Abstract
Angiotensin II increases and decreases arterial pressure by acting at angiotensin type 1 and type 2 receptors respectively. Renovascular hypertensive rats exhibit a high level of activity of the peripheral and central renin-angiotensin system. Therefore, in the present study we evaluated the effect of increasing the expression of angiotensin type 2 receptors in the solitary-vagal complex [nucleus of the solitary tract/dorsal motor nucleus of the vagus], a key brainstem region for cardiovascular regulation, on the development of renovascular hypertension. Holtzman normotensive rats were implanted with a silver clip around the left renal artery to induce 2 kidney-1 clip renovascular hypertension. Three weeks later, rats were microinjected in the solitary-vagal complex with either an adeno-associated virus to increase the expression of angiotensin type 2 receptors, or with a control vector. We observed that increasing angiotensin type 2 receptor expression in the solitary-vagal complex attenuated the development of renovascular hypertension and also reversed the impairment of the baroreflex and the increase in the low frequency component of systolic blood pressure observed in renovascular hypertensive rats. Further, an observed decrease in mRNA levels of angiotensin converting enzyme 2 in the solitary-vagal complex of renovascular hypertensive rats was restored to control levels following viral-mediated increases in angiotensin type 2 receptors at this site. Collectively, these data demonstrate specific and beneficial effects of angiotensin type 2 receptors via the brain of hypertensive rats, and suggest that central angiotensin type 2 receptors may be a potential target for therapeutics in renovascular hypertension.
Keywords: 2K1C, ACE2, baroreflex, angiotensin II, spectral analysis, angiotensin receptor
Introduction
Renovascular hypertension, elicited by ≥ 50% renal artery stenosis or occlusion, affects approximately 6–8% of the hypertensive population, especially the eldery.1 Although an increase in renin-angiotensin system (RAS) activity plays a pivotal role in the raised blood pressure observed in the early phase of renovascular hypertension, patients with renal vascular hypertension also exhibit sympathoexcitation, which contributes to the pathogenesis of renovascular hypertension.2,3 Animal models of renovascular hypertension were demonstrated ~80 years ago using the Goldblatt two kidney 1 clip (2K1C).4 These animals also display impaired baroreflexes 5–7, an increase in sympathetic nerve activity (SNA),7,8 and an overactivity of the RAS.9,10 Considering the impairment of the baroreflex and overactivity of the sympathetic nervous system seen in renovascular hypertension, the role of the central nervous system (CNS) in the development and maintenance of renovascular hypertension has also been demonstrated.8,11
There is great interest in understanding the mechanisms underlying the changes in SNA and baroreflexes, and for developing therapeutic strategies that will lower blood pressure in hypertensive individuals and animal models of neurogenic hypertension. A major contributor to the changes SNA and baroreflexes is the peptide angiotensin II (ANG II) acting via its neuronal type 1 receptors (AT1R) in cardiovascular control centers in the brainstem and forebrain.12,13 Specifically, in the brainstem ANG II acting at AT1R in the nucleus of the solitary tract (NTS) blunts the baroreflex.12,14 Because ANG II injection into the NTS also spread in the dorsal motor nucleus of vagus (DMV), the effects of ANG II in blunting baroreflexes may also involve the DMV.14 It is also important to point out that, in addition to being the first synaptic relay station in CNS for baroreflex regulation, the NTS plays a part in mediating the high blood pressure and sympathoexcitation seen in spontaneously hypertensive rats (SHR).15,16 Thus a potential imbalance between ANG II and/or constituents of the RAS in the solitary-vagal complex may be involved in the development and maintenance of hypertension, including in the 2K1C Goldblatt model. In fact, we recently demonstrated that NTS over-expression of MIF, a protein that inhibits the intracellular actions of the AT1R, decreases arterial pressure in the SHR.16 Thus, mechanisms that appear to oppose the actions of ANG II via AT1R may lead to the development of new anti-hypertensive therapies for people with uncontrolled high blood pressure.
A potential opposing or protective mechanism is the action of ANG II via its type 2 receptors (AT2R). There is evidence that activation of AT2R can oppose the actions of ANG II via AT1R in the cardiovascular system [reviewed by 17]. AT2R knockout mice exhibit elevated basal blood pressure and a greater sensitivity to the vasopressor effect of ANG II.18,19 In addition, co-administration of an AT2R agonist (compound 21) with the AT1R antagonist candesartan induced a greater fall in arterial pressure in SHR compared with candesartan alone in these animals. 20 Besides these peripheral effects, in vitro studies using co-cultures of hypothalamus and brainstem cells demonstrated that AT2R activation stimulates the delayed rectifier K+ current, decreasing the excitability of the cells, whereas AT1R activation exerts an opposite effect.21
Thus, considering that AT2R and AT1R activation exert opposite effects, and the importance of solitary/vagal complex to the regulation of arterial pressure and baroreflex,22 we investigated whether long-term increased expression of AT2R in the solitary vagal complex (NTS/DMV) of 2K1C rats would affect the development/maintenance of renovascular hypertension. Further, we investigated alterations in the expression of various RAS genes (AT1R, AT2R, and angiotensin converting enzyme 2 [ACE2]) at the solitary-vagal complex as a potential mechanism for effects of increased AT2R expression on blood pressure. We were particularly interested in ACE2, as it is known that the ACE2/angiotensin-(1–7)/Mas receptor axis is a cardioprotective arm of the RAS, 23 and chronic activation of AT2R in peripheral tissues activates ACE2.24 In addition, overexpression of the human form of this enzyme in the paraventricular nucleus of hypothalamus (PVN) impaired the development of ANG II-induced hypertension,25 and the increase in SNA observed in mice with chronic heart failure (CHF) was reduced in transgenic mice with overexpression of human ACE2 in the NTS and rostral ventrolateral medulla (RVLM).26
Methods
Detailed descriptions of the animal subjects and all experimental methods are included in the online-only Data Supplement. Given below are the Experimental Protocols used.
