Abstract
Chemotaxis, or directed migration of cells along a chemical gradient, is a highly coordinated process that involves gradient sensing, motility, and polarity. Most of our understanding of chemotaxis comes from studies of cells undergoing amoeboid-type migration, in particular the social amoeba Dictyostelium discoideum and leukocytes. In these amoeboid cells the molecular events leading to directed migration can be conceptually divided into four interacting networks: receptor/G protein, signal transduction, cytoskeleton, and polarity. The signal transduction network occupies a central position in this scheme as it receives direct input from the receptor/G protein network, as well as feedback from the cytoskeletal and polarity networks. Multiple overlapping modules within the signal transduction network transmit the signals to the actin cytoskeleton network leading to biased pseudopod protrusion in the direction of the gradient. The overall architecture of the networks, as well as the individual signaling modules, is remarkably conserved between Dictyostelium and mammalian leukocytes, and the similarities and differences between the two systems are the subject of this review.
Keywords: Chemotaxis, Amoeboid migration, Signal transduction, Cytoskeleton, Polarity, GPCR
Introduction
Role of chemotaxis in health and disease
Directed migration of a cell along a chemical gradient, or chemotaxis, is a fundamental process that is conserved from bacteria to eukaryotes. The ability to sense small differences in the chemoattractant concentration is essential for a variety of physiological and pathophysiological conditions. During embryogenesis, chemotaxis orchestrates the migration of many cells, including neural crest and primordial germ cells [1, 2]. Chemotaxis is also critical for the intricate trafficking of immune cells and their recruitment to sites of inflammation [3–5]. Importantly, inappropriate chemotaxis of leukocytes contributes to chronic inflammatory diseases, including arthritis, asthma, and atherosclerosis [3, 6]. Furthermore, the leading cause of deaths from cancer is metastasis, which is the dissemination of tumor cells to secondary sites, a process that is driven by chemotaxis [7].
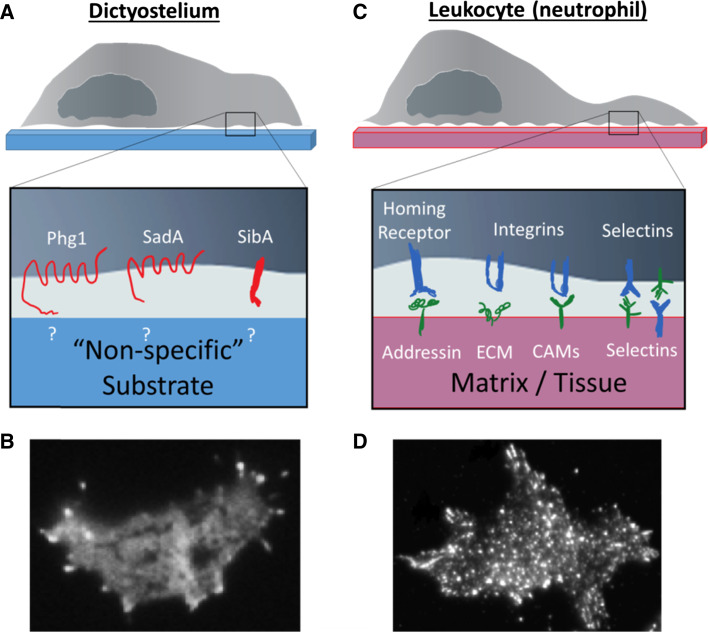
Dictyostelium and leukocytes display amoeboid migration
Most of our understanding of the signaling pathways regulating chemotaxis comes from studies of the soil amoeba Dictyostelium discoideum, which relies on chemotaxis during various stages of its life cycle. Vegetative cells locate food sources by migrating toward products such as folic acid derived from bacteria or yeast. During periods of starvation cells enter a developmental program in which they acquire the ability to sense and migrate toward locally secreted cAMP by upregulating the expression of cAMP receptors (cARs) and related signal transduction components. Chemotaxis toward self-generated cAMP gradients leads to the formation of a multicellular organism that undergoes several morphological changes, eventually resulting in the formation of spores, which can survive unfavorable conditions. The robust chemotactic responses of Dictyostelium cells, combined with the powerful genetic approaches this haploid organism offers, make it a powerful system for the study of chemotaxis.
Eukaryotic cells exhibit several distinct modes of migration. Dictyostelium cells, leukocytes, as well as metastatic tumor cells use amoeboid migration, which is characterized by rapid protrusion and retraction of pseudopods driven by actomyosin contractility, weak cell-substrate interactions, and a lack of matrix degradation [8]. As a result, amoeboid migration is extremely fast, with speeds reaching 10–25 μm/min [9]. In contrast, mesenchymal migration seen in fibroblasts and some tumor cells is slower (~ 0.1-1 μm/min) and requires strong interaction with the substrate, as well as proteolysis of the extracellular matrix [10]. In addition to single-cell migration, cells can migrate as a group in a process known as collective cell migration [11, 12]. Multicellular migration is observed in certain cancers and during neural crest migration, for example, as well as in aggregation-competent Dictyostelium cells, which use “streaming” to relay the chemotactic signal and improve the recruitment range. This review will focus exclusively on amoeboid migration during chemotaxis of individual leukocytes and Dictyostelium cells.
Chemotaxis can be thought of as integrating processes of motility, directional sensing, and polarity. Motility refers to the ability of cells to extend pseudopods and move around randomly in the absence of cues [13–15]. Directional sensing refers to the ability of a cell to sense and move along a gradient, and even when immobilized, to direct its signaling events towards the high side [16–18]. Polarity refers to a semi-stable state where signaling and cytoskeletal events occur preferentially at the front or back of a cell, allowing a cell to move persistently in the same direction even without an external cue. Dictyostelium cells can have more or less intrinsic polarity, with later stages of development having strong polarity similar to neutrophils.
Chemotactic networks of Dictyostelium and leukocytes
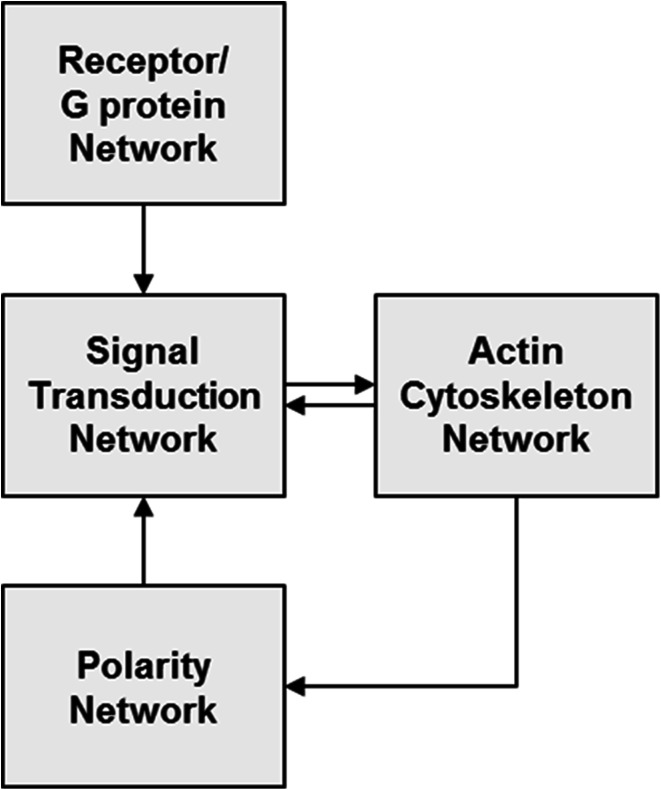
It is convenient to think about the molecular events regulating motility, directional sensing, and polarity in terms of interacting networks. Figure 1 shows the interconnections between the receptor/G protein, signal transduction, actin cytoskeleton, and polarity networks [17]. The receptor/G protein network involves the chemoattractant receptors, G proteins, and additional upstream components that detect the gradient and transmit a bias to the signal transduction network. The signal transduction network consists of a large number of interacting pathways that amplify the directional bias and transmit the signal to the cytoskeleton network. The actin cytoskeleton network generates a protrusive force to move the cell and also provides feedback to the signal transduction network. Finally, the polarity network depends on the cytoskeleton and like the gradient sensing network provides a bias to the signal transduction network. Thus, the signal transduction network occupies a central location among the interacting networks that bring about chemotaxis. Therefore, in this review we focus on the similarities and differences in the topology of the signal transduction networks of Dictyostelium and leukocytes, while only briefly outlining the other networks.
Fig. 1.
Overview of the networks contributing to chemotaxis. The four proposed networks required for amoeboid chemotaxis with arrows representing the interactions between them
Genetic analysis in Dictyostelium and leukocytes has revealed that there are hundreds of proteins involved in chemotaxis. It appears that most of these are in the signal transduction and cytoskeleton networks. The topologies of the networks have been mostly derived from observations of the responses of living cells in a gradient or with uniform stimulation (see Fig. 2). Biosensors for critical activities are compared between wild-type cells and those expressing single or multiple constitutively active or dominant-negative versions of proteins of interest, or cells with reduced amounts of proteins either via knock-down or knock-out approaches. Especially in Dictyostelium multiple genes can be deleted to generate combinations of deficiencies. While the positive interactions are clear, the lack of interaction may be due to the fact that it has not been experimentally examined. Within the more complex networks, such as those for signal transduction and actin cytoskeleton, it is convenient to separate the components into modules. This is clearly an oversimplification as there are numerous points of overlap between the modules. We have chosen to discuss several modules which have the strongest effects on chemotaxis, are the most studied, and have clear homologies across both Dictyostelium and leukocytes.
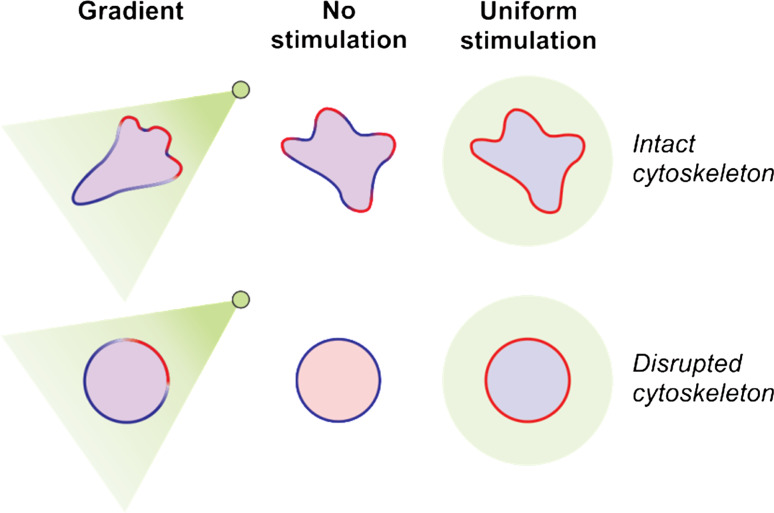
Fig. 2.
Spatiotemporal regulation of “front” and “back” proteins with chemoattractant stimulation. Many proteins involved in chemotaxis are differentially localized to the front or back of migrating cells and this helps establish the balance between protrusion and retraction that leads to directed cellular migration. Thus in a cell exposed to a chemoattractant gradient the “front” proteins (shown in red) localize to the pseudopods, which are oriented toward the gradient, whereas the “back” proteins (shown in blue) line the back and sides of the cell. In a round cell with a disrupted cytoskeleton, for example, via actin-depolymerizing drugs, the “front” proteins localize to the high side of the gradient in a “crescent”, whereas the “back” proteins have opposing localization. In a resting cell or in a cell with a disrupted cytoskeleton in the absence of a chemoattractant the “back” proteins are localized uniformly along the membrane or cortex, whereas the front proteins are in the cytosol. When a cell makes a protrusion, the back proteins dissociate from that region, and the front proteins associate with that extending region of the cell periphery [396, 397]. With a uniform stimulation, the “front” proteins transiently re-localize to the entire membrane and “back” proteins transiently dissociate. These protein translocations after chemoattractant stimulation occur within 10–30 s and then return to basal state after about 30-60 s [19, 85, 397]. The precise kinetics of specific signaling components in different contexts will be addressed further in this review. Chemoattractants also trigger activation of some proteins at the front or the back of a cell without affecting localization of those proteins. In this case, fluorescently-tagged biosensors that recognize activated versions of the protein or their enzymatic products act as “front” or “back” proteins
Receptor/G protein network
Chemoattractants can act through several classes of conserved receptors, each having specific properties and functions in various cell types. In many cases this cellular directional sensing is accomplished through G protein-coupled receptors (GPCRs). Several lines of evidence suggest that these receptors and their functional interactions with the heterotrimeric G proteins (Gα, Gβ, Gγ) share a common ancestor to all eukaryotes [19]. The GPCR systems share even further homologies within the evolutionary clade that includes Animals, Fungi, and Amoebozoans. Within these related phylogenetic Kingdoms, ligand binding to the GPCR activates the Gα guanine nucleotide exchange factor (GEF) activity of the receptor (releasing the Gβγ) and/or G protein-independent signaling leading to the activation of downstream pathways [19–23].
There are many striking similarities when comparing the roles GPCRs play in the amoeboid migration of Dictyostelium and leukocytes, but there are also some differences. First, the pertussis toxin-sensitive Gαi proteins in leukocytes are the predominant heterotrimeric G proteins shared by many receptors, although some evidence suggests that G12/13 proteins may signal to the rear of the cell [23–25]. In Dictyostelium, different receptors for cAMP or folic acid utilize different Gα subunits [26]. Second, leukocytes can utilize numerous combinations of the multiple Gβ/Gγ genes during directed cell migration, whereas Dictyostelium express only one Gβ and one Gγ in their genome [27]. Last, in both cell types ligand binding to chemoattractant GPCRs induces phosphorylation of the intracellular C-terminus of the receptors. Attraction to chemokines in leukocytes has been shown to utilize both β-arrestins and receptor phosphorylating G protein receptor kinases (GRKs) to regulate migration [28, 29]. Receptor phosphorylation is also important in regulating ligand affinity for the cAR1 receptor in Dictyostelium, although there are contradictory reports as to what effects this phosphorylation may have on downstream signal transduction and adaptation [30–32]. Although the intricate details of receptor and G protein function, including receptor-ligand interaction and the regulation of the heterotrimeric G protein cycle, are not addressed here, excellent reviews on these topics can be found in [33–37].
Compared to Dictyostelium, there is a rich repertoire of receptors and ligands controlling directed migration in many different mammalian cells, including leukocytes [38]. As part of the innate immune system neutrophils are the first responders to tissue damage and bacterial infection. N-formylmethionyl-leucyl-phenylalanine (fMLP) is a tripeptide produced by bacteria that serves as a chemoattractant. The GPCR N-formyl peptide receptor, FPR1, responds to and relays this bacterial cue [39]. Stromal cell-derived factor 1 (SDF-1), also known as chemokine 12 (CXCL12), is a strong chemoattractant for lymphocytes and monocytes [40]. Leukocytes also respond to C5a, Interleukin-8 (IL8), PAF, and LTB4 with specific chemokine GPCRs [34]. In contrast, only a few chemoattractants have been identified in Dictyostelium, including folic acid utilized in foraging and cAMP used in cell to cell communication.
The receptor/G protein network provides the initial spatial detection of the extracellular chemoattractant gradient. The directional signal is then transferred inside the cell to the numerous modules in the signal transduction network. The signaling modules downstream of the receptor/G protein network provide the cell with intricate control of its behavior in a chemoattractant gradient. They not only allow for the integration of numerous environmental stimuli simultaneously but also provide the amplification and adaptation mechanisms that provide the great sensitivity that is seen in amoeboid directed migration.
Signal transduction network
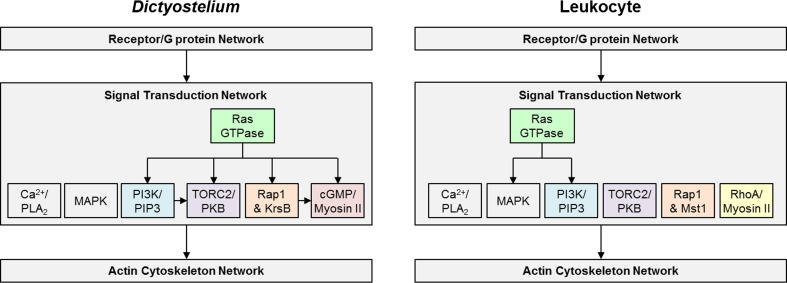
The following signaling modules are downstream of the receptor/G protein network and will be discussed in this section: Ras GTPase, PI3K/PIP3, TORC2/PKB, PLA2, Ca2+, MAPK, Rap1 and KrsB/Mst1, cGMP/Myosin II and RhoA/Myosin II (Fig. 3).
Fig. 3.
Topology of the signal transduction network in Dictyostelium and mammalian leukocytes. The signal transduction network is placed between the receptor/G protein and the actin cytoskeleton networks, while the polarity network is omitted from this figure. The individual modules within the Signal Transduction Network are represented by a specific color and these colors will be used to represent the modules in subsequent figures. The arrows depict interactions between the modules that are strongly supported in the literature
Ras GTPase Module
Dictyostelium
Dictyostelium cells express several members of the Ras small GTPases family that are central to directed cell migration, but the precise functions that Ras proteins play is still under investigation. There are 14 Ras family genes in the Dictyostelium genome and the 5 characterized isoforms RasS, RasD, RasB, RasC, and RasG share similarities with mammalian H-Ras and K-Ras [41, 42]. Evidence suggests that RasS modulates endocytosis and negatively regulates cell speed [43]; RasD mediates multicellular slug thermotaxis and phototaxis [44]; RasB controls the contractile pathway during chemotaxis through myosin heavy chain kinase A (MHCKA) and is also involved in mitosis [45, 46]. In contrast to the other Ras G proteins, RasC and RasG proteins appear to be particularly important for chemotaxis.
Due to the overlapping functions it has been difficult to establish the specific roles of RasC and RasG [42]. Expression of constitutively active RasC leads to the activation of many biochemical pathways, which results in a dramatic increase in cell spreading and cytoskeletal activity, consequently altering migration [47]. Similarly, expression of constitutively active RasG or deletion of its GTPase activating protein (GAP) DdNF1 leads to overactivation of phosphoinositide 3-kinase (PI3K) and a defect in migration [48]. However, while studies of cells with deletions of RasG, RasC, or RasC/G have suggested that these proteins represent an essential basal signaling module necessary for chemotaxis, others indicate that chemotaxis can occur in the absence of these proteins, at least in steep gradients [49]. These discrepancies may be due to possible compensatory expression or activity of many different proteins, for example of RasD in RasG-null cells [44]. In addition to RasC and RasG having both overlapping and unique functions, RasD and RasB also show functional overlap with RasG [42, 50, 51]
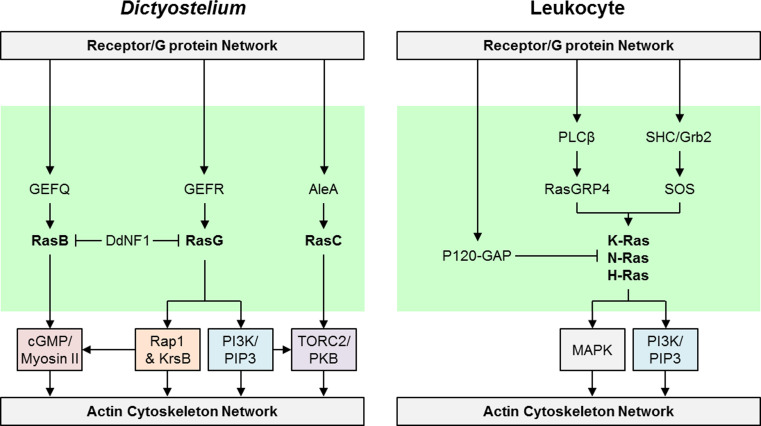
The Ras module is downstream of the receptor and heterotrimeric G proteins, and studies using a combination of genetic mutations and pharmacological inhibitors suggest that it is upstream of several other signal transduction modules (Fig. 3) [42, 52]. First, it has been shown that in strains deficient in cAMP signaling, such as the car1/3¯ and gβ¯ strains, there is no chemoattractant-mediated Ras activation [53]. In gα2¯ cells there is a brief, weak Ras activation [54]. Second, RasC and RasG proteins activate separate downstream pathways (Fig. 4): RasC-GTP activates TORC2, whereas RasG-GTP and RasD-GTP interact with and activate PI3K1 and PI3K2 [47, 55, 56]. In addition, RasG is the major Ras protein that regulates Daydreamer (DydA), which localizes to the leading edge and is required for proper directed cell migration [57, 58]. Thus, RasC/G signaling pathways provide a link between heterotrimeric G proteins and downstream pathways.
Fig. 4.
Ras GTPase module in Dictyostelium and mammalian leukocytes. The arrow and bar lines represent positive and inhibitory links, respectively, and the lines shown depict interactions that are strongly supported in the literature
In many of the studies referenced above Ras activation has been monitored with a tagged Ras-binding domain (RBD) peptide. This probe utilizes the RBD of Raf1, which binds activated Ras-GTP and was originally tagged with GST to monitor mammalian cell cycle progression [59]. In Dictyostelium cells this probe reports localized Ras activity, although it preferentially binds RasG-GTP and not RasC-GTP [53]. During random migration the RBD-GFP probe localizes to sites of membrane protrusions. With uniform cAMP stimulation the probe relocalizes transiently from the cytosol to the entire membrane with a peak at ~ 3-6 s, and in a gradient it localizes to the leading edge, establishing its classification as a “front” protein [42]. When a gradient of chemoattractant is applied to the cell, the activation of Ras goes through phases of an initial uniform response, symmetry breaking of the response to one region of the cell, and then confinement of this activated Ras to a more restricted region of the cell membrane; with the whole process taking up to 90 s to complete [54]. Ras activation is independent of cytoskeletal activity: Patches of active Ras can be observed in unstimulated cells, uniform stimulus results in a uniform response, and cells in a gradient of chemoattractant elicit a crescent towards the high side of the gradient (see Fig. 2) [42].
Precise regulation of Ras activity in Dictyostelium is most likely due to the direct activity of numerous GEF and GAP proteins. In fact, the first suggestion that Ras proteins were downstream of receptor in GPCR-mediated chemotaxis came from the discovery that Aimless (AleA, GEFA), a homolog of mammalian Son of Sevenless (SOS) RasGEF, was required for Dictyostelium cells to aggregate [60, 61]. It is now known that GEFA is part of a complex (Sca1 complex) that includes Sca1, GEFH, PHR, and protein phosphatase 2A (PP2A) [62]. It has been shown in vitro that GEFA is the major activator of RasC nucleotide exchange, while GEFR is required for the activation of RasG [63]. There are 25 genes encoding Ras GEFs in the Dictyostelium genome, and their exact functions are complex and difficult to classify. Based on the studies of null cell lines, different GEFs have distinct roles in the initial uniform response, symmetry breaking, and crescent confinement in RBD-GFP localization experiments [54]. The RasGAP DdNF1 is distributed uniformly on the membrane, negatively regulating both RasB and RasG activity, which helps lead to symmetry breaking and confinement in chemotaxis [48, 54]. Moreover, some evidence suggests RasGAPs might act as global signaling inhibitors that are activated upstream of the Ras G proteins [64].
Leukocytes
In mammalian cells Ras oncogenes are well known to regulate proliferation, cell growth, survival, and energy metabolism; however, Ras involvement in leukocyte chemotaxis has not been extensively studied [65]. The three most commonly studied mammalian Ras genes encode four homologous proteins with slightly different post-translational modifications on their carboxyl terminals: H-Ras, N-Ras, KA-Ras, and KB-Ras [66]. In mice, genetic mutations have shown that only the K-Ras gene is essential for embryonic development, suggesting unique functions of the Ras gene products [67]. Interestingly, K-Ras has previously been shown to be mutated in ~ 30 % of all human tumors [68].
Leukocyte chemokine and fMLP GPCRs have both been shown to activate and utilize Ras protein signaling in directed cell migration. One of the first observations that Ras proteins were acting downstream of a chemoattractant receptor was in neutrophils [69]. Worthern et al. reported that the Ras/Raf/MEK pathway was activated by fMLP stimulation in a pertussis toxin-sensitive manner, with a maximum response in ~ 2 min. In addition, neutrophil chemotaxis mediated by interleukin and integrin signaling was shown to be regulated by Ras activity [70, 71]. In Jurkat T cells, H-Ras is reportedly involved in directed transendothelial migration and integrin signaling in response to SDF-1α [72]. It is also reported that Ras is activated when CCR7 (an additional receptor for SDF-1α) is occupied in primary T cells [73]. In addition to G protein-mediated responses, an SDF-1α receptor CXCR4 transactivates receptor tyrosine kinases (RTKs) leading to the recruitment of a RasGEF SOS to induce chemotaxis responses [74].
The ways in which chemoattractant GPCRs regulate the Ras module is only beginning to be elucidated in mammalian leukocytes. The p110γ subunit of PI3Kγ, which is highly expressed in neutrophils, has a Ras-GTP binding domain and requires H-Ras for optimum activity [75]. This places Ras protein signaling, at least in part, upstream of the PI3K/PIP3 module in leukocytes (Fig. 4). A recent report suggests that PLCβ mediates Ras activation by releasing diacylglycerol, which activates RASGRP4 (Ras GEF). Compared to wild type, fewer RasGRP4-null neutrophils migrate toward the chemoattractant [76]. However, this GEF can also activate another small GTPase Rap1, which may contribute to the observed phenotype. There is additional evidence that suggests that fMLP-stimulated inhibition of Gap120 (Ras GAP) enhances K-Ras and N-Ras signaling in neutrophils, leading to increased directed migration [77]. The Ras GAP neurofibromin (NF1) is highly expressed in leukocytes, but the role NF1 plays in leukocyte cell biology remains to be studied [78].
PI3K/PIP3 Module
Dictyostelium
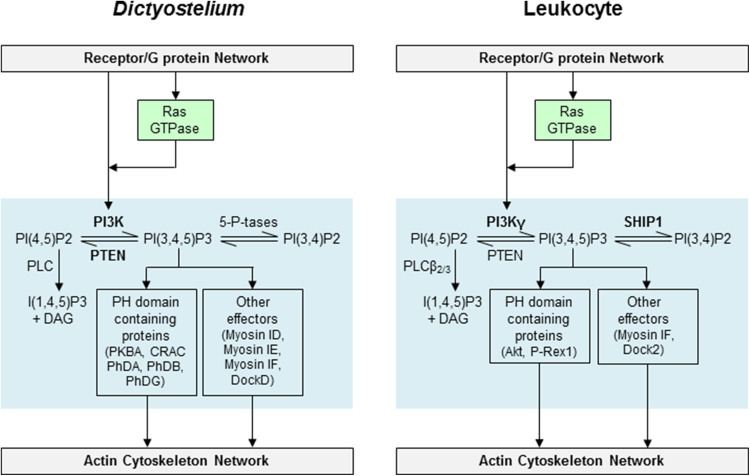
One of the hallmarks of a migrating cell is the establishment of a phosphatidylinositol (3,4,5)-trisphosphate (PIP3) gradient across the cell. PIP3 levels are regulated by the action of PI3K, which converts PI(4,5)P2 into PIP3, and a 3-phosphatase PTEN, which mediates the opposite reaction (Fig. 5). Although PIP3 can be degraded by 5-phosphatases to generate PI(3,4)P2, this process does not appear to play a major role in chemotaxing cells as PI(3,4)P2 does not accumulate in response to cAMP stimulation, nor does deletion of any of the four inositol-5-phosphatases either alone or in combinations of two reduce the cells’ ability to chemotax [79, 80]. Thus, conversion of PIP3 to PI(4,5)P2 by PTEN appears to be the predominant route for PIP3 degradation. In a migrating cell PI3K localizes to and is activated at the leading edge, while PTEN is excluded from the front and instead localizes to the back and sides. Treatment with cAMP stimulates rapid activation of PI3K. This is thought to be achieved by the interaction of membrane-associated PI3K with RasG, as well as F-actin-dependent recruitment of PI3K to the plasma membrane [42, 56]. However, while PI3K recruitment is impaired in cells with a disrupted cytoskeleton due to Latrunculin A treatment, PIP3 is generated effectively. At the same time, PTEN dissociates from the plasma membrane, possibly due to depleted PI(4,5)P2 levels [81]. The main enzyme mediating PI(4,5)P2 degradation is phospholipase C (PLC), which generates inositol (1,4,5)-trisphosphate (IP3) and diacylglycerol in response to cAMP treatment [82]. If PLC were activated at the front of a cell, it could lead to a reduction in PI(4,5)P2, which would favor local PTEN dissociation [83]. Although PLC-null cells do not have defects in chemotaxis, overexpression of PLC mimics the phenotype of PTEN-null cells, as will be discussed below. Together, this spatial and temporal control leads to the accumulation of PIP3 at the leading edge.
Fig. 5.
PI3K/PIP3 module in Dictyostelium and mammalian leukocytes. The arrow and bar lines represent positive and inhibitory links, respectively, and the lines shown depict interactions that are strongly supported in the literature
Aberrant chemotaxis in cells with excessive PIP3 accumulation highlights the importance of the PI3K/PIP3 pathway. PTEN-null cells have increased PIP3 around the cell perimeter. These cells have many protrusions that are not restricted to the leading edge, and consequently they move and chemotax poorly. Disruption of another negative regulator of the PI3K/PIP3 pathway, the dual-specificity kinase SHK1, also results in cells that lack polarity and consequently move slowly [84]. Interestingly, unlike PTEN, these cells do not have increased PIP3 levels basally, but instead have a prolonged peak of cAMP-stimulated PH domain recruitment and PKBA activation. Similarly, expression of PI3K with a myristoylation tag that targets it constitutively to the plasma membrane also leads to additional PIP3 accumulation, extra protrusions, and defective chemotaxis [56].
