Abstract
Here, we have used the green method for synthesis of silver and gold nanoparticles. In the present study the silver (Ag) and gold (Au) nanoparticles (NPs) were synthesized by using the aqueous bark extract of Indian spice dalchini (Cinnamomum zeylanicum) (C. zyelanicum or C. verum J. Presl). Additionally, we have used these synthesized nanoparticles for mosquito control. The larvicidal activity has been tested against the malaria vector Anopheles stephensi and filariasis vector Culex quinquefasciatus. The results were obtained using UV-visible spectrophotometer and the images were recorded with a transmission electron microscope (TEM). The efficacy tests were then performed at different concentrations and varying numbers of hours by probit analysis. The synthesized AgNPs were in spherical shape and average sizes (11.77 nm AgNPs and 46.48 nm AuNPs). The larvae of An. stephensi were found highly susceptible to the synthesized AgNPs and AuNPs than the Cx. quinquefasciatus. These results suggest that the C. zeylanicum synthesized silver and gold nanoparticles have the potential to be used as an ideal ecofriendly approach for the control of mosquito.
1. Introduction
Cinnamon is a small evergreen tree, which belongs to family Lauraceae. It is the native of Sri Lanka and Southern India. It has antioxidant, antimicrobials, mosquito control, and other properties [1]. Mosquitoes are vectors of many diseases, including malaria, filariasis, dengue, and Japanese encephalitis. Among these kinds of malaria, spread by the bite of female Anopheles mosquito, and filariasis, spread by Culex mosquito, are the two vector borne diseases of the tropical region and are considered major public health concerns.
According to WHO, there were about 219 million cases of malaria in 2010 (with an uncertainty range of 154 million to 289 million) and an estimated 660,000 deaths (with an uncertainty range of 490,000 to 836,000). Malaria mortality rate has fallen by more than 25% globally since 2000 and by 33% in the WHO African region. Most deaths occur among children living in Africa, where malaria claims the life of a child every minute. Country-level burden estimates available for 2010 show that an estimated 80% of malaria deaths occur in just 14 countries and about 80% of cases occur in 17 countries. Together, the Democratic Republic of the Congo and Nigeria account for over 40% of the total estimated malaria deaths globally [2].
On the other hand, nearly, 1.4 billion people in 73 countries worldwide are threatened by lymphatic filariasis, commonly known as elephantiasis. Over 120 million people are currently infected, with about 40 million disfigured and incapacitated by the disease [3]. Control or eradication of the mosquito population could significantly restrict the spread of disease.
Synthesizing nanoparticles using plants and microorganisms can eliminate this problem by making the nanoparticles more biocompatible. The synthesis of silver nanoparticles from silver precursor using the bark extract and powder of novel Cinnamon zeylanicum has been reported [4]. The potential of nanocrystalline palladium particle production using Cinnamomum zeylanicum (C. zyelanicum or C. verum J. Presl) bark extract has been studied [5].
The silver and gold nanoparticles using A. calamus [6], E. sativa and S. oleracea [7], T. conoides [8], and A. nilotica [9] have been synthesized.
The larvicidal activity of biosynthesized silver nanoparticles using the leaf extracts of P. pinnata [10] and L. aspera [11] has been tested against the dengue vector Ae. albopictus and Ae. aegypti.
In the present investigation we have synthesized the AgNPs and AuNPs nanoparticles by using the bark extract of C. zeylanicum. Further, the synthesized nanoparticles have also been tested against the larvae of Anopheles stephensi and Culex quinquefasciatus. The C. zeylanicum synthesized AgNPs and AuNPs have the potential to be used as an ideal ecofriendly approach for the control of mosquito.
2. Materials and Methods
2.1. Material
The bark of Cinnamomum zeylanicum (C. zyelanicum or C. verum J. Presl) was collected from the local market of Agra, India. The voucher specimen is maintained in our laboratory for future use.
2.2. Extract Preparation
The bark of Cinnamomum zeylanicum (C. zyelanicum or C. verum J. Presl) was washed with distilled water for removing the dust particles. The bark was air-dried and converted into powder and a bark broth was prepared by placing 10 g of bark powder in a 250 mL of deionized water. This mixture was boiled at 60°C, for 5 min, decanted, or filtered through Whatman-1 filter paper.
