Abstract
Hilar cholangiocarcinomas are common tumors of the bile duct that are often unresectable at presentation. Palliation, therefore, remains the goal in the majority of these patients. Palliative treatment is particularly indicated in the presence of cholangitis and pruritus but is often also offered for high-grade jaundice and abdominal pain. Endoscopic drainage by placing stents at endoscopic retrograde cholangio-pancreatography (ERCP) is usually the preferred modality of palliation. However, for advanced disease, percutaneous stenting has been shown to be superior to endoscopic stenting. Endosonography-guided biliary drainage is emerging as an alternative technique, particularly when ERCP is not possible or fails. Metal stents are usually preferred over plastic stents, both for ERCP and for percutaneous biliary drainage. There is no consensus as to whether it is necessary to place multiple stents within advanced hilar blocks or whether unilateral stenting would suffice. However, recent data have suggested that, contrary to previous belief, it is useful to drain more than 50% of the liver volume for favorable long-term results. In the presence of cholangitis, it is beneficial to drain all of the obstructed biliary segments. Surgical bypass plays a limited role in palliation and is offered primarily as a segment III bypass if, during a laparotomy for resection, the tumor is found to be unresectable. Photodynamic therapy and, more recently, radiofrequency ablation have been used as adjuvant therapies to improve the results of biliary stenting. The exact technique to be used for palliation is guided by the extent of the biliary involvement (Bismuth class) and the availability of local expertise.
Keywords: Cholangiocarcinoma, Hilar cholangiocarcinoma, Klatskin’s tumor, Palliation, Biliary stenting
Core tip: The majority of patients with hilar cholangiocarcinoma present in advanced stages and are candidates for palliation only. The techniques of palliation, primarily at endoscopy or by percutaneous techniques, are evolving as better stents become available. Alternate techniques, such as endosonography-guided procedures, are also becoming popular. Photodynamic therapy and radio-frequency ablation are also used to improve the results of biliary stents. This review article provides a consolidated picture of the present knowledge in this field based on recent data.
INTRODUCTION
Bile duct cancers at or around the confluence of the right and left hepatic ducts are termed as hilar cholangiocarcinomas (HC) or Klatskin’s tumors. HC is the most common type of bile duct cancer throughout the world and constitutes 46%-97% of all bile duct cancers[1,2]. It has a particularly high prevalence in certain Asian countries, such as Thailand and China[3]. This could be related to the liver fluke infestation in these areas. Due to the critical nature and site of the disease, patients with HC suffer greatly from progressive jaundice, anorexia, pruritus, cholangitis and liver failure. Unfortunately, a majority of HC cases manifest late and are diagnosed at a stage when curative resection is not possible[4,5]. Palliation, therefore, is the goal for this subset of patients. This review addresses the indications, techniques and results of various palliative methods. There are a number of “grey zones” in the palliative treatment of HC; these have also been addressed.
INDICATIONS FOR PALLIATION
Only approximately 20%-30% of patients with HC are diagnosed at a stage when surgical resection is possible. Table 1 gives the criteria for surgical non-resectability[6]. Moreover, the associated co-morbidity often precludes any form of surgery. While the median survival for resected patients (R0) can be up to 4 years, for those without the feasible option of resection, survival is almost always less than 1 year[4,7]. Multi-detector CT scan (MDCT) and magnetic resonance imaging (MRI) with MR Cholangiography continue to be the most two accurate modalities in the evaluation of the stages and resectability of HC[8,9]. The accuracy of predicting hepatic artery invasion, portal vein involvement, lymph nodal metastasis and the extent of biliary ductal spread is approximately 85%-95% in both of these approaches[8,9]. Endoscopic ultrasonography (EUS), positron emission tomography (PET) and diagnostic laparoscopy are additional means of evaluating the resectability of HC, but their role is not yet fully established[10-12].
Table 1.
Criteria for non-resectability of hilar cholangiocarcinoma
| Bilateral hepatic duct involvement up to the secondary biliary radicles (Bismuth type IV) |
| Encasement or occlusion of the main portal vein (relative) |
| Unilateral tumor extension to secondary biliary radicles (Bismuth type III) with contralateral portal vein or hepatic artery involvement or encasement |
| Hepatic lobar atrophy with contralateral portal vein or hepatic artery involvement or encasement |
| Hepatic lobar atrophy with contralateral tumor extension to the secondary biliary radicles |
Available from Aljiffry et al[6].
Not every patient with unresectable HC needs palliative intervention. Patients with complications of cholangitis and intractable pruritus are definite candidates for palliation. Palliation is also often performed in patients with abdominal pain and high bilirubin with the hope of ameliorating their pain and sense of well-being, respectively. There are three established methods for the palliation of HC: endoscopic insertion of a stent, percutaneous placement of biliary drainage and surgical bypass. Endosonography-guided procedures have been evolving as alternatives to these standard techniques.
ENDOSCOPIC PALLIATION BY STENTING
Before planning any palliative drainage, either by endoscopy or percutaneously, it is mandatory to obtain a cholangiogram to define the extent of biliary ductal involvement. Magnetic resonance cholangio-pancreatography (MRCP) continues to be the most preferred investigation for this purpose, and HC has been classified as Bismuth type I to type IV at cholangiogram (Figure 1).
Figure 1.
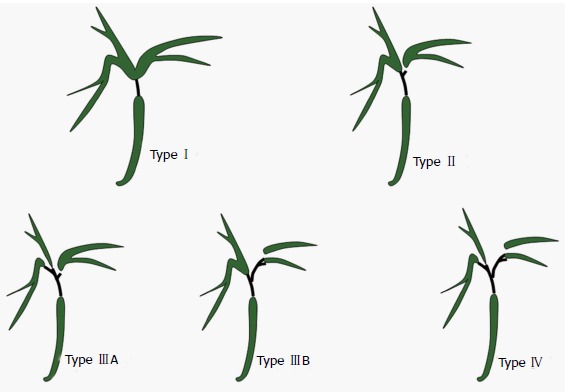
Hilar Cholangicarcinoma-Bismuth classification. Type I: Confluence of right and left hepatic duct is intact; Type II: Right and left hepatic ducts are separated; Type III: Tumor extends into second degree branches of right (IIIA) or left (IIIB) hepatic duct; Type IV: Involvement of both side secondary branches.
Metal vs plastic stents
Both plastic stents (PS) and self-expanding metal stents (SEMS) have been used for biliary drainage (Figure 2). PS have smaller diameters (10-12 Fr), resulting in faster occlusions and a median patency time of only 1.4 to 3 mo[13]. SEMS have a wider diameter (8-10 mm), resulting in longer patency of 6-10 mo[13]. SEMS, which are used for HC, are uncovered with an open mesh, allowing the drainage of side branches. Perdue et al[14], in a multicenter study involving 62 patients, compared metal stents with plastic stents in HC for 30 d outcomes. Adverse effects, including cholangitis, stent occlusion, migration, perforation and the need for reinterventions, occurred in a significantly higher proportion of patients in the plastic stent group (39.3%), compared to metal stents (11.8%). These results were revalidated in a recent study from Thailand[15]. In this randomized study involving 108 patients, Sangchan et al[15] demonstrated that metal stents were better in terms of success rate (70% vs 46%) and patient survival (126 d vs 49 d). In spite of its high initial cost, SEMS are considered more cost-effective than PS. This result could be due to the greater success rate, shorter hospital stays, fewer blockages, fewer re-interventions and lower antibiotic needs associated with SEMS. The superior cost-effectiveness of SEMS compared to PS is particularly apparent if patient survival is expected to be more than 4-6 mo. However, in situations where a decision of palliation has not been made, plastic stents may be preferred because uncovered SEMS used for hilar blocks are not easily removable.