PIP3 interacts with a number of downstream targets with diverse roles. Most proteins interact with PIP3 via pleckstrin homology (PH) domains, including PKBA, CRAC, and the PhD family of proteins. All of these proteins are recruited to the leading edge during chemotaxis, or to the cortex with global cAMP stimulation. Cells lacking PIP3-interacting proteins have variable defects in chemotaxis. For example, one report indicates that cells lacking the Akt homologue PKBA do not polarize and have reduced migration speed in gradients of cAMP [85]. Another indicates that deletion of PKBA has little consequence, but in cells lacking PTEN deleting this kinase rescues the aberrant chemotaxis phenotype [86]. Once recruited to the plasma membrane, PKBA is activated by phosphorylation by phosphoinositide-dependent protein kinase (PDK) and TORC2. A close homolog of PKBA, PKBR1, is myristoylated and thus constitutively membrane-bound, making its activation PIP3-independent but TORC2-dependent. Together the two PKBs transiently phosphorylate downstream substrates, including p21-activated kinase (PakA), talin, Ras GEFs N and S, a scaffold protein supporting another Ras GEF A, Rho GAPs G and Q, and PI4P 5-kinase, possibly providing links to the cytoskeleton and generating feedback loops [55, 62, 86, 87]. PakA, which has been shown to play a role in myosin II dynamics, appears to be an important target of PKBA since its deletion rescues the phenotype of PTEN-null cells [86, 88]. Interestingly, phosphorylated PakA localizes to the back of a migrating cell similarly to PTEN and myosin II. How activated PKBA, which localizes to the leading edge, is able to activate a back protein remains unclear.
Other PH domain-containing proteins have diverse roles in chemotaxis. The PH domain-containing protein cytosolic regulator of adenylyl cyclase (CRAC) was originally identified as a critical regulator of cAMP-stimulated adenylyl cyclase activation [89, 90]. The chemotactic defect of crac¯ cells is independent of their deficiency in AC activity, since C-terminal truncations that lack AC activity are able to rescue the aberrant chemotaxis of crac¯ cells [91]. The Phd family of proteins interact with PIP3 via their PH domains, and four Phd proteins (A, B, G, I) have been identified [92–94]. Cells lacking PhdA, PhdB, and PhdG exhibit chemotaxis defects. Consistent with the PIP3-interacting property of PH domains, PhdA and PhdI localize to the leading edge of a migrating cell, and are recruited to cell cortex with global cAMP stimulation [92, 93]. The affinity of PhdG for PIP3 appears to be quite low; however, it associates with the plasma membrane when PIP3 levels are elevated, for example, in PTEN-null cells [93]. PhdB, which is recruited to the plasma membrane by both PIP3-dependent and PIP3-independent means, is reported to act as a GAP for Rap1 (RapGAP3) [94]. Its localization is not clear since one group reported localization at the leading edge, and another at the lagging edge of a migrating cell [93, 94].
In addition to recruiting downstream targets via PH domains, PIP3 can also interact with several other domains. Actin-based motor proteins myosins ID, IE, and IF are recruited to PIP3 at the leading edge via their tail homology 1 (TH1) domain [95]. Cells lacking all three of these myosins have reduced cAMP-stimulated actin polymerization and defective chemotaxis. Two Dock180-related RacGEFs (DockA and DockD) also regulate actin dynamics, cAMP-stimulated polarization, and motility [96]. Similarly to mammalian Dock180, DockA and DockD possess CDM-zizimin homology (CZH1) domains that interact with phospholipids. In particular, DockD is recruited to the cortex in response to chemoattractant treatment, and this recruitment depends on PIP3 generation. Thus, DockD, together with functionally redundant DockA, might link PIP3 signaling to actin polymerization.
Despite the importance of localized PIP3 generation, the PI3K/PIP3 pathway is not essential for chemotaxis. Dictyostelium possesses five class I PI3K genes, with PI3K1-3 being most similar to mammalian p110 PI3Ks. Hoeller and Kay created cells lacking the five PI3K genes as well as PTEN. PIP3 levels in these cells were undetectable by PH domain recruitment, although PKBA activation in response to cAMP was reported, and PIP3 was biochemically detectable [97, 98]. These gene disruptions minimally impaired the ability to sense a chemotactic gradient, although there was a marked reduction in speed both during chemotaxis and random motility, suggesting that PIP3 signaling plays a critical role in this process [98]. Similar observations were made either using chemical inhibition of PI3K with 60 μM LY294002 or by disrupting PI3K1 and 2, which nearly abolishes cAMP-stimulated PIP3 production. Although both of these conditions reduced motility speed, they did not affect cells’ directionality in a gradient [98–103]. It is important to note that the findings that PI3K is important for motility but not directionality are based on experiments using steep gradients. In contrast, in shallow gradients the PI3K/PIP3 pathway is required for proper cell orientation in the direction of the chemoattractant source [101, 103]. One possible reason for why excessive PI3K/PIP3 pathway can induce pseudopod production but its disruption does not always impair chemotaxis is that cells possess several parallel pathways that transmit input from the receptor to the actin cytoskeleton. Some of these pathways, including those involving TORC2, PLA2 and cGMP, will be discussed below.
Leukocytes
Chemoattractants trigger activation of PI3K and consequent generation of PIP3 in leukocytes (Fig. 5). The PH domain of Akt, which binds PIP3 and its by-product PI(3,4)P2, localizes to the leading edge of migrating neutrophil-like HL-60 cells [104]. Mammalian class I PI3K is divided into two types: IA (α, β, δ) and IB (γ). Although leukocytes express all 4 class I PI3Ks, PI3Kδ and PI3Kγ are predominant. Mammalian PI3Kγ, or p110γ is activated by Gβγ subunit following GPCR activation by a chemoattractant. In addition, PI3Kγ activation depends on interaction with Ras [105]. Neutrophils from PI3Kγ-null mice showed no chemoattractant-stimulated PIP3 accumulation, both biochemically and using the PH-Akt probe, suggesting this is the main isoform activated in this cell type [106–109]. In contrast, class IA PI3Ks are typically activated downstream of RTKs; however, GPCRs can also trigger activation of this class of PI3Ks.
As in Dictyostelium, the PI3K/PIP3 pathway is important, but not essential for leukocyte chemotaxis. The role of the PI3K/PIP3 pathway in leukocytes, including neutrophils, T cells, and natural killer cells, was first examined using general PI3K inhibitors, wortmannin and LY294002 [110–114]. Although these inhibitors resulted in significantly reduced chemoattractant-induced migration, the inhibition was not complete. Similarly, neutrophils and macrophages from PI3Kγ-null mice showed reduced motility, although they were still able to chemotax [106–108, 115, 116]. It appears that PI3Kγ might regulate motility by affecting integrin-based adhesion and F-actin accumulation, but not the ability of cells to sense direction once they are migrating [109]. Even though PI3Kγ is the main GPCR-activated isoform, PI3Kδ is also involved in regulation of directed cell migration, and the importance of a particular PI3K isoform might depend on the cell type examined. Using cells from mice lacking specific PI3K isoforms, PI3Kγ appears to be involved in T cell chemotaxis, whereas PI3Kδ is important in B cells [117]. Other reports indicate that both class 1A and 1B PI3Ks are important for T cell migration [118]. A study using isoform-specific inhibitors suggests that PI3Kδ also plays a significant role in neutrophil migration [119]. Interestingly, one study showed temporal regulation of the different classes. Using dominant negative p85α and p110γ to disrupt class IA and IB PI3K, respectively, Boulven et al. found that PI3Kγ activity is important for the first peak of PIP3 generation (30 s), whereas class IA PI3Ks are important for the second prolonged peak (120 s) in a neutrophil-like cell line [120]. Only the second peak was deemed important for chemotaxis in this system. Another study showed that PI3Kγ and PI3Kδ mediate short-term (90 min) and long-term (several hours) neutrophil emigration, respectively, in vivo [121].
As in Dictyostelium, PI3K activation in leukocytes causes recruitment of PIP3-binding effectors. Some of the effectors, including Akt/PKB, DOCK180-related RacGEFs and myosin I, are clear homologs between the two systems. Others, such as p-Rex1, have only been found in leukocytes so far. Recruitment of Akt, via its PH domain, is often used as a read-out of PIP3 generation [104]; however, despite its robust localization to the leading edge, the exact role of Akt in chemotaxis is not clear. Neutrophil chemotaxis is slightly reduced using a specific Akt inhibitor, but only under certain assay conditions [122]. Mammalian Akt has 3 isoforms (Akt1-3), with Akt1 and Akt2 being most abundant in leukocytes. Using single knockouts, Chen et al. demonstrated that in neutrophils Akt2, but not Akt1, is recruited to the leading edge and is required for migration [123]. It appears that Akt might regulate cell motility by stimulating the production of F-actin in response to fMLP [124]. In fact, some evidence for direct links between Akt and the cytoskeleton is beginning to accumulate. One example is Akt-mediated phosphorylation and inhibition of GSK3β, which leads to activation of an actin-binding and depolymerizing protein cofilin by activation of its phosphatase slingshot2 [125]. Analogously to Dictyostelium, Pak might also be a potential downstream target of Akt. In leukocytes Pak, along with Pak-associated GEF (PIXα), modulates actin polymerization, polarization, adhesion, and chemotaxis [126–128]. Although it is not known whether Pak is an Akt substrate in leukocytes, Akt does phosphorylate and activate Pak in other mammalian cell types [129].
The PI3K/PIP3 pathway can also modulate cytoskeleton via PIP3-mediated recruitment of RacGEFs. P-Rex1, a RacGEF with preference for Rac2, is synergistically activated by PIP3 and Gβγ via interaction with PH and Dbl-homology domains, respectively [130, 131]. DOCK2, a DOCK180-related protein expressed primarily in cells of hematopoietic origin, interacts with PIP3 via its DOCK homology region 1 domain, and this association is enhanced by ELMO1 [132]. Both DOCK2 and P-Rex1 are recruited to the front of chemotaxing neutrophils [132, 133]. Interestingly, DOCK2 slowly accumulates at the leading edge even in the absence of PI3Kγ by associating with phosphatidic acid generated by phospholipase D [134]. Neutrophils from DOCK2-/- or P-Rex1-/- mice have reduced migration speed, although the defects are not complete given the functional redundancy of the two RacGEFs [131, 132]. DOCK2 might have a more prominent role in leukocyte chemotaxis, since DOCK2-/- neutrophils also had defects in directional sensing [132]. Another connection between PIP3 and the cytoskeleton is myosin IF, which is recruited to PIP3 at the leading edge via its TH1 domain, similarly to the homologous myosin ID, IE and IF in Dictyostelium [95].
Negative regulation of PI3K/PIP3 signaling can be achieved by several means, including degradation of the PI3K substrate PI(4,5)P2 and its product PIP3, as well as direct inhibition of PI3Kγ activation. The latter is achieved by a Gβγ-binding protein RACK1, which competes with PI3Kγ and other effectors for binding to Gβγ [135]. Consistent with this role, knock-down of RACK1 enhances, whereas overexpression dampens chemotaxis of Jurkat T cells and differentiated HL-60 cells. Regulation by phosphoinositide degradation is complex and appears to be cell-type and context-dependent. The main PI(4,5)P2-degrading enzyme in leukocytes, PLCβ–2/3, is required for chemotaxis of T lymphocytes, but not neutrophils [115, 136, 137].
PIP3 can be dephosphorylated at the 3’ position by PTEN and at the 5’ position by SHIP1 phosphatases. In Dictyostelium the opposing localization of PI3K and PTEN clearly establishes the PIP3 gradient in a migrating cell. In contrast, in leukocytes there are conflicting reports of PTEN localization to the back of a cell [126, 138–140]. The reasons for the differences in observations are unclear. Disrupting PTEN by knock-out or knock-down approaches leads to slightly enhanced cell speed and very minor, if any, effects on gradient sensing [141–143]. Overexpression of PTEN slightly decreases cell speed without affecting directionality [138]. Consistently, in vivo recruitment of PTEN-null neutrophils is also improved [143, 144]. In contrast to the weak phenotype of PTEN-null leukocytes, disruption of the 5-phosphatase SHIP1, which causes highly elevated levels of PIP3, leads to a phenotype that is similar to the PTEN-null Dictyostelium cells [116]. SHIP1-null neutrophils display a broad flattened morphology, defects in polarization, increased basal F-actin, and reduced migration, and their directionality is not affected [116]. Increased spreading and reduced motility might be due to enhanced adhesion of ship1 -/- cells to the substrate [145]. Similarly to the observations in neutrophils, SHIP1-null macrophages and T lymphocytes also have reduced motility but not directionality [116, 146]. The situation in vivo is complicated by the presence of multiple chemoattractants, and evidence exists that PTEN might be important for prioritizing these signals [140]. Presumably inhibition of PTEN leads to PIP3 localization around the entire cell perimeter, instead of at the leading edge, allowing for activation of parallel pathways, such as PLA2 and p38 MAPK, which are important for the recognition of bacterially derived ‘end-target’ chemoattractants [140, 147].
TORC2/PKB Module
Dictyostelium
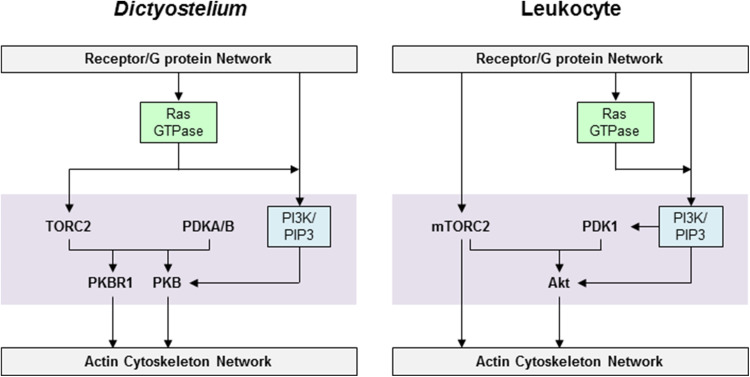
As previously mentioned in the PI3K/PIP3 section, PKBA and PKBR1 are a central part of a signaling hub, are responsible for the relay of extracellular signals in Dictyostelium chemotaxis, and phosphorylate numerous downstream substrates important for migration. These AGC family kinases are homologs of mammalian PKB (also known as Akt) [85, 148]. Akt activation occurs when levels of PIP3 increase causing the N-terminal PH domain to localize the protein to the membrane [149]. PKBA in Dictyostelium also contains a PH domain and transiently translocates to the plasma membrane upon cAMP stimulation [85]. In contrast, the homologous PKBR1 lacks a PH domain but has a myristoylation site that keeps the protein constitutively at the membrane [148]. PKBR1 appears to be more important as pkbR1¯ cells display greater defects in chemotaxis than pkbA¯ cells [85, 148]. Importantly, PKBR1 provides a pathway to phosphorylate PKB substrates independently of PIP3 and thus a rationale for understanding PIP3-independent chemotaxis. In contrast to the stronger activation of PKBR1 induced by cAMP, folate appears to mainly activate PKBA [97]. Expression of PKBA in pkbR1¯ cells and pkbA¯/pkbR1¯ double knockout cells further shows that these two proteins have overlapping and distinct functions for chemotaxis [55, 148].
PKB signaling in Dictyostelium chemotaxis receives input from RasC and RasG heterotrimeric G protein-dependent pathways (Fig. 6). Ras interacting protein 3 (Rip3), so designated because it interacts with human H-Ras in 2-hybrid assays, was isolated in a screen for chemotaxis mutants in Dictyostelium [150]. Since RIP3-null cells share many similar chemotaxis defects as GEFA-null cells, Rip3 appears to be an additional RasG regulatory protein [150]. Another gene isolated in a mutagenesis screen was pianissimo (PiaA), which when disrupted leads to the silencing of chemoattractant-mediated responses [151]. PiaA and Rip3 are highly conserved in evolution and were later discovered to be associated with target of rapamycin (TOR) kinases in yeast and Drosophila, defining a complex designated as TORC2 (TOR Complex 2) [152, 153]. The mammalian homologues of PiaA and Rip3 were designated as Rictor and mSIN1, respectively. The proteins appear to form a complex in Dictyostelium when examined by co-immunoprecipitation, but it is unstable and has not been purified [154]. Evidence suggests that TORC2 is activated by RasC since phosphorylation at specific TORC2 sites on the hydrophobic motifs of PKBs is low in rasC¯ cells [47, 155]. Moreover, the immunoprecipitated complex can restore RasC-activated phosphorylation of the hydrophobic motif in vitro. There is also evidence for negative feedback from PKB signaling to the upstream pathways as TORC2 or PKB signaling disruption leads to an increase in RasC activity [62]. In addition to the importance of the RasC pathway, the previously discussed RasG pathway is essential for the activation of PI3K and initiating PIP3 signaling required for phosphorylation of PKBA [56]. Note that RasC-mediated activation of PKBR1 and phosphorylation of PKBA substrates provide a PIP3-independent pathway from chemoattractant to cytoskeleton activation.
Fig. 6.
TORC2/PKB module in Dictyostelium and mammalian leukocytes. The arrow and bar lines represent positive and inhibitory links, respectively, and the lines shown depict interactions that are strongly supported in the literature
In Dictyostelium the mechanisms of PKBA and PKBR1 activation appear to be more complex than the phosphorylation of the consensus sites in the activation loop and hydrophobic motif required for mammalian Akt activation. While phosphomimetic substitutions in the activation loop and hydrophobic motif of mammalian Akt induce constitutive activation of the kinases, the same mutations in PKBR1 do not [55]. There is also evidence for reciprocal regulation of phosphorylation between the activation loop and hydrophobic motif sites, specifically with the hydrophobic motif phosphorylation by TORC2 being required for optimal phosphorylation of the activation loop site by PDKs [55, 97, 154]. Dictyostelium possesses two orthologous PDKs, PDKA and PDKB, and, surprisingly, their PH domains are not essential for their activity [154]. PDKA translocates to the membrane upon stimulation and phosphorylates both PKBA and PKBR1 on their activation loop sites, while PDKB is always in the cytosol and phosphorylates only PKBR1 on its activation loop site. Interestingly, in pdkA¯/pdkB¯ cells residual activation loop site phosphorylation remains on both PKBA and PKBR1 suggesting additional kinase(s) play a role in regulating these proteins [154].
Leukocytes
The role that Akt and mTORC2 play in leukocyte chemotaxis is only beginning to be defined. In neutrophils phosphorylation of Akt occurs after fMLP stimulation and is lost in PI3K-null and wortmannin-treated cells (Fig. 6) [107]. GFP-tagged PH domain of Akt shows “front” protein localization dynamics in response to fMLP stimulation, and a pertussis toxin sensitivity for this localization demonstrates Gαi dependency [104, 156]. Chemotactic behavior elicited by activating the chemokine receptor CXCR4 in mature dendritic cells also utilizes Akt [157]. As appropriate chemotaxis responses require Akt activity in leukocytes, it is not surprising that the knock-down of PDK1 leads to decreased chemotaxis [158]. The mTORC2 complex has been shown to regulate F-actin production in a Rac/CDC42-dependent manner in neutrophils [159]. Knock-down of Rictor leads to decreased chemotaxis migration speed and F-actin production. In addition, mTORC2 regulates myosin II activity via the protein kinase A (PKA)/RhoA pathway, as will be described in the RhoA/myosin II module [122]. There are some major differences in the observed TORC2 signaling between neutrophils and Dictyostelium. First, Rictor in neutrophils localizes to the leading edge, whereas PiaA in Dictyostelium does not. Second, in neutrophils the kinase activity of mTORC2 is reportedly not required for chemotaxis [159].
PLA2 module
Dictyostelium
The ability of cells to migrate in the absence of PIP3 generation prompted the search for parallel pathways. Phospholipase A2 (PLA2A) was identified as a chemotaxis mediator using a screen for mutants that show increased migration defects in the presence of a PI3K inhibitor LY294002 [160, 161]. Exogenous arachidonic acid can rescue the increased sensitivity of PLA2A-null cells to PI3K inhibition. Interestingly, the PLA2A pathway appears to be regulated by intracellular Ca2+, although this effect is indirect, since similarly to its mammalian group VI PLA2 (iPLA2) homologs, PLA2A is Ca2+-independent.
Leukocytes
Leukocytes have five types of PLA2, of which secretory, cytosolic group IV (cPLA2), and Ca2+-independent PLA2 (iPLA2) have been analyzed with respect to chemotaxis. In neutrophils, both cPLA2 and iPLA2 are implicated in chemotaxis to fMLP [140]. Furthermore, inhibition of cPLA2 reduces IL-8 induced polarization and migration in neutrophils; however, in this situation it is unlikely that cPLA2 is part of a parallel pathway since it lies downstream of PI3K, ERK1/2, and p38 MAPK when regulating integrin-based adhesion [162]. In monocytes, both cPLA2α and iPLA2β regulate MCP-1 induced cell migration by producing arachidonic acid and lysophosphatidic acid, respectively [163–165]. Interestingly, while downregulation of either enzyme reduces cell speed, only iPLA2β, which localizes to the leading edge with stimulation, affects directionality and actin polymerization. Parallel inhibition of PLA2 with other chemotactic pathways has not been tested in leukocytes thus far.
Ca2+ module
Dictyostelium
Although chemoattractants trigger Ca2+ influx and a rise in intracellular Ca2+, the importance of this process for chemotaxis is controversial. Deletion of the homolog of the IP3 receptor (IplA) alone, which abolishes chemoattractant-triggered Ca2+ increase without perturbing resting Ca2+ concentration in the cytosol, does not affect any of the chemotactic responses [166]. However, iplA-null cells might still have very low Ca2+ influx that complicates the conclusion of Ca2+ independence [167]. Furthermore, Ca2+ chelation reduces cell spreading and lowers speed during chemotaxis, and extracellular Ca2+ can improve cAMP-mediated migration at certain concentrations [168–171]. Interestingly, similar concentrations of Ca2+ can induce improvements in cell migration in the absence of cAMP, suggesting that most of the effects on chemotaxis are due to changes in migration. Overall, it is possible that cAMP-mediated chemotaxis, as well as random migration, require low resting levels of Ca2+, for example, for maintenance of proper adhesion; however, Ca2+ flux is likely not essential. Few studies have assessed a role for Ca2+ when other pathways are co-inhibited.
Leukocytes
Similarly to Dictyostelium the role of Ca2+ in leukocyte chemotaxis has been the subject of controversy. Chemoattractants trigger a transient increase in intracellular Ca2+ that depends on PLCβ-mediated generation of IP3 [115]. However, inhibition of the IP3 receptor does not affect T lymphocyte chemotaxis [137]. In contrast, although disruption of Ca2+ transients has no effect on pseudopod extension, it can prevent uropod release and thereby reduce migration of neutrophils on adhesive surfaces (reviewed in [172] ). In addition, intracellular Ca2+ forms a gradient, with the highest concentration in the uropod of basophils [173]. It appears that transient elevation of Ca2+ likely has two roles in leukocytes: disruption of specific integrin-based adhesions and activation of Ca2+/calmodulin-dependent MLCK [174, 175]. Together, this allows for myosin II-mediated uropod retraction. Thus, although Ca2+ is not required for pseudopod extension, it is important for overall cell motility.
MAPK Module
Dictyostelium
Dictyostelium cells express homologs of mitogen-activated protein kinases (MAPKs)/extracellular signal-regulated kinases (ERKs) that in many other eukaryotic cells are induced by extracellular receptors to modify gene expression and behavior. Kinomic analysis of the Dictyostelium genome revealed that they have a simplified collection of the MAPK/ERK family of kinases: two MAPKs (ERK1 and ERK2), one MAPKK (MEK1), and one MAPKKK (MEKK) (compared to 12, 7, and about 14, respectively, in mammals) [176, 177]. This makes Dictyostelium an exceptional simplified model for the study of the MAPK signaling cascades and their role in chemotaxis. Dictyostelium lack homologs for parts of the JNK and p38 pathways, but do possess an ERK7 homolog [176]. Two MEKK-like proteins have also been identified in Dictyostelium: MEKKa and stress-activated protein kinase α (SAPKα). MEKKa localizes to the cortex and is important for developmental morphogenesis [178]. SAPKα is localized to actin-based protrusions, positively regulates F-actin, and when knocked out or overexpressed causes decreased chemotaxis [179]. Neither of these MEKK-like proteins couple to the downstream ERK1 or ERK2 pathways.
The MEK1/ERK1 pathway is essential for proper chemotaxis to cAMP and folic acid in Dictyostelium. A null mutation in the MEK1 gene significantly decreases chemotaxis and leads to lower cAMP-mediated stimulation of both guanylyl cyclase and adenylyl cyclase [180]. MEK1 requires transient stimulated SUMOylation for appropriate localization to the cortex and the leading edge of migrating cells. MEK1 is upstream of ERK1 as overexpression of a constitutively active form of MEK1 does not alter the loss of directed migration phenotype in erk1¯ cells and there is no cAMP-stimulated activation of ERK1 in MEK1-null cells [181]. A MEK1/protein phosphatase 4 complex (PP4C)/SMKA (SMEK) pathway has also been shown to be important for chemotaxis and is independent of, but may interact with, the cAMP-activated MEK1/ERK1 pathway [182, 183]. The erk1¯ cells exhibited similar defects in development and chemotaxis as MEK1-null cells [181]. ERK1 has leading edge localization and has maximal cAMP-stimulated activity at 15 s. A negative regulatory interaction exists between phosphotyrosine phosphatase 2 (PTP2) and ERK1 activity, at least at the genetic level [184].
The ERK2 MAPK pathway is important in chemotaxis relay and the formation of developmental aggregates. ERK2 was originally discovered in a mutagenesis screen for mutants defective in aggregation. The developmental defect in the erk2¯ cells is due to a lack of adenylyl cyclase A (ACA) activity [185, 186]. The cAMP-stimulated activation of ERK2 requires cARs, but there are conflicting reports on its requirement for Gα and Gβ heterotrimeric G proteins [187, 188]. Unlike many chemotaxis ligand-activated pathways, ERK2 appears to be non-adaptive under continuous stimulation by cAMP, since dephosporylation does not occur in the presence of the stimulus [189]. Folic acid also stimulates ERK2 phosphorylation leading to ACA activity [190]. ERK2 has been shown to regulate intracellular cAMP by inhibition of the phosphodiesterase RegA [191]. It is postulated that PKA negatively regulates ERK2 and thus establishes an oscillatory circuit containing ERK2, RegA, ACA, and PKA [191, 192]. RegA is also reported to be important during chemotaxis for the suppression of lateral pseudopods [193].
Leukocytes
Like Dictyostelium, leukocytes utilize MAPK pathways to regulate chemotaxis induced by different GPCRs with cell type specificity. In neutrophils, stimulation of FPR1 with fMLP induces transient phosphorylation and activation of MEK/ERK with a peak ~ 2-5 min, and this activation is reduced by chelation of Ca2+ [194]. MAPK stimulation by fMLP is greater than by other chemoattractants, such as C5a, LTB4, platelet-activating factor (PAF), and IL8, although the dynamics of activation are similar [70, 194–196]. Chemoattractants stimulate activation of the upstream kinase of MEK, Raf (MEKK), in a Gαi- and PI3K-dependent manner [69, 197, 198]. The p38 MAPK pathway is also activated by fMLP/PAF, and this activation is also PI3K-dependent as it is inhibited by wortmannin [199]. Whether p38 MAPK activation is dependent on Gαi heterotrimeric G protein is not clear since there are mixed reports as to the effect of pertussis toxin treatment [197, 199].
Chemotaxis to IL8 utilizes both MEK/ERK and p38 signaling as inhibition of these pathways with PD098059 and SKF86002, respectively, lowers chemotaxis parameters in neutrophils [111, 200]. The p38 MAPK pathway appears to be more important for neutrophil chemotaxis to fMLP than the MEK/ERK pathway since inhibition of p38 with SB20358 has a much stronger effect on chemotaxis than inhibition of MEK/ERK with PD098059 [201]. Moreover, phosphorylated p38 is localized to the leading edge of neutrophils migrating towards fMLP [202]. In eosinophils the effects of the PD098059 inhibitor show that the response to their chemokine eotaxin requires MEK/ERK signaling to increase F-actin levels in vitro [203]. T-lymphocytes require MEK/ERK signaling to chemotax to SDF-1α only when infiltrating interstitial tissue, or, by analogy, in a 3D extracellular matrix culture [204]. A Ras/MEK/LIM domain kinase (LIMK)/Cofilin pathway is believed to be responsible for allowing the lymphocytes to increase actin kinetics to “slide” through a 3D matrix [205]. Cofilin activity is inhibited by LIMK, and LIMK is inhibited by MEK signaling. Therefore, MEK activity enables cofilin to sever and depolymerize actin, generating free barbed ends and increasing G-actin levels, which leads to elevated actin turnover rates [206]. Another potential mechanism for the regulation of chemotaxis by p38 and ERK is activation of map kinase activated protein kinase-2 (MK2) as neutrophils from MK2-/- mice display decreased directionality in fMLP gradients [200, 207].
The importance of the p38 pathway for specific chemoattractants can be clarified when examining its functions in leukocyte migration to a site of infection through hierarchical gradients of chemoattractants. As previously mentioned, end-target chemoattractants, such as fMLP, require p38 signaling, but intermediary chemoattractants, such as IL8, do not. In addition, p38 MAPK signaling from fMLP can inhibit the PI3K/Akt signaling required for intermediary chemoattractants [147]. This creates a hierarchy that allows neutrophils to gradually lose preference for the intermediary chemoattractants, and accumulate at the site of infection.