2.3. Nanoparticles Synthesis
After obtaining the aqueous extract of bark, the filtrates were treated with aqueous 1 mM AgNO3 and HAuCl4 solutions in an Erlenmeyer flask and incubated at room temperature. Formation of AgNPs and AuNPs was indicated by the dark brown and purple coloration of the solutions.
2.4. Characterization of Nanoparticles
Synthesis of AgNPs and AuNPs was confirmed by sampling the reaction mixture at regular intervals, and the absorption maxima were scanned by UV-Vis spectra, at the wavelength of 350–750 nm in a UV-3600 Shimadzu spectrophotometer at 1 nm resolution. The micrographs of AgNPs and AuNPs were obtained by TECHNAI 200 Kv TEM (Fei, Electron Optics) transmission electron microscope. For transmission electron microscopy analysis, samples were prepared on carbon coated copper TEM grids.
2.5. Rearing of Mosquito Larvae
The larvae of Cx. quinquefasciatus and An. stephensi were collected from various localities including urban, rural, and semiurban regions of Agra (27°, 10′N, 78°05′E), India. The larvae were reared in deionized water containing glucose and yeast powder. The colonies of Cx. quinquefasciatus and An. stephensi were maintained in the laboratory at a temperature of 25°C with a relative humidity of 75 ± 5% and 14 h of photoperiod. The larvae of Cx. quinquefasciatus and An. stephensi were maintained in separate enamel containers as per the standard method [12].
2.6. Bioassays, Data Management, and Statistical Analysis
AgNPs and AuNPs synthesized from Cinnamomum zeylanicum (C. zyelanicum or C. verum J. Presl) were tested for their killing activities against the larvae of Cx. quinquefasciatus and An. stephensi and were assessed by using the standard method [13]. All larvae of Cx. quinquefasciatus and An. stephensi were separated and placed in a container in microbe-free deionized water. After that, different test concentrations of AgNPs and AuNPs in 100 mL deionized water were prepared in 250 mL beakers. Bioassays were conducted separately for each instar at five different concentrations of aqueous AgNPs and AuNPs (2, 4, 6, 8, 10 ppm). To test the larvicidal activity of our AgNPs and AuNPs, 20 larvae of each stage were separately exposed to 100 mL of test concentrations. Thereafter, we examined their mortality after different time of treatment during the experimental periods.
The data on the efficacy were subjected to probit analysis [14]. The control mortality was corrected by Abbott's formula [15].
3. Results
3.1. Analysis of UV-Vis Spectra
The aqueous extracts of bark of C. zeylanicum (C. zyelanicum or C. verum J. Presl) were light yellow and brown in colour before immersion in AgNO3 and HAuCl4 solutions. The colour of bark aqueous extract changed into dark red and purple colour after immersing in AgNO3 and HAuCl4 solutions after 72 h of incubation. The change in colour is a signal for the formation of AgNPs and AuNPs. Figures 1(a) and 1(b) show the UV-Vis spectra of synthesized AgNPs and AuNPs using the bark of C. zeylanicum (C. zyelanicum or C. verum J. Presl) recorded from reaction medium before (1) and after immersion of AgNO3 and HAuCl4 (2) after 72 h. Absorption spectra of AgNPs and AuNPs formed in the reaction medium have a broad absorption band centred at 480 nm and 530 nm c.a.
Figure 1.
