Abstract
Acute sleep loss increases pro-inflammatory and synaptic plasticity-related molecules in the brain, including interleukin-1beta (IL-1β), tumor necrosis factor-alpha (TNF-α), and brain-derived neurotrophic factor (BDNF). These molecules enhance non-rapid eye movement sleep slow wave activity (SWA), also known as electroencephalogram delta power, and modulate neurocognitive performance. Evidence suggests that chronic sleep restriction (CSR), a condition prevalent in today's society, does not elicit the enhanced SWA that is seen after acute sleep loss, although it cumulatively impairs neurocognitive functioning. Rats were continuously sleep deprived for 18 h per day and allowed 6 h of ad libitum sleep opportunity for 1 (SR1), 3 (SR3), or 5 (SR5) successive days (i.e., CSR). IL-1β, TNF-α, and BDNF mRNA levels were determined in the somatosensory cortex, frontal cortex, hippocampus, and basal forebrain. Largely, brain IL-1β and TNF-α expression were significantly enhanced throughout CSR. In contrast, BDNF mRNA levels were similar to baseline values in the cortex after 1 day of SR and significantly lower than baseline values in the hippocampus after 5 days of SR. In the basal forebrain, BDNF expression remained elevated throughout the 5 days of CSR, although IL-1β expression was significantly reduced. The chronic elevations of IL-1β and TNF-α and inhibition of BDNF might contribute to the reported lack of SWA responses reported after CSR. Further, the CSR-induced enhancements in brain inflammatory molecules and attenuations in hippocampal BDNF might contribute to neurocognitive and vigilance detriments that occur from CSR.
Keywords: sleep restriction, sleep deprivation, cytokine, interleukin-1 beta, tumor necrosis factor-alpha, brain-derived neurotropic factor
INTRODUCTION
Accumulating evidence indicates that chronic sleep restriction (CSR), a condition prevalent in today's societies, impairs health, immune functioning [1], and cognition [2,3]. These functions are regulated by inflammatory and synaptic plasticity-related molecules [4]. In addition, individuals with various pathologies that are associated with enhanced inflammation, such as insomnia, cancer, and major depression, exhibit disturbed and/or reduced sleep [1,5,6]. Elevated pro-inflammatory molecules, including the cytokines interleukin-1beta (IL-1β) and tumor necrosis factor-alpha (TNF-α), and reduced synaptic plasticity-related molecule brain-derived neurotrophic factor (BDNF) are associated with impairments in performance and cognition, sleepiness, and altered sleep [1,4]. IL-1β, TNF-α, and BDNF enhance NREM sleep electroencephalogram (EEG) delta power [also known as slow-wave activity (SWA); an indicator of sleep intensity]. Unlike SWA after acute sleep loss, SWA is not enhanced after several days of CSR and the exact mechanisms by which this occurs through are currently unknown [7,8].
IL-1β and TNF- α enhance sleep when injected centrally or peripherally [1]. Further, IL-1β and TNF-α injected intracerebroventricularly or locally to the cortex enhance SWA. When these molecules are inhibited via knockout mice or pharmacologically, homeostatic sleep responses to sleep loss are attenuated. In rats, mice, or rabbits, IL-1β and TNF-α messenger ribonucleic acids (mRNAs) and proteins are enhanced in sleep-related brain areas, including the cortex and hippocampus, after acute sleep loss [1]. In humans, acute sleep loss and 5 days of CSR enhance circulating pro-inflammatory cytokines including IL-6 and TNF-α [9]. Nevertheless, the effect of CSR on brain inflammatory molecules has received little attention.
BDNF is a neurotrophin and growth factor found centrally and peripherally that has many functions including activity-dependent synaptic plasticity, cognition, modulating local inflammation, and sleep regulation [4,10]. BDNF enhances spontaneous sleep in rats and rabbits [10] and enhances SWA in the cortical hemisphere where injected relative to the contralateral hemisphere [11]. Acute sleep deprivation enhances BDNF mRNA expression in sleep-related brain areas, such as the cortex [12]. However, reductions in BDNF mRNA expression within the hippocampus after acute sleep deprivation have been reported [13]. Herein, we determined the effects of CSR on cortical and subcortical brain area IL-1β, TNF-α and BDNF mRNA levels in rats.