Signaling at the level of the chemoattractant GPCRs provides additional degrees of regulation in MAPK signaling pathways. MEK/ERK and p38 pathways have opposing roles in the regulation of the FPR1 receptor in neutrophils. The ERK pathway potentiates the activity of the GPCR kinase (GRK) activity, thereby inhibiting neutrophil migration, while p38 phosphorylates the receptor to counteract GRK activity [202]. These two opposing signals most likely lead to an increase in the sensitivity of the system and ensure optimal neutrophil chemotaxis. In T-lymphocytes homo- and heterodimers of CXCR4 and CXCR7 receptors can form to detect SDF-1α. The CXCR4 receptors alone utilize Gαi-induced signaling to activate ERK1/2, but when they are in a complex with CXCR7, the ERK1/2 and p38 pathways are activated through β-arrestin [208]. The CXCR4/CXCR7 signaling through β-arrestin can persist even after receptor internalization, which provides prolonged signaling compared to the more transient heterotrimeric G protein-dependent signaling [209]. The signaling through either the CXCR4/CXCR4 or CXCR4/CXCR7 complexes depends on the expression of each receptor and therefore provides a mechanism to fine tune different MAPK signaling pathways through receptor levels.
Rap1 and KrsB/Mst1 Module
Dictyostelium
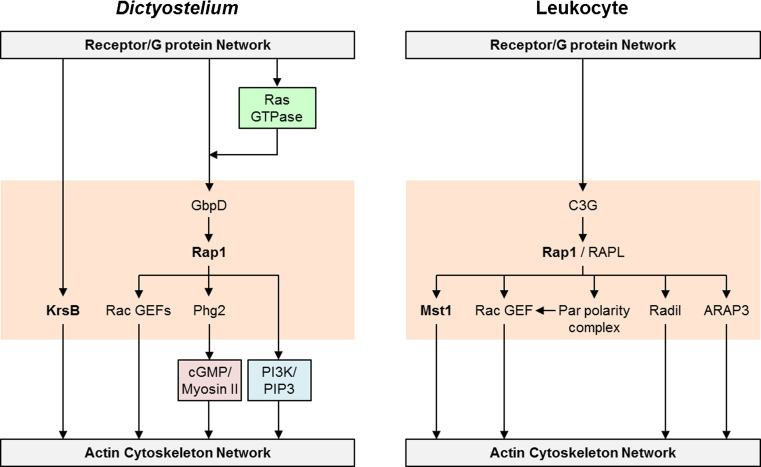
Dictyostelium Rap1 belongs to the Ras family of small GTPases and is very highly conserved with human Rap1 isoforms. Activation of the Rap1 pathway, either by using a constitutively active Rap1 mutant (G12V) or by overexpression of the Rap1 GEF, GbpD, leads to flattened cells that have increased adhesion to the substrate [210–212]. In addition, these cells have many lateral pseudopods, reduced polarity, and consequently poor chemotaxis. Conversely, knockout of gbpD or expression of the dominant-negative Rap1 mutant (S17N) reduces cell-substrate attachment, improves polarity, and enhances chemotaxis [210, 213].
Chemoattractants trigger rapid activation of Rap1 in a heterotrimeric G protein-dependent manner with kinetics only marginally slower than the activation of Ras (Fig. 7) [213, 214]. Consistent with this, the Rap1-GTP binding domain from human RalGDS, which is used as a probe for activated Rap1, transiently translocates to the plasma membrane with uniform chemoattractant stimulation, and localizes to the leading edge and weakly to the sides of cells migrating in a gradient [213]. The localization pattern of Rap1-GTP is broader than for Ras-GTP. RasG may regulate Rap1, since no activation is observed in rasG¯ cells in pulldown assays [215]. Rap1 GTPase activating protein RapGAP1 appears to be a negative regulator of Rap1 function, and also translocates to the cell cortex with chemoattractant stimulation, albeit with slower kinetics than the appearance of Rap1-GTP [216]. The recruitment of a GAP to the front of the cell likely limits the ability of Rap1 to stabilize pseudopods allowing for detachment and forward projection necessary for efficient chemotaxis. Interestingly, the role of Rap1 in regulating chemotaxis might not entirely depend on its function in adhesion, since a second Rap1 GAP (RapGAPB) also contributes to the negative regulation of Rap1 function in adhesion, but not chemotaxis under certain conditions; however, the localization of this GAP has not been examined [217].
Fig. 7.
Rap1 and KrsB/Mst1 pathways in Dictyostelium and mammalian leukocytes. The arrow and bar lines represent positive and inhibitory links, respectively, and the lines shown depict interactions that are strongly supported in the literature
Activated Rap1 interacts genetically or biochemically with a number of proteins; however, it is not yet clear how all of these various interactions are integrated to implement the functions of Rap1. First, a Ser/Thr kinase Phg2, which was originally discovered in a screen for phagocytosis mutants, has a Ras-binding domain that interacts with Rap1-GTP. Phg2 co-localizes with Rap1-GTP and is important for myosin II disassembly at the leading edge [213, 218]. Details of this pathway will be further discussed in the cGMP/Myosin II module. Although there is some controversy in the literature, the most recent study suggests that Phg2-null have increased adhesion and defective chemotaxis [213]. Interestingly, Phg2 appears to mediate the effects of Rap1 on adhesion but not on cell polarity, further highlighting that the role of Rap1 in chemotaxis is not limited to its effects on adhesion [211]. Second, Rap1 interacts with Rac GEFs in vitro, potentially explaining the increased F-actin accumulation observed in cells expressing constitutively active Rap1G12V [219, 220]; however, further studies on the direct involvement of the Rap1 pathway in the cytoskeletal dynamics are warranted. Third, activated Rap1 was shown to directly interact with the RBD of PI3K [221]. Furthermore, overexpression of GbpD leads to increased PIP3 levels both basally and following cAMP stimulation and also fails to enhance adhesion in the absence of PI3K1/2.
Another mediator of Dictyostelium spreading and adhesion that is regulated by chemoattractants is Kinase Responsive to Stress B (KrsB) [222]. KrsB is a Ser/Thr kinase that is homologous to tumor suppressors Hippo and Mst1/2 in Drosophila and mammals, respectively. Chemoattractants positively regulate KrsB function by inducing a transient G protein-dependent increase, like that seen for other “front” responses, in the phosphorylation of a conserved Thr residue (T176) in the activation loop. KrsB-null cells, as well as cells expressing KrsB that cannot be phosphorylated on T176 or is catalytically inactive are very adherent to the substrate, which results in poor chemotaxis. Given the similarity between the phenotypes of KrsB-null and GbpD-overexpressing cells, it is possible that KrsB participates in the Rap1 pathway, although this remains to be tested.
Leukocytes
As in Dictyostelium, Rap1 is an important mediator of leukocyte adhesion, polarity, and migration. In mammalian cells, there are two Rap1 isoforms, Rap1a and Rap1b, although most studies to date do not distinguish between the two [223]. Rap1 is important for random and directed migration of B lymphocytes to SDF-1 [224]. Overexpression of a constitutively active Rap1V12 construct induces polarization and migration of T cells, even in the absence of a chemoattractant [225, 226]. Furthermore, expression of a Rap1-specific GAP, Spa1, completely abrogates chemokine-stimulated adhesion, polarization, and transmigration under shear flow. In neutrophil-like PLB-985 cells, expression of a constitutively active Rap1aQ63E construct causes increased cell adhesion and reduced tail retraction, leading to poor chemotaxis [227]. It should be noted that Rap2, which shares ~ 60 % homology with Rap1, also appears to regulate migration, although this effect might be cell-type or context-dependent [224, 225, 228].
Chemoattractants trigger transient Rap1 activation with the kinetics varying depending on the cell type (Fig. 7). Similarly to the kinetics in Dictyostelium, Rap1-GTP accumulation in fMLP-treated human neutrophils and SDF-1 or CCL21-treated T lymphocytes peaks within seconds [225, 229]. In contrast, in B cell lines the peak activation occurs after several minutes [224]. In T cells, Rap1 activation is sensitive to pertussis toxin suggesting it is mediated by Gi-type heterotrimeric G protein [225]. How the signal is transmitted from the G protein to Rap1 is not known, although several candidates have emerged. In HL-60 cells, fMLP activates a non-RTK Lyn in a Gi protein-dependent manner [230]. Lyn recruits and activates the adaptor protein CrkL, which constitutively associates with a Rap1 GEF C3G. Consistently, depletion of Lyn results in reduced activation of Rap1 and β2 integrin at the leading edge. Furthermore, either Lyn or CrkL knockdown leads to defects in tail retraction and the ability to form stable protrusions, which results in poor chemotaxis. Tyrosine kinases might also be involved in Rap1 activation in T cells, where Abl family kinases Abl and Arg mediate chemokine-stimulated phosphorylation of the adaptor protein human enhancer of filamentation 1 (HEF1), which is required for Rap1 activation and chemotaxis [231].
Two key Rap1 effectors are the regulator for cell adhesion and polarization enriched in lymphoid tissues (RAPL) and Rap1 interacting adapter molecule (RIAM). Overexpression of RIAM increases Jurkat T cell adhesion and spreading, and reduction in RIAM counteracts enhanced integrin-dependent adhesion caused by constitutively active Rap1E63 [232]. Importantly, RIAM appears to be necessary for the localization of Rap1 to the plasma membrane. In addition, RIAM might provide a link between Rap1-GTP and the cytoskeleton since it constitutively associates with VASP and profilin and is necessary for the formation of Rap1-profilin complexes. Although the above studies did not examine chemokine-induced responses, CCL21 stimulates the association of Rap1 with a RIAM-containing complex in human primary T cells [233]. RAPL is essential for integrin-mediated adhesion and migration of T and B lymphocytes and dendritic cells both in vitro and in vivo [234, 235]. Overexpression of RAPL leads to T cell polarization similarly to the overexpression of constitutively active Rap1V12 [234]. In addition, chemoattractant-stimulated Rap1 activation leads to the association of RAPL with LFA-1, which is necessary for RAPL-mediated redistribution of LFA-1 to the leading edge [234].
The effects of Rap1 in lymphocytes also depend on the mammalian Ste20 family kinase Mst1, which is the homolog of D. discoideum KrsB [236]. In T cells CCL21 triggers a rapid accumulation of Mst1 at the leading edge about 30 s after stimulation, as well as a transient increase in Mst1 phosphorylation with a peak at 10 min in a RAPL-dependent manner. Reduction in Mst1 levels by siRNA prevents chemokine or Rap1V12-induced adhesion and polarization. Consistently, lymphocytes from Mst1-/- mice show reduced chemotaxis and interstitial migration in vivo [237, 238].
Other Rap1 effectors that mediate its effects on integrin-dependent adhesion and chemotaxis include Radil, ARAP3, partitioning defective (Par) polarity complex and T lymphoma invasion, and metastasis 1 (Tiam1) [226, 227, 239]. Treatment with fMLP triggers a rapid translocation of the adapter protein Radil to the plasma membrane of neutrophils. Similarly to Rap1E63, overexpression of Radil leads to integrin activation, increased cell-substrate adhesion, and defects in tail retraction [227]. On the other hand, reduction of Radil by siRNA leads to reduced adhesion and chemotaxis. In neutrophils Rap1-GTP also activates ARAP3, a GAP for Arf6 and RhoA, following PIP3-dependent recruitment of ARAP3 to the plasma membrane [239]. ARAP3-deficient cells have elevated RhoA-GTP and LFA-1 clustering, resulting in increased attachment to the substrate and reduced chemotaxis. T cell polarization downstream of Rap1 depends on the activation of the Par polarity complex and a Rac GEF Tiam1, which act together to induce Rac1 activation [226]. Reduction in Tiam1 levels leads to impaired chemokine- or Rap1V12-induced polarization, as well as SDF-1α-mediated chemotaxis. Since all of the effectors have only been examined in one particular cell type thus far, it remains unclear whether the same pathways are conserved between different leukocytes.
cGMP/Myosin II Module
Dictyostelium
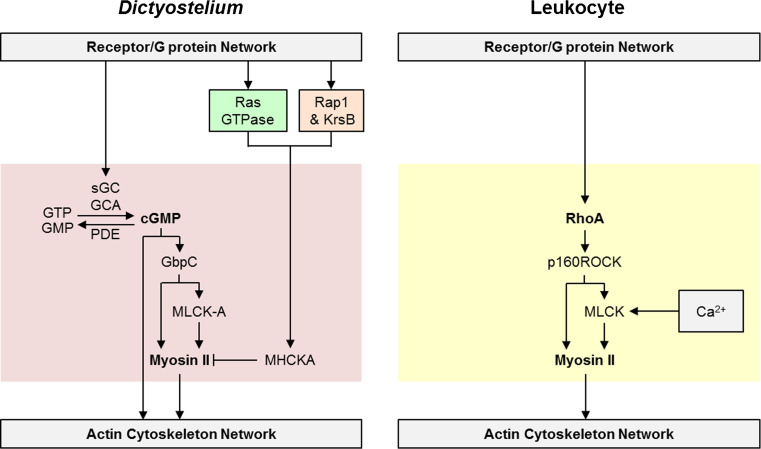
Tail retraction during amoeboid migration is thought to be mediated by conventional non-muscle myosins. Dictyostelium possesses a single conventional non-muscle myosin isoform (myosin II), which is a hexamer composed of two heavy chains (MHC), two essential light chains (ELC), and two regulatory light chains (RLC) [240]. Dictyostelium cells with disrupted myosin II are motile, although their speed and the ability to polarize are reduced to various extents depending on the approach used to perturb myosin II. Disruption of MHC results in the most severe phenotype, including reduction in overall speed, as well as the ability to polarize and move directionally to folic acid or cAMP [241–244]. In contrast, ELC appears to be dispensable for the ability of cells to move directionally, although it affects speed during cAMP, but not folic acid-mediated chemotaxis [244, 245]. Expression of myosin II that lacks RLC or has RLC with a mutation in a key regulatory residue (S13A) does not impair chemotaxis to cAMP, although it affects the ability to track natural cAMP waves due to defects in cell depolarization [246, 247].
Chemoattractants regulate myosin II both spatially and temporally. Chemoattractants stimulate phosphorylation of three Thr residues (T1823, T1833, T2029) in the coiled-coiled tail of MHC by a family of MHC kinases (MHCK) [248, 249]. This phosphorylation favors the monomeric form of myosin II, which is typically found in the cytosol [250, 251]. Mutant myosin that cannot be phosphorylated because the 3 Thr residues have been substituted with Ala (3XAla) constitutively assembles into filaments and localizes at the cortex, whereas the 3XAsp substitutions prevents filament assembly and cortical localization [251]. Cortical localization of myosin II also depends on its association with actin, since Latrunculin A treatment, which disrupts the actin network, prevents myosin II recruitment [252]. Global stimulation with chemoattractants leads to two phases of myosin II regulation. The initial phase, which occurs on the same time scale as cAMP-stimulated actin polymerization, is a rapid MHC phosphorylation, filament disassembly and loss of myosin II at the cortex [253, 254]. The rise in MHC phosphorylation is followed by a broader peak of MHC dephosphorylation, filament overassembly, and localization to the cortex [253, 254].
Transient reduction of myosin II localization at the membrane is similar to the behavior of PTEN and other “back” proteins and, consistently, myosin II, like PTEN, accumulates at the lateral edges and the tail of a moving cell, as well as on retracting pseudopods [244, 246, 255]. Such spatial restriction is thought to be achieved by the activation of MHCK specifically at the front of the cell [256]. This leads to localized myosin II disassembly at the front. Regulation of MHC phosphorylation is critical for myosin II function in chemotaxis, since 3XAla mutant shows reduced speed and polarization in response to cAMP [246, 257]. Interestingly, 3XAla myosin II still localizes to the tail of a moving cell, suggesting an additional mechanism must be involved in restricting myosin II to the back of a cell [246].
Dictyostelium has four MHCK isoforms (A to D). While all MHCKs are capable of phosphorylating MHC, only MHCKA localizes to the front of migrating cells, consistent with its role in restricting myosin II localization to the back [256, 258]. Two independent pathways regulate MHCK A activation (Fig. 8). The first involves activation of Rap1 and its effector Phg2 [213]. Phg2 is a Ser/Thr kinase; whether it directly phosphorylates MHCK A is unclear. Both Rap1 and Phg2 localize to the leading edge of a migrating cell. Cells without Phg2 exhibit increased basal accumulation of cortical myosin II and lack cAMP-stimulated MHC phosphorylation. On the other hand, following cAMP stimulation, cells expressing constitutively active Rap1 (G12V) show a prolonged MHC phosphorylation and a reduction in myosin II overassembly at the cortex. The second pathway participating in MHCK A recruitment to the cortex in response to cAMP stimulation involves RasB and its GEF (RasGEFQ) [45]. Myosin II phosphorylation is reduced leading to its overassembly in gefQ-null cells. Interestingly, although gefQ-null cells have aberrant polarity with more random pseudopods and increased frequency of turning compared to wild-type cells, other chemotactic parameters, such as speed and persistence, are not affected. This might point to the redundancy between the two pathways regulating MHCK A function. MHC phosphorylation is reversible, and dephosphorylation is mediated by the phosphatase PP2A [259]. The importance of this regulation is highlighted in cells that lack the huntingtin protein [260]. These cells have reduced PP2A activity, reduced myosin II disassembly, and aberrant chemotaxis.
Fig. 8.
Dictyostelium cGMP/Myosin II and leukocyte RhoA/Myosin II pathways. The arrow and bar lines represent positive and inhibitory links, respectively, and the lines shown depict interactions that are strongly supported in the literature
Another regulator of myosin II function is cGMP. Chemoattractants trigger a transient increase in cGMP levels via the action of two guanylyl cyclases (sGC and GCA) and two cGMP-specific phosphodiesterases (DdPDE3 and DdPDE5) [261–264]. The association of cGMP with its high-affinity binding protein GbpC (cGMP binding protein C) is important for cAMP-stimulated myosin II accumulation at the cortex, and loss of GbpC or the two GCs leads to reduced chemotaxis [210, 265]. Interestingly, sGC appears to have a dual role in chemotaxing cells: sGC-mediated cGMP production is important for suppressing pseudopod formation at the back, whereas sGC protein, which is localized at the front, helps with reducing the frequency of turning behavior [266]. In addition, cGMP enhances cAMP-stimulated activation of MLC kinase A (MLCK-A), which phosphorylates RLC on S13 and leads to increased ATPase activity of myosin II [267, 268]. Although this regulation appears to be dispensable for myosin II function in growth and development, it is involved in cell depolarization during chemotaxis as mentioned above [247, 268]. A recent report suggests that in addition to its role in myosin II regulation, cGMP can also stimulate actin polymerization [269].
Another protein thought to regulate myosin II dynamics is PakA. This Ste20 family kinase co-localizes with myosin II in the back of a migrating cell and appears to be required for myosin II assembly [88]. pakA-null cells have reduced cortical myosin II and exhibit defects in chemotaxis. PakA likely mediates its effects by negatively regulating MHCK.
RhoA/Myosin II Module
Leukocytes
Assembly of non-muscle myosin II plays multiple roles in leukocyte migration, including the generation of actomyosin contractility and the release of integrin-mediated adhesions involved in tail retraction, as well as maintenance of microtubule stability, which is important for establishing polarity [270–273]. Mammalian non-muscle myosin II is a hexamer composed of 2 MHCs, 2 ELCs and 2 RLCs; however, unlike Dictyostelium myosin II it is primarily regulated by light chain phosphorylation (reviewed in [274] ). There are three isoforms of MHC, which define three types of non-muscle myosin II (A-C). Of these, IIA is the only isoform expressed in T lymphocytes [275]. Chemoattractants promote RLC phosphorylation by activating RhoA, which activates Rho-associated, coiled coil-containing kinase (p160ROCK) (Fig. 8). P160ROCK either phosphorylates MLC directly, or activates another kinase MLC kinase (MLCK), while also inhibiting MLC phosphatase (Protein Phosphatase 1) (reviewed in [274] ).
Pathways leading to RhoA activation during chemotaxis are not well defined, although several possibilities have emerged. For example, LFA-1-mediated migration of T cells to low SDF-1 concentrations depends on pertussis-insensitive Gq, which activates RhoA and mediates contraction [276]. Using the same system Tan et al. demonstrated that activation of the RhoA/p160ROCK/myosin II cascade is independent of Gαi and instead depends on Gα12/13 [277]. Using wild-type and Rac1-null primary neutrophils, Pestonjamasp et al. have demonstrated that Rac1 is required for both activation of RhoA/myosin II pathways in the back and local inhibition of the RhoA pathway at the front of a cell [278].
Myosin II localization is not clear in leukocytes, with some reports of myosin II both at the leading edge and the uropod, whereas others in the uropod only [174, 279, 280]. Interestingly, the two kinases regulating myosin II dynamics have opposing localization in T cells: p160 ROCK is in the uropod and is important for tail detachment, whereas MLCK is at the leading edge and participates in front attachment [280]. How this differential localization leads to specific myosin II function is not clear. One possible pathway that restricts myosin II activity to the back of a migrating neutrophil was described by Liu et al. In this system, mTORC2 activation at the front of a cell leads to the generation of cAMP and consequent activation of PKA, which can inhibit RhoA and MLCK locally [122].
Actin Cytoskeleton network
Dictyostelium
Cell migration in amoeboid cells is largely dependent on the attachments, modifications, and regulatory mechanisms of the actin cytoskeleton. Biochemical analyses have shown that uniform cAMP stimulus in developed cells induces rapid F-actin polymerization followed by disassembly within about 20 s, similarly to the dynamics of typical “front” protein activation and localization [31]. The fluorescently tagged LimE∆coil molecular probe binds newly formed F-actin at the front of protrusions and has thus allowed for the direct monitoring of in vivo spatiotemporal regulation of actin polymerization [281, 282].
At the interphase between the signal transduction and actin cytoskeleton networks are the members of the Rho family of small GTPases, which play essential roles in transmitting upstream signaling to the modulation and reorganization of cortical actin cytoskeleton. In Dictyostelium Rac1b, RacA, RacB, and RacC play a role in directed cell migration by regulating actin polymerization, but the precise role for each of these Rac proteins is still difficult to describe [283–286]. These small G proteins are activated by a number of GEFs that receive different upstream signaling inputs and thus provide a convergence point for several pathways. The Rac GEF GacG has been shown to interact with PKBs in Dictyostelium and thus provides a putative direct connection from the upstream receptor-mediated signaling events to actin cytoskeletal regulation [86]. Small G proteins can also share signaling elements as the GEF GxcC potentially relays Rap1 signaling to the Rac cytoskeletal signaling [287]. The ElmoE and Dock180 proteins (described in the PI3K/PIP3 module) also provide Rac GEF activity from the receptor/heterotrimeric G protein module [96, 288].
Several downstream targets of Rac GTPases act directly on actin to influence its polymerization and dynamics. The SCAR/WAVE complex (containing WAVE, Abi, Nap125, Sra-1, and HSPC-300) is important in the activation of Arp2/3 nucleation and elongation in protrusions [289, 290]. HSPC-300 is of particular importance as it is essential for most of SCAR functions and when labeled with GFP can track localized activation of the SCAR complex [291, 292]. Mutations and knockout cell lines in the SCAR complex itself demonstrate that it is important in Dictyostelium directed motility [293, 294]. The protein PIR121 is a putative relay point from the Rac G proteins to the SCAR complex [293]. Dictyostelium also possess a homolog of the mammalian F-actin-assembling Wiskott-Aldrich syndrome proteins (WASP) [295]. Dictyostelium WASP has been shown to interact with RacC [285]. Although the SCAR/WAVE complex appears to play a predominant role in directing cell migration in Dictyostelium, WASP can complement its loss to regulate pseudopod formation [291]. In addition, a WASP-related protein, WASP-B, has been shown to negativity regulate RacC activity that controls pseudopod extension during chemotaxis to cAMP [296].
Many posttranslational modifications, actin-binding proteins, and signaling pathways alter localized actin polymerization and stabilization. Phosphorylation of actin at Tyr-53 during the later stages of development inhibits F-actin elongation and destabilizes filaments [297]. Arp2 phosphorylation is required for normal chemotaxis towards cAMP as mutations in the threonine and tyrosine phosphorylation sites lead to decreased directionality and speed [298]. The PIP3-dependent, actin-binding proteins myosin ID/E/F, of the Myosin I class, provide spatial regulation of actin polymerization at the leading edge of migrating cells [95]. The actin-binding and nucleating formins, such as Dictyostelium dDia2, are also required for the formation of protrusions [299]. Certain actin-crosslinking proteins, such as filamin and α-actinin, are required for the stabilization of actin polymers during motility [300, 301]. The actin-crosslinking proteins cortexillin I and cortexillin II are also required for directed migration [302, 303]. The IQGAP (DGAP1 in Dictyostelium) and the cortexillin I/II proteins may form a functional quaternary complex with Rac1 GTPase at the trailing edges of migrating cells independent of the integrity of the actin cytoskeleton [286]. This complex is different than the one which contains Rac1 and Pak1, which localizes to the leading edge. Stabilization of actin filaments is specifically important for the development of adhesions required to establish the traction needed to transmit the motility forces [304]. The cross linker α-actinin is known to help form adhesion complexes, but Dictyostelium do not form canonical focal adhesions (see Fig. 9) [305]. Paxillins are also known to localize to focal adhesions in mammalian cells and act as a complex adaptor proteins at the plasma membrane, leading to the reorganization of the actin cytoskeleton [306, 307]. PaxB in Dictyostelium has been shown to be important for maintaining directionality during chemotaxis and localize to punctate regions on the basal surface of Dictyostelium cells (see Fig. 9) [308].
Fig. 9.
Non-specific and specific modes of cell adhesion during migration. To be able to migrate cells must generate traction, which is most commonly achieved by cell adhesion to the substrate. A. Dictyostelium cells possess the ability to navigate over numerous different substrates found in their environment. Their migration is independent of canonical integrin-mediated focal adhesions. Integrins are transmembrane adhesion receptors that comprise a family of 18 α and 8 β subunits in mammals a can associate to form 24 different heterodimers that bind to a variety of ligands [398]. A transmembrane protein similar to integrin beta (SibA) is found in Dictyostelium and has some similarity to integrin β-chains, including the ability to bind talin, but they do not possess the homologous genes for focal adhesion kinase (FAK) or integrin-α chains [305]. The interaction between SibA and talinA is required for proper substrate adhesion and motility in vegetative Dictyostelium cells [399–401]. Although cells lacking SibA have impaired adhesion, they have no defects in cell migration possibly due to the presence of redundant family members [400, 402]. In contrast, lack of talin A/B results in reduced adhesion, as well as migration of vegetative cells; however, it remains unclear which receptor, if any, is necessary for these effects [399]. Two nine transmembrane domain-containing proteins, Phg1 and SadA, positively regulate adhesion to non-specific substrates in Dictyostelium [403, 404]. Recent evidence indicates that an important mechanism for cell-substrate attachment in Dictyostelium is via van der Waals interactions, which allow the cells to adhere to a variety of surfaces within minutes [401]. Although the molecular mechanism of Dictyostelium attachment to the substrate is largely unknown, several signaling pathways that alter cell spreading and adhesion have been identified and are discussed in this review. B. There are focal adhesion-associated proteins, including talin, paxillin, and vinculin, that make transient foci on the basal surface of migrating cells, which may be homologous to mammalian focal adhesions. TIRF microscopy analyzing the basal layer of the cells shows paxillin localizing to these foci [405–407]. C. In 2D environments, leukocytes specifically attach to the tissues via selectins, addressins, and to the extracellular matrix via integrins. L-selectin and P-selectin are cell–cell adhesion molecules that bind glycoproteins, and are utilized by leukocytes and endothelial cells, respectively, to regulate adhesion of leukocytes to specific tissues [408, 409]. Selectins are associated with numerous intracellular signaling proteins to modify cell behavior. Endothelial target localized addressins (such as MAdCAM-1) are bound by leukocyte homing receptors (for example, CD34) to help leukocytes adhere to their targets [410]. Although leukocytes express a number of different integrins, α4β1 (very late antigen-4, VLA-4) and β2-containing integrins αLβ2 (lymphocyte function-associated antigen 1, LFA-1, CD11a/CD18) and αMβ2 (Mac-1, macrophage antigen-1, CD11b/CD18) are particularly important for leukocyte chemotaxis [411, 412]. D. Unlike integrins found in mesenchymal cells, leukocyte integrins do not form well-defined focal adhesions, even though they associate with many proteins typically found in these structures, including talin, paxillin, and focal adhesion kinase (FAK). Paxillin localization in foci at the bottom of a leukocyte under TIRF microscopy is shown [407]. The reason for the absence of well-defined focal adhesions in leukocytes is likely the requirement for very rapid turnover of the cell attachments to the extracellular matrix to allow for the fast migration rates of these cells. Integrins also bind cell adhesion molecules (CAMs), such as VCAM, to aid in targeting appropriate vasculature adhesion [413]. Interestingly, while important for migration on 2D surfaces, in 3D environments, leukocytes switch to integrin-independent migration, where confinement by the extracellular matrix generates enough traction to allow forward propulsion [8]
The negative regulation of actin polymerization and F-actin disassembly is equally important in establishing appropriate actin dynamics for directed cell migration. A coronin protein displays an inhibitory effect on the steady state of F-actin and is partially localized to sites of actin polymerization, specifically the decaying ends of actin tails [309]. The F-actin filament severing protein cofilin helps F-actin form appropriate bundles by increasing turnover rates and localizes at the leading edge of a protrusion within 30–60 s after the 20-s peak [310, 311]. Profilin proteins sequester monomeric actin (G-actin) regulating its polymerization and are required for chemotaxis, as Dictyostelium cells lacking profilin III display defects in directed migration [312, 313].