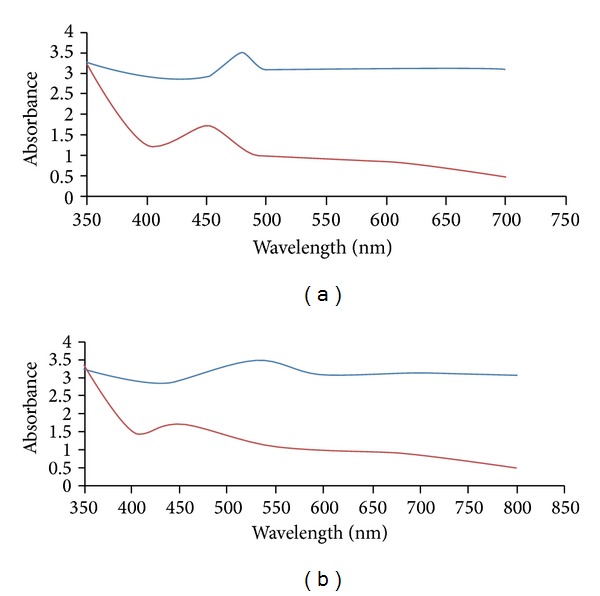
UV-Vis spectra of (a) silver nanoparticles and (b) gold nanoparticles synthesized by using the bark extract of Cinnamomum zeylanicum recorded from reaction medium before (1) and after immersion of AgNO3 (2) after 24 h.
3.2. TEM Analysis
Figures 2(a) and 2(b) show the TEM micrographs of synthesized AgNPs and AuNPs. The average size of AgNPs is 11.77 nm and AuNPs 46.48 nm and they were spherical-shaped.
Figure 2.
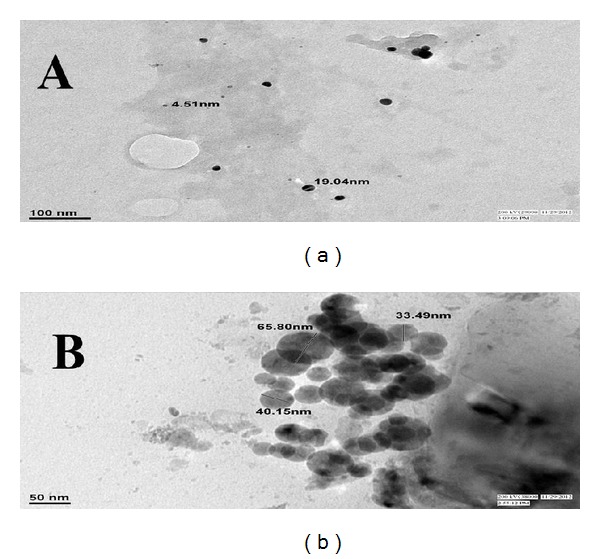
TEM micrographs of Cinnamomum zeylanicum synthesized (a) silver nanoparticles and (b) gold nanoparticles.
3.3. Efficacy of Synthesized AgNPs and AuNPs against An. stephensi Larvae
The efficacy of the synthesized AgNPs and AuNPs was tested against the larvae of An. stephensi. Different concentrations of aqueous AgNPs and AuNPs (2, 4, 6, 8, 10 ppm) were tested against the larvae of An. stephensi.
The larvae of An. stephensi were found highly susceptible to the synthesized AgNPs. First instar larvae (LC50 2, LC90 11, and LC99 12 ppm), second instar larvae (LC50 10, LC90 15, and LC99 17 ppm) after 4 h, third instar larvae (LC50 6, LC90 11, and LC99 13 ppm), and fourth instar larvae (LC50 10, LC90 15, and LC99 17 ppm) after 22 h of exposure were obtained with their confidence limits and χ 2 and r values (Table 1).
Table 1.
Efficacy of silver nanoparticles and gold nanoparticles synthesized by bark extract of Cinnamomum zeylanicum against the larvae of Anopheles stephensi with their LC values, 95% confidential limits (CL), and χ 2 and r values after different time of exposure.
