Abstract
The bioburden (blood, protein, pathogens and biofilm) on flexible endoscopes after use is often high and its removal is essential to allow effective disinfection, especially in the case of peracetic acid-based disinfectants, which are easily inactivated by organic material. Cleaning processes using conventional cleaners remove a variable but often sufficient amount of the bioburden. Some formulations based on peracetic acid are recommended by manufacturers for the cleaning step. We performed a systematic literature search and reviewed the available evidence to clarify the suitability of peracetic acid-based formulations for cleaning flexible endoscopes. A total of 243 studies were evaluated. No studies have yet demonstrated that peracetic acid-based cleaners are as effective as conventional cleaners. Some peracetic acid-based formulations have demonstrated some biofilm-cleaning effects and no biofilm-fixation potential, while others have a limited cleaning effect and a clear biofilm-fixation potential. All published data demonstrated a limited blood cleaning effect and a substantial blood and nerve tissue fixation potential of peracetic acid. No evidence-based guidelines on reprocessing flexible endoscopes currently recommend using cleaners containing peracetic acid, but some guidelines clearly recommend not using them because of their fixation potential. Evidence from some outbreaks, especially those involving highly multidrug-resistant gram-negative pathogens, indicated that disinfection using peracetic acid may be insufficient if the preceding cleaning step is not performed adequately. Based on this review we conclude that peracetic acid-based formulations should not be used for cleaning flexible endoscopes.
Keywords: Peracetic acid, Cleaning, Flexible endoscope, Biofilm, Resistance, Bioburden, Blood, Disinfection, Reprocessing
Core tip: Some formulations based on peracetic acid (PAA) are recommended by manufacturers for cleaning flexible endoscopes. We reviewed 243 studies to analyse the evidence for this recommendation. No study demonstrated that PAA-based cleaners were as effective as conventional cleaners, and some PAA-based formulations had clear biofilm-fixation potential. Dried blood and nerve tissue were substantially fixed by PAA. Some outbreaks, especially of highly multidrug-resistant gram-negative pathogens, indicated that insufficient cleaning could not be compensated for by using PAA in the disinfection step. PAA-based formulations should not be used for cleaning flexible endoscopes.
INTRODUCTION
Flexible endoscopes come into contact with the mucosa and are considered as semi-critical equipment, associated with a high risk of infection[1,2]. Infections, including those due to multidrug-resistant gram-negative pathogens, quite frequently occur after gastrointestinal endoscopy[3,4]. The most common types of infections are primary sepsis or bacteraemia[3], pneumonia[3] and gastroenteritis[3], some of which may be fatal. Blood-borne infections such as hepatitis B or hepatitis C have also been described[3]. Most infections are attributed to inadequate cleaning or disinfection of the endoscope before its use on the next patient[3,5,6]. The cleaning process or disinfection step is usually described as inadequate if it deviates obviously from national evidence-based guidelines[7,8].
The processing protocols for flexible endoscopes have changed over the last few decades, with an increase in the popularity of automatic processing[9]. This is associated with advantages such as better standardization, better process validation compared with manual processing[10-17], better overall reprocessing results[18,19] and similar costs[20]. The choice of active disinfection ingredients has increased at the same time. Glutaraldehyde continues to be the main active ingredient in the disinfection step for several decades[21] and is often used for automatic processing at high temperatures such as 56 °C[22]. It is also used for processing other semi-critical medical devices such as flexible cystoscopes[23], rhinoscopy[24] and bronchoscopes[25]. However, some countries now use peracetic acid-based formulations for the disinfection step[10,14,17,26-30]. Some manufacturers of chemical processing products have recently adapted their processing protocols to recommend the use of peracetic acid-based formulations also for the cleaning step. However, the suitability of peracetic acid for cleaning remains controversial. This study aimed to review the scientific literature on all aspects of the use of peracetic acid-based formulations for cleaning flexible endoscopes, and to provide a clinically relevant summary of the possible implications for patient safety.
STUDY SELECTION
A literature review of the National Library of Medicine was performed on August 19, 2013, using various combinations of the following terms: peracetic acid, cleaning, flexible endoscope, endoscope biofilm, resistance, fixation, infection and outbreak. A total of 471 publications were identified and reviewed for their suitability regarding the topic. A total of 172 studies were considered relevant and evaluated in detail. A further 71 studies not identified by the literature search were also evaluated, e.g., guidelines, reports on side effects, additionally referenced studies or reviews (Figure 1).
Figure 1.
Flow diagram on the study selection process.
STANDARD PROTOCOL FOR PROCESSING FLEXIBLE ENDOSCOPES
Flexible endoscopes are usually processed via several steps (Table 1). The cleaning step itself comprises three steps[31]. Pre-cleaning is usually done immediately after use of the endoscope, e.g., with detergent-soaked gauze and rinsing of all channels with the cleaning agents. Pre-cleaning is a standard procedure and may be omitted only under certain conditions[32]. Secondly, brush-cleaning involves cleaning all accessible channels with a brush suited to each channel, and is followed by chemical cleaning, which involves filling all the channels with the cleaning agent for a few minutes, followed by thorough rinsing. The subsequent disinfection step varies in duration, depending on the chemical formulation used and the required spectrum of antimicrobial activity; if virucidal or mycobactericidal activity is required, the duration may be longer. Finally, the endoscope is rinsed once more and dried[33]. Double cleaning is recommended in some countries, such as France, mainly because of the risk of prion diseases[34,35].
Table 1.
Typical sequence of steps for manual and automatic reprocessing of flexible endoscopes including the typical duration of the various cleaning steps
| Manual processing | Automatic processing |
| Pre-cleaning the outer surface with a detergent-soaked single-use gauze and rinsing all channels with the cleaning agent, usually for 2 min | |
| Brush-cleaning all accessible channels with a suitable brush, usually for 3 min | |
| Rinsing | |
| Chemical cleaning; filling all channels with the cleaning agent, allowing the cleaning agents to persist inside the channel for approximately 5 min | |
| Rinsing, usually for 1 min | |
| Disinfection | |
| Final rinsing | |
| Drying | |
The cleaning step itself is considered to be difficult in flexible endoscopes because of the long, narrow lumens and multiple valves[36]. In addition, endoscope channels should be freely accessible, because limited access is associated with significantly poorer cleaning results (approximately 3%)[37]. Manual cleaning is considered less effective than automatic cleaning[38].
IMPORTANCE OF THE CLEANING STEP
There are two major reasons for performing effective cleaning before the disinfection step. First, organic and inorganic materials left on the inner and outer surfaces interfere with the efficacy of the disinfectants[39,40], given that blocked channels may remain undisinfected[41]; only a clean endoscope with clean channels can be disinfected effectively[34]. Second, cleaning of flexible endoscopes aims to reduce the bioburden as much as possible[41]. It is generally acknowledged that the cleaning, rather than the disinfection or sterilization procedure, controls the success of the endoscope[42,43] or angioscopy reprocessing procedure[44] although cleaning alone does not reduce contamination to a safe level[45].
Inadequate cleaning may reduce the efficacy of the disinfection step[46,47] finally leading to contaminated flexible endoscopes after processing, mainly with gram-negative bacteria[48]. Chemical disinfectants work by direct contact between the disinfectant and the microbe, which may be prevented by residual organic material, resulting in incomplete microbial killing[49,50]. Inadequate cleaning was regarded as a main reason in various outbreaks of nosocomial infections associated with bronchoscopy or endoscopic retrograde cholangiopancreatography (ERCP)[51-53]. The importance of optimal cleaning of flexible endoscopes for the overall reprocessing results is acknowledged as a significant issue by physicians and gastroenterology nurses[54].
CLEANING AGENTS
The cleaning agent is usually a detergent without any biocidal ingredient[35]. Some cleaning agents are enzymatic, others are non-enzymatic[55,56]. The cleaning agent should be compatible with the disinfectant agent. The entire process may then achieve a 9 log10 reduction of microorganisms in a tube simulating an endoscope channel[57]. Other processes using different types of cleaning or disinfection agents have revealed lower overall reductions, e.g., a 7 log10 reduction[58]. Lack of use of a detergent in the cleaning step in an automatic processor did not result in any viral blood-borne infections such as hepatitis B or C in 72 patients[59], indicating that the type of cleaning agent is less important in terms of the overall cleaning result for some enveloped blood-borne viruses.
CHEMICAL CHARACTERIZATION OF PERACETIC ACID
Peracetic acid is an oxygen-releasing compound and has been known as a biocidal agent for decades[60-62]. Its current use is mainly for disinfection, e.g., of flexible endoscopes or surfaces[63], sometimes in combination with 1% hydrogen peroxide[64]. In automatic processing of flexible endoscopes, it is used at concentrations of 0.2%[65], 0.35%[66] or even 1%[45], while in manual procedures it may be used at 0.2%[67]. It degrades rapidly to acetic acid and oxygen[68], and its stability is poor compared with glutaraldehyde[69], but may be prolonged by adding stabilizing agents[68]. In common with all oxygen-releasing compounds, it is inactivated by organic materials such as blood[68,70], serum[71,72], albumin[73] or a combination of organic loads[74]. It may be corrosive for a number of materials such as steel or rubber, whereas glass and some plastics are unaffected[68].
FORMULATIONS BASED ON PERACETIC ACID
Various peracetic-acid-based products for processing flexible endoscopes are available in a number of countries; some are powders, and others are liquids used as a one- or two-component system. A number of products available for manual processing are known to the authors and include: Acecide (Saraya Co. Ltd., Osaka, Japan), Gigasept PAA concentrate (Schülke and Mayr, Norderstedt, Germany), neodisher endo DIS active (Chemische Fabrik Dr. Weigert GmbH and Co. KG, Hamburg, Germany), NU Cidex (ASP, Wokingham, United Kingdom), PeraSafe (Antec International Ltd., Sudbury, United Kingdom), Scotalin (KRD, Busan, South Korea), and Sekusept aktiv (Ecolab Inc., St. Paul, MN, United States). Available products for automatic processing include: neodisher Septo PAC (Chemische Fabrik Dr. Weigert GmbH and Co. KG, Hamburg, Germany), Olympus EndoDis (Olympus Europe Holding GmbH, Hamburg, Germany), or Rapicide PA (Medivators Inc. Minneapolis, MN, United States). All these products are described as suitable for the disinfection of flexible endoscopes, but some of them are also recommended by the manufacturer for the cleaning step (Gigasept PAA concentrate, neodisher endo DIS active, and Sekusept aktiv).
PATHOGENS
Pathogens on flexible endoscopes after use
The total contamination of flexible endoscopes with pathogens is usually highest in colonoscopes, followed by gastroscopes and bronchoscopes[75]. The microbial load after patient examination was found to be between > 103 and 1010 colony-forming units (CFU) per milliliter[48,76], with highest numbers in the suction channel[77-79]. The contamination consisted mainly of gram-negative bacteria (56%) such as Pseudomonas aeruginosa, Klebsiella pneumonia and Escherichia coli, followed by gram-positive bacteria (27%) such as Staphylococcus aureus, coagulase-negative Staphylococcus and Micrococcus luteus, and yeasts (17%) such as Candida albicans and Candida tropicalis[48]. The air and water channels may, however, also be contaminated[80]. If biopsy suction channels are not adequately cleaned, remaining pathogens may contaminate single-use sterile biopsy forceps during passage[81,82].
Infected patients leave their infectious flora on the endoscope. Hepatitis B virus DNA, hepatitis C virus RNA, human immunodeficiency virus DNA and H. pylori have been found after use of endoscopes in infected patients[83-86], especially in the biopsy suction channel[87], and even after cleaning[88]. It is estimated that, on average, 4 in every 1000 endoscopies result in transmission of H. pylori[89].
Pathogens on flexible endoscopes after cleaning
The cleaning step can reduce the bioburden by 4.7 log10 CFU (gastroscopes) and 6.2 log10 CFU (colonoscopes)[76,90]. Automatic cleaning and manual cleaning resulted in a similar reduction in microbial load (4.32 and 4.24, respectively), when measured with E. faecalis and P. aeruginosa[33]. M. chelonae may be reduced by 4 log10-steps by standardized manual cleaning[91]. Automatic cleaning processes may achieve a log10-reduction of 7.0-8.4, depending on the type of washer disinfectant and cleaning agent[92].
In contaminated test tubes the cleaning step during automatic processing of flexible endoscopes shows variable results, depending on the type of process and the cleaning agent[58]. Some cleaning processes using a detergent were significantly less effective (0.3 log10-steps) than water alone (1.1-2.6 log10-steps), indicating that the entire cleaning process needs to be evaluated critically[55,56]. In contrast, other cleaning processes were significantly more effective (4.1 log10-steps)[56].
HCV is usually completely removed from the biopsy suction channel by the cleaning step alone, as demonstrated in 19 upper gastrointestinal endoscopic procedures in patients with chronic replicative hepatitis C[85]. This finding is supported by in vitro data using contaminated high-titre HCV-positive plasma for experimental contamination of flexible endoscopes[93], and by evaluation of flexible endoscopes used in patients with hepatitis C[94]. HIV was a also reduced by at least 99.93% using a detergent cleaning step alone[95].
Overall cleaning effectively reduces or eliminates many pathogens by at least 4 log as recommended[77], but substantial levels of viable bacteria may remain[78]. This suggests that the risk of transmission of nosocomial pathogens cannot be eliminated by cleaning alone[96]. Poor mechanical cleaning may be indicated by a high titre of microorganisms in a surveillance culture[97].
