Abstract
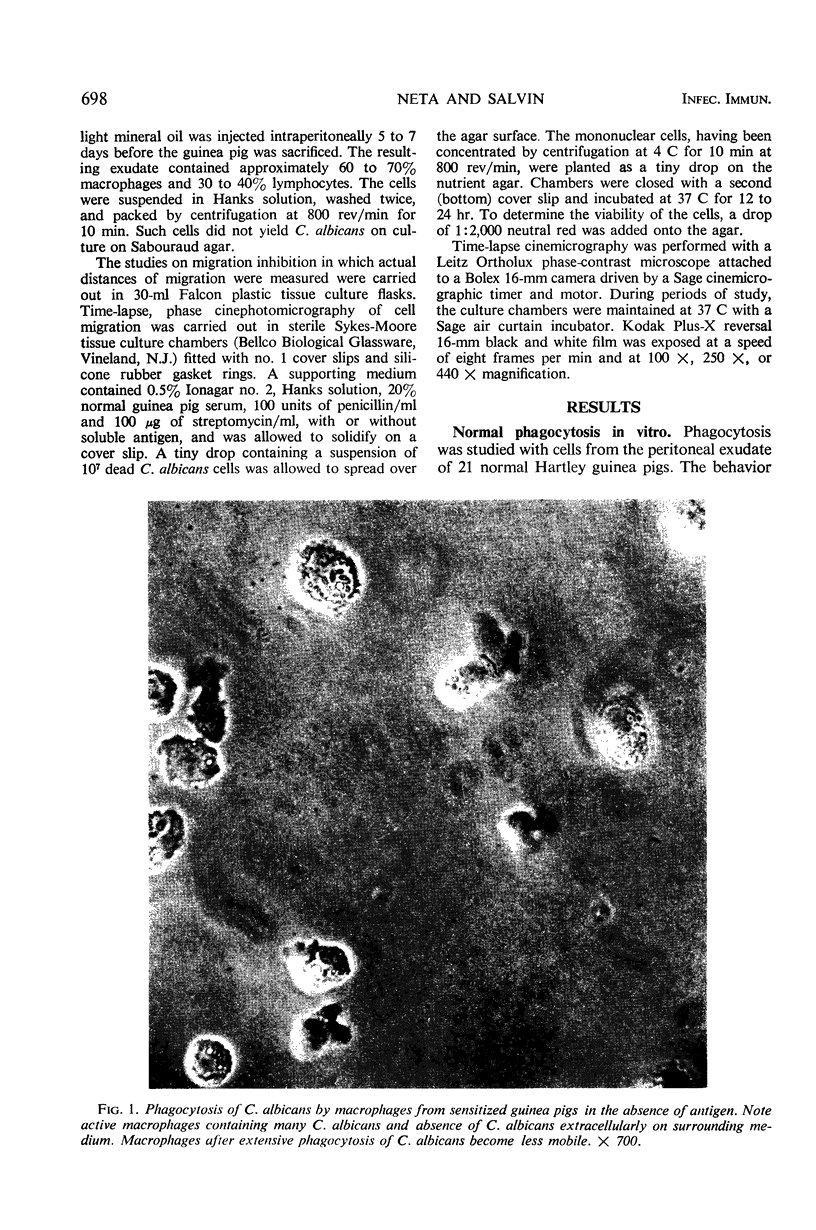
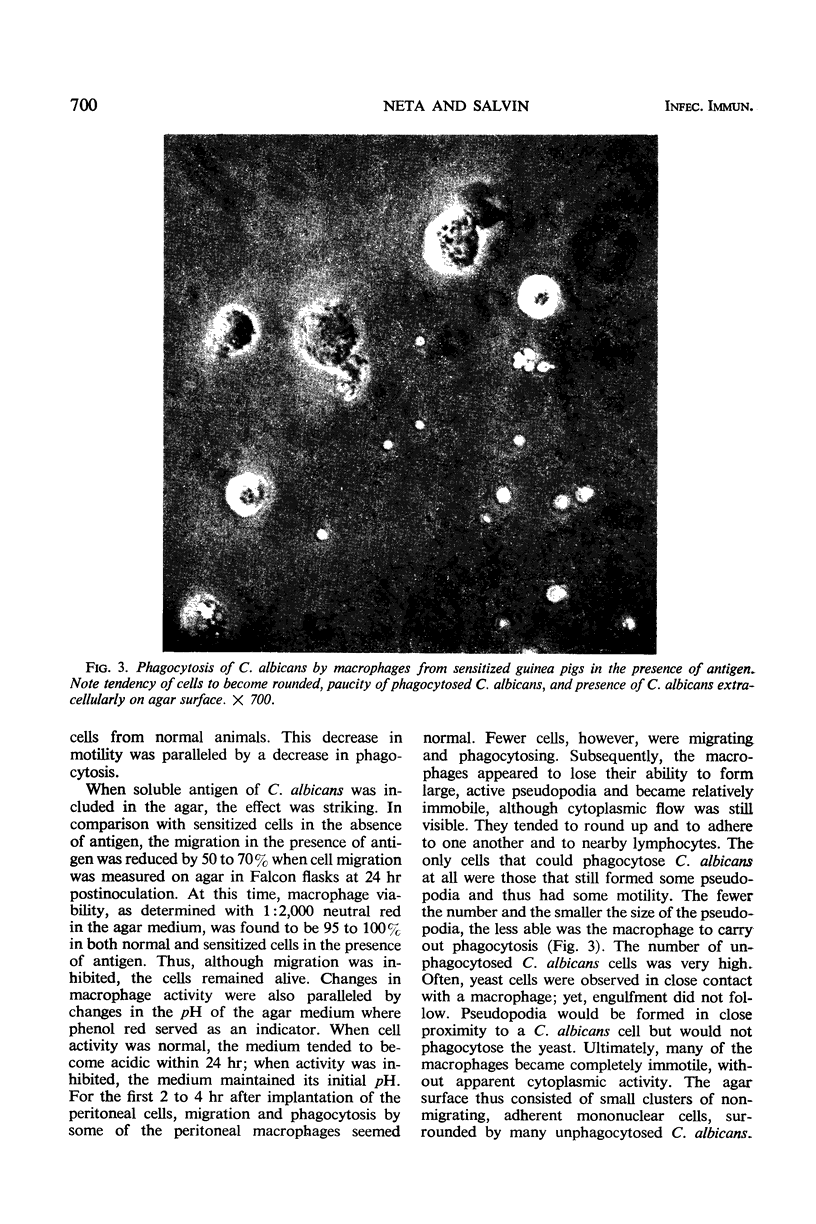
The activity of peritoneal exudate cells from candidin-sensitive and normal guinea pigs with and without antigen was studied in vitro with the aid of time-lapse, phase-contrast cinemicrography. Macrophages from normal animals migrate well on an agar medium and readily phagocytose Merthiolate-killed Candida albicans cells. A reduction in the migration of peritoneal macrophages from guinea pigs sensitized to C. albicans during the first 24 hr after exposure to antigen was accompanied by a decrease in phagocytosis of C. albicans cells. The macrophages were viable but comparatively immotile. Since the sensitized macrophages came from resistant donors, it is possible that the initial stage of cellular immunity to C. albicans is associated with a reduced activity of the phagocytic macrophages, apparently to limit spread of the pathogen from the infected area.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett B., Bloom B. R. Reactions in vivo and in vitro produced by a soluble substance associated with delayed-type hypersensitivity. Proc Natl Acad Sci U S A. 1968 Mar;59(3):756–762. doi: 10.1073/pnas.59.3.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauve R. M., Dekaris D. Macrophage spreading: inhibition in delayed hypersensitivity. Science. 1968 May 17;160(3829):795–796. doi: 10.1126/science.160.3829.795. [DOI] [PubMed] [Google Scholar]
- GEORGE M., VAUGHAN J. H. In vitro cell migration as a model for delayed hypersensitivity. Proc Soc Exp Biol Med. 1962 Nov;111:514–521. doi: 10.3181/00379727-111-27841. [DOI] [PubMed] [Google Scholar]
- Granger G. A., Shacks S. J., Williams T. W., Kolb W. P. Lymphocyte in vitro cytotoxicity: specific release of lymphotoxin-like materials from tuberculin-sensitive lymphoid cells. Nature. 1969 Mar 22;221(5186):1155–1157. doi: 10.1038/2211155a0. [DOI] [PubMed] [Google Scholar]