| NPs | Instar | Time | LC50 (95% CL) | LC90 (95% CL) | LC99 (95% CL) | χ 2 | r |
|---|---|---|---|---|---|---|---|
| AgNPs | 1st | 4 h | 2 (0.86–3.14) | 11 (9.77–12.23) | 12 (10.54–13.46) | 50.29 | 0.82 |
| 2nd | 4 h | 10 (8.86–11.14) | 15 (13.77–16.23) | 17 (15.54–18.46) | 39.43 | 0.95 | |
| 3rd | 22 h | 6 (4.86–7.16) | 11 (9.77–12.23) | 13 (11.54–14.46) | 43.83 | 0.95 | |
| 4th | 22 h | 10 (8.86–11.14) | 15 (13.83–16.17) | 17 (15.77–18.77) | 37.66 | 0.89 | |
|
| |||||||
| AuNPs | 1st | 24 h | ∗∗ | ∗∗ | ∗∗ | ∗∗ | ∗∗ |
| 2nd | 24 h | 1 (0.86–2.14) | 8 (6.83–9.17) | 10.5 (9.27–11.73) | 61.61 | 0.91 | |
| 3rd | 72 h | 1 (0.86–2.14) | 8 (6.83–9.17) | 10.5 (9.27–11.73) | 61.61 | 0.91 | |
| 4th | 72 h | 2 (0.88–3.12) | 10 (8.86–11.14) | 11 (9.87–12.13) | 54.93 | 0.89 | |
∗∗100% mortality.
The larvae of An. stephensi were found susceptible to the synthesized AuNPs. The first instar larvae have shown the 100% mortality after 24 h of exposure. The second instar larvae (LC50 1, LC90 8, and LC99 10.5 ppm) after 24 h, third instar larvae (LC50 1, LC90 8, and LC99 10.5 ppm), and fourth instar larvae (LC50 2, LC90 10, and LC99 11 ppm) after 72 h of exposure were obtained with their confidence limits and χ 2 and r values (Table 1, Figure 3(a)).
Figure 3.
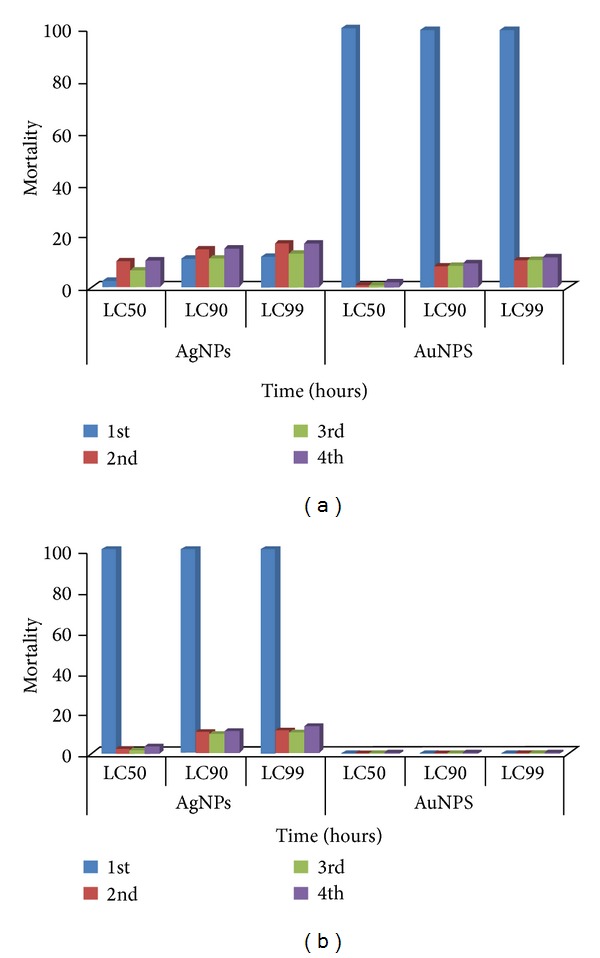
Efficacy of silver and gold nanoparticles against the larvae of Anopheles stephensi at different hours of exposure.
3.4. Efficacy of Synthesized AgNPs and AuNPs against Cx. quinquefasciatus Larvae
The efficacy of the synthesized AgNPs and AuNPs was tested against the larvae of Cx. quinquefasciatus. Different concentrations of aqueous AgNPs and AuNPs (2, 4, 6, 8, 10 ppm) were tested against the larvae of Cx. quinquefasciatus.
The larvae of Cx. quinquefasciatus were found highly susceptible to the synthesized AgNPs. The first instar larvae have shown the 100% mortality after 24 h of exposure. The second instar larvae (LC50 2, LC90 10, and LC99 11 ppm), third instar larvae (LC50 1.5, LC90 9, and LC99 10.5 ppm), and fourth instar larvae (LC50 4, LC90 11, and LC99 13 ppm) after 24 h of exposure were obtained with their confidence limits and χ 2 and r values (Table 2).