Experiment 1
Two months after AAV2-CBA-AT2R or AAV2-CBA-eGFP were injected into the NTS/DMV of naïve male Sprague-Dawley rats (n =3), animals were deeply anesthetized, perfused transcardially and the brainstem was processed for AT2R autoradiography as described in the online-only Data Supplement.
Experiment 2
Male Holtzman rats (n = 5–6/group) underwent 2K1C surgery and implantation of telemetry transducers, and 21 days later animals received microinjections of either AAV2-CBA-eGFP or AAV2-CBA-AT2R into the NTS/DMV. MAP and heart rate (HR) were recorded continuously, for a 24-h period, 18 and 3 days before vector injection, and 7, 14, 21, 26 and 31 days post-vector injection. Rats were euthanized at 31 days post vector injection, brainstems removed, cut and mounted on slides to check the eGFP fluorescence in the NTS/DMV and indicate NTS/DMV injection site.
Experiment 3
Male Holtzman rats (n = 8–9/group) underwent sham or 2K1C surgery. This was followed, 21–24 days later, by microinjections of either AAV2-CBA-eGFP or AAV2-CBA-AT2R into the NTS/DMV. In 2K1C rats, the systolic arterial pressure (SAP) was monitored for 3 weeks by tail cuff starting 1 week after the surgery. Only animals with an increase in SAP after 3 weeks of the 2K1C surgery underwent virus injection in the NTS/DMV. We will refer to Holtzman rats with sham 2K1C surgery as normotensive (NT) rats. Four weeks after the viral transduction, rats were implanted with two catheters: one into the abdominal aorta through the femoral artery for direct recordings of MAP and HR, and another into the femoral vein for drug administration. The next day, MAP and HR were recorded in conscious rats, followed by the analysis of baroreflex function as described in the Methods (online-only Data Supplement). The day following these tests, rats were deeply anesthetized, decapitated, the brains were collected for qRT-PCR analyses of AT1R, AT2R and ACE2 mRNAs in the NTS/DMV, and the kidneys were weighed.
Results
Viral-mediated expression of GFP and AT2R in the NTS/DMV
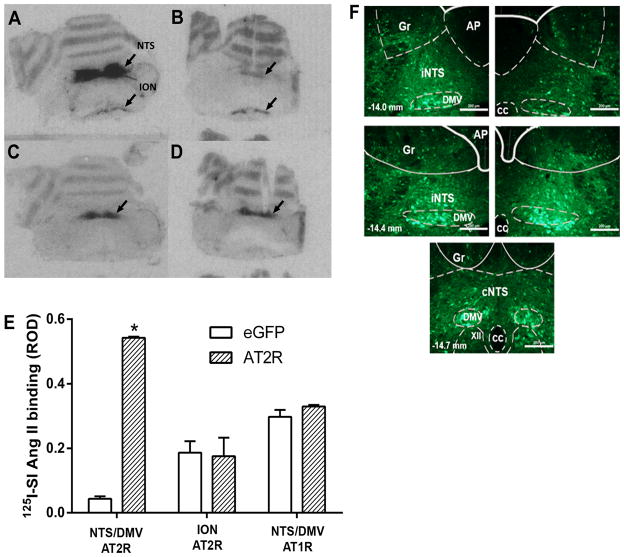
AT2R autoradiography was performed as described in Experiment 1 to verify the effectiveness of AAV2-CBA-AT2R in inducing increased AT2R expression in the NTS/DMV. As shown in the representative autoradiograms in Figure 1A–D there is intense AT2R specific binding in the NTS/DMV of an AAV2-CBA-AT2R injected rat (Figure 1A) when compared with the NTS/DMV of an AAV2-CBA-eGFP injected rat (Figure 1B). Figures 1C and 1D show similar levels of AT1R specific binding in the NTS/DMV of AAV2-CBA-AT2R and AAV2-CBA-eGFP injected rats respectively. Quantification of the autoradiographic data demonstrated that in the eGFP transduced rats, AT2R levels are lower in the NTS/DMV compared to the inferior olivary nucleus, but significantly increase only in the NTS/DMV after AT2R transduction [F(2,6) = 46.89; p < 0.01] (Figure 1E). AT2R transduction in the NTS/DMV did not change AT1R levels at this site (Figure 1E).The representative fluorescence micrographs presented in Figure 1F show eGFP expression at three rostro-caudal levels of the solitary-vagal complex, from bregma, 31 days after microinjection of AAV2-CBA-eGFP as described in Experiments 2 and 3. qRT-PCR analyses, after microinjections of either AAV2-CBA-eGFP or AAV2-CBA-AT2R into the NTS/DMV as described in Experiment 3, showed that the AT2R vector was effective in increasing AT2R levels in the NTS/DMV (Figure 4A).
Figure 1. Viral-mediated transduction of GFP and AT2R into the brainstem.
(A–D) Receptor autoradiography of rat brainstem using 125I-SI-Angiotensin II following microinjection of AAV2-CBA-eGFP or AAV2-CBA-AT2R as described in the Methods. (A) Binding to the AT2R in a representative AT2R-transduced brain; (B) Binding to the AT2R in a representative eGFP-transduced (control) brain; (C) Binding to the AT1R in a representative AT2R-transduced brain; (D) Binding to the AT1R in a representative eGFP-transduced (control) brain. NTS, nucleus of the tractus solitarius; DMV, dorsal motor nucleus of the vagus; ION, inferior olivary nucleus; (E) Quantification of AT2R density in the NTS/DMV and ION and AT1R density in the NTS/DMV of AT2R or eGFP transduced rats. ROD, relative optical density. Data are means + SEM; n = 3/group; *p < 0.05 vs. corresponding eGFP group; (F) Representative fluorescence micrographs taken from coronal sections of the NTS/DMV (−14.0 to −14.7 mm from bregma) from rats that had received microinjections of AAV2-CBA-eGFP as described in the Methods. iNTSi: intermediate nucleus of the solitary tract; cNTS: commissural nucleus of the solitary tract; AP: area postrema, DMV: dorsal motor nucleus of vagus; XII: hypoglossal nucleus; Gr: gracile nucleus; cc: central canal. Scale bar: 200 μm.
