Abstract
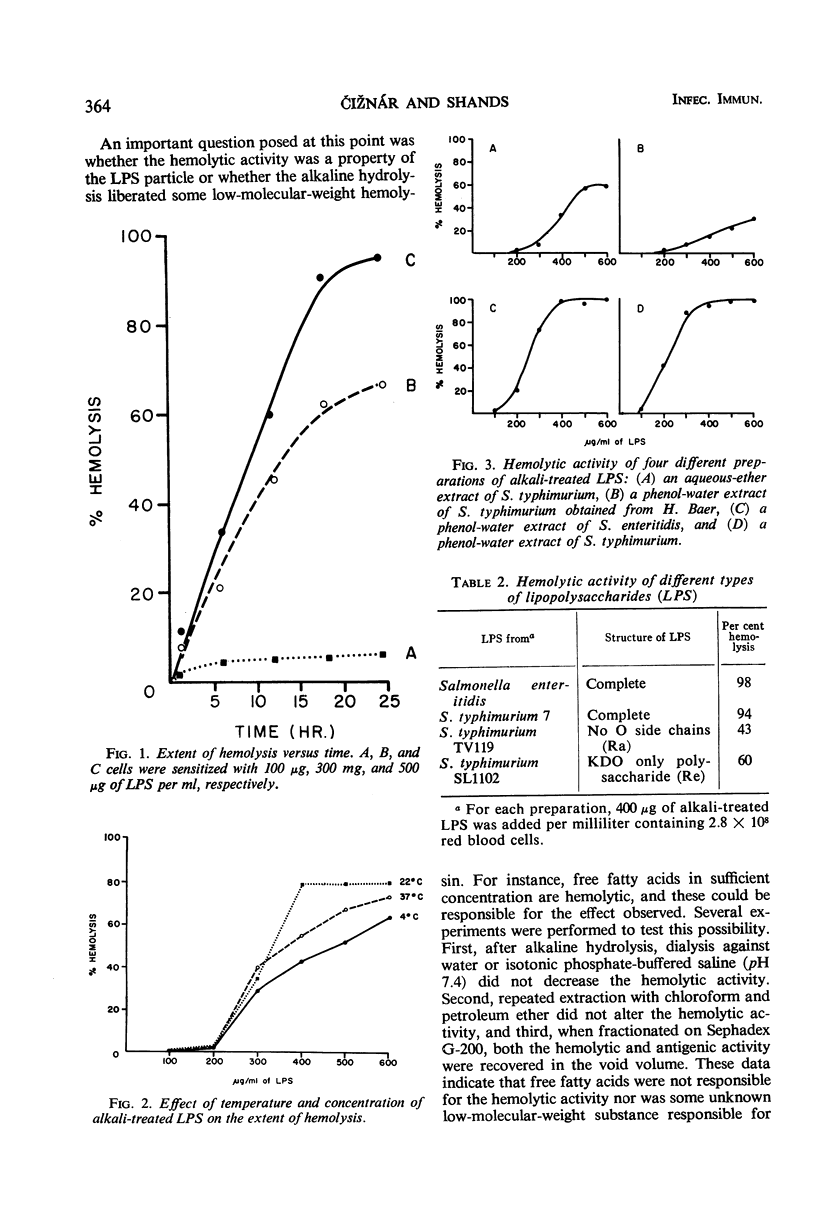
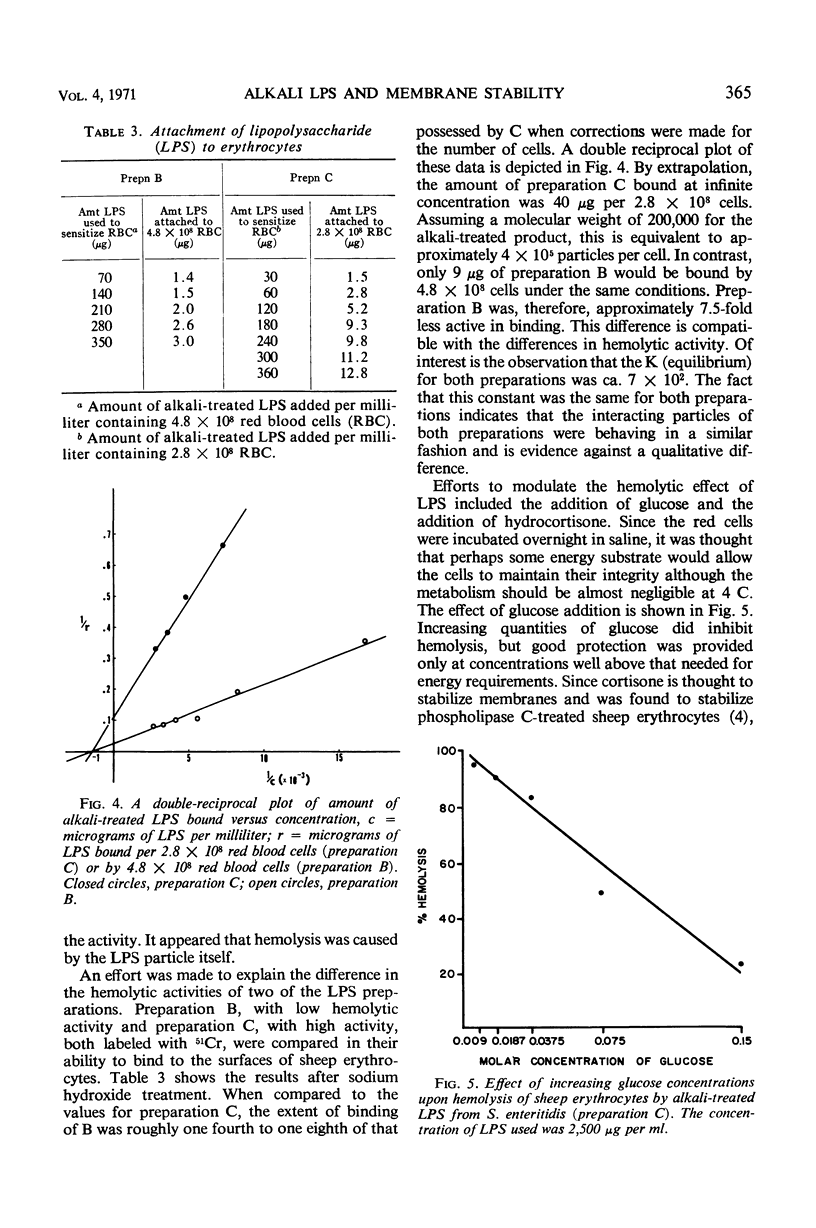
The interaction of various lipopolysaccharides (LPS) with sheep erythrocytes was studied. When subjected to mild alkaline hydrolysis, the affinity of LPS for the red cell surface was greatly increased, as others have reported. In addition, excessive quantities of alkali-treated LPS (but not parent or heated products) were found to cause hemolysis of red cells. Experiments indicated that the hemolysis was caused by the LPS particles themselves and not by liberated free fatty acids.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson E. H. Growth Requirements of Virus-Resistant Mutants of Escherichia Coli Strain "B". Proc Natl Acad Sci U S A. 1946 May;32(5):120–128. doi: 10.1073/pnas.32.5.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAUDE A. I., CAREY F. J., SUTHERLAND D., ZALESKY M. Studies with radioactive endotoxin. I. The use of Cr51 to label endotoxin of Escherichia coli. J Clin Invest. 1955 Jun;34(6):850–857. doi: 10.1172/JCI103140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciznár I., Shands J. W. Effect of Alkali on the Immunological Reactivity of Lipopolysaccharide from Salmonella typhimurium. Infect Immun. 1970 Nov;2(5):549–555. doi: 10.1128/iai.2.5.549-555.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaglstein W. H., Reasor H. S., Hsia S. L. Membrane stabilization: effects of hydrocortisone sodium succinate on phospholipase C-treated sheep erythrocytes. Proc Soc Exp Biol Med. 1969 Dec;132(3):919–923. doi: 10.3181/00379727-132-34337. [DOI] [PubMed] [Google Scholar]
- GORZYNSKI E. A., LUDERITZ O., NETER E., WESTPHAL O. The bacterial hemagglutination test for the demonstration of antibodies to Enterobacteriaceae. Ann N Y Acad Sci. 1956 Aug 10;66(1):141–156. doi: 10.1111/j.1749-6632.1956.tb40113.x. [DOI] [PubMed] [Google Scholar]