Figure 2.
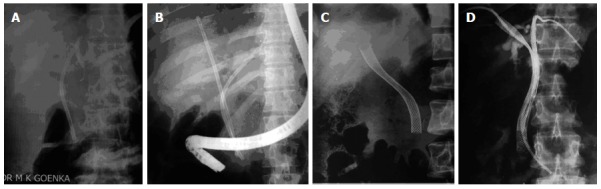
Stenting for hilar cholangiocarcinoma. A: Unilateral plastic stent; B: Bilateral plastic stents; C: Unilateral metal stent; D: Bilateral metal stents (previously placed percutaneous drains are still in place).
Volume of drainage and number of stents (unilateral vs bilateral)
For Bismuth type I HC, it is obvious that one stent is sufficient to drain both lobes of the liver because the confluence is patent. However, the placement of single stent for Bismuth type II to type IV HC would drain only a proportion of liver; instead, multiple stents are often placed in these advanced HC patients. However, no consensus has been reached in terms of the need for multiple stents.
De Palma et al[16] reported better results with unilateral stenting (vs bilateral stenting) in terms of successful stent placement, effective drainage and complication rates (Table 2). However, approximately one-third of the patients in this series had Bismuth type I HC, leading to a bias in the study result. Contrary to this study, Naitoh et al[17] demonstrated a trend towards longer survival and lower cholangitis with bilateral, compared to unilateral, drainage. An important conclusion was drawn in a study by Chang et al[18], which showed that patients with HC faired very poorly, with high cholangitis rates (32%), high 30 d mortality (30%) and poor survival (45 d) if only one stent was placed after opacifying both sides of the liver. This was in contrast to the other two groups, in which the opacified lobes were drained by one or two stents (Table 3). It therefore seems that any contrast placed in an obstructed system must be drained. An experienced endoscopist dealing with hilar blocks is always reluctant to inject any contrast until a guide wire has been negotiated well beyond the site of the biliary obstruction. A study in India[19] demonstrated the importance of contrast-free unilateral endoscopic palliation in Bismuth type II HC. In 18 patients submitting to this technique, successful drainage was achieved in all, with no cholangitis or 30 d mortality.
Table 2.
Unilateral vs bilateral drainage for hilar cholan-giocarcinoma
| Unilateral | Bilateral | P | |
| No. of pts | 79 | 78 | - |
| Stent insertion (%) | 88.6 | 76.9 | 0.041 |
| Successful drainage (%) | 81.0 | 73.0 | 0.049 |
| Early complication (%) | 18.9 | 26.9 | 0.026 |
| Survival (d) | 140 | 142 | 0.482 |
Available from De Palma et al[16].
Table 3.
Malignant hilar obstruction-1 stent or 2
| Group A | Group B | Group C | |
| n | 32 | 29 | 37 |
| Early cholangitis | 6% | 0% | 32% |
| 30-d mortality | 0% | 3% | 30% |
| Survival (d) | 145 | 225 | 45 |
Group A: One lobe opacified and the same lobe drained; Group B: Both lobes opacified and both lobes drained; Group C: Both lobes opacified with just one lobe drained. Available from Chang et al[18].
Conventional teaching suggests that, in the absence of cholangitis, it is only necessary to drain 25% of the liver volume for the adequate palliation of jaundice[20]. However, a recent study by Vienne et al[21] showed better results with the drainage of more than half of the liver volume. More than a 50% drainage of the liver volume was associated with a greater decrease in bilirubin levels, a lower incidence of early cholangitis and longer patient survival compared to patients with less than a 50% drainage of the liver volume. It is known that the right lobe, left lobe and caudate lobes of liver constitute 55%-60%, 30%-35% and approximately 10% of the liver volume, respectively[22]. Incorporating this information in the results of the study by Vienne et al[21], a significant proportion of patients should undergo the draining of at least two segments and the placement of more than one stent to achieve good long-term palliation. However, more data are needed in this respect. It is important to note that, in the presence of cholangitis, all infected ductal systems need to be drained.
It is important to select the appropriate segments of the liver that need to be drained when unilateral or incomplete drainage is planned in patients with advanced HC (Bismuth type II to type IV). It is advisable to select segments with dilated ductal systems and to avoid atrophic segments (Figure 3). MDCT or MRI can provide this useful information prior to palliative stenting[23,24]. A number of studies with both PS and SEMS have shown the usefulness of CT/MRCP-guided, selective biliary drainage (Table 4)[16,25,26]. This CT/MRCP approach can reduce the need for further intervention and has been found to be cost-effective compared to routine bilateral stenting[27]. A recent study[28] with a small number of patients used air cholangiography rather than MRCP to act as a lower cost road map and found air cholangiography to be safe and effective with no cholangitis and no 30 d mortality or morbidity.
Figure 3.
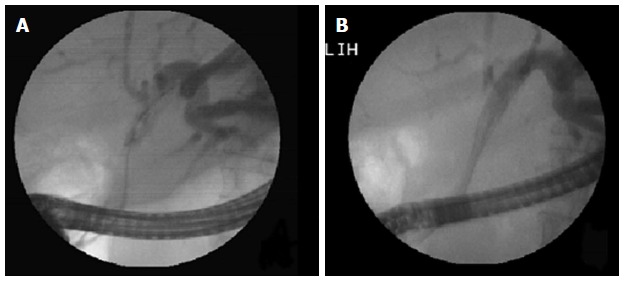
Hilar stricture: Unilateral metal stent placed in dilated left lobe duct. A: Left duct selectively cannulated and showing dilated system; B: Metal stent being deployed in the left ductal system.
Table 4.
Computed tomography/magnetic resonance cholangio-pancreatography -Guided Selective Unilateral Stenting
Endoscopic stenting technique
Stents in HC are mostly introduced at endoscopy[23]. In terms of the complexity of endoscopic procedures, stenting a hilar block is considered one of the most difficult. In a recent consensus, the American Society of Gastrointestinal Endoscopy graded endoscopic hilar stenting as a level 3 ERCP procedure in terms of complexity, with level 1 being the simplest and level 4 being most complex[29]. In general, a higher level of complexity is associated with a lower success rate and a higher complication rate[30]. Therefore, hilar stenting should be practiced only be experienced therapeutic endoscopists.
