, it is possible that the gastric safety of ATB-337 was caused by its enhanced H2S releasing ability. However, it is still unclear why ATB-337 released more H2S than ADT-OH. It is possible that after the conjugation of diclofenac with ADT-OH, the latter’s induction effect changes, making it hydrolyze more easily. Further studies are necessary regarding this question.
In addition to the protective effects in gastric system, other biological effects of HS-NSAIDs have also been observed.128–130 Kashfi and co-workers found that HS-ASA, HS-IBU, HS-SUL and HS-NAP can inhibit the growth of various human cancer cells including breast, prostate, lung, leukemia, pancreas, and colon cancer cells.129 Studies of ATB-429 in a model of postinflammatory hypersensitivity showed that administration of ATB-429 reduces visceral sensitivity and pain perception in conscious healthy and postcolitic hypersensitive rats.131
Although HS-NSAIDs have shown promising H2S-related effects in different tissues and organs, it is still unclear how these molecules release H2S in living systems. Previous studies suggested that the hydrolysis of DTTs releases H2S in aqueous buffer.112–114 However, considering the complexity of biological systems, H2S release from HS-NSAIDs is expected to be more complicated. The hydrolysis of HS-NSAIDs may initiate H2S release. However, due to the abundant amount of cysteine and GSH in biological systems, the resultant thiolactone-like species may continue to react with these thiols to release more H2S. More importantly, it should be noted that perthiols, which belong to reactive sulfane sulfur species, are formed in this process. Therefore, whether the biological effects of HS-NSAIDs are H2S-related or sulfane sulfur-related needs to be further investigated.
2.6. Thiol-activated H2S donors
N-mercapto-based H2S donors
In 2011 Xian and coworkers introduced the concept of controllable H2S donors and reported the first thiol-activated donors.132 Their goal was to develop donors which are stable in aqueous solutions and during sample preparation. Ideally H2S release from these donors should be controlled by factors like biomolecules, pH, light, etc. The first series of thiol-activated donors were based on an N-mercapto (N-SH) template. Since N-SH species are unstable, acyl groups were introduced as SH protecting groups to enhance the stability (Scheme 11a). A series of N-(benzoylthio)benzamide derivatives were prepared in a two-step synthesis: thiocarboxylic acids were treated with hydroxylamine-O-sulfonic acid in basic conditions to yield S-acylthiohydroxylamines, which were further reacted with benzoic anhydride to form the final products (Scheme 11b).
Scheme 11.
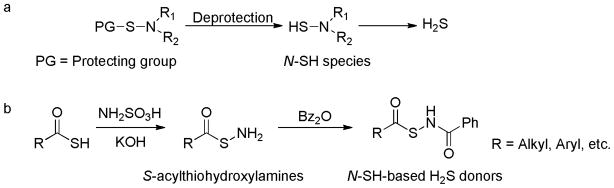
Design and synthesis of N-SH-based H2S donors.
The protected N-SH compounds proved to be stable in aqueous solutions. They did not react with potential cellular nucleophiles, such as −OH and −NH2 groups. However, in the presence of cysteine or GSH, a time-dependent decomposition of the donors accompanied by H2S release was observed. The structure activity relationship studies showed that electron withdrawing groups (EWGs) led to faster H2S release and electron donating groups (EDGs) resulted in slower H2S release, demonstrating the achievement of H2S release regulation by structural modifications. In addition, H2S release from N-SH-based donors was also detected in plasma, suggesting N-SH compounds are active H2S donors in complex systems.
Reaction mechanism studies showed that H2S generation in these donors is initiated by the thiol exchange between cysteine and donors to generate S-acylated cysteine and N-mercaptobenzamide. S-Acylated cysteine then undergoes a native chemical ligation (NCL) to form a stable N-acylated cysteine. The N-mercaptobenzamide intermediate interacts with cysteine to produce cysteine perthiol, which finally reacts with cysteine to form cystine and release H2S (Scheme 12).
Scheme 12.
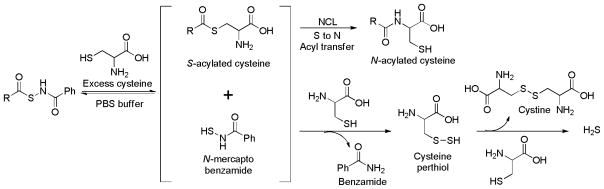
Proposed mechanism of H2S release from N-SH-based donors.
Perthiol-based H2S donors
In the study of N-SH-based donors cysteine perthiol was found to be a key intermediate. It should be noted that cysteine perthiol is also involved in H2S biosynthesis catalyzed by CSE (Scheme 1). This suggests that perthiol (S-SH) could be a useful template for H2S donor design. With this idea in mind Xian and coworkers developed a series of perthiol-based donors.133 They first employed cysteine perthiol (i.e. primary perthiol) as the structure backbone. Since S-SH compounds are very unstable, acyl groups were used again as protecting groups to enhance the stability (Scheme 13).
Scheme 13.
Design of perthiol-based H2S donors.
The synthesis of Cys-S-SH-based donors was achieved in two steps: N-benzoyl cysteine methyl ester was treated with 2-mercapto pyridine disulfide to provide a reactive cysteine-pyridine disulfide intermediate, which then reacted with thioacids to give the desired donor compounds (Scheme 14a). H2S release capabilities of these donors were evaluated. The results indicated that these donors indeed released H2S in the presence of thiols (cysteine or GSH). However, compared to N-SH-based donors, primary perthiol-based donors showed much decreased ability of H2S generation. Only less than 20 μM of H2S was detected from 150 μM of donors. One possible explanation is that thiols can attack the acyldisulfide linkage to form a new disulfide and a thioacid (Scheme 14b, pathway a). This reaction prevents the formation of the key perthiol intermediate (Scheme 14b, pathway b), therefore, diminishing H2S release from these donors.
Scheme 14.
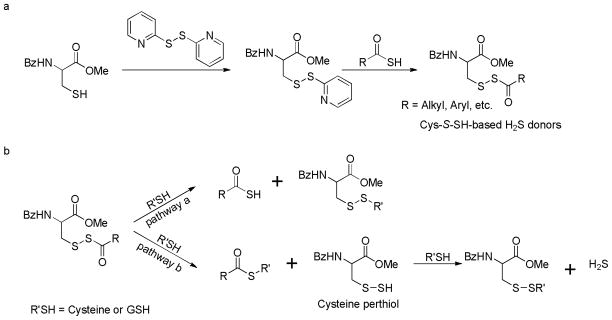
a) Chemical synthesis of cysteine perthiol-based H2S donors; b) explanation of low H2S release from primary perthiol-based donors.
In order to enhance H2S generation capability tertiary perthiol-based donors were synthesized. The acyldisulfide linkage was blocked by two methyl groups on the α-carbon in order to prevent the unwanted disulfide formation. To synthesize these donors, C- and N-protected penicillamine was treated with 2, 2′-dibenzothioazolyl disulfide. The resultant penicillamine-benzothioazolyl disulfide intermediate reacted with different thioacids to furnish the desired donors (Scheme 15).
Scheme 15.
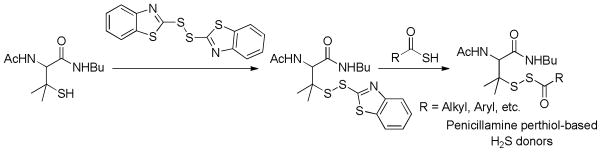
Chemical synthesis of penicillamine perthiol-based H2S donors.
