Abstract
The ability of cells to maintain low levels of Ca2+ under resting conditions and to create rapid and transient increases in Ca2+ upon stimulation is a fundamental property of cellular Ca2+ signaling mechanism. An increase of cytosolic Ca2+ level in response to diverse stimuli is largely accounted for by the inositol 1,4,5-trisphosphate receptor (IP3R) present in the endoplasmic reticulum membranes of virtually all eukaryotic cells. Extensive information is currently available on the function of IP3Rs and their interaction with modulators. Very little, however, is known about their molecular architecture and therefore most critical issues surrounding gating of IP3R channels are still ambiguous, including the central question of how opening of the IP3R pore is initiated by IP3 and Ca2+. Membrane proteins such as IP3R channels have proven to be exceptionally difficult targets for structural analysis due to their large size, their location in the membrane environment, and their dynamic nature. To date, a 3D structure of complete IP3R channel is determined by single-particle cryo-EM at intermediate resolution, and the best crystal structures of IP3R are limited to a soluble portion of the cytoplasmic region representing ~15% of the entire channel protein. Together these efforts provide the important structural information for this class of ion channels and serve as the basis for further studies aiming at understanding of the IP3R function.
Keywords: IP3R, calcium signaling, 3D structure, electron cryomicroscopy, single-particle reconstruction
1. Introduction
Intracellular Ca2+ signaling is a strictly controlled spatial and temporal process guided by the orchestrated mobilization of Ca2+ into the cytoplasm, via Ca2+ channels, either from the extracellular milieu (Ca2+ influx) or from intracellular stores (Ca2+ release). Ca2+ release is mediated by intracellular ligand-gated Ca2+ release channels present in the endoplasmic (ER) and sarcoplasmic (SR) reticulum membranes of all eukaryotic cells. Two closely related families of intracellular Ca2+ release channels have been identified: the ryanodine receptor (RyR) and the inositol 1,4,5-trisphosphate receptor (IP3R). While the RyR represents primary Ca2+ release channel in striated muscle, IP3R channels are detected in the ER of all cell types with the highest densities in the Purkinje cells of cerebellum. Both channels share 30-40 % sequence identity within their C-terminal regions, containing predicted membrane-spanning domains [1,2]. This structural homology accounts for many functional similarities between IP3R and RyR channels and suggests a common molecular architecture for the ion-permission pathway. Functional Ca2+ release channels form large tetrameric structures with a molecular mass of ~1.3 MDa for IP3Rs and ~2.3 MDa for RyRs.
Ca2+ release via IP3R/RyR channels is one of the most ubiquitous and versatile cellular signaling mechanisms that regulates diverse physiological functions, including muscle contraction, fertilization, hormone secretion, gene transcription, metabolic regulation, immune responses, apoptosis, learning and memory. Dysfunction of these channels has been implicated in abnormal intracellular Ca2+ levels associated with many pathological conditions in humans such as cardiac hypertrophy, heart failure, hereditary ataxias, osteoporosis, atherosclerosis, hypertension, some migraines, Alzheimer's disease, Huntington's disease, Malignant Hyperthermia, Central Core and Multi-minicore diseases [3-8]. The focus of this review article is on structural studies of IP3R channels with primary emphasis on structure determination of the tetrameric channel. The long-standing controversy about the 3D structure of complete IP3R has been a critical obstacle substantially slowing progress of the research aiming to understand structure-functional aspects of these key membrane proteins. Recently, the 3D structure of the full-length tetrameric IP3R channel has been unambiguously determined by single-particle electron cryo-microscopy [9,10]. To date, electron cryo-microscopy (cryo-EM) has emerged as the most effective and straightforward technique for the study of macromolecular membrane protein assemblies and their interactions [11]. While X-ray crystallography has recently made strides, only ~2% of PDB entries are related to membrane proteins, whereas they represent an estimated 20-30% of expressed proteins in the genome [12]. Additionally, most of these entries represent only soluble fragments rather than intact integral membrane proteins. Among these are the crystal structures of the N-terminal IP3-binding domains of type 1 IP3R [13-16]. However, a 3D structure of complete channel is critical to be able to trace the coordination of ligand-induced movements throughout the channel assembly and to establish a structural basis for the channel gating. This review summarizes the current knowledge of the 3D structure of IP3R and discusses new insights gained into IP3R channel function.
2. Diversity within the IP3 R channel family
Since it was discovered over two decades ago that specific proteins tightly bound to ER membranes function as Ca2+-permeable ion channels activated by selective ligand inositol 1, 4, 5-trisphosphate (IP3) [17-19], substantial efforts have been made to understand the mechanism of action of these ion channels (Fig. 1). Cloning of receptor proteins established that IP3R is an unusually large membrane protein, comprising four subunits of ~2,700 amino acid residues each (Fig. 2) [1,2]. Furthermore, mammalian genes have been identified encoding three homologous isoforms (types 1-3) of the receptor that share ~70% overall sequence identity which increases to ~90% within the predicted transmembrane (TM) region. Each type shows distinct properties in terms of modulation by endogenous and exogenous ligands and tissue distribution. IP3R diversity is further expanded by alternative gene splicing within the cytoplasmic region (Fig. 2) as well as by the homo- and hetero-tetrameric assembly of the IP3R subunits into functional channels. These gene sub-families correspond to sub-types of Ca2+ release channels that are primarily defined based on the functional and pharmacological criteria rather than rigorous analysis of structure-function relationships (reviewed in [20,21]). However, IP3R protein can be subdivided into four functional regions (Fig. 2): an IP3-binding region comprising ~600 residues at the N-terminus of the receptor protein; a central modulatory region with sites for interaction with regulatory molecules, a C-terminal channel-forming region (residues 2276-2589) containing six putative transmembrane (TM) domains, and the C-terminal tail including the last ~160 residues [22,23]. Predicted membrane topology of IP3R suggests that both the N- and C-termini are intracellular and form a large cytoplasmic portion of the IP3R channel that includes ~90% of the protein mass.