METHODS
Animals
Twenty-eight three-month-old male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) were housed individually and provided water and food ad libitum throughout the experiments. Rats were maintained on a 12:12 h light/dark cycle at 22 ± 3 C°. All experimental protocols were approved by Veteran Affairs Boston Healthcare system Institutional Animal Care and Use Committee and were in compliance with the National Institutes of Health guidelines.
Experimental treatment groups and sleep restriction
Rats were randomly placed into 4 experimental treatment groups (N = 7 per group) that included: 18 h of acute sleep restriction (SR1), 3 consecutive days of CSR (SR3), 5 consecutive days of CSR (SR5), and an ad libitum sleep baseline control (BL) group. Rats were deprived of sleep for 18 h [zeitgeber time (ZT) 6-24] by being placed in a periodically rotating wheel (35.5 cm in diameter x 11 cm in width) (Lafayette Instrument Company, Lafayette, IN, USA), which continuously revolved for 3 m/min for 4 seconds followed by 12 seconds of immobility as previously described [14]. Immediately after the sleep deprivation periods, rats in the SR3 and SR5 treatment groups returned to their home cages allowing 6 h of ad libitum sleep opportunity. This CSR protocol induces at least 93% wakefulness during the SR period and little fragmented sleep during the 12 seconds of wheel immobility [7].
Tissue Collection
At ZT 0 (i.e., immediately after the end of the sleep deprivation), rats were anesthetized with isoflurane, decapitated, and brains were dissected as previously described [15]. Briefly, rat brains were placed on a frozen petri dish, and using a brain punch technique, 4 brain areas were collected: somatosensory cortex, frontal cortex, hippocampus and basal forebrain. The brain tissues were flash frozen in liquid nitrogen, and stored at -80 °C until further analysis.
Real-Time Polymerase Chain Reaction Analysis (RT-PCR)
Brain tissues were homogenized and RNA extracted with Trizol reagent as previously described [16]. A TaqMan Reverse Transcription kit (Applied Biosystems, Foster City, CA, USA) was used to prepare cDNA from each RNA sample as described by the manufacturer. Primer Express Software (Applied Biosystems, Foster City, CA, USA) was used to choose the primers and probes for our genes of interest (IL-1β, TNF-α, BDNF)(Table 1). RT-PCR was used to analyze the mRNA levels of the genes of interest in the selected brain areas. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a housekeeping gene to compare expression levels of mRNAs.
Table 1.
Primers and probes used for RT-PCR analysis.
| Gene | Sequence | Bases | |
|---|---|---|---|
| IL-1β | Primer-f | CACCTCTCAAGCAGAGCACAG | 21 |
| Primer-r | GGGTTCCATGGTGAAGTCAAC | 21 | |
| Probe-FAM/TAMRA | TGTCCCGACCATTGCTGTTTCCTAGG | 26 | |
| TNF-α | Primer-f | CCAGGTTCTCTTCAAGGGACAA | 22 |
| Primer-r | CTCCTGGTATGAAATGGCAAATC | 23 | |
| Probe-FAM/TAMRA | CCCGACTATGTGCTCCTCACCCACA | 25 | |
| BDNF | Primer-f | CCATAAGGACGCGGACTTGTAC | 22 |
| Primer-r | GAGGAGGCTCCAAAGGCACTT | 21 | |
| Probe-FAM/TAMRA | CTTCCCGGGTGATGCTCAGCAGT | 23 | |
| GAPDH | Primer-f | CAATGTGTCCGTCGTGGATCT | 21 |
| Primer-r | GTCCTCAGTGTAGCCCAAGATG | 22 | |
| Probe-FAM/TAMRA | CGTGCCGCCTGGAGAAACCTGCC | 23 |
The delta threshold cycle value method was used to quantify the experimental treatment effects on gene expression as previously described [16, 17]. Briefly, mean cycle threshold values for each rat undergoing baseline experimental treatments were computed for each gene of interest within each particular brain area separately. The change in cycle threshold values were evaluated by subtracting the mean GAPDH cycle threshold value from the baseline experimental treatment cycle threshold value and served as the baseline control mean measure. The gene expression of SR1, SR3, and SR5 experimental treatments was determined using the formula 2^ – (change in cycle threshold for experimental treatment from the baseline control mean) – (change in cycle threshold for the baseline experimental treatment from the baseline control mean).