These tertiary perthiol-based compounds were proved to be potent H2S donors. Up to 80 μM of H2S were generated from 100 μM of donors. The regulation of H2S release from these donors could be achieved by structural modifications. Similar to N-SH-based donors, EWGs caused faster H2S release and EDGs led to slower H2S generation. In addition, steric effects were also observed as more hindered substrates resulted in slower H2S release or even no release at all.
H2S release mechanism of these donors was studied and proved to be similar as that of N-SH-based donors. Briefly, the thiol exchange initiated the reaction and resultant penicillamine perthiol released H2S in two possible pathways: thiol attacked the acyldisulfide linkage to produce a disulfide and generate H2S (Scheme 16, pathway a); the reaction between penicillamine perthiol and thiols would form a new perthiol species (cysteine perthiol or GSH perthiol) and this newly formed perthiol could interact with excess thiol to release H2S (Scheme 16, pathway b).
Scheme 16.
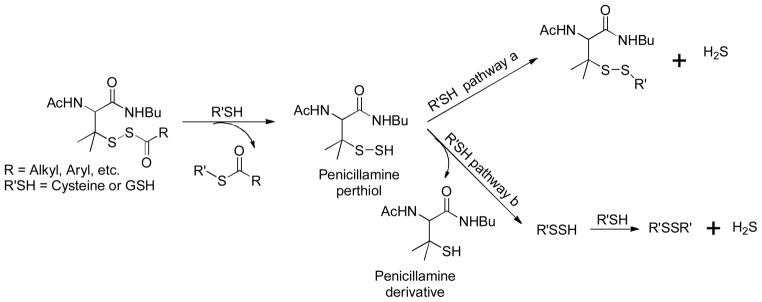
Proposed mechanism of H2S release from S-SH-based donors.
Since H2S exhibited protective effects against myocardial ischemia/reperfusion (MI/R) injury,134–136 perthiol based donors were expected to exhibit similar effects due to H2S release. Myocardial protective effects of selected S-SH-based donors were tested in a murine model of MI/R. In these experiments MI/R injury was induced by subjecting mice to 45-min left ventricular ischemia followed by 24-h reperfusion. Donors or vehicle were administered into left ventricular lumen at 22.5 min of myocardial ischemia. Compared to vehicle-treated mice, mice receiving donors displayed a significant reduction in circulating levels of cardiac troponin I and myocardial infarct size per area-at-risk, suggesting that S-SH-based compounds can exhibit H2S-mediated cardiac protection in MI/R injury and these compounds may have potential therapeutic benefits.
Although S-SH-based donors showed promising biological effects, the exact mechanisms in vivo are to be investigated. It should be noted that the reaction between donors and cysteine yields perthiols, which also belong to reactive sulfane sulfur species. Therefore, further studies on H2S-related and sulfane sulfur-related mechanisms are needed.
Dithioperoxyanhydrides
Similar to N-SH-based and S-SH-based donors, dithioperoxyanhydrides were recently reported as another class of thiol-activated H2S donors.137 These donors were synthesized by either iodine oxidation of the thiocarboxylates138 or reactions between thiocarboxylic acids and methoxycarbonylsulfenyl chloride139 (Scheme 17). H2S release was confirmed in both buffers and cellular lysates. Additionally, one of the donors, CH3C(O)SSC(O)CH3 in Scheme 17, was also shown to induce concentration-dependent vasorelaxation on pre-contracted rat aortic rings.
Scheme 17.
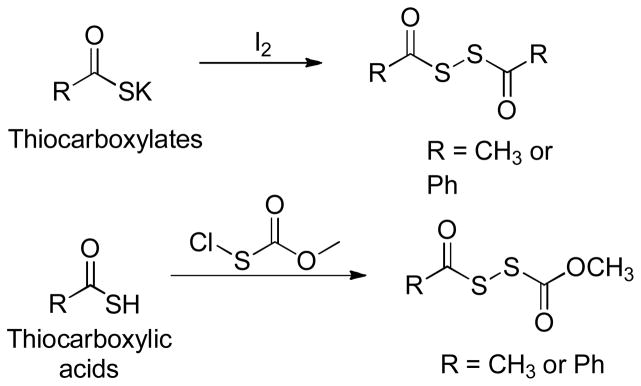
Chemical synthesis of dithioperoxyanhydride-based H2S donors
Acylpersulfides were proposed to be the key intermediates for H2S release from these donors. It is possible that acylpersulfides directly react with thiols (RSH) to give H2S and RSSAc (Scheme 18, pathway a). Alternatively the reaction between acylpersulfides and thiols would produce a new perthiol species (RS-SH), which then reacts with excess thiols to yield H2S (Scheme 18, pathway b).
Scheme 18.
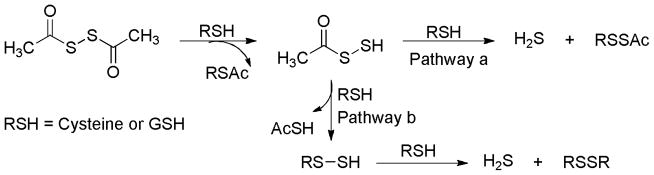
Proposed mechanism for H2S release from dithioperoxyanhydrides.
Arylthioamides
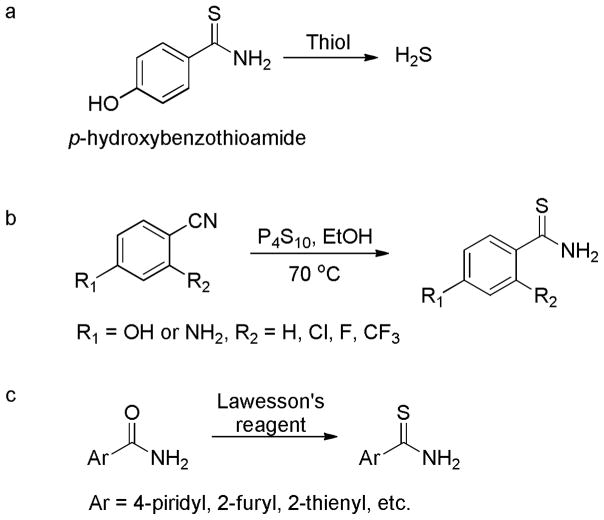
Arylthioamides were classified as the fourth class of thiol-activated H2S donors (Scheme 19a).140 The p-hydroxybenzothioamide was selected as the lead compound and a library of such compounds were synthesized by structural modifications as follows: 1) introduction of EWGs or EDGs at the 2- or 5-position of the phenyl ring; 2) replacement of the 4-hydroxy group with an amino group; and 3) replacement of the phenyl ring with heterocycles. Briefly, the non-heterocyclic compounds were prepared by mixing the corresponding benzonitrile with P4S10 in ethanol at 70 °C for 10 hours (Scheme 19b); the heterocyclic compounds were obtained by the treatment of the amides with Lawesson’s reagent for 12 hours (Scheme 19c).
Scheme 19.
a) H2S release from p-hydroxybenzothioamide; b) chemical synthesis of non-heterocyclic donors; and c) chemical synthesis of heterocyclic donors.
These compounds showed very weak H2S generation in buffers. With 1 mM initial concentration, negligible amounts of H2S were observed from lead donors in the absence of cysteine. When cysteine or GSH (4 mM) was presented, H2S formation was detectable, but at a low level (~1%).
After confirming H2S release ability, Calderone and coworkers tested the effects of the lead donor, p-hydroxybenzothioamide, on vasoconstriction induced by noradrenaline in isolated rat aortic rings. The results suggested that the pretreatment of aortic rings with 1 mM of p-hydroxybenzothioamide almost completely inhibited vasoconstriction. This observation is similar with that obtained in NaHS-treated group, indicating the anti-vasoconstriction effects of p-hydroxybenzothioamide are H2S-related. In addition, the effects of p-hydroxybenzothioamide on blood pressure were tested. As expected, after oral administration of p-hydroxybenzothioamide (0.1 mg/kg) to rats, a decrease of blood pressure (89 ± 1%) was observed.