Figure 4.
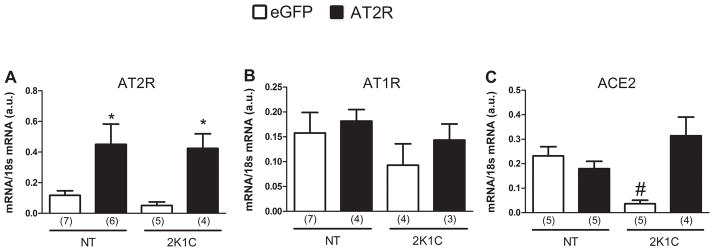
AT2R, AT1R and ACE2 mRNA levels in the NTS of 2K1C and normotensive rats. Following euthanization of the rats used in the Experiment in Figure 3, brains were removed, and mRNA levels of ANG II receptors and ACE2 in the NTS/DMV were assessed by real-time RT-PCR. The results are presented as means ± SEM; n is indicated in parentheses; *p < 0.05 vs. eGFP; # p < 0.05 vs. all groups.
Increasing AT2R expression in the NTS/DMV attenuates the development of 2K1C hypertension
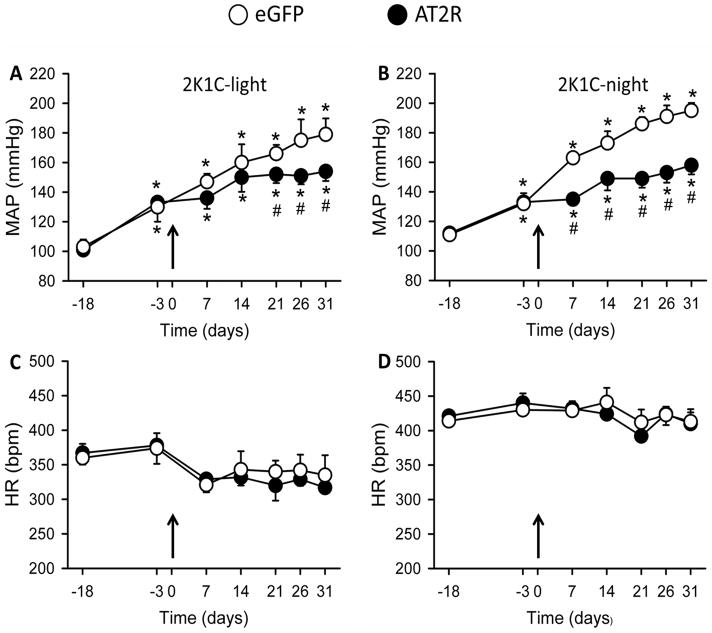
The effects of increasing AT2R expression in the NTS was studied as described in Experiment 2 in the Methods. Baseline levels of MAP (18 days before vector injections) were similar in rats assigned to the AT2R or eGFP groups during the light (AT2R: 101 ± 2 and eGFP: 103 ± 5 mmHg) and dark periods (AT2R: 112 ± 4 and eGFP: 111 ± 5 mmHg). At three days before vector injections, (21 days after 2K1C surgery), rats assigned to the AT2R and eGFP groups exhibited an increase in their MAP compared to baseline levels in the light period [AT2R: 134 ± 3 and eGFP: 130 ± 9 mmHg, vs. respective baselines; F(1,63) = 7.912; p < 0.05] and in the dark period [AT2R: 133 ± 3.7 and eGFP: 132 ± 7 mmHg, vs. respective baselines; F(1,63) = 52.969; p < 0.001], (Figure 2A and 2B). In 2K1C-AT2R rats the development of hypertension was potently attenuated compared with the 2K1C-eGFP animals during both light (2K1C-AT2R: 154 ± 7, vs. 2K1C-eGFP: 179 ± 11 mmHg; at day 31 p < 0.05) and dark periods (2K1C-AT2R: 158 ± 6 vs. 2K1C-eGFP: 195 ± 5 mmHg at day 31; p < 0.05), Figures 2A and 2B. There were no significant differences in HR between the treatment groups either in the light or dark periods in 2K1C rats [F(1,63) = 0.522; p = 0.473 and F(1,63) = 0.0793; p = 0.779, respectively], (Figures 2C and 2D).
Figure 2. Increased AT2R expression in the NTS/DMV decreases the development of hypertension in renovascular hypertensive rats.
Shown here are MAP (mmHg) values from two-kidney one clip (2K1C) rats recorded on the days indicated before and after vector injections (AAV2-CBA-eGFP, open symbols; AAV2-CBA-AT2R, filled symbols) into the NTS/DMV. MAP recordings were made during the light (A and C) and dark periods (B and D), and vector injections were made on Day 0 (indicated by the arrows). The results are presented as means ± SEM; n = 5 and 6 rats for the eGFP and AT2R groups, respectively. *p < 0.05 vs. baseline (Day -18); # p < 0.05 vs. eGFP.