Statistical analysis
Two-way analysis of variance was used to determine differences in gene expression between the number of SR days and brain areas. Independent t-tests were used for post-hoc analysis of gene expression. Data are presented as means ± SEM. Significance differences were set at p < 0.05.
RESULTS
IL-1β
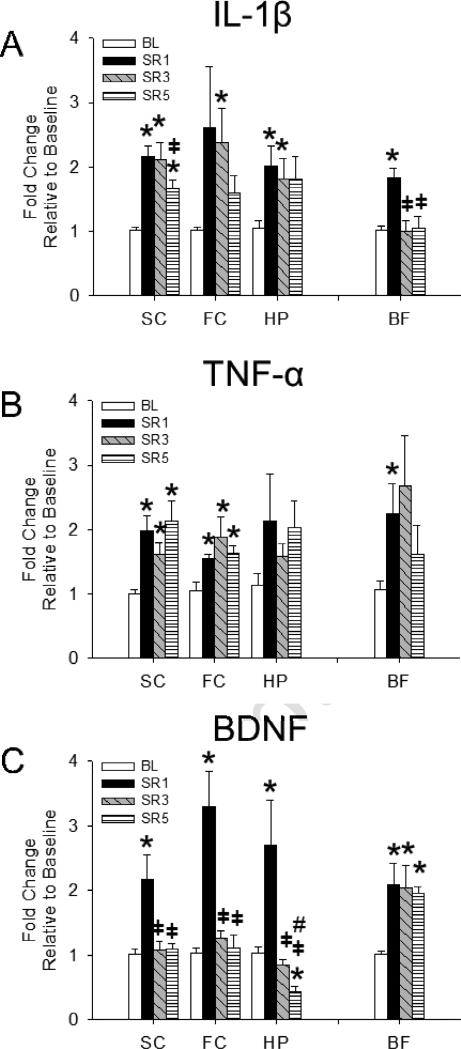
As shown in Fig 1A, a main effect was found for the duration of SR enhancing IL-1β mRNA expression [F(3,96) = 8.257, p < 0.001], although this effect differed depending upon the brain area [number of days of SR × brain area interaction: F(3,96) = 2.938, p = 0.037]. IL-1β mRNA expression in the somatosensory cortex, basal forebrain and hippocampus was significantly greater after 1 day of SR compared to the ad libitum sleep baseline control treatment. IL-1β mRNA expression was also significantly enhanced in the somatosensory cortex, frontal cortex, and hippocampus after 3 days of SR compared to controls, although basal forebrain IL-1β mRNA expression was significantly reduced after 3 days of SR compared to that after 1 day of SR and returned to baseline levels. Compared to baseline levels, IL-1β mRNA expression was significantly enhanced in the somatosensory cortex after 5 days of SR but significantly less than levels found after 1 day post-SR. Frontal cortex and hippocampal mRNA levels were not significantly different from baseline values, although trends were found for their enhancement 5 days post-SR [t(12) = 2.091, p = 0.058 and t(12) = 1.990, p = 0.070, respectively].
Fig 1.