Although these data suggest arylthioamides can release H2S and their biological effects are H2S-related, some puzzling questions remain: 1) H2S release from arylthioamides was only shown for 15 minutes in the paper. It is unclear if H2S concentrations maintained elevated or dropped afterwards. 2) H2S release mechanism (with and without cysteine) is not reported. It is unclear what the active intermediate(s) and final product(s) are. 3) Since the donors release only very limited H2S (~ 1%), the major form of the donors in vivo should still be the donor molecules. Caution should be used in attributing their biological activities to H2S unless careful control experiments using both the active intermediates and final products after H2S release are conducted.
2.7. Photo-induced H2S donors
Gem-dithiol-based-H2S donors
In an effort to develop non-thiol dependent donors, geminal-dithiols (gem-dithiols) were recently identified as a useful structure template.141 Gem-dithiol compounds are unstable in aqueous solutions. H2S can be formed as a decomposition byproduct.142–144 To make stable gem-dithiol-based donors, a photo cleavable 2-nitrobenzyl group145–147 was introduced as the protecting group on SH. Upon light irradiation, the free gem-dithiol intermediates should be formed and subsequent hydrolysis of these intermediates would liberate H2S (Scheme 20).
Scheme 20.
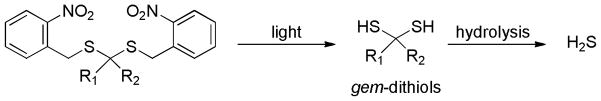
Design of gem-dithiol-based H2S donors.
A three-step synthesis of this type of donors was described as follows: commercially available 2-nitrobenzyl bromide was treated with thiourea in THF to produce the thiouroniumbromide salt. Hydrolysis of this salt in the presence of sodiummetabisulfite provided 2-nitrobenzenemethanethiol in high yield. Finally, 2-nitrobenzenemethanethiol was coupled with ketones in the presence of catalytic amount of titanium tetrachloride to yield the desired donor products (Scheme 21).
Scheme 21.
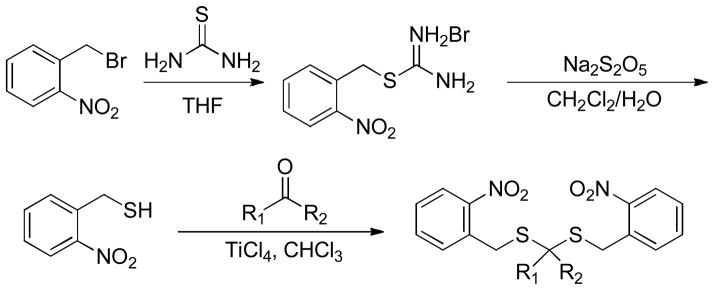
Chemical synthesis of gem-dithiol-based H2S donors.
MB assay indicated that up to 36 μM of H2S was generated after the irradiation of gem-dithiol-based donors (200 μM) at 365 nm. In comparison, no H2S was detected without the exposure of donors under UV light. H2S release of a selected donor was also detected in HeLa cells (under UV irradation) by using an H2S fluorescent probe.
It should be noted that H2S generation from these donors depended on the hydrolysis of gem-dithiol in aqueous solution, which resulted in the difficulties to control the rate of H2S release. Additionally, 2-nitrosobenzaldehyde, the byproduct of donor deprotection, may react with free gem-dithiol, therefore diminishing H2S generation. Considering these drawbacks, 2-nitrobenzyl group may not be suitable in developing photo-induced H2S donors for biological applications. The desired protecting groups should be photoremovable, and meanwhile not form any reactive byproducts.
Ketoprofenate-caged H2S donors
Recently, Nakagawa’s group employed ketoprofenate148 as a photocage to develop photolabile H2S donors.149 The synthesis of this donor was illustrated in Scheme 22a. Briefly, bromination of the nitrile starting material yielded a bromide intermediate in the presence of lithium diisopropylamide (LDA) and dibromomethane. This bromide species was then converted to sulfide intermediate by Na2S and the hydrolysis of this sulfide gave the desired donor. This ketoprofenate-caged donor can release H2S by eliminating 2 equivalents of 2-propenylbenzophenone and CO2 upon the irradiation at 300–350 nm (Scheme 22b). To evaluate H2S releasing capability in a complex biological system, this ketoprofenate-caged donor was applied to fetal bovine serum. No H2S was detected without irradiation. However, approximately 30 μM of H2S was detected from 500 μM of the donor in serum after irradiating for 10 minutes.
Scheme 22.
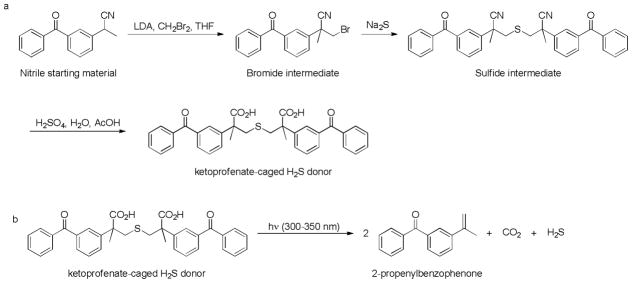
a) Synthesis of ketoprofenate-caged donor and b) H2S release mechanism.
So far two classes of photo-inducible H2S donors have been developed and evaluated. It should be noted that UV photolysis on biological samples such as cells or tissues may have cytotoxicity concerns, therefore limiting the applications of such H2S donors. Nevertheless these works proved the concept that both gem-dithiols and ketoprofenate-caged thioether are viable H2S donor precusors and that non-thiol dependent activation strategies can be used in triggering H2S generation. Based on these results, we expect to see more donors with new H2S release mechanisms in the near future. For example, since near-infrared (NIR) light can penetrate tissues and minimize the damage to biological samples, NIR-activated H2S donors may be developed and be useful.
2.8. Thioamino acids
In 2012 Giannis et al. reported that thioamino acids, such as thioglycine and thiovaline, could be H2S donors.150 They found that in the presence of bicarbonate both compounds were converted to the corresponding amino acid N-carboxyanhydrides with the generation of H2S (Scheme 23).
Scheme 23.
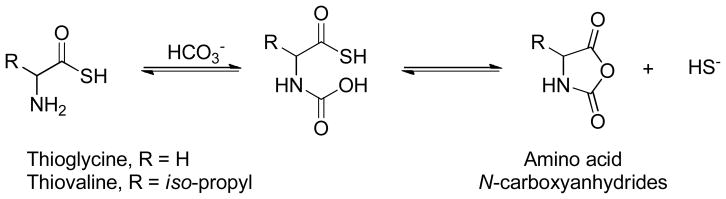
Proposed mechanism for H2S release from thioamino acids.
Considering the high bicarbonate concentration (~ 27 mM) in blood at physiological pH, Giannis et al. envisioned that thioamino acids could be good H2S donors. By using a H2S electrode, H2S releasing capabilities of thioglycine and thiovaline were evaluated and compared with other H2S donors. Results showed that H2S release from these two thioamino acids plateau after 1 hour. In comparison, H2S release from NaHS and Na2S was much faster and completed within 20 minutes. Up to ~50 μM of H2S could be detected from 100 μM of thioglycine, while GYY4137 released much less H2S.
To test the pharmacological benefits of these donors, their effects on intracellular cyclic guanosine monophosphate (cGMP) levels were determined. The results indicated that thioglycine and thiovaline led to a concentration-dependent increase in cGMP levels (~ 10-fold increase). In comparison, NaHS only resulted in a 2-fold increase of cGMP and glycine/valine did not cause any increase of cGMP. In addition, both thioglycine and thiovaline were found to induce significant relaxation of precontracted mouse aortic rings.