A variety of plastic and SEMS are available and have been used for the stenting in HC. In general, 10 Fr plastic stent and uncovered SEMS are preferred. The distal end of stents may be left in the duodenum or in distal bile duct, but the later situation may make reintervention more difficult. When more than one stent (plastic or SEMS) is to be placed, the stents are usually placed side by side (Figure 4). However, in the last few years, a new dual stent design called “stent-in-stent” has been developed for metal stenting[31,32] (Figure 5). In this device, the first stent has an open-cell design, allowing the second stent to pass easily through the first stent. In a recent study by Lee et al[33] from South Korea, stent-in-stent technology for bilateral stenting was evaluated in 84 patients with inoperable, high-grade HC. Technical and clinical success was achieved in 95.2% and 92.9% of patients, respectively. The median survival and patency were noted to be 256 d and 239 d, respectively. Still, this new stent design can be problematic if the first stent becomes occluded. In the study by Lee et al[33], 30.8% patients had an obstruction of the primary biliary stents. For revision stenting, bilateral metal stents could be placed in 55%, while plastic stents were placed in the remaining patients[33].
Figure 4.
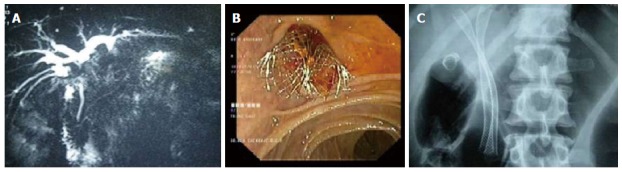
Two metal stents placed side by side in a Type II Bismuth hilar cholangiocarcinoma. A: Magnetic resonance cholangiopancreaticography showing the stricture; B: Endoscopic view showing two metal stents placed side by side; C: X-ray showing two parallel stents with proximal ends in the right or left ductal systems.
Figure 5.
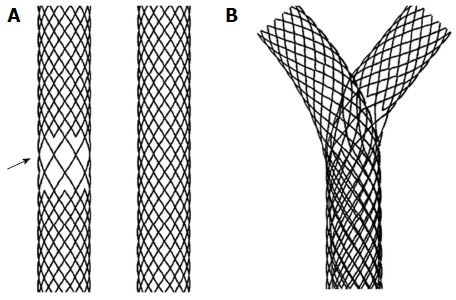
Y stent (stent-in-stent) from Tae Woong Medical (South Korea) for Hilar Cholangiocarcinoma. A: Two stents one with open mesh in the middle (arrow) and other with normal mesh; B: Y stent system in which the normal mesh stent passes through the open mesh of the second stent.
It is important to institute antibiotic prophylaxis in patients with anticipated incomplete biliary drainage by any technique[34]. Antibiotics should be continued in cases of incomplete biliary drainage. The choice of antibiotics should be based on local hospital data.
The occlusion of metal stents, while more common and earlier with plastic stents, can still occur[35]. Blocked plastic stents should always be removed, and the bile ducts should be cleaned of all sludge by a balloon. Then, a new plastic stent can be positioned. Metal stents, however, may be the better choice, particularly in the presence of thick bile or purulent material. It may also be a good idea to temporarily place a nasobiliary drain for repeated flushing prior to restenting[35]. Uncovered SEMS cannot be removed and therefore should be cleaned if they become clogged. However, it is advisable to place another stent through the blocked metal stent. The new stent could be either plastic or SEMS, depending upon the patient’s life expectancy.
PERCUTANEOUS BILIARY DRAINAGE
With technical expertise, it is also possible to perform palliative intervention in HC by percutaneous techniques (Figure 6). Percutaneous procedures have the theoretical advantage of precise lobar selection, which can result in less cholangitis. Moreover, the percutaneous approach can be performed with minimal sedation, which may be beneficial in an unstable patient. Alternatively, the percutaneous approach may be a two-step procedure with external drainage (percutaneous transhepatic biliary drainage) during first session, followed by internalization and subsequent stenting. Pain at the puncture site and the risk of biliary peritonitis are additional concerns with the use of percutaneous approach.
Figure 6.
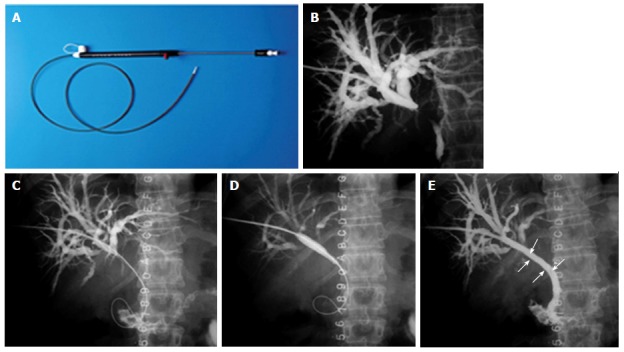
Percutaneous stenting in Bismuth Type I hilar cholangiocarcinoma. A: Stent assembly; B: Cholangiogram showing the block; C: Guide wire being negotiated across the stricture; D: Balloon dilatation being performed; E: Stent (arrow) after deployment.
Just as with the endoscopic data, the advantages of metal stents over plastic stents have been demonstrated in percutaneous approaches as well. Prolonged survival and lower morbidity have been shown with metal stents compared to plastic stents[36]. While using multiple SEMS percutaneously, stents may be placed side by side. However, a T or Y configuration (Figure 7), in which one stent crosses the block and the second stent only reaches the first stent or crosses from the left to the right ducts, have also been used successfully[37]. T or Y configuration stents have been shown to have a median patency of 279 and 218 d and a median survival of 334 and 375 d, respectively[38,39].
Figure 7.
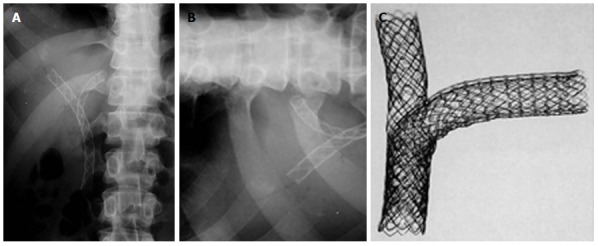
Percutaneous double stenting. A: X-ray showing the Y configuration of the stent (Boston Scientific MA, United States); B: Transverse limb of a T stent (Taewoong Medial, South Korea) showing the open mesh in the center; C: Assembly of a T stent showing the vertical stent passing through the open mesh of the transverse stent.
Few studies have compared percutaneous procedures with endoscopic techniques in HC, and no randomized controlled trials are available addressing this issue. A multicenter retrospective study[40] from South Korea has compared the results of endoscopy vs percutaneous techniques for advanced HC (Bismuth type III and IV). In their 85 patients (endoscopy 44, percutaneous 41), they noted better results for percutaneous procedure, (compared to endoscopy) with a significantly higher success rate (93% vs 77%, P = 0.049) and a trend towards a lesser cholangitis risk (22% vs 29.5%). Similar conclusions were drawn from two other studies from Asia[41,42]. Non-infective complications, such as bleeding and pancreatitis, were more frequent in the percutaneous group. This result suggests that, if infective complication can be avoided by performing contrast-free cannulation, ERCP may have an advantage, even in advanced HC.