Table 2.
Efficacy of silver nanoparticles and gold nanoparticles synthesized by bark extract of Cinnamomum zeylanicum against the larvae of Culex quinquefasciatus with their LC values, 95% confidential limits (CL), and χ 2 and r values after different time of exposure.
| NPs | Instar | Time | LC50 (95% CL) | LC90 (95% CL) | LC99 (95% CL) | χ 2 | r |
|---|---|---|---|---|---|---|---|
| AgNPs | 1st | 24 h | ∗∗ | ∗∗ | ∗∗ | ∗∗ | ∗∗ |
| 2nd | 24 h | 2 (0.88–3.12) | 10 (8.86–11.14) | 11 (9.87–12.13) | 54.93 | 0.89 | |
| 3rd | 24 h | 1.5 (1.36–2.64) | 9 (7.77–10.23) | 10.5 (9.17–11.83) | 58.85 | 0.85 | |
| 4th | 24 h | 4 (2.86–5.14) | 11 (9.77–12.23) | 13 (11.54–14.46) | 49.41 | 0.87 | |
| AuNPs | Larvae | 24 h | — | — | — | — | — |
∗∗100% mortality.
—no mortality.
The larvae of Cx. quinquefasciatus were found less susceptible to the synthesized AuNPs. No mortality was observed after 24 h of exposure (Table 2, Figure 3(b)).
4. Discussion
In the present investigation we have synthesized the AgNPs and AuNPs by using the bark of C. zeylanicum (C. zyelanicum or C. verum J. Presl). The efficacy of synthesized NPs has been tested against the larvae of malaria vector An. stephensi and filariasis vector Cx. quinquefasciatus.
An economically viable and “green chemistry” approach for biological synthesis of silver nanoparticles using aqueous leaf extract of P. dulce has been reported as larvicidal activity against the Cx. quinquefasciatus previously [16]. The larvicidal activity of biogenic nanoparticles against filariasis causing Culex mosquito vector has also been evaluated before [17].
Larvicidal activity of silver nanoparticles (AgNPs) using leaf extract of N. oleander (Apocynaceae) against the first to the fourth instar larvae and pupae of malaria vector, An. stephensi (Diptera: Culicidae), was carried out in an earlier study [18]. The fabrication, characterization, and mosquito larvicidal bioassay of silver nanoparticles synthesized from aqueous fruit extract of Putranjiva and D. roxburghii were observed [19]. Moreover, the activity of silver nanoparticles (AgNPs) synthesized using M. koenigii plant leaf extract against the first to the fourth instars larvae and pupae of An. stephensi and Ae. aegypti was determined, too [20]. Among these previous studies, the silver nanoparticles were synthesized by using the aqueous extract of leaf and other parts of plant extract and nanoparticles were tested against the first and the fourth instar larvae and pupae of mosquitoes. However, in the present study we have synthesized silver and gold nanoparticles by using aqueous extracts of bark of C. zeylanicum (C. zyelanicum or C. verum J. Presl). These nanoparticles were tested as larvicide against An. stephensi and Cx. quinquefasciatus.
Recently, the larvicidal activity of silver nanoparticles synthesized by leaf extract of P. pinnata has been tested against the larvae of dengue vector Ae. albopictus [10]. Further, the larvicidal potential of silver nanoparticles synthesized from L. aspera leaf extract has been tested against the dengue vector Ae. aegypti [11]. However, the larvicidal activity of silver nanoparticles (AgNPs) synthesized using F. elephantum plant leaf extract against late third instar larvae of An. stephensi, Ae. aegypti, and Cx. quinquefasciatus has been determined [21]. Furthermore, activity of aqueous leaf extract and silver nanoparticles (AgNPs) synthesized using H. indicum plant leaves against late third instar larvae of Ae. aegypti, An. Stephensi, and Cx. quinquefasciatus has been studied [22]. Larvicidal activity of silver nanoparticles synthesized from aqueous leaf extract of C. collinus against the larvae of Ae. aegypti has now been determined [23], while in our study we have synthesized silver nanoparticles by using aqueous extracts of bark of C. zeylanicum (C. zyelanicum or C. verum J. Presl). These nanoparticles were tested as larvicide against An. stephensi and Cx. quinquefasciatus.