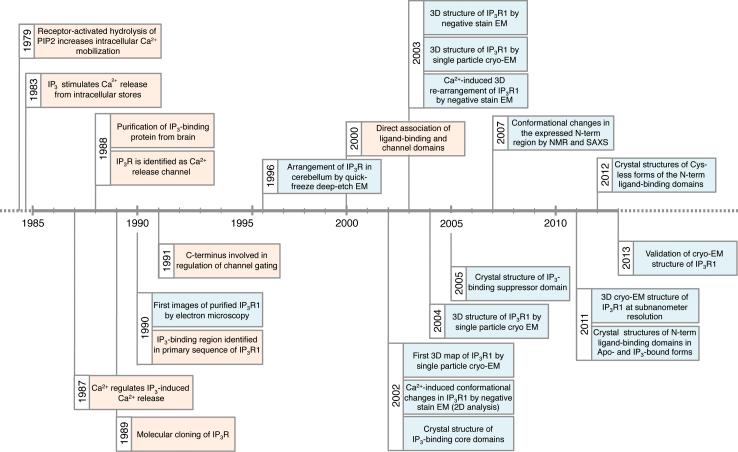
Figure 1. Timeline of IP3R research highlights.
In the 1970s it was found that receptor-activated hydrolysis of PIP2 increases intracellular Ca2+ mobilization [71], and that IP3 is the second messenger stimulating Ca2+ release from intracellular stores [72,73]. It was subsequently demonstrated that Ca2+ regulates IP3-induced Ca2+ release [19,58]. These findings paved the road for intensive research of molecular mechanism underlying this messenger pathway. In 1988, IP3- binding protein was purified from brain [24,25] and was identified as Ca2+ release channel by reconstitution into lipid vesicles [17,18]. The receptor protein was cloned by two research groups in 1989 [1,2]. In the next two decades, much was learned about the structure-function of IP3R: first images of purified IP3R1 [74]; IP3-binding site is identified in the primary sequence of IP3R [75]; involvement of C-terminus in channel gating [76]; native arrangement of IP3R in cerebellum [77]; direct association of lP3-binding and channel domains [59]; first 3D map of IP3R1 by cryo-EM [38]; Ca2+-induced conformational changes in IP3R1 [78]; crystal structure of IP3-binding core domains [13]; 3D structures of IP3R1 by negative stain EM [79] and by cryo-EM [40]; Ca2+-induced re-arrangements of IP3R1 by EM [56]; another 3D structure of IP3R1 by cryo-EM [39]; crystal structure of IP3-binding suppressor domain [14]; ligand-induced conformational changes in the N-terminal region by NMR and small-angle X-ray scattering [55]; 3D cryo-EM structure of IP3R1 in the closed state at subnanometer resolution [9]; crystal structure of N-terminal ligand binding domains in Apo- and IP3-bound forms [15]; Apo and ligand-bound crystal structures of Cys-less forms of the N-terminal ligand-binding domains [16]; validation of IP3R1 cryo-EM structure [10].
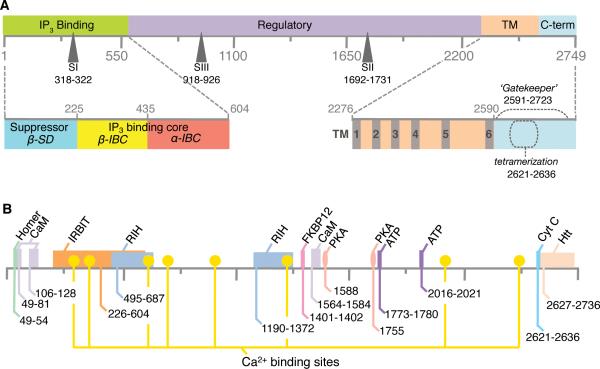
Figure 2. Topology of structure-functional domains in primary sequence of IP3R.
(A) Four key regions defined in the primary structure of IP3R are highlighted: the N-terminal ligand-binding region, the large central modulatory region, the membrane-spanning/pore-forming, and the C-terminal ‘gate-keeping’ region; SI, SII and SIII refer to the three splice sites. Lower panel shows detailed topology of domains within the N- and C-terminal regions (see also Figure 5). (B) Putative binding sites for several modulatory molecules of IP3R are indicated in the primary sequence of the receptor protein: CaM – calmodulin, IRBIT- IP3R binding protein released with IP3, FKBP12 - 12-kDa FK506-binding protein, PKA – cAMP-dependent protein kinase A, Cyt c – cytochrome c; Htt – huntingtin; RIH - RyR/IP3R homology regions. Amino acid residue numbering is the same as for the mouse IP3R1 (GI: 313104120) [1].
A functional hallmark of IP3R channels is that their ion channel activity is regulated by coupled interplay between the binding of its primary ligands, IP3 and Ca2+. Inositol 1,4,5-trisphosphate (IP3) is a second messenger produced through phosphoinositide turnover in response to many extracellular stimuli such as hormones, growth factors, neurotransmitters, neurotrophins, odorants and light [20,21]. The changes in IP3R pore structure that allow it to open are initiated by IP3 binding to the N-terminal IP3-binding region (Fig. 2A) that contains a ligand-binding suppressor domain (SD, residues 1-224). This domain fine-tunes IP3-binding affinity for a particular receptor subtype and for the IP3-binding core (IBC, residues 225-604) comprising two distinct domains and representing the minimum region required for specific IP3 binding. In addition, IP3R channel activity is closely regulated by a wide array of intracellular regulatory molecules that interact with the channel complex in a dynamic manner to provide functional feedback. Through functional analysis and co-immunoprecipitation studies, some of these sites for protein-protein interactions were mapped to the primary structure of IP3R protein (Fig. 2B) (reviewed in [20,21]). While only a single IP3 binding site exists on a single IP3R subunit, there are multiple Ca2+ binding sites (Fig. 2). Thus, the diversity in distribution of regulatory proteins and IP3R isoforms is essential for the versatility of Ca2+ signaling mechanism via IP3R channels. These channels can conceptually be considered as modular scaffold proteins, where binding of IP3 and regulatory components can change the scaffold structure and direct changes in subsequent events leading to generation of intracellular Ca2+ signals. The fact that Ca2+ serves multiple roles raises the possibility of crosstalk between processes, which should otherwise be independent. More information, however, is required for detailed mechanistic understanding of the diverse functions of IP3R.
