Abstract
Dopamine (DA) plays an important role in integrative functions contributing to adaptive behaviors. In support of this essential function, DA modulates synaptic plasticity in different brain areas, including the striatum. Many drugs used for cognitive enhancement are psychostimulants, such as methylphenidate (MPH), which enhance DA levels. MPH treatment is of interest during adolescence, a period of enhanced neurodevelopment during which the DA system is in a state of flux. Recent epidemiological studies report the co-abuse of MPH and ethanol in adolescents and young adults. Although repeated MPH treatment produces enduring changes that affect subsequent behavioral responses to other psychostimulants, few studies have investigated the interactions between MPH and ethanol. Here we addressed whether chronic therapeutic exposure to MPH during adolescence predisposed mice to an altered response to ethanol and whether this was accompanied by altered DA release and striatal plasticity. C57BL/6J mice were administered MPH (3–6 mg/kg/day) via the drinking water between post-natal days 30 and 60. Voltammetry experiments showed that sufficient brain MPH concentrations were achieved during adolescence in mice to increase the DA clearance in adulthood. The treatment also increased long-term depression and reduced the effects of ethanol on striatal synaptic responses. Although the injection of 0.4 or 2 g/kg ethanol dose-dependently decreased locomotion in control mice, only the higher dose decreased locomotion in MPH-treated mice. These results suggested that the administration of MPH during development promoted long-term effects on synaptic plasticity in forebrain regions targeted by DA. These changes in plasticity might, in turn, underlie alterations in behaviors controlled by these brain regions into adulthood.
Keywords: addiction, alcohol, dopamine, dorsal striatum, mouse
Introduction
Brain dopamine (DA) has been implicated in integrative functions contributing to adaptive behaviors such as attention, learning, and memory (Nieoullon, 2002; Cools, 2006). Although DA or DA agonists improve memory, the selective depletion, dysfunction or lesion of forebrain DA systems strongly impairs the acquisition and retention of information, as well as the ability to focus and adapt to environmental changes. In support of its role in cognitive processes, DA modulates the synaptic plasticity in different brain areas, including the striatum. Many of the current drugs used for cognitive enhancement are DA enhancers such as methylphenidate (MPH). These drugs improve memory and reduce impulsive behavior and risky decision-making (Advokat, 2010). The use of MPH has increased substantially in the last few decades and the number of children and adolescents treated with this drug continues to grow (McCabe et al., 2005; Marco et al., 2011; Heal et al., 2012; Sadasivan et al., 2012). Moreover, the rates of non-medical use of prescription stimulants continue to rise and are becoming a matter of public health concern (Kaye & Darke, 2012).
During adolescence, forebrain DA systems undergo prolific remodeling, thus providing a neurobiological substrate to explain the reported behavioral vulnerabilities to psychostimulants (Doremus-Fitzwater et al., 2010; Marco et al., 2011). Although some studies suggest that repeated MPH treatment produces enduring neuronal changes that affect subsequent behavioral responses to other psychostimulants (Volkow & Insel, 2003), few studies have investigated the interactions between MPH and ethanol. Clinical studies report alcohol-related problems to be more prevalent among college populations treated with stimulant medication in the past (Herman et al., 2011; Sepúlveda et al., 2011). Recent work has also established that such interactions are behaviorally relevant (Patrick et al., 2007; Griffin et al., 2010) and that possible co-morbidity issues should be taken into consideration by clinicians prescribing cognitive enhancers.
The dorsal striatum has one of the highest concentrations of DA in the brain, plays a key role in action initiation, learning and memory, and is the locus of neural impairments that contribute to several mal-adaptive behaviors (Lovinger, 2010). Acute and chronic ethanol treatment is known to affect striatal DA levels (Mathews et al., 2006). Long-term changes in the efficacy of striatal synaptic transmission are theorised to contribute to brain development, learning and memory, and addiction (Kauer & Malenka, 2007). The most studied forms of long-lasting synaptic plasticity in the striatum are long-term potentiation and long-term depression (LTD). High-frequency stimulation (HFS) traditionally elicits long-term potentiation in the dorsomedial striatum, whereas LTD is more prevalent in the dorsolateral striatum (Partridge et al., 2000). MPH treatment modifies the expression of striatal genes coding for regulatory proteins implicated in attentional functions and psychostimulant dependence (Brandon & Steiner, 2003; Yano & Steiner, 2007). In the striatum, ethanol alters synaptic responses, reverses the direction of synaptic plasticity and promotes habit formation (Choi et al., 2006; Wang et al., 2007; Yin et al., 2007). Some of the proteins implicated in MPH actions may also be affected by ethanol (Goitia et al., 2013).
The aim of the present study was to investigate the neurobiological consequences of long-term adolescent MPH exposure at levels seen during therapeutic use and how this modifies neural and behavioral responses to ethanol administration in adulthood. Specifically, we studied how chronic peri-adolescence exposure to MPH modifies DA release dynamics and synaptic plasticity, and how ethanol-mediated plasticity was altered following MPH exposure.
Materials and methods
All experiments were approved by the National Institute on Alcohol Abuse and Alcoholism Animal Care and Use Committee and were performed in accordance with National Institutes of Health guidelines.
Adolescent methylphenidate administration
Methylphenidate was administered to male C57Bl/6J mice throughout the peri-adolescence period of post-natal day (P) 30–60 (Fig. 1). This time window was chosen to most closely approximate the entire adolescent developmental period (Spear, 2000; Marco et al., 2011). Mice were individually housed from P25 until the completion of the study, and MPH solution or control water was provided continuously beginning on P30. Body weight and water consumption were monitored for 5 days (i.e. P25–30) prior to MPH administration to provide a baseline level of fluid consumed per day, per animal. Cohorts of control and MPH-treated group-housed mice (three mice per cage) were also set up to control for the possible effects of social isolation. MPH was dissolved in water at appropriate concentrations to ensure that animals consumed a dose of MPH per day that was consistent with techniques used elsewhere in the literature (Moll et al., 2001; Thanos et al., 2007). The target doses of MPH were 3–6 mg/kg per day. Consumption was randomly sampled throughout the experiment to ensure adequate consumption (Fig. 1).
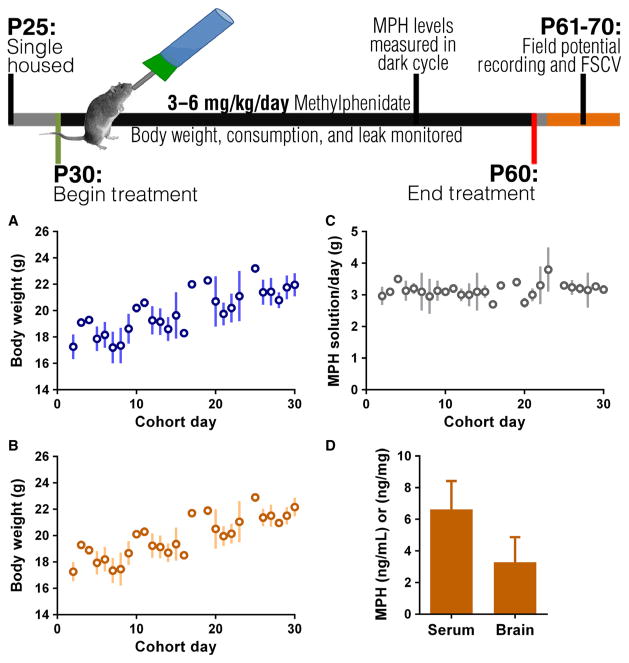
Fig. 1.