Changes in mRNA levels in somatosensory cortex (SC), frontal cortex (FC), hippocampus (HP), and basal forebrain (BF) after chronic sleep restriction (CSR). BL = baseline, SR1 = 18 h acute sleep loss; SR3 = CSR day 3, SR 5 = CSR day 5. Overall, IL-1β and TNF-α mRNA levels were enhanced after both acute sleep loss (SR1) and CSR in all brain areas measured. However, IL-1β mRNA levels in the BF after day 1 of CSR were significantly attenuated after 3 and 5 days of CSR. BDNF mRNA levels were significantly enhanced after acute sleep loss (SR1) in all brain regions but remained elevated on CSR days 3 and 5 only in the BF. Thus, BDNF mRNA levels in the SC and FC returned to basal levels on CSR days 3 and 5 and were significantly lower than BL levels in the HP. Significance was set at p < 0.05. (*) = significant difference from BL. (╪) = significant difference from SR1. (#) = significant difference from SR3.
TNF-α
TNF-α mRNA expression data are displayed in Fig 1B. A main effect was found for the duration of SR enhancing TNF-α mRNA expression [F(3,96) = 5.662, p = 0.001]. TNF-α mRNA expression in the somatosensory cortex, frontal cortex, and basal forebrain, was significantly enhanced after 1 day of SR compared with baseline controls. After 3 days of SR, somatosensory cortex and frontal cortex TNF-α mRNAs were enhanced compared to baseline levels, while basal forebrain TNF-α mRNAs reached near significance [t(12) = 2.045, p = 0.063]. TNF-α mRNA expression was significantly enhanced in the somatosensory cortex and frontal cortex after 5 days of SR compared to baseline controls and approached significance within the hippocampus [t(12) = 2.020, p = 0.066].
BDNF
BDNF mRNA expression data are presented in Fig 1C. A main effect was found for the number of days of SR enhancing BDNF mRNA expression [F(3,96) = 25.201, p < 0.001], although this effect depended upon the brain area [brain area: F(3,96) = 3.150, p = 0.029; number of days of SR x brain area interaction: F(3,96) = 2.954, p = 0.004]. BDNF mRNA expression was significantly enhanced in all brain areas investigated after 18 h of acute sleep loss compared to baseline controls. Basal forebrain BDNF mRNA expression was significantly enhanced after both 3 and 5 days of SR compared to baseline controls. However, in cortical areas assessed and hippocampus, BDNF mRNA expression was significantly reduced after 3 days of SR compared to that after 1 day of SR—an effect that also occurred in the same brain areas when comparing 5 days of SR vs.1 day of SR. Within the hippocampus, however, a CSR-induced attenuation in BDNF mRNA levels was found that was exemplified by the significant reduction in BDNF mRNAs exhibited after 5 days of SR compared to baseline and 3 days of SR levels.
DISCUSSION
Our findings indicate an inverse pattern between pro-inflammatory cytokines and BDNF mRNA levels in the brain after CSR. IL-1β, TNF-α, and BDNF expression was enhanced in the cortical and subcortical areas after 1 day of SR, which is consistent with the literature [1,12]. We now report that cortical IL-1β and TNF-α mRNAs are also enhanced after multiple days of SR, although there is some attenuation in these mRNAs depending upon the brain area. We also found that brain BDNF expression was reduced within the hippocampus after CSR and not enhanced in the cortex after multiple days of SR. Overall, the current findings indicate elevated brain pro-inflammatory cytokines and attenuated hippocampal BDNF expression are induced by CSR, which could potentially contribute to CSR-related impairments in cognition and alterations in SWA [2,7].
Enhanced IL-1β and TNF-α mRNAs observed after 1 day of SR (i.e., 18 h of acute sleep loss) is consistent with studies of shorter acute sleep loss (e.gs. 3-12 h) [1]. Nevertheless, anti-inflammatory molecules, such as IL-10, have anti-somnogenic functions [18]. Thus, the reduced IL-1β expression in the basal forebrain found after CSR could be attributed, in part, to enhanced activation of anti-inflammatory molecules serving in a protective manner to inhibit unbridled brain inflammation. Alternatively, since receptors typically become down-regulated after persistent enhancement of their substrate [19], the reduction of IL-1β expression after CSR could also be attributed to a down-regulation of IL-1β receptors. Indeed, activating IL-1β receptors promotes the IL-1β expression forming a cascade, which supports this hypothesis [20]. Nonetheless, while mRNA expression levels can detect small changes in multiple genes from small brain tissue samples that are not possible in protein, mRNA can be modulated post-transcriptionally [19].
