Obesity is increasingly recognized as a major threat to individual and public health. Unfortunately, it is very difficult to achieve sustained weight loss with current medical approaches. Similarly, optimal treatment of obesity-related comorbidities, including type 2 diabetes, hypertension, nonalcoholic fatty liver disease, and cardiovascular disease remains an elusive goal for the majority of patients. Given these critical unmet needs, both clinicians and patients alike have been buoyed by the results of recent, controlled, clinical trials demonstrating potent effects of bariatric operative procedures to induce sustained weight loss and improve or normalize obesity-related comorbidities, including type 2 diabetes.1–3 Perhaps even more compelling are the 83% reduction in incident diabetes, 30%–40% reduction in myocardial infarction and stroke, 42% reduction in cancer incidence in women, and 30%–40% reduction in overall mortality observed in nonrandomized but controlled studies.1, 4 As with any approach, clinicians need to carefully balance metabolic benefits against both short- and long-term complications of surgery. When surgery is performed at centers of excellence, these benefits are achieved with low operative mortality.1 However, longer term intestinal and nutritional complications can occur, and vary according to the specific procedure. One particularly challenging and sometimes severe complication of roux-en-Y gastric bypass surgery is postprandial hyperinsulinemic hypoglycemia.5, 6 Although it is likely that multiple mechanisms contribute to post-bypass hypoglycemia, the studies of Salehi et al7 reported in this issue of Gastroenterology provide firm evidence for the role of the incretin hormone glucagon-like peptide-1 (GLP-1) as a critical contributor to the inappropriate insulin secretion in this syndrome.
The clinical features of hypoglycemia in patients who have undergone gastric bypass surgery typically emerge gradually over time and are often relatively nonspecific. Thus, recognition of hypoglycemia in post-bypass patients is often delayed. Hypoglycemic symptoms can be broadly classified as autonomic (eg, palpitations, lightheadedness, sweating) or neuroglycopenic (eg, confusion, decreased attentiveness, seizure, loss of consciousness). Symptoms occur for most patients within 1–3 hours after meals, particularly meals rich in simple carbohydrates. Early in the postoperative period hypoglycemia is usually mild, often associated with dumping syndrome, and effectively treated with low glycemic index diets. More severe hypoglycemia associated with neuroglycopenia, loss of consciousness, seizures, and motor vehicle accidents, is rare but typically occurs 1–3 years after gastric bypass. Although prevalence remains uncertain owing to incomplete recognition, documented hypoglycemia occurs in only 0.2% and related diagnoses in about 1% of bypass patients.8 To confirm that symptoms are related to hypoglycemia, venous blood sampling should demonstrate glucose values <70 mg/dL (3.9 mmol/L), and symptoms must resolve quickly with glucose ingestion. Furthermore, plasma insulin concentrations are inappropriately high at the time of hypoglycemia, indicating dysregulation of insulin secretion as an important mechanism. Fasting hypoglycemia is not common with post-bypass hypoglycemia; if this pattern is present, alternative diagnostic strategies need to be considered to exclude autonomous insulin secretion (eg, insulinoma).9
First-line therapeutic approaches to post-bypass hypoglycemia include medical nutrition therapy aimed at reducing intake of high glycemic index carbohydrates,10 and pre-meal treatment with acarbose.11 Both approaches minimize rapid postprandial surges in glucose, which then trigger glucose-dependent insulin secretion. Continuous glucose monitoring can be helpful to improve patient safety, particularly for those with hypoglycemic unawareness.12 Additional therapies that may be considered include octreotide (to reduce incretin and insulin secretion),13 diazoxide (to reduce insulin secretion),14 calcium channel blockade (to reduce insulin secretion),15 gastric restriction or banding (to slow gastric emptying),16 and providing nutrition solely through a gastrostomy tube placed into the bypassed duodenum.17 Surprisingly, reversal of gastric bypass is not uniformly successful,6, 18 suggesting the importance of underlying genetics and/or compensatory mechanisms that persist after surgical reversal. Finally, although pancreatic resection was initially employed for patients with life-threatening hypoglycemia,5, 6 this procedure is not uniformly successful in remitting hypoglycemia and should not be considered for the majority of patients, who can improve frequency and severity of hypoglycemia with medical approaches, often in combination.