Top: treatment and experimental timeline. (A, B) Linear regression of body weight gain for control (A) and MPH (B) groups across days of treatment. Body weight increases throughout study (P < 0001). (C) MPH solution consumed per day corrected for leak. Consumption remains consistent throughout study. (D) MPH levels measured in trunk blood and brain at 6 h into the dark cycle. FSCV, fast-scan cyclic voltammetry.
Methylphenidate levels
The animals reached therapeutically relevant MPH levels, measured in the plasma and brain tissue, during chronic MPH administration (Fig. 1). The MPH levels were measured in trunk blood at 6 h into the dark cycle. The trunk blood was collected and allowed to clot overnight at 4 °C. The blood was then centrifuged at 3000 g for 10 min and serum collected for enzyme-linked immunosorbent assay (Bio-Scientific Max Signal). The serum was diluted 1: 5 with phosphate-buffered saline and the enzyme-linked immunosorbent assay was performed according to the manufacturer’s instructions. Animals not receiving MPH were used as the assay blank.
Fast-scan cyclic voltammetry
Slices were prepared following a 24–48 h withdrawal from MPH treatment. Fast-scan cyclic voltammetry was performed using DEMON VOLTAMMETRY AND ANALYSIS software (Yorgason et al., 2011). Coronal slices (350 μm thick) containing the dorsal striatum were cut in ice-cold modified artificial cerebral spinal fluid (aCSF) containing Kreb’s buffer (John & Jones, 2007). The slices were incubated for at least 1 h at room temperature (25 °C) in fresh aCSF with Kreb’s buffer and were continuously gassed with a 95% O2/5% CO2 mixture. Glass-encased carbon fiber electrodes (100–150 μm exposed length and 7 μm tip diameter) were created by aspirating a 1.2 mm glass capillary with a single carbon fiber. A triangular potential was applied to the carbon fiber at 10 Hz; the carbon fiber was held at −0.4 V, ramped to +1.2 V and back to 0.4 V at 400 V/s. DA release was elicited using single-pulse biphasic electrical stimulation of 300–600 μA lasting 1.2 ms every 5 min. Release was expressed in μM, with the maximal [DA]o level measured at the peak of the DA transient. Relative uptake rates were estimated from single exponential fits of Current vs. Time (I vs. T) traces to obtain the time constant tau, which is the time required (in seconds) for the signal (evoked with a 500 μA stimulus) to decay to 1/e of the initial peak All voltammetry data acquisition and analysis were performed using DEMON VOLTAMMETRY AND ANALYSIS software (Wake Forest University; Yorgason et al., 2011). The pharmacological agents were applied to the slices via the superfusate and responses were recorded throughout the drug application. Following experiments, the electrodes were calibrated using solutions of 1 μM DA in aCSF.
Field potential recording preparation and experiments
Following a 24–48 h withdrawal from MPH, the mice were decapitated and coronal slices (350 μm) containing the dorsal striatum were prepared using a vibratome. Slices were cut in pre-oxygenated ice-cold modified aCSF containing the following (in mM): 194 sucrose, 30 NaCl, 4.5 KCl, 1 MgCl2, 26 NaHCO3, 1.2 NaH2PO4, and 10 glucose. The slices were first incubated for 30 min at 33 °C (± 1 °C), followed by at least 1 h at room temperature, where they were kept until needed. The incubation and recording aCSF contained the following (in mM): 124 NaCl, 4.5 KCl, 1 MgCl2, 26 NaHCO3, 1.2 NaH2PO4, 10 glucose, and 2 CaCl. The aCSF was continuously gassed with a 95% O2/5% CO2 mixture. Glass electrodes with a resistance of 2–5 mΩ were filled with 0.9% NaCl. Hemisected slices were placed in the recording chamber, where aCSF was bath superfused (at a rate of approximately 1.5 mL/min), and kept at a temperature of 30 °C (± 1 °C). A bipolar twisted tungsten stimulating electrode was placed approximately 1 mm away from the recording electrode, in the white matter overlying the dorsolateral subregion of the striatum (for detailed methods, see Partridge et al., 2000). During experiments examining drug effects or plasticity, the stimulus intensity was set to the value required to evoke a population spike (PS) that was half of the maximum PS, and stimulation was delivered every 30 s. Only recordings where the maximum PS amplitude was at least 1 mV were included. Drug or stimulation was administered following a 10 min stable baseline. For slices that had a clear pre-synaptic component (fiber volley, N1), the fiber volley before drug and after drug or stimulation was compared to ensure stability. If the fiber volley differed significantly, the slice was discarded. In the event that multiple slices from the same animal were used for the same experiment, the experiments were averaged (no more than two experiments per animal were used).
Input/output curves were constructed by stimulating every 30 s at an increasing stimulus intensity from 0.1 to 1.5 mA. Each intensity was tested twice and averaged. The maximum PS obtained during the input/output curve was considered as the maximum for subsequent experiments. HFS was used to examine alterations in the plasticity of striatal synapses. The HFS consisted of one set of 4 × 1 s trains of 100 pulses (each pulse, 10 μs at maximum intensity) with a 10 s interval between trains. The effects of drug and HFS were assessed by normalising the PS amplitude to the baseline amplitude.
Two-bottle-choice ethanol drinking
A two-bottle-choice ethanol procedure was used, similar to previously described methods (Boyce-Rustay et al., 2006; Hefner & Holmes, 2007). Single-housed mice (P62–72) received both increasing concentrations of ethanol (3, 5, 10, 15, and 20%) and water (two bottles total) for 48 h each. At 24 h into the presentation of each concentration, the left/right position of the bottles was alternated to control for side bias. Ethanol and water consumption, as well as mouse body weight, were measured every 48 h, accounting for evaporation and spillage with multiple dummy cages. The preference for ethanol relative to water was calculated as ethanol consumption/total fluid consumption.
Locomotor behavior
Locomotor behavior was assessed in a chamber with clear polycarbonate walls (10 × 6.5 inches) and perforated stainless steel flooring. Horizontal activity was detected as infrared beam crosses (1 inch spacing, 10 beams per cage) using Opto M3 activity monitors (Columbus Instruments, OH, USA). Behavior was scored as the number of ambulatory counts during 30 s bins, as measured by beam breaks. For data analysis and graphs, the ambulatory counts are presented in 2 min bins, allowing for clarity in visual interpretation. At 24 h following the completion of MPH treatment, mice were placed in locomotor boxes for four consecutive days for conditioning (1 day of baseline, and 3 days of baseline + intraperitoneal injections of saline vehicle). The effects of ethanol on locomotor behavior were assessed using a latin-square design, ensuring that all mice received all doses of ethanol in a randomised order. Following baseline locomotor assessment, mice randomly received 0.4 or 2 g/kg ethanol in 0.9% saline on non-consecutive days.