BDNF expression was enhanced after 1 day of SR in all brain areas investigated, as similarly reported using protocols of shorter durations of acute sleep loss [12]. Unlike IL-1β and TNF-α, BDNF mRNA expression returned to basal levels in all cortical areas after 3 and 5 days of SR. After 5 days of CSR hippocampal BDNF expression was below baseline values (Fig 1C), a finding that is consistent with our previous finding of reduced BDNF immuno-reactive cells in the hippocampus after a more moderate and persistent amount of CSR in mice (12 h SR for 11 weeks) [21]. Our findings are consistent with a hypothesis by Giese and colleagues who suggested that chronic impairments in sleep are paralleled by reductions in BDNF levels as evidenced by lower plasma BDNF levels exhibited in individuals with insomnia [22]. Further, a recent study of individuals undergoing military training for 9 weeks exhibited reduced sleep quality and quantity and plasma BDNF levels further supporting the idea that CSR induces attenuations in BDNF [23].
It is well-known that chronic stress, such as learned helplessness, attenuates hippocampal BDNF through cyclic adenosine monophosphate response element binding protein-related mechanisms [24]. Consequently, stress-related molecules induced by CSR could, in part, contribute to the attenuations in hippocampal BDNF expression we found in the present study. In fact, the stress-related molecules adrenocorticotropin hormone and corticotrophin releasing hormone remain elevated after CSR, which could serve to enhance downstream corticosterone levels [25]—a stress-related molecule that attenuates hippocampal BDNF expression [26]. Nevertheless, enhanced circulating corticosterone levels are not found in rats after multiple days using either more severe or moderate CSR protocols (i.e., 20 h and 12 h vs. 18 h) [21,25].
The enhanced basal forebrain BDNF but attenuated IL-1β mRNA expression we found after CSR was unlike the cortical and hippocampal findings. The reason for this is unclear, however, an abundance of evidence indicate that the basal forebrain has a distinctive role in regulating sleep homeostasis after enhanced waking activity [27]. For example, IL-1β can function to suppress wake-promoting neurons that are predominant within the basal forebrain [28].
Sleep and other physiologic responses to acute sleep loss typically exhibit homeostatic responses by returning to baseline values. However, allostatic responses are found in various chronic conditions including lack of enhanced SWA and sleep duration responses during periods of recovery sleep after multiple days of CSR and CSR-induced adaptive responses in the noradrenergic system [7,8,14,15]. Our current findings of enhanced IL1-β mRNA levels in the basal forebrain and BDNF mRNA levels in cortical areas after acute sleep loss and a lack of enhanced mRNA expression during CSR suggest these molecules can adapt to chronic sleep loss in brain areas that regulate sleep. Evidence indicates that increased cortical BDNF expression is associated with enhanced SWA after acute sleep deprivation and cortical injections of BDNF enhance SWA [11,12]. Since BDNF enhances SWA [11] and SWA is regulated thalamocortically [27], the lack of cortical BDNF expression we found after CSR could, in part, contribute to the lack of SWA enhancements reported to occur after CSR (i.e., allostatic response) [7,8].