Baroreflex impairment and increased low frequency (LF) of systolic blood pressure (SBP) in 2K1C rats are restored after increasing AT2R expression in the NTS/DMV
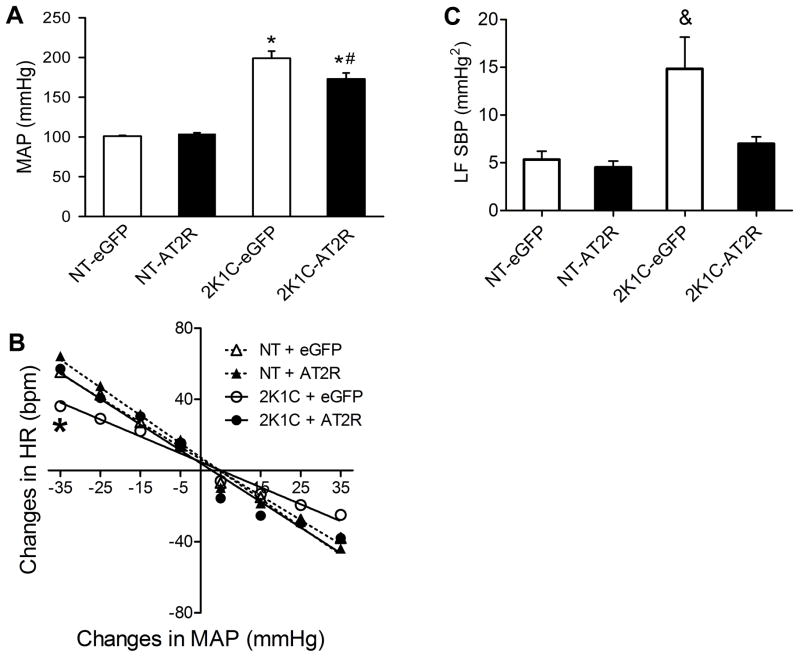
Baseline MAP levels of the animals that underwent baroreflex tests are shown in Figure 3A. The data indicate that the high levels of baseline MAP that occur in the 2K1C-eGFP group, were significantly reduced in the 2K1C-AT2R (Figure 3A), [F(1,31) = 64.841;. p < 0.001]. We also observed that the ratio of the left kidney to the right kidney weight in 2K1C rats was decreased compared to that observed in NT rats, as demonstrated previously,27 and there were no differences between the eGFP- and AT2R treated groups (Table 1). In contrast to the 2K1C animals, there was no change in MAP after AT2R vector injection into the NTS/DMV in NT rats (Figure 3A).
Figure 3. Increased AT2R expression in the NTS/DMV restores baroreflex function and decreases the higher LF of SBP in 2K1C rats.
Normotensive (NT) or 2K1C rats received intra-NTS injections of AAV2-CBA-eGFP or AAV2-CBA-AT2R followed by measurement of MAP, analysis of the SBP variability and baroreflex function as detailed in the Methods. (A) Increased AT2R expression in the NTS/DMV decreases mean arterial pressure in 2K1C rats but not in NT rats. (B) Baroreflex function curves from 2K1C and NT rats. Grouped HR baroreflex slopes in response to each 10 mmHg change in MAP produced by phenylephrine (pressor) or SNP (depressor) as described in the Methods. (C) Power of SBP spectra at the low frequency band (LF). Results in panels A and C are presented as mean ± SEM; NT-eGFP, n = 8; NT-AT2R, n = 9; 2K1C-eGFP, n = 9; 2K1C-AT2R, n = 9. *p< 0.05 vs. respective treatment in NT rats; #p<0.05 vs. 2K1C-eGFP; &p<0.05 vs. all groups.
Table 1.
Left kidney/right kidney ratio (LK/RK) in two-kidney 1-clip (2K1C) rats and normotensive (NT) rats thirty one days after vector transduction with eGFP or AT2R
| Rat | treatment | n | LK/RK |
|---|---|---|---|
| NT | AAV-CBA-eGFP | 9 | 0.97 ± 0.02 |
| NT | AAV-CBA-AT2R | 9 | 0.98 ± 0.01 |
| 2K1C | AAV-CBA-eGFP | 6 | 0.61 ± 0.04* |
| 2K1C | AAV-CBA-AT2R | 9 | 0.67 ± 0.04* |
different from NT; One-way ANOVA, followed by Student-Newman-Keuls, p < 0.05;
n = number of animals
Baroreflexes were assessed using a dose of phenylephrine that elicits a pressor response and a dose of sodium nitroprusside that causes a depressor effect as described in Experiment 3 in the Methods (online-only Data Supplement). The 2K1C-eGFP rats displayed an impairment of baroreflex function [2K1C-eGFP slope: −0.95 ± 0.05, vs. NT-eGFP slope: −1.37 ± 0.06 bpm/mmHg; F(3,31) = 6.175; p<0,05], Figure 3B. Importantly, in 2K1C-AT2R rats the baroreflex function was restored (slope: −1.45 ± 0.07 bmp/mmHg) to the levels observed in normotensive rats (NT-AT2R slope: −1.56 ± 0.07 and NT-eGFP slope: −1.37 ± 0.06 bpm/mmHg), Figure 3B.