Locomotor activity at 2 weeks
At 2 weeks (halfway) into MPH treatment, a separate cohort of mice was placed in locomotor boxes for 30 min. These mice were not reused for later behavioral experiments. Following the locomotor assessment, they were returned to the home-cages and allowed to resume MPH consumption.
Statistics
Field potential experiments
The mean PS amplitude was measured in Clampfit 9.2 and analysed in PRISM (Graphpad Software, La Jolla, CA, USA). The PS amplitudes were averaged per minute and expressed as a percentage of baseline. Group differences were analysed using ANOVA, and individual treatment groups were analysed using a Student’s paired t-test (post-HFS or post-drug effect as compared with baseline).
Two-bottle choice
The ethanol preference data were stored in Microsoft Excel and graphed in PRISM (GraphPad). The data were analysed using ANOVA. The locomotor behavior data were analysed in GRAPHPAD PRISM and compared with two-way ANOVA.
Fast-scan cyclic voltammetry
A linear mixed model with the variance–co-variance matrix set to compound symmetry was used to analyse evoked [DA]o release at various stimulation intensities (PASW18; IBM, New York, NY, USA). A between-subjects twoway ANOVA was used to analyse the quinpirole dose–response curve and the effect of treatment and housing on tau values (PRISM; GraphPad).
Results
Chronic oral methylphenidate intake produced therapeutically relevant drug concentrations in adolescent mice
The C57BL/6J mice were housed individually at P25–30 and consumed MPH or control water orally from P30 to P60. The MPH was dissolved in water. Here, our goal was to establish an oral dose that produced a plasma MPH level of ~6 ng/mL, similar to that achieved in patients treated with MPH (Kuczenski & Segal, 2005). Both groups of animals showed similar body weight gain throughout treatment (Fig. 1A and B) and fluid consumption was stable during the 30 day treatment period (Fig. 1C). This method of chronic MPH administration resulted in therapeutically relevant plasma and brain concentrations of the drug (serum: 6.625 ± 1.796 ng/mL; brain: 3.288 ± 1.560 ng/mg’ Fig. 1D; Swanson & Volkow, 2002; Kuczenski & Segal, 2005).
Increased striatal dopamine clearance following chronic methylphenidate administration during peri-adolescence
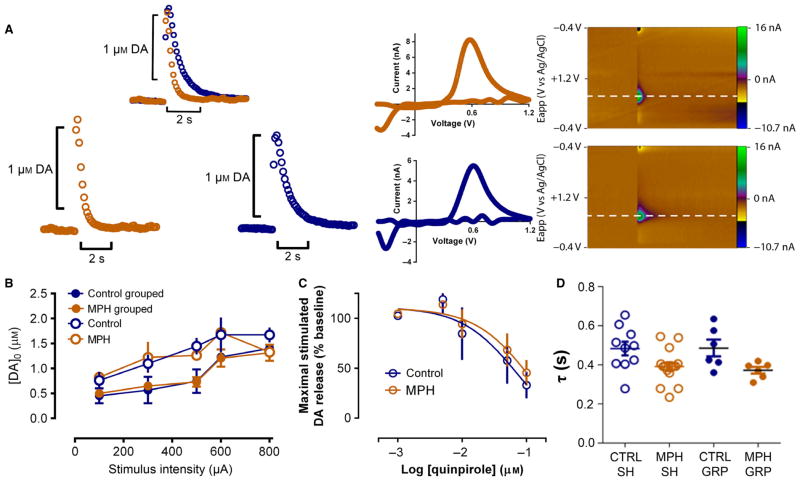
The DA release kinetics were studied following chronic treatment with the drug to evaluate the consequences of long-term MPH exposure during development. Fast-scan cyclic voltammetry was used to evaluate the transient increases in extracellular DA concentrations evoked by single electrical pulse stimulation in slices containing the dorsolateral striatum. Figure 2A shows the representative neurochemical responses in control and MPH-treated mice. The concentration of DA released in response to stimulation did not differ between MPH-treated and control mice when examining input/output curves obtained by applying increasing stimulus intensities (100–800 μA; Fig. 2B). A linear mixed model with the variance–co-variance matrix set to compound symmetry (PASW18; IBM) showed a significant effect on DA release at the different intensities (F4,43.23 = 9.667, P < 0.001), but neither housing (F1,17.07 = 3.22; P = 0.09) nor drug treatment (F1,17.072 = 0.163; P = 0.692) accounted for those differences. There were no significant interactions (F4,43.23 = 0.212; P = 0.930). When group-reared animals (n = 6) received either control or MPH treatment, the DA released did not differ from animals treated in isolation (n = 10–15). When the activity of release-regulating autoreceptors was evaluated, increasing concentrations of the DA D2-like receptor agonist quinpirole (5–100 nM) inhibited DA release to the same extent in control and MPH-treated mice (n = 6; Fig. 2C). However, when DA uptake was evaluated, the decay time constants (tau) were significantly smaller in slices from MPH-treated mice. Two-way ANOVA showed a significant effect of MPH treatment on tau (F1,33 = 11.03; P = 0.0022) with no significant effect of housing condition (F1,33 = 0.3363; P = 0.5659). There was no significant interaction of the housing condition with MPH treatment on tau (F1,33 = 0.2710; P = 0.606). This suggested that peri-adolescent exposure to MPH increased the rate of DA clearance (Fig. 2D) in adult mice.
Fig. 2.
Chronic MPH treatment during peri-adolescence increases striatal DA clearance without changing other measurements of DA function. (A) Representative traces of DA release: blue, control animal; orange, MPH-treated mouse. Left panels: concentration traces; middle panels: characteristic DA voltammograms; right panels: corresponding color plots depicting the voltammetry data with time on the X axis, applied scan potential (Eapp) on the Y axis and background-subtracted faradaic current shown on the Z-axis in pseudocolor for the control (upper) and MPH-treated (bottom) mouse trace. (B) Current-evoked [DA]o at various stimulation intensities. (C) Evoked [DA]o release in the presence of increasing concentrations of bath-applied quinpirole (0, 5, 10, 50 and 100 nM). (D) MPH-treated mice exhibit significantly lower baseline decay time constant (tau) values. GRP, group housed; SH, single housed.