Leukocytes
The majority of spatiotemporally regulated actin dynamics in chemotaxing leukocytes share great similarity to that previously described in Dictyostelium [314]. Using biochemical dyes and in vivo probes it has been observed that the chemoattractant-induced changes in F-actin polymerization and its localized distribution display “front” protein behavior in leukocytes [315]. In neutrophils, a maximal F-actin response in a gradient of fMLP is observed within ~45–60 s using phalloidin staining and fluorescent TMR-actin. This activity shares sequential leading edge co-localization with the Arp2/3 complex that nucleates branching daughter filaments in the direction of fMLP during neutrophil chemotaxis [315, 316]. Using several pharmacological inhibitors, Chodniewicz et al. demonstrated in human neutrophils that two pathways, PI3K/AKT/PKC and Rho/ROCK/Src, were critical for F-actin production in pseudopod extension [124]. T and B cells show peak SDF-1-stimulated F-actin production at about 15 s in flow cytometry experiments with fluorescently tagged phalloidin [317]. The small 17 amino-acid bioprobe Lifeact binds F-actin and does not interfere with actin dynamics. When fluorescently tagged, this probe has helped demonstrate that stable F-actin resides at the tail, whereas dynamic F-actin is at the leading edge of migrating neutrophils in vivo [318, 319].
Like Dictyostelium, Rac small GTPases are critical for activation of the actin nucleating SCAR/WAVE and WASP complexes in mammalian leukocytes [320, 321]. In P-Rex1-/- neutrophils the chemotaxis speed in response to fMLP is significantly decreased since P-Rex1 is the primary GEF for the Rac1/2 proteins [322]. P-Rex1 is activated by both Gβγ and PIP3 to stimulate Rac activation [131, 322]. The Vav proteins are members of the Dbl family of GEFs, possess tandem PH domains, and predominantly provide GEF activity for Rac [323, 324]. Vav1 expression is restricted to hematopoietic cells, while Vav2 and Vav3 have broad expression and possess some, but not completely redundant activity to Vav1 [325, 326]. Vav1 activity regulates adhesion during chemotaxis by increasing attachment of integrins in T lymphocytes in response to CXCL12 [327]. The Zap-70 kinase is a direct upstream regulator of Vav1, and its kinase activity is stimulated when T-cells are exposed to SDF-1 [327]. Lawson et al. have demonstrated that both P-Rex1 and Vav1 synergistically control adhesion and chemotaxis in fMLP-stimulated neutrophil responses [328]. Other RacGEFs, such as Dock2 and Dock180/Elmo, and their regulation by inositol signaling have been previously discussed in the PI3K/PIP3 module. Reduced chemotaxis in T-cells deficient in the Tiam1 RacGEF suggests that the PKC/Tiam1/Rac signaling pathway is necessary for polarization and crawling on endothelial cells [329]. The protein ArhGAP15 is a negative regulator of Rac in both neutrophil and macrophage behavior, but only neutrophils from ArchGAP15 deficient mice show increased chemotaxis [330]. Unlike Dictyostelium, leukocytes possess Cdc42, which is a commonly reported strong inducer of directed actin dynamics [321, 331]. Cdc42 has been shown to have specificity for the N-WASP actin pathway in regulating directed migration of neutrophils towards a gradient of fMLP [321]. In addition to regulating LIMK, Pak1 is also an adaptor protein for the activation of Cdc42 in neutrophils [126].
In addition to the many signaling pathways that feed into actin regulatory mechanisms, this major cytoskeleton component in leukocyte chemotaxis can also modulate upstream pathways to establish feedback loops. If actin polymerization is inhibited by the addition of latrunculin, polarity of the WAVE complex is lost in chemotaxing neutrophils [320]. In addition, there is a significant decrease in the amplification of internal PIP3 gradients in neutrophil cells in a gradient [332]. Moreover, when actin dynamics are stabilized using several pharmacological inhibitors, the actin cytoskeleton positively regulates receptor desensitization in neutrophil fMLP responses [333].
There are several additional proteins that interact with actin and play important regulatory roles on F-actin growth and depolymerization in leukocyte chemotaxis. Like in Dictyostelium, Myosin I class protein Myo1f regulates cell motility in immune cells [334]. The initial steps of filamentous actin formation are regulated by the direct nucleation of actin monomers from the profilin-sequestered pool. First, as in Dictyostelium, many chemotaxis signaling cascades feed into SCAR/WAVE and WASP complexes, which are activators of Arp2/3 induced actin filament branching. In contrast, formin proteins nucleate the production of linear unbranched actin filaments [335, 336]. The formin mDia1 is a RhoA effector and localizes to the leading edge of migrating T cells to control filamentous actin production during chemotaxis to SDF-1 [337]. Moreover, mDia1 interacts with WASP at the leading edge of migrating neutrophils, and neutrophils from mDia1-/- mice show strong defects in chemotaxis to both fMLP and MIP2 [338]. Second, profilin, which helps actin polymerization by catalyzing the exchange for ATP and delivering G-actin to barbed ends of a growing filament, has been shown to enhance Cdc42-induced actin polymerization in neutrophils [339]. Cofilin/ADF can be induced by Rac2 and, as in Dictyostelium, is necessary for the amplification of barbed F-actin ends, which is critical for fMLP-directed migration in neutrophils [340, 341]. Chemoattractant-mediated activation of cofilin also depends on the inhibition of the phosphatase slingshot2 downstream of Akt/GSK3, as mentioned previously [125]. Third, coronin-1 is another actin-associated protein that accumulates at the leading edge of migrating neutrophils, and most likely enhances barbed-end production via its association with Arp2/3 [342]. Fourth, the actin crosslinker FilaminA is important for the negative regulation of integrin activity, as well as uropod retraction through RhoA during neutrophil chemotaxis [343, 344]. Last, the actin fiber associated α-actinin is a cortex-localized protein that works to reduce adhesion in pseudopods and establish the structure of the retraction fiber network in neutrophils [345].
Polarity network
Amoeboid cells, whether randomly migrating or in a chemoattractant gradient, form and maintain a dominant leading edge. This polarized active front can be established with chemoattractant stimuli or spontaneously arise to varying extents in numerous amoeboid cell types and conditions. Cells display both temporary and intrinsic polarity. Temporary polarity is characterized by the presence of more dynamic and easily outcompeted “front” and “back” restricted localizations of specific biomarkers and is expanded upon in Fig. 2. Intrinsic polarity can occur without external stimuli, but can be maintained even in the face of an opposing chemoattractant gradient. Dictyostelium cells that have progressed through their developmental cycle to the social stages have increased intrinsic polarity. These fully differentiated Dictyostelium cells will maintain the leading edge and when exposed to a shallow gradient of chemoattractant will make a U-turn rather than create a new leading edge facing the chemoattractant source. Neutrophils also possess intrinsic polarity as after they are exposed to uniform chemoattractant they will create and maintain a single leading edge.
There are numerous molecular components that have been implicated in the intrinsic polarity state of amoeboid cells. In Dictyostelium cells the microtubule network is involved in polarity, as depolarization with benomyl or genetic manipulations of Lissencephaly protein I (LisI), Dynein, or Tsunami (TsuA) all cause defects in polarity [346, 347]. Surprisingly, there appears to be an opposite trend in neutrophils, where disruption of the microtubule network with nocodazole induces polarity [348]. In addition to the role of microtubules, genetic disruptions of MEK1, Tortoise (TorA), and a NA–H exchanger (Nhe1) all lead to decreased polarity in Dictyostelium [182, 349, 350]. Not surprisingly, the actin cytoskeleton is also required for intrinsic polarity as treatment with Latrunculin abolishes the spatial localization of leading edge proteins in these amoeboid cells [332, 351].
Most models for polarity balance positive feedback elements at the front with some global inhibitor that prevents the formation of other fronts. Some have suggested that localized recruitment of cytosolic components at the protrusion inhibits additional protrusions at other regions due to depletion of these components [352, 353]. Another model suggests that membrane tension plays a role as the inhibitor. Houk et al. demonstrated in neutrophils that a leading edge can be abolished if tension of another region of the membrane is increased through pipette aspiration [354]. Furthermore, cell-severing experiments and uniform reduction of membrane tension can establish new regions of actin assembly, suggesting diffusion mechanisms are not necessary. It is likely all of these models play a role in the complex processes of maintaining polarity in migrating amoeboid cells.
Computational models explaining chemotaxis
A series of computational models have been proposed to explain various aspects of chemotaxis. Some of the models seek to explain single aspects of chemotaxis such as directional sensing, motility, or polarity (see Fig. 1). Some of these models are conceptual, while others describe the interactions of specific molecules. Some of the conceptual models are able to describe the overall behavior of chemotaxing cells but the parameters represent the aggregate behavior of groups of components that change dynamically. Most of the molecular models focus on a particular set of results and describe the temporal and spatial changes in the components involved but do not take into account the full complexity of the networks mediating chemotaxis. Ultimately, models are needed that simulate all aspects of chemotaxis with accurate molecular detail.
Whether specific or conceptual, the most promising models are those that focus on biochemical excitability. Clearly there is evidence for biochemical excitability in chemotactic signaling systems. In the past decade, there have been increasing observations of propagating waves of cytoskeletal components. For example, oscillations and propagating waves of subunits of the SCAR/WAVE complex and actin-binding proteins have been reported on the basal surface of Dictyostelium amoebae, human neutrophils, and mouse fibroblasts [281, 355–362]. Furthermore, oscillations and propagating waves of signal transduction events, such as PIP3 accumulation and Ras activation, have been observed in Dictyostelium [358, 359, 363–369]. Further evidence for excitability has come from stimulation experiments where short and longer stimuli produce an “all-or-none” response of Ras and PI3K activation, which displays absolute and relative refractory periods [366]. This may suggest that the entire signal transduction network we are describing here is excitable.
Several models have incorporated excitability to explain the stochastic nature of pseudopodia production, the observations of propagating waves, or both. Predating all of the observations, Meinhardt proposed a local activator–inhibitor system that provided excitability and stochastic response together with a global inhibitor that confined the activity to a region [370]. Similar excitable systems have been proposed to explain the stochastic nature of pseudopodia [368, 371]. The Meinhardt scheme can be guided by providing input to the activator, constituting what was later termed a “biased excitable network” (BEN). This system was later shown to simulate chemotactic behavior [372]. Arai et al. proposed a molecular model involving reciprocal feedback loops to explain oscillations of PI3K and PTEN that were observed in immobilized Dictyostelium cells [363]. Xiong et al. employed an upstream local-excitation, global-inhibition (LEGI) module to bias a downstream excitable network (LEGI-BEN) to account for the adaptive spatiotemporal behavior of the signal transduction network [368]. Shi et al. extended this model using level set methods to simulate chemotactic behavior and added a polarity module that derived from and fed back to the excitable network [17].
The networks regulating chemotaxis in Dictyostelium and leukocytes share many similarities
Having examined the details of the networks regulating chemotaxis we see that the overall topologies are remarkably similar between Dictyostelium and mammalian leukocytes. In both systems the receptor/G protein network provides an input to various modules in the signal transduction network, ultimately all of which lead to changes in the cytoskeletal dynamics. Feedback from the cytoskeleton network further amplifies the responses of the signal transduction module, and contributes to the polarity network. A direct comparison of the major molecular events within each network, which is presented in Table 1, reveals remarkable conservation of the core components, either by sequence or functional homology, between the two cell types.
Table 1.
Molecular components in Dictyostelium and leukocyte chemotaxis
| Molecular Component | References | |||
|---|---|---|---|---|
| Dictyostelium | Leukocytes | Dictyostelium | Leukocytes | |
| Receptor/G protein Network | ||||
| Receptors | GPCRs(cAR1-4) | GPCRs(FPR1, C5aR, PAFR, chemokine receptors) | [383] | [34, 39, 384] |
| Heterotrimeric G proteins | Gα(2,4), Gβγ | Gα(I, 12/13, q), Gβγ’s | [26] | [24, 76, 259, 385] |
| Signaling Network | ||||
| Ras Module | RasS,RasD,RasB, RasC, RasG | H-Ras, N-Ras, KA-Ras, and KB-Ras | [43, 44, 47, 55] [41, 42] | |
|
Sca1 complex: AleA/GEFA,Sca1, GEFH, PHR, PP2A GEFR |
SOS RASGRP4 |
[54] |
[74] [76] | |
| DdNF1 | NF1 |
[48] [54] |
[78] | |
| – | Gap120 | – | [77] | |
| PI3K/PIP3 Module | PI3K1-5 | PI3Kγ, δ | [56, 98, 101–103] | [106–109, 117–121] |
| PTEN | PTEN | [81] | [138, 140–144] | |
| SHK1 | MAP3K7* | [84] | – | |
|
5-phosphatase (Dd5P1-4) |
SHIP1 | [80] | [116, 145, 146, 386] | |
| PLC | PLCβ-2/3 | [83] | [115, 136, 137] | |
| GpbB | RACK1 | – | [135] | |
| PKBA, PKBR1 | Akt | [55, 85, 86, 148] | [122, 123] | |
| PakA | Pak | [86, 88] | [126–128] | |
| CRAC | – | [91] | – | |
|
PhdA PhdB (RapGAP3) PhdG |
– | [92–94] | – | |
| Myosin ID, IE, IF | Myosin IF | [95] | [95] | |
|
Rac GEFs (DockA, DockD) |
Rac GEFs (DOCK2, P-Rex1) |
[96] | [131, 132] | |
| TORC2/PKB Module |
TORC2 complex: TOR, RIP3, PiaA, LST8 |
mTORC2 complex: mTOR, mSIN1, Rictor, LST8 |
[47, 150, 151, 154, 155] | [153, 159, 387] |
| PDKA, PDKB | PDK1 | [55, 97, 154] | [158] | |
| PLA2 Module | PLA2A (via AA) | iPLA2-β (via LPA) | [160] | [140, 163, 164] |
| – | cPLA2α (via AA) | – | [140, 163–165] | |
| Ca2+ Module | Ca2+ | Ca2+ | [168–171] | [172, 174, 175] |
| MAPK Module | ERK1/2 | ERK | [181] [185, 186, 189] | [194, 195]. |
| MEK1 | MEK | [180, 182, 183] | [194, 195]. | |
| MEKK | MEKK/Raf | [177] | [69, 197] | |
| – | p38 | – |
[147] [201] [202] |
|
| Rap1 and KrsB/Mst1 Module | – | Tyrosine kinases (Lyn, Abl, Arg) | – | [230, 231] |
| – |
Adaptor proteins (CrkL, HEF1) |
– | [230, 231] | |
|
Rap1 GEF (GbpD) |
Rap1 GEF (C3G) |
[210, 211] | [230] | |
| Rap1 GAP (RapGAP1, RapGAPB) |
Rap1 GAP (Spa1) |
[216, 217] | [225, 226] | |
| Rap1 | Rap1 | [213] | [224–227] | |
| Phg2 | – | [211, 213] | – | |
| – | RAPL | – | [234, 235] | |
| KrsB | Mst1 | [222] | [236–238] | |
| – | Radil | – | [227] | |
| – | ARAP3 | – | [239] | |
|
Rac GEFs (RacGEF1, GxcC) |
Rac GEF (Tiam1) |
[219, 220] | [226] | |
| – | Par polarity complex | – | [226] | |
|
cGMP/Myosin II and RhoA/Myosin II Modules |
guanylyl cyclase (sGC, GCA) |
guanylyl cyclase (sGC) |
[261, 265, 266] | [373, 376] |
|
cGMP phosphodiesterase (DdPDE3, DdPDE5) |
– | [262, 263] | – | |
| GbpC | – | [210] | – | |
| MHCKA | – | [246, 256, 257] | – | |
| MHC phosphatase (PP2A) | – | [260, 388] | – | |
| PakA | Pak | [88] | [126–128] | |
| – | RhoA | – | [271, 272, 280, 389] | |
| – | P160ROCK | – | [271, 272, 280, 389, 390] | |
| – | Ca2+/calmodulin | – | [174, 280] | |
| MLCK-A | MLCK | [247, 267] | [174, 272, 280, 391] | |
| Myosin II | Myosin II | [241–244, 246] | [174, 270, 272] | |
| Actin Cytoskeleton Network |
G/F-Actin (Act8) |
G/F-Actin (ActB) |
[380, 381] | [392, 393] |
|
Rac GEFs (GacQ, GxcC) |
Rac GEFs (P-Rex1,Vav1-3, Tiam1) |
[86, 287] | [130, 131, 322–324, 326, 327, 329] | |
| Rac1, A, B, C | Rac1/2 | [283–286] | [322] | |
| SCAR/WAVE | SCAR/WAVE | [289–294] | [320] | |
| N-Wasp | N-Wasp | [285, 295] | [321, 338] | |
| – | RhoA | – | [337] | |
| – | Cdc42 | – | [321] | |
| Filamin | Filamin | [301] | [343] | |
| α-actinin | α-actinin | [300] | [345] | |
| Paxillin | Paxillin | [308] | [307] | |
| Coronin | Coronin | [309] | [342] | |
| Cofilin | Cofilin | [310, 311] | [125, 340, 341] | |
| Profilin | Profilin | [312, 313] | [339] | |
| Cortexillin I/II | – | [302, 303] | – | |
| IQGAP | IQGAP | [394] | – | |
| PakA | Pak | [286] | [126–128] | |
| Myosin ID, IE, IF | Myosin IF | [95] | [334] | |
| dDia1 | mDia1 | [395] | [337, 338] | |
| dDia2 | mDia2 | [299] | – | |
| Arp2/3 | Arp2/3 | [298] | [315, 316] | |
* The proteins in italics are direct homologues, although their function in chemotaxis has not been studied
As outlined above the receptor/G protein networks are very similar in Dictyostelium and leukocytes, except that the leukocytes are known to sense a wider variety of chemoattractants. Accordingly, the known chemoattractant receptors in Dictyostelium comprise a small family of cAMP receptors, whereas in leukocytes a large family of chemokine receptors mediates their chemotactic responses. Most signaling downstream of chemoattractant receptors is transduced by heterotrimeric G proteins in both Dictyostelium and leukocytes, although the specific subunits and complexity differ between the two systems.
There is symmetry in the overall organization of the signal transduction networks in Dictyostelium and leukocytes, although in Dictyostelium the Ras GTPase module relays the input from the receptor/G protein network to several downstream modules, including PI3K/PIP3, TORC2/PKB, Rap1 and KrsB, and cGMP/Myosin II, whereas in leukocytes studies to date have demonstrated Ras GTPase involvement upstream of PI3K/PIP3 and MAPK modules only.
The similarities between Dictyostelium and leukocytes continue beyond the overall topology of their signal transduction networks and are clearly evident within the individual modules as well. For example, Ras or Rap1 GTPases are highly conserved between Dictyostelium and mammalian cells; however, the GEF and GAP proteins that regulate these GTPases, while present in both systems, are often not direct orthologs. Many protein kinases, including PKB, PDK, Pak, TOR (within the TORC2 complex), ERK, MEK, KrsB/Mst1, as well as lipid-modifying enzymes, including PI3K, PLC, and PLA2, are implicated in directed migration for both Dictyostelium and leukocytes. Perhaps, one interesting exception is in the roles that PIP3-degrading enzymes play in the two cell types. In Dictyostelium PTEN degrades PIP3 to PI(4,5)P2, and deleting this enzyme leads to excessive spreading and the inability to restrict PIP3 signal to the side facing the gradient, resulting in defects in chemotaxis. In contrast, in leukocytes degradation of PIP3 to PI(3,4)P2 by SHIP1 appears to play a more dominant role, with SHIP1-null cells showing excessive adhesion and spreading, as well as impaired motility. The reason for why Dictyostelium and leukocytes preferentially utilize PTEN vs. SHIP1, respectively, is not known. It is possible that 5-phosphatases are involved in Dictyostelium chemotaxis, since all four putative 5-phosphatases have not been deleted together.
One notable difference between Dictyostelium and leukocytes is the organization of the modules regulating myosin II dynamics. First, in Dictyostelium, the main regulatory mechanism for myosin II function is its phosphorylation by MHCK, whereas in leukocytes it is phosphorylation by MLCK. Interestingly, unlike Dictyostelium MLCK-A, MLCK activity in neutrophils is Ca2+-dependent [174]. Second, a cGMP signaling pathway plays a prominent role in myosin II dynamics and localization in Dictyostelium, whereas RhoA/p160ROCK is the major regulator of myosin II in leukocytes. Interestingly, it has been reported that cGMP levels increase following chemoattractant treatment and that cGMP can modulate chemotaxis in neutrophils and monocytes [373–376]. However, the mechanism of cGMP action in leukocytes remains very ambiguous. First, cGMP has been reported to both enhance and inhibit chemotaxis, possibly reflecting concentration dependence of this process [376]. Second, although the direct target of cGMP appears to be cGMP-dependent protein kinase, downstream effectors are not known, although some candidates include cytoskeletal and focal adhesion proteins, including vimentin, vasodilator-stimulated phosphoprotein, Rap1 and Rap2 [374, 377, 378]. Although it is not known if cGMP affects myosin II dynamics in leukocytes as it does in Dictyostelium, cGMP has been shown to inhibit myosin II via the RhoA/ROCK pathway in other mammalian cells, for example, vascular smooth muscle cells [379].
A curious observation is that cell-substrate attachment is regulated by the Rap1 and KrsB/Mst1 module in both systems, even though the mechanism of adhesion itself appears to be different between Dictyostelium and leukocytes (see Fig. 9). In addition to the same core components (Rap1, Rap1 GEFs and GAPs), the two systems also share similarities in some of the downstream targets, for example, Rac GEFs. However, in leukocytes, but not Dictyostelium, several Rap1 effectors, including ARAP3, Radil and Mst1, modify adhesion by specifically affecting integrins. Interestingly, a Dictyostelium homolog of Mst1, KrsB, also alters cell-substrate attachment, although the mechanism of this process, as well as whether KrsB is an effector of Rap1, is not known. The effects of Rap1 itself on Dictyostelium adhesion appear to be mediated by Phg2, which affects myosin II dynamics. Whether an analogous mechanism exists in leukocytes is not known.
The overall organization of the actin cytoskeleton network, as well as the mechanisms of regulation of actin dynamics, is highly conserved between Dictyostelium and leukocytes. In the Dictyostelium genome, duplication events have created 17 separate actin genes, but the act8 proteins comprise 95 % of total actin in the cell [380]. Out of the numerous cell type-specific actin genes in humans, act8 in Dictyostelium has the closest homology to ACTB, ACTG, ACTA, and ACTC in mammalian cells [381]. In both systems Arp2/3 proteins are utilized to nucleate actin polymerization. Both Dictyostelium and leukocytes utilize SCAR/WAVE and WASP actin nucleating complexes activated by Rac-type Rho GTPases. In contrast, Dictyostelium does not possess Cdc42 or RhoA GTPases that are known to regulate WASP and mDia, respectively, in leukocytes [382]. Leukocytes do not possess the actin-binding cortexillins, but like Dictyostelium they possess IQGAP actin crosslinkers [286, 302]. The function of IQGAPs in leukocytes has not been explored.
The molecular networks examined in this review do not include the numerous feedback loops that add to the overall complexity of the signaling events involved in chemotaxis. Future studies will likely reveal further parallels between the mechanisms of chemotactic signaling not only in Dictyostelium and leukocytes, but also in other cells undergoing amoeboid migration, including metastatic tumor cells.
Acknowledgments
This work was supported by the American Cancer Society fellowship to TL, and National Institutes of Health grants GM34933 and GM28007 to PND.