Using spectral analysis of SBP, we observed that the LF of SBP, an index of vasomotor sympathetic activity, was significantly higher in the 2K1C-eGFP transduced rats (14.8 ± 3.3 mmHg2), but was reduced in 2K1C-AT2R rats (7.0 ± 0.7 mmHg2), returning to values comparable to those seen in normotensive animals (NT- eGFP: 5.4 ± 0.9 and NT- AT2R: 4.5 ± 0.6), F[(3,26) = 6.613; p < 0.01], Figure 3C. LF and HF of pulse interval (PI) were not different amongst the groups (Figure S1, Supplemental Data), although there was a tendency for LF of PI to increase and for HF of PI to decrease in the 2K1C-eGFP group. Indeed, when the LF/HF ratio is calculated, a higher ratio was found in 2K1C-eGFP (0.23 ± 0.024, vs. other groups; p < 0.05), probably due to a combination of higher LF and decreased HF of PI in these animals Figure S1 (Supplemental Data)
Reduced ACE 2 mRNA levels in the NTS/DMV of 2K1C rats are restored in the AAV2-CBA-AT2R-injected rats
As expected, the NT-AT2R and 2K1C-AT2R groups displayed a higher level of AT2R mRNA in the NTS compared with the eGFP-injected rats [NT-AT2R: 0.45 ± 0.13 and 2K1C-AT2R: 0.425 ± 0.9, vs. NT-eGFP: 0.117 ± 0.03 and 2K1C-AT2R: 0.05 ± 0.023; F(3,18) = 6.013; p < 0.05], (Figure 4A). However, AT2R over-expression did not influence AT1R mRNA levels [F(3,14) = 0.750; p > 0.05], Figure 4B. The 2K1C-eGFP rats displayed decreased levels of ACE2 mRNA in the NTS compared with the NT rats (0.037 ± 0.014, vs. other groups; F(3,15) = 7.775; p < 0.05]. Interestingly, the lower levels of ACE2 mRNA found in 2K1C-eGFP were restored in 2K1C-AT2R rats to those observed in the NT rats (2K1C-AT2R: 0.314 ± 0.076, vs. NT-eGFP: 0.232 ± 0.037 and NT-AT2R: 0.180 ± 0.0304; p > 0.05), Figure 4C.
Discussion
The role of the RAS and particularly AT1R in the development and maintenance of renovascular hypertension is well established. 9,10,28 In addition to the peripheral RAS, higher levels of ANG II and AT1R are present in different brain areas in 2K1C hypertensive rats, including in the PVN in the hypothalamus and the RVLM in the brainstem, 8,29,30 suggesting that the central RAS has a pivotal role in the development/maintenance of hypertension of the 2K1C model. In contrast to the established role of central and peripheral AT1R, both central and peripheral, in renovascular hypertension, thus far there have been no studies implicating a role of central AT2R in this form of high blood pressure. In fact, studies from mice suggested that AT2R do not have a counter-regulatory effect against the hypertensive action of ANG II acting via AT1AR in 2K1C hypertension. 28 In contrast, the present study has demonstrated, unequivocally and for the first time an effect of AT2R in the solitary-vagal complex, a brainstem region that is important in the control of arterial pressure and for the development and maintenance of renovascular hypertension. Our current data demonstrate that increased expression of AT2R in the solitary-vagal complex attenuates the increase in arterial pressure observed in 2K1C rats.
In the last decade, studies have begun to shed light on the role of AT2R in cardiovascular control [reviewed in 17]. Despite low levels of endogenous AT2R in several brainstem areas of adult rats, including the NTS/DMV as demonstrated in the present study and others, 31 functional data indicate that central AT2R can influence blood pressure. Specifically, chronic intracerebroventricular infusion of the selective AT2R agonist Compound 21 reduced arterial pressure and norepinephrine excretion in normotensive rats, suggesting that SNA has been decreased. 32 Another study from the same group 33 demonstrated that adenoviral-mediated increases in the expression of AT2R in the RVLM of normotensive rats elicited a reduction in nocturnal MAP and norepinephrine excretion, and an increase in urine excretion, possibly as a result of sympathoinhibition. This effect of AT2R overexpression on arterial pressures of normotensive rats seems to be specific for the RVLM, since in the present study 30 days after AT2R transduction in the NTS/DMV of normotensive rats there was no change in MAP (Figure 3A).
It is well established that the baroreflex is attenuated in 2K1C rats and impairment of the baroreflex seems to be critical to the development and maintenance of 2K1C hypertension 5–7 since a reduction in the baroreflex gain might also contribute to the increase in sympathetic outflow and high blood pressure.34 Evidence suggests that in 2K1C rats ANG II acting via its AT1R in the NTS is responsible for dampening the baroreflexes in renovascular hypertension. For example, circulating ANG II levels are high, especially during phase I (first 4 weeks) of 2K1C renovascular hypertension,9,10 and ANG II acting at AT1R within the NTS has been reported to attenuate baroreflex function. 12,14 Similar to the findings in the present study, Oliveira-Sales 7 demonstrated that during phase I of 2K1C hypertension there is an impairment of the baroreflex, and this may be related to the high levels of ANG II.
The present study demonstrated, in a very striking manner, that after increasing AT2R expression in the solitary-vagal complex of 2K1C rats, baroreflex function was restored to control levels. The mechanism involved in the restoration of the baroreflex remains to be determined, but it may involve the ACE2/Ang-(1–7)/Mas receptor axis based on the following evidence. ACE2 has high catalytic activity to convert ANG II to angiotensin 1–7 (reviewed in 35) and this enzyme is present in rat NTS as observed previously 36 and also in the present study. Angiotensin 1–7 has been shown to increase baroreflex sensitivity in the NTS, 37 and overexpression of human ACE2 in the NTS and RVLM of mice enhances baroreflex function. 26 Conversely, microinjection of an ACE2 inhibitor into the NTS decreased baroreflex sensitivity.38 In the present study, we observed a significant decrease in ACE2 mRNA levels in the solitary-vagal complex of 2K1C-eGFP rats, but not in the 2K1C-AT2R animals. Thus, it is possible that in the 2K1C-eGFP rats the observed decrease in baroreflex sensitivity and altered sympathovagal balance (indicated by the ratio of LF to HF of PI) was due to an imbalance of ACE2 in the solitary-vagal complex. Restoring the levels of ACE2 [and therefore Ang-(1–7)] via overexpression of AT2R at the NTS/DMV may have ameliorated the loss of baroreflex function and the altered sympathovagal balance normally seen in 2K1C rats, consequently reducing their hypertension. In accordance with this idea, Ali et al. 24 demonstrated that CGP42112A, an AT2R agonist, increase the expression of ACE2 and Mas receptors in the HK-2 cell line. In vivo data, in the same study, demonstrated that the lower expression levels and activity of ACE2 in obese Zucker rats were restored after 2 weeks treatment with CGP42112A. Therefore, it seems that the activation of AT2R leads to an increase in ACE2 expression, similar to that observed in the present study. However, the molecular mechanisms involved the regulation of ACE2 mRNA expression in the NTS by AT2R levels are yet to be determined.