Although both thioglycine and thiovaline were proved to release H2S, thioamino acids are highly reactive molecules. Under aerobic conditions, they can rapidly carry out amidation reactions151–156 and can be easily oxidized to dithioperoxyanhydrides, which were also reported as H2S donors.137 All of these may lead to unwanted side-effects. Therefore, caution should be taken when using thioamino acids to study H2S-related effects.
3. CONLUSIONS AND FUTURE DIRECTIONS
In this review, we summarized the information on currently available H2S donors, with a focus on fundamental chemistry and some biological applications (Table 1). Given the importance of H2S in biomedical research, H2S donors are not only useful research tools, but also potential therapeutic agents. Although a number of donors have been developed and shown to release H2S both in vitro and in vivo, it is hard to define a universal “best” donor. All the donors have their own advantages, as well as problems. A major problem is that H2S release from many donors (i.e. sulfide salts, GYY4137, and DTTs) is not controllable and cannot mimic biological/endogenous H2S generation. This fast and uncontrollable H2S release can cause severe problems and sometimes can even be lethal. In addition, the byproducts associated with H2S release from some widely used donors such as GYY4137 and DTTs are still unclear. It is necessary to identify these products and study their biological activities. As for controllable donors, current efforts have been mostly put on thiol-activated donors and four types of such donors (N-SH-based donors, S-SH-based donors, dithioperoxyanhydrides, and arylthioamides) have been developed. These donors require the consumption of biological thiols to promote H2S generation. Considering biological levels of H2S, it is anticipated that the active dose of these donors should be at low micromolar level. Therefore the consumption of free thiols by donors may not cause significant changes in thiol redox balance. However, it is possible that in some cases, free thiols’ level might be low due to disulfide formation or binding to proteins. Caution should be taken when using these donors in such conditions or careful control experiments are needed. Last but not least, since reactive sulfane sulfur species, such as perthiols, are possibly involved in some donors (i.e. garlic-derived sulfur compounds, DTTs, dithioperoxyanhydrides, N-SH-based and S-SH-based donors), whether the biological activities of these donors are H2S-dependent or sulfane sulfur-dependent needs to be further investigated. In conclusion, H2S is a potential therapeutic molecule. The development of useful donors is critical. In our opinion future efforts should be focused on developing controllable H2S donors and different H2S releasing mechanisms should be explored. We expect to see more work coming out in this exciting field.
Table 1.
Summary of H2S donors.
| H2S donors | Structures | H2S release mechanism |
Representative bioactivities |
Key ref. |
|---|---|---|---|---|
| H2S (gas) | H2S | Authentic H2S resource | Hibernation induction Type II diabetes amelioration | 67–70 |
| Sulfide salts | NaHS & Na2S | Hydrolysis | Heart failure reduction Anti-inflammation | 75–83 |
| Garlic-derived sulfur compounds | R-S-Sn-S-R | Thiol activation | Vasodilation | 86 |
| Lawesson’s reagent |
|
Hydrolysis | Anti-inflammation Ion channel regulation | 97, 98 |
| GYY4137 |
|
Hydrolysis | Vasodilation Anti-inflammation Anti-cancer | 99, 105, 106 |
| Phosphorodithioates |
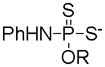
|
Hydrolysis | Prevention against oxidative damages | 107 |
| DTTs |
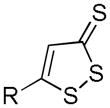
|
Hydrolysis | Anti-inflammation Anti-cancer | 114, 124–126, 129 |
| N-(acylthio)-benzamides |
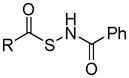
|
Thiol activation | No biological effects were reported to date. | 132 |
| S-SH compounds |
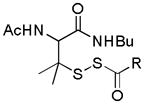
|
Thiol activation | MI/R protection | 133 |
| Dithioperoxyanhydrides |
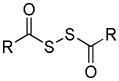
|
Thiol activation | Vasodilation | 137 |
| Arylthioamides |
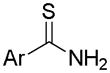
|
Thiol activation | Vasodilation | 140 |
| Gem-dithiol compounds |
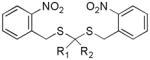
|
Light activation | No biological effects were reported to date. | 141 |
| Ketoprofenate-caged compound |
|
Light activation | No biological effects were reported to date. | 149 |
| Thioamino acids |
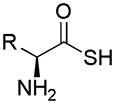
|
Bicarbonate activation | Vasodilation | 150 |
Acknowledgments
This work is supported by ACS-Teva USA Scholar Award and NIH (R01HL116571).
Abbreviations
- H2S
Hydrogen sulfide
- NO
Nitric oxide
- CO
Carbon monoxide
- CBS
Cystathionine β-synthase
- CSE
Cystathionine γ-lyase
- MPST
3-Mercaptopyruvate sulfur transferase
- CAT
Cysteine amino transferase
- ONOO−
Peroxynitrite
- O2.−
Superoxide
- H2O2
Hydrogen peroxide
- HSNO
Thionitrous acid
- HCN
Hydrogen cyanide
- MB
Methylene blue
- ISE
Ion selective electrodes
- N3
Azide
- O2
Oxygen
- CO2
Carbon dioxide
- MR
Metabolic rate
- CBT
Core body temperature
- Na2S
Sodium sulfide
- NaHS
Sodium hydrogen sulfide
- LV
Left ventricular
- OA
Osteoarthritis
- DAS
Diallyl sulfide
- DADS
Diallyl disulfide
- DATS
Diallyl trisulfide
- RBCs
Red blood cells
- PBS
Phosphate buffered saline
- GSH
Glutathione
- AMS
Allyl methyl sulfide
- DPDS
Dipropyl disulfide
- S8
Elemental sulfur
- P4S10
Phosphorus pentasulfide
- TNBS
Trinitrobenzene sulfonic acid
- DTNB
5,5′-Dithiobis-(2-nitrobenzoic acid)
- DBU
1,8-Diazobicyclo[5.4.0]undec-7-ene
- DNS-Az
Dansyl azide
- DTTs
1,2-Dithiole-3-thiones
- CS2
Carbon disulfide
- HMDT
Hexamethyldisilathiane
- NCS
N-chlorosuccinimide
- NSAIDs
Nonsteroidal anti-inflammatory drugs
- HS-NSAIDs
HS-hybrid NSAIDs
- ADT-OH
5-(4-Hydroxyphenyl)-3H-1,2-dithiole-3-thione
- DCC
Dicyclohexylcarbodiimide
- DMAP
4-Dimethylaminopyridine
- EWGs
Electron withdrawing groups
- EDGs
Electron donating groups
- NCL
Native chemical ligation