In contrast to these results, endoscopy in general is the preferred approach for less advanced Bismuth type (Type I and II) HCs, mainly because endoscopy is a less invasive and faster procedure. However, no comparative data are available, and local expertise usually guides the treatment approach in these patients. Occasionally, palliation after endoscopic stenting may be suboptimal due to the presence of pus flakes, mucus or blood clots in the biliary ductal system. A temporary placement of a percutaneous catheter can allow repeated saline irrigations and may enable effective biliary drainage. It must be recognized that HC requires a multidisciplinary approach, with close co-ordination between the endoscopist and radiologist. For example, a failed endoscopic drainage after ductal opacification may result in suppurative cholangitis, and an emergency percutaneous drainage may became mandatory.
ENDOSONOGRAPHY GUIDED BILIARY DRAINAGE
Endosonography (EUS)-guided biliary drainage is emerging as an alternative to ERCP and percutaneous biliary stenting[43-46]. In the present scenario, an EUS-guided procedure is performed only if the ERCP fails in the presence of a tight hilar block or distorted duodenal anatomy. It remains unclear if EUS-guided drainage should be the second preferred modality or if it should be performed only when the percutaneous procedure fails or is contraindicated. Prospective randomized trials are needed to solve this issue.
The EUS technique involves the puncture of the left hepatic ducts, usually through the gastric wall, i.e., hepaticogastrostomy. A guide wire is then passed through this tract. Stenting can then be achieved in one of the three ways: (1) negotiating the wire across the hilar stricture and then passing it through the ampulla into the duodenum; the rendezvous procedure is then performed by catching the wire at the papilla, positioning the stent as in the ERCP procedure[43,44]; (2) negotiating the wire across the hilar stricture and then placing the stent across the stricture[45]; or (3) placing the stent across the hepatico-jejunostomy itself without negotiating the wire across the stricture[46].
In experienced hands, EUS-guided biliary drainage has a technical success rate of 70%-98%. However, up to 20% of patients can have complications, such as bile leak, biliary peritonitis, pneumoperitoneum, bleeding, pain or stent migration[43-50]. This complication rate is higher than that reported historically with ERCP. All types of stents, including plastic, uncovered SEMS and covered (partial or total) SEMS, have been used. However, covered SEMS are usually preferred. Between the three techniques described above, the rendezvous technique is more natural and is preferred; however, there are no comparative data with other techniques, and the procedure is more demanding[43,44].
EUS-guided procedures are exciting but still evolving. Because most of the data are in the form of a case series, prospective randomized studies are required to position the modality correctly in the management algorithm of hilar blocks.
SURGICAL BYPASS
Due to its invasiveness, surgical bypass has a very limited role to play in palliating hilar tumors. It is only accepted when all of the other techniques described above have failed or are not available, as well as occasionally when laparotomy has been performed for curative intent but an unresectable tumor is revealed[51,52]. The various surgical drainage procedures that can be carried out include segment III hepaticojejunostomy, stent placement across the tumor and sectoral duct (i.e., right anterior, right posterior or left hepatic duct) bypass. However, segment III bypass is the preferred choice because it resolves jaundice in approximately two-thirds of HC patients and has a median survival of approximately 6.3 mo[53,54].
LOCAL ABLATIVE THERAPY
A few exciting techniques have been used to improve the results of biliary stenting with the aim of delaying stent blockage and prolonging patient survival. These techniques include photodynamic therapy, radiofrequency ablation and chemo/radiotherapy.
Photodynamic therapy
Photodynamic therapy (PDT) is a relatively new technique that has evolved over the last 10 years[55]. PDT uses a photosensitive agent that concentrates preferentially in malignant tumors. Subsequent photoactivation with red laser lights of a specific wavelength[56] creates reactive oxygen, leading to selective tumor cell death. Table 5 summarizes some of the studies[56-58] that have shown improved patient survival by the addition of PDT to biliary stenting. Studies have also shown improved quality of life after PDT[56,58]. In HC patients who have been treated with plastic stents, the stents should be removed temporarily for light delivery during PDT. In cases of SEMS, PDT can be performed through the stent by adjusted the required light dose to compensate for the decreased transmission of light[59]. A further moot point that should be addressed is whether these results can be reproduced in certain Asian countries, in which the sun does not affect skin photosensitivity to the same extent as in the Western white population[57,60].
Table 5.
Photodynamic therapy as an adjunct to biliary stenting: Improved survival
| Year | No. of pts |
Median survival (mo) |
P value | ||
| Stenting alone | Stenting with PDT | ||||
| Ortner et al[56] | 2003 | 39 | 3 | 16 | < 0.0001 |
| Cheon et al[57] | 2004 | 47 | 10 | 18 | 0.0143 |
| Zoepf et al[58] | 2005 | 32 | 7 | 21 | 0.0109 |
PDT: Photodynamic therapy.
In general, PDT is preferred through the percutaneous route [compared to the endoscopic] because of the lower chance of laser tip fracture, the easy repeatability of the procedure and the feasibility of obtaining cholangiogram. Shim et al[61] showed that PDT using percutaneous cholangioscopy is safe and effective, improving quality of life with a good median survival time (i.e., 558 ± 178.8 d). Cholangitis, hemobilia and photosensitivity are known complications of PDT.
A recent study by Wagner et al[62] used temoporfin rather than conventional porfimer for PDT in 10 patients with HC. Temoporfin PDT was highly tumoricidal and had double the depth of local tumor ablative effect, compared to porfimer PDT. Infectious complications and skin photosensitivity were similar with both agents[62].
Radiofrequency ablation
Radiofrequency ablation (RFA) is an ablative procedure that is well established for treating small liver cancers. With the introduction of an endoscopic probe for RFA and the use of a lower power setting with the existing generators, it is now possible to treat pancreatico-biliary cancers with this modality[63]. Within the bile duct, it has the potential of improving stent patency by decreasing tumor ingrowth and benign epithelial hyperplasias. Steel et al[64] were the first to demonstrate its safety and efficacy in 22 patients with biliary malignancy (pancreatic/cholangiocarcinoma). A recent study involving 20 patients with pancreatico-biliary malignancy (11 with cholangiocarcinoma) showed a significant increase in bile duct diameter after RFA[65]. Further studies are required to revalidate these results, but the preliminary data are interesting.
Chemo/radiotherapy
Radiotherapy has a very limited role in HC. Intraluminal brachytherapy has been delivered to cholangiocarcinoma using Ir-S192 seeds, with a total dose of 30-50 Gy through the percutaneous route. Good short-term effects were demonstrated, showing prolonged stent patency and improved survival[66]. A number of studies from Japan have shown better SEMS patency (10-18 mo vs 4-12 mo) after external beam radiotherapy[67,68].