In addition to the baroreceptor impairment in 2K1C rats, another mechanism that could account for the hypertension in 2K1C rats is the increase in SNA observed in these animals. 7,8 It has been reported that NTS inhibition reduces arterial pressure and SNA in SHR.15 Although we have not directly measured SNA, we observed that in 2K1C-eGFP there was in increase in LF of SBP. Since LF of SBP is an index of vasomotor sympathetic activity, it is possible that AT2R overexpression in the NTS/DMV induced a decrease in the sympathoexcitation in 2K1C rats. As pointed out earlier in the discussion, MAP did not totally recover to normotensive values after AT2R overexpression in the NTS/DMV. Viral transduction was made between phases I and II (5–8 weeks) of 2K1C hypertension [see 10]. In phase II, both SNA and MAP are increased by volume retention, decreased sodium excretion, and an increase in ANG II sensitivity. 7,9,10 all of which might be not directly affected by the AT2R overexpression in the solitary-vagal complex.
In conclusion, the current results indicate that increased expression of AT2R within the NTS/DMV of 2K1C rats impairs the full development of hypertension in these animals. The restoration of baroreflex function and in the increase of LF of SBP produced by increased AT2R expression is associated with increased ACE2 mRNA levels in the solitary-vagal complex, and this may be an important mechanism that contributed to these responses.
Perspectives
Despite a variety of treatment regimens hypertension, and in particular high blood pressure associated with enhanced sympathetic outflow, remains a worldwide problem. While it is well known that sympathoexcitation is involved in renovascular hypertension, the underlying mechanisms including a role for AT2R, and whether they antagonize AT1R actions in CNS cardiovascular control centers, are yet to be established. The current study demonstrates that increasing AT2R expression in the solitary-vagal complex exerts a profound attenuation of the development of renovascular hypertension, and restores baroreflex function and sympathetic modulation of arterial pressure to normal values in 2K1C rats. As such, the present data demonstrate a specific and beneficial effect of AT2R in a CNS cardiovascular control center, and suggest that AT2R may be a potential target for therapeutics in hypertension that involves enhanced sympathetic outflow/impaired baroreflex function. Whether or not this beneficial action of AT2R is a specific antagonism of AT1R effects in the NTS/DMV or simply an opposing effect remains to be determined.
Supplementary Material
Novelty and Significance.
What’s new
Increased expression of angiotensin type 2 receptors (AT2R) in the nucleus of the solitary tract (NTS)/dorsal motor nucleus of vagus (DMV) attenuated the development two kidney 1 clip (2K1C) hypertension.
Increased AT2R expression in the NTS/DMV of 2K1C rats also restored baroreflex function and sympathetic modulation of arterial pressure to normal in 2K1C rats.
These beneficial effects of AT2R in 2K1C rats are associated with increased expression of ACE2 mRNA at the NTS/DMV.
What’s relevant
AT2R exerts opposite effects compared with ANG II via its AT1R.
Increasing AT2R levels in the NTS/DMV may be a mechanism that can be targeted as a potential anti-hypertensive strategy.
Summary
Increased expression of AT2R within the solitary-vagal complex of 2K1C rats impairs the full development of hypertension in these animals, and restores of baroreflex function and the sympathetic modulation of the SBP to normal values. The normalization of ACE2 mRNA levels in the solitary-vagal complex after AT2R overexpression might be an important mechanism for these beneficial effects of AT2R.
Acknowledgments
The authors thank Reginaldo C. Queiroz, Silas P. Barbosa and Silvia Fóglia for expert technical assistance, Silvana A. D. Malavolta and Carla Molina for secretarial assistance, Malaika Jean-Baptiste for graphical assistance and Adriano P. Oliveira e Ana V. Oliveira for animal care.
Sources of Funding
This research was supported by Brazilian public funding from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (304918/2011-3 and 473108/2011-9), Fundação de Amparo a Pesquisa do Estado de São Paulo (2010/17218-0 and 2013/50121-9), Programa Nacional de Pós Doutorado/Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (PNPD/CAPES 2748/2010) and NIH grant R01 HL076803.
Footnotes
Conflits of Interest/Disclosures
None.