Chronic methylphenidate administration in adolescence decreased synaptic excitation and potentiated synaptic plasticity in the dorsolateral striatum
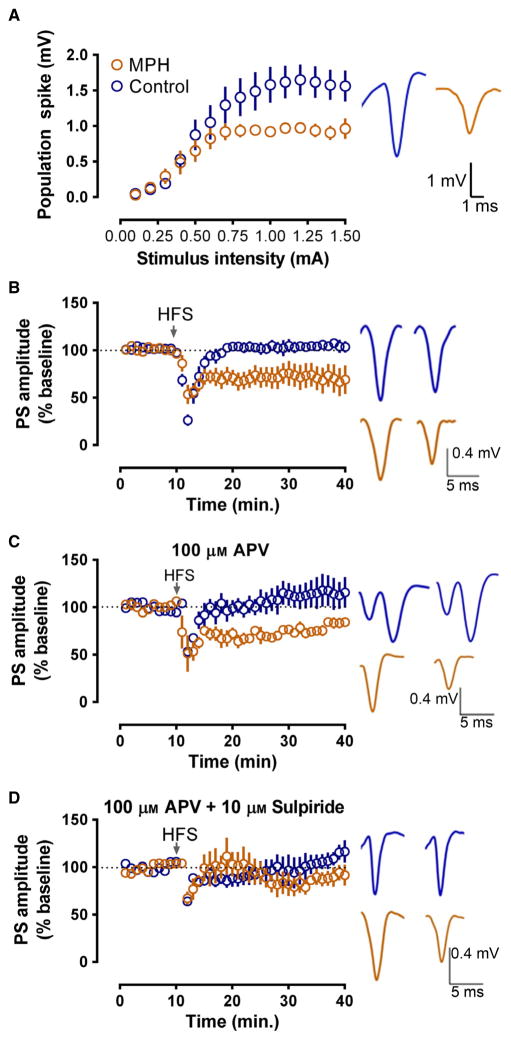
Field potential recordings were performed in adulthood to examine whether peri-adolescent exposure to MPH modified the responses to synaptic activation as well as synaptic plasticity in the dorsolateral striatum. Synaptically-driven PSs with stable amplitudes were evoked in slices from the dorsolateral striatum. Input/output curves indicated a decrease in net synaptically-driven activity between MPH-treated and control mice (Fig. 3A). Due to the inherent variability in PS amplitude across slices, the maximum and half-maximum spike amplitudes were compared using a non-parametric Mann–Whitney U test. The MPH-treated mice had significantly smaller maximum PSs (control: n = 8; MPH: n = 8; U = 7, P < 0.05; Fig. 3A) and half-maximum PSs (U = 10, P < 0.05). Linear regression analysis indicated that the slopes of input/output curves differed significantly across the two groups (F1,223 = 12.4745, P < 0.001), with MPH-treated mice reaching a plateau at lower stimulus intensity.
Fig. 3.
MPH exposure alters dorsolateral striatal plasticity via a D2-dependent mechanism. (A) Left: slices from MPH-treated mice show decreased evoked PS amplitude at multiple stimulus intensities as compared with control mice. Right: representative traces illustrating PS amplitude. (B) Left: in the absence of any drug, MPH-treated mice show significantly greater HFS-induced LTD when compared with control mice. Right: representative traces before HFS and after HFS in control and MPH slices. (C) Left: LTD seen in slices from MPH-treated mice is N-methyl-D-aspartate receptor (NMDAR)-independent, as 100 μM of the NMDAR antagonist, DL-2-amino-5-phosphonovaleric acid (APV) does not block the depression. Right: representative traces before HFS and after HFS in control and MPH slices. (D) Left: application of 100 μM DL-2-amino-5-phos-phonovaleric acid in conjunction with 10 μM sulpiride blocks HFS-induced LTD in MPH slices, demonstrating a D2-dependent mechanism. Right: representative traces before HFS and after HFS in control and MPH slices. Blue, control mice; orange, MPH-treated mice.
Following HFS, slices from MPH-treated mice showed significantly greater HFS-induced LTD compared with control mice (t = 36–40 min, F1,12 = 6.297, P < 0.05). Whereas control mice showed no LTD [PS amplitude at t = 36–40 min, 104 ± 6% of baseline; t(4) = 4.276, P > 0.1], MPH-treated mice showed robust HFS-induced LTD [Fig. 3B; PS amplitude at t = 36–40 min, 70 ± 6% of baseline; t(4) = 30.42, P < 0.0001]. Next, we elucidated the role of N-methyl-D-aspartate and DA D2 receptors in the dorsolateral striatum synaptic plasticity changes elicited by chronic MPH during peri-adolescence. LTD in the dorsolateral striatum involves a retrograde signaling mechanism in which DA and glutamate are implicated (for review, see Lovinger, 2010). In addition, an N-methyl-D-aspartate receptor-dependent form of long-term potentiation can also be evoked with this protocol (Dang et al., 2006). When the competitive N-methyl-D-aspartate receptor antagonist (±)-DL-2-amino-5-phosphonovaleric acid (100 μM) was present during HFS, significant differences in plasticity were observed in control vs. MPH slices (Fig. 3C; F4,4 = 2.749, P < 0.05). Specifically, control slices showed a small but significant HFS-induced potentiation in PS amplitude compared with control baseline [PS amplitude at t = 36–40 min, 115 ± 16% of baseline; t(4) = 9.814, P < 0.005]. Slices from MPH-treated mice, in contrast, showed HFS-induced depression of PS amplitude [PS amplitude at t = 36–40 min, 84 ± 5% of baseline; t(4) = 6.130, P < 0.05], indicating that, even if N-methyl-D-aspartate receptor blockade has modest effects on plasticity in control and MPH slices, this treatment did not eliminate the difference in effects of HFS between the two groups (Fig. 3C). In addition, when the D2-like DA antagonist sulpiride (10 μM) was bath applied in combination with DL-2-amino-5-phosphonovaleric acid (100 μM), HFS did not produce lasting alterations in PS amplitude in either group (Fig. 3D), and the effects of HFS in the two groups did not differ significantly from one another (F1,3 = 0.10, P > 0.1). This finding indicated that the plasticity observed in these experiments, especially the LTD observed in the MPH group, was mediated by D2 receptors.