Pharmaceutical studies provide evidence that pro-inflammatory cytokines can inhibit BDNF expression within the brain [29,30]. For instance, central injections of TNF-α inhibit cortical BDNF expression in rats [29]. Interestingly, acute central injections of IL-1β enhance hippocampal BDNF expression in rats [30]; however, multiple days of central injections of IL-1β enhance TNF-α expression while inhibiting BDNF in the hippocampus. Although speculative, the current findings support our hypothesis that CSR-induced brain inflammation inhibits BDNF. It is plausible that the lack of enhanced IL-1β mRNA expression in the basal forebrain after CSR could allow sleep loss enhancements in BDNF expression to persist during CSR. The mechanisms that mediate inflammation-induced reduction in BDNF are currently not known, although stress-related molecules modulated by CSR that can enhance pro-inflammatory cytokines and impair synaptic-plasticity-related molecules, including BDNF, are likely involved [32,33].
Elevated pro-inflammatory cytokine mRNA expression is associated with lower BDNF mRNA expression and impairments in cognition [30]. Since we found enhanced cortical IL-1β and TNF-α expression corresponding with reductions in hippocampal BDNF expression after CSR, and activity-dependent BDNF expression in the hippocampus is reported to be associated with altered memory [31], our findings suggests a possible link between CSR-induced brain inflammation and impairments in cognition. Notwithstanding, differences exist in the type of enhanced behavioral activity that modulate BDNF in the brain. For example, enhanced waking activity associated with CSR attenuates hippocampal BDNF immuno-positive reactive cells, while exercise is well-known to enhance BDNF levels [34]. Nevertheless, a number of receptors and cells that are modulated by inflammatory cytokines, including adrenergic receptors [15] and sleep-active cortical neurons expressing nitric oxide synthase [14], are enhanced after CSR and also likely contribute to the detriments of CSR.
In conclusion, we provide evidence that CSR enhances the brain inflammatory molecules IL-1β and TNF-α and attenuates the synaptic plasticity-related molecule BDNF. These molecular effects in the brain could contribute to the detriments of CSR including sleepiness and impairments in neurocognitive performance and health, and lack of SWA responses to CSR. Further experimental studies are necessary to confirm that enhanced pro-inflammatory cytokines and attenuations in BDNF contribute to the detriments of CSR including sleepiness, neurocognitive performance, SWA, and health.
HIGHLIGHTS.
Chronic sleep restriction enhances cortical IL-1β and TNF-α mRNAs
Chronic sleep restriction attenuates acute sleep loss elevations in BDNF mRNA
Chronic sleep restriction reduces hippocampal BDNF mRNA levels below baseline levels
ACKNOWLEDGEMENTS
This work was supported by the National Institutes of Health/National Institute of Neurological Disorders and Stroke NS064193 awarded to DG, Department of Veterans Affairs Medical Research Service Award (RES), MH039683 (RWM), HL060292 and HL095491 (RWM & RES). We also thank Xiaomei Yang for her contributions to the molecular analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Zielinski MR, Krueger JM. Sleep and innate immunity. Front Biosci (Schol Ed) 2011;3:632–42. doi: 10.2741/s176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCoy JG, Christie MA, Kim Y, Brennan R, Poeta DL, McCarley RW, Strecker RE. Chronic sleep restriction impairs spatial memory in rats. Neuroreport. 2013;24:91–5. doi: 10.1097/WNR.0b013e32835cd97a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCoy JG, Strecker RE. The cognitive cost of sleep lost. Neurobiol Learn Mem. 2011;96:564–82. doi: 10.1016/j.nlm.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun. 2011;25:181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 5.Martin C, Tansey KE, Schalkwyk LC, Powell TR. The inflammatory cytokines: molecular biomarkers for major depressive disorder? Biomark Med. 2014 doi: 10.2217/bmm.14.29. (in press) [DOI] [PubMed] [Google Scholar]