Effect of peracetic acid on pathogens
Antimicrobial activity: Peracetic acid is very reactive and has strong antimicrobial activity. Depending on its concentration and pH value[98], it is effective against bacteria including H. pylori, fungi, mycobacteria, viruses including hepatitis B virus, and bacterial spores[35,66,68,99-112], though for specific isolates, such as Mycobacterium gordonae, the exposure time may have to be prolonged to 20 min to achieve the required efficacy[67]. However, despite its broad spectrum of antimicrobial activity it is not suitable for sterilizing surgical instruments[113]. In combination with copper, peracetic acid is also considered to be suitable for prion decontamination[114]. The optimal pH value for its antimicrobial activity is between 2.5 and 4[68]. It is also assumed that exposure of gram-positive species such as Bacillus subtilis to chlorine dioxide enhances a stable cross-resistance to other oxidizing agents, such as peracetic acid[74], as confirmed by Bridier et al[115]. The efficacies of different formulations differ remarkably compared with solutions of the active ingredient alone[116].
Cellular changes to sublethal concentrations: Bacterial resistance to biocides is apparently increasing, although peracetic acid has not been implicated in the selection and persistence of bacterial strains with low-level antibiotic resistance[117]. Exposure of nosocomial pathogens to peracetic acid at a sublethal concentration (e.g., 1 mmol/L) has been reported to induce a cellular response in S. aureus. This response includes the induction of many virulence-factor genes upon exposure, suggesting stimulation of pathogenesis in response to peracetic acid[118]. Other effects included significant alterations in the regulation of membrane-transport genes, selective induction of DNA-repair and -replication genes, and differential repression of primary metabolism-related genes between the two growth states[118]. Similar reactions were observed after exposure of P. aeruginosa to a sublethal concentration (e.g., 1 mmol/L) of peracetic acid: many genes associated with cellular protective processes were induced, while transcription of genes involved in primary metabolic pathways was repressed, and that of genes encoding membrane proteins and small molecule transporters was altered[119]. In terms of E. coli O157:H7, a sublethal concentration of peracetic acid (0.1%) induced a substantial increase in peroxidative tolerance[120]. Finally, a strain of Salmonella typhimurium exposed to a sublethal concentration of peracetic acid (e.g., 15 mg/L) showed modified physiological characteristics: the cells remained viable but were unable to be cultured, but retained their virulence, as shown by their adhesive and invasive capacities[121]. A higher concentration of peracetic acid (e.g., 20 mg/L) resulted in bacterial death. This study indicated that a negative culture result from an endoscope does not exclude the presence of pathogens on the endoscope, and transmission may occur if the bacterial cells modify their physiological characteristics, e.g., by exposure to sublethal concentrations of peracetic acid.
BIOFILM
General background
Biofilms are communities of cells that are attached to an abiotic or living surface embedded in an extracellular polymeric substance[122,123]. They are preferentially formed in wet environments (e.g., insufficient drying of endoscopes before storage[124,125]), can form under different flow conditions[126,127] and can be potential sources of contamination and infection[128]. Virtually all bacterial species can form biofilm including clinically-relevant ones such as P. aeruginosa, S. aureus, E. coli and Clostridium difficile[123,129,130]. Under natural environmental conditions, biofilms are likely to be composed of a mixture of different species[131,132]. In the laboratory, they can be grown on various materials and devices, including polystyrene microtitre plates[133-136], haemolysis glass tubes[137,138], stainless steel coupons[134,139] and also in Teflon tubes[140-143], similar to endoscope channels.
Resistance of biofilm bacteria
One feature of many biofilm bacteria is their resistance to some antibiotics and disinfectants ([144-147] and reviewed in[148,149]). Artificial P. aeruginosa biofilms resisted treatment with various biocidal agents including peracetic acid, compared with their planktonic counterparts[150-152]. Biofilms composed of E. coli[152,153], S. aureus[152,154,155], Mycobacterium fortuitum[156] or Listeria monocytogenes[157] also resisted treatment with diverse biocides compared with planktonic cells. Bacteria in mature (old) biofilms were more resistant to killing than those in young biofilms[153,158,159]. An older biofilm of P. aeruginosa required up to 20-fold higher concentrations of peracetic acid (0.2%) to be eradicated, compared with their planktonic counterparts (0.01%)[151]. Similar results were found with an E. coli biofilm and peracetic acid/H2O2[153]. The resistance of biofilms can often further increase when the communities are composed of more than one bacterial species[134,136,160-163] which may include resistance against 0.35% peracetic acid, which is a concentration used in many formulations[133]. Especially “build-up” biofilms mimicking repeated endoscope reprocessing cycles exhibited a significantly higher survival rate than ‘traditional’ biofilms[158]. The mechanisms underlying disinfectant-resistant phenotypes appear to be multifactorial[133,148,151,153,164].
Biofilm on flexible endoscopes
Direct evidence for extensive biofilm contamination was provided in 1 of 13 investigated biopsy suction channels and 5 of 12 air/water channels of reprocessed endoscopes[165]. Some reports showed persistent levels of bacteria in endoscope channels, despite reprocessing according to published guidelines, providing indirect evidence for contamination by biofilms[166-168]. Residual biofilm can be seen in Figure 2. In one case, a colonoscope was contaminated with a total of 195 bacteria despite six rounds of reprocessing[168]. Treatment with a cleaning agent that had previously been shown to remove biofilms from endoscope tubes[142] was capable of eradicating the microbes almost completely, indicating that the presence of biofilm was the main reason for ongoing bacterial contamination[168]. Biofilms were also found in washer disinfectors resulting in contamination of automatically-processed endoscopes, e.g., with Mycobacterium chelonae[169,170], Methylobacterium mesophilicum[170] or P. aeruginosa[171], some giving rise to nosocomial infections[171]. Biofilm formation and fixation should therefore also be avoided in washer disinfectors[172]. If biofilms are not thoroughly removed from endoscope channels by cleaning, subsequent disinfection might fail, enabling microorganisms to persist. Further, efficient interchange of plasmids might occur in biofilms, including those coding for antibiotic resistance such as cefotaxime- or aminoglycoside-resistance[173-176].
Figure 2.
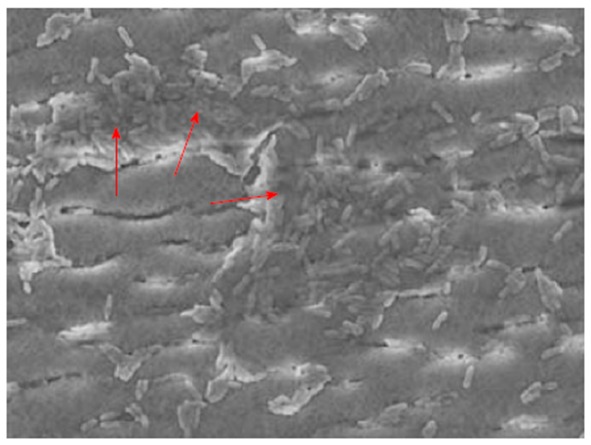
Residual biofilm after exposure to 0.09%-0.15% peracetic acid, as shown by Balsamo et al[141]. Reproduced by kind permission of the publisher.
Biofilm on flexible endoscopes after cleaning
Shear stress was found to remove some biofilms, though 24% and 47% of the biofilm masses, respectively, remained attached[177]. Brushing a silicone tube 10 times with a sterile brush was found to completely remove a multispecies biofilm that had developed over a period of 50 d[178].
Commercial detergents show variable results on biofilm removal[179]. A non-enzymatic detergent yielded a significantly higher log10-reduction (4.13 to 4.17 log10-reduction) of residual wall E. coli biofilm bacteria than the enzymatic detergents (0.74 to 0.88 log10-reduction), whilst contact time (3, 5 or 7 min) had no significant impact[180]. Similar results on different cleaners were reported by Fang et al[181] and Vickery et al[182]. Quantification of endotoxin levels also revealed better results for a non-enzymatic cleaner in terms of biofilm reduction[183]. A non-enzymatic cleaner continued to remove more biofilm with an increasing number of wash/contamination cycles: by the 20th cycle, 90% of the tubing was biofilm-free[184].
New cleaning formulations based on phosphates, hydrates, minerals and surfactants were developed several years ago[142]. These formulations effectively removed multispecies biofilms from Teflon tubes, prevented the growth of new biofilms in endoscopes, and established biofilms were completely removed from endoscopes by sequential washing with an enzymatic solution and a bleach-enriched version of the new cleaning formulations[142]. Three repeats of a reprocessing of more than 1 h using sequential application of these cleaning components almost completely removed biofilms from flexible endoscopes that had been used in patients, and were persistently contaminated with bacteria despite six rounds of reprocessing[168]. The practicality of this procedure, however, remains doubtful.
Effect of peracetic acid on biofilm
Treatments with aldehyde, peracetic acid plus detergent, or chlorine failed to disturb or remove biofilm, despite a significant log reduction in biofilm bacteria[178]. Biofilm in a water line in a dental unit with permanent water contact was effectively removed by a peracetic acid flush (0.26%)[185], but this has no correlate in endoscope processing. P. aeruginosa biofilms remained in an endoscope prototype in 76.2% of tested tube segments after cleaning followed by manual peracetic acid (0.09%-0.15%) processing and in 23.8% after cleaning followed by automatic peracetic acid processing[141]. The same processes with glutaraldehyde (2%) revealed lower rates of 71.4% after manual processing and 4.8% after automatic processing[141]. Protein in a P. aeruginosa biofilm could be removed by peracetic acid by 41%. The removal is much lower from mature biofilms or biofilms subjected to repeated peracetic acid treatments, which may modify biofilm structure[143]. At the same time, the biofilm was partially fixed and accumulated after exposure to two peracetic acid-based formulations[143]. Fixation rates varied between formulations within the same chemical group[143]. Four peracetic acid-based products were reported, two of which fixed artificial biofilms quite strongly, while the other two containing additional quaternary ammonium compounds showed no biofilm fixation[138]. An E. coli biofilm exposed to three different peracetic acid-based formulations (one with peracetic acid, one with additional non-ionic surfactant, and one with additional cationic surfactant) was partly removed by two formulations, and not fixed by any of the three formulations[137].
Finally, sublethal concentrations of chlorine dioxide, an active compound used for disinfection of endoscopes, may accelerate formation of B. subtilis or P. aeruginosa biofilms compared with biofilms grown in the absence of chlorine dioxide[186]. A similar effect can be expected with other oxygen-releasing compounds.
BLOOD
Blood on flexible endoscopes after use
Contamination of flexible endoscopes with blood is to be expected, e.g., after biopsy or in the case of variceal gastrointestinal bleeding. It is also common in other types of endoscopic procedures[187]. After different types of endoscopic procedures, suction channels contain haemoglobin at a concentration of 85 μg/cm2[78]. Residual blood may contain blood-borne viral pathogens[83,84,87,88] and may impair the efficacy of the subsequent disinfection step[44,68,70,188].
Blood on flexible endoscopes after cleaning
Detergent-based formulations are capable to remove between 88% and 95% of dried blood while peracetic acid-based formulations only removed 8%-59% depending on the type of formulation[183,189]. These results indicate that dried blood is not removed as easily by peracetic acid-based formulations compared with detergent-based formulations.
Effect of peracetic acid on blood
At the same time, however, the rate of fixation of blood exposed to the same peracetic acid-based formulations was between 19% and 78%[189], indicating that the remaining blood is fixed and cannot be easily removed. A similar effect can be seen on clinically used endoscopes containing organic contamination fixed by glutaraldehyde disinfectant solution: 20 cleaning cycles using a buffered peracetic acid procedure removed 30%-50% of the contamination[190]. These data highlight the need to avoid contact between organic contaminant and agents with fixation properties, because subsequent removal may be difficult.
OTHER ORGANIC CONTAMINATION
Organic contamination on flexible endoscopes after use
Suction channels may contain proteins at a concentration of 115 μg/cm2 after endoscopic procedures[78].
Organic contamination on flexible endoscopes after cleaning
Organic contamination may remain after cleaning. It was reported that 95 out of 504 samples obtained before disinfection and tested for adenosine triphosphate were above the benchmark values (200 relative light units [RLUs])[191], indicating inadequate cleaning[192]. Levels may be as high as 10417 RLUs on the exterior endoscope surface, or 30281 RLUs on the biopsy suction channel rinsates[193].
Haemoglobin and protein may also remain after cleaning. A channel is considered clean if the haemoglobin level is < 2.2 μg/cm2 and the protein level is < 6.4 μg/cm2[194]. If all these parameters are fulfilled, the ATP level will be < 200 RLUs[191] which can be considered a validated benchmark from patient endoscopes[195].
Overall, most of the organic contamination is usually removed below benchmark by detergent-based cleaning procedures, although exceptions may occur[196].
Effect of peracetic acid on organic contamination
Peracetic acid used for high-level disinfection of duodenoscopes yielded significantly lower levels of protein (4.2 μg/mL vs 10.1 μg/mL), carbohydrate (18.5 μg/mL vs 111.1 μg/mL) and endotoxin (2.8 EU/mL vs 44.5 EU/mL) in the biopsy suction channels compared with processes using glutaraldehyde[197]. Despite the differences between the two active agents used only for the disinfection step, the authors concluded there may be a cumulative build-up of organic material components on the inner lumen of the biopsy suction channels of endoscopic retrograde cholangiopancreatography scopes in use[197]. An outbreak of eight fatal cases of Serratia odorifera septicemia was caused by contaminated parenteral nutrition fluid due to inadequate cleaning of the surfaces prior to the use of peracetic acid[198]. Dialyzers cleaned with peracetic acid showed significantly lower clearance of larger dextrans as a result of the presence of residual proteins on or within the membrane[199]. Similar findings were reported with a product containing hydrogen peroxide and peroxyacetic acid, compared with one containing sodium hypochlorite[200].