Chemotherapy has been used in HC as a palliative therapy for both locally advanced cancers and metastatic disease[69]. However, most of these studies involved case series with a heterogeneous mixture of patients with HC or distal bile duct cancers, as well as pancreatic or gall bladder cancers. A retrospective study comparing a gemcitabine-based regimen with a cisplatin-based therapy and a fluoropyrimidine-based therapy clearly showed the gemcitabine-based regimen to be superior in terms of the lowest fatality[70]. Gemcitabine has been used in combination with cisplatin, oxaliplatin or capecitabine to improve its efficacy[69]. Valle et al[71,72] compared gemcitabine with a combination of gemcitabine with cisplatine in their two ABC trials, which were published sequentially. ABC-02 trial by Valle et al[72], which involved 410 patients with advanced biliary cancers in a multicenter randomized phase III study, clearly showed the superiority of the combination over gemcitabine alone. The combination improved progression-free survival and overall survival by 30% over gemcitabine alone. Another multicenter trial from Japan[73] also demonstrated the superiority of gemcitabine combined with cisplatin over gemcitabine alone in terms of one year survival, median survival time, median progression-free survival time and overall response rate. These data have resulted in this combination being used as standard of care chemotherapy for advanced cholangiocarcinoma, including HC. Gemcitabine-free combinations, such as capecitabine with oxaliplatin, capecitabine with cisplatin and 5 fluorouracil with cisplatin, have also been used for bile duct cancers with modest results[69]. However, newer drugs are being investigated for bile duct cancers; these include erlotinib, cetuximab and bevacizumab[69].
CONCLUSION
HC is the most common type of malignancy of the bile duct and is highly prevalent in the Eastern Hemisphere. Unfortunately, the majority of patients with HC manifest too late for resection. Therefore, palliation is the goal in the majority of patients. Palliation is usually performed with ERCP or through a percutaneous route. Figure 8 shows our approach and suggested guidelines based on Bismuth types. For advanced HC (Bismuth type III and IV), we prefer the percutaneous route because this has been shown to be superior to the endoscopic approach. For Bismuth type I and II, ERCP is usually preferred in view of its being less invasive and faster. Generally, we prefer metal stents over plastic stents because of the former’s documented better median patency and patient survival. Plastic stents are only offered when the expected survival is very short. Controversy continues as to whether single or multiple stents should be preferred for advanced HC. Recent data suggesting that better outcomes will be obtained if > 50% of the liver is drained have made us change our policy such that we offer two stents in the majority of patients. EUS-guided biliary drainage is also gradually emerging as an alternative modality. Percutaneous or EUS-guided biliary drainage is at present offered for all patients who fail ERCP or for patients who cannot undergo ERCP due to their altered anatomy or duodenal obstructions from tumors. A few reports showed that photodynamic therapy and radio-frequency ablation could improve the patency of biliary stenting and patient survival, suggesting that this procedure should be considered in advanced tumors (Bismuth type III and IV). Local expertise is often the deciding factor for choosing one modality over the other.
Figure 8.
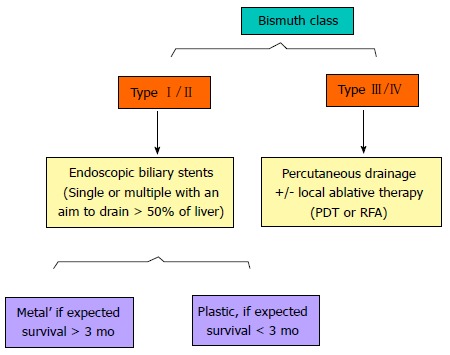
A simplified algorithm suggested for the palliation of Hilar Cholangiocarcinoma, based on Bismuth type. Additional percutaneous procedures may be required if the endoscopic stenting results are suboptimal. PDT: Photodynamic therapy; RFA: Radiofrequency ablation.
Footnotes
P- Reviewer: Cheon YK, Fusai G, Shim CS S- Editor: Song XX L- Editor: A E- Editor: Wu HL
References
- 1.Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24:115–125. doi: 10.1055/s-2004-828889. [DOI] [PubMed] [Google Scholar]
- 2.Yusoff AR, Siti ZM, Muzammil AR, Yoong BK, Vijeyasingam R. Cholangiocarcinoma: a 10-year experience of a single tertiary centre in the multi ethnicity-Malaysia. Med J Malaysia. 2012;67:45–51. [PubMed] [Google Scholar]
- 3.Shin HR, Oh JK, Masuyer E, Curado MP, Bouvard V, Fang YY, Wiangnon S, Sripa B, Hong ST. Epidemiology of cholangiocarcinoma: an update focusing on risk factors. Cancer Sci. 2010;101:579–585. doi: 10.1111/j.1349-7006.2009.01458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Otani K, Chijiiwa K, Kai M, Ohuchida J, Nagano M, Tsuchiya K, Kondo K. Outcome of surgical treatment of hilar cholangiocarcinoma. J Gastrointest Surg. 2008;12:1033–1040. doi: 10.1007/s11605-007-0453-z. [DOI] [PubMed] [Google Scholar]
- 5.Li H, Qin Y, Cui Y, Chen H, Hao X, Li Q. Analysis of the surgical outcome and prognostic factors for hilar cholangiocarcinoma: a Chinese experience. Dig Surg. 2011;28:226–231. doi: 10.1159/000327361. [DOI] [PubMed] [Google Scholar]
- 6.Aljiffry M, Walsh MJ, Molinari M. Advances in diagnosis, treatment and palliation of cholangiocarcinoma: 1990-2009. World J Gastroenterol. 2009;15:4240–4262. doi: 10.3748/wjg.15.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hemming AW, Reed AI, Fujita S, Foley DP, Howard RJ. Surgical management of hilar cholangiocarcinoma. Ann Surg. 2005;241:693–699; discussion 699-702. doi: 10.1097/01.sla.0000160701.38945.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masselli G, Manfredi R, Vecchioli A, Gualdi G. MR imaging and MR cholangiopancreatography in the preoperative evaluation of hilar cholangiocarcinoma: correlation with surgical and pathologic findings. Eur Radiol. 2008;18:2213–2221. doi: 10.1007/s00330-008-1004-z. [DOI] [PubMed] [Google Scholar]
- 9.Lee MG, Park KB, Shin YM, Yoon HK, Sung KB, Kim MH, Lee SG, Kang EM. Preoperative evaluation of hilar cholangiocarcinoma with contrast-enhanced three-dimensional fast imaging with steady-state precession magnetic resonance angiography: comparison with intraarterial digital subtraction angiography. World J Surg. 2003;27:278–283. doi: 10.1007/s00268-002-6701-1. [DOI] [PubMed] [Google Scholar]