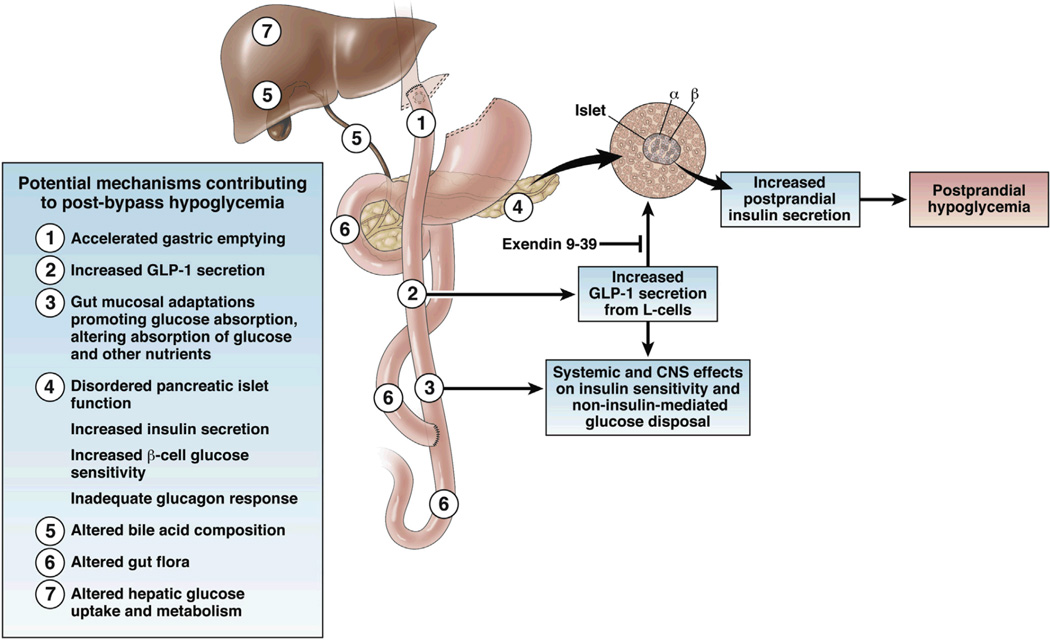
The etiology of post-bypass hyperinsulinemic hypoglycemia remains incompletely understood, but likely arises from the profound alterations in glycemic and hormonal patterns in the postprandial state occurring with gastric bypass anatomy and profound weight loss (Figure 1). Food intake and rapid emptying of the gastric pouch triggers a brisk and excessive rise in glucose and parallel increases in insulin secretion, with subsequent rapid decline in glucose levels. Although initial reports suggested that pancreatic islet hypertrophy might play a major role, pancreatic resection does not provide cure of hypoglycemia,6, 18 and excessive islet number has not been consistently observed in the few pathologic specimens available for examination. 5, 6, 19 Thus, hyperinsulinemic hypoglycemia may be owing to dysregulation of islet function rather than solely an increase in mass. One candidate mediator of increased insulin secretion in post-bypass hypoglycemia is GLP-1, a peptide released from intestinal neuroendocrine L-cells in response to meals. GLP-1 binds to specific receptors on b-cells, stimulating insulin secretion in a glucose-dependent manner. Consistent with this hypothesis, postprandial GLP-1 levels are increased by >10-fold in post-bypass patients, are higher in those with hyperinsulinemic hypoglycemia and neuroglycopenia, and correlate inversely with postprandial glucose levels.20, 21 Furthermore, pharmacologic blockade of the GLP-1 receptor markedly attenuates insulin secretion and b-cell glucose sensitivity in post-bypass individuals.22
Figure 1.
Schematic of potential mechanisms contributing to post-bypass hypoglycemia. Infusion of exendin9–39 attenuates the impact of GLP-1 on insulin secretion and hypoglycemia.