Special case: effect of peracetic acid on nerve tissue
Exposure of brain homogenate to peracetic acid (1500 ppm for 20 min) is associated with a very high protein fixation rate of 96%, which is much higher than with exposure to glutaraldehyde (19%)[201]. Mice inoculated with variant Creutzfeld-Jacob disease (vCJD)-infective brain homogenate previously exposed to peracetic acid survived on average 291 d, which was significantly shorter than mice inoculated with negative control homogenate (> 450 d). Mice inoculated with vCJD-infective brain homogenate previously exposed to glutaraldehyde (2% for 20 min) survived longer compared with the peracetic acid group (mean: 324 d), demonstrating a clinical correlate of the almost complete fixation of brain homogenate protein by peracetic acid[201].
OUTBREAKS AND PSEUDO-OUTBREAKS
Outbreaks and pseudo-outbreaks connected with peracetic acid-based processing of flexible endoscopes are summarized in Table 2. In some outbreaks peracetic acid was used for the cleaning step[202], the cleaning and disinfection step[203], the disinfection step[124,125,203-205] or generally for processing/washing[206,207]. The reasons for the infections were insufficient (initial) cleanin[124,125,202-204], inadequate drying prior to storage[124,125,207], shortening of the immersion time and brushing time[124], insufficient channel flushing[124], a problem with the washer disinfector[203], presence of biofilm on undamaged channels[205], an endoscope defect[203], delayed pre-wash resulting in drying of the gastroscope[207], and incorrect connectors joining the bronchoscope suction channel to the STERIS SYSTEM 1 processor[206]. Strict adherence to infection control guidelines for reprocessing endoscopes is therefore the key element for prevention of endoscope-associated outbreaks[203].
Table 2.
Outbreaks and pseudo-outbreaks reported in connection with biofilm or peracetic acid-based processing of flexible endoscopes
| Number/type of infection(s) | Pathogen(s) | Type of endoscopic procedure | Reason for outbreak / pseudo-outbreak | Peracetic acid-based formulations were used for | Ref. |
| None (pseudo-outbreak) | Pseudomonas aeruginosa | Gastroscopy, bronchoscopy | Suboptimal duration of glutaraldehyde application during disinfection; “resistance” to glutaraldehyde may have been enhanced by manual cleaning with peracetic acid-based disinfectant[214] | Cleaning step | [202] |
| 2: infection (not further specified)3: colonization | OXA-48 Klebsiella pneumoniae | Bronchoscopy | A problem with the washer disinfector or the cleaning procedure was assumed as the reason | Cleaning step and disinfection step (Gastmeier P, personal communication) | [203] |
| 4: pneumonia (3 cases); colonization (1 case) | MDR Pseudomonas aeruginosa | Gastroscopy | Insufficient initial cleaning, shortening of the immersion time and brushing time, insufficient channel flushing, and inadequate drying prior to storage | Disinfection step | [124] |
| 4: bacteraemia, biliary tract infection, respiratory tract infection9: colonisation | KPC-2 Klebsiella pneumoniae | Duodenoscopy | Contaminated duodenoscope; reason for outbreak: inadequate cleaning | Disinfection step | [204] |
| 8: bloodstream infection4: biliary tract infection4: colonization | ESBL Klebsiella pneumoniae (CTX-M-15) | ERCP | Insufficient manual cleaning, insufficient drying after processing | Disinfection step | [125] |
| 3: sepsis | Pseudomonas aeruginosa | ERCP | Presence of biofilm on undamaged channels | Disinfection step (Kovaleva J; personal communication) | [205] |
| 5: infection (not further specified) 9: colonization | OXA-48 Klebsiella pneumoniae | Duodenoscopy | One endoscope had probably a defect resulting in insufficient disinfection | Disinfection step (Gastmeier P, personal communication) | [203] |
| 18: pulmonary infection (4 cases, one of them died); colonization (14 cases) | Imipenem-resistant Pseudomonas aeruginosa | Bronchoscopy | Incorrect connectors joining the bronchoscope suction channel to the STERIS SYSTEM 1 processor | “Automatic processing” | [206] |
| 2: bacteremia and biliary tract infection 4: colonization | KPC-2 Klebsiella pneumoniae | Gastroscopy | Delayed pre-wash resulting in drying of the gastroscope; short drying period after the peracetic acid treatment resulting in incomplete drying | “Wash” | [207] |
ERCP: Endoscopic retrograde cholangiopancreatography.
CLINICAL SIDE EFFECTS OF PERACETIC ACID
The potential health risks associated with all high-level disinfectants are considered to be serious, though little is known about the risks to humans, especially employees, from glutaraldehyde alternatives[208,209]. Gutterman et al[209] identified only eight studies “which reported numerous adverse outcomes to healthcare personnel associated with endoscope reprocessing”, including one case report with asthma for workers using a peracetic acid and hydrogen peroxide based product. The most commonly-reported side effect of peracetic acid in patients is a form of colitis, previously known as pseudolipomatosis[210], which is commonly induced by hydrogen peroxide and peracetic acid but occasionally also by glutaraldehyde[211]. The colitis is often self-limiting but sometimes requires medical treatment. The frequency of colitis caused by peracetic acid might be underestimated[212]. An overview of all reported cases is summarized in Table 3.
Table 3.
Adverse effects after processing with peracetic acid after endoscopy
| Number of cases | Type of reaction | Possible explanation | Ref. |
| 10 | Colitis | Unclear, reprocessing with PAA, but afterwards channels were flushed with hydrogen peroxide | [210] |
| 1 | Colitis | PAA residues in the biopsy suction channel | [215] |
| 2 | Colitis | Defect of automatic rinsing of a channel | [216] |
| 1 | Colitis | Channel not flushed | [217] |
| 1 | Colitis | Inadequate rinsing of a channel | [212] |
| No number provided | Pseudolipomatosis | Air channels not rinsed | [218] |
| 4 | Colitis | Programming error in the automatic disinfection device, related to the air/water channels | [219] |
| 12 | Colonic mucosal pseudolipomatosis | Rinsing was not done as recommended | [220] |
REVIEW OF NATIONAL AND INTERNATIONAL GUIDELINES
An overview of 17 guidelines from 14 different institutions is given in Table 4. Most institutions make no statement on the suitability of peracetic acid for cleaning flexible endoscopes, but there seems to be a recent trend in a few institutions to either skip their earlier recommendations of peracetic acid (ESGE/ESGNA and WGO/WEO) or to state that it is not suitable for cleaning (RKI).
Table 4.
Overview of evidence-based guidelines for processing flexible endoscopes, focusing on the use of peracetic acid during the cleaning step
| Institution | Guidelines | Year | Use of peracetic acid for cleaning |
| AORN | Recommended practices for cleaning and processing endoscopes and endoscope accessories[221,222] | 2012 | No recommendation |
| APIC | APIC guidelines for infection prevention and control in flexible endoscopy. Association for Professionals in Infection Control[223] | 2000 | No recommendation |
| APSIC | The ASEAN Guidelines for disinfection and sterilization of instruments in health care facilities[224] | 2012 | No recommendation |
| ASGE | Multisociety guidelines on reprocessing flexible gastrointestinal endoscopes: 2011[225,226] | 2011 | No recommendation |
| BC Ministry of Health | Best Practice Guidelines For Cleaning, Disinfection and Sterilization of Critical and Semi-critical Medical Devices[227] | 2011 | No recommendation |
| BSG | BSG Guidelines for Decontamination of Equipment for Gastrointestinal Endoscopy[228] | 2008 | No recommendation |
| CDC | Guidelines for Disinfection and Sterilization in Healthcare Facilities, 2008[229] | 2008 | No recommendation |
| ESGE/ESGENA | 1ESGE/ESGENA Technical Note on Cleaning and Disinfection[230] | 2003 | Recommended |
| ESGE/ESGENA | ESGE-ESGENA guideline: Cleaning and disinfection in gastrointestinal endoscopy, update 2008[231] | 2008 | No recommendation |
| HPS | Endoscope Reprocessing: Guidance on the Requirements for Decontamination Equipment, Facilities and Management[232] | 2007 | No recommendation |
| JGETS | Guidelines for cleaning and disinfecting endoscopes - Second edition[233] | 2004 | No recommendation |
| Public Health Agency of Canada | Infection Prevention and Control Guideline for Flexible Gastrointestinal Endoscopy and Flexible Bronchoscopy[234] | 2010 | No recommendation |
| RKI | 2Hygiene requirements for reprocessing of medical devices[235] | 2001 | No recommendation |
| RKI | Hygiene requirements for reprocessing of medical devices[236] | 2012 | Not recommended |
| SGNA | Standards of Infection Control in Reprocessing of Flexible Gastrointestinal Endoscopes[237] | 2013 | No recommendation |
| WGO/OMED | WGO/OMED Practice Guideline Endoscope Disinfection[238] | 2005 | Recommended |
| WGO/WEO | Endoscope disinfection - a resource-sensitive approach[239] | 2011 | No recommendation |
These guidelines were updated in 2008 by guidelines[231];
These guidelines were updated in 2012 by guidelines[236]. AORN: Association of periOperative Registered Nurses; APIC: Association for Professionals in Infection Control and Epidemiology; APSIC: Asia Pacific Society of Infection Control; ASGE: American Society for Gastrointestinal Endoscopy; BSG: British Society of Gastroenterology; CDC: Centers for Disease Control and Prevention; ESGE: European Society of Gastrointestinal Endoscopy; ESGENA: European Society of Gastroenterology and Endoscopy Nurses and Associates; HPS: Health Protection Scotland; JGETS: Japanese Gastroenterological Endoscopy Technicians Society; OMED: Organisation Mondiale d'Endoscopie Digestive/World Organization for Digestive Endoscopy; RKI: Robert Koch Institute; SGNA: Society of Gastroenterology Nurses and Associates, Inc; WEO: World Endoscopy Organization (former OMED); WGO: World Gastroenterology Organisation.
CONCLUSION
Few national and international guidelines highlight the need for the cleaning of flexible endoscopes to be carried out using formulations without any fixation potential, but use of peracetic acid for cleaning is discouraged. Some peracetic acid-based formulations have some cleaning capacity. However, we found no conclusive evidence to suggest that the cleaning capacity of any peracetic acid-based formulation was as good as that of detergent-based cleaning agents without biocidal agents. Different peracetic acid-based formulations have been shown to enhance surface fixation of dried blood (all tested formulations), biofilm (some tested formulations) and brain tissue (all tested formulations). Fixed blood and biofilm are likely to impair the efficacy of the disinfection step, given that peracetic acid is known to lose its antimicrobial activity in the presence of various types of organic load. Fixed biofilm will reduce the susceptibility of microorganisms present in the biofilm, making it more difficult to achieve the required log-reduction during the disinfection phase. Even if the bacteria within a biofilm are killed by a disinfectant, microorganisms are likely to adhere to any residual biofilm structure within the endoscope more easily during the next endoscopic procedure.
Published research suggests that peracetic acid-based agents are not suitable for use in the cleaning step during the processing of flexible endoscopes (Table 5). However, some practical tips may help to improve the quality of the cleaning step (Table 6). This review highlights that protocols for processing flexible endoscopes should be evidence-based, rather than being based on convenience[213].
Table 5.
Effects and possible outcomes of peracetic acid use for cleaning flexible endoscopes
| Characteristic, reason for cleaning step | Effect of peracetic acid | Possible outcome, compared with classical cleaning |
| Removal of biofilm | Variable1 | Insufficient removal of biofilm |
| Fixation of biofilm | Possible1 | Fixation of biofilm to variable degrees |
| Removal of dried blood | Partial removal1 | Insufficient removal of dried blood |
| Fixation of dried blood | Very likely | Fixation of dried blood to variable degrees |
| Fixation of brain tissue | Very likely | Strong fixation of nerve tissue, including prions |
| Adaptation of microorganisms surviving the cleaning step | Likely, especially in gram-negative bacteria | Insufficient efficacy of disinfection step, persistence of pathogens, beginning of biofilm formation |
| Cross-resistance to other biocidal compounds as a result of exposure to sublethal peracetic acid concentrations | Possible | Insufficient efficacy of disinfection step, persistence of pathogens, beginning of biofilm formation |
Depending on the formulation.
Table 6.