The larvicidal activities of synthesized cobalt nanoparticles using the biocontrol agent, B. thuringiensis, have been investigated against the malaria vector An. subpictus and dengue vector, Ae. aegypti [24]. Furthermore, the larvicidal activity of silver nanoparticles synthesized by using B. thuringiensis has been revealed against the Ae. aegypti [25], whereas in the present study we have tested the larvicidal activity of silver and gold nanoparticles synthesized by using aqueous extracts of bark of C. zeylanicum (C. zyelanicum or C. verum J. Presl). These nanoparticles were tested as larvicide against An. stephensi and Cx. quinquefasciatus.
Efficacy of fungus mediated silver and gold nanoparticles has been tested against the larvae of An. stephensi, Cx. Quinquefasciatus, and Ae. aegypti [26–28]. Furthermore, the larvicidal and pupicidal activities of silver and gold nanoparticles synthesized by fungi have also been investigated against An. stephensi, Cx. Quinquefasciatus, and Ae. aegypti [29–31]. Recently the silver nanoparticles have been synthesized by using the leaf and stem of Piper nigrum for their antibacterial activity against agriculture plant pathogens [32]. The silver nanoparticles have synthesized by using the leaf of Pimenta dioica [33]. However, in the present study the silver and gold nanoparticles have been synthesized by bark of C. zeylanicum against the larvae of An. stephensi and Cx. quinquefasciatus.
5. Conclusion
In this study we have synthesized the silver and gold nanoparticles by using the bark of Cinnamomum zeylanicum (C. zyelanicum or C. verum J. Presl). The larvicidal activity of the synthesized nanoparticles has been tested against the larvae of malaria vector Anopheles stephensi and filariasis vector Culex quinquefasciatus. The synthesized AgNPs were in spherical shape and average sizes (11.77 nm AgNPs, 46.48 nm AuNPs). The synthesized nanoparticles were found effective against the larvae of mosquito species. The results suggest that the C. zeylanicum (C. zyelanicum or C. verum J. Presl) synthesized silver and gold nanoparticles have the potential to be used as an ideal ecofriendly approach for the control of mosquito.
Acknowledgments
The authors sincerely thank Professor P. S. Satsangi Sahab, Chairman, of Advisory Committee on Education, Dayalbagh Educational Institute, and Professor P. K. Kalra, Director, Dayalbagh Educational Institute, for providing support and encouragements for the work. They also thank to UGC, New Delhi, Major Research Project (39-599/2010), for financial support.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Lal SS, Nayak PL. Green synthesis of gold nanoparticles using various extract of plants and species. International Journal of Science Innovations and Discoveries. 2012;2(23):325–350. [Google Scholar]
- 2.World Health Organization. Malaria. 2013, http://www.who.int/mediacentre/factsheets/fs094/en/index.html.