Despite these provocative associations between GLP-1 and post-bypass hypoglycemia, it has previously been difficult to determine whether elevated GLP-1 concentrations are simply associated with altered intestinal anatomy post-bypass, or actually contribute to the pathophysiology of hyperinsulinemic hypoglycemia. To test the role of GLP-1 in this syndrome in humans in vivo, Salehi et al performed an elegant series of studies in controls (no prior bariatric surgery) and 2 groups of post-bypass patients: Individuals with severe recurrent hypoglycemia post-bypass, defined as neuroglycopenia with documented glucose levels <50 mg/dL (2.8 mmol/L), or asymptomatic post-bypass patients. Glycemia and insulin secretion patterns were assessed over 5 hours in response to an oral mixed meal in the presence or absence of a peptide antagonist to the GLP-1 receptor (exendin9–39). As expected, patients with a history of hypoglycemia had not only lower postprandial glucose nadir, but also higher glucose-stimulated insulin secretion during late phases of the meal tolerance test. Using the dual-tracer technique (constant intravenous infusion of [6,6-2H2] glucose, together with [U-13C]-labeled glucose in oral meal), the investigators found that hypoglycemic patients also had increased rate of appearance of meal-derived glucose compared with controls, whereas hepatic glucose production did not differ significantly between groups.
Infusion of exendin9–39 to block GLP-1 action increased both fasting and postprandial plasma glucose concentrations in all subjects, an effect mediated through reduced insulin secretion. exendin9–39 also reduced dumping syndrome symptom scores. Notably, the effects of exendin9–39 on glycemia, insulin secretion, and b-cell sensitivity to glucose were much greater for post-bypass patients with hypoglycemia than for patients without hypoglycemia. The disproportionately greater response to GLP-1 receptor blockade in hypoglycemia patients strongly supports GLP-1 as a major contributor to excessive insulin secretion and hypoglycemia in the late postprandial state in post-bypass patients with neuroglycopenia.
Several key questions about the pathophysiology of post-bypass hypoglycemia remain unanswered. Which factors are responsible for interindividual variability in apparent sensitivity to GLP-1 and the development of hypoglycemia? It is interesting that Salehi et al21 previously reported effects of GLP-1 receptor inhibition were similar in patients with hypoglycemia compared with asymptomatic post-bypass individuals. Indeed, glycemic patterns in asymptomatic patients in the present cohort do not mirror the major glycemic excursions typically observed in post-bypass patients, because the current cohort was selected from patients with no postprandial glucose of <50 mg/dL (2.8 mmol/L) to unequivocally represent individuals without hypoglycemia. Additional differences in study design may also contribute; in the former study, insulin secretion and responses to GLP-1 receptor inhibition were assessed at stable levels of hyperglycemia, whereas in the present study, patients were assessed during dynamic changes in glucose in the postprandial period. Differences between the responses to exendin9–39 in the 2 studies and the relatively small number of subjects suggest substantial interindividual variability in the relative contributions of incretin levels or responses, islet secretory function, or other metabolic factors in patients with post-bypass hypoglycemia.
Could increases in GLP-1 responsiveness also contribute to post-bypass hypoglycemia? Our group previously assessed GLP-1 receptor density in pancreatic specimens from patients with severe hypoglycemia, finding no differences compared with controls.23 However, it is possible that GLP-1 receptor-mediated signaling pathways or other modifiers of GLP-1 effects on insulin secretion and glucose disposal could differ in those individuals with hypoglycemia post-bypass.