Practical tips to ensure optimal cleaning of flexible endoscopes
| Clinical practice tip | Major advantage | Ref. |
| Clean promptly after use | No drying of organic material such as blood | [77,207] |
| Follow the instructions of the endoscope manufacturer as closely as possible (e.g., type of brush or cleaning adapter) | Optimum cleaning of an entire channel | |
| Prefer washer disinfectors with a monitoring system indicating channel blockage | A blocked channel cannot be cleaned adequately and is immediately identified; targeted brush cleaning may be necessary | |
| Do not switch off the monitoring system for detection of blocked channels | Channels may be blocked and inadequately cleaned; personnel may not detect blocked channels with all possible implications for patient safety | |
| Support by gastroenterologist | It is strongly recommended that the clinician fully understands the cleaning and disinfection steps and does not inhibit his or her staff's ability to perform them correctly | [240] |
| Allow external audits by local health authorities on the quality of processing including cleaning | Implementation of guidelines may be more successful if the local health authorities visit the endoscopy units and compare current practices with the relevant guidelines. This effect seems to be more easily achieved in in-patient rather than in out-patient endoscopy units | [241-243] |
Footnotes
P- Reviewer: Albuquerque A, Camellini L, Sofi A, Wang YH S- Editor: Song XX L- Editor: A E- Editor: Zhang DN
References
- 1.Rutala WA, Weber DJ. Sterilization, high-level disinfection, and environmental cleaning. Infect Dis Clin North Am. 2011;25:45–76. doi: 10.1016/j.idc.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 2.Leiss O, Niebel J, Exner M. [Risk of infection in endoscopy] Leber Magen Darm. 1995;25:198–202. [PubMed] [Google Scholar]
- 3.Kovaleva J, Peters FT, van der Mei HC, Degener JE. Transmission of infection by flexible gastrointestinal endoscopy and bronchoscopy. Clin Microbiol Rev. 2013;26:231–254. doi: 10.1128/CMR.00085-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franchi D, Bahrani A, Ober JF, Edmond MB. Preventing nosocomial infections from gastrointestinal endoscopy. Curr Gastroenterol Rep. 2000;2:294–298. doi: 10.1007/s11894-000-0021-0. [DOI] [PubMed] [Google Scholar]
- 5.Barbosa JM, Souza AC, Tipple AF, Pimenta FC, Leão LS, Silva SR. Endoscope reprocessing using glutaraldehyde in endoscopy services of Goiânia, Brazil: a realidade em serviços de endoscopia de Goiânia, GO. Arq Gastroenterol. 2010;47:219–224. doi: 10.1590/s0004-28032010000300002. [DOI] [PubMed] [Google Scholar]
- 6.Spach DH, Silverstein FE, Stamm WE. Transmission of infection by gastrointestinal endoscopy and bronchoscopy. Ann Intern Med. 1993;118:117–128. doi: 10.7326/0003-4819-118-2-199301150-00008. [DOI] [PubMed] [Google Scholar]
- 7.Nelson DB. Recent advances in epidemiology and prevention of gastrointestinal endoscopy related infections. Curr Opin Infect Dis. 2005;18:326–330. doi: 10.1097/01.qco.0000171925.47452.8f. [DOI] [PubMed] [Google Scholar]
- 8.Nelson DB. Infection control during gastrointestinal endoscopy. J Lab Clin Med. 2003;141:159–167. doi: 10.1067/mlc.2003.24. [DOI] [PubMed] [Google Scholar]
- 9.Exner M, Leiss O, Tuschewitzki GJ. [Hygienic measures in endoscopy] Z Gastroenterol. 1990;28:635–643. [PubMed] [Google Scholar]
- 10.Soares JB, Gonçalves R, Banhudo A, Pedrosa J. Reprocessing practice in digestive endoscopy units of district hospitals: results of a Portuguese National Survey. Eur J Gastroenterol Hepatol. 2011;23:1064–1068. doi: 10.1097/MEG.0b013e328348d5d6. [DOI] [PubMed] [Google Scholar]
- 11.Pineau L, Roques C, Luc J, Michel G. Automatic washer disinfector for flexible endoscopes: a new evaluation process. Endoscopy. 1997;29:372–379. doi: 10.1055/s-2007-1004218. [DOI] [PubMed] [Google Scholar]
- 12.Desilets D, Kaul V, Tierney WM, Banerjee S, Diehl DL, Farraye FA, Kethu SR, Kwon RS, Mamula P, Pedrosa MC, et al. Automated endoscope reprocessors. Gastrointest Endosc. 2010;72:675–680. doi: 10.1016/j.gie.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 13.Ofstead CL, Wetzler HP, Snyder AK, Horton RA. Endoscope reprocessing methods: a prospective study on the impact of human factors and automation. Gastroenterol Nurs. 2010;33:304–311. doi: 10.1097/SGA.0b013e3181e9431a. [DOI] [PubMed] [Google Scholar]
- 14.Spinzi G, Fasoli R, Centenaro R, Minoli G. Reprocessing in digestive endoscopy units in Lombardy: results of a regional survey. Dig Liver Dis. 2008;40:890–896. doi: 10.1016/j.dld.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 15.Heeg P. Reprocessing endoscopes: national recommendations with a special emphasis on cleaning--the German perspective. J Hosp Infect. 2004;56 Suppl 2:S23–S26. doi: 10.1016/j.jhin.2003.12.034. [DOI] [PubMed] [Google Scholar]
- 16.Leiss O, Exner M, Niebel J. [Preventing transmission of infection in endoscopy: hygienic maintainance of flexible endoscopes and measures for personal protection] Leber Magen Darm. 1995;25:251–257. [PubMed] [Google Scholar]
- 17.Fraser VJ, Zuckerman G, Clouse RE, O’Rourke S, Jones M, Klasner J, Murray P. A prospective randomized trial comparing manual and automated endoscope disinfection methods. Infect Control Hosp Epidemiol. 1993;14:383–389. doi: 10.1086/646766. [DOI] [PubMed] [Google Scholar]
- 18.Birkner BR, Bader L, Blumenstock G, Riemann JF, Selbmann HK. [Quality of hygiene in endoscope reprocessing--the fundamentals of indicator-assisted quality management in gastroenterology] Z Arztl Fortbild Qualitatssich. 2003;97:227–232. [PubMed] [Google Scholar]
- 19.Bader L, Blumenstock G, Birkner B, Leiss O, Heesemann J, Riemann JF, Selbmann HK. [HYGEA (Hygiene in gastroenterology--endoscope reprocessing): Study on quality of reprocessing flexible endoscopes in hospitals and in the practice setting] Z Gastroenterol. 2002;40:157–170. doi: 10.1055/s-2002-22326. [DOI] [PubMed] [Google Scholar]
- 20.Shields N. A survey of the costs of flexible endoscope cleaning and disinfection. Gastroenterol Nurs. 1993;16:53–60. doi: 10.1097/00001610-199310000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X, Kong J, Tang P, Wang S, Hyder Q, Sun G, Zhang R, Yang Y. Current status of cleaning and disinfection for gastrointestinal endoscopy in China: a survey of 122 endoscopy units. Dig Liver Dis. 2011;43:305–308. doi: 10.1016/j.dld.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 22.Zühlsdorf B, Winkler A, Dietze B, Floss H, Martiny H. Gastroscope processing in washer-disinfectors at three different temperatures. J Hosp Infect. 2003;55:276–282. doi: 10.1016/j.jhin.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 23.Malavaud S, Boiteux JP, Coloby P, Bugel H, Verine JL, Conquy S, Doublet JD, Bruyère F. [Flexible cystoscopes: disinfection and microbiological surveillance practices among French urologists] Prog Urol. 2012;22:731–735. doi: 10.1016/j.purol.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 24.Kutter J, Blanc D, Lang FJ. [Residual bacterial contamination of rhinoscopes used in ENT consultation after cleaning with a pad impregnated with a disinfectant] Schweiz Med Wochenschr. 2000;Suppl 125:48S–51S. [PubMed] [Google Scholar]
- 25.Wallace CG, Agee PM, Demicco DD. Liquid chemical sterilization using peracetic acid. An alternative approach to endoscope processing. ASAIO J. 1995;41:151–154. [PubMed] [Google Scholar]
- 26.Mannion PT. The use of peracetic acid for the reprocessing of flexible endoscopes and rigid cystoscopes and laparoscopes. J Hosp Infect. 1995;29:313–315. doi: 10.1016/0195-6701(95)90281-3. [DOI] [PubMed] [Google Scholar]
- 27.Babb JR, Bradley CR. Endoscope decontamination: where do we go from here? J Hosp Infect. 1995;30 Suppl:543–551. doi: 10.1016/0195-6701(95)90061-6. [DOI] [PubMed] [Google Scholar]
- 28.Gorse GJ, Messner RL. Infection control practices in gastrointestinal endoscopy in the United States: a national survey. Gastroenterol Nurs. 1991;14:72–79. doi: 10.1097/00001610-199110000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Tandon RK. Disinfection of gastrointestinal endoscopes and accessories. J Gastroenterol Hepatol. 2000;15 Suppl:G69–G72. doi: 10.1046/j.1440-1746.2000.02268.x. [DOI] [PubMed] [Google Scholar]
- 30.Baker K, McCullagh L. Comparison of actual and recommended ENT endoscope disinfection practices, by geographical regions in the United States. ORL Head Neck Nurs. 1997;15:14–17. [PubMed] [Google Scholar]
- 31.Gillespie EE, Kotsanas D, Stuart RL. Microbiological monitoring of endoscopes: 5-year review. J Gastroenterol Hepatol. 2008;23:1069–1074. doi: 10.1111/j.1440-1746.2007.05264.x. [DOI] [PubMed] [Google Scholar]
- 32.Alfa MJ, DeGagne P, Olson N, Fatima I. EVOTECH endoscope cleaner and reprocessor (ECR) simulated-use and clinical-use evaluation of cleaning efficacy. BMC Infect Dis. 2010;10:200. doi: 10.1186/1471-2334-10-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alfa MJ, Olson N, DeGagne P. Automated washing with the Reliance Endoscope Processing System and its equivalence to optimal manual cleaning. Am J Infect Control. 2006;34:561–570. doi: 10.1016/j.ajic.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 34.Darbord JC. Importance of cleaning for reprocessing endoscopes and thermolabile sterile medical devices: French use and regulations. J Hosp Infect. 2004;56 Suppl 2:S40–S43. doi: 10.1016/j.jhin.2003.12.028. [DOI] [PubMed] [Google Scholar]
- 35.Mignard JP. [Endoscope disinfection] Ann Urol (Paris) 2006;40 Suppl 3:S91–S93. doi: 10.1016/s0003-4401(06)80031-8. [DOI] [PubMed] [Google Scholar]
- 36.Moreno Fernández M, Sancliment Guitart S. [Cleaning and disinfecting flexible endoscopes] Rev Enferm. 2004;27:60–62. [PubMed] [Google Scholar]
- 37.Dietze B, Kircheis U, Schwarz I, Martiny H. Freely accessible endoscope channels improve efficacy of cleaning. Endoscopy. 2001;33:523–528. doi: 10.1055/s-2001-14959. [DOI] [PubMed] [Google Scholar]
- 38.Wu MS, Wang JT, Yang JC, Wang HH, Sheu JC, Chen DS, Wang TH. Effective reduction of Helicobacter pylori infection after upper gastrointestinal endoscopy by mechanical washing of the endoscope. Hepatogastroenterology. 1996;43:1660–1664. [PubMed] [Google Scholar]
- 39.Knieler R. Manual cleaning and disinfection of flexible endoscopes--an approach to evaluating a combined procedure. J Hosp Infect. 2001;48 Suppl A:S84–S87. doi: 10.1016/s0195-6701(01)90020-9. [DOI] [PubMed] [Google Scholar]
- 40.Hanson PJ. AIDS: practising safe endoscopy. Baillieres Clin Gastroenterol. 1990;4:477–494. doi: 10.1016/0950-3528(90)90013-7. [DOI] [PubMed] [Google Scholar]
- 41.Martiny H, Floss H, Zühlsdorf B. The importance of cleaning for the overall results of processing endoscopes. J Hosp Infect. 2004;56 Suppl 2:S16–S22. doi: 10.1016/j.jhin.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 42.Chu NS, Favero M. The microbial flora of the gastrointestinal tract and the cleaning of flexible endoscopes. Gastrointest Endosc Clin N Am. 2000;10:233–244. [PubMed] [Google Scholar]
- 43.Leiss O, Bader L, Mielke M, Exner M. [Five years of the Robert Koch Institute guidelines for reprocessing of flexible endoscopes. A look back and a look forward] Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2008;51:211–220. doi: 10.1007/s00103-008-0451-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chaufour X, Deva AK, Vickery K, Zou J, Kumaradeva P, White GH, Cossart YE. Evaluation of disinfection and sterilization of reusable angioscopes with the duck hepatitis B model. J Vasc Surg. 1999;30:277–282. doi: 10.1016/s0741-5214(99)70138-2. [DOI] [PubMed] [Google Scholar]
- 45.Baas EU. [Automatic disinfection of fiberendoscopes (author’s transl)] Zentralbl Bakteriol Orig B. 1977;165:458–463. [PubMed] [Google Scholar]
- 46.Burdick JS, Hambrick D. Endoscope reprocessing and repair costs. Gastrointest Endosc Clin N Am. 2004;14:717–724, ix-x. doi: 10.1016/j.giec.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 47.Corcoran GD, Holton J, Ridgway GL. Endoscope decontamination: a comparison of the Wolf 35100 and DSD-91 systems. J Hosp Infect. 1994;27:307–315. doi: 10.1016/0195-6701(94)90118-x. [DOI] [PubMed] [Google Scholar]
- 48.Machado AP, Pimenta AT, Contijo PP, Geocze S, Fischman O. Microbiologic profile of flexible endoscope disinfection in two Brazilian hospitals. Arq Gastroenterol. 2006;43:255–258. doi: 10.1590/s0004-28032006000400002. [DOI] [PubMed] [Google Scholar]