- 6.Felger JC, Lotrich FE. Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience. 2013;246:199–229. doi: 10.1016/j.neuroscience.2013.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim Y, Bolortuya Y, Chen L, Basheer R, McCarley RW, Strecker RE. Decoupling of sleepiness from sleep time and intensity during chronic sleep restriction: evidence for a role of the adenosine system. Sleep. 2012;35:861–69. doi: 10.5665/sleep.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deurveilher S, Rusak B, Semba K. Time-of-day modulation of homeostatic and allostatic sleep responses to chronic sleep restriction in rats. Am J Physiol Regul Integr Comp Physiol. 2012;302:R1411–25. doi: 10.1152/ajpregu.00678.2011. [DOI] [PubMed] [Google Scholar]
- 9.Lekander M, Andreasson AN, Kecklund G, Ekman R, Ingre M, Akerstedt T, Axelsson J. Subjective health perception in healthy young men changes in response to experimentally restricted sleep and subsequent recovery sleep. Brain Behav Immun. 2013;34:43–6. doi: 10.1016/j.bbi.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Kushikata T, Fang J, Krueger JM. Brain-derived neurotrophic factor enhances spontaneous sleep in rats and rabbits. Am J Physiol. 1999;276:R1334–8. doi: 10.1152/ajpregu.1999.276.5.R1334. [DOI] [PubMed] [Google Scholar]
- 11.Faraguna U, Vyazovskiy VV, Nelson AB, Tononi G, Cirelli C. A causal role for brain-derived neurotrophic factor in the homeostatic regulation of sleep. J Neurosci. 2008;28:40088–95. doi: 10.1523/JNEUROSCI.5510-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cirelli C, Tononi G. Differential expression of plasticity-related genes in waking and sleep and their regulation by the noradrenergic system. J Neurosci. 2000;20:9187–94. doi: 10.1523/JNEUROSCI.20-24-09187.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guzman-Marin R, Ying Z, Suntsova N, Methippara M, Bashir T, Szymusiak R, Gomez-Pinilla F, McGinty D. Suppression of hippocampal plasticity-related gene expression by sleep deprivation in rats. J Physiol. 2006;575:807–19. doi: 10.1113/jphysiol.2006.115287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zielinski MR, Kim Y, Karpova SA, Winston S, McCarley RW, Strecker RE, Gerashchenko D. Sleep active cortical neurons expressing neuronal nitric oxide synthase are active after both acute sleep deprivation and chronic sleep restriction. Neuroscience. 2013;247:35–42. doi: 10.1016/j.neuroscience.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim Y, Chen L, McCarley RW, Strecker RE. Sleep allostasis in chronic sleep restriction: the role of the norepinephrine system. Brain Res. 2013;1531:9–16. doi: 10.1016/j.brainres.2013.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zielinski MR, Dunbrasky DL, Taishi P, Souza G, Krueger JM. Vagotomy attenuates brain cytokines and sleep induced by peripherally administered tumor necrosis factor-alpha and Lipopolysaccharide in mice. Sleep. 2013;36:1227–38. doi: 10.5665/sleep.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alt JA, Bohnet S, Taishi P, Duricka D, Obal F, Jr, Traynor T, Majde JA, Krueger JM. Influenza virus-induced glucocorticoid and hypothalamic and lung cytokine mRNA responses in dwarf lit/lit mice. Brain Behav Immun. 2007;21:60–7. doi: 10.1016/j.bbi.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Opp MR, Smith EM, Hughes TK., Jr Interleukin-10 (cytokine synthesis inhibitory factor) acts in the central nervous system of rats to reduce sleep. J Neuroimmunol. 1995;60:165–8. doi: 10.1016/0165-5728(95)00066-b. [DOI] [PubMed] [Google Scholar]
- 19.Nussey S, Whithead S. Endocrinology: an integrated approach. BIOS Scientific Publishers; Oxford: 2001. [PubMed] [Google Scholar]