- MI/R
Myocardial ischemia/reperfusion
- gem-dithiol
Geminal-dithiols
- LDA
Lithium diisopropylamide
- NIR
Near infrared
- cGMP
Cyclic guanosine monophosphate
Notes and references
- 1.Vandiver MS, Snyder SH. J Mol Med. 2012;90:255. doi: 10.1007/s00109-012-0873-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang R. Physiol Rev. 2012;92:791. doi: 10.1152/physrev.00017.2011. [DOI] [PubMed] [Google Scholar]
- 3.Li L, Moore PK. Annu Rev Pharmacol Toxicol. 2011;51:169. doi: 10.1146/annurev-pharmtox-010510-100505. [DOI] [PubMed] [Google Scholar]
- 4.Szabó C. Nat Rev Drug Discovery. 2007;6:917. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- 5.Kolluru GK, Shen X, Bir SC, Kevil CG. Nitric Oxide. 2013;35:5. doi: 10.1016/j.niox.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fukuto JM, Carrington SJ, Tantillo DJ, Harrison JG, Ignarro LJ, Freeman BA, Chen A, Wink DA. Chem Res Toxicol. 2012;25:769. doi: 10.1021/tx2005234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olson KR, Donald JA, Dombkowski RA, Perry SF. Respir Physiol Neurobiol. 2012;184:117. doi: 10.1016/j.resp.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Olson KR. Antioxid Redox Signal. 2012;17:32. doi: 10.1089/ars.2011.4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.King BS. Free Radic Biol Med. 2013;55:1. doi: 10.1016/j.freeradbiomed.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martelli A, Testai L, Breschi MC, Blandizzi C, Virdis A, Taddei S, Calderone V. Med Res Rev. 2012;32:1093. doi: 10.1002/med.20234. [DOI] [PubMed] [Google Scholar]
- 11.Szabo C. Antioxid Redox Signal. 2012;17:68. doi: 10.1089/ars.2011.4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paul BD, Snyder SH. Nat Rev Mol Cell Biol. 2012;13:499. doi: 10.1038/nrm3391. [DOI] [PubMed] [Google Scholar]
- 13.(a) Hu LF, Lu M, Hon Wong PT, Bian JS. Antioxid Redox Signal. 2011;15:405. doi: 10.1089/ars.2010.3517. [DOI] [PubMed] [Google Scholar]; (b) Prabhakar NR. Respir Physiol Neurobiol. 2012;184:165. doi: 10.1016/j.resp.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whiteman M, Le Trionnaire S, Chopra M, Fox B, Whatmore J. Clin Sci (Lond) 2011;121:459. doi: 10.1042/CS20110267. [DOI] [PubMed] [Google Scholar]
- 15.Shibuya N, Tanaka M, Yoshida M, Ogasawara Y, Togawa T, Ishii K, Kimura H. Antioxid Redox Signal. 2009;11:703. doi: 10.1089/ars.2008.2253. [DOI] [PubMed] [Google Scholar]
- 16.Shibuya N, Koike S, Tanaka M, Ishigami-Yuasa M, Kimura Y, Ogasawara Y, Fukui K, Nagahara N, Kimura H. Nat Comm. 2013;4:1366. doi: 10.1038/ncomms2371. [DOI] [PubMed] [Google Scholar]
- 17.Abe K, Kimura H. J Neurosci. 1996;16:1066. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Köhn C, Dubrovska G, Huang Y, Gollasch M. Int J Biomed Sci. 2012;8:81. [PMC free article] [PubMed] [Google Scholar]
- 19.Jackson-Weaver O, Osmond JM, Riddle MA, Naik JS, Gonzalez Bosc LV, Walker BR, Kanagy NL. Am J Physiol Heart Circ Physiol. 2013;304:H1446. doi: 10.1152/ajpheart.00506.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ariyaratnam P, Loubani M, Morice AH. Microvasc Res. 2013;90:135. doi: 10.1016/j.mvr.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Predmore BL, Lefer DJ, Gojon G. Antioxid Redox Signal. 2012;17:119. doi: 10.1089/ars.2012.4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.King AL, Lefer DJ. Exp Physiol. 2011;96:840. doi: 10.1113/expphysiol.2011.059725. [DOI] [PubMed] [Google Scholar]
- 23.Calvert JW, Coetzee WA, Lefer DJ. Antioxid Redox Signal. 2010;12:1203. doi: 10.1089/ars.2009.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kimura Y, Goto Y, Kimura H. Antioxid Redox Signal. 2010;12:1. doi: 10.1089/ars.2008.2282. [DOI] [PubMed] [Google Scholar]
- 25.Kimura Y, Kimura H. FASEB J. 2004;18:1165. doi: 10.1096/fj.04-1815fje. [DOI] [PubMed] [Google Scholar]
- 26.Wen YD, Wang H, Kho SH, Rinkiko S, Sheng X, Shen HM, Zhu YZ. PLoS One. 2013;8:e53147. doi: 10.1371/journal.pone.0053147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Filipovic MR, Miljkovic J, Allgauer A, Chaurio R, Shubina T, Herrmann M, Ivanovic-Burmazovic I. Biochem J. 2012;441:609. doi: 10.1042/BJ20111389. [DOI] [PubMed] [Google Scholar]
- 28.Jones CM, Lawrence A, Wardman P, Burkitt MJ. Free Radic Biol Med. 2002;32:982. doi: 10.1016/s0891-5849(02)00791-8. [DOI] [PubMed] [Google Scholar]
- 29.Carballal S, Trujillo M, Cuevasanta E, Bartesaghi S, Moller MN, Folkes LK, Garcia-Bereguian MA, Gutierrez-Merino C, Wardmann P, Denicola A, Radi R, Alvarez B. Free Radic Biol Med. 2011;50:196. doi: 10.1016/j.freeradbiomed.2010.10.705. [DOI] [PubMed] [Google Scholar]
- 30.Haouzi P, Bell H, Philmon M. Respir Physiol Neurobiol. 2011;177:273. doi: 10.1016/j.resp.2011.04.025. [DOI] [PubMed] [Google Scholar]
- 31.Paulsen CE, Carroll KS. Chem Rev. 2013;113:4633. doi: 10.1021/cr300163e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pan J, Carroll KS. ACS Chem Biol. 2013;8:1110. doi: 10.1021/cb4001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang D, Macinkovic I, Devarie-Baez NO, Pan J, Park CM, Carroll KS, Filipovic MR, Xian M. Angew Chem Int Ed. 2014;53:575. doi: 10.1002/anie.201305876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mustafa AK, Gadalla MM, Sen N, Kim S, Mu W, Gazi SK, Barrow RK, Yang G, Wang R, Snyder SH. Sci Signal. 2009;2:ra72. doi: 10.1126/scisignal.2000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gadalla MM, Snyder SH. J Neurochem. 2010;113:14. doi: 10.1111/j.1471-4159.2010.06580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sen N, Paul BD, Gadalla MM, Mustafa AK, Sen T, Xu R, Kim S, Snyder SH. Mol Cell. 2012;45:13. doi: 10.1016/j.molcel.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krishnan N, Fu C, Pappin DJ, Tonks NK. Sci Signaling. 2011;4:ra86. doi: 10.1126/scisignal.2002329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vandiver MS, Paul BD, Xu R, Karuppagounder S, Rao F, Snowman AM, Ko HS, Lee YI, Dawson VL, Dawson TM, Sen N, Snyder SH. Nat Commun. 2013;4:1626. doi: 10.1038/ncomms2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paul BD, Snyder SH. Nat Rev Mol Cell Biol. 2012;13:499. doi: 10.1038/nrm3391. [DOI] [PubMed] [Google Scholar]