- 49.Deva AK, Vickery K, Zou J, West RH, Selby W, Benn RA, Harris JP, Cossart YE. Detection of persistent vegetative bacteria and amplified viral nucleic acid from in-use testing of gastrointestinal endoscopes. J Hosp Infect. 1998;39:149–157. doi: 10.1016/s0195-6701(98)90329-2. [DOI] [PubMed] [Google Scholar]
- 50.Buss AJ, Been MH, Borgers RP, Stokroos I, Melchers WJ, Peters FT, Limburg AJ, Degener JE. Endoscope disinfection and its pitfalls--requirement for retrograde surveillance cultures. Endoscopy. 2008;40:327–332. doi: 10.1055/s-2007-995477. [DOI] [PubMed] [Google Scholar]
- 51.Allen JI, Allen MO, Olson MM, Gerding DN, Shanholtzer CJ, Meier PB, Vennes JA, Silvis SE. Pseudomonas infection of the biliary system resulting from use of a contaminated endoscope. Gastroenterology. 1987;92:759–763. doi: 10.1016/0016-5085(87)90029-1. [DOI] [PubMed] [Google Scholar]
- 52.Michele TM, Cronin WA, Graham NM, Dwyer DM, Pope DS, Harrington S, Chaisson RE, Bishai WR. Transmission of Mycobacterium tuberculosis by a fiberoptic bronchoscope. Identification by DNA fingerprinting. JAMA. 1997;278:1093–1095. [PubMed] [Google Scholar]
- 53.Classen DC, Jacobson JA, Burke JP, Jacobson JT, Evans RS. Serious Pseudomonas infections associated with endoscopic retrograde cholangiopancreatography. Am J Med. 1988;84:590–596. doi: 10.1016/0002-9343(88)90141-6. [DOI] [PubMed] [Google Scholar]
- 54.Foss D, Monagan D. A national survey of physicians’ and nurses’ attitudes toward endoscope cleaning and the potential for cross-infection. Gastroenterol Nurs. 1992;15:59–65. doi: 10.1097/00001610-199210000-00003. [DOI] [PubMed] [Google Scholar]
- 55.Zühlsdorf B, Emmrich M, Floss H, Martiny H. Cleaning efficacy of nine different cleaners in a washer-disinfector designed for flexible endoscopes. J Hosp Infect. 2002;52:206–211. doi: 10.1053/jhin.2002.1284. [DOI] [PubMed] [Google Scholar]
- 56.Zühlsdorf B, Floss H, Martiny H. Efficacy of 10 different cleaning processes in a washer-disinfector for flexible endoscopes. J Hosp Infect. 2004;56:305–311. doi: 10.1016/j.jhin.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 57.Zühlsdorf B, Kampf G. Evaluation of the effectiveness of an enzymatic cleaner and glutaraldehyde-based disinfectant for chemothermal processing of flexible endoscopes in washer-disinfectors in accordance with prEN ISO 15 883. Endoscopy. 2006;38:586–591. doi: 10.1055/s-2006-925133. [DOI] [PubMed] [Google Scholar]
- 58.Foliente RL, Kovacs BJ, Aprecio RM, Bains HJ, Kettering JD, Chen YK. Efficacy of high-level disinfectants for reprocessing GI endoscopes in simulated-use testing. Gastrointest Endosc. 2001;53:456–462. doi: 10.1067/mge.2001.113380. [DOI] [PubMed] [Google Scholar]
- 59.Méan M, Mallaret MR, Bichard P, Shum J, Zarski JP. Gastrointestinal endoscopes cleaned without detergent substance following an automated endoscope washer/disinfector dysfunction. Gastroenterol Clin Biol. 2006;30:665–668. doi: 10.1016/s0399-8320(06)73258-4. [DOI] [PubMed] [Google Scholar]
- 60.Flemming HC. [Peracetic acid as disinfectant--a review] Zentralbl Bakteriol Mikrobiol Hyg B. 1984;179:97–111. [PubMed] [Google Scholar]
- 61.Krzywicka H. [Desinfectant activity of peracetic acid on the vegetative forms of bacteria] Rocz Panstw Zakl Hig. 1970;21:427–433. [PubMed] [Google Scholar]
- 62.Russell AD. Introduction of biocides into clinical practice and the impact on antibiotic-resistant bacteria. J Appl Microbiol. 2002;92 Suppl:121S–135S. [PubMed] [Google Scholar]
- 63.Mücke H, Wutzler P, Recknagel S. [Odorless surface disinfection with peracetic acid] Z Arztl Fortbild (Jena) 1989;83:1125–1127. [PubMed] [Google Scholar]
- 64.Rutala WA, Weber DJ. Disinfection of endoscopes: review of new chemical sterilants used for high-level disinfection. Infect Control Hosp Epidemiol. 1999;20:69–76. doi: 10.1086/501544. [DOI] [PubMed] [Google Scholar]
- 65.Bradley CR, Babb JR, Ayliffe GA. Evaluation of the Steris System 1 Peracetic Acid Endoscope Processor. J Hosp Infect. 1995;29:143–151. doi: 10.1016/0195-6701(95)90196-5. [DOI] [PubMed] [Google Scholar]
- 66.Griffiths PA, Babb JR, Fraise AP. Mycobactericidal activity of selected disinfectants using a quantitative suspension test. J Hosp Infect. 1999;41:111–121. doi: 10.1016/s0195-6701(99)90048-8. [DOI] [PubMed] [Google Scholar]
- 67.Jackson J, Leggett JE, Wilson DA, Gilbert DN. Mycobacterium gordonae in fiberoptic bronchoscopes. Am J Infect Control. 1996;24:19–23. doi: 10.1016/s0196-6553(96)90049-8. [DOI] [PubMed] [Google Scholar]
- 68.Kramer A, Reichwagen S, Heldt P, Widulle H, Nürnberg W. Oxidanzien. In: Kramer A, Assadian O, editors. Wallhäußers Praxis der Sterilisation, Desinfektion, Antiseptik und Konservierung. Stuttgart: Georg Thieme Verlag; 2008. pp. 713–745. [Google Scholar]
- 69.Holton J, Shetty N. In-use stability of Nu-Cidex. J Hosp Infect. 1997;35:245–248. doi: 10.1016/s0195-6701(97)90214-0. [DOI] [PubMed] [Google Scholar]
- 70.Spicher G, Peters J. [The activity of formaldehyde, glutardialdehyde, peracetic acid, chloramine T (N-chlor-4-toluolsulfonamide), m-cresol, ethanol and benzyldimethyldodecylammonium bromide against bacteria which are found in coagulated blood. (Model studies for chemical disinfection of instruments] Zentralbl Hyg Umweltmed. 1991;191:457–477. [PubMed] [Google Scholar]
- 71.Penna TC, Mazzola PG, Silva Martins AM. The efficacy of chemical agents in cleaning and disinfection programs. BMC Infect Dis. 2001;1:16. doi: 10.1186/1471-2334-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sagripanti JL, Bonifacino A. Effects of salt and serum on the sporicidal activity of liquid disinfectants. J AOAC Int. 1997;80:1198–1207. [PubMed] [Google Scholar]
- 73.Urata M, Isomoto H, Murase K, Wada A, Yanagihara K, Hirakata Y, Takeshima F, Omagari K, Mizuta Y, Murata I, et al. Comparison of the microbicidal activities of superoxidized and ozonated water in the disinfection of endoscopes. J Int Med Res. 2003;31:299–306. doi: 10.1177/147323000303100407. [DOI] [PubMed] [Google Scholar]
- 74.Martin DJ, Denyer SP, McDonnell G, Maillard JY. Resistance and cross-resistance to oxidising agents of bacterial isolates from endoscope washer disinfectors. J Hosp Infect. 2008;69:377–383. doi: 10.1016/j.jhin.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 75.Alfa MJ, Sitter DL. In-hospital evaluation of orthophthalaldehyde as a high level disinfectant for flexible endoscopes. J Hosp Infect. 1994;26:15–26. doi: 10.1016/0195-6701(94)90075-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vesley D, Melson J, Stanley P. Microbial bioburden in endoscope reprocessing and an in-use evaluation of the high-level disinfection capabilities of Cidex PA. Gastroenterol Nurs. 1999;22:63–68. doi: 10.1097/00001610-199903000-00007. [DOI] [PubMed] [Google Scholar]
- 77.Rutala WA, Weber DJ. Reprocessing endoscopes: United States perspective. J Hosp Infect. 2004;56 Suppl 2:S27–S39. doi: 10.1016/j.jhin.2003.12.035. [DOI] [PubMed] [Google Scholar]
- 78.Alfa MJ, Degagne P, Olson N. Worst-case soiling levels for patient-used flexible endoscopes before and after cleaning. Am J Infect Control. 1999;27:392–401. doi: 10.1016/s0196-6553(99)70004-0. [DOI] [PubMed] [Google Scholar]
- 79.Chu NS, McAlister D, Antonoplos PA. Natural bioburden levels detected on flexible gastrointestinal endoscopes after clinical use and manual cleaning. Gastrointest Endosc. 1998;48:137–142. doi: 10.1016/s0016-5107(98)70154-3. [DOI] [PubMed] [Google Scholar]
- 80.Ishino Y, Ido K, Koiwai H, Sugano K. Pitfalls in endoscope reprocessing: brushing of air and water channels is mandatory for high-level disinfection. Gastrointest Endosc. 2001;53:165–168. doi: 10.1067/mge.2001.112195. [DOI] [PubMed] [Google Scholar]
- 81.Kinney TP, Kozarek RA, Raltz S, Attia F. Contamination of single-use biopsy forceps: a prospective in vitro analysis. Gastrointest Endosc. 2002;56:209–212. doi: 10.1016/s0016-5107(02)70179-x. [DOI] [PubMed] [Google Scholar]
- 82.Lee RM, Kozarek RA, Sumida SE, Raltz SL. Risk of contamination of sterile biopsy forceps in disinfected endoscopes. Gastrointest Endosc. 1998;47:377–381. doi: 10.1016/s0016-5107(98)70222-6. [DOI] [PubMed] [Google Scholar]
- 83.Hanson PJ, Gor D, Clarke JR, Chadwick MV, Gazzard B, Jeffries DJ, Gaya H, Collins JV. Recovery of the human immunodeficiency virus from fibreoptic bronchoscopes. Thorax. 1991;46:410–412. doi: 10.1136/thx.46.6.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hanson PJ, Gor D, Clarke JR, Chadwick MV, Nicholson G, Shah N, Gazzard B, Jeffries DJ, Gaya H, Collins JV. Contamination of endoscopes used in AIDS patients. Lancet. 1989;2:86–88. doi: 10.1016/s0140-6736(89)90323-1. [DOI] [PubMed] [Google Scholar]
- 85.Deflandre J, Cajot O, Brixko C, Crine M, Labalue J, Senterre JM. [Risk of contamination by hepatitis C of endoscopes utilized in gastroenterology hospital service] Rev Med Liege. 2001;56:696–698. [PubMed] [Google Scholar]
- 86.Nürnberg M, Schulz HJ, Rüden H, Vogt K. Do conventional cleaning and disinfection techniques avoid the risk of endoscopic Helicobacter pylori transmission? Endoscopy. 2003;35:295–299. doi: 10.1055/s-2003-38149. [DOI] [PubMed] [Google Scholar]
- 87.Bécheur H, Harzic M, Colardelle P, Deny P, Coste T, Dubeaux B, Chochon M, Roussin-Bretagne S, Doll J, Andrieu J. [Hepatitis C virus contamination of endoscopes and biopsy forceps] Gastroenterol Clin Biol. 2000;24:906–910. [PubMed] [Google Scholar]
- 88.Ishino Y, Ido K, Sugano K. Contamination with hepatitis B virus DNA in gastrointestinal endoscope channels: risk of infection on reuse after on-site cleaning. Endoscopy. 2005;37:548–551. doi: 10.1055/s-2005-861316. [DOI] [PubMed] [Google Scholar]
- 89.Tytgat GN. Endoscopic transmission of Helicobacter pylori. Aliment Pharmacol Ther. 1995;9 Suppl 2:105–110. [PubMed] [Google Scholar]
- 90.Cronmiller JR, Nelson DK, Salman G, Jackson DK, Dean RS, Hsu JJ, Kim CH. Antimicrobial efficacy of endoscopic disinfection procedures: a controlled, multifactorial investigation. Gastrointest Endosc. 1999;50:152–158. doi: 10.1016/s0016-5107(99)70217-8. [DOI] [PubMed] [Google Scholar]
- 91.Kovacs BJ, Chen YK, Kettering JD, Aprecio RM, Roy I. High-level disinfection of gastrointestinal endoscopes: are current guidelines adequate? Am J Gastroenterol. 1999;94:1546–1550. doi: 10.1111/j.1572-0241.1999.01142.x. [DOI] [PubMed] [Google Scholar]
- 92.Kircheis U, Martiny H. Comparison of the cleaning and disinfecting efficacy of four washer-disinfectors for flexible endoscopes. J Hosp Infect. 2007;66:255–261. doi: 10.1016/j.jhin.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 93.Chanzy B, Duc-Bin DL, Rousset B, Morand P, Morel-Baccard C, Marchetti B, Fauconnier J, Mallaret MR, Calop J, Zarski JP, et al. Effectiveness of a manual disinfection procedure in eliminating hepatitis C virus from experimentally contaminated endoscopes. Gastrointest Endosc. 1999;50:147–151. doi: 10.1016/s0016-5107(99)70216-6. [DOI] [PubMed] [Google Scholar]
- 94.Rey JF, Halfon P, Feryn JM, Khiri H, Masseyeff MF, Ouzan D. [Risk of transmission of hepatitis C virus by digestive endoscopy] Gastroenterol Clin Biol. 1995;19:346–349. [PubMed] [Google Scholar]
- 95.Hanson PJ, Gor D, Jeffries DJ, Collins JV. Elimination of high titre HIV from fibreoptic endoscopes. Gut. 1990;31:657–659. doi: 10.1136/gut.31.6.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ribeiro MM, de Oliveira AC, Ribeiro SM, Watanabe E, de Resende Stoianoff MA, Ferreira JA. Effectiveness of flexible gastrointestinal endoscope reprocessing. Infect Control Hosp Epidemiol. 2013;34:309–312. doi: 10.1086/669518. [DOI] [PubMed] [Google Scholar]
- 97.Moses FM, Lee J. Surveillance cultures to monitor quality of gastrointestinal endoscope reprocessing. Am J Gastroenterol. 2003;98:77–81. doi: 10.1111/j.1572-0241.2003.07165.x. [DOI] [PubMed] [Google Scholar]
- 98.Dusart G, Zuccarelli M, Ossia-Ongagna Y, Simeon de Buochberg M. [Kinetics of bactericidal and sporicidal effects of a disinfectant against bacteria isolated from hospital units] Pathol Biol (Paris) 1992;40:523–528. [PubMed] [Google Scholar]
- 99.Sattar SA, Kibbee RJ, Tetro JA, Rook TA. Experimental evaluation of an automated endoscope reprocessor with in situ generation of peracetic acid for disinfection of semicritical devices. Infect Control Hosp Epidemiol. 2006;27:1193–1199. doi: 10.1086/508830. [DOI] [PubMed] [Google Scholar]