Ethanol impaired synaptic plasticity but not dopamine release following chronic methylphenidate administration during adolescence
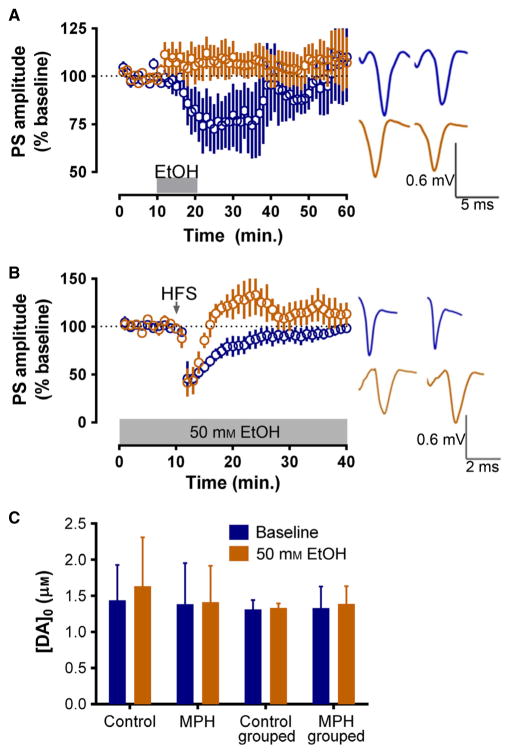
Field potential recordings were again performed to evaluate whether ethanol differentially altered synaptic plasticity changes observed in the adult dorsolateral striatum following MPH treatment during adolescence. The baseline PS amplitude was monitored over time before and following ethanol application. MPH vs. control mice were compared at t = 21–25 min, or the point of maximal effect. We found that the responses in slices from mice treated with MPH and control showed a marginal significance in the effects of ethanol when compared with each other (F4,1 = 4.346, P = 0.06). Although MPH slices showed a small but significant potentiation in response to 50 mM ethanol [PS amplitude at t = 30–35 min, 110 ± 6% of baseline; t(4) = 4.970, P > 0.1], its effects were diminished when compared with controls; ethanol induced a significant depression of PS amplitude in slices from control mice [PS amplitude at t = 30–35 min, 71 ± 11% of baseline; t(4) = 10.32, P < 0.005] (Fig. 4A). Interestingly, the bath application of 50 mM ethanol in combination with the HFS protocol significantly reversed the direction of plasticity observed in MPH-treated mice, consistent with the bath application of ethanol alone. Although no main effect of treatment group was seen (F1,6 = 4.24, P > 0.05), a significant time × treatment group interaction was noted (F39,234 = 2.41, P < 0.0001). This effect was underscored by a lack of plasticity in control mice; control slices did not show significant LTD [PS amplitude at t = 36–40 min, 90 ± 8% of baseline; t(4) = 3.601, P > 0.01], whereas MPH slices showed significant HFS+ethanol-induced LTD [PS amplitude at t = 36–40 min, 125 ± 10% of baseline; t(4) = 5.857, P < 0.005] (Fig. 4B). The application of 50 mM ethanol did not alter DA release in either control or MPH-treated mice reared in isolation (n = 6) or groups (n = 10–15; Fig. 4C).
Fig. 4.
MPH-treated mice lack ethanol-induced LTD and exhibit reversed HFS-induced plasticity under ethanol (EtOH). (A, B) Field potential recordings in the dorsolateral striatum evoked by stimulation that induces half-maximal PS amplitudes. (A) A 10 min application of 50 mM EtOH inhibits PS amplitude in control but not MPH-treated mouse slices. (B) Bath-applied 50 mM EtOH reverses the direction of HFS-induced plasticity seen in MPH-treated mice. (C) Voltammetry shows that evoked [DA]o is similar across treatment groups both before and during the bath application of 50 mM EtOH. Blue, control mice; orange, MPH-treated mice.
Altered effects of ethanol on locomotor activity following adolescent methylphenidate were not accompanied by altered consumption
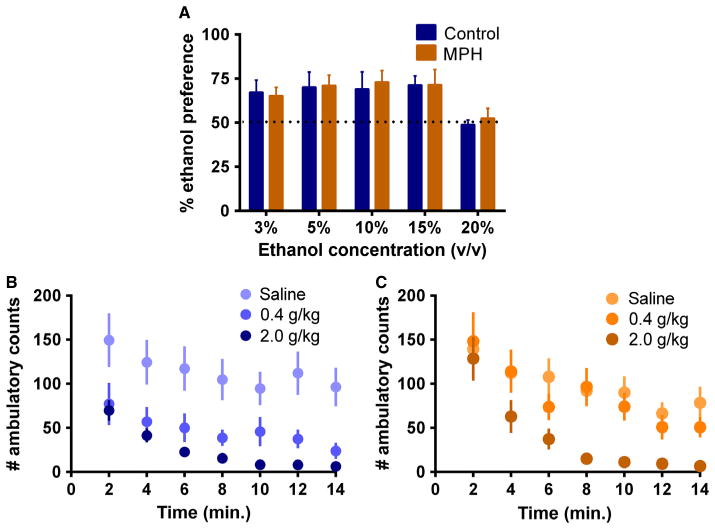
Following chronic treatment during peri-adolescence, MPH-treated and control mice showed similar consumption of and preference for ethanol compared with water when tested in adulthood. The daily consumption and preference for ethanol remained stable over the 3–15% concentrations, and decreased in both groups at 20%. There were no significant differences in ethanol consumption across MPH-treated and control groups (F1,28 = 0.1002, P > 0.05). An expected significant main effect for ethanol concentration was observed, indicating that both MPH and control mice consumed less ethanol at the highest concentrations (F1,4 = 3.458, P < 0.05). Similarly, there was no significant interaction between treatment group and ethanol concentration (F1,4 = 0.06930, P > 0.05). These results indicated that MPH exposure did not influence ethanol consumption or preference compared with control mice (Fig. 5a).
Fig. 5.
Ethanol preference and locomotor activity following i.p. ethanol. (A) Two-bottle choice of water and increasing concentrations of ethanol (v/v) shows no differences between control and MPH-treated animals. Preference values reflect ethanol consumed as a percentage of total liquid consumption. (B, C) Decreased locomotor depressant effect of a low dose of ethanol specifically in animals treated with MPH in adolescence. Locomotor activity reflected as ambulatory counts following a 0.4 g/kg (B) or 2 g/kg (C) i.p. injection of ethanol. Blue, control mice; orange, MPH-treated mice.
No differences in baseline locomotor activity were observed when control or MPH-treated mice were placed in a novel environment at 2 weeks into MPH or control treatment (P43; data not shown) or at the end of treatment (P62). However, following a challenge with two different doses of ethanol (0.4 and 2 g/kg), MPH-treated and control mice responded differently to ethanol-induced locomotor activity behavior. Specifically, the lower dose of ethanol (0.4 g/kg) did not produce a significant decrease in locomotor activation in MPH-treated mice as it did in control mice (F2,27 = 9.012, P < 0.001; n = 10 per group; Fig. 5B and C), suggestive of different sensitivity to the effect of ethanol in animals treated with MPH in adolescence.
Discussion
Here, we show that chronic MPH treatment with therapeutically relevant concentrations given during peri-adolescence in mice results in increased DA clearance, reduced overall synaptic responsiveness, increased HFS-evoked LTD, reduced effects of ethanol on responses in brain slices as well as reduced sensitivity to the locomotor effects of ethanol.