References
- 1.Hansen KJ, Edwards MS, Craven TE, Cherr GS, Jackson SA, Appel RG, Burke GL, Dean RH. Prevalence of renovascular disease in the elderly: a population-based study. J Vasc Surg. 2002;36:443–451. doi: 10.1067/mva.2002.127351. [DOI] [PubMed] [Google Scholar]
- 2.Remuzzi G. Sympathetic overactivity in hypertensive patients with chronic renal disease. N Engl J Med. 1999;340:1360–1361. doi: 10.1056/NEJM199904293401711. [DOI] [PubMed] [Google Scholar]
- 3.Johansson M, Elam M, Rundqvist B, Eisenhofer G, Herlitz H, Lambert G, Friberg P. Increased sympathetic nerve activity in renovascular hypertension. Circulation. 1999;99:2537–2542. doi: 10.1161/01.cir.99.19.2537. [DOI] [PubMed] [Google Scholar]
- 4.Goldblatt H, Lynch J, Hanzal RF, Summerville WW. Studies on experimental hypertension : I. The production of persistent elevation of systolic blood pressure by means of renal ischemia. J Exp Med. 1934;59:347–379. doi: 10.1084/jem.59.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berenguer LM, Garcia-Estan J, Ubeda M, Ortiz AJ, Quesada T. Role of renin-angiotensin system in the impairment of baroreflex control of heart rate in renal hypertension. J Hypertens. 1991;9:1127–1133. [PubMed] [Google Scholar]
- 6.Tsyrlin VA, Galagudza MM, Kuzmenko NV, Pliss MG, Rubanova NS, Shcherbin YI. Arterial baroreceptor reflex counteracts long-term blood pressure increase in the rat model of renovascular hypertension. PLoS ONE. 2013;8:e64788. doi: 10.1371/journal.pone.0064788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oliveira-Sales EB, Toward MA, Campos RR, Paton JFR. Revealing the role of the autonomic nervous system in the development and maintenance of Goldblatt hypertension in rats. Autonomic Neuroscience. 2014 doi: 10.1016/j.autneu.2014.02.001. ( http://dx.doi.org/10.1016/j.autneu.2014.02.001) [DOI] [PMC free article] [PubMed]
- 8.Oliveira-Sales EB, Nishi EE, Carillo BA, Boim MA, Dolnikoff MS, Bergamaschi CT, Campos RR. Oxidative stress in the sympathetic premotor neurons contributes to sympathetic activation in renovascular hypertension. Am J Hypertens. 2009;22:484–492. doi: 10.1038/ajh.2009.17. [DOI] [PubMed] [Google Scholar]
- 9.Navar LG, Zou L, Von Thun A, Tarng Wang C, Imig JD, Mitchell KD. Unraveling the Mystery of Goldblatt Hypertension. Physiology. 1998;13:170–176. doi: 10.1152/physiologyonline.1998.13.4.170. [DOI] [PubMed] [Google Scholar]
- 10.Martinez-Maldonado M. Pathophysiology of renovascular hypertension. Hypertension. 1991;17:707–719. doi: 10.1161/01.hyp.17.5.707. [DOI] [PubMed] [Google Scholar]
- 11.Marson O, Saragoca MA, Ribeiro AB, Bossolan D, Tufik S, Ramos OL. Anteroventral third ventricle and renin-angiotensin system interaction in the two-kidney, one clip hypertensive rat. Hypertension. 1983;5:V90–V93. doi: 10.1161/01.hyp.5.6_pt_3.v90. [DOI] [PubMed] [Google Scholar]
- 12.Matsumura K, Averill DB, Ferrario CM. Angiotensin II acts at AT1receptors in the nucleus of the solitary tract to attenuate the baroreceptor reflex. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 1998;275:R1611–R1619. doi: 10.1152/ajpregu.1998.275.5.R1611. [DOI] [PubMed] [Google Scholar]
- 13.Toney GM, Porter JP. Effects of blockade of AT1 and AT2 receptors in brain on the central angiotensin II pressor response in conscious spontaneously hypertensive rats. Neuropharmacology. 1993;32:581–589. doi: 10.1016/0028-3908(93)90054-7. [DOI] [PubMed] [Google Scholar]
- 14.Michelini LC, Bonagamba LG. Angiotensin II as a modulator of baroreceptor reflexes in the brainstem of conscious rats. Hypertension. 1990;15:I45–I50. doi: 10.1161/01.hyp.15.2_suppl.i45. [DOI] [PubMed] [Google Scholar]
- 15.Sato MA, Colombari E, Morrison SF. Inhibition of neurons in commissural nucleus of solitary tract reduces sympathetic nerve activity in SHR. Am J Physiol Heart Circ Physiol. 2002;282:H1679–H1684. doi: 10.1152/ajpheart.00619.2001. [DOI] [PubMed] [Google Scholar]
- 16.Freiria-Oliveira AH, Blanch GT, Li H, Colombari E, Colombari DSA, Sumners C. Macrophage migration inhibitory factor in the nucleus of solitary tract decreases blood pressure in SHRs. Cardiovasc Res. 2013;97:153–160. doi: 10.1093/cvr/cvs297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallinat S, Busche S, Raizada MK, Sumners C. The angiotensin II type 2 receptor: an enigma with multiple variations. American Journal of Physiology - Endocrinology And Metabolism. 2000;278:E357–E374. doi: 10.1152/ajpendo.2000.278.3.E357. [DOI] [PubMed] [Google Scholar]
- 18.Ichiki T, Labosky PA, Shiota C, Okuyama S, Imagawa Y, Fogo A, Niimura F, Ichikawa I, Hogan BL, Inagami T. Effects on blood pressure and exploratory behaviour of mice lacking angiotensin II type-2 receptor. Nature. 1995;377:748–750. doi: 10.1038/377748a0. [DOI] [PubMed] [Google Scholar]
- 19.Gross V, Milia AF, Plehm R, Inagami T, Luft FC. Long-term blood pressure telemetry in AT2 receptor-disrupted mice. J Hypertens. 2000;18:955–961. doi: 10.1097/00004872-200018070-00018. [DOI] [PubMed] [Google Scholar]