3. Overall 3D structure of tetrameric IP3R
Historically, 3D structure determination of Ca2+ release channels did not start until IP3R protein was successfully purified in detergent-solubilized form (Fig. 1) [24,25]. Structural studies have been focused primary on type 1 IP3R (IP3R1), which is the predominant isoform in the ER of cerebellar Purkinje cells and the best characterized of the mammalian isoforms. The structural analysis of the full-length tetrameric IP3R channels has been hampered by their enormous size (over 1.2 MDa), interaction with lipid membranes in their native state and their dynamic nature, making X-ray or NMR techniques difficult to apply. Single-particle cryo-EM has been a powerful method providing the capabilities to determine reliable structures in 3-5 Å range and to evaluate the dynamic aspects of macromolecular assemblies [26-36]. It is noteworthy, however, that the quaternary structure of IP3R1 channel has remained controversial (reviewed in [37]) and not until recently has a 3D structure of tetrameric functional IP3R1 channel in the closed state been unambiguously determined [9], Its accuracy was to ~17 Å resolution clearly identifying structural flexibility, rather than imaging or specimen problems, as the resolution-limiting factor (Fig. 3)[10]. This implies that higher resolutions should, indeed, be possible with sufficently large data set while also subclassifying particles. Moreover, these recent cryo-EM studies [9,10] demonstrated that the root of controversy between earlier cryo-EM reconstructions of IP3R1 [38-40] is likely due to insufficient contrast in the cryo-EM raw data underlying these structures. The EM community is now beginning to establish tools and standards for better identifying such errors [10,41,42], and if best practices were followed now, it is doubtful that any of the earlier cryo-EM structures of IP3R1 [38-40] would have been published.
Figure 3. 3D structure of tetrameric IP3R1 channel determined by single-particle Cryo-EM.
Surface representation of cryo-EM density map of IP3R1 determined by single-particle cryo-EM at intermediate resolution (EMD-5278) [9,10]. IP3R1 protein has been visualized under conditions that favor the closed state of the channel, i.e. in the absence of its primary ligands, IP3 and Ca2+ [9]. Surface representation of the structure is shown in three orthogonal views: from the cytoplasmic side (A), from the luminal side (B) and along membrane plane (C, D). One putative subunit is depicted in color; (D) two opposing subunits are shown to allow visualization of internal features in the channel structure. Scale bar is 100 Å.
We have currently reasonable knowledge about the quaternary structure of entire IP3R1 channel that is based solely on single-particle cryo-EM studies. The 3D structure of the tetrameric IP3R1 channel exhibits an overall characteristic mushroom shape: the large cytoplasmic (CY) region is connected via stalk-like densities with the smaller transmembrane (TM) region (Fig. 3). The delineated subunit boundaries confirm common architectural arrangement of Ca2+ release channels where the four identical or highly homologous subunits form a single central ion conduction pathway arranged around the four-fold symmetry axis normal to the membrane plane (Fig. 3). The TM regions of RyR1 and IP3R1 channels are quite similar, although the cytoplasmic portions, aside from the difference in their overall dimensions, have quite different architectural arrangements with the most notable features such as a central plug in the IP3R1 structure prominent enough to be visible directly in 2D images [9]. These observations are consistent with the idea that functional diversity of Ca2+ release channels and their ability to respond to various cellular stimuli are encoded in the CY region of the channel protein. The CY domains are targets of protein-protein interactions and interactions with a large array of intracellular small molecules that are linked to the channel gating activity (Fig. 2B)(reviewed in [20,21,43]).
4. Architecture of channel gating machinery
Understanding the channel gating machinery at an atomic level remains the most challenging issue in ion channel biology fascinating structural biologists for many years. Recent cryo-EM structure of IP3R1 provides initial insights into the 3D arrangement of the Ca2+-permission pathway across the membrane (Fig. 4)[9]. The TM structure reveals six putative α-helices. Four helices (one from each subunit) form a twisted bundle around the central axis. These helices are long enough to span the lipid bilayer shaping the ion-conduction pathway like a teepee-like structure with the apex pointing toward the cytoplasmic region. The position and geometry of these helices is strikingly similar to that of the inner (pore-lining) helices in closed-state K+ channels (Fig. 4) [44-46]. One α-helix is identified at the interface between the TM and CY structures and runs nearly parallel to the membrane plane (Fig. 4). This helix is similarly placed to the interfacial helix identified earlier in RyR1 [47] and Kir channels [45,46]. Other short helices are most likely represent small segments of longer helices that were not yet entirely resolved due to the resolution limits in this cryo-EM map [9]. The position of one of these helices is similar to that of the outer helix in the K+ channels [44-46]. Moreover, the lumenal face of IP3R1 has prominent densities that surround the pore entrance similar to the highly structured turrets of Kir channels [46]. While such overall similarity between the TM structure of Ca2+ release channels and that of certain K+ channels can be observed at the current resolution (12-17 Å) [9,47,48], it is evident based on cryo-EM studies of other ion channels, such as the nicotinic acetylcholine receptor [49,50], that precise geometry of the current secondary structure elements will be further refined and adjusted with improving resolution.
Figure 4. Close-up view of the TM region of IP3R1.
(A) Cryo-EM densities of two opposing subunits of IP3R1 are shown as a side view. Identified α-helices are annotated with cylinders and labeled as in [9]. Scale bar is 50 Å. (B-D) X-ray structure of the eukaryotic Kir2.2 (PDB ID:3JYC) is shown as a ribbon and superimposed with the secondary structure elements identified in the TM region of IP3R1. (B) Two subunits are viewed along the membrane plane. Four subunits are viewed along the channel central axis from the luminal side (C) and from the cytoplasmic side (D).