Faster extracellular DA clearance is hypothesised to decrease extracellular DA and this may underlie the changes in HFS-evoked LTD. Previous studies have shown that 6-hydroxydopamine lesions block HFS-evoked LTD in the striatum (Picconi et al., 2005; Belujon et al., 2010). Furthermore, DA itself decreases dopaminergic influences on corticostriatal synaptic plasticity depending on the level of depletion (Paillé et al., 2010). It is perhaps surprising that shorter DA-evoked signals in the slice would increase the magnitude of LTD. However, previous findings indicate that strict spatial and temporal patterns of DA release are required for LTD induction because DA reuptake blockers restore LTD (Partridge et al., 2002). Thus, a tighter regulation of the duration of DA release might contribute to the enhanced LTD observed following MPH treatment.
In sharp contrast to control mice, MPH-treated mice showed smaller net synaptically-driven responses and robust HFS–LTD. The finding that control mice displayed little to no LTD could be explained, in part, by the age of the animals at the time of study (Partridge et al., 2000). In addition to age, the need for social isolation to achieve control over liquid consumption could be a confounding factor in our design, and could perhaps affect LTD in control animals. Previous studies have shown that social isolation decreases synaptic plasticity in different brain regions (Roberts & Greene, 2005; Conrad et al., 2011), changes the expression of molecules related to inhibitory neurotransmission and structural plasticity (Roberts & Greene, 2005; Gilabert-Juan et al., 2012), and alters the ability to learn and perform decision-making tasks (Zeeb et al., 2012). If isolation-rearing indeed contributes to the lack of LTD observed in control mice, this would suggest that treatment with low doses of MPH could theoretically reverse this effect. This should be an area of future investigation. It has been reported that isolation changes DA release (Yorgason et al., 2013) and, although we saw a trend for DA release to be higher in the isolation-housed mice vs. the group-housed mice, this difference was not significant.
Methylphenidate is structurally related to amphetamine and produces DA overflow in the striatum in a manner similar to cocaine (Volkow et al., 1995). It exhibits the characteristics of both a DA blocker and releaser, depending on the concentration applied (Ferris et al., 2012) as it binds to both the cocaine/blocker and DA/substrate sites on the DA transporter (Wayment et al., 1999; Dar et al., 2005). This concentration-dependent effect may underlie the differential effects of MPH when administered at low (therapeutic doses) or higher (McCabe et al., 2005; Kaloyanides et al., 2007) doses. Studies examining the long-term effects of early exposure to cognitive enhancers during critical developmental periods remain inconsistent. The age of onset of MPH exposure as well as the route of administration (Brandon et al., 2001; Andersen et al., 2002; Kuczenski & Segal, 2002) may be critical factors in explaining the conflicting results of these behavioral studies. Furthermore, clinical studies have shown that individuals medicated with MPH are more likely to smoke cigarettes and have higher rates of cocaine use (Lambert & Hartsough, 1998). Other studies have reported that individuals treated with psychostimulants are at lower risk of (Biederman et al., 1999) or are even protected from (Wilens et al., 2003) substance abuse. Two recent reports in non-human primates have shown that chronic MPH during peri-adolescence (Soto et al., 2012) and young adulthood (Gill et al., 2012) at clinically relevant doses does not significantly alter synaptic DA markers and does not render animals vulnerable to cocaine self-administration (Gill et al., 2012). Although these recent findings in non-human primates are a significant contribution to the field, they do not fully address the limitations encountered in rodent studies related to the age of onset and length of treatment.
The clarification of whether exposure to MPH during the peri-pubertal transition to adulthood promotes neuroadaptations that could modify future responses to other drugs of abuse such as ethanol is still of paramount importance. Here, we find that exposure to MPH in adolescence reverses the ethanol-induced changes in plasticity from LTD to long-term potentiation (Fig. 4B). Pre-synaptic DA mobilisation is unlikely to modulate this effect because ethanol did not alter electrically evoked release in slices from control or MPH-treated mice. However, interactions between ethanol and the effects of increased DA uptake in MPH-treated animals might contribute to this change in plasticity. Our finding that lower doses of ethanol do not produce inhibition in locomotor behavior in MPH-treated mice may reflect an interaction between the effects of low-dose ethanol on intrastriatal DA levels and the increased rate of DA reuptake produced by chronic MPH exposure observed with voltammetry. For example, shortening of the time course of evoked [DA]o during movement in a novel environment may increase context-induced movement. Interestingly, these mice showed no alteration in ethanol consumption in a two-bottle-choice task (Fig. 5A). Although these data would agree with recent literature showing a lack of vulnerability to self-administer abused drugs following adolescent MPH exposure (Gill et al., 2012), it is noteworthy that home-cage drinking measures do not always predict the consumption of ethanol in an operant procedure. Thus, more work is needed to assess whether adolescent MPH enhances the motivation to self-administer ethanol.
The results of this study reinforce the idea that the administration of MPH at therapeutic doses during development can have long-term effects on synaptic plasticity in forebrain regions targeted by DA. These changes in plasticity may, in turn, underlie alterations in behaviors controlled by these brain regions even into adulthood.
Abbreviations
- aCSF
artificial cerebral spinal fluid
- DA
dopamine
- HFS
high-frequency stimulation
- LTD
long-term depression
- MPH
methylphenidate
- P
post-natal day
- PS
population spike
Footnotes
Conflict of interest
The authors declare that, except for income received from the primary employer, no financial support or compensation has been received from any individual or corporate entity for research or professional service, and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
References
- Advokat C. What are the cognitive effects of stimulant medications? Emphasis on adults with attention-deficit/hyperactivity disorder (ADHD) Neurosci Biobehav R. 2010;34:1256–1266. doi: 10.1016/j.neubiorev.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Arvanitogiannis A, Pliakas AM, LeBlanc C, Carlezon WA., Jr Altered responsiveness to cocaine in rats exposed to methylphenidate during development. Nat Neurosci. 2002;5:13–14. doi: 10.1038/nn777. [DOI] [PubMed] [Google Scholar]