Beyond GLP-1, additional mechanisms could contribute to the severity of post-bypass hypoglycemia. For example, individuals who are more insulin sensitive could be at higher risk for insulin-induced hypoglycemia. Conversely, disruptions in physiologic feedback loops, which typically limit severe hypoglycemia, could also increase risk; these could include inadequate secretion of glucagon and other counter regulatory hormones in response to acute hypoglycemia, inadequate glycogen stores, or reductions in gluconeogenic substrates.24 With repeated episodes of hypoglycemia, awareness may be attenuated, leading to more severe hypoglycemia. Additional gastrointestinal factors, which could modify systemic metabolism, include dietary composition, gut microbiota,25 bile acid composition,26 and intestinal adaptive responses27; these could influence absorption of glucose and other nutrients, intestinally derived hormonal responses, and the magnitude of neurologic–gut–liver regulatory loops. Finally, genetic variation could also contribute to altered hormonal responses and sensitivity, as has been demonstrated for incretins and insulin alike.28
More broadly, the results from Salehi et al provide optimism that GLP-1 receptor inhibition could ultimately provide a new therapeutic strategy for severely affected patients with hypoglycemia. However, we do not yet have data regarding the effects of long-term responses to GLP-1 receptor inhibition. It is interesting to note that exendin9–39 infusion increases GLP-1, gastric inhibitory polypeptide, and glucagon levels.22 Incomplete or intermittent inhibition, or desensitization, might exacerbate hypoglycemia. Nevertheless, efforts to develop oral or parenterally effective strategies to normalize glucose metabolism in affected individuals should be undertaken. Further studies of individuals with hypoglycemia, in whom normalization of metabolism is “extreme,” may also allow us to better understand the complexities of gut regulation of systemic metabolism and to elucidate the mechanisms by which bariatric procedures normalize the hyperglycemia of type 2 diabetes.
ACKNOWLEDGMENTS
Acknowledgements: Supported by NIH R56 DK095451 and DK036836, and support for the Joslin Clinical Research Center from its philanthropic donors.
Footnotes
The authors declare no conflicts of interest related to this manuscript.
References
- 1.Sjostrom L. Review of the key results from the Swedish Obese Subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273:219–234. doi: 10.1111/joim.12012. [DOI] [PubMed] [Google Scholar]
- 2.Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366:1567–1576. doi: 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577–1585. doi: 10.1056/NEJMoa1200111. [DOI] [PubMed] [Google Scholar]
- 4.Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357:753–761. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 5.Service GJ, Thompson GB, Service FJ, et al. Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med. 2005;353:249–254. doi: 10.1056/NEJMoa043690. [DOI] [PubMed] [Google Scholar]
- 6.Patti ME, McMahon G, Mun EC, et al. Severe hypoglycaemia post-gastric bypass requiring partial pancreatectomy: evidence for inappropriate insulin secretion and pancreatic islet hyperplasia. Diabetologia. 2005;48:2236–2240. doi: 10.1007/s00125-005-1933-x. [DOI] [PubMed] [Google Scholar]
- 7.Salehi M, Gastaldelli A, D’Alessio DA. Blockade of glucagon-like peptide 1 receptor corrects post-prandial hypoglycemia after gastric bypass. Gastroenterology. 2014;146:000–000. doi: 10.1053/j.gastro.2013.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marsk R, Jonas E, Rasmussen F, et al. Nationwide cohort study of post-gastric bypass hypoglycaemia including 5,040 patients undergoing surgery for obesity in 1986–2006 in Sweden. Diabetologia. 53:2307–2311. doi: 10.1007/s00125-010-1798-5. 010. [DOI] [PubMed] [Google Scholar]
- 9.Zagury L, Moreira RO, Guedes EP, et al. Insulinoma misdiagnosed as dumping syndrome after bariatric surgery. Obes Surg. 2004;14:120–123. doi: 10.1381/096089204772787419. [DOI] [PubMed] [Google Scholar]
- 10.Kellogg TA, Bantle JP, Leslie DB, et al. Postgastric bypass hyperinsulinemic hypoglycemia syndrome: characterization and response to a modified diet. Surg Obes Relat Dis. 2008;4:492–499. doi: 10.1016/j.soard.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Valderas JP, Ahuad J, Rubio L, et al. Acarbose improves hypoglycaemia following gastric bypass surgery without increasing glucagon-like peptide 1 levels. Obes Surg. 2012;22:582–586. doi: 10.1007/s11695-011-0581-0. [DOI] [PubMed] [Google Scholar]