- 3.World Health Organization. Lymphatic filariasis. 2013, http://www.who.int/mediacentre/factsheets/fs102/en/
- 4.Sathishkumar M, Sneha K, Won SW, Cho C-W, Kim S, Yun Y-S. Cinnamon zeylanicum bark extract and powder mediated green synthesis of nano-crystalline silver particles and its bactericidal activity. Colloids and Surfaces B: Biointerfaces. 2009;73(2):332–338. doi: 10.1016/j.colsurfb.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Sathishkumar M, Sneha K, Kwak IS, Mao J, Tripathy SJ, Yun Y-S. Phyto-crystallization of palladium through reduction process using Cinnamom zeylanicum bark extract. Journal of Hazardous Materials. 2009;171(1–3):400–404. doi: 10.1016/j.jhazmat.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 6.Dhanashekaran S, Karunakaran S, Amutha R, Suruthipariyadarshini S, Jayalakshmi K. Biosynthesis of silver nanoparticles using Arocus calamus and its antibacterial activity. International Journal of Nanomaterials and Biostructures. 2014;4(1):16–20. [Google Scholar]
- 7.Alaraidh IA, Ibrahim MM, El-Gaaly GA. Evaluation of green synthesis of Ag nanoparticles using Eruca sativa and Spinacia oleracea leaf extracts and their antimicrobial activity. Iranian Journal of Biotechnology. 2014;12(1)e12392 [Google Scholar]
- 8.Vijayan SR, Santhiyagu P, Singamuthu M, Ahila NK, Jayaraman R, Ehiraj K. Synthesis and characterization of silver and gold nanoparticles using aqueous extract of seaweed, Turbinaria conoides, and their antimicrofouling activity. The Scientific World Journal. 2014;2014:10 pages. doi: 10.1155/2014/938272.938272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prasad CH, Gangadhara S, Venkateswarlu S, Jyothi NVV, Venkateswarlu P. Green synthesis of silver nanoparticles/ microparticles using extract of Acacia nilotica seeds and their characterization. International Journal of Nanomaterials and Biostructures. 2014;4(1):1–4. [Google Scholar]
- 10.Naik BR, Gowreeswari GS, Singh Y, Satyavathi R, Daravath RR, Reddy PR. Bio-synthesis of silver nanoparticles from leaf extract of Pongamia pinnata as an effective larvicide on dengue vector Aedes albopictus (Skuse) (Diptera: Culicidae) Advances in Entomology. 2014;2:93–101. [Google Scholar]
- 11.Suganya G, Karthi S, Shivakumar MS. Larvicidal potential of silver nanoparticles synthesized from Lucas aspera leaf extracts against dengue vector Aedes aegypti . Parasitology Research. 2014;113(5):1673–1679. doi: 10.1007/s00436-014-3811-2. [DOI] [PubMed] [Google Scholar]
- 12.Gerberg EJ, Barnard DR, Ward RA. Manual for mosquito rearing and experimental techniques. Journal of American Mosquito Control Association. 1994;5:p. 98. [Google Scholar]
- 13.World Health Organization. Guidelines for Laboratory and Field Testing of Mosquito Larvicides. 2005. (WHO/CDS/WHOPES/GCDPP/13). [Google Scholar]
- 14.Finney DJ. Probit Analysis. 3rd edition. Cambridge, UK: Cambridge University Press; 1971. [Google Scholar]
- 15.Abbott WS. A method of computing the effectiveness of an insecticide. Journal of Economic Entomology. 1925;18(2):265–267. [Google Scholar]
- 16.Raman N, Sudharsan S, Veerakumar V, Pravin N, Vithiya K. Pithecellobium dulce mediated extra-cellular green synthesis of larvicidal silver nanoparticles. Spectrochimica Acta A: Molecular and Biomolecular Spectroscopy. 2012;96:1031–1037. doi: 10.1016/j.saa.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 17.Dhanasekaran D, Thangaraj R. Evaluation of larvicidal activity of biogenic nanoparticles against filariasis causing Culex mosquito vector. Asian Pacific Journal of Tropical Disease. 2013;3(3):174–179. [Google Scholar]
- 18.Roni M, Murugan K, Panneerselvam C, Subramaniam J, Hwang J-S. Evaluation of leaf aqueous extract and synthesized silver nanoparticles using Nerium oleander against Anopheles stephensi (Diptera: Culicidae) Parasitology Research. 2013;112(3):981–990. doi: 10.1007/s00436-012-3220-3. [DOI] [PubMed] [Google Scholar]