- Belujon P, Lodge DJ, Grace AA. Aberrant striatal plasticity is specifically associated with dyskinesia following levodopa treatment. Movement Disord. 2010;25:1568–1576. doi: 10.1002/mds.23245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Wilens T, Mick E, Spencer T, Faraone SV. Pharmacotherapy of attention-deficit/hyperactivity disorder reduces risk for substance use disorder. Pediatrics. 1999;104:1–5. doi: 10.1542/peds.104.2.e20. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Wiedholz LM, Millstein RA, Carroll J, Murphy DL, Daws LC, Holmes A. Ethanol-related behaviors in serotonin transporter knockout mice. Alcohol Clin Exp Res. 2006;30:1957–1965. doi: 10.1111/j.1530-0277.2006.00241.x. [DOI] [PubMed] [Google Scholar]
- Brandon CL, Steiner H. Repeated methylphenidate treatment in adolescent rats alters gene regulation in the striatum. Eur J Neurosci. 2003;18:1584–1592. doi: 10.1046/j.1460-9568.2003.02892.x. [DOI] [PubMed] [Google Scholar]
- Brandon CL, Marinelli M, Baker LK, White FJ. Enhanced reactivity and vulnerability to cocaine following methylphenidate treatment in adolescent rats. Neuropsychopharmacol. 2001;25:651–661. doi: 10.1016/S0893-133X(01)00281-0. [DOI] [PubMed] [Google Scholar]
- Choi SJ, Kim KJ, Cho HS, Kim SY, Cho YJ, Hahn SJ, Sung KW. Acute inhibition of corticostriatal synaptic transmission in the rat dorsal striatum by ethanol. Alcohol. 2006;40:95–101. doi: 10.1016/j.alcohol.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Conrad KL, Louderback KM, Gessner CP, Winder DG. Stress-induced alterations in anxiety-like behavior and adaptations in plasticity in the bed nucleus of the stria terminalis. Physiol Behav. 2011;104:248–256. doi: 10.1016/j.physbeh.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R. Dopaminergic modulation of cognitive function-implications for L-DOPA treatment in Parkinson’s disease. Neurosci Biobehav R. 2006;30:1–23. doi: 10.1016/j.neubiorev.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Dang MT, Yokoi F, Yin HH, Lovinger DM, Wang Y, Li Y. Disrupted motor learning and long-term synaptic plasticity in mice lacking NMDAR1 in the striatum. Proc Natl Acad Sci USA. 2006;103:15254–15259. doi: 10.1073/pnas.0601758103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dar DE, Mayo C, Uhl GR. The interaction of methylphenidate and benztropine with the dopamine transporter is different than other substrates and ligands. Biochem Pharmacol. 2005;70:461–469. doi: 10.1016/j.bcp.2005.04.032. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Motivational systems in adolescence: possible implications for age differences in substance abuse and other risk-taking behaviors. Brain Cognition. 2010;72:114–123. doi: 10.1016/j.bandc.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris MJ, Calipari ES, Mateo Y, Melchior JR, Roberts DC, Jones SR. Cocaine self-administration produces pharmacodynamic tolerance: differential effects on the potency of dopamine transporter blockers, releasers, and methylphenidate. Neuropsychopharmacol. 2012;37:1708–1716. doi: 10.1038/npp.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilabert-Juan J, Molto MD, Nacher J. Post-weaning social isolation rearing influences the expression of molecules related to inhibitory neurotransmission and structural plasticity in the amygdala of adult rats. Brain Res. 2012;1448:129–136. doi: 10.1016/j.brainres.2012.01.073. [DOI] [PubMed] [Google Scholar]
- Gill KE, Pierre PJ, Daunais J, Bennett AJ, Martelle S, Gage HD, Swanson JM, Nader MA, Porrino LJ. Chronic treatment with extended release methylphenidate does not alter dopamine systems or increase vulnerability for cocaine self-administration: a study in nonhuman primates. Neuropsychopharmacol. 2012;37:2555–2565. doi: 10.1038/npp.2012.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goitia B, Raineri M, González LE, Rozas JL, Garcia-Rill E, Bisagno V, Urbano FJ. Differential effects of methylphenidate and cocaine on GABA transmission in sensory thalamic nuclei. J Neurochem. 2013;124:602–612. doi: 10.1111/jnc.12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC, III, Novak AJ, Middaugh LD, Patrick KS. The interactive effects of methylphenidate and ethanol on ethanol consumption and locomotor activity in mice. Pharmacol Biochem Be. 2010;95:267–272. doi: 10.1016/j.pbb.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heal DJ, Smith SL, Findling RL. ADHD: Current and Future Therapeutics. In: Stanford C, Tannock R, editors. Behavioral Neuroscience of Attention Deficit Hyperactivity Disorder and Its Treatment. Springer; Berlin: 2012. pp. 361–390. [DOI] [PubMed] [Google Scholar]
- Hefner K, Holmes A. An investigation of the behavioral actions of ethanol across adolescence in mice. Psychopharmacology. 2007;191:311–322. doi: 10.1007/s00213-006-0646-2. [DOI] [PubMed] [Google Scholar]
- Herman L, Shtayermman O, Aksnes B, Anzalone M, Cormerais A, Liodice C. The use of prescription stimulants to enhance academic performance among college students in health care programs. J Physician Assist Educ. 2011;22:15–22. doi: 10.1097/01367895-201122040-00003. [DOI] [PubMed] [Google Scholar]
- John CE, Jones SR. Voltammetric characterization of the effect of monoamine uptake inhibitors and releasers on dopamine and serotonin uptake in mouse caudate-putamen and substantia nigra slices. Neuropharmacology. 2007;52:1596–1605. doi: 10.1016/j.neuropharm.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaloyanides KB, McCabe SE, Cranford JA, Teter CJ. Prevalence of illicit use and abuse of prescription stimulants, alcohol, and other drugs among college students: relationship with age at initiation of prescription stimulants. Pharmacotherapy. 2007;27:666–674. doi: 10.1592/phco.27.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Kaye S, Darke S. The diversion and misuse of pharmaceutical stimulants: what do we know and why should we care? Addiction. 2012;107:467–477. doi: 10.1111/j.1360-0443.2011.03720.x. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Exposure of adolescent rats to oral methylphenidate: preferential effects on extracellular norepinephrine and absence of sensitization and cross-sensitization to methamphetamine. J Neurosci. 2002;22:7264–7271. doi: 10.1523/JNEUROSCI.22-16-07264.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Stimulant actions in rodents: implications for attention-deficit/hyperactivity disorder treatment and potential substance abuse. Biol Psychiat. 2005;57:1391–1396. doi: 10.1016/j.biopsych.2004.12.036. [DOI] [PubMed] [Google Scholar]
- Lambert NM, Hartsough CS. Prospective study of tobacco smoking and substance dependencies among samples of ADHD and non-ADHD participants. J Learn Disabil. 1998;31:533–544. doi: 10.1177/002221949803100603. [DOI] [PubMed] [Google Scholar]
- Lovinger DM. Neurotransmitter roles in synaptic modulation, plasticity and learning in the dorsal striatum. Neuropharmacology. 2010;58:951–961. doi: 10.1016/j.neuropharm.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco EM, Adriani W, Ruocco LA, Canese R, Sadile AG, Lavio-la G. Neurobehavioral adaptations to methylphenidate: the issue of early adolescent exposure. Neurosci Biobehav R. 2011;35:1722–1739. doi: 10.1016/j.neubiorev.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Mathews TA, John CE, Lapa GB, Budygin EA, Jones SR. No role of the dopamine transporter in acute ethanol effects on striatal dopamine dynamics. Synapse. 2006;60:288–294. doi: 10.1002/syn.20301. [DOI] [PubMed] [Google Scholar]