- 40.Yang G, Zhao K, Ju Y, Mani S, Cao Q, Puukila S, Khaper N, Wu L, Wang R. Antioxid Redox Signal. 2013;18:1906. doi: 10.1089/ars.2012.4645. [DOI] [PubMed] [Google Scholar]
- 41.Filipovic MR, Miljkovic JL, Nauser T, Royzen M, Klos K, Shubina T, Koppenol WH, Lippard SJ, Ivanovic-Burmazovic I. J Am Chem Soc. 2012;134:12016. doi: 10.1021/ja3009693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Q, Lancaster JR., Jr Nitric Oxide. 2013;35:21. doi: 10.1016/j.niox.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lindenmann J, Matzi V, Neuboeck N, Ratzenhofer-Komenda B, Maier A, Smolle-Juettner FM. Diving Hyperb Med. 2010;40:213. [PubMed] [Google Scholar]
- 44.Evans CL. J Exp Physiol. 1967;52:231. doi: 10.1113/expphysiol.1967.sp001909. [DOI] [PubMed] [Google Scholar]
- 45.Mark G, Naumov S, von Sonntag C. Ozone Sci Eng. 2011;33:37. [Google Scholar]
- 46.Kabil O, Banerjee R. J Biol Chem. 2010;285:21903. doi: 10.1074/jbc.R110.128363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vorobets VS, Kovach SK, Kolvasov GY. Russian J Appl Chem. 2002;75:229. [Google Scholar]
- 48.Ubuka T. J Chromatogr B. 2002;781:227. doi: 10.1016/s1570-0232(02)00623-2. [DOI] [PubMed] [Google Scholar]
- 49.Doeller JE, Isbell TS, Benavides G, Koenitzer J, Patel H, Patel RP, Lancaster JR., Jr Anal Biochem. 2005;341:40. doi: 10.1016/j.ab.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 50.Nagata T, Kage S, Kimura K, Kudo K, Noda M. J Forensic Sci. 1990;35:706. [PubMed] [Google Scholar]
- 51.Olson KR. Biochim Biophys Acta. 2009;1787:856. doi: 10.1016/j.bbabio.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 52.Lippert AR, New EJ, Chang CJ. J Am Chem Soc. 2011;133:10078. doi: 10.1021/ja203661j. [DOI] [PubMed] [Google Scholar]
- 53.Liu C, Pan J, Li S, Zhao Y, Wu LY, Berkman CE, Whorton AR, Xian M. Angew Chem Int Ed. 2011;50:10327. doi: 10.1002/anie.201104305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qian Y, Karpus J, Kabil O, Zhang SY, Zhu HL, Banerjee R, Zhao J, He C. Nat Comm. 2011;2:495. doi: 10.1038/ncomms1506. [DOI] [PubMed] [Google Scholar]
- 55.Peng H, Cheng Y, Dai C, King AL, Predmore BL, Lefer DJ, Wang B. Angew Chem Int Ed. 2011;50:9672. doi: 10.1002/anie.201104236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sasakura K, Hanaoka K, Shibuya N, Mikami Y, Kimura Y, Komatsu T, Ueno T, Terai T, Kimura H, Nagano T. J Am Chem Soc. 2011;133:18003. doi: 10.1021/ja207851s. [DOI] [PubMed] [Google Scholar]
- 57.Chan J, Dodani SC, Chang CJ. Nat Chem. 2012;4:973. doi: 10.1038/nchem.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lin VS, Chang CJ. Curr Opin Chem Biol. 2012;16:595. doi: 10.1016/j.cbpa.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xuan W, Sheng C, Cao Y, He W, Wang W. Angew Chem Int Ed. 2012;51:2282. doi: 10.1002/anie.201107025. [DOI] [PubMed] [Google Scholar]
- 60.Duan C, Liu Y. Curr Med Chem. 2013;20:2929. doi: 10.2174/09298673113209990005. [DOI] [PubMed] [Google Scholar]
- 61.Kumar N, Bhalla V, Kumar M. Coord Chem Rev. 2013;257:2335. [Google Scholar]
- 62.(a) Sun W, Fan J, Hu C, Cao J, Zhang H, Xiong X, Wang J, Cui S, Sun S, Peng X. Chem Comm. 2013;49:3890. doi: 10.1039/c3cc41244j. [DOI] [PubMed] [Google Scholar]; (b) Zhang J, Sun Y, Liu J, Shi Y, Guo W. Chem Comm. 2013;49:11305. doi: 10.1039/c3cc46932h. [DOI] [PubMed] [Google Scholar]; (c) Liu C, Peng B, Li S, Park CM, Whorton AR, Xian M. Org Lett. 2012;14:2184. doi: 10.1021/ol3008183. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Xu Z, Xu L, Zhou J, Xu Y, Zhu W, Qian X. Chem Comm. 2012;48:10871. doi: 10.1039/c2cc36141h. [DOI] [PubMed] [Google Scholar]; (e) Wan Q, Song Y, Li Z, Gao X, Ma H. Chem Comm. 2013;49:502. doi: 10.1039/c2cc37725j. [DOI] [PubMed] [Google Scholar]; (f) Wang B, Li P, Yu F, Chen J, Qu Z, Han K. Chem Comm. 2013;49:5790. doi: 10.1039/c3cc42313a. [DOI] [PubMed] [Google Scholar]; (g) Peng B, Chen W, Liu C, Rosser EW, Pacheco A, Zhao Y, Aguilar HC, Xian M. Chem Eur J. 2014;20:1010. doi: 10.1002/chem.201303757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martelli A, Testai L, Marino A, Breschi MC, Da Settimo F, Calderone V. Curr Med Chem. 2012;19:3325. doi: 10.2174/092986712801215928. [DOI] [PubMed] [Google Scholar]
- 64.Kashfi K, Olson KR. Biochem Pharmacol. 2013;85:689. doi: 10.1016/j.bcp.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li L, Bhatia M, Zhu YZ, Zhu YC, Ramnath RD, Wang ZJ, Anuar FBM, Whiteman M, Salto-Tellez M, Moore PK. Faseb J. 2005;19:1196. doi: 10.1096/fj.04-3583fje. [DOI] [PubMed] [Google Scholar]
- 66.Tamizhselvi R, Moore PK, Bhatia M. Pancreas. 2008;36:E24. doi: 10.1097/MPA.0b013e31816857bb. [DOI] [PubMed] [Google Scholar]
- 67.Xue R, Hao D, Sun J, Li W, Zhao M, Li X, Chen Y, Zhu J, Ding Y, Liu J, Zhu Y. Antioxid Redox Signal. 2013;19:5. doi: 10.1089/ars.2012.5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Blackstone E, Morrison M, Roth MB. Science. 2005;308:518. doi: 10.1126/science.1108581. [DOI] [PubMed] [Google Scholar]
- 69.Collman JP, Ghosh S, Dey A, Decréau RA. Proc Natl Acad Sci USA. 2009;106:22090. doi: 10.1073/pnas.0904082106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Blackstone E, Roth MB. Shock. 2007;27:370. doi: 10.1097/SHK.0b013e31802e27a0. [DOI] [PubMed] [Google Scholar]
- 71.Li J, Zhang G, Cai S. Pediatr Crit Care Med. 2008;9:110. doi: 10.1097/01.PCC.0000298639.08519.0C. [DOI] [PubMed] [Google Scholar]
- 72.Haouzi P, Notet V, Chenuel B, Chalon B, Sponne I, Ogier V, Bihain B. Respir Physiol Neurobiol. 2008;160:109. doi: 10.1016/j.resp.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 73.Wagner K, Georgieff M, Asfar P, Calzia E, Knoferl MW, Radermacher P. Crit Care. 2011;15:146. doi: 10.1186/cc10079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Derwall M, Francis RCE, Kida K, Bougaki M, Crimi E, Adrie C, Zapol WM, Ichinose F. Crit Care. 2011;15:R51. doi: 10.1186/cc10016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zanardo RC, Brancaleone V, Distrutti E, Fiorucci S, Cirino G, Wallace JL. FASEB J. 2006;20:2118. doi: 10.1096/fj.06-6270fje. [DOI] [PubMed] [Google Scholar]