- 100.Middleton AM, Chadwick MV, Gaya H. Disinfection of bronchoscopes, contaminated in vitro with Mycobacterium tuberculosis, Mycobacterium avium-intracellulare and Mycobacterium chelonae in sputum, using stabilized, buffered peracetic acid solution (‘Nu-Cidex’) J Hosp Infect. 1997;37:137–143. doi: 10.1016/s0195-6701(97)90183-3. [DOI] [PubMed] [Google Scholar]
- 101.Fantry GT, Zheng QX, James SP. Conventional cleaning and disinfection techniques eliminate the risk of endoscopic transmission of Helicobacter pylori. Am J Gastroenterol. 1995;90:227–232. [PubMed] [Google Scholar]
- 102.Sauerbrei A, Schacke M, Glück B, Egerer R, Wutzler P. Validation of biocides against duck hepatitis B virus as a surrogate virus for human hepatitis B virus. J Hosp Infect. 2006;64:358–365. doi: 10.1016/j.jhin.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 103.Block C. The effect of Perasafe and sodium dichloroisocyanurate (NaDCC) against spores of Clostridium difficile and Bacillus atrophaeus on stainless steel and polyvinyl chloride surfaces. J Hosp Infect. 2004;57:144–148. doi: 10.1016/j.jhin.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 104.Sauerbrei A, Sehr K, Eichhorn U, Reimer K, Wutzler P. Inactivation of human adenovirus genome by different groups of disinfectants. J Hosp Infect. 2004;57:67–72. doi: 10.1016/j.jhin.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 105.Sauerbrei A, Sehr K, Brandstädt A, Heim A, Reimer K, Wutzler P. Sensitivity of human adenoviruses to different groups of chemical biocides. J Hosp Infect. 2004;57:59–66. doi: 10.1016/j.jhin.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 106.Hernández A, Martró E, Matas L, Ausina V. In-vitro evaluation of Perasafe compared with 2% alkaline glutaraldehyde against Mycobacterium spp. J Hosp Infect. 2003;54:52–56. doi: 10.1016/s0195-6701(03)00037-9. [DOI] [PubMed] [Google Scholar]
- 107.Wang GQ, Zhang CW, Liu HC, Chen ZB. Comparison of susceptibilities of M. tuberculosis H37Ra and M. chelonei subsp. abscessus to disinfectants. Biomed Environ Sci. 2005;18:124–127. [PubMed] [Google Scholar]
- 108.Ernst C, Schulenburg J, Jakob P, Dahms S, Lopez AM, Nychas G, Werber D, Klein G. Efficacy of amphoteric surfactant- and peracetic acid-based disinfectants on spores of bacillus cereus in vitro and on food premises of the German armed forces. J Food Prot. 2006;69:1605–1610. doi: 10.4315/0362-028x-69.7.1605. [DOI] [PubMed] [Google Scholar]
- 109.Hernández A, Martró E, Puzo C, Matas L, Burgués C, Vázquez N, Castella J, Ausina V. In-use evaluation of Perasafe compared with Cidex in fibreoptic bronchoscope disinfection. J Hosp Infect. 2003;54:46–51. doi: 10.1016/s0195-6701(03)00072-0. [DOI] [PubMed] [Google Scholar]
- 110.Stanley PM. Efficacy of peroxygen compounds against glutaraldehyde-resistant mycobacteria. Am J Infect Control. 1999;27:339–343. doi: 10.1016/s0196-6553(99)70054-4. [DOI] [PubMed] [Google Scholar]
- 111.Grand I, Bellon-Fontaine MN, Herry JM, Hilaire D, Moriconi FX, Naïtali M. The resistance of Bacillus atrophaeus spores to the bactericidal activity of peracetic acid is influenced by both the nature of the solid substrates and the mode of contamination. J Appl Microbiol. 2010;109:1706–1714. doi: 10.1111/j.1365-2672.2010.04799.x. [DOI] [PubMed] [Google Scholar]
- 112.Sagripanti JL, Eklund CA, Trost PA, Jinneman KC, Abeyta C, Kaysner CA, Hill WE. Comparative sensitivity of 13 species of pathogenic bacteria to seven chemical germicides. Am J Infect Control. 1997;25:335–339. doi: 10.1016/s0196-6553(97)90026-2. [DOI] [PubMed] [Google Scholar]
- 113.de Melo EM, Leão Cde S, Andreto LM, de Mello MJ. Surgical infection in a videolaparoscopic cholecystectomy when using peracetic acid for the sterilization of instruments. Rev Col Bras Cir. 2013;40:208–214. doi: 10.1590/s0100-69912013000300008. [DOI] [PubMed] [Google Scholar]
- 114.Lehmann S, Pastore M, Rogez-Kreuz C, Richard M, Belondrade M, Rauwel G, Durand F, Yousfi R, Criquelion J, Clayette P, et al. New hospital disinfection processes for both conventional and prion infectious agents compatible with thermosensitive medical equipment. J Hosp Infect. 2009;72:342–350. doi: 10.1016/j.jhin.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 115.Bridier A, Briandet R, Thomas V, Dubois-Brissonnet F. Comparative biocidal activity of peracetic acid, benzalkonium chloride and ortho-phthalaldehyde on 77 bacterial strains. J Hosp Infect. 2011;78:208–213. doi: 10.1016/j.jhin.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 116.Bordas JM, Marcos-Maeso MA, Perez MJ, Llach J, Gines A, Pique JM. GI flexible endoscope disinfection: “in use” test comparative study. Hepatogastroenterology. 2005;52:800–807. [PubMed] [Google Scholar]
- 117.Russell AD. Bacterial resistance to disinfectants: present knowledge and future problems. J Hosp Infect. 1999;43 Suppl:S57–S68. doi: 10.1016/s0195-6701(99)90066-x. [DOI] [PubMed] [Google Scholar]
- 118.Chang W, Toghrol F, Bentley WE. Toxicogenomic response of Staphylococcus aureus to peracetic acid. Environ Sci Technol. 2006;40:5124–5131. doi: 10.1021/es060354b. [DOI] [PubMed] [Google Scholar]
- 119.Chang W, Small DA, Toghrol F, Bentley WE. Microarray analysis of toxicogenomic effects of peracetic acid on Pseudomonas aeruginosa. Environ Sci Technol. 2005;39:5893–5899. doi: 10.1021/es0503534. [DOI] [PubMed] [Google Scholar]
- 120.Zook CD, Busta FF, Brady LJ. Sublethal sanitizer stress and adaptive response of Escherichia coli O157: H7. J Food Prot. 2001;64:767–769. doi: 10.4315/0362-028x-64.6.767. [DOI] [PubMed] [Google Scholar]
- 121.Jolivet-Gougeon A, Sauvager F, Bonnaure-Mallet M, Colwell RR, Cormier M. Virulence of viable but nonculturable S. Typhimurium LT2 after peracetic acid treatment. Int J Food Microbiol. 2006;112:147–152. doi: 10.1016/j.ijfoodmicro.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 122.Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15:167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2:95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 124.Bajolet O, Ciocan D, Vallet C, de Champs C, Vernet-Garnier V, Guillard T, Brasme L, Thiefin G, Cadiot G, Bureau-Chalot F. Gastroscopy-associated transmission of extended-spectrum beta-lactamase-producing Pseudomonas aeruginosa. J Hosp Infect. 2013;83:341–343. doi: 10.1016/j.jhin.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 125.Aumeran C, Poincloux L, Souweine B, Robin F, Laurichesse H, Baud O, Bommelaer G, Traoré O. Multidrug-resistant Klebsiella pneumoniae outbreak after endoscopic retrograde cholangiopancreatography. Endoscopy. 2010;42:895–899. doi: 10.1055/s-0030-1255647. [DOI] [PubMed] [Google Scholar]
- 126.den Aantrekker ED, Vernooij WW, Reij MW, Zwietering MH, Beumer RR, van Schothorst M, Boom RM. A biofilm model for flowing systems in the food industry. J Food Prot. 2003;66:1432–1438. doi: 10.4315/0362-028x-66.8.1432. [DOI] [PubMed] [Google Scholar]
- 127.Perni S, Jordan SJ, Andrew PW, Shama G. Biofilm development by Listeria innocua in turbulent flow regimes. Food Control. 2006;17:875–83. [Google Scholar]
- 128.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 129.López D, Vlamakis H, Kolter R. Biofilms. Cold Spring Harb Perspect Biol. 2010;2:a000398. doi: 10.1101/cshperspect.a000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dawson LF, Valiente E, Faulds-Pain A, Donahue EH, Wren BW. Characterisation of Clostridium difficile biofilm formation, a role for Spo0A. PLoS One. 2012;7:e50527. doi: 10.1371/journal.pone.0050527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zijnge V, van Leeuwen MB, Degener JE, Abbas F, Thurnheer T, Gmür R, Harmsen HJ. Oral biofilm architecture on natural teeth. PLoS One. 2010;5:e9321. doi: 10.1371/journal.pone.0009321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lyautey E, Lacoste B, Ten-Hage L, Rols JL, Garabetian F. Analysis of bacterial diversity in river biofilms using 16S rDNA PCR-DGGE: methodological settings and fingerprints interpretation. Water Res. 2005;39:380–388. doi: 10.1016/j.watres.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 133.Bridier A, Sanchez-Vizuete Mdel P, Le Coq D, Aymerich S, Meylheuc T, Maillard JY, Thomas V, Dubois-Brissonnet F, Briandet R. Biofilms of a Bacillus subtilis hospital isolate protect Staphylococcus aureus from biocide action. PLoS One. 2012;7:e44506. doi: 10.1371/journal.pone.0044506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kostaki M, Chorianopoulos N, Braxou E, Nychas GJ, Giaouris E. Differential biofilm formation and chemical disinfection resistance of sessile cells of listeria monocytogenes strains under monospecies and dual-species (with Salmonella enterica) conditions. Appl Environ Microbiol. 2012;78:2586–2595. doi: 10.1128/AEM.07099-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kovaleva J, Degener JE, van der Mei HC. Mimicking disinfection and drying of biofilms in contaminated endoscopes. J Hosp Infect. 2010;76:345–350. doi: 10.1016/j.jhin.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 136.van der Veen S, Abee T. Mixed species biofilms of Listeria monocytogenes and Lactobacillus plantarum show enhanced resistance to benzalkonium chloride and peracetic acid. Int J Food Microbiol. 2011;144:421–431. doi: 10.1016/j.ijfoodmicro.2010.10.029. [DOI] [PubMed] [Google Scholar]
- 137.Loukili NH, Granbastien B, Faure K, Guery B, Beaucaire G. Effect of different stabilized preparations of peracetic acid on biofilm. J Hosp Infect. 2006;63:70–72. doi: 10.1016/j.jhin.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 138.Henoun Loukili N, Becker H, Harno J, Bientz M, Meunier O. Effect of peracetic acid and aldehyde disinfectants on biofilm. J Hosp Infect. 2004;58:151–154. doi: 10.1016/j.jhin.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 139.Królasik J, Zakowska Z, Krepska M, Klimek L. Resistance of bacterial biofilms formed on stainless steel surface to disinfecting agent. Pol J Microbiol. 2010;59:281–287. [PubMed] [Google Scholar]
- 140.Aumeran C, Thibert E, Chapelle FA, Hennequin C, Lesens O, Traoré O. Assessment on experimental bacterial biofilms and in clinical practice of the efficacy of sampling solutions for microbiological testing of endoscopes. J Clin Microbiol. 2012;50:938–942. doi: 10.1128/JCM.06221-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Balsamo AC, Graziano KU, Schneider RP, Antunes Junior M, Lacerda RA. [Removing biofilm from a endoscopic: evaluation of disinfection methods currently used] Rev Esc Enferm USP. 2012;46 Spec No:91–98. doi: 10.1590/s0080-62342012000700014. [DOI] [PubMed] [Google Scholar]
- 142.Marion K, Freney J, James G, Bergeron E, Renaud FN, Costerton JW. Using an efficient biofilm detaching agent: an essential step for the improvement of endoscope reprocessing protocols. J Hosp Infect. 2006;64:136–142. doi: 10.1016/j.jhin.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 143.Pineau L, Desbuquois C, Marchetti B, Luu Duc D. Comparison of the fixative properties of five disinfectant solutions. J Hosp Infect. 2008;68:171–177. doi: 10.1016/j.jhin.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 144.Stewart PS, Rayner J, Roe F, Rees WM. Biofilm penetration and disinfection efficacy of alkaline hypochlorite and chlorosulfamates. J Appl Microbiol. 2001;91:525–532. doi: 10.1046/j.1365-2672.2001.01413.x. [DOI] [PubMed] [Google Scholar]
- 145.Nett JE, Guite KM, Ringeisen A, Holoyda KA, Andes DR. Reduced biocide susceptibility in Candida albicans biofilms. Antimicrob Agents Chemother. 2008;52:3411–3413. doi: 10.1128/AAC.01656-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Smith K, Hunter IS. Efficacy of common hospital biocides with biofilms of multi-drug resistant clinical isolates. J Med Microbiol. 2008;57:966–973. doi: 10.1099/jmm.0.47668-0. [DOI] [PubMed] [Google Scholar]
- 147.Wong HS, Townsend KM, Fenwick SG, Trengove RD, O’Handley RM. Comparative susceptibility of planktonic and 3-day-old Salmonella Typhimurium biofilms to disinfectants. J Appl Microbiol. 2010;108:2222–2228. doi: 10.1111/j.1365-2672.2009.04630.x. [DOI] [PubMed] [Google Scholar]
- 148.Bridier A, Briandet R, Thomas V, Dubois-Brissonnet F. Resistance of bacterial biofilms to disinfectants: a review. Biofouling. 2011;27:1017–1032. doi: 10.1080/08927014.2011.626899. [DOI] [PubMed] [Google Scholar]