4. Gating by conformational coupling
A central mechanistic question of IP3R gating is how IP3 binding in the N-terminal sequence of the channel protein is communicated through the membrane in order to open the pore formed near the C-terminus (Fig. 2). Furthermore, how does the channel change its conformation to allow Ca2+ translocation across the membrane? IP3R channels open transiently upon stimulation by IP3 and Ca2+ that allows Ca2+ ions to flow through them from the ER, ultimately leading to a change in intracellular Ca2+ levels. How this activation is accomplished is not known, yet it is fundamental to our understanding of Ca2+ signaling mechanisms via IP3Rs. A number of functional models have been proposed to describe the interplay between IP3 and Ca2+ in the modulation of IP3R gating (reviewed in [20,21,51]). These models differ in terms of consequences of IP3 and Ca2+ binding and interdependence (or lack thereof) in agonist binding. However, lack of high-resolution 3D structure of the entire functional IP3R channel limits our ability to discriminate between these models.
Several lines of evidence support a model of IP3R gating by conformational coupling between the IP3-binding and TM domains. Changes in the pore structure that allow it to open are initiated by IP3 binding to the IBC region (Fig. 2). However, IP3 fails to open the pore without the SD domain, although it still binds to IP3R [52,53]. Moreover, the SD decreases the affinity of IP3R1 for IP3 [53,54]. The N-terminal ligand-binding domains (i.e. SD and IBC domains) of IP3R1 have been expressed as soluble proteins and crystallized in Apo- and IP3-bound states [13-16]. Some studies demonstrated that the IBC adopts a more constrained structure when it binds IP3. Moreover, IP3 binding causes twisting of the SD around the flexible hinge connecting it to the IBC (Fig. 5A, B) [15,16]. Other evidence for interactions between the ligand-binding domains and IP3-induced conformational changes comes from NMR and small-angle X-ray scattering studies performed when these domains are expressed as independent entities [55]. It has, therefore, been suggested that the SD is the essential link between IP3 binding to the IBC and the subsequent conformational changes that lead to opening of the pore in IP3R1. However, it is not yet clear whether similar rearrangements take place in the full-length protein, and if they are related to channel opening. Although low-resolution negative staining EM studies showed two distinct structures of IP3R1 - one is square-shaped and the other is pinwheel-like, the relative abundance of each seems to be Ca2+-dependent [56] and the precise nature and structural basis of these differences were not established due to the fundamental resolution limit. It is clear that higher-resolution structures of the entire IP3R1 in the Apo- and ligand-bound states are urgently needed to assess conformational changes underlying IP3-induced gating of the channel.
Figure 5. Structure of the N-terminal ligand-binding region of IP3R1.
(A-B) Ribbon representations of the Apo- and IP3-bound crystal structures of the ligand-binding domains of IP3R1 channel (PDB ID: 3T8S): β trefoil fold of the IP3-binding suppressor domain (β SD), β and armadillo repeat folds of the IP3-binding core domains (β IBC and β IBC) are shown in cyan, yellow and red, respectively; IP3 molecule is green; N- and C-termini are shown with spheres and labeled. The cleft between β IBC and α IBC domains undergoes conformational changes upon IP3-binding (indicated with arrows in B) [15,16]. (C-E) Rigid body fitting of the crystal structure of N-terminal ligand binding region in the Apo conformation (PDB ID: 3TBS) into the cryo-EM density map of tetrameric IP3R1 channel (EMD-5278) [9]. (C) The tetrameric CY region is viewed from the cytoplasmic side along the four-fold axis. Green color indicates a putative IP3-binding pocket within the tetrameric channel assembly. (D) Zoomed-in view of the region marked by dashed line in (C). EM densities are shown only for one subunit; the loops making contacts between neighboring subunits (purple arrows) are colored with purple for the SD and with orange for the IBC. Position of Y167 ‘hotspot’ residue is indicated with purple sphere within the SD loop. Residues involved in IP3-binding are shown with green, green arrow indicates IP3-binding pocket.
Ultimately, channel opening requires a structural re-arrangement of the ion conduction pathway. However, the precise molecular nature of such conformational changes and how ligand-binding is communicated to the channel pore remain largely speculative at the moment without a sufficiently high-resolution reconstruction of the full-length tetrameric IP3R [57].
5. Building a quasi-atomic model by putting pieces together
The atomic-resolution structure of the complete IP3R Ca2+ release channel is not yet available, but recent crystallographic studies have begun to provide high-resolution structures for soluble cytoplasmic domains of the IP3R1 [13-16]. It has become common practice to build pseudoatomic models for the quaternary structure by fitting crystal structures of molecular fragments into lower-resolution cryo-EM reconstruction. A recently solved crystal structure of the N-terminal ligand-binding region of IP3R1 was localized into the central cytoplasmic domains of the tetrameric IP3R by fitting into the cryo-EM density map of IP3R1 resolved at intermediate resolution by single-particle cryo-EM [9,16]. The top solutions derived by several docking programs (unpublished results from our group) place the N-terminal IP3-binding domains in the same position within the channel tetramer: domains are arranged around the central plug and form the apical surface of the CY region distal to the TM domains (Fig. 5 C, Graphical abstract). Furthermore, this location is consistent with subunit boundaries identified in the cryo-EM structure of IP3R1 and with the accessibility of binding sites for IP3 within the tetrameric structure (Fig. 5C) [9]. Docking studies suggest that inter-subunit contacts between the N-terminal ligand-binding domains within the tetrameric channel are likely formed by the SD loop (residues 165-180) and a flexible loop of the IBC (residues 421-429) (Fig. 5D). It is important to note that Y167A mutation within the SD loop completely impairs IP3-induced Ca2+ release but does not affect the IP3-binding activity [58]. This implies that this region is critical for the functional coupling between ligand binding and channel opening. However, the precise molecular mechanism of this coupling remains unclear. The consensus view that has emerged from co-immunoprecipitation and cross-linking studies suggests that the SD of one IP3R subunit may interact directly with the cytoplasmic TM4-5 loop of another subunit [54,59,60]. However, in light of the docking studies, it is difficult to imagine that the SD and TM domains can interact directly since they are located at least 70 Å apart in the tetrameric IP3R1 (Graphical abstract). Another attractive possibility is that the C-terminal tail may mediate interactions between the pore and IP3-binding domains [58,61]. It is clear that these models have to be verified by direct structural analysis of the entire IP3R1 at resolutions sufficient to unambiguously establish spatial relationships between the structure-functional domains.