Footnotes
Y. Artemenko, T. J. Lampert contributed equally to this work.
References
- 1.Theveneau E, Mayor R (2012) Neural crest delamination and migration: From epithelium-to-mesenchyme transition to collective cell migration. Developmental Biology 366 (1):34-54. doi: 10.1016/j.ydbio.20112.041 [DOI] [PubMed]
- 2.Richardson BE, Lehmann R (2010) Mechanisms guiding primordial germ cell migration: strategies from different organisms. Nat Rev Mol Cell Biol 11 (1):37-49. doi:http://www.nature.com/nrm/journal/v11/n1/suppinfo/nrm2815_S1.html [DOI] [PMC free article] [PubMed]
- 3.Sadik CD, Luster AD. Lipid-cytokine-chemokine cascades orchestrate leukocyte recruitment in inflammation. J Leukoc Biol. 2012;91(2):207–215. doi: 10.1189/jlb.0811402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13(3):159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 5.Sixt M (2011) Interstitial locomotion of leukocytes. Immunology Letters 138 (1):32-34. doi: 10.1016/j.imlet.2011.02.013 [DOI] [PubMed]
- 6.Zernecke A, Weber C. Chemokines in the vascular inflammatory response of atherosclerosis. Cardiovasc Res. 2010;86(2):192–201. doi: 10.1093/cvr/cvp391. [DOI] [PubMed] [Google Scholar]
- 7.Bravo-Cordero JJ, Hodgson L, Condeelis J (2012) Directed cell invasion and migration during metastasis. Current Opinion in Cell Biology 24 (2):277-283. doi: 10.1016/j.ceb.2011.12.004 [DOI] [PMC free article] [PubMed]
- 8.Lammermann T, Bader BL, Monkley SJ, Worbs T, Wedlich-Soldner R, Hirsch K, Keller M, Forster R, Critchley DR, Fassler R, Sixt M (2008) Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature 453 (7191):51-55. doi:http://www.nature.com/nature/journal/v453/n7191/suppinfo/nature06887_S1.html [DOI] [PubMed]
- 9.Friedl P, Zänker KS, Bröcker E-B. Cell migration strategies in 3-D extracellular matrix: Differences in morphology, cell matrix interactions, and integrin function. Microsc Res Tech. 1998;43(5):369–378. doi: 10.1002/(SICI)1097-0029(19981201)43:5<369::AID-JEMT3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 10.Roussos ET, Condeelis JS, Patsialou A (2011) Chemotaxis in cancer. Nat Rev Cancer 11 (8):573-587. doi:http://www.nature.com/nrc/journal/v11/n8/suppinfo/nrc3078_S1.html [DOI] [PMC free article] [PubMed]
- 11.Rorth P. Fellow travellers: emergent properties of collective cell migration. EMBO Rep. 2012;13(11):984–991. doi: 10.1038/embor.2012.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedl P, Locker J, Sahai E, Segall JE. Classifying collective cancer cell invasion. Nat Cell Biol. 2012;14(8):777–783. doi: 10.1038/ncb2548. [DOI] [PubMed] [Google Scholar]
- 13.Li L, Norrelykke SF, Cox EC. Persistent cell motion in the absence of external signals: a search strategy for eukaryotic cells. PLoS ONE. 2008;3(5):e2093. doi: 10.1371/journal.pone.0002093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takagi H, Sato MJ, Yanagida T, Ueda M. Functional analysis of spontaneous cell movement under different physiological conditions. PLoS ONE. 2008;3(7):e2648. doi: 10.1371/journal.pone.0002648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li L, Cox EC, Flyvbjerg H. ‘Dicty dynamics’: Dictyostelium motility as persistent random motion. Phys Biol. 2011;8(4):046006. doi: 10.1088/1478-3975/8/4/046006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu B, Fuller D, Loomis WF, Levine H, Rappel WJ. Phenomenological approach to eukaryotic chemotactic efficiency. Phys Rev E: Stat, Nonlin, Soft Matter Phys. 2010;81(3 Pt 1):031906. doi: 10.1103/PhysRevE.81.031906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi C, Huang CH, Devreotes PN, Iglesias PA. Interaction of motility, directional sensing, and polarity modules recreates the behaviors of chemotaxing cells. PLoS Comput Biol. 2013;9(7):e1003122. doi: 10.1371/journal.pcbi.1003122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang CH, Tang M, Shi C, Iglesias PA, Devreotes PN. An excitable signal integrator couples to an idling cytoskeletal oscillator to drive cell migration. Nat Cell Biol. 2013;15(11):1307–1316. doi: 10.1038/ncb2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fredriksson R, Schioth HB. The repertoire of G-protein-coupled receptors in fully sequenced genomes. Mol Pharmacol. 2005;67(5):1414–1425. doi: 10.1124/mol.104.009001. [DOI] [PubMed] [Google Scholar]
- 20.Milne JL, Wu L, Caterina MJ, Devreotes PN. Seven helix cAMP receptors stimulate Ca2+ entry in the absence of functional G proteins in Dictyostelium. J Biol Chem. 1995;270(11):5926–5931. doi: 10.1074/jbc.270.11.5926. [DOI] [PubMed] [Google Scholar]
- 21.Milne JL, Kim JY, Devreotes PN. Chemoattractant receptor signaling: G protein-dependent and -independent pathways. Adv Second Messenger Phosphoprotein Res. 1997;31:83–104. doi: 10.1016/s1040-7952(97)80011-0. [DOI] [PubMed] [Google Scholar]
- 22.Shukla AK, Manglik A, Kruse AC, Xiao K, Reis RI, Tseng WC, Staus DP, Hilger D, Uysal S, Huang LY, Paduch M, Tripathi-Shukla P, Koide A, Koide S, Weis WI, Kossiakoff AA, Kobilka BK, Lefkowitz RJ. Structure of active beta-arrestin-1 bound to a G-protein-coupled receptor phosphopeptide. Nature. 2013;497(7447):137–141. doi: 10.1038/nature12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brandt SJ, Dougherty RW, Lapetina EG, Niedel JE. Pertussis toxin inhibits chemotactic peptide-stimulated generation of inositol phosphates and lysosomal enzyme secretion in human leukemic (HL-60) cells. Proc Natl Acad Sci U S A. 1985;82(10):3277–3280. doi: 10.1073/pnas.82.10.3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neptune ER, Bourne HR. Receptors induce chemotaxis by releasing the betagamma subunit of Gi, not by activating Gq or Gs. Proc Natl Acad Sci U S A. 1997;94(26):14489–14494. doi: 10.1073/pnas.94.26.14489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Keymeulen A, Wong K, Knight ZA, Govaerts C, Hahn KM, Shokat KM, Bourne HR. To stabilize neutrophil polarity, PIP3 and Cdc42 augment RhoA activity at the back as well as signals at the front. J Cell Biol. 2006;174(3):437–445. doi: 10.1083/jcb.200604113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kesbeke F, Vanhaastert PJM, Dewit RJW, Snaarjagalska BE. Chemotaxis to Cyclic-Amp and Folic-Acid Is Mediated by Different G-Proteins in Dictyostelium-Discoideum. J Cell Sci. 1990;96:669–673. [Google Scholar]
- 27.Zhang N, Long Y, Devreotes PN. Ggamma in dictyostelium: its role in localization of gbetagamma to the membrane is required for chemotaxis in shallow gradients. Mol Biol Cell. 2001;12(10):3204–3213. doi: 10.1091/mbc.12.10.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeFea KA. Stop that cell! Beta-arrestin-dependent chemotaxis: a tale of localized actin assembly and receptor desensitization. Annu Rev Physiol. 2007;69:535–560. doi: 10.1146/annurev.physiol.69.022405.154804. [DOI] [PubMed] [Google Scholar]
- 29.Gao YJ. Desensitization of vascular endothelin receptors by G protein-coupled receptor kinase 2. Cardiovasc Res. 2010;85(3):405–406. doi: 10.1093/cvr/cvp392. [DOI] [PubMed] [Google Scholar]
- 30.Xiao Z, Yao Y, Long Y, Devreotes P. Desensitization of G-protein-coupled receptors. agonist-induced phosphorylation of the chemoattractant receptor cAR1 lowers its intrinsic affinity for cAMP. J Biol Chem. 1999;274(3):1440–1448. doi: 10.1074/jbc.274.3.1440. [DOI] [PubMed] [Google Scholar]
- 31.Kim JY, Soede RD, Schaap P, Valkema R, Borleis JA, Van Haastert PJ, Devreotes PN, Hereld D. Phosphorylation of chemoattractant receptors is not essential for chemotaxis or termination of G-protein-mediated responses. J Biol Chem. 1997;272(43):27313–27318. doi: 10.1074/jbc.272.43.27313. [DOI] [PubMed] [Google Scholar]
- 32.Brzostowski JA, Sawai S, Rozov O, Liao XH, Imoto D, Parent CA, Kimmel AR. Phosphorylation of chemoattractant receptors regulates chemotaxis, actin re-organization, and signal-relay. J Cell Sci. 2013 doi: 10.1242/jcs.122952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Janetopoulos C, Jin T, Devreotes P. Receptor-mediated activation of heterotrimeric G-proteins in living cells. Science. 2001;291(5512):2408–2411. doi: 10.1126/science.1055835. [DOI] [PubMed] [Google Scholar]
- 34.Viola A, Luster AD. Chemokines and their receptors: drug targets in immunity and inflammation. Annu Rev Pharmacol Toxicol. 2008;48:171–197. doi: 10.1146/annurev.pharmtox.48.121806.154841. [DOI] [PubMed] [Google Scholar]
- 35.Mellado M, Rodriguez-Frade JM, Manes S, Martinez AC. Chemokine signaling and functional responses: the role of receptor dimerization and TK pathway activation. Annu Rev Immunol. 2001;19:397–421. doi: 10.1146/annurev.immunol.19.1.397. [DOI] [PubMed] [Google Scholar]
- 36.Xu X, Meckel T, Brzostowski JA, Yan J, Meier-Schellersheim M, Jin T (2010) Coupling mechanism of a GPCR and a heterotrimeric G protein during chemoattractant gradient sensing in Dictyostelium. Science signaling 3 (141):ra71. doi:10.1126/scisignal.2000980 [DOI] [PubMed]
- 37.Van Haastert PJM. Chemotaxis: insights from the extending pseudopod. J Cell Sci. 2010;123(18):3031–3037. doi: 10.1242/jcs.071118. [DOI] [PubMed] [Google Scholar]
- 38.Jin T, Xu X, Hereld D. Chemotaxis, chemokine receptors and human disease. Cytokine. 2008;44(1):1–8. doi: 10.1016/j.cyto.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boulay F, Tardif M, Brouchon L, Vignais P. The human N-formylpeptide receptor. Characterization of two cDNA isolates and evidence for a new subfamily of G-protein-coupled receptors. Biochemistry. 1990;29(50):11123–11133. doi: 10.1021/bi00502a016. [DOI] [PubMed] [Google Scholar]
- 40.Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1) J Exp Med. 1996;184(3):1101–1109. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lim CJ, Spiegelman GB, Weeks G. Cytoskeletal regulation by Dictyostelium Ras subfamily proteins. J Muscle Res Cell Motil. 2002;23(7–8):729–736. doi: 10.1023/a:1024471527153. [DOI] [PubMed] [Google Scholar]
- 42.Sasaki AT, Chun C, Takeda K, Firtel RA. Localized Ras signaling at the leading edge regulates PI3K, cell polarity, and directional cell movement. The Journal of Cell Biology. 2004;167(3):505–518. doi: 10.1083/jcb.200406177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chubb JR, Wilkins A, Thomas GM, Insall RH. The Dictyostelium RasS protein is required for macropinocytosis, phagocytosis and the control of cell movement. J Cell Sci. 2000;113(Pt 4):709–719. doi: 10.1242/jcs.113.4.709. [DOI] [PubMed] [Google Scholar]
- 44.Wilkins A, Khosla M, Fraser DJ, Spiegelman GB, Fisher PR, Weeks G, Insall RH. Dictyostelium RasD is required for normal phototaxis, but not differentiation. Genes Dev. 2000;14(11):1407–1413. [PMC free article] [PubMed] [Google Scholar]
- 45.Mondal S, Bakthavatsalam D, Steimle P, Gassen B, Rivero F, Noegel AA. Linking Ras to myosin function: RasGEF Q, a Dictyostelium exchange factor for RasB, affects myosin II functions. The Journal of Cell Biology. 2008;181(5):747–760. doi: 10.1083/jcb.200710111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sutherland BW, Spiegelman GB, Weeks G. A Ras subfamily GTPase shows cell cycle-dependent nuclear localization. EMBO Rep. 2001;2(11):1024–1028. doi: 10.1093/embo-reports/kve222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cai H, Das S, Kamimura Y, Long Y, Parent CA, Devreotes PN. Ras-mediated activation of the TORC2-PKB pathway is critical for chemotaxis. J Cell Biol. 2010;190(2):233–245. doi: 10.1083/jcb.201001129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang S, Charest PG, Firtel RA. Spatiotemporal regulation of Ras activity provides directional sensing. Current biology : CB. 2008;18(20):1587–1593. doi: 10.1016/j.cub.2008.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Srinivasan K, Wright GA, Hames N, Housman M, Roberts A, Aufderheide KJ, Janetopoulos C. Delineating the core regulatory elements crucial for directed cell migration by examining folic-acid-mediated responses. J Cell Sci. 2013;126(Pt 1):221–233. doi: 10.1242/jcs.113415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bolourani P, Spiegelman G, Weeks G. Determinants of RasC specificity during Dictyostelium aggregation. J Biol Chem. 2010;285(53):41374–41379. doi: 10.1074/jbc.M110.181115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khosla M, Spiegelman GB, Insall R, Weeks G. Functional overlap of the dictyostelium RasG, RasD and RasB proteins. J Cell Sci. 2000;113(Pt 8):1427–1434. doi: 10.1242/jcs.113.8.1427. [DOI] [PubMed] [Google Scholar]
- 52.Kortholt A, Kataria R, Keizer-Gunnink I, Van Egmond WN, Khanna A, Van Haastert PJ. Dictyostelium chemotaxis: essential Ras activation and accessory signalling pathways for amplification. EMBO Rep. 2011;12(12):1273–1279. doi: 10.1038/embor.2011.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kae H, Lim CJ, Spiegelman GB, Weeks G. Chemoattractant-induced Ras activation during Dictyostelium aggregation. EMBO Rep. 2004;5(6):602–606. doi: 10.1038/sj.embor.7400151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kortholt A, Keizer-Gunnink I, Kataria R, Van Haastert PJ. Ras activation and symmetry breaking during Dictyostelium chemotaxis. J Cell Sci. 2013 doi: 10.1242/jcs.132340. [DOI] [PubMed] [Google Scholar]
- 55.Kamimura Y, Xiong Y, Iglesias PA, Hoeller O, Bolourani P, Devreotes PN (2008) PIP3-Independent Activation of TorC2 and PKB at the Cell’s Leading Edge Mediates Chemotaxis. Current Biology 18 (14):1034-1043. doi: 10.1016/j.cub.2008.06.068 [DOI] [PMC free article] [PubMed]
- 56.Funamoto S, Meili R, Lee S, Parry L, Firtel RA (2002) Spatial and Temporal Regulation of 3-Phosphoinositides by PI 3-Kinase and PTEN Mediates Chemotaxis. Cell 109 (5):611-623. doi: 10.1016/S0092-8674(02)00755-9 [DOI] [PubMed]
- 57.Kolsch V, Shen Z, Lee S, Plak K, Lotfi P, Chang J, Charest PG, Romero JL, Jeon TJ, Kortholt A, Briggs SP, Firtel RA. Daydreamer, a Ras effector and GSK-3 substrate, is important for directional sensing and cell motility. Mol Biol Cell. 2013;24(2):100–114. doi: 10.1091/mbc.E12-04-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Teo R, Lewis KJ, Forde JE, Ryves WJ, Reddy JV, Rogers BJ, Harwood AJ. Glycogen synthase kinase-3 is required for efficient Dictyostelium chemotaxis. Mol Biol Cell. 2010;21(15):2788–2796. doi: 10.1091/mbc.E09-10-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Taylor SJ, Shalloway D. Cell cycle-dependent activation of Ras. Current biology : CB. 1996;6(12):1621–1627. doi: 10.1016/s0960-9822(02)70785-9. [DOI] [PubMed] [Google Scholar]
- 60.Insall RH, Borleis J, Devreotes PN. The aimless RasGEF is required for processing of chemotactic signals through G-protein-coupled receptors in Dictyostelium. Current biology : CB. 1996;6(6):719–729. doi: 10.1016/s0960-9822(09)00453-9. [DOI] [PubMed] [Google Scholar]
- 61.Charest PG, Firtel RA. Big roles for small GTPases in the control of directed cell movement. Biochem J. 2007;401(2):377–390. doi: 10.1042/BJ20061432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Charest PG, Shen Z, Lakoduk A, Sasaki AT, Briggs SP, Firtel RA. A Ras signaling complex controls the RasC-TORC2 pathway and directed cell migration. Dev Cell. 2010;18(5):737–749. doi: 10.1016/j.devcel.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kae H, Kortholt A, Rehmann H, Insall RH, Van Haastert PJ, Spiegelman GB, Weeks G. Cyclic AMP signalling in Dictyostelium: G-proteins activate separate Ras pathways using specific RasGEFs. EMBO Rep. 2007;8(5):477–482. doi: 10.1038/sj.embor.7400936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takeda K, Shao D, Adler M, Charest PG, Loomis WF, Levine H, Groisman A, Rappel WJ, Firtel RA (2012) Incoherent feedforward control governs adaptation of activated ras in a eukaryotic chemotaxis pathway. Science signaling 5 (205):ra2. doi:10.1126/scisignal.2002413 [DOI] [PMC free article] [PubMed]
- 65.Pylayeva-Gupta Y, Grabocka E, Bar-Sagi D. RAS oncogenes: weaving a tumorigenic web. Nat Rev Cancer. 2011;11(11):761–774. doi: 10.1038/nrc3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- 67.Johnson L, Greenbaum D, Cichowski K, Mercer K, Murphy E, Schmitt E, Bronson RT, Umanoff H, Edelmann W, Kucherlapati R, Jacks T. K-ras is an essential gene in the mouse with partial functional overlap with N-ras. Genes Dev. 1997;11(19):2468–2481. doi: 10.1101/gad.11.19.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Khosravi-Far R, Der CJ. The Ras signal transduction pathway. Cancer metastasis reviews. 1994;13(1):67–89. doi: 10.1007/BF00690419. [DOI] [PubMed] [Google Scholar]
- 69.Worthen GS, Avdi N, Buhl AM, Suzuki N, Johnson GL. FMLP activates Ras and Raf in human neutrophils. Potential role in activation of MAP kinase. J Clin Invest. 1994;94(2):815–823. doi: 10.1172/JCI117401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Knall C, Young S, Nick JA, Buhl AM, Worthen GS, Johnson GL. Interleukin-8 regulation of the Ras/Raf/mitogen-activated protein kinase pathway in human neutrophils. J Biol Chem. 1996;271(5):2832–2838. doi: 10.1074/jbc.271.5.2832. [DOI] [PubMed] [Google Scholar]
- 71.Zheng L, Sjolander A, Eckerdal J, Andersson T. Antibody-induced engagement of beta 2 integrins on adherent human neutrophils triggers activation of p21ras through tyrosine phosphorylation of the protooncogene product Vav. Proc Natl Acad Sci U S A. 1996;93(16):8431–8436. doi: 10.1073/pnas.93.16.8431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weber KS, Ostermann G, Zernecke A, Schroder A, Klickstein LB, Weber C. Dual role of H-Ras in regulation of lymphocyte function antigen-1 activity by stromal cell-derived factor-1alpha: implications for leukocyte transmigration. Mol Biol Cell. 2001;12(10):3074–3086. doi: 10.1091/mbc.12.10.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thelen M, Stein JV. How chemokines invite leukocytes to dance. Nat Immunol. 2008;9(9):953–959. doi: 10.1038/ni.f.207. [DOI] [PubMed] [Google Scholar]
- 74.Patrussi L, Ulivieri C, Lucherini OM, Paccani SR, Gamberucci A, Lanfrancone L, Pelicci PG, Baldari CT. p52Shc is required for CXCR4-dependent signaling and chemotaxis in T cells. Blood. 2007;110(6):1730–1738. doi: 10.1182/blood-2007-01-068411. [DOI] [PubMed] [Google Scholar]
- 75.Pacold ME, Suire S, Perisic O, Lara-Gonzalez S, Davis CT, Walker EH, Hawkins PT, Stephens L, Eccleston JF, Williams RL. Crystal structure and functional analysis of Ras binding to its effector phosphoinositide 3-kinase gamma. Cell. 2000;103(6):931–943. doi: 10.1016/s0092-8674(00)00196-3. [DOI] [PubMed] [Google Scholar]
- 76.Suire S, Lecureuil C, Anderson KE, Damoulakis G, Niewczas I, Davidson K, Guillou H, Pan D, Jonathan C, Phillip TH, Stephens L. GPCR activation of Ras and PI3Kc in neutrophils depends on PLCb2/b3 and the RasGEF RasGRP4. EMBO J. 2012;31(14):3118–3129. doi: 10.1038/emboj.2012.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zheng L, Eckerdal J, Dimitrijevic I, Andersson T. Chemotactic peptide-induced activation of Ras in human neutrophils is associated with inhibition of p120-GAP activity. J Biol Chem. 1997;272(37):23448–23454. doi: 10.1074/jbc.272.37.23448. [DOI] [PubMed] [Google Scholar]
- 78.Trovo-Marqui AB, Tajara EH. Neurofibromin: a general outlook. Clin Genet. 2006;70(1):1–13. doi: 10.1111/j.1399-0004.2006.00639.x. [DOI] [PubMed] [Google Scholar]
- 79.Dormann D, Weijer G, Dowler S, Weijer CJ. In vivo analysis of 3-phosphoinositide dynamics during Dictyostelium phagocytosis and chemotaxis. J Cell Sci. 2004;117(26):6497–6509. doi: 10.1242/jcs.01579. [DOI] [PubMed] [Google Scholar]
- 80.Loovers HM, Veenstra K, Snippe H, Pesesse X, Erneux C, van Haastert PJM. A Diverse Family of Inositol 5-Phosphatases Playing a Role in Growth and Development in Dictyostelium discoideum. J Biol Chem. 2003;278(8):5652–5658. doi: 10.1074/jbc.M208396200. [DOI] [PubMed] [Google Scholar]
- 81.Iijima M, Huang YE, Luo HR, Vazquez F, Devreotes PN. Novel Mechanism of PTEN Regulation by Its Phosphatidylinositol 4,5-Bisphosphate Binding Motif Is Critical for Chemotaxis. J Biol Chem. 2004;279(16):16606–16613. doi: 10.1074/jbc.M312098200. [DOI] [PubMed] [Google Scholar]
- 82.Drayer AL, Van der Kaay J, Mayr GW, Van Haastert PJ. Role of phospholipase C in Dictyostelium: formation of inositol 1,4,5-trisphosphate and normal development in cells lacking phospholipase C activity. EMBO J. 1994;13(7):1601–1609. doi: 10.1002/j.1460-2075.1994.tb06423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kortholt A, King JS, Keizer-Gunnink I, Harwood AJ, Van Haastert PJM. Phospholipase C Regulation of Phosphatidylinositol 3,4,5-trisphosphate-mediated Chemotaxis. Mol Biol Cell. 2007;18(12):4772–4779. doi: 10.1091/mbc.E07-05-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moniakis J, Funamoto S, Fukuzawa M, Meisenhelder J, Araki T, Abe T, Meili R, Hunter T, Williams J, Firtel RA. An SH2-domain-containing kinase negatively regulates the phosphatidylinositol-3 kinase pathway. Genes Dev. 2001;15(6):687–698. doi: 10.1101/gad.871001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Meili R, Ellsworth C, Lee S, Reddy TBK, Ma H, Firtel RA. Chemoattractant-mediated transient activation and membrane localization of Akt/PKB is required for efficient chemotaxis to cAMP in Dictyostelium. EMBO J. 1999;18(8):2092–2105. doi: 10.1093/emboj/18.8.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tang M, Iijima M, Kamimura Y, Chen L, Long Y, Devreotes P. Disruption of PKB signaling restores polarity to cells lacking tumor suppressor PTEN. Mol Biol Cell. 2011;22(4):437–447. doi: 10.1091/mbc.E10-06-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chung CY, Potikyan G, Firtel RA (2001) Control of Cell Polarity and Chemotaxis by Akt/PKB and PI3Kinase through the Regulation of PAKa. Molecular Cell 7 (5):937-947. doi: 10.1016/S1097-2765(01)00247-7 [DOI] [PubMed]
- 88.Chung CY, Firtel RA. Paka, a Putative Pak Family Member, Is Required for Cytokinesis and the Regulation of the Cytoskeleton in Dictyostelium discoideum Cells during Chemotaxis. The Journal of Cell Biology. 1999;147(3):559–576. doi: 10.1083/jcb.147.3.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lilly PJ, Devreotes PN. Identification of CRAC, a cytosolic regulator required for guanine nucleotide stimulation of adenylyl cyclase in Dictyostelium. J Biol Chem. 1994;269(19):14123–14129. [PubMed] [Google Scholar]
- 90.Insall R, Kuspa A, Lilly PJ, Shaulsky G, Levin LR, Loomis WF, Devreotes P. CRAC, a cytosolic protein containing a pleckstrin homology domain, is required for receptor and G protein-mediated activation of adenylyl cyclase in Dictyostelium. The Journal of Cell Biology. 1994;126(6):1537–1545. doi: 10.1083/jcb.126.6.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Comer FI, Lippincott CK, Masbad JJ, Parent CA (2005) The PI3K-Mediated Activation of CRAC Independently Regulates Adenylyl Cyclase Activation and Chemotaxis. Current Biology 15 (2):134-139. doi: 10.1016/j.cub.2005.01.007 [DOI] [PubMed]
- 92.Funamoto S, Milan K, Meili R, Firtel RA. Role of Phosphatidylinositol 3′ Kinase and a Downstream Pleckstrin Homology Domain-Containing Protein in Controlling Chemotaxis inDictyostelium. The Journal of Cell Biology. 2001;153(4):795–810. doi: 10.1083/jcb.153.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang P, Wang Y, Sesaki H, Iijima M. Proteomic identification of phosphatidylinositol (3,4,5) triphosphate-binding proteins in Dictyostelium discoideum. Proc Natl Acad Sci. 2010;107(26):11829–11834. doi: 10.1073/pnas.1006153107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jeon TJ, Lee S, Weeks G, Firtel RA (2009) Regulation of Dictyostelium morphogenesis by RapGAP3. Developmental Biology 328 (2):210-220. doi: 10.1016/j.ydbio.2009.01.016 [DOI] [PubMed]
- 95.Chen C-L, Wang Y, Sesaki H, Iijima M (2012) Myosin I Links PIP3 Signaling to Remodeling of the Actin Cytoskeleton in Chemotaxis. Sci Signal 5 (209):ra10-. doi:10.1126/scisignal.2002446 [DOI] [PMC free article] [PubMed]
- 96.Para A, Krischke M, Merlot S, Shen Z, Oberholzer M, Lee S, Briggs S, Firtel RA. Dictyostelium Dock180-related RacGEFs Regulate the Actin Cytoskeleton during Cell Motility. Mol Biol Cell. 2009;20(2):699–707. doi: 10.1091/mbc.E08-09-0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liao XH, Buggey J, Kimmel AR. Chemotactic activation of Dictyostelium AGC-family kinases AKT and PKBR1 requires separate but coordinated functions of PDK1 and TORC2. J Cell Sci. 2010;123(Pt 6):983–992. doi: 10.1242/jcs.064022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hoeller O, Kay RR (2007) Chemotaxis in the Absence of PIP3 Gradients. Current Biology 17 (9):813-817. doi: 10.1016/j.cub.2007.04.004 [DOI] [PubMed]
- 99.Loovers HM, Postma M, Keizer-Gunnink I, Huang YE, Devreotes PN, van Haastert PJM. Distinct Roles of PI(3,4,5)P3 during Chemoattractant Signaling in Dictyostelium: A Quantitative In Vivo Analysis by Inhibition of PI3-Kinase. Mol Biol Cell. 2006;17(4):1503–1513. doi: 10.1091/mbc.E05-09-0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen L, Janetopoulos C, Huang YE, Iijima M, Borleis J, Devreotes PN. Two Phases of Actin Polymerization Display Different Dependencies on PI(3,4,5)P3 Accumulation and Have Unique Roles during Chemotaxis. Mol Biol Cell. 2003;14(12):5028–5037. doi: 10.1091/mbc.E03-05-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bosgraaf L, Keizer-Gunnink I, Van Haastert PJM. PI3-kinase signaling contributes to orientation in shallow gradients and enhances speed in steep chemoattractant gradients. J Cell Sci. 2008;121(21):3589–3597. doi: 10.1242/jcs.031781. [DOI] [PubMed] [Google Scholar]
- 102.Huang YE, Iijima M, Parent CA, Funamoto S, Firtel RA, Devreotes P. Receptor-mediated Regulation of PI3Ks Confines PI(3,4,5)P3 to the Leading Edge of Chemotaxing Cells. Mol Biol Cell. 2003;14(5):1913–1922. doi: 10.1091/mbc.E02-10-0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Takeda K, Sasaki AT, Ha H, Seung H-A, Firtel RA. Role of Phosphatidylinositol 3-Kinases in Chemotaxis in Dictyostelium. J Biol Chem. 2007;282(16):11874–11884. doi: 10.1074/jbc.M610984200. [DOI] [PubMed] [Google Scholar]
- 104.Servant G, Weiner OD, Herzmark P, Balla T, Sedat JW, Bourne HR. Polarization of Chemoattractant Receptor Signaling During Neutrophil Chemotaxis. Science. 2000;287(5455):1037–1040. doi: 10.1126/science.287.5455.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Suire S, Condliffe AM, Ferguson GJ, Ellson CD, Guillou H, Davidson K, Welch H, Coadwell J, Turner M, Chilvers ER, Hawkins PT, Stephens L (2006) G[β][γ]s and the Ras binding domain of p110[γ] are both important regulators of PI3K[γ] signalling in neutrophils. Nat Cell Biol 8 (11):1303-1309. doi:http://www.nature.com/ncb/journal/v8/n11/suppinfo/ncb1494_S1.html [DOI] [PubMed]
- 106.Hirsch E, Katanaev VL, Garlanda C, Azzolino O, Pirola L, Silengo L, Sozzani S, Mantovani A, Altruda F, Wymann MP. Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science. 2000;287(5455):1049–1053. doi: 10.1126/science.287.5455.1049. [DOI] [PubMed] [Google Scholar]
- 107.Sasaki T, Irie-Sasaki J, Jones RG, Oliveira-dos-Santos AJ, Stanford WL, Bolon B, Wakeham A, Itie A, Bouchard D, Kozieradzki I, Joza N, Mak TW, Ohashi PS, Suzuki A, Penninger JM. Function of PI3Kγ in Thymocyte Development, T Cell Activation, and Neutrophil Migration. Science. 2000;287(5455):1040–1046. doi: 10.1126/science.287.5455.1040. [DOI] [PubMed] [Google Scholar]