- 20.Bosnyak S, Welungoda IK, Hallberg A, Alterman M, Widdop RE, Jones ES. Stimulation of angiotensin AT2 receptors by the non-peptide agonist, Compound 21, evokes vasodepressor effects in conscious spontaneously hypertensive rats. Br J Pharmacol. 2010;159:709–716. doi: 10.1111/j.1476-5381.2009.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang J, Sumners C, Posner P. Modulation of net outward current in cultured neurons by angiotensin II: involvement of AT1 and AT2 receptors. Brain Res. 1992;580:317–324. doi: 10.1016/0006-8993(92)90960-h. [DOI] [PubMed] [Google Scholar]
- 22.Dampney RAL. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev. 1994;74:323–364. doi: 10.1152/physrev.1994.74.2.323. [DOI] [PubMed] [Google Scholar]
- 23.Santos RAS, Ferreira AJ, Verano-Braga T, Bader M. Angiotensin-converting enzyme 2, angiotensin-(1–7) and Mas: new players of the renin-angiotensin system. Journal of Endocrinology. 2013;216:R1–R17. doi: 10.1530/JOE-12-0341. [DOI] [PubMed] [Google Scholar]
- 24.Ali Q, Wu Y, Hussain T. Chronic AT2 receptor activation increases renal ACE2 activity, attenuates AT1 receptor function and blood pressure in obese Zucker rats. Kidney Int. 2013;84:931–939. doi: 10.1038/ki.2013.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sriramula S, Cardinale JP, Lazartigues E, Francis J. ACE2 overexpression in the paraventricular nucleus attenuates angiotensin II-induced hypertension. Cardiovasc Res. 2011;92:401–408. doi: 10.1093/cvr/cvr242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiao L, Gao L, Lazartigues E, Zucker IH. Brain-Selective Overexpression of Angiotensin-Converting Enzyme 2 Attenuates Sympathetic Nerve Activity and Enhances Baroreflex Function in Chronic Heart Failure. Hypertension. 2011;58:1057–1065. doi: 10.1161/HYPERTENSIONAHA.111.176636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith SH, Bishop SP. Selection criteria for drug-treated animals in two-kidney, one clip renal hypertension. Hypertension. 1986;8:700–705. doi: 10.1161/01.hyp.8.8.700. [DOI] [PubMed] [Google Scholar]
- 28.Cervenka L, Horacek V, Vaneckova I, Hubacek JA, Oliverio MI, Coffman TM, Navar LG. Essential role of AT1A receptor in the development of 2K1C hypertension. Hypertension. 2002;40:735–741. doi: 10.1161/01.hyp.0000036452.28493.74. [DOI] [PubMed] [Google Scholar]
- 29.Morishita R, Higaki J, Nakamura Y, Aoki M, Yamada K, Moriguchi A, Rakugi H, Tomita N, Tomita S, Yu H. Effect of an antihypertensive drug on brain angiotensin II levels in renal and spontaneously hypertensive rats. Clin Exp Pharmacol Physiol. 1995;22:665–669. doi: 10.1111/j.1440-1681.1995.tb02085.x. [DOI] [PubMed] [Google Scholar]
- 30.Chen AD, Zhang SJ, Yuan N, Xu Y, De W, Gao XY, Zhu GQ. Angiotensin AT1 receptors in paraventricular nucleus contribute to sympathetic activation and enhanced cardiac sympathetic afferent reflex in renovascular hypertensive rats. Exp Physiol. 2011;96:94–103. doi: 10.1113/expphysiol.2010.054353. [DOI] [PubMed] [Google Scholar]
- 31.Lenkei Z, Palkovits M, Corvol P, Llorens-Cortes C. Expression of angiotensin type-1 (AT1) and type-2 (AT2) receptor mRNAs in the adult rat brain: a functional neuroanatomical review. Front Neuroendocrinol. 1997;18:383–439. doi: 10.1006/frne.1997.0155. [DOI] [PubMed] [Google Scholar]
- 32.Gao J, Zhang H, Le KD, Chao J, Gao L. Activation of central angiotensin type 2 receptors suppresses norepinephrine excretion and blood pressure in conscious rats. Am J Hypertens. 2011;24:724–730. doi: 10.1038/ajh.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao L, Wang W, Wang W, Li H, Sumners C, Zucker IH. Effects of angiotensin type 2 receptor overexpression in the rostral ventrolateral medulla on blood pressure and urine excretion in normal rats. Hypertension. 2008;51:521–527. doi: 10.1161/HYPERTENSIONAHA.107.101717. [DOI] [PubMed] [Google Scholar]
- 34.Lohmeier TE, Irwin ED, Rossing MA, Serdar DJ, Kieval RS. Prolonged activation of the baroreflex produces sustained hypotension. Hypertension. 2004;43:306–311. doi: 10.1161/01.HYP.0000111837.73693.9b. [DOI] [PubMed] [Google Scholar]
- 35.Fraga-Silva RA, Ferreira AJ, Dos Santos RA. Opportunities for targeting the angiotensin-converting enzyme 2/angiotensin-(1–7)/mas receptor pathway in hypertension. Curr Hypertens Rep. 2013;15:31–38. doi: 10.1007/s11906-012-0324-1. [DOI] [PubMed] [Google Scholar]
- 36.Sakima A, Averill DB, Gallagher PE, Kasper SO, Tommasi EN, Ferrario CM, Diz DI. Impaired heart rate baroreflex in older rats: role of endogenous angiotensin-(1–7) at the nucleus tractus solitarii. Hypertension. 2005;46:333–340. doi: 10.1161/01.HYP.0000178157.70142.33. [DOI] [PubMed] [Google Scholar]
- 37.Chaves GZ, Caligiorne SM, Santos RA, Khosla MC, Campagnole-Santos MJ. Modulation of the baroreflex control of heart rate by angiotensin-(1–7) at the nucleus tractus solitarii of normotensive and spontaneously hypertensive rats. J Hypertens. 2000;18:1841–1848. doi: 10.1097/00004872-200018120-00019. [DOI] [PubMed] [Google Scholar]
- 38.Diz DI, Garcia-Espinosa MA, Gegick S, Tommasi EN, Ferrario CM, Ann Tallant E, Chappell MC, Gallagher PE. Injections of angiotensin-converting enzyme 2 inhibitor MLN4760 into nucleus tractus solitarii reduce baroreceptor reflex sensitivity for heart rate control in rats. Exp Physiol. 2008;93:694–700. doi: 10.1113/expphysiol.2007.040261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.