- 10.Petrowsky H, Wildbrett P, Husarik DB, Hany TF, Tam S, Jochum W, Clavien PA. Impact of integrated positron emission tomography and computed tomography on staging and management of gallbladder cancer and cholangiocarcinoma. J Hepatol. 2006;45:43–50. doi: 10.1016/j.jhep.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 11.Tamada K, Ido K, Ueno N, Ichiyama M, Tomiyama T, Nishizono T, Wada S, Noda T, Tano S, Aizawa T. Assessment of portal vein invasion by bile duct cancer using intraductal ultrasonography. Endoscopy. 1995;27:573–578. doi: 10.1055/s-2007-1005760. [DOI] [PubMed] [Google Scholar]
- 12.Ruys AT, Busch OR, Gouma DJ, van Gulik TM. Staging laparoscopy for hilar cholangiocarcinoma: is it still worthwhile? Ann Surg Oncol. 2011;18:2647–2653. doi: 10.1245/s10434-011-1576-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raju RP, Jaganmohan SR, Ross WA, Davila ML, Javle M, Raju GS, Lee JH. Optimum palliation of inoperable hilar cholangiocarcinoma: comparative assessment of the efficacy of plastic and self-expanding metal stents. Dig Dis Sci. 2011;56:1557–1564. doi: 10.1007/s10620-010-1550-5. [DOI] [PubMed] [Google Scholar]
- 14.Perdue DG, Freeman ML, DiSario JA, Nelson DB, Fennerty MB, Lee JG, Overby CS, Ryan ME, Bochna GS, Snady HW, et al. Plastic versus self-expanding metallic stents for malignant hilar biliary obstruction: a prospective multicenter observational cohort study. J Clin Gastroenterol. 2008;42:1040–1046. doi: 10.1097/MCG.0b013e31815853e0. [DOI] [PubMed] [Google Scholar]
- 15.Sangchan A, Kongkasame W, Pugkhem A, Jenwitheesuk K, Mairiang P. Efficacy of metal and plastic stents in unresectable complex hilar cholangiocarcinoma: a randomized controlled trial. Gastrointest Endosc. 2012;76:93–99. doi: 10.1016/j.gie.2012.02.048. [DOI] [PubMed] [Google Scholar]
- 16.De Palma GD, Galloro G, Siciliano S, Iovino P, Catanzano C. Unilateral versus bilateral endoscopic hepatic duct drainage in patients with malignant hilar biliary obstruction: results of a prospective, randomized, and controlled study. Gastrointest Endosc. 2001;53:547–553. doi: 10.1067/mge.2001.113381. [DOI] [PubMed] [Google Scholar]
- 17.Naitoh I, Ohara H, Nakazawa T, Ando T, Hayashi K, Okumura F, Okayama Y, Sano H, Kitajima Y, Hirai M, et al. Unilateral versus bilateral endoscopic metal stenting for malignant hilar biliary obstruction. J Gastroenterol Hepatol. 2009;24:552–557. doi: 10.1111/j.1440-1746.2008.05750.x. [DOI] [PubMed] [Google Scholar]
- 18.Chang WH, Kortan P, Haber GB. Outcome in patients with bifurcation tumors who undergo unilateral versus bilateral hepatic duct drainage. Gastrointest Endosc. 1998;47:354–362. doi: 10.1016/s0016-5107(98)70218-4. [DOI] [PubMed] [Google Scholar]
- 19.Singh V, Singh G, Verma GR, Singh K, Gulati M. Contrast-free unilateral endoscopic palliation in malignant hilar biliary obstruction: new method. J Gastroenterol Hepatol. 2004;19:589–592. doi: 10.1111/j.1440-1746.2003.03313.x. [DOI] [PubMed] [Google Scholar]
- 20.Dowsett JF, Vaira D, Hatfield AR, Cairns SR, Polydorou A, Frost R, Croker J, Cotton PB, Russell RC, Mason RR. Endoscopic biliary therapy using the combined percutaneous and endoscopic technique. Gastroenterology. 1989;96:1180–1186. doi: 10.1016/0016-5085(89)91639-9. [DOI] [PubMed] [Google Scholar]
- 21.Vienne A, Hobeika E, Gouya H, Lapidus N, Fritsch J, Choury AD, Chryssostalis A, Gaudric M, Pelletier G, Buffet C, et al. Prediction of drainage effectiveness during endoscopic stenting of malignant hilar strictures: the role of liver volume assessment. Gastrointest Endosc. 2010;72:728–735. doi: 10.1016/j.gie.2010.06.040. [DOI] [PubMed] [Google Scholar]
- 22.Bismuth H. Surgical anatomy and anatomical surgery of the liver. World J Surg. 1982;6:3–9. doi: 10.1007/BF01656368. [DOI] [PubMed] [Google Scholar]
- 23.Khan SA, Davidson BR, Goldin R, Pereira SP, Rosenberg WM, Taylor-Robinson SD, Thillainayagam AV, Thomas HC, Thursz MR, Wasan H. Guidelines for the diagnosis and treatment of cholangiocarcinoma: consensus document. Gut. 2002;51 Suppl 6:VI1–VI9. doi: 10.1136/gut.51.suppl_6.vi1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan SA, Davidson BR, Goldin RD, Heaton N, Karani J, Pereira SP, Rosenberg WM, Tait P, Taylor-Robinson SD, Thillainayagam AV, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: an update. Gut. 2012;61:1657–1669. doi: 10.1136/gutjnl-2011-301748. [DOI] [PubMed] [Google Scholar]
- 25.Hintze RE, Abou-Rebyeh H, Adler A, Veltzke-Schlieker W, Felix R, Wiedenmann B. Magnetic resonance cholangiopancreatography-guided unilateral endoscopic stent placement for Klatskin tumors. Gastrointest Endosc. 2001;53:40–46. doi: 10.1067/mge.2001.111388. [DOI] [PubMed] [Google Scholar]
- 26.Freeman ML, Overby C. Selective MRCP and CT-targeted drainage of malignant hilar biliary obstruction with self-expanding metallic stents. Gastrointest Endosc. 2003;58:41–49. doi: 10.1067/mge.2003.292. [DOI] [PubMed] [Google Scholar]
- 27.Harewood GC, Baron TH. Cost analysis of magnetic resonance cholangiography in the management of inoperable hilar biliary obstruction. Am J Gastroenterol. 2002;97:1152–1158. doi: 10.1111/j.1572-0241.2002.05682.x. [DOI] [PubMed] [Google Scholar]
- 28.Singh V, Singh G, Gupta V, Gupta R, Kapoor R. Contrast-free air cholangiography-assisted unilateral plastic stenting in malignant hilar biliary obstruction. Hepatobiliary Pancreat Dis Int. 2010;9:88–92. [PubMed] [Google Scholar]
- 29.Cotton PB, Eisen G, Romagnuolo J, Vargo J, Baron T, Tarnasky P, Schutz S, Jacobson B, Bott C, Petersen B. Grading the complexity of endoscopic procedures: results of an ASGE working party. Gastrointest Endosc. 2011;73:868–874. doi: 10.1016/j.gie.2010.12.036. [DOI] [PubMed] [Google Scholar]