Moreover, while commonplace, it is important to note that the success and accuracy of docking attempts are limited by the size and shape of the crystal structure domain as well as by the resolution of the EM map. Although this approach can definitely provide valuable structural insights and can expand horizons for functional interpretations, establishing the uniqueness and quality of the fit using only computational operations remains problematic [62]. At the marginal resolution limit (12-15 Å), when no secondary structure elements can be identified in the majority of the density map as in the case of IP3R1 Ca2+ release channel, no mechanism exists to validate fits of relatively small domains into the large cryo-EM map without additional supporting data such as specific labelling and these limitations can further lead to ambiguities in fitting.
6. Conclusions
Understanding molecular machinery of ion channels at the atomic level remains a major challenge that attracts many structural biologists. The current round of cryo-EM structural data [9,10] in combination with recent crystallographic studies of small soluble domains [13-16], has provided an important structural basis for posing new hypotheses that can be tested in experiments aiming to understand how IP3-gated Ca2+ release channels work at the molecular level. However, lack of atomic details for the entire Ca2+ release channel molecule limits the conclusions that can currently be drawn leaving significant obscurity of functional and structural nature.
Integral membrane proteins are the most difficult targets for structural analysis due to their tight association with the lipid membranes in vivo and their intrinsic dynamic nature. Despite recent strides made by x-ray crystallography, it remains unclear to what extent the 3D structure of detergent-solubilized channels represents the structure of the physiologically active channel in a biological membrane environment. To overcome limitations present in structural studies of membrane proteins in detergent-solubilized state, new cryo-EM approaches are currently being developed. These include structural analysis of ion channels reconstituted into a lipid membrane environment [63] and substitution of detergents with new surfactants such as ‘amphipols’, for better structural preservation in the absence of a bilayer, while keeping the membrane proteins soluble and in functional form in aqueous solution [64,65]. Notably, recent technical advances in the cryo-EM field that include the use of the direct electron detection cameras and improved image-processing software packages [36,66-69], allowed to resolve the structure of the heat-sensitive TRPV1 channel at 3.4 Å resolution [65,70]. Thus, research aiming at detailed understanding of IP3R function is now poised to move forward toward structure determination of the entire IP3R channel at atomic resolution.
Highlights.
The 3D structure of the tetrameric IP3R is unambiguously determined by single-particle cryo-EM
Structural evidence for allosteric coupling between the ligand-binding and channel domains of IP3R
Advances in the cryo-EM field allow for near-atomic resolution structures of ion channels
Acknowledgments
I would like to thank Mariah Baker for her help with the preparation of Figures 1 and 2. This work is supported by grants from the National Institutes of Health (R01GM079429, R01GM072804, R21AR063255) and the America Heart Association (12GNT10510002, 14RNT1980029).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Furuichi T, Yoshikawa S, Miyawaki A, Wada K, Maeda N, Mikoshiba K. Primary structure and functional expression of the inositol 1,4,5- trisphosphate-binding protein P400. Nature. 1989;342:32–38. doi: 10.1038/342032a0. [DOI] [PubMed] [Google Scholar]
- 2.Mignery GA, Sudhof TC, Takei K, De Camilli P. Putative receptor for inositol 1,4,5-trisphosphate similar to ryanodine receptor. Nature. 1989;342:192–195. doi: 10.1038/342192a0. [DOI] [PubMed] [Google Scholar]
- 3.Bezprozvanny I. Inositol 1,4,5-tripshosphate receptor, calcium signalling and Huntington's disease. Subcell Biochem. 2007;45:323–335. doi: 10.1007/978-1-4020-6191-2_11. [DOI] [PubMed] [Google Scholar]
- 4.Jacobsen AN, Du XJ, Lambert KA, Dart AM, Woodcock EA. Arrythmogenic action of thrombin during myocardial reperfusion via release of inositol 1,4,5-triphosphate. Circulation. 1996;93:23–26. doi: 10.1161/01.cir.93.1.23. [DOI] [PubMed] [Google Scholar]
- 5.Marks AR. Intracellular calcium-release channels: regulators of cell life and death. Am J Physiol. 1997;272:H597–605. doi: 10.1152/ajpheart.1997.272.2.H597. [DOI] [PubMed] [Google Scholar]
- 6.Matsumoto M, Nakagawa T, Innoe T, et al. Ataxia and epileptic seizures in mice lacking type 1 inositol 1,4,5-triphosphate receptor. Nature. 1996;379:168–171. doi: 10.1038/379168a0. [DOI] [PubMed] [Google Scholar]
- 7.Durham W, Wehrens X, Sood S, Hamilton SL. Diseases associated with altered ryanodine activity Calcium Signalling and Disease. In: Carfoli E, Brini M, editors. Spinger; 2007. pp. 273–321. [DOI] [PubMed] [Google Scholar]
- 8.Treves S, Jungbluth H, Muntoni F, Zorzato F. Congenital muscle disorders with cores: the ryanodine receptor calcium channel paradigm. Curr Opin Pharmacol. 2008;8:319–326. doi: 10.1016/j.coph.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Ludtke SJ, Tran TP, Ngo QT, Moiseenkova-Bell VY, Chiu W, Serysheva II. Flexible architecture of IP3R1 by Cryo-EM. Structure. 2011;19:1192–1199. doi: 10.1016/j.str.2011.05.003. doi: http://dx.doi.org/10.1016/j.str.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murray SC, Flanagan J, Popova OB, Chiu W, Ludtke SJ, Serysheva II. Validation of cryo-EM structure of IP(3)R1 channel. Structure. 2013;21:900–909. doi: 10.1016/j.str.2013.04.016. doi: http://dx.doi.org/10.1016/j.str.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cong Y, Ludtke SJ. Single particle analysis at high resolution. Methods Enzymol. 2010;482:211–235. doi: 10.1016/S0076-6879(10)82009-9. doi: http://dx.doi.org/10.1016/S0076-6879(10)82009-9. [DOI] [PubMed] [Google Scholar]