- 108.Hannigan M, Zhan L, Li Z, Ai Y, Wu D, Huang C-K. Neutrophils lacking phosphoinositide 3-kinase γ show loss of directionality during N-formyl-Met-Leu-Phe-induced chemotaxis. Proc Natl Acad Sci. 2002;99(6):3603–3608. doi: 10.1073/pnas.052010699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ferguson GJ, Milne L, Kulkarni S, Sasaki T, Walker S, Andrews S, Crabbe T, Finan P, Jones G, Jackson S, Camps M, Rommel C, Wymann M, Hirsch E, Hawkins P, Stephens L (2007) PI(3)K[gamma] has an important context-dependent role in neutrophil chemokinesis. Nat Cell Biol 9 (1):86-91. doi:http://www.nature.com/ncb/journal/v9/n1/suppinfo/ncb1517_S1.html [DOI] [PubMed]
- 110.Coffer PJ, Geijsen N, M’rabet L, Schweizer RC, Maikoe T, Raaijmakers JA, Lammers JW, Koenderman L. Comparison of the roles of mitogen-activated protein kinase kinase and phosphatidylinositol 3-kinase signal transduction in neutrophil effector function. Biochem J. 1998;329(1):121–130. doi: 10.1042/bj3290121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Knall C, Worthen GS, Johnson GL. Interleukin 8-stimulated phosphatidylinositol-3-kinase activity regulates the migration of human neutrophils independent of extracellular signal-regulated kinase and p38 mitogen-activated protein kinases. Proc Natl Acad Sci. 1997;94(7):3052–3057. doi: 10.1073/pnas.94.7.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Niggli V, Keller H (1997) The phosphatidylinositol 3-kinase inhibitor wortmannin markedly reduces chemotactic peptide-induced locomotion and increases in cytoskeletal actin in human neutrophils. European Journal of Pharmacology 335 (1):43-52. doi: 10.1016/S0014-2999(97)01169-2 [DOI] [PubMed]
- 113.Vicente-Manzanares M, Rey M, Jones DR, Sancho D, Mellado M, Rodriguez-Frade JM, del Pozo MA, Yáñez-Mó M, de Ana AM, Martínez-A C, Mérida I, Sánchez-Madrid F. Involvement of Phosphatidylinositol 3-Kinase in Stromal Cell-Derived Factor-1α-Induced Lymphocyte Polarization and Chemotaxis. J Immunol. 1999;163(7):4001–4012. [PubMed] [Google Scholar]
- 114.Al-Aoukaty A, Rolstad B, Maghazachi AA. Recruitment of Pleckstrin and Phosphoinositide 3-Kinase γ into the Cell Membranes, and Their Association with Gβγ After Activation of NK Cells with Chemokines. J Immunol. 1999;162(6):3249–3255. [PubMed] [Google Scholar]
- 115.Li Z, Jiang H, Xie W, Zhang Z, Smrcka AV, Wu D. Roles of PLC-β2 and -β3 and PI3Kγ in Chemoattractant-Mediated Signal Transduction. Science. 2000;287(5455):1046–1049. doi: 10.1126/science.287.5455.1046. [DOI] [PubMed] [Google Scholar]
- 116.Nishio M, Watanabe K-i, Sasaki J, Taya C, Takasuga S, Iizuka R, Balla T, Yamazaki M, Watanabe H, Itoh R, Kuroda S, Horie Y, Forster I, Mak TW, Yonekawa H, Penninger JM, Kanaho Y, Suzuki A, Sasaki T (2007) Control of cell polarity and motility by the PtdIns(3,4,5)P3 phosphatase SHIP1. Nat Cell Biol 9 (1):36-44. doi:http://www.nature.com/ncb/journal/v9/n1/suppinfo/ncb1515_S1.html [DOI] [PubMed]
- 117.Reif K, Okkenhaug K, Sasaki T, Penninger JM, Vanhaesebroeck B, Cyster JG. Cutting Edge: Differential Roles for Phosphoinositide 3-Kinases, p110γ and p110δ, in Lymphocyte Chemotaxis and Homing. J Immunol. 2004;173(4):2236–2240. doi: 10.4049/jimmunol.173.4.2236. [DOI] [PubMed] [Google Scholar]
- 118.Curnock AP, Sotsios Y, Wright KL, Ward SG. Optimal Chemotactic Responses of Leukemic T Cells to Stromal Cell-Derived Factor-1 Requires the Activation of Both Class IA and IB Phosphoinositide 3-Kinases. J Immunol. 2003;170(8):4021–4030. doi: 10.4049/jimmunol.170.8.4021. [DOI] [PubMed] [Google Scholar]
- 119.Sadhu C, Masinovsky B, Dick K, Sowell CG, Staunton DE. Essential Role of Phosphoinositide 3-Kinase δ in Neutrophil Directional Movement. J Immunol. 2003;170(5):2647–2654. doi: 10.4049/jimmunol.170.5.2647. [DOI] [PubMed] [Google Scholar]
- 120.Boulven I, Levasseur S, Marois S, Paré G, Rollet-Labelle E, Naccache PH. Class IA Phosphatidylinositide 3-Kinases, rather than p110γ, Regulate Formyl-Methionyl-Leucyl-Phenylalanine-Stimulated Chemotaxis and Superoxide Production in Differentiated Neutrophil-Like PLB-985 Cells. J Immunol. 2006;176(12):7621–7627. doi: 10.4049/jimmunol.176.12.7621. [DOI] [PubMed] [Google Scholar]
- 121.Liu L, Puri KD, Penninger JM, Kubes P. Leukocyte PI3Kγ and PI3Kδ have temporally distinct roles for leukocyte recruitment in vivo. Blood. 2007;110(4):1191–1198. doi: 10.1182/blood-2006-11-060103. [DOI] [PubMed] [Google Scholar]
- 122.Liu L, Das S, Losert W, Parent CA (2010) mTORC2 Regulates Neutrophil Chemotaxis in a cAMP- and RhoA-Dependent Fashion. Developmental Cell 19 (6):845-857. doi: 10.1016/j.devcel.2010.11.004 [DOI] [PMC free article] [PubMed]
- 123.Chen J, Tang H, Hay N, Xu J, Ye RD. Akt isoforms differentially regulate neutrophil functions. Blood. 2010;115(21):4237–4246. doi: 10.1182/blood-2009-11-255323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chodniewicz D, Zhelev DV. Chemoattractant receptor-stimulated F-actin polymerization in the human neutrophil is signaled by 2 distinct pathways. Blood. 2003;101(3):1181–1184. doi: 10.1182/blood-2002-05-1435. [DOI] [PubMed] [Google Scholar]
- 125.Tang W, Zhang Y, Xu W, Harden TK, Sondek J, Sun L, Li L, Wu D (2011) A PLCβ/PI3Kγ-GSK3 Signaling Pathway Regulates Cofilin Phosphatase Slingshot2 and Neutrophil Polarization and Chemotaxis. Developmental Cell 21 (6):1038-1050. doi: 10.1016/j.devcel.2011.10.023 [DOI] [PMC free article] [PubMed]
- 126.Li Z, Hannigan M, Mo Z, Liu B, Lu W, Wu Y, Smrcka AV, Wu G, Li L, Liu M, Huang C-K, Wu D (2003) Directional Sensing Requires Gβγ-Mediated PAK1 and PIXα-Dependent Activation of Cdc42. Cell 114 (2):215-227. doi: 10.1016/S0092-8674(03)00559-2 [DOI] [PubMed]
- 127.Itakura A, Aslan JE, Kusanto BT, Phillips KG, Porter JE, Newton PK, Nan X, Insall RH, Chernoff J, McCarty OJT. p21-Activated Kinase (PAK) Regulates Cytoskeletal Reorganization and Directional Migration in Human Neutrophils. PLoS ONE. 2013;8(9):e73063. doi: 10.1371/journal.pone.0073063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Volinsky N, Gantman A, Yablonski D. A Pak- and Pix-dependent branch of the SDF-1α signalling pathway mediates T cell chemotaxis across restrictive barriers. Biochem J. 2006;397(1):213–222. doi: 10.1042/BJ20051655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhou G-L, Zhuo Y, King CC, Fryer BH, Bokoch GM, Field J. Akt Phosphorylation of Serine 21 on Pak1 Modulates Nck Binding and Cell Migration. Mol Cell Biol. 2003;23(22):8058–8069. doi: 10.1128/MCB.23.22.8058-8069.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Welch HCE, Coadwell WJ, Ellson CD, Ferguson GJ, Andrews SR, Erdjument-Bromage H, Tempst P, Hawkins PT, Stephens LR (2002) P-Rex1, a PtdIns(3,4,5)P3- and Gβγ-Regulated Guanine-Nucleotide Exchange Factor for Rac. Cell 108 (6):809-821. doi: 10.1016/S0092-8674(02)00663-3 [DOI] [PubMed]
- 131.Dong X, Mo Z, Bokoch G, Guo C, Li Z, Wu D (2005) P-Rex1 Is a Primary Rac2 Guanine Nucleotide Exchange Factor in Mouse Neutrophils. Current Biology 15 (20):1874-1879. doi: 10.1016/j.cub.2005.09.014 [DOI] [PubMed]
- 132.Kunisaki Y, Nishikimi A, Tanaka Y, Takii R, Noda M, Inayoshi A, Watanabe KI, Sanematsu F, Sasazuki T, Sasaki T, Fukui Y. DOCK2 is a Rac activator that regulates motility and polarity during neutrophil chemotaxis. The Journal of Cell Biology. 2006;174(5):647–652. doi: 10.1083/jcb.200602142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhao T, Nalbant P, Hoshino M, Dong X, Wu D, Bokoch GM. Signaling requirements for translocation of P-Rex1, a key Rac2 exchange factor involved in chemoattractant-stimulated human neutrophil function. J Leukoc Biol. 2007;81(4):1127–1136. doi: 10.1189/jlb.0406251. [DOI] [PubMed] [Google Scholar]
- 134.Nishikimi A, Fukuhara H, Su W, Hongu T, Takasuga S, Mihara H, Cao Q, Sanematsu F, Kanai M, Hasegawa H, Tanaka Y, Shibasaki M, Kanaho Y, Sasaki T, Frohman MA, Fukui Y. Sequential Regulation of DOCK2 Dynamics by Two Phospholipids During Neutrophil Chemotaxis. Science. 2009;324(5925):384–387. doi: 10.1126/science.1170179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chen S, Lin F, Shin ME, Wang F, Shen L, Hamm HE. RACK1 Regulates Directional Cell Migration by Acting on Gβγ at the Interface with Its Effectors PLCβ and PI3Kγ. Mol Biol Cell. 2008;19(9):3909–3922. doi: 10.1091/mbc.E08-04-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bach TL, Chen Q-M, Kerr WT, Wang Y, Lian L, Choi JK, Wu D, Kazanietz MG, Koretzky GA, Zigmond S, Abrams CS. Phospholipase Cβ Is Critical for T Cell Chemotaxis. J Immunol. 2007;179(4):2223–2227. doi: 10.4049/jimmunol.179.4.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cronshaw DG, Kouroumalis A, Parry R, Webb A, Brown Z, Ward SG. Evidence that phospholipase C-dependent, calcium-independent mechanisms are required for directional migration of T lymphocytes in response to the CCR4 ligands CCL17 and CCL22. J Leukoc Biol. 2006;79(6):1369–1380. doi: 10.1189/jlb.0106035. [DOI] [PubMed] [Google Scholar]
- 138.Lacalle RA, Gómez-Moutón C, Barber DF, Jiménez-Baranda S, Mira E, Martínez-A C, Carrera AC, Mañes S. PTEN regulates motility but not directionality during leukocyte chemotaxis. J Cell Sci. 2004;117(25):6207–6215. doi: 10.1242/jcs.01545. [DOI] [PubMed] [Google Scholar]
- 139.Xu J, Wang F, Van Keymeulen A, Herzmark P, Straight A, Kelly K, Takuwa Y, Sugimoto N, Mitchison T, Bourne HR (2003) Divergent Signals and Cytoskeletal Assemblies Regulate Self-Organizing Polarity in Neutrophils. Cell 114 (2):201-214. doi: 10.1016/S0092-8674(03)00555-5 [DOI] [PubMed]
- 140.Heit B, Robbins SM, Downey CM, Guan Z, Colarusso P, Miller BJ, Jirik FR, Kubes P (2008) PTEN functions to ‘prioritize’ chemotactic cues and prevent ‘distraction’ in migrating neutrophils. Nat Immunol 9 (7):743-752. doi:http://www.nature.com/ni/journal/v9/n7/suppinfo/ni.1623_S1.html [DOI] [PubMed]
- 141.Fox JA, Ung K, Tanlimco SG, Jirik FR. Disruption of a Single Pten Allele Augments the Chemotactic Response of B Lymphocytes to Stromal Cell-Derived Factor-1. J Immunol. 2002;169(1):49–54. doi: 10.4049/jimmunol.169.1.49. [DOI] [PubMed] [Google Scholar]
- 142.Gao P, Wange RL, Zhang N, Oppenheim JJ, Howard OMZ. Negative regulation of CXCR4-mediated chemotaxis by the lipid phosphatase activity of tumor suppressor PTEN. Blood. 2005;106(8):2619–2626. doi: 10.1182/blood-2004-08-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Subramanian KK, Jia Y, Zhu D, Simms BT, Jo H, Hattori H, You J, Mizgerd JP, Luo HR. Tumor suppressor PTEN is a physiologic suppressor of chemoattractant-mediated neutrophil functions. Blood. 2007;109(9):4028–4037. doi: 10.1182/blood-2006-10-055319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sarraj B, Massberg S, Li Y, Kasorn A, Subramanian K, Loison F, Silberstein LE, von Andrian U, Luo HR. Myeloid-Specific Deletion of Tumor Suppressor PTEN Augments Neutrophil Transendothelial Migration during Inflammation. J Immunol. 2009;182(11):7190–7200. doi: 10.4049/jimmunol.0802562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mondal S, Subramanian KK, Sakai J, Bajrami B, Luo HR. Phosphoinositide lipid phosphatase SHIP1 and PTEN coordinate to regulate cell migration and adhesion. Mol Biol Cell. 2012;23(7):1219–1230. doi: 10.1091/mbc.E11-10-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Harris SJ, Parry RV, Foster JG, Blunt MD, Wang A, Marelli-Berg F, Westwick J, Ward SG. Evidence That the Lipid Phosphatase SHIP-1 Regulates T Lymphocyte Morphology and Motility. J Immunol. 2011;186(8):4936–4945. doi: 10.4049/jimmunol.1002350. [DOI] [PubMed] [Google Scholar]
- 147.Heit B, Tavener S, Raharjo E, Kubes P. An intracellular signaling hierarchy determines direction of migration in opposing chemotactic gradients. The Journal of Cell Biology. 2002;159(1):91–102. doi: 10.1083/jcb.200202114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Meili R, Ellsworth C, Firtel RA (2000) A novel Akt/PKB-related kinase is essential for morphogenesis in Dictyostelium. Current Biology 10 (12):708-717. doi: 10.1016/S0960-9822(00)00536-4 [DOI] [PubMed]
- 149.Bhaskar PT, Hay N. The two TORCs and Akt. Dev Cell. 2007;12(4):487–502. doi: 10.1016/j.devcel.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 150.Lee S, Parent CA, Insall R, Firtel RA. A novel Ras-interacting protein required for chemotaxis and cyclic adenosine monophosphate signal relay in Dictyostelium. Mol Biol Cell. 1999;10(9):2829–2845. doi: 10.1091/mbc.10.9.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chen MY, Long Y, Devreotes PN. A novel cytosolic regulator, Pianissimo, is required for chemoattractant receptor and G protein-mediated activation of the 12 transmembrane domain adenylyl cyclase in Dictyostelium. Genes Dev. 1997;11(23):3218–3231. doi: 10.1101/gad.11.23.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Cybulski N, Hall MN. TOR complex 2: a signaling pathway of its own. Trends Biochem Sci. 2009;34(12):620–627. doi: 10.1016/j.tibs.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 153.Lee S, Comer FI, Sasaki A, McLeod IX, Duong Y, Okumura K, Yates JR, 3rd, Parent CA, Firtel RA. TOR complex 2 integrates cell movement during chemotaxis and signal relay in Dictyostelium. Mol Biol Cell. 2005;16(10):4572–4583. doi: 10.1091/mbc.E05-04-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kamimura Y, Devreotes PN. Phosphoinositide-dependent protein kinase (PDK) activity regulates phosphatidylinositol 3,4,5-trisphosphate-dependent and -independent protein kinase B activation and chemotaxis. J Biol Chem. 2010;285(11):7938–7946. doi: 10.1074/jbc.M109.089235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lim CJ, Spiegelman GB, Weeks G. RasC is required for optimal activation of adenylyl cyclase and Akt/PKB during aggregation. EMBO J. 2001;20(16):4490–4499. doi: 10.1093/emboj/20.16.4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Thelen M. Dancing to the tune of chemokines. Nat Immunol. 2001;2(2):129–134. doi: 10.1038/84224. [DOI] [PubMed] [Google Scholar]
- 157.Delgado-Martin C, Escribano C, Pablos JL, Riol-Blanco L, Rodriguez-Fernandez JL. Chemokine CXCL12 uses CXCR4 and a signaling core formed by bifunctional Akt, extracellular signal-regulated kinase (ERK)1/2, and mammalian target of rapamycin complex 1 (mTORC1) proteins to control chemotaxis and survival simultaneously in mature dendritic cells. J Biol Chem. 2011;286(43):37222–37236. doi: 10.1074/jbc.M111.294116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yagi M, Kantarci A, Iwata T, Omori K, Ayilavarapu S, Ito K, Hasturk H, Van Dyke TE. PDK1 regulates chemotaxis in human neutrophils. J Dent Res. 2009;88(12):1119–1124. doi: 10.1177/0022034509349402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.He Y, Li D, Cook SL, Yoon MS, Kapoor A, Rao CV, Kenis PJ, Chen J, Wang F. Mammalian Target of Rapamycin and Rictor control neutrophil chemotaxis by regulating Rac/Cdc42 activity and the actin cytoskeleton. Mol Biol Cell. 2013 doi: 10.1091/mbc.E13-07-0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chen L, Iijima M, Tang M, Landree MA, Huang YE, Xiong Y, Iglesias PA, Devreotes PN (2007) PLA2 and PI3K/PTEN Pathways Act in Parallel to Mediate Chemotaxis. Developmental Cell 12 (4):603-614. doi: 10.1016/j.devcel.2007.03.005 [DOI] [PMC free article] [PubMed]
- 161.van Haastert PJM, Keizer-Gunnink I, Kortholt A. Essential role of PI3-kinase and phospholipase A2 in Dictyostelium discoideum chemotaxis. The Journal of Cell Biology. 2007;177(5):809–816. doi: 10.1083/jcb.200701134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Meliton AY, Munoz NM, Meliton LN, Binder DC, Osan CM, Zhu X, Dudek SM, Leff AR. Cytosolic group IVa phospholipase A2 mediates IL-8/CXCL8-induced transmigration of human polymorphonuclear leukocytes in vitro. J Inflamm (Lond) 2010;7:14. doi: 10.1186/1476-9255-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Carnevale KA, Cathcart MK. Calcium-Independent Phospholipase A2 Is Required for Human Monocyte Chemotaxis to Monocyte Chemoattractant Protein 1. J Immunol. 2001;167(6):3414–3421. doi: 10.4049/jimmunol.167.6.3414. [DOI] [PubMed] [Google Scholar]
- 164.Mishra RS, Carnevale KA, Cathcart MK. iPLA2β: front and center in human monocyte chemotaxis to MCP-1. J Exp Med. 2008;205(2):347–359. doi: 10.1084/jem.20071243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Locati M, Lamorte G, Luini W, Introna M, Bernasconi S, Mantovani A, Sozzani S. Inhibition of Monocyte Chemotaxis to C-C Chemokines by Antisense Oligonucleotide for Cytosolic Phospholipase A. J Biol Chem. 1996;271(11):6010–6016. doi: 10.1074/jbc.271.11.6010. [DOI] [PubMed] [Google Scholar]
- 166.Traynor D, Milne JLS, Insall RH, Kay RR. Ca2+ signalling is not required for chemotaxis in Dictyostelium. EMBO J. 2000;19(17):4846–4854. doi: 10.1093/emboj/19.17.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Schaloske R, Lusche D, Bezares-Roder K, Happle K, Malchow D, Schlatterer C. Ca2+ regulation in the absence of the iplA gene product in Dictyostelium discoideum. BMC Cell Biology. 2005;6(1):13. doi: 10.1186/1471-2121-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lusche DF, Wessels D, Soll DR. The effects of extracellular calcium on motility, pseudopod and uropod formation, chemotaxis, and the cortical localization of myosin II in Dictyostelium discoideum. Cell Motil Cytoskelet. 2009;66(8):567–587. doi: 10.1002/cm.20367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Van Duijn B, Van Haastert PJ. Independent control of locomotion and orientation during Dictyostelium discoideum chemotaxis. J Cell Sci. 1992;102(4):763–768. doi: 10.1242/jcs.102.4.763. [DOI] [PubMed] [Google Scholar]
- 170.Unterweger N, Schlatterer C (1995) Introduction of calcium buffers into the cytosol of Dictyostelium discoideum amoebae alters cell morphology and inhibits chemotaxis. Cell Calcium 17 (2):97-110. doi: 10.1016/0143-4160(95)90079-9 [DOI] [PubMed]
- 171.Lusche D, Bezares-Roder K, Happle K, Schlatterer C. cAMP controls cytosolic Ca2+ levels in Dictyostelium discoideum. BMC Cell Biology. 2005;6(1):12. doi: 10.1186/1471-2121-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Maxfield FR (1993) Regulation of leukocyte locomotion by Ca2+. Trends in Cell Biology 3 (11):386-391. doi: 10.1016/0962-8924(93)90088-I [DOI] [PubMed]
- 173.Brundage RA, Fogarty KE, Tuft RA, Fay FS. Calcium gradients underlying polarization and chemotaxis of eosinophils. Science. 1991;254(5032):703–706. doi: 10.1126/science.1948048. [DOI] [PubMed] [Google Scholar]
- 174.Eddy RJ, Pierini LM, Matsumura F, Maxfield FR. Ca2+ -dependent myosin II activation is required for uropod retraction during neutrophil migration. J Cell Sci. 2000;113(7):1287–1298. doi: 10.1242/jcs.113.7.1287. [DOI] [PubMed] [Google Scholar]
- 175.Hendey B, Klee CB, Maxfield FR. Inhibition of neutrophil chemokinesis on vitronectin by inhibitors of calcineurin. Science. 1992;258(5080):296–299. doi: 10.1126/science.1384129. [DOI] [PubMed] [Google Scholar]
- 176.Goldberg JM, Manning G, Liu A, Fey P, Pilcher KE, Xu Y, Smith JL. The dictyostelium kinome–analysis of the protein kinases from a simple model organism. PLoS Genet. 2006;2(3):e38. doi: 10.1371/journal.pgen.0020038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev. 1999;79(1):143–180. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- 178.Chung CY, Reddy TB, Zhou K, Firtel RA. A novel, putative MEK kinase controls developmental timing and spatial patterning in Dictyostelium and is regulated by ubiquitin-mediated protein degradation. Genes Dev. 1998;12(22):3564–3578. doi: 10.1101/gad.12.22.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sun B, Ma H, Firtel RA. Dictyostelium stress-activated protein kinase alpha, a novel stress-activated mitogen-activated protein kinase kinase kinase-like kinase, is important for the proper regulation of the cytoskeleton. Mol Biol Cell. 2003;14(11):4526–4540. doi: 10.1091/mbc.E03-01-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ma H, Gamper M, Parent C, Firtel RA. The Dictyostelium MAP kinase kinase DdMEK1 regulates chemotaxis and is essential for chemoattractant-mediated activation of guanylyl cyclase. EMBO J. 1997;16(14):4317–4332. doi: 10.1093/emboj/16.14.4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sobko A, Ma H, Firtel RA. Regulated SUMOylation and ubiquitination of DdMEK1 is required for proper chemotaxis. Dev Cell. 2002;2(6):745–756. doi: 10.1016/s1534-5807(02)00186-7. [DOI] [PubMed] [Google Scholar]
- 182.Mendoza MC, Booth EO, Shaulsky G, Firtel RA. MEK1 and protein phosphatase 4 coordinate Dictyostelium development and chemotaxis. Mol Cell Biol. 2007;27(10):3817–3827. doi: 10.1128/MCB.02194-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Mendoza MC, Du F, Iranfar N, Tang N, Ma H, Loomis WF, Firtel RA. Loss of SMEK, a novel, conserved protein, suppresses MEK1 null cell polarity, chemotaxis, and gene expression defects. Mol Cell Biol. 2005;25(17):7839–7853. doi: 10.1128/MCB.25.17.7839-7853.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Gaskins C, Maeda M, Firtel RA. Identification and functional analysis of a developmentally regulated extracellular signal-regulated kinase gene in Dictyostelium discoideum. Mol Cell Biol. 1994;14(10):6996–7012. doi: 10.1128/mcb.14.10.6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Gaskins C, Clark AM, Aubry L, Segall JE, Firtel RA. The Dictyostelium MAP kinase ERK2 regulates multiple, independent developmental pathways. Genes Dev. 1996;10(1):118–128. doi: 10.1101/gad.10.1.118. [DOI] [PubMed] [Google Scholar]
- 186.Segall JE, Kuspa A, Shaulsky G, Ecke M, Maeda M, Gaskins C, Firtel RA, Loomis WF. A MAP kinase necessary for receptor-mediated activation of adenylyl cyclase in Dictyostelium. J Cell Biol. 1995;128(3):405–413. doi: 10.1083/jcb.128.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Knetsch ML, Epskamp SJ, Schenk PW, Wang Y, Segall JE, Snaar-Jagalska BE. Dual role of cAMP and involvement of both G-proteins and ras in regulation of ERK2 in Dictyostelium discoideum. EMBO J. 1996;15(13):3361–3368. [PMC free article] [PubMed] [Google Scholar]
- 188.Maeda M, Aubry L, Insall R, Gaskins C, Devreotes PN, Firtel RA. Seven helix chemoattractant receptors transiently stimulate mitogen-activated protein kinase in Dictyostelium. Role of heterotrimeric G proteins. J Biol Chem. 1996;271(7):3351–3354. doi: 10.1074/jbc.271.7.3351. [DOI] [PubMed] [Google Scholar]
- 189.Brzostowski JA, Kimmel AR. Nonadaptive regulation of ERK2 in Dictyostelium: implications for mechanisms of cAMP relay. Mol Biol Cell. 2006;17(10):4220–4227. doi: 10.1091/mbc.E06-05-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Maeda M, Firtel RA. Activation of the mitogen-activated protein kinase ERK2 by the chemoattractant folic acid in Dictyostelium. J Biol Chem. 1997;272(38):23690–23695. doi: 10.1074/jbc.272.38.23690. [DOI] [PubMed] [Google Scholar]
- 191.Thomason PA, Traynor D, Cavet G, Chang WT, Harwood AJ, Kay RR. An intersection of the cAMP/PKA and two-component signal transduction systems in Dictyostelium. EMBO J. 1998;17(10):2838–2845. doi: 10.1093/emboj/17.10.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Maeda M, Lu S, Shaulsky G, Miyazaki Y, Kuwayama H, Tanaka Y, Kuspa A, Loomis WF. Periodic signaling controlled by an oscillatory circuit that includes protein kinases ERK2 and PKA. Science. 2004;304(5672):875–878. doi: 10.1126/science.1094647. [DOI] [PubMed] [Google Scholar]
- 193.Wessels DJ, Zhang H, Reynolds J, Daniels K, Heid P, Lu S, Kuspa A, Shaulsky G, Loomis WF, Soll DR. The internal phosphodiesterase RegA is essential for the suppression of lateral pseudopods during Dictyostelium chemotaxis. Mol Biol Cell. 2000;11(8):2803–2820. doi: 10.1091/mbc.11.8.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Grinstein S, Butler JR, Furuya W, L’Allemain G, Downey GP. Chemotactic peptides induce phosphorylation and activation of MEK-1 in human neutrophils. J Biol Chem. 1994;269(30):19313–19320. [PubMed] [Google Scholar]
- 195.Thompson HL, Marshall CJ, Saklatvala J. Characterization of two different forms of mitogen-activated protein kinase kinase induced in polymorphonuclear leukocytes following stimulation by N-formylmethionyl-leucyl-phenylalanine or granulocyte-macrophage colony-stimulating factor. J Biol Chem. 1994;269(13):9486–9492. [PubMed] [Google Scholar]
- 196.Knall C, Worthen GS, Buhl AM, Johnson GL. IL-8 signal transduction in human neutrophils. Ann N Y Acad Sci. 1995;766:288–291. doi: 10.1111/j.1749-6632.1995.tb26679.x. [DOI] [PubMed] [Google Scholar]
- 197.Nick JA, Avdi NJ, Young SK, Knall C, Gerwins P, Johnson GL, Worthen GS. Common and distinct intracellular signaling pathways in human neutrophils utilized by platelet activating factor and FMLP. J Clin Invest. 1997;99(5):975–986. doi: 10.1172/JCI119263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Marshall CJ. Ras effectors. Curr Opin Cell Biol. 1996;8(2):197–204. doi: 10.1016/s0955-0674(96)80066-4. [DOI] [PubMed] [Google Scholar]
- 199.Rane MJ, Carrithers SL, Arthur JM, Klein JB, McLeish KR. Formyl peptide receptors are coupled to multiple mitogen-activated protein kinase cascades by distinct signal transduction pathways: role in activation of reduced nicotinamide adenine dinucleotide oxidase. Journal of immunology. 1997;159(10):5070–5078. [PubMed] [Google Scholar]
- 200.Coxon PY, Rane MJ, Uriarte S, Powell DW, Singh S, Butt W, Chen Q, McLeish KR. MAPK-activated protein kinase-2 participates in p38 MAPK-dependent and ERK-dependent functions in human neutrophils. Cell Signal. 2003;15(11):993–1001. doi: 10.1016/s0898-6568(03)00074-3. [DOI] [PubMed] [Google Scholar]
- 201.Zu YL, Qi J, Gilchrist A, Fernandez GA, Vazquez-Abad D, Kreutzer DL, Huang CK, Sha’afi RI. p38 mitogen-activated protein kinase activation is required for human neutrophil function triggered by TNF-alpha or FMLP stimulation. Journal of immunology. 1998;160(4):1982–1989. [PubMed] [Google Scholar]
- 202.Liu X, Ma B, Malik AB, Tang H, Yang T, Sun B, Wang G, Minshall RD, Li Y, Zhao Y, Ye RD, Xu J. Bidirectional regulation of neutrophil migration by mitogen-activated protein kinases. Nat Immunol. 2012;13(5):457–464. doi: 10.1038/ni.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Boehme SA, Sullivan SK, Crowe PD, Santos M, Conlon PJ, Sriramarao P, Bacon KB. Activation of mitogen-activated protein kinase regulates eotaxin-induced eosinophil migration. Journal of immunology. 1999;163(3):1611–1618. [PubMed] [Google Scholar]
- 204.Weber M, Sixt M. MEK signalling tunes actin treadmilling for interstitial lymphocyte migration. EMBO J. 2010;29(17):2861–2863. doi: 10.1038/emboj.2010.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Klemke M, Kramer E, Konstandin MH, Wabnitz GH, Samstag Y. An MEK-cofilin signalling module controls migration of human T cells in 3D but not 2D environments. EMBO J. 2010;29(17):2915–2929. doi: 10.1038/emboj.2010.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112(4):453–465. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- 207.Hannigan MO, Zhan L, Ai Y, Kotlyarov A, Gaestel M, Huang CK. Abnormal migration phenotype of mitogen-activated protein kinase-activated protein kinase 2-/- neutrophils in Zigmond chambers containing formyl-methionyl-leucyl-phenylalanine gradients. Journal of immunology. 2001;167(7):3953–3961. doi: 10.4049/jimmunol.167.7.3953. [DOI] [PubMed] [Google Scholar]