- 30.Ragunath K, Thomas LA, Cheung WY, Duane PD, Richards DG. Objective evaluation of ERCP procedures: a simple grading scale for evaluating technical difficulty. Postgrad Med J. 2003;79:467–470. doi: 10.1136/pmj.79.934.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park do H, Lee SS, Moon JH, Choi HJ, Cha SW, Kim JH, Seo DW, Lee SK, Park SH, Lee MS, et al. Newly designed stent for endoscopic bilateral stent-in-stent placement of metallic stents in patients with malignant hilar biliary strictures: multicenter prospective feasibility study (with videos) Gastrointest Endosc. 2009;69:1357–1360. doi: 10.1016/j.gie.2008.12.250. [DOI] [PubMed] [Google Scholar]
- 32.Chahal P, Baron TH. Expandable metal stents for endoscopic bilateral stent-within-stent placement for malignant hilar biliary obstruction. Gastrointest Endosc. 2010;71:195–199. doi: 10.1016/j.gie.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 33.Lee TH, Moon JH, Kim JH, Park DH, Lee SS, Choi HJ, Cho YD, Park SH, Kim SJ. Primary and revision efficacy of cross-wired metallic stents for endoscopic bilateral stent-in-stent placement in malignant hilar biliary strictures. Endoscopy. 2013;45:106–113. doi: 10.1055/s-0032-1325928. [DOI] [PubMed] [Google Scholar]
- 34.Banerjee S, Shen B, Baron TH, Nelson DB, Anderson MA, Cash BD, Dominitz JA, Gan SI, Harrison ME, Ikenberry SO, et al. Antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2008;67:791–798. doi: 10.1016/j.gie.2008.02.068. [DOI] [PubMed] [Google Scholar]
- 35.Dumonceau JM, Tringali A, Blero D, Devière J, Laugiers R, Heresbach D, Costamagna G. Biliary stenting: indications, choice of stents and results: European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline. Endoscopy. 2012;44:277–298. doi: 10.1055/s-0031-1291633. [DOI] [PubMed] [Google Scholar]
- 36.Hii MW, Gibson RN, Speer AG, Collier NA, Sherson N, Jardine C. Role of radiology in the treatment of malignant hilar biliary strictures 2: 10 years of single-institution experience with percutaneous treatment. Australas Radiol. 2003;47:393–403. doi: 10.1046/j.1440-1673.2003.01209.x. [DOI] [PubMed] [Google Scholar]
- 37.Ahn SJ, Bae JI, Han TS, Won JH, Kim JD, Kwack KS, Lee JH, Kim YC. Percutaneous biliary drainage using open cell stents for malignant biliary hilar obstruction. Korean J Radiol. 2012;13:795–802. doi: 10.3348/kjr.2012.13.6.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gwon DI, Ko GY, Yoon HK, Kim JH, Lee JM, Ohm JY, Sung KB. Prospective evaluation of a newly designed T-configured stent graft system for palliative treatment of advanced hilar malignant biliary obstructions. J Vasc Interv Radiol. 2010;21:1410–1418. doi: 10.1016/j.jvir.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 39.Gwon DI, Ko GY, Yoon HK, Kim YJ, Kim TH, Lee WH, Sung KB. Safety and efficacy of percutaneous Y-configured covered stent placement for malignant hilar biliary obstruction: a prospective, pilot study. J Vasc Interv Radiol. 2012;23:528–534. doi: 10.1016/j.jvir.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 40.Paik WH, Park YS, Hwang JH, Lee SH, Yoon CJ, Kang SG, Lee JK, Ryu JK, Kim YT, Yoon YB. Palliative treatment with self-expandable metallic stents in patients with advanced type III or IV hilar cholangiocarcinoma: a percutaneous versus endoscopic approach. Gastrointest Endosc. 2009;69:55–62. doi: 10.1016/j.gie.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 41.Rerknimitr R, Kladcharoen N, Mahachai V, Kullavanijaya P. Result of endoscopic biliary drainage in hilar cholangiocarcinoma. J Clin Gastroenterol. 2004;38:518–523. doi: 10.1097/01.mcg.0000123204.36471.be. [DOI] [PubMed] [Google Scholar]
- 42.Lee SH, Park JK, Yoon WJ, Lee JK, Ryu JK, Yoon YB, Kim YT. Optimal biliary drainage for inoperable Klatskin’s tumor based on Bismuth type. World J Gastroenterol. 2007;13:3948–3955. doi: 10.3748/wjg.v13.i29.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dhir V, Bhandari S, Bapat M, Maydeo A. Comparison of EUS-guided rendezvous and precut papillotomy techniques for biliary access (with videos) Gastrointest Endosc. 2012;75:354–359. doi: 10.1016/j.gie.2011.07.075. [DOI] [PubMed] [Google Scholar]
- 44.Kim YS, Gupta K, Mallery S, Li R, Kinney T, Freeman ML. Endoscopic ultrasound rendezvous for bile duct access using a transduodenal approach: cumulative experience at a single center. A case series. Endoscopy. 2010;42:496–502. doi: 10.1055/s-0029-1244082. [DOI] [PubMed] [Google Scholar]
- 45.Nguyen-Tang T, Binmoeller KF, Sanchez-Yague A, Shah JN. Endoscopic ultrasound (EUS)-guided transhepatic anterograde self-expandable metal stent (SEMS) placement across malignant biliary obstruction. Endoscopy. 2010;42:232–236. doi: 10.1055/s-0029-1243858. [DOI] [PubMed] [Google Scholar]
- 46.Park do H, Song TJ, Eum J, Moon SH, Lee SS, Seo DW, Lee SK, Kim MH. EUS-guided hepaticogastrostomy with a fully covered metal stent as the biliary diversion technique for an occluded biliary metal stent after a failed ERCP (with videos) Gastrointest Endosc. 2010;71:413–419. doi: 10.1016/j.gie.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 47.Shami VM, Kahaleh M. Endoscopic ultrasound-guided cholangiopancreatography and rendezvous techniques. Dig Liver Dis. 2010;42:419–424. doi: 10.1016/j.dld.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 48.Eum J, Park do H, Ryu CH, Kim HJ, Lee SS, Seo DW, Lee SK, Kim MH. EUS-guided biliary drainage with a fully covered metal stent as a novel route for natural orifice transluminal endoscopic biliary interventions: a pilot study (with videos) Gastrointest Endosc. 2010;72:1279–1284. doi: 10.1016/j.gie.2010.07.026. [DOI] [PubMed] [Google Scholar]
- 49.Park do H, Koo JE, Oh J, Lee YH, Moon SH, Lee SS, Seo DW, Lee SK, Kim MH. EUS-guided biliary drainage with one-step placement of a fully covered metal stent for malignant biliary obstruction: a prospective feasibility study. Am J Gastroenterol. 2009;104:2168–2174. doi: 10.1038/ajg.2009.254. [DOI] [PubMed] [Google Scholar]