- McCabe SE, Knight JR, Teter CJ, Wechsler H. Non-medical use of prescription stimulants among US college students: prevalence and correlates from a national survey. Addiction. 2005;100:96–106. doi: 10.1111/j.1360-0443.2005.00944.x. [DOI] [PubMed] [Google Scholar]
- Moll GH, Hause S, Ruther E, Rothenberger A, Huether G. Early methylphenidate administration to young rats causes a persistent reduction in the density of striatal dopamine transporters. J Child Adol Psychop. 2001;11:15–24. doi: 10.1089/104454601750143366. [DOI] [PubMed] [Google Scholar]
- Nieoullon A. Dopamine and the regulation of cognition and attention. Prog Neurobiol. 2002;67:53–83. doi: 10.1016/s0301-0082(02)00011-4. [DOI] [PubMed] [Google Scholar]
- Paillé V, Picconi B, Bagetta V, Ghiglieri V, Sgobio C, Di Filippo M, Viscomi MT, Giampà C, Fusco FR, Gardoni F, Bernardi G, Green-gard P, Di Luca M, Calabresi P. Distinct levels of dopamine denervation differentially alter striatal synaptic plasticity and NMDA receptor subunit composition. J Neurosci. 2010;30:14182–14193. doi: 10.1523/JNEUROSCI.2149-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge JG, Tang KC, Lovinger DM. Regional and postnatal heterogeneity of activity-dependent long-term changes in synaptic efficacy in the dorsal striatum. J Neurophysiol. 2000;84:1422–1429. doi: 10.1152/jn.2000.84.3.1422. [DOI] [PubMed] [Google Scholar]
- Partridge JG, Apparsundaram S, Gerhardt GA, Ronesi J, Lovinger DM. Nicotinic acetylcholine receptors interact with dopamine in induction of striatal long-term depression. J Neurosci. 2002;22:2541–2549. doi: 10.1523/JNEUROSCI.22-07-02541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick KS, Straughn AB, Minhinnett RR, Yeatts SD, Herrin AE, DeVane CL, Malcolm R, Janis GC, Markowitz JS. Influence of ethanol and gender on methylphenidate pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2007;81:346–353. doi: 10.1038/sj.clpt.6100082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picconi B, Pisani A, Barone I, Bonsi P, Centonze D, Bernardi G, Calabresi P. Pathological synaptic plasticity in the striatum: implications for Parkinson’s disease. Neurotoxicology. 2005;26:779–783. doi: 10.1016/j.neuro.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Roberts L, Greene JR. Hyperpolarization-activated current (Ih): a characterization of subicular neurons in brain slices from socially and individually housed rats. Brain Res. 2005;1040:1–13. doi: 10.1016/j.brainres.2004.11.065. [DOI] [PubMed] [Google Scholar]
- Sadasivan S, Pond BB, Pani AK, Qu C, Jiao Y, Smeyne RJ. Methylphenidate exposure induces dopamine neuron loss and activation of microglia in the basal ganglia of mice. PLoS One. 2012;7:e33693. doi: 10.1371/journal.pone.0033693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepúlveda DR, Thomas LM, McCabe SE, Cranford JA, Boyd CJ, Teter CJ. Misuse of prescribed stimulant medication for ADHD and associated patterns of substance use: preliminary analysis among college students. J Pharm Pract. 2011;24:551–560. doi: 10.1177/0897190011426558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto PL, Wilcox KM, Zhou Y, Kumar A, Ator NA, Riddle MA, Wong DF, Weed MR. Long-term exposure to oral methylphenidate or DL-amphetamine mixture in peri-adolescent Rhesus monkeys: effects on physiology, behavior, and dopamine system development. Neuropsychopharmacol. 2012;37:2566–2579. doi: 10.1038/npp.2012.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear L. Modeling adolescent development and alcohol use in animals. Alcohol Res Health. 2000;24:115–123. [PMC free article] [PubMed] [Google Scholar]
- Swanson JM, Volkow ND. Pharmacokinetic and pharmacodynamic properties of stimulants: implications for the design of new treatments for ADHD. Behav Brain Res. 2002;130:73–78. doi: 10.1016/s0166-4328(01)00433-8. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Michaelides M, Benveniste H, Wang GJ, Volkow ND. Effects of chronic oral methylphenidate on cocaine self-administration and striatal dopamine D2 receptors in rodents. Pharmacol Biochem Be. 2007;87:426–433. doi: 10.1016/j.pbb.2007.05.020. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Insel TR. What are the long-term effects of methylphenidate treatment? Biol Psychiat. 2003;54:1307–1309. doi: 10.1016/j.biopsych.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Ding YS, Fowler JS, Wang GJ, Logan J, Gatley JS, Dewey S, Ashby C, Liebermann J, Hitzemann R, Wolf AP. Is methylphenidate like cocaine? Studies on their pharmacokinetics and distribution in the human brain. Arch Gen Psychiat. 1995;52:456–463. doi: 10.1001/archpsyc.1995.03950180042006. [DOI] [PubMed] [Google Scholar]
- Wang J, Carnicella S, Phamluong K, Jeanblanc J, Ronesi JA, Chaudhri N, Janak PH, Lovinger DM, Ron D. Ethanol induces long-term facilitation of NR2B-NMDA receptor activity in the dorsal striatum: implications for alcohol drinking behavior. J Neurosci. 2007;27:3593–3602. doi: 10.1523/JNEUROSCI.4749-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayment HK, Deutsch H, Schweri MM, Schenk JO. Effects of methylphenidate analogues on phenethylamine substrates for the striatal dopamine transporter: potential as amphetamine antagonists? J Neurochem. 1999;72:1266–1274. doi: 10.1046/j.1471-4159.1999.0721266.x. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Faraone SV, Biederman J, Gunawardene S. Does stimulant therapy of attention-deficit/hyperactivity disorder beget later substance abuse? A meta-analytic review of the literature. Pediatrics. 2003;111:179–185. doi: 10.1542/peds.111.1.179. [DOI] [PubMed] [Google Scholar]
- Yano M, Steiner H. Methylphenidate and cocaine: the same effects on gene regulation? Trends Pharmacol Sci. 2007;28:588–596. doi: 10.1016/j.tips.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Yin HH, Park BS, Adermark L, Lovinger DM. Ethanol reverses the direction of long-term synaptic plasticity in the dorsomedial striatum. Eur J Neurosci. 2007;25:3226–3232. doi: 10.1111/j.1460-9568.2007.05606.x. [DOI] [PubMed] [Google Scholar]
- Yorgason JT, España RA, Jones SR. Demon voltammetry and analysis software: analysis of cocaine-induced alterations in dopamine signaling using multiple kinetic measures. J Neurosci Meth. 2011;202:158–164. doi: 10.1016/j.jneumeth.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorgason JT, España RA, Konstantopoulos JK, Weiner JL, Jones SR. Enduring increases in anxiety-like behavior and rapid nucleus accumbens dopamine signaling in socially isolated rats. Eur J Neurosci. 2013;37:1022–1031. doi: 10.1111/ejn.12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeb FD, Wong AC, Winstanley CA. Differential effects of environmental enrichment, social-housing, and isolation-rearing on a rat gambling task: dissociations between impulsive action and risky decision-making. Psychopharmacology. 2012;225:381–395. doi: 10.1007/s00213-012-2822-x. [DOI] [PubMed] [Google Scholar]