- 208.Decaillot FM, Kazmi MA, Lin Y, Ray-Saha S, Sakmar TP, Sachdev P. CXCR7/CXCR4 heterodimer constitutively recruits beta-arrestin to enhance cell migration. J Biol Chem. 2011;286(37):32188–32197. doi: 10.1074/jbc.M111.277038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Sanchez-Martin L, Sanchez-Mateos P, Cabanas C. CXCR7 impact on CXCL12 biology and disease. Trends in molecular medicine. 2013;19(1):12–22. doi: 10.1016/j.molmed.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 210.Bosgraaf L, Waijer A, Engel R, Visser AJWG, Wessels D, Soll D, van Haastert PJM. RasGEF-containing proteins GbpC and GbpD have differential effects on cell polarity and chemotaxis in Dictyostelium. J Cell Sci. 2005;118(9):1899–1910. doi: 10.1242/jcs.02317. [DOI] [PubMed] [Google Scholar]
- 211.Kortholt A, Rehmann H, Kae H, Bosgraaf L, Keizer-Gunnink I, Weeks G, Wittinghofer A, Van Haastert PJM. Characterization of the GbpD-activated Rap1 Pathway Regulating Adhesion and Cell Polarity in Dictyostelium discoideum. J Biol Chem. 2006;281(33):23367–23376. doi: 10.1074/jbc.M600804200. [DOI] [PubMed] [Google Scholar]
- 212.Rebstein PJ, Cardelli J, Weeks G, Spiegelman GB (1997) Mutational Analysis of the Role of Rap1 in Regulating Cytoskeletal Function inDictyostelium. Experimental Cell Research 231 (2):276-283. doi: 10.1006/excr.1996.3466 [DOI] [PubMed]
- 213.Jeon TJ, Lee D-J, Merlot S, Weeks G, Firtel RA. Rap1 controls cell adhesion and cell motility through the regulation of myosin II. The Journal of Cell Biology. 2007;176(7):1021–1033. doi: 10.1083/jcb.200607072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Cha I, Lee S, Jeon T. Chemoattractant-mediated Rap1 activation requires GPCR/G proteins. Mol Cells. 2010;30(6):563–567. doi: 10.1007/s10059-010-0153-5. [DOI] [PubMed] [Google Scholar]
- 215.Bolourani P, Spiegelman GB, Weeks G. Rap1 Activation in Response to cAMP Occurs Downstream of Ras Activation during Dictyostelium Aggregation. J Biol Chem. 2008;283(16):10232–10240. doi: 10.1074/jbc.M707459200. [DOI] [PubMed] [Google Scholar]
- 216.Jeon TJ, Lee D-J, Lee S, Weeks G, Firtel RA. Regulation of Rap1 activity by RapGAP1 controls cell adhesion at the front of chemotaxing cells. The Journal of Cell Biology. 2007;179(5):833–843. doi: 10.1083/jcb.200705068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Parkinson K, Bolourani P, Traynor D, Aldren NL, Kay RR, Weeks G, Thompson CRL. Regulation of Rap1 activity is required for differential adhesion, cell-type patterning and morphogenesis in Dictyostelium. J Cell Sci. 2009;122(3):335–344. doi: 10.1242/jcs.036822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Gebbie L, Benghezal M, Cornillon S, Froquet R, Cherix N, Malbouyres M, Lefkir Y, Grangeasse C, Fache S, Dalous J, Brückert F, Letourneur F, Cosson P. Phg2, a Kinase Involved in Adhesion and Focal Site Modeling in Dictyostelium. Mol Biol Cell. 2004;15(8):3915–3925. doi: 10.1091/mbc.E03-12-0908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Mun H, Jeon T. Regulation of actin cytoskeleton by Rap1 binding to RacGEF1. Mol Cells. 2012;34(1):71–76. doi: 10.1007/s10059-012-0097-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Plak K, Veltman D, Fusetti F, Beeksma J, Rivero F, Van Haastert P, Kortholt A. GxcC connects Rap and Rac signaling during Dictyostelium development. BMC Cell Biology. 2013;14(1):6. doi: 10.1186/1471-2121-14-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Kortholt A, Bolourani P, Rehmann H, Keizer-Gunnink I, Weeks G, Wittinghofer A, Van Haastert PJM. A Rap/Phosphatidylinositol 3-Kinase Pathway Controls Pseudopod Formation. Mol Biol Cell. 2010;21(6):936–945. doi: 10.1091/mbc.E09-03-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Artemenko Y, Batsios P, Borleis J, Gagnon Z, Lee J, Rohlfs M, Sanséau D, Willard SS, Schleicher M, Devreotes PN. Tumor suppressor Hippo/MST1 kinase mediates chemotaxis by regulating spreading and adhesion. Proc Natl Acad Sci. 2012;109(34):13632–13637. doi: 10.1073/pnas.1211304109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Bos JL, de Rooij J, Reedquist KA. Rap1 signalling: adhering to new models. Nat Rev Mol Cell Biol. 2001;2(5):369–377. doi: 10.1038/35073073. [DOI] [PubMed] [Google Scholar]
- 224.McLeod SJ, Li AHY, Lee RL, Burgess AE, Gold MR. The Rap GTPases Regulate B Cell Migration Toward the Chemokine Stromal Cell-Derived Factor-1 (CXCL12): Potential Role for Rap2 in Promoting B Cell Migration. J Immunol. 2002;169(3):1365–1371. doi: 10.4049/jimmunol.169.3.1365. [DOI] [PubMed] [Google Scholar]
- 225.Shimonaka M, Katagiri K, Nakayama T, Fujita N, Tsuruo T, Yoshie O, Kinashi T. Rap1 translates chemokine signals to integrin activation, cell polarization, and motility across vascular endothelium under flow. The Journal of Cell Biology. 2003;161(2):417–427. doi: 10.1083/jcb.200301133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Gérard A, Mertens AEE, van der Kammen RA, Collard JG. The Par polarity complex regulates Rap1- and chemokine-induced T cell polarization. The Journal of Cell Biology. 2007;176(6):863–875. doi: 10.1083/jcb.200608161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Liu L, Aerbajinai W, Ahmed SM, Rodgers GP, Angers S, Parent CA. Radil controls neutrophil adhesion and motility through β2-integrin activation. Mol Biol Cell. 2012;23(24):4751–4765. doi: 10.1091/mbc.E12-05-0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Miertzschke M, Stanley P, Bunney TD, Rodrigues-Lima F, Hogg N, Katan M. Characterization of Interactions of Adapter Protein RAPL/Nore1B with RAP GTPases and Their Role in T Cell Migration. J Biol Chem. 2007;282(42):30629–30642. doi: 10.1074/jbc.M704361200. [DOI] [PubMed] [Google Scholar]
- 229.M’Rabet L, Coffer P, Zwartkruis F, Franke B, Segal AW, Koenderman L, Bos JL. Activation of the Small GTPase Rap1 in Human Neutrophils. Blood. 1998;92(6):2133–2140. [PubMed] [Google Scholar]
- 230.He Y, Kapoor A, Cook S, Liu S, Xiang Y, Rao CV, Kenis PJA, Wang F. The non-receptor tyrosine kinase Lyn controls neutrophil adhesion by recruiting the CrkL–C3G complex and activating Rap1 at the leading edge. J Cell Sci. 2011;124(13):2153–2164. doi: 10.1242/jcs.078535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Gu JJ, Lavau CP, Pugacheva E, Soderblom EJ, Moseley MA, Pendergast AM (2012) Abl Family Kinases Modulate T Cell-Mediated Inflammation and Chemokine-Induced Migration Through the Adaptor HEF1 and the GTPase Rap1. Sci Signal 5 (233):ra51-. doi:10.1126/scisignal.2002632 [DOI] [PMC free article] [PubMed]
- 232.Lafuente EM, van Puijenbroek AAFL, Krause M, Carman CV, Freeman GJ, Berezovskaya A, Constantine E, Springer TA, Gertler FB, Boussiotis VA (2004) RIAM, an Ena/VASP and Profilin Ligand, Interacts with Rap1-GTP and Mediates Rap1-Induced Adhesion. Developmental Cell 7 (4):585-595. doi: 10.1016/j.devcel.2004.07.021 [DOI] [PubMed]
- 233.Kliche S, Worbs T, Wang X, Degen J, Patzak I, Meineke B, Togni M, Moser M, Reinhold A, Kiefer F, Freund C, Förster R, Schraven B. CCR7-mediated LFA-1 functions in T cells are regulated by 2 independent ADAP/SKAP55 modules. Blood. 2012;119(3):777–785. doi: 10.1182/blood-2011-06-362269. [DOI] [PubMed] [Google Scholar]
- 234.Katagiri K, Maeda A, Shimonaka M, Kinashi T (2003) RAPL, a Rap1-binding molecule that mediates Rap1-induced adhesion through spatial regulation of LFA-1. Nat Immunol 4 (8):741-748. doi:http://www.nature.com/ni/journal/v4/n8/suppinfo/ni950_S1.html [DOI] [PubMed]
- 235.Katagiri K, Ohnishi N, Kabashima K, Iyoda T, Takeda N, Shinkai Y, Inaba K, Kinashi T (2004) Crucial functions of the Rap1 effector molecule RAPL in lymphocyte and dendritic cell trafficking. Nat Immunol 5 (10):1045-1051. doi:http://www.nature.com/ni/journal/v5/n10/suppinfo/ni1111_S1.html [DOI] [PubMed]
- 236.Katagiri K, Imamura M, Kinashi T (2006) Spatiotemporal regulation of the kinase Mst1 by binding protein RAPL is critical for lymphocyte polarity and adhesion. Nat Immunol 7 (9):919-928. doi:http://www.nature.com/ni/journal/v7/n9/suppinfo/ni1374_S1.html [DOI] [PubMed]
- 237.Dong Y, Du X, Ye J, Han M, Xu T, Zhuang Y, Tao W. A Cell-Intrinsic Role for Mst1 in Regulating Thymocyte Egress. J Immunol. 2009;183(6):3865–3872. doi: 10.4049/jimmunol.0900678. [DOI] [PubMed] [Google Scholar]
- 238.Katagiri K, Katakai T, Ebisuno Y, Ueda Y, Okada T, Kinashi T (2009) Mst1 controls lymphocyte trafficking and interstitial motility within lymph nodes. EMBO J 28 (9):1319-1331. doi:http://www.nature.com/emboj/journal/v28/n9/suppinfo/emboj200982a_S1.html [DOI] [PMC free article] [PubMed]
- 239.Gambardella L, Anderson KE, Nussbaum C, Segonds-Pichon A, Margarido T, Norton L, Ludwig T, Sperandio M, Hawkins PT, Stephens L, Vermeren S. The GTPase-activating protein ARAP3 regulates chemotaxis and adhesion-dependent processes in neutrophils. Blood. 2011;118(4):1087–1098. doi: 10.1182/blood-2010-10-312959. [DOI] [PubMed] [Google Scholar]
- 240.Bosgraaf L, van Haastert PJM (2006) The regulation of myosin II in Dictyostelium. European Journal of Cell Biology 85 (9–10):969-979. doi: 10.1016/j.ejcb.2006.04.004 [DOI] [PubMed]
- 241.Peters DJM, Knecht DA, Loomis WF, De Lozanne A, Spudich J, Van Haastert PJM (1988) Signal transduction, chemotaxis, and cell aggregation in Dictyostelium discoideum cells without myosin heavy chain. Developmental Biology 128 (1):158-163. doi: 10.1016/0012-1606(88)90278-3 [DOI] [PubMed]
- 242.Wessels D, Soll DR, Knecht D, Loomis WF, De Lozanne A, Spudich J (1988) Cell motility and chemotaxis in Dictyostelium amebae lacking myosin heavy chain. Developmental Biology 128 (1):164-177. doi: 10.1016/0012-1606(88)90279-5 [DOI] [PubMed]
- 243.Heid PJ, Wessels D, Daniels KJ, Gibson DP, Zhang H, Voss E, Soll DR. The role of myosin heavy chain phosphorylation in Dictyostelium motility, chemotaxis and F-actin localization. J Cell Sci. 2004;117(20):4819–4835. doi: 10.1242/jcs.01358. [DOI] [PubMed] [Google Scholar]
- 244.Laevsky G, Knecht DA. Cross-linking of actin filaments by myosin II is a major contributor to cortical integrity and cell motility in restrictive environments. J Cell Sci. 2003;116(18):3761–3770. doi: 10.1242/jcs.00684. [DOI] [PubMed] [Google Scholar]
- 245.Chen TL, Kowalczyk PA, Ho G, Chisholm RL. Targeted disruption of the Dictyostelium myosin essential light chain gene produces cells defective in cytokinesis and morphogenesis. J Cell Sci. 1995;108(10):3207–3218. doi: 10.1242/jcs.108.10.3207. [DOI] [PubMed] [Google Scholar]
- 246.Yumura S, Uyeda TQP. Myosin II can be localized to the cleavage furrow and to the posterior region of Dictyostelium amoebae without control by phosphorylation of myosin heavy and light chains. Cell Motil Cytoskelet. 1997;36(4):313–322. doi: 10.1002/(SICI)1097-0169(1997)36:4<313::AID-CM2>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 247.Zhang H, Wessels D, Fey P, Daniels K, Chisholm RL, Soll DR. Phosphorylation of the myosin regulatory light chain plays a role in motility and polarity during Dictyostelium chemotaxis. J Cell Sci. 2002;115(8):1733–1747. doi: 10.1242/jcs.115.8.1733. [DOI] [PubMed] [Google Scholar]
- 248.Lück-Vielmetter D, Schleicher M, Grabatin B, Wippler J, Gerisch G (1990) Replacement of threonine residues by serine and alanine in a phosphorylatable heavy chain fragment of Dictyostelium myosin II. FEBS Letters 269 (1):239-243. doi: 10.1016/0014-5793(90)81163-I [DOI] [PubMed]
- 249.Vaillancourt JP, Lyons C, Côté GP. Identification of two phosphorylated threonines in the tail region of Dictyostelium myosin II. J Biol Chem. 1988;263(21):10082–10087. [PubMed] [Google Scholar]
- 250.Kuczmarski ER, Tafuri SR, Parysek LM. Effect of heavy chain phosphorylation on the polymerization and structure of Dictyostelium myosin filaments. The Journal of Cell Biology. 1987;105(6):2989–2997. doi: 10.1083/jcb.105.6.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Egelhoff TT, Lee RJ, Spudich JA (1993) Dictyostelium myosin heavy chain phosphorylation sites regulate myosin filament assembly and localization in vivo. Cell 75 (2):363-371. doi: 10.1016/0092-8674(93)80077-R [DOI] [PubMed]
- 252.Levi S, Polyakov MV, Egelhoff TT. Myosin II dynamics in Dictyostelium: Determinants for filament assembly and translocation to the cell cortex during chemoattractant responses. Cell Motil Cytoskelet. 2002;53(3):177–188. doi: 10.1002/cm.10068. [DOI] [PubMed] [Google Scholar]
- 253.Berlot CH, Spudich JA, Devreotes PN (1985) Chemoattractant-elicited increases in myosin phosphorylation in dictyostelium. Cell 43 (1):307-314. doi: 10.1016/0092-8674(85)90036-4 [DOI] [PubMed]
- 254.Liu G, Newell PC. Evidence of cyclic GMP may regulate the association of myosin II heavy chain with the cytoskeleton by inhibiting its phosphorylation. J Cell Sci. 1991;98(4):483–490. doi: 10.1242/jcs.98.4.483. [DOI] [PubMed] [Google Scholar]
- 255.Moores SL, Sabry JH, Spudich JA. Myosin dynamics in live Dictyostelium cells. Proc Natl Acad Sci. 1996;93(1):443–446. doi: 10.1073/pnas.93.1.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Steimle PA, Yumura S, Côté GP, Medley QG, Polyakov MV, Leppert B, Egelhoff TT (2001) Recruitment of a myosin heavy chain kinase to actin-rich protrusions in Dictyostelium. Current Biology 11 (9):708-713. doi: 10.1016/S0960-9822(01)00182-8 [DOI] [PubMed]
- 257.Stites J, Wessels D, Uhl A, Egelhoff T, Shutt D, Soll DR. Phosphorylation of the Dictyostelium myosin II heavy chain is necessary for maintaining cellular polarity and suppressing turning during chemotaxis. Cell Motil Cytoskelet. 1998;39(1):31–51. doi: 10.1002/(SICI)1097-0169(1998)39:1<31::AID-CM4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 258.Liang W, Licate L, Warrick H, Spudich J, Egelhoff T. Differential localization in cells of myosin II heavy chain kinases during cytokinesis and polarized migration. BMC Cell Biology. 2002;3(1):19. doi: 10.1186/1471-2121-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Brakefield PM, Kesbeke F, Koch PB. The regulation of phenotypic plasticity of eyespots in the butterfly Bicyclus anynana. Am Nat. 1998;152(6):853–860. doi: 10.1086/286213. [DOI] [PubMed] [Google Scholar]
- 260.Wang Y, Steimle PA, Ren Y, Ross CA, Robinson DN, Egelhoff TT, Sesaki H, Iijima M. Dictyostelium huntingtin controls chemotaxis and cytokinesis through the regulation of myosin II phosphorylation. Mol Biol Cell. 2011;22(13):2270–2281. doi: 10.1091/mbc.E10-11-0926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Roelofs J, Van Haastert PJM. Characterization of Two Unusual Guanylyl Cyclases fromDictyostelium. J Biol Chem. 2002;277(11):9167–9174. doi: 10.1074/jbc.M111437200. [DOI] [PubMed] [Google Scholar]
- 262.Kuwayama H, Snippe H, Derks M, Roelofs J, Van Haastert PJ. Identification and characterization of DdPDE3, a cGMP-selective phosphodiesterase from Dictyostelium. Biochem J. 2001;353(3):635–644. doi: 10.1042/0264-6021:3530635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Bosgraaf L, Russcher H, Snippe H, Bader S, Wind J, Van Haastert PJM. Identification and Characterization of Two Unusual cGMP-stimulated Phoshodiesterases in Dictyostelium. Mol Biol Cell. 2002;13(11):3878–3889. doi: 10.1091/mbc.E02-05-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Bader S, Kortholt A, Van haastert PJM. Seven Dictyostelium discoideum phosphodiesterases degrade three pools of cAMP and cGMP. Biochem J. 2007;402(1):153–161. doi: 10.1042/BJ20061153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Bosgraaf L, Russcher H, Smith JL, Wessels D, Soll DR, Van Haastert PJ. A novel cGMP signalling pathway mediating myosin phosphorylation and chemotaxis in Dictyostelium. EMBO J. 2002;21(17):4560–4570. doi: 10.1093/emboj/cdf438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Veltman DM, Van Haastert PJM. Guanylyl Cyclase Protein and cGMP Product Independently Control Front and Back of Chemotaxing Dictyostelium Cells. Mol Biol Cell. 2006;17(9):3921–3929. doi: 10.1091/mbc.E06-05-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Goldberg JM, Wolpin ES, Bosgraaf L, Clarkson BK, Van Haastert PJM, Smith JL (2006) Myosin light chain kinase A is activated by cGMP-dependent and cGMP-independent pathways. FEBS Letters 580 (8):2059-2064. doi: 10.1016/j.febslet.2006.03.008 [DOI] [PubMed]
- 268.Ostrow BD, Chen P, Chisholm RL. Expression of a myosin regulatory light chain phosphorylation site mutant complements the cytokinesis and developmental defects of Dictyostelium RMLC null cells. The Journal of Cell Biology. 1994;127(6):1945–1955. doi: 10.1083/jcb.127.6.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Pfannes EK, Anielski A, Gerhardt M, Beta C. Intracellular photoactivation of caged cGMP induces myosin II and actin responses in motile cells. Integr Biol (Camb) 2013;5(12):1456–1463. doi: 10.1039/c3ib40109j. [DOI] [PubMed] [Google Scholar]
- 270.Morin NA, Oakes PW, Hyun Y-M, Lee D, Chin YE, King MR, Springer TA, Shimaoka M, Tang JX, Reichner JS, Kim M. Nonmuscle myosin heavy chain IIA mediates integrin LFA-1 de-adhesion during T lymphocyte migration. J Exp Med. 2008;205(1):195–205. doi: 10.1084/jem.20071543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Worthylake RA, Lemoine S, Watson JM, Burridge K. RhoA is required for monocyte tail retraction during transendothelial migration. The Journal of Cell Biology. 2001;154(1):147–160. doi: 10.1083/jcb.200103048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Vicente-Manzanares M, Cabrero JR, Rey M, Pérez-Martínez M, Ursa A, Itoh K, Sánchez-Madrid F. A Role for the Rho-p160 Rho Coiled-Coil Kinase Axis in the Chemokine Stromal Cell-Derived Factor-1α-Induced Lymphocyte Actomyosin and Microtubular Organization and Chemotaxis. J Immunol. 2002;168(1):400–410. doi: 10.4049/jimmunol.168.1.400. [DOI] [PubMed] [Google Scholar]
- 273.Takesono A, Heasman SJ, Wojciak-Stothard B, Garg R, Ridley AJ. Microtubules Regulate Migratory Polarity through Rho/ROCK Signaling in T Cells. PLoS ONE. 2010;5(1):e8774. doi: 10.1371/journal.pone.0008774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol. 2009;10(11):778–790. doi: 10.1038/nrm2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Jacobelli J, Chmura SA, Buxton DB, Davis MM, Krummel MF (2004) A single class II myosin modulates T cell motility and stopping, but not synapse formation. Nat Immunol 5 (5):531-538. doi:http://www.nature.com/ni/journal/v5/n5/suppinfo/ni1065_S1.html [DOI] [PubMed]
- 276.Soede RDM, Zeelenberg IS, Wijnands YM, Kamp M, Roos E. Stromal Cell-Derived Factor-1-Induced LFA-1 Activation During In Vivo Migration of T Cell Hybridoma Cells Requires Gq/11, RhoA, and Myosin, as well as Gi and Cdc42. J Immunol. 2001;166(7):4293–4301. doi: 10.4049/jimmunol.166.7.4293. [DOI] [PubMed] [Google Scholar]
- 277.Tan W, Martin D, Gutkind JS. The Gα13-Rho Signaling Axis Is Required for SDF-1-induced Migration through CXCR4. J Biol Chem. 2006;281(51):39542–39549. doi: 10.1074/jbc.M609062200. [DOI] [PubMed] [Google Scholar]
- 278.Pestonjamasp KN, Forster C, Sun C, Gardiner EM, Bohl B, Weiner O, Bokoch GM, Glogauer M. Rac1 links leading edge and uropod events through Rho and myosin activation during chemotaxis. Blood. 2006;108(8):2814–2820. doi: 10.1182/blood-2006-01-010363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.del Pozo MA, Nieto M, Serrador JM, Sancho D, Vicente-Manzanares M, Martínez C, Sánchez-Madrid F. The two poles of the lymphocyte: specialized cell compartments for migration and recruitment. Cell Adhes Commun. 1998;6(2–3):125–133. doi: 10.3109/15419069809004468. [DOI] [PubMed] [Google Scholar]
- 280.Smith A, Bracke M, Leitinger B, Porter JC, Hogg N. LFA-1-induced T cell migration on ICAM-1 involves regulation of MLCK-mediated attachment and ROCK-dependent detachment. J Cell Sci. 2003;116(15):3123–3133. doi: 10.1242/jcs.00606. [DOI] [PubMed] [Google Scholar]
- 281.Gerisch G, Bretschneider T, Muller-Taubenberger A, Simmeth E, Ecke M, Diez S, Anderson K. Mobile actin clusters and traveling waves in cells recovering from actin depolymerization. Biophys J. 2004;87(5):3493–3503. doi: 10.1529/biophysj.104.047589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Schneider N, Weber I, Faix J, Prassler J, Muller-Taubenberger A, Kohler J, Burghardt E, Gerisch G, Marriott G. A Lim protein involved in the progression of cytokinesis and regulation of the mitotic spindle. Cell Motil Cytoskeleton. 2003;56(2):130–139. doi: 10.1002/cm.10139. [DOI] [PubMed] [Google Scholar]
- 283.Chung CY, Lee S, Briscoe C, Ellsworth C, Firtel RA. Role of Rac in controlling the actin cytoskeleton and chemotaxis in motile cells. Proc Natl Acad Sci U S A. 2000;97(10):5225–5230. doi: 10.1073/pnas.97.10.5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Park KC, Rivero F, Meili R, Lee S, Apone F, Firtel RA. Rac regulation of chemotaxis and morphogenesis in Dictyostelium. EMBO J. 2004;23(21):4177–4189. doi: 10.1038/sj.emboj.7600368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Han JW, Leeper L, Rivero F, Chung CY. Role of RacC for the regulation of WASP and phosphatidylinositol 3-kinase during chemotaxis of Dictyostelium. J Biol Chem. 2006;281(46):35224–35234. doi: 10.1074/jbc.M605997200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Filic V, Marinovic M, Faix J, Weber I. A dual role for Rac1 GTPases in the regulation of cell motility. J Cell Sci. 2012;125(Pt 2):387–398. doi: 10.1242/jcs.089680. [DOI] [PubMed] [Google Scholar]
- 287.Plak K, Veltman D, Fusetti F, Beeksma J, Rivero F, Van Haastert PJ, Kortholt A. GxcC connects Rap and Rac signaling during Dictyostelium development. BMC Cell Biol. 2013;14:6. doi: 10.1186/1471-2121-14-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Yan J, Mihaylov V, Xu X, Brzostowski JA, Li H, Liu L, Veenstra TD, Parent CA, Jin T. A Gbetagamma effector, ElmoE, transduces GPCR signaling to the actin network during chemotaxis. Dev Cell. 2012;22(1):92–103. doi: 10.1016/j.devcel.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Seastone DJ, Harris E, Temesvari LA, Bear JE, Saxe CL, Cardelli J. The WASp-like protein scar regulates macropinocytosis, phagocytosis and endosomal membrane flow in Dictyostelium. J Cell Sci. 2001;114(Pt 14):2673–2683. doi: 10.1242/jcs.114.14.2673. [DOI] [PubMed] [Google Scholar]
- 290.Caracino D, Jones C, Compton M, Saxe CL., 3rd The N-terminus of Dictyostelium Scar interacts with Abi and HSPC300 and is essential for proper regulation and function. Mol Biol Cell. 2007;18(5):1609–1620. doi: 10.1091/mbc.E06-06-0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Veltman DM, King JS, Machesky LM, Insall RH. SCAR knockouts in Dictyostelium: WASP assumes SCAR’s position and upstream regulators in pseudopods. J Cell Biol. 2012;198(4):501–508. doi: 10.1083/jcb.201205058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Pollitt AY, Insall RH. Loss of Dictyostelium HSPC300 causes a scar-like phenotype and loss of SCAR protein. BMC Cell Biol. 2009;10:13. doi: 10.1186/1471-2121-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Blagg SL, Stewart M, Sambles C, Insall RH. PIR121 regulates pseudopod dynamics and SCAR activity in Dictyostelium. Current biology : CB. 2003;13(17):1480–1487. doi: 10.1016/s0960-9822(03)00580-3. [DOI] [PubMed] [Google Scholar]
- 294.Ura S, Pollitt AY, Veltman DM, Morrice NA, Machesky LM, Insall RH. Pseudopod growth and evolution during cell movement is controlled through SCAR/WAVE dephosphorylation. Current biology : CB. 2012;22(7):553–561. doi: 10.1016/j.cub.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Myers SA, Han JW, Lee Y, Firtel RA, Chung CY. A Dictyostelium homologue of WASP is required for polarized F-actin assembly during chemotaxis. Mol Biol Cell. 2005;16(5):2191–2206. doi: 10.1091/mbc.E04-09-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Chung CY, Feoktistov A, Hollingsworth RJ, Rivero F, Mandel NS. An attenuating role of a WASP-related protein, WASP-B, in the regulation of F-actin polymerization and pseudopod formation via the regulation of RacC during Dictyostelium chemotaxis. Biochem Biophys Res Commun. 2013;436(4):719–724. doi: 10.1016/j.bbrc.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Shu S, Liu X, Kriebel PW, Hong MS, Daniels MP, Parent CA, Korn ED. Expression of Y53A-actin in Dictyostelium disrupts the cytoskeleton and inhibits intracellular and intercellular chemotactic signaling. J Biol Chem. 2010;285(36):27713–27725. doi: 10.1074/jbc.M110.116277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Choi CH, Thomason PA, Zaki M, Insall RH, Barber DL. Phosphorylation of actin-related protein 2 (Arp2) is required for normal development and cAMP chemotaxis in Dictyostelium. J Biol Chem. 2013;288(4):2464–2474. doi: 10.1074/jbc.M112.435313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Schirenbeck A, Bretschneider T, Arasada R, Schleicher M, Faix J. The Diaphanous-related formin dDia2 is required for the formation and maintenance of filopodia. Nat Cell Biol. 2005;7(6):619–625. doi: 10.1038/ncb1266. [DOI] [PubMed] [Google Scholar]
- 300.Rivero F, Koppel B, Peracino B, Bozzaro S, Siegert F, Weijer CJ, Schleicher M, Albrecht R, Noegel AA. The role of the cortical cytoskeleton: F-actin crosslinking proteins protect against osmotic stress, ensure cell size, cell shape and motility, and contribute to phagocytosis and development. J Cell Sci. 1996;109(Pt 11):2679–2691. doi: 10.1242/jcs.109.11.2679. [DOI] [PubMed] [Google Scholar]
- 301.Khaire N, Muller R, Blau-Wasser R, Eichinger L, Schleicher M, Rief M, Holak TA, Noegel AA. Filamin-regulated F-actin assembly is essential for morphogenesis and controls phototaxis in Dictyostelium. J Biol Chem. 2007;282(3):1948–1955. doi: 10.1074/jbc.M610262200. [DOI] [PubMed] [Google Scholar]
- 302.Faix J, Steinmetz M, Boves H, Kammerer RA, Lottspeich F, Mintert U, Murphy J, Stock A, Aebi U, Gerisch G. Cortexillins, major determinants of cell shape and size, are actin-bundling proteins with a parallel coiled-coil tail. Cell. 1996;86(4):631–642. doi: 10.1016/s0092-8674(00)80136-1. [DOI] [PubMed] [Google Scholar]
- 303.Shu S, Liu X, Kriebel PW, Daniels MP, Korn ED. Actin cross-linking proteins cortexillin I and II are required for cAMP signaling during Dictyostelium chemotaxis and development. Mol Biol Cell. 2012;23(2):390–400. doi: 10.1091/mbc.E11-09-0764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Parsons JT, Horwitz AR, Schwartz MA. Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat Rev Mol Cell Biol. 2010;11(9):633–643. doi: 10.1038/nrm2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Sebe-Pedros A, Roger AJ, Lang FB, King N, Ruiz-Trillo I. Ancient origin of the integrin-mediated adhesion and signaling machinery. Proc Natl Acad Sci U S A. 2010;107(22):10142–10147. doi: 10.1073/pnas.1002257107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Brown MC, West KA, Turner CE. Paxillin-dependent paxillin kinase linker and p21-activated kinase localization to focal adhesions involves a multistep activation pathway. Mol Biol Cell. 2002;13(5):1550–1565. doi: 10.1091/mbc.02-02-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Fuortes M, Jin WW, Nathan C. Beta 2 integrin-dependent tyrosine phosphorylation of paxillin in human neutrophils treated with tumor necrosis factor. J Cell Biol. 1994;127(5):1477–1483. doi: 10.1083/jcb.127.5.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Duran MB, Rahman A, Colten M, Brazill D. Dictyostelium discoideum paxillin regulates actin-based processes. Protist. 2009;160(2):221–232. doi: 10.1016/j.protis.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Heinrich D, Youssef S, Schroth-Diez B, Engel U, Aydin D, Blummel J, Spatz JP, Gerisch G. Actin-cytoskeleton dynamics in non-monotonic cell spreading. Cell adhesion & migration. 2008;2(2):58–68. doi: 10.4161/cam.2.2.6190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Ichetovkin I, Han J, Pang KM, Knecht DA, Condeelis JS. Actin filaments are severed by both native and recombinant dictyostelium cofilin but to different extents. Cell Motil Cytoskeleton. 2000;45(4):293–306. doi: 10.1002/(SICI)1097-0169(200004)45:4<293::AID-CM5>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 311.Aizawa H, Fukui Y, Yahara I. Live dynamics of Dictyostelium cofilin suggests a role in remodeling actin latticework into bundles. J Cell Sci. 1997;110(Pt 19):2333–2344. doi: 10.1242/jcs.110.19.2333. [DOI] [PubMed] [Google Scholar]