- 12.Tusnady GE, Dosztanyi Z, Simon I. Transmembrane proteins in the Protein Data Bank: identification and classification. Bioinformatics. 2004;20:2964–2972. doi: 10.1093/bioinformatics/bth340. doi: http://dx.doi.org/10.1093/bioinformatics/bth340. [DOI] [PubMed] [Google Scholar]
- 13.Bosanac I, Alattia JR, Mal TK, et al. Structure of the inositol 1,4,5-trisphosphate receptor binding core in complex with its ligand. Nature. 2002;420:696–700. doi: 10.1038/nature01268. [DOI] [PubMed] [Google Scholar]
- 14.Bosanac I, Yamazaki H, Matsu-Ura T, Michikawa T, Mikoshiba K, Ikura M. Crystal structure of the ligand binding suppressor domain of type 1 inositol 1,4,5-trisphosphate receptor. Mol Cell. 2005;17:193–203. doi: 10.1016/j.molcel.2004.11.047. [DOI] [PubMed] [Google Scholar]
- 15.Lin CC, Baek K, Lu Z. Apo and InsP-bound crystal structures of the ligand-binding domain of an InsP receptor. Nat Struct Mol Biol. 2011;18:1172–1174. doi: 10.1038/nsmb.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seo MD, Velamakanni S, Ishiyama N, et al. Structural and functional conservation of key domains in InsP3 and ryanodine receptors. Nature. 2012;483:108–112. doi: 10.1038/nature10751. doi: http://dx.doi.org/10.1038/nature10751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ehrlich BE, Watras J. Inositol 1,4,5-trisphosphate activates a channel from smooth muscle sarcoplasmic reticulum. Nature. 1988;336:583–586. doi: 10.1038/336583a0. doi: http://dx.doi.org/10.1038/336583a0. [DOI] [PubMed] [Google Scholar]
- 18.Ferris CD, Huganir RL, Supattapone S, Snyder SH. Purified inositol 1,4,5-trisphosphate receptor mediates calcium flux in reconstituted lipid vesicles. Nature. 1989;342:87–89. doi: 10.1038/342087a0. [DOI] [PubMed] [Google Scholar]
- 19.Iino M. Calcium dependent inositol trisphosphate-induced calcium release in the guinea-pig taenia caeci. Biochem Biophys Res Commun. 1987;142:47–52. doi: 10.1016/0006-291x(87)90449-9. [DOI] [PubMed] [Google Scholar]
- 20.Foskett JK, White C, Cheung KH, Mak DO. Inositol trisphosphate receptor Ca2+ release channels. Physiol Rev. 2007;87:593–658. doi: 10.1152/physrev.00035.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fedorenko OA, Popugaeva E, Enomoto M, et al. Itracellular calcium channels: Inositol-1,4,5-trisphosphate receptors. Eur J Pharm. 2014;739:39–48. doi: 10.1016/j.ejphar.2013.10.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mignery GA, Newton CL, Archer BT, Sudhof TC. Structure and expression of the rat inositol 1,4,5-trisphosphate receptor. J Biol Chem. 1990;265:12679–12685. [PubMed] [Google Scholar]
- 23.Yoshikawa S, Tanimura T, Miyawaki A, et al. Molecular cloning and characterization of the inositol 1,4,5-trisphosphate receptor in Drosophila melanogaster. J Biol Chem. 1992;267:16613–16619. [PubMed] [Google Scholar]
- 24.Supattapone S, Worley PF, Baraban JM, Snyder SH. Solubilization, purification and characteriztion of an inositol triphosphate receptor. J Biol Chem. 1988;263:1530–1534. [PubMed] [Google Scholar]
- 25.Maeda N, Niinobe M, Nakahira K, Mikoshiba K. Purification and characterization of P400 protein, a glycoprotein characteristic of Purkinje cell, from mouse cerebellum. J Neurochem. 1988;51:1724–1730. doi: 10.1111/j.1471-4159.1988.tb01151.x. [DOI] [PubMed] [Google Scholar]
- 26.Jiang W, Baker ML, Jakana J, Weigele PR, King J, Chiu W. Backbone structure of the infectious epsilon15 virus capsid revealed by electron cryomicroscopy. Nature. 2008;451:1130–1134. doi: 10.1038/nature06665. [DOI] [PubMed] [Google Scholar]
- 27.Zhang X, Settembre E, Xu C, et al. Near-atomic resolution using electron cryomicroscopy and single-particle reconstruction. Proc Natl Acad Sci U S A. 2008;105:1867–1872. doi: 10.1073/pnas.0711623105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ludtke SJ, Baker ML, Chen DH, Song JL, Chuang DT, Chiu W. De novo backbone trace of GroEL from single particle electron cryomicroscopy. Structure. 2008;16:441–448. doi: 10.1016/j.str.2008.02.007. doi: http://dx.doi.org/10.1016/j.str.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 29.Yu X, Jin L, Zhou ZH. 3.88 Å structure of cytoplasmic polyhedrosis virus by cryo-electron microscopy. Nature. 2008;453:415–419. doi: 10.1038/nature06893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cong Y, Baker ML, Jakana J, et al. 4.0-Å resolution cryo-EM structure of the mammalian chaperonin TRiC/CCT reveals its unique subunit arrangement. Proc Natl Acad Sci U S A. 2010;107:4967–4972. doi: 10.1073/pnas.0913774107. doi: http://dx.doi.org/10.1073/pnas.0913774107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang J, Baker ML, Schroder GF, et al. Mechanism of folding chamber closure in a group II chaperonin. Nature. 2010;463:379–383. doi: 10.1038/nature08701. doi: http://dx.doi.org/10.1038/nature08701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng L, Zhu J, Hui WH, et al. Backbone model of an aquareovirus virion by cryo-electron microscopy and bioinformatics. J Mol Biol. 2010;397:852–863. doi: 10.1016/j.jmb.2009.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu X, Zhang Q, Murata K, et al. Structural changes in a marine podovirus associated with release of its genome into Prochlorococcus. Nat Struct Mol Biol. 2010;17:830–836. doi: 10.1038/nsmb.