- Gimber P. E., Rafter G. W. The interaction of Escherichia coli endotoxin with leukocytes. Arch Biochem Biophys. 1969 Dec;135(1):14–20. doi: 10.1016/0003-9861(69)90510-4. [DOI] [PubMed] [Google Scholar]
- Hämmerling U., Westphal O. Synthesis and use of O-stearoyl polysaccharides in passive hemagglutination and hemolysis. Eur J Biochem. 1967 Mar;1(1):46–50. doi: 10.1007/978-3-662-25813-2_9. [DOI] [PubMed] [Google Scholar]
- NETER E. Bacterial hemagglutination and hemolysis. Bacteriol Rev. 1956 Sep;20(3):166–188. doi: 10.1128/br.20.3.166-188.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NETER E., WESTPHAL O., LUDERITZ O., GORZYNSKI E. A., EICHENBERGER E. Studies of enterobacterial lipopolysaccharides; effects of heat and chemicals on erythrocyte-modifying, antigenic, toxic and pyrogenic properties. J Immunol. 1956 May;76(5):377–385. [PubMed] [Google Scholar]
- RIBI E., MILNER K. C., PERRINE T. D. Endotoxic and antigenic fractions from the cell wall of Salmonella enteritidis; methods for separation and some biologic activities. J Immunol. 1959 Jan;82(1):75–84. [PubMed] [Google Scholar]
- Tenney S. R., Rafter G. W. Leukocyte adenosine triphosphatases and the effect of endotoxin on their activity. Arch Biochem Biophys. 1968 Jul;126(1):53–58. doi: 10.1016/0003-9861(68)90558-4. [DOI] [PubMed] [Google Scholar]
- Tripodi D., Nowotny A. Relation of structure to function in bacterial O-antigens. V. Nature of active sites in endotoxic lipopolysaccharides of Serratia marcescens. Ann N Y Acad Sci. 1966 Jun 30;133(2):604–621. doi: 10.1111/j.1749-6632.1966.tb52392.x. [DOI] [PubMed] [Google Scholar]