- 149.Mah TF. Biofilm-specific antibiotic resistance. Future Microbiol. 2012;7:1061–1072. doi: 10.2217/fmb.12.76. [DOI] [PubMed] [Google Scholar]
- 150.Grobe KJ, Zahller J, Stewart PS. Role of dose concentration in biocide efficacy against Pseudomonas aeruginosa biofilms. J Ind Microbiol Biotechnol. 2002;29:10–15. doi: 10.1038/sj.jim.7000256. [DOI] [PubMed] [Google Scholar]
- 151.Bridier A, Dubois-Brissonnet F, Greub G, Thomas V, Briandet R. Dynamics of the action of biocides in Pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother. 2011;55:2648–2654. doi: 10.1128/AAC.01760-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Surdeau N, Laurent-Maquin D, Bouthors S, Gellé MP. Sensitivity of bacterial biofilms and planktonic cells to a new antimicrobial agent, Oxsil 320N. J Hosp Infect. 2006;62:487–493. doi: 10.1016/j.jhin.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 153.Ntsama-Essomba C, Bouttier S, Ramaldes M, Dubois-Brissonnet F, Fourniat J. Resistance of Escherichia coli growing as biofilms to disinfectants. Vet Res. 1997;28:353–363. [PubMed] [Google Scholar]
- 154.Campanac C, Pineau L, Payard A, Baziard-Mouysset G, Roques C. Interactions between biocide cationic agents and bacterial biofilms. Antimicrob Agents Chemother. 2002;46:1469–1474. doi: 10.1128/AAC.46.5.1469-1474.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Luppens SB, Reij MW, van der Heijden RW, Rombouts FM, Abee T. Development of a standard test to assess the resistance of Staphylococcus aureus biofilm cells to disinfectants. Appl Environ Microbiol. 2002;68:4194–4200. doi: 10.1128/AEM.68.9.4194-4200.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bardouniotis E, Ceri H, Olson ME. Biofilm formation and biocide susceptibility testing of Mycobacterium fortuitum and Mycobacterium marinum. Curr Microbiol. 2003;46:28–32. doi: 10.1007/s00284-002-3796-4. [DOI] [PubMed] [Google Scholar]
- 157.Saá Ibusquiza P, Herrera JJ, Cabo ML. Resistance to benzalkonium chloride, peracetic acid and nisin during formation of mature biofilms by Listeria monocytogenes. Food Microbiol. 2011;28:418–425. doi: 10.1016/j.fm.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 158.Alfa MJ, Howie R. Modeling microbial survival in buildup biofilm for complex medical devices. BMC Infect Dis. 2009;9:56. doi: 10.1186/1471-2334-9-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Shen Y, Stojicic S, Haapasalo M. Antimicrobial efficacy of chlorhexidine against bacteria in biofilms at different stages of development. J Endod. 2011;37:657–661. doi: 10.1016/j.joen.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 160.Simões M, Simões LC, Vieira MJ. Species association increases biofilm resistance to chemical and mechanical treatments. Water Res. 2009;43:229–237. doi: 10.1016/j.watres.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 161.Kara D, Luppens SB, Cate JM. Differences between single- and dual-species biofilms of Streptococcus mutans and Veillonella parvula in growth, acidogenicity and susceptibility to chlorhexidine. Eur J Oral Sci. 2006;114:58–63. doi: 10.1111/j.1600-0722.2006.00262.x. [DOI] [PubMed] [Google Scholar]
- 162.Burmølle M, Webb JS, Rao D, Hansen LH, Sørensen SJ, Kjelleberg S. Enhanced biofilm formation and increased resistance to antimicrobial agents and bacterial invasion are caused by synergistic interactions in multispecies biofilms. Appl Environ Microbiol. 2006;72:3916–3923. doi: 10.1128/AEM.03022-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Simões LC, Simões M, Vieira MJ. Influence of the diversity of bacterial isolates from drinking water on resistance of biofilms to disinfection. Appl Environ Microbiol. 2010;76:6673–6679. doi: 10.1128/AEM.00872-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sagripanti JL, Bonifacino A. Resistance of Pseudomonas aeruginosa to liquid disinfectants on contaminated surfaces before formation of biofilms. J AOAC Int. 2000;83:1415–1422. [PubMed] [Google Scholar]
- 165.Pajkos A, Vickery K, Cossart Y. Is biofilm accumulation on endoscope tubing a contributor to the failure of cleaning and decontamination? J Hosp Infect. 2004;58:224–229. doi: 10.1016/j.jhin.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 166.Bisset L, Cossart YE, Selby W, West R, Catterson D, O’hara K, Vickery K. A prospective study of the efficacy of routine decontamination for gastrointestinal endoscopes and the risk factors for failure. Am J Infect Control. 2006;34:274–280. doi: 10.1016/j.ajic.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 167.Miner N, Harris V, Ebron T, Cao TD. Sporicidal activity of disinfectants as one possible cause for bacteria in patient-ready endoscopes. Gastroenterol Nurs. 2007;30:285–290. doi: 10.1097/01.SGA.0000287201.98483.43. [DOI] [PubMed] [Google Scholar]
- 168.Perret-Vivancos C, Marion K, Renaud FN, Freney J. Efficient removal of attached biofilm in a naturally contaminated colonoscope using detachment-promoting agents. J Hosp Infect. 2008;68:277–278. doi: 10.1016/j.jhin.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 169.Shimoide H, Anzai E, Murata Y, Kusajima K, Ichihara H, Takano T, Hirayama N, Sato N, Kobayashi Y. [Contamination of flexible fiberoptic bronchoscopes with Mycobacterium chelonae linked to an automated endoscope disinfection machine--on the relationship between the presence of the organism in the intestinal tract and contamination of disinfection machine, and a case of gallbladder and bile duct infection with M. chelonae] Kekkaku. 1995;70:571–577. [PubMed] [Google Scholar]
- 170.Kressel AB, Kidd F. Pseudo-outbreak of Mycobacterium chelonae and Methylobacterium mesophilicum caused by contamination of an automated endoscopy washer. Infect Control Hosp Epidemiol. 2001;22:414–418. doi: 10.1086/501926. [DOI] [PubMed] [Google Scholar]
- 171.Alvarado CJ, Stolz SM, Maki DG. Nosocomial infections from contaminated endoscopes: a flawed automated endoscope washer. An investigation using molecular epidemiology. Am J Med. 1991;91:272S–280S. doi: 10.1016/0002-9343(91)90381-7. [DOI] [PubMed] [Google Scholar]
- 172.Rosengarten D, Block C, Hidalgo-Grass C, Temper V, Gross I, Budin-Mizrahi A, Berkman N, Benenson S. Cluster of pseudoinfections with Burkholderia cepacia associated with a contaminated washer-disinfector in a bronchoscopy unit. Infect Control Hosp Epidemiol. 2010;31:769–771. doi: 10.1086/653611. [DOI] [PubMed] [Google Scholar]
- 173.Hennequin C, Aumeran C, Robin F, Traore O, Forestier C. Antibiotic resistance and plasmid transfer capacity in biofilm formed with a CTX-M-15-producing Klebsiella pneumoniae isolate. J Antimicrob Chemother. 2012;67:2123–2130. doi: 10.1093/jac/dks169. [DOI] [PubMed] [Google Scholar]
- 174.Molin S, Tolker-Nielsen T. Gene transfer occurs with enhanced efficiency in biofilms and induces enhanced stabilisation of the biofilm structure. Curr Opin Biotechnol. 2003;14:255–261. doi: 10.1016/s0958-1669(03)00036-3. [DOI] [PubMed] [Google Scholar]
- 175.Hausner M, Wuertz S. High rates of conjugation in bacterial biofilms as determined by quantitative in situ analysis. Appl Environ Microbiol. 1999;65:3710–3713. doi: 10.1128/aem.65.8.3710-3713.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Savage VJ, Chopra I, O’Neill AJ. Staphylococcus aureus biofilms promote horizontal transfer of antibiotic resistance. Antimicrob Agents Chemother. 2013;57:1968–1970. doi: 10.1128/AAC.02008-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Simões M, Cleto S, Pereira MO, Vieira MJ. Influence of biofilm composition on the resistance to detachment. Water Sci Technol. 2007;55:473–480. doi: 10.2166/wst.2007.293. [DOI] [PubMed] [Google Scholar]
- 178.Exner M, Tuschewitzki GJ, Scharnagel J. Influence of biofilms by chemical disinfectants and mechanical cleaning. Zentralbl Bakteriol Mikrobiol Hyg B. 1987;183:549–563. [PubMed] [Google Scholar]
- 179.Cheetham NWH, Berentsveig V. Relative efficacy and activity of medical instrument cleaning agents. Australian Infection Control. 2002;7:105–112. [Google Scholar]
- 180.Ren W, Sheng X, Huang X, Zhi F, Cai W. Evaluation of detergents and contact time on biofilm removal from flexible endoscopes. Am J Infect Control. 2013;41:e89–e92. doi: 10.1016/j.ajic.2013.01.027. [DOI] [PubMed] [Google Scholar]
- 181.Fang Y, Shen Z, Li L, Cao Y, Gu LY, Gu Q, Zhong XQ, Yu CH, Li YM. A study of the efficacy of bacterial biofilm cleanout for gastrointestinal endoscopes. World J Gastroenterol. 2010;16:1019–1024. doi: 10.3748/wjg.v16.i8.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Vickery K, Pajkos A, Cossart Y. Removal of biofilm from endoscopes: evaluation of detergent efficiency. Am J Infect Control. 2004;32:170–176. doi: 10.1016/j.ajic.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 183.Bloss R, Kampf G. Test models to determine cleaning efficacy with different types of bioburden and its clinical correlation. J Hosp Infect. 2004;56 Suppl 2:S44–S48. doi: 10.1016/j.jhin.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 184.Vickery K, Ngo QD, Zou J, Cossart YE. The effect of multiple cycles of contamination, detergent washing, and disinfection on the development of biofilm in endoscope tubing. Am J Infect Control. 2009;37:470–475. doi: 10.1016/j.ajic.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 185.Montebugnoli L, Chersoni S, Prati C, Dolci G. A between-patient disinfection method to control water line contamination and biofilm inside dental units. J Hosp Infect. 2004;56:297–304. doi: 10.1016/j.jhin.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 186.Shemesh M, Kolter R, Losick R. The biocide chlorine dioxide stimulates biofilm formation in Bacillus subtilis by activation of the histidine kinase KinC. J Bacteriol. 2010;192:6352–6356. doi: 10.1128/JB.01025-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Spaun GO, Goers TA, Pierce RA, Cassera MA, Scovil S, Swanstrom LL. Use of flexible endoscopes for NOTES: sterilization or high-level disinfection? Surg Endosc. 2010;24:1581–1588. doi: 10.1007/s00464-009-0815-6. [DOI] [PubMed] [Google Scholar]
- 188.Vickery K, Pajkos A, Cossart Y. Evaluation of the effectiveness of decontamination of dental syringes. Br Dent J. 2000;189:620–624. doi: 10.1038/sj.bdj.4800847. [DOI] [PubMed] [Google Scholar]
- 189.Kampf G, Bloss R, Martiny H. Surface fixation of dried blood by glutaraldehyde and peracetic acid. J Hosp Infect. 2004;57:139–143. doi: 10.1016/j.jhin.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 190.Tucker RC, Lestini BJ, Marchant RE. Surface analysis of clinically used expanded PTFE endoscopic tubing treated by the STERIS PROCESS. ASAIO J. 1996;42:306–313. [PubMed] [Google Scholar]
- 191.Alfa MJ, Fatima I, Olson N. Validation of adenosine triphosphate to audit manual cleaning of flexible endoscope channels. Am J Infect Control. 2013;41:245–248. doi: 10.1016/j.ajic.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 192.Obee PC, Griffith CJ, Cooper RA, Cooke RP, Bennion NE, Lewis M. Real-time monitoring in managing the decontamination of flexible gastrointestinal endoscopes. Am J Infect Control. 2005;33:202–206. doi: 10.1016/j.ajic.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 193.Fushimi R, Takashina M, Yoshikawa H, Kobayashi H, Okubo T, Nakata S, Kaku M. Comparison of adenosine triphosphate, microbiological load, and residual protein as indicators for assessing the cleanliness of flexible gastrointestinal endoscopes. Am J Infect Control. 2013;41:161–164. doi: 10.1016/j.ajic.2012.02.030. [DOI] [PubMed] [Google Scholar]
- 194.Alfa MJ, Olson N, Degagné P, Simner PJ. Development and validation of rapid use scope test strips to determine the efficacy of manual cleaning for flexible endoscope channels. Am J Infect Control. 2012;40:860–865. doi: 10.1016/j.ajic.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 195.Alfa MJ, Fatima I, Olson N. The adenosine triphosphate test is a rapid and reliable audit tool to assess manual cleaning adequacy of flexible endoscope channels. Am J Infect Control. 2013;41:249–253. doi: 10.1016/j.ajic.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 196.Hervé R, Keevil CW. Current limitations about the cleaning of luminal endoscopes. J Hosp Infect. 2013;83:22–29. doi: 10.1016/j.jhin.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 197.Alfa MJ, Olson N, DeGagne P, Jackson M. A survey of reprocessing methods, residual viable bioburden, and soil levels in patient-ready endoscopic retrograde choliangiopancreatography duodenoscopes used in Canadian centers. Infect Control Hosp Epidemiol. 2002;23:198–206. doi: 10.1086/502035. [DOI] [PubMed] [Google Scholar]