- 19.Haldar KM, Haldar B, Chandra G. Fabrication, characterization and mosquito larvicidal bioassay of silver nanoparticles synthesized from aqueous fruit extract of putranjiva, Drypetes roxburghii (Wall.) Parasitology Research. 2013;112(4):1451–1459. doi: 10.1007/s00436-013-3288-4. [DOI] [PubMed] [Google Scholar]
- 20.Suganya A, Murugan K, Kovendan K, Mahesh Kumar P, Hwang J-S. Green synthesis of silver nanoparticles using Murraya koenigii leaf extract against Anopheles stephensi and Aedes aegypti . Parasitology Research. 2013;112(4):1385–1397. doi: 10.1007/s00436-012-3269-z. [DOI] [PubMed] [Google Scholar]
- 21.Veerakumar K, Govindrajan M, Rajeswary M, Muthukumaran U. Low-cost and eco-friendly green synthesis of silver nanoparticles using Feronia elephantum (Rutaceae) against Culex quinquefasciatus, Anopheles stephensi, and Aedes aegypti (Diptera: Culicidae) Parasitology Research. 2014;113:1775–1785. doi: 10.1007/s00436-014-3823-y. [DOI] [PubMed] [Google Scholar]
- 22.Veerakumar K, Govindrajan M, Rajeswary M, Muthukumaran U. Mosquito larvicidal properties of silver nanoparticles synthesized using Heliotropium indicum (Boraginaceae) against Aedes aegypti, Anopheles stephensi, and Culex quinquefasciatus (Diptera: Culicidae) Parasitology Research. 2014;113(6):2363–2373. doi: 10.1007/s00436-014-3895-8. [DOI] [PubMed] [Google Scholar]
- 23.Ramar G, Suman T, Elangomathavan R, Jeyasankar A. Larvicidal activity of biologically synthesised silver nanoparticles against dengue vector Aedes Aegypti (Culicidae) Discovery. 2014;9:65–68. [Google Scholar]
- 24.Marimuthu S, Rahuman AA, Kirthi AV, Santhoshkumar T, Jayaseelan C, Rajakumar G. Eco-friendly microbial route to synthesize cobalt nanoparticles using Bacillus thuringiensis against malaria and dengue vectors. Parasitology Research. 2013;112(12):4105–4112. doi: 10.1007/s00436-013-3601-2. [DOI] [PubMed] [Google Scholar]
- 25.Banu AN, Balasubramanian C, Moorthi PV. Biosynthesis of silver nanoparticles using Bacillus thuringiensis against dengue vector, Aedes aegypti (Diptera: Culicidae) Parasitology Research. 2014;113(1):311–316. doi: 10.1007/s00436-013-3656-0. [DOI] [PubMed] [Google Scholar]
- 26.Soni N, Prakash S. Efficacy of fungus mediated silver and gold nanoparticles against Aedes aegypti larvae. Parasitology Research. 2012;110(1):175–184. doi: 10.1007/s00436-011-2467-4. [DOI] [PubMed] [Google Scholar]
- 27.Soni N, Prakash S. Synthesis of gold nanoparticles by the fungus Aspergillus niger and its efficacy against mosquito larvae. Reports in Parasitology. 2012;2:1–7. [Google Scholar]
- 28.Soni N, Prakash S. Entomopathogenic fungus generated Nanoparticles for enhancement of efficacy in Culex quinquefasciatus and Anopheles stephensi . Asian Pacific Journal of Tropical Disease. 2012;2(1):S356–S361. [Google Scholar]
- 29.Soni N, Prakash S. Possible mosquito control by silver nanoparticles synthesized by soil fungus (Aspergillus niger 2587) Advances in Nanoparticles. 2013;2:125–132. [Google Scholar]
- 30.Soni N, Prakash S. Fungus generated novel nanoparticles: a new prospective for mosquito control. International Jornal of Recent Scientific Research. 2013;4:1481–1487. [Google Scholar]
- 31.Soni N, Prakash S. Microbial synthesis of spherical nanosilver and nanogold for mosquito control. Annals of Microbiology. 2014;64(3):1099–1111. [Google Scholar]
- 32.Paulkumar K, Gnanajobitha G, Vanaja M, et al. Piper nigrum leaf and stem assisted green synthesis of silver nanoparticles and evaluation of its antibacterial activity against agricultural plant pathogens. The Scientific World Journal. 2014;2014:9 pages. doi: 10.1155/2014/829894.829894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geetha AR, George E, Srinivasan A, Shaik J. Optimization of green synthesis of silver nanoparticles from leaf of Pimenta dioica (Allspice) The Sciemtific World Journal. 2013;2013:5 pages. doi: 10.1155/2013/362890.362890 [DOI] [PMC free article] [PubMed] [Google Scholar]