- 12.Halperin F, Patti ME, Skow M, et al. Continuous glucose monitoring for evaluation of glycemic excursions after gastric bypass. J Obes. 2011;2011:869536. doi: 10.1155/2011/869536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Myint KS, Greenfield JR, Farooqi IS, et al. Prolonged successful therapy for hyperinsulinaemic hypoglycaemia after gastric bypass: the pathophysiological role of GLP1 and its response to a somatostatin analogue. Eur J Endocrinol. 2012;166:951–955. doi: 10.1530/EJE-11-1065. [DOI] [PubMed] [Google Scholar]
- 14.Spanakis E, Gragnoli C. Successful medical management of statuspost-Roux-en-Y-gastric-bypass hyperinsulinemic hypoglycemia. Obes Surg. 2009;19:1333–1334. doi: 10.1007/s11695-009-9888-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moreira RO, Moreira RB, Machado NA, et al. Postprandial hypoglycemia after bariatric surgery: pharmacological treatment with verapamil and acarbose. Obes Surg. 2008;18:1618–1621. doi: 10.1007/s11695-008-9569-9. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez-Esparrach G, Lautz DB, Thompson CC. Peroral endoscopic anastomotic reduction improves intractable dumping syndrome in Roux-en-Y gastric bypass patients. Surg Obes Relat Dis. 2010;6:36–40. doi: 10.1016/j.soard.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 17.McLaughlin T, Peck M, Holst J, et al. Reversible hyperinsulinemic hypoglycemia after gastric bypass: a consequence of altered nutrient delivery. J Clin Endocrinol Metab. 2010;95:1851–1855. doi: 10.1210/jc.2009-1628. [DOI] [PubMed] [Google Scholar]
- 18.Lee CJ, Brown T, Magnuson TH, et al. Hormonal response to a mixed-meal challenge after reversal of gastric bypass for hypoglycemia. J Clin Endocrinol Metab. 2013;98:E1208–E1212. doi: 10.1210/jc.2013-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meier JJ, Butler AE, Galasso R, et al. Hyperinsulinemic hypoglycemia after gastric bypass surgery is not accompanied by islet hyperplasia or increased {beta}-cell turnover. Diabetes Care. 2006;29:1554–1559. doi: 10.2337/dc06-0392. [DOI] [PubMed] [Google Scholar]
- 20.Goldfine AB, Mun EC, Devine E, et al. Patients with neuroglycopenia after gastric bypass surgery have exaggerated incretin and insulin secretory responses to a mixed meal. J Clin Endocrinol Metab. 2007;92:4678–4685. doi: 10.1210/jc.2007-0918. [DOI] [PubMed] [Google Scholar]
- 21.Salehi M, Prigeon RL, D’Alessio DA. Gastric bypass surgery enhances glucagon-like Peptide 1-stimulated postprandial insulin secretion in humans. Diabetes. 2011;60:2308–2314. doi: 10.2337/db11-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jorgensen NB, Dirksen C, Bojsen-Moller KN, et al. Exaggerated glucagon-like peptide 1 response is important for improved beta-cell function and glucose tolerance after Roux-en-Y gastric bypass in patients with type 2 diabetes. Diabetes. 2013;62:3044–3052. doi: 10.2337/db13-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reubi JC, Perren A, Rehmann R, et al. Glucagon-like peptide-1 (GLP-1) receptors are not overexpressed in pancreatic islets from patients with severe hyperinsulinaemic hypoglycaemia following gastric bypass. Diabetologia. 2010;53:2641–2645. doi: 10.1007/s00125-010-1901-y. [DOI] [PubMed] [Google Scholar]
- 24.Laferrere B, Reilly D, Arias S, et al. Differential metabolic impact of gastric bypass surgery versus dietary intervention in obese diabetic subjects despite identical weight loss. Sci Transl Med. 2011;3:80re2. doi: 10.1126/scitranslmed.3002043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liou AP, Paziuk M, Luevano JM, Jr, et al. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med. 2013;5:178ra41. doi: 10.1126/scitranslmed.3005687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patti ME, Houten SM, Bianco AC, et al. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity (Silver Spring) 2009;17:1671–1677. doi: 10.1038/oby.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hansen CF, Bueter M, Theis N, et al. Hypertrophy dependent doubling of L-cells in Roux-en-Y gastric bypass operated rats. PLoS ONE. 2013;8:e65696. doi: 10.1371/journal.pone.0065696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mussig K, Staiger H, Machicao F, et al. Genetic variants affecting incretin sensitivity and incretin secretion. Diabetologia. 2010;53:2289–2297. doi: 10.1007/s00125-010-1876-8. [DOI] [PubMed] [Google Scholar]