- 20.Vitkovic L, Konsman JP, Bockaert J, Dantzer R, Homburger V, Jacque C. Cytokine signals propagate through the brain. Mol Psychiatry. 2000;5:604–15. doi: 10.1038/sj.mp.4000813. [DOI] [PubMed] [Google Scholar]
- 21.Zielinski MR, Davis JM, Fadel JR, Youngstedt SD. Influence of chronic moderate sleep restriction and exercise on inflammation and carcinogenesis in mice. Brain Behav Immun. 2012;26:672–9. doi: 10.1016/j.bbi.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giese M, Unternaher E, Huttig H, Beck J, Brand S, Calabrese P, Holsboer-trachsler E, Eckert A. BDNF:an indicator of insomnia? Mol Psychiatry. 2014;19:151–2. doi: 10.1038/mp.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suzuki G, Tokuno S, Nibuya M, Ishida T, Yamamoto T, Mukai Y, Mitani K, Tsumatori G, Scott D, Shimizu K. Decreased plasma brain-derived neurotrophic factor and vascular endothelial growth factor concentrations during military training. PLoS One. 2014;9:e89455. doi: 10.1371/journal.pone.0089455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song L, Che W, Min-Wei W, Murakami Y, Matsumoto K. Impairment of the spatial learning and memory induced by learned helplessness and chronic mild stress. Pharmacol Biochem Behav. 2006;83:186–93. doi: 10.1016/j.pbb.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Meerlo P, Koehl M, van der Borght K, Turek FW. Sleep restriction alters the hypothalamic-pituitary-adrenal response to stress. J Neuroendocrinol. 2002;14:397–402. doi: 10.1046/j.0007-1331.2002.00790.x. [DOI] [PubMed] [Google Scholar]
- 26.Schaaf MJ, DeKloet ER, Vreugdenhil E. Corticosterone effects on BDNF expression in the hippocampus.Implications for memory formation. Stress. 2000;3:201–8. doi: 10.3109/10253890009001124. [DOI] [PubMed] [Google Scholar]
- 27.Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW. Control of sleep and wakefulness. Physiol Rev. 2012;92:1087–187. doi: 10.1152/physrev.00032.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alam MN, McGinty D, Bashir T, Kumar S, Imeri L, Opp MR, Szymusiak R. Interleukin-1beta modulates state-dependent discharge activity of preoptic area and basal forebrain neurons: role in sleep regulation. Eur J Neurosci. 2004;20:207–16. doi: 10.1111/j.1460-9568.2004.03469.x. [DOI] [PubMed] [Google Scholar]
- 29.Churchill L, Taishi P, Wang M, Brandt J, Cearley C, Rehman A, Krueger JM. Brain distribution of cytokine mRNA induced by systemic administration of interleukin-1 beta and tumor necrosis factor alpha. Brain Res. 2006;1120:64–73. doi: 10.1016/j.brainres.2006.08.083. [DOI] [PubMed] [Google Scholar]
- 30.Song C, Zhang Y, Dong Y. Acute and subacute IL-1beta administrations differentially modulate neuroimmune and neurotrophic systems: possible implications for neuroprotection and neurodegeneration. J Neuroinflammation. 2013;10:59. doi: 10.1186/1742-2094-10-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakata K, Martinowich K, Woo NH, Schloesser RJ, Jimenez DV, Ji Y, Shen L, Lu B. Role of activity-dependent BDNF expression in hippocampal-prefrontal cortical regulation of behavioral perseverance. Proc Natl Acad Sci U S A. 2013;110:15103–8. doi: 10.1073/pnas.1222872110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anisman H. Cascading effects of stressors and inflammatory immune system activation: implications for major depressive disorder. J Psychiatry Neurosci. 2009;34:4–20. [PMC free article] [PubMed] [Google Scholar]
- 33.Schaff MJ, Hoetelmans RW, de Kloet ER, Vreugdenhil E. Corticosterone regulates expression of BDNF and trkB but not NT-3 and trkC mRNA in the rat hippocampus. J Neurosci Res. 1997;48:334–41. [PubMed] [Google Scholar]
- 34.Zielinski MR, Davis JM, Fadel JR, Youngstedt SD. Influence of chronic moderate sleep restriction and exercise training on anxiety, spatial memory, and associated neurobiological measures in mice. Behav Brain Res. 2013;250C:74–80. doi: 10.1016/j.bbr.2013.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]