- 50.Yamao K, Hara K, Mizuno N, Sawaki A, Hijioka S, Niwa Y, Tajika M, Kawai H, Kondo S, Shimizu Y, et al. EUS-Guided Biliary Drainage. Gut Liver. 2010;4 Suppl 1:S67–S75. doi: 10.5009/gnl.2010.4.S1.S67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Witzigmann H, Lang H, Lauer H. Guidelines for palliative surgery of cholangiocarcinoma. HPB (Oxford) 2008;10:154–160. doi: 10.1080/13651820801992567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang BH, Cheng QB, Luo XJ, Zhang YJ, Jiang XQ, Zhang BH, Yi B, Yu WL, Wu MC. Surgical therapy for hiliar cholangiocarcinoma: analysis of 198 cases. Hepatobiliary Pancreat Dis Int. 2006;5:278–282. [PubMed] [Google Scholar]
- 53.Guthrie CM, Banting SW, Garden OJ, Carter DC. Segment III cholangiojejunostomy for palliation of malignant hilar obstruction. Br J Surg. 1994;81:1639–1641. doi: 10.1002/bjs.1800811125. [DOI] [PubMed] [Google Scholar]
- 54.Li HM, Dou KF, Sun K, Gao ZQ, Li KZ, Fu YC. Palliative surgery for hilar cholangiocarcinoma. Hepatobiliary Pancreat Dis Int. 2003;2:110–113. [PubMed] [Google Scholar]
- 55.Lee TY, Cheon YK, Shim CS. Current status of photodynamic therapy for bile duct cancer. Clin Endosc. 2013;46:38–44. doi: 10.5946/ce.2013.46.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ortner ME, Caca K, Berr F, Liebetruth J, Mansmann U, Huster D, Voderholzer W, Schachschal G, Mössner J, Lochs H. Successful photodynamic therapy for nonresectable cholangiocarcinoma: a randomized prospective study. Gastroenterology. 2003;125:1355–1363. doi: 10.1016/j.gastro.2003.07.015. [DOI] [PubMed] [Google Scholar]
- 57.Cheon YK, Cho YD, Baek SH, Cha SW, Moon JH, Kim YS, Lee JS, Lee MS, Shim CS, Kim BS. Comparison of survival of advanced hilar cholangiocarcinoma after biliary drainage alone versus photodynamic therapy with external drainage. Korean J Gastroenterol. 2004;44:280–287. [PubMed] [Google Scholar]
- 58.Zoepf T, Jakobs R, Arnold JC, Apel D, Riemann JF. Palliation of nonresectable bile duct cancer: improved survival after photodynamic therapy. Am J Gastroenterol. 2005;100:2426–2430. doi: 10.1111/j.1572-0241.2005.00318.x. [DOI] [PubMed] [Google Scholar]
- 59.Wang LW, Li LB, Li ZS, Chen YK, Hetzel FW, Huang Z. Self-expandable metal stents and trans-stent light delivery: are metal stents and photodynamic therapy compatible? Lasers Surg Med. 2008;40:651–659. doi: 10.1002/lsm.20680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tomizawa Y, Tian J. Photodynamic therapy for unresectable cholangiocarcinoma. Dig Dis Sci. 2012;57:274–283. doi: 10.1007/s10620-011-1957-7. [DOI] [PubMed] [Google Scholar]
- 61.Shim CS, Cheon YK, Cha SW, Bhandari S, Moon JH, Cho YD, Kim YS, Lee LS, Lee MS, Kim BS. Prospective study of the effectiveness of percutaneous transhepatic photodynamic therapy for advanced bile duct cancer and the role of intraductal ultrasonography in response assessment. Endoscopy. 2005;37:425–433. doi: 10.1055/s-2005-861294. [DOI] [PubMed] [Google Scholar]
- 62.Wagner A, Kiesslich T, Neureiter D, Friesenbichler P, Puespoek A, Denzer UW, Wolkersdörfer GW, Emmanuel K, Lohse AW, Berr F. Photodynamic therapy for hilar bile duct cancer: clinical evidence for improved tumoricidal tissue penetration by temoporfin. Photochem Photobiol Sci. 2013;12:1065–1073. doi: 10.1039/c3pp25425a. [DOI] [PubMed] [Google Scholar]
- 63.Monga A, Gupta R, Ramchandani M, Rao GV, Santosh D, Reddy DN. Endoscopic radiofrequency ablation of cholangiocarcinoma: new palliative treatment modality (with videos) Gastrointest Endosc. 2011;74:935–937. doi: 10.1016/j.gie.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 64.Steel AW, Postgate AJ, Khorsandi S, Nicholls J, Jiao L, Vlavianos P, Habib N, Westaby D. Endoscopically applied radiofrequency ablation appears to be safe in the treatment of malignant biliary obstruction. Gastrointest Endosc. 2011;73:149–153. doi: 10.1016/j.gie.2010.09.031. [DOI] [PubMed] [Google Scholar]
- 65.Figueroa-Barojas P, Bakhru MR, Habib NA, Ellen K, Millman J, Jamal-Kabani A, Gaidhane M, Kahaleh M. Safety and efficacy of radiofrequency ablation in the management of unresectable bile duct and pancreatic cancer: a novel palliation technique. J Oncol. 2013;2013:910897. doi: 10.1155/2013/910897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Larghi A, Tringali A, Lecca PG, Giordano M, Costamagna G. Management of hilar biliary strictures. Am J Gastroenterol. 2008;103:458–473. doi: 10.1111/j.1572-0241.2007.01645.x. [DOI] [PubMed] [Google Scholar]
- 67.Isayama H, Tsujino T, Nakai Y, Sasaki T, Nakagawa K, Yamashita H, Aoki T, Koike K. Clinical benefit of radiation therapy and metallic stenting for unresectable hilar cholangiocarcinoma. World J Gastroenterol. 2012;18:2364–2370. doi: 10.3748/wjg.v18.i19.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shinchi H, Takao S, Nishida H, Aikou T. Length and quality of survival following external beam radiotherapy combined with expandable metallic stent for unresectable hilar cholangiocarcinoma. J Surg Oncol. 2000;75:89–94. doi: 10.1002/1096-9098(200010)75:2<89::aid-jso3>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 69.Ramírez-Merino N, Aix SP, Cortés-Funes H. Chemotherapy for cholangiocarcinoma: An update. World J Gastrointest Oncol. 2013;5:171–176. doi: 10.4251/wjgo.v5.i7.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yonemoto N, Furuse J, Okusaka T, Yamao K, Funakoshi A, Ohkawa S, Boku N, Tanaka K, Nagase M, Saisho H, et al. A multi-center retrospective analysis of survival benefits of chemotherapy for unresectable biliary tract cancer. Jpn J Clin Oncol. 2007;37:843–851. doi: 10.1093/jjco/hym116. [DOI] [PubMed] [Google Scholar]
- 71.Valle JW, Wasan H, Johnson P, Jones E, Dixon L, Swindell R, Baka S, Maraveyas A, Corrie P, Falk S, et al. Gemcitabine alone or in combination with cisplatin in patients with advanced or metastatic cholangiocarcinomas or other biliary tract tumours: a multicentre randomised phase II study - The UK ABC-01 Study. Br J Cancer. 2009;101:621–627. doi: 10.1038/sj.bjc.6605211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 73.Okusaka T, Nakachi K, Fukutomi A, Mizuno N, Ohkawa S, Funakoshi A, Nagino M, Kondo S, Nagaoka S, Funai J, et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer. 2010;103:469–474. doi: 10.1038/sj.bjc.6605779. [DOI] [PMC free article] [PubMed] [Google Scholar]