- Mackaness G. B. The monocyte in cellular immunity. Semin Hematol. 1970 Apr;7(2):172–184. [PubMed] [Google Scholar]
- Nathan C. F., Karnovsky M. L., David J. R. Alterations of macrophage functions by mediators from lymphocytes. J Exp Med. 1971 Jun 1;133(6):1356–1376. doi: 10.1084/jem.133.6.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. The uptake of particulate antigens. J Reticuloendothel Soc. 1968 Jun;5(3):203–229. [PubMed] [Google Scholar]
- Salvin S. B., Cheng S. L. Lymphoid Cells in Delayed Hypersensitivity II. In Vitro Phagocytosis and Cellular Immunity. Infect Immun. 1971 Apr;3(4):548–552. doi: 10.1128/iai.3.4.548-552.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvin S. B., Nishio J. Communications. "In vitro" cell reactions in delayed hypersensitivity. J Immunol. 1969 Jul;103(1):138–141. [PubMed] [Google Scholar]
- Salvin S. B., Nishio J., Gribik M. Lymphoid cells in delayed hypersensitivity. 1. In vitro vs. in vivo responses. Cell Immunol. 1970 May;1(1):62–77. doi: 10.1016/0008-8749(70)90061-4. [DOI] [PubMed] [Google Scholar]
- Salvin S. B., Sell S., Nishio J. Activity in vitro of lymphocytes and macrophages in delayed hypersensitivity. J Immunol. 1971 Sep;107(3):655–662. [PubMed] [Google Scholar]
- WAKSMAN B. H., MATOLTSY M. The effect of tuberculin on peritoneal exudate cells of sensitized guinea pigs in surviving cell culture. J Immunol. 1958 Sep;81(3):220–234. [PubMed] [Google Scholar]
- Ward P. A., Remold H. G., David J. R. The production by antigen-stimulated lymphocytes of a leukotactic factor distinct from migration inhibitory factor. Cell Immunol. 1970 Jul;1(2):162–174. doi: 10.1016/0008-8749(70)90003-1. [DOI] [PubMed] [Google Scholar]