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu H, Jin L, Koh SB, et al. Atomic structure of human adenovirus by cryo-EM reveals interactions among protein networks. Science. 2010;329:1038–1043. doi: 10.1126/science.1187433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen JZ, Settembre EC, Aoki ST, et al. Molecular interactions in rotavirus assembly and uncoating seen by high-resolution cryo-EM. Proc Natl Acad Sci U S A. 2009;106:10644–10648. doi: 10.1073/pnas.0904024106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bai XC, Martin TG, Scheres SH, Dietz H. Cryo-EM structure of a 3D DNA-origami object. Proc Natl Acad Sci U S A. 2012;109:20012–20017. doi: 10.1073/pnas.1215713109. doi: http://dx.doi.org/10.1073/pnas.1215713109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Serysheva II, Ludtke SJ. 3D Structure of IP(3) Receptor. Curr Top Membr. 2010;66C:171–189. doi: 10.1016/S1063-5823(10)66008-5. doi: http://dx.doi.org/10.1016/S1063-5823(10)66008-5. [DOI] [PubMed] [Google Scholar]
- 38.Jiang QX, Thrower EC, Chester DW, Ehrlich BE, Sigworth FJ. Three-dimensional structure of the type 1 inositol 1,4,5-trisphosphate receptor at 24 Å resolution. Embo J. 2002;21:3575–3581. doi: 10.1093/emboj/cdf380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sato C, Hamada K, Ogura T, et al. Inositol 1,4,5-trisphosphate receptor contains multiple cavities and L-shaped ligand-binding domains. J Mol Biol. 2004;336:155–164. doi: 10.1016/j.jmb.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 40.Serysheva II, Bare DJ, Ludtke SJ, Kettlun CS, Chiu W, Mignery GA. Structure of the type 1 inositol 1,4,5-trisphosphate receptor revealed by electron cryomicroscopy. J Biol Chem. 2003;278:21319–21322. doi: 10.1074/jbc.C300148200. [DOI] [PubMed] [Google Scholar]
- 41.Scheres SH, Chen S. Prevention of overfitting in cryo-EM structure determination. Nat Methods. 2012;9:853–854. doi: 10.1038/nmeth.2115. doi: http://dx.doi.org/10.1038/nmeth.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Henderson R, Chen S, Chen JZ, et al. Tilt-pair analysis of images from a range of different specimens in single-particle electron cryomicroscopy. J Mol Biol. 2011;413:1028–1046. doi: 10.1016/j.jmb.2011.09.008. doi: http://dx.doi.org/10.1016/j.jmb.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choe CU, Ehrlich BE. The inositol 1,4,5-trisphosphate receptor (IP3R) and its regulators: sometimes good and sometimes bad teamwork. Sci STKE. 2006;2006:re15. doi: 10.1126/stke.3632006re15. [DOI] [PubMed] [Google Scholar]
- 44.Doyle DA, Morais Cabral J, Pfuetzner RA, et al. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- 45.Kuo A, Gulbis JM, Antcliff JF, et al. Crystal structure of the potassium channel KirBac1.1 in the closed state. Science. 2003;300:1922–1926. doi: 10.1126/science.1085028. [DOI] [PubMed] [Google Scholar]
- 46.Tao X, Avalos JL, Chen J, MacKinnon R. Crystal structure of the eukaryotic strong inward-rectifier K+ channel Kir2.2 at 3.1 Å resolution. Science. 2009;326:1668–1674. doi: 10.1126/science.1180310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ludtke SJ, Serysheva II, Hamilton SL, Chiu W. The pore structure of the closed RyR1 channel. Structure (Camb) 2005;13:1203–1211. doi: 10.1016/j.str.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Samso M, Wagenknecht T, Allen PD. Internal structure and visualization of transmembrane domains of the RyR1 calcium release channel by cryo-EM. Nat Struct Mol Biol. 2005;12:539–544. doi: 10.1038/nsmb938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Unwin N. Nicotinic acetylcholine receptor at 9 Å resolution. J Mol Biol. 1993;229:1101–1124. doi: 10.1006/jmbi.1993.1107. [DOI] [PubMed] [Google Scholar]
- 50.Unwin N. Refined structure of the nicotinic acetylcholine receptor at 4 Å resolution. J Mol Biol. 2005;346:967–989. doi: 10.1016/j.jmb.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 51.Taylor CW, Tovey SC. IP(3) receptors: toward understanding their activation. Cold Spring Harb Perspect Biol. 2010;2:a004010. doi: 10.1101/cshperspect.a004010. doi: http://dx.doi.org/10.1101/cshperspect.a004010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Szlufcik K, Bultynck G, Callewaert G, Missiaen L, Parys JB, De Smedt H. The suppressor domain of inositol 1,4,5-trisphosphate receptor plays an essential role in the protection against apoptosis. Cell Calcium. 2006;39:325–336. doi: 10.1016/j.ceca.2005.11.007. doi: http://dx.doi.org/10.1016/j.ceca.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 53.Uchida K, Miyauchi H, Furuichi T, Michikawa T, Mikoshiba K. Critical regions for activation gating of the inositol 1,4,5-trisphosphate receptor. J Biol Chem. 2003;278:16551–16560. doi: 10.1074/jbc.M300646200. [DOI] [PubMed] [Google Scholar]
- 54.Yoshikawa F, Iwasaki H, Michikawa T, Furuichi T, Mikoshiba K. Trypsinized cerebellar inositol 1,4,5-trisphosphate receptor. Structural and functional coupling of cleaved ligand binding and channel domains. J Biol Chem. 1999;274:316–327. doi: 10.1074/jbc.274.1.316. [DOI] [PubMed] [Google Scholar]
- 55.Chan J, Whitten AE, Jeffries CM, et al. Ligand-induced conformational changes via flexible linkers in the amino-terminal region of the inositol 1,4,5-trisphosphate receptor. J Mol Biol. 2007;373:1269–1280. doi: 10.1016/j.jmb.2007.08.057. [DOI] [PubMed] [Google Scholar]
- 56.Hamada K, Terauchi A, Mikoshiba K. Three-dimensional rearrangements within inositol 1, 4, 5-trisphosphate receptor by calcium. J Biol Chem. 2003;278:52881–52889. doi: 10.1074/jbc.M309743200. [DOI] [PubMed] [Google Scholar]