- 76.Zhang Q, Fu H, Zhang H, Xu F, Zou Z, Liu M, Wang Q, Miao M, Shi X. PLoS One. 2013;8:e74422. doi: 10.1371/journal.pone.0074422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhao WM, Zhang J, Lu YJ, Wang R. Embo J. 2001;20:6008. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu L, Liu H, Sun D, Qiao W, Qi Y, Sun H, Yan C. Circ J. 2012;76:1012. doi: 10.1253/circj.cj-11-0890. [DOI] [PubMed] [Google Scholar]
- 79.Calvert JW, Elston M, Nicholson CK, Gundewar S, Jha S, Elrod JW, Ramachandran A, Lefer DJ. Circulation. 2010;122:11. doi: 10.1161/CIRCULATIONAHA.109.920991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Calvert JW, Jha S, Gundewar S, Elrod JW, Ramachandran A, Pattillo CB, Kevil CG, Lefer DJ. Circ Res. 2009;105:365. doi: 10.1161/CIRCRESAHA.109.199919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sodha NR, Clements RT, Feng J, Liu Y, Bianchi C, Horvath EM, Szabo C, Sellke FW. Eur J Cardiothorac Surg. 2008;33:906. doi: 10.1016/j.ejcts.2008.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.(a) Esechie A, Kiss L, Olah G, Horvath EM, Hawkins H, Szabo C, Traber DL. Clin Sci. 2008;115:91. doi: 10.1042/CS20080021. [DOI] [PubMed] [Google Scholar]; (b) Kloesch B, Liszt M, Steiner G, Bröll J. Rheumatol Int. 2012;32:729. doi: 10.1007/s00296-010-1682-0. [DOI] [PubMed] [Google Scholar]
- 83.DeLeon ER, Stoy GF, Olson KR. Anal Biochem. 2012;421:203. doi: 10.1016/j.ab.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 84.Greiner R, Palinkas Z, Basell K, Becher D, Antelmann H, Nagy P, Dick TP. Antioxid Redox Signal. 2013;19:1749. doi: 10.1089/ars.2012.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Amagase H. J Nutr. 2006;136:716S. doi: 10.1093/jn/136.3.716S. [DOI] [PubMed] [Google Scholar]
- 86.Benavides GA, Squadrito GL, Mills RW, Patel HD, Isbell TS, Patel RP, Darley-Usmar VM, Doeller JE, Kraus DW. Proc Natl Acad Sci USA. 2007;104:17977. doi: 10.1073/pnas.0705710104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tangerman A. J Chromato B. 2009;877:3366. doi: 10.1016/j.jchromb.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 88.Gruhlke MCH, Slusarenko AJ. Plant Physiol Biochem. 2012;59:98. doi: 10.1016/j.plaphy.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 89.Toohey JI. Biochem J. 1989;264:625. doi: 10.1042/bj2640625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Toohey JI. Anal Biochem. 2011;413:1. doi: 10.1016/j.ab.2011.01.044. [DOI] [PubMed] [Google Scholar]
- 91.Francoleon NE, Carrington SJ, Fukuto JM. Arch Biochem Biophys. 2011;516:146. doi: 10.1016/j.abb.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 92.Kimura Y, Mikami Y, Osumi K, Tsugane M, Oka JI, Kimura H. FASEB J. 2013;27:2451. doi: 10.1096/fj.12-226415. [DOI] [PubMed] [Google Scholar]
- 93.Jacob C, Anwar A, Burkholz T. Plant Med. 2008;74:1580. doi: 10.1055/s-0028-1088299. [DOI] [PubMed] [Google Scholar]
- 94.Ozturk T, Ertas E, Mert O. Chem Rev. 2007;107:5210. doi: 10.1021/cr040650b. [DOI] [PubMed] [Google Scholar]
- 95.Lecher HZ, Greenwood RA, Whitehouse KC, Chao TH. J Am Chem Soc. 1956;78:5018. [Google Scholar]
- 96.Thomsen I, Clausen K, Scheibye S, Lawesson SO. Org Synth. 1984;62:158. [Google Scholar]
- 97.Spiller F, Orrico MI, Nascimento DC, Czaikoski PG, Souto FO, Alves-Filho JC, Freitas A, Carlos D, Mentenegro MF, Neto AF, Ferreira SH, Rossi MA, Hothersall JS, Assreuy J, Cunha FQ. Am J Respir Crit Care Med. 2010;182:360. doi: 10.1164/rccm.200907-1145OC. [DOI] [PubMed] [Google Scholar]
- 98.Wallace JL, Vong L, Mcknight W, Dicay M, Martin GR. Gastroenterology. 2009;137:569. doi: 10.1053/j.gastro.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 99.Li L, Whiteman M, Guan YY, Neo KL, Cheng Y, Lee SW, Zhao Y, Baskar R, Tan CH, Moore PK. Circulation. 2008;117:2351. doi: 10.1161/CIRCULATIONAHA.107.753467. [DOI] [PubMed] [Google Scholar]
- 100.Baskar R, Li L, Moore PK. FASEB J. 2007;21:247. doi: 10.1096/fj.06-6255com. [DOI] [PubMed] [Google Scholar]
- 101.Yang G, Sun X, Wang R. FASEB J. 2004;18:1782. doi: 10.1096/fj.04-2279fje. [DOI] [PubMed] [Google Scholar]
- 102.Campolo M, Esposito E, Ahmad A, Paola RD, Wallace JL, Cuzzocrea S. FASEB J. 2013;27:4489. doi: 10.1096/fj.13-234716. [DOI] [PubMed] [Google Scholar]
- 103.Wallace JL. Trends Pharmacol Sci. 2007;28:501. doi: 10.1016/j.tips.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 104.Wallace JL, Caliendo G, Santagada V, Cirino G, Fiorucci S. Gastroenterology. 2007;132:261. doi: 10.1053/j.gastro.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 105.Whiteman M, Li L, Rose P, Tan CH, Parkinson DB, Moore PK. Antioxid Redox Signal. 2010;12:1147. doi: 10.1089/ars.2009.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lee ZW, Zhou J, Chen CS, Zhao Y, Tan CH, Li L, Moore PK, Deng LW. PLoS One. 2011;6:e21077. doi: 10.1371/journal.pone.0021077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Park C, Zhao Y, Zhu Z, Pacheco A, Peng B, Devarie-Baez NO, Bagdon P, Zhang H, Xian M. Mol Biosyst. 2013;9:2430. doi: 10.1039/c3mb70145j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Szabo G, Veres G, Radovits T, Gero D, Modis K, Miesel-Groschel C, Horkay F, Karck M, Szabo C. Nitric Oxide. 2011;25:201. doi: 10.1016/j.niox.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Marshall WS, Caruthers MH. Science. 1993;259:1564. doi: 10.1126/science.7681216. [DOI] [PubMed] [Google Scholar]
- 110.Nielsen J, Brill WKD, Caruthers MH. Tetrahedron Lett. 1988;29:2911. [Google Scholar]
- 111.Marshall WS, Beaton G, Stein CA, Matsukura M, Caruthers MH. Proc Natl Acad Sci USA. 1992;89:6265. doi: 10.1073/pnas.89.14.6265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Qandil AM. Int J Mol Sci. 2012;13:17244. doi: 10.3390/ijms131217244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zanatta SD, Jarrott B, Williams SJ. Aust J Chem. 2010;63:946. [Google Scholar]