- 198.Frean JA, Arntzen L, Rosekilly I, Isaäcson M. Investigation of contaminated parenteral nutrition fluids associated with an outbreak of Serratia odorifera septicaemia. J Hosp Infect. 1994;27:263–273. doi: 10.1016/0195-6701(94)90114-7. [DOI] [PubMed] [Google Scholar]
- 199.Shao J, Wolff S, Zydney AL. In vitro comparison of peracetic acid and bleach cleaning of polysulfone hemodialysis membranes. Artif Organs. 2007;31:452–460. doi: 10.1111/j.1525-1594.2007.00387.x. [DOI] [PubMed] [Google Scholar]
- 200.Caillou S, Boonaert CJ, Dewez JL, Rouxhet PG. Oxidation of proteins adsorbed on hemodialysis membranes and model materials. J Biomed Mater Res B Appl Biomater. 2008;84:240–248. doi: 10.1002/jbm.b.30866. [DOI] [PubMed] [Google Scholar]
- 201.Vadrot C, Darbord JC. Quantitative evaluation of prion inactivation comparing steam sterilization and chemical sterilants: proposed method for test standardization. J Hosp Infect. 2006;64:143–148. doi: 10.1016/j.jhin.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 202.Tschudin-Sutter S, Frei R, Kampf G, Tamm M, Pflimlin E, Battegay M, Widmer AF. Emergence of glutaraldehyde-resistant Pseudomonas aeruginosa. Infect Control Hosp Epidemiol. 2011;32:1173–1178. doi: 10.1086/662624. [DOI] [PubMed] [Google Scholar]
- 203.Gastmeier P, Vonberg RP. Klebsiella spp. in endoscopy-associated infections: we may only be seeing the tip of the iceberg. Infection. 2014;42:15–21. doi: 10.1007/s15010-013-0544-6. [DOI] [PubMed] [Google Scholar]
- 204.Carbonne A, Thiolet JM, Fournier S, Fortineau N, Kassis-Chikhani N, Boytchev I, Aggoune M, Seguier JC, Senechal H, Tavolacci MP, et al. Control of a multi-hospital outbreak of KPC-producing Klebsiella pneumoniae type 2 in France, September to October 2009. Euro Surveill. 2010;15 doi: 10.2807/ese.15.48.19734-en. [DOI] [PubMed] [Google Scholar]
- 205.Kovaleva J, Meessen NE, Peters FT, Been MH, Arends JP, Borgers RP, Degener JE. Is bacteriologic surveillance in endoscope reprocessing stringent enough? Endoscopy. 2009;41:913–916. doi: 10.1055/s-0029-1215086. [DOI] [PubMed] [Google Scholar]
- 206.Sorin M, Segal-Maurer S, Mariano N, Urban C, Combest A, Rahal JJ. Nosocomial transmission of imipenem-resistant Pseudomonas aeruginosa following bronchoscopy associated with improper connection to the Steris System 1 processor. Infect Control Hosp Epidemiol. 2001;22:409–413. doi: 10.1086/501925. [DOI] [PubMed] [Google Scholar]
- 207.Naas T, Cuzon G, Babics A, Fortineau N, Boytchev I, Gayral F, Nordmann P. Endoscopy-associated transmission of carbapenem-resistant Klebsiella pneumoniae producing KPC-2 beta-lactamase. J Antimicrob Chemother. 2010;65:1305–1306. doi: 10.1093/jac/dkq117. [DOI] [PubMed] [Google Scholar]
- 208.Rideout K, Teschke K, Dimich-Ward H, Kennedy SM. Considering risks to healthcare workers from glutaraldehyde alternatives in high-level disinfection. J Hosp Infect. 2005;59:4–11. doi: 10.1016/j.jhin.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 209.Gutterman E, Jorgensen L, Mitchell A, Fua S. Adverse staff health outcomes associated with endoscope reprocessing. Biomed Instrum Technol. 2013;47:172–179. doi: 10.2345/0899-8205-47.2.172. [DOI] [PubMed] [Google Scholar]
- 210.Cammarota G, Cesaro P, Cazzato A, Fedeli P, Riccioni ME, Sparano L, Vitale G, Costamagna G, Gasbarrini G, Larocca LM. Hydrogen peroxide-related colitis (previously known as “pseudolipomatosis”): a series of cases occurring in an epidemic pattern. Endoscopy. 2007;39:916–919. doi: 10.1055/s-2007-966652. [DOI] [PubMed] [Google Scholar]
- 211.Ahishali E, Uygur-Bayramiçli O, Dolapçioğlu C, Dabak R, Mengi A, Işik A, Ermiş E. Chemical colitis due to glutaraldehyde: case series and review of the literature. Dig Dis Sci. 2009;54:2541–2545. doi: 10.1007/s10620-008-0630-2. [DOI] [PubMed] [Google Scholar]
- 212.Coriat R, Chaput U, Ismaili Z, Chaussade S. What induces colitis? Hydrogen peroxide or peracetic acid. Endoscopy. 2008;40:231. doi: 10.1055/s-2007-995417. [DOI] [PubMed] [Google Scholar]
- 213.Tremain SC, Orientale E, Rodney WM. Cleaning, disinfection, and sterilization of gastrointestinal endoscopes: approaches in the office. J Fam Pract. 1991;32:300–305. [PubMed] [Google Scholar]
- 214.Kampf G, Ostermeyer C, Tschudin-Sutter S, Widmer AF. Resistance or adaptation? How susceptible is a ‚glutaraldehyde-resistant‘ Pseudomonas aeruginosa isolate in the absence of selection pressure? J Hosp Infect. 2013;84:316–318. doi: 10.1016/j.jhin.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 215.Zullo A, Hassan C, Guarini A, Lorenzetti R, Campo S, Morini S. Chemical colitis due to peracetic acid: A case report and review of literature. JDE. 2011;2:15–17. [Google Scholar]
- 216.Morini S, Campo SM, Zullo A, Guarini A, Ridola L, Hassan C. Chemical colitis induced by peracetic acid: further evidence. Endoscopy. 2009;41:383. doi: 10.1055/s-0029-1214493. [DOI] [PubMed] [Google Scholar]
- 217.Coton T, Bohand X, Guisset M, Carre D, Delpy R, Valette M, Debonne JM. [Acute colitis induced by a peracetic acid based solution used to disinfect endoscopes] Gastroenterol Clin Biol. 2003;27:556–558. [PubMed] [Google Scholar]
- 218.Lapeyre B. The “frost sign” and the “snow white sign”: intramucosal air injection or peroxide colitis? Endoscopy. 2005;37:679; author reply 680. doi: 10.1055/s-2005-861332. [DOI] [PubMed] [Google Scholar]
- 219.Kara M, Turan I, Polat Z, Dogru T, Bagci S. Chemical colitis caused by peracetic acid or hydrogen peroxide: a challenging dilemma. Endoscopy. 2010;42 Suppl 2:E3–E4. doi: 10.1055/s-0029-1215260. [DOI] [PubMed] [Google Scholar]
- 220.Kim SJ, Baek IH. Colonic mucosal pseudolipomatosis: disinfectant colitis? Gastroenterol Nurs. 2012;35:208–213. doi: 10.1097/SGA.0b013e3182562bde. [DOI] [PubMed] [Google Scholar]
- 221.Association of periOperative Registered Nurses. Recommended Practices for Cleaning and Processing Flexible Endoscopes and Endoscope Accessories. Perioperative Standards and Recommended Practices. Stuttgart: Georg Thieme Verlag; 2013. pp. 473–484. [Google Scholar]
- 222.Association of perioperative registered nurses. 2012. Recommended Practices for Cleaning and Processing Flexible Endoscopes and Endoscope Accessories. Available from: http://aornstandards.org/content/1/SEC32.extract. Accessed on Oct, 2013.
- 223.Alvarado CJ, Reichelderfer M. APIC guideline for infection prevention and control in flexible endoscopy. Association for Professionals in Infection Control. Am J Infect Control. 2000;28:138–155. [PubMed] [Google Scholar]
- 224.Asia pacific society of infection control. 2012. The ASEAN Guidelines for disinfection and sterilization of instruments in health care facilities. Available from: http://apsic.info/documents/The-ASEAN-Guidelines-for-Disinfection-and-Sterilisation-of-Instruments-in-Health-Care-Facilities.pdf. Accessed on Oct, 2013. [DOI] [PMC free article] [PubMed]
- 225.Petersen BT, Chennat J, Cohen J, Cotton PB, Greenwald DA, Kowalski TE, Krinsky ML, Park WG, Pike IM, Romagnuolo J, et al. Multisociety guideline on reprocessing flexible gastrointestinal endoscopes: 2011. Gastrointest Endosc. 2011;73:1075–1084. doi: 10.1016/j.gie.2011.03.1183. [DOI] [PubMed] [Google Scholar]
- 226.Petersen BT, Chennat J, Cohen J, Cotton PB, Greenwald DA, Kowalski TE, Krinsky ML, Park WG, Pike IM, Romagnuolo J, et al. Multisociety guideline on reprocessing flexible GI endoscopes: 2011. Infect Control Hosp Epidemiol. 2011;32:527–537. doi: 10.1086/660676. [DOI] [PubMed] [Google Scholar]
- 227.BC Ministry of Health. Best Practice Guidelines For Cleaning, Disinfection and Sterilization of Critical and Semi-critical Medical Devices 2011. Available from: http://www.health.gov.bc.ca/library/publications/year/2011/Best-practice-guidelines-cleaning.pdf. Accessed on Oct, 2013.
- 228.British Society of Gastroenterology. 2008. BSG Guidelines for Decontamination of Equipment for Gastrointestinal Endoscopy. Available from: http://www.bsg.org.uk/images/stories/docs/clinical/guidelines/endoscopy/decontamination_2008.pdf. Accessed on Oct, 2013.
- 229.Rutala WA, Weber DJ, Healthcare Infection Control Practices Advisory Committee (HICPAC) Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008. Available from: http://www.cdc.gov/hicpac/pdf/guidelines/disinfection_nov_2008.pdf. Accessed on Oct, 2013.
- 230.Rey JF, Kruse A, Neumann C. ESGE/ESGENA technical note on cleaning and disinfection. Endoscopy. 2003;35:869–877. doi: 10.1055/s-2003-42626. [DOI] [PubMed] [Google Scholar]
- 231.Beilenhoff U, Neumann CS, Rey JF, Biering H, Blum R, Cimbro M, Kampf B, Rogers M, Schmidt V. ESGE-ESGENA Guideline: cleaning and disinfection in gastrointestinal endoscopy. Endoscopy. 2008;40:939–957. doi: 10.1055/s-2008-1077722. [DOI] [PubMed] [Google Scholar]
- 232.Health Protection Scotland. 2007. Endoscope Reprocessing: Guidance on the Requirements for Decontamination Equipment, Facilities and Management. Available from: http://www.documents.hps.scot.nhs.uk/hai/decontamination/publications/end-001-01-v1.1.pdf. Accessed on Oct, 2013.
- 233.Society JGET. 2004. "Guidelines for cleaning and disinfecting endoscopes" Second edition. Available from: http://www.aspjj.com/us/sites/default/files/pdf/JGETS-Guidelines-gastroenterological-endoscopy-(Japan).pdf. Accessed on Oct, 2013.
- 234.Public Health Agency of Canada. 2010. Infection and Prevention and Control Guideline for Flexible Gastrointestinal Endoscopy and Flexible Bronchoscopy. Available from: http://www.phac-aspc.gc.ca/nois-sinp/guide/endo/pdf/endo-eng.pdf. Accessed on Oct, 2013.
- 235.Robert Koch Institute. Hygiene requirements for the reprocessing of medical devices. Recommendation of the Commission for Hospital Hygiene and Infection Prevention (KRINKO) at the Robert Koch Institute (RKI) and the Federal Institute for Drugs and Medical Devices (BfArM) Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2001;44:1115–1126. doi: 10.1007/s00103-012-1548-6. [DOI] [PubMed] [Google Scholar]
- 236.Commission for Hospital Hygiene and Infection Prevention (KRINKO); Federal Institute for Drugs and Medical Devices (BfArM) [Hygiene requirements for the reprocessing of medical devices. Recommendation of the Commission for Hospital Hygiene and Infection Prevention (KRINKO) at the Robert Koch Institute (RKI) and the Federal Institute for Drugs and Medical Devices (BfArM)] Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2012;55:1244–1310. doi: 10.1007/s00103-012-1548-6. [DOI] [PubMed] [Google Scholar]
- 237.Society of Gastroenterology Nurses and Associates. Standards of infection control in reprocessing of flexible gastrointestinal endoscopes. Gastroenterol Nurs. 2013;36:293–303. doi: 10.1097/SGA.0b013e31829c6d5b. [DOI] [PubMed] [Google Scholar]
- 238.World Gastroenterology Organisation. Organisation Mondiale d'Endoscopie Digestive. 2005. WGO/OMED Practice Guideline Endoscope Disinfection. Available from: http://www.worldgastroenterology.org/assets/downloads/en/pdf/guidelines/09_endoscope_disinfection_en.pdf.
- 239.World Gastroenterology Organisation, World Endoscopy Organization. 2011. Endoscope disinfection- a resource-sensitive approach. Available from: http://www.worldendo.org/assets/downloads/pdf/guidelines/wgo_weo_endoscope_disinfection.pdf. Accessed on Oct, 2013.
- 240.Tremain SC. Cleaning and disinfection of lower gastrointestinal endoscopes. Prim Care. 1995;22:471–478. [PubMed] [Google Scholar]
- 241.Heudorf U, Hofmann H, Kutzke G, Otto U, Exner M. [Current hygiene status in endoscopic practice. Results from monitoring the reprocessing of flexible endoscopes in hospitals and private practices in Frankfurt on the Main, Germany, 2003/4] Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2005;48:1265–1272. doi: 10.1007/s00103-005-1155-x. [DOI] [PubMed] [Google Scholar]
- 242.Bischoff H. [Public health measures in endoscopy--routine maintenance of endoscopes] Gesundheitswesen. 1994;56:338–342. [PubMed] [Google Scholar]
- 243.Heudorf U, Hofmann H, Kutzke G, Otto U, Exner M. [Hygiene in endoscopy in the clinic and practice, 2003: Results of infection hygiene survey on endoscopy services in Frankfurt am Main by the public health service] Z Gastroenterol. 2004;42:669–676. doi: 10.1055/s-2004-813285. [DOI] [PubMed] [Google Scholar]