- 312.Haugwitz M, Noegel AA, Karakesisoglou J, Schleicher M. Dictyostelium amoebae that lack G-actin-sequestering profilins show defects in F-actin content, cytokinesis, and development. Cell. 1994;79(2):303–314. doi: 10.1016/0092-8674(94)90199-6. [DOI] [PubMed] [Google Scholar]
- 313.Arasada R, Gloss A, Tunggal B, Joseph JM, Rieger D, Mondal S, Faix J, Schleicher M, Noegel AA. Profilin isoforms in Dictyostelium discoideum. Biochim Biophys Acta. 2007;1773(5):631–641. doi: 10.1016/j.bbamcr.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 314.Lammermann T, Sixt M. Mechanical modes of ‘amoeboid’ cell migration. Curr Opin Cell Biol. 2009;21(5):636–644. doi: 10.1016/j.ceb.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 315.Howard TH, Oresajo CO. The kinetics of chemotactic peptide-induced change in F-actin content, F-actin distribution, and the shape of neutrophils. J Cell Biol. 1985;101(3):1078–1085. doi: 10.1083/jcb.101.3.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Weiner OD, Servant G, Welch MD, Mitchison TJ, Sedat JW, Bourne HR. Spatial control of actin polymerization during neutrophil chemotaxis. Nat Cell Biol. 1999;1(2):75–81. doi: 10.1038/10042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Fukui Y, Hashimoto O, Sanui T, Oono T, Koga H, Abe M, Inayoshi A, Noda M, Oike M, Shirai T, Sasazuki T. Haematopoietic cell-specific CDM family protein DOCK2 is essential for lymphocyte migration. Nature. 2001;412(6849):826–831. doi: 10.1038/35090591. [DOI] [PubMed] [Google Scholar]
- 318.Riedl J, Crevenna AH, Kessenbrock K, Yu JH, Neukirchen D, Bista M, Bradke F, Jenne D, Holak TA, Werb Z, Sixt M, Wedlich-Soldner R. Lifeact: a versatile marker to visualize F-actin. Nat Methods. 2008;5(7):605–607. doi: 10.1038/nmeth.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Yoo SK, Deng Q, Cavnar PJ, Wu YI, Hahn KM, Huttenlocher A. Differential regulation of protrusion and polarity by PI3K during neutrophil motility in live zebrafish. Dev Cell. 2010;18(2):226–236. doi: 10.1016/j.devcel.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Millius A, Dandekar SN, Houk AR, Weiner OD. Neutrophils establish rapid and robust WAVE complex polarity in an actin-dependent fashion. Current biology : CB. 2009;19(3):253–259. doi: 10.1016/j.cub.2008.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Kumar S, Xu J, Perkins C, Guo F, Snapper S, Finkelman FD, Zheng Y, Filippi MD. Cdc42 regulates neutrophil migration via crosstalk between WASp, CD11b, and microtubules. Blood. 2012;120(17):3563–3574. doi: 10.1182/blood-2012-04-426981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Welch HC, Condliffe AM, Milne LJ, Ferguson GJ, Hill K, Webb LM, Okkenhaug K, Coadwell WJ, Andrews SR, Thelen M, Jones GE, Hawkins PT, Stephens LR. P-Rex1 regulates neutrophil function. Current biology : CB. 2005;15(20):1867–1873. doi: 10.1016/j.cub.2005.09.050. [DOI] [PubMed] [Google Scholar]
- 323.Reynolds LF, Smyth LA, Norton T, Freshney N, Downward J, Kioussis D, Tybulewicz VL. Vav1 transduces T cell receptor signals to the activation of phospholipase C-gamma1 via phosphoinositide 3-kinase-dependent and -independent pathways. J Exp Med. 2002;195(9):1103–1114. doi: 10.1084/jem.20011663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Hornstein I, Alcover A, Katzav S. Vav proteins, masters of the world of cytoskeleton organization. Cell Signal. 2004;16(1):1–11. doi: 10.1016/s0898-6568(03)00110-4. [DOI] [PubMed] [Google Scholar]
- 325.Tedford K, Nitschke L, Girkontaite I, Charlesworth A, Chan G, Sakk V, Barbacid M, Fischer KD. Compensation between Vav-1 and Vav-2 in B cell development and antigen receptor signaling. Nat Immunol. 2001;2(6):548–555. doi: 10.1038/88756. [DOI] [PubMed] [Google Scholar]
- 326.Doody GM, Bell SE, Vigorito E, Clayton E, McAdam S, Tooze R, Fernandez C, Lee IJ, Turner M. Signal transduction through Vav-2 participates in humoral immune responses and B cell maturation. Nat Immunol. 2001;2(6):542–547. doi: 10.1038/88748. [DOI] [PubMed] [Google Scholar]
- 327.Garcia-Bernal D, Wright N, Sotillo-Mallo E, Nombela-Arrieta C, Stein JV, Bustelo XR, Teixido J. Vav1 and Rac control chemokine-promoted T lymphocyte adhesion mediated by the integrin alpha4beta1. Mol Biol Cell. 2005;16(7):3223–3235. doi: 10.1091/mbc.E04-12-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Lawson CD, Donald S, Anderson KE, Patton DT, Welch HC. P-Rex1 and Vav1 cooperate in the regulation of formyl-methionyl-leucyl-phenylalanine-dependent neutrophil responses. Journal of immunology. 2011;186(3):1467–1476. doi: 10.4049/jimmunol.1002738. [DOI] [PubMed] [Google Scholar]
- 329.Gerard A, van der Kammen RA, Janssen H, Ellenbroek SI, Collard JG. The Rac activator Tiam1 controls efficient T-cell trafficking and route of transendothelial migration. Blood. 2009;113(24):6138–6147. doi: 10.1182/blood-2008-07-167668. [DOI] [PubMed] [Google Scholar]
- 330.Costa C, Germena G, Martin-Conte EL, Molineris I, Bosco E, Marengo S, Azzolino O, Altruda F, Ranieri VM, Hirsch E. The RacGAP ArhGAP15 is a master negative regulator of neutrophil functions. Blood. 2011;118(4):1099–1108. doi: 10.1182/blood-2010-12-324756. [DOI] [PubMed] [Google Scholar]
- 331.Wilkins A, Insall RH. Small GTPases in Dictyostelium: lessons from a social amoeba. Trends in genetics : TIG. 2001;17(1):41–48. doi: 10.1016/s0168-9525(00)02181-8. [DOI] [PubMed] [Google Scholar]
- 332.Wang F, Herzmark P, Weiner OD, Srinivasan S, Servant G, Bourne HR. Lipid products of PI(3)Ks maintain persistent cell polarity and directed motility in neutrophils. Nat Cell Biol. 2002;4(7):513–518. doi: 10.1038/ncb810. [DOI] [PubMed] [Google Scholar]
- 333.Dandekar SN, Park JS, Peng GE, Onuffer JJ, Lim WA, Weiner OD. Actin dynamics rapidly reset chemoattractant receptor sensitivity following adaptation in neutrophils. Philos Trans R Soc Lond B Biol Sci. 2013;368(1629):20130008. doi: 10.1098/rstb.2013.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Kim SV, Mehal WZ, Dong X, Heinrich V, Pypaert M, Mellman I, Dembo M, Mooseker MS, Wu D, Flavell RA. Modulation of cell adhesion and motility in the immune system by Myo1f. Science. 2006;314(5796):136–139. doi: 10.1126/science.1131920. [DOI] [PubMed] [Google Scholar]
- 335.Goode BL, Eck MJ. Mechanism and function of formins in the control of actin assembly. Annu Rev Biochem. 2007;76:593–627. doi: 10.1146/annurev.biochem.75.103004.142647. [DOI] [PubMed] [Google Scholar]
- 336.Pruyne D, Evangelista M, Yang C, Bi E, Zigmond S, Bretscher A, Boone C. Role of formins in actin assembly: nucleation and barbed-end association. Science. 2002;297(5581):612–615. doi: 10.1126/science.1072309. [DOI] [PubMed] [Google Scholar]
- 337.Vicente-Manzanares M, Rey M, Perez-Martinez M, Yanez-Mo M, Sancho D, Cabrero JR, Barreiro O, de la Fuente H, Itoh K, Sanchez-Madrid F. The RhoA effector mDia is induced during T cell activation and regulates actin polymerization and cell migration in T lymphocytes. Journal of immunology. 2003;171(2):1023–1034. doi: 10.4049/jimmunol.171.2.1023. [DOI] [PubMed] [Google Scholar]
- 338.Shi Y, Zhang J, Mullin M, Dong B, Alberts AS, Siminovitch KA. The mDial formin is required for neutrophil polarization, migration, and activation of the LARG/RhoA/ROCK signaling axis during chemotaxis. Journal of immunology. 2009;182(6):3837–3845. doi: 10.4049/jimmunol.0803838. [DOI] [PubMed] [Google Scholar]
- 339.Yang C, Huang M, DeBiasio J, Pring M, Joyce M, Miki H, Takenawa T, Zigmond SH. Profilin enhances Cdc42-induced nucleation of actin polymerization. J Cell Biol. 2000;150(5):1001–1012. doi: 10.1083/jcb.150.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Sun CX, Magalhaes MA, Glogauer M. Rac1 and Rac2 differentially regulate actin free barbed end formation downstream of the fMLP receptor. J Cell Biol. 2007;179(2):239–245. doi: 10.1083/jcb.200705122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Oser M, Condeelis J. The cofilin activity cycle in lamellipodia and invadopodia. J Cell Biochem. 2009;108(6):1252–1262. doi: 10.1002/jcb.22372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Yan M, Di Ciano-Oliveira C, Grinstein S, Trimble WS. Coronin function is required for chemotaxis and phagocytosis in human neutrophils. Journal of immunology. 2007;178(9):5769–5778. doi: 10.4049/jimmunol.178.9.5769. [DOI] [PubMed] [Google Scholar]
- 343.Das M, Ithychanda SS, Qin J, Plow EF. Migfilin and filamin as regulators of integrin activation in endothelial cells and neutrophils. PLoS ONE. 2011;6(10):e26355. doi: 10.1371/journal.pone.0026355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Sun C, Forster C, Nakamura F, Glogauer M. Filamin-A Regulates Neutrophil Uropod Retraction through RhoA during Chemotaxis. PLoS ONE. 2013;8(10):e79009. doi: 10.1371/journal.pone.0079009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Yuruker B, Niggli V. Alpha-actinin and vinculin in human neutrophils: reorganization during adhesion and relation to the actin network. J Cell Sci. 1992;101(Pt 2):403–414. doi: 10.1242/jcs.101.2.403. [DOI] [PubMed] [Google Scholar]
- 346.Rehberg M, Kleylein-Sohn J, Faix J, Ho TH, Schulz I, Graf R. Dictyostelium LIS1 is a centrosomal protein required for microtubule/cell cortex interactions, nucleus/centrosome linkage, and actin dynamics. Mol Biol Cell. 2005;16(6):2759–2771. doi: 10.1091/mbc.E05-01-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Tang L, Franca-Koh J, Xiong Y, Chen MY, Long Y, Bickford RM, Knecht DA, Iglesias PA, Devreotes PN. tsunami, the Dictyostelium homolog of the Fused kinase, is required for polarization and chemotaxis. Genes Dev. 2008;22(16):2278–2290. doi: 10.1101/gad.1694508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Xu J, Wang F, Van Keymeulen A, Rentel M, Bourne HR. Neutrophil microtubules suppress polarity and enhance directional migration. Proc Natl Acad Sci U S A. 2005;102(19):6884–6889. doi: 10.1073/pnas.0502106102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.van Es S, Wessels D, Soll DR, Borleis J, Devreotes PN. Tortoise, a novel mitochondrial protein, is required for directional responses of Dictyostelium in chemotactic gradients. The Journal of cell biology. 2001;152(3):621–632. doi: 10.1083/jcb.152.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Lusche DF, Wessels D, Ryerson DE, Soll DR. Nhe1 is essential for potassium but not calcium facilitation of cell motility and the monovalent cation requirement for chemotactic orientation in Dictyostelium discoideum. Eukaryot Cell. 2011;10(3):320–331. doi: 10.1128/EC.00255-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Janetopoulos C, Borleis J, Vazquez F, Iijima M, Devreotes P. Temporal and spatial regulation of phosphoinositide signaling mediates cytokinesis. Dev Cell. 2005;8(4):467–477. doi: 10.1016/j.devcel.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 352.Postma M, Van Haastert PJ. A diffusion-translocation model for gradient sensing by chemotactic cells. Biophys J. 2001;81(3):1314–1323. doi: 10.1016/S0006-3495(01)75788-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Weiner OD, Neilsen PO, Prestwich GD, Kirschner MW, Cantley LC, Bourne HR. A PtdInsP(3)- and Rho GTPase-mediated positive feedback loop regulates neutrophil polarity. Nat Cell Biol. 2002;4(7):509–513. doi: 10.1038/ncb811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Houk AR, Jilkine A, Mejean CO, Boltyanskiy R, Dufresne ER, Angenent SB, Altschuler SJ, Wu LF, Weiner OD. Membrane tension maintains cell polarity by confining signals to the leading edge during neutrophil migration. Cell. 2012;148(1–2):175–188. doi: 10.1016/j.cell.2011.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Vicker MG (2002) F-actin assembly in Dictyostelium cell locomotion and shape oscillations propagates as a self-organized reaction–diffusion wave. FEBS Letters 510 (1–2):5-9. doi: 10.1016/S0014-5793(01)03207-0 [DOI] [PubMed]
- 356.Vicker MG (2002) Eukaryotic Cell Locomotion Depends on the Propagation of Self-Organized Reaction–Diffusion Waves and Oscillations of Actin Filament Assembly. Experimental Cell Research 275 (1):54-66. doi: 10.1006/excr.2001.5466 [DOI] [PubMed]
- 357.Bretschneider T, Anderson K, Ecke M, Müller-Taubenberger A, Schroth-Diez B, Ishikawa-Ankerhold HC, Gerisch G (2009) The Three-Dimensional Dynamics of Actin Waves, a Model of Cytoskeletal Self-Organization. Biophysical journal 96 (7):2888-2900. doi: 10.1016/j.bpj.2008.12.3942 [DOI] [PMC free article] [PubMed]
- 358.Gerisch G, Ecke M, Schroth-Diez B, Gerwig S, Engel U, Maddera L, Clarke M. Self-organizing actin waves as planar phagocytic cup structures. Cell adhesion & migration. 2009;3(4):373–382. doi: 10.4161/cam.3.4.9708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Gerisch G. Self-organizing actin waves that simulate phagocytic cup structures. PMC Biophys. 2010;3(1):7. doi: 10.1186/1757-5036-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Weiner OD, Marganski WA, Wu LF, Altschuler SJ, Kirschner MW. An Actin-Based Wave Generator Organizes Cell Motility. PLoS Biol. 2007;5(9):e221. doi: 10.1371/journal.pbio.0050221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Case LB, Waterman CM. Adhesive F-actin Waves: A Novel Integrin-Mediated Adhesion Complex Coupled to Ventral Actin Polymerization. PLoS ONE. 2011;6(11):e26631. doi: 10.1371/journal.pone.0026631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Westendorf C, Negrete J, Jr, Bae AJ, Sandmann R, Bodenschatz E, Beta C. Actin cytoskeleton of chemotactic amoebae operates close to the onset of oscillations. Proc Natl Acad Sci USA. 2013;110(10):3853–3858. doi: 10.1073/pnas.1216629110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Arai Y, Shibata T, Matsuoka S, Sato MJ, Yanagida T, Ueda M. Self-organization of the phosphatidylinositol lipids signaling system for random cell migration. Proc Natl Acad Sci. 2010;107(27):12399–12404. doi: 10.1073/pnas.0908278107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Taniguchi D, Ishihara S, Oonuki T, Honda-Kitahara M, Kaneko K, Sawai S. Phase geometries of two-dimensional excitable waves govern self-organized morphodynamics of amoeboid cells. Proc Natl Acad Sci. 2013;110(13):5016–5021. doi: 10.1073/pnas.1218025110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Asano Y, Nagasaki A, Uyeda TQP. Correlated waves of actin filaments and PIP3 in Dictyostelium cells. Cell Motil Cytoskelet. 2008;65(12):923–934. doi: 10.1002/cm.20314. [DOI] [PubMed] [Google Scholar]
- 366.Huang CH, Tang M, Shi C, Iglesias PA, Devreotes PN. An excitable signal integrator couples to an idling cytoskeletal oscillator to drive cell migration. Nat Cell Biol. 2013 doi: 10.1038/ncb2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Shibata T, Nishikawa M, Matsuoka S, Ueda M (2013) Intracellular Encoding of Spatiotemporal Guidance Cues in a Self-Organizing Signaling System for Chemotaxis in Dictyostelium Cells. Biophysical journal 105 (9):2199-2209. doi: 10.1016/j.bpj.2013.09.024 [DOI] [PMC free article] [PubMed]
- 368.Xiong Y, Huang CH, Iglesias PA, Devreotes PN. Cells navigate with a local-excitation, global-inhibition-biased excitable network. Proc Natl Acad Sci U S A. 2010;107(40):17079–17086. doi: 10.1073/pnas.1011271107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Hecht I, Skoge ML, Charest PG, Ben-Jacob E, Firtel RA, Loomis WF, Levine H, Rappel W-J. Activated Membrane Patches Guide Chemotactic Cell Motility. PLoS Comput Biol. 2011;7(6):e1002044. doi: 10.1371/journal.pcbi.1002044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Meinhardt H. Orientation of chemotactic cells and growth cones: models and mechanisms. J Cell Sci. 1999;112(17):2867–2874. doi: 10.1242/jcs.112.17.2867. [DOI] [PubMed] [Google Scholar]
- 371.Hecht I, Kessler DA, Levine H. Transient Localized Patterns in Noise-Driven Reaction-Diffusion Systems. Phys Rev Lett. 2010;104(15):158301. doi: 10.1103/PhysRevLett.104.158301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Neilson MP, Veltman DM, van Haastert PJM, Webb SD, Mackenzie JA, Insall RH. Chemotaxis: A Feedback-Based Computational Model Robustly Predicts Multiple Aspects of Real Cell Behaviour. PLoS Biol. 2011;9(5):e1000618. doi: 10.1371/journal.pbio.1000618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Stephens CG, Snyderman R. Cyclic nucleotides regulate the morphologic alterations required for chemotaxis in monocytes. J Immunol. 1982;128(3):1192–1197. [PubMed] [Google Scholar]
- 374.Wyatt TA, Lincoln TM, Pryzwansky KB. Vimentin is transiently co-localized with and phosphorylated by cyclic GMP-dependent protein kinase in formyl-peptide-stimulated neutrophils. J Biol Chem. 1991;266(31):21274–21280. [PubMed] [Google Scholar]
- 375.Belenky SN, Robbins RA, Rubinstein I. Nitric oxide synthase inhibitors attenuate human monocyte chemotaxis in vitro. J Leukoc Biol. 1993;53(5):498–503. doi: 10.1002/jlb.53.5.498. [DOI] [PubMed] [Google Scholar]
- 376.Elferink JGR, Vanuffelen BE (1996) The role of cyclic nucleotides in neutrophil migration. General Pharmacology: The Vascular System 27 (2):387-393. doi: 10.1016/0306-3623(95)00070-4 [DOI] [PubMed]
- 377.Lawrence DW, Pryzwansky KB. The Vasodilator-Stimulated Phosphoprotein Is Regulated by Cyclic GMP-Dependent Protein Kinase During Neutrophil Spreading. J Immunol. 2001;166(9):5550–5556. doi: 10.4049/jimmunol.166.9.5550. [DOI] [PubMed] [Google Scholar]
- 378.Jenei V, Deevi RK, Adams CA, Axelsson L, Hirst DG, Andersson T, Dib K. Nitric oxide produced in response to engagement of beta2 integrins on human neutrophils activates the monomeric GTPases Rap1 and Rap2 and promotes adhesion. J Biol Chem. 2006;281(46):35008–35020. doi: 10.1074/jbc.M601335200. [DOI] [PubMed] [Google Scholar]
- 379.Kato M, Blanton R, Wang GR, Judson TJ, Abe Y, Myoishi M, Karas RH, Mendelsohn ME. Direct binding and regulation of RhoA protein by cyclic GMP-dependent protein kinase Ialpha. J Biol Chem. 2012;287(49):41342–41351. doi: 10.1074/jbc.M112.421040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Vandekerckhove J, Weber K. Vegetative Dictyostelium cells containing 17 actin genes express a single major actin. Nature. 1980;284(5755):475–477. doi: 10.1038/284475a0. [DOI] [PubMed] [Google Scholar]
- 381.Joseph JM, Fey P, Ramalingam N, Liu XI, Rohlfs M, Noegel AA, Muller-Taubenberger A, Glockner G, Schleicher M. The actinome of Dictyostelium discoideum in comparison to actins and actin-related proteins from other organisms. PLoS ONE. 2008;3(7):e2654. doi: 10.1371/journal.pone.0002654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Wherlock M, Mellor H. The Rho GTPase family: a Racs to Wrchs story. J Cell Sci. 2002;115(Pt 2):239–240. doi: 10.1242/jcs.115.2.239. [DOI] [PubMed] [Google Scholar]
- 383.Saxe CL, 3rd, Johnson R, Devreotes PN, Kimmel AR. Multiple genes for cell surface cAMP receptors in Dictyostelium discoideum. Dev Genet. 1991;12(1–2):6–13. doi: 10.1002/dvg.1020120104. [DOI] [PubMed] [Google Scholar]
- 384.Unnewehr H, Rittirsch D, Sarma JV, Zetoune F, Flierl MA, Perl M, Denk S, Weiss M, Schneider ME, Monk PN, Neff T, Mihlan M, Barth H, Gebhard F, Ward PA, Huber-Lang M. Changes and regulation of the C5a receptor on neutrophils during septic shock in humans. Journal of immunology. 2013;190(8):4215–4225. doi: 10.4049/jimmunol.1200534. [DOI] [PubMed] [Google Scholar]
- 385.Oostra V, de Jong MA, Invergo BM, Kesbeke F, Wende F, Brakefield PM, Zwaan BJ. Translating environmental gradients into discontinuous reaction norms via hormone signalling in a polyphenic butterfly. Proceedings Biological sciences/The Royal Society. 2011;278(1706):789–797. doi: 10.1098/rspb.2010.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.P-y Lam, Yoo SK, Green JM, Huttenlocher A. The SH2-domain-containing inositol 5-phosphatase (SHIP) limits the motility of neutrophils and their recruitment to wounds in zebrafish. J Cell Sci. 2012;125(21):4973–4978. doi: 10.1242/jcs.106625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Liu L, Luo Y, Chen L, Shen T, Xu B, Chen W, Zhou H, Han X, Huang S. Rapamycin inhibits cytoskeleton reorganization and cell motility by suppressing RhoA expression and activity. J Biol Chem. 2010;285(49):38362–38373. doi: 10.1074/jbc.M110.141168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Murphy MB, Egelhoff TT. Biochemical characterization of a Dictyostelium myosin II heavy-chain phosphatase that promotes filament assembly. Eur J Biochem. 1999;264(2):582–590. doi: 10.1046/j.1432-1327.1999.00670.x. [DOI] [PubMed] [Google Scholar]
- 389.Alblas J, Ulfman L, Hordijk P, Koenderman L. Activation of RhoA and ROCK Are Essential for Detachment of Migrating Leukocytes. Mol Biol Cell. 2001;12(7):2137–2145. doi: 10.1091/mbc.12.7.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Niggli V (1999) Rho-kinase in human neutrophils: a role in signalling for myosin light chain phosphorylation and cell migration. FEBS Letters 445 (1):69-72. doi: 10.1016/S0014-5793(99)00098-8 [DOI] [PubMed]
- 391.Adachi T, Stafford S, Kayaba H, Chihara J, Alam R (2003) Myosin light chain kinase mediates eosinophil chemotaxis in a mitogen-activated protein kinase–dependent manner. Journal of Allergy and Clinical Immunology 111 (1):113-116. doi: 10.1067/mai.2003.27 [DOI] [PubMed]
- 392.Perrin BJ, Ervasti JM. The actin gene family: function follows isoform. Cytoskeleton. 2010;67(10):630–634. doi: 10.1002/cm.20475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Howard TH, Watts RG. Actin polymerization and leukocyte function. Curr Opin Hematol. 1994;1(1):61–68. [PubMed] [Google Scholar]
- 394.Ku CJ, Wang Y, Weiner OD, Altschuler SJ, Wu LF. Network crosstalk dynamically changes during neutrophil polarization. Cell. 2012;149(5):1073–1083. doi: 10.1016/j.cell.2012.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Winterhoff M, Junemann A, Nordholz B, Linkner J, Schleicher M, Faix J. The Diaphanous-related formin dDia1 is required for highly directional phototaxis and formation of properly sized fruiting bodies in Dictyostelium. Eur J Cell Biol. 2013 doi: 10.1016/j.ejcb.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 396.Parent CA, Blacklock BJ, Froehlich WM, Murphy DB, Devreotes PN (1998) G Protein Signaling Events Are Activated at the Leading Edge of Chemotactic Cells. Cell 95 (1):81-91. doi: 10.1016/S0092-8674(00)81784-5 [DOI] [PubMed]
- 397.Iijima M, Devreotes P (2002) Tumor Suppressor PTEN Mediates Sensing of Chemoattractant Gradients. Cell 109 (5):599-610. doi: 10.1016/S0092-8674(02)00745-6 [DOI] [PubMed]
- 398.Hynes RO (2002) Integrins: Bidirectional, Allosteric Signaling Machines. Cell 110 (6):673-687. doi: 10.1016/S0092-8674(02)00971-6 [DOI] [PubMed]
- 399.Tsujioka M, Yoshida K, Nagasaki A, Yonemura S, Müller-Taubenberger A, Uyeda TQP. Overlapping Functions of the Two Talin Homologues in Dictyostelium. Eukaryot Cell. 2008;7(5):906–916. doi: 10.1128/EC.00464-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Cornillon S, Gebbie L, Benghezal M, Nair P, Keller S, Wehrle-Haller B, Charette SJ, Bruckert F, Letourneur F, Cosson P (2006) An adhesion molecule in free-living Dictyostelium amoebae with integrin [beta] features. EMBO Rep 7 (6):617-621. doi:http://www.nature.com/embor/journal/v7/n6/suppinfo/7400701_S1.html [DOI] [PMC free article] [PubMed]
- 401.Loomis WF, Fuller D, Gutierrez E, Groisman A, Rappel W-J. Innate Non-Specific Cell Substratum Adhesion. PLoS ONE. 2012;7(8):e42033. doi: 10.1371/journal.pone.0042033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Cornillon S, Froquet R, Cosson P. Involvement of Sib Proteins in the Regulation of Cellular Adhesion in Dictyostelium discoideum. Eukaryot Cell. 2008;7(9):1600–1605. doi: 10.1128/EC.00155-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Fey P, Stephens S, Titus MA, Chisholm RL. SadA, a novel adhesion receptor in Dictyostelium. J Cell Biol. 2002;159(6):1109–1119. doi: 10.1083/jcb.200206067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Cornillon S, Pech E, Benghezal M, Ravanel K, Gaynor E, Letourneur F, Bruckert F, Cosson P. Phg1p is a nine-transmembrane protein superfamily member involved in dictyostelium adhesion and phagocytosis. J Biol Chem. 2000;275(44):34287–34292. doi: 10.1074/jbc.M006725200. [DOI] [PubMed] [Google Scholar]
- 405.Bukahrova T, Weijer G, Bosgraaf L, Dormann D, van Haastert PJ, Weijer CJ. Paxillin is required for cell-substrate adhesion, cell sorting and slug migration during Dictyostelium development. J Cell Sci. 2005;118(18):4295–4310. doi: 10.1242/jcs.02557. [DOI] [PubMed] [Google Scholar]
- 406.Patel H, König I, Tsujioka M, Frame MC, Anderson KI, Brunton VG. The multi-FERM-domain-containing protein FrmA is required for turnover of paxillin-adhesion sites during cell migration of Dictyostelium. J Cell Sci. 2008;121(8):1159–1164. doi: 10.1242/jcs.021725. [DOI] [PubMed] [Google Scholar]
- 407.Sampaio NG, Yu W, Cox D, Wyckoff J, Condeelis J, Stanley ER, Pixley FJ. Phosphorylation of CSF-1R Y721 mediates its association with PI3K to regulate macrophage motility and enhancement of tumor cell invasion. J Cell Sci. 2011;124(Pt 12):2021–2031. doi: 10.1242/jcs.075309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Burns AR, Bowden RA, Abe Y, Walker DC, Simon SI, Entman ML, Smith CW. P-selectin mediates neutrophil adhesion to endothelial cell borders. J Leukoc Biol. 1999;65(3):299–306. doi: 10.1002/jlb.65.3.299. [DOI] [PubMed] [Google Scholar]
- 409.Stadtmann A, Germena G, Block H, Boras M, Rossaint J, Sundd P, Lefort C, Fisher CI, Buscher K, Gelschefarth B, Urzainqui A, Gerke V, Ley K, Zarbock A. The PSGL-1-L-selectin signaling complex regulates neutrophil adhesion under flow. J Exp Med. 2013;210(11):2171–2180. doi: 10.1084/jem.20130664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Ulbrich H, Eriksson EE, Lindbom L. Leukocyte and endothelial cell adhesion molecules as targets for therapeutic interventions in inflammatory disease. Trends Pharmacol Sci. 2003;24(12):640–647. doi: 10.1016/j.tips.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 411.Heit B, Colarusso P, Kubes P. Fundamentally different roles for LFA-1, Mac-1 and α4-integrin in neutrophil chemotaxis. J Cell Sci. 2005;118(22):5205–5220. doi: 10.1242/jcs.02632. [DOI] [PubMed] [Google Scholar]
- 412.Hogg N, Laschinger M, Giles K, McDowall A. T-cell integrins: more than just sticking points. J Cell Sci. 2003;116(23):4695–4705. doi: 10.1242/jcs.00876. [DOI] [PubMed] [Google Scholar]
- 413.Lomakina EB, Waugh RE. Adhesion between human neutrophils and immobilized endothelial ligand vascular cell adhesion molecule 1: divalent ion effects. Biophys J. 2009;96(1):276–284. doi: 10.1016/j.bpj.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]