- 114.Caliendo G, Cirino G, Santagada V, Wallace JL. J Med Chem. 2010;53:6275. doi: 10.1021/jm901638j. [DOI] [PubMed] [Google Scholar]
- 115.Landis PS. Chem Rev. 1965;65:237. [Google Scholar]
- 116.Landis PS, Hamilton LA. J Org Chem. 1960;25:1742. [Google Scholar]
- 117.Spindt RS, Stevens DR, Baldwin WE. J Am Chem Soc. 1951;73:3693. [Google Scholar]
- 118.Curphey TJ, Joyner HH. Tetrahedron Lett. 1993;34:7231. [Google Scholar]
- 119.Curphey TJ, Libby AA. Tetrahedron Lett. 2000;41:6977. [Google Scholar]
- 120.Pedersen BS, Lawesson SO. Tetrahedron. 1979;35:2433. [Google Scholar]
- 121.Schnitzer TJ, Burmester GR, Mysler E, Hochberg MC, Doherty M, Ehrsam E, Gitton X, Krammer G, Mellein B, Matchaba P, Gimona A, Hawkey CJ. Lancet. 2004;364:665. doi: 10.1016/S0140-6736(04)16893-1. [DOI] [PubMed] [Google Scholar]
- 122.Sigh G, Fort JG, Goldstein JL, Levy RA, Hanrahan PS, Bello AE, Andrade-Ortega L, Wallemark C, Agrawal NM, Eisen GM, Stenson WF, Triadafilopoulos G. Am J Med. 2006;119:255. doi: 10.1016/j.amjmed.2005.09.054. [DOI] [PubMed] [Google Scholar]
- 123.Kurahara K, Matsumoto T, Iida M, Honda K, Yao T, Fujishima M. Am J Gastroenterol. 2001;96:473. doi: 10.1111/j.1572-0241.2001.03530.x. [DOI] [PubMed] [Google Scholar]
- 124.Gargallo CJ, Lanas A. J Dig Dis. 2013;14:55. doi: 10.1111/1751-2980.12019. [DOI] [PubMed] [Google Scholar]
- 125.Sparetore A, Santus G, Giustarini D, Rossi R, Del Soldato P. Expert Rev Clin Pharmacol. 2011;4:109. doi: 10.1586/ecp.10.122. [DOI] [PubMed] [Google Scholar]
- 126.Chan MV, Wallace JL. Am J Physiol. 2013;305:G467. doi: 10.1152/ajpgi.00169.2013. [DOI] [PubMed] [Google Scholar]
- 127.Li L, Rossoni G, Sparatore A, Lee LC, Del Soldato P, Moore PK. Free Radic Biol Med. 2007;42:706. doi: 10.1016/j.freeradbiomed.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 128.Zhang Y, Munday R. Mol Cancer Ther. 2008;7:3470. doi: 10.1158/1535-7163.MCT-08-0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chattopadhyay M, Kodela R, Nath N, Dastagirzada YM, Velazquez-Martinez CA, Boring D, Kashfi K. Biochem Pharmacol. 2012;83:715. doi: 10.1016/j.bcp.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 130.Xie L, Hu L, Teo XQ, Tiong CX, Tazzari V, Sparatore A, Del Soldato P, Dawe GS, Bian JS. PLoS One. 2013;8:e60200. doi: 10.1371/journal.pone.0060200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Distrutti E, Sediari L, Mencarelli A, Renga B, Orlandi S, Russo G, Caliendo G, Santagada V, Cirino G, Wallace JL, Fiorucci S. J Pharmacol Exp Ther. 2006;319:447. doi: 10.1124/jpet.106.106435. [DOI] [PubMed] [Google Scholar]
- 132.Zhao Y, Wang H, Xian M. J Am Chem Soc. 2011;133:15. doi: 10.1021/ja1085723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhao Y, Bhushan S, Yang C, Otsuka H, Stein JD, Pacheco A, Peng B, Devarie-Baez NO, Aguilar HC, Lefer DJ, Xian M. ACS Chem Biol. 2013;8:1283. doi: 10.1021/cb400090d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kimura H, Shibuya N, Kimura Y. Antioxid Redox Signal. 2012;17:45. doi: 10.1089/ars.2011.4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dongo E, Hornyak I, Benko Z, Kiss L. Acta Physiol Hung. 2011;98:369. doi: 10.1556/APhysiol.98.2011.4.1. [DOI] [PubMed] [Google Scholar]
- 136.Peake BF, Nicholson CK, Lambert JP, Hood RL, Amin H, Amin S, Calvert JW. Am J Physiol. 2013;304:H1215. doi: 10.1152/ajpheart.00796.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Roger T, Raynaud F, Bouillaud F, Ransy C, Simonet S, Crespo C, Bourguignon MP, Villeneuve N, Vilaine JP, Artaud I, Galardon E. ChemBioChem. 2013;14:2268. doi: 10.1002/cbic.201300552. [DOI] [PubMed] [Google Scholar]
- 138.Frank RL, Blegen JR. Org Synth. 1948;28:16. [Google Scholar]
- 139.Heimer NE, Field L, Neal RA. J Org Chem. 1981;46:1374. [Google Scholar]
- 140.Martelli A, Testai L, Citi V, Marino A, Pugliesi I, Barresi E, Nesi G, Rapposelli S, Taliani S, Da Settimo F, Breschi MC, Calderone V. ACS Med Chem Lett. 2013;4:904. doi: 10.1021/ml400239a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Devarie-Baez NO, Bagdon PW, Peng B, Zhao Y, Park CM, Xian M. Org Lett. 2013;15:2786. doi: 10.1021/ol401118k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cairns TL, Evans GL, Larchar AW, Mckusick BC. J Am Chem Soc. 1952;74:3982. [Google Scholar]
- 143.Berchtold GA, Edwards BE, Campaigne E, Carmack M. J Am Chem Soc. 1959;81:3148. [Google Scholar]
- 144.Voronkov M, Shagun L, Ermolyuk L, Timokhina L. J Sulfur Chem. 2004;25:131. [Google Scholar]
- 145.Kotzur N, Briand B, Beyermann M, Hagen V. J Am Chem Soc. 2009;131:16927. doi: 10.1021/ja907287n. [DOI] [PubMed] [Google Scholar]
- 146.Smith AB, Savinov SN, Manjappara UV, Chaiken IM. Org Lett. 2002;4:4041. doi: 10.1021/ol026736d. [DOI] [PubMed] [Google Scholar]
- 147.Clarke KM, La Clair JJ, Burkart MD. J Org Chem. 2005;70:3709. doi: 10.1021/jo0481396. [DOI] [PubMed] [Google Scholar]
- 148.Lukeman M, Scaiano JC. J Am Chem Soc. 2005;127:7698. doi: 10.1021/ja0517062. [DOI] [PubMed] [Google Scholar]
- 149.Fukushima N, Ieda N, Sasakura K, Nagano T, Hanaoka K, Suzuki T, Miyata N, Nakagawa H. Chem Comm. 2014;50:587. doi: 10.1039/c3cc47421f. [DOI] [PubMed] [Google Scholar]
- 150.Zhou Z, von Wantoch Rekowski M, Coletta C, Szabo C, Bucci M, Cirino G, Topouzis S, Papapetropoulos A, Giannis A. Bioorg Med Chem. 2012;20:2675. doi: 10.1016/j.bmc.2012.02.028. [DOI] [PubMed] [Google Scholar]
- 151.Pan J, Devarie-Baez NO, Xian M. Org Lett. 2011;13:1092. doi: 10.1021/ol1031393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bao Y, Li X, Danishefshy SJ. J Am Chem Soc. 2009;131:12924. doi: 10.1021/ja906005j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang P, Danishefshy SJ. J Am Chem Soc. 2010;132:17045. doi: 10.1021/ja1084628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Liu R, Orgel LE. Nature. 1997;389:52. doi: 10.1038/37944. [DOI] [PubMed] [Google Scholar]
- 155.Dawson PE, Muir TW, Clark-Lewis I, Kent SBH. Science. 1994;266:776. doi: 10.1126/science.7973629. [DOI] [PubMed] [Google Scholar]
- 156.Crich D, Sharma I. Angew Chem Int Ed. 2009;48:2355. doi: 10.1002/anie.200805782. [DOI] [PubMed] [Google Scholar]