- 57.Baker ML, Zhang J, Ludtke SJ, Chiu W. Cryo-EM of macromolecular assemblies at near-atomic resolution. Nat Protoc. 2010;5:1697–1708. doi: 10.1038/nprot.2010.126. doi: http://dx.doi.org/10.1038/nprot.2010.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamazaki H, Chan J, Ikura M, Michikawa T, Mikoshiba K. Tyr-167/Trp-168 in type 1/3 inositol 1,4,5-trisphosphate receptor mediates functional coupling between ligand binding and channel opening. J Biol Chem. 2010;285:36081–36091. doi: 10.1074/jbc.M110.140129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boehning D, Joseph SK. Direct association of ligand-binding and pore domains in homo-and heterotetrameric inositol 1,4,5-trisphosphate receptors. Embo J. 2000;19:5450–5459. doi: 10.1093/emboj/19.20.5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schug ZT, Joseph SK. The role of the S4-S5 linker and C-terminal tail in inositol 1,4,5-trisphosphate receptor function. J Biol Chem. 2006;281:24431–24440. doi: 10.1074/jbc.M604190200. [DOI] [PubMed] [Google Scholar]
- 61.Chan J, Yamazaki H, Ishiyama N, et al. Structural studies of inositol 1,4,5-trisphosphate receptor: coupling ligand binding to channel gating. J Biol Chem. 2010;285:36092–36099. doi: 10.1074/jbc.M110.140160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Egelman EH. Problems in fitting high resolution structures into electron microscopic reconstructions. HFSP J. 2008;2:324–331. doi: 10.2976/1.2992221. doi: http://dx.doi.org/10.2976/1.2992221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang L, Sigworth FJ. Structure of the BK potassium channel in a lipid membrane from electron cryomicroscopy. Nature. 2009;461:292–295. doi: 10.1038/nature08291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Popot JL, Althoff T, Bagnard D, et al. Amphipols from A to Z. Annu Rev Biophys. 2011;40:379–408. doi: 10.1146/annurev-biophys-042910-155219. doi: http://dx.doi.org/10.1146/annurev-biophys-042910-155219. [DOI] [PubMed] [Google Scholar]
- 65.Liao M, Cao E, Julius D, Cheng Y. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature. 2013;504:107–112. doi: 10.1038/nature12822. doi: http://dx.doi.org/10.1038/nature12822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bammes BE, Rochat RH, Jakana J, Chen DH, Chiu W. Direct electron detection yields cryo-EM reconstructions at resolutions beyond 3/4 Nyquist frequency. J Struct Biol. 2012 doi: 10.1016/j.jsb.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Campbell MG, Cheng A, Brilot AF, et al. Movies of Ice-Embedded Particles Enhance Resolution in Electron Cryo-Microscopy. Structure. 2012 doi: 10.1016/j.str.2012.08.026. doi: http://dx.doi.org/10.1016/j.str.2012.08.026. [DOI] [PMC free article] [PubMed]
- 68.Li X, Zheng SQ, Egami K, Agard DA, Cheng Y. Influence of electron dose rate on electron counting images recorded with the K2 camera. J Struct Biol. 2013 doi: 10.1016/j.jsb.2013.08.005. doi: http://dx.doi.org/10.1016/j.jsb.2013.08.005. [DOI] [PMC free article] [PubMed]
- 69.Li X, Mooney P, Zheng S, et al. Electron counting and beam-induced motion correction enable near-atomic-resolution single-particle cryo-EM. Nat Methods. 2013;10:584–590. doi: 10.1038/nmeth.2472. doi: http://dx.doi.org/10.1038/nmeth.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cao E, Liao M, Cheng Y, Julius D. TRPV1 structures in distinct conformations reveal activation mechanisms. Nature. 2013;504:113–118. doi: 10.1038/nature12823. doi: http://dx.doi.org/10.1038/nature12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Berridge MJ, Fain JN. Inhibition of phosphatidylinositol synthesis and the inactivation of calcium entry after prolonged exposure of the blowfly salivary gland to 5-hydroxytryptamine. Biochem J. 1979;178:59–69. doi: 10.1042/bj1780059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Berridge MJ. Rapid accumulation of inositol trisphosphate reveals that agonists hydrolyse polyphosphoinositides instead of phosphatidylinositol. Biochem J. 1983;212:849–858. doi: 10.1042/bj2120849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Streb H, Irvine RF, Berridge MJ, Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983;306:67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- 74.Chadwick CC, Saito A, Fleischer S. Isolation and characteriztion of the inositol triphosphate receptor from smooth muscle. Proceedings of the National Academy of Science. 1990;87:2132–2136. doi: 10.1073/pnas.87.6.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mignery GA, Sudhof TC. The ligand binding site and transduction mechanism in the inositol-1,4,5-triphosphate receptor. Embo J. 1990;9:3893–3898. doi: 10.1002/j.1460-2075.1990.tb07609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nakade S, Maeda N, Mikoshiba K. Involvement of the C-terminus of the inositol 1,4,5-trisphosphate receptor in Ca2+ release analysed using region-specific monoclonal antibodies. Biochem J. 1991;277(Pt 1):125–131. doi: 10.1042/bj2770125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Katayama E, Funahashi H, Michikawa T, et al. Native structure and arrangement of inositol-1,4,5-triphosphate receptor molecules in bovine cerebellar Purkinje cells as studied by quick-freeze deep-etch electron microscopy. EMBO J. 1996;15:4844–4851. [PMC free article] [PubMed] [Google Scholar]
- 78.Hamada K, Miyata T, Mayanagi K, Hirota J, Mikoshiba K. Two-state conformational changes in inositol 1,4,5-trisphosphate receptor regulated by calcium. J Biol Chem. 2002;277:21115–21118. doi: 10.1074/jbc.C200244200. [DOI] [PubMed] [Google Scholar]
- 79.da Fonseca PC, Morris SA, Nerou EP, Taylor CW, Morris EP. Domain organization of the type 1 inositol 1,4,5-trisphosphate receptor as revealed by single-particle analysis. Proc Natl Acad Sci U S A. 2003;100:3936–3941. doi: 10.1073/pnas.0536251100. [DOI] [PMC free article] [PubMed] [Google Scholar]